Chapter 15 Congenital Heart Disease
In patients with congenital heart disease, a complete and accurate description of the morphologic abnormalities is crucial for determining appropriate management strategies. Currently, two-dimensional echocardiography (2DE) allows a complete description of the intracardiac anatomy of different congenital lesions. Because of its high spatial and temporal resolution, 2DE is still the mainstay technique for the diagnosis and follow-up of patients with congenital defects. However, the representation of a three-dimensional (3D) structure by a 2D technique has intrinsic limitations, which explains why 3DE, particularly real-time (RT) 3DE, has potential applications in congenital heart disease. The development of RT3DE with matrix transducers and a high-frequency pediatric matrix 3D transducer has especially sparked growth and interest in the congenital field. RT3DE has emerged as a valuable additional tool because it provides a direct representation of morphology and volumetric calculations. The real additional diagnostic value still remains to be proven, but recently published data suggest potential use in the following three areas1:
1. Improved visualization and understanding of the 3D nature of congenital heart defects
Three-Dimensional Visualization of Anatomy
Atrial and Ventricular Septa
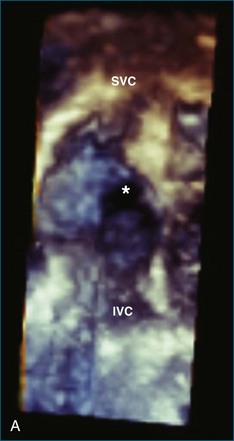
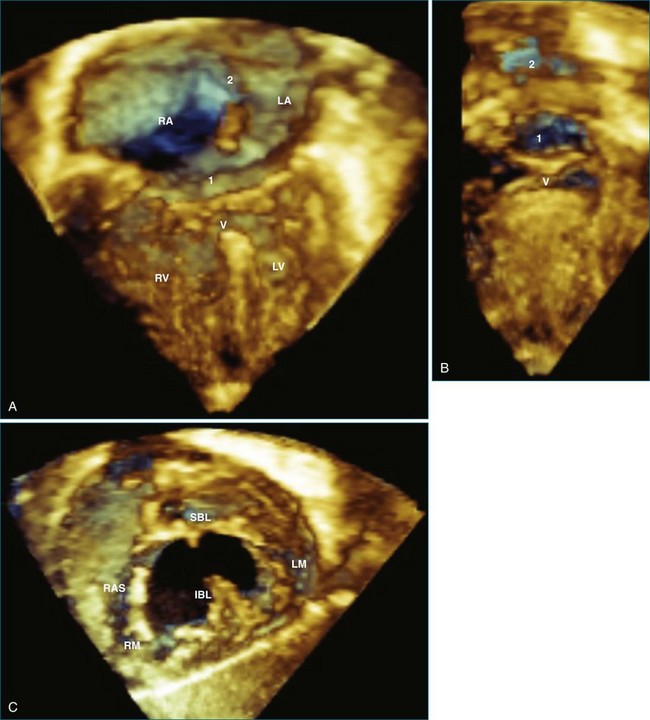
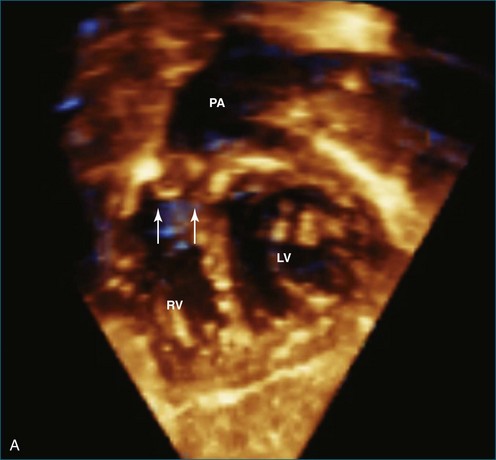
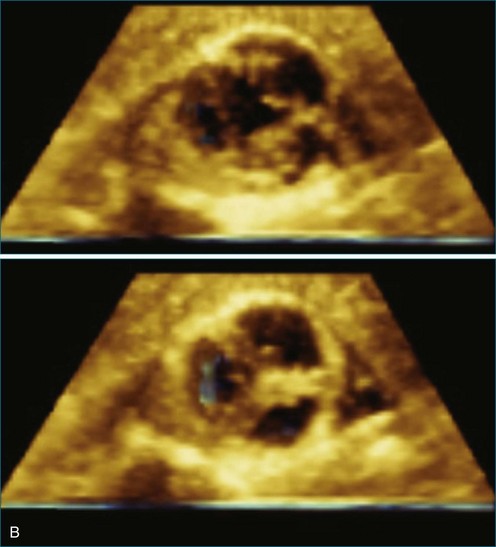
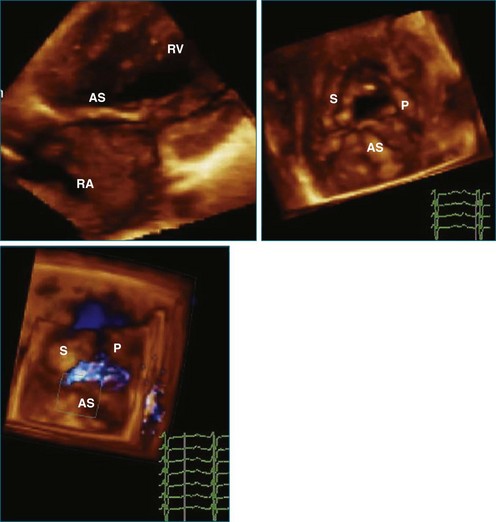
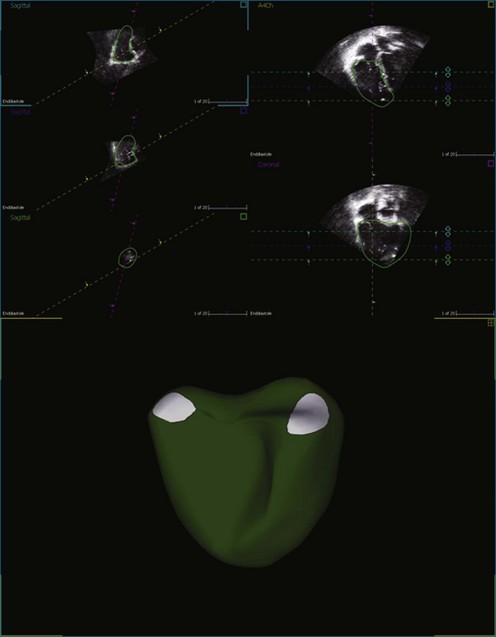
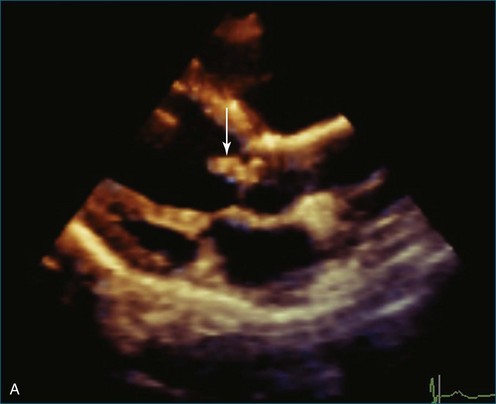
Atrial and ventricular septal defects typically are not circular “holes,” but instead are complex structures, often irregularly shaped or fenestrated, that are difficult to visualize in two dimensions. RT3DE enables improved visualization of septal defects using unique en face representations of the interatrial and interventricular septa. The interatrial septum can be represented as viewed from the left or right atrium and the interventricular septum as viewed from the left or right ventricular side. This allows the creation of surgical views of the defects as well as improved understanding of their shape and relationships to surrounding intracardiac structures. These views also allow a more accurate measurement of defect size, including their dynamic shape change during the cardiac cycle.2–4 The importance of viewing the defects from both sides of the septum cannot be overemphasized; this aids in understanding their anatomy and particularly facilitates the determination of both the right and left border edges and shapes.5 These measurements are useful for surgical or interventional planning in the catheterization laboratory (Figures 15-1 and 15-2; Videos 15-1 to 15-3).
Atrioventricular Valves
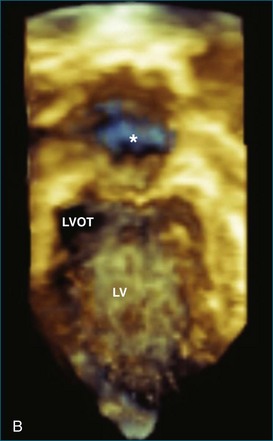
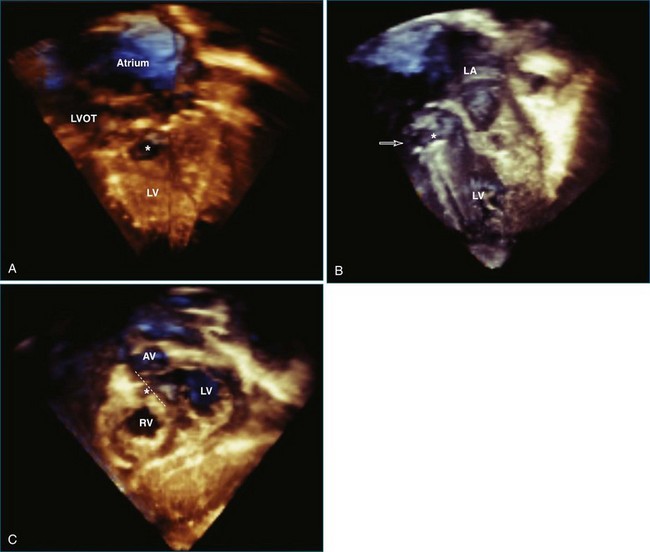
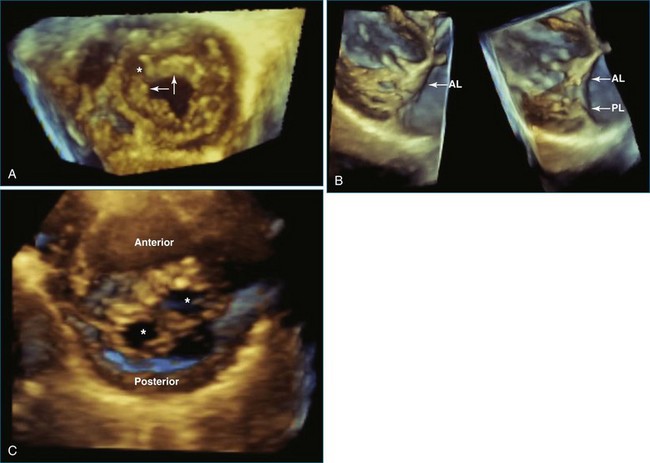
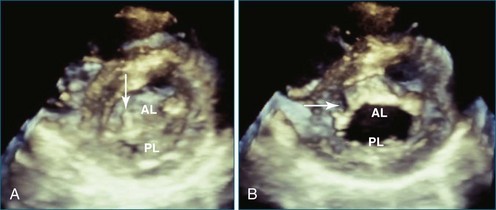
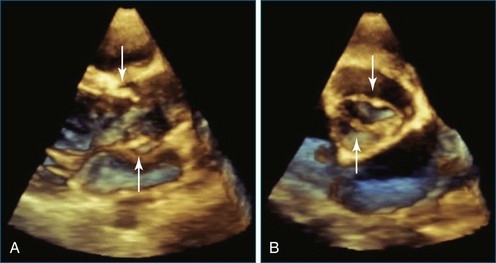
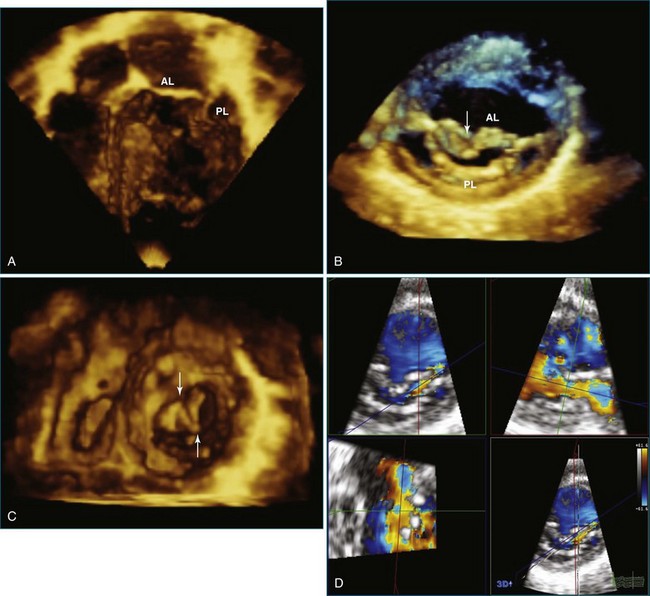
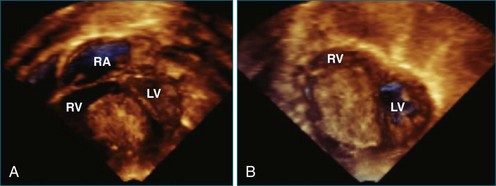
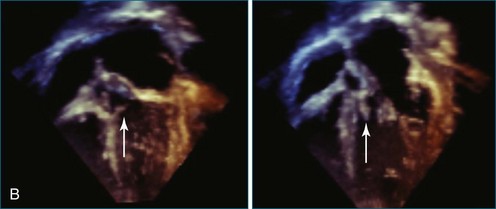
Transthoracic RT3DE is complementary to 2D transesophageal echocardiography (2DTEE) and 2D transthoracic echocardiography (2DTTE) in detecting anatomic and functional abnormalities of atrioventricular (AV) valves in patients with congenital heart disease.6–8 This is mainly due to the unique 3D visualization and representation of the AV valves. RT3DE facilitates viewing of the mitral valve with its complex annular shape and interactions with the left ventricular shape and function and the subchordal apparatus.7,9 RT3DE also facilitates increased understanding of the maturation of the dynamic function of the mitral valve. The mitral valve is saddle shaped, and its motion and dynamic function during the cardiac cycle are complex. In adults, the mitral valve annulus has its largest dimension at end systole and is smallest at end diastole.10,11 However, RT3DE has demonstrated that in children, the mitral annular motion is somewhat different and that it has its largest area in systole and decreases in diastole.12,13 The main advantage of 3DE is that the individual scallops of the mitral valve can be imaged in the same view, which helps define the surgical anatomy and enhances communication between the echocardiographer and the surgeon.
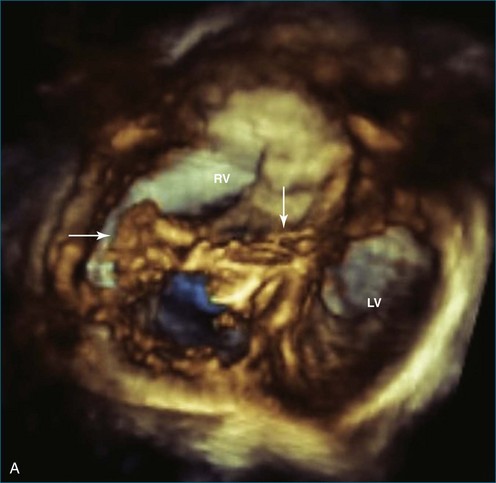
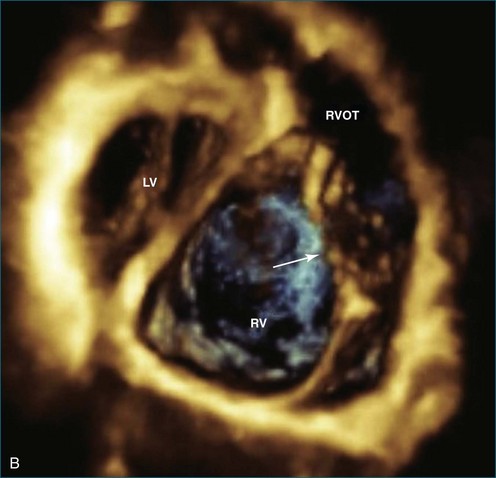
RT3DE has a clear advantage for imaging the tricuspid valve because the three leaflets are very difficult to view together by cross-sectional imaging. For congenital abnormalities of the tricuspid valve, RT3DE can be useful for a better representation of the valvar anatomy. A typical example is imaging of the tricuspid valve in Ebstein’s anomaly. In this lesion, RT3DE can provide better visualization of the morphology of the tricuspid valve leaflets, their attachments, their degree of coaptation, and the mechanism of regurgitation.14 The multiplanar review mode also facilitates appreciation of the degree of displacement and rotation of the tricuspid valve annulus, which is the key feature of this anomaly.7 Potentially, RT3DE could also help determine the right ventricular stroke volume, although no validation data on right ventricular volumes have been published yet in this disease (Figures 15-2 to 15-5; Videos 15-2 to 15-9).
Atrioventricular Septal Defects
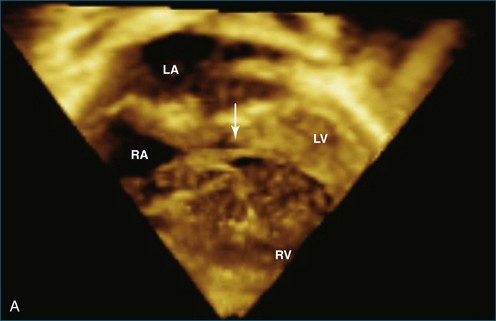
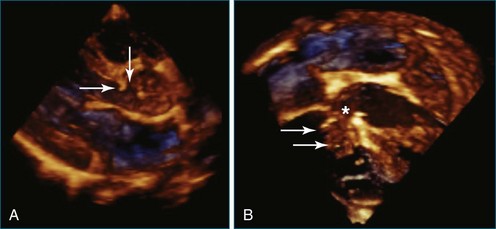
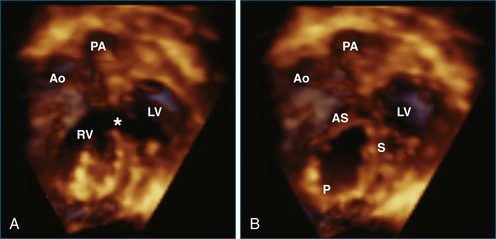
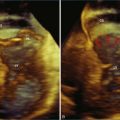
Patients with AV septal defects have a common AV junction with a single AV valve at the entrance of both ventricles. RT3DE can provide clear visualization of the anatomic variability, which is crucial in the preoperative planning. Studies have demonstrated that RT3DE can provide additional anatomic information to cross-sectional imaging in patients before and after surgical repair.15–17 In preoperative assessment, the anatomy of the superior and inferior bridging leaflets, as well as that of the left-sided mural leaflet, provides useful clinical information. In postoperative patients with residual AV valve regurgitation, the mechanisms contributing to the regurgitation can be complex, and RT3DE can be useful in clarifying the origin of the regurgitant jets and in guiding surgical decision making by demonstrating defects such as residual cleft, central regurgitation, leaflet prolapse, dysplasia, annular dilation, and so on (Figures 15-6 and 15-7; Videos 15-10 to 15-12).
Outflow Tracts
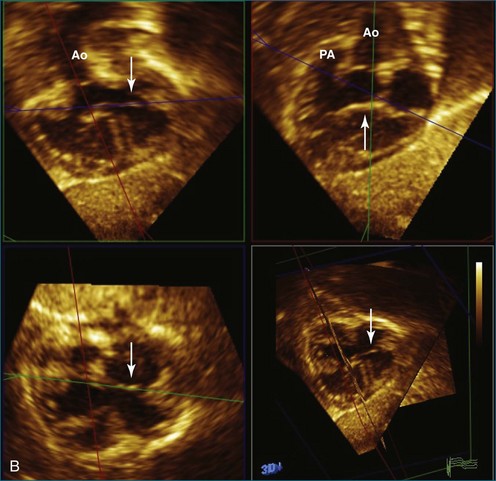
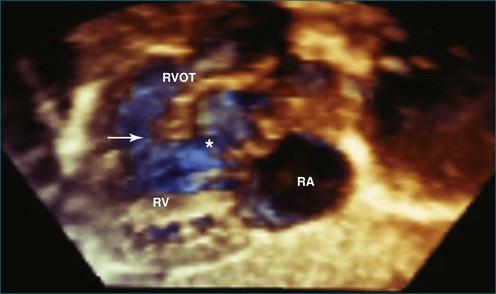
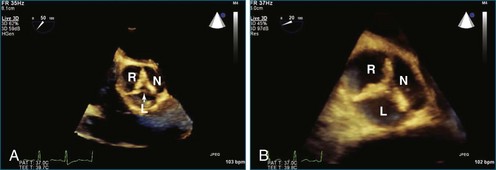
RT3DE allows accurate anatomic visualization of both left and right outflow tracts and is helpful in defining the mechanisms contributing to complex outflow tract obstruction.18 In both outflow tracts, the obstruction can be present at different levels (subvalvar, valvar, and supravalvar). In the left ventricular outflow tract, a subvalvar obstruction can be caused by a subvalvar membrane, abnormal AV valve tissue with septal attachments, or a tunnel-like obstruction. Here, RT3DE with multiplanar reconstructions can be helpful in defining the exact anatomic mechanisms. Semilunar valve abnormalities may, however, be well delineated by RT3DE, which has been demonstrated to correlate well with surgical findings.19
Subaortic obstructions usually are incompletely visualized by the surgeon, who approaches the lesion through the aortic valve. Therefore, accurate preoperative imaging is beneficial for surgical planning and may demonstrate additional lesions, such as abnormal mitral chordal attachments in subaortic stenosis.20
The right ventricular outflow tract can be more difficult to visualize with RT3DE because of its anterior position. Right ventricular outflow tract obstruction also can be complex with subvalvar, valvar, and supravalvar components. The additional value of RT3DE still remains to be proven for this indication (Figures 15-8 to 15-11; Videos 15-13 to 15-18).
Measurement of Size and Function
Valve Orifice
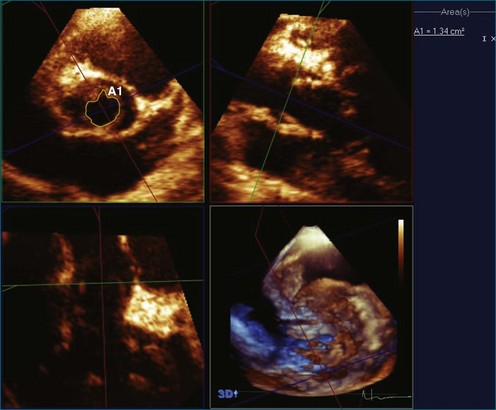
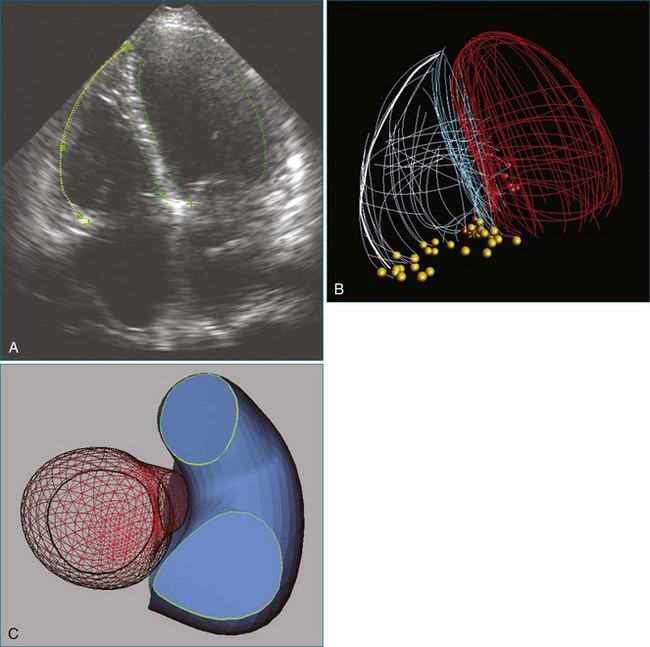
In certain congenital heart diseases, accurate assessment of valve size may be important in determining surgical management. RT3DE enables the operator to view the true plane of the valve orifice more accurately. For stenotic lesions, this allows direct measurement of the effective orifice using planimetry on 3D images.21 RT3DE for area calculation has been shown to be more reliable, with a lower interobserver variability, compared with cross-sectional imaging (Figure 15-12).22
Regurgitant Jets
Valvar regurgitation in patients with congenital heart disease may have complex mechanisms that are difficult to evaluate fully by 2DE; RT3DE helps elucidate these details. The regurgitant jet may have an irregular shape, which makes it more readily appreciated in 3D than in 2D (Figures 15-13 and 15-14; Video 15-19).
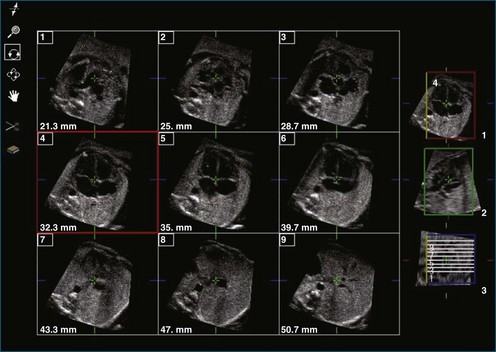
Ventricular Volume and Mass Quantification
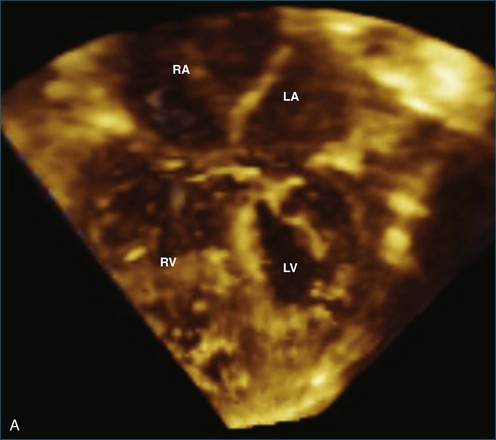
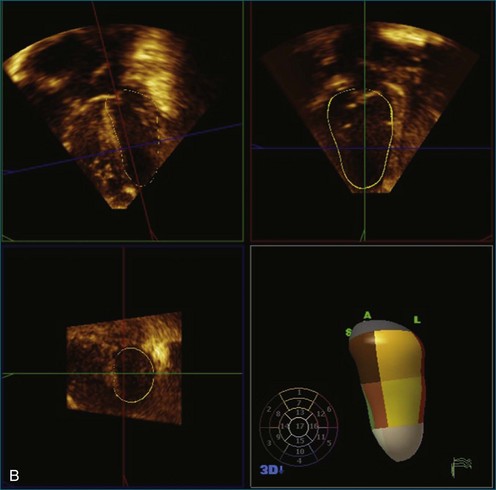
There are many congenital lesions in which quantification of left ventricular volume or mass is important. RT3DE demonstrates excellent intraobserver and interobserver reproducibility in left ventricular quantification.23,24 This can be a useful adjunctive investigation in patients in whom determination of left ventricular adequacy is important for clinical decision making, such as in those with borderline left ventricles when the choice is either two-ventricle repair or single-ventricle palliation. In patients with a functional single ventricle, RT3DE measurements of mass and volume correlate well with cardiac magnetic resonance imaging (MRI) measurement and allow monitoring of ventricular size and function over time.25
Evaluation of right ventricular function is extremely important in congenital heart disease, particularly in patients with right-sided lesions, such as tetralogy of Fallot, or in patients in whom the right ventricle is the systemic ventricle, such as in congenitally corrected transposition of the great arteries, or after an atrial switch procedure. 3DE has been demonstrated to be a sensitive tool to identify right ventricular dysfunction and also may be useful to assess which patients would benefit from quantitative analysis, for which cardiac MRI remains the gold standard.26–28
RT3DE also allows excellent visualization of intracardiac masses, which may be monitored by RT3DE assessment of the volume of the mass (Figures 15-15 to 15-17; Videos 15-20 and 15-21).
Dyssynchrony Assessment: Regional Left Ventricular Volumes
As in adults, RT3DE has been used to demonstrate left ventricular dyssynchrony in children with left ventricular dysfunction.29 In congenital heart disease, dyssynchrony is an important mechanism that can contribute to ventricular dysfunction. Data on the potential use of timing of the regional volume changes for the diagnosis of dyssynchrony in patients with congenital heart disease are still limited.
Interventional Planning
Presurgical Imaging
RT3DE can be used to enhance preoperative planning of procedures by enhancing understanding of intracardiac anatomy. RT3DE reduces operation time by giving improved anatomic detail before surgery in some patients, such as those with complex outflow tract obstruction.18 Findings on RT3DE correlate well with anatomic and surgical findings in congenital heart disease in both simple and complex defects.18,20,30 New, clinically important information that substantially alters the course of management may be discovered with RT3DE analysis in a small subset of patients.20
Intraprocedural Imaging
Both intraoperative epicardial RT3DE and intraoperative RT3DTEE have been demonstrated to be useful in the immediate postbypass evaluation of surgical repair of congenital heart defects.31 Both techniques may provide particular additive value in the intraoperative imaging of AV valves.32 Transcatheter closure of atrial septal defects has traditionally been guided by 2DTEE or intracardiac echocardiography, but RT3DE is increasingly being used either alone or as an adjunct to 2DE. RT3DE may minimize the use of fluoroscopy in some procedures; for example, it has been successfully used in guiding endomyocardial biopsy of the right ventricle in children (Figures 15-18 to 15-20; Videos 15-22 to 15-24).33
Fetal Echocardiography
Although 2DE remains the primary imaging modality for antenatal diagnosis of congenital heart disease, 3DE may provide additional information in a small subgroup of patients (Figure 15-21).34–36
1 Shirali GS. Three dimensional echocardiography in congenital heart defects. Ann Pediatr Cardiol. 2008;1(1):8–17.
2 Cheng TO, Xie MX, Wang XF, et al. Real-time 3-dimensional echocardiography in assessing atrial and ventricular septal defects: an echocardiographic-surgical correlative study. Am Heart J. 2004;148(6):1091–1095.
3 Maeno YV, Benson LN, McLaughlin PR, Boutin C. Dynamic morphology of the secundum atrial septal defect evaluated by three dimensional transoesophageal echocardiography. Heart. 2000;83(6):673–677.
4 Handke M, Schafer DM, Muller G, et al. Dynamic changes of atrial septal defect area: New insights by three-dimensional volume-rendered echocardiography with high temporal resolution. Eur J Echocardiogr. 2001;2(1):46–51.
5 Saric M, Perk G, Purgess JR, Kronzon I. Imaging atrial septal defects by real-time three-dimensional transesophageal echocardiography: Step-by-step approach. J Am Soc Echocardiogr. 2010;23(11):1128–1135.
6 Takahashi K, Mackie AS, Rebeyka IM, et al. Two-dimensional versus transthoracic real-time three-dimensional echocardiography in the evaluation of the mechanisms and sites of atrioventricular valve regurgitation in a congenital heart disease population. J Am Soc Echocardiogr. 2010;23(7):726–734.
7 Bharucha T, Anderson RH, Lim ZS, Vettukattil JJ. Multiplanar review of three-dimensional echocardiography gives new insights into the morphology of Ebstein’s malformation. Cardiol Young. 2010;20(1):49–53.
8 van den Bosch AE, van Dijk VF, McGhie JS, et al. Real-time transthoracic three-dimensional echocardiography provides additional information of left-sided AV valve morphology after AVSD repair. Int J Cardiol. 2006;106(3):360–364.
9 Barrea C, Levasseur S, Roman K, et al. Three-dimensional echocardiography improves the understanding of left atrioventricular valve morphology and function in atrioventricular septal defects undergoing patch augmentation. J Thorac Cardiovasc Surg. 2005;129(4):746–753.
10 Pai RG, Jintapakorn W, Tanimoto M, Shah PM. Role of papillary muscle position and mitral valve structure in systolic anterior motion of the mitral leaflets in hyperdynamic left ventricular function. Am J Cardiol. 1995;76(8):623–628.
11 Salgo IS, Gorman JH, 3rd., Gorman RC, et al. Effect of annular shape on leaflet curvature in reducing mitral leaflet stress. Circulation. 2002;106(6):711–717.
12 Bharucha T, Sivaprakasam MC, Roman KS, Vettukattil JJ. A multiplanar three dimensional echocardiographic study of mitral valvar annular function in children with normal and regurgitant valves. Cardiol Young. 2008;18(4):379–385.
13 Nii M, Roman KS, Macgowan CK, Smallhorn JF. Insight into normal mitral and tricuspid annular dynamics in pediatrics: A real-time three-dimensional echocardiographic study. J Am Soc Echocardiogr. 2005;18(8):805–814.
14 Vettukattil JJ, Bharucha T, Anderson RH. Defining Ebstein’s malformation using three-dimensional echocardiography. Interact Cardiovasc Thorac Surg. 2007;6(6):685–690.
15 Singh A, Romp RL, Nanda NC, et al. Usefulness of live/real time three-dimensional transthoracic echocardiography in the assessment of atrioventricular septal defects. Echocardiography. 2006;23(7):598–608.
16 Hlavacek AM, Crawford FA, Jr., Chessa KS, Shirali GS. Real-time three-dimensional echocardiography is useful in the evaluation of patients with atrioventricular septal defects. Echocardiography. 2006;23(3):225–231.
17 Miller AP, Nanda NC, Aaluri S, et al. Three-dimensional transesophageal echocardiographic demonstration of anatomical defects in AV septal defect patients presenting for reoperation. Echocardiography. 2003;20(1):105–109.
18 Dall’Agata A, Cromme-Dijkhuis AH, Meijboom FJ, et al. Use of three-dimensional echocardiography for analysis of outflow obstruction in congenital heart disease. Am J Cardiol. 1999;83(6):921–925.
19 Sadagopan SN, Veldtman GR, Sivaprakasam MC, et al. Correlations with operative anatomy of real time three-dimensional echocardiographic imaging of congenital aortic valvar stenosis. Cardiol Young. 2006;16(5):490–494.
20 Bharucha T, Ho SY, Vettukattil JJ. Multiplanar review analysis of three-dimensional echocardiographic datasets gives new insights into the morphology of subaortic stenosis. Eur J Echocardiogr. 2008;9(5):614–620.
21 Poh KK, Levine RA, Solis J, et al. Assessing aortic valve area in aortic stenosis by continuity equation: A novel approach using real-time three-dimensional echocardiography. Eur Heart J. 2008;29(20):2526–2535.
22 Binder TM, Rosenhek R, Porenta G, et al. Improved assessment of mitral valve stenosis by volumetric real-time three-dimensional echocardiography. J Am Coll Cardiol. 2000;36(4):1355–1361.
23 Baker G, Flack E, Hlavacek A, et al. Variability and resource utilization of bedside three-dimensional echocardiographic quantitative measurements of left ventricular volume in congenital heart disease. Congenit Heart Dis. 2006;1(6):309–314.
24 Hascoet S, Brierre G, Caudron G, et al. Assessment of left ventricular volumes and function by real time three-dimensional echocardiography in a pediatric population: A TomTec versus QLAB comparison. Echocardiography. 2010;27(10):1263–1273.
25 Soriano BD, Hoch M, Ithuralde A, et al. Matrix-array 3-dimensional echocardiographic assessment of volumes, mass, and ejection fraction in young pediatric patients with a functional single ventricle: A comparison study with cardiac magnetic resonance. Circulation. 2008;117(14):1842–1848.
26 van der Zwaan HB, Helbing WA, Boersma E, et al. Usefulness of real-time three-dimensional echocardiography to identify right ventricular dysfunction in patients with congenital heart disease. Am J Cardiol. 2010;106(6):843–850.
27 van der Zwaan HB, Helbing WA, McGhie JS, et al. Clinical value of real-time three-dimensional echocardiography for right ventricular quantification in congenital heart disease: Validation with cardiac magnetic resonance imaging. J Am Soc Echocardiogr. 2010;23(2):134–140.
28 Mertens LL, Friedberg MK. Imaging the right ventricle-current state of the art. Nat Rev Cardiol. 2010;7(10):551–563.
29 Baker GH, Hlavacek AM, Chessa KS, et al. Left ventricular dysfunction is associated with intraventricular dyssynchrony by 3-dimensional echocardiography in children. J Am Soc Echocardiogr. 2008;21(3):230–233.
30 van den Bosch AE, Ten Harkel DJ, McGhie JS, et al. Surgical validation of real-time transthoracic 3D echocardiographic assessment of atrioventricular septal defects. Int J Cardiol. 2006;112(2):213–218.
31 Suradi H, Byers S, Green-Hess D, et al. Feasibility of using real time “live 3D” echocardiography to visualize the stenotic aortic valve. Echocardiography. 2010;27(8):1011–1020.
32 Rawlins DB, Austin C, Simpson JM. Live three-dimensional paediatric intraoperative epicardial echocardiography as a guide to surgical repair of atrioventricular valves. Cardiol Young. 2006;16(1):34–39.
33 Scheurer M, Bandisode V, Ruff P, et al. Early experience with real-time three-dimensional echocardiographic guidance of right ventricular biopsy in children. Echocardiography. 2006;23(1):45–49.
34 Meyer-Wittkopf M, Cooper S, Vaughan J, Sholler G. Three-dimensional (3D) echocardiographic analysis of congenital heart disease in the fetus: Comparison with cross-sectional (2D) fetal echocardiography. Ultrasound Obstet Gynecol. 2001;17(6):485–492.
35 Cohen L, Mangers K, Grobman WA, et al. Three-dimensional fast acquisition with sonographically based volume computer-aided analysis for imaging of the fetal heart at 18 to 22 weeks’ gestation. J Ultrasound Med. 2010;29(5):751–757.
36 Nelson TR, Pretorius DH, Sklansky M, Hagen-Ansert S. Three-dimensional echocardiographic evaluation of fetal heart anatomy and function: Acquisition, analysis, and display. J Ultrasound Med. 1996;15(1):1–9.