CHAPTER 18 Constipation
DEFINITION AND PRESENTING SYMPTOMS
The definition of constipation varies among people, and it is important to ask patients what they mean when they say “I am constipated.” Most persons are describing a perception of difficulty with bowel movements or a discomfort related to bowel movements. The most common terms used by young healthy adults to define constipation are straining (52%), hard stools (44%), and the inability to have a bowel movement (34%).1
The definition of constipation also varies among physicians and other health care providers. The traditional medical definition of constipation, based on the 95% lower confidence limit for healthy adults in North America and the United Kingdom,2 has been three or fewer bowel movements/week. Reports of stool frequency, however, are often inaccurate and do not correlate with complaints of constipation.3 In an attempt to standardize the definition of constipation, a consensus definition was initially developed by international experts in 1992 (Rome I criteria)4 and was revised in 1999 and in 2006 (Rome II and III criteria, respectively; Table 18-1).5,6
| Two or more of the following six must be present*: |
* Criteria fulfilled for the previous three months with symptom onset at least six months prior to diagnosis. In addition, loose stools should rarely be present without the use of laxatives, abdominal pain is not required, and there should be insufficient criteria for irritable bowel syndrome. These criteria may not apply when the patient is taking laxatives.
The Rome criteria incorporate the multiple symptoms of constipation, of which stool frequency is only one, and require that a minimum of two symptoms be present at least 25% of the time. Unlike the Rome I criteria, the Rome II criteria include symptoms suggestive of outlet obstruction (e.g., a sensation of anorectal blockage or obstruction and use of maneuvers to facilitate defecation). The Rome III criteria allow patients to have occasional loose stools and require that symptoms be present during the previous three months, with an onset at least six months earlier. When abdominal pain or discomfort is the predominant symptom, irritable bowel syndrome (IBS), rather than constipation, should be considered to be the diagnosis (see Chapter 118). Intermittently loose stools unrelated to laxative use also suggest a diagnosis of IBS. Although distinguishing IBS from constipation alone is important, the symptoms and pathophysiology of these entities overlap substantially.
EPIDEMIOLOGY
PREVALENCE
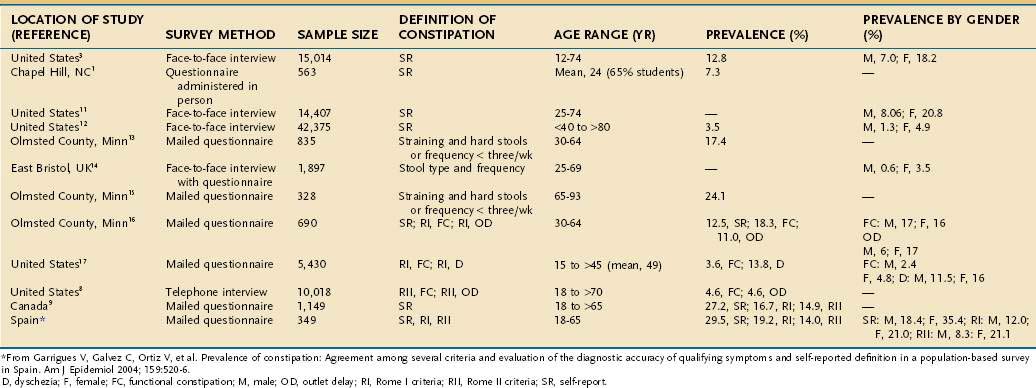
The prevalence of constipation ranges from 2% to 28% of the population in Western countries (Table 18-2)7–17 and varies depending on the demographics of the population, definition of constipation (e.g., self-reported symptoms, fewer than three bowel movements/week, or the Rome criteria), and method of questioning (e.g., postal questionnaire, interview). Some studies have attempted to identify subcategories of constipation based on the symptom pattern. In general, the prevalence is highest when constipation is self-reported9 and lowest when the Rome II criteria for constipation are applied. When the Rome II criteria are used to diagnose constipation, the effects of gender, race, socioeconomic status, and level of education on the prevalence of constipation are reduced.10
INCIDENCE
Little is known about the incidence of constipation in the general population. Talley and colleagues studied 690 nonelderly residents of Olmsted County, Minnesota, at baseline and after 12 to 20 months.18 Constipation, defined as frequent straining at stool and passing hard stool, a stool frequency of fewer than three stools/week, or both, was present in 17% of respondents on the first survey and 15% on the second survey. The rate of new constipation in this study was 50/1000 person-years, whereas the disappearance rate was 31/1000 person-years. Robson and colleagues found that 12.5% of older persons (mean age, 83 years) entering a nursing home had constipation and that constipation developed in 7% over three months of follow-up.19
PUBLIC HEALTH PERSPECTIVE
Constipation results in more than 2.5 million physician visits, 92,000 hospitalizations, and several hundred million dollars of laxative sales/year in the United States.20 Eighty-five percent of physician visits for constipation lead to a prescription for laxatives or cathartics.21 The cost of testing alone in patients with constipation has been estimated to be $6.9 billion annually.22 Among patients with constipation seen in a tertiary referral center, the average cost of a medical evaluation was $2,252, with the greatest cost attributed to colonoscopy.23
In an analysis of physician visits for constipation in the United States between 1958 and 1986, 31% of patients who required medical attention were seen by general and family practitioners, followed by internists (20%), pediatricians (15%), surgeons (9%), and obstetricians-gynecologists (9%). Only 4% of patients were seen by gastroenterologists, suggesting that few such patients were deemed to need advice from a specialist.20,21 In a National Canadian Survey, 34% of persons who reported constipation had seen a physician for their symptoms.9
RISK FACTORS
Risk factors for constipation in the United States include female gender, advanced age, nonwhite ethnicity, low levels of income and education, and low level of physical activity.3,8,11,24 Other risk factors include use of certain medications and particular underlying medical disorders (see later). Diet and lifestyle also may play a role in the development of constipation (Table 18-3).
Table 18-3 Risk Factors for Constipation
GENDER
The prevalence of self-reported constipation is two to three times higher in women than in men,10–1216 and infrequent bowel movements (e.g., once a week) are reported almost exclusively by women.25 In one study of 220 normal subjects eating their normal diets, 17% of women, but only 1% of men, passed less than 50 g of stool daily.26 The reason for the female predominance is unknown. A reduction in levels of steroid hormones has been observed in women with severe idiopathic constipation, although the clinical significance of this finding is dubious.27 An overexpression of progesterone receptors on colonic smooth muscle cells has been reported to down-regulate contractile G proteins and up-regulate inhibitory G proteins.28
In addition, overexpression of progesterone receptor B on colonic muscle cells, thereby making them more sensitive to physiologic concentrations of progesterone, has been proposed as an explanation for severe slow-transit constipation in some women.29
AGE
The prevalence of self-reported constipation among older adults ranges from 15% to 30%, with most,7,21,24,30,31 but not all,8,9,12,17 studies showing an increase in prevalence with age. Constipation is particularly problematic in nursing home residents, among whom constipation is reported in almost half and 50% to 74% use laxatives on a daily basis.32,33 Similarly, hospitalized older patients appear to be at high risk of developing constipation. A study of patients on a geriatrics ward in the United Kingdom showed that up to 42% had a fecal impaction.34
Older adults also tend to seek medical assistance for constipation more commonly than their younger counterparts. In an analysis of physician visits for constipation in the United States between 1958 and 1986, the frequency was about 1% in persons younger than 60, between 1% and 2% in those 60 to 65, and between 3% and 5% in those older than 65 years.21
Constipation in older adults is most commonly the result of excessive straining and hard stools30 rather than a decrease in stool frequency. In a community sample of 209 people ages 65 to 93 years, the main symptom used to describe constipation was the need to strain at defecation; 3% of men and 2% of women reported that their average bowel frequencies were less than three/week.29 Possible causes for the increased frequency of straining in older adults include decreased food intake, reduced mobility, weakening of abdominal and pelvic wall muscles, chronic illness, psychological factors, and medications, particularly pain-relieving drugs.19,32
Constipation is also common in children younger than 4 years.33 For example, in Great Britain, the frequency of a consultation for constipation in general practice was 2% to 3% for children ages 0 to 4, approximately 1% for women ages 15 to 64, 2% to 3% for both genders ages 65 to 74, and 5% to 6% for patients ages 75 years or older. Fecal retention with fecal soiling is a common cause of impaired quality of life and the need for medical attention in childhood.
ETHNICITY
In North America, constipation is reported more commonly by nonwhites than whites. In a survey of 15,014 persons, the frequency of constipation in whites was 12.2%, compared with 17.3% in nonwhites.3 Both groups demonstrate similar age-specific increases in prevalence.7 In developing countries, constipation is less common among the native populations, in whom stool weights are three to four times more than the median of 106 g daily in Britain.26 In rural Africa, constipation appears to be rare.
SOCIOECONOMIC CLASS AND EDUCATION LEVEL
The prevalence of constipation is influenced by socioeconomic status. In population-based surveys, subjects with a lower income status have higher rates of constipation as compared with those who have a higher income.3,6–8 In a survey of approximately 9000 Australians, men and women of lower socioeconomic status were more likely to report constipation than those of higher socioeconomic status.35 Similarly, persons who have less education tend to have an increased prevalence of constipation as compared with those who have more education.3,8,9,11,16
DIET AND PHYSICAL ACTIVITY
Cross-sectional studies have not linked low intake of fiber with constipation,29,36 yet data suggest that increased consumption of fiber decreases colonic transit time and increases stool weight and frequency.22 An analysis from the Nurses Health Study, which assessed the self-reported bowel habits of 62,036 women between the ages of 36 and 61 years, demonstrated that women who were in the highest quintile of fiber intake and who exercised daily were 68% less likely to report constipation than women who were in the lowest quintile of fiber intake and exercised less than once a week.24 Although other observational studies have supported a protective effect of physical activity on constipation, results from trials designed to test this hypothesis are conflicting. In a trial designed to assess the effect of regular exercise on chronic constipation, symptoms did not improve after a four-week exercise program.37 In healthy sedentary subjects, a nine-week program of progressively increasing exercise had no consistent effect on whole-gut transit time or stool weight.38
Dehydration has been identified as a potential risk factor for constipation. Some but not all observational studies have found an association between slowed intestinal transit time and dehydration.36,39 Although patients with constipation are advised routinely to increase their intake of fluid, the benefit of increased fluid intake has not been investigated thoroughly.
MEDICATION USE
Persons who use certain medications are at a substantially higher risk of constipation. In a review of 7251 patients with chronic constipation (and nonconstipated controls) from a general practice database, medications that were significantly associated with constipation were opioids, diuretics, antidepressants, antihistamines, antispasmodics, anticonvulsants, and aluminum antacids (Table 18-4).40 The use of aspirin or other nonsteroidal anti-inflammatory drugs in the older population is associated with a small but significantly increased risk of constipation.14
| Mechanical Obstruction |
COLONIC FUNCTION
LUMINAL CONTENTS
The main contents of the colonic lumen are food residue, water and electrolytes, bacteria, and gas. Unabsorbed food entering the cecum contains carbohydrates that are resistant to digestion and absorption by the small intestine, such as starches and nonstarch polysaccharides (NSPs). Some of the unabsorbed carbohydrate serves as substrate for bacterial proliferation and fermentation, yielding short-chain fatty acids and gas (see Chapter 16). On average, bacteria represent approximately 50% of stool weight.41 In an analysis of feces from nine healthy subjects on a metabolically controlled British diet, bacteria constituted 55% of the total solids, and fiber represented approximately 17% of the stool weight.42
A meta-analysis of the effect of wheat bran on colonic function has suggested that bran increases stool weight and decreases mean colonic transit time in healthy volunteers.42 The effect of bran may be the result primarily of increased bulk within the colonic lumen; the increased bulk stimulates propulsive motor activity. The particulate nature of some fibers also may stimulate the colon. For example, ingestion of coarse bran, 10 g twice daily, was shown to reduce colonic transit time by about one third, whereas ingestion of the same quantity of fine bran led to no significant decrease.41 Similarly, ingestion of inert plastic particles similar in size to coarse bran increased fecal output by almost three times their own weight and decreased colonic transit time.43
ABSORPTION OF WATER AND SODIUM
The colon avidly absorbs sodium and water (see Chapter 99). Increased water absorption can lead to smaller, harder stools. The colon extracts most of the 1000 to 1500 mL of fluid that crosses the ileocecal valve, and leaves only 100 to 200 mL of fecal water daily. Less reabsorption of electrolytes and nutrients takes place in the colon than in the small intestine, and sodium-chloride exchange and short-chain fatty acid transport are the principal mechanisms for stimulating water absorption. Colonic absorptive mechanisms remain intact in patients with constipation. One proposed pathophysiologic mechanism in slow-transit constipation is that the lack of peristaltic movement of contents through the colon allows more time for bacterial degradation of stool solids and increased NaCl and water absorption, thereby decreasing both stool weight and frequency.44 The volume of stool water and quantity of stool solids seem to be reduced proportionally in constipated persons.45
DIAMETER AND LENGTH
A wide or long colon may lead to a slow colonic transit rate (see Chapter 96). Although only a small fraction of patients with constipation have megacolon or megarectum, most patients with dilatation of the colon or rectum report constipation. Colonic width can be measured on barium enema films. A width of more than 6.5 cm at the pelvic brim is abnormal and has been associated with chronic constipation.46
MOTOR FUNCTION
Colonic muscle has four main functions (see also Chapter 98): (1) delays passage of the luminal contents so as to allow time for the absorption of water; (2) mixes the contents and allows contact with the mucosa; (3) allows the colon to store feces between defecations; and (4) propels the contents toward the anus. Muscle activity is affected by sleep and wakefulness, eating, emotion, the contents of the colon, and drugs. Nervous control is partly intrinsic and partly extrinsic by the sympathetic nerves and the parasympathetic sacral outflow.
Transit of contents along the colon takes hours or days (longer than transit in other portions of the gastrointestinal tract). In a study of 73 healthy subjects, the mean colonic transit time was 35 hours.47 In another similar study, the mean colonic transit time in healthy volunteers was 34 hours, with an upper limit of normal of 72 hours.48
Scintigraphic studies in constipated subjects have shown that overall transit of colonic contents is slow. In some patients, the rate of movement of contents is approximately normal in the ascending colon and hepatic flexure but delayed in the transverse and left colon. Other patients show slow transit in the right and left sides of the colon.49
Colonic propulsions are of two basic types, low-amplitude propagated contractions (LAPCs) and high-amplitude propagated contractions (HAPCs).50 The frequency and duration of HAPCs are reduced in some patients with constipation. In one study, 14 chronically constipated patients with proved slow transit of intestinal contents and one or fewer bowel movements weekly were compared with 18 healthy subjects. Four of the patients had no peristaltic movement, whereas peristaltic movement was normal in all the healthy subjects during a 24-hour period. Peristaltic movements in other subjects with constipation were fewer in number and shorter in duration, and thus passed for a shorter distance along the colon, as compared with the findings in the healthy controls. All the healthy subjects reported abdominal discomfort or an urge to defecate during peristaltic movements, and two defecated, whereas only four of the 14 subjects with constipation experienced any sensation during such movements, and none defecated.51
INNERVATION AND THE INTERSTITIAL CELLS OF CAJAL
Proximal colonic motility is under the involuntary control of the enteric nervous system, whereas defecation is voluntary. Slow-transit constipation may be related to autonomic dysfunction.52,53 Histologic studies have shown abnormal numbers of myenteric plexus neurons involved in excitatory or inhibitory control of colonic motility, thereby resulting in decreased amounts of the excitatory transmitter substance P54 and increased amounts of the inhibitory transmitters vasoactive intestinal polypeptide (VIP) or nitric oxide (NO).55
Interstitial cells of Cajal (ICCs) are the intestinal pacemaker cells and play an important role in regulating gastrointestinal motility. They facilitate the conduction of electrical current and mediate neural signaling between enteric nerves and muscles. ICCs initiate slow waves throughout the gastrointestinal tract. Confocal images of ICCs in patients with slow-transit constipation show not only reduced numbers but also abnormal morphology of ICCs, with irregular surface markings and a decreased number of dendrites. In patients with slow-transit constipation, the number of ICCs has been shown to be decreased in the sigmoid colon56 or the entire colon.57,58 Pathologic examination of colectomy specimens of 14 patients with severe intractable constipation has revealed decreased numbers of ICCs and myenteric ganglion cells throughout the colon.59
DEFECATORY FUNCTION
The process of defecation in healthy persons begins with a predefecatory period, during which the frequency and amplitude of propagating sequences (three or more successive pressure waves) are increased. Stimuli such as waking and meals (gastroileal reflex, also referred to as gastrocolic reflex) can stimulate this process. This predefecatory period is blunted, and may be absent, in patients with slow-transit constipation.50 The gastroileal reflex also is diminished in persons with slow-transit constipation. Stool is often present in the rectum before the urge to defecate arises. The urge to defecate is usually experienced when stool comes into contact with receptors in the upper anal canal. When the urge to defecate is resisted, retrograde movement of stool may occur and transit time increases throughout the colon (see Chapter 98).60
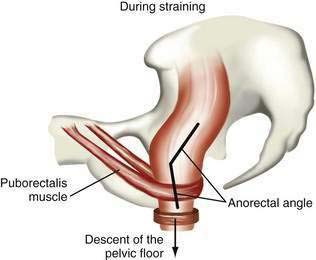
Although the sitting or squatting position seems to facilitate defecation, the benefit of squatting has not been studied in patients with constipation. Full flexion of the hips stretches the anal canal in an anteroposterior direction and straightens the anorectal angle, thereby promoting emptying of the rectum.61 Contraction of the diaphragm and abdominal muscles raises intrapelvic pressure, and the pelvic floor relaxes simultaneously. Striated muscular activity expels rectal contents, with little contribution from colonic or rectal propulsive waves. Coordinated relaxation of the puborectalis muscle, which maintains the anorectal angle, and external anal sphincter at a time when pressure is increasing in the rectum results in expulsion of stool (Fig. 18-1).
The length of the colon emptied during spontaneous defecation varies but most commonly extends from the descending colon to the rectum.62 When the propulsive action of smooth muscle is normal, defecation usually requires minimal voluntary effort. If colonic and rectal waves are infrequent or absent, however, the normal urge to defecate may not occur.51
SIZE AND CONSISTENCY OF STOOL
In a study of normal subjects who were asked to expel single hard spheres of different sizes from the rectal ampulla, the intrarectal pressure and time needed to pass the objects varied inversely with their diameters. Small hard stools are more difficult to pass than large soft stools. When larger stimulated stools were tested, a hard stool took longer to expel than a soft silicone rubber object of approximately the same shape and volume. Similarly, more subjects were able to expel a 50-mL water-filled compressible balloon than a hard 1.8-cm sphere.63
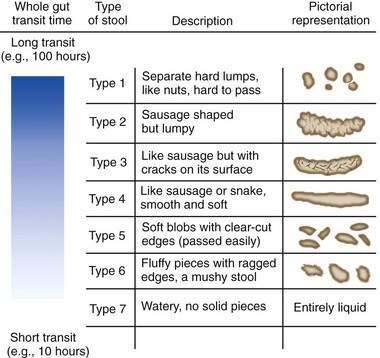
Human stools may vary in consistency from small hard lumps to liquid. The water content of stool determines consistency. Rapid colonic transit of fecal residue leads to diminished water absorption and (perhaps counterintuitively) an increase in the bacterial content of the stool. The Bristol Stool Scale25 is used in the assessment of constipation and is regarded as the best descriptor of stool form and consistency (Fig. 18-2). Stool consistency appears to be a better predictor of whole-gut transit time than of defecation frequency or stool volume.64
CLASSIFICATION
Mechanical small and large bowel obstruction, medications, and systemic illnesses can cause constipation, and these causes of secondary constipation must be excluded, especially in patients presenting with a new onset of constipation (see Table 18-4). Most often, however, constipation is caused by disordered function of the colon or rectum (functional constipation). Functional constipation can be divided into three broad categories—normal-transit constipation, slow-transit constipation, and defecatory or rectal evacuation disorders (Table 18-5). In a study of more than 1000 patients with functional constipation who were evaluated at the Mayo Clinic, 59% were found to have normal-transit constipation, 25% had defecatory disorders, 13% had slow-transit constipation, and 3% had a combination of a defecatory disorder and slow-transit constipation.65
Table 18-5 Clinical Classification of Functional Constipation
| CATEGORY | FEATURES | CHARACTERISTIC FINDINGS |
|---|---|---|
| Normal-transit constipation | Incomplete evacuation; abdominal pain may be present but not a predominant feature | Normal physiologic test results |
| Slow-transit constipation | Infrequent stools (e.g., ≤1/wk); lack of urge to defecate; poor response to fiber and laxatives; generalized symptoms, including malaise and fatigue; more prevalent in young women | Retention in colon of >20% of radiopaque markers five days after ingestion |
| Defecatory disorders (pelvic floor dysfunction, anismus, descending perineum syndrome, rectal prolapse) | Frequent straining; incomplete evacuation; need for manual maneuvers to facilitate defecation | Abnormal balloon expulsion test and/or rectal manometry |
PATHOPHYSIOLOGY
NORMAL-TRANSIT CONSTIPATION
In normal-transit constipation, stool travels along the colon at a normal rate.66 Patients with normal-transit constipation may have misperceptions about their bowel frequencies and often exhibit psychosocial distress.67 Some patients have abnormalities of anorectal sensory and motor function indistinguishable from those in patients with slow-transit constipation.68 Whether increased rectal compliance and reduced rectal sensation are effects of chronic constipation or contribute to the failure of the patients to experience an urge to defecate is unclear. Most patients, however, have normal physiologic testing. IBS with constipation differs from normal-transit constipation in that abdominal pain is the predominant symptom in IBS (see Chapter 118).
SLOW-TRANSIT CONSTIPATION
Slow-transit constipation is most common in young women and is characterized by infrequent bowel movements (less than one bowel movement/week). Associated symptoms include abdominal pain, bloating, and malaise. Symptoms are often intractable, and conservative measures such as fiber supplements and osmotic laxatives are usually ineffective.69,70 The onset of symptoms is gradual and usually occurs around the time of puberty. Slow-transit constipation arises from disordered colonic motor function. Patients who have mild delays in colonic transit have symptoms similar to those seen in persons with IBS.71 In patients with more severe symptoms, the pathophysiology includes delayed emptying of the proximal colon and fewer HAPCs after meals. Colonic inertia is a term used to describe the disorder in patients with symptoms at the severe end of the spectrum. In this condition, colonic motor activity fails to increase after a meal,72 ingestion of bisacodyl,73 or administration of a cholinesterase inhibitor such as neostigmine.74
DEFECATORY DISORDERS
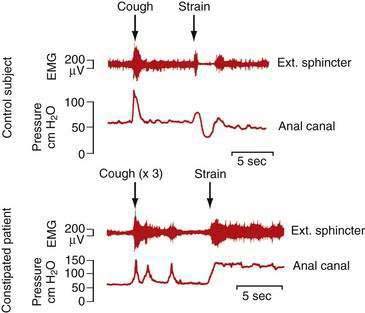
Defecatory disorders arise from failure to empty the rectum effectively because of an inability to coordinate the abdominal, rectoanal, and pelvic floor muscles. Many patients with defecatory disorders also have slow-transit constipation75 Defecatory disorders are also known as anismus, dyssynergia, pelvic floor dyssynergia, spastic pelvic floor syndrome, obstructive defecation, or outlet obstruction. These disorders appear to be acquired and may start in childhood. They may be a learned behavior to avoid some discomfort associated with the passage of large hard stools or pain associated with attempted defecation in the setting of an active anal fissure or inflamed hemorrhoids. Patients with defecatory disorders commonly have inappropriate contraction of the anal sphincter when they bear down (Fig. 18-3). This phenomenon can occur in asymptomatic subjects but is more common among patients who complain of difficult defecation.76 Some patients with a defecatory disorder are unable to raise intrarectal pressure to a level sufficient to expel stool, a disturbance that manifests clinically as failure of the pelvic floor to descend on straining.77
Defecatory disorders are particularly common in older patients with chronic constipation and excessive straining, many of whom do not respond to standard medical treatment.78 Defecatory disorders rarely are associated with structural abnormalities such as rectal intussusception, an obstructing rectocele, megarectum, or excessive perineal descent.79
Patients with defecatory disorders may report infrequent bowel movements, ineffective and excessive straining, and the need for manual disimpaction; however, symptoms, particularly in the case of pelvic floor dysfunction, do not correlate with physiologic findings.80 For a diagnosis of a defecatory disorder, a Rome working group81 has specified the criteria listed in Table 18-6. In patients with this disorder, constipation is functional and caused by dysfunction of the pelvic floor muscles as determined by physiologic tests. Pelvic floor dyssynergia is a subset of these patients in which the anal sphincter fails to relax more than 20% of its basal resting pressure during attempted defecation, despite the presence of adequate propulsive forces in the rectum.
Table 18-6 Rome III Criteria for Functional Defecation Disorders81*
| The patient must satisfy diagnostic criteria for functional constipation (see Table 18-1). |
| During repeated attempts to defecate, the patient must have at least two of the following: |
EMG, electromyography.
* Criteria fulfilled for the previous three months with symptom onset at least six months prior to diagnosis.
Functional fecal retention (FFR) is the most common defecatory disorder in children. It is a learned behavior that results from withholding defecation, often because of fear of a painful bowel movement.82 The symptoms are common and may result in secondary encopresis (fecal incontinence) because of leakage of liquid stool around a fecal impaction. FFR is the most common cause of encopresis in childhood (see Chapter 17).83
DISORDERS OF THE ANORECTUM AND PELVIC FLOOR
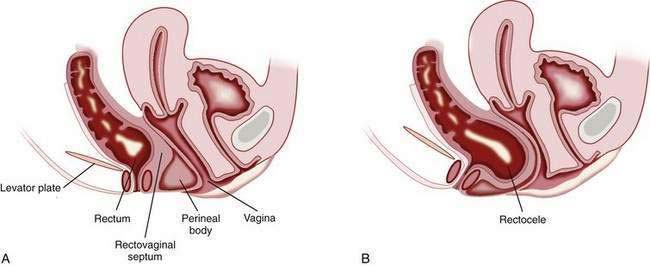
RECTOCELE
A rectocele is the bulging or displacement of the rectum through a defect in the anterior rectal wall. In women, the perineal body supports the anterior rectal (posterior vaginal) wall above the anorectal junction, and a layer of fascia runs from the rectovaginal pouch of Douglas to the perineal body and adheres to the posterior vaginal wall. The anterior rectal wall is unsupported above the level of the perineal body, and the rectovaginal septum can bulge anteriorly to form a rectocele (Fig. 18-4). Rectoceles can arise from damage to the rectovaginal septum or its supporting structures during vaginal childbirth. These injuries are exacerbated by repetitive increases in intra-abdominal pressure and the long-term effects of gravity. Prolapse of other pelvic organs may be present. For example, urinary incontinence, as well as a previous hysterectomy, has been reported to be more common in patients with a rectocele than in patients with difficult defecation but no demonstrable rectocele.84
Studies using defecating proctography (see later) have shown that rectoceles are common in symptomless healthy women and may protrude as much as 4 cm from the line of the anterior rectal wall without causing bowel symptoms, although 2 cm is the generally accepted lower limit of a rectocele that may be regarded as clinically significant.85 Symptomatic patients report the inability to complete fecal evacuation, perineal pain, sensation of local pressure, and appearance of a bulge at the vaginal opening on straining. Women may report the need to use their thumb or fingers to support the posterior vaginal wall to complete defecation.84 Women also may report the need to use a finger to evacuate the rectum digitally.
Defecating proctography can be used to demonstrate a rectocele, measure its size, and determine whether barium becomes trapped within the rectocele. In one study, trapping of barium in rectoceles changed with the degree of rectal emptying and was related to the size of the rectocele86; however, the size of the rectocele or degree of emptying on defecation has not been shown to correlate with the outcome of surgical repair.87,88
Asymptomatic women with rectoceles do not require surgical treatment. Kegel exercises (designed to strengthen the pelvic floor muscles that support the urethra, bladder, uterus, and rectum) and instructions to avoid repetitive increases in intra-abdominal pressure may help prevent progression of the rectocele. Surgery should be considered only for patients in whom contrast is retained during defecography and patients in whom constipation is relieved with digital vaginal pressure to facilitate defecation.89 Surgical repair can be performed by endorectal, transvaginal, or transperineal approaches. Other types of genital prolapse may also be present, and collaboration between the surgeon and gynecologist may be appropriate. In carefully selected patients surgical repair benefits approximately 75% of patients. In a review of 89 women who underwent a combined transvaginal and transanal rectocele repair for symptoms of obstructive defecation, the repair was successful in 71% of patients, as assessed by the absence of symptoms after one year.90 Reduction in the size of the rectocele, as judged by defecating proctography, does not appear to correlate clearly with improvement in symptoms.88
DESCENDING PERINEUM SYNDROME
In the descending perineum syndrome, the pelvic floor descends to a greater extent than normal (1 to 4 cm) when the patient strains during defecation, and rectal expulsion is difficult. The anorectal angle is widened as a result of pelvic floor weakness, and the rectum is more vertical than normal. The perineal body is weak (thereby facilitating formation of a rectocele), and the lax muscular support favors intrarectal mucosal intussusception or rectal prolapse. The pelvic floor may not provide the resistance necessary for extrusion of solid stool through the anal canal. A common reason for pelvic floor weakness is trauma or stretching during parturition. In some cases, repeated and prolonged defecation appears to be a damaging factor. Symptoms include constipation, incomplete rectal evacuation, excessive straining and, less commonly, digital rectal evacuation.91 Electrophysiologic studies show partial denervation of the striated muscle and evidence of pudendal nerve damage. Histologic examination of operative specimens of the pelvic floor muscles confirms loss of muscle fibers.
DIMINISHED RECTAL SENSATION
The urge to defecate depends in part on tension within the rectal wall (determined by the tone of the circular muscle of the rectal wall), rate and volume of rectal distention, and size of the rectum. Some patients with constipation appear to feel pain normally as the rectum is distended to the maximal tolerable volume, but they fail to experience an urge to defecate with intermediate volumes.92 In a study of women with severe idiopathic constipation, a higher than normal electrical stimulation current applied to the rectal mucosa was required to elicit pain, thereby suggesting a possible rectal sensory neuropathy.93
Rectal hyposensitivity (RH) is defined as insensitivity of the rectum to balloon distention on anorectal physiologic investigation, although the pathophysiology of RH is not entirely clear. Constipation is the most common presenting symptom of RH. In an investigation of 261 patients with RH, 38% had a history of pelvic surgery, 22% had a history of anal surgery, and 13% had a history of spinal trauma.94
RECTAL PROLAPSE AND SOLITARY RECTAL ULCER SYNDROME
Rectal prolapse refers to complete protrusion of the rectum through the anus (see Chapter 125). Occult (asymptomatic) rectal prolapse has been found in 33% of patients with clinically recognized rectoceles and defecatory dysfunction.95 Rectal prolapse can be detected easily on physical examination by asking the patient to strain as if to defecate. A laparoscopic rectopexy—in which the prolapsed rectum is raised and secured with sutures to the adjacent fascia—is the recommended treatment.96
Solitary rectal ulcer syndrome is a rare disorder characterized by erythema or ulceration generally of the anterior rectal wall as a result of chronic straining (see Chapter 115). Mucus and blood may be passed when the patient strains during defecation.97,98 Endoscopic findings may include erythema, hyperemia, mucosal ulceration, and polypoid lesions. Misdiagnosis may occur because of the heterogeneous findings and misleading name of the syndrome (an ulcer need not be present). In a study of 98 patients with solitary rectal ulcer syndrome, 26% were initially diagnosed incorrectly. In patients with a rectal ulcer or mucosal hyperemia, the most common misdiagnoses were Crohn’s disease and ulcerative colitis. In those with a polypoid lesion, the most common misdiagnosis was a neoplastic polyp.99 Histology of full-thickness specimens of the lesion reveals extension of the muscularis mucosa between crypts and disorganization of the muscularis propria. Defecography, transrectal ultrasonography, and anorectal manometry are helpful in the diagnosis.
Varying degrees of rectal prolapse exist in association with solitary rectal ulcer syndrome. Rectal prolapse and paradoxical contraction of the puborectalis muscle can lead to rectal trauma because of the high pressures generated within the rectum. In addition, rectal mucosal blood flow is reduced.100
Medical treatment may be difficult, and a single optimal therapy does not exist. The patient should be advised to resist the urge to strain. Bulk laxatives and dietary fiber may be of some benefit.101 Surgery may be required; rectopexy is performed most commonly. Of patients who undergo surgery for solitary rectal ulcer syndrome with rectal prolapse, 55% to 60% report long-term satisfaction, although a colostomy is eventually required in approximately one third of patients.102 Repair of a rectal prolapse may aggravate constipation. Biofeedback appears to be a promising mode of therapy for patients with solitary rectal ulcer syndrome.103
SYSTEMIC DISORDERS
HYPOTHYROIDISM
Constipation is the most common gastrointestinal complaint in patients with hypothyroidism. The pathologic effects are caused by an alteration of intestinal motor function and possible infiltration of the intestine by myxedematous tissue. The basic electrical rhythm that generates peristaltic waves in the duodenum decreases in hypothyroidism, and small bowel transit time is increased.104 Myxedema megacolon is rare but can result from myxedematous infiltration of the muscle layers of the colon. Symptoms include abdominal pain, flatulence, and constipation.105
DIABETES MELLITUS
The mean colonic transit time is longer in diabetics than in healthy controls. In one study, the mean total colonic transit time in 28 diabetic patients (34.9 ± 29.6 hours; mean ± SD) was significantly longer than that in 28 healthy subjects (20.4 ± 15.6 hours; P < 0.05).106 Among the 28 diabetic patients, 9 of 28 (32%) met the Rome II criteria for constipation and 14 of 28 (50%) had cardiovascular autonomic neuropathy. The mean colonic transit times in diabetic patients with and without cardiovascular autonomic neuropathy were similar. By contrast, a previous study reported that asymptomatic diabetic patients with cardiovascular autonomic neuropathy had significantly longer whole-gut transit times (although still within the range of normal) than a control group without evidence of neuropathy.107 In another study, diabetic patients with mild constipation demonstrated delayed colonic myoelectrical and motor responses after ingestion of a standard meal, whereas diabetics with severe constipation had no increases in these responses after food. Neostigmine increased colonic motor activity in all diabetic patients, suggesting that the defect was neural rather than muscular (see Chapter 35).108
NERVOUS SYSTEM DISEASE
PARKINSON’S DISEASE
Constipation occurs frequently in patients with Parkinson’s disease (PD). In a study of 12 patients with PD compared with normal controls, slow colonic transit, decreased phasic rectal contractions, weak abdominal wall muscle contraction, and paradoxical anal sphincter contraction on defecation were all features in patients with PD and frequent constipation.110 Loss of dopamine-containing neurons in the central nervous system is the underlying defect in PD; a defect in dopaminergic neurons in the enteric nervous system also may be present. Histopathologic studies of the myenteric plexuses of the ascending colon in 11 patients with PD and constipation revealed that in 9 patients, the number of dopamine-positive neurons was one tenth or less the number in control subjects. Dopamine concentrations in the muscularis externa were significantly lower in patients with PD than in controls (P < 0.01).111
Another possible contributor to constipation is the inability of some patients with PD to relax the striated muscles of the pelvic floor on defecation. This finding is a local manifestation of the extrapyramidal motor disorder that affects skeletal muscle. Preliminary observations suggest that injection of botulinum toxin into the puborectalis muscle is a potential therapy for this type of outlet dysfunction constipation in patients with PD.112,113
MULTIPLE SCLEROSIS
Constipation is common among patients with multiple sclerosis (MS). In an unselected group of 280 patients with MS, the frequency of constipation (defined as diminished bowel frequency, digitation to facilitate defecation, or the use of laxatives) was approximately 43%. Almost 25% of the subjects passed fewer than three stools/week, and 18% used a laxative more than once a week. Constipation correlated with the duration of MS but preceded the diagnosis of MS in 45% of subjects. Constipation did not correlate with immobility or the use of medications.114 In another questionnaire study of 221 patients with MS, the frequency of constipation was as high as 54%.115 Constipation in patients with MS can be multifactorial and related to a reduction in postprandial colonic motor activity, limited physical activity, and medications with constipating side effects.
Patients with advanced MS and constipation have evidence of a visceral neuropathy. In a group of patients with advanced MS and severe constipation, all had evidence of disease in the lumbosacral spinal cord and decreased compliance of the colon. Motor and electrophysiologic measurements have shown that the usual increase in colonic motor activity after meals is absent. Among less severely affected patients, slow colonic transit and manometric evidence of pelvic floor muscular and anal sphincter dysfunction have been demonstrated. Patients may have fecal incontinence.116,117 Therapy with biofeedback has been reported to relieve constipation and fecal incontinence, although in a study of 13 patients with MS who underwent biofeedback for either constipation or incontinence, only 38% improved (see Chapter 17).118
SPINAL CORD LESIONS
Lesions Above the Sacral Segments
Spinal cord lesions or injury above the sacral segments lead to an upper motor neuron disorder, with severe constipation. The resulting delay in colonic transit affects the rectosigmoid colon primarily.119,120 In a study of patients with severe thoracic spinal cord injury, colonic compliance was abnormal, with a rapid rise in colonic pressure on instillation of relatively small volumes of fluid. Motor activity after meals did not increase, but the colonic response to neostigmine was normal, thereby suggesting absence of myopathy.
Studies of anorectal function in patients with severe traumatic spinal cord injury have shown that rectal sensation to distention is abolished, although a dull pelvic sensation is experienced by some patients at maximum levels of rectal balloon distention. Anal relaxation on rectal distention is exaggerated and occurs at a lower balloon volume than in normal subjects. Distention of the rectum leads to a linear increase in rectal pressure, without the plateau at intermediate values seen in normal subjects, and ends in high-pressure rectal contractions after a relatively small volume (100 mL) has been instilled into the balloon. As expected, the rectal pressure generated by straining is lower in patients than in control subjects and is less with higher than lower spinal cord lesions. Patients demonstrate a loss of conscious external anal sphincter control, and the sphincter does not relax on straining, suggesting that in normal subjects, descending inhibitory pathways are present.121 These findings explain why some patients with spinal cord lesions experience not only constipation, but also sudden uncontrollable rectal expulsion with incontinence. Other patients cannot empty the rectum in response to laxatives or enemas, possibly because of failure of the external anal sphincter to relax, and they may require manual evacuation.
Electrical stimulation of anterior sacral nerve roots S2, S3, and S4 via electrodes implanted for urinary control in paraplegic patients leads to a rise in pressure within the sigmoid colon and rectum and contraction of the external anal sphincter. Contraction of the rectum and relaxation of the internal anal sphincter persist for a short time after the stimulus ceases. By appropriate adjustment of the stimulus in one study, it was possible for 5 of 12 paraplegic patients to evacuate feces completely and for most of the others to increase the frequency of defecation and reduce the time spent emptying the rectum.122 In another series, left-sided colonic transit time decreased with regular sacral nerve stimulation.123
Lesions of the Sacral Cord, Conus Medullaris, Cauda Equina, and Nervi Erigentes (S2 to S4)
Neural integration of anal sphincter control and rectosigmoid propulsion occurs in the sacral segments of the spinal cord. The motor neurons that supply the striated sphincter muscles are grouped in Onuf’s nucleus at the level of S2. There is evidence that efferent parasympathetic nerves that arise in the sacral segments enter the colon at the region of the rectosigmoid junction and extend distally in the intermuscular plane to reach the level of the internal anal sphincter and proximally to the midcolon via the ascending colonic nerves, which retain the structure of peripheral nerves (see Chapter 98).124
Damage to sacral segments of the spinal cord or to efferent nerves leads to severe constipation. Fluoroscopic studies show a loss of progression of contractions in the left colon. When the colon is filled with fluid, the intraluminal pressure generated is lower than normal, in contrast with the situation after higher lesions of the spinal cord. The distal colon and rectum may dilate, and feces may accumulate in the distal colon. Spasticity of the anal canal can occur. Loss of sensation of the perineal skin may extend to the anal canal, and rectal sensation may be diminished. Rectal wall tone depends on the level of the spinal lesion. In a study of 25 patients with spinal cord injury, rectal tone was significantly higher than normal in patients with acute and chronic supraconal lesions but significantly lower than normal in patients with acute and chronic conal or cauda equina lesions.125
STRUCTURAL DISORDERS OF THE COLON, RECTUM, ANUS, AND PELVIC FLOOR
OBSTRUCTION
Anal atresia in infancy, anal stenosis later in life, or obstruction of the colon may manifest as constipation. Obstruction of the small intestine generally manifests as abdominal pain and distention, but constipation and inability to pass flatus also may be features (see Chapters 96 and 119).
DISORDERS OF SMOOTH MUSCLE
Myopathy Affecting Colonic Muscle
Congenital or acquired myopathy of the colon usually manifests as pseudo-obstruction. The colon is hypotonic and inert (see Chapter 120).
Hereditary Internal Anal Sphincter Myopathy
Hereditary internal anal sphincter myopathy is a rare condition characterized by constipation with difficulty in rectal expulsion and episodes of severe proctalgia fugax, defined as the sudden onset of brief episodes of pain in the anorectal region.126–128 Three affected families have been reported. The mode of inheritance appears to be autosomal dominant with incomplete penetrance. In symptomatic persons, the internal anal sphincter muscle is thickened, and resting anal pressure is increased greatly. In two of the described patients, treatment with a calcium channel blocker improved pain but had no effect on constipation. In another family, two patients were treated by internal anal sphincter strip myectomy; one showed marked improvement and one had improvement in the constipation but only slight improvement in the pain. Examination of the muscle strips showed myopathic changes with polyglucosan bodies (glucose polymers) in the smooth muscle fibers and increased endomysial fibrosis.
Progressive Systemic Sclerosis
Progressive systemic sclerosis (scleroderma) may lead to constipation. In patients with progressive systemic sclerosis and constipation, 9 of 10 had no increase in colonic motor activity after ingestion of a 1000-kcal meal. Histologic examination of colonic specimens from these subjects revealed smooth muscle atrophy of the colonic wall (see Chapter 35).129
Muscular Dystrophies
Muscular dystrophies usually are regarded as disorders of striated muscle, but visceral smooth muscle also may be abnormal. In myotonic muscular dystrophy, a condition in which skeletal muscle fails to relax normally, megacolon may be found, and abnormal function of the anal sphincter is demonstrable.130 Cases associated with intestinal pseudo-obstruction have been reported (see Chapter 120).131
DISORDERS OF ENTERIC NERVES
Congenital Aganglionosis or Hypoganglionosis
Congenital absence or reduction in the number of ganglia in the colon leads to functional colonic obstruction with proximal dilatation, as seen in Hirschsprung’s disease and related conditions (see Chapter 96). In Hirschsprung’s disease, ganglion cells in the distal colon are absent because of an arrest in the caudal migration of neural crest cells in the intestine during embryonic development. Although most patients present during early childhood, often with delayed passage of meconium, some patients with a relatively short segment of involved colon present later in life.132 Typically, the colon narrows at the area that lacks ganglion cells, and the bowel proximal to the narrowing is usually dilated. Two genetic defects have been identified in patients with Hirschsprung’s disease—a mutation in the RET (rearranged during transfection) proto-oncogene, which is involved in the development of neural crest cells, and a mutation in the gene that encodes the endothelin B receptor, which affects intracellular calcium levels.133,134
Hypoganglionosis is reported when small, sparse myenteric ganglia are seen. Neuronal counts can be made on full-thickness tissue specimens and compared with published reference values obtained from autopsy material. Establishing the diagnosis of hypoganglionosis is not easy, because of variations in the normal density of neurons.135 Quantitative declines in the number of neurons in the enteric nervous system also are seen in patients with severe slow-transit constipation and characterized morphologically as oligoneuronal hypoganglionosis.136
Congenital Hyperganglionosis (Intestinal Neuronal Dysplasia)
Congenital hyperganglionosis, or intestinal neuronal dysplasia, is a developmental defect characterized by hyperplasia of the submucosal nerve plexus. Clinical manifestations of the disease are similar to those seen in Hirschsprung’s disease and include young age of onset and symptoms of intestinal obstruction (see Chapter 96). In contrast to functional constipation, affected children do not have symptoms of soiling or evidence of a fecaloma.137 A multicenter study of interobserver variation in the histologic interpretation of findings in children with constipation caused by abnormalities of the enteric nervous system has shown complete agreement in the diagnosis of Hirschsprung’s disease but accord in only 14% of children with colonic motility disorders other than aganglionosis. Some of the clinical features and histologic changes previously associated with congenital hyperganglionosis may be age-related and evolve to normal as children age.135 A diagnosis of congenital hyperganglionosis can be made on the basis of hyperganglionosis of the submucous plexus with giant ganglia and at least one of the following features in rectal biopsy specimens: (1) ectopic ganglia; (2) increased acetylcholinesterase (AChE) activity in the lamina propria; and (3) increased AChE nerve fibers around the submucosal blood vessels. Most patients with congenital hyperganglionosis respond to conservative treatment, including laxatives. Internal anal sphincter myectomy may be performed if conservative management fails.138
Acquired Neuropathies
Chagas’ disease, which results from infection with Trypanosoma cruzi, is the only known infectious neuropathy. The reason for neuronal degeneration in this disorder is unclear but may have an immune basis.139 Patients present with progressively worsening symptoms of constipation and abdominal distention resulting from a segmental megacolon that may be complicated by sigmoid volvulus (see Chapter 109).
Paraneoplastic visceral neuropathy may be associated with malignant tumors outside the gastrointestinal tract, particularly small cell carcinoma of the lung and carcinoid tumors. Pathologic examination of the affected intestine reveals neuronal degeneration or myenteric plexus inflammation.140 An antibody against a component of myenteric neurons has been identified in some patients with this disorder (see Chapter 120).141 Disruption of the ICCs has been associated with a case of small cell lung carcinoma–related paraneoplastic colonic motility disorder.142
MEDICATIONS
Constipation may be a side effect of a drug or preparation taken long term. Drugs commonly implicated are listed in Table 18-4. Common offenders include opioids used for chronic pain, anticholinergic agents including antispasmodics, calcium supplements, some tricyclic antidepressants, phenothiazines used as long-term neuroleptics, and antimuscarinic drugs used for parkinsonism.
PSYCHOLOGICAL DISORDERS
Constipation may be a symptom of a psychiatric disorder or a side effect of its treatment (see Chapter 21). Healthy men who are socially outgoing, energetic, and optimistic—and not anxious—and who described themselves in more favorable terms than others have heavier stools than men without these personality characteristics.143 Psychological factors associated with a prolonged colonic transit time in constipated patients include a highly depressed mood state and frequent control of anger.144 In one study, women with constipation had higher somatization and anxiety scores than healthy controls, and the psychological scores correlated inversely with rectal mucosal blood flow (used as an index of innervation of the distal colon).145 In a study that assessed psychological characteristics of older persons with constipation, a delayed colonic transit time was related significantly to symptoms of somatization, obsessive-compulsiveness, depression, and anxiety.36 In a study of 28 consecutive female patients undergoing psychological assessment for intractable constipation, 60% had evidence of a current affective disorder. One third reported distorted attitudes toward food. Patients with slow-transit constipation reported more psychosocial distress on rating scales than those with normal-transit constipation.146
DEPRESSION
For some patients, constipation can be a somatic manifestation of an affective disorder. In a study of patients with depression, 27% said that constipation developed or became worse at the onset of the depression.147 Constipation can occur in the absence of other typical features of severe depression, such as anorexia or psychomotor retardation with physical inactivity. Psychological factors are likely to influence intestinal function via autonomic efferent neural pathways.145 In an analysis of 4 million discharge records of U.S. military veterans, major depression was associated with constipation, and schizophrenia was associated with both constipation and megacolon.148
EATING DISORDERS
Patients with anorexia nervosa or bulimia often complain of constipation, and a prolonged whole-gut transit time has been demonstrated in patients with these disorders.149 Colonic transit time returns to normal in most patients with anorexia nervosa once they are consuming a balanced diet and gaining weight for at least three weeks.150 Pelvic floor dysfunction is found in some patients with an eating disorder and does not improve with weight gain and a balanced diet.151
Anorexia nervosa should be considered as a possible diagnosis in a young underweight woman who presents with constipation. Patients with an eating disorder often resort to the regular use of laxatives as treatment for constipation or to facilitate weight loss or relieve the presumed consequences of binge eating. Treatment of such patients is directed at the underlying eating disorder (see Chapter 8).
CLINICAL ASSESSMENT
HISTORY
A dietary history should be obtained. The amount of daily fiber and fluid consumed should be assessed. Many patients tend to skip breakfast,152 and this practice may exacerbate constipation, because the postprandial increase in colonic motility is greatest after breakfast.72,153,154 Although caffeinated coffee (150 mg of caffeine) stimulates colonic motility, the ingestion of a meal has a greater effect.155
A detailed social history may provide useful information as to why the patient has sought help for constipation at this point in time; potentially relevant behavioral background information also may be obtained. In patients with IBS, the frequency of a history of sexual abuse is increased as compared with healthy controls.156 In a survey of 120 patients with dyssynergia, 22% reported a history of sexual abuse, and 32% reported a history of physical abuse. Bowel dysfunction adversely affected sexual life in 56% and social life in 76% of patients.157 The physician should be alert to manifestations of depression, such as insomnia, lack of energy, loss of interest in life, loss of confidence, and a sense of hopelessness. A history of physical or sexual abuse may not emerge during the initial visit, but if the physician evinces no surprise at whatever is revealed, indicates that distressing events are common in patients with intestinal symptoms, and maintains a sensitive, encouraging attitude, the full story often gradually emerges during subsequent visits, provided that there is privacy, confidentiality, and adequate time (see Chapters 21 and 118).
PHYSICAL EXAMINATION
The rectal examination is paramount in evaluating a patient with constipation. Placing the patient in the left lateral position is most convenient for performing a thorough rectal examination. Painful perianal conditions and rectal mucosal disease should be excluded, and defecatory function should be evaluated. The perineum should be observed both at rest and after the patient strains as if to have a bowel movement. Normally, the perineum descends between 1 and 4 cm during straining. Descent of the perineum with the patient in the left lateral position below the plane of the ischial tuberosities (i.e., >4 cm) usually suggests excessive perineal descent. A lack of descent may indicate the inability to relax the pelvic floor muscles during defecation, whereas excessive perineal descent may indicate descending perineum syndrome. Patients with descending perineum syndrome strain excessively and achieve only incomplete evacuation because of lack of straightening of the anorectal angle. Excessive laxity or descent of the perineum usually results from previous childbirth or excessive straining. Eventually, excessive descent of the perineum may result in injury to the sacral nerves from stretching, a reduction in rectal sensation, and ultimately incontinence resulting from denervation.91 Rectal prolapse may be detected when the patient is asked to strain.
The perianal area should be examined for scars, fistulas, fissures, and external hemorrhoids. A digital rectal examination should be performed to evaluate the patient for the presence of a fecal impaction, anal stricture, and rectal mass. A patulous anal sphincter may suggest prior trauma to the anal sphincter or a neurologic disorder that impairs sphincter function. Other important functions that should be assessed during the digital examination are summarized in Table 18-7. Specifically, the inability to insert the examining finger into the anal canal may suggest an elevated anal sphincter pressure, and tenderness on palpation of the pelvic floor as it traverses the posterior aspect of the rectum may suggest pelvic floor spasm. The degree of descent of the perineum during attempts to strain and expel the examining finger provides another way of assessing the degree of perineal descent. A thorough history and physical examination can exclude most secondary causes of constipation (see Table 18-4).
| History |
DIAGNOSTIC TESTS
TESTS TO EXCLUDE STRUCTURAL DISEASE OF THE INTESTINE
A barium enema study reveals the width and length of the colon and excludes an obstructing lesion severe enough to cause constipation. When fecal impaction is present, a limited enema study with a water-soluble contrast agent outlines the colon and fecal mass without aggravating the condition. A barium examination of the small bowel is indicated only if obstruction or pseudo-obstruction involving the small bowel is suspected (see Chapters 119 and 120). Endoscopy allows direct visualization of the colonic mucosa. The yield of colonoscopy in the absence of “alarm” symptoms in patients with chronic constipation is low and is comparable with that for asymptomatic patients who undergo colonoscopy for colon cancer screening. Therefore, a colonoscopy is recommended only when there has been a recent change in bowel habits, blood in stools, or other alarming symptoms (e.g., weight loss, fever).158,159 All adults older than 50 years who present with constipation should undergo a colonoscopy, flexible sigmoidoscopy and barium enema, or computed tomographic colonography to screen for colorectal cancer, as widely recommended. A flexible sigmoidoscopy is probably sufficient for the evaluation of constipation in patients younger than 50 years without alarm symptoms (e.g., weight loss, recent onset of severe constipation, rectal bleeding) or a family history of colon cancer.
PHYSIOLOGIC MEASUREMENTS
Physiologic testing is unnecessary for most patients with constipation and is reserved for patients with refractory symptoms who do not have an identifiable secondary cause of constipation or in whom a trial of a high-fiber diet and laxatives has not been effective. An American Gastroenterological Association Technical Review on Anorectal Testing Techniques160 has recommended the following investigations in patients with refractory constipation: symptom diaries to establish a diagnosis of constipation and monitor the efficacy of treatment; colonic transit study to confirm the patient’s complaint of constipation and assess colonic motility for slow transit and regional delay; anorectal manometry to exclude Hirschsprung’s disease and to complement other tests of pelvic floor dysfunction; and surface electromyography (EMG) to evaluate anal sphincter function and facilitate biofeedback training. Tests of possible value include the following: defecation proctography to document the patient’s inability to defecate; balloon expulsion test to document the inability to defecate; and rectal sensory testing to help distinguish functional from neurologic disorders as a cause of constipation.
Measurement of Colonic Transit Time
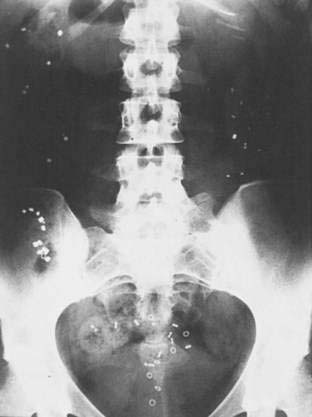
Radiopaque Markers
The normal colonic transit time is less than 72 hours. Measurement of colonic transit time is performed only when objective evidence of slow transit is needed to confirm a patient’s history or as a prelude to surgical treatment. Colonic transit time is measured by performing abdominal radiography 120 hours after the patient has ingested radiopaque markers in a gelatin capsule (Fig. 18-5). Before the study, patients should be maintained on a high-fiber diet and should avoid laxatives, enemas, or medications that may affect bowel function. Retention of more than 20% of the markers at 120 hours is indicative of prolonged colonic transit. Because the markers are eliminated only with defecation, the process of measuring colonic transit is discontinuous, and the result of a transit measurement should be regarded with caution, taking recent defecation into account. If the markers are retained exclusively in the sigmoid colon and rectum, the patient may have a defecatory disorder. The presence of markers throughout the colon, however, does not exclude the possibility of a defecatory disorder because delayed colonic transit can result from a defecatory disorder. Measurements of transit through different segments of the colon are of doubtful value in planning treatment, except for megarectum, in which all the markers move rapidly to the rectum and are retained there.
Wireless Motility Capsule
The wireless pH and pressure recording capsule (SmartPill, Buffalo, NY) is a novel ambulatory technique for assessing colonic transit without radiation. Colonic transit measurements with the wireless capsule technique have been shown to correlate well with the radiopaque marker test and also allow an assessment of gastric and small bowel transit.161
If surgical treatment for severe constipation is being considered (see later), studies of gastric emptying, small bowel transit, and segmental colonic transit times are valuable for confirming slow transit and correlating abnormalities with therapeutic outcome. In particular, scintigraphic studies of gastrointestinal transit are indicated.50 Generally, abnormal gastric or small bowel motility precludes surgical treatment of constipation.
Tests to Assess the Physiology of Defecation
Defecography
Defecography is performed by instilling thickened barium into the rectum. With the patient sitting on a radiolucent commode, films or videos are taken during fluoroscopy with the patient resting, deferring defecation, and straining to defecate. This procedure evaluates the rate and completeness of rectal emptying, anorectal angle, and amount of perineal descent. In addition, defecography can identify structural abnormalities, such as a large rectocele, internal mucosal prolapse, or intussusception. A rectocele represents a herniation, usually of the anterior rectal wall into the lumen of the vagina, and usually results from trauma during childbirth or an episiotomy.161 Paradoxical anal sphincter contraction is common in patients with a rectocele, suggesting that straining and attempts at emptying against a contracted pelvic floor may facilitate development of a rectocele. The limitations of defecography include variability among radiologists in interpreting studies, inhibition of normal rectal emptying because of patient embarrassment, and differences in texture between barium paste and stool. Confirmatory studies are needed before a decision about management can be made on the basis of the radiographic findings alone. Importantly, identified anatomic abnormalities are not always functionally relevant. For example, a rectocele is only relevant if it fills preferentially (i.e., instead of the rectal ampulla) and fails to empty after simulated defecation. Magnetic resonance defecography may offer advantages over standard barium defecography162 but is not yet widely available.
Balloon Expulsion Test
When the rectum is distended with a balloon, the internal anal sphincter relaxes. The inability to evacuate a 50- to 60-mL inflated balloon in the rectum163 while sitting on the toilet for two minutes, with the addition of 200 g of weight to the end of the balloon,93 suggests a defecatory disorder. The balloon expulsion test is an effective and useful screening tool for identifying patients with a defecatory disorder who do not have pelvic floor dyssynergia. In one study of 359 patients with constipation, the balloon expulsion test was abnormal in 21 of 24 patients with pelvic floor dyssynergia and an additional 12 of 106 patients without pelvic floor dyssynergia. (The diagnosis of pelvic floor dyssynergia was confirmed by manometric and defecographic findings according to the Rome II criteria.164)
Anorectal Manometry
Anorectal manometry can provide useful information about patients with severe constipation by assessing the resting and maximum squeeze pressure of the anal sphincters, presence or absence of relaxation of the anal sphincter during balloon distention of the rectum (rectoanal inhibitory reflex), rectal sensation, and ability of the anal sphincter to relax during straining.93,158,165 Patients with a defecatory disorder commonly have inappropriate contraction of the anal sphincter when they bear down. The absence of the rectoanal inhibitory reflex raises the possibility of Hirschsprung’s disease. A high resting anal pressure suggests the presence of an anal fissure or anismus, the paradoxical contraction of the external anal sphincter in response to straining or pressure within the anal canal. Rectal hyposensitivity suggests a neurologic disorder; however, the volume of rectal content needed to induce rectal urgency also may be increased in patients with fecal retention, and the results of rectal sensitivity testing need to be interpreted with caution.
Rectal Sensitivity and Sensation Testing
Rectal sensitivity to distention can be measured by introducing successive volumes of air into a rectal balloon and recording the volume at which the stimulus is first perceived, the volume that produces an urge to defecate, and the volume above which further addition of air can no longer be tolerated owing to discomfort. These measurements are not of value in the routine investigation of constipation but are of research interest. The threshold current needed to elicit sensation when the rectal mucosa is stimulated electrically by a current passed between bipolar electrodes can be used as a test of sensory nerve function, but the test has not been established for general use.93
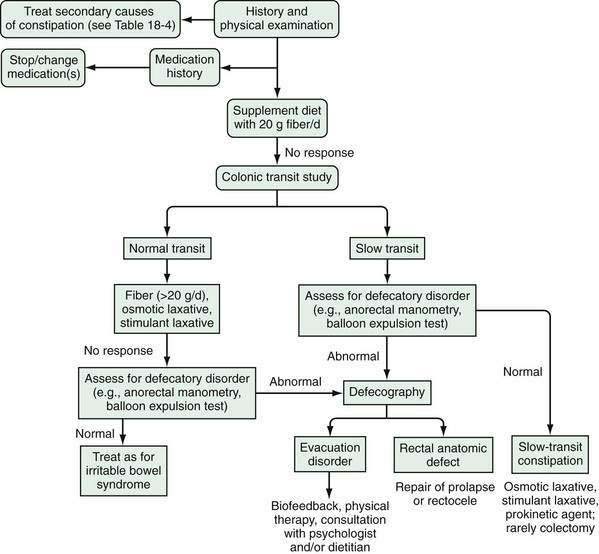
TREATMENT
Initial treatment of constipation is based on nonpharmacologic interventions. If these measures fail, then pharmacologic agents may be used. Figure 18-6 provides an algorithm for the evaluation and treatment of patients with severe constipation. If a defecatory disorder is present, initial treatment should include biofeedback; up to 75% of patients with disordered evacuation respond to biofeedback, and many do not respond well to fiber supplementation or oral laxatives. Otherwise, the initial treatment should include increased fluid, exercise, and intake of fiber, either through changes in diet or use of commercial fiber supplements. Patients who do not improve with fiber should be given an osmotic laxative, such as milk of magnesia or polyethylene glycol. The dose of the osmotic laxative should be adjusted until soft stools are attained. Stimulant agents, such as bisacodyl or senna derivatives, should be reserved for patients who do not respond to fiber or osmotic laxatives.
GENERAL MEASURES
Psychological Support
Constipation may be aggravated by stress or may be a manifestation of emotional disturbance (e.g., previous sexual abuse; see Chapter 21). For such patients, an assessment of the person’s circumstances, personality, and background and supportive advice may help more than any physical measures of treatment. Behavioral treatment (see later) offers a physical approach with a psychological component and is often acceptable and beneficial. Psychological treatment is needed only when it would be indicated in any circumstance, not specifically for constipation.
Fluid Intake
Dehydration or salt depletion is likely to lead to increased salt and water absorption by the colon, leading in turn to the passage of small hard stools. Although dehydration is generally accepted as a risk factor for constipation, unless a person is clinically dehydrated, no data support the notion that increasing fluid intake improves constipation.166
Dietary Changes and Fiber Supplementation
After studying the dietary and stool patterns of rural Africans in the early 1970s, Burkitt and colleagues speculated that a deficiency in dietary fiber was contributing to constipation and other colonic diseases in Western societies.167 Since then, studies have shown that when nonconstipated persons increase their intake of dietary fiber, stool weight increases in proportion to their baseline stool weight and frequency of defecation and correlates with a decrease in colonic transit time.168 Every gram of wheat fiber ingested yields approximately 2.7 g of stool expelled. It follows that when an increased intake of dietary fiber leads to an increase in stool weight in constipated subjects who pass small stools, the resulting stool weight may still be lower than normal. For this reason, the therapeutic results of a high-fiber diet are often disappointing as a treatment for constipation. In a study of 10 constipated women who took a supplement of wheat bran, 20 g/day, average daily stool weight increased from approximately 30 to 60 g/day, with only half of patients achieving a normal average stool weight. Bowel frequency increased from a mean of two to three bowel movements weekly.169 In a controlled, crossover trial, 24 patients took 20 g of bran or placebo daily for four weeks. Although bran was more effective than placebo in improving bowel frequency and oroanal transit rate, the occurrence and severity of constipation experienced by the patients did not differ between the two treatment periods.170 This result probably reflects the observation that patients complain mainly of difficulty in defecation, rather than a decreased frequency of bowel movements. In a series of constipated patients, about half were reported to have gained some benefit from a bran supplement of 20 g daily.171
Dietary fiber appears to be effective in relieving mild to moderate43 but not severe constipation,69 especially if severe constipation is associated with slow colonic transit, evacuation disorders, or medications. Although dietary modification may not succeed, all constipated subjects should be advised initially to increase their dietary fiber intake as the simplest, most physiologic, and cheapest form of treatment. Patients should be encouraged to take about 25 g of NSPs daily by eating whole-wheat bread, unrefined cereals, plenty of fruit and vegetables and, if necessary, a supplement of raw bran, either in breakfast cereals or with cooked foods. Specific dietary counseling often is needed to achieve a satisfactory increase in dietary fiber.
Because of side effects, adherence with fiber supplementation is poor, especially during the first several weeks of therapy. Side effects include abdominal distention, bloating, flatulence, and poor taste. Most controlled studies of the effect of fiber have shown that the minimum supplementation needed to consistently alter bowel function or colonic transit time significantly is 12 g/day. To improve adherence, patients should be instructed to increase their dietary fiber intake gradually over several weeks to approximately 20 to 25 g/day. If results of therapy are disappointing, commercially packaged fiber supplements should be tried (Table 18-8). Fiber and bulking agents are concentrated forms of NSPs based on wheat, plant seed mucilage (ispaghula), plant gums (sterculia), or synthetic methylcellulose derivatives (methylcellulose, carboxymethylcellulose; see later).
| AGENT | STARTING DAILY DOSE (G) | COMMENTS |
|---|---|---|
| Methylcellulose | 4-6 | Semisynthetic cellulose fiber that is relatively resistant to colonic bacterial degradation and tends to cause less bloating and flatus than psyllium |
| Psyllium | 4-6 | Made from ground seed husk of the ispaghula plant; forms a gel when mixed with water, so an ample amount of water should be taken with psyllium to avoid intestinal obstruction; undergoes bacterial degradation, which may contribute to side effects of bloating and flatus; allergic reactions such as anaphylaxis and asthma have been reported but are rare |
| Polycarbophil | 4-6 | Synthetic fiber made of polymer of acrylic acid, which is resistant to bacterial degradation |
| Guar gum | 3-6 | Soluble fiber extracted from seeds of the leguminous shrub Cyamopsis tetragonoloba |
SPECIFIC THERAPEUTIC AGENTS
Commercial Fiber Products
Methylcellulose
Methylcellulose is a semisynthetic NSP of varying chain length and degree of methylation. Methylation reduces bacterial degradation in the colon. One study of constipated patients with an average daily fecal weight of only 35 g showed an increase in fecal solids with 1, 2, and 4 g of methylcellulose/day, but fecal water increased only with the 4-g dose. Bowel frequency in this group of patients increased from an average of two to four stools weekly, but the patients did not report marked improvement in consistency or ease of passage of stools (see Table 18-8).172
Ispaghula (Psyllium)
Ispaghula (3.4 g as Metamucil) has been shown to increase fecal bulk to the same extent as methylcellulose 1 to 4 g daily in constipated subjects. Although both stool dry and wet weights increased, the total weekly weights remained less than those of a healthy control group without treatment. In an observational study, 149 patients were treated with psyllium in the form of Plantago ovata seeds, 15 to 30 g daily, for a period of at least 6 weeks. The response to treatment was poor among patients with slow colonic transit or a disorder of defecation, whereas 85% of patients without abnormal physiologic testing results improved or became symptom-free. Nevertheless, the authors recommend that a trial of dietary fiber be undertaken before diagnostic testing is performed.69
Ispaghula taken by mouth can cause an acute allergic immunoglobulin E–mediated response, with facial swelling, urticaria, tightness in the throat, cough, and asthma.173 Workers who inhale the compound during manufacture or preparation can have a similar reaction174 (see Table 18-8).
Calcium Polycarbophil
Calcium polycarbophil is a hydrophilic polyacrylic resin that is resistant to bacterial degradation and thus may be less likely to cause gas and bloating. In patients with IBS, calcium polycarbophil appears to improve global symptoms and ease of stool passage175 but not abdominal pain (see Table 18-8).
Other Laxatives
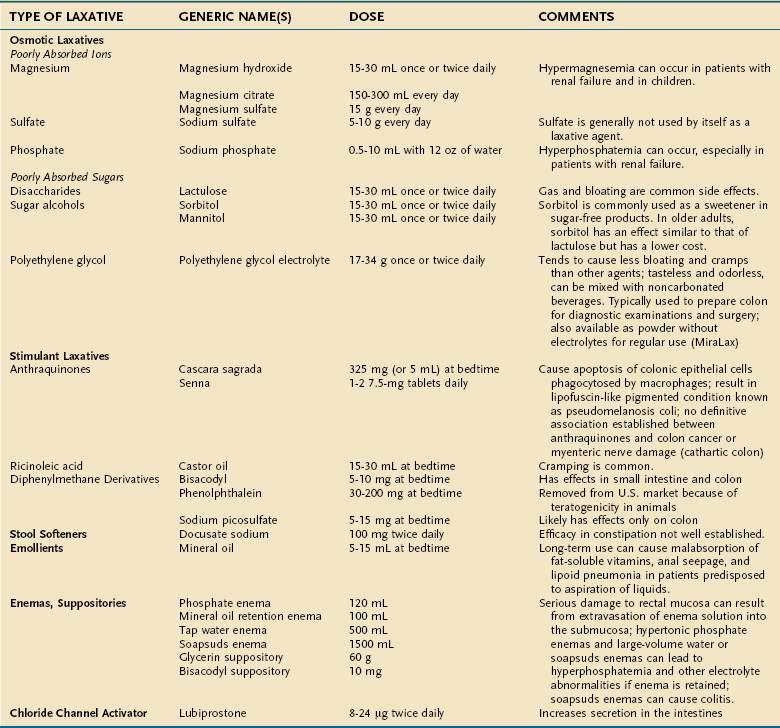
The main groups of laxatives other than fiber are osmotic agents and stimulatory laxatives; stool softeners and emollients are additional therapeutic agents (see later) (Tables 18-9 and 18-10).
Table 18-10 Grade of Evidence for the Use of Laxatives According to the American College of Gastroenterology Task Force on Chronic Constipation
| LAXATIVE | GRADE OF EVIDENCE* |
|---|---|
| Bulking agents | |
| Psyllium | B |
| Calcium polycarbophil | B |
| Bran | † |
| Stool softeners | B |
| Lubricants | C |
| Osmotic laxatives | |
| PEG | A |
| Lactulose | A |
| Milk of magnesia | † |
| Stimulant laxatives | B |
| Prokinetic agent | |
| Tegaserod‡ | A |
| Chloride channel activator | |
| Lubiprostone | § |
PEG, polyethylene glycol.
* Grade A: Based on two or more randomized controlled trials (RCTs) with adequate sample sizes and appropriate methodology. Grade B: Based on evidence from a single RCT of high quality or conflicting results from high-quality RCTs or two or more RCTs of lesser quality. Grade C: Based on noncontrolled trials or case reports.
‡ Removed from the U.S. market.
Data from Brandt LJ, Prather CM, Quigley EM, et al. Systematic review on the management of chronic constipation in North America. Am J Gastroenterol 2005; 100(Suppl 1):S5-21.
Poorly Absorbed Ions
Magnesium, sulfate, and phosphate ions are poorly absorbed by the gut and thereby create a hyperosmolar intraluminal environment. Their primary mode of action appears to be osmotic, but they may have other possible effects with unclear consequences, such as increasing prostaglandin concentrations in the stool.176 In mildly constipated patients, regular use of magnesium hydroxide is a useful and safe laxative. Stool weight increases by 7.3 g for each additional millimole of soluble magnesium excreted.177 Standard doses of magnesium hydroxide (see Table 18-9) contain 40 to 80 mmol of magnesium ion and typically produce a bowel movement within 6 hours. Magnesium sulfate is a more potent laxative that tends to produce a large volume of liquid stool. Patients may complain about this compound because it often leads to abdominal distention and the sudden passage of a liquid foul-smelling stool. Use of magnesium in older adults is limited by adverse effects such as flatulence, abdominal cramps, and magnesium toxicity.
A small percentage of magnesium is actively absorbed in the small intestine; the remainder draws water into the intestine along an osmotic gradient.178 Hypermagnesemia can occur in patients with renal failure and in children. Hypermagnesemia-induced paralytic ileus is a rare complication,179 and hypermagnesemia with coma has occurred in a normal 6-week-old infant given 16 2-mL doses of milk of magnesia.180 Severe toxicity with coma also has occurred in a chronically constipated child given an enema containing 32.5 mg of magnesium sulfate.181 Patients with renal insufficiency or cardiac dysfunction can experience electrolyte and volume overload from the absorption of magnesium or phosphorus. Even patients who are otherwise healthy can experience these complications, in addition to dehydration, as a result of excessive use.
Phosphate can be absorbed by the small intestine, and a substantial dose must be ingested to produce an osmotic laxative effect. One commercial preparation, Fleet Phospho Soda, contains 48 g (400 mmol) of monobasic sodium phosphate and 18 g (130 mmol) of dibasic sodium phosphate/100 mL, resulting in a hypertonic solution. Hyperphosphatemia can occur, especially in patients with renal insufficiency. In addition, a rare but serious form of acute kidney injury has been associated with sodium phosphate solution used before colonoscopy, even in patients with normal baseline renal function. Risk factors include hypertension, advanced age, volume depletion, and use of angiotensin-converting enzyme inhibitors or nonsteroidal anti-inflammatory drugs.182,183 The preparation is no longer available over the counter in the United States.
Poorly Absorbed Sugars
Lactulose
The recommended dose of lactulose for adults is 15 to 30 mL once or twice daily. The time to onset of action is longer than that for other osmotic laxatives, and two or three days are required for lactulose to achieve an effect. Some patients report that lactulose is effective initially but then loses its effect, perhaps because the intestinal flora are altered in response to the medication.184 Adverse effects related to lactulose include abdominal distention or discomfort, presumably as a result of colonic gas production. Cases of lactulose-induced megacolon have been reported.
In a group of young, chronically constipated volunteers who reported fewer than three stools a week, lactulose increased bowel frequency and percentage of stool moisture and softened the stools when compared with a control syrup that contained only sucrose. The effectiveness of lactulose was dose-dependent.185
The effect of lactulose in older patients has been studied in two double-blind, placebo-controlled trials. In one trial, only about half of patients were found to be truly constipated, and, among these patients, lactulose was effective in 80%, as compared with 33% of those who received placebo (glucose) (P < 0.01).186 The second trial was conducted in a nursing home over 8 to 12 weeks in 42 older patients with constipation.187 The initial dose of lactulose was 30 mL/day, and the dose was reduced temporarily or permanently to 15 mL, depending on bowel frequency. Lactulose showed an advantage over placebo (a 50% glucose syrup) by increasing the mean number of bowel movements each day and markedly reducing episodes of fecal impaction (P < 0.015) and the need for enemas.
Sorbitol
Sorbitol is used widely in the food industry as an artificial sweetener. Ingestion of as little as 5 g causes a rise in breath hydrogen, and 20 g produces diarrhea in about half of normal subjects.188 Sorbitol is as effective as lactulose and less expensive. A randomized, double-blind, crossover trial of lactulose, 20 g/day, and sorbitol, 21 g/day, in ambulant older men with chronic constipation showed no difference between the two compounds with regard to frequency or normality of bowel movements or patient preference.189 The frequency of side effects was similar except for nausea, which was more common with lactulose. Mannitol is another sugar alcohol that can be used as a laxative.
Polyethylene Glycol
Polyethylene glycol (PEG) has emerged as a safe and effective treatment for chronic constipation. It is an isosmotic laxative that is metabolically inert and able to bind water molecules, thereby increasing intraluminal water retention.190 PEG is not metabolized by colonic bacteria. Solutions containing PEG and electrolytes typically are administered orally to prepare the colon for diagnostic examinations or surgery. Ingestion of PEG leads to an increase in stool volume and softer stools, which may become liquid depending on the volume of PEG consumed. PEG is excreted unchanged in the feces. Electrolytes are added to PEG solutions used for colonic lavage before colonoscopy to avoid the potential adverse effects associated with drinking large volumes of a fluid, such as dehydration and electrolyte imbalance. PEG (with electrolytes) is also available as a powder that is mixed in smaller doses with water for regular use to treat constipation.
Several studies have demonstrated the efficacy of PEG in the treatment of chronic constipation. In a trial in which 70 ambulatory outpatients were treated for four weeks with a PEG-electrolyte solution, 250 mL once or twice daily, at the end of the four weeks, bowel frequency had increased to normal, hard stools were uncommon, and straining on defecation was experienced by fewer than 20% of patients, compared with 80% before treatment. The patients were then randomized to continue PEG or a placebo for 20 weeks in a dose of one or two packets daily, as determined to give the best result. In every parameter examined, the active treatment gave significantly improved results over placebo without adverse clinical or laboratory events. At the end of follow-up, complete remission of constipation was reported by 77% and 20% of patients randomized to PEG and placebo, respectively. The dropout rate of 46% in the placebo group, mostly secondary to treatment failure, was notable.191
In another randomized multicenter trial that compared standard and maximum doses of two PEG formulations of different molecular weights, PEG 3350 and PEG 4000, in 266 outpatients, most patients had their first stool within one day of initiating PEG treatment, and stool consistency improved in both treatment groups. The lowest dose of PEG produced the most normal stool consistency, whereas higher doses produced more liquid stools.192 Low-dose PEG appears to be more effective than lactulose in the treatment of chronic constipation.193,194 A study of 307 patients with chronic constipation who were randomized to 17 g of PEG or placebo for six months showed continued benefit of PEG compared with placebo and no electrolyte abnormalities or intestinal malabsorption.194
PEG is approved by the FDA for children, and a dose-ranging trial in children with constipation, ages six months to 15 years, has suggested that PEG is a potentially useful treatment, provided that the dose is adjusted to the child’s age.195
PEG solutions may be useful for the short-term treatment of fecal impaction. In one study,196 16 severely ill patients, ages 26 to 87 years, who, despite treatment with various laxatives, had not had a bowel movement in the hospital for 5 to 23 days, were treated with PEG. All had a fecal impaction on clinical examination. They were advised to drink 1 L of a PEG-electrolyte solution, taken as two portions of 500 mL, each over 4 to 6 hours. The regimen was repeated on a second and third day, if necessary. The full dose was taken by 12 patients on the first day, and the remainder took at least one half of the recommended dose; only 8 patients needed treatment on the second day and 2 patients on the third day. The treatment was highly effective and, after the last dose, most patients were passing moderate or large volumes of soft stool, with resolution of impaction. No adverse side effects, apart from abdominal rumbling, occurred, and only 1 patient, who was paraplegic, experienced fecal incontinence. Successful treatment with PEG has been described in outpatients with refractory constipation, older adults (with administration of PEG by mouth or by a nasogastric tube), and children with fecal impaction,197 although children have had difficulty drinking the large volume of fluid.
The most common adverse effects of PEG include abdominal bloating and cramps.190 The medication is generally well tolerated, but cases of fulminant pulmonary edema have been reported after administration of PEG solution by nasogastric tube, with one fatality.198,199 In each case, the patient had emesis, suggesting aspiration of PEG. PEG also may delay gastric emptying.200
Stimulant Laxatives
Stimulant laxatives sometimes are abused, especially in patients with an eating disorder,201 even though at high doses they have only a modest effect on calorie absorption. Although a cathartic colon (i.e., a colon with reduced motility) has been attributed to prolonged use of stimulant laxatives, no animal or human data support this effect. Rather, cathartic colon, as seen on a barium enema examination, is probably a primary motility disorder.
Anthraquinones
Anthraquinones cause apoptosis of colonic epithelial cells, which then are phagocytosed by macrophages and appear as a lipofuscin-like pigment that darkens the colonic mucosa, a condition termed pseudomelanosis coli202 (see Chapter 124 and Figures 124-7 and 124-8). Whether anthraquinone laxatives given over the long term cause adverse functional or structural changes in the intestine is controversial. Animal studies have shown neither damage to the myenteric plexus after long-term administration of sennosides203 nor a functional defect in motility.204 A case-control study in which multiple colonic mucosal biopsy specimens were examined by electron microscopy showed no differences in the submucosal plexuses between patients taking an anthraquinone laxative regularly for one year and those not taking one.205 An association between use of anthraquinones and colon cancer or myenteric nerve damage and the development of cathartic colon has not been established.206
Senna has been shown in controlled trials to soften stools207 and to increase the frequency and wet and dry weights of stool. The formulations available for clinical use vary from crude vegetable preparations to purified and standardized extracts to a synthetic compound.
Castor Oil
Castor oil comes from the castor bean. After oral ingestion, it is hydrolyzed by lipase in the small intestine to ricinoleic acid, which inhibits intestinal water absorption and stimulates intestinal motor function by damaging mucosal cells and releasing neurotransmitters.206 Cramping is a common side effect.
Diphenylmethane Derivatives
The effects of bisacodyl, and presumably sodium picosulfate, on the colon are similar to those of the anthraquinone laxatives. When applied to the colonic mucosa, bisacodyl induces an almost immediate, powerful, propulsive motor activity in healthy and constipated subjects, although the effect is sometimes reduced in the latter.208 The drugs also stimulate colonic secretion.
Like the anthraquinone laxatives, bisacodyl leads to apoptosis of colonic epithelial cells, the remnants of which accumulate in phagocytic macrophages, but these cellular remnants are not pigmented.209 Aside from these changes, bisacodyl does not appear to cause adverse effects with long-term use.210
Stool Softeners and Emollients
Docusate Sodium
Although the detergent dioctyl sodium sulfosuccinate (docusate sodium) is available as a stool softener, further study of its efficacy is needed. The compound stimulates fluid secretion by the small and large intestines but does not increase the volume of ileostomy output or the weight of stools in normal subjects.211,212 A double-blind, crossover trial has shown benefit in 5 of 15 older constipated subjects, as judged by patients and their caregivers, and a significant increase in bowel frequency.213 In a multicenter, double-blind, randomized trial in adults, docusate sodium was less effective than psyllium for the treatment of chronic idiopathic constipation.214
Enemas and Suppositories
Compounds may be introduced into the rectum to stimulate contraction by distention or chemical action, soften hard stools, or both. Serious damage to the rectal mucosa can result from extravasation of the enema solution into the submucosal plane. The anterior rectal mucosa is the site most vulnerable to trauma from the tip of a catheter introduced through the backward-angulated anal canal (see Chapter 125). The enema nozzle should be directed posteriorly after the anal canal has been passed.
Phosphate Enemas
Hypertonic sodium phosphate enemas are often effective. They cause distention and stimulation of the rectum. A histologic study in normal subjects showed that a single hypertonic phosphate enema caused disruption of the surface epithelium in 17 of 21 biopsy specimens. Scanning electron microscopy showed patchy denudation of the surface epithelium, with exposure of the lamina propria and absence of goblet cells. The proctoscopic appearance of the mucosa was abnormal in every case but returned to normal within one week.215 Therefore, superficially damaged mucosa appears to heal rapidly. Phosphate enemas are used widely, but no conclusive evidence supports their use.
Phosphate enemas, if given to a patient who cannot evacuate it promptly, can lead to dangerous hyperphosphatemia and hypocalcemic tetany; one patient, age 91 years, died after a single phosphate enema,216 and coma developed in an adult who was given six phosphate enemas at hourly intervals without evacuation.217 Severe hyperphosphatemia, hypocalcemia, and seizure have been reported in a 4-year-old child with normal renal function after retention of two phosphate enemas.218 The use of phosphate enemas in children 3 years and younger is not recommended.219,220
Saline, Tap Water, and Soapsuds Enemas
Saline, tap water, or soapsuds enemas can be effective mainly by distending the rectum and softening feces. Stool evacuation typically occurs two to five minutes following administration. A saline enema does no damage to the rectal mucosa and may be effective.215 Water enemas and soapsuds enemas also may be used, but with large volumes, dangerous water intoxication can occur if the enema is retained. Large-volume water or soapsuds enemas also can lead to hyperphosphatemia and other electrolyte disturbances if the enema is retained. Soapsuds enemas can cause rectal mucosal damage and necrosis.
Stimulant Suppositories and Enemas
Glycerin can be administered as a suppository and is often clinically effective. The rectum is stimulated by an osmotic effect. The effect of glycerin, if any, on the rectal mucosa is unknown. Bisacodyl, 10 mg, is available as a suppository that appears to act topically by stimulating enteric neurons.182 In normal subjects, a single bisacodyl suppository or an enema containing 19 mg of bisacodyl in 100 or 200 mL of water produced marked changes in 23 of 25 rectal mucosal biopsy specimens. The epithelium of the surface and within the crypt was altered; with use of the enema, the surface epithelium was absent.215 The regular use of bisacodyl suppositories thus appears unwise. Oxyphenisatin (Veripaque), which is no longer available in the United States, is a stimulant enema that was used in the past mainly before diagnostic procedures. When given by mouth, this compound led to some cases of chronic hepatitis.
Chloride Channel Activator
Lubiprostone is a novel bicyclic fatty acid that activates the chloride 2 channel, thereby increasing intestinal fluid secretion and transit221 without altering serum electrolyte levels. In two phase III randomized, placebo-controlled trials, lubiprostone, 24 µg twice daily, increased the number of spontaneous bowel movements in patients with chronic constipation as defined by the Rome II criteria. Lubiprostone also significantly decreased straining, improved stool consistency, and reduced overall severity of symptoms. The frequency of spontaneous bowel movements increased in men and women, as well as older patients, who took the drug. A rebound effect after withdrawal of the drug was not evident.222 The most common side effects were nausea, headache, and diarrhea. Lubiprostone, 24 µg twice daily, was approved by the FDA in 2006 for the treatment of men and women with chronic constipation and in 2008, in a dose of 8 µg twice daily, for women who have IBS with constipation.
Prokinetic Agents
Prokinetic agents induce increased contractility in a segment of the gastrointestinal tract. Stimulation of the 5-hydroxytryptamine4 (5-HT4) receptor on afferent nerves in the wall of the gastrointestinal tract induces peristaltic contraction of the intestine. Several 5-HT4 agonists have been tested for the treatment of constipation. Cisapride, a benzodiazepine, has had variable results in treating constipation.223 Potentially lethal cardiac dysrhythmias led to its withdrawal from the commercial market in the United States in July 2000, although it still remains available through a limited access program. Newer 5-HT4 agonists such as prucalopride and TD-5108 appear promising as future treatments for chronic constipation.
Tegaserod
Tegaserod, a partial 5-HT4 agonist, is an aminoguanidine indole derivative of serotonin that is structurally different from cisapride. Because of cardiovascular safety concerns, tegaserod was withdrawn from the market in April 2007. The frequency of cardiovascular events in previous clinical trials was 13 in 13,614 (0.11%) compared with 1 in 7031 (0.01%) in control subjects. The cardiovascular events reported were myocardial infarction (n = 3), sudden cardiac death (n = 1), unstable angina (n = 6), and stroke (n = 3). The decision of the U.S. Food and Drug Administration (FDA) to withdraw the drug has been the subject of debate.224
In a randomized, double-blind, placebo-controlled trial, 1348 subjects with chronic constipation were randomized to receive tegaserod 2 mg twice daily, tegaserod 6 mg twice daily, or placebo for 12 weeks. Response was defined as an increase in the number of complete spontaneous bowel movements (CSBMs) of one or more/week as compared with baseline. During the first 4 weeks of treatment, response rates were 41.4%, 43.2%, and 25.1% for tegaserod 2 mg twice daily, tegaserod 6 mg twice daily, and placebo recipients, respectively. The effect was maintained over 12 weeks. The median time to first CSBM was significantly shorter in patients treated with tegaserod 6 mg twice daily (73 ± 45 hours) and 2 mg twice daily (117 ± 66 hours) than in those treated with placebo (229 ± 123 hours). Tegaserod also improved the frequency and consistency of stools and reduced straining. No rebound effect was seen after withdrawal of tegaserod. Diarrhea was more common with tegaserod 2 and 6 mg twice daily (4.5% and 7.3%, respectively) than with placebo (3.8%). Most cases of diarrhea occurred within the first week of therapy and lasted a median of 2.0 days. Treatment with tegaserod did not result in any cases of electrolyte imbalance.225 Tegaserod had also been used in women with constipation-predominant IBS (see Chapter 118).226
Prucalopride
Prucalopride, a full 5-HT4 agonist, is a benzofuran derivative that induces strong contractions in the proximal colon in dogs and accelerates colonic transit in healthy humans and in patients with functional constipation.227 Three large, 12-week, randomized, placebo-controlled phase III trials of similar design that evaluated the efficacy and safety of prucalopride 2 mg or 4 mg once daily versus placebo in patients with chronic constipation have been published.228–230 Patients enrolled in these studies were required to have at least two CSBMs/week, in combination with straining, a sensation of incomplete evacuation, or hard stools, at least 25% of the time. In one of these studies, the percentage of patients achieving more than three CSBMs/week was 30.9% for those receiving prucalopride 2 mg and 28.4% for those receiving prucalopride 4 mg, compared with 12.0% in the placebo group (P < 0.001 for both comparisons). All other secondary efficacy endpoints, including patients’ satisfaction with their bowel function and treatment and their perception of the severity of their constipation symptoms, were improved significantly at week 12 with the use of 2 or 4 mg of prucalopride as compared with placebo. When the results of the phase III studies (N = 1924) were combined, the percentage of patients with an average of at least three CSBMs/week over the 12-week treatment period was 23.6%, 24.7%, and 11.3% for prucalopride 2 mg, prucalopride 4 mg, and placebo, respectively (P < 0.005). The most frequent adverse effects were headaches, nausea, and diarrhea. No cardiovascular side effects were observed, nor were any electrocardiographic abnormalities reported.
TD-5108
TD-5108 is also a full 5-HT4 agonist. In a four-week phase II trial, 401 patients with chronic constipation (less than three CSBMs/week during a two-week baseline period) were randomized to receive TD-5108, 15, 30, or 50 mg or placebo, once daily. The percentages of patients achieving three or more CSBMs for all four weeks were 27%, 19%, 21%, and 5%, respectively (P = 0.0006 for all TD-5108 groups compared with placebo).231
Peripheral Mu-Opioid Antagonists
Peripherally acting opioid antagonists have been shown to reverse opioid-induced bowel dysfunction without reversing analgesia or precipitating central nervous system withdrawal signs. Preclinical data suggest that these agents may be effective in the treatment of constipation. Methylnaltrexone is a peripherally acting mu-opioid receptor antagonist that was approved by the FDA in 2008 for the treatment of opioid-induced constipation in patients with a late-stage, advanced illness who are receiving an opioid on a continuous basis to relieve pain. Approval was based on two phase III trials. One of the studies involved 133 patients with a life expectancy of less than six months and fewer than three bowel movements in the week prior to treatment or no bowel movements for more than two days. Patients were randomized to receive methylnaltrexone, 0.15 mg/kg subcutaneously, or placebo every other day for two weeks, followed by a three-month open-label treatment period. In this study, 47% of patients reported having a bowel movement within four hours of starting methylnaltrexone compared with 15% of those who received placebo (P < 0.001). Methylnaltrexone did not appear to precipitate opioid withdrawal symptoms or affect central analgesia.232 Results of long-term studies are awaited.
Alvimopan is another mu-opioid receptor antagonist approved by the FDA to accelerate bowel recovery following surgery. Alvimopam also appears to improve symptoms of opioid-induced constipation. Patients who received alvimopam, 0.5 mg or 1 mg twice daily or 1 mg once daily, had significantly more spontaneous bowel movements than those receiving placebo.233 Other symptoms of constipation also improved. The most common side effects were abdominal pain, nausea, and diarrhea.
Other Agents
Colchicine, a drug used for gout, and misoprostol, a prostaglandin analog, have been used to treat patients with severe chronic constipation. Treatment of chronic constipation with colchicine has been studied in a randomized, placebo-controlled, double-blind crossover trial in which colchicine increased the frequency of bowel movements as compared with placebo; however, abdominal pain was greater during administration of colchicine than placebo.234 Data for misoprostol are limited, and side effects of the drug are common.235
Cholinergic Agents
Cholinergic agents also have been used to treat constipation. Bethanechol, a cholinergic agonist, appears to benefit patients in whom constipation results from therapy with tricyclic antidepressants; however, data to support its use in patients with other causes of constipation are limited. A single intravenous dose of neostigmine, a cholinesterase inhibitor, has been shown to be remarkably effective in decompressing the colon in patients with acute colonic pseudo-obstruction236 (see Chapter 120), but controlled studies of this class of drugs have not been completed in patients with normal-transit or slow-transit constipation. Side effects, such as bradycardia, increased salivation, vomiting, and abdominal cramping, are common.
Botulinum Toxin
Clostridium botulinum toxin type A (Botox), a potent neurotoxin that inhibits presynaptic release of acetylcholine, has been injected intramuscularly into the puborectalis muscle to treat defecatory disorders. Preliminary data suggest that botulinum toxin may be effective for treating patients with defecatory disorders in which spastic pelvic floor dysfunction causes outlet delay,237 including those who also have Parkinson’s disease.112,113 One study showed that 19 of 24 patients reported improvement in symptoms and physiologic measurements of pelvic floor function at two months.238 Controlled trials have not yet been performed, however, and this approach is not recommended in lieu of biofeedback, for which clinical experience is greater (see later).
Newer Agents
A newer approach to treating constipation involves using neurotrophins, a multigene family of proteins that includes nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and neurotrophin-3 (NT-3).239,240 These factors promote growth of subpopulations of sensory neurons and modulate synaptic transmission at developing neuromuscular junctions in Xenopus nerve muscle cultures.241 A specific factor, R-metHuNT-3, has been shown to increase stool frequency and facilitate passage of stool when administered to constipated patients. In healthy persons, R-metHuNT-3 administered subcutaneously has been shown to accelerate gastric, small bowel, and colonic transit. The effects on stool frequency are observed within three days of the start of treatment and last up to five days after cessation of treatment. R-metHuNT-3 has been well tolerated, although half of patients treated in the two studies experienced injection site reactions or paresthesias, presumably by stimulating noncholinergic excitation and suppressing nitrergic inhibition.
Linaclotide is a novel, investigational, minimally absorbed guanylate cyclase C agonist that has been shown to reduce visceral pain and promote intestinal secretion and colonic transit in animal models. In health volunteers, linaclotide was safe and well tolerated. In women with IBS with constipation, oral linaclotide, 1000 µg daily, significantly accelerated ascending colon transit and improved bowel function.241 In a four-week phase IIb study, 307 patients with chronic constipation with less than three spontaneous bowel movements/week were randomized to placebo or linaclotide, 75, 150, 300, or 600 µg. Within 24 hours, 55% of patients who received 300 µg and 59% of patients who received all the linaclotide doses had a spontaneous bowel movement, compared with 37% of those who received placebo. By week four, the mean number of spontaneous bowel movements/week was 5.5 in those who received 300 µg of linaclotide, compared with 3.9 for those who received placebo. Linaclotide was well tolerated; diarrhea was the only dose-dependent adverse effect. No evidence of rebound constipation was noted in the post-treatment period.242
OTHER FORMS OF THERAPY
Anorectal Biofeedback
Anorectal biofeedback typically follows defecation training. During anorectal biofeedback, patients receive visual or auditory feedback, or both, on the functioning of their anal sphincter and pelvic floor muscles. Biofeedback can be used to train patients to relax their pelvic floor muscles during straining and to coordinate this relaxation with abdominal maneuvers to enhance entry of stool into the rectum. Biofeedback can be performed with an electromyographic or anorectal manometry catheter. Simulated evacuation with a balloon or silicone-filled artificial stool is commonly taught to patients to emphasize normal coordination of successful defecation.243 Patient education and rapport between the therapist and the patient are integral components of successful biofeedback.244 Patients typically complete from six sessions in six weeks to three sessions/day for 10 successive days.
A systematic review of biofeedback studies performed up to 1993 revealed an overall success rate of 67%, although controlled studies were lacking.245 Biofeedback may be less effective for patients with descending perineum syndrome than for those with spastic pelvic floor disorders.91 In a review of 38 biofeedback studies, psychological factors were found to influence the response to biofeedback.246 More recently, several controlled trials have supported the efficacy of biofeedback.247–249 Patients with pelvic floor dyssynergia who failed fiber, 20 g/day, plus enemas or suppositories were randomized to five weekly biofeedback sessions (n = 54) or PEG, 14.6 to 29.2 g/day, plus five weekly counseling sessions on preventing constipation (n = 55). At six months, major improvement was reported by 80% of patients who underwent biofeedback compared with 22% of the laxative-treated patients (P < 0.001). The benefits of biofeedback were sustained at 12 and 24 months and produced greater reductions in straining, sensations of incomplete evacuation and anorectal blockage, use of enemas and suppositories, and abdominal pain (all P < 0.01). Stool frequency increased in both groups. All biofeedback-treated patients reporting major improvement were able to relax the pelvic floor and defecate a 50-mL balloon at 6 and 12 months.248 In another controlled trial, 77 patients with dyssynergic defecation were randomized to biofeedback, sham therapy, or standard therapy for three months. Patients who received biofeedback were significantly more likely to correct dyssynergia, improve the defecation index, decrease the balloon expulsion time, increase the number of CSBMs/week, and decrease the use of digital maneuvers. Global bowel satisfaction was also higher for patients treated with biofeedback than for those in the other groups.249
Originally, biofeedback training was intensive and initiated during admission to the hospital,250 but subsequent experience has shown that training as an outpatient is satisfactory. A small comparative trial has shown no difference in outcome with or without use of an intrarectal balloon or with home training.251 Results are similar when training is conducted with or without access to a visual display of muscular activity. In the absence of a visual display, the instructor gives continuous information and encouragement to the patient and assesses the effect of instruction by observing how the patient strains and by sensing the effectiveness of straining through gentle tension on a rectal balloon.
Many patients do not complete defecation training. Of those who do complete the training, most continue to report improvement in symptoms up to two years after completion of training.250,251 Symptoms reported to improve with defecation training include bowel frequency, straining, abdominal pain, bloating, and need for laxatives.252 Physiologic measurements before and after treatment have shown that training results in appropriate relaxation of the puborectalis and external anal sphincter muscles,253–255 increase in intrarectal pressure,75 a widened rectoanal angle on straining during defecation, an increased rate of rectal emptying, an increased rate of colonic transit, and increased rectal mucosal blood flow.
Role of Physiologic and Anatomic Investigation
Most published series have restricted defecation training and anorectal biofeedback to patients with a defecatory disorder (i.e., paradoxical contraction of the pelvic floor muscles). At one center, however, such training appeared to benefit a high proportion of unselected patients with idiopathic constipation, regardless of the results of investigation of colonic transit or pelvic floor dysfunction, including patients with slow colonic transit.254,256 In another series, the results of treatment did not depend on the presence or absence of a rectocele, intussusception, or perineal descent.252 Other investigators, however, have shown that patients who fail to respond to defecation training and biofeedback have a greater degree of perineal descent than those who respond.91 Defecation training has benefited some patients in whom constipation developed after hysterectomy257 and some patients with solitary rectal ulcer syndrome.258
Complementary and Alternative Medical Therapies
Many complementary and alternative therapies are used by patients with constipation,259 but clinical studies are limited and generally of poor quality (see Chapter 127). A systematic review of approximately 90 trials of acupuncture for the treatment of chronic constipation identified in the Chinese Biomedical Database is in progress.260
Sacral Nerve Stimulation
Uncontrolled data suggest that sacral nerve stimulation may be helpful for patients with severe constipation.261 Further investigation is in progress.
Surgery
Colectomy
Colectomy for constipation produces variable results. A review of 32 published studies of surgery for chronic constipation has found considerable variability in rates of patient satisfaction (39% to 100%).262 The most common complications following surgery are small bowel obstruction, diarrhea, and incontinence; however, diarrhea and incontinence tend to improve after the first year following surgery.
Selection of Patients for Colectomy
Preoperative psychological assessment is essential, because poor results are common among patients who are psychologically disturbed.263 Because the aim of surgery is to increase bowel frequency, slow colonic transit must be demonstrated by an objective method. Also, defecatory function must be assessed, inasmuch as the inability to expel stool from the rectum may be a major factor in causing symptoms. Finally, a generalized intestinal dysmotility or pseudo-obstruction syndrome should be excluded, as much as possible, by appropriate radiographic study of the small intestine and, when available, studies of gastric emptying and small bowel transit.
Series in which these steps have been taken to select a homogeneous group of patients have shown the best results, although longer follow-up is awaited. For example, at one center, only 74 of 1009 patients referred for possible surgical treatment of chronic constipation underwent surgery. Measurement of intestinal transit and tests of pelvic floor function revealed that 597 patients had no quantifiable abnormality and that 249 patients had pelvic floor dysfunction without slow colonic transit. Colectomy with an ileorectal anastomosis was performed in 52 patients with demonstrable slow colonic transit and normal defecatory function. The operation also was performed in 22 patients with slow colonic transit and pelvic floor dysfunction after the latter had been treated by a training program. Of the 74 patients treated surgically, 97% were satisfied with the result, and 90% had a good or improved quality of life after a mean follow-up of 56 months. There was no operative mortality, but 7 patients had a subsequent episode of small bowel obstruction.41
Type of Operation
Several series have shown that the results of colectomy with cecorectal or ileosigmoid anastomosis are inferior to those for a subtotal colectomy with an ileorectal anastomosis.264 Occasional reports have described proctocolectomy with ileoanal anastomosis and construction of an ileal pouch, usually following failure of colectomy and ileorectal anastomosis.265 In one patient, ileorectal anastomosis failed because the rectum had a larger than normal capacity.266 Laparoscopic subtotal colectomy appears to be as effective as an open approach.267,268
Construction of a Stoma
A colostomy is occasionally performed for slow-transit constipation because it is reversible and the results of colectomy are uncertain. Most patients report subjective improvement after a colostomy performed as a primary procedure for slow-transit constipation or for neurologic disease.89 Many patients, however, continue to require laxatives or regular colonic irrigation.
Creation of a continent catheterizable appendicostomy, through which antegrade enemas can be administered, can sometimes benefit patients with paraplegia and severe constipation and incontinence. Such a procedure can decrease the time and medication needed for bowel care; most of the experience is in children.269
Operations for Defecatory Disorders
The stapled transanal rectal resection (STARR) procedure has been used with some success, particularly for patients who also have a rectocele and intussusception.270–272 Puborectalis or internal anal sphincter muscle division is unsuccessful in patients with slow-transit constipation.273 Procedures to correct a rectocele should be considered only for patients who have evidence of retained contrast during defecating proctography or in women in whom constipation is relieved with digital vaginal pressure.89
Bharucha AE, Wald A, Enck P, Rao S. Functional anorectal disorders. Gastroenterology. 2006;130:1510-18. (Ref 81.)
Camilleri M, Bharucha AE, Ueno R, et al. Effect of a selective chloride channel activator, lubiprostone, on gastrointestinal transit, gastric sensory, and motor functions in healthy volunteers. Am J Physiol Gastrointest Liver Physiol. 2006;290:G942-7. (Ref 221.)
Camilleri M, Kerstens R, Rykx A, Vandeplassche L. A placebo-controlled trial of prucalopride for severe chronic constipation. N Engl J Med. 2008;358:2344-54. (Ref 228.)
Chiarioni G, Whitehead WE, Pezza V, et al. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology. 2006;130:657-64. (Ref 248.)
Dipalma JA, Cleveland MV, McGowan J, Herrera JL. A randomized, multicenter, placebo-controlled trial of polyethylene glycol laxative for chronic treatment of chronic constipation. Am J Gastroenterol. 2007;102:1436-41. (Ref 194.)
Fletcher JG, Busse RF, Riederer SJ, et al. Magnetic resonance imaging of anatomic and dynamic defects of the pelvic floor in defecatory disorders. Am J Gastroenterol. 2003;98:399-411. (Ref 162.)
Higgins PD, Johanson JF. Epidemiology of constipation in North America: A systematic review. Am J Gastroenterol. 2004;99:750-9. (Ref 10.)
Holzer B, Rosen HR, Novi G, et al. Sacral nerve stimulation in patients with severe constipation. Dis Colon Rectum. 2008;51:524-9. (Ref 261.)
Lembo A, Camilleri M. Chronic constipation. N Engl J Med. 2003;349:1360-8. (Ref 2.)
Locke GR3rd, Pemberton JH, Phillips SF. AGA technical review on constipation. American Gastroenterological Association. Gastroenterology. 2000;119:1766-78. (Ref 22.)
Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology. 2006;130:1480-91. (Ref 6.)
Markowitz GS, Stokes MB, Radhakrishnan J, D’Agati VD. Acute phosphate nephropathy following oral sodium phosphate bowel purgative: An underrecognized cause of chronic renal failure. J Am Soc Nephrol. 2005;16:3389-96. (Ref 183.)
Rao SS, Seaton K, Miller M, et al. Randomized controlled trial of biofeedback, sham feedback, and standard therapy for dyssynergic defecation. Clin Gastroenterol Hepatol. 2007;5:331-8. (Ref 249.)
Steinman TI, Samir AE, Cornell LD. Case records of the Massachusetts General Hospital. Case 27-2008. A 64-year-old man with abdominal pain, nausea, and an elevated level of serum creatinine. N Engl J Med. 2008;359:951-60. (Ref 182.)
Thomas J, Karver S, Cooney GA, et al. Methylnaltrexone for opioid-induced constipation in advanced illness. N Engl J Med. 2008;358:2332-43. (Ref 232.)
1. Sandler RS, Drossman DA. Bowel habits in young adults not seeking health care. Dig Dis Sci. 1987;32:841-5.
2. Lembo A, Camilleri M. Chronic constipation. N Engl J Med. 2003;349:1360-8.
3. Sandler RS, Jordan MC, Shelton BJ. Demographic and dietary determinants of constipation in the U.S. population. Am J Public Health. 1990;80:185-9.
4. Whitehead WE, Chaussade E, Corazziari E. Report of an international workshop on management of constipation. Gastroenterol Int. 1991;4:99-113.
5. Drossman DA. The functional gastrointestinal disorders and the Rome II process. Gut. 1999;45(Suppl 2):II1-5.
6. Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology. 2006;130:1480-91.
7. Johanson JF, Sonnenberg A, Koch TR. Clinical epidemiology of chronic constipation. J Clin Gastroenterol. 1989;11:525-36.
8. Stewart WF, Liberman JN, Sandler RS, et al. Epidemiology of constipation (EPOC) study in the United States: Relation of clinical subtypes to socio-demographic features. Am J Gastroenterol. 1999;94:3530-40.
9. Pare P, Ferrazzi S, Thompson WG, et al. An epidemiological survey of constipation in Canada: Definitions, rates, demographics, and predictors of health care seeking. Am J Gastroenterol. 2001;96:3130-7.
10. Higgins PD, Johanson JF. Epidemiology of constipation in North America: A systematic review. Am J Gastroenterol. 2004;99:750-9.
11. Everhart JE, Go VL, Johannes RS, et al. A longitudinal survey of self-reported bowel habits in the United States. Dig Dis Sci. 1989;34:1153-62.
12. Harari D, Gurwitz JH, Avorn J, et al. Bowel habit in relation to age and gender. Findings from the National Health Interview Survey and clinical implications. Arch Intern Med. 1996;156:315-20.
13. Talley NJ, Zinsmeister AR, Van Dyke C, et al. Epidemiology of colonic symptoms and the irritable bowel syndrome. Gastroenterology. 1992;101:927-34.
14. Heaton KW, Cripps HA. Straining at stool and laxative taking in an English population. Dig Dis Sci. 1993;38:1004-8.
15. Talley NJ, O’Keefe EA, Zinsmeister AR, Melton LJ3rd. Prevalence of gastrointestinal symptoms in the elderly: A population-based study. Gastroenterology. 1992;102:895.
16. Talley NJ, Weaver AL, Zinsmeister AR, et al. Functional constipation and outlet delay: A population-based study. Gastroenterology. 1993;105:781-90.
17. Drossman DA, Li Z, Andruzzi E, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38:1569-80.
18. Talley NJ, Weaver AL, Zinsmeister AR, Melton LJ3rd. Onset and disappearance of gastrointestinal symptoms and functional gastrointestinal disorders. Am J Epidemiol. 1992;136:165-77.
19. Robson KM, Kiely DK, Lembo T. Development of constipation in nursing home residents. Dis Colon Rectum. 2000;43:940-3.
20. Sonnenberg A, Koch TR. Epidemiology of constipation in the United States. Dis Colon Rectum. 1989;32:1-8.
21. Sonnenberg A, Koch TR. Physician visits in the United States for constipation: 1958 to 1986. Dig Dis Sci. 1989;34:606-11.
22. Locke GR3rd, Pemberton JH, Phillips SF. AGA technical review on constipation. American Gastroenterological Association. Gastroenterology. 2000;119:1766-78.
23. Rantis PCJr, Vernava AM3rd, Daniel GL, Longo WE. Chronic constipation–is the work-up worth the cost? Dis Colon Rectum. 1997;40:280-6.
24. Dukas L, Willett WC, Giovannucci EL. Association between physical activity, fiber intake, and other lifestyle variables and constipation in a study of women. Am J Gastroenterol. 2003;98:1790-6.
25. Heaton KW, Radvan J, Cripps H, et al. Defecation frequency and timing, and stool form in the general population: A prospective study. Gut. 1992;33:818-24.
26. Cummings JH, Bingham SA, Heaton KW, Eastwood MA. Fecal weight, colon cancer risk, and dietary intake of nonstarch polysaccharides (dietary fiber). Gastroenterology. 1992;103:1783-9.
27. Kamm MA, Farthing MJ, Lennard-Jones JE, et al. Steroid hormone abnormalities in women with severe idiopathic constipation. Gut. 1991;32:80-4.
28. Xiao Z-L, Pricolo V, Brancani P, Behar J. Role of progesterone signaling in the regulation of G-protein levels in female chronic constipation. Gastroenterology. 2005;128:667-75.
29. Cheng L, Pricolo VE, Biancani P, Behar J. Overexpression of progesterone receptor B increases the sensitivity of human colon muscle cells to progesterone. Am J Physiol Gastrointest Liver Physiol. 2008;295:G493-G502.
30. Talley NJ, Fleming KC, Evans JM, et al. Constipation in an elderly community: A study of prevalence and potential risk factors. Am J Gastroenterol. 1996;91:19-25.
31. Campbell AJ, Busby WJ, Horwath CC. Factors associated with constipation in a community-based sample of people aged 70 years and over. J Epidemiol Commun Health. 1993;47:23-6.
32. Merkel IS, Locher J, Burgio K, et al. Physiologic and psychologic characteristics of an elderly population with chronic constipation. Am J Gastroenterol. 1993;88:1854-9.
33. Rasquin-Weber A, Hyman PE, Cucchiara S, et al. Childhood functional gastrointestinal disorders. Gut. 1999;45(Suppl 2):60-8.
34. Read NW, Celik AF, Katsinelos P. Constipation and incontinence in the elderly. J Clin Gastroenterol. 1995;20:61-70.
35. Bytzer P, Howell S, Leemon M, et al. Low socioeconomic class is a risk factor for upper and lower gastrointestinal symptoms: A population-based study in 15,000 Australian adults. Gut. 2001;49:66-72.
36. Towers AL, Burgio KL, Locher JL, et al. Constipation in the elderly: Influence of dietary, psychological, and physiological factors. J Am Geriatr Soc. 1994;42:701-6.
37. Meshkinpour H, Selod S, Movahedi H, et al. Effects of regular exercise in management of chronic idiopathic constipation. Dig Dis Sci. 1998;43:2379-83.
38. Bingham SA, Cummings JH. Effect of exercise and physical fitness on large intestinal function. Gastroenterology. 1989;97:1389-99.
39. van Nieuwenhoven MA, Vriens BE, Brummer RJ, Brouns F. Effect of dehydration on gastrointestinal function at rest and during exercise in humans. Eur J Appl Physiol. 2000;83:578-84.
40. Talley NJ, Jones M, Nuyts G, Dubois D. Risk factors for chronic constipation based on a general practice sample. Am J Gastroenterol. 2003;98:1107-11.
41. Kirwan WO, Smith AN, McConnell AA, et al. Action of different bran preparations on colonic function. Br Med J. 1974;4:187-9.
42. Muller-Lissner SA. Effect of wheat bran on weight of stool and gastrointestinal transit time: A meta analysis. Br Med J (Clin Res Ed). 1988;296:615-17.
43. Tomlin J, Read NW. Laxative properties of indigestible plastic particles. BMJ. 1988;297:1175-6.
44. Schiller LR. Review article: The therapy of constipation. Aliment Pharmacol Ther. 2001;15:749-63.
45. Aichbichler BW, Werzl HH, Santa Ana CA, et al. A comparison of stool characteristics from normal and constipated people. Dig Dis Sci. 1998;43:2353-62.
46. Preston DM, Lennard-Jones JE, Thomas BM. Towards a radiologic definition of idiopathic megacolon. Gastrointest Radiol. 1985;10:167-9.
47. Metcalf AM, Phillips SF, Zinsmeister AR, et al. Simplified assessment of segmental colonic transit. Gastroenterology. 1987;92:40-7.
48. Chaussade S, Roche H, Khyari A, et al. Measurement of colonic transit time: Description and validation of a new method. Gastroenterol Clin Biol. 1986;10:385-9.
49. van der Sijp JR, Kamm MA, Nightingale JM, et al. Radio-isotope determination of regional colonic transit in severe constipation: Comparison with radio opaque markers. Gut. 1993;34:402-8.
50. Bassotti G, Imtorno G, Fiorella S, et al. Colonic motility in man: Features in normal subjects and in patients with chronic idiopathic constipation. Am J Gastroenterol. 1999;94:1760-70.
51. Bassotti G, Gaburri M, Imbimbo BP, et al. Colonic mass movements in idiopathic chronic constipation. Gut. 1988;29:1173-9.
52. Knowles CH, Scott SM, Wellmer A, et al. Sensory and autonomic neuropathy in patients with idiopathic slow-transit constipation. Br J Surg. 1999;86:54-60.
53. Emmanuel AV, Kamm MA. Laser Doppler flowmetry as a measure of extrinsic colonic innervation in functional bowel disease. Gut. 2000;46:212-17.
54. Tzavella K, Riepl RL, Klauser AG, et al. Decreased substance P levels in rectal biopsies from patients with slow transit constipation. Eur J Gastroenterol Hepatol. 1996;8:1207-11.
55. Cortesini C, Cianchi F, Infantino A, Lise M. Nitric oxide synthase and VIP distribution in enteric nervous system in idiopathic chronic constipation. Dig Dis Sci. 1995;40:2450-5.
56. He CL, Burgart L, Wang L, et al. Decreased interstitial cell of Cajal volume in patients with slow-transit constipation. Gastroenterology. 2000;118:14-21.
57. Lyford GL, He CL, Soffer E, et al. Pan-colonic decrease in interstitial cells of Cajal in patients with slow transit constipation. Gut. 2002;51:496-501.
58. Wedel T, Spiegler J, Soellner S, et al. Enteric nerves and interstitial cells of Cajal are altered in patients with slow-transit constipation and megacolon. Gastroenterology. 2002;123:1459-67.
59. Yu CS, Kim HC, Hong HK, et al. Evaluation of myenteric ganglion cells and interstitial cells of Cajal in patients with chronic idiopathic constipation. Int J Colorectal Dis. 2002;17:253-8.
60. Klauser AG, Voderholzer WA, Heinrich CA, et al. Behavioral modification of colonic function. Can constipation be learned? Dig Dis Sci. 1990;35:1271-5.
61. Tagart RE. The anal canal and rectum: Their varying relationship and its effect on anal continence. Dis Colon Rectum. 1966;9:449-52.
62. Kamm MA, van der Sijp JR, Lennard-Jones JE. Colorectal and anal motility during defaecation. Lancet. 1992;339:820.
63. Bannister JJ, Davison P, Timms JM, et al. Effect of stool size and consistency on defecation. Gut. 1987;28:1246-50.
64. Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920-4.
65. Nyam DC, Pemberton JH, Ilstrup DM, Rath DM. Long-term results of surgery for chronic constipation. Dis Colon Rectum. 1997;40:273-9.
66. Heaton KW, O’Donnell LJ. An office guide to whole-gut transit time. Patients’ recollection of their stool form. J Clin Gastroenterol. 1994;19:28-30.
67. Ashraf W, Park F, Lof J, Quigley EM. An examination of the reliability of reported stool frequency in the diagnosis of idiopathic constipation. Am J Gastroenterol. 1996;91:26-32.
68. Mertz H, Naliboff B, Mayer E. Physiology of refractory chronic constipation. Am J Gastroenterol. 1999;94:609-15.
69. Voderholzer WA, Schatke W, Muhldorfer BE, et al. Clinical response to dietary fiber treatment of chronic constipation. Am J Gastroenterol. 1997;92:95-8.
70. Wald A. Slow transit constipation. Curr Treat Options Gastroenterol. 2002;5:279-83.
71. Bharucha AE. Treatment of severe and intractable constipation. Curr Treat Options Gastroenterol. 2004;7:291-8.
72. Bassotti G, Imbimbo BP, Betti C, et al. Impaired colonic motor response to eating in patients with slow-transit constipation. Am J Gastroenterol. 1992;87:504-8.
73. O’Brien MD, Camiller M, van der Ohe MR, et al. Motility and tone of the left colon in constipation: A role in clinical practice? Am J Gastroenterol. 1996;91:2532-8.
74. Bassotti G, Chiarioni G, Imbimbo BP, et al. Impaired colonic motor response to cholinergic stimulation in patients with severe chronic idiopathic (slow transit type) constipation. Dig Dis Sci. 1993;38:1040-5.
75. Rao SS, Welcher KD, Leistikow JS. Obstructive defecation: A failure of rectoanal coordination. Am J Gastroenterol. 1998;93:1042-50.
76. Rao SS, Hatfield R, Soffer E, et al. Manometric tests of anorectal function in healthy adults. Am J Gastroenterol. 1999;94:773-83.
77. Roberts JP, Womack NR, Hallan RI, et al. Evidence from dynamic integrated proctography to redefine anismus. Br J Surg. 1992;79:1213-15.
78. Cheskin LJ, Kamal N, Crawell MD, et al. Mechanisms of constipation in older persons and effects of fiber compared with placebo. J Am Geriatr Soc. 1995;43:666-9.
79. Karlbom U, Pahlman L, Nilsson S, Graf W. Relationships between defecographic findings, rectal emptying, and colonic transit time in constipated patients. Gut. 1995;36:907-12.
80. Mertz H, Naliboff B, Mayer EA. Symptoms and physiology in severe chronic constipation. Am J Gastroenterol. 1999;94:131-8.
81. Bharucha AE, Wald A, Enck P, Rao S. Functional anorectal disorders. Gastroenterology. 2006;130:1510-18.
82. Di Lorenzo C, Benninga MA. Pathophysiology of pediatric fecal incontinence. Gastroenterology. 2004;126(Suppl 1):S33-40.
83. Loening-Baucke V. Functional fecal retention with encopresis in childhood. J Pediatr Gastroenterol Nutr. 2004;38:79-84.
84. Siproudhis L, Dartreme S, Ropert A, et al. Dyschezia and rectocele—a marriage of convenience? Physiologic evaluation of the rectocele in a group of 52 women complaining of difficulty in evacuation. Dis Colon Rectum. 1993;36:1030-6.
85. Shorvon PJ, McHugh S, Diamant NE, et al. Defecography in normal volunteers: Results and implications. Gut. 1989;30:1737-49.
86. Greenberg T, Kelvin FM, Maglinte DD. Barium trapping in rectoceles: Are we trapped by the wrong definition? Abdom Imaging. 2001;26:587-90.
87. van Dam JH, Ginai AZ, Gossclink MJ, et al. Role of defecography in predicting clinical outcome of rectocele repair. Dis Colon Rectum. 1997;40:201-7.
88. Van Laarhoven CJ, Kamm MA, Bartram CI, et al. Relationship between anatomic and symptomatic long-term results after rectocele repair for impaired defecation. Dis Colon Rectum. 1999;42:204-10.
89. Sarles JC, Arnavd A, Selezneff I, Olivier S. Endo-rectal repair of rectocele. Int J Colorectal Dis. 1989;4:167-71.
90. van Dam JH, Hop WC, Schouten WR. Analysis of patients with poor outcome of rectocele repair. Dis Colon Rectum. 2000;43:1556-60.
91. Harewood GC, Coulie B, Camilleri M, et al. Descending perineum syndrome: Audit of clinical and laboratory features and outcome of pelvic floor retraining. Am J Gastroenterol. 1999;94:126-30.
92. Read NW, Timms JM, Barfield LJ, et al. Impairment of defecation in young women with severe constipation. Gastroenterology. 1986;90:53-60.
93. Kamm MA, Lennard-Jones JE. Rectal mucosal electrosensory testing—evidence for a rectal sensory neuropathy in idiopathic constipation. Dis Colon Rectum. 1990;33:419-23.
94. Gladman MA, Scott SM, Williams NS, Lunniss PJ. Clinical and physiological findings, and possible aetiological factors of rectal hyposensitivity. Br J Surg. 2003;90:860-6.
95. Thompson JR, Chen AH, Pettit PD, Bridges MD. Incidence of occult rectal prolapse in patients with clinical rectoceles and defecatory dysfunction. Am J Obstet Gynecol. 2002;187:1494-9.
96. Felt-Bersma RJ, Cuesta MA. Rectal prolapse, rectal intussusception, rectocele, and solitary rectal ulcer syndrome. Gastroenterol Clin North Am. 2001;30:199-222.
97. Simsek A, Yagci G, Gorgulu S, et al. Diagnostic features and treatment modalities in solitary rectal ulcer syndrome. Acta Chir Belg. 2004;104:92-6.
98. Vaizey CJ, van der Bogaerde JB, Emmanuel AV, et al. Solitary rectal ulcer syndrome. Br J Surg. 1998;85:1617-23.
99. Tjandra JJ, Fazio VW, Petras RE, et al. Clinical and pathologic factors associated with delayed diagnosis in solitary rectal ulcer syndrome. Dis Colon Rectum. 1993;36:146-53.
100. Halligan S, Nicholls RJ, Bartram CI. Evacuation proctography in patients with solitary rectal ulcer syndrome: Anatomic abnormalities and frequency of impaired emptying and prolapse. AJR Am J Roentgenol. 1995;164:91-5.
101. Bishop PR, Nowicki MJ. Nonsurgical therapy for solitary rectal ulcer syndrome. Curr Treat Options Gastroenterol. 2002;5:215-23.
102. Sitzler PJ, Kamm MA, Nicholls RJ, McKee RF. Long-term clinical outcome of surgery for solitary rectal ulcer syndrome. Br J Surg. 1998;85:1246-50.
103. Jarrett ME, Emmanuel AV, Vaizey CJ, Kamm MA. Behavioural therapy (biofeedback) for solitary rectal ulcer syndrome improves symptoms and mucosal blood flow. Gut. 2004;53:368-70.
104. Shafer RB, Prentiss RA, Bond JH. Gastrointestinal transit in thyroid disease. Gastroenterology. 1984;86(Pt 1):852-5.
105. Patel R, Hughes RWJr. An unusual case of myxedema megacolon with features of ischemic and pseudomembranous colitis. Mayo Clin Proc. 1992;67:369-72.
106. Jung HK, Kim DY, Moon IH, Hong YS. Colonic transit time in diabetic patients—comparison with healthy subjects and the effect of autonomic neuropathy. Yonsei Med J. 2003;44:265-72.
107. Werth B, Meyer-Wyss B, Spinas GA, et al. Non-invasive assessment of gastrointestinal motility disorders in diabetic patients with and without cardiovascular signs of autonomic neuropathy. Gut. 1992;33:1199-203.
108. Battle WM, Snape WJJr, Alavi A, et al. Colonic dysfunction in diabetes mellitus. Gastroenterology. 1980;79:1217-21.
109. Sharma S, Longo WE, Baniadam B, Vermva AM3rd. Colorectal manifestations of endocrine disease. Dis Colon Rectum. 1995;38:318-23.
110. Sakakibara R, Odaka T, Uchiyama T, et al. Colonic transit time and rectoanal videomanometry in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2003;74:268-72.
111. Singaram C, Ashraf W, Gaumnitz EA, et al. Dopaminergic defect of enteric nervous system in Parkinson’s disease patients with chronic constipation. Lancet. 1995;346:861-4.
112. Albanese A, Maria G, Bentivoglio AR, et al. Severe constipation in Parkinson’s disease relieved by botulinum toxin. Mov Disord. 1997;12:764-6.
113. Albanese A, Brisinda G, Bentivoglio AR, Maria G. Treatment of outlet obstruction constipation in Parkinson’s disease with botulinum neurotoxin A. Am J Gastroenterol. 2003;98:1439-40.
114. Hinds JP, Eidelman BH, Wald A. Prevalence of bowel dysfunction in multiple sclerosis. A population survey. Gastroenterology. 1990;98:1538-42.
115. Hennessey A, Robertson NP, Swingter R, Compston DA. Urinary, faecal and sexual dysfunction in patients with multiple sclerosis. J Neurol. 1999;246:1027-32.
116. Weber J, Grise P, Roquebert M, et al. Radiopaque markers transit and anorectal manometry in 16 patients with multiple sclerosis and urinary bladder dysfunction. Dis Colon Rectum. 1987;30:95-100.
117. Sorensen M, Lorentzen M, Petersen J, Christiansen J. Anorectal dysfunction in patients with urologic disturbance due to multiple sclerosis. Dis Colon Rectum. 1991;34:136-9.
118. Wiesel PH, Norton C, Roy AJ, et al. Gut-focused behavioural treatment (biofeedback) for constipation and faecal incontinence in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2000;69:240-3.
119. Weber JL. Constipation in spinal cord lesions, multiple sclerosis, and diabetes mellitus. In: Kamm MA, Lennard Jones JE, editors. Constipation. Petersfield, England: Wrightson Biomedical; 1994:273.
120. Glick ME, Meshkinpour H, Haldemm S, et al. Colonic dysfunction in patients with thoracic spinal cord injury. Gastroenterology. 1984;86:287-94.
121. MacDonagh R, Sun WM, Thomas DG, et al. Anorectal function in patients with complete supraconal spinal cord lesions. Gut. 1992;33:1532-8.
122. MacDonagh RP, Sun WM, Smallwood R, et al. Control of defecation in patients with spinal injuries by stimulation of sacral anterior nerve roots. BMJ. 1990;300:1494-7.
123. Binnie NR, Smith AN, Creasey GH, Edmond P. Constipation associated with chronic spinal cord injury: The effect of pelvic parasympathetic stimulation by the Brindley stimulator. Paraplegia. 1991;29:463-9.
124. Christensen J. Morphology of the innervation of the large intestine and the neuropathology of constipation. In: Kamm MA, Lennard-Jones JE, editors. Constipation. Petersfield, England: Wrightson Biomedical; 1994:33.
125. Krogh K, Mosdal C, Gregersen H, Laurberg S. Rectal wall properties in patients with acute and chronic spinal cord lesions. Dis Colon Rectum. 2002;45:641-9.
126. Kamm MA, Hoyle CH, Burleigh DE, et al. Hereditary internal anal sphincter myopathy causing proctalgia fugax and constipation. A newly identified condition. Gastroenterology. 1991;100:805-10.
127. Konig P, Ambrose NS, Scott N. Hereditary internal anal sphincter myopathy causing proctalgia fugax and constipation: Further clinical and histological characterization in a patient. Eur J Gastroenterol Hepatol. 2000;12:127-8.
128. Celik AF, Katsinelos P, Read NW, et al. Hereditary proctalgia fugax and constipation: Report of a second family. Gut. 1995;36:581-4.
129. Battle WM, Snape WJJr, Wright S, et al. Abnormal colonic motility in progressive systemic sclerosis. Ann Intern Med. 1981;94:749-52.
130. Eckardt VF, Nix W. The anal sphincter in patients with myotonic muscular dystrophy. Gastroenterology. 1991;100:424-30.
131. Fuger K, Barnert J, Hopfner W, Wienbeck M. Intestinal pseudoobstruction as a feature of myotonic muscular dystrophy. Z Gastroenterol. 1995;33:534-8.
132. Barnes PR, Lennard-Jones JE, Hawley PR, Todd IP. Hirschsprung’s disease and idiopathic megacolon in adults and adolescents. Gut. 1986;27:534-41.
133. Puffenberger EG, Hosoda K, Washington SS, et al. A missense mutation of the endothelin-B receptor gene in multigenic Hirschsprung’s disease. Cell. 1994;79:1257-66.
134. Edery P, Lyonnet S, Mulligon LM, et al. Mutations of the RET proto-oncogene in Hirschsprung’s disease. Nature. 1994;367:378-80.
135. Milla PJ. Aganglionosis, hypoganglionosis, and hyperganglionosis: Clinical presentation and histopathology. In: Kamm MA, Lennard-Jones JE, editors. Constipation. Petersfield, England: Wrightson Biomedical; 1994:183.
136. Wedel T, Roblich UJ, Ott V, et al. Oligoneuronal hypoganglionosis in patients with idiopathic slow-transit constipation. Dis Colon Rectum. 2002;45:54-62.
137. Montedonico S, Acevedo S, Fadda B. Clinical aspects of intestinal neuronal dysplasia. J Pediatr Surg. 2002;37:1772-4.
138. Gillick J, Tazawa H, Puri P. Intestinal neuronal dysplasia: Results of treatment in 33 patients. J Pediatr Surg. 2001;36:777-9.
139. Miles M. Chagas disease and chagasic megacolon. In: Kamm MA, Lennard-Jones JE, editors. Constipation. Petersfield, England: Wrightson Biomedical; 1994:205.
140. Chinn JS, Schuffler MD. Paraneoplastic visceral neuropathy as a cause of severe gastrointestinal motor dysfunction. Gastroenterology. 1988;95:1279-86.
141. Lennon VA, Sas DF, Busk MF, et al. Enteric neuronal autoantibodies in pseudoobstruction with small-cell lung carcinoma. Gastroenterology. 1991;100:137-42.
142. Pardi DS, Miller SM, Miller DL, et al. Paraneoplastic dysmotility: Loss of interstitial cells of Cajal. Am J Gastroenterol. 2002;97:1828-33.
143. Tucker DM, Sandstead HH, Logan GMJr, et al. Dietary fiber and personality factors as determinants of stool output. Gastroenterology. 1981;81:879-83.
144. Bennett EJ, Evans P, Scott AM, et al. Psychological and sex features of delayed gut transit in functional gastrointestinal disorders. Gut. 2000;46:83-7.
145. Emmanuel AV, Mason HJ, Kamm MA. Relationship between psychological state and level of activity of extrinsic gut innervation in patients with a functional gut disorder. Gut. 2001;49:209-13.
146. Dykes S, Smilgin-Humphreys S, Bass C. Chronic idiopathic constipation: A psychological enquiry. Eur J Gastroenterol Hepatol. 2001;13:39-44.
147. Garvey M, Noyes RJr, Yates W. Frequency of constipation in major depression: Relationship to other clinical variables. Psychosomatics. 1990;31:204-6.
148. Sonnenberg A, Tsou VT, Muller AD. The “institutional colon”: A frequent colonic dysmotility in psychiatric and neurologic disease. Am J Gastroenterol. 1994;89:62-6.
149. Kamal N, Chami T, Andersen A, et al. Delayed gastrointestinal transit times in anorexia nervosa and bulimia nervosa. Gastroenterology. 1991;101:1320-4.
150. Chun AB, Sokol MS, Kaye WH, et al. Colonic and anorectal function in constipated patients with anorexia nervosa. Am J Gastroenterol. 1997;92:1879-83.
151. Chiarioni G, Bassotti G, Monsignori A, et al. Anorectal dysfunction in constipated women with anorexia nervosa. Mayo Clin Proc. 2000;75:1015-9.
152. Rao SS. Constipation: Evaluation and treatment. Gastroenterol Clin North Am. 2003;32:659-83.
153. Rao SS, Kavelock R, Beaty J, et al. Effects of fat and carbohydrate meals on colonic motor response. Gut. 2000;46:205-11.
154. Rao SS, Sadeghi P, Beaty J, et al. Ambulatory 24-hr colonic manometry in healthy humans. Am J Physiol Gastrointest Liver Physiol. 2001;280:G629-39.
155. Rao SS, Welcher K, Zimmerman B, Stumbo P. Is coffee a colonic stimulant? Eur J Gastroenterol Hepatol. 1998;10:113-8.
156. Drossman DA. Do psychosocial factors define symptom severity and patient status in irritable bowel syndrome? Am J Med. 1999;107:415-505.
157. Rao SS, Tuteja AK, Vellema T, et al. Dyssynergic defecation: Demographics, symptoms, stool patterns, and quality of life. J Clin Gastroenterol. 2004;38:680-5.
158. Pepin C, Ladabaum U. The yield of lower endoscopy in patients with constipation: Survey of a university hospital, a public county hospital, and a Veterans Administration medical center. Gastrointest Endosc. 2002;56:325-32.
159. Qureshi W, Adler DG, Davila RE, et al. ASGE guideline: Guideline on the use of endoscopy in the management of constipation. Gastrointest Endosc. 2005;62:199-201.
160. Diamant NE, Kamm MA, Wald A, Whitehead WE. AGA technical review on anorectal testing techniques. Gastroenterology. 1999;116:735-60.
161. Rao SS, Kuo B, McCallum RW, et al. Investigation of colonic and whole-gut transit with wireless motility capsule and radioopaque markers in constipation. Clin Gastroenterol Hepatol. 2009;7:537-44.
162. Fletcher JG, Busse RF, Riederer SJ, et al. Magnetic resonance imaging of anatomic and dynamic defects of the pelvic floor in defecatory disorders. Am J Gastroenterol. 2003;98:399-411.
163. Grotz RL, Pemberton JH, Talley NJ, et al. Discriminant value of psychological distress, symptom profiles, and segmental colonic dysfunction in outpatients with severe idiopathic constipation. Gut. 1994;35:798-802.
164. Minguez M, Herreros B, Sanchiz V, et al. Predictive value of the balloon expulsion test for excluding the diagnosis of pelvic floor dyssynergia in constipation. Gastroenterology. 2004;126:57-62.
165. Rao SS, Patel RS. How useful are manometric tests of anorectal function in the management of defecation disorders? Am J Gastroenterol. 1997;92:469-75.
166. Lindeman RD, Romero LJ, Liang HC, et al. Do elderly persons need to be encouraged to drink more fluids? J Gerontol A Biol Sci Med Sci. 2000;55:M361-5.
167. Burkitt DP, Walker AR, Painter NS. Dietary fiber and disease. JAMA. 1974;229:1068-74.
168. Cummings JH. Constipation, dietary fibre and the control of large bowel function. Postgrad Med J. 1984;60:811-19.
169. Graham DY, Moser SE, Estes MK. The effect of bran on bowel function in constipation. Am J Gastroenterol. 1982;77:599-603.
170. Badiali D, Corazziari E, Habib FI, et al. Effect of wheat bran in treatment of chronic nonorganic constipation. A double-blind controlled trial. Dig Dis Sci. 1995;40:349-56.
171. Chaussade S, Khyari A, Roche H, et al. Determination of total and segmental colonic transit time in constipated patients. Results in 91 patients with a new simplified method. Dig Dis Sci. 1989;34:1168-72.
172. Hamilton JW, Wagner J, Burdich BB, Bass P. Clinical evaluation of methylcellulose as a bulk laxative. Dig Dis Sci. 1988;33:993-8.
173. Lantner RR, Espiritv BR, Zumerchih P, Tobin MC. Anaphylaxis following ingestion of a psyllium-containing cereal. JAMA. 1990;264:2534-6.
174. Busse WW, Schoenwetter WF. Asthma from psyllium in laxative manufacture. Ann Intern Med. 1975;83:361-2.
175. Toskes PP, Connery KL, Ritchey TW. Calcium polycarbophil compared with placebo in irritable bowel syndrome. Aliment Pharmacol Ther. 1993;7:87-92.
176. Donowitz M, Rood RP. Magnesium hydroxide: New insights into the mechanism of its laxative effect and the potential involvement of prostaglandin E2. J Clin Gastroenterol. 1992;14:20-6.
177. Fine KD, Santa Ana CA, Fordtran JS. Diagnosis of magnesium-induced diarrhea. N Engl J Med. 1991;324:1012-17.
178. Fine KD, Santa Ana CA, Porter JL, Fordtran JS. Intestinal absorption of magnesium from food and supplements. J Clin Invest. 1991;88:396-402.
179. Golzarian J, Scott HWJr, Richards WO. Hypermagnesemia-induced paralytic ileus. Dig Dis Sci. 1994;39:1138-42.
180. Alison LH, Bulugahapitiya D. Laxative-induced magnesium poisoning in a 6-week-old infant. BMJ. 1990;300:125.
181. Ashton MR, Sutton D, Nielsen M. Severe magnesium toxicity after magnesium sulphate enema in a chronically constipated child. BMJ. 1990;300:541.
182. Steinman TI, Samir AE, Cornell LD. Case records of the Massachusetts General Hospital. Case 27-2008. A 64-year-old man with abdominal pain, nausea, and an elevated level of serum creatinine. N Engl J Med. 2008;359:951-60.
183. Markowitz GS, Stokes MB, Radhakrishnan J, D’Agati VD. Acute phosphate nephropathy following oral sodium phosphate bowel purgative: An underrecognized cause of chronic renal failure. J Am Soc Nephrol. 2005;16:3389-96.
184. Wright RA. Lactulose-induced megacolon. Gastrointest Endosc. 1988;34:489-90.
185. Bass P, Dennis S. The laxative effects of lactulose in normal and constipated subjects. J Clin Gastroenterol. 1981;3(Suppl 1):23-8.
186. Wesselius-De Casparis A, Braadbaart S, Bergh-Bohlker GE, Mimica M. Treatment of chronic constipation with lactulose syrup: Results of a double-blind study. Gut. 1968;9:84-6.
187. Sanders JF. Lactulose syrup assessed in a double-blind study of elderly constipated patients. J Am Geriatr Soc. 1978;26:236-9.
188. Hyams JS. Sorbitol intolerance: An unappreciated cause of functional gastrointestinal complaints. Gastroenterology. 1983;84:30-3.
189. Lederle FA, Busch DL, Mattox KM, et al. Cost-effective treatment of constipation in the elderly: A randomized double-blind comparison of sorbitol and lactulose. Am J Med. 1990;89:597-601.
190. Corazziari E, Badiali D, Habib FI, et al. Small-volume isosmotic polyethylene glycol electrolyte balanced solution (PMF-100) in treatment of chronic nonorganic constipation. Dig Dis Sci. 1996;41:1636-42.
191. Corazziari E, Badiali D, Bazzocchi G, et al. Long term efficacy, safety, and tolerability of low daily doses of isosmotic polyethylene glycol electrolyte balanced solution (PMF-100) in the treatment of functional chronic constipation. Gut. 2000;46:522-6.
192. Chaussade S, Minic M. Comparison of efficacy and safety of two doses of two different polyethylene glycol-based laxatives in the treatment of constipation. Aliment Pharmacol Ther. 2003;17:165-72.
193. Attar A, Lemann M, Ferguson A, et al. Comparison of a low dose polyethylene glycol electrolyte solution with lactulose for treatment of chronic constipation. Gut. 1999;44:226-30.
194. Dipalma JA, Cleveland MV, McGowan J, Herrera JL. A randomized, multicenter, placebo-controlled trial of polyethylene glycol laxative for chronic treatment of chronic constipation. Am J Gastroenterol. 2007;102:1436-41.
195. Dupont C, Ammar F, Leluyer B. Polyethylene glycol (PEG) 4000 in constipated children: A dose determination study. Gastroenterology. 2000;118:A846.
196. Culbert P, Gillett H, Ferguson A. Highly effective new oral therapy for faecal impaction. Br J Gen Pract. 1998;48:1599-600.
197. Tolia V, Lin CH, Elitsur Y. A prospective randomized study with mineral oil and oral lavage solution for treatment of faecal impaction in children. Aliment Pharmacol Ther. 1993;7:523-9.
198. Argent A, Hatherill M, Reynolds L, Purves L. Fulminant pulmonary oedema after administration of a balanced electrolyte polyethylene glycol solution. Arch Dis Child. 2002;86:209.
199. Marschall HU, Bartels F. Life-threatening complications of nasogastric administration of polyethylene glycol-electrolyte solutions (GoLYTELY) for bowel cleansing. Gastrointest Endosc. 1998;47:408-10.
200. Coremans G, Vos R, Margaritis V, et al. Small doses of the unabsorbable substance polyethylene glycol 3350 accelerate oro-caecal transit, but slow gastric emptying in healthy subjects. Dig Liver Dis. 2005;37:97-101.
201. Neims DM, McNeill J, Gikes TR, Todd F. Incidence of laxative abuse in community and bulimic populations: A descriptive review. Int J Eat Disord. 1995;17:211-28.
202. Badiali D, Marcheggiano A, Pallone F, et al. Melanosis of the rectum in patients with chronic constipation. Dis Colon Rectum. 1985;28:241-5.
203. Rudolph RL, Mengs U. Electron microscopical studies on rat intestine after long-term treatment with sennosides. Pharmacology. 1988;36(Suppl 1):188-93.
204. Fioramonti J, Dupuy C, Bueno L. In vivo motility of rat colon chronically pretreated with sennosides. Pharmacology. 1993;47(Suppl 1):155-61.
205. Riecken EO, Zeitz M, Emde C, et al. The effect of an anthraquinone laxative on colonic nerve tissue: A controlled trial in constipated women. Z Gastroenterol. 1990;28:660-4.
206. van Gorkom BA, de Vries EG, Karrenbeld A, Kleibeuker JH. Review article: Anthranoid laxatives and their potential carcinogenic effects. Aliment Pharmacol Ther. 1999;13:443-52.
207. Exton-Smith AN, Bendall MJ, Kent F. A new technique for measuring the consistency of faeces: A report on its application to the assessment of Senokot therapy in the elderly. Age Ageing. 1975;4:58-62.
208. Kamm MA, Lennard-Jones JE, Thompson DG, et al. Dynamic scanning defines a colonic defect in severe idiopathic constipation. Gut. 1988;29:1085-92.
209. Mengs U, Rudolph RL. Light and electron-microscopic changes in the colon of the guinea pig after treatment with anthranoid and non-anthranoid laxatives. Pharmacology. 1993;47(Suppl 1):172-7.
210. Flig E, Hermann TW, Zabel M. Is bisacodyl absorbed at all from suppositories in man? Int J Pharm. 2000;196:11-20.
211. Saunders DR, Sillery J, Rachmilewitz D. Effect of dioctyl sodium sulfosuccinate on structure and function of rodent and human intestine. Gastroenterology. 1975;69:380-6.
212. Chapman RW, Sillery J, Fontana DD, et al. Effect of oral dioctyl sodium sulfosuccinate on intake-output studies of human small and large intestine. Gastroenterology. 1985;89:489-93.
213. Hyland CM, Foran JD. Dioctyl sodium sulphosuccinate as a laxative in the elderly. Practitioner. 1968;200:698-9.
214. McRorie JW, Daggy BP, Morel JG, et al. Psyllium is superior to docusate sodium for treatment of chronic constipation. Aliment Pharmacol Ther. 1998;12:491-7.
215. Meisel JL, Bergman D, Groney D, et al. Human rectal mucosa: Proctoscopic and morphological changes caused by laxatives. Gastroenterology. 1977;2:1274-9.
216. Spinrad S, Sztern M, Grosskopf Y, et al. Treating constipation with phosphate enema: An unnecessary risk. Isr J Med Sci. 1989;25:237-8.
217. Rohack JJ, Mehta BR, Subramanyam K. Hyperphosphatemia and hypocalcemic coma associated with phosphate enema. South Med J. 1985;78:1241-2.
218. Marraffa JM, Hui A, Stork CM. Severe hyperphosphatemia and hypocalcemia following the rectal administration of a phosphate-containing Fleet pediatric enema. Pediatr Emerg Care. 2004;20:453-6.
219. Walton DM, Thomas DC, Aly HZ, Short BL. Morbid hypocalcemia associated with phosphate enema in a six-week-old infant. Pediatrics. 2000;106:E37.
220. Ismail EA, Al-Mutairi G, Al-Anzy H. A fatal small dose of phosphate enema in a young child with no renal or gastrointestinal abnormality. J Pediatr Gastroenterol Nutr. 2000;30:220-1.
221. Camilleri M, Bharucha AE, Ueno R, et al. Effect of a selective chloride channel activator, lubiprostone, on gastrointestinal transit, gastric sensory, and motor functions in healthy volunteers. Am J Physiol Gastrointest Liver Physiol. 2006;290:G942-7.
222. Johanson JF, Morton D, Geenen J, Ueno R. Multicenter, 4-week, double-blind, randomized, placebo-controlled trial of lubiprostone, a locally-acting type-2 chloride channel activator, in patients with chronic constipation. Am J Gastroenterol. 2008;103:170-7.
223. Muller-Lissner SA. Treatment of chronic constipation with cisapride and placebo. Gut. 1987;28:1033-8.
224. Brandt LJ. The FDA’s decision-making process: Isn’t it time to temper the principle of protective paternalism? Am J Gastroenterol. 2008;103:1226-7.
225. Johanson JF, Wald A, Tougas G, et al. Effect of tegaserod in chronic constipation: A randomized, double-blind, controlled trial. Clin Gastroenterol Hepatol. 2004;2:796-805.
226. Muller-Lissner SA, Fumagalli I, Bardhan KD, et al. Tegaserod, a 5-HT(4) receptor partial agonist, relieves symptoms in irritable bowel syndrome patients with abdominal pain, bloating and constipation. Aliment Pharmacol Ther. 2001;15:1655-66.
227. Bouras EP, Camilleri M, Burton DD, et al. Prucalopride accelerates gastrointestinal and colonic transit in patients with constipation without a rectal evacuation disorder. Gastroenterology. 2001;120:354-60.
228. Camilleri M, Kerstens R, Rykx A, Vandeplassche L. A placebo-controlled trial of prucalopride for severe chronic constipation. N Engl J Med. 2008;358:2344-54.
229. Quigley EM, Vandeplassche L, Kerstens R, Ausma J. Clinical trial: The efficacy, impact on quality of life, and safety and tolerability of prucalopride in severe chronic constipation—a 12-week, randomised, double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2009;29:315-28.
230. Tack J, Van Outryve M, Beyens G, et al. Prucalopride (resolor(R)) in the treatment of severe chronic constipation in patients dissatisfied with laxatives. Gut. 2009;58:357-65.
231. Goldberg MR, Li YP, Pitzer K, et al. TD-5108, a selective 5-HT4 agonist, is consistently better than placebo regardless of response definition in patients with chronic constipation. Gastroenterology. 2008;134:A545.
232. Thomas J, Karver S, Cooney GA, et al. Methylnaltrexone for opioid-induced constipation in advanced illness. N Engl J Med. 2008;358:2332-43.
233. Webster L, Jansen JP, Peppin J, et al. Alvimopan, a peripherally acting mu-opioid receptor (PAM-OR) antagonist for the treatment of opioid-induced bowel dysfunction: Results from a randomized, double-blind, placebo-controlled, dose-finding study in subjects taking opioids for chronic non-cancer pain. Pain. 2008;137:428-40.
234. Verne GN, Davis RH, Robinson ME, et al. Treatment of chronic constipation with colchicine: Randomized, double-blind, placebo-controlled, crossover trial. Am J Gastroenterol. 2003;98:1112-16.
235. Roarty TP, Weber F, Soykan I, McCallum RW. Misoprostol in the treatment of chronic refractory constipation: Results of a long-term open label trial. Aliment Pharmacol Ther. 1997;11:1059-66.
236. Ponec RJ, Saunders MD, Kimmey MB. Neostigmine for the treatment of acute colonic pseudo-obstruction. N Engl J Med. 1999;341:137-41.
237. Ron Y, Avni Y, Lukovetski A, et al. Botulinum toxin type-A in therapy of patients with anismus. Dis Colon Rectum. 2001;44:1821-6.
238. Maria G, Cadeddu F, Brandara F, et al. Experience with type A botulinum toxin for treatment of outlet-type constipation. Am J Gastroenterol. 2006;101:2570-5.
239. Coulie B, Szarka LA, Camilleri M, et al. Recombinant human neurotrophic factors accelerate colonic transit and relieve constipation in humans. Gastroenterology. 2000;119:41-50.
240. Parkman HP, Rao SS, Reynolds JC, et al. Neurotrophin-3 improves functional constipation. Am J Gastroenterol. 2003;98:1338-47.
241. Andresen V, Camilleri M, Busciglio IA, et al. Effect of 5 days linaclotide on transit and bowel function in females with constipation-predominant irritable bowel syndrome. Gastroenterology. 2007;133:761-8.
242. Lembo A JJ, MacDougall JE, Lavins BJ, et al. Linaclotide significantly improved bowel habits and relieved abdominal symptoms in adults with chronic constipation: Data from a large four-week, randomized, double-blind, placebo-controlled study. Gastroenterology. 2008;134:682f.
243. Pelsang RE, Rao SS, Welcher K. FECOM: A new artificial stool for evaluating defecation. Am J Gastroenterol. 1999;94:183-6.
244. Koutsomanis D, Lennard-Jones JE, Roy AJ, Kamm MA. Controlled randomised trial of visual biofeedback versus muscle training without a visual display for intractable constipation. Gut. 1995;37:95-9.
245. Enck P. Biofeedback training in disordered defecation. A critical review. Dig Dis Sci. 1993;38:1953-60.
246. Heymen S, Jones KR, Scarlett Y, Whitehead WE. Biofeedback treatment of constipation: A critical review. Dis Colon Rectum. 2003;46:1208-17.
247. Van Outryve M, Pelckmans P. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology. 2006;131:333-4.
248. Chiarioni G, Whitehead WE, Pezza V, et al. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology. 2006;130:657-64.
249. Rao SS, Seaton K, Miller M, et al. Randomized controlled trial of biofeedback, sham feedback, and standard therapy for dyssynergic defecation. Clin Gastroenterol Hepatol. 2007;5:331-8.
250. Bleijenberg G, Kuijpers HC. Treatment of the spastic pelvic floor syndrome with biofeedback. Dis Colon Rectum. 1987;30:108-11.
251. Heymen S, Wexner SD, Vickers D, et al. Prospective, randomized trial comparing four biofeedback techniques for patients with constipation. Dis Colon Rectum. 1999;42:1388-93.
252. Lau CW, Heymen S, Alabaz O, et al. Prognostic significance of rectocele, intussusception, and abnormal perineal descent in biofeedback treatment for constipated patients with paradoxical puborectalis contraction. Dis Colon Rectum. 2000;43:478-82.
253. Papachrysostomou M, Smith AN. Effects of biofeedback on obstructive defecation-reconditioning of the defecation reflex? Gut. 1994;35:252-6.
254. Emmanuel AV, Kamm MA. Response to a behavioural treatment, biofeedback, in constipated patients is associated with improved gut transit and autonomic innervation. Gut. 2001;49:214-19.
255. Dahl J, Lindquist BL, Tysk C, et al. Behavioral medicine treatment in chronic constipation with paradoxical anal sphincter contraction. Dis Colon Rectum. 1991;34:769-76.
256. Chiotakakou-Faliakou E, Kamm MA, Roy AJ, et al. Biofeedback provides long-term benefit for patients with intractable, slow and normal transit constipation. Gut. 1998;42:517-21.
257. Roy AJ, Emmanuel AV, Storrie JB, et al. Behavioural treatment (biofeedback) for constipation following hysterectomy. Br J Surg. 2000;87:100-5.
258. Vaizey CJ, Roy AJ, Kamm MA. Prospective evaluation of the treatment of solitary rectal ulcer syndrome with biofeedback. Gut. 1997;41:817-20.
259. van Tilburg MA, Palsson OS, Levy RL, et al. Complementary and alternative medicine use and cost in functional bowel disorders: A six month prospective study in a large HMO. BMC Complement Altern Med. 2008;8:46.
260. Zhao H, Liu JP, Liv ZS, Peng WN. Acupuncture for chronic constipation. Cochrane Library, Oxford: Update Software; 2004.
261. Holzer B, Rosen HR, Novi G, et al. Sacral nerve stimulation in patients with severe constipation. Dis Colon Rectum. 2008;51:524-9.
262. Knowles CH, Scott M, Lunniss PJ. Outcome of colectomy for slow transit constipation. Ann Surg. 1999;230:627-38.
263. Redmond JM, Smith GW, Barofsky I, et al. Physiological tests to predict long-term outcome of total abdominal colectomy for intractable constipation. Am J Gastroenterol. 1995;90:748-53.
264. Pemberton JH, Rath DM, Ilstrup DM. Evaluation and surgical treatment of severe chronic constipation. Ann Surg. 1991;214:403-11.
265. Nicholls RJ, Kamm MA. Proctocolectomy with restorative ileoanal reservoir for severe idiopathic constipation. Report of two cases. Dis Colon Rectum. 1988;31:968-9.
266. Christiansen J, Rasmussen OO. Colectomy for severe slow-transit constipation in strictly selected patients. Scand J Gastroenterol. 1996;31:770-3.
267. Ho YH, Tan M, Eu KW, et al. Laparoscopic-assisted compared with open total colectomy in treating slow transit constipation. Aust N Z J Surg. 1997;67:562-5.
268. Young-Fadok TM. Raising the bar. Laparoscopic resection of colorectal cancer. Surg Endosc. 2001;15:911-12.
269. Teichman JM, Barber DB, Rogenes VJ, Harris JM. Malone antegrade continence enemas for autonomic dysreflexia secondary to neurogenic bowel. J Spinal Cord Med. 1998;21:245-7.
270. Pechlivanides G, Tsiaoussis J, Athanasakis E, et al. Stapled transanal rectal resection (STARR) to reverse the anatomic disorders of pelvic floor dyssynergia. World J Surg. 2007;31:1329-35.
271. Arroyo A, Pérez-Vicente F, Serrano P, et al. Evaluation of the stapled transanal rectal resection technique with two staplers in the treatment of obstructive defecation syndrome. J Am Coll Surg. 2007;204:56-63.
272. Boccasanta P, Venturi M, Stuto A, et al. Stapled transanal rectal resection for outlet obstruction: A prospective, multicenter trial. Dis Colon Rectum. 2004;47:1285-96.
273. Kamm MA, Hawley PR, Lennard-Jones JE. Lateral division of the puborectalis muscle in the management of severe constipation. Br J Surg. 1988;75:661-3.