CHAPTER 22 Congenital Scoliosis
Embryology
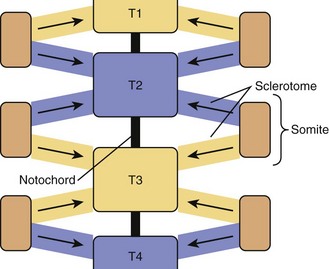
Paraxial mesoderm on either side of the notochord condenses to form somites, in a process known as somitogenesis. Each somite subdivides further into ventral sclerotome and dorsolateral dermomyotome. During the 4th week of development, cells from the sclerotome region of the somite on each side of the body migrate ventrally and surround the notochord and the neural tube. Each vertebra is formed by sclerotome cells from two somite levels (Fig. 22–1). The cranial and caudal parts of adjacent sclerotomes, which are not ossified yet, fuse with each other.1 The ventral part of each vertebra forms the body around the notochord, and the dorsal part forms costal processes laterally and the vertebra arch dorsally.
Ossification begins during the 6th week from three primary ossification centers: one in the body (or centrum, formed by early fusion of two centers) and one in each half of the vertebral arch. During the 6th week of development, mesenchymal cells between cranial and caudal parts of the original sclerotome fill the space between two vertebral bodies to contribute to formation of the intervertebral structures.2 This stage is called the segmentation stage.
Somitogenesis relies on the Notch signaling pathway and its interactions with FGF and Wnt signaling; however the precise mechanisms remain unclear.3–5 Mutations in downstream components and targets of the Notch signaling pathway, such as Dll3, Mesp2, and Lfng, result in abnormal vertebral development in mouse models and are associated with characteristic vertebral defects seen in patients with spondylocostal dysostosis.3–7 More recent experimental evidence in vertebrate animal models and human stem cells has revealed the oscillatory nature of gene expression in paraxial mesoderm during somitogenesis.3,8,9 These findings support the concept that a putative segmentation clock triggers the cyclic expression of genes in the Notch, FGF, and Wnt signaling pathways and is essential for normal vertebral development.
Classification
Two types of basic vertebral anomalies can occur: failures of formation and failures of segmentation.10
Failures of Formation
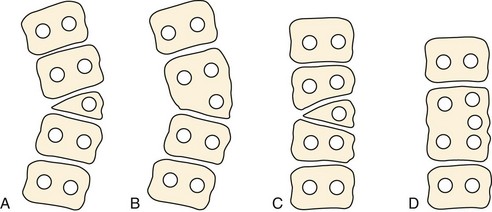
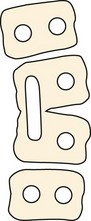
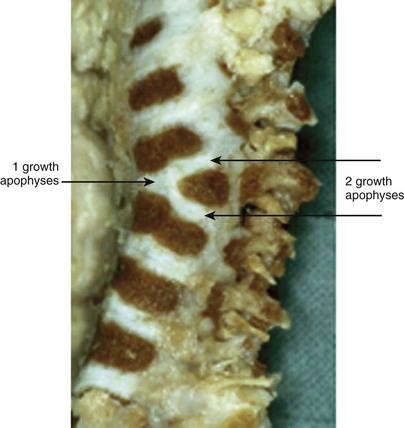
Failure of vertebral formation (type I deformity) can be partial, causing a wedged vertebra with intact pedicles (Fig. 22–2), or complete, causing a hemivertebra with a unilateral pedicle (Fig. 22–3). Hemivertebrae are classified according to their longitudinal growth potential, which in normal vertebrae is provided by growth apophyses on both vertebral ends.
Failures of Segmentation
Failure of segmentation (type II deformity) can be partial, causing a bar (Fig. 22–4), or complete, causing a block vertebra. A congenital bar can be anterior, posterior, lateral, or mixed. In many cases, vertebral anomalies owing to failures of formation and failures of segmentation coexist,11 occasionally on several levels, and form a mixed deformity (type III deformity).
Associated Anomalies
Embryologic development of the spine coincides with the development of many other organ systems. It is not rare to have associated anomalies with vertebral deficiencies. These anomalies occur in 30% to 60% of children with congenital spinal anomalies.11–13 The most common coexistent anomalies involve the spinal cord and the genitourinary tract. Intraspinal anomalies include problems such as tethered cord, diastematomyelia, and syringomyelia. The most common genitourinary defects are renal agenesis, ectopic kidney, duplication, and reflux.
Many of these anomalies are part of the VATER association. The acronym VATER14 includes the following deficiencies: vertebral defects (V), anal atresia (A), tracheoesophageal fistula (TE), radial limb reduction, and renal defects (R). The acronym VATER was modified in 197515 to VACTERL by adding cardiac defect (C) and limb defect (L) (Fig. 22–5).
Congenital vertebral anomalies are also found with a high incidence in Klippel-Feil syndrome,16,17 which is characterized by the combination of cervical fusion, limited neck range of motion, short neck, and low hairline. More recently, congenital scoliosis has been associated with Sprengel deformity, Mayer-Rokitansky-Küster-Hauser syndrome, Jarcho-Levin syndrome, Goldenhar syndrome, and Genoa syndrome.18–21
Etiology
Congenital scoliosis is uncommon in the general population. Its true incidence is unknown, but the familial incidence in the congenital scoliosis population is typically 1% to 5%.13,22–24 It is slightly more common in girls than in boys, with a ratio of 3 : 2.
The precise etiology of congenital scoliosis is unclear. Although most cases seem to be sporadic, in contrast to idiopathic scoliosis,25 the role of genetic and environmental factors is often reported.6,26,27 The genetic role has been reported in cases of congenital scoliosis in twins,28–30 but, more recently, several studies have isolated gene mutations.25,27,31
Environmental factors have also been implicated in the genesis of congenital scoliosis. Maternal acute carbon monoxide exposure during somite formation induces vertebral anomalies in the offspring of mouse and rabbit models.26,32 The mechanism of carbon monoxide action remains vague, however. Carbon monoxide could act directly on the cartilaginous spine via resulting hypoxia or a gene mutation.26 The etiologic theories are clouded further by the finding of an increased incidence of idiopathic scoliosis in families with congenital scoliosis.
Natural History
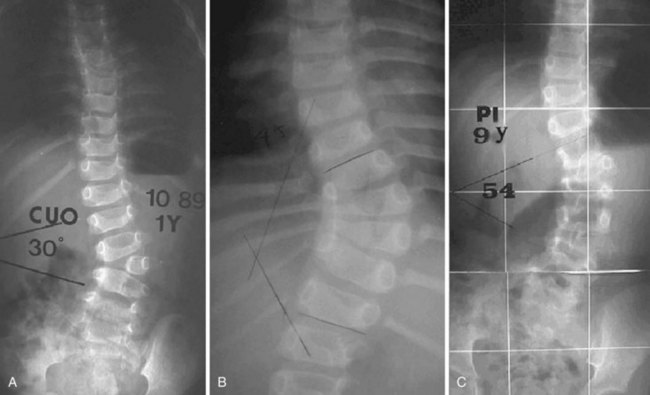
As with scoliosis of any etiology, congenital scoliosis progresses in 70% of patients during growth. The potential for increase in curvature is related to imbalances in the number of growth apophyses and the location of vertebral anomalies.33 Without any treatment, about 85% of patients with congenital scoliosis have a curve greater than 41 degrees by maturity.33 Curves with segmented hemivertebrae are at risk for progression during growth because segmented hemivertebrae act as enlarging wedges (Fig. 22–6). The most progressive anomaly is a convex segmented hemivertebra associated with a concave unilateral bar because there is absolutely no growth potential on the side of the bar. Conversely, a wedge vertebra has only a slight risk of worsening, whereas a complete block or an incarcerated hemivertebra does not cause any progressive scoliosis.
The natural history of congenital scoliosis has to take several factors into account:
More recent work has focused on the role of the spine and chest wall in lung development, which predominantly occurs by age 5. Congenital defects in the development of the ribs and vertebrae often occur together. Concurrent scoliosis and rib fusions may constrict the thorax and compromise pulmonary development. The inability of the thorax to support normal respiration is termed thoracic insufficiency syndrome.34 Thoracic insufficiency syndrome can be assessed clinically by respiratory rate and the thumb excursion test and radiographically by plain radiographs and computed tomography (CT) volumetric studies. Early fusion of scoliotic deformity before age 9, especially in patients requiring more than four levels of fusion and patients with proximal fusions, also puts these patients at risk for the development of restrictive pulmonary disease.35 The increasing awareness of the need to preserve pulmonary function has led to a surge in growth-preserving surgical techniques for complex multilevel congenital scoliosis.
Assessment
Imaging
Radiographs
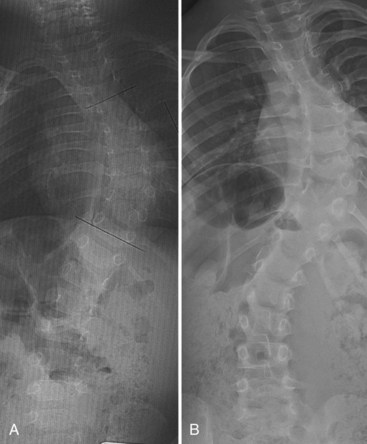
For patients with congenital scoliosis, early plain radiographs are helpful to determine the type of vertebral abnormality. The best period to categorize the deformity is before 4 years of age. If a patient is seen by the orthopaedic surgeon after this age, valuable information may sometimes be gained by examining prior chest radiographs and abdominal or renal films. The spine surgeon learns to check for subtle clues, such as the presence and spacing of pedicles and fused or absent ribs. Later films make it difficult to assess the type of anomaly present because the vertebrae are too ossified, especially in the area of a fusion or bar (Fig. 22–7).
Anteroposterior and lateral films allow one to check the type and the location of deficiency, to measure the spine curvature, and to assess the pedicle width. Measurement of curvature according to the Cobb method36 can be a challenge, however.37 It has been shown that there is an increase in measurement error38 in congenital scoliosis owing to irregular vertebral landmarks and difficulty in numbering them. It is important always to compare current films against original ones rather than rely on curvature measurement itself. Assessment of curve evolution and compensatory curve development helps to confirm or to refute scoliosis progression. Because compensatory curves involve normally formed vertebrae, they can be more reproducibly measured. If a compensatory curve has not progressed, it is less likely that significant progression has occurred in a congenital curve.
Computed Tomography
CT with three-dimensional reconstruction can be used to identify spinal abnormalities in complex cases in which radiographs are often difficult to interpret.39 CT scans can also help visualize spinal deformities that are obscured by overlying structures on plain radiographs.
Magnetic Resonance Imaging
Intraspinal anomalies are often associated with congenital scoliosis, but their incidence is variable. Before the introduction of magnetic resonance imaging (MRI), myelography and CT were the procedures of choice, and the incidence of intraspinal anomalies in this context varied widely from 5% to 58%.17,40 The advent of MRI has led to more accurate discovery of intraspinal anomalies in 30% to 41% of cases.41–43 The most common anomalies reported are tethered cord, syringomyelia, and diastematomyelia.
Finally, a genitourinary assessment is useful at the time of initial diagnosis of congenital vertebral anomaly. This assessment can be performed accurately with renal ultrasonography. Often, MRI of the spine also shows the presence or absence of renal anomalies.44 Further consultation may be indicated based on the results of this study.
Treatment
Operative Treatment
Posterior Spine Fusion
Posterior in situ fusion is the simplest and the safest technique (Fig. 22–8). This seemingly simple surgical exposure must be performed with caution, however, because failure to recognize potential posterior laminar defects can lead to neurologic damage. Imaging is useful before the exposure is made because the anomalous area is difficult to localize. After exposure of the posterior elements, the target region should be reconfirmed with radiographs because the hemivertebra or bar seen anteriorly may not have corresponding posterior elements. Fusion must include all vertebrae involved in the congenital curve and should extend laterally to the transverse processes. A postoperative cast or a rigid brace is required for 4 to 6 months to achieve fusion and curve correction.
With this technique, problems may occur, as follows:
To avoid pseudarthrosis and to obtain greater intraoperative correction, posterior instrumentation can be used. The development of pediatric-sized instrumentation has limited the problem of implant prominence in young children; however, concern for potential neurologic injury persists. Although a more recent review reported no significant increase in neurologic injury,46 careful intraoperative spinal cord monitoring and possibly an intraoperative wake-up test is recommended to avoid such complications. The surgeon also must carefully consider anatomy because anomalous pedicles and laminae do not always lend themselves well to fixation. Instrumentation becomes more feasible with increasing age, especially after 2 years of age.
Combined Anterior and Posterior Spine Fusion
Performing an anterior approach allows a surgeon to perform discectomy and remove vertebral endplates.47 Better spine flexibility can be obtained, allowing for better deformity correction. Anterior bone graft is placed for fusion. This bone graft is most appropriate when the anomalous segment is lordotic.
Convex Hemiepiphysiodesis
The principle of convex hemiepiphysiodesis is the same as that commonly employed for deformity of growing long bones. Convex hemiepiphysiodesis slows convex side growth while the concave curve still grows, allowing for safe progressive deformity correction. Prerequisites for this procedure include a patient young enough for significant corrective growth (<6 years old), the presence of less than seven involved vertebrae, and significant concave growth potential.48 This technique requires a combined anterior and posterior exposure, followed by corrective rigid spinal immobilization for 6 months until fusion is complete. The anterior procedure consists of removal of the convex portion of discs and vertebral endplates and fusion of the convex portion with bone graft. A longitudinal rib inlay functions as an effective peripheral tether. The posterior exposure consists of unilateral removal of the facet joints and fusion. The correction is usually modest, on the order of 0 to 20 degrees by maturity. A single posterior approach with transpedicular convex anterior hemiepiphysiodesis has been reported; however, it seems less long-term correction is maintained.49
Instrumentation can be used to achieve concave posterior distraction50 and convex posterior compression.50,51 This technique allows a surgeon to obtain better intraoperative correction. Because this technique relies on remaining growth to achieve correction, it is most useful in children with intact spinal growth potential in whom involved vertebrae are limited.
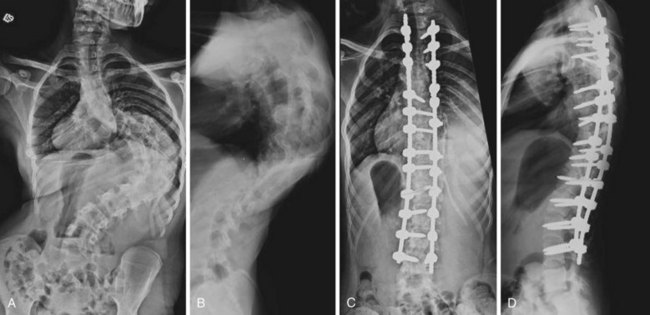
Hemivertebra Excision
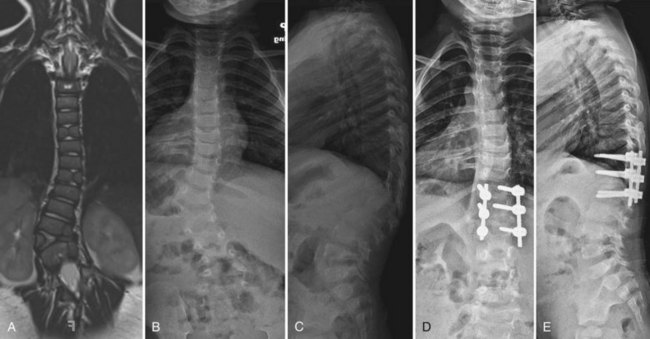
The operation for hemivertebra excision consists of combined anterior and posterior excision of the hemivertebra, followed by anterior and posterior fusion (Fig. 22–9). Anterior structural graft is useful on the concave side to maintain a normal sagittal contour. If the patient is old enough, instrumentation may be useful anteriorly and posteriorly to maintain good correction and to apply compression. Unless the construct is rigid, postoperative immobilization is usually necessary. Instrumentation may allow the patient to use a brace, however, instead of a cast.
Similar to convex hemiepiphysiodesis, hemivertebra excision can be performed via a single posterior approach.52–57 This is becoming the most common approach for hemivertebra excision because of improved imaging and monitoring.
Guided Growth Procedures
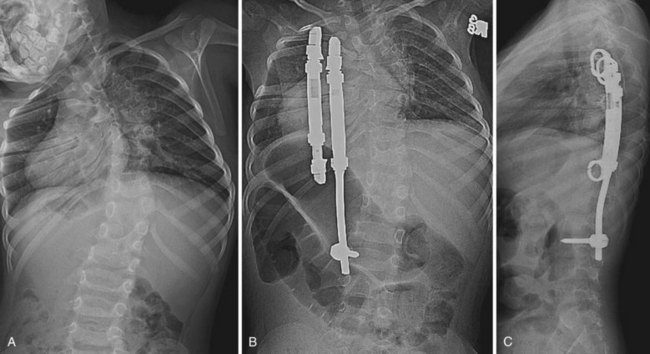
More recent concern regarding thoracic insufficiency syndrome in patients with congenital scoliosis, especially after early fusion with traditional growth-arresting surgical techniques, has led to the development of guided growth procedures using growing rods and VEPTR devices.58 Growing rods allow for continued growth in spinal height before definitive fusion; this presumably optimizes the thoracic volume available for pulmonary development and function. Although complications associated with growing rod procedures are manageable, it is important to inform patients of the risks inherent to undergoing multiple surgical spinal procedures. In a study of early-onset scoliosis patients, dual growing rods lengthened at 6-month intervals were shown to achieve greater curve correction and allow for greater spinal growth compared with single growing rods lengthened after 15 to 20 degrees of curve progression.59
Expansion thoracoplasty using VEPTR aims to relieve a constricted concave hemithorax in patients with congenital scoliosis associated with fused ribs (Fig. 22–10).60 When surgery is performed before 2 years of age, which coincides with the period of rapid lung growth, pulmonary function at 5-year follow-up is significantly better compared with children who undergo surgery at a later age.61 Significant curve correction can also be indirectly achieved.61 The use of VEPTR continues to evolve.
Indications
The problem in congenital scoliosis is usually not whether surgery is needed, but rather when and what kind of surgery is needed. In contrast to idiopathic scoliosis, for which a definitive fusion is delayed until near skeletal maturity, early deformity correction is generally desired to avoid structural spine decompensation52 and to fuse the fewest vertebrae possible in congenital scoliosis. Patient height at maturity is not significantly diminished by early surgical intervention because in allowing a progressive curve to grow, the only growth that occurs is deformed growth (with increasing rotation and development of compensatory curves) and not vertical growth. Performing early corrective surgery, even with traditional growth-arresting procedures, ultimately allows the child to be taller and straighter.
Key Points
1 Loder RT, Urquhart A, Steen H, et al. Variability in Cobb angle measurements in children with congenital scoliosis. J Bone Joint Surg Br. 1995;77:768-770.
The measurement variation in congenital scoliosis was found to be 19 degrees in this study.
2 McMaster MJ, Ohtsuka K. The natural history of congenital scoliosis: A study of two hundred and fifty-one patients. J Bone Joint Surg Am. 1982;64:1128-1147.
3 Ruf M, Harms J. Hemivertebra resection by a posterior approach: Innovative operative technique and first results. Spine. 2002;27:1116-1123.
4 Giampietro PF, Dunwoodie SL, Kusumi K, et al. Progress in the understanding of the genetic etiology of vertebral segmentation disorders in humans. Ann N Y Acad Sci. 2009;1151:38-67.
5 Campbell RMJr, Smith MD, Mayes TC, et al. The characteristics of thoracic insufficiency syndrome associated with fused ribs and congenital scoliosis. J Bone Joint Surg Am. 2003;85:399-408.
1 Larsen WJ, Sherman LS, Potter SS, et al. The fourth week. In: Human Embryology, 3rd ed. New York: Churchill Livingstone; 2001:pp 79-112.
2 Sadler TW, Langman J. Skeletal system. In: Langman’s Medical Embryology, 8th ed. Philadelphia: Lippincott Williams & Wilkins; 2000.
3 Dunwoodie SL. Mutation of the fucose-specific beta1,3 N-acetylglucosaminyltransferase LFNG results in abnormal formation of the spine. Biochim Biophys Acta. 2009;1792:100-111.
4 Dequeant ML, Pourquie O. Segmental patterning of the vertebrate embryonic axis. Nat Rev Genet. 2008;9:370-382.
5 Giampietro PF, Dunwoodie SL, Kusumi K, et al. Progress in the understanding of the genetic etiology of vertebral segmentation disorders in humans. Ann N Y Acad Sci. 2009;1151:38-67.
6 Bulman MP, Kusumi K, Frayling TM, et al. Mutations in the human delta homologue, DLL3, cause axial skeletal defects in spondylocostal dysostosis. Nat Genet. 2000;24:438-441.
7 Turnpenny PD, Alman B, Cornier AS, et al. Abnormal vertebral segmentation and the notch signaling pathway in man. Dev Dyn. 2007;236:1456-1474.
8 William DA, Saitta B, Gibson JD, et al. Identification of oscillatory genes in somitogenesis from functional genomic analysis of a human mesenchymal stem cell model. Dev Biol. 2007;305:172-186.
9 Aulehla A, Wehrle C, Brand-Saberi B, et al. Wnt3a plays a major role in the segmentation clock controlling somitogenesis. Dev Cell. 2003;4:395-406.
10 Winter RB, Moe JH, Eilers VE. Congenital scoliosis: A study of 234 patients treated and untreated. J Bone Joint Surg Am. 1968;50:1-47.
11 Jaskwhich D, Ali RM, Patel TC, et al. Congenital scoliosis. Curr Opin Pediatr. 2000;12:61-66.
12 Jog S, Patole S, Whitehall J. Congenital scoliosis in a neonate: Can a neonatologist ignore it? Postgrad Med J. 2002;78:469-472.
13 Shahcheraghi GH, Hobbi MH. Patterns and progression in congenital scoliosis. J Pediatr Orthop. 1999;19:766-775.
14 Quan L, Smith DW. The VATER association: Vertebral defects, Anal atresia, T-E fistula with esophageal atresia, Radial and Renal dysplasia. A spectrum of associated defects. J Pediatr. 1973;82:104-107.
15 Nora AH, Nora JJ. A syndrome of multiple congenital anomalies associated with teratogenic exposure. Arch Environ Health. 1975;30:17-21.
16 Chaumien JP, Rigault P, Maroteaux P, et al. [The so-called Klippel-Feil syndrome and its orthopaedic incidences]. Rev Chir Orthop Reparatrice Appar Mot. 1990;76:30-38.
17 Winter RB, Moe JH, Lonstein JE. The incidence of Klippel-Feil syndrome in patients with congenital scoliosis and kyphosis. Spine. 1984;9:363-366.
18 Fisher K, Esham RH, Thorneycroft I. Scoliosis associated with typical Mayer-Rokitansky-Küster-Hauser syndrome. South Med J. 2000;93:243-246.
19 Lapunzina P, Musante G, Pedraza A, et al. Semilobar holoprosencephaly, coronal craniosynostosis, and multiple congenital anomalies: A severe expression of the Genoa syndrome or a newly recognized syndrome? Am J Med Genet. 2001;102:258-260.
20 Larson AR, Josephson KD, Pauli RM, et al. Klippel-Feil anomaly with Sprengel anomaly, omovertebral bone, thumb abnormalities, and flexion-crease changes: Novel association or syndrome? Am J Med Genet. 2001;101:158-162.
21 Mooney JF3rd, Emans JB. Progressive kyphosis and neurologic compromise complicating spondylothoracic dysplasia in infancy (Jarcho-Levin syndrome). Spine. 1995;20:1938-1942.
22 Purkiss SB, Driscoll B, Cole WG, et al. Idiopathic scoliosis in families of children with congenital scoliosis. Clin Orthop Relat Res. 2002;401:27-31.
23 Winter RB. Congenital scoliosis. Orthop Clin North Am. 1988;19:395-408.
24 Wynne-Davies R. Congenital vertebral anomalies: Aetiology and relationship to spina bifida cystica. J Med Genet. 1975;12:280-288.
25 Giampietro PF, Raggio CL, Blank RD. Synteny-defined candidate genes for congenital and idiopathic scoliosis. Am J Med Genet. 1999;83:164-177.
26 Farley FA, Loder RT, Nolan BT, et al. Mouse model for thoracic congenital scoliosis. J Pediatr Orthop. 2001;21:537-540.
27 Imaizumi K, Masuno M, Ishii T, et al. Congenital scoliosis (hemivertebra) associated with de novo balanced reciprocal translocation, 46,XX,t(13;17)(q34;p11.2). Am J Med Genet. 1997;73:244-246.
28 McKinley LM, Leatherman KD. Idiopathic and congenital scoliosis in twins. Spine. 1978;3:227-229.
29 Ogden JA, Southwick WO. Congenital and infantile scoliosis in triplets. Clin Orthop Relat Res. 1978;136:176-178.
30 Pool RD. Congenital scoliosis in monozygotic twins: Genetically determined or acquired in utero? J Bone Joint Surg Br. 1986;68:194-196.
31 Goshu E, Jin H, Fasnacht R, et al. Sim2 mutants have developmental defects not overlapping with those of Sim1 mutants. Mol Cell Biol. 2002;22:4147-4157.
32 Schwetz BA, Smith FA, Leong BK, et al. Teratogenic potential of inhaled carbon monoxide in mice and rabbits. Teratology. 1979;19:385-392.
33 McMaster MJ, Ohtsuka K. The natural history of congenital scoliosis: A study of two hundred and fifty-one patients. J Bone Joint Surg Am. 1982;64:1128-1147.
34 Campbell RMJr, Smith MD, Mayes TC, et al. The characteristics of thoracic insufficiency syndrome associated with fused ribs and congenital scoliosis. J Bone Joint Surg Am. 2003;85:399-408.
35 Karol LA, Johnston C, Mladenov K, et al. Pulmonary function following early thoracic fusion in non-neuromuscular scoliosis. J Bone Joint Surg Am. 2008;90:1272-1281.
36 Cobb JR. Outline for the study of scoliosis. Edwards JW, trans. Instructional Course Lecture Vol 5. Ann Arbor, MI: American Academy of Orthopaedic Surgeons; 1948:pp 261-275.
37 Facanha-Filho FA, Winter RB, Lonstein JE, et al. Measurement accuracy in congenital scoliosis. J Bone Joint Surg Am. 2001;83:42-45.
38 Loder RT, Urquhart A, Steen H, et al. Variability in Cobb angle measurements in children with congenital scoliosis. J Bone Joint Surg Br. 1995;77:768-770.
39 Newton PO, Hahn GW, Fricka KB, et al. Utility of three-dimensional and multiplanar reformatted computed tomography for evaluation of pediatric congenital spine abnormalities. Spine. 2002;27:844-850.
40 Blake NS, Lynch AS, Dowling FE. Spinal cord abnormalities in congenital scoliosis. Ann Radiol (Paris). 1986;29(3-4):377-379.
41 Bradford DS, Heithoff KB, Cohen M. Intraspinal abnormalities and congenital spine deformities: A radiographic and MRI study. J Pediatr Orthop. 1991;11:36-41.
42 Prahinski JR, Polly DWJr, McHale KA, et al. Occult intraspinal anomalies in congenital scoliosis. J Pediatr Orthop. 2000;20:59-63.
43 Suh SW, Sarwark JF, Vora A, et al. Evaluating congenital spine deformities for intraspinal anomalies with magnetic resonance imaging. J Pediatr Orthop. 2001;21:525-531.
44 Riccio AI, Guille JT, Grissom L, et al. Magnetic resonance imaging of renal abnormalities in patients with congenital osseous anomalies of the spine. J Bone Joint Surg Am. 2007;89:2456-2459.
45 Kesling KL, Lonstein JE, Denis F, et al. The crankshaft phenomenon after posterior spinal arthrodesis for congenital scoliosis: A review of 54 patients. Spine. 2003;28:267-271.
46 Hedequist DJ, Hall JE, Emans JB. The safety and efficacy of spinal instrumentation in children with congenital spine deformities. Spine. 2004;29:2081-2086. discussion 2087
47 McMaster MJ, Singh H. The surgical management of congenital kyphosis and kyphoscoliosis. Spine. 2001;26:2146-2154. discussion 2155
48 Winter RB, Lonstein JE, Denis F, et al. Convex growth arrest for progressive congenital scoliosis due to hemivertebrae. J Pediatr Orthop. 1988;8:633-638.
49 Keller PM, Lindseth RE, DeRosa GP. Progressive congenital scoliosis treatment using a transpedicular anterior and posterior convex hemiepiphysiodesis and hemiarthrodesis: A preliminary report. Spine. 1994;19:1933-1939.
50 Shono Y, Abumi K, Kaneda K. One-stage posterior hemivertebra resection and correction using segmental posterior instrumentation. Spine. 2001;26:752-757.
51 Cheung KM, Zhang JG, Lu DS, et al. Ten-year follow-up study of lower thoracic hemivertebrae treated by convex fusion and concave distraction. Spine. 2002;27:748-753.
52 Klemme WR, Polly DWJr, Orchowski JR. Hemivertebral excision for congenital scoliosis in very young children. J Pediatr Orthop. 2001;21:761-764.
53 Lazar RD, Hall JE. Simultaneous anterior and posterior hemivertebra excision. Clin Orthop Relat Res. 1999;364:76-84.
54 Nakamura H, Matsuda H, Konishi S, et al. Single-stage excision of hemivertebrae via the posterior approach alone for congenital spine deformity: Follow-up period longer than ten years. Spine. 2002;27:110-115.
55 Ruf M, Harms J. Hemivertebra resection by a posterior approach: Innovative operative technique and first results. Spine. 2002;27:1116-1123.
56 Ruf M, Harms J. Posterior hemivertebra resection with transpedicular instrumentation: Early correction in children aged 1 to 6 years. Spine. 2003;28:2132-2138.
57 Suk SI, Chung ER, Kim JH, et al. Posterior vertebral column resection for severe rigid scoliosis. Spine. 2005;30:1682-1687.
58 Vitale MG, Matsumoto H, Bye MR, et al. A retrospective cohort study of pulmonary function, radiographic measures, and quality of life in children with congenital scoliosis: An evaluation of patient outcomes after early spinal fusion. Spine. 2008;33:1242-1249.
59 Thompson GH, Akbarnia BA, Campbell RMJr. Growing rod techniques in early-onset scoliosis. J Pediatr Orthop. 2007;27:354-361.
60 Campbell RMJr, Smith MD, Hell-Vocke AK. Expansion thoracoplasty: The surgical technique of opening-wedge thoracostomy. Surgical technique. J Bone Joint Surg Am. 2004;86(Suppl 1):51-64.
61 Campbell RMJr, Smith MD, Mayes TC, et al. The effect of opening wedge thoracostomy on thoracic insufficiency syndrome associated with fused ribs and congenital scoliosis. J Bone Joint Surg Am. 2004;86:1659-1674.