CHAPTER 11 Contrast Agents and Medications in Cardiac Computed Tomography
Earlier generations of multidetector CT scanners provided the foundation for cardiac multidetector CT angiography, and the implementation of 64-multidetector CT technologies made it a reliable tool with a high degree of diagnostic efficacy.1–3 In contrast to CT angiography applications using intravenous contrast medium (CM) administration elsewhere in the body, adequate cardiac opacification is not always simple to achieve, particularly in light of continuously evolving CT technology, with scan times becoming substantially shorter, but yielding improved spatial and temporal resolution. Empiric injection protocols are no longer adequate because they do not take into account the complexity of all confounding variables that allow consistent, robust cardiac imaging.4 These confounding variables include the requirement for a controlled and regular heart rate and carefully timed arterial enhancement for optimal cardiac and coronary vessel enhancement during minimal cardiac motion. Physiologic and pharmacokinetic constraints of cardiac enhancement in addition to inherent technical scanner properties have to be considered when building an integrated scanning and injection protocol for cardiac CT angiography.
TECHNICAL REQUIREMENTS
Medications for Cardiac Imaging
Although newer generations of multidetector CT scanners have shown a substantial improvement in temporal resolution—and with this, the capability to “freeze” cardiac motion—it is still desirable for most cardiac applications to slow the heart rate for various reasons. Slow heart rates prolong the cardiac phases with the least cardiac motion, specifically end-diastolic relaxation and end-systolic contraction. Best image quality is achieved when the data reconstruction window fits temporally into these phases.5 Slower heart rates also decrease heart rate variability during data acquisition and allow optimal efficacy of ECG-gated dose modulation techniques to control patient radiation dose.6
In our practice, we aim for a target heart rate less than 65 beats/min. To slow the heart rate, β blockers are ubiquitously used, and different means of β blocker administration for cardiac imaging are described, depending on patient, workflow, and logistics-related factors. Knowledge of the pharmacologic properties of the various β blockers is key to ensure safe and efficient patient prescan preparation. First-generation agents, such as propranolol, nonselectively block all β receptors (β1 and β2 receptors). Second-generation agents, such as atenolol, metoprolol, acebutolol, and others, have a relative selectivity, when given in low doses, for β1 (largely cardiac) receptors. The administration of β1 adrenoreceptor antagonists is favored for cardiac CT because of fewer side effects and fewer contraindications. Table 11-1 summarizes the effects mediated by β1 and β2 adrenoreceptors, and Figure 11-1 shows the important effects of nonselective and selective β blockers.
Diverse cardiac CT imaging centers have proposed different protocols of β blocker administration. Some centers routinely use an oral β blocker (e.g., atenolol [Tenormin]) to slow the heart rate and add an intravenous β blocker (e.g., metoprolol tartrate [Lopressor]) only if rate control is unsatisfactory. Other centers performing cardiac CT suggest different medication protocols (i.e., no combination of intravenous and oral β blocker administration) based on their experience and available operational logistics. Table 11-2 shows the sequence of our routine β blocker administration in the absence of any contraindications. Any patient preparation protocol should include a checklist for contraindications to the use of β blockade. The general contraindications for the therapeutic use of β blockers also apply for a “one-time” use for cardiac CT. Table 11-3 summarizes the most important contraindications for the drugs involved in cardiac CT. In case of questionable contraindications, the responsible imaging specialist should confer with the referring physician and determine if the administration of a β blocker is justified, or if alternatives should be applied.
Table 11-3 Contraindications for the Use of β Blocker, CA Channel Blockers, and Nitroglycerin for Cardiac CT
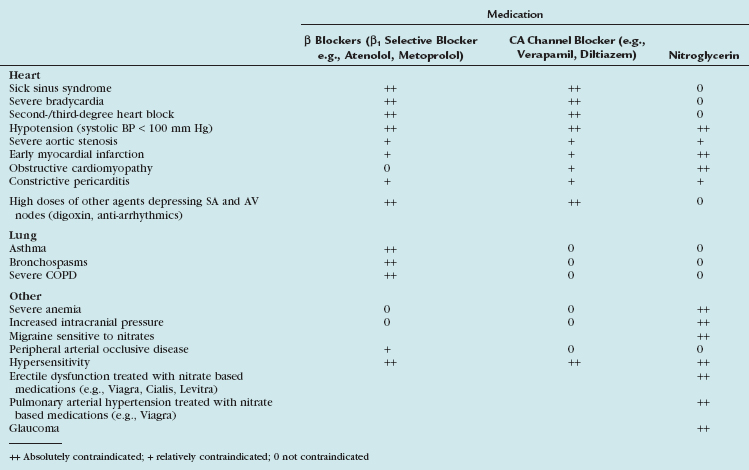
Calcium channel blockers embody an alternative heart rate-controlling medication. In patients with contraindications to β blockers (i.e., asthma), an attempt with calcium channel blockers may be worthwhile. There is less experience with calcium channel blockers in the setting of cardiac CT, and the imaging physician should be familiar with the medication, its administration (see Table 11-2), and its contraindications (see Table 11-3). Consultation with the referring physician is suggested to obtain an optimal and safe result.
Organic nitrates are widely used to treat angina and alleviate symptoms of myocardial ischemia through various mechanisms.7 Intracoronary injection of nitroglycerin has long been used to obtain maximal arterial dilation during conventional coronary angiography.8 Similarly, oral administration of short-acting nitrates (sublingual nitroglycerin tablets or sublingual nitroglycerin spray) is an accepted practice to dilate the lumen of the coronary arteries during coronary CT angiography.9 Its vasodilative effect on the coronary arterial bed allows improved visualization of smaller arterial branches.10 The significant impact on the diameter of coronary arteries seems to be more pronounced in nondiseased/nonstenotic coronary segments when compared with diseased/stenotic coronary segments.11 This additional pharmacologic end-organ response is a welcome side effect because it accentuates further the difference between diseased and nondiseased coronary segments, and improves depiction of subtle coronary arterial disease. In addition to dilation of the vascular bed, nitroglycerin suppresses potential coronary artery spasm that may mimic stenosis at coronary CT angiography, especially in younger patients.
Our rationale of nitroglycerin administration is summarized in Table 11-2. Because the blood level peaks around 2 minutes, and the half life is around 7 minutes, we suggest waiting approximately 5 minutes after sublingual nitroglycerin administration to ensure maximal effect of coronary artery vasodilation.
The side effects of nitroglycerin are harmful; severe decreases in blood pressure and death have been reported in patients given nitroglycerin within 24 hours because of a pharmacologic interaction with phosphodiesterase type 5 inhibitors (e.g., sildenafil citrate [Viagra] or tadalafil [Cialis]). A careful check for any contraindications before administration of nitroglycerin must be a part of any patient premedication protocol (see Table 11-3).
Contrast Media
CT contrast agents are water-soluble derivates of iodinated benzene rings, which are either charged (ionic contrast agent) or not charged (nonionic contrast agent) monomeric or dimeric chemical structures. The x-ray absorption is proportional to the concentration of the iodine captured within the agent.12 Other important physicochemical properties of CM are their osmolality (high-osmolar, low-osmolar, iso-osmolar) and viscosity. Higher flow rates are necessary for cardiac CT imaging. Warming the contrast agent to body temperature reduces viscosity and maintains high injection flow rates.13 The viscosity of CM decreases about 50% by heating it from 20° C to body temperature.
Safety Issues of Contrast Media
Nonionic contrast agents for CT angiography are considered to be very safe with an adverse event rate of approximately 3%.14 Most adverse effects are mild to moderate, but severe reactions may occur in approximately 0.04%.15 Non–dose-dependent, (idiosyncratic) allergy-like and dose-dependent (nonidiosyncratic) reactions are the two major categories of adverse effects. Guidelines for patient screening, premedication, and management of acute adverse reactions are available in the literature.16 There is also a substantial incidence of delayed cutaneous reactions occurring 24 hours or more after CM injection.17 Because these events tend to recur with reinjection of the same CM, screening and documentation of these reactions is of paramount importance.17 Another absolute contraindication for iodinated CM is manifest hyperthyroidism (i.e., Graves disease) with the risk of triggering a thyrotoxic crisis.18
Important dose-dependent effects include CM extravasation, cardiovascular effects, drug interactions, and contrast-induced nephropathy (CIN). CM extravasation is a well-known problem that occurs with an incidence of approximately 0.2% to 0.6% with the use of mechanical power injectors.19 Injection flow rates of 5 to 6 mL/s are routinely used for cardiac CT imaging and are considered to be safe.20 Only large volumes (>30 mL) of CM extravasation are at risk of severe side effects such as skin necrosis, ulceration, or compartment syndrome. Although adverse effects are more common and severe with ionic CM, they have been described to occur occasionally with nonionic agents as well. Conservative management of extravasation injuries (applying warm or cold compresses) is often adequate. For more severe injuries, plastic or hand surgery consultation is recommended to determine optimal treatment. Any adverse effects should be documented for future therapeutic planning.
To avoid any extravasation, intravenous access should be tested for stable and correct cannula position by a preliminary rapid manual injection of saline with the patient’s arm in the scanning position. Most extravasation events happen within the first seconds of injection; however, they may also occur later during mid or late phase of injection. Checking the intravenous access during early CM administration does not entirely prevent any extravasation. Extravasation detection devices linked to the power injector provide monitoring during the entire CM administration and may reduce the risk of late extravasation.21 Their use comes, however, with increased cost and risk of erroneous abortion of CM administration because of false-positive alarms.
Cardiovascular adverse events after intravenous administration of nonionic agents are probably rare; the literature suggests that severe reactions are more likely to be due to cardiopulmonary decompensation than to allergy-like reactions.22 Rapid injection of CM volume may lead to acute decompensation in patients with preexisting substantial cardiopulmonary compromise. Reducing the CM volume and injection rate seems to be one rational technique to reduce this specific risk.
CIN resulting from nonionic CM is rare (<2%) in the general population, but it may be substantial in the subset of patients at increased risk (>25%).23 CIN is defined as a greater than 25% increase of serum creatinine from baseline value within 3 days after CM injection in the absence of other causes.24 Controversy exists currently as to which features of the CM abet the development of CIN. In most cases, CIN is reversible, but it may be associated with considerable morbidity and mortality.25 Primary risk factors include preexisting renal insufficiency, diabetes, volume depletion, and interaction with nephrotoxic drugs.
Screening for patients at risk and taking subsequent renoprotective measures to reduce the risk of CIN is mandatory in any patients referred for cardiac CT angiography. Different operational logistics between inpatient and outpatient clinics determines the practice of screening. In an outpatient setting, routine measurement of serum creatinine is unnecessary in patients younger than 70 years if a simple questionnaire designed to elicit a history of renal disorders and exclude risk factors for CIN is used.26 In patients with prior kidney disease (including renal transplantation), patients with diabetes, patients with congestive heart failure, and patients older than 70 years, serum creatinine levels should be obtained. We request a serum creatinine level in all inpatients before CM administration. Because serum creatinine levels only partially reflect renal function, it has been suggested to calculate the patient’s estimated glomerular filtration rate based on patient’s body surface, age, sex, race, and serum creatinine. Convenient World Wide Web–based calculators are available (e.g., http://www.kidney.org/kls/professionals/gfr_calculator.cfm). Any estimated glomerular filtration rate equal to or less than 60 mL/min/1.73 m2 indicates a patient at risk for CIN.
Renoprotective measures in at-risk patients in whom CT angiography is desired include the use of the lowest possible dose of low-osmolar or iso-osmolar nonionic contrast agent, discontinuation of any nephrotoxic drugs (i.e., nonsteroidal anti-inflammatory drugs) 24 hours before the scan, and, importantly, volume expansion with intravenous fluid for several hours before and after the scan.27 Intravenous hydration with sodium bicarbonate 1 hour before the scan seems to have a similar protective effect,28 as does oral hydration. Other regimens with various drugs—although with initially very promising results—do not seem to offer consistent protection against CIN. Post–CT angiography serum creatinine levels should be obtained to monitor any change in renal function and with this, the development of CIN.
Interactions with other drugs also have to be expected with the administration of CM and have been addressed in several reports.29 Nephrotoxic drugs, loop diuretics, interleukin-2, metformin, and hydralazine are some medication categories with known interaction. Most important in the context of cardiac CT angiography is the potential interaction with β blockers. Anaphylactic reactions and reduced effectiveness of epinephrine in a case of shock are more common in patients who received intravenous CM than in matched controls.16 Effects of CM on coagulation, fibrinolytic drugs, and calcium channel blockers are usually not relevant for cardiac CT angiography.
TECHNIQUES
Early Arterial Contrast Media Dynamics
Early arterial enhancement describes the relationship between intravenous CM injection and the corresponding arterial enhancement.30 Figure 11-2 schematically shows the early arterial enhancement and its dependence on two user-selectable parameters, the administrated iodine flux (IF) and contrast injection duration (ID). Every CM injection results in a “first-pass” peak of enhancement at the CM transit time (TT) and in a lower “tail” of enhancement, owing to bolus broadening and recirculation. The TT is variable among individuals and is an important parameter for scan timing. The bolus broadening occurs within the cardiac and pulmonary circulation and is also due to a recirculation effect of opacified venous blood from highly perfused organs, such as kidney and brain.
Enhancement response is proportional to the administrated IF (milligrams of iodine injected per second [mg I/s]), referred to as the first “primary” injection parameter. IF (mg I/s) is a function of contrast injection flow rate (IR) (mL/s) and iodine concentration (IC) (mg I/mL) of the CM. Doubling the IF approximately doubles the degree of enhancement (HU), whereas the TT does not change substantially.30
ID (s) is the second “primary” injection parameter. CM volume (CV)—as we used it intuitively in the past—is just a secondary (dependent) injection parameter and is derived by simple multiplication of IR and ID. The effect of increasing the ID is not proportional to the degree of enhancement (HU) and is less intuitive. The degree of increased enhancement is mainly based on the recirculation phenomena. A schematic based on the assumption of a time-invariant linear system, as shown in Figure 11-2, may help to explain the effect of a prolonged injection (ID) on arterial enhancement (HU). Generally, the arterial enhancement (HU) continuously increases over time with longer ID. The shape of the enhancement curve is characterized by a steep enhancement increase at the TT time, followed by a more gradual increase of enhancement (shoulder) for approximately the ID and finally by a rapid decrease of enhancement. Consequently, shorter ID leads to lower enhancement (HU), and not, as widely believed, to an equal enhancement for a shorter time. Only longer ID increases the arterial enhancement (HU) if the IF is kept constant.30
The high variability of arterial enhancement among individuals for a given injection protocol makes it difficult to ensure robust enhancement across the entire patient population. Individual arterial enhancement is mainly based on cardiac output (CO) and central blood volume (CBV). Arterial enhancement varies inversely with CO and to some degree with CBV (CBV influences mainly tissue enhancement and recirculation).31 Patients with high CO dilute the injected CM more, and have less arterial enhancement (HU), despite their shorter TT time, compared with patients with normal or lower CO.32 Body weight allows an approximation of CO and CBV in the daily routine.33 Adjusting the CM IR to the body weight allows reduction in the interindividual differences of arterial enhancement, and should be an integral part in any injection protocol.
Basic rules of early arterial CM dynamics can be summarized as follows:
Timing of Contrast Media Injection
There are basically two timing issues to be considered when designing a CT angiography injection protocol: ID and scan delay (shown in Fig. 11-3). The scan time itself ultimately depends on the requested anatomic coverage (i.e., only cardiac coverage for coronary artery CT angiography; cardiac and great vessel coverage for coronary artery bypass graft assessment), the scan mode (helical vs. nonhelical technique), and proprietary scanner specifications such as detector bank width or pitch. The scanner console provides the scan time after choosing the above-mentioned parameters. Normal scan times range from 5 to 20 seconds depending on the specific clinical question and type of CT system.
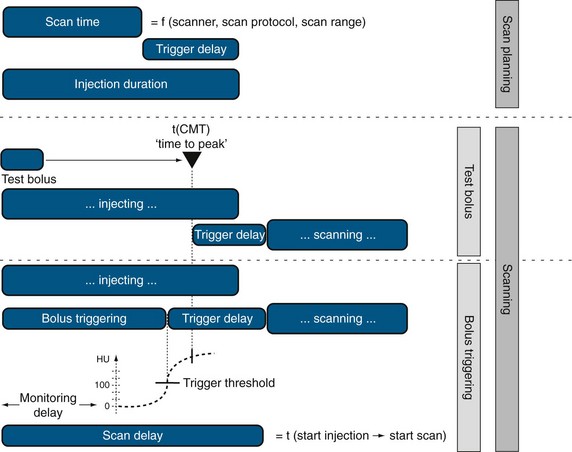
 FIGURE 11-3 Injection duration and scan delay need to be considered when designing a CT angiography injection protocol.
FIGURE 11-3 Injection duration and scan delay need to be considered when designing a CT angiography injection protocol.
When the scan time is determined, the ID has to be determined to ensure a sufficient contrast filling of the vascular territory of interest (Fig. 11-4). Normally, this time for injection is longer than the effective scan time, which necessitates adding a scan delay to allow for longer CM injection before data acquisition is initiated. This scan delay refers to the time between the start of CM injection and the start of the CT data acquisition, and includes the trigger event to time the start of the data acquisition relative to the patient’s individual CM arrival time (TT). TTs are determined by either a test bolus injection or an automatic bolus-triggering technique. The test bolus experiment provides the “time to peak” time by injection of a small test bolus (20 mL) before the final scan, whereas the automated bolus-triggering technique initiates the scan automatically after reaching a specific threshold enhancement (e.g., 100 HU) in the target vessel (e.g., the ascending aorta) during the CM injection.
Saline Flushing
Saline flushing allows use of the remaining CM in the upper extremity at the end of the CM injection bolus. Without use of a saline flush, ±15 mL of CM is normally trapped in the arm veins, outside the target imaging field. Probably more importantly, the use of a saline flush reduces the perivenous streak artifacts by “washing out” the superior vena cava and clearing the right atrium and ventricle to reduce the artifacts near the right coronary artery. Initially, complete clearance of the right heart chambers was suggested for coronary CT angiography, but complete lack of CM in the right heart may compromise morphologic and functional analysis of the right heart and potentially left heart (e.g., interventricular septum thickness inaccessible). Newer power injectors allow “splitting” the bolus and admixing contrast agent and saline at the end of the injection. This dilute contrast material (e.g. 30% contrast agent, 70% saline) at the end of the injection significantly reduces the above-mentioned artifacts, but still provides the assessment of the right heart chambers (Figs. 11-5 and 11-6).34
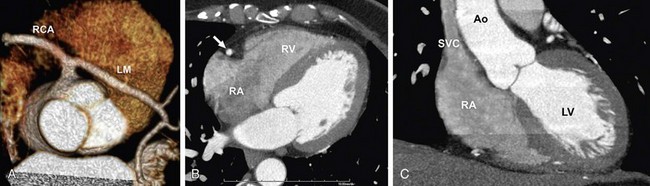
 FIGURE 11-6 A 59-year-old administrative associate with chest pain is referred for coronary artery CT angiography after abnormal catheter coronary angiography (64 × 0.6 mm detector configuration, scan time 12 seconds, trigger delay 8 seconds, injection duration 20 seconds, injection flow rate 4.5 mL/s, contrast volume 90 mL, contrast agent 370 mg/mL), flushing with 50 mL diluted contrast material (30% contrast agent/70% saline) followed by 30 mL saline. A, Volume rendering view with strong enhancement of the coronary arteries shows anomalous origin of left main (LM) coronary artery taking off the right coronary artery (RCA). B and C, Four-chamber view (B) and modified short-axis view (C) show strong enhancement of the left cardiac chambers (left ventricle [LV] and aorta [Ao]). Flushing with a split bolus of diluted contrast material at the end of the injection allows an improved morphologic and functional assessment of the right heart (right atrium [RA], right ventricle [RV]) compared with Figure 11-5. Note the lack of streak artifacts within the superior vena cava (SVC) and over the right atrioventricular groove carrying the RA (arrow in B).
FIGURE 11-6 A 59-year-old administrative associate with chest pain is referred for coronary artery CT angiography after abnormal catheter coronary angiography (64 × 0.6 mm detector configuration, scan time 12 seconds, trigger delay 8 seconds, injection duration 20 seconds, injection flow rate 4.5 mL/s, contrast volume 90 mL, contrast agent 370 mg/mL), flushing with 50 mL diluted contrast material (30% contrast agent/70% saline) followed by 30 mL saline. A, Volume rendering view with strong enhancement of the coronary arteries shows anomalous origin of left main (LM) coronary artery taking off the right coronary artery (RCA). B and C, Four-chamber view (B) and modified short-axis view (C) show strong enhancement of the left cardiac chambers (left ventricle [LV] and aorta [Ao]). Flushing with a split bolus of diluted contrast material at the end of the injection allows an improved morphologic and functional assessment of the right heart (right atrium [RA], right ventricle [RV]) compared with Figure 11-5. Note the lack of streak artifacts within the superior vena cava (SVC) and over the right atrioventricular groove carrying the RA (arrow in B).
Clinical Contrast Media Injection Protocols
Although injection protocols have to take into account inherent scanner-specific properties, basic rules apply for any scanner type. Table 11-4 summarizes clinical injection protocols for coronary CT angiography, coronary artery bypass graft CT angiography, and left atrial and coronary vein mapping.
KEY POINTS
 Contrast media administration embodies an integral part in every protocol designed for cardiac CT angiography.
Contrast media administration embodies an integral part in every protocol designed for cardiac CT angiography.Bae KT, Heiken JP, Brink JA. Aortic and hepatic contrast medium enhancement at CT. Part II: effect of reduced cardiac output in a porcine model. Radiology. 1998;207:657-662.
Fleischmann D. Use of high-concentration contrast media in multiple-detector-row CT: principles and rationale. Eur Radiol. 2003;13(Suppl 5):M14-M20.
Hallett RL, Fleischmann D. Tools of the trade for CTA: MDCT scanners and contrast medium injection protocols. Tech Vasc Interv Radiol. 2006;9:134-142.
Schoepf UJ, Zwerner PL, Savino G, et al. Coronary CT angiography. Radiology. 2007;244:48-63.
1 Gallagher MJ, Ross MA, Raff GL, et al. The diagnostic accuracy of 64-slice computed tomography coronary angiography compared with stress nuclear imaging in emergency department low-risk chest pain patients. Ann Emerg Med. 2007;49:125-136.
2 Raff GL, Gallagher MJ, O’Neill WW, et al. Diagnostic accuracy of noninvasive coronary angiography using 64-slice spiral computed tomography. J Am Coll Cardiol. 2005;46:552-557.
3 Raff GL, Goldstein JA. Coronary angiography by computed tomography: coronary imaging evolves. J Am Coll Cardiol. 2007;49:1830-1833.
4 Fleischmann D, Hittmair K. Mathematical analysis of arterial enhancement and optimization of bolus geometry for CT angiography using the discrete fourier transform. J Comput Assisted Tomogr. 1999;23:474-484.
5 Giesler T, Baum U, Ropers D, et al. Noninvasive visualization of coronary arteries using contrast-enhanced multidetector CT: influence of heart rate on image quality and stenosis detection. AJR Am J Roentgenol. 2002;179:911-916.
6 Jakobs TF, Becker CR, Ohnesorge B, et al. Multislice helical CT of the heart with retrospective ECG gating: reduction of radiation exposure by ECG-controlled tube current modulation. Eur Radiol. 2002;12:1081-1086.
7 Parker JD, Parker JO. Nitrate therapy for stable angina pectoris. N Engl J Med. 1998;338:520-531.
8 Feldman RL, Marx JD, Pepine CJ, et al. Analysis of coronary responses to various doses of intracoronary nitroglycerin. Circulation. 1982;66:321-327.
9 Schoepf UJ, Zwerner PL, Savino G, et al. Coronary CT angiography. Radiology. 2007;244:48-63.
10 Decramer I, Vanhoenacker PK, Sarno G, et al. Effects of sublingual nitroglycerin on coronary lumen diameter and number of visualized septal branches on 64-MDCT angiography. AJR Am J Roentgenol. 2008;190:219-225.
11 Schnaar RL, Sparks HV. Response of large and small coronary arteries to nitroglycerin, NaNO2, and adenosine. Am J Physiol. 1972;223:223-228.
12 Dawson P, Blomley MJ. Contrast media as extracellular fluid space markers: adaptation of the central volume theorem. Br J Radiol. 1996;69:717-722.
13 Knopp M, Kauczor HU, Knopp MA, et al. [Effects of viscosity, cannula size and temperature in mechanical contrast media administration in CT and magnetic resonance tomography]. Rofo. 1995;163:259-264.
14 Caro JJ, Trindade E, McGregor M. The risks of death and of severe nonfatal reactions with high- vs low-osmolality contrast media: a meta-analysis. AJR Am J Roentgenol. 1991;156:825-832.
15 Katayama H, Yamaguchi K, Kozuka T, et al. Adverse reactions to ionic and nonionic contrast media. A report from the Japanese Committee on the Safety of Contrast Media. Radiology. 1990;175:621-628.
16 Thomsen HS, Morcos SK. Contrast Media Safety Committee of European Society of Urogenital Radiology. Management of acute adverse reactions to contrast media. Eur Radiol. 2004;14:476-481.
17 Webb JA, Stacul F, Thomsen HS, et al. Members of the Contrast Media Safety Committee of the European Society of Urogenital Radiology. Late adverse reactions to intravascular iodinated contrast media. Eur Radiol. 2003;13:181-184.
18 van der Molen AJ, Thomsen HS, Morcos SK. Contrast Media Safety Committee, European Society of Urogenital Radiology (ESUR). Effect of iodinated contrast media on thyroid function in adults. Eur Radiol. 2004;14:902-907.
19 Bellin MF, Jakobsen JA, Tomassin I, et al. Contrast Media Safety Committee of the European Society of Urogenital Radiology. Contrast medium extravasation injury: guidelines for prevention and management. Eur Radiol. 2002;12:2807-2812.
20 Jacobs JE, Birnbaum BA, Langlotz CP. Contrast media reactions and extravasation: relationship to intravenous injection rates. Radiology. 1998;209:411-416.
21 Birnbaum BA, Nelson RC, Chezmar JL, et al. Extravasation detection accessory: clinical evaluation in 500 patients. Radiology. 1999;212:431-438.
22 Cochran ST, Bomyea K, Sayre JW. Trends in adverse events after IV administration of contrast media. AJR Am J Roentgenol. 2001;176:1385-1388.
23 Parfrey PS, Griffiths SM, Barrett BJ, et al. Contrast material-induced renal failure in patients with diabetes mellitus, renal insufficiency, or both: a prospective controlled study. N Engl J Med. 1989;320:143-149.
24 Morcos SK. Contrast media-induced nephrotoxicity—questions and answers. Br J Radiol. 1998;71:357-365.
25 Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality: a cohort analysis. JAMA. 1996;275:1489-1494.
26 Choyke PL, Cady J, DePollar SL, et al. Determination of serum creatinine prior to iodinated contrast media: is it necessary in all patients? Tech Urol. 1998;4:65-69.
27 Morcos SK. Prevention of contrast media nephrotoxicity—the story so far. Clin Radiol. 2004;59:381-389.
28 Merten GJ, Burgess WP, Gray LV, et al. Prevention of contrast-induced nephropathy with sodium bicarbonate: a randomized controlled trial. JAMA. 2004;291:2328-2334.
29 Morcos SK, Thomsen HS, Exley CM. Contrast media: interactions with other drugs and clinical tests. Eur Radiol. 2005;15:1463-1468.
30 Fleischmann D. Use of high-concentration contrast media in multiple-detector-row CT: principles and rationale. Eur Radiol. 2003;13(Suppl 5):M14-M20.
31 Sheiman RG, Raptopoulos V, Caruso P, et al. Comparison of tailored and empiric scan delays for CT angiography of the abdomen. AJR Am J Roentgenol. 1996;167:725-729.
32 Bae KT, Heiken JP, Brink JA. Aortic and hepatic contrast medium enhancement at CT. Part II: effect of reduced cardiac output in a porcine model. Radiology. 1998;207:657-662.
33 Fleischmann D, Rubin GD, Bankier AA, et al. Improved uniformity of aortic enhancement with customized contrast medium injection protocols at CT angiography. Radiology. 2000;214:363-371.
34 Kerl JM, Ravenel JG, Nguyen SA, et al. Right heart: split-bolus injection of diluted contrast medium for visualization at coronary CT angiography. Radiology. 2008;247:356-364.

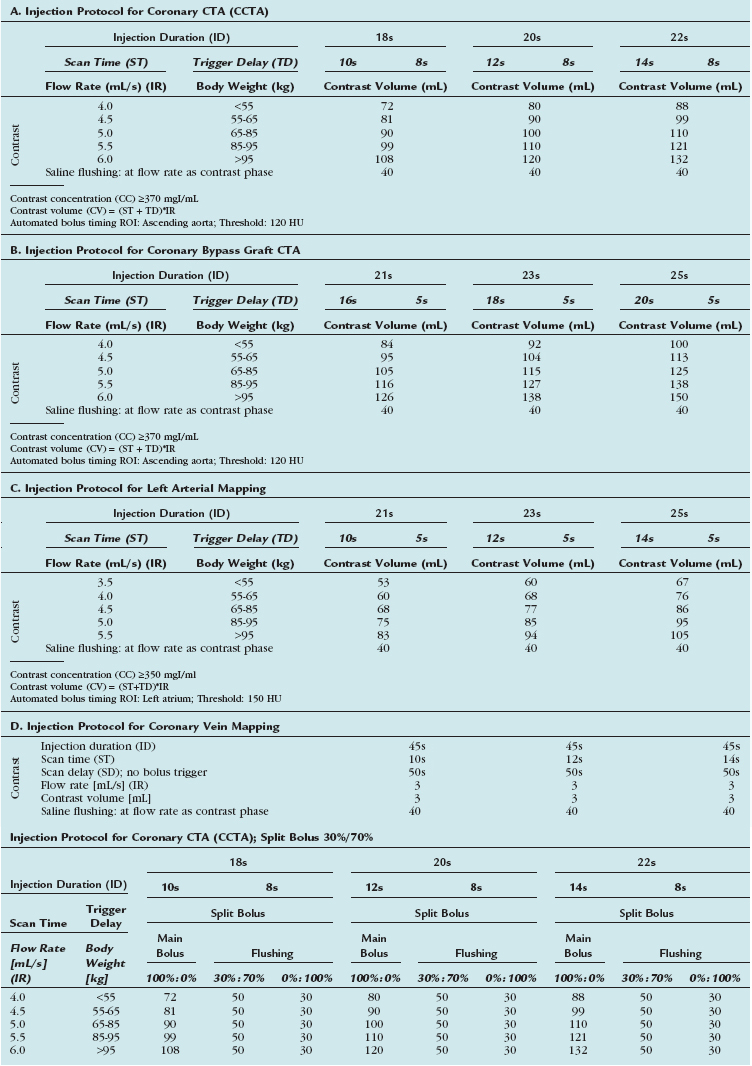
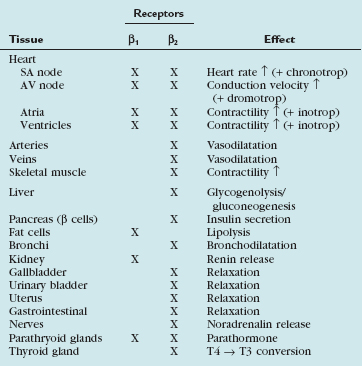
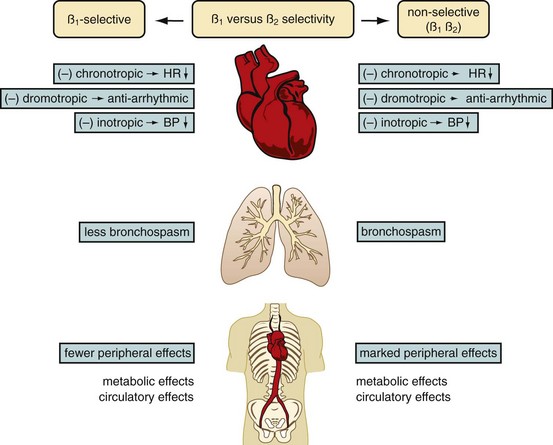
 FIGURE 11-1
FIGURE 11-1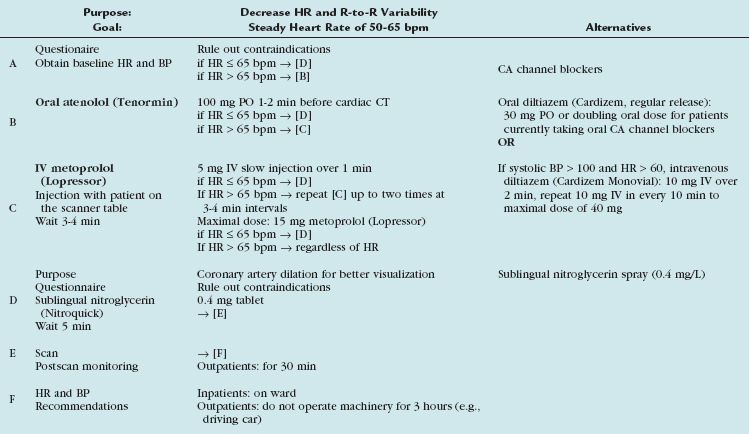
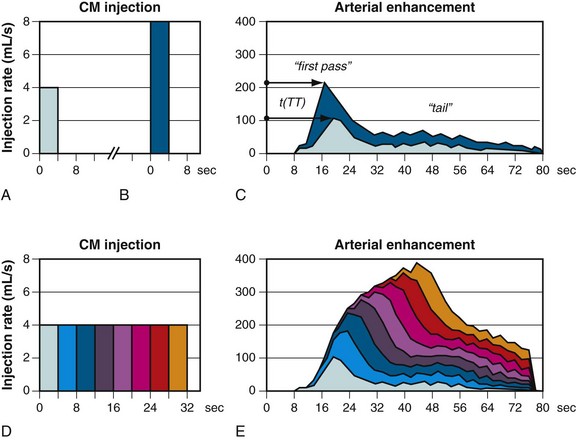
 FIGURE 11-2
FIGURE 11-2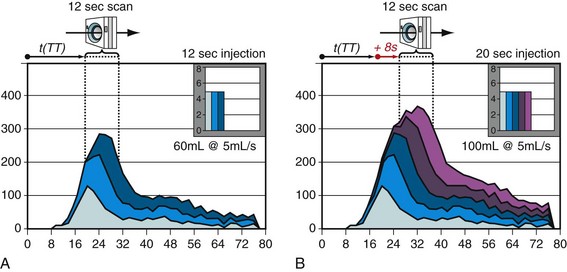
 FIGURE 11-4
FIGURE 11-4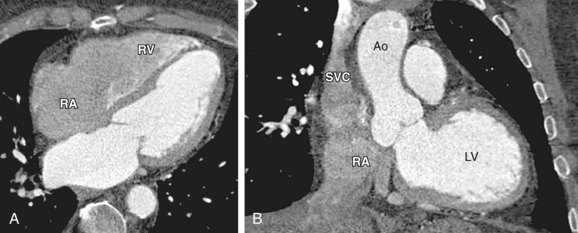
 FIGURE 11-5
FIGURE 11-5

