Controlled Tissue Expansion in Facial Reconstruction
History
Tissue expansion can be a natural physiologic process encountered in normal life, pregnancy and obesity being the most obvious examples. This process has been used for centuries in certain cultures to modify facial features as illustrated on the pages of National Geographic magazine. Modern use of tissue expansion was first introduced in the early 1900s. A technique was described that used progressively larger objects to expand tissue with multiple operations required.1 Because of the number of procedures required and the high morbidity, the procedure was abandoned. The practical application of tissue expansion was not possible until the introduction of plastic materials later in the century. In 1957, Neumann published “The Expansion of an Area of Skin by Progressive Distension of a Subcutaneous Balloon.” This article described the use of a latex balloon to expand the skin for reconstruction of an ear.2 He placed the balloon above the ear in a subcutaneous tissue pocket and brought a polyethylene tube out through an independent skin incision. He gradually injected air into the implant through a stopcock. His procedure was a success, but the potential impact of his work was not recognized at the time. No other work was performed on tissue expansion for almost 20 years.
In the early 1970s, Radovan and Austed independently began studying the use of tissue expansion to repair soft tissue defects. Radovan’s early expanders consisted of a balloon and two separate tubing systems for injection and removal of saline. The entire system was placed under the skin.3 His first experiments were performed in 1976 and were met with skepticism when his results were presented to the medical community later that year. This was most likely because of his unscientific methodology. He was not discouraged by the skepticism and continued his clinical trials. The results of Radovan’s experiments met wider acceptance 3 years later accompanied by Austed’s work using an osmotically driven, self-inflating expander.4 Austed, unaware of Neumann’s and Radovan’s work, first suggested the idea of implanting a device beneath the skin to induce tissue expansion in 1975. His work established the basis for understanding of the tissue response to expansion. His early laboratory experience and histologic studies confirmed that tissue expansion is safe and effective.5
Physical Properties and Morphologic Changes
It is important for surgeons performing tissue expansion to understand the physiologic and morphologic changes associated with the process. This knowledge will help the surgeon understand the potential benefits, limitations, and complications of this procedure. When the technique of tissue expansion was first introduced, the unanswered question was whether skin is stretched or new skin is created. If skin is simply stretched, a permanent gain in terms of surface area would not occur. Gibson6 studied the properties of skin and described its inherent extensibility. He noted that skin could stretch beyond its inherent extensibility and called this mechanical creep. The beneficial effects of mechanical creep are attributed to displacement of interstitial fluids and mucopolysaccharide ground substance, parallel alignment of randomly oriented collagen fibers, and migration of tissue into the field by the stretching force.
He referred to biologic creep as the gradual stretching of skin overlying a slowly expanding subcutaneous structure. This is not simple stretching because the skin is affected by metabolic activity, including the creation of epithelium, blood vessels, nerves, lymphatics, collagen, and elastin fibers. All of this occurs during sustained prolonged expansion of the skin.
The first experimental studies of skin expansion were performed by Austed et al5 and Pasyk et al7 using guinea pigs. They used self-inflating expanders, causing gradual uninterrupted expansion. Unlike humans, guinea pigs have loose skin, allowing it to move freely over the muscle beneath and to be picked up in large folds. These qualities make the guinea pig model less than ideal for understanding the effects of expansion on human skin. However, even with these limitations, their studies provided valuable information about tissue expansion. Because pig skin lacks a well-developed panniculus carnosus and has elastic tissue comparable with that of humans, it has been used in place of guinea pig skin for subsequent laboratory studies of skin expansion.
Epidermis
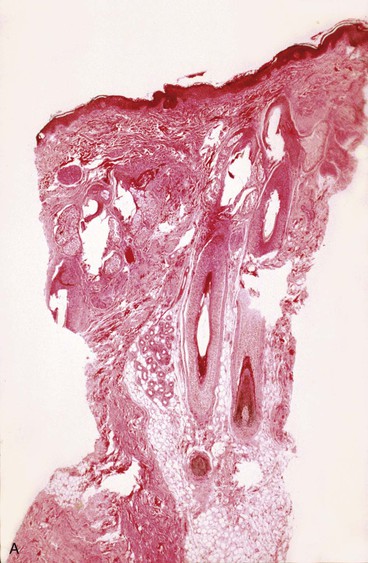
The epidermis is composed of stratified squamous epithelium of the keratinized type. The epidermis shows no thinning during or after expansion. In fact, most studies have noted an increase in thickness during skin expansion. The active basal layer of the epidermis increases in density and thickness. The basal layer normally composes 10% of the thickness of the epidermis but during expansion increases to 40%. van Rappard et al8 described the histologic changes found in expanded pig skin. They observed that during skin expansion, epidermal thickness increases; however, there is no histologic change in the layers of the epidermis. Other studies have also demonstrated that epidermal thickness increases or remains unchanged during expansion in spite of an obvious increase in surface area (Fig. 25-1). These findings would indicate that the epidermis is not merely being stretched. Using tritiated thymidine labeling, Austed et al9 demonstrated an increase in the cellular mitotic rate in the stratum basale of expanded skin and showed a net gain in donor tissue. van Rappard et al10 confirmed these findings in the pig model. They demonstrated that the number of epidermal cells for a given surface area remained the same in expanded and nonexpanded skin. Initial stretching of the epidermis from inflation of an expander increases the intercellular spaces, which in turn probably signals the cells to increase mitotic activity and restore the epidermis to a normal cellular density and thickness. The increase in mitotic activity caused by tissue expansion eventually returns to normal but remains active for as long as 2 months after expansion is discontinued.
Dermis
The dermis, which is a sheet of connective tissue underlying and supporting the epidermis, is composed of the papillary layer and the reticular layer. The papillary layer contains finer collagen fibers than the reticular layer and contains numerous elastic fibers. The reticular layer is composed of dense, irregular collagen fibers. The reticular layer is responsible for the lines of tension. The dermis does not tolerate expansion as well as the epidermis. During tissue expansion, a rapid decrease in dermal thickness is observed and averages 20%.11 Total restoration of dermal thickness after skin expansion has not been demonstrated. The amount of thinning depends on the rate of expansion. Greater thinning is noted with more rapid expansion. The expanded dermis contains large bundles of collagen fibers and active fibroblasts. An increase in collagen synthesis occurs in the dermis during tissue expansion.5 Normal collagen fibers contain bundles cross-linked at angles of 70°. With expansion, these angles gradually decrease until the collagen bundles are almost parallel to the skin surface. This effect is noted for 6 months after cessation of the expansion process.8 Melanin production increases during skin expansion and can cause a temporary hyperpigmentation of the skin. This disappears, in most cases, within a few months. Olenius et al12 described an increase in the amount of melanin pigment granules in the basal layers of expanded human skin.
Subcutaneous Tissue
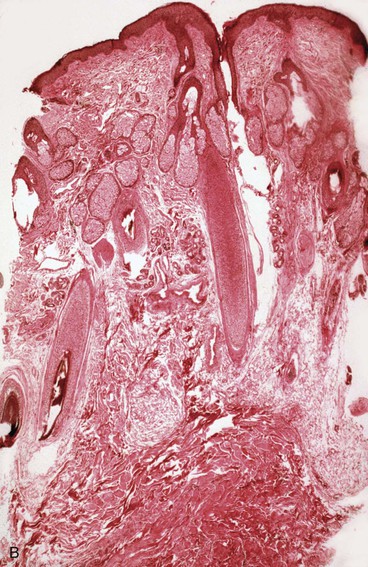
The subcutaneous tissue is composed of fat, loose connective tissue, collagen, arteries, veins, nerves, lymphatics, hair follicles, and sebaceous glands. The thickness of the subcutaneous layer is diminished as much as 50% during prolonged expansion. However, Pasyk et al11 noted the return to greater than normal thickness of the subcutaneous tissue within 2 years after removal of expanders in a series of three patients. During skin expansion, there is a marked decrease in the number of fat cells within the subcutaneous tissue, and those that remain have less lipid content (Fig. 25-2).11 There is also an increase in the interlobular spaces and an increase in the collagen content within these spaces. A varying amount of fat necrosis is also noted. The more rapid the skin expansion, the greater is the fat necrosis. Restoration of normal-appearing fat cells after skin expansion is not observed for as long as 12 months after expansion.8
Skeletal Muscle
Skeletal muscle is sensitive to tissue expansion. Ultrastructural changes of expanded muscle occur. An increase in the number and size of mitochondria, number of vesicles, and amount of sarcoplasm is observed. The number of muscle cells remains unchanged, but significant thinning of muscle occurs during tissue expansion.8 The striated pattern of muscle cells decreases, and some cells may become necrotic. Hyalinization of muscle cells is also observed and may be followed by calcification in some cases. The significance of these changes is not fully understood, but expansion may lead to a decrease in muscle function and strength in the region of tissue expansion. Sasaki13 has reported visible muscle atrophy and weakness after tissue expansion, but permanent sequelae are rare.
Blood Vessels
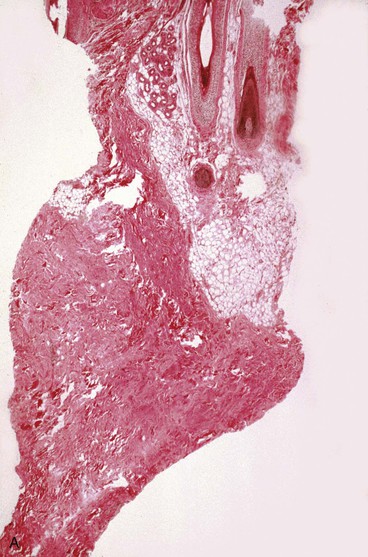
Blood vessels undergo some of the most important changes during tissue expansion. Tissue expansion stimulates the proliferation of blood vessels, creating highly vascular tissue (Fig. 25-3). Distention of capillaries is first noted during tissue expansion, followed by an increase in the number of venules and arterioles within a few days. Barium angiography demonstrates a dense vascular pattern in expanded tissue.14 Stark et al15 demonstrated a rapid elongation of arteries and veins with no loss of vessel wall diameter or intimal integrity. They also found no difference in tolerance to tissue expansion between arteries and veins. Studies of flap survival, which is dependent on skin vascularity, reveal that flaps raised from expanded tissue have survival rates comparable to those of delayed flaps. Cherry et al14 demonstrated that flaps raised from expanded tissue have a 117% increase in survival length compared with random pattern flaps raised in nonexpanded skin. Other authors have described similar findings.16,17 In essence, expanded skin has characteristics similar to delayed flaps. These studies indicate that tissue expansion enhances the vascularity of skin and improves flap survival.
Nerves
Nerve tissue appears to tolerate expansion well without demyelination or necrosis of nerve tissue. There are no clinical reports of nerve deficits attributed to tissue expansion. The potential benefit of nerve expansion for nerve grafting or transposition is promising. Elongation of peripheral nerves by use of an expander has been successful. Manders et al18 described their experience with expansion of peripheral nerves and made some important observations. They monitored the intraluminal pressure of expanders while measuring nerve response to expansion with electroneuromyography. They observed no neurologic changes in response to expansion if the intraluminal pressure of expanders remained below 40 mm Hg. If higher pressures were used, reduction in axon potential was immediately noted. They recommend monitoring of intraluminal pressure of all expanders used in patients undergoing expansion of peripheral nerves.
Capsule
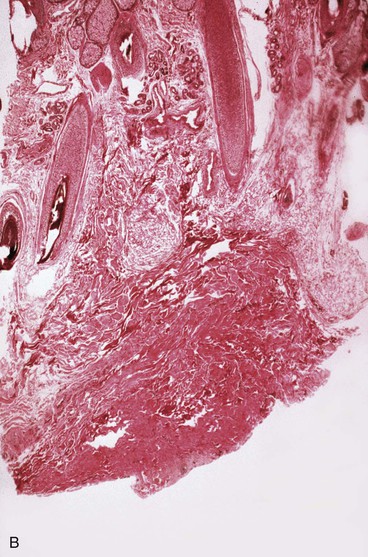
A dense fibrous capsule develops around tissue expanders (Fig. 25-4). The capsule is composed of elongated fibroblasts that are active in collagen production.5 Granulation tissue consisting of macrophages, fibroblasts, and lymphocytes is first observed, followed by development of a double-layered capsule within 7 days after an expander is implanted. The inner layer consists of macrophages and the outer layer eventually becomes rich in collagen fibers. Histologic remnants of the capsule can be found up to 1 year after removal of an expander, but grossly the capsule disappears within a few weeks after removal of an expander. The contribution the capsule makes to the blood supply of expanded skin is not fully understood, but in flaps in which capsulectomies have been performed, there appears to be no difference in flap survival compared with those with intact capsules.19 Myofibroblasts are also present in the capsule, and some believe that leaving the capsule intact may add to stretchback. The significance and function of the myofibroblasts are not fully understood.
Tissue Expansion Devices
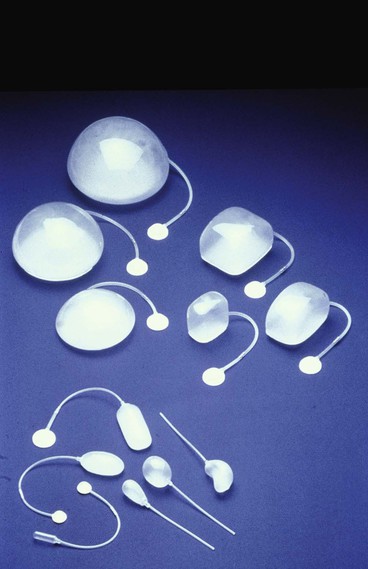
Because nearly every part of the body has been considered for tissue expansion, a wide variety of expander styles, sizes, and shapes have been introduced (Fig. 25-5). Custom-made expanders can also be used for irregular defects or unusual areas. The standard Radovan expander is composed of a Silastic reservoir, a self-sealing injection port, and connecting tubing. This design remains the most commonly used system. Expanders with self-contained injection ports are useful in some situations, but the injection port may be more difficult to identify, leading to a greater risk of damage to the expander during inflation. The necessary multiple percutaneous injections may also traumatize the expanding skin, leading to a greater risk of complications.
A directional expander has been developed for breast reconstruction. Becker20 developed a permanent expander prosthesis for the same purpose. Manders and Friedman21 introduced a differential expander for the treatment of male pattern baldness. Despite many manufacturers’ modifications, the high technical standards for the devices have persisted. Their durability has been thoroughly tested, and their safety is well established.
Hallock22,23 was the first to study durability of expanders in a scientific way. He demonstrated in vitro that expanders can be overinflated at least 15 times the amount recommended by the manufacturer before rupture occurs. From in vivo studies, he concluded that expanders in the body could be safely overinflated to at least twice the amount recommended by the manufacturer without risk of rupture. However, the greater the pressure within the expander, the greater is the risk for leakage from the injection port. Nordstrom found that the average pressure necessary for leakage of the injection port is 32 mm Hg. This may explain reports of volume loss of saline injected into some expanders. To avoid such leakage, it is recommended to use the largest expander that can reasonably be inserted beneath the planned expansion area.
Expander Selection
When the shape of an expander is selected, the area to be reconstructed should be considered. For breast reconstruction, a round, pear-shaped, or directional expander is most helpful. For covering a large defect on the head or neck area, a rectangular expander is preferred. A crescent-shaped expander has been advocated for reconstruction of round defects. van Rappard et al24 compared the three most commonly used expander shapes (i.e., rectangular, round, and crescent) for their effectiveness. By mathematic calculations, rectangular expanders provided the most effective gain of surface area in expanded skin. In clinical situations, the gain in surface area compared with the area of the expander base is 38% for rectangular expanders, 32% for crescent-shaped expanders, and 25% for round expanders.24 Compared with conventionally shaped expanders, Brobmann and Huber25 found that less expansion is required to gain sufficient skin for repair when custom-made expanders are used for reconstruction of irregularly shaped defects.
In determining the appropriate size of an expander, Radovan26 initially suggested that the expander base be the same size as the defect to be reconstructed. It was thought that the surface area of expanded skin would be twice the area of the base of the expander used. However, with clinical experience, van Rappard demonstrated that only a fraction of the expected surface area is actually gained.24 Gibney27 recommended that the expander base be 2.3 to 3 times the surface area of the defect to be closed. This ratio of base size to expected increase in skin surface area is the generally accepted guideline and has been supported by van Rappard et al.24
In addition to the size of an expander, there are other factors that influence the amount of skin gained from expansion. These factors, described by Gibson6 and Sugihara et al,28 include the amount of undermining performed and the inherent extensibility of the skin. The response of skin to expansion differs according to the area of the body expanded and between individuals.
Technique
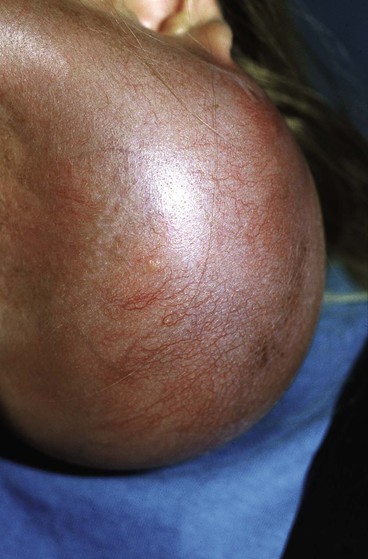
It is important to create a sufficiently large subcutaneous tissue pocket to accommodate the implant without causing folds in the expander. The ridges made by folds may compromise the overlying tissue by creating focal points of increased pressure, leading to implant extrusion (Fig. 25-6). If these folds are noticed during inflation, it is recommended to gently manipulate them to dissipate the folds. If the area for expansion cannot accommodate the ideal-sized expander, a smaller expander can be used and overinflated to compensate for the smaller size.
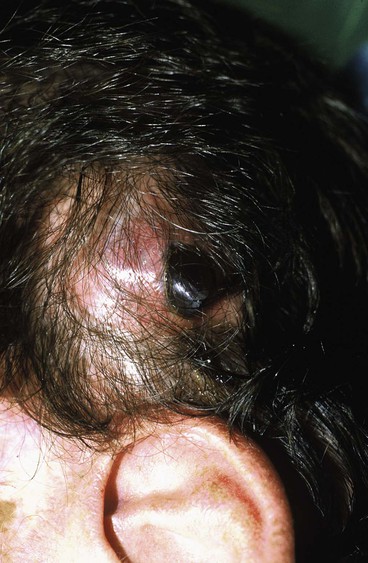
FIGURE 25-6 Exposure of tissue expander resulting from fold in expander, causing internal pressure point.
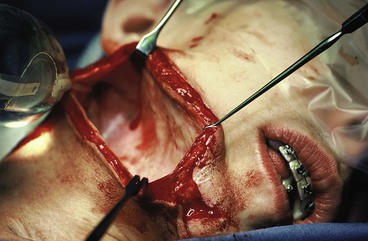
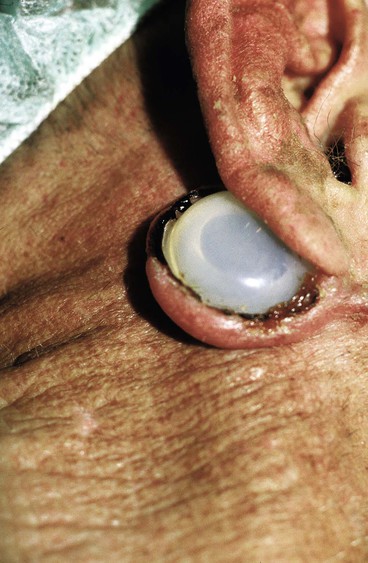
The injection port of the tissue expander is placed through the same incision used for insertion of the expander. A separate subcutaneous tissue pocket several centimeters removed from the pocket created for the expander is developed to accommodate the port. Placement should be sufficiently removed from the expander so that the port can be easily palpated and accessible for saline injections. Placement must be such that inflation of the expander will not result in the expander’s covering the port, preventing further injections. The port should be placed in an area where the overlying tissue has sufficient thickness to avoid breakdown from repeated injections. The postauricular skin area over the mastoid should be avoided. The skin in this area is thin, and there is a high incidence of tissue breakdown and extrusion when the port is placed beneath this skin (Fig. 25-7).
The access incision for implanting a tissue expander is closed in layers with a strong permanent suture for the deep wound closure. This assists with counteracting wound closure tension on the incision line during tissue expansion. After closure of the incision, the expander may be injected with saline to obliterate the remaining dead space in the subcutaneous pocket. The volume injected should not create tension on the suture line. To allow sufficient healing, inflation of the expander is commenced 2 weeks after implantation. Injections of isotonic saline are performed every 1 to 7 days, depending on the volume injected and the response of the tissue. Too rapid inflation may thin the tissue and potentially compromise the final reconstruction.8 Because the goal of expansion is to create additional skin, sufficient time is required for proper tissue response.
The second surgical stage is performed when sufficient additional skin has been created. Expanded skin appears to act like a delayed flap, so use of epinephrine should be avoided in removing expanders and proceeding with reconstruction. This recommendation is based on studies by Reinisch and Myers,29 who studied the effect of epinephrine on delayed and nondelayed random pattern flaps. The authors found that when 1 : 200,000 concentration of epinephrine was used in dissecting a flap, the surviving length of nondelayed flaps was unaffected, whereas that of delayed flaps was significantly reduced.
Scalp Expansion
The scalp is unique because of its density and the presence of hair. Defects of the scalp secondary to burns, those due to avulsion-type injuries (Fig. 25-8), and those resulting from cancer or tumor removal have often been difficult to correct. The scalp is well suited for expansion because of its vascularity and thickness. The abundant vascularity of the scalp allows expedient healing and a low rate of complications from expansion. Large defects of the scalp have traditionally been reconstructed with skin grafts or flaps with little or no hair-bearing skin. Although this provides coverage of the area, it results in an unacceptable aesthetic appearance (Fig. 25-9). Tissue expansion has enabled the correction of such defects with hair-bearing skin and has revolutionized scalp reconstruction, offering results that were previously impossible to achieve (Figs. 25-10 and 25-11). Defects or deformities of up to half of the surface area of the scalp can be reconstructed with expansion of the remaining scalp. Serial expansion is sometimes necessary. In such instances, the expander is immediately replaced in a subgaleal pocket and the expansion process continued after an appropriate time for the wounds resulting from the most recent procedure to heal.
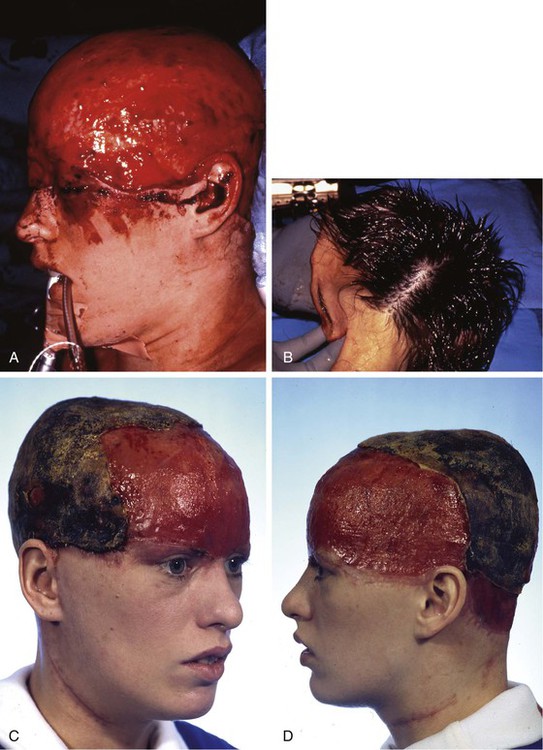
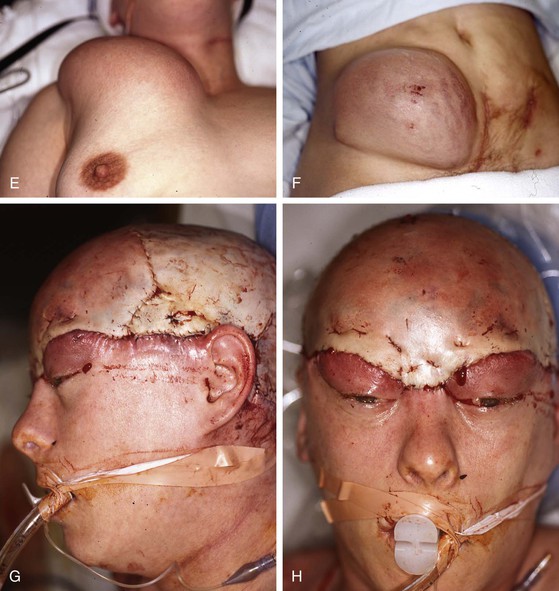
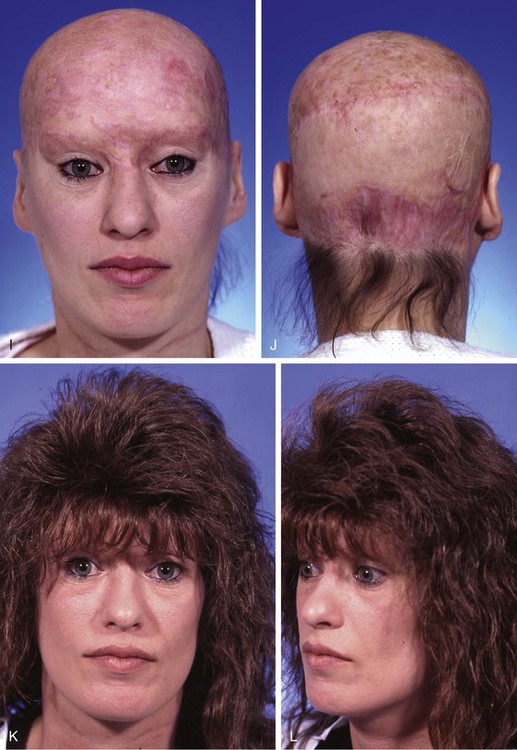
FIGURE 25-8 A, Complete avulsion of scalp and forehead skin. B, Avulsed scalp was replaced as full-thickness graft over pericranium after removal of hair, galea, and subcutaneous fat. C, D, Entire scalp graft dies, leaving eschar. Eschar débrided anteriorly. E, F, Supraclavicular fossa and groin skin expanded to provide source for harvesting of full-thickness skin graft used to cover cranium. G, H, Intraoperative view immediately after covering of cranium with full-thickness skin graft. I, J, Healed skin graft provided stable covering over cranium to enable patient to wear wig. K, L, Patient fitted with wig. (Courtesy of Shan R. Baker, MD.)
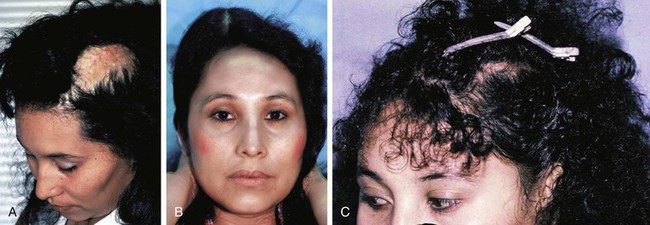
FIGURE 25-9 A, Scalp scarring resulting from burn. Multiple surgeries had previously been attempted to remove burn scar. Area was scarred tightly to skull with no mobility of tissue. B, A 250-mL rectangular expander used to create additional hair-bearing scalp. Patient was able to work full-time during expansion because expander was camouflaged by hairstyle. C, Six months after removal of expander, resection of scar and advancement of expanded scalp to close defect.
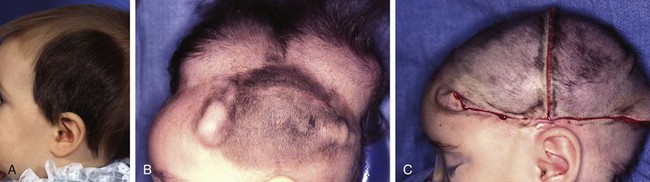
FIGURE 25-10 A, Giant pigmented hairy scalp nevus in a 17-month-old child. B, Two 250-mL rectangular tissue expanders used to expand contralateral scalp in anticipation of excision of nevus. C, Nevus completely excised. Resulting defect repaired with expanded scalp advancement flaps. (Courtesy of Shan R. Baker, MD.)
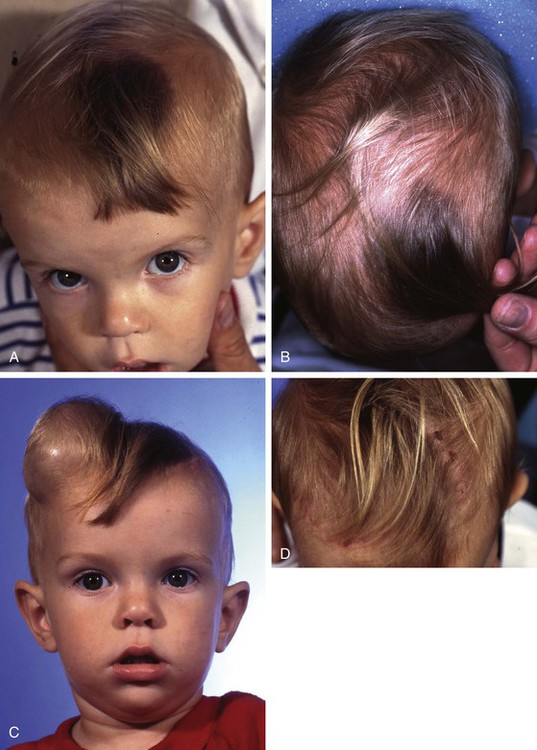
FIGURE 25-11 A, B, Giant pigmented hairy scalp nevus in a 20-month-old child. C, Contralateral scalp expanded with 250-mL rectangular tissue expander. D, Two weeks after complete excision of nevus and repair with expanded scalp advancement flap. (Courtesy of Shan R. Baker, MD.)
Kabaker et al30 described the use of expanders for the treatment of male pattern baldness. They described a technique for implanting expanders and design of expanded flaps that created a double Y-shaped wound closure (Fig. 25-12). This procedure can offer dramatic results (Figs. 25-13 and 25-14). Other authors have also described their successful results with this technique, but proper patient selection is imperative.31 A minimum of 6 weeks to 2 months of scalp expansion is required before the transfer of expanded advancement flaps. The patient must understand the interval required for use of this method and the physical appearance during the expansion process.
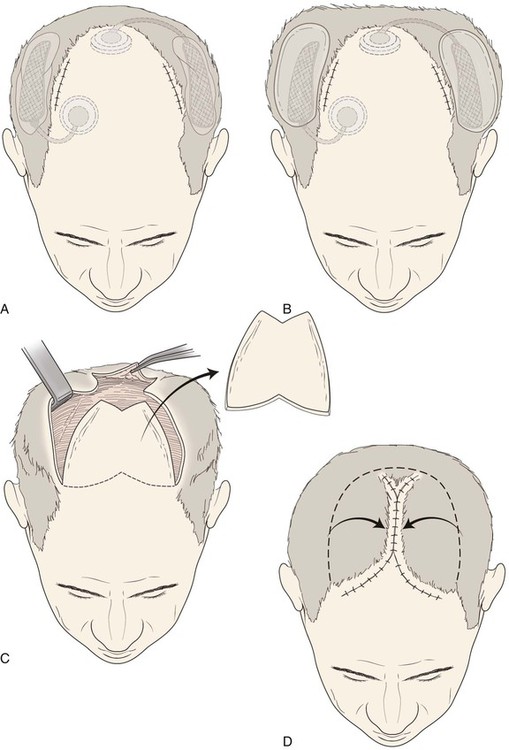
FIGURE 25-12 A, Expanders implanted beneath hair-bearing parietal scalp to treat male pattern baldness. Access incisions are at border of balding areas. B, Injection ports are positioned in accessible areas. C, After scalp expansion, expanders removed and flaps undermined. D, Bald scalp removed and expanded advancement flaps used to close wound.
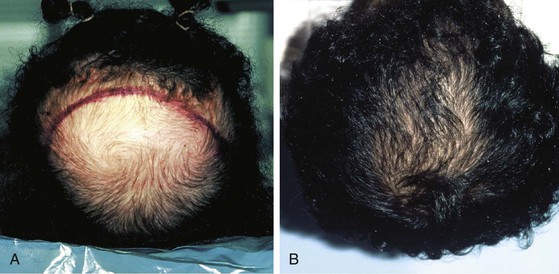
FIGURE 25-13 A, Preoperative photograph of patient with baldness of crown. B, One year after excision of bald scalp and repair with expanded advancement scalp flap created with 1000-mL horseshoe-shaped tissue expander.
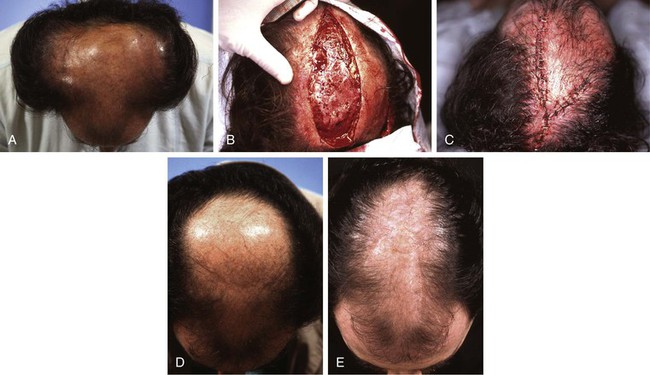
FIGURE 25-14 A, Male pattern central baldness. Peripheral hair-bearing scalp expanded with bilateral 250-mL rectangular tissue expanders. B, Expanders removed and large central area of baldness excised. C, Resulting defect repaired with bilateral expanded scalp advancement flaps. D, E, Preoperative and postoperative views. (Courtesy of Shan R. Baker, MD.)
Forehead Expansion
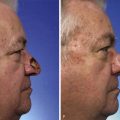
Forehead skin can tolerate expansion well because of its thickness and vascularity. However, expansion of the forehead usually produces more pain than expansion of other areas of the head and neck. This limits the rate at which expansion can be performed. Expansion of the forehead and scalp often creates a depression or “bathtub” deformity in the underlying soft tissue and bone because of pressure from the expander and the effect of the capsule. Resorption of bone with thinning of the outer layer of the calvarium is sometimes observed, creating a depression after removal of an expander. This deformity resolves with time (Fig. 25-15).
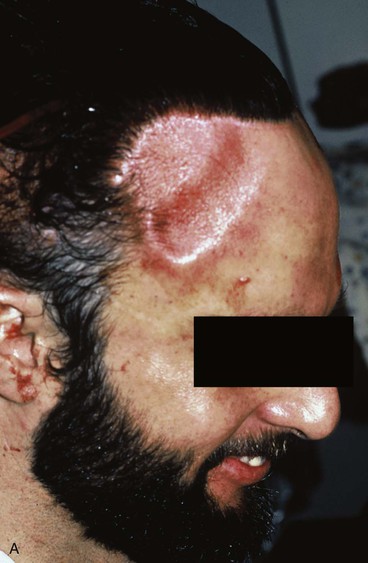
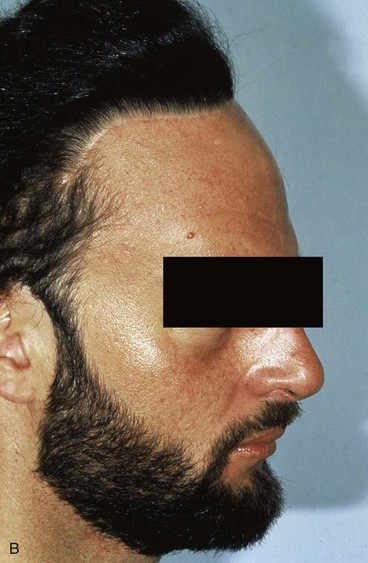
FIGURE 25-15 A, Immediately after removal of tissue expander demonstrating “bathtub” deformity. B, Same patient 4 months later showing complete resolution of deformity.
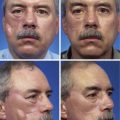
Figure 25-16 shows a patient who was reconstructed with use of tissue expansion in multiple surgical stages for excision of a skin graft on the lateral forehead, temple, and superior cheek. The skin graft was deforming and had a poor color match with adjacent facial skin. The patient was also troubled with breakdown of the graft, resulting in recurrent ulcerations and poor tissue healing. Initially, two 250-mL rectangular expanders were implanted, one beneath the skin of the forehead and one beneath the parietal scalp. During the expansion process, the expander under the scalp became exposed because of a fold in the expander (see Fig. 25-6). Because of this, the scalp expander was removed prematurely, but sufficient scalp expansion had been achieved to enable the anterior advancement of the parietal scalp to restore a normal-appearing temporal hairline. The forehead expander was left in place and expansion continued to complete the expansion process. A total of 300 mL of saline was injected into the expander. The expander was then removed and an advancement flap was created and used to cover the left side of the forehead after removal of the skin graft.
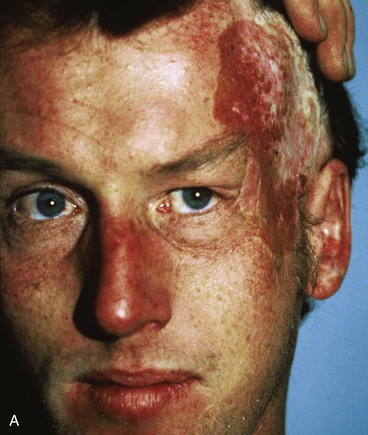
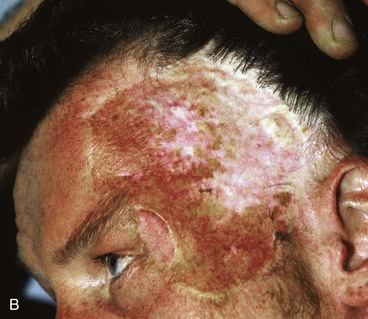
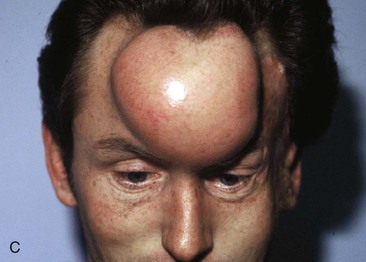
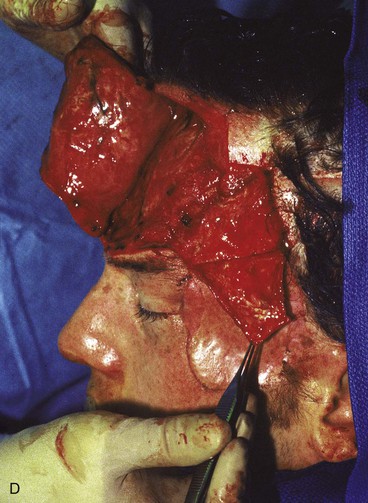
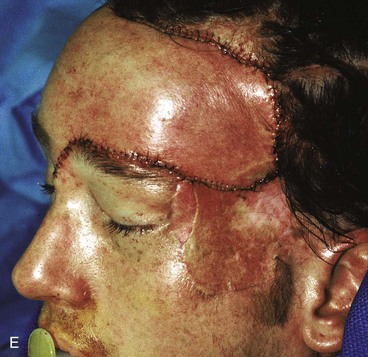
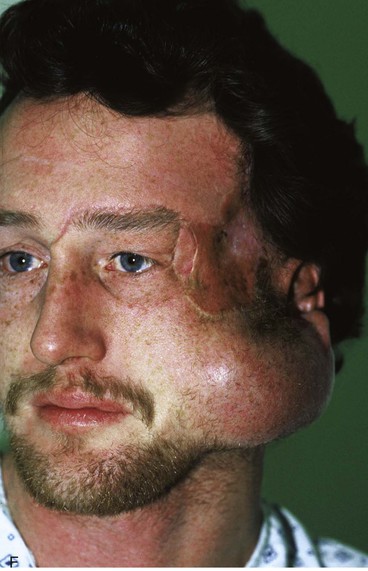
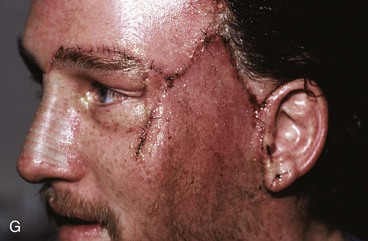
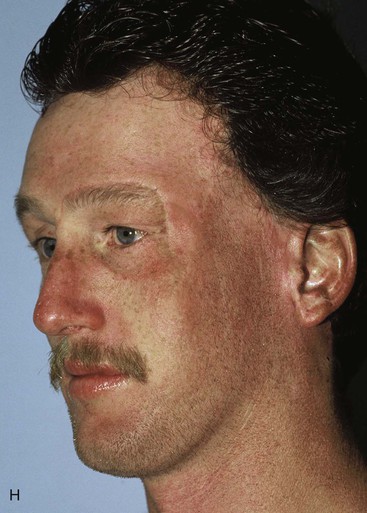
FIGURE 25-16 A, B, Skin graft covering temple, anterior parietal scalp, and lateral cheek. C, Expanded forehead skin. D, After tissue expansion, expanded forehead skin used to cover defect created by partial resection of skin graft. E, Expansion provided sufficient skin to cover temple. F, Tissue expander beneath lateral cheek skin. G, Six days after removal of skin graft from cheek and reconstruction with expanded cheek advancement flap. H, Postoperative result at 6 months.
The forehead and scalp expanders were effective in providing sufficient skin to enable the removal of the skin graft covering the left side of the forehead and to restore a normal temporal hairline. The patient still had a skin graft covering the zygomatic area. A third tissue expander was placed beneath cheek skin to create an expanded cheek advancement flap. The cheek flap was used to resurface the zygomatic area, enabling the complete resection of the skin graft covering the area. A similar case is shown in Figure 25-17, in which a large skin graft was excised from the temple aided by expanded forehead and scalp flaps.
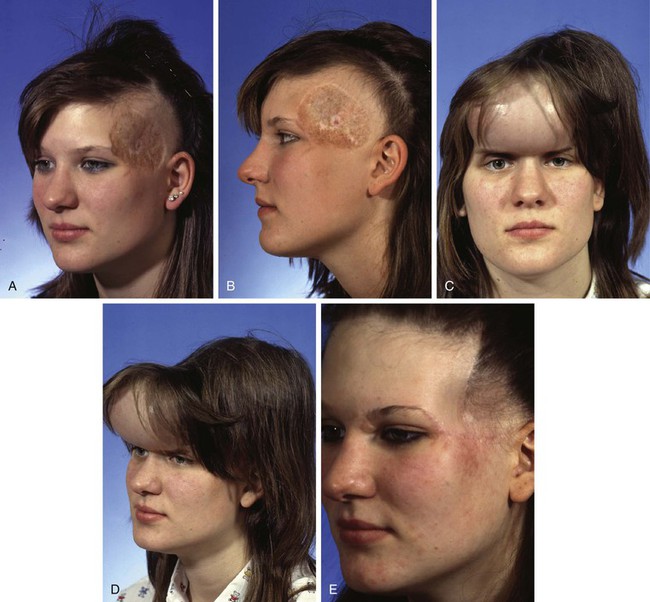
FIGURE 25-17 A, B, Unsightly split-thickness skin graft and large area of radiation alopecia. C, D, In anticipation of excision of skin graft and area of alopecia, forehead skin and posterior scalp on ipsilateral side of skin graft expanded with 250-mL rectangular tissue expanders. E, Postoperative result after complete excision of skin graft and area of alopecia. Resulting defect repaired with expanded forehead skin and scalp advancement flaps. (Courtesy of Shan R. Baker, MD.)
Face and Neck Expansion
Expanders can be used to expand skin of the face, neck, and chest.32 The expander is generally placed in a more superficial tissue plane in the face and neck than it is in the scalp and forehead. Because many of the deformities to be corrected are large scars from burns and other trauma, dissection can be difficult when expanders are implanted and advancement flaps are created from the expanded skin. In the region of the cheek, extreme care is required to avoid damage to the facial nerve during dissection. The expander may be placed directly over the vicinity of the facial nerve and expansion performed without nerve impairment. When possible, incisions are placed in natural creases to allow the best long-term aesthetic results. When the skin of the cheek or neck is expanded, a periauricular facelift-type incision is often used for implanting the expander (Fig. 25-18).
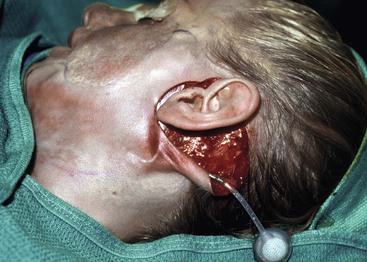
FIGURE 25-18 Preoperative planning of incision location is important to avoid compromising subsequent design of expanded flap. Facelift-type incision is useful for implanting expanders beneath cheek and upper neck skin.
Because the expander is placed more superficially in the face and the neck compared with the forehead and because the skin is thinner in these areas, the expanded skin may become very thin during the process (Fig. 25-19). The more rapid the expansion, the thinner the skin becomes and the greater the risk of tissue breakdown. The expander may be placed deep to the platysmal muscle in the neck. The author has not encountered problems when placing tissue expanders over vital structures such as the carotid artery, trachea, or larynx, but the surgeon is still obligated to observe patients closely for possible signs and symptoms of complications (Fig. 25-20).
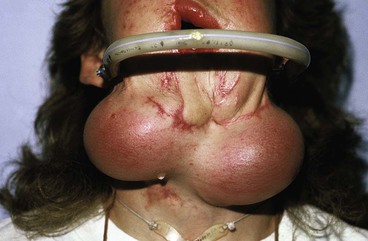
FIGURE 25-20 Expanders beneath neck skin in preparation for reconstruction of mandible, lower lip, and chin.
Tissue expansion has been used to reconstruct traumatic defects of the mandible.33 Successful bone grafting of the mandible has been used in conjunction with expanded neck flaps and expanded oral mucosa to provide an improvement in the form and function of the reconstructed mandible . The patient in Figure 25-21 sustained a gunshot wound to the face, resulting in the loss of the anterior mandible and overlying soft tissue in addition to a portion of the tongue, maxilla, and nose. Before the author’s care of this patient, he had multiple reconstructive procedures including skin grafts, myocutaneous flaps, and bone grafts, many of which failed because of poor vascularity of the traumatized tissue. Tissue expansion of the neck skin was recommended for the purpose of replacing the traumatized tissue and to add additional skin and soft tissue to the area of the chin. Two 250-mL rectangular expanders were implanted on either side of the neck through postauricular incisions. The expanders were placed superficial to the platysmal muscle, and expansion was continued for 2 months. Each expander was inflated to a total volume of 300 mL of saline. Once expansion was completed, the expanders were removed, advancement flaps were developed, and the deformity was reconstructed with the expanded tissue. At the time of repair, a small expander was inserted in a soft tissue pocket in the area of the missing section of the mandible. The expander served as a “spacer” preserving a cavity for subsequent placement of a bone graft. The expander also served as a stimulus to create additional soft tissue as a vascularized recipient site for the bone graft. Two previous bone grafts had failed because of poor vascularity and lack of sufficient soft tissue coverage of the grafts.
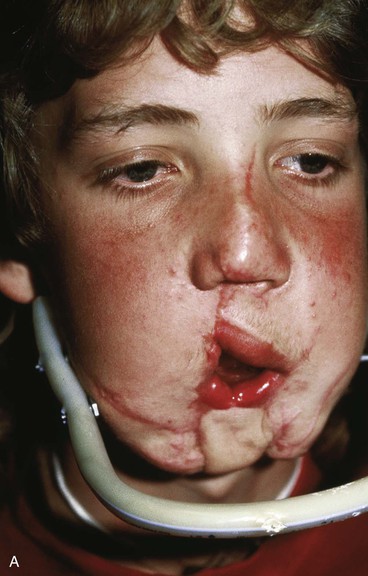
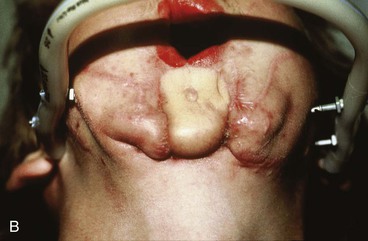
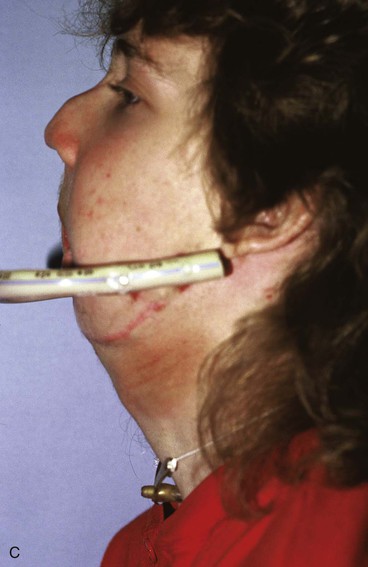
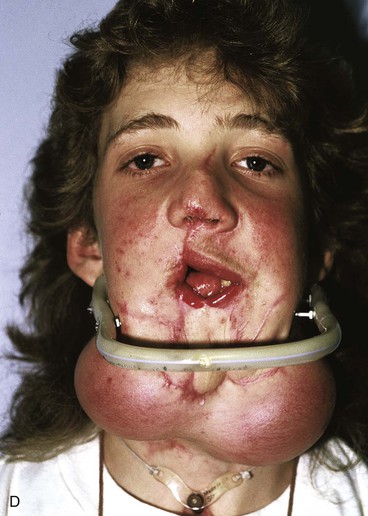
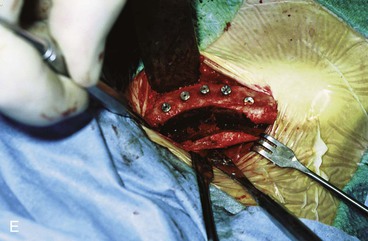
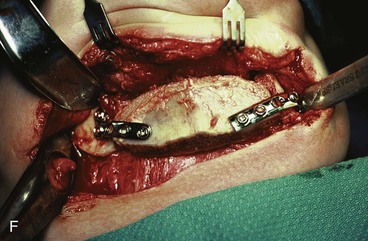
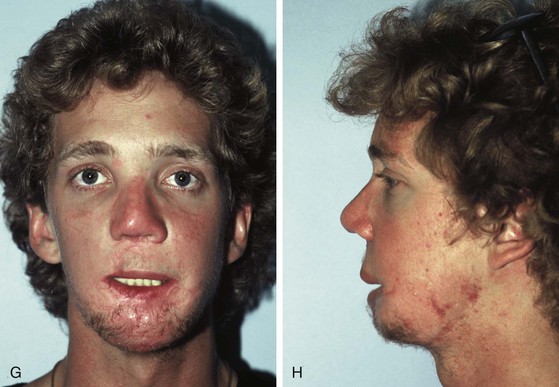
FIGURE 25-21 A, B, Patient with healed gunshot wound of mandible, lower lip, and chin. Several reconstructive procedures previously performed. Functional and aesthetic deformity persisted. C, External biphasic apparatus used for two years because of lack of anterior mandibular bone replacement. D, Two 250-mL expanders beneath neck skin implanted through postauricular incisions. E, Titanium implants placed in iliac crest in preparation for bone grafting of anterior mandible. F, Iliac crest bone graft in place to restore anterior mandible. G, H, Four years after reconstruction. In addition to mandible reconstruction, scar revisions of nose, lip, and commissure performed.
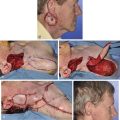
The patient shown in Figure 25-22 was a 12-year-old girl with a giant pigmented hairy nevus covering the left side of her face. She was referred to the author after having laser treatment, dermabrasion, and electrolysis. It was elected to use tissue expansion before attempting surgical excision. A 500-mL crescent-shaped expander was inserted beneath the cheek skin through a facelift-type incision. A crescent-shaped expander was selected because it allowed maximum skin expansion across the central portion of the expander and minimized expansion at the ends of the expander. This reduced the size of standing cutaneous deformities after advancement of expanded skin. The crescent-shaped expander is most useful for repair of round defects, and the pigmented nevus was circular in configuration. The injection port was placed externally behind the ear (Fig. 25-23). The patient was frightened of needles and lived 2 hours in traveling time from the office. To relieve the patient from traveling such long distances for inflation of the expander, her father was instructed in how to perform sterile injections of saline through the external port. The external port provided an easy access for her father to inflate the expander and avoided the need for percutaneous needle injections. The patient tolerated tissue expansion well and was checked periodically to ensure that infection had not occurred.
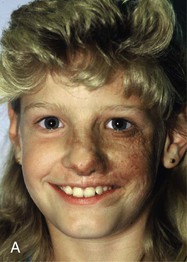
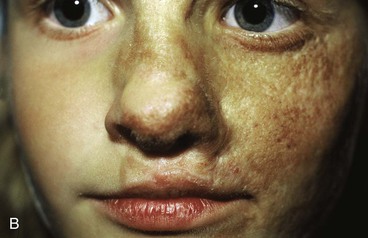
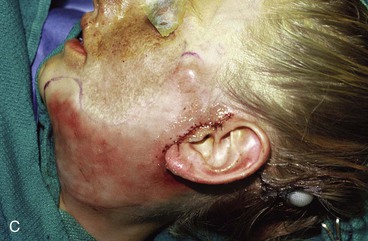
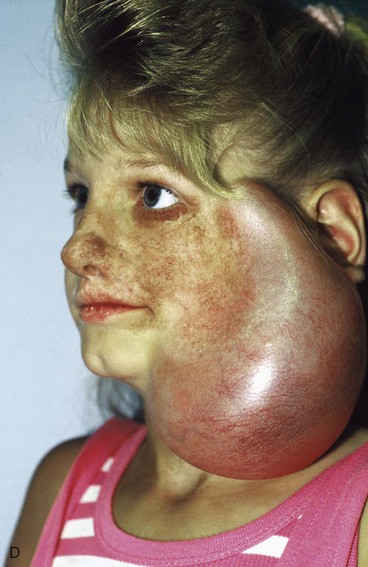
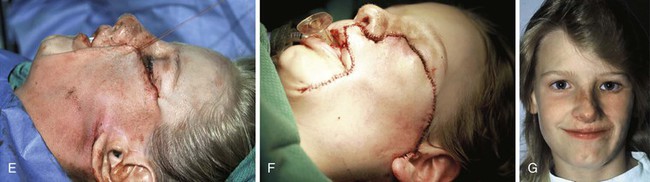
FIGURE 25-22 A, Patient with giant pigmented hairy nevus of left side of face. B, Persistent pigment and scarring present after treatment by laser, dermabrasion, and electrolysis. C, A 500-mL expander implanted through facelift-type incision. Injection port placed externally. D, During a 10-week period, 750 mL of saline was used to inflate expander. E, Expanded flap measured to ensure adequate coverage. F, Patient immediately after complete excision of nevus and advancement of expanded flap. G, Postoperative result at 1 year.
Expanders for Nasal and Ear Reconstruction
Myocutaneous flaps remain the preferred method for reconstruction of large deformities in which bulk and coverage of an open wound are required. Such is the case when nasal defects require a paramedian forehead flap. Expanders can be used as an aid to nasal reconstruction when forehead flaps are required.21 Forehead skin expansion provides the advantage of increasing the availability of skin and the ability to close the donor site without wound closure tension.
Tissue expanders have also been used for ear reconstruction.34 In fact, the first use of an expander was for reconstruction of an auricle. The expander is usually inserted beneath the postauricular skin. Skin is expanded until there is sufficient tissue to cover a framework for the ear.
External Injection Port
The first reported series using external ports showed no increased risk of infections.35 Subsequently, other authors have reported infections, but the incidence appears to be statistically the same for internal and external ports. An external injection port should not be used when the expander is replaced by prostheses such as breast implants.
Intraoperative Skin Expansion
In 1987, Sasaki reported his experience with the use of rapid intraoperative skin expansion.36 Expansion is performed at the time of the surgery rather than leaving the expander implanted for a prolonged time. Sasaki first observed that with long-term expansion, intraoperative expansion just before removal of the expander created an additional 1 to 3 cm of available skin for construction of a flap. He also observed that this rapid expansion produced no adverse effect on the chronically expanded flap. These observations led to the use of rapid expansion for immediate closure of smaller defects.
The technique of intraoperative tissue expansion consists of developing a subcutaneous pocket to accommodate the expander adjacent to the defect to be closed. The expander is inserted, and a series of inflations are performed. Repetitive inflation allows cyclic loading of the tissue as described by Gibson.6 Gibson noted that when tension is placed on skin, the initial stretch of skin is from displacement of fluid and ground substance in the dermis. The maximum amount of stretch obtained does not occur on the first stress load but after serial repetitive expansions of the tissue. After three or four load cyclings, microfragmentation of elastin fibers occurs, and collagen fibers realign in a more parallel orientation. The expander is inflated for 2 to 3 minutes with sufficient saline to cause tissue pallor and firmness. The expander is then deflated for 2 to 3 minutes. With each subsequent cycle, more saline can be injected and the measurement across the dome of the expander increases. After completion of intraoperative expansion, the expander is removed and the skin is advanced for a trial closure. It is important to observe bleeding from the edge of the expanded skin before creating a flap and transferring it. This ensures that tissue ischemia has not occurred from the expansion.
The histologic changes in the skin associated with prolonged expansion leading to biologic creep (described earlier in this chapter) are not observed with intraoperative expansion. On histologic evaluation, there are no significant changes in the epidermis, dermis, subcutaneous tissue, or muscle. The origin of additional skin gained by intraoperative expansion is not known. Hayden37 suggested that it is not a true gain. He suggested that the gain is from a more efficient undermining of the skin provided by expansion than is provided by simple subcutaneous dissection alone.
Complications
The rate of complications associated with tissue expansion is high, but most are minor. Manders et al38 studied the rate of complications in patients undergoing tissue expansion to treat a wide variety of deformities. They defined a major complication as one that required specific treatment and altered the planned procedure. By use of these criteria, 25% of patients suffered major complications. Antonyshyn et al39 reported an overall complication rate of 48%. However, sufficient tissue was generated to complete the planned reconstruction in 70% of the patients.
Infection associated with tissue expanders is rare in the head and neck area because of the abundant blood supply of facial skin.38 Perioperative antibiotics are recommended when an expander is implanted initially and at the first sign of infection. If infection occurs during the expansion process and is not quickly controlled, the expander should be removed and the infection treated before re-expansion of the skin is attempted.
Mechanical failure of the expander is rare and is usually associated with physician error. The expander can be accidentally punctured when the expander is inserted or when sutures are placed during closure of the access incision. The most common problem is leakage of the tubing attaching the expander to the injection port when the tubing has been shortened with a connector to adjust the length. It is important to tie a suture on both sides of the connector used to shorten the tubing to ensure a tight seal and to prevent leakage. Leakage does not always necessitate removal or replacement of the expander. Several surgeons have reported successful expansion despite leakage from the expander.40
Expanding irradiated, scarred, or acutely traumatized skin may lead to failure. These skin conditions are not absolute contraindications for tissue expansion but may increase the risk of expander extrusion before adequate skin gain has occurred to complete the planned procedure. However, when it is used with caution, expansion has been shown to be successful under these conditions.41
The patient’s mental condition must be considered before the use of tissue expansion is recommended. Patient selection is important because of the change in appearance and length of time required to complete the expansion process. The surgeon should use this procedure with caution in a psychologically unstable patient. Even mentally stable patients may undergo psychological stress leading to a wide range of reactions, making it difficult to complete the expansion process.42 All patients must thoroughly understand the temporary deformity that expansion will cause as well as the risks and complications of skin expansion.
References
1. Versaci, AD, Balkovich, ME. Tissue expansion. In: Habal M, ed. Advances in plastic and reconstructive surgery. Chicago: Year Book Medical Publishers, 1984.
2. Neumann, CG. The expansion of an area of skin by progressive distension of a subcutaneous balloon. Plast Reconstr Surg. 1957; 19:124.
3. Radovan, C, Adjacent flap development using expandable Silastic implants Presented at the annual meeting of the American Society of Plastic and Reconstructive Surgeons, Boston, September, 1976.
4. Austed, E, A self-inflating tissue expander Presented at the annual meeting of the American Society of Plastic and Reconstructive Surgeons, Toronto, October 25, 1979.
5. Austed, ED, Pasyk, KA, McClatchey, KD, et al. Histomorphologic evaluation of guinea pig skin and soft tissue after controlled tissue expansion. Plast Reconstr Surg. 1982; 70:704.
6. Gibson, T, The physical properties of skin. Reconstructive plastic surgery. Converse, JM, eds. Reconstructive plastic surgery; vol. 1. WB Saunders, Philadelphia, 1977.
7. Pasyk, KA, Austed, ED, McClatchey, KD, et al. Electron microscopic evaluation of guinea pig skin and soft tissues “expanded” with a self-inflating silicone implant. Plast Reconstr Surg. 1982; 70:37.
8. van Rappard, JHA, Grubben, MJAL, Jerusalem, C, et al. Histological changes in expanded soft tissue. In: van Rappard JHA, ed. Controlled tissue expansion of reconstructive surgery. Nijmegen, Netherlands: SSN, 1988.
9. Austed, ED, Thomas, SB, Pasyk, K. Tissue expansion: dividend or loan? Plast Reconstr Surg. 1986; 78:63.
10. van Rappard, JHA, Bauer, FW, Grubben, MJAL, et al. Epidermopoiesis in controlled tissue expansion. In: van Rappard JHA, ed. Controlled tissue expansion in reconstructive surgery. Nijmegan, Netherlands: SSN, 1988.
11. Pasyk, KA, Argenta, LC, Hassett, C. Quantitative analysis of the thickness of a silicone implant. Plast Reconstr Surg. 1988; 81:516.
12. Olenius, M, Jerell, G, Froslind, B, Histomorphologic changes in expanded human tissue Presented at the Plastic Surgery Educational Foundation International Tissue Expansion Symposium, San Francisco, October, 1987.
13. Sasaki, GH, Expansion of extremities Presented at the Plastic Surgery Educational Foundation International Tissue Expansion Symposium, San Francisco, October, 1987.
14. Cherry, GW, Austed, ED, Pasyk, KA, et al. Increased survival and vascularity of random pattern skin flaps elevated in controlled expanded skin. Plast Reconstr Surg. 1983; 72:680.
15. Stark, GB, Hong, C, Futrell, JW. Rapid elongation of arteries and veins in rats with a tissue expander. Plast Reconstr Surg. 1987; 80:570.
16. Leighton, WD, Russell, RC, Marcus, DE, et al. Experimental pretransfer expansion of free-flap donor sites. II. Physiology, histology and clinical correlation. Plast Reconstr Surg. 1988; 82:76.
17. Saxby, PJ. Survival of island flaps after tissue expansion: a pig model. Plast Reconstr Surg. 1988; 81:30.
18. Manders, EK, Saggers, GC, Diaz-Alonso, P, et al. Elongation of peripheral nerve and viscera containing smooth muscle. Clin Plast Surg. 1987; 14:551.
19. Morris, SF, Pang, CY, Mahoney, J, et al. Effect of capsulectomy on the hemodynamics and viability of random-pattern skin flaps raised on expanded skin of the pig. Plast Reconstr Surg. 1989; 84:314.
20. Becker, H. The permanent tissue expander. Clin Plast Surg. 1987; 14:519.
21. Manders, EK, Friedman, M, The treatment of male pattern baldness utilizing a differential expander Presented at the annual meeting of the American Society of Aesthetic Plastic Surgery, Boston, 1985.
22. Hallock, GG. Maximum overinflation of tissue expansion. Plast Reconstr Surg. 1987; 80:567.
23. Hallock, GG, Rice, DO. Objective monitoring for safe tissue expansion. Plast Reconstr Surg. 1986; 77:416.
24. van Rappard, JHA, Sonneveld, GJ, Borghouts, JMHM. Geometric planning and the shape of the expander. Facial Plast Surg. 1988; 5:287.
25. Brobmann, GF, Huber, J. Effects of different-shaped tissue expanders on transluminal pressure, oxygen tension, histologic changes, and skin expansion in pigs. Plast Reconstr Surg. 1985; 76:731.
26. Radovan, C. Tissue expansion in soft tissue reconstruction. Plast Reconstr Surg. 1984; 74:482.
27. Gibney, J, Tissue expansion in reconstructive surgery Presented at the American Society of Plastic and Reconstructive Surgeons annual scientific meeting, Las Vegas, October, 1984.
28. Sugihara, T, Ohura, T, Kim, C, et al, The extensibility in human skin Presented at the Plastic Surgery Educational Foundation International Tissue Expansion Symposium, San Francisco, 1987.
29. Reinisch, J, Myers, B. The effect of local anesthesia with epinephrine on skin flap survival. Plast Reconstr Surg. 1974; 54:324.
30. Kabaker, SS, Kridel, RWH, Krugman, ME, et al. Tissue expansion in the treatment of alopecia. Arch Otyolaryngol. 1986; 112:720.
31. Anderson, RD, Argenta, LC. Tissue expansion for the treatment of alopecia. In Unger WP, ed.: Hair transplantation, 2nd ed, New York: Marcel Dekker, 1987.
32. Hayden, RE, Expansion of a pectoralis myocutaneous flap Presented at the annual meeting of the American Academy of Facial Plastic and Reconstructive Surgery, San Antonio, Texas, 1986.
33. Swenson, RW, Expansion assisted reconstruction of gunshot wounds to the mandible Presented at the annual meeting of the American Academy of Facial Plastic and Reconstructive Surgery, San Antonio, Texas, 1986.
34. Nordstrom, REA, Salo, HP, Rintala, AE. Auricle reconstruction with the help of tissue expansion. Facial Plast Surg. 1988; 5:338.
35. Jackson, IT, Sharpe, DT, Polley, J, et al. Use of external reservoirs in tissue expansion. Plast Reconstr Surg. 1987; 80:266.
36. Sasaki, GH. Intraoperative sustained limited expansion (ISLE) as an immediate reconstructive technique. Clin Plast Surg. 1987; 14:563.
37. Hayden, RE, Intraoperative tissue expansion: fact or fiction? Presented at the annual meeting of the American Academy of Facial Plastic and Reconstructive Surgery, New Orleans, September, 1989.
38. Manders, EK, Schenden, MJ, Furrey, JA, et al. Soft tissue expansion: concepts and complications. Plast Reconstr Surg. 1984; 74:493.
39. Antonyshyn, O, Gruss, JS, Zuker, R, et al. Tissue expansion in head and neck reconstruction. Plast Reconstr Surg. 1988; 82:58.
40. Manders, EK, Sasaki, G, Austed, E, Tissue expansion today A symposium sponsored by the Plastic Surgery Educational Foundation, Hershey, Pa, September, 1986.
41. Fritz, M, Swenson, RW, Tissue expanders for reconstruction within irradiated head and neck tissues TorontoStucker FJ, ed. Plastic and reconstructive surgery of the head and neck: proceedings of the American Academy of Facial Plastic and Reconstructive Surgery Fifth International Symposium. 1991.
42. Goin, M, Psychological aspects of tissue expansion Presented at the American Society of Plastic and Reconstructive Surgery Symposium, Pasadena, November, 1984.