Conception and nidation
Oogenesis
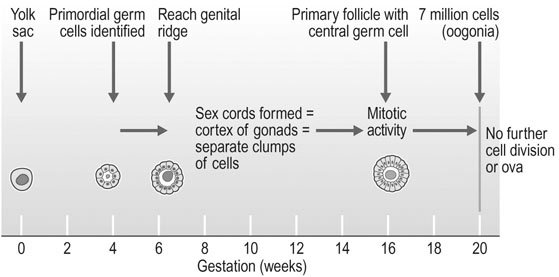
Primordial germ cells originally appear in the yolk sac and can be identified by the fourth week of fetal development (Fig. 2.1). These cells migrate through the dorsal mesentery of the developing gut and finally reach the genital ridge between 44 and 48 days post-conception. Migration occurs into a genital tubercle consisting of mesenchymal cells that appear over the ventral part of the mesonephros. The germ cells form sex cords and become the cortex of the ovary.
Meiosis
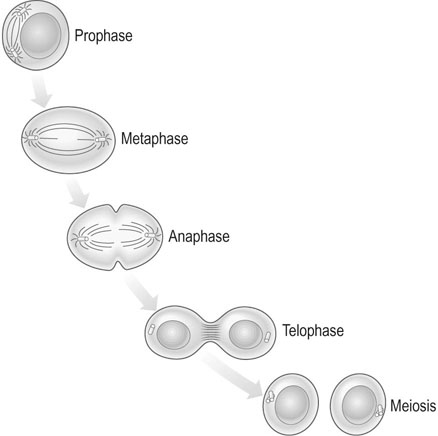
In meiosis, two cell divisions occur in succession, each of which consists of prophase, metaphase, anaphase and telophase. The first of the two cell divisions is a reduction division and the second is a modified mitosis in which the prophase is usually lacking (Fig. 2.2). At the end of the first meiotic prophase, the double chromosomes undergo synapsis, producing a group of four homologous chromatids called a tetrad. The two centrioles move to opposite poles. A spindle forms in the middle and the membrane of the nucleus disappears. During this prophase period of meiosis I the double chromosomes, which are closely associated in pairs along their entire length, undergo synapsis, crossing over and undergoing chromatid exchange, with these processes accounting for the differences seen between two same sex siblings despite the fact the female gametes came from the same mother.
Follicular development in the ovary
The gross structure and the blood supply and nerve supply of the ovary have been described in Chapter 1. However, the microscopic anatomy of the ovary is important in understanding the mechanism of follicular development and ovulation.
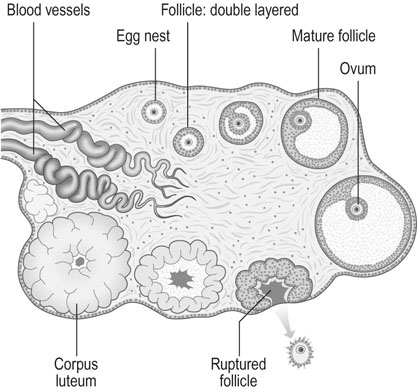
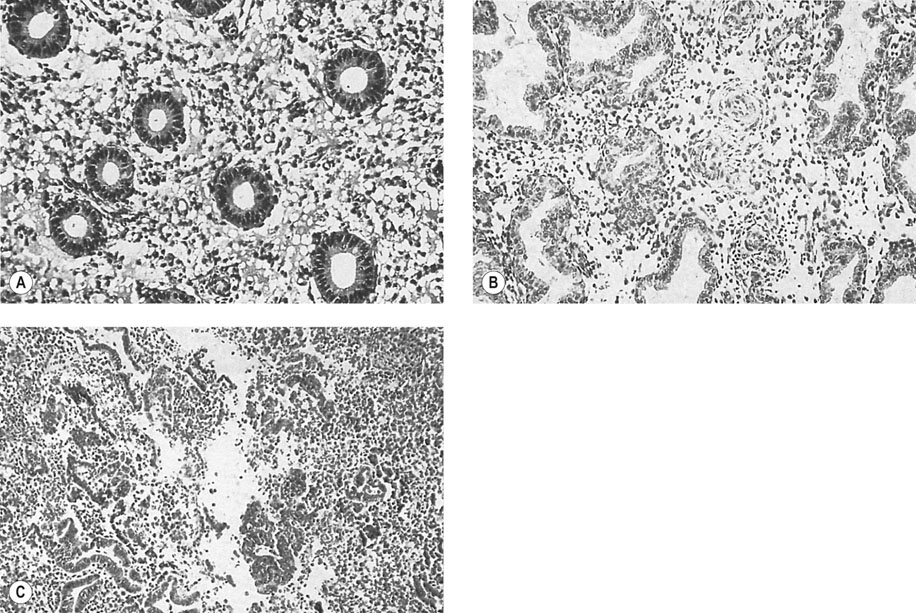
The surface of the ovary is covered by a single layer of cuboidal epithelium. The cortex of the ovary contains a large number of oogonia surrounded by follicular cells that become granulosa cells. The remainder of the ovary consists of a mesenchymal core. Most of the ova in the cortex never reach an advanced stage of maturation and become atretic early in follicular development. At any given time, follicles can be seen in various stages of maturation and degeneration (Fig. 2.3). About 800 primary follicles are ‘lost’ during each month of life from soon after puberty until the menopause, with only one or two of these follicles resulting in release of a mature ovum each menstrual cycle in the absence of ovarian hyperstimulation therapy. This progressive loss occurs irrespective of whether the patient is pregnant, on the oral contraceptive pill, having regular cycles or is amenorrhoeic, with the menopause occurring at the same time irrespective of the number of pregnancies or cycle characteristics. The vast majority of the follicles lost have undergone minimal or no actual maturation.
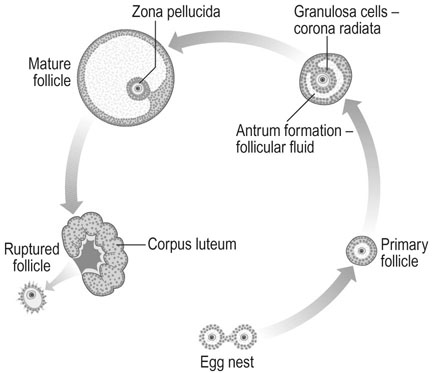
The cavity of the follicle often fills with blood but, at the same time, the granulosa cells and the theca interna cells undergo the changes of luteinization to become filled with yellow carotenoid material. The corpus luteum in its mature form shows intense vascularization and pronounced vacuolization of the theca and granulosa cells with evidence of hormonal activity. This development reaches its peak approximately seven days after ovulation and thereafter the corpus luteum regresses unless implantation occurs, when β-human chorionic gonadotropin (β-hCG) production by the implanting embryo prolongs corpus luteum function until the placenta takes over this role at about 10 weeks of gestation. The corpus luteum degeneration is characterized by increasing vacuolization of the granulosa cells and the appearance of increased quantities of fibrous tissue in the centre of the corpus luteum. This finally develops into a white scar known as the corpus albicans (Fig. 2.4).
Hormonal events associated with ovulation
The maturation of oocytes, ovulation and the endometrial and tubal changes of the menstrual cycle are all regulated by a series of interactive hormonal changes (Fig. 2.5).
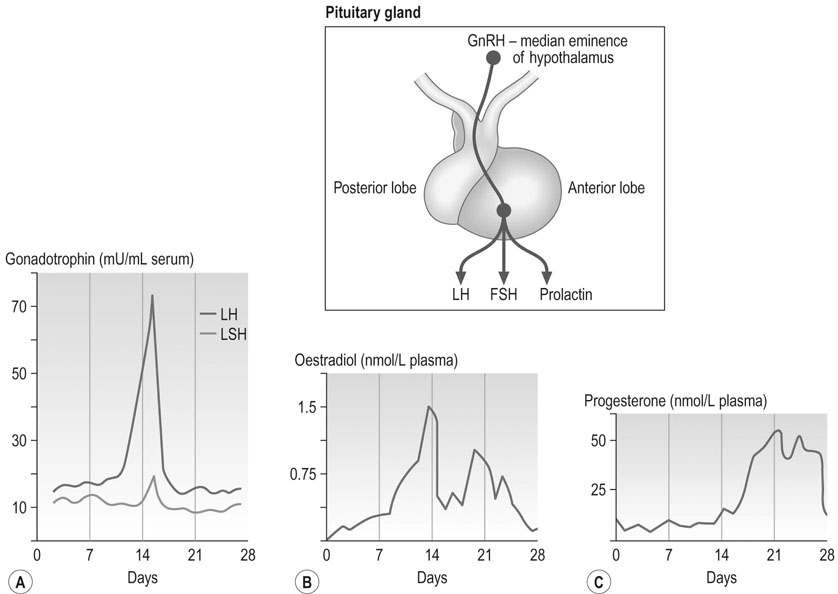
The three major hormones involved in reproduction are produced by the anterior lobe of the pituitary gland or adenohypophysis, and include FSH, LH and prolactin. Blood levels of FSH are slightly higher during menses and subsequently decline due to the negative feedback effect of the oestrogen production by the dominant follicle. LH levels appear to remain at a relatively constant level in the first half of the cycle, however there is a marked surge of LH 35–42 hours before ovulation and a smaller coincidental FSH peak (Fig. 2.5). The LH surge is, in fact, made up of two proximate surges and a peak in plasma oestradiol precedes the LH surge. Plasma LH and FSH levels are slightly lower in the second half of the cycle than in the pre-ovulatory phase, but continued LH release by the pituitary is necessary for normal corpus luteum function. Pituitary gonadotrophins influence the activity of the hypothalamus by a short-loop feedback system between the gonadotrophins themselves and the effect of the ovarian hormones produced due to FSH and LH action on the ovaries.
Oestrogen production increases in the first half of the cycle, falls to about 60% of its follicular phase peak following ovulation and a second peak occurs in the luteal phase. Progesterone levels are low prior to ovulation but then become elevated throughout most of the luteal phase. These features are shown in Figure 2.5.
The action of gonadotrophins
LH stimulates the process of ovulation, the reactivation of meiosis I and sustains development of the corpus luteum; receptors for LH are found in the theca and granulosa cells and in the corpus luteum. There is a close interaction between FSH and LH in follicular growth and maturation. The corpus luteum produces oestrogen and progesterone until it begins to deteriorate in the late luteal phase (Fig. 2.4).
The endometrial cycle
The normal endometrium responds in a cyclical manner to the fluctuations in ovarian steroids. The endometrium consists of three zones and it is the two outer zones that are shed during menstruation (Fig. 2.6).
1. Menstrual phase. This occupies the first 4 days of the cycle and results in shedding of the outer two layers of the endometrium. The onset of menstruation is preceded by segmental vasoconstriction of the spiral arterioles. This leads to necrosis and shedding of the functional layers of the endometrium. The vascular changes are associated with a fall in both oestrogen and progesterone levels but the mechanism by which these vascular changes are mediated is still not understood. What is clear clinically is that the menstruation due to the shedding of the outer layers of the endometrium occurs whether oestrogen or progesterone, or both, fall with the loss generally being less if both the oestrogen and progesterone levels fall (as at the end of an ovulatory cycle), and heavier when only the oestrogen level falls as in an anovulatory cycle.
2. Phase of repair. This phase extends from day 4 to day 7 and is associated with the formation of a new capillary bed arising from the arterial coils and with the regeneration of the epithelial surface.
3. Follicular or proliferative phase. This is the period of maximal growth of the endometrium and is associated with elongation and expansion of the glands and with stromal development. This phase extends from day 7 until the day of ovulation (generally day 14 of the cycle).
4. Luteal or secretory phase. This follows ovulation and continues until 14 days later when menstruation starts again. During this phase, the endometrial glands become convoluted and ‘saw-toothed’ in appearance. The epithelial cells exhibit basal vacuolation and, by the mid-luteal phase (about day 20 of a 28 day cycle), there is visible secretion in these cells. The secretion subsequently becomes inspissated and, as menstruation approaches, there is oedema of the stroma and a pseudodecidual reaction. Within 2 days of menstruation, there is infiltration of the stroma by leukocytes.
Production of sperm
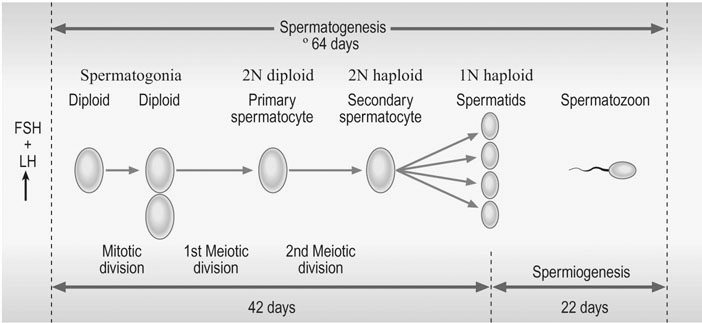
Spermatogenesis
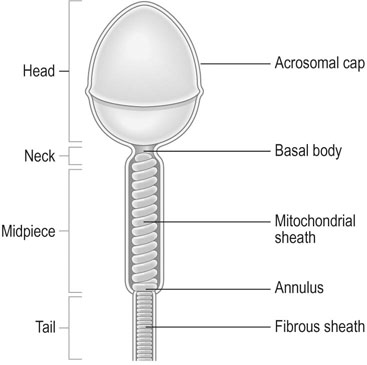
The full maturation of spermatozoa takes about 64-70 days (Fig. 2.7). All phases of maturation can be seen in the testis. Mitotic proliferation produces large numbers of cells (called spermatogonia) after puberty until late in life. These spermatogonia are converted to spermatocytes within the testis, and then the first meiotic division commences. As in the female, during this phase chromatid exchange occurs resulting in all gametes being different despite coming from the same original cell. Spermatocytes and spermatids are produced from the spermatogonia. Spermatozoa are finally produced and released into the lumen of the seminiferous tubules and then into the vas deferens. At the time of this final release meiosis II has been completed. Full capacitation of the sperm, to enable fertilization to occur, is not achieved until the sperm have passed through the epididymis and seminal vesicles, augmented by a suitable endocrine environment in the uterus or Fallopian tube and finally when the spermatozoon becomes adherent to the oocyte.
Fertilization
Fertilization and implantation
Only a small number of spermatozoa reach the oocyte in the ampulla of the tube and surround the zona pellucida. The adherence of the sperm to the oocyte initiates the acrosome reaction, which involves the loss of plasma membrane over the acrosomal cap (Fig. 2.9A).
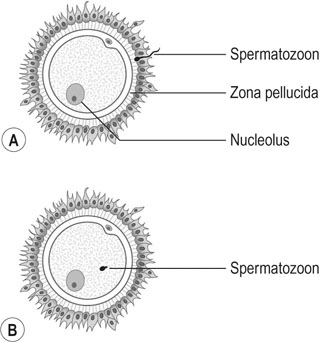
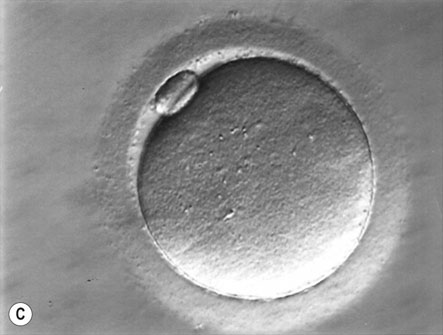
The sperm head decondenses to form the male pronucleus and eventually becomes apposed to the female pronucleus in the female egg to form the zygote. The membranes of the pronuclei break down to facilitate the fusion of male and female chromosomes. This process is known as syngamy (Fig. 2.9B, C) and is followed almost immediately by the first cleavage division.
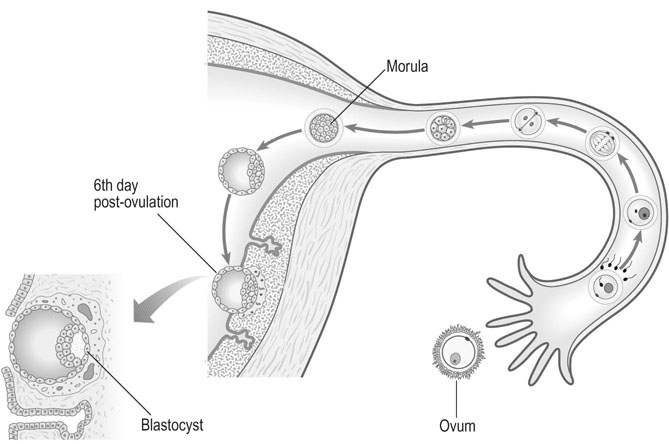
During the 36 hours after fertilization, the conceptus is transported through the tube by muscular peristaltic action. The zygote undergoes cleavage and at the 16-cell stage, becomes a solid ball of cells known as a morula. A fluid-filled cavity develops within the morula to form the blastocyst (Fig. 2.10). Six days after ovulation, the embryonic pole of the blastocyst attaches itself to the endometrium, usually near to the mid-portion of the uterine cavity. By the seventh post-ovulatory day, the blastocyst has penetrated deeply into the endometrium.
The processes of fertilization and implantation are now complete.
The physiology of coitus
The orgasmic phase is induced by stimulation of the glans penis and by movement of penile skin on the penile shaft. There are reflex contractions of the bulbocavernosus and ischiocavernosus muscles and ejaculation of semen in a series of spurts. Specific musculoskeletal activity occurs that is characterized by penile thrusting. The systemic changes of hyperventilation and rapid respiration persist.
During the resolution phase, penile erection rapidly subsides, as does the hyperventilation and tachycardia. There is a marked sweating reaction in some 30–40% of individuals. During this phase, the male becomes refractory to further stimulation. The plateau phase may be prolonged if ejaculation does not occur.