CHAPTER 30 Congenital Anomalies of the Cervical Spine
Basilar Impression
Basilar impression (or invagination) is a deformity of the bones of the base of the skull at the margin of the foramen magnum. The floor of the skull appears to be indented by the upper cervical spine, and the tip of the odontoid is more cephalad, sometimes protruding into the opening of the foramen magnum, and it may encroach on the brainstem. This deformity increases the risk of neurologic damage from injury, circulatory embarrassment, or impairment of cerebrospinal fluid flow. In 1939, Chamberlain1 first called attention to the clinical significance of this anomaly with the following vivid description:
There are two types of basilar impression: (1) primary, a congenital abnormality often associated with other vertebral defects, such as atlanto-occipital fusion, hypoplasia of the atlas, bifid posterior arch of the atlas, odontoid abnormalities, Klippel-Feil syndrome, and Goldenhar syndrome,2 and (2) secondary, a developmental condition usually attributed to softening of the osseous structures at the base of the skull, with the deformity developing later in life. Secondary basilar invagination is a potentially devastating sequelae of osteogenesis imperfecta, Hajdu-Cheney syndrome, and other osteochondrodysplasias.3 Although the precise etiology remains unclear, it has been suggested that the axial load from the cranium and its contents lead to recurrent microfractures in the region of the foramen magnum and precipitate flattening and infolding of the posterior skull base. This theory has been supported by intraoperative findings of thickened, proliferative callus at the skull base in patients with osteogenesis imperfecta.4,5 This thickened callus is also seen in conditions such as osteomalacia, rickets, Paget disease, renal osteodystrophy, rheumatoid arthritis, neurofibromatosis, and ankylosing spondylitis.6
Radiographic Features
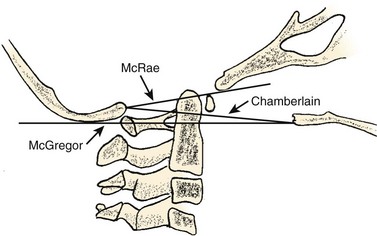
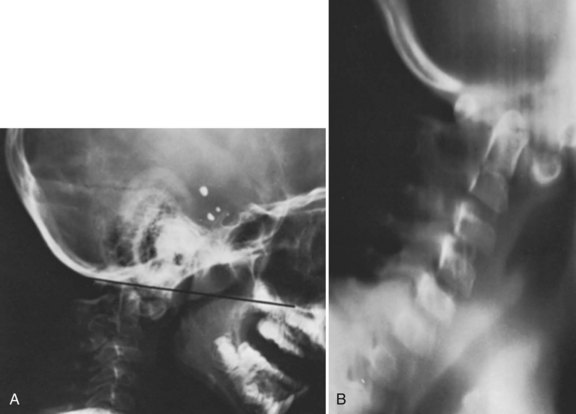
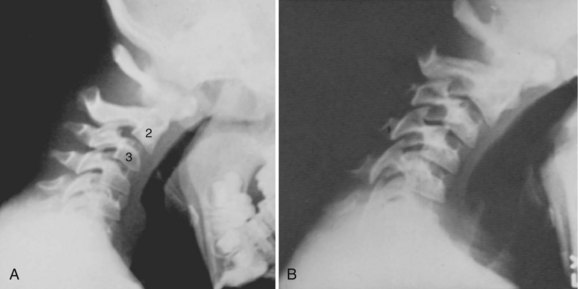
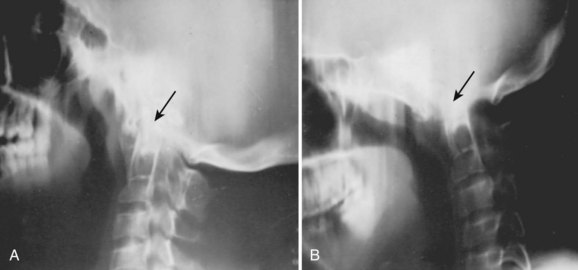
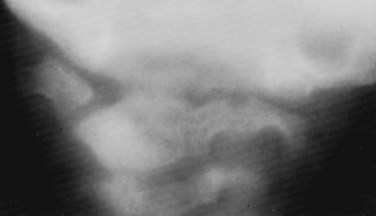
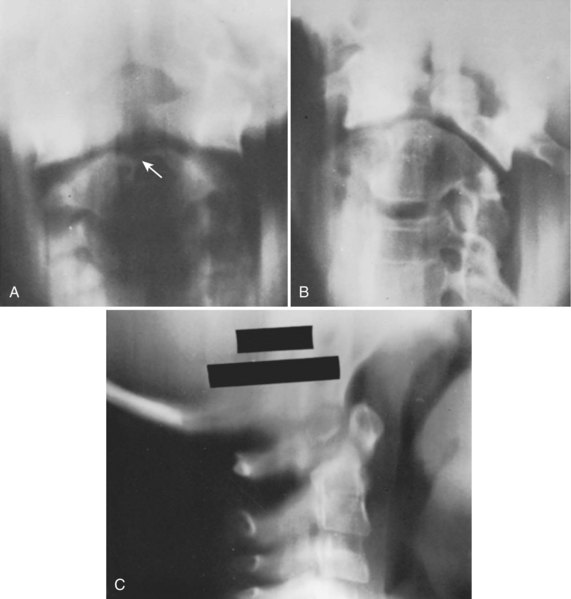
Basilar impression is difficult to assess radiographically. Many measurement schemes have been proposed; those most commonly referred to are the Chamberlain,1 McGregor,7 and McRae lines in the lateral radiograph (Fig. 30–1) and the Fischgold-Metzger line in the anteroposterior projection. The Chamberlain line1 (a line drawn from the dorsal marginal hard palate to the posterior lip of the foramen magnum) is seldom used because the posterior lip of the foramen magnum (opisthion) is difficult to define on a standard radiograph (Fig. 30–2A) and is often itself invaginated in basilar impressions.
The McGregor line (a line drawn from the upper surface of the posterior edge of the hard palate to the most caudal point of the occipital curve of the skull) is easier to identify (see Figs. 30-1 and 30-2) and is preferable. The position of the tip of the odontoid is measured in relation to this baseline, and a distance of 4.5 mm above McGregor line is considered to be on the extreme edge of normality.7 The study of normal variations by Hinck and colleagues8 showed a wide range of normality, however, and difference between males and females.
A criticism of the lateral lines (McGregor and Chamberlain) is that the hard palate is not actually a part of the skull and may be distorted by an abnormal facial configuration or a highly arched palate, independent of a craniovertebral anomaly. In addition, the patient may have an abnormally long or short odontoid or an abnormality of the axis or occipital facets, which can diminish the value of the measurements. Upper cervical spine measurements on plain radiographs have an unacceptable interobserver reliability and intraobserver reproducibility. Although there are multiple factors for the wide variation between observers, many authors have suggested that the basion and opisthion are difficult to localize on radiographs and may be altered significantly by small changes in patient position.9
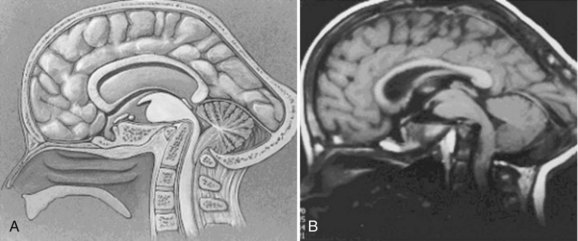
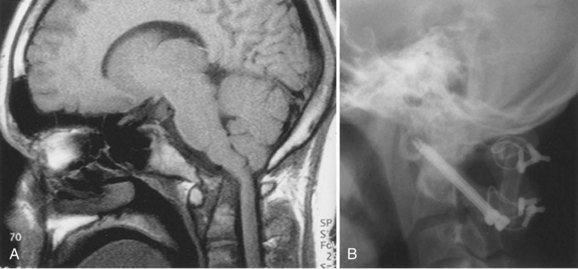
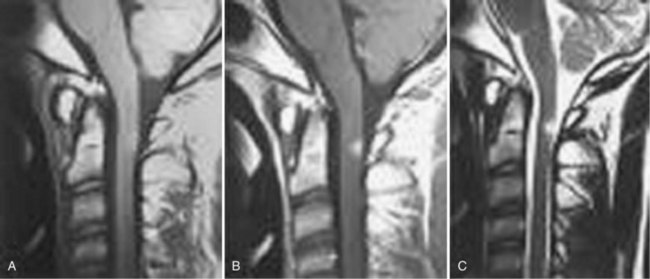
Since the advent of CT and MRI, the diagnosis of basilar invagination can be made directly rather than based on craniometric lines.10 These techniques have largely replaced the older tomography techniques, particularly the anteroposterior measurement of Fischgold and Metzger,10,11 which was previously the most accurate method. The mean and range of normal distances from the odontoid peg to the most frequently used skull baseline employing MRI was 1.2 mm (median 1.5 mm, SD 3 mm) below Chamberlain line; 0.9 mm (median 1.1, SD 3 mm) below McGregor line; and 4.6 mm (median 4.8, SD 2.6) below McRae line.10 MRI also allows direct assessment of the degree of neural compression and identifies the presence of associated pathologic processes, such as Chiari malformation, hydrocephalus, syringomyelia, or cerebellar herniation (Fig. 30–3).4 The lateral reference lines on plain radiographs are still very useful screening tools, however.12,13
Clinical Features
Patients with basilar impression frequently have a deformity of the skull or neck (e.g., a short neck in 78%, asymmetry of the face or skull or torticollis in 68%).14 These physical findings are often noted in patients without basilar impression (Klippel-Feil syndrome, occipitalization) and are not considered pathognomonic.
The symptoms (or lack of them) even in severe basilar impression are difficult to explain.13,14 The more segments involved in a patient with Klippel-Feil syndrome, the more likely it is that the odontoid will be superior.13 Basilar impression is frequently associated with anomalous neurologic conditions, such as Arnold-Chiari malformation14 and syringomyelia,15,16 which can cloud the clinical picture further. Symptoms are generally due to crowding of the neural structures at the level of the foramen magnum, particularly the medulla oblongata. The neurologic sequelae of basilar invagination are determined by the degree of cephalad migration of the clivus-atlas-dens complex. In addition to the direct compression of the ventral brainstem, the dens creates a fulcrum for traction on the cervical cord. Cerebellar dysfunction may arise from direct compression or reflect secondary herniation from the posterior fossa.
There is an unusually high incidence of basilar impression in northeast Brazil,17 and the work of De Barros and colleagues14 has been helpful in delineating the symptoms and signs. Patients who were symptomatic with pure basilar impression had the dominant complaints of motor and sensory disturbances, and 85% had weakness and paresthesia of the limbs. In contrast, patients who were symptomatic with pure Chiari malformation were more likely to have cerebellar and vestibular disturbances (unsteadiness of gait, dizziness, and nystagmus). In both conditions, there may be impingement of the lower cranial nerves as they emerge from the medulla oblongata, particularly the trigeminal (V), glossopharyngeal (IX), vagus (X), and hypoglossal (XII) nerves.14 Headache and pain in the nape of the neck, in the distribution of the greater occipital nerve, are common findings.14,18
Posterior encroachment may cause blockage of the aqueduct of Sylvius, and the presenting symptoms may be from increased intracranial pressure or hydrocephalus.7,14 Compression of the cerebellum with vestibular involvement or herniation of the cerebellar tonsils (Chiari malformation) is a frequent finding,14 leading to vertical or lateral nystagmus in 65% of cases. These symptoms may be caused not by direct pressure from the posterior rim of the foramen magnum but rather by a thickened band of dura invisible on plain radiographs; this has prompted several authors to recommend routine MRI evaluation. If this situation is unrecognized, bony decompression alone (without opening the dura) would be unsuccessful in obtaining remission of symptoms or halting progression of the neurologic injury.14
There is a high incidence of vertebral artery anomalies in basilar impression, atlanto-occipital fusion, and absence of the C1 facet.19 In addition, the vertebral arteries may be compressed as they pass through the crowded foramen magnum, causing symptoms suggestive of vertebral artery insufficiency, such as dizziness, seizures, mental deterioration, and syncope.7,19,20 These symptoms may occur alone or in combination with symptoms of spinal cord compression.7,14,19,21 Michie and Clark22 and Bachs and colleagues23 theorized that one explanation for the frequent association of syringomyelia or syringobulbia and basilar impression is that the vertebral arteries and the anterior spinal artery are compromised in the region of the foramen magnum, with subsequent degeneration of the spinal cord and medulla. Arteriographic studies are unavailable to confirm this thesis. Children with occipitocervical anomalies may be more susceptible to vertebral artery injury and brainstem ischemia, particularly children who undergo skull traction for correction of scoliosis. Moderate amounts of traction (<15 lb) that normally would be well tolerated may compromise these abnormal vessels. Careful radiographic evaluation of the occipitocervical junction should precede any use of skull traction, even if only minimal traction forces are planned.24
Although this condition is congenital, many patients do not develop symptoms until the 2nd or 3rd decade of life.13,14 These delayed symptoms may be due to a gradually increasing instability from ligamentous laxity caused by aging, similar to the delayed myelopathies reported after atlantoaxial dislocations or the increasing instability of C1 and C2 in patients with odontoid agenesis.25 These individuals often develop premature cervical osteoarthritis, as found in the family studies of Gunderson and colleagues.21 Chamberlain1 and others theorized that the young developing brain may be more tolerant of compressive effects, which later prove deleterious to older tissues. Similarly, arteriosclerotic changes in the vertebral arteries may make these vessels more susceptible to minor constrictions. Symptoms frequently occur in older patients in whom a congenital anomaly would not ordinarily be considered. Patients with this malformation have been mistakenly diagnosed as having multiple sclerosis, posterior fossa tumors, amyotrophic lateral sclerosis, or traumatic injury. It is important to survey this area whenever such a diagnosis is considered and whenever this malformation is suspected.
Treatment
Treatment depends on the cause of the symptoms and often requires the teamwork of an orthopaedist, neurosurgeon, neurologist, and radiologist. It is possible to have a severe basilar impression without neurologic symptoms, and a search for associated conditions must be conducted. Hydrocephalus from aqueductal stenosis, if present, must be addressed first with ventricular shunting before any other surgical interventions.4
Treatment of symptomatic basilar invagination is dictated by the severity of ventral neural compression and reducibility of the deformity. An initial attempt should be made to realign the cervical spine and decompress the neural elements by head positioning and, in some cases, halo traction. Plain radiographs and MRI may be used to determine the adequacy of reduction and neural decompression. Patients who respond to such closed reduction methods may be treated with suboccipital craniectomy and upper cervical laminectomy, followed by occipitocervical arthrodesis. More recent reports suggest that many of the physical changes can be reversed by atlantoaxial distraction fixation surgery.26,27 Most authors suggest opening the dura to look for a tight posterior dural band.14 Brainstem compression not relieved by traction demands anterior decompression and resection of the offending anterior atlas, odontoid, or distal clivus. Posterior decompression and fusion are also necessary and may be performed immediately after or as a staged procedure.28
Posterior fossa decompression alone for symptomatic secondary basilar invagination typically provides only transient relief of symptoms, followed by recurrent neurologic deterioration months to years after the index procedure. Studies have shown that secondary basilar invagination in patients with osteochondrodysplasia tends to progress even after occipitocervical fusion with infolding of the skull base around the fusion mass and progressive forward bending.3 Prolonged bracing with an orthotic device and lifelong surveillance may be prudent to halt further recurrence or symptomatic progression.4 These recommendations are generalizations regarding treatment, and appropriate references should be consulted in the evaluation of individual patients.
Atlantoaxial Instability
The clinical significance of a bony anomaly in the region of the atlantoaxial joint is primarily related to its influence on the stability of this articulation. The precipitating factor may be an abnormal odontoid, atlanto-occipital fusion, or laxity of the transverse atlantal ligament, but the end result is narrowing of the spinal canal (encroachment) and impingement on the neural elements.29 It is important not to lose sight of this basic problem, but frequently it becomes obscured in radiographic detail, conflicting reports, and unusual clinical symptoms and signs. It is important to review atlantoaxial instability before a detailed discussion of the individual anomalies that may be contributory.
Pathomechanics
The articulation between C1 and C2 is the most mobile part of the vertebral column and normally has the least stability of any of the vertebral articulations. The normal cervical spine permits about 90 degrees of rotatory motion; 50% of this motion occurs in the atlantoaxial joint. Considerable shifting from side to side (lateral slide) also occurs as a component of this rotatory motion. Flexion and extension are permitted to a limited degree (normally about 10 degrees of extension and 5 degrees of forward flexion); more than 10 degrees of flexion indicates subluxation.30 The odontoid acts as a bony buttress to prevent hyperextension, but the remaining normal range of motion is maintained and is solely dependent on the integrity of the surrounding ligaments and capsular structures.
Motion of the atlantoaxial articulation is usually accentuated in patients with bony anomalies of the occipitocervical junction. An excellent example is a patient with atlanto-occipital fusion, who is frequently found to have compensatory hypermobility of the atlantoaxial joint. If this same patient has an associated synostosis of C2-3, it is reasonable to expect that this additional stress on the atlantoaxial articulation may eventually lead to significant instability.28,29,31,32 This assumption has clinical support in that 60% of patients with symptomatic atlanto-occipital fusion have associated fusion of C2-3.29,32,33
More likely, the degenerative changes of aging cause the lower cervical articulations to become more rigid. This gradual restriction of motion below places an increased demand on the ligaments and capsular structures of the atlantoaxial articulation, with the development of instability.34 With aging, the central nervous system itself becomes less tolerant of intermittent compression, and its ability to recover is diminished. There is evidence to suggest that intermittent compression is more harmful or irritating to the spinal cord than static, constant compression.35 Arteriosclerosis and loss of elasticity from aging affect the vertebral arteries, making them more sensitive to compression at the foramen magnum.36
In patients with basilar impression or atlanto-occipital fusion, the clinical findings suggest that the major damage is occurring anteriorly from the odontoid. The symptoms and signs of pyramidal tract irritation, muscle weakness and wasting, ataxia, spasticity, hyperreflexia, and pathologic reflexes are commonly found.33,37 Autopsy findings consistently show that the brainstem is indented by the abnormal odontoid.37,38 Other, less common complaints include diplegia, tinnitus, earaches, dysphasia, and poor phonation, all of which are due to cranial nerve or bulbar irritation from direct pressure of the odontoid on the medulla.
If the primary area of impingement is posterior from the rim of the foramen magnum, the dural band, or the posterior ring of the atlas (typical of odontoid anomalies), symptoms are referable to the posterior columns with alterations in deep pain and vibratory responses and proprioception. If there is also an associated cerebellar herniation, nystagmus, ataxia, and incoordination may be observed. Symptoms referable to vertebral artery compression—dizziness, seizures, mental deterioration, and syncope—may occur alone or in combination with symptoms of spinal cord compression.34
In children, the presenting symptoms may be quite subtle and nonspecific. Perovic and colleagues39 reported that in most of their patients the only presenting symptom was generalized weakness, manifested as lack of physical endurance, a history of frequent falling, or the child’s asking to be carried. Pyramidal tract signs appeared later, and posterior column signs and sphincter disturbances were less frequently encountered.39 Similarly, in achondroplasia, retardation of motor skills and increasing hydrocephalus may be the only clinical manifestation of impending quadriparesis secondary to basilar stenosis.
Atlantoaxial Instability and Down Syndrome
Spitzer and colleagues40 were the first to describe occipitoatlantal instability and atlantal hypoplasia in Down syndrome. Generalized ligamentous laxity and flat facets predispose to hypermobility and pathologic motion at the craniovertebral and atlantoaxial articulations. Depending on patient age at the time of the study, 10% to 40% of these patients have radiologically detectable atlantoaxial instability.40,41
The natural history of atlantoaxial instability in patients with Down syndrome is not well known. Burke and colleagues42 reported 7 of 32 patients developing atlantoaxial instability over a 13-year period. Morton and colleagues43 found no evidence of de novo atlantoaxial instability in 67 patients with Down syndrome over a 5-year period. In addition, a direct correlation between neurologic deterioration and atlantoaxial instability has not been established. Ferguson and colleagues44 identified 17 of 84 patients with atlantoaxial instability on plain radiographs but could not correlate any difference in neurologic symptoms with radiographic findings. Taggard and colleagues45 did not find such a benign relationship between C1-2 instability and neurologic function, however, reporting acute neurologic deterioration in six patients after a minor fall or intubation for a general anesthetic.
Radiographic Features
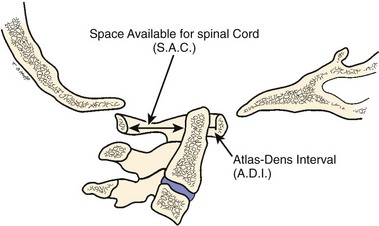
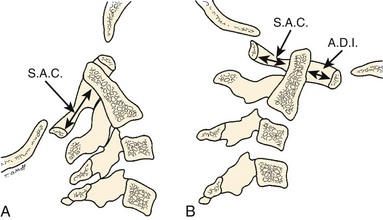
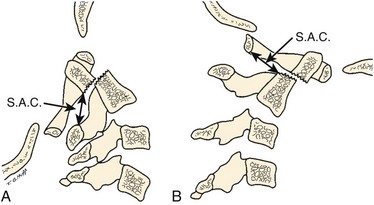
The atlas-dens interval (ADI) is the space seen on the lateral radiograph between the anterior aspect of the dens and the posterior aspect of the anterior ring of the atlas (Fig. 30–4). In children, the ADI should be no greater than 4 mm,30 particularly in flexion, where the greatest distance can be noted. The upper limit of normal in adults is less than 3 mm. Occasionally, the space between the odontoid and the ring of C1 forms a “V” shape in flexion; in this situation, the ADI is measured at the mid-portion of the odontoid. A subtle increase in the ADI in the neutral position may indicate disruption of the transverse atlantal ligament. The ADI is a valuable aid in the evaluation of acute injury, when standard flexion-extension views would be potentially hazardous.30,46 Fielding and colleagues47 noted that the shift of C1-2 does not exceed 3 mm in adults if the transverse ligament is intact. The transverse ligament ruptures within the range of 5 mm.48 Similar data for children are unavailable.
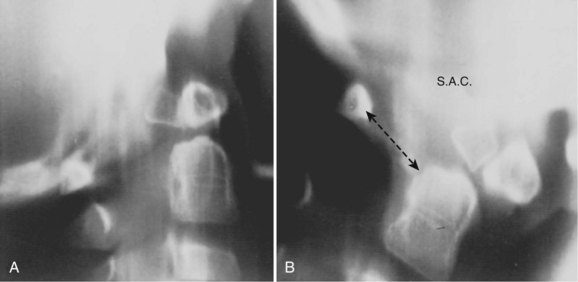
The ADI is of limited value in evaluating chronic atlantoaxial instability owing to congenital anomalies, rheumatoid arthritis, or Down syndrome. In these conditions, the odontoid is frequently found to be hypermobile with a widened ADI, particularly in flexion (Fig. 30–5), but not all are symptomatic, and all do not require surgical stabilization.42,48 In this situation, attention should be directed to the amount of space available for the spinal cord (SAC). Determining the SAC is accomplished by measuring the distance from the posterior aspect of the odontoid or axis to the nearest posterior structure (foramen magnum or posterior ring of the atlas).29,32,49 This measurement is particularly helpful in evaluating a patient with nonunion of the odontoid or os odontoideum because in both conditions the ADI may be normal, yet in flexion or extension there may be considerable reduction in the space available for the spinal cord (Fig. 30–6).50 Lateral flexion-extension views should be conducted voluntarily by the patient, particularly a patient with a neurologic deficit (Fig. 30–7). Most symptomatic patients exhibit significant instability.
Patients with a normal odontoid process and an attenuated or ruptured transverse atlantal ligament are particularly at risk; with anterior shift of the atlas over the axis, the spinal cord is easily damaged by direct impingement against the intact odontoid process,50,51 such as in atlanto-occipital fusion. The situation is less dangerous if the odontoid process is absent or fractured and is carried forward with the atlas (os odontoideum).47 In a large series of patients with os odontoideum reported by Fielding and colleagues,47 the average displacement was 1 cm, mostly either anterior or posterior, but some were unstable in all directions.
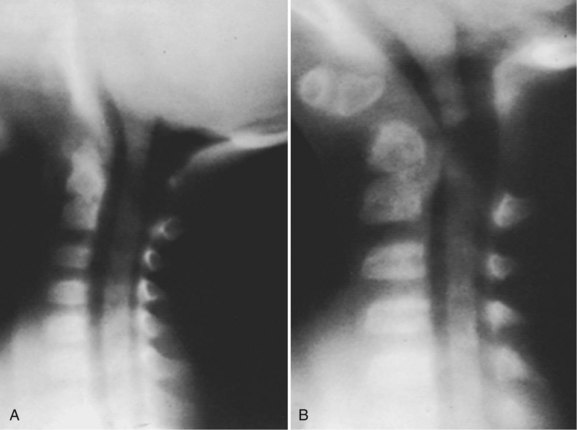
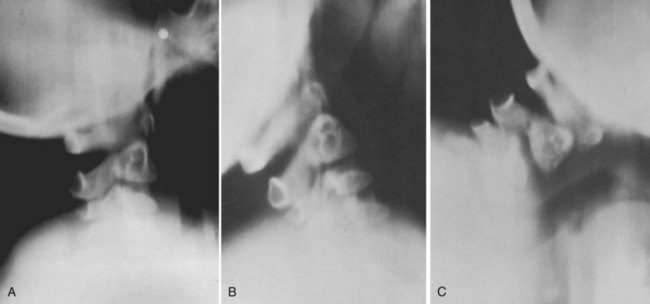
In patients with multiple anomalies, the usual radiographic views are not always reliable in confirming the presence or absence of an odontoid.39 Similarly, in patients with abnormal bone, such as patients with Morquio syndrome or spondyloepiphyseal dysplasia, the odontoid may be present but dysplastic, blending with the surrounding abnormal bone, and cannot be differentiated. In these situations, adequate visualization can be obtained by using lateral laminagraphic techniques (see Fig. 30–2) or with a CT scan with reconstruction views.52,53 When the exact cut at the level of the odontoid is determined, it is repeated in flexion-extension to ascertain the stability of the atlantoaxial articulation. Extension views should not be ignored. Many patients have been found to have significant posterior subluxation.29,47,54 Dynamic (flexion-extension) CT scans have been used to show this area, particularly with lateral reconstruction, and have replaced the laminagraphs.51–53
Dynamic MRI provides direct vision of the neurologic structures and in many cases the site of bony impingement and cord compression that may not be appreciated on routine views.52,55–57 CT scans have been extremely helpful in evaluating anomalies of the upper cervical spine. Three-dimensional reconstruction aids in visualizing anatomic relationships. Dynamic CT or MRI flexion and extension views are difficult to perform and time-consuming, but they can provide graphic evidence of looseness.58 MRI similarly has been helpful in showing the relationship of the neurologic structures to the bony anatomy, such as the Chiari malformation and syringomyelia.55,58 Also, the transverse atlantal ligament can be visualized with MRI.55
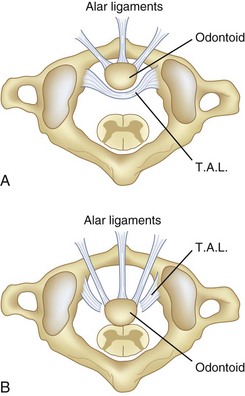
Steel59 called attention to the checkrein effect of the alar ligaments and how they form the second line of defense after disruption of the transverse atlantal ligament (Fig. 30–8). This secondary stability no doubt plays an important role in patients with chronic atlantoaxial instability.60 Steel’s anatomic studies provided a simple rule that is helpful to physicians evaluating this area. He defined the “rule of thirds”: The area of the vertebral canal at C1 can be divided into one third odontoid; one third spinal cord; and one third “space,” which represents a safe zone in which displacement can occur without neurologic impingement and is roughly equivalent to the transverse diameter of the odontoid (usually 1 cm).59
In chronic atlantoaxial instability, it is of prime importance to recognize when the patient has exceeded the “safe zone” of Steel and enters the area of impending spinal cord compression. At this point, the second line of defense, the alar ligaments, has failed, and there is no longer a margin of safety (see Fig. 30–8).48 Experimentally, Fielding and colleagues48 found that after rupture of the transverse ligament, the alar ligaments are usually inadequate to prevent further displacement of C1-2 when a force similar to that which ruptured the transverse ligament is applied. Although the alar ligaments appear thick and strong, they stretch with relative ease and permit significant displacement.
McRae33 was first to call attention to the relationship of neurologic symptoms and the sagittal diameter of the spinal canal (SAC) (see Fig. 30–4). He noted that his patients with atlanto-occipital fusion with less than 10 mm of available space behind the odontoid or atlas were always symptomatic. With the availability of more clinical data, this measurement has been more specifically defined. After an extensive review of the literature, Greenberg34 determined that in adults spinal cord compression always occurs if the sagittal diameter of the cervical canal behind the dens is 14 mm or less. Cord compression is possible between 15 mm and 17 mm and never occurs if the distance is 18 mm or more. Variations in patient size and the presence or absence of soft tissue elements affect the clinical significance of the measurement, however. Jauregui and colleagues56 refined these measurements further for children. MRI may be helpful in assessing the integrity of the transverse atlantal ligament.55 In some patients, myelography has shown a ventral impingement secondary to a thick wad of radiolucent soft tissue posterior to the dysplastic odontoid and the body of the axis.39
Data on normal variations in sagittal and transverse diameter of the cervical spine have been collected for infants, children, and young adults.46,61,62 As might be expected, these measurements generally are smaller than in adults and follow a predictable growth curve. Clinical significance is determined via two parameters: (1) comparison of the absolute diameter with the known norms for that vertebral level and age, and (2) comparison of successive vertebral levels within the same individual. Variations in the latter are more sensitive in determining an abnormality involving a single vertebra because there is good correlation between adjacent levels in the same child. These measurements should be readily available when evaluating the growing spine for pathologic narrowing and expansion.63 The transparencies developed by Haworth and Keillor61 are particularly helpful in this regard and provide an efficient screening test for assessing the transverse diameter of the spinal canal in the growing child. The sagittal diameter of the cervical canal is largest at C1 and gradually narrows in size until C5-7. The appearance is similar to a funnel, and enlargement in the outline suggests an intraspinal mass, even if the absolute measurements do not exceed the upper limits of normal.63
In children, there are several normal variations in cervical spine mobility that can be alarming to physicians who are unaware of them. Cattell and Filtzer64 called attention to the frequent (20%) finding of overriding of the anterior arch of the atlas on the odontoid with extension of the neck. This variation is due to normal elasticity of ligaments and diminishes with growth; it is not present after age 7 years. Pseudosubluxation of 3 mm of C2 on C3 can be found in more than one half of children younger than age 8 years (Fig. 30–9).64–66 Swischuk66 suggested a posterior cervical line that is useful in differentiating physiologic from pathologic anterior displacement of C2 on C3. Less frequently, hypermobility occurs at the C3-4 interspace. Recognition becomes particularly important when evaluating a young child who has Klippel-Feil syndrome or has undergone recent trauma.
The normal pattern of ossification of the cervical spine in children can pose problems in radiographic interpretation. At birth, the odontoid is separated from the body of the axis by a wide cartilaginous band, which represents the vestigial disc space, referred to as the neurocentral synchondrosis (see section on anomalies of odontoid). On the lateral radiograph, this lucent line is similar in appearance to an epiphyseal growth plate. This line may be confused with the jointlike articulation between the odontoid and the body of the atlas found in os odontoideum; this is present in nearly all children at age 3 and absent in most by age 6. Consequently, the diagnosis of os odontoideum in children must be confirmed by showing motion between the odontoid and the body of the axis. Atlantoaxial fusion may be difficult to diagnose in a young child because a significant portion of the ring of C1 is unossified at birth. There is usually a 5- to 9-mm gap posteriorly, which ossifies by age 4.31 The anterior arch of the atlas is invisible in 80% of infants, and the entire ring may not be completely ossified until age 10 years.31,53
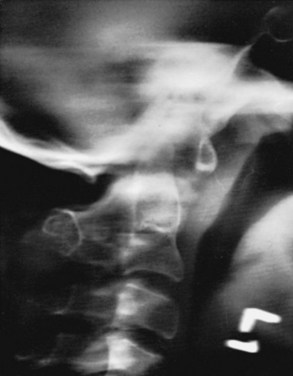
Myelography can be helpful in defining an area of constriction. In this regard, gas or water-soluble contrast agent (metrizamide) myelography should be used in preference to myelography with oil contrast media (Fig. 30–10).33,39,67,68 CT in conjunction with metrizamide myelography is particularly helpful in evaluating children with rotational deformities or compromise of the neural canal in the upper cervical spine.51,67,68
MRI allows a more complete and accurate examination of the soft tissues and brainstem and assists in identifying the area of direct impingement in individuals with occipitocervical anomalies.57 Dynamic flexion-extension MRI allows for improved selection of patients for cervical arthrodesis and allows direct determination of the need for concurrent anterior or posterior decompression of neural elements.52,58 Surgical stabilization may be avoided or delayed in individuals with mild radiographic instability without spinal cord compromise.
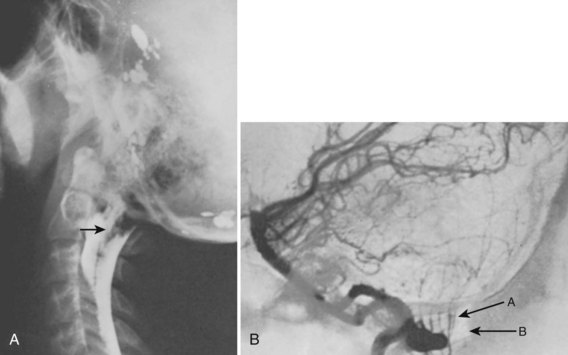
Excessive motion at the occiput-C1 may lead to compression of the vertebral arteries.53 Vertebral arteriography is helpful in evaluating patients who exhibit symptoms of transient brainstem ischemia.24,69,70 If cerebellar herniation (Chiari malformation) is suspected, arteriography combined with myelography can show the anomaly (Fig. 30–11).68
Treatment
Effective treatment generally can be provided only if the exact cause of symptoms has been determined by a careful correlation of the clinical and radiologic findings. Before surgical intervention, reduction of the atlantoaxial articulation should be achieved by either positioning or traction.28,60,65,71 Operative reduction should be avoided, if possible, because it is associated with increased morbidity and mortality rates.72,73 The patient should be maintained in the reduced position preoperatively until spinal cord edema and local irritation resolve; this is usually accompanied by improvement of the neurologic status and often remission of symptoms. If neurologic symptoms are unchanged with reduction and rest, the physician should carefully seek other causes.
Various techniques for surgical stabilization of atlantoaxial instability have been used in pediatric patients with good results.60,74 Sublaminar wiring, C1-2 transarticular screws, transoral decompression with posterior plating, and laminectomy with Steinmann pin occipitocervical fusion all have been reported with good results (Fig. 30–12).28,45 Choice of surgical procedure for stabilization must be individualized based on clinical presentation and surgeon expertise.28,71
Too little attention has been paid in the past to occult respiratory dysfunction in patients with atlantoaxial instability.74,75 Many patients have an unrecognized decrease in vital capacity and chronic alveolar hypoventilation as a result of the neurologic injury to the brainstem.18,76 Similarly, gag and cough reflexes are often depressed,74 and the patient may experience a deterioration of pulmonary function in the postoperative period.76 Periods of apnea and respiratory distress during surgery or in the immediate postoperative period have frequently resulted in death or have required prolonged respiratory support.76 Preoperative pulmonary evaluation can be helpful in limiting the severity of respiratory complications. If pulmonary function is significantly reduced or if gag and cough reflexes are depressed, consideration should be given to preoperative tracheostomy.74 Equipment for mechanical respiratory support must be immediately available during the postoperative period.76
Laxity of Transverse Atlantal Ligament
Laxity of the transverse atlantal ligament is a diagnosis of exclusion suggested by the clinical occurrence of chronic atlantoaxial dislocation without a predisposing cause.33 There is no history of trauma, congenital anomaly, infection, or rheumatoid arthritis to account for the radiologic finding. Most patients with this condition have the typical symptoms of atlantoaxial instability and require surgical stabilization.
Laxity of the transverse atlantal ligament is unusually common in patients with Down syndrome, with a reported incidence of 15% (Fig. 30–13).42,77 The lesion may be found in all age groups, with no age preponderance.42 These patients seem to have rupture or attenuation of the transverse atlantal ligament with encroachment of the safe zone of Steel (see Fig. 30–8), but at least initially they are protected by the checkrein action of the alar ligaments from spinal cord compression (see Fig. 30–13). In other words, many patients have excessive motion, but relatively few are symptomatic, and most cases are discovered only by radiologic survey.78 If radiographs of the upper cervical spine indicate an ADI of more than 5 mm, instability is considered to be present. Usually, if symptoms are present, instability of greater than 7 to 10 mm is found. MRI can be helpful in assessing the integrity of the transverse atlantal ligament.55
Complete data are unavailable regarding the best way to manage this problem.42,77,78 Very little is known about the natural history, despite radiographic examination of hundreds of children.78 Studies have indicated that some children experience progressive looseness with time; others who manifest small degrees of instability can occasionally become stable.42 Currently, radiographic examination on a routine basis is recommended for children with Down syndrome, particularly children who compete in athletics.77 Any child with Down syndrome who has a musculoskeletal complaint, such as subluxing patella, dislocating hips, or unsteady gait, should be investigated. In addition, evaluation of the cervical spine should be a part of the preoperative assessment in these cases.
A patient with Down syndrome and no evidence of radiographic instability requires no activity restrictions other than avoidance of collision sports.42–44 With current knowledge, prophylactic stabilization is not indicated. When a patient develops atlantoaxial instability and is neurologically intact, any sport with the potential to stress the cervical spine should be avoided. The Special Olympics has identified gymnastics, high jump, diving, butterfly and breast stroke swimming, and pentathlon to be at-risk activities.42 The use of a cervical collar in these patients is controversial. Progressive instability, neurologic symptoms, or both are indications for surgical stabilization.34,42
Occipitoatlantal Instability
Occipitoatlantal instability is a rare condition often caused by trauma or, less commonly, congenital abnormalities.79,80 Previously, most patients did not survive this injury, but with improved resuscitative measures, the problem has been reported more frequently. Clinical and neurologic manifestations include cardiorespiratory arrest, motor weakness, quadriplegia, torticollis, pain in the neck, vertigo, and projectile vomiting.79 Surgical stabilization is usually necessary. Anomalies of the upper cervical spine can also lead to this condition, and it has been reported in association with Down syndrome. The curvature of the occipital condyle in healthy children increases by 60% from infancy to adolescence.80 Patients who have Down syndrome and occipitoatlantal instability fail to develop this curved architecture in the occipital condyle.80
Atlanto-occipital Fusion (Occipitalization, Occipitocervical Synostosis, Assimilation of Atlas)
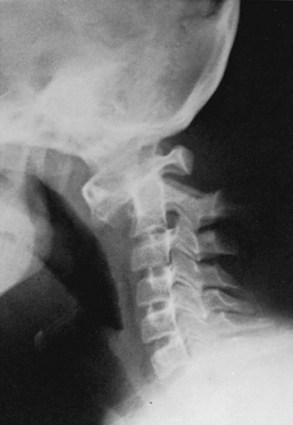
Atlanto-occipital fusion is characterized by a partial or complete congenital union between the atlas and the base of the occiput (Fig. 30–14). It ranges from total incorporation of the atlas into the occipital bone to a bony or fibrous band uniting one small area of the atlas to the occiput.81 Basilar impression is commonly associated with occipitocervical synostosis; other associated anomalies include Klippel-Feil syndrome, occipital vertebrae, and condylar hypoplasia.31,32
Occipitocervical synostosis, basilar impression, and odontoid anomalies are the most common developmental malformations of the occipitocervical junction. Incidence ranges from 1.4 to 2.5 per 1000 children.31,82 Gholve and colleagues32 found a predominance in boys of 80%.
Clinical Features
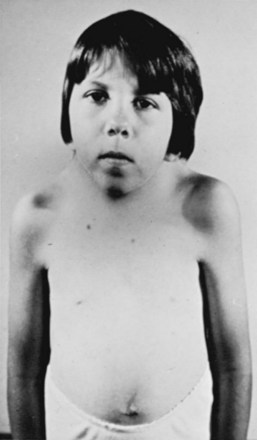
Most patients have an appearance similar to that in Klippel-Feil syndrome, with a short, broad neck; a low hairline; torticollis; a high scapula; and restricted neck movements.35,37,83 The skull may be deformed and shaped like a “tower.” Kyphosis and scoliosis are frequent. Other associated anomalies occasionally seen include dwarfism, funnel chest, pes cavus, syndactylies, jaw anomalies, cleft palate, congenital ear deformities, hypospadias, and sometimes genitourinary tract defects.
Neurologic symptoms do not usually occur until the 3rd or 4th decade but can manifest during childhood.32,84 Symptoms progress in a slow, unrelenting manner and may be initiated by traumatic or inflammatory processes. Symptoms rarely begin dramatically, but they have been reported as a cause of sudden death.85 It is difficult to explain why neurologic problems develop so late and progress so slowly in these patients. It may be that the frequently associated atlantoaxial instability progresses with age and the resultant added demands placed on this articulation, producing gradual spinal cord or vertebral artery compromise.
McRae and Barnum83 suggested that the key to development of neurologic manifestations lies with the odontoid and its position, an indication of the degree of actual or relative basilar impression. If the odontoid lies below the foramen magnum, the patient is usually asymptomatic.33 With the decrease in vertical height of the atlas, the odontoid may project well into the foramen magnum, producing brainstem pressure, a fact well documented by autopsy.37,38
Anterior compression of the brainstem from the backward-projecting odontoid is most common (see Fig. 30–14). This compression produces various findings, depending on the location and the degree of pressure. Pyramidal tract signs and symptoms (spasticity, hyperreflexia, muscle weakness and wasting, and gait disturbances) are most common, but cranial nerve involvement (diplopia, tinnitus, dysphagia, and auditory disturbances) may be seen less often. Compression from the posterior lip of the foramen magnum or the constricting band of dura may disturb the posterior columns, resulting in loss of proprioception, vibration, and tactile discrimination. Nystagmus, a common occurrence, is probably due to posterior cerebellar compression.
Vascular disturbances from vertebral artery involvement may occasionally result in syncope, seizures, vertigo, and unsteady gait, among other signs and symptoms of brainstem ischemia.15,53 Disturbed mechanics of the cervical spine may result in a dull aching pain in the posterior occiput and neck with episodic neck stiffness and torticollis.15 Tenderness noted in the area of the posterior scalp may be due to irritation of the greater occipital nerve. The following most common signs and symptoms occur, in decreasing order of frequency: pain in the occiput and neck, vertigo, unsteady gait, paresis of the limbs, paresthesias, speech disturbances, hoarseness, double vision, syncope, auditory noise or disturbance, and interference with swallowing. Acute respiratory failure secondary to hypoventilation and sleep apnea may occur.15 All these may be manifestations of underlying atlantoaxial instability, which, as an isolated lesion, may produce neck pain, headaches, and neurologic deficits from cord or root irritation and, rarely, sudden death.34,73
Radiographic Features
Standard radiographs of this area can be difficult to interpret. Tomography, CT, and MRI may be necessary to clarify the pathologic condition (see Fig. 30–14).15,52,53,84,85 Most commonly, the anterior arch of the atlas is assimilated into the occiput, usually in association with a hypoplastic posterior arch. The condition may range from total incorporation of the atlas into the occipital bone to a bony or fibrous band uniting one small area of the atlas to the occiput. CT scans are often necessary to show bony continuity of the anterior arch of the atlas with the occiput. Posterior fusion is not usually evident because this portion of the ring may be represented only by a short bony fringe on the edge of the foramen magnum. Despite its innocuous radiologic appearance, this fringe is frequently directed downward and inward, can compromise the spinal canal posteriorly, and has been found to create a groove in the spinal cord. It is usually assumed that the assimilated atlas is fused symmetrically to the occipital opening, but several autopsy specimens have shown a posterior positioning of the atlas.83 This position, in effect, pushes the odontoid posteriorly, narrowing the spinal canal and the space available for the spinal cord.
Ossification of the atlas proceeds from paired centers—one for each of the lateral masses. These progress posteriorly into the neural arches, which are fully ossified at birth except for a gap of 5 to 9 mm posteriorly that closes by the 4th year.53 The anterior arch of the atlas is invisible in 80% of neonates. This area most commonly ossifies from a single center, which appears during the 1st year of life and fuses to the remainder of the atlas by the 3rd year.31,34,53,86,87 A persistent bifid anterior and posterior arch of the atlas beyond age 4 years is observed in skeletal dysplasias, Goldenhar syndrome, Down syndrome, Conradi syndrome, and atlas assimilation.88 In an extensive review of CT scans from 1104 patients, Senoglu and colleagues89 found a 3.35% incidence. None had neurologic deficits or needed intervention. Rarely, hyperplasia of the posterior arch occurs and has been associated with myelopathy.90
There is varying loss of height of the atlas, allowing the odontoid to project upward into the foramen magnum and creating a “relative” basilar impression (see Fig. 30–14). The position of the odontoid relative to the foramen magnum was described earlier (see section on basilar impression). McRae38,83 measured the distance from the posterior aspect of the odontoid to either the posterior arch of the atlas or the posterior lip of the foramen magnum (SAC), which was closer. He stated that a neurologic deficit would be present if this distance was less than 19 mm. This distance should be determined in flexion because this position most dramatically reduces the space available for the cord.
Flexion-extension stress films (see Fig. 30–14) often show posterior displacement of the odontoid from the anterior arch of the atlas of 12 mm.83 Associated atlantoaxial instability has been reported to develop eventually in 50% of patients29,31; this is determined by measuring the distance from the anterior border of the odontoid to the posterior aspect of the anterior arch of the atlas. A distance greater than 4 mm in young children who probably have considerable cartilage present and 3 mm in older children and adults is considered pathologic.86,87,91 The odontoid itself often has an abnormal shape and direction; it frequently is longer, and its angle with the body of the axis is directed more posteriorly.73,83
McRae33 was the first to note the frequent occurrence of congenital fusion of C2-3 (70%) and C1-2 instability in patients with atlanto-occipital fusion (Fig. 30–15). This occurrence suggests that greater demands are placed on the atlantoaxial articulation, particularly in flexion and extension when the joints above and below are fused.37,83 von Torklus and Gehle31 noted that approximately 50% of patients develop late onset of atlantoaxial instability and the resultant potential for compromise of the spinal cord.29
Gholve and colleagues32 used a morphologic classification: zone 1, a fused anterior arch; zone 2, fused lateral masses; zone 3, a fused posterior arch; and a combination of fused zones. In their study, 57% had atlantoaxial instability, with almost half of those having an associated C2-3 fusion. Spinal canal encroachment occurred in 37%, with almost half of these patients having clinical findings of myelopathy. The highest prevalence of spinal canal encroachment (63%) was noted in patients with occipitalization in zone 2.
Another commonly associated abnormality is the presence of a constricting band of dura posteriorly. This band has been found to create a groove in the spinal cord and may be the primary cause of symptoms. The band cannot be visualized on routine radiographs, and it does not correlate with the presence or absence of the posterior bony fringe of the atlas. Consequently, CT and MRI should be an integral part of the evaluation. Water-soluble contrast material (metrizamide) alone or in conjunction with CT can visualize the spinal canal and its contents. A properly performed study yields valuable information regarding the presence of a dural band; tonsillar herniation; and the size, shape, and position of the spinal cord in the spinal canal.35,92
Treatment
Management of this uncommon problem may be hazardous. In contrast to anomalies of the odontoid, surgical intervention carries a much higher risk of morbidity and mortality.37,73,93 Nonoperative methods such as cervical collars, braces, plaster, and traction should be attempted initially in some patients. These methods are often helpful in patients with persistent complaints of head and neck pain and are particularly helpful if symptoms follow minor trauma or infection. If neurologic deficits are present, immobilization may achieve only temporary relief. Patients presenting with evidence of a compromised situation in the upper cervical area must take precautions not to expose themselves to undue trauma.
With anterior spinal cord signs and symptoms owing to an unstable atlantoaxial complex, an occiput-to-C2 fusion is suggested, with preliminary traction to attempt reduction, if necessary. If reduction is possible and there are no neurologic signs, surgical intervention carries an improved prognosis.34,37,73 Operative reduction should be avoided because this has frequently resulted in death.72,73
Transoral resection of the odontoid should be considered if there is irreducible ventral compression.28,94 Resection of the odontoid through an anterior approach may destabilize the upper cervical spine. In a large study, Dickman and colleagues95 noted that 11 of 19 patients (40%) developed craniovertebral junction instability after transoral removal of the odontoid, 8 of whom did not have clinical or radiographic evidence of instability preoperatively. Instability was more common if there was a congenital bony malformation or if the patient required posterior decompression for an associated condition, such as Chiari malformation or syrinx.95
Posterior signs and symptoms and myelographic evidence of bony or dural compression, depending on the degree of neurologic involvement, may be indications for a posterior decompression and stabilization of the occiput to C2.71,96–99 Satisfactory occipital cervical stabilization and fusion can be obtained by using a halo brace in the postoperative period.98 Internal fixation can facilitate postoperative management for certain patients, however. Internal fixation of the occipital cervical junction can pose problems, and several fixation techniques have been found helpful.27,28,71 The addition of a cervical laminectomy may increase C1-2 instability or lead to the late development of a cervical kyphosis, particularly if the laminectomy involves several levels.96,100 In these cases, the patient should be managed expectantly with appropriate internal stabilization or a halo vest.82,96
Anomalies of Ring of C1
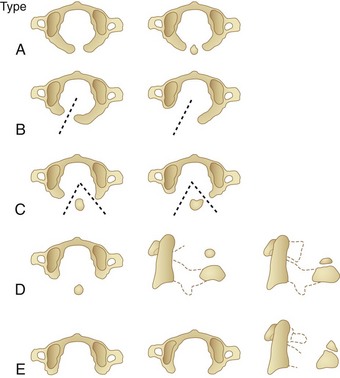
Anomalies of the posterior arch can be characterized as median clefts or hypoplasia. Geipel101 found defects in the posterior arch, most of which were median clefts, in 4% of 1613 autopsies. Currarino and colleagues102 developed a classification scheme (types A to E) for congenital defects of the posterior arch of C1 (Fig. 30–16). Greater than 90% are the type A median cleft variant. These patients are usually asymptomatic, and their anomaly is incidentally identified on radiographic studies.89 Types C and D are accompanied by a free-floating posterior tubercle. These patients may experience neck pain and neurologic symptoms before discovery of the anomaly. The free posterior tubercle has been shown to move with neck extension and in some cases may migrate anteriorly and traumatize the spinal cord with neck extension. MRI studies have shown signal abnormality of the posterior spinal cord at the C1-2 level consistent with this mechanism of injury (Fig. 30–17).102
Dubousset103 called attention to a previously unrecognized problem with the ring of C1, the hemiatlas. Although there had been an occasional case report, Dubousset103 presented the first large study to review the problem in depth. He reported 17 patients in whom he was able to document absence of the facet of C1, which led to a severe and progressive torticollis in young children (Fig. 30–18). Initially, the deformity is flexible and can be passively corrected. As the child ages, the torticollis becomes more severe and eventually fixed. Radiographic diagnosis using tomography or CT has been helpful in identifying this deformity. Use of traction to align the head and neck at the time of the study assists in highlighting the problem. If the patient is passively correctable, a single posterior fusion, occiput to C2, is performed. A halo cast is applied postoperatively to maintain the head in a satisfactory alignment until the fusion is complete.
Although the time at which this becomes a fixed deformity was not defined, Dubousset103 reported good results in teenagers. This deformity can accompany Klippel-Feil syndrome with anomalies of the lower cervical spine as well. Dubousset103 found an increased incidence of anomalies of the vertebral vessels in these children and suggested arteriographic evaluation before the use of traction or surgical intervention, which could further compromise a precarious blood supply to the midbrain and spinal cord.
Anomalies of Odontoid (Dens)
Congenital anomalies of the odontoid can lead to an unstable atlantoaxial complex, with potential neurologic sequelae and death owing to spinal cord pressure.50 Several gradations or variations of anomalies of the odontoid exist, ranging from aplasia (complete absence) to hypoplasia (partial absence) to os odontoideum (Fig. 30–19).
Aplasia or agenesis of the odontoid is a complete absence of development. Hypoplasia is a partially developed odontoid, ranging in size from a short, stubby, peglike projection to an odontoid of almost normal size.104 Os odontoideum is an anomaly in which the odontoid process is divided by a wide transverse gap, leaving the apical segment without support from the base.105 Distinguishing aplasia or hypoplasia from os odontoideum is of limited importance because these anomalies usually lead to atlantoaxial instability and the clinical signs, symptoms, and treatment are identical. The only distinctive features are radiographic.
Incidence
In the authors’ experience, aplasia is extremely rare. Many previous reports have confused aplasia for hypoplasia, and, as previously emphasized, aplasia probably has been a misnomer because it almost never describes an associated absence of the portion below the articular facets that contributes to the body of the axis. Hypoplasia and os odontoideum are infrequently reported and can be considered rare.54,104,106–109 With more recent awareness, these lesions are being recognized more commonly, however, than previous literature might indicate—especially os odontoideum.47 In a large series reported by Wollin,109 the average age of diagnosis was 30 years, but in a later series it was 18.9 years, suggesting earlier recognition.47 An increasing number of children with these anomalies are being discovered.
In conditions such as Down syndrome, Morquio syndrome, Klippel-Feil syndrome, and some skeletal dysplasias, odontoid anomalies in association with ligamentous laxity producing atlantoaxial instability are much more common than in the general population.40,42,50,110–115 Associated regional malformations may occur, but in contrast to many congenital cervical anomalies, they are rare.50,104,108,116
Development of Odontoid
The body of the odontoid is derived from the mesenchyme of the first cervical sclerotome and is actually the centrum of the first cervical vertebra, which becomes separated from the atlas during development to fuse with the remainder of the axis.82,114,117 The apex of the odontoid process is derived from the mesenchyme of the most caudal occipital sclerotome or proatlas. Ossification of these two segments of the odontoid proceeds along separate lines.
Between the 1st and 5th prenatal months, the dens begins to ossify from two centers, one on each side of the midline. By the time of birth, they have fused into a single mass.86,87,91 Occasionally, the right and left halves of the odontoid are not fused at birth, and a longitudinal midline cleft may be seen. At birth, the tip of the odontoid has not ossified, is V-shaped, and is known as a dens bicornis (Fig. 30–20). A separate ossification center within the “V,” known as a summit ossification center or ossiculum terminale, usually appears at age 3 years and fuses with the remainder of the dens by age 12.86,87,118 Cattell and Filtzer64 found an ossiculum terminale in 26% of 70 normal children 5 to 11 years old.
An ossiculum terminale may never appear or may occasionally fail to fuse with the dens; it is then called an ossiculum terminale persistens. It is occasionally discernible as either a cyst or an area of increased density. These developmental anomalies are of little clinical significance.50,119 Sherk and Nicholson114 reported a rare case of quadriplegia and death, however, in a child with Down syndrome that was directly attributable to atlantoaxial instability secondary to an ossiculum terminale. A similar occurrence in an otherwise normal child has been reported.120 The ossiculum terminale usually is firmly bound to the main body of the dens by cartilage and consequently is seldom the source of instability.
At birth, the dens is separated from the body of the axis by a cartilaginous band (see Fig. 30–20) that represents the epiphyseal growth plate. This plate does not run across the base of the dens at the level of the superior articular facets of the axis but lies well below this level within the body of the axis (see Fig. 30–20B). The part of the odontoid below the articular facets contributes to the body of the axis. On the open-mouth view, the odontoid fits like a “cork in a bottle,” lying sandwiched between the neural arches (see Fig. 30–20B). This epiphyseal line is present in almost all children by age 3 years and in 50% of children by age 4, but it is absent in most by age 6.64,91 It rarely persists into adolescence and adult life. If present, the line is not seen at the base of the dens where a fracture would be anticipated but lies well below the level of the superior articular facets within the body of the axis. In a young child, the unossified portions of the odontoid may give the false impression of odontoid hypoplasia. Similarly, one may erroneously conclude that the child has C1-2 instability because the anterior arch of the atlas commonly may slide upward and may protrude beyond the ossified portion of the odontoid64 on the lateral extension radiograph (Fig. 30–21).
Etiology and Pathogenesis
A congenital etiology for os odontoideum has been assumed, and two theories have been advanced:
There is more often an associated short, stubby projection or hypoplastic odontoid remnant in the area of the odontoid base.105,109 Hypoplasia and os odontoideum can be acquired secondary to trauma or, rarely, infection.33,47,109,118,121–123 Several cases of “os odontoideum” that developed several years after trauma when a normal odontoid was initially present have been reported (see Fig. 30–21).41,122–124 Most patients have a significant episode of trauma before the diagnosis of os odontoideum.47
Fielding and colleagues47 suggested that most evidence favors an unrecognized fracture in the region of the base of the odontoid as the most common cause and less often a congenital origin. They postulated that after fracture of the odontoid there may be only slight separation of the fragments, but, with time, contracture of the alar ligaments, which attach to the tip of the odontoid, exerts a distraction force that pulls the fragment away from the base and closer to their origin at the occiput (Fig. 30–22). The blood supply to the odontoid is precarious because it passes up along the sides of the odontoid, is easily traumatized, and contributes to poor fracture healing or callus formation that may retard retraction of the fragment. The position of the odontoid adjacent to the ring of C1 is maintained by the intact transverse atlantal ligament.
The blood supply to the fragment is maintained by the proximal arterial arcade, from the carotid to the alar ligaments, and may be sufficient to maintain only a portion of the odontoid. Similarly, the blood supply to the proximal portion of the odontoid can be interrupted by excessive traction on these ligaments.47 A familial form125 and os odontoideum in identical twins with no history of trauma have been reported.126,127 It is probable that congenital and post-traumatic forms of hypoplasia and os odontoideum exist.115 Failure of fusion of the apex of the odontoid (derived from the proatlas) to the main body of the atlas (derived from the first cervical sclerotome) is thought to result in the congenital form of os odontoideum.
The free ossicle of the os odontoideum usually appears fixed to the anterior arch of the atlas and moves with it in flexion and extension (see Fig. 30–7).49,115 Instability can reduce the SAC but not the ADI.30 The instability may be predominantly anterior or posterior or grossly unstable in all directions.47,49,60 In a group of patients requiring surgery, the average displacement was 11 mm.
There is controversy regarding the presence of associated bony anomalies in the area of the hypoplastic dens or os odontoideum. In the authors’ experience, these changes are occasionally present, but with improved awareness they will undoubtedly be found more often. The posterior arch of C1 may be hypoplastic, whereas the anterior arch is hypertrophied.34,49,54 Hypertrophy of the C1 anterior arch can be a useful sign of a chronic pathologic condition of C1-2.53,115,128 If the posterior ring of C1 is narrow and there is abnormal anterior displacement of C1, there is less available space for the cord and an increased danger of neurologic sequelae.
Clinical Features
Patients may present clinically with no symptoms, local neck symptoms, transitory episodes of paresis after trauma, or frank myelopathy secondary to cord compression.50,73,129 Minor trauma is commonly associated with the onset of symptoms, often of sufficient degree to warrant radiologic evaluation of the cervical spine. Symptoms may be mechanical owing to local irritation of the atlantoaxial articulation, such as neck pain, torticollis, or headache. Neurologic symptoms are due to C1-2 displacement and spinal cord compression. An important factor differentiating os odontoideum from other anomalies of the occipitovertebral junction is that these patients seldom have symptoms referable to cranial nerves107 because the area of the spinal cord impingement is below the foramen magnum.
If clinical manifestations are limited to neck pain and torticollis (local joint irritation) without neurologic involvement (40% of cases),49,106 the prognosis is excellent.47,107,130,131 Similarly, patients who exhibit only transient weakness of the extremities and dysesthesia after trauma usually have complete return of function. Patients in whom there is an insidious onset and slowly progressive neurologic impairment have a greater potential for permanent deficit.47,116 Sudden death has been reported.108 Damage may be mixed, with involvement of the anterior and the posterior spinal cord structures. Weakness and ataxia are more common complaints than sensory loss. Spasticity, increased deep tendon reflexes, clonus, loss of proprioception, and sphincter disturbances in various combinations have all been described, however.49
A few patients may have symptoms and signs of cerebral and brainstem ischemia, seizures, mental deterioration, syncope, vertigo, and visual disturbances.60,117,131,132 In these patients, there typically is a paucity of cervical spinal cord signs and symptoms, and it is presumed that the patients are experiencing vertebral artery compression at the foramen magnum or just below it.133–135 The diagnosis can be confusing, and many patients are misdiagnosed or thought to have progressive neurologic illness. Whenever Friedreich ataxia, multiple sclerosis, or other unexplained neurologic complaint is encountered, survey of the occipitocervical junction is suggested.
Radiographic Features
Recommended radiographic views are open-mouth, anteroposterior, lateral, flexion-extension, and CT reconstructions.42 Dynamic flexion-extension CT scans are valuable because plain films do not always show the anomaly or the extent of motion (Fig. 30–23; see Fig. 30–7).
Cineradiography has been valuable for understanding odontoid anomalies, particularly anomalies that cause atlantoaxial instability,129,136 but owing to high radiation exposure it has been largely replaced by dynamic CT scans in flexion and extension and CT reconstruction.51,57 Most children with these lesions have a predominance of either anterior or posterior instability, but these can exist together.49
Normal Variations
At birth, the normal odontoid can be visualized in the lateral view with its epiphyseal plate (see Fig. 30–20). A mistaken impression of hypoplasia may be given by a lateral extension radiograph because the anterior arch of the atlas may slide upward and protrude beyond the ossified tip of the dens, especially in very young patients.137
Agenesis or Hypoplasia of Odontoid
Agenesis or hypoplasia of odontoid is an extremely rare anomaly that may be recognized from birth onward and is best seen in the open-mouth view, which is sometimes difficult to obtain in infancy (see Fig. 30–19). The diagnostic feature is the absence of the basilar portion of the odontoid, which normally dips down into and contributes to the body of the axis. This basilar portion is well below the level of the superior articular facets of the axis. The lateral view is of little help in distinguishing this anomaly from hypoplasia.
The most common form of hypoplasia manifests as a short, stubby peg of odontoid projecting just above the lateral facet articulations (see Fig. 30–19). Tomography is necessary to confirm whether an os odontoideum is present in addition to the hypoplasia.
In patients with multiple anomalies, the usual radiographic views are not always reliable in confirming the presence or absence of an odontoid. Similarly, in patients with abnormal bone, such as in Morquio syndrome and spondyloepiphyseal dysplasia,50 the odontoid may be present but dysplastic, blending with the surrounding abnormal bone, and cannot be differentiated. In these situations, good results have been obtained by using lateral laminagraphic techniques (see Fig. 30–23) or dynamic CT scans in flexion and extension to ascertain the stability of the atlantoaxial articulation. The extension view should not be ignored. Many patients have been found with significant posterior subluxation.47,54 Giannestras and colleagues54 reported a youngster who became quadriparetic from prolonged hyperextension while lying prone watching television.
Os Odontoideum
In os odontoideum, there is a jointlike articulation between the odontoid and the body of the axis that appears radiologically as a wide radiolucent gap (see Fig. 30–19B and C). This gap may be confused with the normal neurocentral synchondrosis (see Fig. 30–19) before age 5 years. In children, the diagnosis of os odontoideum is confirmed by showing motion between the odontoid and the body of the axis. In adults, the diagnosis of os odontoideum is suggested by observing a radiolucent defect between the dens and the body of the axis.
The radiologic appearance of os odontoideum may be similar to a traumatic nonunion, and often they cannot be differentiated.33,109 In os odontoideum, the gap between the free ossicle and the axis usually extends above the level of the superior facets and is wide with a smooth edge. The ossicle is usually approximately one half the normal size of the odontoid and is round or oval, and the cortex is of uniform thickness. In traumatic nonunion, the gap between the fragments is characteristically narrow and irregular and frequently extends into the body of the axis below the level of the superior facets of the axis. The bone fragments appear to “match,” and there is no marginal cortex at the level of the fracture or the rounded-off appearance found with os odontoideum.118 The odontoid ossicle is fixed firmly to the anterior ring of the atlas and moves with it in flexion, extension, and lateral slide. The anterior portion of the atlas is usually hypertrophied, and the posterior portion of the ring may be hypoplastic or absent.47,107,128
Recommended radiographic views are open-mouth and lateral flexion-extension views. The literature supports that plain radiographs are sufficient to establish the diagnosis of os odontoideum. Spierings and Braakman138 and Watanabe and colleagues129 studied flexion-extension radiographs in patients with os odontoideum and attempted to correlate radiographic measurements with myelopathy. The degree of C1-2 instability measured by anteroposterior translation did not correlate with neurologic deficits, but the space available for the cord with neck extension (SAC) was significantly smaller in the myelopathic group. A critical anteroposterior diameter for cervical myelopathy was identified as 13 mm.129,138
CT reconstructions are indicated when routine views are unsatisfactory in showing the anomaly. Lateral flexion-extension stress views should be conducted voluntarily by patients, particularly patients with a neurologic deficit. The degree of anteroposterior displacement of the atlas on the axis should be documented (Fig. 30–24). The os odontoideum moves with the ring of C1, and consequently measurements of its relationship to C1 are of little value. Measurements can be made using a line projected superiorly from the posterior border of the body of the axis to a line projected inferiorly from the posterior border of the anterior arch of the atlas. Measurements greater than 3 mm should be considered pathologic. Most symptomatic patients exhibit significant instability.47 In a large series reported by Fielding and colleagues,47 the average measurement was 1 cm; most were either anterior or posterior, but some were unstable in all directions.129
When instability has been long-standing, it becomes multidirectional, and the C1-2 articulation is very unstable.129 Watanabe and colleagues129 noted that patients who have more than 20 degrees of sagittal plane rotation have a higher rate of myelopathy (86%), and patients with myelopathy have a high rate of instability (40%). The greater the sagittal plane rotation and looseness, the more likely is spinal cord compression.
CT scan can help to define the anatomy of the craniocervical junction, including the completeness of the atlas ring and the position of the transverse foramina at C1 and C2. CT scan has also been shown to aid in distinguishing os odontoideum from a post-traumatic odontoid nonunion (Fig. 30–25).139
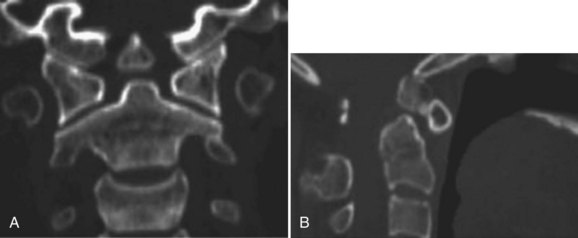
FIGURE 30–25 A and B, Coronal (A) and sagittal (B) CT scans of upper cervical spine clearly show os odontoideum.
MRI is helpful for evaluating the SAC. MRI studies have shown a direct correlation between cord signal abnormality and the degree of myelopathy measured clinically.140 Dynamic MRI as described by Hughes and colleagues141 allows direct visualization of motion of the os odontoideum, atlas, and axis and surrounding motion throughout the full range of motion of the cervical spine (Fig. 30–26). New open MRI configurations allow analysis of the cervical spine in the physiologic upright position, supporting the weight of the head; this may assist in identifying subtle pathology that is not apparent in the nonphysiologic, supine position.
Treatment
Patients with congenital anomalies of the odontoid lead a precarious existence. The concern is that a trivial insult superimposed on an already weakened and compromised structure may be catastrophic. In the authors’ experience, patients with these problems either have or develop gross atlantoaxial instability and with it the possibility of progressive myelopathies or even death.49
The natural history of os odontoideum is variable, and predictive factors for instability have not been identified.49 Indications for surgical stabilization include an os odontoideum in association with occipitocervical pain or os odontoideum with myelopathy. Other factors that may assist in surgical decision making are the severity of C1-2 instability and other associated osseous anomalies. Neural decompression must address anterior bony or soft tissue impingement of the spinal cord or compression posteriorly from the dorsal arch of C1.
Patients with local symptoms or transient myelopathies may expect recovery, at least temporarily.107,130,131 Cervical traction or immobilization may be helpful in such circumstances. Surgical stabilization is indicated if there is neurologic involvement (even if transient), if there is instability of 10 mm or more in flexion and extension, if there is progressive instability, or if there are persistent neck complaints associated with atlantoaxial instability.
Considerable controversy exists over the role of prophylactic stabilization in asymptomatic patients with instability.49,65,107,130,135 Surgical treatment is not required for every patient in whom an os odontoideum is identified. Patients who have no neurologic symptoms or instability at C1-2 can be managed with periodic observation. The lack of C1-2 instability at initial diagnosis does not guarantee that instability will not develop in these patients, however, or that they are not at higher risk for spinal cord injury with trauma. For this reason, longitudinal follow-up with flexion-extension radiographs of these patients is recommended.
Klimo and colleagues49 recommended surgical stabilization for all patients with os odontoideum. The safety of stability, and with it the ability to lead a normal active life, must cause one to weigh the possible complications of surgery against the catastrophic dangers of instability with secondary cord pressure. In the pediatric age group, it may be difficult or impossible to curtail activity, even in the presence of marked instability.47,60,142 If possible, reduction of the atlantoaxial articulation must be accomplished before surgery either by careful positioning of the patient or by skull traction. Manipulative reduction during surgery is discouraged because it has proved extremely hazardous and may result in respiratory distress, apnea, or death.65 Ideally, the patient should be maintained in the reduced position several days before surgery to allow recovery of neurologic function and to lessen spinal cord irritation. When fusion is undertaken, regardless of the indication, preoperative halo traction is often required to achieve reduction, which may have to be continued during surgery and postoperatively until transfer to a suitable immobilization device.107,143
The suggested method of stabilization is posterior cervical fusion of C1-2. Posterior C1-2 arthrodesis in the treatment of os odontoideum provides effective stabilization in most patients. Posterior Gallie and Brook sublaminar wiring techniques with iliac crest bone graft and halo immobilization have been reported with favorable fusion results.71,144–153 This method is not without risk because slight flexion is often required to pass the wire beneath the posterior ring of the atlas, and it can have tragic results.107,144 When attempting posterior stabilization using a wire technique in small children, the spinous process of C2 may be small or poorly developed or not yet completely ossified. In this situation, a threaded Kirschner wire through the spinous process, as described by Mah and colleagues,154 can be very helpful in improving the stability of the wiring technique.
In a patient with a marginally functioning neurologic status, it may be wiser to perform an occiput-to-C2 arthrodesis and plan to maintain immobilization in extension during the postoperative period.107 In this regard, the halo vest is helpful. Incomplete development of the posterior ring of C1 is uncommon but is reported to occur with increased frequency in patients with os odontoideum.47 The completeness of the C1 arch should be evaluated preoperatively because a large gap may preclude wire fixation.148 If wire fixation is employed, excessive tightening of the wire should be avoided. The articulation is frequently unstable in flexion and extension, and posterior dislocations may occur, owing to overcorrection, with disastrous results.
In patients who have a highly mobile but reducible C1-2 articulation, several authors have reported good results using transarticular screws.41,71,144,149,150,152 Atlantoaxial transarticular screws have been shown to be feasible in pediatric patients, and the superior biomechanical stability may eliminate the need for prolonged postoperative halo vest immobilization (see Fig. 30–12).149,155 A preoperative CT scan with reconstruction views is essential with this technique to define the C1-2 anatomy and course of the vertebral arteries.41 An aberrant path of the vertebral vessels that are at risk with screw placement is a contraindication for the procedure.41 Other techniques have been described. Lateral mass screws and transarticular screws have been used with good success being reported.152,156–158 Lateral mass screws and fixation to the skull occiput have comparable strength.156 The carotid artery can be hit with a lateral mass screw.159
With transarticular fixation of C1-2, the lamina of C1 can be removed without the necessity of extending the fusion to the occiput. The cervical fusion of C1-2 is generally reliable and safe; however, in patients with spinal cord compression, fixed dislocations, or congenital ligamentous laxity, particularly in patients with Down syndrome, extra caution should be employed.148,160 Smith and colleagues160 reported significant problems with C1-2 stabilization, particularly in patients who are very unstable or have a myelopathy. Patients with failed fusions or irreducible dislocations were at high risk for perioperative neurologic complications.
Patients in whom the C1-2 dislocation is unreducible after an adequate trial of traction pose a difficult management problem.161 In this situation, posterior decompression by laminectomy has been associated with increased morbidity and mortality.107 In addition, posterior decompression alone may potentiate C1-2 instability, and if performed it must be accompanied by occiput-to-C2 arthrodesis.47,161 For patients with no neurologic deficit, a simple in situ posterior fusion is the least hazardous procedure. If reduction of the C1-2 dislocation is considered necessary or if the clinical situation precludes posterior stabilization, an anterior approach should be considered.28,34,162 The lateral retropharyngeal approach described by Whitesides and McDonald162 provides anterior exposure of the C1-2 articulation adequate to perform decompression, reduction, and stabilization. This route may be used in place of the transoral or mandibular and tongue-splitting approaches, which are associated with an increased incidence of infection.28,162 Associated myelopathy can be addressed with reduction of the deformity, dorsal or ventral decompression, and occipitocervical fusion.
A common clinical problem is differentiation between a fractured odontoid and os odontoideum. Discovery is usually made after trauma in both conditions, and an accurate diagnosis may be impossible by radiographic techniques alone. In this situation, a period of immobilization (skull traction or cast) is recommended. Overdistraction, particularly in children, should be avoided because it can lead to os odontoideum.47 If the lesion represents an acute fracture, healing usually occurs. If a congenital or traumatic nonunion is present, surgical stabilization is necessary if atlantoaxial instability is shown.
Klippel-Feil Syndrome (Congenital Synostosis of Cervical Vertebrae, Brevicollis)
In 1912, Klippel and Feil163 published the first complete description of the clinical aspects and pathology of the syndrome that bears their name. Their attention was attracted to a patient with the unusual clinical findings of marked shortening of the neck, a low posterior hairline, and severe restriction of neck motion. The patient died, and at the postmortem examination Klippel and Feil discovered a complete fusion of the cervical vertebrae. Subsequently, Feil collected 13 additional examples and published a thesis164 in 1919 that included his findings from this larger group and a review of the literature. The term Klippel-Feil syndrome in its present usage refers to all patients with congenital fusion of the cervical vertebrae, whether it involves two segments, congenital block vertebrae (Fig. 30–27), or the entire cervical spine (Fig. 30–28).
Feil originally suggested a system of classification based on the extent and type of the cervical fusion, but except for the area of genetics,165,166 this classification has not proved clinically useful. As additional patients were discovered and radiographic techniques improved, it became apparent that certain anomalies of the occipitocervical junction (see sections on basilar impression, atlanto-occipital fusion, and anomalies of odontoid) should be considered separately from the original syndrome. Although these conditions occur commonly in conjunction with fusion of the lower cervical vertebrae, their significance depends on how they influence the atlantoaxial joint. Their prognostic and therapeutic implications are distinctly different, and they occur with sufficient frequency to warrant individual analysis.
Between 20 and 30 days’ gestational age, the paraxial mesoderm undergoes cephalad-to-caudal segmentation into spherical, discrete somites. As somites mature, they subdivide into sclerotomes, myotomes, and dermatomes. The sclerotomes, precursors of the adult vertebral bodies, undergo resegmentation such that the caudal section of one somite fuses with the cephalad segment of the adjacent one to form a vertebral body (Fig. 30–29).167
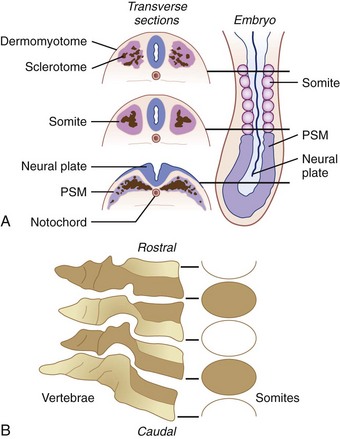
(Adapted from Tracy MR, Dormans JP, Kusumi K: Klippel-Feil syndrome. Clin Orthop 424:183-190, 2004.)
Congenital cervical fusion is the result of failure of normal segmentation of the cervical somites during the 3rd to 8th weeks of life. With the exception of a few patients in whom this condition is inherited,21,166,168,169 the etiology is as yet undetermined. Klippel-Feil syndrome has been theorized to result from disruption of this embryologic segmentation process. Pedigree analysis and molecular studies in animal models are ongoing to define specific genetic abnormalities that may explain these congenital spinal anomalies. Pedigree analysis of a large affected family has identified the first human Klippel-Feil locus (SGM1). This gene is disrupted by a heritable paracentric inversion of chromosome 8. The function of this locus remains to be defined, however. Drosophila and mouse models have identified genes in the HOX, PAX, and Notch signaling pathways to play a key role in segmentation and somitogenesis. In particular, Pax1 and Pax9 are strongly expressed in the vertebral column in mice and play central roles in somite segmentation. Future molecular genetics studies will continue to define the genetic basis for these congenital cervical anomalies.167 Klippel-Feil anomaly has been associated with fetal alcohol syndrome, but it is unlikely that this is a common mechanism.170
The effect of this embryologic abnormality is not limited to the cervical spine—the entire fetus may be adversely affected. Patients with Klippel-Feil syndrome, even patients with minor cervical lesions, may have other, less apparent or even occult defects in the genitourinary,112,171,172 nervous,92,173 and cardiopulmonary systems174–176 and even hearing impairment.112,177–179 Many of these “hidden” abnormalities may be more detrimental to the patient’s general well-being than the obvious deformity of the neck. In the review by Hensinger and colleagues,112 a high incidence of related congenital anomalies was found (Table 30–1), emphasizing that all patients with Klippel-Feil syndrome should be thoroughly investigated.
TABLE 30–1 Abnormalities Associated with Klippel-Feil Syndrome
| % | |
|---|---|
| Common Abnormalities | |
| Scoliosis | 60 |
| Renal abnormalities | 35 |
| Sprengel deformity | 30 |
| Deafness | 30 |
| Synkinesis | 20 |
| Congenital heart disease | 14 |
| Less Common Abnormalities | |
| Ptosis | |
| Duane contracture | |
| Lateral rectus palsy | |
| Facial nerve palsy | |
| Syndactyly | |
| Hypoplastic thumb | |
| Upper extremity hypoplasia | |
| Neurenteric cyst |
Clinical Features
The classic clinical description of Klippel-Feil syndrome is a triad—low posterior hairline, short neck, and limitation of neck motion—but less than half of patients have all three signs (Figs. 30-30 and 30-31).112,180 The presence of these signs is directly related to the degree of cervical spine involvement. Clinically, the most consistent finding is limitation of neck motion.166 If fewer than three vertebrae are fused or if only the lower cervical segments are fused, the patient generally has no detectable limitation.166 In addition, many patients with marked cervical involvement are able to compensate with hypermobility at the unfused joints and to maintain a deceptively good range of motion.112 Several patients seen by the authors have 90 degrees of flexion-extension, occurring at the only open interspace (Fig. 30–32). Generally, flexion-extension is better preserved than rotation or lateral bending. Rarely, patients have no detectable motion and fixed hyperextension of the neck; this is usually associated with iniencephaly (absence of the posterior cervical laminae and an enlarged foramen magnum) (see Fig. 30–28).181
Unless extreme, shortening of the neck is a subtle finding. Similarly, the low posterior hairline is not constant (see Figs. 30-30 and 30-31). Less than 20% of patients with Klippel-Feil syndrome have obvious facial asymmetry, torticollis, or webbing of the neck.112,166 When extreme, webbing of the neck is called pterygium colli and consists of large skin folds extending from the mastoid to the acromion (see Fig. 30–31).182 The underlying muscles may be involved, but surgical release generally does not result in improved neck motion.
Sprengel deformity occurs in 15% to 35% of cases unilaterally or bilaterally (Fig. 30–33).112,165,166,171,183,184 At the 3rd week of gestation, the scapula develops from mesodermal tissue high in the neck at the level of C4. It descends into the thoracic position by the 8th week, or approximately at the same time that the Klippel-Feil lesion is thought to occur.165,166 It is logical to expect a significant relationship between these two anomalies. Occasionally, there is a bony bridge between the cervical spine and scapula, an omovertebral bone, or connection to the clavicle.185 Its removal may permit an increase in neck and shoulder motion.
Probably for the same embryologic reasons, other clinical findings are occasionally apparent (see Table 30–1), including ptosis of the eye, Duane contracture (contracture of the lateral rectus muscle),166 lateral rectus palsy, facial nerve palsy, neurenteric cyst,186 and cleft or high-arched palate.187,188 Abnormalities of the upper extremities include syndactyly, hypoplastic thumb, supernumerary digits, and hypoplasia of the upper extremity, which may represent an interruption of the embryonic blood supply.189 Abnormalities of the lower extremities are infrequent.
With the exception of the anomalies that involve the atlantoaxial joint, no symptoms can be directly attributed to the fused cervical vertebrae. All symptoms commonly associated with Klippel-Feil syndrome originate at the open segments where the remaining free articulations may become compensatorily hypermobile.180,190,191 Owing to the increased demands placed on these joints or in response to trauma, this hypermobility can lead to frank instability or early degenerative arthritis.180,183,190
Symptoms may arise from two sources: (1) mechanical symptoms owing to irritation of the joint and (2) neurologic symptoms owing to root irritation or spinal cord compression. Patients with a short segment fusion are less likely to develop symptoms166,192 because the loss of motion is adequately compensated by the remaining free segments. Patients with synostosis of the lower cervical spine are at less risk because the limitation is minimal and can be adequately compensated by the more normally mobile joints above. Most patients who develop symptoms are in the 2nd or 3rd decade of life,166,180,190 suggesting that the instability is in part a function of time with increasing ligament laxity.
Neurologic symptoms are generally localized to the head, neck, and upper extremities and result from direct irritation or impingement of the cervical nerve roots with radicular symptoms in the upper extremities.22 The symptoms can usually be localized to the hypermobile joints adjacent to the fused segments.193 There may be constriction and narrowing of the nerve root at the foramen from osteophytic spurring.22,180 If joint instability is progressive or if there is appropriate trauma, the spinal cord may be involved to varying degrees, ranging from mild spasticity, hyperreflexia, and muscular weakness to sudden complete quadriplegia after minor trauma.166,183,194,195 Syncope and neurologic compromise may be associated with mechanical compromise of the vertebral arteries secondary to hypermobility, with ischemic episodes or emboli.196,197
Radiographic Features
In a child with severe involvement, an adequate radiographic evaluation can be difficult. Fixed bony deformities frequently prevent proper positioning, and overlapping shadows from the mandible, occiput, or foramen magnum may obscure the upper vertebrae (Fig. 30–34). In this situation, flexion-extension views may provide the information necessary to assess stability. Another technique that has gained wide acceptance in the diagnosis of cervical instability is CT.51,180 This technique coupled with flexion-extension of the cervical spine can delineate more precisely the presence or absence of spinal cord compression. CT can be enhanced further by contrast myelography. With MRI, the relationship between the bony elements and neurologic structures can be viewed directly in flexion and extension to show the origin of neural compression.57,187,198 Use of MRI is particularly helpful for patients who have abnormal bone, such as patients with Morquio disease.199
With these techniques, the space available for the spinal cord can be measured directly rather than inferred by use of the ADI.57 Knowledge of the normal variations in cervical spine mobility, particularly in children, is important in evaluating patients with Klippel-Feil syndrome.64,200 Pseudosubluxation of C2 on C3 with flexion can be observed in 45% of normal children younger than 8 years of age (see Fig. 30–9).64 Marked angulation at a single interspace during flexion, rather than a uniform arc of vertebral motion, can be observed in normal children (16%)201 and may be misinterpreted as vertebral fusion below.
Fusion of cervical vertebrae is the hallmark of Klippel-Feil syndrome. This fusion may be simply synostosis of two bodies (congenital block vertebrae) (Fig. 30–35; see Fig. 30–27) or massive fusion of vertebrae, which was found in Klippel and Feil’s first patient (see Fig. 30–28).163
Aside from vertebral fusion, flattening (wasp waist) and widening of the involved vertebral bodies, an increase in the space available for the spinal cord, and absent disc spaces are the most common findings.202,203 Hypoplasia of the disc space or remnants of it can often be seen (see Fig. 30–35). In a young child, narrowing of the cervical disc space cannot always be appreciated because the ossification of the vertebral body is incomplete, and the unossified endplates may give the false impression of a normal disc space (Fig. 30–36). With continued growth, the ossification of the vertebral bodies is completed, however, and the fusion becomes obvious.191 If fusion is suspected in a child, it may be confirmed by flexion-extension views (Fig. 30–37). Juvenile rheumatoid arthritis (Fig. 30–38), rheumatoid spondylitis, and infection can mimic the radiographic findings, but usually the clinical history and physical examination indicate the correct diagnosis.
Hemivertebrae are common (Fig. 30–39); they occurred in 74% of patients in the review of Gray and colleagues,166 and the incidence increases with the number of segments fused. Fusion of posterior elements usually parallels fusion of the vertebral bodies. In a young child, particular attention should be paid to the laminae because fusion posteriorly is often more apparent than anteriorly in early life (Fig. 30–40).112,191
The sagittal and transverse diameters of the spinal canal are usually normal. Narrowing of the spinal canal, if it occurs, is usually seen in adult life and is due to degenerative changes (osteoarthritic spurs) or hypermobility.* Enlargement of the cervical canal is uncommon; if found, it may indicate conditions such as syringomyelia, hydromyelia, or Arnold-Chiari malformation.62,206 The intervertebral foramina are usually smooth in contour but are frequently smaller than normal and oval rather than circular (Fig. 30–41). Posterior spina bifida is common (45%), but anterior spina bifida is rare. Rarely, there is complete absence of the posterior elements. This condition is usually accompanied by enlargement of the foramen magnum and fixed hyperextension of the neck, referred to as iniencephaly (see Fig. 30–28).181
Patterns of Cervical Motion
Pizzutillo and colleagues190 reviewed patients from the Alfred I. DuPont Institute and patients reported in the literature to determine the long-term problems found in Klippel-Feil syndrome. In their classification, these investigators noted that patients who have upper segment instability were more likely to be younger and to have neurologic problems. Conversely, degenerative changes were more common in older patients with low segment hypermobility. This finding emphasizes the importance of screening the upper cervical spine in a young child for instability. There are three high-risk patterns of cervical spinal motion that potentially have a poor prognosis, from either early instability or late degenerative osteoarthritis.207
Pattern 1 is fusion of C2 and C3 with occipitalization of the atlas. Complications associated with this pattern were first reported by McRae in 1953,33 and they received substantial support in the literature.22,208 Flexion-extension is concentrated in the area of C1 and C2. With aging, an odontoid can become hypermobile, narrowing the spinal canal and compromising the spinal cord and brainstem.
Pattern 2 is a long fusion with an abnormal occipitocervical junction (see Fig. 30–28). This is similar to the C2-3 fusion of McRae and could be reviewed as a more elaborate variation. The force of flexion-extension and rotation is concentrated in the area of the abnormal odontoid or poorly developed ring of C1, which cannot withstand the wear and tear of aging.204,207,208 It is important to differentiate this pattern from the pattern in a patient with a long fusion and a normal C1-2 articulation (Fig. 30–42), which is usually compatible with a normal life expectancy.
Pattern 3 is a single open interspace between two fused segments (Fig. 30–43). In this situation, cervical spine motion is concentrated at the single open articulation. In some patients, this hypermobility may lead to frank instability or degenerative osteoarthritis (Fig. 30–44).* This pattern can be easily recognized because the cervical spine appears to angle or hinge at the open segment. Samartzis and colleagues203 studied cervical fusion patterns in patients with Klippel-Feil syndrome. They developed a classification system in which type I described a single fusion; type II described multiple, noncontiguous fusions; and type III described multiple, contiguous fusions. Samartzis and colleagues203 found that type I was associated with axial neck symptoms, and types II and III were associated with radicular and myelopathic symptoms.
Associated Conditions
Scoliosis
Scoliosis is the most frequent anomaly found in association with the syndrome.112,171,209,210 Of these patients, 60% have a significant degree of scoliosis (>15 degrees by the Cobb method).112,210 Most of these patients require treatment and should be followed through the growth years. Radiographic examinations should include lateral views of the spine because increasing kyphosis may make the need for treatment of the scoliosis more urgent. If the deformity is recognized early, many children can be successfully managed with standard spinal orthoses. At present, most of these patients have required posterior spinal stabilization, partly owing to late recognition.112,171,173,194
Two types of scoliosis can be identified: congenital scoliosis, owing to vertebral anomalies and differential growth patterns (Fig. 30–45), and compensatory scoliosis, below the area of vertebral involvement. In the authors’ series, congenital scoliosis is more common (55%), and in more than half of the children, the curvature was progressive and required treatment.112 Progressive curves are more likely associated with children who have extensive fusions.210 Most children (75%) required posterior spinal fusion to arrest an increasing deformity, and the remainder were controlled with a brace or cast.112 Progressive scoliosis frequently occurred in the normal-appearing vertebrae below the primary congenital curve. If only the congenitally involved segments are examined in follow-up, an increasing compensatory scoliosis in the lower vertebrae may not be recognized, and its significance may not be appreciated until serious deformity results.207
Documented progression of scoliosis, whether in the congenitally distorted elements or in the compensatory curve below, demands immediate and appropriate treatment to prevent serious additional deformity. Progressive scoliosis in the thoracic spine may seriously compromise pulmonary function.112,174 More subtle occult abnormalities can lead to respiratory difficulty in some patients with Klippel-Feil syndrome.211 Abnormal rib spacing, congenital fusion of the ribs, and deformed costovertebral joints may inhibit full expansion of the rib cage during respiration.212 Although not causing an angular deformity, fusion of the thoracic vertebrae may decrease the size of the thoracic cage. A dwarf with spondylothoracic deformity may represent a severe form of this problem, leading to early respiratory death.213
Also, Krieger and colleagues75 reported on the relationship of occult respiratory dysfunction and craniovertebral anomalies. They noted that in addition to the obvious problems of bony impingement or traction on the brainstem, these patients may have subtle hydrocephalus, which may adversely affect respiratory function. This information has particular application when cervical distraction devices are contemplated in the treatment of scoliosis (halo-femoral or halo-pelvic traction). When considering the use of such devices, the physician should be aware that children with Klippel-Feil syndrome may be more susceptible to neurologic or vascular injury and that the presence of cervical anomalies may preclude the use of cervical distraction.112
Renal Abnormalities
More than one third of children with Klippel-Feil syndrome can be expected to have a significant urinary tract anomaly. These anomalies are often asymptomatic in young children. Previously, the authors recommended that such anomalies be evaluated with intravenous pyelography (Fig. 30–46).112 It has been found, however, that ultrasonography offers a noninvasive way to screen adequately for the anomalies associated with this syndrome.201 The pronephros, the embryologic tissue destined to become the genitourinary tract, develops between the 7th and 14th somites, in the same region and at the same time as the cervical spine,212,214 quite similar to the scapulae in Sprengel deformity. The most frequent abnormality is unilateral absence of a kidney. Other abnormalities include a double collecting system, renal ectopia, horseshoe kidney, and hydronephrosis from ureteropelvic obstruction. In the authors’ series, 2 of 50 patients developed severe pyelonephritis in the remaining kidney, requiring renal transplantation.112 In Klippel and Feil’s original case report, the patient died of nephritis.163
Cardiovascular Abnormalities
The literature notes the association of Klippel-Feil syndrome with congenital heart disease (4.2% to 14%).112,175,176,194 The most common lesion reported has been an interventricular septal defect occurring alone or in combination with other defects, such as patent ductus arteriosus and abnormal position of the heart and aorta.
Deafness
The association of hearing impairment and deafness in Klippel-Feil syndrome (>30%) has been reported in the otology literature,177–179205 but it is seldom mentioned in orthopaedic reports.112,171 Other defects include absence of the auditory canal and microtia.
Jalladeau205 published the first report of deafness. Stark and Borton179 noted that detailed audiologic data were not yet available, and the precise defect is often unknown. There is no characteristic audiologic anomaly, and all types of hearing loss (conductive, sensorineural, and mixed) have been described. These patients should undergo a complete audiometric evaluation when they are discovered. The relationship between hearing loss and speech-language retardation is well documented, and early detection of hearing impairment can lessen the retardation by permitting early initiation of speech and language training.179
Mirror Motions (Synkinesis)
Synkinesis consists of involuntary paired movements of the hands and occasionally the arms. The patient is unable to move one hand without similar reciprocal motion of the opposite hand. Mirror motion was first described by Bauman,173 who found it in four of six patients with Klippel-Feil syndrome. This condition has been noted to occur occasionally in normal preschool children and patients with cerebral palsy or Parkinson disease, but most patients with this condition have Klippel-Feil syndrome.165 Approximately 20% exhibit mirror motions clinically.112 Baird and colleagues,92 using electromyography to examine 13 patients with Klippel-Feil syndrome, found 10 patients with electrically detectable paired motion in the opposite extremity. This finding suggests that many patients may be subclinically affected and may be more clumsy at two-handed activities. Some authors have suggested that synkinesis should be included as part of the syndrome.92,165
The etiology of synkinesis is unknown, but it seems to be a separate congenital neurologic defect not caused by bony impingement or irritation of the spinal cord.212,215 The examination of two autopsy specimens suggests that the clinical findings are due to inadequate or incomplete decussation of the pyramidal tracts in conjunction with a dysraphic cervical spinal cord. As a consequence, cerebral control over the upper extremities must follow less direct pathways located in the extrapyramidal system, and affected patients require more extensive practice to dissociate the movements of the individual extremities.
Synkinesis is most pronounced in young children, particularly children younger than 5 years. The condition tends to decrease with age. Occupational therapy has been helpful in teaching control over the extremities, or at least in disguising the reciprocal motion to a tolerable cosmetic level. Still, many patients may find discriminating two-handed activity difficult, such as playing the piano, typing, sewing, or ladder climbing.216
Treatment
As discussed, sudden neurologic compromise or death after minor trauma has been reported in patients with Klippel-Feil syndrome and is usually due to disruption at the hypermobile articulation.22,166,183 The role of prophylactic surgical stabilization in asymptomatic patients has not yet been defined. There is no satisfactory definition of when the risk of instability warrants further reduction of neck motion.
For symptomatic patients with mechanical problems, the usual treatment measures for degenerative osteoarthritis are applicable and include traction, a cervical collar, and analgesics. Symptoms that suggest neurologic compromise require careful consideration and evaluation by a neurologist, neurosurgeon, and orthopaedist (see Fig. 30–44). The exact area of irritation must be determined before surgical intervention. Attempts should be made preoperatively to obtain reduction of the bony architecture in advance of surgical stabilization. Also, the anesthesiologist may need to plan for airway management and be prepared for difficult intubation.217 The physician must be mindful that there are other associated abnormalities in the brainstem and in the spinal cord itself that may be contributing to the symptoms.
Treatment of the cosmetic aspects of this deformity has met with limited success. Occasionally, children with the fixed torticollis posture may be improved with bracing. Bracing requires long-term application, however, and excellent patient cooperation. Correction of the bony deformity by direct means, such as wedge osteotomy or hemivertebrae excision, has been done on a limited basis.218 Ruf and colleagues218 reported hemivertebrae resection for correction of head tilt. Occasionally, carefully selected patients who have cervical congenital scoliosis may obtain some correction and improvement of appearance by use of the halo vest combined with posterior cervical fusion. Bonola219 described a method of rib resection to attain apparent increase in neck length and motion. This procedure is an extensive surgical experience, however, and is a great risk to the patient. No subsequent reports have appeared in the literature. More recently, the vertical expandable prosthetic titanium rib (VEPTR) has been used to correct cervical tilt along with congenital thoracic scoliosis in thoracic insufficiency syndrome.220 Early results of this treatment have been promising.
Soft tissue procedures, Z-plasty, and muscle resection may achieve cosmetic improvement in properly selected patients.171 These procedures can restore a more natural contour to the shoulders and neck and an apparent increase in neck length. Neck motion is generally not increased, and the scars may be extensive, particularly in a patient with a large skin web. If an omovertebral bone is present or an abnormal connection to the clavicle,185 its removal may permit an increase in neck and shoulder motion. The risk of brachial plexus injury from traction is higher in patients with Klippel-Feil syndrome because there are likely to be anomalous origins of the cervical nerve roots in these patients. Iniencephaly and absence of the posterior cervical elements (see Fig. 30–28) may be associated with Sprengel deformity and must be identified if surgical correction is considered.181
Muscular Torticollis (Wry Neck)
Muscular torticollis is a common condition usually discovered in the first 6 to 8 weeks of life. The deformity is caused by contracture of the sternocleidomastoid muscle, with the head tilted toward the involved side and the chin rotated toward the contralateral shoulder (Fig. 30–47A). If the infant is examined within the first 4 weeks of life, a mass or “tumor” is usually palpable in the neck (Fig. 30–48).221 It is generally a nontender, soft enlargement that is mobile beneath the skin and attached to or located within the body of the sternocleidomastoid muscle. Ultrasonography may be helpful in confirming the mass.222 The mass attains maximal size within the 1st month of life and then gradually regresses. If the child is examined after 4 to 6 months of age, the mass is usually absent, and the contracture of the sternocleidomastoid muscle and the torticollis posture are the only clinical findings (Fig. 30–49). The mass is frequently unrecognized and was undetected in 80% of the patients of Coventry and Harris.223

FIGURE 30–49 This 18-month-old child had torticollis that was resistant to stretching exercises and required surgical release.
If the condition is progressive, deformities of the face and skull can result and are usually apparent within the 1st year.224 Flattening of the face on the side of the contracted sternocleidomastoid muscle may be particularly impressive (see Fig. 30–47A). The deformity is probably due to the position the child assumes when sleeping (see Fig. 30–47B). In a study of children in the United States, it was found that they generally sleep prone,225 and in this position it is more comfortable to have the affected side down. As a consequence, the face remodels to conform to the bed. In children who sleep supine, reverse modeling of the contralateral aspect of the skull is evident. The Back to Sleep Campaign has altered sleep patterns.226 If the condition remains untreated during the growth years, the level of the eyes and ears becomes distorted and may result in considerable cosmetic deformity. More recent reports suggest that infants with torticollis are at an increased risk for early gross motor delay, but most normalize in the 1st year of life. The delay seemed more strongly associated with little or no time prone when awake than with congenital muscular torticollis.227
Etiology
At present, congenital muscular torticollis is believed to be the result of local compression of the soft tissues of the neck at the time of delivery.228 It is usually unilateral, but bilateral torticollis has been reported.224 Birth records of affected children show a preponderance of breech or difficult deliveries or primiparous births.162,229 The deformity has occurred after otherwise normal deliveries, however, and has been reported in infants born by cesarean section.221,229 Davids and colleagues228 found that MRI studies of the sternocleidomastoid muscle in infants with congenital muscular torticollis have signal changes similar to changes observed in the forearm and leg after compartment syndrome. That finding, coupled with cadaveric dissections, shows that there is a fascial covering of the entire sternocleidomastoid muscle; a review of the children treated by Davids and colleagues228 strongly suggests that congenital muscular torticollis represents a compartment syndrome.
Microscopic examinations of resected surgical specimens and experimental work with dogs230 suggest that the lesion is due to occlusion of the venous outflow of the sternocleidomastoid muscle. This occlusion results in edema, degeneration of muscle fibers, and eventual fibrosis of the muscle body. Coventry and Harris223 suggested that the clinical deformity is related to the ratio of fibrosis to remaining functional muscle. If sufficient normal muscle is present, the sternocleidomastoid stretches with growth, and the child is not likely to develop the torticollis posture, whereas if there is a predominance of fibrosis, there is very little elastic potential.
Pathologic studies showed that with time the fibrosis of the sternal head may entrap and compromise the branch of the accessory nerve to the clavicular head of the muscle (progressive denervation), leading to a late increase in the deformity.231 Some evidence suggests that the problem may be due to uterine crowding or “packaging syndrome” because in three of four children the lesion is on the right side.221,228,229 Also, 5% to 20% of children with congenital muscular torticollis have congenital dysplasia of the hip, which is believed to be due to restriction of infant movement in the tight maternal space.232–234 Boys with developmental dysplasia of the hip were nearly five times more likely to have congenital muscular torticollis.233 Radiographs of the cervical spine should be obtained to rule out congenital anomaly of the cervical spine or C1-2 rotatory subluxation.235,236
Ultrasonography is now widely accepted to be one of the most sensitive tests for the diagnosis of congenital muscular torticollis and developmental hip dysplasia. Tien and colleagues237 found that a homogeneous, hyperechoic mass within the sternocleidomastoid muscle was diagnostic of congenital muscular torticollis. They found the coexistence of developmental hip dysplasia and torticollis to be 17%; 8.5% of children with identifiable hip dysplasia required some treatment.
Treatment
Conservative Measures
Excellent results can be obtained with conservative measures in most patients.221,223,229,238,239 Of the patients reported by Coventry and Harris,223 90% responded to stretching exercises alone. Cheng and colleagues240 showed that controlled manual stretching was safe and effective in the treatment of greater than 95% of patients with congenital muscular torticollis when seen before the age of 1 year. The most important prognostic factors for success were minimal initial rotational deficit, age younger than 1 year at presentation, and lack of a palpable tight band or tumor in the affected sternocleidomastoid muscle.
Exercises are performed by the patient, with guidance from the physical therapist and physician. Standard maneuvers include positioning of the ear opposite the contracted muscle to the shoulder and touching the chin to the shoulder on the affected side. When adequate stretching has been obtained in the neutral position, these maneuvers should be repeated with the head hyperextended, to achieve maximal stretching and prevent positions of the crib and toys so that the neck is stretched when the infant tries to reach and grasp. Children should be examined for plagiocephaly because the findings may be subtle.241 The use of a “sleeping helmet” has been suggested to reduce the deformity and hasten face and skull remodeling.242
Surgery
If the condition persists beyond 1 year of age, nonoperative measures are rarely successful.238 Similarly, established facial asymmetry and limitation of normal motion of more than 30 degrees usually preclude a good result and lead to surgical intervention and poor cosmesis (Fig. 30–50).238 A good (but not perfect) cosmetic result can be obtained, however, in children 5 to 7 years of age (Fig. 30–51).223,236,239,243,244 Asymmetry of the skull and face improves as long as adequate growth potential remains after the deforming pull of the sternocleidomastoid is removed (Fig. 30–52).223,236,243 Shim and colleagues245 showed that patients older than 8 years, including patients who were skeletally mature, benefit from unipolar or bipolar release of the sternocleidomastoid and postoperative physical therapy. These authors preferred to wait until the child was old enough to comply with the postoperative exercise and bracing.246 All patients had improved functional and cosmetic results postoperatively.
Surgery consists of resection of a portion of the distal sternocleidomastoid muscle (see Fig. 30–50). At least a 1-cm segment of the tendon should be removed to guard against recurrence of the deformity. A transverse incision is made low in the neck to coincide with a normal skin fold.247 It is important not to place the incision near or very near the clavicle because scars in this area tend to spread and are cosmetically unacceptable (see Fig. 30–51).121 Similarly, closure with a subcuticular suture is preferred.166
The most common postoperative complaint is disfiguring scarring.221,229,247 The two heads of the sternocleidomastoid are identified, and both are sectioned (see Fig. 30–50). It is important to release the investing fascia around the sternocleidomastoid because this, too, is frequently contracted.221 Rotation of the chin and head at this point generally reveals the adequacy of the surgery, and palpitation of the neck shows any extraneous tight bands that could lead to partial recurrence of incomplete correction (see Fig. 30–52).229 In an older child, an accessory incision (bipolar) is often required to section the muscle at its origin on the mastoid process. The whole muscle should not be excised because this may lead to reverse torticollis229 or additional deformity from asymmetry in the contour of the neck.221
The postoperative regimen includes passive stretching exercises performed in the same manner as those done preoperatively. The exercises should begin as soon as the patient can tolerate manipulation of the neck. Occasionally, head traction at night is helpful, particularly in an older child. Bracing or cast correction may be necessary if the deformity has been of long duration or if the torticollis posture has become a strong habit.248 Results of surgery have been uniformly good, with a low incidence of complications or recurrence, and almost all patients are pleased with the results.221,223,229,238 Slight restriction of the neck motion and anomalous reattachment occur frequently229,247 but are generally unnoticed by the patient. If the patient is young, the facial asymmetry can be expected to resolve completely, unless there is persistence of the torticollis, particularly from residual fascial bands (Fig. 30–53; see Fig. 30–52).229
Differential Diagnosis
Torticollis is a common childhood complaint. The etiology is diverse, and identifying the cause can pose a difficult diagnostic problem (Tables 30-2 and 30-3).
| Congenital | Acquired |
|---|---|
| Occipitocervical anomalies | Neurogenic |
| Basilar impressions | Spinal cord tumors |
| Atlanto-occipital fusion | Cerebellar tumors (posterior fossa) |
| Odontoid anomalies | Syringomyelia |
| Hemiatlas | Ocular dysfunction |
| Pterygium colli (skin web) | Bulbar palsies |
| Congenital muscular torticollis | Traumatic (particularly C1-2) |
| Klippel-Feil syndrome | Subluxations |
| Dislocations | |
| Fractures | |
| Inflammatory | |
| Cervical adenitis | |
| Spontaneous hyperemic atlantoaxial rotatory subluxation | |
| Tuberculosis | |
| Typhoid | |
| Rheumatoid arthritis | |
| Acute calcification of disc | |
| Miscellaneous | |
| Sandifer syndrome (hiatal hernia with esophageal reflux) |
TABLE 30–3 Torticollis Caused by Bony Anomalies
| Congenital Anomalies of Craniocervical Junction | Acquired Anomalies of Craniocervical Junction |
|---|---|
| Klippel-Feil syndrome | Traumatic |
| Atlanto-occipital synostosis (unilateral) | Subluxations |
| Odontoid anomalies | Dislocations |
| Aplasia | Fractures |
| Hypoplasia | Inflammatory |
| Os odontoideum | Rheumatoid arthritis |
| Occipital vertebra | Idiopathic |
| Asymmetry of occipital condyles (hypoplasia) | Atlantoaxial rotatory displacement |
| Subluxation | |
| Fixation |
Gyorgyi249 examined 20 cases of congenital torticollis and found the following coexisting anomalies: congenital cervical fusions, asymmetrical facet joints, basilar impression, atlantoaxial dislocation, assimilation of the atlas, and deformities of the odontoid process. Gyorgyi249 noted that in children with congenital torticollis, 40% had a history of breech presentation. Many occipitocervical malformations manifest as torticollis.31,33,38,112 De Barros and colleagues14 noted that 68% had basilar impression, most commonly unilaterally. Approximately 20% of patients with Klippel-Feil syndrome have associated torticollis.112,250 With asymmetrical development of the occipital condyles or the facets of C1, the head tilt may result in a torticollis unless compensated for by a tilt of the lower cervical spine similar to that which occurs in the milder forms.14,103,166
Inflammatory conditions include local irritation from cervical lymphadenitis, which may lead to the appearance of a wry neck or tilt of the head. Another less frequent cause is a retropharyngeal abscess after inflammation of the posterior pharynx or tonsillitis.251 Children with polyarticular juvenile rheumatoid arthritis frequently develop involvement of the cervical joints. Torticollis and limitation of cervical motion may be the only clinical signs. Spontaneous atlantoaxial rotatory subluxation may follow acute pharyngitis.250,252 Radiographic confirmation is difficult, and the CT methods for evaluation suggested by Phillips and Hensinger252 should be used. Early diagnosis and reduction of the displacement are important.252,253 If it becomes fixed, it poses a considerable treatment problem.252 A rare inflammatory cause is acute calcification of a cervical disc,254,255 which can be visualized on routine radiographic study of the neck (Fig. 30–54).
Traumatic causes should always be considered and carefully excluded early in the evaluation. If unrecognized, they may have serious neurologic consequences. Generally, torticollis most commonly follows injury to the C1-2 articulation. Minor trauma can lead to spontaneous C1-2 subluxation. Fractures or dislocation of the odontoid may not be apparent in the initial radiographic views (see Fig. 30–18), and consequently a high index of suspicion and careful follow-up are required. Children with bone dysplasia, Morquio syndrome, spondyloepiphyseal dysplasia, and Down syndrome have a high incidence of C1-2 instability and should be evaluated routinely.
Intermittent torticollis can occur in a young child. A seizurelike disorder termed benign paroxysmal torticollis of infancy has many neurologic causes, including drug intoxication. Similarly, Sandifer syndrome, involving gastroesophageal reflux with sudden posturing of the trunk and torticollis, is being recognized more often, particularly in neurologically handicapped children, such as children with cerebral palsy.256
Neurologic disorders, particularly space-occupying lesions of the central nervous system, such as tumors of the posterior fossa or spinal column, chordoma, and syringomyelia, are often accompanied by torticollis. Generally, there are additional neurologic findings, such as long tract signs and weakness in the upper extremities. Uncommon neurologic causes include dystonia musculorum deformans and problems of hearing and vision that can result in head tilt.257 Although uncommon, hysterical and psychogenic causes exist, but these should be diagnosed only after other causes are carefully excluded.
Radiographic Features
Rotatory subluxation of C1 and C2 presents a unique problem. Plain radiographs seldom differentiate the position of C1 and C2 during subluxation from that in a normal child whose head is rotated because both give the same picture. Open-mouth views have been difficult to obtain and interpret. Lack of cooperation and decreased neck motion on the part of the child can make it impossible to obtain these special views. Fielding and Hawkins250 recommended cineradiography, but the radiation dosage is relatively high, and patient cooperation may be difficult to obtain because of muscle spasm.
The normal relationship between the occiput and the atlas is thought to be rarely affected in atlantoaxial rotatory subluxation. A lateral radiograph of the skull may show the relative position of the atlas and axis more clearly than a lateral radiograph of the cervical spine, in which tilting of the head also tilts the atlas, and overlapping shadows make interpretation difficult (Fig. 30–55). Similarly, if during CT scans the child is in the torticollis position, the image may be interpreted by the radiologist as showing rotation of C1 and C2. Conversely, in a child with rotatory subluxation, the rotation of C1 and C2 may be within the range of normal, as is usually the case. Early in this condition, the radiologist may attribute the finding to patient positioning.245 This dilemma can be resolved by paying close attention to proper positioning of the patient, obtaining CT cuts at the level of C1, and rotating the head to the right and left and showing the facets to be locked in that position.245
Key Points
1 Aronson DD, Kahn RH, Canady A, et al. Instability of the cervical spine after decompression in patients who have Arnold-Chiari malformation. J Bone Joint Surg Am. 1991;73:898-906.
2 Chamberlain WE. Basilar impression (platybasia): A bizarre developmental anomaly of the occipital bone and upper cervical spine with striking and misleading neurologic manifestations. Yale J Biol Med. 1939;11:487.
3 Currarino G, Rollins N, Diehl JT. Congenital defects of the posterior arch of the atlas: A report of seven cases including an affected mother and son. AJNR Am J Neuroradiol. 1994;15:249-254.
4 De Barros MC, Faria W, Ataide L, et al. Basilar impression and Arnold-Chiari malformation: A study of 66 cases. J Neurol Neurosurg Psychiatry. 1968;1:596.
5 Fielding JW, Griffin PO. Os odontoideum: An acquired lesion. J Bone Joint Surg Am. 1974;56:187.
The authors postulated the concept of a traumatic and a congenital origin of os odontoideum.
6 Fielding JW, Hawkins RJ, Ratzan S. Fusion for atlantoaxial instability. J Bone Joint Surg Am. 1976;58:400.
In this landmark article, the authors discussed fusion for atlantoaxial instability.
7 Hall JE, Simmons ED, Danylchuk K, et al. Instability of the cervical spine and neurological involvement in Klippel-Feil syndrome: A case report. J Bone Joint Surg Am. 1990;92:460-462.
8 Hensinger RN, Lang JR, MacEwen GD. The Klippel-Feil syndrome: A constellation of related anomalies. J Bone Joint Surg Am. 1974;56:1246.
9 Locke GR, Gardner JI, Van Epps EF. Atlas-dens interval (ADI) in children: A survey based on 200 normal cervical spines. AJR Am J Roentgenol. 1966;97:135.
These authors were the first to define the ADI in children.
10 McAfee PC, Bohlman HH, Han JS, et al. Comparison of nuclear magnetic resonance imaging and computed tomography in the diagnosis of upper cervical spinal cord compression. Spine. 1986;11:295.
These authors helped to define the new role of CT and MRI in evaluating basilar invagination.
11 McRae DL. Bony abnormalities in the region of the foramen magnum: Correlation of the anatomic and neurologic findings. Acta Radiol. 1953;40:335.
12 McRae DL, Barnum AS. Occipitalization of the atlas. AJR Am J Roentgenol Radium Ther Nucl Med. 1953;70:23.
13 Sawin PD, Menezes AH. Basilar invagination in osteogenesis imperfecta and related osteochondral dysplasias: Medical and surgical management. J Neurosurg. 1997;86:950-960.
1 Chamberlain WE. Basilar impression (platybasia): A bizarre developmental anomaly of the occipital bone and upper cervical spine with striking and misleading neurologic manifestations. Yale J Biol Med. 1939;11:487.
2 Gosain AK, McCarthy JG, Pinto RS. Cervicovertebral anomalies and basilar impression in Goldenhar syndrome. Plast Reconstr Surg. 1994;93:498-506.
3 Menezes AH. Specific entities affecting the craniocervical region. Childs Nerv Syst. 2008;24:1169-1172.
4 Sawin PD, Menezes AH. Basilar invagination in osteogenesis imperfecta and related osteochondral dysplasias: Medical and surgical management. J Neurosurg. 1997;86:950-960.
5 Brooks BL, Gall C, Wang AM, et al. Osteogenesis imperfecta associated with basilar impression and cerebral atrophy: A case report. Comput Med Imaging Graph. 1989;13:363-367.
6 Epstein BS, Epstein JA. The association of cerebellar tonsillar herniation with basilar impression incident to Paget’s disease. AJR Am J Roentgenol Radium Ther Nucl Med. 1969;107:535.
7 McGregor M. The significance of certain measurements of the skull in the diagnosis of basilar impression. Br J Radiol. 1948;21:171.
8 Hinck VC, Hopkins CE, Savara BS. Diagnostic criteria of basilar impression. Radiology. 1961;76:572.
9 Clay WC, Sturm PF, Hatch RS, et al. Intraobserver reproducibility and interobserver reliability of cervical spine measurements. J Pediatr Orthop. 2000;20:66-74.
10 Cronin CG, Lohan DG, Mhuircheartigh JN, et al. MRI evaluation and measurement of the normal odontoid ped position. Clin Radiol. 2007;62:897-903.
11 Fischgold H, Metzger J. Étude radiotomographique de l’impression basilaire. Rev Rhum Mal Osteoartic. 1952;19:261.
12 Adam AM. Skull radiographic measurements of normals and patients with basilar impression: Use of Landzert’s angle. Surg Radiol Anat. 1987;9:225.
13 Samartzis D, Kalluri P, Herman J, et al. Superior odontoid migration in the Klippel-Feil patient. Eur Spine J. 2007;16:1489-1497.
14 De Barros MC, Faria W, Ataide L, et al. Basilar impression and Arnold-Chiari malformation: A study of 66 cases. J Neurol Neurosurg Psychiatry. 1968;1:596.
15 Bassi P, Corona C, Contri P, et al. Congenital basilar impression: Correlated neurologic syndromes. Eur Neurol. 1992;32:238-243.
16 Kohno K, Sakaki S, Nakamura H, et al. Foramen magnum decompression for syringomyelia associated with basilar impression and Chiari I malformation: Report of three cases. Neurol Med Chir. 1991;31:715-719.
17 da Silva JA. Basilar impression and Arnold-Chiari malformation: Surgical findings in 209 cases. Neurochirurgia. 1992;35:189-195.
18 Ali MM, Russell N, Awada N, et al. A craniocervical malformation presenting as acute respiratory failure. J Emerg Med. 1996;14:569-572.
19 Bernini F, Elefante R, Smaltino F, et al. Angiographic study on the vertebral artery in cases of deformities of the occipitocervical joint. AJR Am J Roentgenol Radium Ther Nucl Med. 1969;107:526.
20 Dickinson LD, Tuite GF, Colon GP, et al. Vertebral artery dissection related to basilar impression: Case report. Neurosurgery. 1995;36:835-838.
21 Gunderson CH, Greenspan RH, Glaser GH, et al. Klippel-Feil syndrome: Genetic and clinical re-evaluation of cervical fusion. Medicine. 1967;46:491-511.
22 Michie I, Clark M. Neurological syndromes associated with cervical and craniocervical anomalies. Arch Neurol. 1968;18:241.
23 Bachs A, Barraquer-Bordas L, Barraquer-Ferre L, et al. Delayed myelopathy following atlantoaxial dislocations by separated odontoid process. Brain. 1955;78:537.
24 Barker R, Fareedi S, Thompson D, et al. The use of CT angiography in the preoperative planning of cervical spine surgery in children. Childs Nerv Syst. 2009;25:955-959.
25 Fromm GH, Pitner SE. Late progressive quadriparesis due to odontoid agenesis. Arch Neurol. 1963;9:291.
26 Goel A, Shah A. Reversal of longstanding musculoskeletal changes in basilar invagination after surgical decompression and stabilization. J Neurosurg Spine. 2009;10:220-227.
27 Goel A, Shah A, Rajan S. Vertical mobile and reducible atlantoaxial dislocation. J Neurosurg Spine. 2009;11:9-14.
28 Kumar R, Kalra SK, Mahapatra AK. A clinical scoring system for neurological assessment of high cervical myelopathy: Measurements in pediatric patients with congenital atlantoaxial dislocations. Neurosurgery. 2007;61:987-994.
29 Hosalkar HS, Sankar WN, Wills BPD, et al. Congenital osseous anomalies of the upper cervical spine. J Bone Joint Surg Am. 2008;90:337-348.
30 Locke GR, Gardner JI, Van Epps EF. Atlas-dens interval (ADI) in children: A survey based on 200 normal cervical spines. AJR Am J Roentgenol. 1966;97:135.
31 von Torklus D, Gehle W. The Upper Cervical Spine. New York: Grune & Stratton; 1972.
32 Gholve PA, Hosalkar HS, Ricchetti ET, et al. Occipitalization of the atlas in children. J Bone Joint Surg Am. 2007;89:571-578.
33 McRae DL. Bony abnormalities in the region of the foramen magnum: Correlation of the anatomic and neurologic findings. Acta Radiol. 1953;40:335.
34 Greenberg AD. Atlantoaxial dislocations. Brain. 1968;91:655.
35 Spillane JD, Pallis C, Jones AM. Developmental abnormalities in the region of the foramen magnum. Brain. 1957;80:11.
36 Nagashima C. Atlanto-axial dislocation due to agenesis of the os odontoideum or odontoid. J Neurosurg. 1970;33:270.
37 Bharucha EP, Dastur HM. Craniovertebral anomalies (a report on 40 cases). Brain. 1964;87:469.
38 McRae DL. The significance of abnormalities of the cervical spine. AJR Am J Roentgenol. 1960;84:3.
39 Perovic NM, Kopits SE, Thompson RC. Radiologic evaluation of the spinal cord in congenital atlanto-axial dislocations. Radiology. 1973;109:713.
40 Spitzer R, Rabinowitch JY, Wybar KC. A study of the abnormalities of the skull, teeth, and lenses in mongolism. Can Med Assoc J. 1961;84:567-572.
41 Reilly CW, Choit RL. Transarticular screws in the management of C1-C2 instability in children. J Pediatr Orthop. 2006;26:582-588.
42 Burke SW, French HG, Roberts JM, et al. Chronic atlanto-axial instability in Down syndrome. J Bone Joint Surg Am. 1985;67:1356-1360.
43 Morton RE, Khan MA, Murray-Leslie C, et al. Atlantoaxial instability in Down syndrome: A five-year follow up study. Arch Dis Child. 1995;72:115-119.
44 Ferguson RL, Putney ME, Allen BL. Comparison of neurological deficits with atlanto-dens intervals in patients with Down syndrome. J Spinal Disord. 1997;10:246-252.
45 Taggard DA, Menezes AH, Ryken TC. Treatment of Down syndrome-associated craniovertebral junction anomalies. J Neurosurg Spine. 2000;93:205-213.
46 Hinck VC, Hopkins CE, Savara BS. Sagittal diameter of the cervical spinal canal in children. Radiology. 1962;79:97.
47 Fielding JW, Hensinger RN, Hawkins RJ. Os odontoideum. J Bone Joint Surg Am. 1980;62:376.
48 Fielding JW, Cochran GV, Lawsing JFIII, et al. Tears of the transverse ligament of the atlas. J Bone Joint Surg Am. 1974;56:1683.
49 Klimo PJr, Kan P, Rao G, et al. Os odentoideum: Presentation, diagnosis, and treatment in a series of 78 patients. J Neurosurg Spine. 2008;9:332-342.
50 Hensinger RN. Osseous anomalies of the craniovertebral junction. Spine. 1986;111:323.
51 Roach JW, Duncan D, Wenger DR, et al. Atlanto-axial instability and spinal cord compression in children: Diagnosis by computerized tomography. J Bone Joint Surg Am. 1984;66:708.
52 Gupta V, Khandelwal N, Mathuria SN, et al. Dynamic magnetic resonance imaging evaluation of craniovertebral junction abnormalities. J Comput Assist Tomogr. 2007;31:354-359.
53 Smoker WRK, Khanna G. Imaging the craniocervical junction. Childs Nerv Syst. 2008;24:1123-1145.
54 Giannestras NJ, Mayfield FH, Provencio FP, et al. Congenital absence of the odontoid process: A case report. J Bone Joint Surg Am. 1964;46:839.
55 Dickman CA, Mamourian A, Sonntag VK, et al. Magnetic resonance imaging of the transverse atlantal ligament for the evaluation of atlantoaxial instability. J Neurosurg. 1991;75:221-227.
56 Jauregui N, Lincoln T, Mubarak S, et al. Surgically related upper cervical spine canal anatomy in children. Spine. 1993;18:1939-1944.
57 McAfee PC, Bohlman HH, Han JS, et al. Comparison of nuclear magnetic resonance imaging and computed tomography in the diagnosis of upper cervical spinal cord compression. Spine. 1986;11:295.
58 Weng MS, Haynes RJ. Flexion and extension cervical MRI in pediatric population. J Pediatr Orthop. 1996;16:359-363.
59 Steel HH. Anatomical and mechanical considerations of the atlanto-axial articulations. J Bone Joint Surg Am. 1968;50:1481.
60 Fielding JW, Hawkins RJ, Ratzan SA. Spine fusion for atlanto-axial instability. J Bone Joint Surg Am. 1976;58:400.
61 Haworth JB, Keillor GW. Use of transparencies in evaluating the width of the spinal canal in infants, children and adults. Radiology. 1962;79:109.
62 Naik DR. Cervical spinal canal in normal infants. Clin Radiol. 1970;21:323.
63 Dolan KD. Expanding lesions of the cervical spinal canal. Radiol Clin North Am. 1977;15:203.
64 Cattell HS, Filtzer DL. Pseudosubluxation and other normal variations in the cervical spine in children. J Bone Joint Surg Am. 1965;47:1295.
65 Garber JN. Abnormalities of the atlas and axis vertebrae, congenital and traumatic. J Bone Joint Surg Am. 1964;46:1782.
66 Swischuk LE. Anterior displacement of C2 in children: Physiologic or pathologic? Radiology. 1977;12:759-763.
67 Geehr RB, Rothman SLG, Kier EL. The role of computed tomography in the evaluation of upper cervical spine pathology. Comput Tomogr. 1978;2:79.
68 Resjo M, Harwood-Nash DC, Fitz CR. Normal cord in infants and children examined with computed tomographic metrizamide myelography. Radiology. 1979;130:691.
69 Bhatnagar M, Sponseller PD, Carroll CIV, et al. Pediatric atlantoaxial instability presenting as cerebral and cerebellar infarcts. J Pediatr Orthop. 1991;11:103-107.
70 Miyata I, Imaoka T, Masaoka T, et al. Pediatric cerebellar infarction caused by atlantoaxial subluxation: Case report. Neurol Med Chir. 1994;34:241-245.
71 Ahmed R, Trynelis VC, Menezes AH. Fusions at the craniovertebral junction. Childs Nerv Syst. 2008;24:1209-1224.
72 Sinh G, Pandya SK. Treatment of congenital atlanto-axial dislocations. Proc Aust Assoc Neurol. 1968;5:507.
73 Wadia NH. Myelopathy complicating congenital atlanto-axial dislocation (a study of 28 cases). Brain. 1967;90:449.
74 Grantham SA, Dick HM, Thompson RCJr, et al. Occipito-cervical arthrodesis: Indications, technic and results. Clin Orthop. 1969;65:118.
75 Krieger AJ, Rosomoff HL, Kuperman AS, et al. Occult respiratory dysfunction in a craniovertebral anomaly. J Neurosurg. 1969;31:15.
76 Reddy KR, Rao GS, Devi BI, et al. Pulmonary function after surgery for congenital atlantoaxial dislocation. J Neurosurg Anesthesiol. 2009;21:196-201.
77 Pueschel SM, Scola FH. Atlantoaxial instability in individuals with Down’s syndrome: Epidemiologic, radiographic, and clinical studies. Pediatrics. 1987;80:555.
78 Davidson RG. Atlantoaxial instability in individuals with Down’s syndrome: A fresh look at the evidence. J Bone Joint Surg Am. 1965;47:1295.
79 Georgopoulos G, Pizzutillo PD, Lee MS. Occipitoatlantal instability in children: A report of five cases and review of the literature. J Bone Joint Surg Am. 1987;69:429.
80 Browd SR, McIntyre JS, Brockmeyer D. Failed age-dependent maturation of the occipital condyle in patients with congenital occipitoatlantal instability and Down syndrome: A preliminary analysis. J Neurosurg Pediatrics. 2008;2:359-364.
81 Chandraraj S, Briggs CA. Failure of somite differentiation at the craniovertebral region as a cause of occipitalization of the atlas. Spine. 1992;17:1249-1251.
82 Macalister A. Notes on the development and variations of the atlas. J Anat Physiol. 1983;27:519.
83 McRae DL, Barnum AS. Occipitalization of the atlas. AJR Am J Roentgenol Radium Ther Nucl Med. 1953;70:23.
84 Malhotra V, Leeds NE. Case report 277. Skeletal Radiol. 1984;12:55-58.
85 Hadley LA. The Spine. Springfield, IL: Charles C Thomas; 1956.
86 Bailey DK. The normal cervical spine in infants and children. Radiology. 1962;59:712.
87 Caffey J. Paediatric X-ray Diagnosis. Chicago: Year Book Medical Publishers; 1967.
88 Menezes AH. Craniocervical developmental anatomy and its implications. Childs Nerv Syst. 2008;24:1109-1122.
89 Senoglu M, Safavi-Abbasi S, Theodore N, et al. The frequency and clinical significance of congenital defects of the posterior and anterior arch of the atlas. J Neurosurg Spine. 2007;7:399-402.
90 Musha Y, Mizutani K. Cervical myelopathy accompanied with hypoplasia of the posterior arch of the atlas. J Spinal Disord Tech. 2009;22:228-232.
91 Fielding JW. The cervical spine in the child. Curr Pract Orthop Surg. 1973;5:31.
92 Baird PA, Robinson GC, Buckler WS. Klippel-Feil syndrome. Am J Dis Child. 1967;113:546.
93 Nicholson JS, Sherk HH. Anomalies of the occipitocervical articulation. J Bone Joint Surg Am. 1968;50:295.
94 DiLorenzo N. Craniocervical junction malformation treated by transoral approach: A survey of 25 cases with emphasis on postoperative instability and outcome. Acta Neurochir. 1992;118:112-116.
95 Dickman CA, Locantro J, Fessler RG. The influence of transoral odontoid resection on stability of the craniovertebral junction. J Neurosurg. 1992;77:525-530.
96 Aronson DD, Kahn RH, Canady A, et al. Instability of the cervical spine after decompression in patients who have Arnold-Chiari malformation. J Bone Joint Surg Am. 1991;73:898-906.
97 Fehlings MG, Errico T, Cooper P, et al. Occipitocervical fusion with a five-millimeter malleable rod and segmental fixation. Neurosurgery. 1993;32:198-207.
98 Letts M, Slutsky D. Occipitocervical arthrodesis in children. J Bone Joint Surg Am. 1990;72:1166-1170.
99 Rea GL, Mullin BB, Mervis LJ, et al. Occipitocervical fixation in nontraumatic upper cervical spine instability. Surg Neurol. 1993;40:255-261.
100 McLaughlin MR, Wahlig JB, Pollack IF. Incidence of postlaminectomy kyphosis after Chiari decompression. Spine. 1997;22:613-617.
101 Geipel P. Zur Kenntnis der Spina bifida das Atlas. Forstschr Rontgenstr. 1930;42:583-589.
102 Currarino G, Rollins N, Diehl JT. Congenital defects of the posterior arch of the atlas: A report of seven cases including an affected mother and son. AJNR Am J Neuroradiol. 1994;15:249-254.
103 Dubousset J. Torticollis in children caused by congenital anomalies of the atlas. J Bone Joint Surg Am. 1986;68:178.
104 Stevens JM, Chong WK, Barber C, et al. A new appraisal of abnormalities of the odontoid process associated with atlantoaxial subluxation and neurological disability. Brain. 1994;117:133-148.
105 Michaels L, Prevost MJ, Crong DF. Pathological changes in a case of os odontoideum (separate odontoid process). J Bone Joint Surg Am. 1969;51:965.
106 Gwinn JL, Smith JL. Acquired and congenital absence of the odontoid process. Am J Roentgenol Radium Ther Nucl Med. 1962;88:424.
107 Minderhoud JM, Braakman R, Penning L. Os odontoideum: Clinical, radiological, and therapeutic aspects. J Neurol Sci. 1969;89:521.
108 Schiller F, Nieda I. Malformations of the odontoid process: Report of a case and clinical survey. Calif Med. 1957;86:394.
109 Wollin DG. The os odontoideum: Separate odontoid process. J Bone Joint Surg Am. 1963;45:1459.
110 Curtis BH, Blank S, Fisher RL. Atlantoaxial dislocation in Down’s syndrome. JAMA. 1968;205:464.
111 Dzenitis AJ. Spontaneous atlantoaxial dislocation in a mongoloid child with spinal cord compression: Case report. J Neurosurg. 1966;25:458.
112 Hensinger RN, Lang JR, MacEwen GD. The Klippel-Feil syndrome: A constellation of related anomalies. J Bone Joint Surg Am. 1974;56:1246.
113 Martel W, Fishler JM. Observation of the spine in mongoloidism. Am J Roentgenol Radium Ther Nucl Med. 1966;97:630.
114 Sherk HH, Nicholson JL. Ossiculum terminale and mongolism. J Bone Joint Surg Am. 1969;51:957.
115 Sankar WN, Wills BPD, Dormans JP, et al. Os odentoideum revisited: The case for a multifactorial etiology. Spine. 2006;31:979-984.
116 Basset FH, Goldner JL. Aplasia of the odontoid process. Proc Am Acad Orthop Surg. 1968;50:833.
117 Shapiro R, Youngsberg AS, Rothman SLG. The differential diagnosis of traumatic lesions of the occipito-atlanto-axial segment. Radiol Clin North Am. 1971;3:505.
118 Rothman RH, Simeone FA. The Spine, 2nd ed. Philadelphia: WB Saunders; 1982.
119 Evarts CM, Lonsdale D. Ossiculum terminale: An anomaly of the odontoid process: Report of a case of atlantoaxial dislocation with cord compression. Cleve Clin Q. 1970;37:73.
120 Swoboda B, Hirschfelder H, Hohmann D. Atlantoaxial instability in a 7-year-old boy associated with traumatic disrupture of the ossiculum terminale (apical odontoid epiphysis). Eur Spine J. 1995;4:248-251.
121 Ahlback I, Collert S. Destruction of the odontoid process due to axial pyogenic spondylitis. Acta Radiol [Diagn] (Stockh). 1970;10:394.
122 Fielding JW. Disappearance of the central portion of the odontoid process. J Bone Joint Surg Am. 1965;47:1228.
123 Fielding JW, Griffin PO. Os odontoideum: An acquired lesion. J Bone Joint Surg Am. 1974;56:187.
124 Freiberger RH, Wilson PDJr, Nicholas JA. Acquired absence of the odontoid process. J Bone Joint Surg Am. 1965;47:1231.
125 Phillips PC, Lorentsen KJ, Shropshire LC, et al. Congenital odontoid aplasia and posterior circulation stroke in childhood. Ann Neurol. 1988;23:410-413.
126 Kirlew KA, Hathout GM, Reiter SD, et al. Os odontoideum in identical twins: Perspectives on etiology. Skeletal Radiol. 1993;22:525-527.
127 Morgan MK, Onofrio BM, Bender CE. Familial os odontoideum: Case report. J Neurosurg. 1989;70:636-639.
128 Holt RG, Helms CA, Munk PL, et al. Hypertrophy of C-1 anterior arch: Useful sign to distinguish os odontoideum from acute dens fracture. Radiology. 1989;173:207-209.
129 Watanabe M, Toyama Y, Fujimura Y. Atlantoaxial instability in os odontoideum with myelopathy. Spine. 1996;21:1435-1439.
130 McKeever FM. Atlantoaxial instability. Surg Clin North Am. 1968;48:1375.
131 Rowland LP, Shapiro JH, Jacobson HG. Neurological syndromes associated with congenital absence of the odontoid process. Arch Neurol Psychiatry. 1958;80:286.
132 Ford FK. Syncope, vertigo, and disturbances of vision resulting from intermittent obstruction of the vertebral arteries due to a defect in the odontoid process and excessive mobility of the axis. Bull Johns Hopkins Hosp. 1952;91:168.
133 Kikuchi K, Nakagawa H, Watanabe K, et al. Bilateral vertebral artery occlusion secondary to atlantoaxial dislocation with os odontoideum: Implication for prophylactic cervical stabilization by fusion: Case report. Neurol Med Chir. 1993;33:769-773.
134 Takakuwa T, Hiroi S, Hasegawa H, et al. Os odontoideum with vertebral artery occlusion. Spine. 1994;19:460-462.
135 Gillman CL. Congenital absence of the odontoid process of the axis: Report of a case. J Bone Joint Surg Am. 1959;41:340.
136 Hosono N, Yonenobu K, Ebara S, et al. Cineradiographic motion analysis of atlantoaxial instability in os odontoideum. Spine. 1991;16(Suppl):S480-S482.
137 Elliott S. The odontoid process in children: Is it hypoplastic? Clin Radiol. 1988;39:391-393.
138 Spierings EL, Braakman R. The management of os odontoideum: Analysis of 37 cases. J Bone Joint Surg Br. 1982;64:422-428.
139 Fagan AB, Askin GN, Earwaker JW. The jigsaw sign: A reliable indicator of congenital etiology in os odontoideum. Eur Spine J. 2004;13:295-300.
140 Yamashita Y, Takahashi M, Sakamoto Y, et al. Atlantoaxial subluxation: Radiography and magnetic resonance imaging correlated to myelopathy. Acta Radiol. 1989;30:135-140.
141 Hughes TB, Richman JD, Rothfus WE. Diagnosis of os odontoideum using kinematic magnetic resonance imaging. Spine. 1999;24:715-718.
142 Shepard CN. Familial hypoplasia of the odontoid process. J Bone Joint Surg Am. 1966;48:1224.
143 Greenberg AD, Scovillo WB, Davey LM. Transoral decompression of atlantoaxial dislocation due to odontoid hypoplasia: Report of two cases. J Neurosurg. 1968;28:266.
144 Levy ML, McComb JG. C1-C2 fusion in children with atlantoaxial instability and spinal cord compression: Technical note. Neurosurgery. 1996;38:211-215.
145 Huang CI, Chen IH. Atlantoaxial arthrodesis using Halifax interlaminar clamps reinforced by halo vest immobilization: A long-term follow-up experience. Neurosurgery. 1996;38:1153-1156.
146 Moskovich R, Crockard HA. Atlantoaxial arthrodesis using interlaminar clamps: An improved technique. Spine. 1992;17:261-267.
147 Dickman CA, Crawford NR, Paramore CG. Biomechanical characteristics of C1-2 cable fixations. J Neurosurg. 1996;85:316-322.
148 Smith MD, Phillips WA, Hensinger RN. Fusion of the upper cervical spine in children and adolescents: An analysis of 17 patients. Spine. 1991;16:695-701.
149 Brockmeyer D, Apfelbaum R, Tippets R, et al. Pediatric cervical spine instrumentation using screw fixation. Pediatr Neurosurg. 1995;22:147-157.
150 Coyne TJ, Fehlings MG, Wallace MC, et al. C1-C2 posterior cervical fusion: Long-term evaluation of results and efficacy. Neurosurgery. 1995;37:688-693.
151 Stabler CL, Eismont FJ, Brown MD, et al. Failure of posterior cervical fusions using cadaveric bone graft in children. J Bone Joint Surg Am. 1985;67:370-375.
152 Menendez JA. Techniques of posterior C1-C2 stabilization. Neurosurgery. 60(Suppl 1), 2007. s1-103–s1-s111
153 Visocchi M, Fernandez E, Ciampini A, et al. Reducible and irreducible os odentoideum in childhood treated with posterior wiring, instrumentation and fusion. Past or present? Acta Neurochir (Wien). 2009;151:1265-1274.
154 Mah JY, Thometz J, Emans J, et al. Threaded K-wire spinous process fixation of the axis for modified Gallie fusion in children and adolescents. J Pediatr Orthop. 1989;9:675-679.
155 Grob D, Crisco JJIII, Panjabi MM, et al. Biomechanical evaluation of four different posterior atlantoaxial fixation techniques. Spine. 1992;17:480-490.
156 Bambakidis NC, Feiz-Erfan I, Horn EM, et al. Biomechanical comparison of occipitoatlantal screw fixation techniques. J Neurosurg Spine. 2008;8:143-152.
157 Haque A, Price AV, Sklar FH, et al. Screw fixation of the upper cervical spine in the pediatric population. J Neurosurg Pediatrics. 2009;3:529-533.
158 Chamoun RB, Relyea KM, Johnson KK, et al. Use of axial and subaxial translaminar screw fixation in the management of upper cervical spinal instability in a series of 7 children. Neurosurgery. 2009;64:734-739.
159 Hoh DJ, Maya M, Jung A, et al. Anatomical relationship of the internal carotid artery to C-1: Clinical implications for screw fixation of the atlas. J Neurosurg Spine. 2008;8:335-340.
160 Smith MD, Phillips WA, Hensinger RN. Complications of fusion to the upper cervical spine. Spine. 1991;16:702-705.
161 Dyck P. Os odontoideum in children: Neurological manifestations and surgical management. Neurosurgery. 1978;2:93.
162 Whitesides TE, McDonald AP. Lateral retropharyngeal approach to the upper cervical spine. Orthop Clin North Am. 1978;9:1115.
163 Klippel M, Feil A. Un cas d’absence des vertèbres cervicales avec cage thoracique remontant jusqu’a la base du crane. Nouv Icon Salpet. 1912;25:223.
164 Feil A. L’absence et la diminution des vertèbres cervicales (étude clinique et pathogenique): Le syndrome de réduction numérique cervicale. Thèses de Paris. 1919.
165 Erskine CA. An analysis of the Klippel-Feil syndrome. Arch Pathol. 1946;41:269.
166 Gray SW, Romaine CB, Skandalakis JF. Congenital fusion of the cervical vertebrae. Surg Gynecol Obstet. 1964;118:373.
167 Tracy MR, Dormans JP, Kusumi K. Klippel-Feil syndrome: Clinical features and current understanding of etiology. Clin Orthop. 2004;424:183-190.
168 Clarke RA, Kearsley JH, Walsh DA. Patterned expression in familial Klippel-Feil syndrome. Teratology. 1996;53:152-157.
169 Clarke RA, Singh S, McKenzie H, et al. Familial Klippel-Feil syndrome and paracentric inversion inv(8)(q22.2q.23.3). Am J Hum Genet. 1995;57:1364-1370.
170 Schilgen M, Loeser H. Klippel-Feil anomaly combined with fetal alcohol syndrome. Eur Spine J. 1994;3:289-290.
171 McElfresh E, Winter R. Klippel-Feil syndrome. Minn Med. 1973;56:353.
172 Ramsey J, Bliznak J. Klippel-Feil syndrome with renal agenesis and other anomalies. AJR Am J Roentgenol. 1971;113:460.
173 Bauman GI. Absence of the cervical spine: Klippel-Feil syndrome. JAMA. 1932;98:129.
174 Baga N, Chusid EL, Miller A. Pulmonary disability in the Klippel-Feil syndrome. Clin Orthop. 1969;67:105.
175 Morrison SG, Perry LW, Scott LP. Congenital brevicollis (Klippel-Feil syndrome) and cardiovascular anomalies. Am J Dis Child. 1968;115:614.
176 Nora JJ, Cohen M, Maxwell GM. Klippel-Feil syndrome with congenital heart disease. Am J Dis Child. 1961;102:858.
177 McLay K, Maran AG. Deafness and the Klippel-Feil syndrome. J Laryngol Otol. 1969;83:175.
178 Palant DI, Carter BL. Klippel-Feil syndrome and deafness. Am J Dis Child. 1972;123:218.
179 Stark EW, Borton TE. Hearing loss and the Klippel-Feil syndrome. Am J Dis Child. 1972;123:233.
180 Ulmer JL, Elster AD, Ginsberg LE, et al. Klippel-Feil syndrome: CT and MRI of congenital abnormalities of cervical spine and cord. J Comput Assist Tomogr. 1993;17:215-224.
181 Sherk HH, Shut L, Chung S. Iniencephalic deformity of cervical spine with Klippel-Feil anomalies and congenital elevation of the scapula. J Bone Joint Surg Am. 1974;56:1254.
182 Frawley JM. Congenital webbing. Am J Dis Child. 1925;29:799.
183 Shoul MI, Ritvo M. Clinical and roentgenological manifestations of the Klippel-Feil syndrome (congenital fusion of the cervical vertebrae, brevicollis): Report of eight additional cases and review of the literature. AJR Am J Roentgenol. 1952;68:369.
184 Samartzis D, Herman J, Lubicky JP, Shen FH. Sprengel’s deformity in Klippel-Feil syndrome. Spine. 2007;32:E512-E516.
185 Mooney JFIII, White DR, Glazier S. Previously unreported structure associated with Sprengel deformity. J Pediatr Orthop. 2009;29:26-28.
186 Gumerlock MK, Spollen LE, Nelson MJ, et al. Cervical neurenteric fistula causing recurrent meningitis in Klippel-Feil sequence: Case report and literature review. Pediatr Infect Dis J. 1991;10:532-535.
187 Guille JT, Miller A, Bowen JR, et al. The natural history of Klippel-Feil syndrome: Clinical, roentgenographic, and magnetic resonance imaging findings at adulthood. J Pediatr Orthop. 1995;15:617-626.
188 Prasad VS, Reddy DR, Murty JM. Cervicothoracic neurenteric cyst: Clinicoradiological correlation with embryogenesis. Childs Nerv Syst. 1996;12:48-51.
189 Bavinck JN, Weaver DD. Subclavian artery supply disruption sequence: Hypothesis of a vascular etiology for Poland, Klippel-Feil, and Mobius anomalies. Am J Med Genet. 1986;23:903-918.
190 Pizzutillo PD, Woods MW, Nicholson L. Risk factors in the Klippel-Feil syndrome. Orthop Trans. 1987;11:473.
191 Samartzis D, Kalluri P, Herman J, et al. The extent of fusion within the congenital Klippel-Feil segment. Spine. 2008;33:1637-1642.
192 Samartzis D, Herman J, Lubicky JP, et al. Classification of congenitally fused cervical patters in Klippel-Feil patients. Spine. 2006;31:E798-E804.
193 Hall JE, Simmons ED, Danylchuk K, et al. Instability of the cervical spine and neurological involvement in Klippel-Feil syndrome: A case report. J Bone Joint Surg Am. 1990;92:460-462.
194 Forney WR, Robinson SJ, Pascoe DJ. Congenital heart disease, deafness, and skeletal malformations: A new syndrome? J Pediatr. 1966;68:14.
195 Illingworth RS. Attacks of unconsciousness in association with fused cervical vertebrae. Arch Dis Child. 1956;31:8.
196 Born CT, Petrik M, Freed M, et al. Cerebrovascular accident complicating Klippel-Feil syndrome: A case report. J Bone Joint Surg Am. 1988;79:1412-1415.
197 Ross CA, Curnes JT, Greenwood RS. Recurrent vertebrobasilar embolism in an infant with Klippel-Feil anomaly. Pediatr Neurol. 1987;3:181-183.
198 Ritterbusch JF, McGinty LD, Spar J, et al. Magnetic resonance imaging for stenosis and subluxation in Klippel-Feil syndrome. Spine. 1991;16(Suppl):S539-S541.
199 Kulkarni MV, Williams JC, Yeakley JW, et al. Magnetic resonance imaging in the diagnosis of the cranio-cervical manifestations of the mucopolysaccharidoses. Magn Res Imaging. 1987;5:317.
200 Sullivan RC, Bruwer AJ, Harris L. Hypermobility of the cervical spine in children: A pitfall in the diagnosis of cervical dislocation. Am J Surg. 1958;95:636.
201 Drvaric DM, Ruderman RJ, Conrad RW, et al. Congenital scoliosis and urinary tract abnormalities: Are intravenous pyelograms necessary? J Pediatr Orthop. 1987;7:441.
202 Nguyen VD, Tyrrel R. Klippel-Feil syndrome: Patterns of bony fusion and a wasp-waist sign. Skeletal Radiol. 1993;22:519-523.
203 Samartzis D, Kalluri P, Herman J, et al. The role of congenitally fused cervical segments upon the space available for the cord and associated symptoms in Klippel-Feil patients. Spine. 2008;33:1442-1450.
204 Baba H, Maezawa Y, Furusawa N, et al. The cervical spine in the Klippel-Feil syndrome: A report of 57 cases. Int Orthop. 1995;19:204-208.
205 Jalladeau J. Malformations congénitales associées syndrome de Klippel-Feil. Thèse de Paris. 1936.
206 Yousefzadeh DK, El-Khoury GY, Smith WL. Normal sagittal diameter and variation in the pediatric cervical spine. Pediatr Radiol. 1982;144:319.
207 Hensinger RG. Congenital anomalies of the cervical spine. Clin Orthop. 1991;264:16-38.
208 Shen FH, Samartzis D, Herman J, et al. Radiographic assessment of segmental motion at the atlantoaxial junction in the Klippel-Feil patient. Spine. 2006;31:171-177.
209 Theiss SM, Smith MD, Winter RB. The long-term follow-up of patients with Klippel-Feil syndrome with congenital scoliosis. Spine. 1997;22:1219-1222.
210 Thomsen MN, Schneider U, Weber M, et al. Scoliosis and congenital anomalies associated with Klippel-Feil syndrome types I-III. Spine. 1997;22:396-401.
211 Rosen CL, Novotny EJ, D’Andrea LA, et al. Klippel-Feil sequence and sleep-disordered breathing in two children. Am Rev Respir Dis. 1993;147:202-204.
212 Avery LW, Rentfro CC. The Klippel-Feil syndrome: A pathologic report. Arch Neurol Psychiatry. 1936;36:1068.
213 Moseley JE, Bonforte RJ. Spondylothoracic dysplasia: A syndrome with congenital heart disease. Am J Dis Child. 1961;102:858.
214 Moore WB, Matthews TJ, Rabinowitz R. Genitourinary anomalies associated with Klippel-Feil syndrome. J Bone Joint Surg Am. 1975;57:355.
215 Gunderson CH, Solitare GB. Mirror movements in patients with the Klippel-Feil syndrome: Neuropathologic observations. Arch Neurol. 1968;18:675.
216 Notermans SLH, Go KG, Boonstra S. EMG studies of associated movements in a patient with Klippel-Feil syndrome. Psychiatr Neurol Neurochir. 1970;73:257.
217 Stallmer ML, Vanaharam V, Mashour GA. Congenital cervical spine fusion and airway management: A case series of Klippel-Feil syndrome. J Clin Anesth. 2008;20:447-451.
218 Ruf M, Jenson R, Harms J. Hemivertebra resection in the cervical spine. Spine. 2005;30:380-385.
219 Bonola A. Surgical treatment of the Klippel-Feil syndrome. J Bone Joint Surg Br. 1956;38:440.
220 Campbell RM, Adcox BM, Smith MD, et al. The effect of mid-thoracic VEPTR opening wedge thoracostomy on cervical tilt associated with congenital thoracic scoliosis in patients with thoracic insufficiency syndrome. Spine. 2007;32:2171-2177.
221 Ling CM, Low YS. Sternomastoid tumor and muscular torticollis. Clin Orthop. 1972;86:144.
222 Chan YL, Cheng JC, Metreweil LC. Ultrasonography of congenital muscular torticollis. Pediatr Radiol. 1992;22:356-360.
223 Coventry MB, Harris LE. Congenital muscular torticollis in infancy: Some observations regarding treatment. J Bone Joint Surg Am. 1959;41:815.
224 Babu MKV, Lee P, Mahadev A, et al. Congenital bilateral sternocleidomastoid contracture: A case report. J Pediatr Orthop B. 2009;18:145-147.
225 Brackbill Y, Douthitt TC, West H. Psychophysiological effects in the neonate of prone versus supine placement. J Pediatr. 1973;82:2.
226 National Institutes of Health. National Institute of Child Health and Human Development. Back to Sleep Campaign. Available at http://www.nichd.nih.gov/sids/, July 30, 2009. Accessed
227 Ohman A, Nilsson S, Lagerkvist A-L, et al. Are infants with torticollis at risk of a delay in early motor milestones compared with a control group of healthy infants? Dev Med Child Neurol. 2009;51:545-550.
228 Davids JR, Wenger DR, Mubarak SJ. Congenital muscular torticollis: Sequela of intrauterine or perinatal compartment syndrome. J Pediatr Orthop. 1993;13:141-147.
229 MacDonald C. Sternomastoid tumor and muscular torticollis. J Bone Joint Surg Br. 1969;51:432.
230 Brooks B. Pathologic changes in muscle as a result of disturbances of circulation. Arch Surg. 1922;5:188.
231 Sarant JB, Morrissy RT. Idiopathic torticollis: Sternocleidomastoid myopathy and accessory neuropathy. Muscle Nerve. 1981;4:374.
232 Hummer DCJr, MacEwen GD. The coexistence of torticollis and congenital dysplasia of the hip. J Bone Joint Surg Am. 1972;54:1255.
233 von Heideken J, Green DW, Burke SW, et al. The relationship between developmental dysplasia of the hip and congenital muscular torticollis. J Pediatr Orthop. 2006;26:805-808.
234 Minihane KP, Grayhack JJ, Simmons TD, et al. Developmental dysplasia of the hip in infants with congenital muscular torticollis. Am J Orthop. 2008;37:E155-E158.
235 Brougham DI, Cole WG, Dickens DR, et al. Torticollis due to a combination of sternomastoid contracture and congenital vertebral anomalies. J Bone Joint Surg Br. 1989;71:404.
236 Slate RK, Posnick JC, Armstrong DC, et al. Cervical spine subluxation associated with congenital muscular torticollis and craniofacial asymmetry. Plast Reconstr Surg. 1993;91:1187.
237 Tien YC, Su JY, Lin GT, et al. Ultrasonographic study of the coexistence of muscular torticollis and dysplasia of the hip. J Pediatr Orthop. 2001;21:343-347.
238 Canale ST, Griffin DW, Hubbard CN. Congenital muscular torticollis: Long-term follow-up. J Bone Joint Surg Am. 1982;64:810.
239 de Chalain TM, Katz A. Idiopathic muscular torticollis in children: The Cape Town experience. Br J Plast Surg. 1992;45:397.
240 Cheng JC, Wong MW, Tang SP, et al. Clinical determinants of the outcome of manual stretching in the treatment of congenital muscular torticollis in infants. J Bone Joint Surg Am. 2001;83:679-687.
241 Rogers GF, Oh AK, Mulliken JB. The role of congenital muscular torticollis in the development of deformational plagiocephaly. Plast Reconstr Surg. 2009;123:643-652.
242 Clarren FA. Muscular torticollis. J Bone Joint Surg Am. 1948;30:556.
243 Wirth CJ, Hagena FW, Wuelker N, et al. Biterminal tenotomy for the treatment of congenital muscular torticollis: Long-term results. J Bone Joint Surg Am. 1992;74:427.
244 Lee IJ, Lim SY, Song HS, et al. Complete tight fibrous band release and resection in congenital muscular torticollis. J Plastic Reconstr Aesthet Surg. 2010;63:947-953.
245 Shim JS, Noh KC, Park SJ. Treatment of congenital muscular torticollis in patients older than 8 years. J Pediatr Orthop. 2004;24:683-688.
246 Shim JS, Jang HP. Operative treatment of congenital torticollis. J Bone Joint Surg Br. 2008;90:934-939.
247 Staheli LT. Muscular torticollis: Late results of operative treatment. Surgery. 1971;69:469.
248 Akazawa H, Nakatsuka Y, Miyake Y, et al. Congenital muscular torticollis: Long-term follow-up of thirty-eight partial resections of the sternocleidomastoid muscle. Arch Orthop Trauma Surg. 1993;112:205-209.
249 Gyorgyi G. Les changements morphologiques de la region occipitocervicale associés au torticollis. J Radiol Electrol Med Nucl. 1965;45:797.
250 Fielding JW, Hawkins RJ. Atlanto-axial rotatory fixation (fixed rotatory subluxation of the atlanto-axial joint.). J Bone Joint Surg Am. 1977;59:37.
251 Bredenkamp JR, Maceri DR. Inflammatory torticollis in children. Arch Otolaryngol Head Neck Surg. 1990;116:310.
252 Phillips WA, Hensinger RN. The management of rotatory atlanto-axial subluxation in children. J Bone Joint Surg Am. 1989;71:664.
253 Been HD, Kerkhoffs GM, Maas M. Suspected atlantoaxial rotatory fixation-subluxation. Spine. 2007;32:E163-E167.
254 Melnick JC, Silverman FN. Intervertebral disk calcification in childhood. Radiology. 1963;80:399.
255 Schechter LS, Smith A, Pearl M. Intervertebral disk calcification in childhood. Am J Dis Child. 1972;123:608.
256 Sutcliff J. Torsion spasms and abnormal postures in children with hiatus hernia: Sandifer’s syndrome. Prog Pediatr Radiol. 1969;2:190.
257 Williams CR, O’Flynn E, Clarke NM, et al. Torticollis secondary to ocular pathology. J Bone Joint Surg Br. 1996;78:620.