Conjunctival Anatomy and Physiology
Introduction
The conjunctiva is the mucosal surface that extends from the corneoscleral limbus to the eyelid margins and caruncle.1–4 Often overlooked, conjunctival tissue’s complex functions are necessary to maintain ocular surface homeostasis.
Many important functions are performed by the conjunctiva including: (1) protection of the soft tissues of the orbit and the eyelid, (2) provision of the tear film’s aqueous and mucous layers, (3) supply of immune tissue, and (4) facilitation of independent globe movement. The conjunctiva can be divided into three distinct regions: bulbar, forniceal, and palpebral. The total surface area of the conjunctiva and cornea in an average adult measures approximately 16 cm2 per eye.2–4
Anatomy and Histology
The non-keratinized stratified secretory epithelium interfaces with a basement membrane and substantia propria below to create the blanket-like covering of the globe. Bulbar conjunctiva has a preponderance of cuboidal epithelial cells around goblet cells, Langerhans cells, melanocytes, and lymphocytes. In the normal bulbar conjunctiva, epithelial thickness can be more than six cell layers. Apical cell tight junctions, gap junctions, and desmosomes exist to create selective permeability, whereas the epithelial cell microvilli–glycocalyx complex encourages tear film adherence due to hydrophilicity.1–4
Mucous-secreting goblet cells constitute 5–10% of the conjunctival epithelial basal cells. The highest density of goblet cells occurs in the inferonasal bulbar conjunctiva and tarsal conjunctiva. Goblet cells are a likely apocrine in nature. Release of secretory granules results from parasympathetic activation.2,3
The underlying epithelial basement membrane is primarily composed of type IV collagen. The substantia propria, located beneath the epithelial basement membrane, is a highly vascularized, loose connective tissue. The substantia propria in the limbal conjunctiva is thin and compact.2–4
The bulbar conjunctiva is relatively loosely adherent to the underlying Tenon’s capsule. The conjunctiva and Tenon’s fascia are less mobile within the first few millimeters adjacent to the limbus, where the epithelium transitions to flatter epithelial cell morphology. Radiating infolds at the limbus are known as the palisades of Vogt. The stem cells of the cornea are located here.2,3
The dimensions of the bulbar conjunctiva vary with age, race, eye position, inherent redundancies of tissue and method of measurement. The adult chord length from limbus to fornix averages between approximately 13 and 16 mm superiorly. The inferior fornix is typically between 10 and 12 mm in normals and decreases with age. Cicatrizing conditions can create a foreshortened fornix, thereby decreasing the area of measurable bulbar conjunctiva.5 Temporally, the bulbar conjunctiva extends for more than 12 mm from the limbus and with a significant portion hidden by the lateral canthus. The nasal bulbar conjunctiva covers the smallest area, limited by the presence of the caruncle and the medial wall of the orbit.2
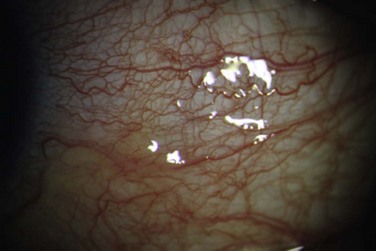
The vascular supply of the bulbar conjunctiva comes principally from anterior ciliary arteries and the peripheral tarsal arcades of the eyelid. The arteries eventually anastomose to create an arteriolar plexus near the limbus to ensure redundancy of oxygenation (Fig. 4.1). The majority of the blood supply for the bulbar conjunctiva near the limbus is derived from the anterior ciliary arteries. The venous drainage is similar: conjunctiva drains into anterior ciliary veins and into many peripheral conjunctival veins that connect to the eyelid’s venous plexus, before joining the superior and inferior ophthalmic veins. Bulbar conjunctival veins can become dilated and prominent along with those of the episclera in primary pulmonary hypertension, carotid cavernous fistulas, and other vascular malformations.2
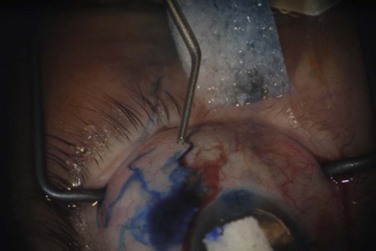
The lymphatics of the nasal bulbar conjunctiva drain to the submandibular nodes. Temporal bulbar conjunctival lymphatics drain to preauricular nodes. Bulbar conjunctival lymphatic channels can be seen with injection of dyes at or near the limbus.2 The darker dye contrasts the lymphatic channel against the white background of sclera (Fig. 4.2).
The ophthalmic branch of the trigeminal nerve contains sensory afferent fibers for the bulbar conjunctiva. Afferent nerves do not synapse until the fifth nerve nucleus. Autonomic efferent nerves supply vessels, accessory lacrimal glands, and the epithelia.2
Fornix
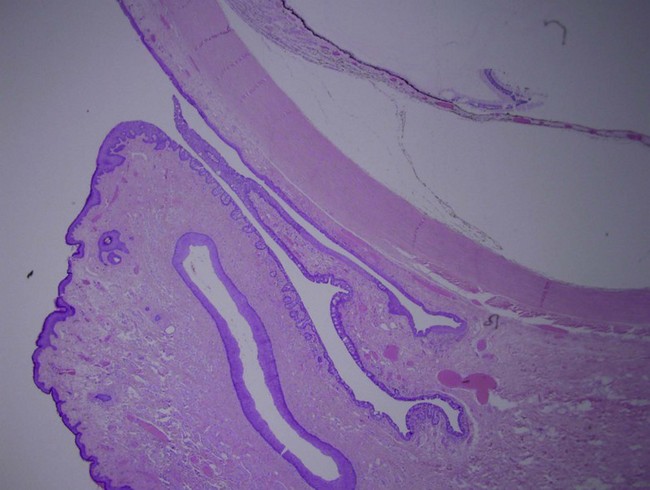
The conjunctiva of the fornix is continuous with the skin and lies between bulbar and palpebral conjunctiva (Fig. 4.3). It contains a nonkeratinized stratified squamous epithelium that is typically three layers thick.2 The superficial layer is cylindrical, the middle layer is polyhedral, and the deep layer is cuboidal. Within the epithelium, goblet cells, melanocytes and dendritic cells are often encountered.
The substantia propria is thickest in the conjunctival fornix and is anatomically split into two sections: a superficial lymphoid layer and a deeper fibrous layer. The superficial lymphoid layer is microscopically comprised of a loose connective tissue with an admixture of lymphocytes (primarily T lymphocytes), mast cells, plasma cells and neutrophils. The deeper fibrous layer contains the vessels, nerves and glands of Krause. The glands of Krause are accessory lacrimal glands deep within the superior and inferior fornix where they number approximately 42 and 6–8, respectively. These glands collectively form an intricate duct system which opens into the fornices. Like the main lacrimal gland, these glands help produce the aqueous component of the tear film.2,3
Two specialized modifications of this conjunctival tissue are present medially: the plica semilunaris and the caruncle. The plica semilunaris (or semilunar fold), a vestigial remnant of the nictitating membrane, is a crescentic fold in the medial fornix. The caruncle, which is medial to the plica semilunaris, is a modified tissue type which contains features of both the conjunctival fornix and of the adjacent cutaneous structures which includes pilosebaceous units and fibroadipose tissue.2 These structures are around 7 mm from the nasal limbus.
The superior forniceal cul-de-sac is maintained without collapse due to the presence of fine smooth muscle attachments to the levator palpebrae superioris. Unlike the superior fornix, the inferior forniceal cul-de-sac is visible with simple eversion of the lower eyelid. The lateral fornix extends between the lateral canthus and globe and is maintained by fibrous attachments to the lateral rectus tendon. Medially, the fornix is the shallowest and contains the plica semilunaris and caruncle. The medial fornix only exists during adduction due to fibrous attachments to the medial rectus tendon.2
Perfusion, innervation, and lymphatic drainage mirror that of the bulbar tissue. The medial fornix has sensory afferents from the maxillary division of the trigeminal nerve. The preponderance of lymphocytes in this region and their role are discussed below.2
Palpebral
The marginal mucocutaneous junction marks the transition from eyelid keratinized stratified squamous epithelium to nonkeratinized, stratified squamous epithelium of the palpebral conjunctiva. The palpebral conjunctiva contains cuboidal epithelial cells, similar to the bulbar conjunctiva, and columnar epithelial cells overlying the tarsus. The epithelial cells of the palpebral conjunctiva are smaller compared to the bulbar conjunctiva. The thickness of the epithelium varies from 2–3 cell layers over the upper tarsus to 4–5 over the lower tarsus. Similar to the bulbar and forniceal epithelium, Langerhans cells and goblet cells are present. The substantia propria is thin, compact, and firmly attached over the tarsus.1–4
The palpebral conjunctiva lines the inner surfaces of the eyelids. It extends from the mucocutaneous junction of the eyelid margin to the fornices.2 It is subdivided into marginal, tarsal and orbital conjunctiva.
The marginal conjunctiva measures approximately 2 mm wide. It extends from the mucocutaneous junction to the subtarsal groove, a shallow sulcus that runs parallel to the eyelid margin along the tarsal surface. The transition from nonkeratinized stratified epithelium of the eyelid margin to the cuboidal epithelium of the tarsal conjunctiva occurs at this site.2
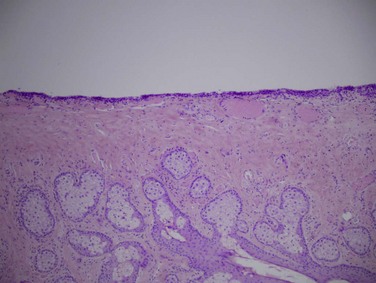
The tarsal conjunctiva is thin, vascular and firmly adherent to the underlying tarsus, particularly the upper tarsus (Fig. 4.4). This tight adherence provides a smooth tarsal surface, a critical function given its intimate relationship with the cornea. The palpebral conjunctiva contains the accessory lacrimal glands, glands of Wolfring, which are located above or within the tarsus. Epithelial infolds with abundant goblet cells, known as pseudoglands of Henle, are also located here (Fig. 4.5).2
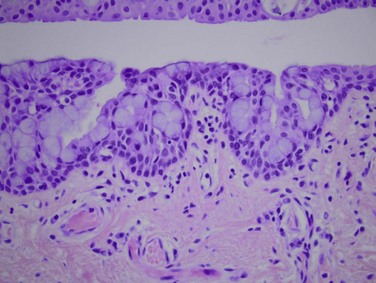
Figure 4.5 Pseudoglands of Henle – H&E, 40× magnification. (Image courtesy of Daniel M. Albert, M.D., M.S.)
There is a dual blood supply for the palpebral conjunctiva. The main vascular supply arises from the terminal branches of the ophthalmic artery: dorsal, nasal, frontal, supraorbital, and lacrimal arteries. The facial, superficial, temporal, and infraorbital branches of the facial artery provide the supplemental blood supply. Venous drainage occurs through post-tarsal veins of the eyelids, deep facial branches of the anterior facial vein, and the pterygoid plexus.2
The lymphatics of the palpebral conjunctiva join the eyelid lymphatics, draining medially to the submandibular lymph nodes and laterally to the preauricular lymph nodes.2
Similar to the bulbar and forniceal conjunctiva, the palpebral conjunctiva is mainly innervated by branches of the ophthalmic division of the trigeminal nerve, i.e. the lacrimal, supraorbital, supratrochlear and infraorbital. Additionally, VIP-containing nerve fibers have been shown to innervate accessory lacrimal glands and goblet cells, as well as glands of Moll at the eyelid margin.2
Conjunctival Function
In addition to the supportive role of accessory lacrimal glands (Krause and Wolfring), arguably the conjunctiva’s greatest contribution to the tear film is the production of hydrophilic mucins. Mucins are well-studied products of mucus membranes that are critical for conjunctival health. Mucins are large heavily glycosylated proteins, exhibiting extensive tandem amino acid repeats, and multifunctional utility. Recent assays have helped clarify the mucins’ role in: (1) clearance of allergens, pathogens, and debris, (2) lubrication, (3) antimicrobial activity. Their O-glycans have hydrophilic properties to help keep the tear film in contact with the epithelia.6
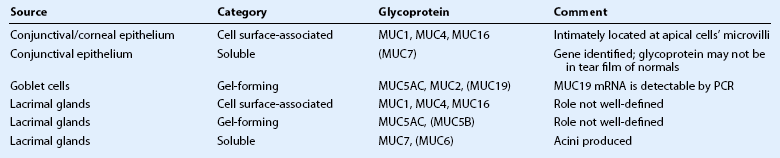
Mucins can be categorized as secreted or cell surface-associated. The secreted mucins are either soluble (located closer to the tear film lipid layer) or gel-forming (located closer to the conjunctival apical cells). Cell surface-associated mucins (also called ‘membrane-associated’) form the glycocalyx. The gel-forming mucins appear to work together with cell surface-associated mucins to maximally protect the epithelium and limit desiccation. Additionally, shed cell surface-associated mucins contribute to tear fluid.3 The various ocular surface mucins are described in Table 4.1.
Secreted mucins have been described as having critical ‘cleaning’ capabilities, addressing unwanted debris, allergens, and microbes. Combined with efficient tear clearance, lymphatics, inherent immunologic proteins, and secondary immune responses, mucins help the ocular surface to maintain optimal health.6
Decreased gel-forming mucin gene expression (e.g. Sjögren’s syndrome – MUC5A) and decreased glycosylation of cell surface-associated mucin (e.g. non-Sjögren sicca – MUC16) are two known examples of mucin abnormalities that negatively affect tear film.4,6
Conjunctival apical epithelial cell microvilli are integral for proper cell membrane-associated mucin presence.4 Recent work has shown that conjunctival epithelial microvilli are fewer and smaller (in size) in graft-versus-host disease sicca versus normals and Sjögren’s syndrome sicca. Other findings of interest in graft-versus-host disease were abundant CD8+ T cells in the basal epithelium with decreased goblet cell secretory vesicles.
Immunology
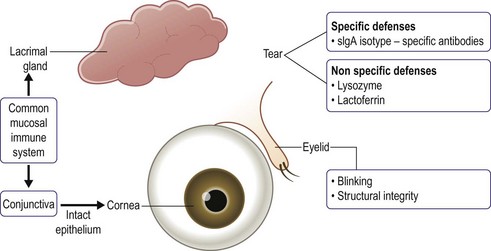
The conjunctiva is equipped with several distinct defense mechanisms: anatomical, mechanical, antimicrobial and immunologic. An intact epithelium provides an anatomic defense against pathogen invasion. Eyelid blinking mechanically removes pathogens and foreign substances.7 Tears contain a variety of antimicrobial proteins, including: lysozyme, immunoglobulins, and lactoferrin. Lysozyme provides protection against Gram-positive organisms through lysis of bacterial cell walls. Immunoglobulins, particularly IgG, neutralize viruses and lyse bacteria.8 Lactoferrin has bacteriostatic and bactericidal properties (Fig. 4.6).
The conjunctiva’s immunologic defense is complex and consists of an innate, adaptive and mucosal component. The innate immune system is a non-specific early host response against pathogens. Pathogens, through pathogen-associated molecular patterns (PAMPs), are recognized by toll-like receptors (TLRs), specific innate immune-recognition receptors. After pathogen recognition by TLRs, an immune response is triggered, leading to inflammation and induction of the adaptive immune system. Recent studies have shown that the conjunctiva expresses β-defensins, important components of the innate immune system, TLR mRNA and proteins.9
The adaptive immune system is a delayed host response containing humoral and cellular arms. Immunoglobulins are the main component of the humoral arm whereas T lymphocytes form the cellular arm. T lymphocytes, cytotoxic and helper T cells, are found in both the conjunctival epithelium and substantia propria; B cells are found rarely in the substantia propria. The adaptive and innate immune systems work together to provide an integrated conjunctival immune system.8
There is increasing evidence that the conjunctiva has a specific mucosal immune system, termed conjunctival associated lymphoid tissue (CALT). CALT has previously been described in several different animals; recent studies have shown its existence in humans. CALT is thought to be part of the larger common secretory immune system comprising mucosa-associated lymphoid tissue (MALT) from the gastrointestinal, respiratory and genitourinary tracts.8,10
The secretory immune system’s main humoral mediator is IgA.8,10 IgA can provide a protective layer to the mucosa by preventing bacterial binding to mucosal epithelial, binding to antigen to prevent absorption, and neutralizing viruses.7,8 High endothelial venules, specialized vessels for migration of lymphoid cells between integrated mucosal systems, along with lymphocytes, lymphoid follicles, IgA positive plasma cells and their associated transporter molecule, secretory component (SC), have all been found in the human conjunctiva, further supporting the presence of CALT.7,9,10
References
1. Calonge M, Stern ME, Pflugfelder SC, Beuerman RW, Stern ME, eds., eds. Dry eye and ocular surface disorders. 1st ed. Marcel Dekker: New York, 2004:89–109.
2. Nelson, J, Cameron, J. The conjunctiva: anatomy and physiology. In: Krachmer JH, Mannis MJ, Holland EJ, eds. Cornea: fundamentals, diagnosis and management. 3rd ed. Philadelphia: Elsevier-Mosby; 2011:25–31.
3. Tsubota, K, Tseng, SCG, Nordlund, ML. Anatomy and physiology of the ocular surface. In: Holland EJ, Mannis MJ, eds. Ocular surface disease: medical and surgical management. 1st ed. New York: Springer-Verlag; 2002:3–15.
4. Gipson, IK, Joyce, N, Zieske, J. The anatomy and cell biology of the human cornea, limbus, conjunctiva, and adnexa. In: Foster CS, Azar D, Dohlman C, eds. Smolin and Thoft’s: The cornea. 4th ed. Philadelphia: Lippincott WIlliams & Wilkins; 2005:3–37.
5. Williams, GP, Saw, VPJ, Saeed, T, et al. Validation of a fornix depth measurer: a putative tool for the assessment of progressive cicatrising conjunctivitis. Br J Ophthalmol. 2011;95:842–847.
6. Mantelli, F, Argüeso, P. Functions of ocular surface mucins in health and disease. Curr Opin Allergy Clin Immunol. 2008;8:477–483.
7. McClellan, KA. Mucosal defense of the outer eye. Surv Ophthalmol. 1997;42:233–246.
8. Foster, CS, Streilein, J. Basic immunology. In: Foster CS, Azar D, Dohlman C, eds. Smolin and Thoft’s: The cornea. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2005:91–93.
9. Lambiase, A, Micera, A, Sacchetti, M, et al. Toll-like receptors in ocular surface diseases: overview and new findings. Clin Sci. 2011;120:441–450.
10. Knop, E, Knop, N. The role of eye-associated lymphoid tissue in corneal immune protection. J Anat. 2005;206:271–285.