Contact Lenses for Ocular Surface Disease
History of Contact Lenses and Innovations Allowing for Therapeutic Use
The first description of contact lenses in the medical literature was a report on contact lenses by Adolf Eugene Fick in 1888.1 Pearson,2 in his review of the history of contact lenses, reports Karl Otto Himmler as the first manufacturer of contact lenses. Pearson describes reports of contact lenses ranging in diameter from 15 to 22 mm in 1888, 1888, and 1889 by Fick, Kalt, and Muller, respectively, all made of glass. Examining these early glass and then polymethyl methacrylate (PMMA) large-diameter lens designs retrospectively exposes various reasons for failure, most prominently, hypoxia related to the impervious nature of glass or PMMA. This problem was circumvented in the middle of the last century through the use of small diameter PMMA corneal lenses that allowed the majority of the cornea access to atmospheric oxygen, with some transmission to the area under the mobile contact lens via the tear film. Overwear syndrome could arise, due to hypoxia under a tight or immobile lens. These lenses were used primarily for treatment of refractive error because there was invariably corneal touch and underlying hypoxia, both of which would be a challenge in the setting of ocular surface disease. Two innovations in material science allowed for contact lens to enter the therapeutic armamentarium: rigid gas-permeable polymers and soft hydrogels.
The introduction of rigid gas-permeable polymers into contact lens manufacturing allowed for better physiological tolerance of corneal lenses and the reintroduction of large-diameter scleral lens designs in the early 1980s.3 Wide use of lenses made of these materials has been limited by challenges of corneal RGP lens fitting and the perception that a ‘hard’ lens cannot serve as a ‘bandage.’ Rigid gas-permeable scleral lenses that vault the cornea entirely can play a role in the treatment of ocular surface disease, as presented throughout the remainder of the chapter.
The introduction of hydrophilic gels for biologic use, and in particular for contact lens, by Czech chemist Otto Wichterle in the 1960s led to the availability of ‘soft’ lenses within a decade. Soft lenses are easily fitted, with greater range of tolerance for a given lens profile. The potential for therapeutic use was recognized contemporaneously with the introduction of soft contact lenses. In the decades that followed, modifications to materials, including increased water content or silicone for the sake of increased oxygen permeability, or modifications to improve wetting, were introduced in the field of soft contact lenses. Oxygen transmission has been held as the paragon for therapeutic lens, but mechanical interaction with the surface is also key to tolerance and clinical effectiveness. A North American survey of ‘bandage’ soft lens use by optometrists and ophthalmologist in 2002 found that 72% of respondents had prescribed soft contact lenses for therapeutic purposes, most typically for corneal wound healing and management of postoperative complications.4
In the 2007 Report of the International Dry Eye Workshop,5 contact lens wear is among the treatment recommendations by severity level among level 3 interventions along with autologous serum tears and permanent punctal occlusion, with systemic treatment and surgery listed as level 4 interventions. Some are skeptical that a contact lens for ocular surface disease is therapeutic. One might ask how an object ‘hard’ or ‘soft’ can help an eye that is dry (and inflamed). Clinical observations are that an appropriately chosen, well-fitted therapeutic contact lens can:
Consideration of mechanisms underlying these observations, some of which remain part hypothetical, is beyond the scope of this chapter.
Characteristics of Soft Lenses Used for Treatment of Ocular Surface Disease
The prototype therapeutic contact lens was the Bausch & Lomb Plano T, a hydrophilic hydrogel introduced in the 1970s specifically as a therapeutic lens. It had low water content (38%), low oxygen permeability (Dk 8.4), and was thick. Clinically, it reduced pain, promoted epithelialization, sealed leaks, and induced corneal edema (which was good for leaks but bad for other indications). The Permalens (CooperVision Inc, Fairport, NY) represented a significant advance with higher Dk value of 42. It was developed as an extended-wear lens for aphakia to be changed monthly, but its properties in low powers were advantageous over a Plano T lens for therapeutic indications. When programmed replacement and disposable lenses for correction of refractive error entered the marketplace, clinicians chose these for OSD because of immediate availability among trial inventories and relative low cost,6,7 even though they were not labeled for therapeutic use. The Silsoft (Bausch & Lomb Inc, Rochester, NY) is the most gas-permeable soft lens (Dk 340); it is specifically approved for extended wear for the therapeutic indication of correction of pediatric aphakia. These lenses typically have required high lens plus power and larger diameter to support that power, all reducing availability of atmospheric oxygen to the cornea.8
In the last decade, very high Dk silicone hydrogel (SiHy) material and lenses have been developed and labeled specifically for therapeutic use in addition to cosmetic use. There have been reports of the utility of SiHy lenses as a therapeutic option across the spectrum of ocular surface disease.9,10 Epidemiologic studies have not found a lower rate of infection with these newer materials,11,12 but there should be advantages conferred in OSD simply because of their higher oxygen transmission.
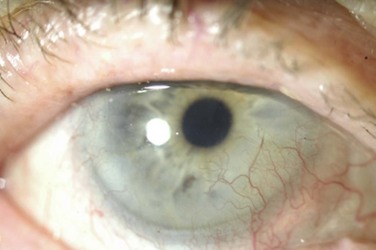
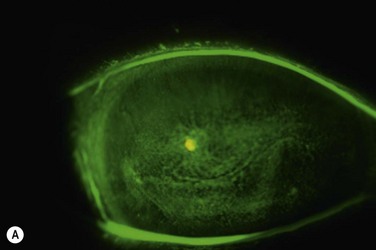
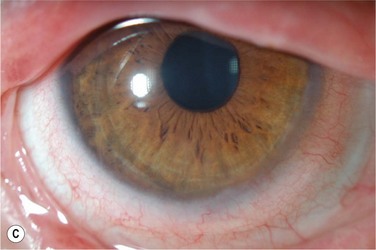
The authors recommend that clinicians choose a high Dk lens labeled for extended wear and/or therapeutic use as a first choice for therapeutic use in OSD (Fig. 35.1). Potential complications, including lens loss, lens deposits, discomfort, infectious keratitis and ulceration, and tight lens syndrome7 should be reviewed. The risks and benefits of overnight wear should be reviewed with the patient and weighed against the alternatives for the treatment of their particular disease process. It is probably not advisable to use a daily disposable lens on an extended wear or therapeutic basis as they were not developed or manufactured for those uses.
Table 35.1 presents some lenses of historical and current interest, with their Dk, percentage water content, and labeling. Some soft lenses are labeled for therapeutic use on an extended wear basis. Others are labeled for extended wear, but not specifically for therapeutic use. Lenses labeled for daily wear could be used ‘off- label’ on an extended wear basis. Lenses labeled for extended wear on a cosmetic basis could be used ‘off-label’ on a therapeutic basis.
Very Large Diameter Soft Lenses
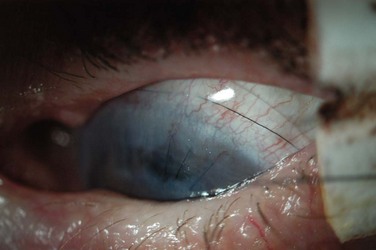
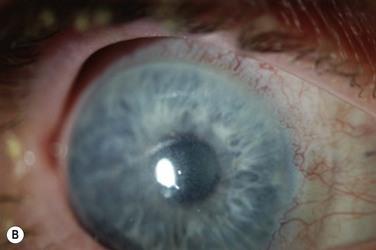
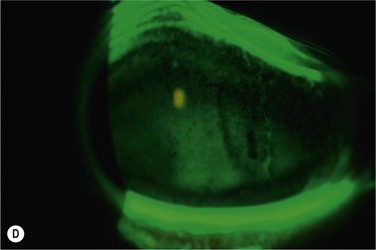
There are very-large-diameter hydrogel lenses (16–24 mm diameter) that have special utility in instances of OSD in which there is history or likelihood of poor soft lens retention. Profound aqueous deficiency, incomplete blink, lid abnormalities, eye rubbing, and exposure can all be contributory to poor retention (Fig. 35.2). Large-diameter soft lenses sold in range of base curves for a given diameter and the lenses with larger diameters will have central and peripheral zones of different diameters. Ideally, an assortment of diameters and curvatures are kept on hand, and best fit, tolerance, and retention are assessed with empiric trials. Large-diameter soft lenses in SiHy materials have recently become available on a custom-order basis.
Characteristics of Scleral Lenses Used for Treatment of Ocular Surface Disease
In 1983, Ezekiel3 reported the use of gas-permeable material in a scleral or ‘haptic’ lens solving the problem of hypoxia in a large-diameter contact lens. This innovation was applied successfully in that decade in innovative large-diameter RGP lens designs at centers of excellence around the world.13–15 The Dk of RGP materials has exceeded that of soft lens materials until the advent of SiHy materials for soft lenses in the past decade. Once hypoxia was conquered by these high-Dk RGP materials, lens suction was the next challenge. Suction was circumvented, literally, by fenestration, which allows for air ventilation, or by haptic design with channels or contours that allow for fluid ventilation with no intrusion of air bubbles. Air ventilation is often satisfactory for eyes with optical indications, such as keratoconus or post-keratoplasty astigmatism, but air bubble(s) are typically not well tolerated in ocular surface disease. Reports of utility of RGP scleral lenses for OSD in general have emerged over the past two decades.13,14,16–19 Success with this modality is dependent on the motivation and skill set of the clinician, as well as access to specialty lens manufacturing lab. A true scleral lens, well-fitted, with features, including a fluid reservoir, little movement, and no corneal touch, can provide support and protection for the corneal epithelium beyond that offered by any soft lens, and can be tolerated and retained in setting in which a soft lens is not.
There is a category of specialty contact lenses that is called ‘mini-scleral,’ ‘semi-scleral,’ and ‘corneoscleral’ lenses. The definitions and distinctions are evolving, and classifications may be based on the diameter, characteristics of fit or both. Generally, these lenses are 13–16 mm, made of high-Dk materials, may contact the cornea at the apex or peripherally, and the principles of fit are similar to those for an RGP corneal lens rather than a true scleral lens.20 These larger RGP lenses are sometimes included among reports of scleral lenses. Although they may be more easily fitted and initially well tolerated, a reduced bearing zone and deliberate or unrecognized corneal touch centrally and at the limbus make these lenses less appropriate for patients with ocular surface disease as they may induce erosion, scarring and neovascularization in areas of contact, compression, or impingement.
Typically, scleral lenses are inserted and removed on a daily-wear basis with overnight disinfection. Newer materials developed in the past decade for overnight orthokeratology and FDA approved for that use, might be used in a large-diameter lens on a daily or overnight regimen on an off-label basis. The challenges of fitting the eye and training the patient or caretaker in daily insertion and removal limit the use of scleral lenses as a short-term ‘bandage lens.’ In certain circumstances, continuous wear coupled with daily removal, disinfection, and reinsertion may be appropriate for support of the ocular surface.21,22
PROSE Treatment
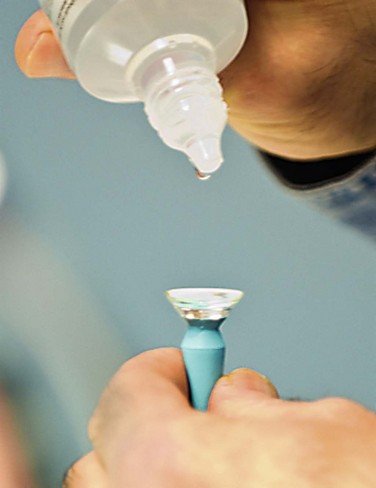
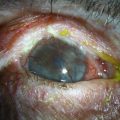
The critical design and fitting features in PROSE treatment are that the device is fitted with no corneal contact, with fluid ventilation, and with minimal movement. One of the design features that distinguishes a PROSE device from a conventional scleral lens is that the contour of a PROSE device is defined mathematically by software that uses spline functions rather than by a superposition of base curves, yielding front and back surface profiles that are malleable and junctionless. At insertion, the device is filled with sterile saline or artificial tears, which remains oxygenated, due to the gas permeability of the material (Fig. 35.3). This fluid exchanges minimally with the precorneal tear film because of the precision of the fit. PROSE devices are distinguished from conventional scleral lenses in the degree to which they are customized per eye in order to eliminate movement, conjunctival impingement, and tissue compression that might ultimately reduce patient comfort and physiological tolerance.
A PROSE device creates a transparent and smooth optical surface over the irregular, damaged or diseased cornea. It reestablishes a healthy ocular surface environment by creating an expanded artificial tear reservoir that provides constant lubrication while maintaining necessary oxygen supply. A PROSE device then supports the fragile ocular surface by shielding the cornea and conjunctiva against insult from the environment and lids. PROSE treatment promotes healing, improves vision by masking surface corneal irregularities, and mitigates pain and photophobia by supporting and stabilizing the ocular surface (Fig. 35.4).
A 2000 report17 reviewed the experience with PROSE for treatment of OSD in 49 patients (76 eyes). Improvement in best-corrected visual acuity (defined as a gain of two or more Snellen lines) was observed in 40 (53%) of the eyes. Forty-five (92%) of 49 patients reported improvement in quality of life from reduction in photophobia and discomfort. In a report from 2010,19 101 consecutive patients seen in consultation for PROSE treatment in 2006 were reviewed. The full spectrum of corneal disease, including corneal ectasia and postoperative astigmatism is represented, but nearly half of patients (38 of 80) who completed fitting were referred for treatment of OSD. Mean visual acuity (VA) in the OSD cohort improved by −0.22 (logMAR). The authors report an increase in mean NEI VFQ-2523,24 scores for the entire cohort from 57.0 to 77.8. Findings were similar for the OSD group, with only two patients recording a decrease in VFQ score in this group. Greater benefits in the VFQ subcategories of eye pain, photophobia, and role difficulties were seen in the OSD group than in the ectasia and astigmatism subgroup. Cost effectiveness25 of PROSE treatment in this cohort was also reported. As availability of PROSE treatment has increased, reports of clinical impact in cohorts with subgroups of OSD have arisen both in the United States15 and abroad.26
Prevention and Treatment of Complications
Microbial keratitis, sterile melt, and corneal neovascularization are potential complications of contact lenses for ocular surface disease. In the absence of ocular surface disease, it is established that rates of infection is highest with extended wear, compared to daily wear regardless of soft lens type, and that RGP lens wear has the lowest infection rate.11,12 Lens wear in the presence of punctate keratopathy or frank geographical defect of the corneal epithelium can be presumed to put the cornea at higher risk of infection, as would concurrent steroid use. There are no good data on prophylaxis or infection rate for contact lens use in the setting of OSD.
Corneal neovascularization is a subacute complication of therapeutic contact lens wear, particularly in the setting of persistent epithelial defects, inflammatory processes, tight lens, or low Dk values. When neovascularization occurs, consideration must be given as to whether it is related to lens material, design and/or fit, or to the underlying disease, because treatment approaches vary, and lens discontinuation is not necessarily required. Vigilance and interdisciplinary collaboration between ophthalmology and optometry is required to avoid or treat this complication of contact lens wear when it arises in the setting of OSD. Maneuvers, such as switching to a higher-Dk lens, avoiding lens care products with epithelial toxicity, and introduction of topical steroid may allow for maintenance of the surface and regression of vessels without requiring the discontinuation of contact lens wear. At the authors’ center, VEG-F inhibitors have been used as adjunct to PROSE treatment in the treatment of corneal neovascularization associated with OSD.27
An area of particular challenge is OSD in the setting of advanced glaucoma. Glaucoma patients may have surface breakdown resulting from either toxicity of topical agents, preservatives used in their formulation, and/or limbal stem cell deficiency (LSCD) from prior surgical interventions. Presence of a filtering bleb or tube shunt is a relative contraindication to contact lens wear in general and scleral lens fitting in particular because of risk of erosion and/or infection in an eye with direct communication between the anterior or posterior chambers and the subconjunctival space. The presence of the tube and plate(s) of a valve shunt may preclude a stable contact lens fit and may put the overlying conjunctiva at risk of erosion. Large-diameter hydrogel lenses are sometimes used for bleb leaks, and might be an appropriate choice for OSD in the presence of a filtering bleb or shunt. If a tube shunt is required for a patient with pre-existing cornea or OSD, it may be advisable to suggest to the surgeon pars plana entry for the tube and very posterior placement of the plates, using a long tube to facilitate subsequent-contact lens fitting and wear. PROSE devices can be fitted, with difficulty, in some patients with glaucoma tubes and trabeculectomies, which are usually relative contraindications to contact lens wear in general, particularly with scleral lens wear. Device modification after manufacture is the primary approach to dealing with limbal variants that are related to previous surgery.28
Contact Lens for Specific Ocular Surface diseases
Soft contact lenses, on an extended wear basis, can result in satisfactory resolution of recurrent erosion syndrome (RES). A therapeutic lens is sometimes abandoned in favor of stromal micropuncture, superficial keratectomy with or without diamond burr polishing, or phototherapeutic keratectomy after a single trial of a suboptimal lens, or of well-fitted and tolerated lens worn for insufficient duration. Typically, months of wear, rather than weeks are required. Duration of wear in reports of effectiveness include 3 months,29 and mean of 6 months.30 If symptoms recur after discontinuation of lens wear it is this author’s practice to double duration of wear prior to trial without lens. Lens selection, duration of therapy, frequency of exchange or disinfection, and use of antibiotic in the setting of treatment of RES vary from setting to setting.
Persistent Corneal Epithelial Defect (PED)
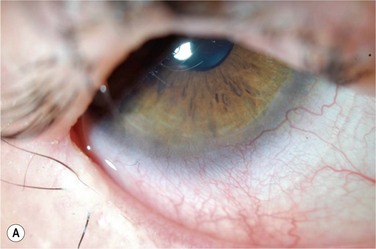
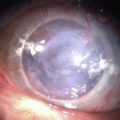
Contact lens wear is an important option in the treatment of a persistent corneal epithelial defect (PED).31 Therapeutic soft lens wear is used in normal eyes postoperatively in refractive surgery after surface ablation and in the case of traumatic and surgical wound leaks; in the latter cases, the local edema induced by a low-Dk lens might be desirable. In instances of a frank epithelial defect in the absence of a wound leak, a higher-Dk lens designed, manufactured, and labeled for extended wear or therapeutic use is advisable (Fig. 35.5). Appropriate fit may be a higher priority than material or labeling to achieve clinical tolerance. If prophylactic antibiotics are used, care should be taken to avoid drugs that are toxic. It is this author’s opinion that topical steroids are not absolutely contraindicated in cases of PED treated with therapeutic contact lens, and that the risks of rebound inflammation must be weighed against the known risks of topical steroid use. Combination treatment with therapeutic lens and autologous serum eye drops may be more effective in PED than either treatment individually.32,33
Effectiveness of PROSE treatment for PED has been reported.15,21 Recent experience with the use of non-preserved fluoroquinolones in the device reservoir for overnight wear in the setting of PED and daily monitoring have reduced the apparent rate of infection in PROSE treatment of PED. Extended wear of a PROSE device is effective in promoting the healing of a PED in some eyes that fail to heal after other therapeutic measures.21,34 Infection was associated with extended wear, until the introduction of non-preserved fourth-generation fluoroquinolone antibiotics and the codification of device wear and hygiene regimens. Re-epithelialization appears to be supported by a combination of oxygenation, moisture, and protection of the fragile epithelium provided by the device.
Limbal Stem Cell Deficiency (LSCD)
Therapeutic contact lenses have been reported as an adjunct to the treatment of LSCD of various etiologies. Soft contact lens therapy for aniridic ocular surface disease after penetrating keratoplasty has been reported.35 Scleral lenses are reported as beneficial in LSCD of uncertain etiology36 and in a case of ocular cicatricial pemphigoid.37 PROSE treatment has been reported effective in the treatment of LSCD after treatment of conjunctival melanoma.38 The specific type of lens for the specific entity is probably less important than the skill and experience of the clinician who is fitting lenses in these inflamed and sensitive eyes. All three reports involving epitheliopathy ascribed to LSCD, point to the capacity of a well-fitted scleral lens or PROSE device to improve clinical function by improving the environment at the ocular surface. Contact lens wear holds promise as a platform for expansion and transplantation of expanded stem cell populations. There is an early report on the use of an FDA-approved contact lens as the substrate and carrier of corneal stem cells in treatment of ocular surface melanoma and aniridia.39
Stevens–Johnson syndrome (SJS)
A retrospective review from France, published in 2009 by Tougeron-Brousseau et al.,40 details the authors’ experience fitting scleral RGP lenses in a cohort of 39 consecutive patients. Sixty-seven eyes of the 39 patients diagnosed with Stevens–Johnson syndrome (SJS) or toxic epidermal necrolysis (TEN) were deemed candidates for scleral RGP wear at consultation. OSDI, NEI-VFQ-25, and VA were all outcome measures with the investigators finding significant improvement in all three categories. Mean follow-up was 33.3 months (range 16 to 54 months). The authors did not report on the patients they classified as non-candidates.
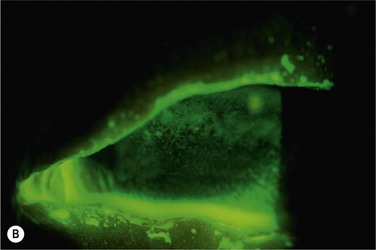
Patients with SJS/TEN have been described in the major reports17,19,41 on PROSE treatment from the past two decades, although there has not been specific analysis of this disease subgroup. It is the authors’ experience that PROSE treatment is life changing for patients suffering from the ocular sequelae of SJS. Typically, there is marked improvement in surface breakdown and debilitating photophobia (Fig. 35.6). PROSE treatment can heal a PED in SJS on full-time-wear basis, and then support the healed surface on a daily-wear basis.21 The limiting factors for PROSE treatment in SJS are presence of symblephara to the cornea or limbus, and poor cooperation in children.
Rathi et al.26 from India report their experience with PROSE treatment for 20 eyes in SJS patients who were treated primarily for relief of pain and photophobia and secondarily for improvement in vision. Although no method was taken to quantify the symptomatic relief, all patients reported a reduction in symptoms, and the authors concluded that their findings regarding PROSE treatment of SJS patients was encouraging.
Chronic Graft-versus-Host disease (cGVHD)
PROSE treatment has high impact in patients suffering from the ocular graft-versus-host disease (GVHD) as indicated in two reports from 2007. Jacobs and Rosenthal42 report on the impact of PROSE treatment in cGVHD with benefit in comfort, vision, reading, and driving, and Takahide et al.43 report on benefit on vision, clinical findings, and OSDI. Jupiter lenses (Medlens Innovations, Front Royal, VA, or Essilor Contact Lens, Inc., Dallas, TX)44 and silicone hydrogel soft-contact lenses (Air Optix Night & Day, Alcon, Forth Worth, TX)45 can also play a role in the management of GVHD. All four studies found improvement in symptoms, with Russo et al.45 and Takahide et al.43 both noting significant changes in OSDI scores. Ocular cGVHD is a disease for which scleral or soft lenses or PROSE treatment is an important option, since little else provides symptomatic relief for these patients. Until ocular cGVHD can be prevented or medically suppressed, therapeutic lens, and PROSE treatment in particular, is an important approach to improved quality of life in patients with cGVHD.
Allergy
Ocular surface disease associated with allergy can be treated with therapeutic contact lens wear. Large-diameter soft hydrogel lens wear contributed to the resolution of vernal ulcer in children.46 Scleral RGP lenses had favorable response in management of the ocular surface and visual rehabilitation of 10 patients with medically controlled advanced atopic keratoconjunctivitis.47 Atopic keratoconjunctivitis is a cause of disabling ocular surface disease and is not infrequently associated with keratoconus. PROSE treatment with its high degree of customization allows for the optical correction of keratoconus when there is coexistent atopy, which typically reduces contact lens tolerance.
Exposure Keratopathy
Contact lens wear can reduce desiccation and resultant corneal surface breakdown in instances of anatomic or paralytic exposure keratitis. Underlying etiologies include cranial nerve VII dysfunction from Bell’s palsy and tumor impingement or nerve damage following tumor resection, as with acoustic neuroma, schwannoma, or parotid gland tumors. Additional causes include trauma, history of cosmetic blepharoplasty or ptosis surgery, and thermal injury. Soft lenses are prone to the same desiccation as the ocular surface, so retention can be problematic. Hydrogel lenses of very large diameter offer increased retention. The benefits of PROSE treatment for exposure keratitis have been reported.22,48,49
A PROSE device is a valuable option for treatment of corneal exposure. Rosenthal and Croteau50 reported success of PROSE treatment in 4 of 10 eyes with exposure (both paralytic and anatomic) when other measures, short of complete tarsorrhaphy, failed. In 2008, Lin et al.49 described a case study in which PROSE treatment proved to be a useful adjunct in a patient with history of lymphoma and cGVHD. The patient was treated for a secondary facial sarcoma with surgical and radiation therapy. PROSE treatment allowed for preservation of the ocular surface and the globe. PROSE treatment is an alternative to tarsorrhaphy in the management of both anatomic and paralytic exposure keratitis.
References
1. Fick, AE. A contact lens. 1888. Arch Ophthalmol. 1997;115:120–121.
2. Pearson, RM. Karl Otto Himmler, manufacturer of the first contact lens. Cont Lens Anterior Eye. 2007;30:11–16.
3. Ezekiel, D. Gas permeable haptic lenses. Br Contact Lens Assoc. 1983;6:158–161.
4. Karlgard, CC, Jones, LW, Moresoli, C. Survey of bandage lens use in North America, October-December 2002. Eye Contact Lens. 2004;30:25–30.
5. Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5:163–178.
6. Lindahl, KJ, DePaolis, MD, Aquavella, JV, et al. Applications of hydrophilic disposable contact lenses as therapeutic bandages. CLAO J. 1991;17:241–243.
7. Bouchard, CS, Trimble, SN. Indications and complications of therapeutic disposable Acuvue contact lenses. CLAO J. 1996;22:106–108.
8. Bendoriene, J, Vogt, U. Therapeutic use of silicone hydrogel contact lenses in children. Eye Contact Lens. 2006;32:104–108.
9. Kanpolat, A, Uçakhan, OO. Therapeutic use of focus Night & Day contact lenses. Cornea. 2003;22:726–734.
10. Lim, L, Tan, DT, Chan, WK. Therapeutic use of Bausch & Lomb PureVision contact lenses. CLAO J. 2001;27:179–185.
11. Stapleton, F, Keay, L, Edwards, K, et al. The incidence of contact lens-related microbial keratitis in Australia. Ophthalmology. 2008;115:1655–1662.
12. Dart, JK, Radford, CF, Minassian, D, et al. Risk factors for microbial keratitis with contemporary contact lenses: a case-control study. Ophthalmology. 2008;115:1647–1654.
13. Visser, ES, Visser, R, van Lier, HJ, et al. Modern scleral lenses part I: clinical features. Eye Contact Lens. 2007;33:13–20.
14. Visser, ES, Visser, R, van Lier, HJ, et al. Modern scleral lenses part II: patient satisfaction. Eye Contact Lens. 2007;33:21–25.
15. Gumus, K, Gire, A, Pflugfelder, SC. The successful use of Boston ocular surface prosthesis in the treatment of persistent corneal epithelial defect after herpes zoster ophthalmicus. Cornea. 2010;29:1465–1468.
16. Pullum, K, Buckley, R. Therapeutic and ocular surface indications for scleral contact lenses. Ocul Surf. 2007;5:40–48.
17. Romero-Rangel, T, Stavrou, P, Cotter, J, et al. Gas-permeable scleral contact lens therapy in ocular surface disease. Am J Ophthalmol. 2000;130:25–32.
18. Severinsky, B, Millodot, M. Current applications and efficacy of scleral contact lenses – a retrospective study. J Optometry. 2010;03:158–163.
19. Stason, WB, Razavi, M, Jacobs, DS, et al. Clinical benefits of the Boston Ocular Surface Prosthesis. Am J Ophthalmol. 2010;149:54–61.
20. Ye, P, Sun, A, Weissman, BA. Role of mini-scleral gas-permeable lenses in the treatment of corneal disorders. Eye Contact Lens. 2007;33:111–113.
21. Rosenthal, P, Cotter, JM, Baum, J. Treatment of persistent corneal epithelial defect with extended wear of a fluid-ventilated gas-permeable scleral contact lens. Am J Ophthalmol. 2000;130:33–41.
22. Kalwerisky, K, Davies, B, Mihora, L, et al. Use of the Boston ocular surface prosthesis in the management of severe periorbital thermal injuries: a case series of 10 patients. Ophthalmology. 2012;119:516–521.
23. Mangione, CM, Lee, PP, Gutierrez, PR, et al. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119:1050–1058.
24. Raphael, BA, Galetta, KM, Jacobs, DA, et al. Validation and test characteristics of a 10-item neuro-ophthalmic supplement to the NEI-VFQ-25. Am J Ophthalmol. 2006;142:1026–1035.
25. Shepard, DS, Razavi, M, Stason, WB, et al. Economic appraisal of the Boston Ocular Surface Prosthesis. Am J Ophthalmol. 2009;148:860-868 e2.
26. Rathi, VM, Mandathara, PS, Dumpati, S, et al. Boston ocular surface prosthesis: An Indian experience. Ind J Ophthalmol. 2011;59:279–281.
27. Jacobs, DS, Lim, M, Carrasquillo, KG, et al. Bevacizumab for corneal neovascularization. Ophthalmology. 2009;116:592–593. [author reply 3–4].
28. Tanhehco, T, Jacobs, DS. Technological advances shaping scleral lenses: the Boston ocular surface prosthesis in patients with glaucoma tubes and trabeculectomies. Semin Ophthalmol. 2010;25:233–238.
29. Fraunfelder, FW, Cabezas, M. Treatment of recurrent corneal erosion by extended-wear bandage contact lens. Cornea. 2011;30:164–166.
30. Moutray, TN, Frazer, DG, Jackson, AJ. Recurrent erosion syndrome – the patient’s perspective. Cont Lens Anterior Eye. 2011;34:139–143.
31. Blackmore, SJ. The use of contact lenses in the treatment of persistent epithelial defects. Cont Lens Anterior Eye. 2010;33:239–244.
32. Choi, JA, Chung, SH. Combined application of autologous serum eye drops and silicone hydrogel lenses for the treatment of persistent epithelial defects. Eye Contact Lens. 2011;37:370–373.
33. Schrader, S, Wedel, T, Moll, R, et al. Combination of serum eye drops with hydrogel bandage contact lenses in the treatment of persistent epithelial defects. Graefes Arch Clin Exp Ophthalmol. 2006;244:1345–1349.
34. Rosenthal, P, Cotter, J. The Boston Scleral Lens in the management of severe ocular surface disease. Ophthalmol Clin N Am. 2003;16:89–93.
35. Ozbek, Z, Raber, IM. Successful management of aniridic ocular surface disease with long-term bandage contact lens wear. Cornea. 2006;25:245–247.
36. Schornack, MM. Limbal stem cell disease: management with scleral lenses. Clin Exp Optom. 2011.
37. Schornack, MM, Baratz, KH. Ocular cicatricial pemphigoid: the role of scleral lenses in disease management. Cornea. 2009;28:1170–1172.
38. Grover, S, Jacobs, DS, Colby, KA. Boston Ocular Surface Prosthesis for persistent epitheliopathy after treatment of conjunctival melanoma. Cornea. 2010;29:459–461.
39. Di Girolamo, N, Bosch, M, Zamora, K, et al. A contact lens-based technique for expansion and transplantation of autologous epithelial progenitors for ocular surface reconstruction. Transplantation. 2009;87:1571–1578.
40. Tougeron-Brousseau, B, Delcampe, A, Gueudry, J, et al. Vision-related function after scleral lens fitting in ocular complications of Stevens–Johnson syndrome and toxic epidermal necrolysis. Am J Ophthalmol. 2009;148:852-859 e2.
41. Schein, OD, Rosenthal, P, Ducharme, C. A gas-permeable scleral contact lens for visual rehabilitation. Am J Ophthalmol. 1990;109:318–322.
42. Jacobs, DS, Rosenthal, P. Boston scleral lens prosthetic device for treatment of severe dry eye in chronic graft-versus-host disease. Cornea. 2007;26:1195–1199.
43. Takahide, K, Parker, PM, Wu, M, et al. Use of fluid-ventilated, gas-permeable scleral lens for management of severe keratoconjunctivitis sicca secondary to chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2007;13:1016–1021.
44. Schornack, MM, Baratz, KH, Patel, SV, et al. Jupiter scleral lenses in the management of chronic graft versus host disease. Eye Contact Lens. 2008;34:302–305.
45. Russo, PA, Bouchard, CS, Galasso, JM. Extended-wear silicone hydrogel soft contact lenses in the management of moderate to severe dry eye signs and symptoms secondary to graft-versus-host disease. Eye Contact Lens. 2007;33:144–147.
46. Quah, SA, Hemmerdinger, C, Nicholson, S, et al. Treatment of refractory vernal ulcers with large-diameter bandage contact lenses. Eye Contact Lens. 2006;32:245–247.
47. Margolis, R, Thakrar, V, Perez, VL. Role of rigid gas-permeable scleral contact lenses in the management of advanced atopic keratoconjunctivitis. Cornea. 2007;26:1032–1034.
48. Williams, ZR, Aquavella, JV. Management of exposure keratopathy associated with severe craniofacial trauma. J Cataract Refract Surg. 2007;33:1647–1650.
49. Lin, SJ, Jacobs, DS, Frankenthaler, R, et al. An ocular surface prosthesis as an innovative adjunct in patients with head and neck malignancy. Otolaryngol Head Neck Surg. 2008;139:589–591.
50. Rosenthal, P, Croteau, A. Fluid-ventilated, gas-permeable scleral contact lens is an effective option for managing severe ocular surface disease and many corneal disorders that would otherwise require penetrating keratoplasty. Eye Contact Lens. 2005;31:130–134.
51. Pushker, N, Dada, T, Vajpayee, RB, et al. Neurotrophic keratopathy. CLAO J. 2001;27:100–107.