Conization of the Cervix
The term cone biopsy has come to refer not only to biopsy of the geometric cone but also to cylinder and disc biopsies (loop excision of the T-zone). Over the past two decades, a great deal of research as well as discussion has focused on the specifications for conization of the cervix. Principally, the goal of the gynecologic surgeon is to obtain a clear (i.e., non-neoplastic) cell margin at the ectocervical, endocervical, lateral, and depth perimeters of the specimen. The strategy is to couple a diagnostic procedure to a therapeutic one. An additional goal of the operation should be maintenance of fertility because most patients who undergo this operation are within the reproductive age group. When performed in the pregnant patient, the procedure should not lead to pregnancy loss.
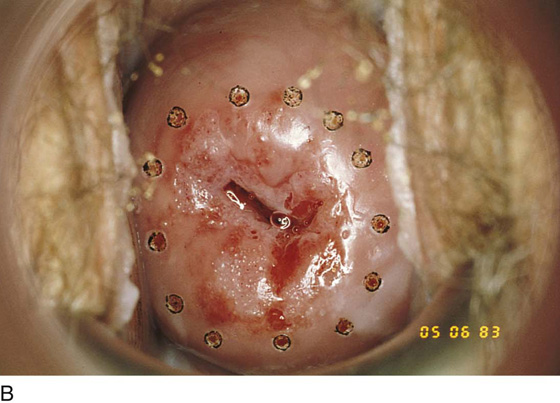
With the exception of adenocarcinoma in situ, which makes up a minority of premalignant neoplastic disorders of the cervix, neoplastic cells spread by direct continuity from the squamocolumnar junction by tracking a course into the endocervical canal or outward onto the portio. The former course is by far more common. Additionally, spread onto the ectocervix is visible by colposcopy, whereas movement into the canal is not. Squamous intraepithelial neoplasia (dysplasia, cervical intraepithelial neoplasia) rarely progresses more than 1 to 1.5 cm up into the endocervical canal. Similarly, when these lesions involve plunging into the endocervical clefts (glands), they penetrate the stroma to a depth of 3 to 3.5 mm and rarely to a depth of up to 6 mm. Thus, the height of the cone should be specified at no more than 15 mm and the peripheral margin around the canal 3 to 3.5 mm. This will encompass and cure 95% of high-grade lesions, including squamous intraepithelial neoplasia stages I (moderate dysplasia) and III (severe dysplasia, carcinoma in situ). An even more conservative approach should be adopted for low-grade squamous neoplasia (mild dysplasia, condylomatous atypia, cervical intraepithelial neoplasia stage I) because its propensity to spread into the canal is less than that of high-grade disorders. Low-grade disease should be excised to a maximal height of 8 to 10 mm with a 3-mm peripheral margin at the transformation zone.
On the basis of these facts, several methods can be used to perform a cone biopsy. This chapter does not describe ablative techniques because they do not provide a specimen for the pathologist (the only exception is a description of the unique combination cone).
Cold-Knife Conization
As with other biopsy and therapeutic techniques, use of the colposcope throughout cold-knife conization provides great advantage because the surgeon’s view of the field is magnified, permitting greater precision, the light is excellent and focused onto the field, and the instrument does not take up any space in the operative field.
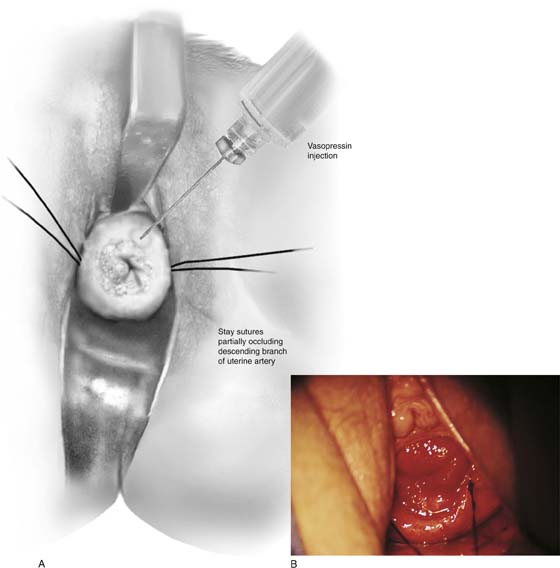
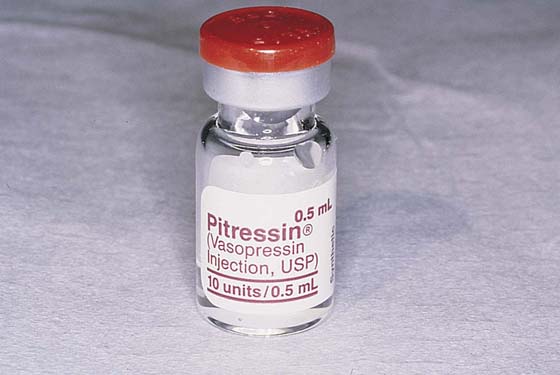
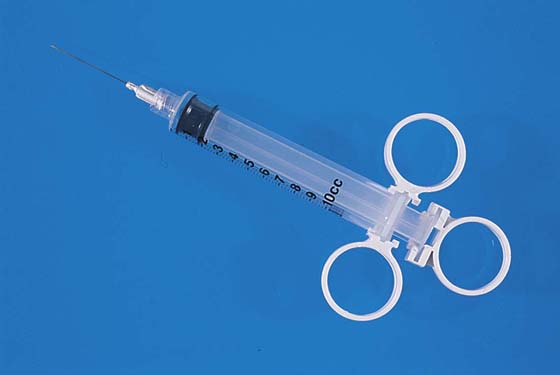
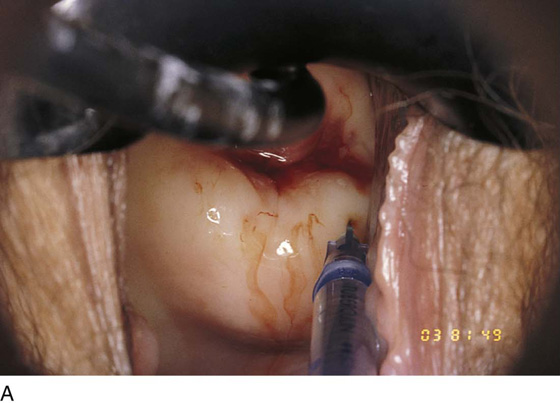
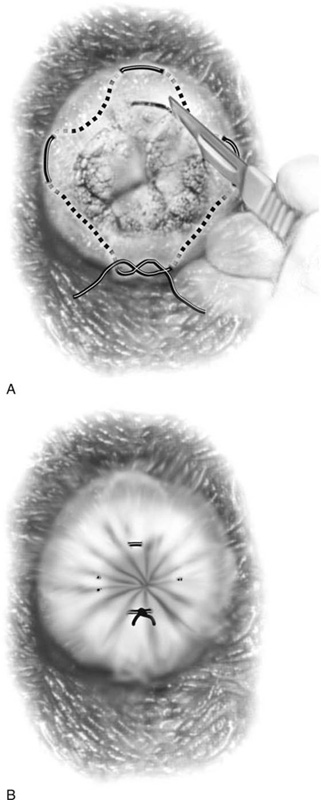
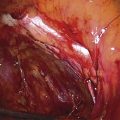
Hemostasis is a key element in the performance of a knife conization. Stay sutures of 0 Vicryl are placed into the cervix at 9 o’clock or 3 o’clock to partially occlude the descending branch of the uterine artery and to provide stabilization of the cervix (Fig. 46–1A, B). The goal is better exposure of the operative field. Injection of vasoconstrictors into the cervical substance provides additional hemostasis (see Fig. 46–1A). The most potent vasoconstrictor is vasopressin, which must be diluted. Vasopressin is supplied as a powder; when mixed with sterile water, this agent contains 20 units per milliliter. An alternative preparation when mixed with sterile water contains 10 units of vasopressin per 0.5 mL (Fig. 46–2). For injection into the cervix, vasopressin should be diluted 1 : 100 (i.e., add 99 mL of diluent to 1 mL of reconstituted vasopressin solution such that each milliliter of the diluted solution will contain 0.2 unit). Typically, 10 mL of the solution is injected into the cervix. If 1% lidocaine without epinephrine is used to dilute the vasopressin, the resultant solution provides vasoconstriction and local anesthesia simultaneously when injected into the cervix (Fig. 46–3).
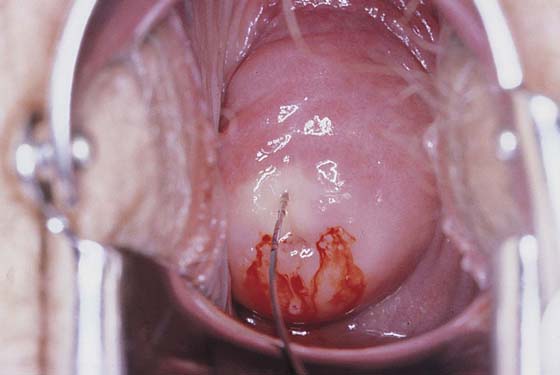
Before injection, however, a colposcopy should be performed, and the peripheral margins of the cone should be marked. Once the vasopressin is injected, the abnormal transformation zone (ATZ) will be difficult to see (Fig. 46–4).
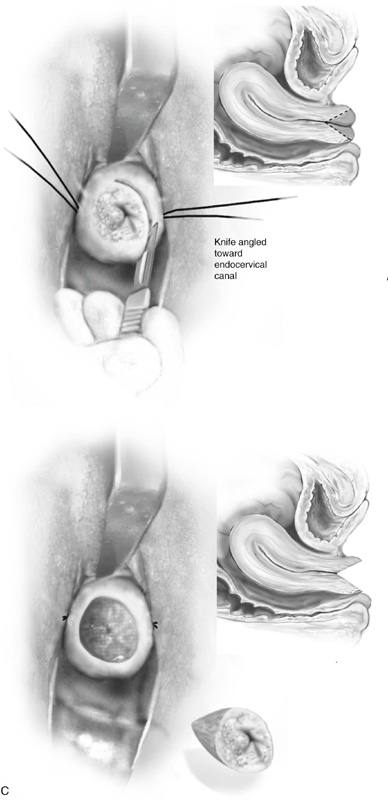
The colposcope is set on scanning power. A circular knife cut is made 3 mm peripheral to the AZT. The knife is angled toward the endocervical canal and cuts deeper into the stroma to a height of 1.5 cm. The endocervical margin is cut (Fig. 46–5A through E). Hemostasis is carried out with a ball electrode utilizing spray or forced coagulation at a setting of 50 W. The stay sutures are cut close to the knot (intact), and the field is inspected for hemostasis. No sponges or packs are placed in the vagina or the crater. Performance of endocervical curettage is optional. If the surgeon wishes to curette the remaining endocervical canal, this should be done at the conclusion of the conization but before hemostatic coagulation occurs.
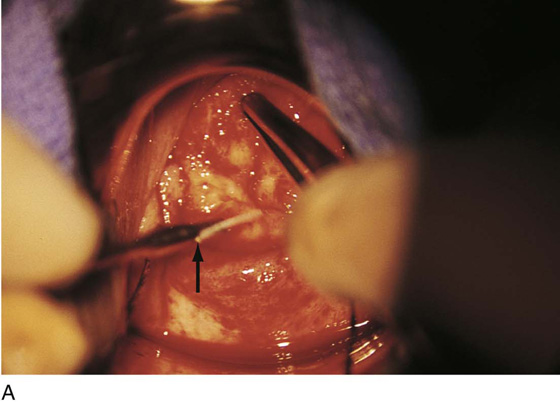
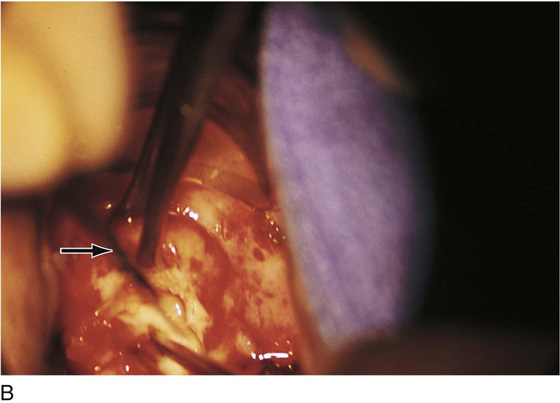
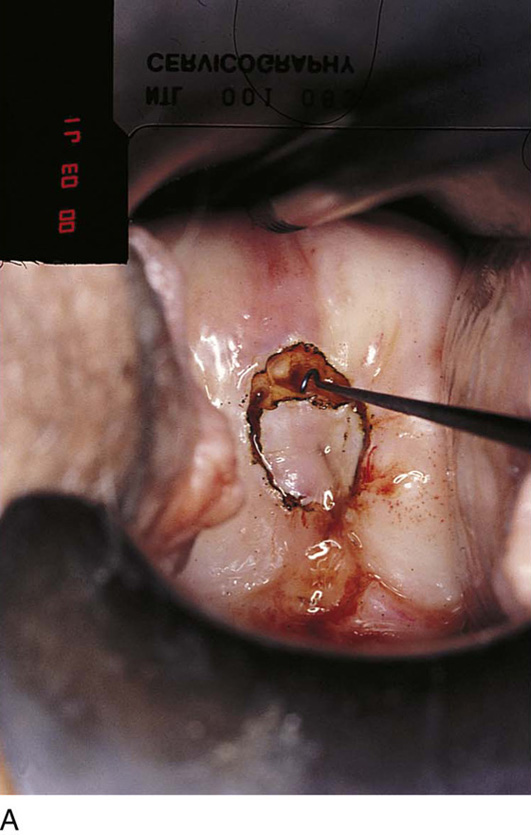
FIGURE 46–1 A. Two sutures of 0 Vicryl have been placed into the lateral aspect of the cervix at the 9 o’clock and 3 o’clock locations. These are placed to diminish bleeding and to stabilize the cervix during surgery. B. The stay sutures are pulled downward to better expose the cervix. Even with a deep retractor placed posteriorly, the vagina bulges beneath the posterior cervix.
FIGURE 46–2 Vasopressin is diluted such that 1 mL (20 units) is diluted 100-fold. In the case illustrated, each 0.5 mL contains 10 units. Therefore, if 0.5 mL of this solution were mixed with 50 mL of sterile water, the resultant solution would be equivalent.
FIGURE 46–3 The vasopressin mixture is injected by using a 10-mL syringe with a 1½-inch, 25-gauge needle attached.
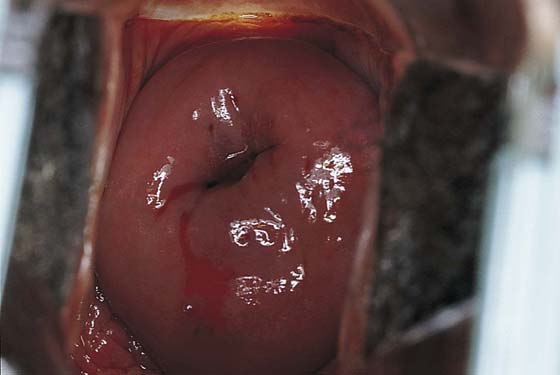
FIGURE 46–4 A very superficial needle stick is made into the cervix, and the vasopressin mixture is injected under pressure. As the vasopressin infiltrates, the tissue blanches.
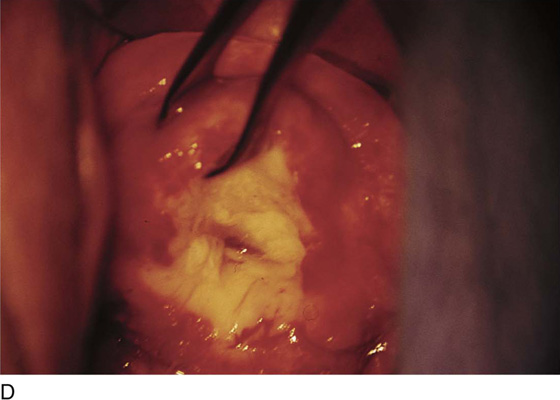
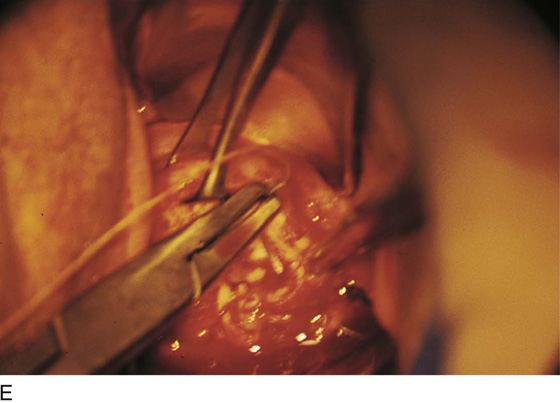
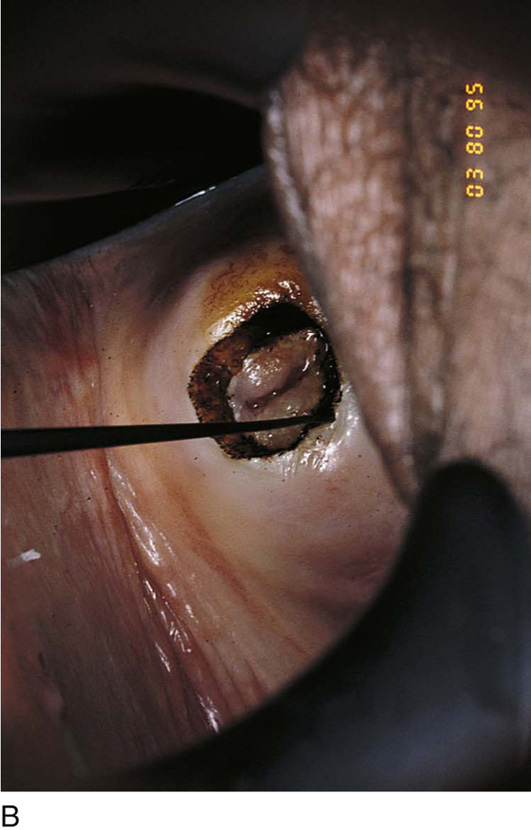
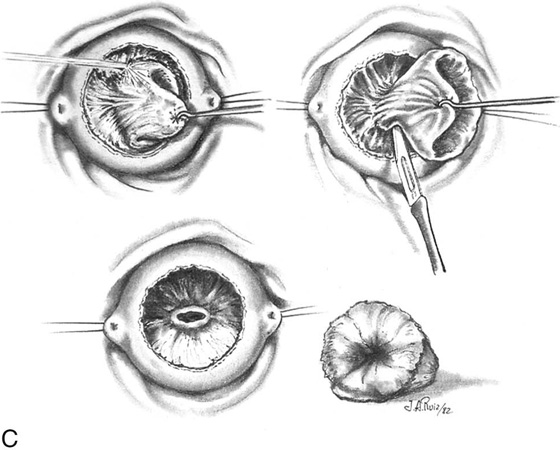
FIGURE 46–5 A. The knife cuts into the cervix at 6 o’clock with a margin 3 mm peripheral to the abnormal transformation zone (ATZ) (arrow at knife). B. The knife cut extends deep as traction is placed on the edge of the cut with an Allis clamp (arrow at knife). C. The knife is angled inward toward an imaging line 1.5 cm superior to the external os. The coned-out specimen is sent to pathology in a saline-soaked sponge. Hemostasis may be obtained with a ball electrode or a simple suture. D. The specimen has been excised, leaving a defect where the tissue once was located. Note the relatively dry field. E. The edge of the cone margin is sutured with a 0 Vicryl continuous suture.
Laser Conization
This technique is similar to a knife conization with the exception that a superpulsed carbon dioxide (CO2) laser beam is substituted for the scalpel (Fig. 46–6A through C). The advantage of the laser is that it is coupled to the microscope, thereby permitting a more precise cone (Fig. 46–7A through C). Additionally, the thermal action of the laser promotes better hemostasis. The disadvantages of the laser are that the procedure requires more time to finish and the laser may cause thermal injury (artifact) to the specimen (Fig. 46–8).
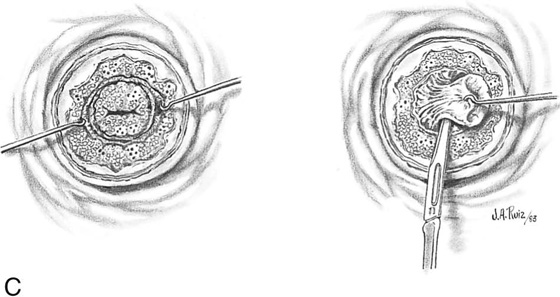
FIGURE 46–6 A. In the case of carbon dioxide (CO2) laser conization, vasopressin is again injected into the cervix to create hemostasis. B. The laser beam traces a series of marking spots around the abnormal transformation zone (ATZ) to identify the outer margin(s) for excision. C. The laser beam diameter is reduced to a 1- to 1.5-mm spot. Power is set at 40 to 60 W. The dots are connected, and a peripheral crater is created.
FIGURE 46–7 A. A laser titanium manipulating hook creates traction on the edge of the cervical incision, and the beam continues to cut deeper. B. The incision is focused inward to create the cone-shaped specimen. C. When the cervix has been cut to a sufficient height, the endocervical margin is cut and the specimen removed.
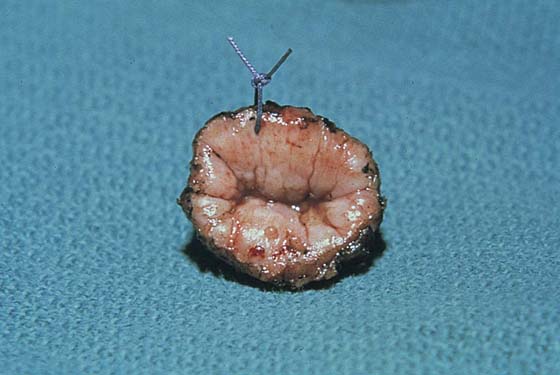
FIGURE 46–8 The specimen is marked at 12 o’clock with a stitch and is sent to pathology for section.
Conization During Pregnancy
As with punch biopsy, conization during pregnancy is associated with greater risk of bleeding. Therefore, the conization must be restricted to the lowest height possible while necessary information is secured to exclude or include the diagnosis of invasive cancer. A purse string or 0 Vicryl suture identical to that placed for the treatment of an incompetent cervix is placed (Fig. 46–9A). Next, with the use of a knife or energy device, the cone is taken. The purse string is tightened and tied (Fig. 46–9B).
Loop Electrical Excision Conization
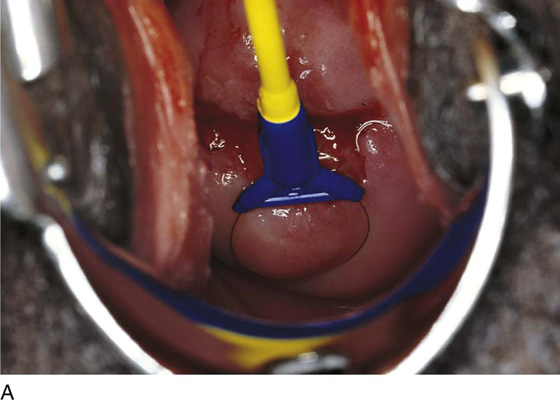
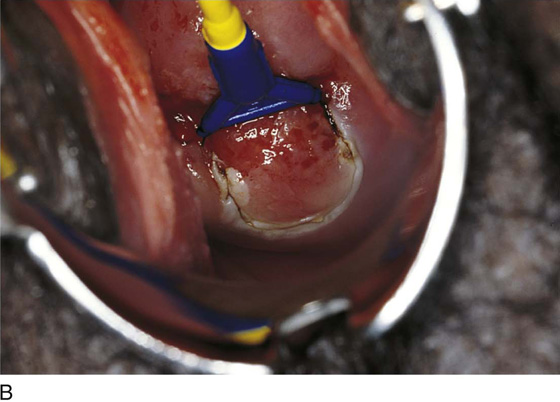
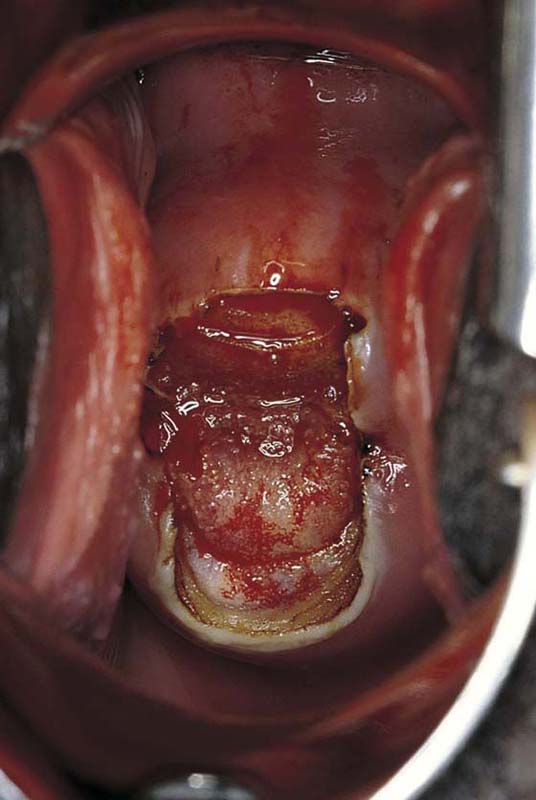
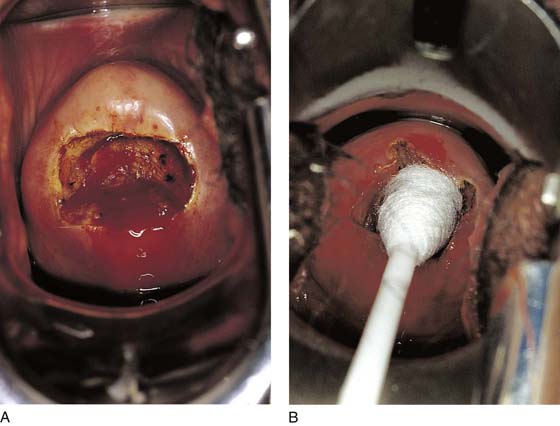
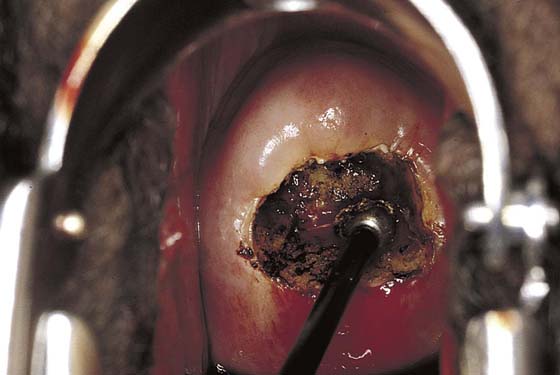
This technique is an office-based procedure. After the ATZ is marked, a 1 : 100 diluted vasopressin/lidocaine solution is injected into the cervix circumferentially (Fig. 46–10). Next, the loop electrode of proper size is selected. The electrosurgical unit is set to 50 to 60 W of cutting power. The electrode makes light contact with the cervix as power is applied (Fig. 46–11A, B). It sinks deep into the cervical matrix to a depth of 10 mm. The loop is swept across the entire T-zone by following a horizontal or a vertical pathway (Figs. 46–11C and 46–12). The loop is removed, and a large cotton swab is placed in the crater to absorb blood (Fig. 46–13A, B). The specimen is sent to pathology. The cutting loop electrode is removed from the handpiece, and a ball electrode is substituted for it. As the large swab is removed, the electrode is placed in the crater and is electrically activated to coagulate bleeding vessels and sinuses (Fig. 46–14). When hemostasis is complete, a small cotton-tipped applicator soaked with Monsel’s solution may be utilized to stanch any persistent small vessel bleeding (Fig. 46–15).
Loop Electrical Excision by Selective Double-Excision (“Top Hat”) Technique
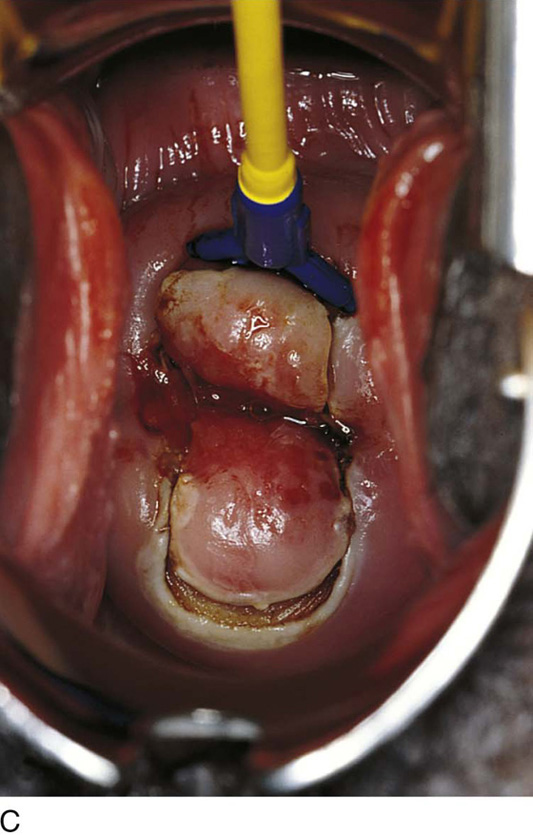
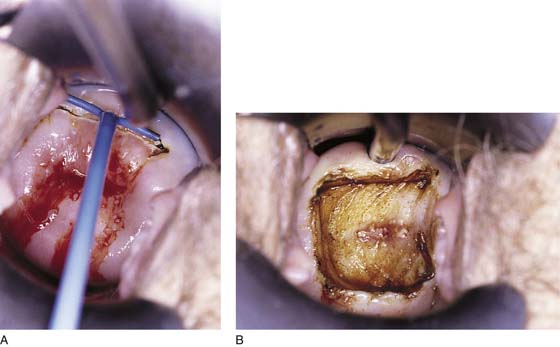
The selective double-excision technique is used for the treatment of high-grade lesions. Its goal is to conserve cervical stroma while removing an extra margin of cervical canal to provide clear margins and thus a high cure rate (Fig. 46–16A, B). Essentially, the first part of this operation is identical to the loop electrical excision conization described earlier (Fig. 46–17A, B). However, following specimen removal and the attainment of hemostasis, a small (4–5 mm) loop is placed into the handpiece and (by using 30–40 W of cutting current) a 5-mm endocervical sample is obtained. The sample then is marked and sent with the first specimen to pathology (Fig. 46–18A, B).
Combination Conization
A young patient with extensive ectocervical intraepithelial neoplasia that additionally extends into the cervical canal beyond the view of the colposcope presents a dilemma for the gynecologist (Fig. 46–19). If adequate margins and depth were maintained, then the cervix would be more or less amputated by conventional cone techniques (Fig. 46–20A, B). The combination conization eliminates disease but preserves the stroma and volume of cervical tissue. This technique must be done with a superpulsed CO2 laser for optimal results.
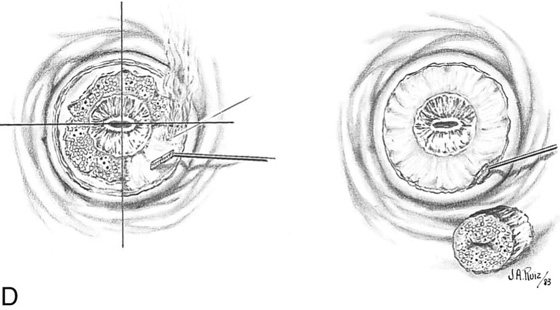
Two sets of trace spots are fired into the cervix: one set 3 mm beyond the ectocervical margin and a second set at the squamocolumnar junction. A narrow cylindrical excisional conization is performed to a height of 1.0 to 1.5 cm (Fig. 46–21A through C).
Next, a shallow 4- to 5-mm vaporization of the ectocervical disease is performed. (The lesion has been previously sampled, and its intraepithelial nature has been established via histopathologic diagnosis) (see Fig. 46–21D). The wound is copiously irrigated with saline.
The patient is seen at biweekly intervals for 4 to 6 weeks and returns 6 weeks later for a final check (Fig. 46–22).
FIGURE 46–9 A. During pregnancy, a conization can be a very bloody procedure. To better control the bleeding, a purse string suture is placed high up on the cervix and is held with mosquito clamps. B. Immediately after completion of the operation, the stitch is snugged down and then tied. The constriction of the cervix will stop or significantly diminish bleeding secondary to conization.
FIGURE 46–10 The cervix has been injected with 1 : 100 vasopressin in preparation for loop excision. The injection was made deeper into the tissue, which accounts for the absence of blanching.
FIGURE 46–11 A. The loop electrode is placed at the 6 o’clock position (just before electrical activation). B. The electric current is activated, and the excision is initiated by moving the electrode vertically from 6 to 12 o’clock. C. In a single sweeping motion, the electrode has finished its excursion.
FIGURE 46–12 The excision of the abnormal transformation zone (ATZ) is complete.
FIGURE 46–13 A. In this loop excision patient, bleeding was sufficient to warrant coagulation. B. A large cotton-tipped swab (proctoswab) tamponades the bleeding site while the loop electrode is changed to a ball electrode. The generator has already been set for coagulation mode.
FIGURE 46–14 The ball electrode coagulates the bleeding vessels by using forced or spray coagulation at 40 to 50 W of power.
FIGURE 46–15 The field is dry and the procedure is terminated. No packs are placed in the cervix. Any small additional bleeding may be staunched with a small cotton swab saturated with ferric subsulfate (Monsel’s solution).
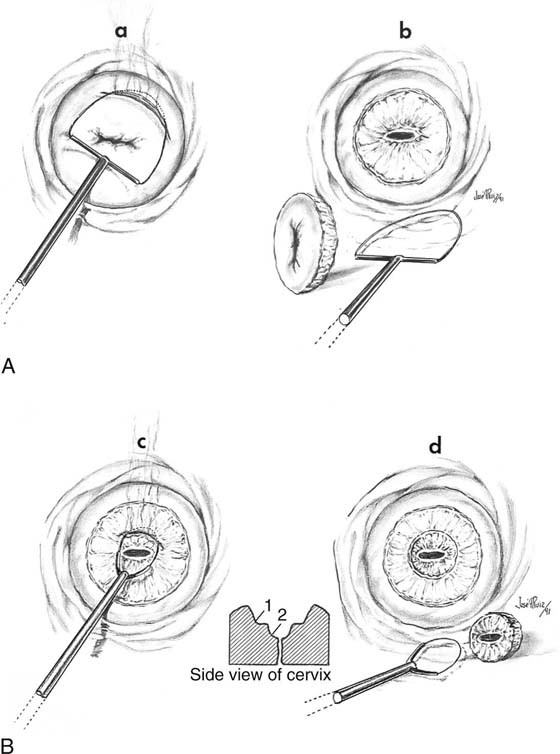
FIGURE 46–16 A. The technique of selective double excision is shown schematically. A loop excision of the T-zone is carried out to a depth not to exceed 10 mm (a, b). B. Next, a second smaller electrode is attached to the handpiece (control unit). This electrode measures 5 × 5 mm. A 5-mm excision of the endocervical canal is performed, and the specimen is submitted in a separate bottle to pathology. The defect produced resembles a cone.
FIGURE 46–17 A. The loop electrode has been activated and cuts into the cervix at 12 o’clock. B. The T-zone has been excised to a 10-mm depth. Note the excellent hemostasis produced by injecting 12 to 15 mL of 1 : 100 vasopressin very superficially into the cervix before loop excision is performed.
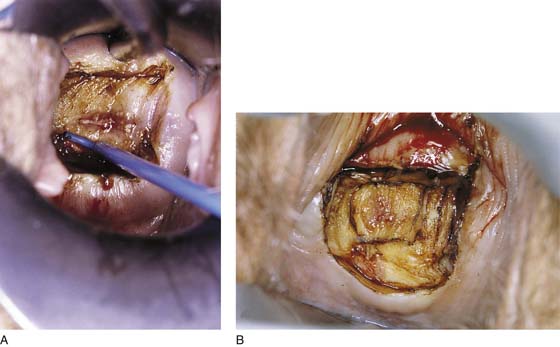
FIGURE 46–18 A. A 5-mm loop electrode is placed into the stroma just beneath the endocervical mucosa (6 o’clock). B. The second excision (5 mm) has been performed. The specimen is placed in a separate container of fixative. The defect has a top hat or a roughly conical shape and measures 15 mm in height.
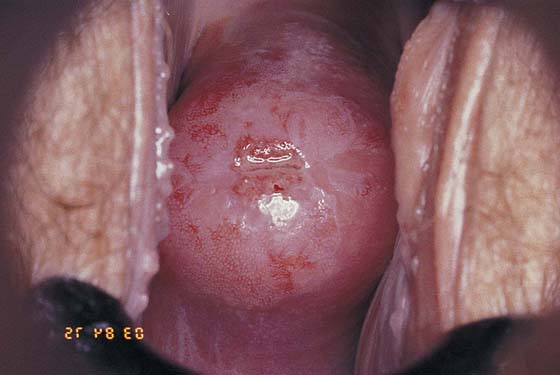
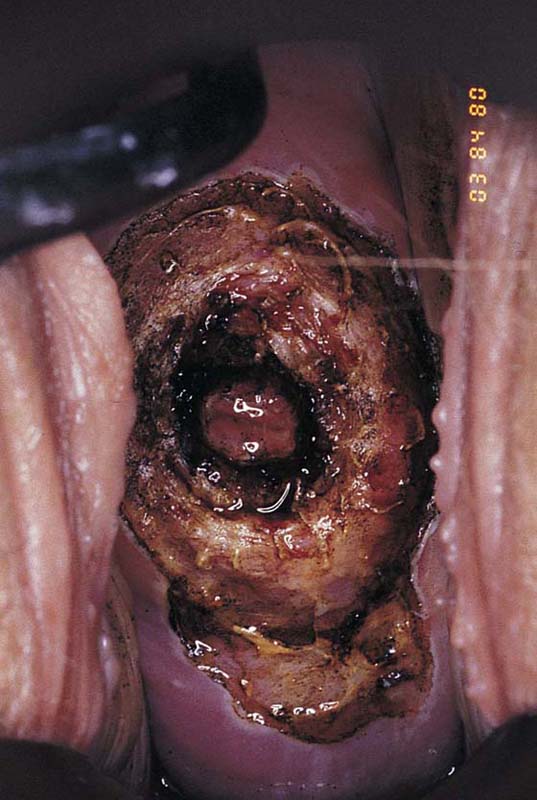
FIGURE 46–19 This cervix shows a very extensive abnormal transformation zone (ATZ). The abnormal vessels are set in a white epithelial background and extend into the canal and out onto the portio, even to the vaginal fornices. Conventional conization performed by any means would eventuate in virtual amputation of the cervix.
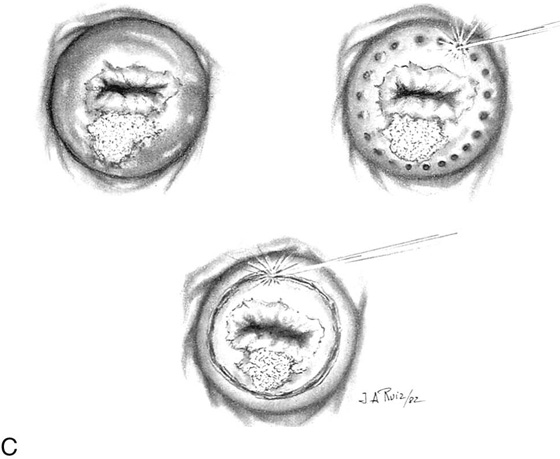
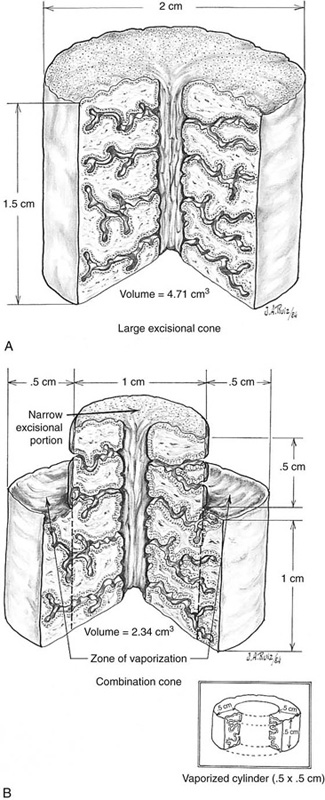
FIGURE 46–20 A. The situation described and shown in Figure 46–19 is quantified. A 1.5- × 2.0-cm cylinder conization results in tissue loss of 4.73 cm3. B. In contrast, a laser combination conization, which combines a narrow cylinder conization with superficial peripheral vaporization, calculates to a volume loss of cervix of 2.43 cm3. The combination of excision and vaporization thus preserves cervical integrity.
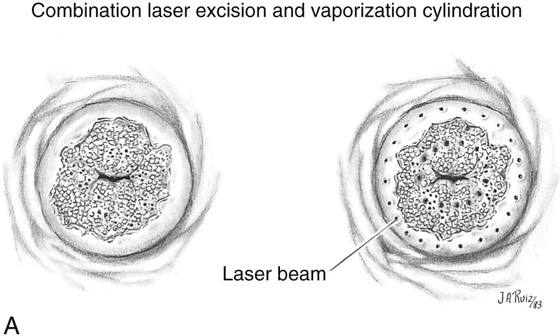
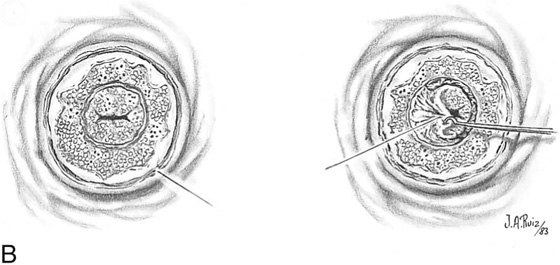
FIGURE 46–21 A. To perform a combination conization, a carbon dioxide (CO2) laser is required. Two sets of laser trace spots are placed. The inner ring outlines the narrow excisional cone. The outer row is made peripheral to the ectocervical extension of the abnormal transformation zone (ATZ). B. The dots are connected by continuous firing of the laser beam, producing the inner and outer circular outlines. Excisional conization is performed by using the laser to cut the tissue (superpulsed and tightly focused beam), as described in Figures 46–4 through 46–8. C. The coned-out tissue (narrow cylinder) measures 1.5 cm in height. Its endocervical margin is cut with a scalpel, and the specimen is placed into fixative solution. D. The ectocervix is vaporized to a depth of 5 mm centrally, tapering to 2 mm peripherally. Vaporization eliminates the ectocervical disease. Note that the excised cylinder is pictured underlying the vaporized exocervix.
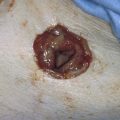
FIGURE 46–22 A completed combination conization. Note the shallow but total ectocervical vaporization area and the deeper excised central cone (cylinder) cavity. Note also that peripheral vaporization of the abnormal transformation zone (ATZ) extends into the posterior fornix of the vagina.