Congenital Abdominal Wall Defects
Abdominal wall defects are divided into omphalocele and gastroschisis. While often considered together, they are distinct and separate entities. Differences between gastroschisis and omphalocele are illustrated in Figure 48-1 and summarized in Table 48-1.
TABLE 48-1
Differentiating Characteristics between Gastroschisis and Omphalocele
| Characteristic | Omphalocele | Gastroschisis |
| Herniated viscera | Bowel ± liver | Bowel only |
| Sac | Present | Absent |
| Associated anomalies | Common (50%) | Uncommon (<10%) |
| Location of defect | Umbilicus | Right of umbilicus |
| Mode of delivery | Vaginal/cesarean | Vaginal |
| Surgical management | Nonurgent | Urgent |
| Prognostic factors | Associated anomalies | Condition of bowel |
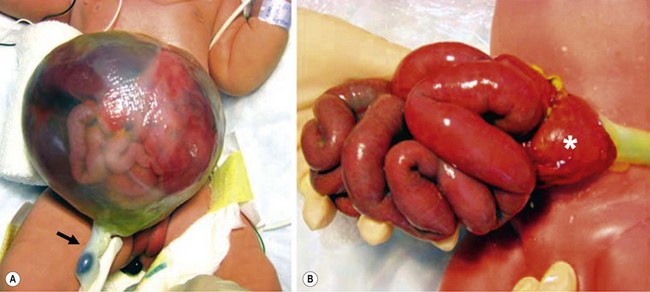
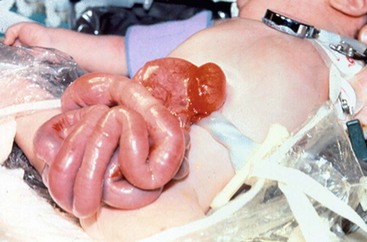
FIGURE 48-1 These two photographs nicely depict the differences between an omphalocele and gastroschisis. (A) In an omphalocele both the liver and bowel can be herniated. A sac is always present and the umbilical cord (arrow) inserts onto the sac. Moreover, this is always a midline defect. (B) With a gastroschisis, the liver is never herniated and a sac is absent. The location of the fascial defect is to the right of the umbilicus, and the umbilical cord is attached to the umbilicus. In addition to the large and small intestine, the stomach (asterisk) can sometimes be herniated as well.
Embryology and Etiology
The etiology for gastroschisis is less clear. One theory suggests that gastroschisis results from failure of the mesoderm to form in the anterior abdominal wall. Currently, the ventral body folds theory, which suggests failure of migration of the lateral folds (more frequent on the right side), is most widely accepted.1 This implies a gastroschisis develops early in gestation and prior to development of an omphalocele. Due to the increasing incidence of gastroschisis, there are a number of possible causative factors including tobacco, certain environmental exposures, lower maternal age and low socioeconomic status, all suggested by epidemiologic studies, but not proven.1–4
Gastroschisis
Prenatal Management and Diagnosis
Gastroschisis occurs in 1 in 4,000 live births.5 An increased incidence in mothers younger than 21 years of age has been widely documented.6 There has also been a significant worldwide increase in the incidence of gastroschisis in all age groups over the past two decades.7 Preterm delivery is more frequent in infants with gastroschisis, with an incidence of 28% compared with only 6% in babies without an abdominal wall defect.8
The majority of pregnancies complicated by gastroschisis are diagnosed sonographically by 20 weeks’ gestation.2,3 Often an ultrasound (US) evaluation is performed because of an abnormal maternal serum α-fetoprotein (AFP) level, which is universally elevated in the presence of gastroschisis.9,10 Detection of bowel loops freely floating in the amniotic fluid and a defect in the abdominal wall to the right of a normal umbilical cord are diagnostic of gastroschisis. Intrauterine growth retardation (IUGR) has been noted in a large number of these fetuses as well.11
Some authors advocate selective preterm delivery based on the finding of bowel distention and thickening on prenatal ultrasound.12 Dilated fetal bowel has been shown to correlate with a worse outcome, including fetal distress and demise in some series, but not in others.13 One problem with using bowel dilatation to predict outcome is the lack of a definition of ‘dilated,’ with ranges from 7 to 25 mm being considered abnormal.14,15 Moreover, there is also variability in the part of the intestine that is being measured. Studies in animal models have shown that the duration of amniotic fluid exposure is correlated with the degree of the inflammatory peel and intestinal dysmotility.16–19 Efforts to reduce this exposure by either amniotic fluid exchange or intrauterine furosemide treatment, which induces fetal diuresis, have shown to be beneficial in animals.19–22 These studies spurred early human trials with amniotic fluid exchange in Paris and Italy, but these trials have proven inconclusive.20,23
Concomitant bowel atresia is the most common associated anomaly in patients with gastroschisis, with rates ranging from 6.9–28% in several series (Table 48-2).24,25 A recent literature review noted associated anomalies in the cardiac, pulmonary, nervous, musculoskeletal, genitourinary systems, as well as chromosomal abnormalities in babies with gastroschisis.33
Perinatal Care
The optimal mode and timing of delivery for a fetus with gastroschisis has been debated for many years. Proponents of routine cesarean delivery (C-section) argue that the process of vaginal birth results in injury or increased risks for infection and sepsis.34 However, the literature suggests that both vaginal delivery and C-section are safe.35,36 A recent meta-analysis failed to demonstrate a difference in outcomes for infants delivered either vaginally or by C-section.37 Therefore, the delivery method should be at the discretion of the obstetrician and the mother, with C-section reserved for obstetric indications or fetal distress.
Preterm delivery of the fetus with gastroschisis has been advocated to limit exposure of the bowel to the amniotic fluid.12 Interleukin-6, interleukin-8, and ferritin are elevated in the amniotic fluid in fetuses with gastroschisis when compared with controls.18,38 Damage to the pacemaker cells and nerve plexi may contribute to the profound dysmotility and malabsorption seen in these infants.39 Early delivery may mitigate these effects, but the literature is mixed.12 A randomized trial from the UK found no benefit after induced early delivery with the only trends being an improvement in length of hospitalization and earlier initiation of feeding.40 Another study demonstrated that birth weight less than 2 kg was associated with increased morbidity.41 Currently available evidence does not support elective preterm delivery for gastroschisis.42
Neonatal Resuscitation and Management
Neonates with gastroschisis have significant evaporative water losses from the open abdominal cavity and exposed bowel. Appropriate intravenous access should be obtained and fluid resuscitation initiated after birth. Nasogastric (NG) decompression is important to prevent further gastric and intestinal distention. Routine endotracheal intubation is not necessary. The bowel should be wrapped in warm saline-soaked gauze and placed in a central position on the abdominal wall. The neonate should be positioned on the right side to prevent kinking of the mesentery with resultant bowel ischemia. The bowel should be wrapped with plastic wrap or the infant placed partially in a plastic bag to reduce evaporative losses and improve temperature homeostasis (Fig. 48-2). Although gastroschisis most often is an isolated anomaly, thorough examination of the neonate is important. In addition, the bowel must be carefully examined for intestinal atresia, necrosis, or perforation (Fig. 48-3). Recent evidence suggests that excess fluid resuscitation is detrimental and results in edema, an increase in time to closure, and an increased risk of abdominal compartment syndrome.43
Surgical Management
The primary goal is to return the viscera to the abdominal cavity while minimizing the risk of damage due to trauma or increased intra-abdominal pressure. The two most commonly used treatment options are placement of a silo followed by serial reductions and delayed closure, or attempted primary closure.44 The timing and location of surgical intervention is also controversial.45 In all cases, inspection of the bowel for obstructing bands, perforation, or atresia must be undertaken. Bands crossing the bowel loops should be lysed before silo placement or primary abdominal closure to avoid subsequent intestinal obstruction.
Primary Closure
Historically, urgent primary closure of gastroschisis was advocated in all cases. This approach is still commonly practiced in neonates in whom reduction of the herniated viscera appears possible.46 Attempted primary closure has traditionally been performed in the operating room, but some authors have advocated primary closure at the bedside without general anesthesia.47–49 Some surgeons prefer to close the skin only and leave the fascia separated. Others have described the use of the umbilicus as an allograft.50,51 Prosthetic options for fascial closure include nonabsorbable mesh or bioprosthetic materials such as porcine small intestinal submucosa mesh.52 In the past, most surgeons have excised the umbilicus during closure. However, preservation of the umbilicus has been shown to lead to an excellent cosmetic result (Fig. 48-4).53,54
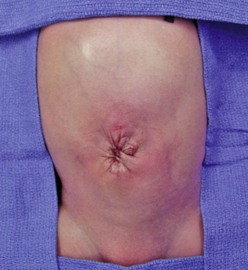
FIGURE 48-4 An excellent cosmetic result after gastroschisis repair in which the umbilicus was preserved.
Intra-abdominal pressure approximated from either the bladder pressure or stomach pressure can be used to guide the surgeon during reduction.55 Pressures higher than 10–15 mmHg are often associated with decreased renal and intestinal perfusion, and a silo or patch may be needed.56 Pressures higher than 20 mmHg can lead to renal failure and bowel ischemia.57 Similarly, an increase in central venous pressure greater than 4 mmHg has been correlated with the need for silo placement or patch closure.58 Splanchnic perfusion pressure, the difference between mean arterial pressure and intra-abdominal pressure, has also been used to guide the reduction. A splanchnic perfusion pressure less than 44 mmHg implies a decrease in intestinal blood flow.59
Staged Closure
In the mid-1990s, a prefabricated silo was developed with a circular spring that is positioned under the fascial opening, without the need for sutures or general anesthesia.60 This has made it possible to insert the silo in the delivery room or at the bedside (Fig. 48-5). After placement, the bowel is reduced daily into the abdominal cavity as the silo is shortened by sequential ligation. When the contents are entirely reduced, fascial and skin closure are performed. This process usually takes between one and 14 days with the majority being ready within a week, depending on the condition of the bowel and the infant.
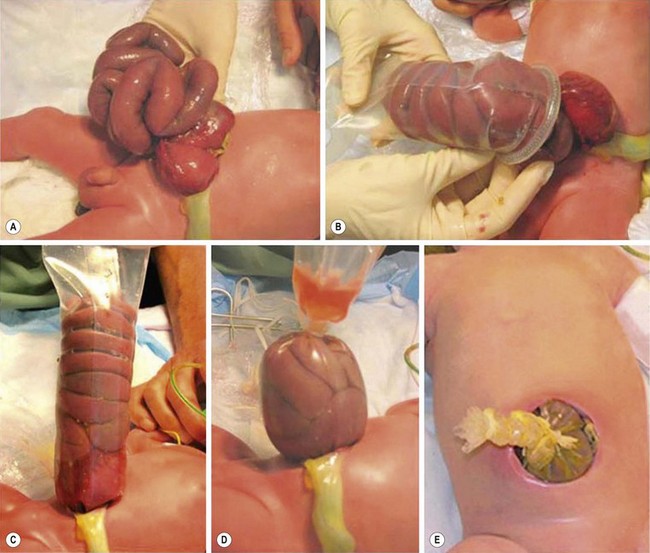
FIGURE 48-5 The use of a spring-loaded prefabricated silo is shown in these photographs. (A) The gastroschisis defect is seen. (B) An appropriate-sized spring-loaded silo is then placed over the eviscerated intestine. (C) The ring of the silo has been positioned under the fascial defect and attached to an overhead support to keep the bowel from torquing, which may result in intestinal ischemia. (D) Gradual reduction of the silo is performed. (E) Finally, the bowel has been completely returned to the abdominal cavity and the neonate is ready for transport to the operating room for closure of the fascia and skin.
Definitive closure in the operating room consists of raising small skin flaps around the fascial defect followed by fascial closure in a horizontal or vertical direction. Closure of the skin in a transverse direction creates a ‘keyhole’ appearance with a horizontal scar to the right of the umbilicus. This is why some surgeons advocate a vertical closure to allow for a central umbilicus. Also, a purse-string skin closure around the umbilicus can be performed to create a circular scar for improved cosmesis. Recently, the ‘plastic closure’ method has been described in which the umbilical cord is tailored to fill the gastroschisis defect and is then covered with an adhesive dressing.61,62 If the umbilical cord is not salvageable, the bowel can be covered with the dressing. Ingrowth of granulation tissue and epithelialization occurs over time. With this technique, an operation and general anesthesia can be avoided in many infants. Residual ventral hernia rates are reported to be 60–84%, the majority of which close spontaneously.54
The routine use of a preformed silo has increasingly come into favor, with the theory being that avoidance of high intra-abdominal pressure will avoid ischemic injury to the viscera and allow earlier extubation.60,63 One study reported fewer days on mechanical ventilation for patients undergoing silo reduction when compared to primary closure.47 However, there was no difference in time to full feeds or days on parenteral nutrition. Two reports noted a similar time to full enteral feedings but found that primary closure was associated with higher mean airway pressures, oxygen requirement, vasopressor requirement, and decreased urine output.64,65 However, other reviews have suggested that the trend to use a silo instead of immediate closure may have swung too far.66,67 Also, recent data from the Canadian Pediatric Surgeons Network (CAPSNet) database showed that infants who are able to undergo primary closure require less parenteral nutrition and hospitalization when compared with those who required staged reduction and repair.68 An attempt at a prospective randomized multicenter trial looking at silo vs. attempted primary closure that centered on ventilator days did not meet accrual targets and failed to show any significant difference.69 A similar trial has begun in the UK.70 Current data suggests that there is no significant difference in outcome with either approach (silo vs. immediate closure) for patients with uncomplicated gastroschisis.
Management of Associated Intestinal Atresia
Up to 10% of neonates with gastroschisis have an associated atresia, most commonly jejunal or ileal. In a database review of 4,344 infants with gastroschisis, a 5% incidence of small bowel atresia and a 2% incidence of large bowel atresia was noted.24 These atresias can be treated at the time of abdominal wall closure with resection and primary anastomosis in cases where there is minimal inflammatory peel. If the condition of the bowel makes primary anastomosis inadvisable, the bowel is reduced with the atresia intact and repair is undertaken four to six weeks after the initial abdominal wall closure.71 Some surgeons have chosen to create a stoma, particularly in the case of a distal atresia, to allow for enteral feeding while awaiting repair.72 If perforation is found, the perforated segment can be resected and a primary anastomosis performed if the inflammation is minimal (Fig. 48-6). Alternatively, an ostomy can be created followed by ostomy closure at a later date.73 There is no consensus about the optimal management for these complicated problems (see Table 48-2). Patients with an atresia are considered to be ‘complex’ to differentiate them from the patients without any such association (simple). Most authors note worse outcomes with complex cases.24,70,74
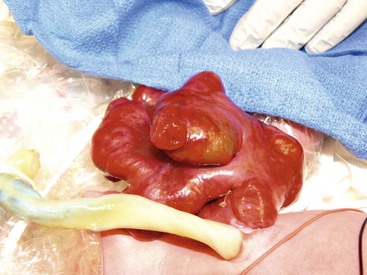
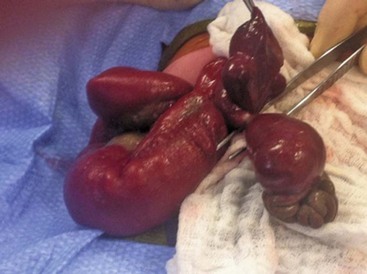
FIGURE 48-6 This newborn presented with gastroschisis and intestinal perforation. Note the two lumens in the exposed segment of intestine. Because there was not significant inflammation, this perforation was closed primarily and the bowel was placed in a silo. The baby recovered uneventfully.
An intestinal atresia should be differentiated from ‘vanishing bowel’ in infants with gastroschisis. This condition is usually associated with a very small abdominal wall defect and is characterized by necrosis and disappearance of some or all of the intestine (Fig. 48-7). Although this is a rare finding, it usually results in short bowel syndrome.75,76
Postoperative Course
Prokinetic medication may be helpful. In a rabbit model of gastroschisis, cisapride improved contractility of newborn intestine whereas erythromycin improved motility in control adult tissue only.77 However, a randomized controlled trial of erythromycin versus placebo found that enterally administered erythromycin did not improve time to achieve full enteral feedings.78 A similar randomized trial examining the use of cisapride in postoperative neonates, most of whom had gastroschisis, showed a beneficial effect, but this drug is not available in the USA.79
Long-Term Outcomes
Long-term outcomes for patients born with gastroschisis are generally excellent. The presence of complex disease is the most important prognostic determinant for a poor outcome.80,81 A number of authors have attempted to stratify patients with gastroschisis according to risk.82 A recent population-based cohort study from the UK with 301 patients noted that babies with complex gastroschisis took a median of 21 days longer to reach full enteral feedings, had a longer total parenteral nutrition (TPN) use, and had almost 2 months longer length of hospitalization.70 In addition, they were twice as likely to develop intestinal failure and six times more likely to develop liver disease. Another study noted an increased incidence of central venous catheter-related sepsis, a longer time to full enteral feeding, and a longer hospital stay in infants with gastroschisis complicated by atresia or necrotic bowel.83 Gastroschisis is the most common reason for intestinal transplantation.84 The ability to risk-stratify gastroschisis patients with respect to increased morbidity and mortality has utility in counseling families, predicting hospital utilization, and identifying a group of patients who would benefit from further strategies to improve outcomes.
Necrotizing enterocolitis (NEC) has been found in full-term infants with gastroschisis in higher than expected frequencies (up to 18.5%).85 Significant bowel loss from NEC can predispose to short bowel syndrome and its associated hepatic and infectious complications. On the other hand, another group found that the clinical course of babies with gastroschisis who developed NEC often had an uncomplicated course.86 There is a report suggesting that infants with gastroschisis who were fed breast milk had a lower incidence of NEC than those fed with formula.87
Cryptorchidism is associated with gastroschisis in 15–30% of cases.88–90 Several retrospective analyses have shown that placement of the herniated testis into the abdominal cavity will result in normal testicular descent into the scrotum in most cases.88 Most centers recommend allowing a year for spontaneous descent and then performing an orchiopexy if needed.89
If the umbilicus is sacrificed during the repair of the gastroschisis defect, up to 60% of children report psychosocial stress from not having an umbilicus.91
Omphalocele
Prenatal Diagnosis And Management
Elevation of maternal serum AFP is also present in many pregnancies complicated by omphalocele, although not as commonly as with gastroschisis. The diagnosis of omphalocele can be made by two-dimensional ultrasound at the time of the normal 18-week ultrasound evaluation for dates. Early first trimester detection is possible if three-dimensional ultrasound is utilized.92 The incidence of omphalocele seen at 14–18 weeks is as high as 1 in 1,100, but the incidence at birth drops to 1 in 4,000–6,000.93 Thus there is a considerable ‘hidden’ mortality for a fetus with an omphalocele. One review noted that requests for termination of pregnancy in omphalocele cases were as high as 83%.94
Ultrasound evaluation is very useful for the detection of associated anomalies in these infants. This is important as an isolated omphalocele has a survival rate of over 90%, but is reduced with other defects.93 Prenatal ultrasound and karyotyping are able to identify only 60–70% of the associated defects that are found postnatally.94 One study that reviewed associated defects in omphalocele infants noted anomalies that involved every organ system, while another study found that only 14% of omphaloceles were truly isolated anomalies.33,93 Prenatal screening in an infant with an omphalocele requires a detailed evaluation of the cardiac (14–47% incidence of anomalies) and central nervous (3–33% anomalies) systems as severe defects may lead to a discussion about termination of the pregnancy.26 Recently, there has been some attention towards developing a reliable sonographic predictor of postnatal morbidity and survival.95–97 Unfortunately, the prenatal finding of a ‘giant’ omphalocele has not been accurate in predicting outcomes. Investigators have studied ratios between the greatest omphalocele diameter compared to abdominal circumference (O/AC), the femur length (O/FL), and the head circumference (O/HC), and have attempted to correlate that with postnatal morbidity and mortality.98,99 Of these variables, the most useful may be the O/HC.98 Prospective studies are needed to assess the usefulness of this information.
Perinatal Care
The route of delivery of infants with an omphalocele should be dictated by obstetric considerations as C-section has not been shown to be superior.100 Pregnancies are usually allowed to come to term, and spontaneous labor and vaginal delivery is preferred. However, despite the lack of data, many neonates with giant omphaloceles are delivered by C-section because of the fear of liver injury.
Neonatal Resuscitation And Management
In some cases, the omphalocele sac may have ruptured either prenatally, during delivery, or postnatally (Fig. 48-8). A large, prenatally ruptured omphalocele is a special situation that represents one of the most difficult problems in pediatric surgery. The goal is to cover the exposed abdominal viscera, which can be challenging. There is one small series that has good outcomes in terms of survival, but there was a high incidence of intestinal fistulas, sepsis, and pulmonary hypoplasia.101
Surgical Management
There are a large variety of repairs described as a ‘one size fits all’ option does not exist. In a recent survey, authors of reports from 1967–2009 discussing closure of ‘giant’ omphaloceles were asked to see if they were still using the same approach, or whether they had modified their techniques.102 Interestingly, 42% of the authors no longer use the approach that they favored in their original article. They concluded that there is currently no completely accepted technique to treat ‘giant’ omphaloceles, and two methods are used the most: staged closure or delayed closure. Also, defining a giant defect is variable as some surgeons use size alone, others consider the presence or absence of the liver, while others use an estimate of the amount of intestinal contents, and still others have used a combination of the amount of liver and intestine in the sac. This lack of an accepted definition has resulted in an inability to arrive at a consensus for management.44
Immediate Primary Closure
Treatment options in infants with omphalocele depend on the size of the defect, the baby’s gestational age, and the presence of associated anomalies. Defects that are less than 1.5 cm in diameter are referred to as hernia of the cord and are repaired shortly after birth without any issues as long as there are no associated anomalies.103 The defects that are larger, but still easy to close without much loss of abdominal domain can also be closed soon after birth. Primary closure consists of excision of the sac and closure of the fascia and skin over the abdominal contents. It is not unusual for an omphalomesenteric duct remnant to be associated with a small omphalocele (Fig. 48-9). When dealing with a medium-sized omphalocele, care must be taken when excising the portion of the sac covering the liver, because the hepatic veins are located just under the epithelium/sac interface in the midline and can be injured. The sac is often adherent to the liver and significant hemorrhage can result from tears in the Glissen capsule. Therefore, it is usually best to leave part of the sac on the liver. The inferior portion of the sac covering the bladder can be quite thin, and excision of the sac in this area can lead to bladder injury as well. The intra-abdominal pressure can be elevated during reduction, leading to abdominal compartment syndrome.
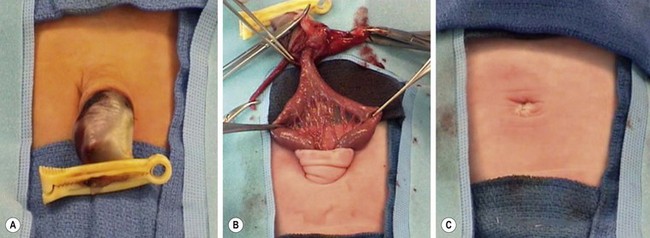
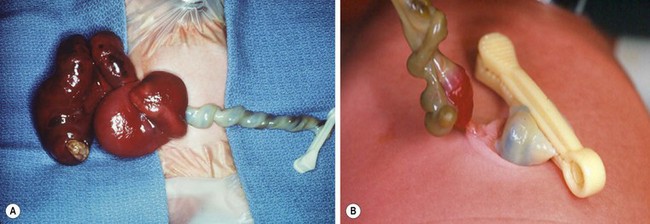
FIGURE 48-9 (A, B) In patients with small omphaloceles, it is not unusual for an omphalomesenteric duct remnant to be found. (C) The diverticulum was excised primarily and the fascia and skin closed. This neonate recovered uneventfully.
There are a number of reports of primary closure of a ‘giant’ omphalocele shortly after birth with good results. In a report from London, 12 of 24 babies with a large defect had an immediate repair without any mortality. Compared to the other cases, these patients had a shorter ventilator requirement and time to full feeding.79 However, this trial was not prospective and there was significant selection bias with using immediate repair for the full-term and normal birth weight neonates.
Staged Neonatal Closure
In many cases, the loss of domain in the peritoneal cavity prevents primary closure without an undue increase in intra-abdominal pressure (Fig. 48-10). Multiple methods have been proposed to obtain primary abdominal wall closure in these babies.
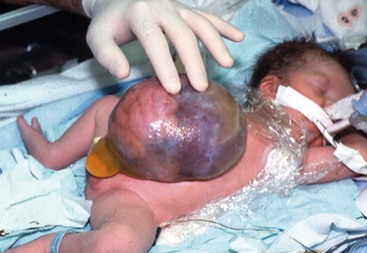
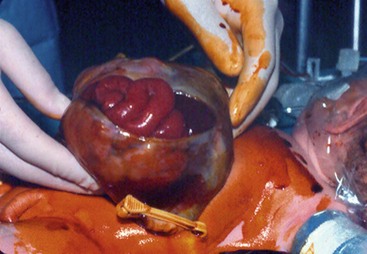
FIGURE 48-10 This neonate was born with a large omphalocele. As is evident, the abdominal cavity is quite small. Primary closure is not possible in such a patient.
Staged closure in the neonatal period involves the use of different techniques. These can be classified into methods that utilize the existing amnion sac with serial inversion, and those in which the sac is excised and replaced with mesh and then closed over time. Amnion inversion allows gradual reduction of the sac followed by sac excision and primary or mesh closure.104,105 Methods involving primary repair with mesh require removal of the amnion sac with the mesh used to bridge the fascial gap followed by skin closure. Repeated procedures to excise central portions of the mesh may eventually allow native fascial closure, or mesh may be left in situ with skin over it.44 Some authors have advocated the use of biologic mesh that allows vascular and tissue ingrowth.106 Vacuum-assisted closure of these defects has also been described as has a novel external skin closure system.101,107 There are many different techniques because no one method is uniformly applicable or successful.
Delayed Staged Closure
Historically, children with large omphaloceles were managed using skin flaps that were mobilized to cover the exposed viscera, leaving a large ventral hernia that could, hopefully, be closed later. In 1967, Schuster first described the use of a silastic ‘silo’ to allow staged reduction for children with an omphalocele.108 With this method, the omphalocele sac is excised and the silastic sheeting is sewn to the rectus fascia. Alternatively, the silo can be sewn to the full thickness of the abdominal wall. In our experience, the use of preformed spring-loaded silos is usually unsuccessful in babies with omphalocele as the relatively large size of the defect allows the silo to become easily displaced. Serial reductions, similar to that for gastroschisis, are performed on a once- to twice-daily schedule until definitive closure can be obtained. If the fascial edges cannot be approximated in a reasonable time period, prosthetic closure can be utilized.
Scarification Treatment
Nonoperative techniques have in common the use of an agent that allows an eschar to develop over the intact amnion sac. This eschar epithelializes over time, leaving a ventral hernia that will likely require repair later in life. This approach is employed when the surgeon considers the defect too large to allow for a safe primary repair, or if the neonate has significant cardiac or respiratory issues. This is not a new concept, having evolved from the time of Dr Robert Gross who described using skin flaps in 1948.109 The primary concern in a baby with a large omphalocele is that an initial repair will result in potential life-threatening abdominal compartment syndrome or the inability to provide skin coverage. Initial reports described mercurochrome, alcohol, and silver nitrate as the eschar-producing agents, which were very effective, but were associated with toxicity (Fig. 48-11).110–112 Subsequently there have been reports of a number of substances including silver sulfadiazine, povidone-iodine solution, silver-impregnated dressings, neomycin, and polymixin/bacitracin ointments.113 The eschar and epithelialization may take 4-10 weeks. There are also reports that combine the use of an agent listed above with compression dressing, which helps in sequentially reducing the contents into the abdomen and facilitates subsequent closure. Rarely, operative closure may not be needed as the defect contracts and closes similar to an umbilical hernia. However, most patients will eventually require closure of a ventral hernia between 1 and 5 years of age (Fig. 48-12).114 The ventral hernia repair is performed using a variety of techniques: primary fascial closure, autologous repair with component separation, or mesh repair.115 Although all may be successful, the number of patients in each report is small and failures are rarely reported. In some cases, innovative techniques have been utilized to recreate the abdominal domain including the use of tissue expanders (Fig. 48-13).116 While the initial reports of the staged Gross operation had significant mortality and morbidity, current results are better.114
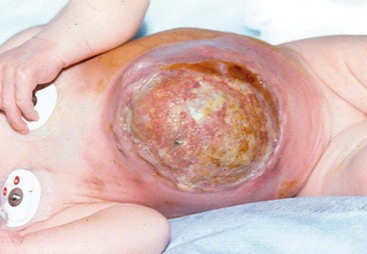
FIGURE 48-11 An infant with a large omphalocele that was treated by scarification using mercurochrome. Note the intense inflammation on the abdominal wall surrounding the scarred sac. Reports of death due to mercury poisoning led to abandonment of this method.
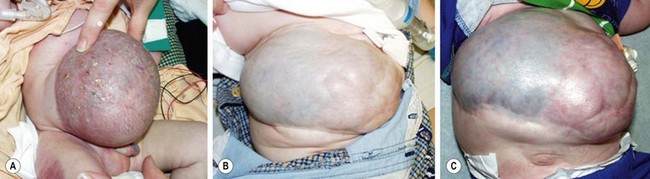
FIGURE 48-12 This baby presented with a large omphalocele and multiple medical problems, including significant lung disease. (A) Initial management was with compression therapy. Subsequently, skin grafts were placed. (B) The baby is seen at 9-months-old and again at (C) 2-years-old. The baby underwent several operations to repair the large ventral hernia.
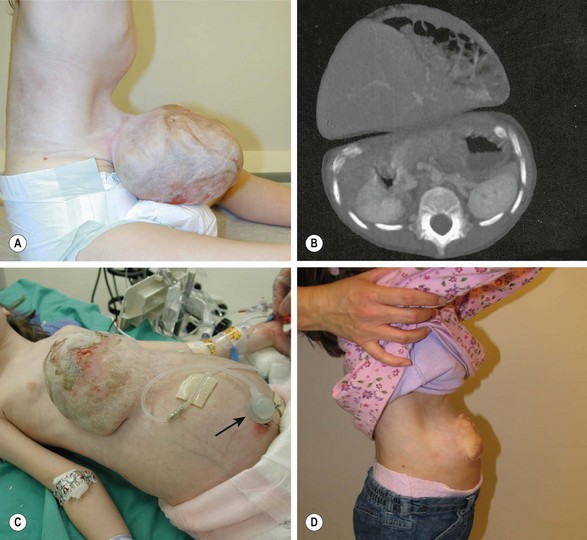
FIGURE 48-13 A 4-year-old girl was born with a number of anomalies including a diaphragmatic hernia, pulmonary hypoplasia and pulmonary hypertension, atrial septal defect, and a large omphalocele. She underwent repair of the diaphragmatic hernia shortly after birth, but no attempt was made to repair the large omphalocele because of its massive size and the disproportion between the extraperitoneal viscera and the peritoneal cavity. (A, B) At 4 years old, she was found to have loss of abdominal domain, a narrow neck to the omphalocele sac, and most of the viscera in the sac. (C) She underwent placement of an intraperitoneal tissue expander in the pelvis. As an outpatient, the tissue expander was gradually filled to 900 mL of volume, through a catheter emanating from the expander (arrow). Over time, it was possible to expand the peritoneal cavity to the point that the abdominal muscles and fascia could be approximated. However, a biosynthetic patch was needed to complete the fascial closure. (D) She has recovered uneventfully and has a very reasonable appearing abdomen. (A and B reprinted with permission from Foglia R, Kane A, Becker D, et al. Management of giant omphalocele with rapid creation of abdominal domain. J Pediatr Surg 2006;41:704–9. Photos courtesy of Dr. Robert Foglia.)
Postoperative Course
The method used for closure (primary vs. staged with delayed primary closure or mesh) has not been shown to affect length of hospitalization likely because the patient numbers are very small.117 The time to enteral feeding may be shorter with primary closure, though this likely reflects a more favorable defect. In a review of treatment of omphaloceles at one institution, the authors reported a 12% incidence of complications of increased intra-abdominal pressure after closure, including acute hepatic congestion requiring reoperation, renal failure requiring dialysis, and bowel infarction.118 In this retrospective review, wound complications including skin and fascial dehiscence occurred in up to 25% of patients undergoing primary closure.
Long-Term Outcomes
A number of long-term medical problems develop in patients with large omphaloceles.119–121 These include gastroesophageal reflux (GERD), pulmonary insufficiency, recurrent lung infections or asthma, and feeding difficulty with failure to thrive.122 In 23 patients with omphalocele, 43% were found to have GERD by esophageal biopsy or pH monitoring. Patients younger than 2 years had an increased rate of reflux compared with those older than two years of age.123 Patients with large defects also had an increased incidence of reflux. In this study, only one child required a fundoplication, suggesting that reflux improves as the child ages.
Feeding difficulties can occur in up to 60% of infants with a giant omphalocele.115 Many of these children require a gastrostomy for feeding. These difficulties seem to resolve by childhood, with height and weight measurements becoming similar to their peer group. The respiratory insufficiency associated with giant omphaloceles may be secondary to abnormal thoracic development with a narrow thorax and small lung area leading to pulmonary hypoplasia.124 The cause of this hypoplasia is unclear as it has been noted in omphaloceles of varying size, but appears more common in the larger defects. Also, it may be associated with poor intrauterine diaphragmatic motion and altered chest wall development. Prolonged respiratory difficulties can occur in up to 20% of infants with giant omphaloceles, leading to increased time of mechanical ventilation and the need for supplemental oxygen during the neonatal period. Some neonates may require a tracheostomy.125,126 Interestingly, in a study looking at the long-term cardiopulmonary consequences of large abdominal wall defects, lung volumes and oxygen consumption were found to be normal.127
References
1. Feldkamp, ML, Carey, JC, Sadler, TW. Development of gastroschisis: Review of hypotheses, a novel hypothesis, and implications for research. Am J Med Genet A. 2007; 143:639–652.
2. Feldkamp, ML, Carmichael, SL, Shaw, GM, et al. Maternal nutrition and gastroschisis: Findings from the National Birth Defects Prevention Study. Am J Obstet Gynecol. 2011; 204:404.
3. Feldkamp, ML, Alder, SC, Carey, JC. A case control population-based study investigating smoking as a risk factor for gastroschisis in Utah, 1997–2005. Birth Defects Res A Clin Mol Teratol. 2008; 82:768–775.
4. Browne, ML, Hoyt, AT, Feldkamp, ML, et al. Maternal caffeine intake and risk of selected birth defects in the National Birth Defects Prevention Study. Birth Defects Res A Clin Mol Teratol. 2011; 91:93–101.
5. Baird, PA, MacDonald, EC. An epidemiologic study of congenital malformations of the anterior abdominal wall in more than half a million consecutive live births. Am J Hum Genet. 1981; 33:470–478.
6. Forrester, MB, Merz, RD. Impact of demographic factors on prenatal diagnosis and elective pregnancy termination because of abdominal wall defects, Hawaii, 1986–1997. Fetal Diagn Ther. 1999; 14:206–211.
7. Castilla, EE, Mastroiacovo, P, Orioli, IM. Gastroschisis: International epidemiology and public health perspectives. Am J Med Genet C Semin Med Genet. 2008; 148:162–179.
8. Lausman, AY, Langer, JC, Tai, M, et al. Gastroschisis: What is the average gestational age of spontaneous delivery? J Pediatr Surg. 2007; 42:1816–1821.
9. Cerekja, A, Piazze, J, Cozzi, D. Early prenatal sonographic diagnosis of gastroschisis. J Clin Ultrasound. 2012; 40:526–528.
10. David, AL, Tan, A, Curry, J. Gastroschisis: Sonographic diagnosis, associations, management and outcome. Prenat Diagn. 2008; 28:633–644.
11. Juhasz-Boss, I, Goelz, R, Solomayer, EF, et al. Fetal and neonatal outcome in patients with anterior abdominal wall defects (gastroschisis and omphalocele). J Perinat Med. 2012; 40:85–90.
12. Moir, CR, Ramsey, PS, Ogburn, PL, et al. A prospective trial of elective preterm delivery for fetal gastroschisis. Am J Perinatol. 2004; 21:289–294.
13. Mills, JA, Lin, Y, Macnab, YC, et al. Perinatal predictors of outcome in gastroschisis. J Perinatol. 2010; 30:809–813.
14. Alsulyman, OM, Monteiro, H, Ouzounian, JG, et al. Clinical significance of prenatal ultrasonographic intestinal dilatation in fetuses with gastroschisis. Am J Obstet Gynecol. 1996; 175:982–984.
15. Piper, HG, Jaksic, T. The impact of prenatal bowel dilation on clinical outcomes in neonates with gastroschisis. J Pediatr Surg. 2006; 41:897–900.
16. Langer, JC, Longaker, MT, Crombleholme, TM, et al. Etiology of intestinal damage in gastroschisis. I: Effects of amniotic fluid exposure and bowel constriction in a fetal lamb model. J Pediatr Surg. 1989; 24:992–997.
17. Olguner, M, Akgur, FM, Api, A, et al. The effects of intraamniotic human neonatal urine and meconium on the intestines of the chick embryo with gastroschisis. J Pediatr Surg. 2000; 35:458–461.
18. Caglar, M, Hakguder, G, Ates, O, et al. Amniotic fluid ferritin as a marker of intestinal damage in gastroschisis: A time course experimental study. J Pediatr Surg. 2007; 42:1710–1715.
19. Hakguder, G, Ates, O, Olguner, M, et al. Induction of fetal diuresis with intraamniotic furosemide increases the clearance of intraamniotic substances: An alternative therapy aimed at reducing intraamniotic meconium concentration. J Pediatr Surg. 2002; 37:1337–1342.
20. Marder, AL, Moise, K, Jr., Chuang, A, et al. Amnioexchange for the treatment of gastroschisis–an in vitro study to determine the volume and number of exchanges needed. Fetal Diagn Ther. 2008; 23:95–99.
21. Luton, D, Guibourdenche, J, Vuillard, E, et al. Prenatal management of gastroschisis: The place of the amnioexchange procedure. Clin Perinatol. 2003; 30:551–572.
22. Hakguder, G, Olguner, M, Gurel, D, et al. Induction of fetal diuresis with intraamniotic furosemide injection reduces intestinal damage in a rat model of gastroschisis. Eur J Pediatr Surg. 2011; 21:183–187.
23. Midrio, P, Stefanutti, G, Mussap, M, et al. Amnioexchange for fetuses with gastroschisis: Is it effective? J Pediatr Surg. 2007; 42:777–782.
24. Arnold, MA, Chang, DC, Nabaweesi, R, et al. Risk stratification of 4344 patients with gastroschisis into simple and complex categories. J Pediatr Surg. 2007; 42:1520–1525.
25. Dixon, JC, Penman, DM, Soothill, PW. The influence of bowel atresia in gastroschisis on fetal growth, cardiotocograph abnormalities and amniotic fluid staining. BJOG. 2000; 107:472–475.
26. Amoury, RA, Ashcraft, KW, Holder, TM. Gastroschisis complicated by intestinal atresia. Surgery. 1977; 82:373–381.
27. Pokorny, WJ, Harberg, FJ, McGill, CW. Gastroschisis complicated by intestinal atresia. J Pediatr Surg. 1981; 16:261–263.
28. Gornall, P. Management of intestinal atresia complicating gastroschisis. J Pediatr Surg. 1989; 24:522–524.
29. Shah, R, Woolley, MM. Gastroschisis and intestinal atresia. J Pediatr Surg. 1991; 26:788–790.
30. Hoehner, JC, Ein, SH, Kim, PC. Management of gastroschisis with concomitant jejuno-ileal atresia. J Pediatr Surg. 1998; 33:885–888.
31. Fleet, MS, de la Hunt, MN. Intestinal atresia with gastroschisis: A selective approach to management. J Pediatr Surg. 2000; 35:1323–1325.
32. Emil, S, Canvasser, N, Chen, T, et al. Contemporary 2 year outcomes of complex gastroschisis. J Pediatr Surg. 2012; 47:1521–1528.
33. Frolov, P, Alali, J, Klein, MD. Clinical risk factors for gastroschisis and omphalocele in humans: A review of the literature. Pediatr Surg Int. 2010; 26:1135–1148.
34. Snyder, CL, St Peter, SD. Trends in mode of delivery for gastroschisis infants. Am J Perinatol. 2005; 22:391–396.
35. Salihu, HM, Emusu, D, Aliyu, ZY, et al. Mode of delivery and neonatal survival of infants with isolated gastroschisis. Obstet Gynecol. 2004; 104:678–683.
36. Puligandla, PS, Janvier, A, Flageole, H, et al. Routine cesarean delivery does not improve the outcome of infants with gastroschisis. J Pediatr Surg. 2004; 39:742–745.
37. Segel, SY, Marder, SJ, Parry, S, et al. Fetal abdominal wall defects and mode of delivery: A systematic review. Obstet Gynecol. 2001; 98:867–873.
38. Guibourdenche, J, Berrebi, D, Vuillard, E, et al. Biochemical investigations of bowel inflammation in gastroschisis. Pediatr Res. 2006; 60:565–568.
39. Vargun, R, Aktug, T, Heper, A, et al. Effects of intrauterine treatment on interstitial cells of Cajal in gastroschisis. J Pediatr Surg. 2007; 42:783–787.
40. Logghe, HL, Mason, GC, Thornton, JG, et al. A randomized controlled trial of elective preterm delivery of fetuses with gastroschisis. J Pediatr Surg. 2005; 40:1726–1731.
41. Charlesworth, P, Njere, I, Allotey, J, et al. Postnatal outcome in gastroschisis: Effect of birth weight and gestational age. J Pediatr Surg. 2007; 42:815–818.
42. Maramreddy, H, Fisher, J, Slim, M, et al. Delivery of gastroschisis patients before 37 weeks of gestation is associated with increased morbidities. J Pediatr Surg. 2009; 44:1360–1366.
43. Jansen, LA, Safavi, A, Lin, Y, et al. Preclosure fluid resuscitation influences outcome in gastroschisis. Am J Perinatol. 2012; 29:307–312.
44. Mortellaro, VE, St Peter, SD, Fike, FB, et al. Review of the evidence on the closure of abdominal wall defects. Pediatr Surg Int. 2011; 27:391–397.
45. Aldrink, JH, Caniano, DA, Nwomeh, BC. Variability in gastroschisis management: A survey of North American pediatric surgery training programs. J Surg Res. 2012; 176:159–163.
46. Alali, JS, Tander, B, Malleis, J, et al. Factors affecting the outcome in patients with gastroschisis: How important is immediate repair? Eur J Pediatr Surg. 2011; 21:99–102.
47. Owen, A, Marven, S, Jackson, L, et al. Experience of bedside preformed silo staged reduction and closure for gastroschisis. J Pediatr Surg. 2006; 41:1830–1835.
48. Hassan, SF, Pimpalwar, A. Primary suture-less closure of gastroschisis using negative pressure dressing (wound vacuum). Eur J Pediatr Surg. 2011; 21:287–291.
49. Bianchi, A, Dickson, AP. Elective delayed reduction and no anesthesia: ‘minimal intervention management’ for gastroschisis. J Pediatr Surg. 1998; 33:1338–1340.
50. Wesson, DE, Baesl, TJ. Repair of gastroschisis with preservation of the umbilicus. J Pediatr Surg. 1986; 21:764–765.
51. Houben, CH, Patel, S. Gastroschisis closure: A technique for improved cosmetic repair. Pediatr Surg Int. 2008; 24:1057–1060.
52. Hernandez Siverio, N, M López-Tomassetti Fernández, E, Mario Troyano Luque, J. Gastroschisis: Primary closure using umbilical cord strengthened by a polypropylene mesh. J Perinat Med. 2007; 35:249–251.
53. Uceda, J. Umbilical preservation in gastroschisis. J Pediatr Surg. 1996; 31:1367–1368.
54. Bonnard, A, Zamakhshary, M, de Silva, N, et al. Non-operative management of gastroschisis: A case-matched study. Pediatr Surg Int. 2008; 24:767–771.
55. Olesevich, M, Alexander, F, Khan, M, Cotman, K. Gastroschisis revisited: Role of intraoperative measurement of abdominal pressure. J Pediatr Surg. 2005; 40:789–792.
56. Lacey, SR, Carris, LA, Beyer, AJ, 3rd., et al. Bladder pressure monitoring significantly enhances care of infants with abdominal wall defects: A prospective clinical study. J Pediatr Surg. 1993; 28:1370–1374.
57. Ein, SH, Superina, R, Bagwell, C, et al. Ischemic bowel after primary closure for gastroschisis. J Pediatr Surg. 1988; 23:728–730.
58. Yaster, M, Scherer, TL, Stone, MM, et al. Prediction of successful primary closure of congenital abdominal wall defects using intraoperative measurements. J Pediatr Surg. 1989; 24:1217–1220.
59. McGuigan, RM, Mullenix, PS, Vegunta, R, et al. Splanchnic perfusion pressure: A better predictor of safe primary closure than intraabdominal pressure in neonatal gastroschisis. J Pediatr Surg. 2006; 41:901–904.
60. Kidd, JN, Jr., Jackson, RJ, Smith, SD, et al. Evolution of staged versus primary closure of gastroschisis. Ann Surg. 2003; 237:759–764.
61. Sandler, A, Lawrence, J, Meehan, J, et al. A “plastic” sutureless abdominal wall closure in gastroschisis. J Pediatr Surg. 2004; 39:738–741.
62. Orion, KC, Krein, M, Liao, J, et al. Outcomes of plastic closure in gastroschisis. Surgery. 2011; 150:177–1785.
63. Jensen, AR, Waldhausen, JH, Kim, SS. The use of a spring-loaded silo for gastroschisis: Impact on practice patterns and outcomes. Arch Surg. 2009; 144:516–519.
64. Chiu, B, Lopoo, J, Hoover, JD, et al. Closing arguments for gastroschisis: Management with silo reduction. J Perinat Med. 2006; 34:243–245.
65. Schlatter, M, Norris, K, Uitvlugt, N, et al. Improved outcomes in the treatment of gastroschisis using a preformed silo and delayed repair approach. J Pediatr Surg. 2003; 38:459–464.
66. Banyard, D, Ramones, T, Phillips, SE, et al. Method to our madness: An 18-year retrospective analysis on gastroschisis closure. J Pediatr Surg. 2010; 45:579–584.
67. Weil, BR, Leys, CM, Rescorla, FJ. The jury is still out: Changes in gastroschisis management over the last decade are associated with both benefits and shortcomings. J Pediatr Surg. 2012; 47:119–124.
68. Weinsheimer, RL, Yanchar, NL, Bouchard, SB, et al. Gastroschisis closure–does method really matter? J Pediatr Surg. 2008; 43:874–878.
69. Pastor, AC, Phillips, JD, Fenton, SJ, et al. Routine use of a SILASTIC spring-loaded silo for infants with gastroschisis: A multicenter randomized controlled trial. J Pediatr Surg. 2008; 43:1807–1812.
70. Bradnock, TJ, Marven, S, Owen, A, et al. Gastroschisis: One year outcomes from national cohort study. BMJ. 2011; 343.
71. Fleet, MS, de la Hunt, MN. Intestinal atresia with gastroschisis: A selective approach to management. J Pediatr Surg. 2000; 35:1323–1325.
72. Phillips, JD, Raval, MV, Redden, C, et al. Gastroschisis, atresia, dysmotility: Surgical treatment strategies for a distinct clinical entity. J Pediatr Surg. 2008; 43:2208–2212.
73. Kronfli, R, Bradnock, TJ, Sabharwal, A. Intestinal atresia in association with gastroschisis: A 26-year review. Pediatr Surg Int. 2010; 26:891–894.
74. Vachharajani, AJ, Dillon, PA, Mathur, AM. Outcomes in neonatal gastroschisis: An institutional experience. Am J Perinatol. 2007; 24:461–465.
75. Houben, C, Davenport, M, Ade-Ajayi, N, et al. Closing gastroschisis: Diagnosis, management, and outcomes. J Pediatr Surg. 2009; 44:343–347.
76. Barsoom, MJ, Prabulos, A, Rodis, JF, et al. Vanishing gastroschisis and short-bowel syndrome. Obstet Gynecol. 2000; 96:818–819.
77. Langer, JC, Bramlett, G. Effect of prokinetic agents on ileal contractility in a rabbit model of gastroschisis. J Pediatr Surg. 1997; 32:605–608.
78. Curry, JI, Lander, AD, Stringer, MD. A multicenter, randomized, double-blind, placebo-controlled trial of the prokinetic agent erythromycin in the postoperative recovery of infants with gastroschisis. J Pediatr Surg. 2004; 39:565–569.
79. Rijhwani, A, Davenport, M, Dawrant, M, et al. Definitive surgical management of antenatally diagnosed exomphalos. J Pediatr Surg. 2004; 40:516–522.
80. Arnold, MA, Chang, DC, Nabaweesi, R, et al. Development and validation of a risk stratification index to predict death in gastroschisis. J Pediatr Surg. 2007; 42:950–955.
81. Kassa, AM, Lilja, HE. Predictors of postnatal outcome in neonates with gastroschisis. J Pediatr Surg. 2011; 46:2108–2114.
82. Molik, KA, Gingalewski, CA, West, KW, et al. Gastroschisis: A plea for risk categorization. J Pediatr Surg. 2001; 36:51–55.
83. Lao, OB, Larison, C, Garrison, MM, et al. Outcomes in neonates with gastroschisis in USA children’s hospitals. Am J Perinatol. 2010; 27:97–101.
84. Wada, M, Kato, T, Hayashi, Y, et al. Intestinal transplantation for short bowel syndrome secondary to gastroschisis. J Pediatr Surg. 2006; 41:1841–1845.
85. Oldham, KT, Coran, AG, Drongowski, RA, et al. The development of necrotizing enterocolitis following repair of gastroschisis: A surprisingly high incidence. J Pediatr Surg. 1988; 23:945–949.
86. Kurbegov, AC, Sondheimer, JM. Pneumatosis intestinalis in non-neonatal pediatric patients. Pediatrics. 2001; 108:402–406.
87. Jayanthi, S, Seymour, P, Puntis, JW, et al. Necrotizing enterocolitis after gastroschisis repair: A preventable complication? J Pediatr Surg. 1998; 33:705–707.
88. Lawson, A, de La Hunt, MN. Gastroschisis and undescended testis. J Pediatr Surg. 2001; 36:366–367.
89. Hill, SJ, Durham, MM. Management of cryptorchidism and gastroschisis. J Pediatr Surg. 2011; 46:1798–1803.
90. Berger, AP, Hager, J. Management of neonates with large abdominal wall defects and undescended testis. Urology. 2006; 68:175–178.
91. Davies, BW, Stringer, MD. The survivors of gastroschisis. Arch Dis Child. 1997; 77:158–160.
92. Solerte, L. Three-dimensional multiplanar ultrasound in a limb-body wall complex fetus: Clinical evidence for counseling. J Matern Fetal Neonatal Med. 2006; 19:109–112.
93. Brantberg, A, Blaas, HG, Haugen, SE, et al. Characteristics and outcome of 90 cases of fetal omphalocele. Ultrasound Obstet Gynecol. 2005; 26:527–537.
94. Cohen-Overbeek, TE, Tong, WH, Hatzmann, TR, et al. Omphalocele: Comparison of outcome following prenatal or postnatal diagnosis. Ultrasound Obstet Gynecol. 2010; 36:687–692.
95. Nicholas, SS, Stamilio, DM, Dicke, JM, et al. Predicting adverse neonatal outcomes in fetuses with abdominal wall defects using prenatal risk factors. Am J Obstet Gynecol. 2009; 201:383.
96. Kamata, S, Usui, N, Sawai, T, et al. Prenatal detection of pulmonary hypoplasia in giant omphalocele. Pediatr Surg Int. 2008; 24:107–111.
97. Hidaka, N, Murata, M, Yumoto, Y, et al. Characteristics and perinatal course of prenatally diagnosed fetal abdominal wall defects managed in a tertiary center in Japan. J Obstet Gynaecol Res. 2009; 35:40–47.
98. Montero, FJ, Simpson, LL, Brady, PC, et al. Fetal omphalocele ratios predict outcomes in prenatally diagnosed omphalocele. Am J Obstet Gynecol. 2011; 205:284.
99. Kleinrouweler, CE, Kuijper, CF, van Zalen-Sprock, MM, et al. Characteristics and outcome and the omphalocele circumference/abdominal circumference ratio in prenatally diagnosed fetal omphalocele. Fetal Diagn Ther. 2011; 30:60–69.
100. Lurie, S, Sherman, D, Bukovsky, I. Omphalocele delivery enigma: The best mode of delivery still remains dubious. Eur J Obstet Gynecol Reprod Biol. 1999; 82:19–22.
101. Baird, R, Gholoum, S, Laberge, JM, Puligandla, P. Management of a giant omphalocele with an external skin closure system. J Pediatr Surg. 2010; 45:E17–E20.
102. van Eijck, FC, Aronson, DA, Hoogeveen, YL, et al. Past and current surgical treatment of giant omphalocele: Outcome of a questionnaire sent to authors. J Pediatr Surg. 2011; 46:482–488.
103. Islam, S. Clinical care outcomes in abdominal wall defects. Curr Opin Pediatr. 2008; 20:305–310.
104. Delorimier, AA, Adzick, NS, Harrison, MR. Amnion inversion in the treatment of giant omphalocele. J Pediatr Surg. 1991; 26:804–807.
105. Yokomori, K, Ohkura, M, Kitano, Y, et al. Advantages and pitfalls of amnion inversion repair for the treatment of large unruptured omphalocele: Results of 22 cases. J Pediatr Surg. 1992; 27:882–884.
106. Alaish, SM, Strauch, ED. The use of Alloderm in the closure of a giant omphalocele. J Pediatr Surg. 2006; 41:e37–e39.
107. Kilbride, KE, Cooney, DR, Custer, MD. Vacuum-assisted closure: A new method for treating patients with giant omphalocele. J Pediatr Surg. 2006; 41:212–215.
108. Brown, AL, II., Roty, AR, Jr., Kilway, JB. Increased survival with new techniques in treatment of gastroschisis. Am Surg. 1978; 44:417–420.
109. Gross, RE. A new method for surgical treatment of a large omphalocele. Surgery. 1948; 24:277–292.
110. Mullins, ME, Horowitz, BZ. Iatrogenic neonatal mercury poisoning from Mercurochrome treatment of a large omphalocele. Clin Pediatr. 1999; 38:111–112.
111. Festen, C, Severijnen, RS, vd Staak, FH. Nonsurgical (conservative) treatment of giant omphalocele. A report of 10 cases. Clin Pediatr. 1987; 26:35–39.
112. Whitehouse, JS, Gourlay, DM, Masonbrink, AR, et al. Conservative management of giant omphalocele with topical povidone-iodine and its effect on thyroid function. J Pediatr Surg. 2010; 45:1192–1197.
113. Almond, S, Reyna, R, Barganski, N, et al. Nonoperative management of a giant omphalocele using a silver impregnated hydrofiber dressing: A case report. J Pediatr Surg. 2010; 45:1546–1549.
114. Lee, SL, Beyer, TD, Kim, SS, et al. Initial nonoperative management and delayed closure for treatment of giant omphaloceles. J Pediatr Surg. 2006; 41:1846–1849.
115. van Eijck, FC, de Blaauw, I, Bleichrodt, RP, et al. Closure of giant omphaloceles by the abdominal wall component separation technique in infants. J Pediatr Surg. 2008; 43:246–250.
116. De Ugarte, DA, Asch, MJ, Hedrick, MH, et al. The use of tissue expanders in the closure of a giant omphalocele. J Pediatr Surg. 2004; 39:613–615.
117. Islam, S. Advances in surgery for abdominal wall defects: Gastroschisis and omphalocele. Clin Perinatol. 2012; 39:375–386.
118. Maksoud-Filho, JG, Tannuri, U, da Silva, MM, et al. The outcome of newborns with abdominal wall defects according to the method of abdominal closure: The experience of a single center. Pediatr Surg Int. 2006; 22:503–507.
119. Dunn, JCY, Fonkalsrud, EW. Improved survival of infants with omphalocele. Am J Surg. 1997; 173:284–287.
120. Wilson, RD, Biard, JM, Johnson, MP, et al. Giant omphalocele: Prenatal diagnosis, short, and long-term outcomes. Am J Hum Genet. 2003; 73:594.
121. Lakasing, L, Cicero, S, Davenport, M, et al. Current outcome of antenatally diagnosed exomphalos: An 11 year review. J Pediatr Surg. 2006; 41:1403–1406.
122. Biard, JM, Wilson, RD, Johnson, MP, et al. Prenatally diagnosed giant omphaloceles: Short- and long-term outcomes. Prenat Diagn. 2004; 24:434–439.
123. Koivusalo, A, Rintala, R, Lindahl, H. Gastroesophageal reflux in children with a congenital abdominal wall defect. J Pediatr Surg. 1999; 34:1127–1129.
124. Argyle, JC. Pulmonary hypoplasia in infants with giant abdominal wall defects. Pediatr Pathol. 1989; 9:43–55.
125. Hershenson, MB, Brouillette, RT, Klemka, L, et al. Respiratory insufficiency in newborns with abdominal wall defects. J Pediatr Surg. 1985; 20:348–353.
126. Edwards, EA, Broome, S, Green, S, et al. Long-term respiratory support in children with giant omphalocele. Anaesth Intensive Care. 2007; 35:94–98.
127. Zaccara, A, Iacobelli, BD, Calzolari, A, et al. Cardiopulmonary performances in young children and adolescents born with large abdominal wall defects. J Pediatr Surg. 2003; 38:478–481.