Chapter 3 Conception and placental development
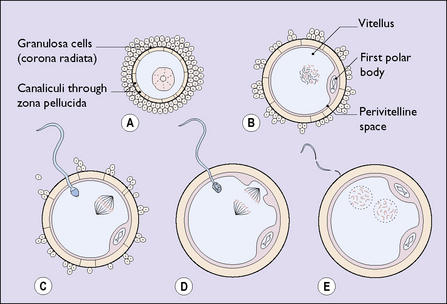
In contrast to other body cells, the oocyte has only 23 chromosomes. At some stage of its growth it undergoes a meiotic division of its nucleus and an unequal division of its cytoplasm, to become a secondary oocyte. The smaller cell is expelled into the perivitelline space and is termed a polar body (Fig. 3.1B). The oocyte and its polar body both contain 23 chromosomes.
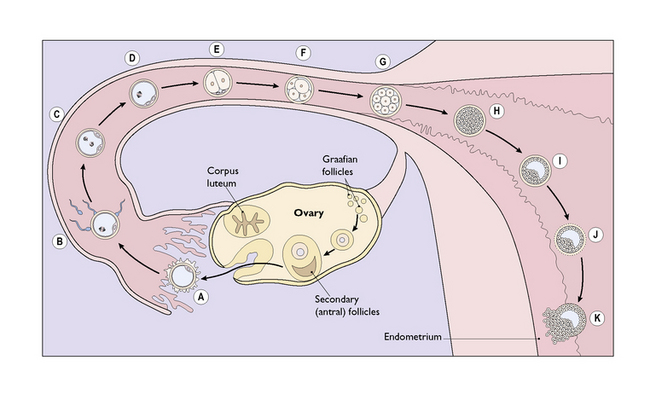
One of the 20 follicles (occasionally two or more, particularly if the ovaries are hyperstimulated in an in-vitro fertilization programme) outstrips the others, developing a large fluid-filled antrum and migrating to bulge through the thickened surface of the ovary (Fig. 3.2). With the release of the luteinizing hormone (LH) surge by the pituitary at midcycle the follicle bursts, expelling the ovum, which is gathered into the Fallopian tube by the fimbriae that project from its proximal end. The ovum, surrounded by the perivitelline, is contained in a condensed opaque substance 5–10 μm thick called the zona pellucida. Adherent to the zona pellucida are theca granulosa cells derived from the mature follicle.
Once the ovum has been expelled the follicle collapses and turns yellow, forming the corpus luteum (Fig. 3.2). The ovum is now ready to be fertilized should it be reached by a sperm.
Of the 60–100 million sperm ejaculated into the vagina at the time of ovulation, several million will negotiate the helical channels in the cervical mucus to reach the uterine cavity. Several hundred will pass through the narrow entrance to the fallopian tubes, and a few will survive to reach the ovum in the fimbrial end of the Fallopian tube. One sperm may penetrate the zona pellucida of the ovum, its head entering the substance of the ovum. When this occurs a chemical reaction prevents the entry of any other sperm. At the same time the oocyte undergoes another division of its chromosomes and a second polar body is formed (see Fig. 3.1D).
Once inside the cytoplasm of the ovum the sperm’s nuclear membrane dissolves, leaving a naked male pronucleus. The ovum, having divided to produce a second polar body, also loses its nuclear membrane. The two naked nuclei approach each other and fuse (see Fig. 3.1E). Fertilization and conception have occurred.
Within a few hours of fertilization, the fused nuclei divide to form two cells (see Fig. 3.2E). Once this has occurred, further cell division proceeds rapidly until, within 3–4 days, a solid mass of cells (the morula) has formed (see Fig. 3.2G, H).
IMPLANTATION
Implantation occurs 8–10 days after ovulation in most healthy pregnancies.
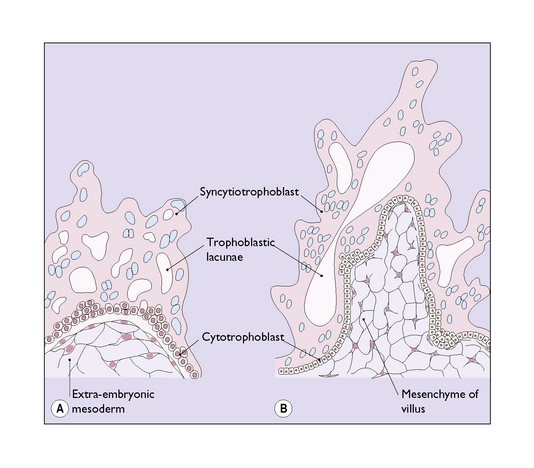
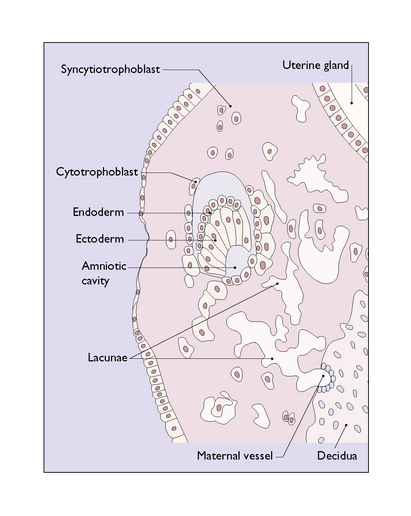
The morula is rapidly propelled along the Fallopian tube to enter the uterine cavity. During its passage, fluid passes through canaliculae in the zona pellucida to create a central fluid-filled cavity in the morula, forming a blastocyst (see Fig. 3.2I). On reaching the uterine cavity the zona pellucida becomes distended and thin. It soon disappears, leaving the surface cells of the blastocyst in contact with the endometrial stroma. About 50% of blastocysts adhere to the endometrium. The surface trophoblastic cells of the adhering blastocyst differentiate into an inner cellular layer, the cytotrophoblast, and an outer syncytiotrophoblast.
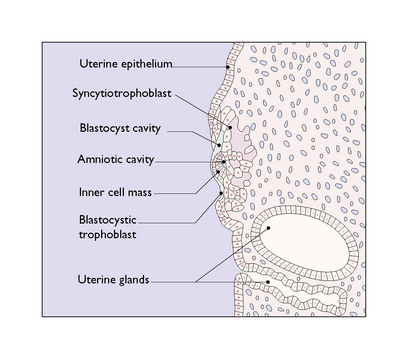
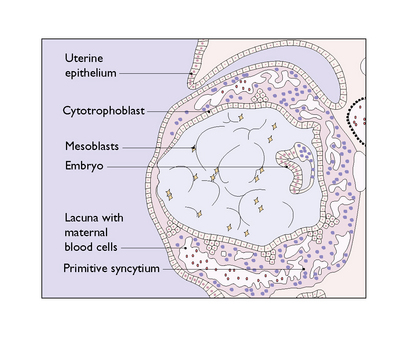
Knobs of trophoblast rapidly form and invade the endometrial stroma in a controlled manner (Fig. 3.3). By the 10th day after fertilization the knobs of trophoblastic tissue have developed a mesodermal core and have pushed deep into the endometrial stroma (Fig. 3.4). The stromal cells react to the invasion by becoming polyhedral in shape and filled with glycogen and lipid, converting into a decidua, which supplies the energy needed by the invading trophoblast. At the same time a number of deep cells at one pole of the blastocyst differentiate to become an inner cell mass, from which the embryo will develop.
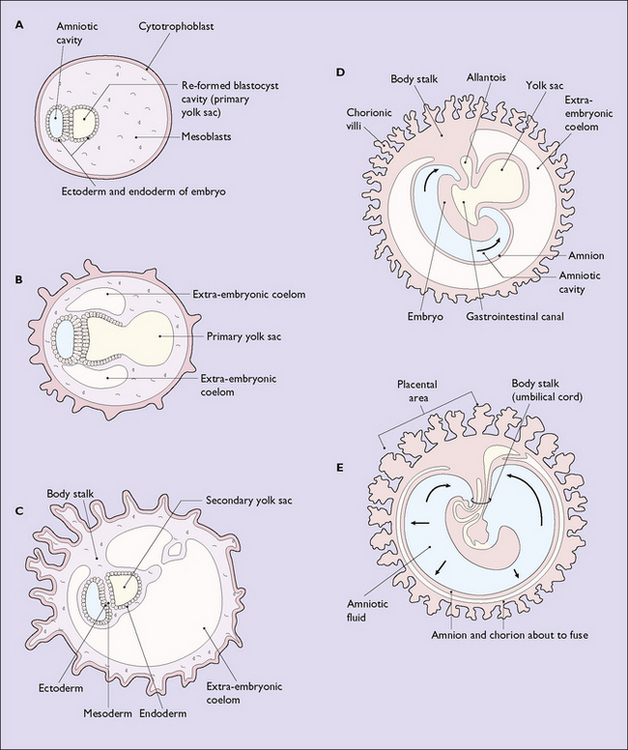
By the 9th to 10th day after fertilization the inner cell mass has differentiated into an ectodermal layer, a mesodermal layer and an endodermal layer, in which a small fluid-filled cavity, the amniotic sac, has formed (Fig. 3.5). The further development of the amniotic sac, in which the fetus will float relatively weightless until it is born, is shown in Figure 3.6.
The inner cell mass projects into the original blastocystic cavity, the walls of which are formed from cytotrophoblast, and the cavity is filled with mesoderm (Fig. 3.7). Quickly, the surface layer of ectoderm divides to surround a fluid-filled cavity in the mesoderm – the yolk sac (see Fig. 3.6A–C).
With further development the yolk sac shrinks in size and a second fluid-filled cavity, the amniotic sac, surrounds the growing embryo (see Fig. 3.6D, E).
FORMATION OF THE PLACENTA
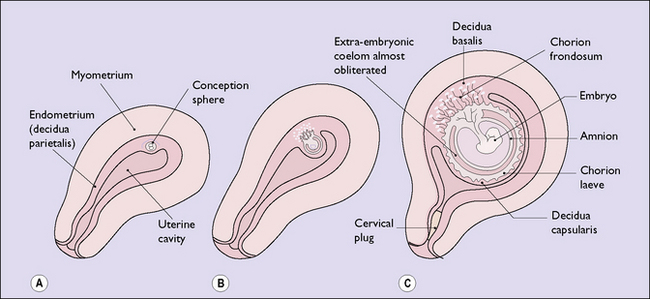
By the 19th day after fertilization the entire conceptus is covered by growing chorionic villi, some attached to the decidua (anchoring villi) but most floating freely in the blood lakes. At this stage further penetration of the decidua ceases, by immunological or chemical mechanisms, and a collagen layer appears through which the spiral arteries and veins pass. As the blood supply to the chorionic villi is greatest on the deep surface of the conceptus the villi grow profusely here, resembling leafy trees (chorion frondosum). The chorionic villi covering the remainder of the conceptus degenerate, forming the chorion laeve. The chorion frondosum forms the placenta, which is the functional union between the conceptus and the maternal tissues. Connection between the fetal and placental circulation is established by day 35, and by the 70th day the placental development is complete (Fig. 3.8).
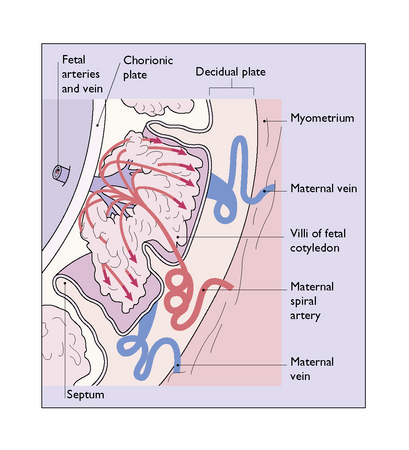
As the blood lakes coalesce, a large blood-filled space is created in which the villi covering each fetal cotyledon float, moved by the motion of the blood. The roof of the space is formed by chorion (the chorionic plate) and its base by trophoblast and decidua (the decidual plate). Septa of varying heights and sizes grow from the decidual plate to separate each fetal cotyledon, each forming an intervillous space. Each intervillous space is supplied by a number of separate arteries, which enter the space at the base of the septa. During maternal systole arterial blood spurts into the space, like a fountain, at a pressure of 80 mmHg. The pressure of the blood pushes the villi aside and the blood hits the chorionic plate and then flows laterally and downwards, bathing the villi, to escape slowly through the veins in the decidual plate (Figs 3.9 and 3.10).
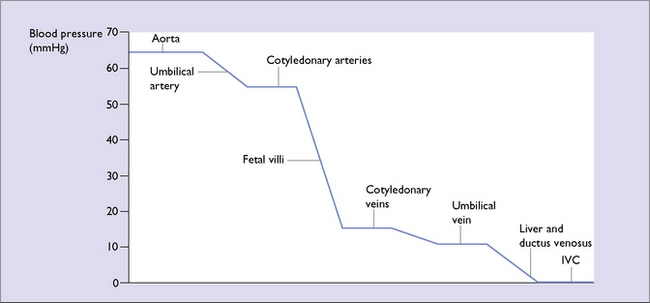
Each villus has a complex anastomosis of capillaries (Fig. 3.10), so there is ample opportunity for the exchange of gases and nutrients. The exchange is enhanced by the vascular resistance in the vessels of the chorionic villi (Fig. 3.11).
FUNCTION OF THE PLACENTA
The placenta acts for the fetus as:
Transport mechanisms through the placenta
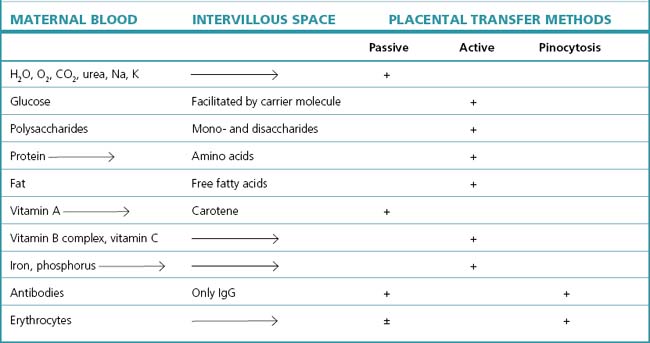
Transport of substances through the placenta takes place by:
These mechanisms require the expenditure of energy, and the rate of placental metabolism has been calculated as comparable to that of the liver or kidney. The transfer of substances is shown in Table 3.1.
Respiratory function
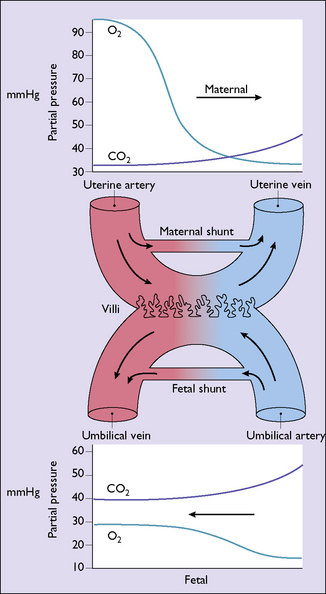
The extensive vasculature in the villi and the relatively slow passage of maternal blood through the intervillous space permits a good exchange of oxygen and carbon dioxide between the fetal and the maternal blood by passive diffusion. The exchange is further enhanced by the maternal blood entering the intervillous space having a 90–100% saturation and a Po2 of 90–100 mmHg. After the metabolic needs of the placenta have been met the fetal erythrocytes take up the oxygen, which is 70% saturated and has a Po2 of 30–40 mmHg, sufficient to meet all fetal needs. Carbon dioxide, like oxygen, diffuses passively across the placenta (Fig. 3.12).
The efficiency of these exchanges depends on a good maternal blood supply to the spiral arteries and a well-functioning placenta. Should the maternal blood supply to the arteries be reduced, as may occur in severe hypertensive states (see Ch. 14), placental ageing (see Ch. 19), uterine hyperactivity or cord compression (see Ch. 20), fetal ketoacidosis may occur independently of maternal acidosis.
Nutrient transfer
Most nutrients are transferred from mother to fetus by active transfer methods, involving enzyme processes. Complex nutrients are broken down into simple components before transfer and are reconstituted in the fetal chorionic villi. Glucose is particularly important as it is the major source of energy for the growing fetus, supplying over 90% of its requirements (10% deriving from amino acids). The quantity of glucose transferred increases after the 30th gestational week. Towards the end of pregnancy about 10 g of glucose per kilogram weight is retained daily, and the excess over metabolic requirements is converted into glycogen and fat. The glycogen is stored in the liver and the fat is deposited around the heart and behind the scapula. In the last quarter of pregnancy 2 g of fat are synthesized daily, and thus by 40 weeks’ gestation 15% of the fetal body weight is fat. This provides an energy store of 21 000 kJ and provides for the metabolic functions and the regulation of body temperature in the days after birth. In preterm and dysmature babies the energy stores are lower, which may cause problems (see Ch. 19).
Drug transfer
Illegal drugs (narcotics, cocaine and amphetamines) taken by the mother pass through the placenta and may affect fetal development. The extent of their effect is difficult to determine, as most drug abusers also smoke and drink alcohol. The fetus tends to be growth restricted, may have congenital abnormalities and is likely to be born preterm, and if the mother takes narcotics the infant may have withdrawal symptoms (see pp. 51–52).
Hormone synthesis
Human chorionic gonadotrophin
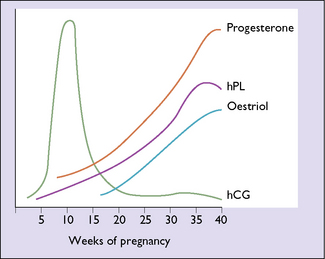
Human chorionic gonadotrophin (hCG) is synthesized from the early days of placentation. Its blood level reaches a peak between the 60th and 80th days of pregnancy, when 500 000–1 000 000 IU are secreted each day. The amount secreted then falls to between 80 000 and 120 000 IU/day, a level which is maintained until term (Fig. 3.13). The peak is higher and lasts longer in multiple pregnancy and in gestational trophoblastic disease. After childbirth the level falls rapidly. Initially the function of hCG is to maintain the corpus luteum’s secretion of progesterone and oestrogen. Once the placenta takes over this function, the level of hCG declines. Human chorionic gonadotrophin may also regulate oestrogen production by the placenta and suppress maternal immunological reactions directed against the fetus.
Oestrogen
In pregnancy the main source of oestrogen is the placenta. The synthesis of oestrogen requires the intervention of the fetus, as the placenta lacks specific enzymes (C17, 20-desmolase and 16-hydroxylase), which are required in the synthesis of oestrogen from acetate and cholesterol. Of the three classic oestrogens, oestriol (E3), oestradiol (E2) and oestrone (E1), the placenta produces more oestriol and retains it selectively. The result is that compared to a non-pregnant circulating E3 : E2 : E1 ratio of 3 : 2 : 1, in pregnancy the ratio is 30 : 2: 1. This means that over 90% of the oestrogen secreted in pregnancy is oestriol, and the level rises progressively throughout pregnancy (Fig. 3.13).
Progesterone
In pregnancy, progesterone is secreted initially by the corpus luteum but, by the 35th gestational day, the placental cytotrophoblast takes over all significant production from maternally supplied precursors, mainly cholesterol. At this stage of pregnancy the plasma progesterone level is 50 ng/mL. From now on the level rises in a linear manner to reach 150 ng/mL by term (see Fig. 3.13).
Protein hormones
The placenta secretes a number of pregnancy-specific hormones, of which human placental lactogen (hPL), or human chorionic somatomammotropin, is the best understood. Its secretion is the reverse of that of hCG: when hCG falls from its peak, hPL continues to rise (see Fig. 3.13). The most important pregnancy-preserving function of hPL is to mobilize free fatty acids from maternal body stores. This lipolytic effect reduces utilization of maternal glucose, which is then diverted for fetal energy needs. Human placental lactogen also stimulates insulin secretion, but inhibits its effects at peripheral sites, and aids in the transfer of amino acids to the fetus.