Congenital abnormalities and assessment of fetal wellbeing
Congenital abnormalities
Fetal abnormality is found in:
• 15% of deaths between 20 weeks gestation and 1 year postnatal
• 1–2% of births, including major and minor anomalies (a major abnormality is an abnormality or abnormalities that result in the death of the baby or severe disability)
The overall incidence in the UK has fallen over the past three decades due to the introduction of screening programmes in pregnancy, the resultant greater success at diagnosis during pregnancy and parents opting to terminate a pregnancy once a severe abnormality has been diagnosed.
The commonest four groups of defects are neural tube defects (3–7/1000), congenital cardiac defects (6/1000), Down’s syndrome (1.5/1000) and cleft lip/palate (1.5/1000) (Table 10.1).
Table 10.1
Major congenital abnormalities
| Abnormality | Approximate incidence (per 1000 births) |
| Neural tube defects | 3–7 |
| Congenital heart disease | 6 |
| Severe mental retardation | 4 |
| Down’s syndrome | 1.5 |
| Cleft lip/palate | 1.5 |
| Talipes | 1–2 |
| Abnormalities of limbs | 1–2 |
| Deafness | 0.8 |
| Blindness | 0.2 |
| Others, including urinary tract anomalies | 2 |
| Total | 15–30 |
Neural tube defects
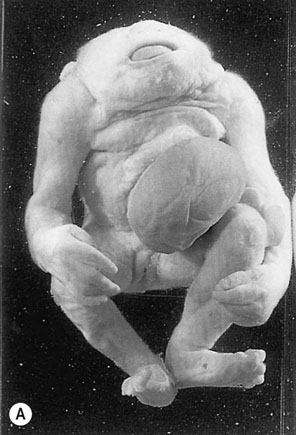
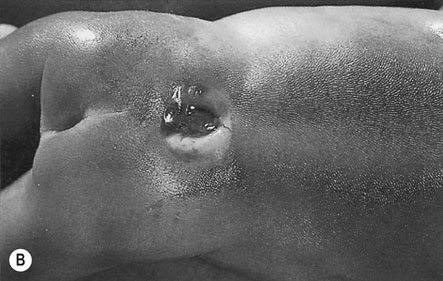
The neural tube defects are the commonest of the major congenital abnormalities and include anencephaly, microcephaly, spina bifida with or without myelomeningocele, encephalocele, holoprosencephaly and hydranencephaly (Fig. 10.1). The incidence is approximately 1/200 and the chance of having an affected child after one previous abnormal child is 1/20. Infants with anencephaly or microcephaly do not usually survive. Many die during labour and the remainder within the first week of life. Infants with open neural tube defects often survive, particularly where it is possible to cover the lesion surgically with skin. However, the defect may result in paraplegia and bowel and bladder incontinence. The child often has normal intelligence and becomes aware of the problems posed for the parents. Closed lesions generally do not cause problems and may escape detection until after birth.
Congenital cardiac defects
Some of these infants present with intrauterine growth retardation and oligohydramnios, but in many cases the diagnosis is recognised and diagnosed after delivery. With improvements in real-time ultrasound imaging, recognition of many cardiac defects has become possible; however, early recognition is essential if any action is to be taken. A four-chamber view of the fetal heart is shown in Figure 10.2.
Defects of the abdominal wall
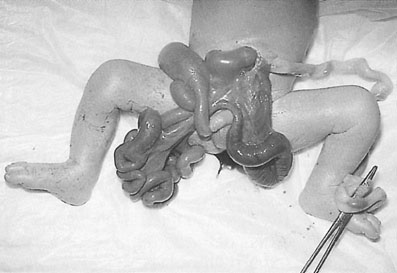
Defects of the abdominal wall can be diagnosed by ultrasound imaging. They include gastroschisis and exomphalos (Fig. 10.3). In both cases, the bowel extrudes outside the abdominal cavity. The main differences between the two are that a gastroschisis is a defect that is separate from the umbilical cord (usually 2–3 cm below and to the right), does not have a peritoneal covering and is usually an isolated problem. In contrast, an exomphalos is essentially a large hernia of the umbilical cord with a peritoneal covering and an increased risk of an underlying chromosomal abnormality,
Chromosomal abnormalities
Down’s syndrome
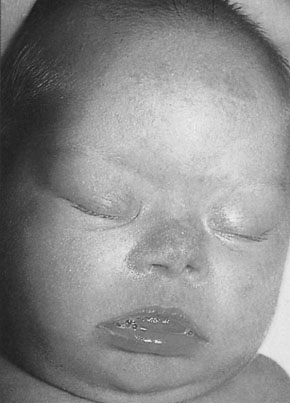
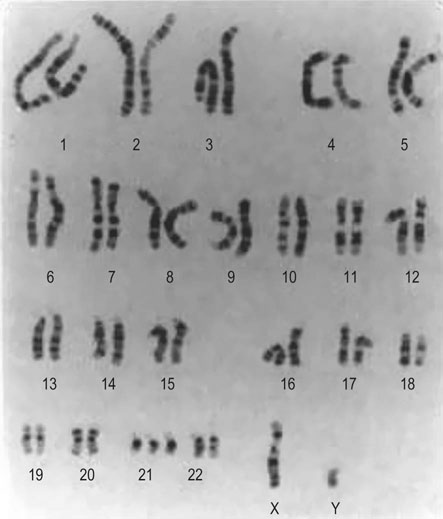
Down’s syndrome (DS) is characterized by the typical abnormal facial features (Fig 10.4), mental retardation of varying degrees of severity and congenital heart disease. The karyotype includes an additional chromosome on group 21 (‘trisomy 21’; Fig 10.5). The incidence overall is 1.5/1000 births. However, the risk increases with advancing maternal age (see below). The underlying reason is thought to be an increased frequency of non-disjunction at meiosis.
Assessing fetal normality
Screening
Clinical risk factors: early pregnancy
• maternal age and risk of aneuploidy especially DS (see Tables 10.2 and 10.3).
Table 10.2
The risk of having a pregnancy affected by Down’s syndrome according to maternal age at the time of birth
| Maternal age at delivery (years) | Risk of Down’s syndrome |
| 15 | 1 : 1578 |
| 20 | 1 : 1528 |
| 25 | 1 : 1351 |
| 30 | 1 : 909 |
| 31 | 1 : 796 |
| 32 | 1 : 683 |
| 33 | 1 : 574 |
| 34 | 1 : 474 |
| 35 | 1 : 384 |
| 36 | 1 : 307 |
| 37 | 1 : 242 |
| 38 | 1 : 189 |
| 39 | 1 : 146 |
| 40 | 1 : 112 |
| 41 | 1 : 85 |
| 42 | 1 : 65 |
| 43 | 1 : 49 |
| 44 | 1 : 37 |
| 45 | 1 : 28 |
| 46 | 1 : 21 |
| 47 | 1 : 15 |
| 48 | 1 : 11 |
| 49 | 1 : 8 |
| 50 | 1 : 6 |
Reproduced with permission from James D, Steer, P, Weiner, C, Gonik et al., eds. High Risk Pregnancy: Management Options, 4th edn. WB Saunders, © 2010 Elsevier.
Table 10.3
Chromosomal abnormalities by maternal age at the time of amniocentesis performed at 16 weeks’ gestation (expressed as rate per 1000)
| Maternal age (years) | Trisomy 21 | Trisomy 18 | Trisomy 13 | XXY | All chromosomal anomalies |
| 35 | 3.9 | 0.5 | 0.2 | 0.5 | 8.7 |
| 36 | 5.0 | 0.7 | 0.3 | 0.6 | 10.1 |
| 37 | 6.4 | 1.0 | 0.4 | 0.8 | 12.2 |
| 38 | 8.1 | 1.4 | 0.5 | 1.1 | 14.8 |
| 39 | 10.4 | 2.0 | 0.8 | 1.4 | 18.4 |
| 40 | 13.3 | 2.8 | 1.1 | 1.8 | 23.0 |
| 41 | 16.9 | 3.9 | 1.5 | 2.4 | 29.0 |
| 42 | 21.6 | 5.5 | 2.1 | 3.1 | 37.0 |
| 43 | 27.4 | 7.6 | 4.1 | 45.0 | |
| 44 | 34.8 | 5.4 | 50.0 | ||
| 45 | 44.2 | 7.0 | 62.0 | ||
| 46 | 55.9 | 9.1 | 77.0 | ||
| 47 | 70.4 | 11.9 | 96.0 |
 anticonvulsant drugs (e.g. phenytoin, carbamazepine and sodium valproate) that can produce defects of the central nervous system especially neural tube defects
anticonvulsant drugs (e.g. phenytoin, carbamazepine and sodium valproate) that can produce defects of the central nervous system especially neural tube defects
 cytotoxic agents used in cancer therapy or for immunosuppression with organ transplantation are associated with an increased risk of fetal growth restriction
cytotoxic agents used in cancer therapy or for immunosuppression with organ transplantation are associated with an increased risk of fetal growth restriction
 warfarin is teratogenic when used in the first trimester and can produce a fetal bleeding disorder when used later in pregnancy
warfarin is teratogenic when used in the first trimester and can produce a fetal bleeding disorder when used later in pregnancy
• previous history of fetal abnormality:
 if, for example, a woman has had a DS baby in the past she is at greater risk of recurrence than the risk given by her age alone
if, for example, a woman has had a DS baby in the past she is at greater risk of recurrence than the risk given by her age alone
 however, not all fetal abnormalities are associated with a greater risk of recurrence in a subsequent pregnancy
however, not all fetal abnormalities are associated with a greater risk of recurrence in a subsequent pregnancy
• maternal disease (see Chapter 9) including:
 diabetes: the reported risks of fetal abnormality vary between 3–8%. This figure is reduced significantly if the diabetes is well controlled before and during the first trimester
diabetes: the reported risks of fetal abnormality vary between 3–8%. This figure is reduced significantly if the diabetes is well controlled before and during the first trimester
 congenital heart cardiac disease: a woman who has a congenital cardiac defect has a 1–2% risk of a cardiac abnormality in her fetus.
congenital heart cardiac disease: a woman who has a congenital cardiac defect has a 1–2% risk of a cardiac abnormality in her fetus.
Clinical risk factors: late pregnancy
The following are risk factors associated with a higher likelihood of fetal abnormality:
• persistent breech presentation or abnormal lie
• vaginal bleeding, though the majority of pregnant women with vaginal bleeding in pregnancy do not have a fetal abnormality
• abnormal fetal movements, both increased and decreased, though for women to be aware of this perhaps subtle difference they usually have to have had a pregnancy previously
• abnormal amniotic fluid volume: both polyhydramnios (which is commonly associated with abnormalities of the gastrointestinal system especially obstruction) and oligohydramnios (which is commonly associated with abnormalities of the renal tract such as urethral valves or renal agenesis)
• growth restriction though the majority of fetuses that are growth retarded do not have an abnormality.
Ultrasound
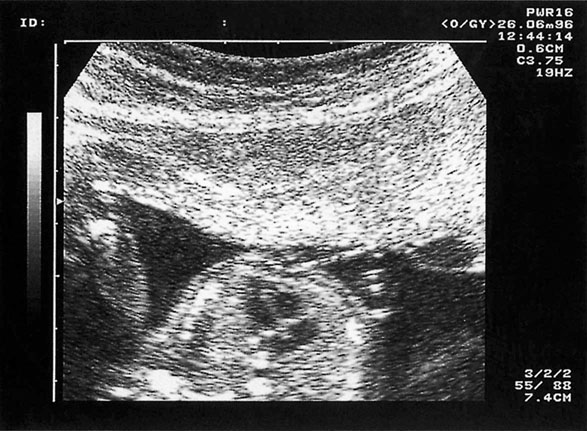
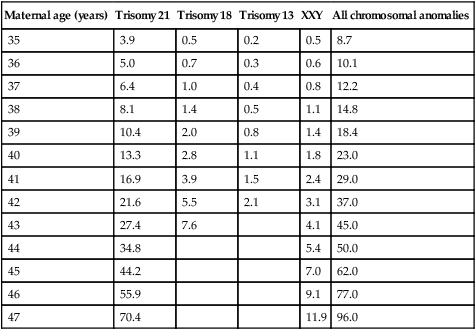
Most pregnant women in the UK present for their first visit to a health professional in the first trimester. This means that they can be offered two early ultrasound (US) scans. The first scan, ideally between 11w+0d and 13w+6d, allows fetal viability and number to be confirmed, gestation to be confirmed by crown–rump length (CRL) (see Chapter 4) and, if the woman wishes screening for DS, she can have a measurement of fetal nuchal translucency (NT) (Fig. 10.6) as part of the ‘combined testing’ programme (see next section).
• confirmation of fetal viability
• measurement of fetal head and abdominal circumferences, biparietal diameter, and femur length
• anatomical survey which seeks to confirm a normal appearance in a number of organ systems listed below. The success at identifying structural abnormalities in these systems at about 20 weeks varies and the approximate rates of detection with US reported in 2000 are:
Biochemistry
The recommendation from the UK National Screening Committee is that a DS risk of greater than 1 : 150 indicates a ‘high’ risk and that further assessment in the form of chorionic villus sampling (CVS) in the first trimester or amniocentesis in the second trimester should be discussed with the woman and her partner. In practice, many prefer to present the risk estimate to the woman and allow her to make her own decision. For example, a woman aged 40 years who has a DS risk of 1 in 130 from a quadruple test undertaken at 16 weeks may consider that to be an acceptable risk compared to her background age risk of 1 : 75 at that stage of pregnancy (Table 10.2), especially when there is a risk of 1 : 100 of losing the pregnancy from an amniocentesis (see below). Conversely, a woman aged 20 years with a background risk of delivering a baby with DS of over 1 : 1500 (Table 10.2) may consider a 1 : 180 risk estimate in the first trimester to be too high and might want an invasive test (e.g. CVS, see below).
Management options with an ‘abnormal’ or ‘positive’ test
Further counselling
Further assessment
It may be appropriate for the couple to consider further assessment in the form of:
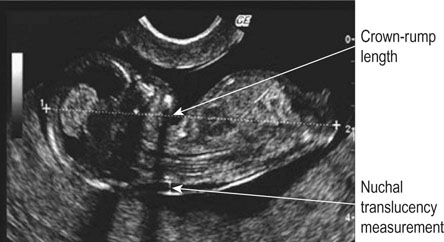
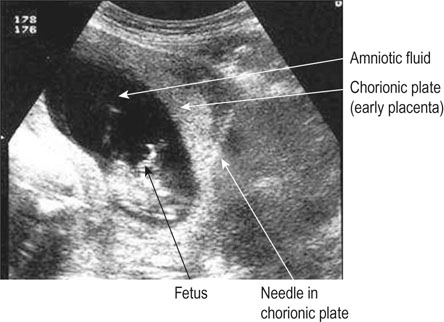
• For women with an increased risk of a chromosomal abnormality, an invasive test such as a chorionic villus biopsy/sampling (Fig. 10.7) if the woman presents in the first trimester or an amniocentesis (Fig. 10.8) if the woman presents in the second trimester. Before undertaking the procedure, the woman should be informed that it is undertaken aseptically, will provide information about chromosome number and structure but that it carries a risk of miscarriage (about 1%).
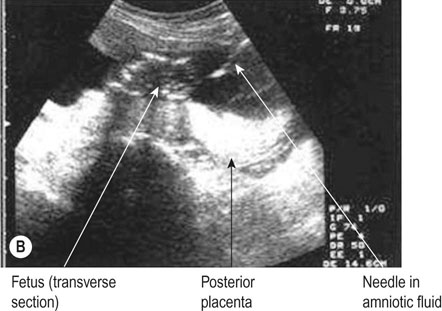
• For women suspected to have a structural fetal abnormality, further imaging to clarify the diagnosis either in the form of further US examinations after 1–2 weeks (to allow for fetal growth and better visualization of fetal anatomy) or an MRI scan (especially useful with abnormalities of the central nervous system). If the anatomical appearances suggest the fetus has a chromosomal abnormality, a CVS (which is more accurately termed placental biopsy at this stage of pregnancy) or amniocentesis could be offered.
Delivery issues
• In advance of the delivery deciding on the place of birth on the basis of the baby’s likely need of resources after birth, e.g. neonatal intensive care or surgery, and arranging for the relevant neonatal medical professionals to meet the parents.
• Arranging elective delivery if it is considered desirable that the baby would benefit from delivery during the working day and week when full neonatal resources are available.
• With some fetal abnormalities it is preferable to avoid a vaginal birth, e.g. there may be a greater risk of fetal trauma such as in a case of fetal hydrocephalus.
Assessing the health of a normally formed fetus
Screening for fetal health
• Maternal vigilance for fetal activity over second half of pregnancy.
• Fundal height measurement at every antenatal clinic visit. This involves measuring the distance between the maternal symphysis pubis and uterine fundus (see Fig. 6.12). The reverse blank side of tape measure uppermost and the distance (in cm) is read after it has been determined by turning the tape measure over. The normal range between 16–36 weeks is (gestational age in ±3 cm). Thus at 32 weeks the normal range is 32 ± 3 cm.
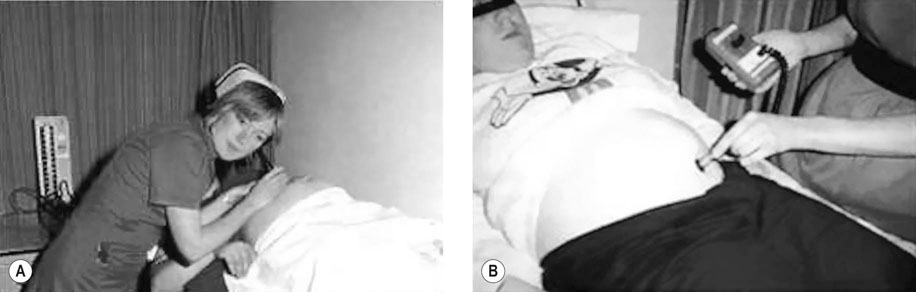
• Auscultation of the fetal heart at every antenatal clinic visit. This is undertaken either using a pinard stethoscope (Fig. 10.9A) or a handheld Doppler US device (Fig. 10.9B). In routine practice, the rate is not recorded just a note that the fetal heart is beating. Thus, abnormalities of the fetal baseline heart rate may be missed.
Women with risk factors (‘high risk’) have customized surveillance that is determined by the presumed underlying pathophysiological process. The more common examples are shown in Table 10.4.
Table 10.4
Risk factors for fetal compromise
| Type of risk | Risk factor/problem | Presumed pathophysiology | Surveillance |
| Specific | Maternal vascular disease, e.g. hypertension, antiphospholipid or lupus antibodies | Uteroplacental vascular disease (UPVD), i.e. poor blood flow to and within the placenta with associated reduced gaseous and nutritional transfer | Maternal vigilance for fetal movements Umbilical artery Doppler recordings US monitoring of fetal growth Biophysical assessment if any of these not normal |
| Maternal diabetes | The pathophysiology of fetal risk is uncertain | The same package of surveillance is used as in women with UPVD, but it is less effective at predicting fetal death | |
| Twins | Main risks are: For all twins: fetal growth restriction form UPVD For monochorionic twins: the twin–twin transfusion syndrome (TTTS), i.e. shared placental circulation with risk of one fetus being the ‘donor’ and the other being the ‘recipient’ |
For fetal growth restriction:
For TTTS, monitoring of monochorionic twins to look for signs of TTTS: |
|
| Isoimmunization due to Rhesus antibodies | Transplacental passage of maternal antibodies (anti-D or Kell or Duffy) causing severe fetal anaemia | With fetal anaemia the fetal middle cerebral artery blood flow is raised with anaemia and confirmed by fetal blood sampling (FBS) | |
| Non-specific | Previous fetal death Previous fetal growth restriction Maternal perception of reduced fetal movements Vaginal bleeding Abdominal pain |
A variety of pathologies result in fetal death, fetal growth restriction, reduced movements, vaginal bleed and abdominal pain. Unless the cause is known the normal approach initially is to assume it is UPVD | Fetal surveillance as in women at risk of UPVD (see above) In women with reduced FM, ongoing surveillance is only needed where the FM do not return to normal In women with vaginal bleeding or abdominal pain ongoing surveillance is needed only where the symptoms persist |
| Abnormal uterine size and/or growth (larger or smaller than normal) | Many cases of clinically suspected abnormal fetal growth are not confirmed with US. If confirmed, a variety of pathologies result in fetal growth restriction. Unless cause is known the normal approach is to assume it is UPVD | If abnormal fetal size/growth confirmed with US, fetal assessment as in women at risk of UPVD (see above) |
Surveillance of fetal health in at-risk pregnancies
Doppler recordings of blood flow in the umbilical artery (UA)
This investigation has been shown to significantly improve fetal outcome in high-risk pregnancies. Figure 10.10A shows a normal recording.
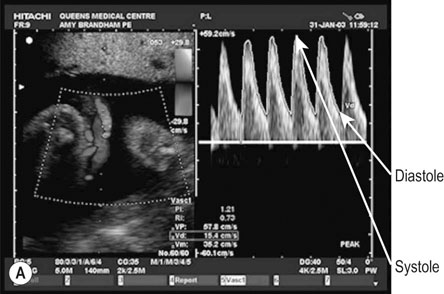
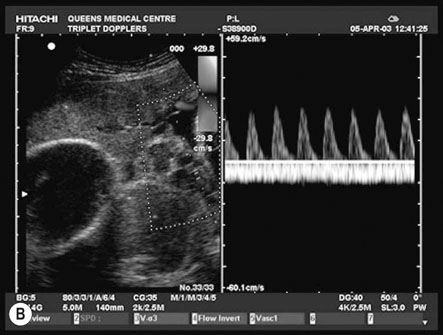
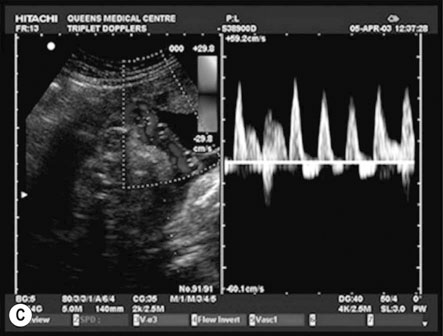
Figure 10.10B shows an example of ‘absent end-diastolic flow’ (AEDV). The commonest explanation is an increase in placental vascular resistance (‘downstream’ from the point of recording), which is typical of umbilical placental vascular disease (UPVD). The prognosis for the fetus with AEDV is worse with growth restriction, hypoxia and death all being commoner. However, the risk is not usually immediate and management options include continued close surveillance with biophysical testing until a viable gestational age is reached or the biophysical testing is abnormal. If this abnormality occurs at 34 weeks or beyond most would offer the woman elective preterm delivery rather than continuing the pregnancy and the possibility of fetal death.
Figure 10.10C shows an example of ‘reversed diastolic flow’. This is an even more ominous feature and is associated with a much higher chance of imminent fetal death. Management will depend on gestational age. If the pregnancy is at 26 weeks or more then elective preterm delivery (with the attendant risks of prematurity) compared with continuing the pregnancy (and the high risk of fetal death) has to be discussed with the parents. If the pregnancy is less than 26 weeks the discussions are more difficult and the parents may opt for no intervention.
Fetal growth
Small fetuses
Figure 10.11A illustrates a constitutionally small fetus. Genetic factors contribute to this pattern. Typically the mother will be short and/or of Asian ethnicity. If multiparous, her previous baby(ies) may have been small. Whilst this fetus is not at such a high risk of complications as in the pathologically small fetus (see below), those risks are still greater compared to a normally grown fetus.
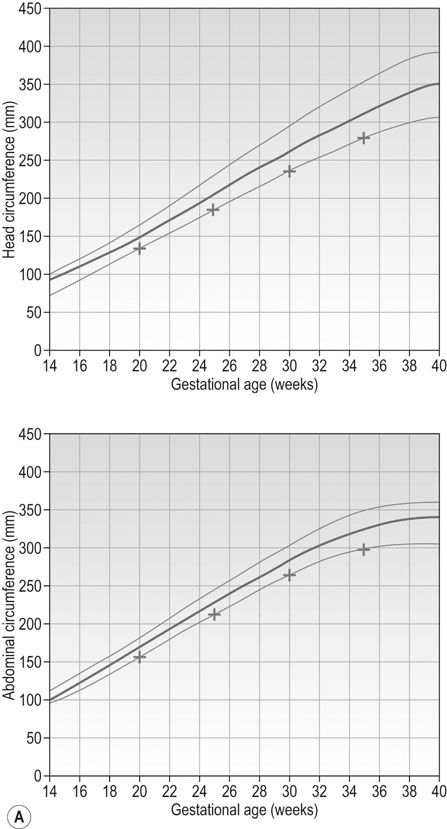
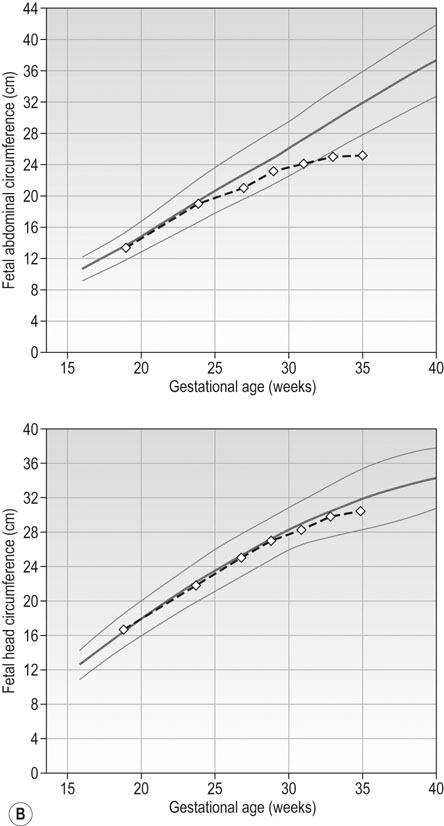
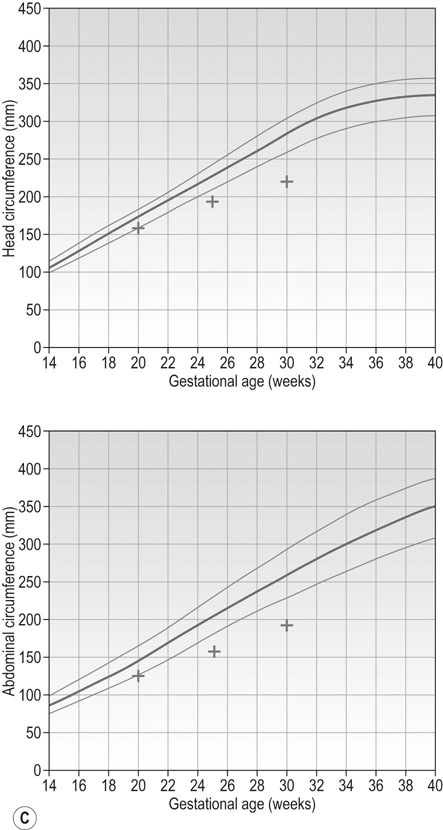
Figure 10.11B and C represent fetal growth patterns that are due to a pathological cause. They represent different points on the spectrum of fetal growth restriction. The asymmetrical type tends to occur later in pregnancy and is more commonly associated with conditions such as UPVD in the last trimester, whereas the symmetrical type tends to represent a pathological insult that operated from an early point in pregnancy, e.g. fetal abnormality, severe early onset pre-eclampsia. Both are associated with a higher risk of fetal death and hypoxia, preterm delivery and placental bleeding.
Big fetuses
In an analogous way to small fetuses, large fetuses can either be:
• Constitutionally large (‘large-for-dates’) with the HC and AC growth trajectories both following the top centile line. Typically the woman would be tall and/or of Afro-Caribbean ethnicity.
• Pathologically large (‘macrosomia’) with the HC growth trajectory following a centile in the normal range but the AC growth trajectory demonstrates accelerated growth upwards across centiles. This pattern of growth is most commonly seen in fetuses of diabetic women.
Amniotic fluid volume (AFV)
The most accurate estimate of AFV is with US. Two methods are used:
• Single deepest pocket (the normal range is 2–8 cm).
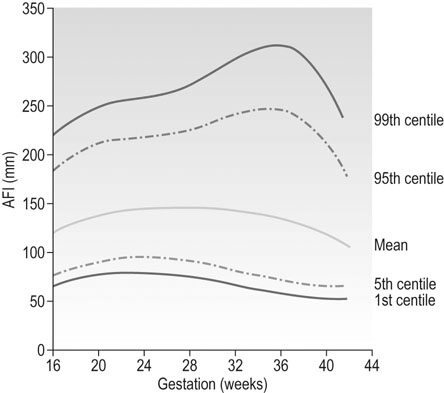
• Amniotic fluid index (AFI) which is the sum of the depths of the pools of amniotic fluid in each of the 4 quadrants of the uterus (top right and left and bottom right and left). Figure 10.12 shows the normal range of the AFI during pregnancy.
Causes of reduced and increased amniotic fluid are discussed in Chapter 4.
Biophysical measurements
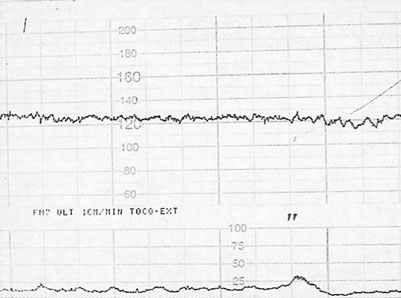
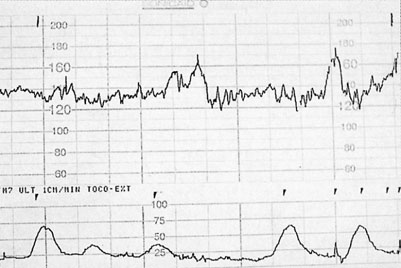
• Fetal heart rate (FHR): this is recorded with a cardiotocograph (CTG) (as in labour). The maximum recording time is 40 minutes and in that time there should be at least 2 accelerations of the FHR by 15 beats/min or more and lasting for at least 15 seconds. The patterns of heart rate change are similar to those described in labour (see Chapter 11) with the difference that uterine activity is minimal and more emphasis is therefore placed on the interpretation of baseline heart rate. An example of a normal antenatal CTG is shown in Figure 10.13. This shows a baseline variability of more than 5 beats/min with accelerations and no decelerations. Figure 10.14 shows a normal baseline rate but reduced baseline variability.
• Fetal movements: there should be at least 3 separate/discreet movements in 40 minutes of fetal observation with US.
• Fetal tone: at least one of these fetal movements should demonstrate a full 90° flexion–extension–flexion cycle.
• Fetal breathing: there should be a sustained 30 second period of regular fetal breathing movements during the 40 minute observation period.
• AFV: there should be at least one vertical pool measuring between 2 and 8 cm.
The use of all five parameters in combination is called ‘the biophysical profile’ or ‘score’ (BPP or BPS). A normal response is for the fetus to exhibit at least 4 of these parameters in a period of up to 40 minutes (they may be seen in a much shorter period). The original BPS was recorded over 30 minutes but that did not take account of the possibility of normal fetal ‘sleep’ which can last up to 40 minutes and during which no movements, accelerations, or breathing may be seen, hence the change to an observation window of 40 minutes.
Interventions
Non-specific risk
• Elective delivery: if the risk is identified at 34 or more weeks then there is usually no reason to delay the delivery. Where the risk is identified before 34 weeks the management is determined by the assessment of immediate risk of fetal death. Thus, if the BPS or CTG (acute measures of health) are abnormal and/or there is reversed end diastolic flow in the umbilical artery then delivery without delay is discussed with the parents. Where there is no abnormality in these parameters, then continued close monitoring could continue to ‘gain’ time in the pregnancy and allow the maternal administration of steroids.
• Maternal steroids: if it is clear that elective preterm delivery is likely to occur in an at risk pregnancy but only the chronic measures of fetal health are abnormal, e.g. suboptimal growth and absent umbilical artery Doppler diastolic recordings, then the woman would be advised to have a course of betamethasone.
Conclusions
• Most, though not all, fetuses with structural or chromosomal abnormality are identified during pregnancy with current screening programmes.
• In normally formed fetuses, once risk is identified, both specific and non-specific, the current methods of surveillance coupled with the judicious use of maternal steroid administration and elective delivery are effective in the sense that most fetuses identified to be ‘at risk’ will not die in utero.
• In normally formed fetuses that are apparently at no risk the current method of routine surveillance during pregnancy (maternal perception of fetal movements, fundal height measurement and auscultation of the fetal heart) is limited and does not identify all fetuses that are genuinely at risk.