4 CONNECTIVE TISSUE
Classification
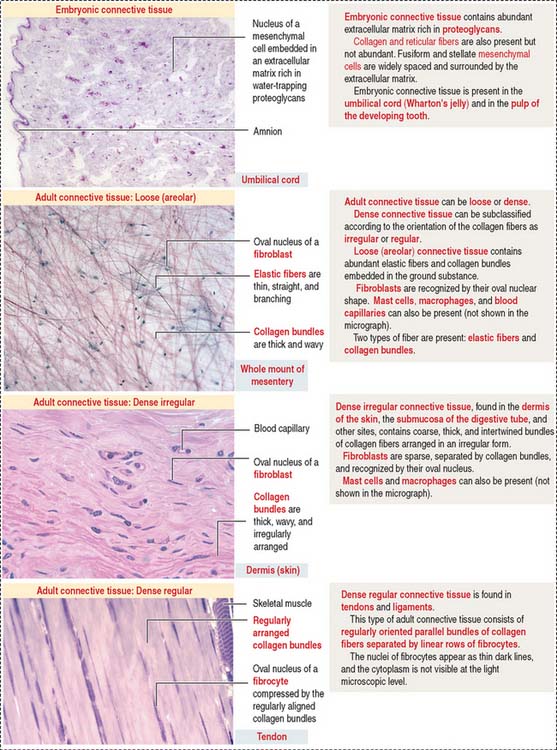
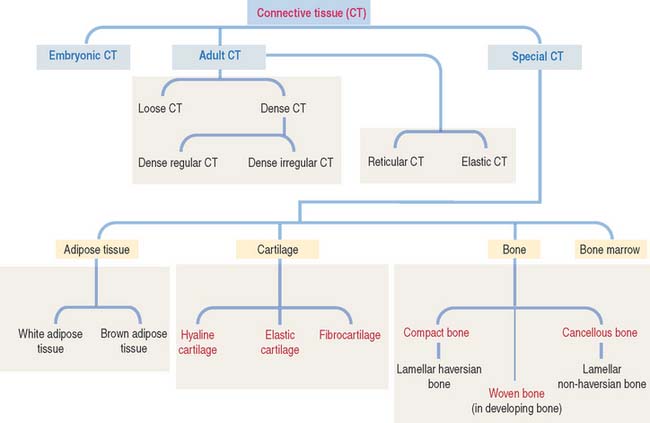
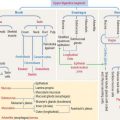
Connective tissue can be classified into three major groups (Figure 4-1): embryonic connective tissue, adult connective tissue, and special connective tissue.
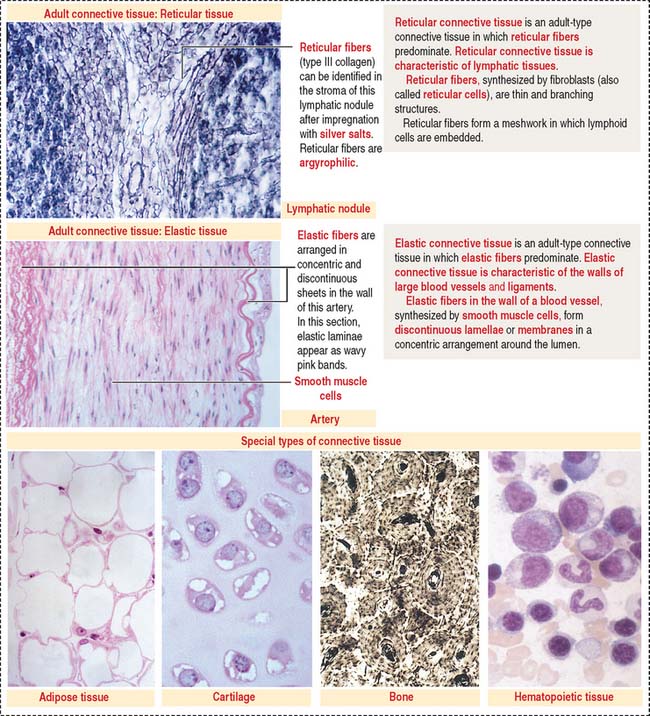
In addition, reticular and elastic fibers predominate in irregular connective tissue.
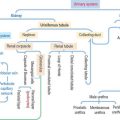
The special connective tissue comprises types of connective tissue with special properties not observed in the embryonic or adult connective tissue proper. There are four types of special connective tissue (Figure 4-2):
Adipose tissue has more cells (called adipose cells or adipocytes) than collagen fibers and ground substance. This type of connective tissue is the most significant energy storage site of the body.
The hematopoietic tissue is found in the marrow of selected bones. This type of connective tissue is discussed in Chapter 6, Blood and Hematopoiesis.
Cartilage and bone are also regarded as special connective tissue but are traditionally placed in separate categories. Essentially, cartilage and bone are dense connective tissues with specialized cells and ground substance. An important difference is that cartilage has a noncalcified ECM, whereas the ECM of bone is calcified. These two types of specialized connective tissue fulfill weight-bearing and mechanical functions that are discussed later (see Cartilage and Bone).
Cell components of connective tissue
The fibroblast synthesizes and continuously secretes mature proteoglycans and glycoproteins and the precursor molecules of various types of collagens and elastin. Different types of collagen proteins and proteoglycans can be recognized as components of the basement membrane. As you may remember, type IV collagen is found in the basal lamina and type III collagen appears in the reticular lamina as a component of reticular fibers (see Boxes 4-A and 4-B). Heparan sulfate proteoglycans and the glycoprotein fibronectin are two additional products of the fibroblast that appear in the basement membrane. The protein collagen is a component of collagen and reticular fibers. However, elastic fibers do not contain collagen.
Box 4-A Distribution of collagen
Observed in hyaline and elastic cartilage as fibrils thinner than type I collagen.
Box 4-B Cell types making collagen
Collagen: Synthesis, secretion, and assembly
Collagens are generally divided into two categories: fibrillar collagens (forming fibrils with a characteristic banded pattern), and nonfibrillar collagens (see Box 4-C).
Box 4-C Characteristics of collagens
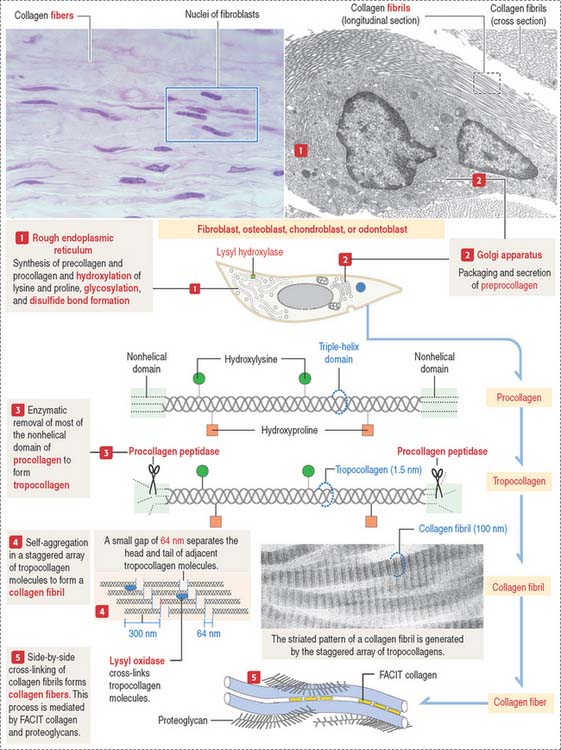
The synthesis of collagen starts in the rough endoplasmic reticulum (RER) following the typical pathway of synthesis for export from the cell (Figure 4-3).
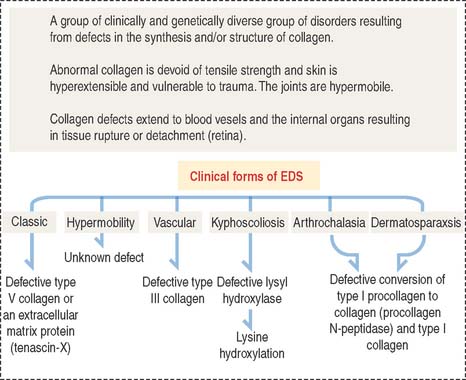
Clinical significance: Ehlers-Danlos syndrome
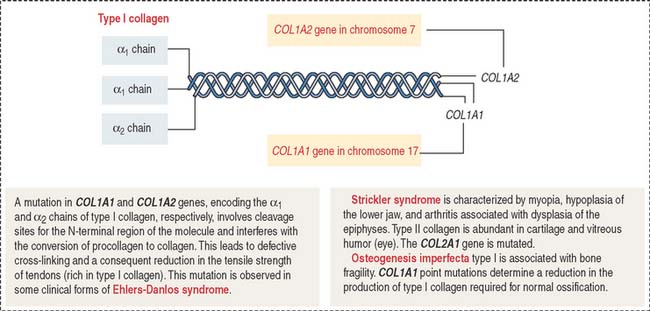
Ehlers-Danlos syndrome is clinically characterized by hyperelasticity of the skin (Figure 4-4) and hypermobility of the joints. The major defect resides in the synthesis, processing, and assembly of collagen. Several clinical subtypes are observed. They are classified by the degree of severity and the mutations in the collagen genes. For example, the vascular type form of Ehlers-Danlos syndrome—caused by a mutation in the COL3A1 gene—is associated with severe vascular alterations leading to the development of varicose veins and spontaneous rupture of major arteries. A deficiency in the synthesis of type III collagen, prevalent in the walls of blood vessels, is the major defect. Arthrochalasia and dermatosparaxsis types of Ehlers-Danlos syndrome display congenital dislocation of the hips and marked joint hypermobility. Mutations in the COL1A1 and COL1A2 genes (Figure 4-5), encoding type I collagen, and procollagen N-peptidase gene disrupt the cleavage site at the N-terminal of the molecule and affect the conversion of procollagen to collagen in some individuals.
Elastic fibers: Synthesis, secretion, and assembly
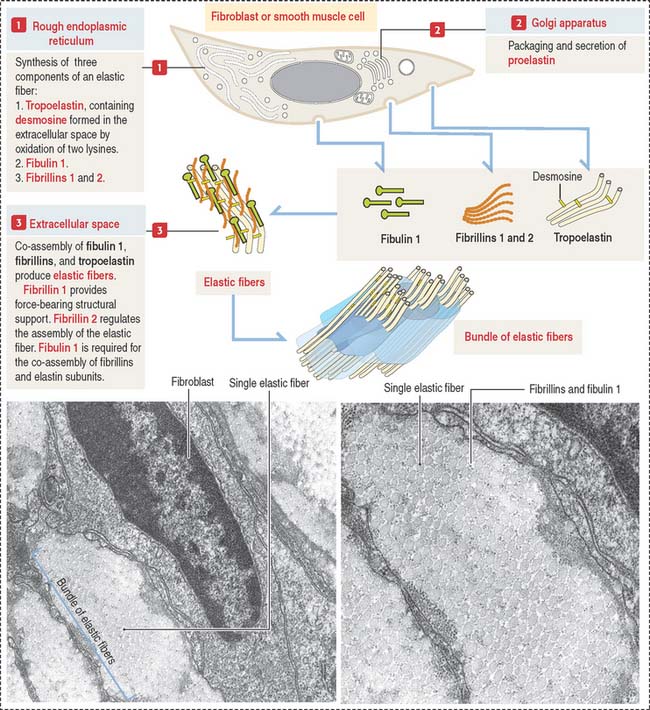
Like collagen, the synthesis of elastic fibers involves both the RER and the Golgi apparatus (Figure 4-6).
Elastic fibers are synthesized by the fibroblast (in skin and tendons), the chondroblast, the chondrocyte (in elastic cartilage of the auricle of the ear, epiglottis, larynx, and auditory tubes), and smooth muscle cells (in large blood vessels like the aorta and in the respiratory tree).
Proelastin, the precursor of elastin, is cleaved and secreted as tropoelastin. In the extracellular space, tropoelastin interacts with fibrillins and fibulin 1 to organize elastic fibers, which aggregate to form bundles of elastic fibers. Tropoelastin contains a characteristic but uncommon amino acid: desmosine. Two lysine residues of tropoelastin are oxidized by lysyl oxidase to form a desmosine ring that cross-links two tropoelastin molecules. Cross-linking enables the stretching and recoil of tropoelastin, like rubber bands. Elastic fibers do not contain collagen. Elastic fibers are produced during embryonic development and in adolescence but not so much in adults. Although elastic fibers are resilient during human life, many tissues decrease elasticity with age, in particular the skin, which develops wrinkles.
Clinical significance: Marfan syndrome
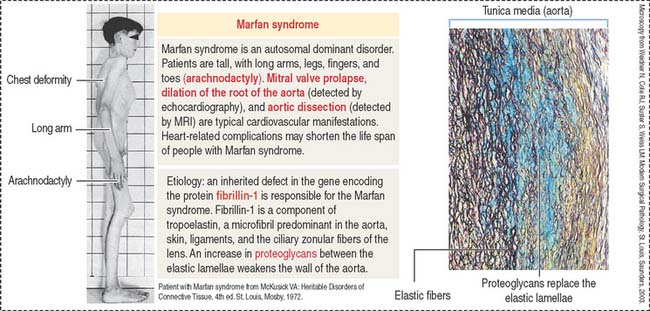
Marfan syndrome is an autosomal dominant disorder in which the elastic tissue is weakened. Defects are predominantly observed in three systems: the ocular, skeletal, and cardiovascular systems. The ocular defects include myopia and detached lens (ectopia lentis). The skeletal defects (Figure 4-7) include long and thin arms and legs (dolichostenomelia), hollow chest (pectus excavatum), scoliosis, and elongated fingers (arachnodactyly).
Defects observed in Marfan syndrome are caused by poor recoiling of the elastic lamellae dissociated by an increase in proteoglycans (see Figure 4-7). In the skeletal system, the periosteum, a relatively rigid layer covering the bone, is abnormally elastic and does not provide an oppositional force during bone development, resulting in skeletal defects.
A mutation of the fibrillin 1 gene on chromosome 15 is responsible for Marfan syndrome. Fibrillin is present in the aorta, suspensory ligaments of the lens (see Chapter 9, Sensory Organs: Vision and Hearing), and the periosteum (see Bone). A homologous fibrillin 2 gene is present on chromosome 5. Mutations in the fibrillin 2 gene cause a disease called congenital contractural arachnodactyly. This disease affects the skeletal system, but ocular and cardiovascular defects are not observed.
Macrophages
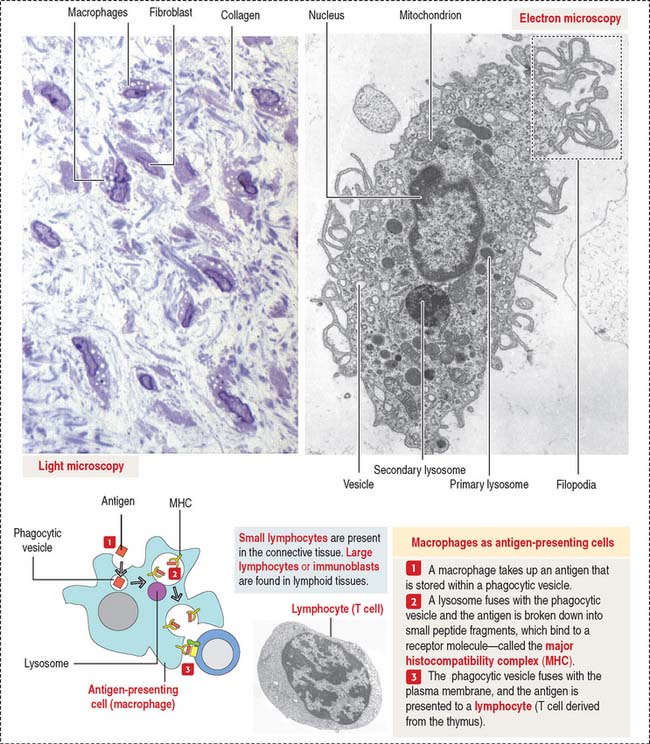
Macrophages have phagocytic properties and derive from monocytes, cells formed in the bone marrow (Figure 4-8).
Monocytes circulate in blood and migrate into the connective tissue, where they differentiate into macrophages. Macrophages have specific names in certain organs; for example, they are called Kupffer cells in the liver, osteoclasts in bone, and microglial cells in the central nervous system. Macrophages migrate to the site of inflammation, attracted by certain mediators, particularly C5a (a member of the complement cascade; see Chapter 10, Immune-Lymphatic System). Macrophages in the connective tissue have the following structural features:
Macrophages of the connective tissue have three major functions:
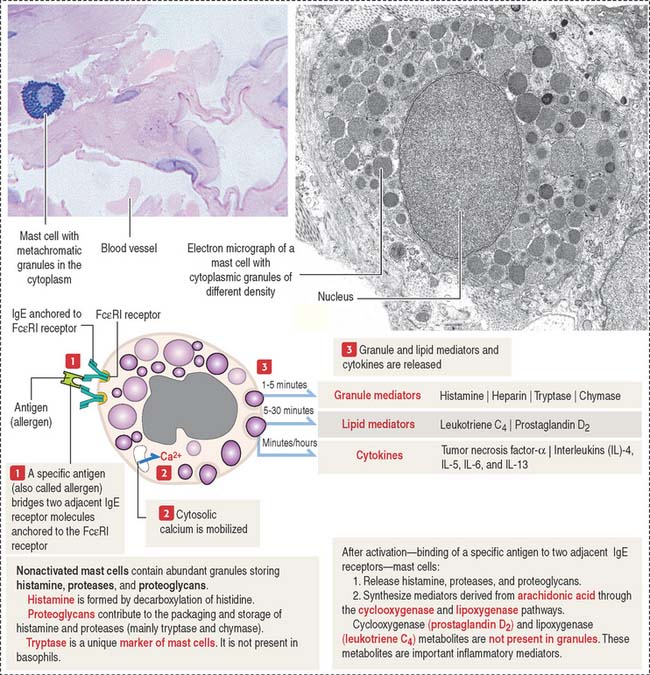
Mast cells
The mast cell is the source of vasoactive mediators contained in cytoplasmic granules (Figure 4-9). These granules contain histamine, heparin, and chemotactic mediators to attract monocytes, neutrophils, and eosinophils circulating in blood to the site of mast cell activation.
Connective tissue mast cells differ from mucosal mast cells in the number and size of metachromatic (see Box 4-D) cytoplasmic granules, which tend to be more abundant in connective tissue mast cells. Although these two cell populations have the same cell precursor, the definitive structural and functional characteristics of mast cells depend on the site of differentiation (mucosa or connective tissue).
Box 4-D Metachromasia
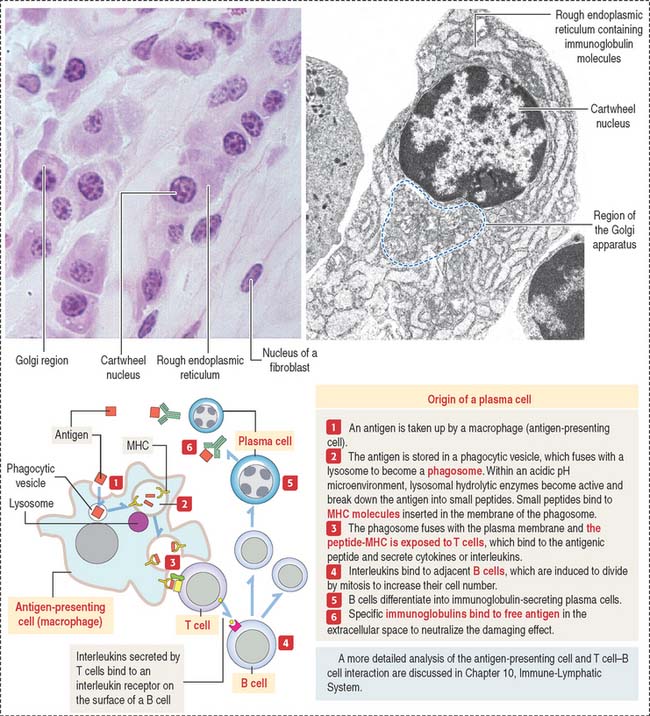
Plasma cells
The plasma cell, which derives from the differentiation of B lymphocytes (also called B cells), synthesizes and secretes a single class of immunoglobulin (Figure 4-10). We discuss in Chapter 10, Immune-Lymphatic System, details of the origin of plasma cells.
At the light microscopic level, most of the cytoplasm of a plasma cell is basophilic because of the large amount of ribosomes associated with the endoplasmic reticulum. A clear area near the nucleus is slightly acidophilic and represents the Golgi apparatus. The nucleus has a characteristic cartwheel configuration created by the particular distribution of heterochromatin.
Extracellular matrix
Recall that the basement membrane contains several ECM components such as laminin, fibronectin, various types of collagen, and heparan sulfate proteoglycan. In addition, epithelial and nonepithelial cells have receptors for ECM constituents. An example is the family of integrins with binding affinity for laminin and fibronectin. Integrins interact with the cytoskeleton, strengthening cell interactions with the ECM by establishing focal contacts or modifying cell shape or adhesion.
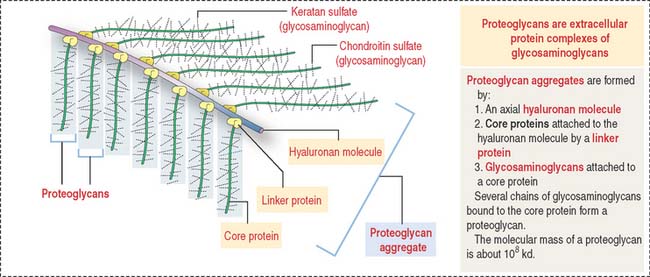
Proteoglycan aggregates (Figure 4-11) are the major components of the ECM. Each proteoglycan consists of glycosaminoglycans (GAGs), proteins complexed with polysaccharides. GAGs are linear polymers of disaccharides with sulfate residues. GAGs control the biological functions of proteoglycans by establishing links with cell surface components, growth factors, and other ECM constituents.
Degradation of the extracellular matrix
The expression of matrix metalloproteinase genes is regulated by cytokines, growth factors, and cell contact with the ECM.
Members of the family of matrix metalloproteinases include:
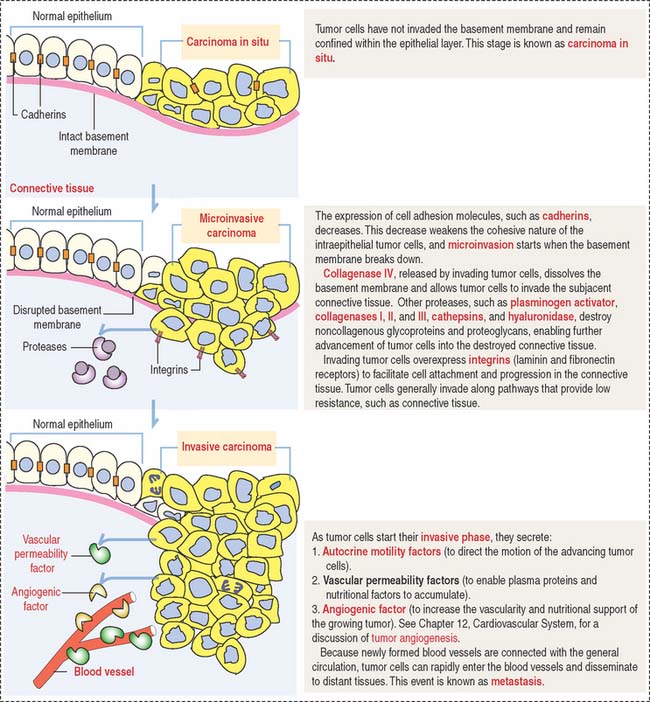
Clinical significance: Molecular biology of tumor invasion
Invasion is defined by the breakdown of the basement membrane by tumor cells and implies the transition from precancer to cancer. Metastasis is the spread of tumor cells throughout the body through blood and lymphatic vessels, generally leading to death. Figure 4-12 illustrates and describes the initial events of tumor cell invasion.
One critical event during metastasis is angiogenesis, the development of blood vessels. Blood vessels supply oxygen and nutrients required for tumor growth. Angiogenesis is stimulated by tumor cells, in particular the proliferation of capillary endothelial cells forming new capillaries in the tumoral growth. In Chapter 12, Cardiovascular System, we discuss the mechanism of action and targets of endostatin and angiostatin, two new proteins that inhibit angiogenesis.
ADIPOSE TISSUE OR FAT
There are two classes of adipose tissue:
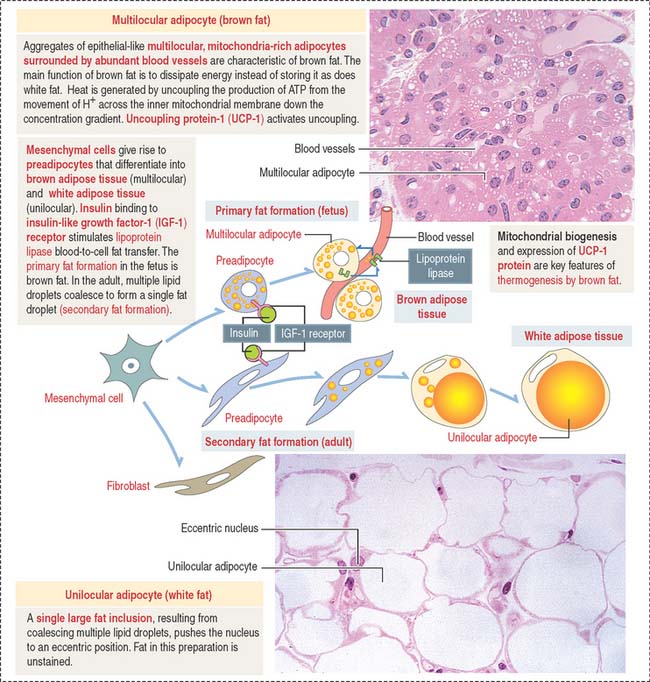
Under the influence of insulin—bound to insulin-like growth factor-1 (IGF-1) receptor—preadipocytes synthesize lipoprotein lipase and begin to accumulate fat in small droplets. Small droplets fuse to form a single large lipid-storage droplet, a characteristic of mature unilocular (Latin unus, single; loculus, small place) adipocytes (also called adipose cell) (Figure 4-13). The single lipid-storage droplet pushes the nucleus to an eccentric position and the adipocyte assumes a “signet-ring” appearance. In histologic sections, capillaries appear as single structures that may contain blood cell elements, whereas adipocytes form aggregates.
Lipid droplets contain about 95% triglycerides rich in carotene, a lipid-soluble pigment that gives the so-called white fat a yellowish color. Each lipid droplet is in direct contact with the cytosol and is not surrounded by a cytomembrane. Therefore, lipid droplets can be classified as cell inclusions (see Box 4-E).
Box 4-E Fat in histologic sections
The main function of white fat is storage of energy. Unlike brown fat, white fat responds slightly to cold and acts as an insulator. The blood supply to white fat, mainly capillaries, is not as extensive as in brown fat. Adipose tissue also insulates the body against heat loss, fills spaces, and cushions certain anatomic parts, behaving as a shock-absorber in the soles of the feet, around the kidneys, and in the orbit around the eye. Most adipose tissues form at sites where loose connective tissue is present, such as the subcutaneous layer—or hypodermis—of the skin.
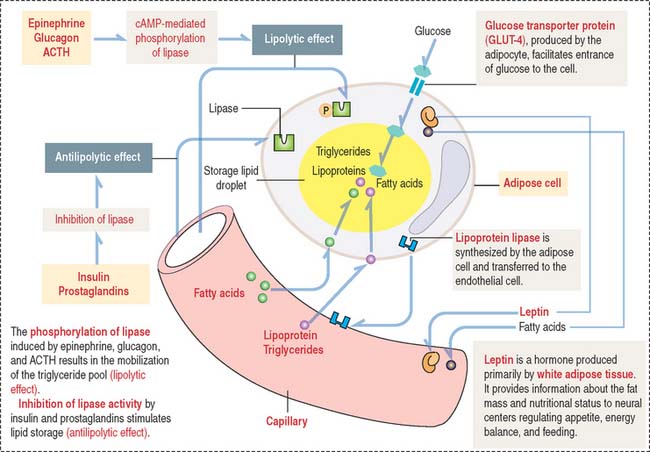
The storage of lipids by mature adipocytes are regulated by insulin and prostaglandins. The breakdown and release of lipids is regulated by epinephrine, glucagon, and adrenocorticotropic hormone (ACTH) (Figure 4-14). Adipose tissue is innervated by the sympathetic nervous system.
As stated initially, the main function of brown fat is to dissipate energy in the form of heat (thermogenesis) in cold environments as a protective mechanism in the newborn. Thermogenesis by brown fat cells has two requirements (see Figure 4-13):
As we briefly mentioned in Chapter 2, Epithelial Glands, in our discussion on UCP transporters in mitochondria, UCP-1 dissipates the proton gradient established across the inner mitochondrial membrane when electrons pass along the electron-transport chain. Thermogenesis takes place because UCP-1 allows the reentry of protons down their concentration gradient into the mitochondrial matrix and uncouples respiration from ATP production.
Clinical significance: Obesity
Obesity is a disorder of energy balance. It occurs when energy intake exceeds energy expenditure. Protection against obesity without consideration of energy intake results in an increase in circulating levels of triglycerides, and excessive accumulation of fat in liver (steatosis). The metabolic activities of adipocytes have very significant clinical consequences. An increase in visceral adiposity is associated with a higher risk of insulin resistance (see Chapter 19, Endocrine System), dyslipidemia (alteration in blood fat levels), and cardiovascular disease.
CARTILAGE
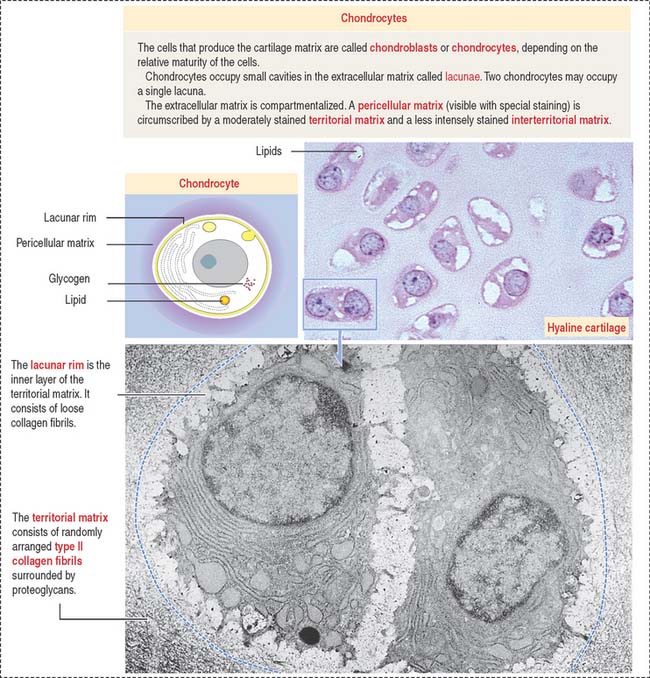
In contrast to typical connective tissue, the cartilage is avascular and cells receive nutrients by diffusion through the ECM. At all ages, chondrocytes have significant nutritional requirements. Although they rarely divide in the adult cartilage, they continuously synthesize molecules to replace a constantly turned-over ECM, in particular, proteoglycans (Figure 4-15; see Box 4-F).
Box 4-F Cartilage repair after injury
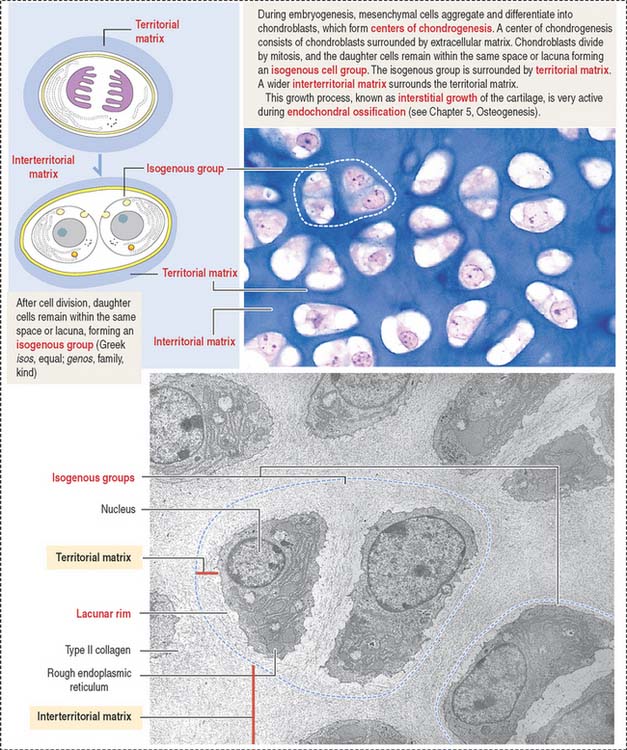
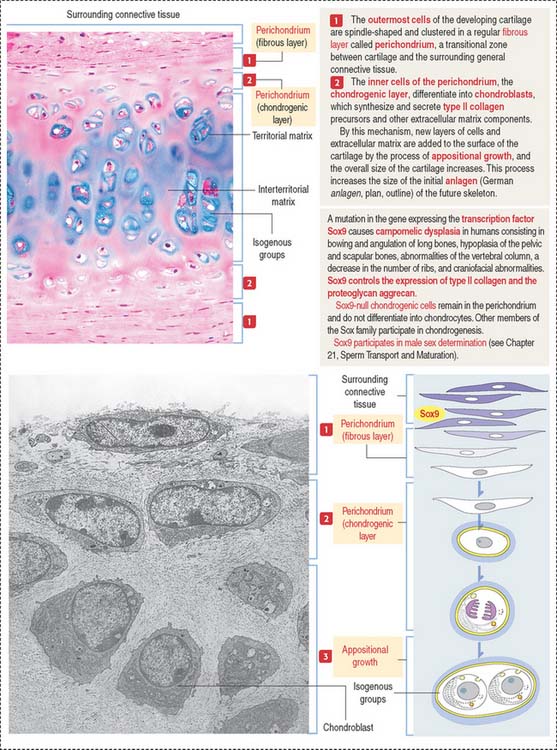
Growth of cartilage (chondrogenesis)
Cartilage grows by two mechanisms (Figures 4-16 and 4-17):
The matrix in close contact with each chondrocyte forms a bluish (with hematoxylin and eosin), metachromatic (see Box 4-D), or PAS-positive basketlike structure called the territorial matrix.
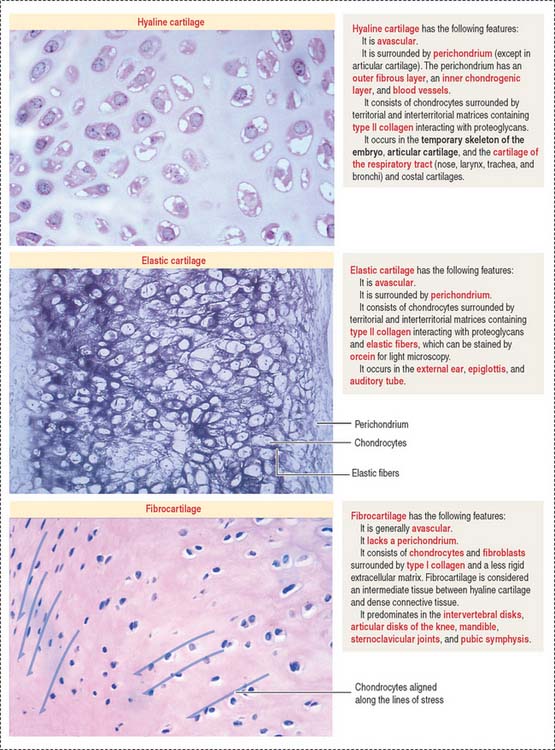
Types of cartilage
There are three major types of cartilage (Figure 4-18):
Articular surfaces are not lined by an epithelium. The hyaline cartilage contains:
Chondrocytes have the structural characteristics of a protein-secreting cell (well-developed RER and Golgi apparatus, and large nucleolus) and store lipids and glycogen in the cytoplasm. Chondrocytes are coated by a pericellular matrix, surrounded by the territorial and interterritorial matrices, respectively. A lacunar rim separates the cell from the territorial matrix.
The perichondrium consists of two layers:
The ECM contains hyaluronic acid, proteoglycans (rich in the GAGs chondroitin sulfate and keratan sulfate), and a high water content (70% to 80% of its weight). Aggrecan is a large proteoglycan characteristic of cartilage (see Boxes 4-G and 4-H).
Box 4-G Cartilage repair after injury
Box 4-H How chondrocytes survive
The transcription factor Sox9 is required for expression of cartilage-specific ECM components such as type II collagen and the proteoglycan aggrecan. Sox9 activates the expression of collagen by the C0L2A1 gene. A lack of Sox9 expression prevents the chondrogenic layer to differentiate into chondrocytes. Mutations in the Sox9 gene cause the rare and severe dwarfism campomelic dysplasia (Figure 4-17).
BONE
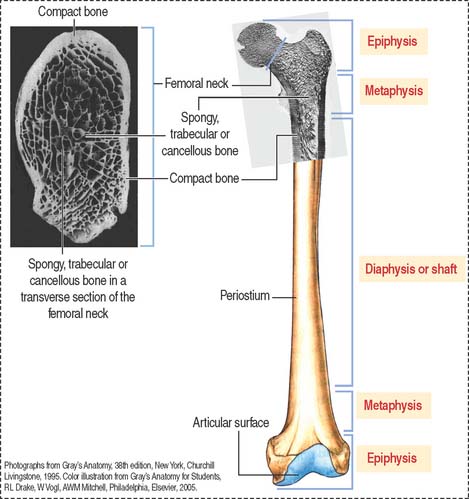
Macroscopic structure of mature bone
Based on its gross appearance (Figure 4-19), two forms of bone are distinguished:
In long bones, such as the femur, the shaft or diaphysis consists of compact bone forming a hollow cylinder with a central marrow space, called the medullary or marrow cavity.
Microscopic structure of mature bone
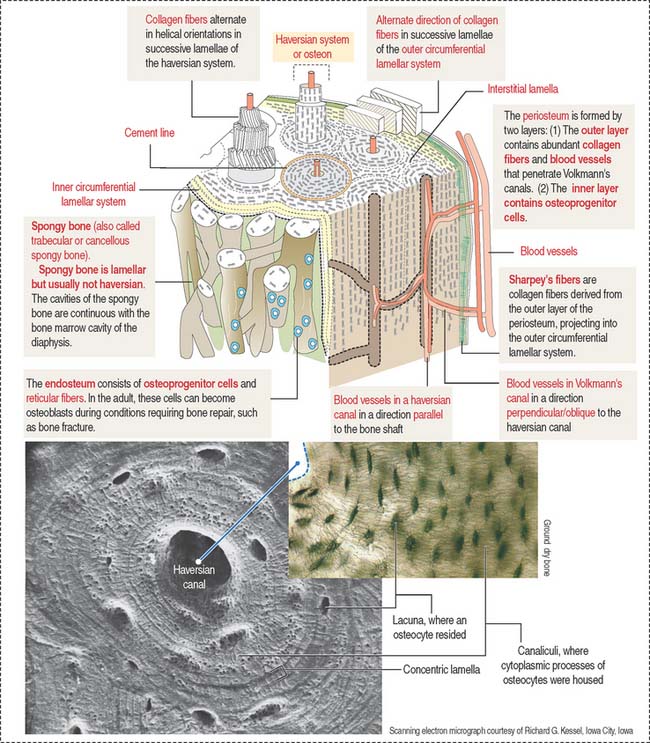
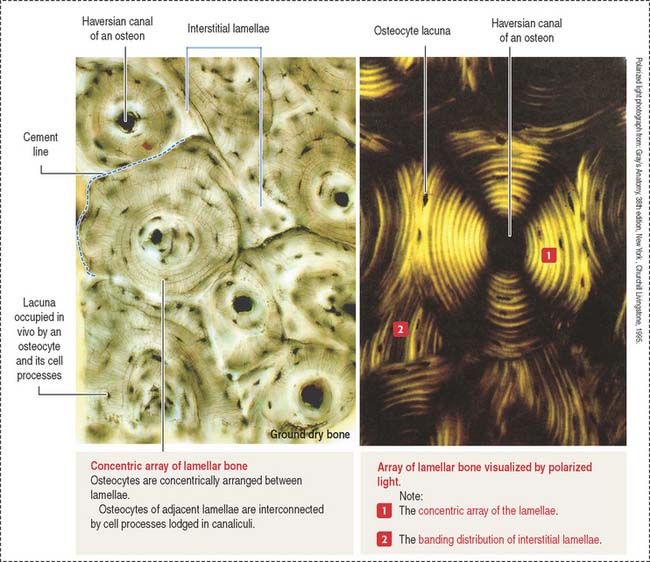
The lamellar bone consists of lamellae, largely composed of bone matrix, a mineralized substance deposited in layers or lamellae, and osteocytes, each one occupying a cavity or lacuna with radiating and branching canaliculi that penetrate the lamellae of adjacent lacunae. The lamellar bone displays four distinct patterns (Figure 4-20):
The vascular channels in compact bone have two orientations with respect to the lamellar structures:
Periosteum and endosteum
The outer layer is rich in blood vessels, some of them entering Volkmann’s canals, and thick anchoring collagen fibers, called Sharpey’s fibers, that penetrate the outer circumferential lamellae deep in the bone (see Figure 4-20).
Bone matrix
Type I collagen is the predominant protein of the bone matrix. In mature lamellar bone, collagen fibers have a highly ordered arrangement with changing orientations with respect to the axis of the haversian canal in successive concentric lamellae (see Figure 4-20).
Cellular components of bone
Actively growing bone contains cells of two different lineages:
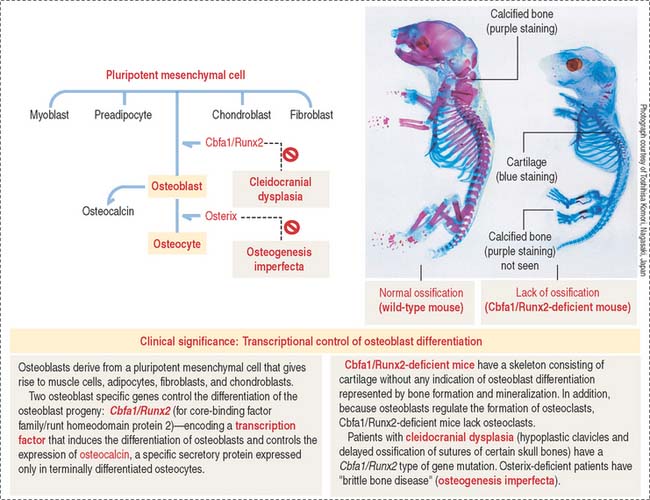
Osteoblasts differentiate into osteocytes after they are trapped inside lacunae within the mineralized matrix they produce. Their differentiation involves the participation of two transcription factors: Cbfa1/Runx2 and osterix (see Box 4-I).
Box 4-I How osteocytes differentiate
The osteoclast lineage derives from the monocyte-macrophage lineage in the bone marrow.
Osteoblasts and osteocytes
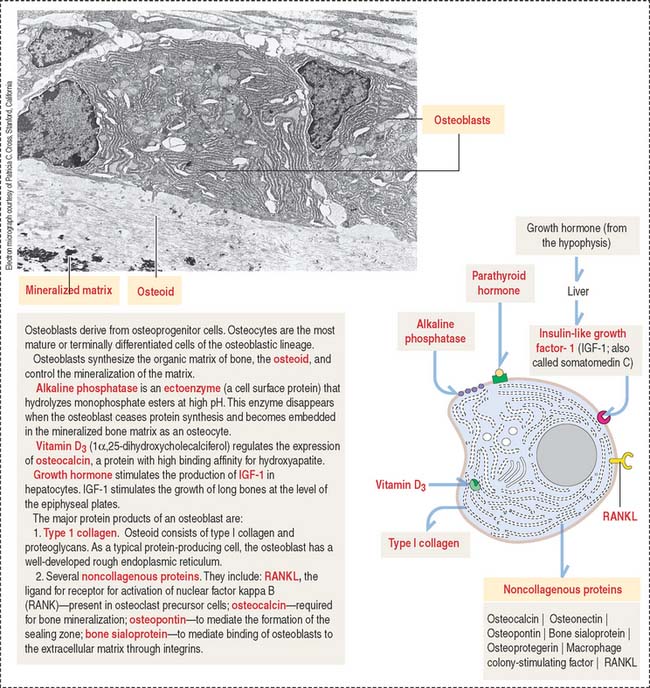
In electron micrographs, osteoblasts display the typical features of cells actively engaged in protein synthesis, glycosylation, and secretion. Their specific products are type I collagen, osteocalcin, osteopontin, and bone sialoprotein (Figure 4-23). Osteoblasts give a strong cytochemical reaction for alkaline phosphatase that disappears when the cells become embedded in the matrix as osteocytes. In addition, osteoblasts produce growth factors, in particular members of the bone morphogenetic protein family, with bone-inductive activities.
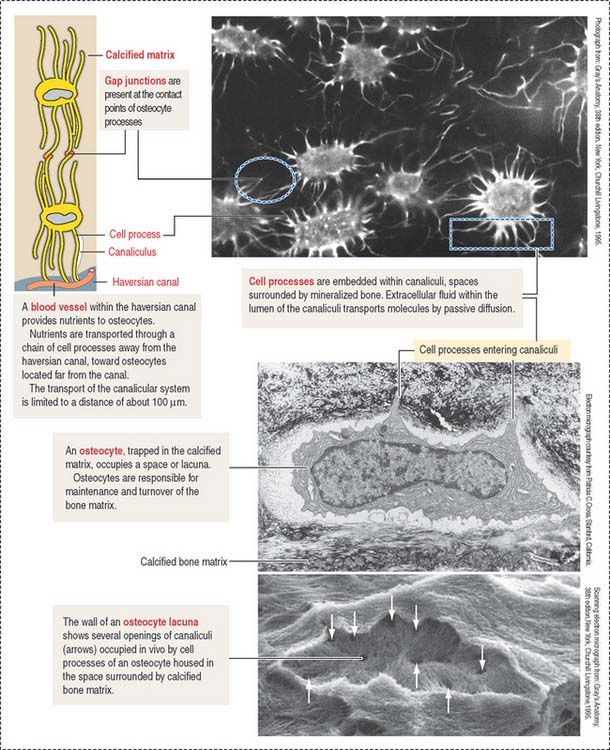
When bone formation is completed, osteoblasts flatten out and transform into osteocytes. Osteocytes are highly branched cells with their body occupying small spaces between lamellae, called lacunae. Small channels, the canaliculi, course through the lamellae and interconnect neighboring lacunae. Adjacent cell processes, found within canaliculi, are connected by gap junctions (see Figure 4-22). Nutrient materials diffuse from a neighboring blood vessel, within the haversian canal, through the canaliculi into the lacunae. As you can see, the dense network of osteocytes depends not only on intracellular communication across gap junctions but also on the mobilization of nutrients and signaling molecules along the extracellular environment facilitated by canaliculi running from lacuna to lacuna.
Clinical significance: Osteoblast to osteocyte differentiation
Osteoblast-specific genes modulate the differentiation of the osteoblast progeny (Figure 4-24): Cbfa1/Runx2 (a member of the core-binding factor family) encodes a transcription factor that induces the differentiation of osteoblasts and controls the expression of osteocalcin. Cbfa1/Runx2 is the earliest and most specific indicator of osteogenesis and its expression is induced by BMP7, followed by the expression of osteocalcin and osteopontin. Osteocalcin is a specific secretory protein expressed only in terminally differentiated osteoblasts under the control of Cbfa1/Runx2 (see Box 4-I).
Osteoclasts
Osteoclasts play an essential role in bone remodeling and renewal. This process involves removal of bone matrix at several sites, followed by its replacement with new bone by osteoblasts.
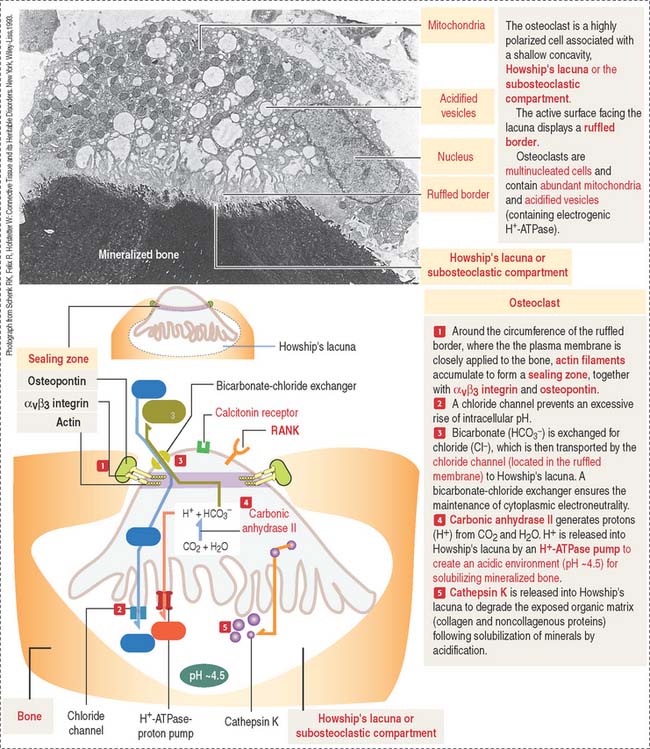
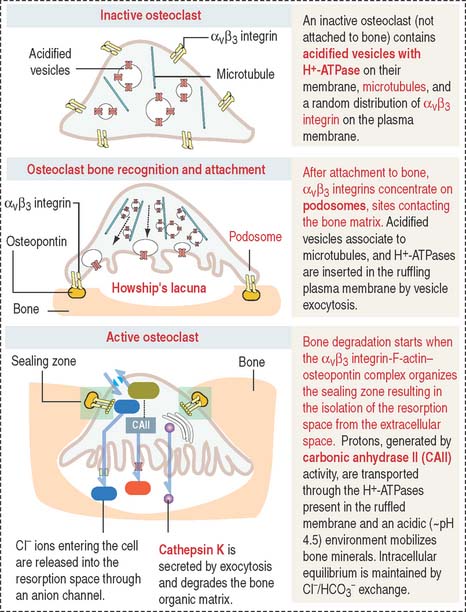
The osteoclast is a large (up to 100 μm in diameter) and highly polarized cell that occupies a shallow concavity called Howship’s lacuna or the subosteoclastic compartment (Figures 4-25 and 4-26).
The cytoplasm of the osteoclast is very rich in mitochondria and acidified vesicles. The membrane of the acidified vesicles contains H+-ATPase; mitochondria are the source of adenosine triphosphate (ATP) to drive the H+-ATPase pumps required for the acidification of the subosteoclastic compartment for the subsequent activation of the enzyme cathepsin K. Cathepsin K breaks down the bone organic matrix following removal of the mineral component of bone. Figure 4-26 provides a step-by-step sequence of the activation of an osteoclast. We discuss in Chapter 15, Upper Digestive Segment, that the mechanism of production of HCl in the stomach is very similar to the acidification of Howship’s lacuna.
Osteoclasts are transiently active in response to a metabolic demand for the mobilization of calcium from bone into blood. Osteoclast activity is directly regulated by calcitonin (synthesized by neural crest derived parafollicular or C cells of the thyroid follicle), vitamin D3, and regulatory molecules produced by osteoblasts and stromal cells of the bone marrow (see Osteoclastogenesis).
Osteoclastogenesis (osteoclast differentiation)
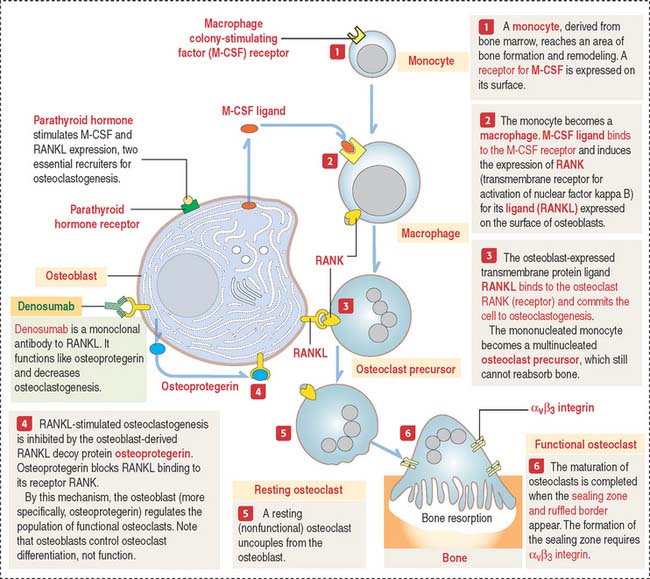
Osteoclastogenesis is triggered by two relevant molecules produced by the osteoblast: (1) macrophage colony-stimulating factor (M-CSF), and (2) nuclear factor kappa B (NF-κB) ligand (RANKL).
The osteoclast precursor, the monocyte, responds to M-CSF, a secretory product of osteoblasts. M-CSF is required for the survival and proliferation of the osteoclast precursor (Figure 4-27). Its role was established by studies of the op/op mouse, which does not express M-CSF, lacks osteoclasts, and has an increase in bone mass (osteopetrosis; Greek osteon, bone; petra, stone; osis, condition). In humans, osteopetrosis is characterized by high-density bone due to absent osteoclastic activity. In long bones, this condition leads to the occlusion of marrow spaces and to anemia.
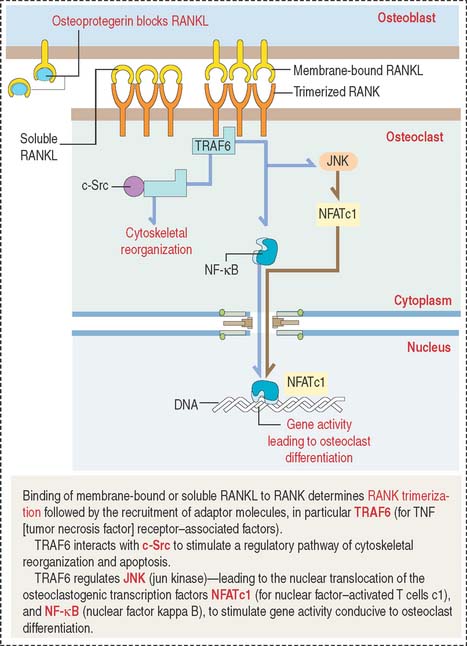
TRAF6 stimulates a downstream signaling cascade, including the nuclear relocation of two transcription factors: NF-κB and NFATc1 (for nuclear factor—activated T cells c1). In the nucleus, these two transcription factors activate genes leading to osteoclast differentiation. We discuss in Chapter 3, Cell Signaling (see Figure 3-8), that NF-κB is a critical transcription factor heterodimer activated in response to inflammatory or immunologic signaling.
TRAF6 also interacts with c-Src to stimulate a pathway leading to cytoskeletal reorganization and prevention of apoptosis. Figure 4-28 summarizes the relevant signaling steps following RANKL binding to RANK.
Parathyroid hormone stimulates the expression of osteoclastogenic RANKL. By this mechanism, the pool of RANKL increases relative to osteoprotegerin. An excess of parathyroid hormone enhances osteoclastogenesis (see Chapter 19, Endocrine System).
Clinical significance: Osteoporosis and osteomalacia
The diagnosis of osteoporosis is made radiologically or, preferentially, by measuring bone density by dual-energy x-ray absorptiometry (DEXA). DEXA measures photon absorption from an x-ray source to estimate the amount of bone mineral content.
Osteomalacia (Greek osteon, bone; malakia, softness) is a disease characterized by a progressive softening and bending of the bones. Softening occurs because of a defect in the mineralization of the osteoid due to lack of vitamin D or renal tubular dysfunction (see Chapter 14, Urinary System). In the young, a defect in mineralization of cartilage in the growth plate (see Chapter 5, Osteogenesis), causes a defect called rickets (juvenile osteomalacia). Osteomalacia can result from a deficiency of vitamin D (for example, intestinal malabsorption) or heritable disorders of vitamin D activation (for example, renal 1α-hydroxylase deficiency in which calciferol is not converted to the active form of vitamin D, calcitriol; see vitamin D in Chapter 19, Endocrine System).
Connective Tissue
Mesenchymal cells give rise to preadipocytes. Preadipocytes, under control of insulin, bound to insulin-like growth factor-1 (IGF-1) receptor, synthesize lipoprotein lipase. Lipoprotein lipase is transferred to endothelial cells in the adjacent blood vessels to enable the passage of fatty acids and triglycerides into the adipocytes.
The function of osteoclasts is regulated by calcitonin, produced by C cells located in the thyroid gland. Active osteoclasts, involved in bone resorption, are highly polarized cells. The free domain has a sealing zone, a tight belt consisting of ανβ3 integrin with its intracellular domain linked to F-actin and the extracellular domain attached to osteopontin on the bone surface. The domain associated to the subosteoclastic compartment (Howship’s lacunae) displays a ruffled plasma membrane (ruffled border). The cytoplasm contains two relevant structures: mitochondria and acidified vesicles. The osteoclast is a multinucleated cell resulting from the fusion of several monocytes during osteoclastogenesis. You should be aware that the bone marrow contains megakaryocytes that may be confused with osteoclasts. Osteoclasts are intimately associated to bone and are multinucleated; megakaryocytes are surrounded by hematopoietic cells and their nucleus is multilobulated.