CHAPTER 11 Computer-Assisted Surgery for Femoroacetabular Impingement
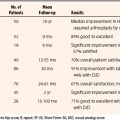
Arthroscopic treatment of FAI has grown in popularity during the last decade. Advantages include minimally invasive access to the hip joint, peripheral compartments, and associated soft tissues. Furthermore, arthroscopy allows for a dynamic intraoperative assessment and correction of the offending lesions. Although initially met with skepticism, the early results of arthroscopic treatment of FAI have shown favorable outcomes. A recent systematic review reported 67% to 100% good to excellent short-term clinical outcomes after arthroscopic treatment of hip impingement.1 The long-term viability of these outcomes, however, is predicated on meticulous intraoperative evaluation and a thorough and accurate correction of impingement lesions on both the femoral and acetabular sides. Failure of arthroscopic techniques for FAI is most commonly associated with incomplete decompression of the associated bony anatomy.2
REQUIREMENTS FOR A COMPUTER-ASSISTED SYSTEM
Preoperative Considerations
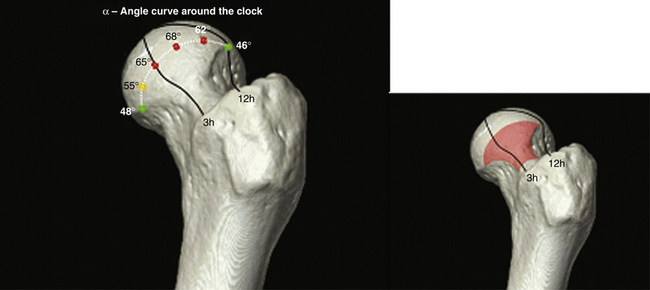
Cam impingement is the result of a loss of the normal sphericity of the femoral head from congenital, developmental, or post-traumatic changes in the shape of the proximal femur. The deformity typically occurs at the anterolateral aspect of the junction between the femoral head and neck, but can occur in any location around the circumference of the head-neck junction. Cam impingement results in a characteristic injury pattern to the transition zone cartilage of the acetabulum, where the labrum loses its structural attachment to the adjacent hyaline cartilage.
Pincer impingement results from overcoverage of the acetabulum, resulting in a specific pattern of labral degeneration as the overhanging bone on the acetabulum crushes the labrum during movement; this produces one or more cleavage planes of variable depth within the substance of the labrum. Subtypes of pincer morphology have been identified—focal anterosuperior overcoverage, coxa profunda, acetabular protrusio, and acetabular retroversion.3 Identification of the specific type and location of pincer pathology is critical to developing an appropriate treatment plan that will reliably eliminate mechanical impingement postoperatively.
Intraoperative Considerations
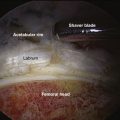
Localization and Resection of Impingement Lesions
Even if the locations of the cam and/or pincer lesions are accurately defined, the magnitude of resection that should be performed is perhaps even more difficult to define. Unfortunately, current techniques rely on the surgeon’s judgment to define an adequate resection that will relieve mechanical impingement without compromising normal structural anatomy. Less experienced hip arthroscopists will tend to perform a less aggressive resection to avoid the risk of a femoral neck fracture or iatrogenic acetabular dysplasia from an overzealous resection. Unfortunately, this tendency leads to a high risk for residual impingement and unfavorable long-term outcomes. On the other hand, aggressive resections that extend beyond the margins of the cam lesion can compromise the cortical support of the native femoral neck and precipitate femoral neck fracture, a significant and potentially devastating postoperative complication. In addition, errant overresection of a pincer lesion on the acetabular side can result in iatrogenic undercoverage and dysplasia.
STATE OF THE ART OF COMPUTER-ASSISTED SURGERY
Methods
Many CAS technologies have been developed over the last 20 years, with applications in orthopedic surgery since the early 1990s.4–6 First, CAS systems depend on the type of patient information that is used. Most systems for total knee and hip surgery use image-free technology in which an optical or magnetic localization system is used to collect bony anatomic reference points intraoperatively. No computed tomography (CT) or magnetic resonance imaging (MRI) scanning is necessary. An extension of those methods considers the use of deformable statistical models to fit the patient anatomy from a collection of surface points.7 In the domain of FAI, none of those image-free techniques have yet given satisfactory results. Because of the complex pathology, preoperative three-dimensional images seem to be necessary. Both CT and MRI have pros and cons, but technically the use of preoperative CT scans provides reliable and accurate contours of bones that make possible automatic processing. So far, extraction of bone and cartilage surfaces from MRI scans can be only achieved manually with semiautomated tools. In the ideal world, fused CT and MRI scans in three dimensions would be the best option, CT because it is accurate and reliable and can be processed automatically, and MRI because it visualizes soft tissues and can be registered automatically to CT images. In practice, CT seems to be a sufficient means to address the requirements indicated at reasonable cost but the debate remains open.
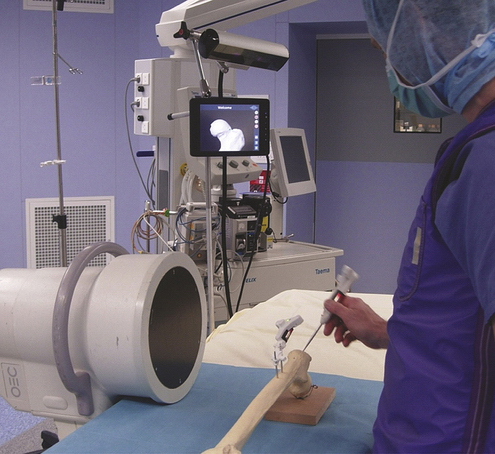
Third, a registration method must be used to match the preoperative images and planning with the intraoperative position of the femur and acetabulum during surgery.8 A mandatory step of any navigation system is to attach trackers to the bones; this is usually accomplished by inserting one 6- or two 3-mm pins in the greater trochanter for the femur and iliac crest for the pelvis. With most ergonomic systems, this takes only a few seconds and has no associated significant risk. These reference trackers can be placed anywhere but must not move during surgery. At that stage, the registration process can start. There are several known methods, including the following:
 Digitization of bone surface landmarks or surface areas using a calibrated probe equipped with a tracker.
Digitization of bone surface landmarks or surface areas using a calibrated probe equipped with a tracker.
 Fluoroscopy can be used to acquire intraoperative images and match them with the preoperative data set.
Fluoroscopy can be used to acquire intraoperative images and match them with the preoperative data set.
 Ultrasound is a noninvasive modality that enables access to difficult areas.
Ultrasound is a noninvasive modality that enables access to difficult areas.
 Intraoperative three-dimensional fluoroscopic imaging is a recent technique that can be used to register the CT or MRI scan based on planning in a stable, precise, and robust manner.
Intraoperative three-dimensional fluoroscopic imaging is a recent technique that can be used to register the CT or MRI scan based on planning in a stable, precise, and robust manner.
 The final stage is the intraoperative navigation, which can be done once the registration process has succeeded.
The final stage is the intraoperative navigation, which can be done once the registration process has succeeded.
Preoperative Assistance
A group at the Müller Institute in Bern, Switzerland, has developed a platform to analyze the hip joint in three dimensions, using two-dimensional x-rays of the pelvis.10 A combination of anteroposterior (AP) and lateral radiographs are integrated into a projection model to create a virtual three-dimensional reconstruction of the pelvis. The goal of this computation is to standardize the measured radiographic parameters, especially the caudocranial femoral head coverage used in the diagnosis of impingement. The main contribution of this work is a standardization of the pelvis orientation that was developed to take into account individual tilt and rotation variability. This absolute reference is computed from a correction of the radiographic orientation based on anatomic and radiographic parameters describing the morphology of the hip. A dedicated software (Hip²Norm) has been developed for that purpose. A validation study was conducted to evaluate the results statistically and quantitatively on 60 cadavers and qualitatively on 51 patients. Depending on the measured parameters, the interpretation of the results showed a satisfactory analysis. Except for the restriction of being applicable only to spherical joint configurations, the method was proved accurate and valuable to help in the diagnosis of the acetabular morphology from two-dimensional radiographs.
A group from Geneva University has addressed this preoperative planning issue with a four-stage method.11 The method developed by this group was intended to help the surgeon in diagnosis, localization of the impingement area, and determination of the area and amount of bone to be resected to regain a satisfactory range of motion. The first stage is the reconstruction of a three-dimensional surface model of the joint, femur, and acetabulum. MRI was used and different slice thicknesses were considered, according to the requested accuracy at different levels of the joint. Manual segmentation of bony contours was performed and three-dimensional surface models were reconstructed. The second stage is the integration of joint kinematics in the surface model to simulate circumduction motion. The main issue with this step is the correct determination of the hip center of rotation, which is done with an iterative procedure. The simulation of the bone reshaping is the third stage of the planning and involves three procedures.
The femoral head is first reshaped by fitting a sphere on its three-dimensional surface, which defines the areas out of the sphere as the impingement zone to be resected. An impingement test simulation is performed to determine the acetabular rim that produces overcoverage of the femoral head. The simulation of the acetabular resection is done manually by intersecting the three-dimensional surface model with planes until the impingement has been reduced. Periacetabular osteotomy lines can also be simulated manually by fitting a controlled curve over the surface of the model. The last step is the computation of the range of motion (ROM). Several joint motions including flexion and extension, rotation, and abduction and adduction can be combined and impingement detection tools can predict the postoperative range of motion after virtual surgery. The developed system is based only on the MRI data from a group of healthy volunteers. It provides the main hip kinematic factors defining the ROM. However, the team that implemented this program does not claim any cadaveric validation or clinical trials.
Another important work on planning and simulation of the treatment has been done by another group from Bern, Switzerland.12 The aim of this system is to provide a tool to help the surgeon better assess the preoperative situation of the hip joint and to plan the most appropriate correction to solve the patient’s femoroacetabular impingement. The software developed, HipMotion, is based on a patient’s preoperative CT scans, enabling a real three-dimensional analysis of the joint. As in the work discussed earlier,11 the patient’s three-dimensional model is reconstructed from preoperative three-dimensional image data. A collision detection algorithm is applied during simulated joint motion to determine the regions of impingement. A quantification of the trimming of bony structures is displayed and can be adjusted. The simulation of postoperative ROM is then computed after these virtual resections have been applied. The automatic computation of the range of motion from a reconstructed three-dimensional joint model has been validated on sawbones and cadaveric hips by comparing the simulations given by the preoperative analysis HipMotion software with actual measurements obtained using a commercially available navigation system from BrainLAB (Feldkirchen, Germany). Reproducibility and reliability were assessed from the results, except for external rotation measurements that showed a moderate similarity. The software had then been used for a pilot clinical study, in which the ROM of a control group was compared with FAI patients. From that quantitative study, it could be shown that the ROM measurements did correspond to what was described more qualitatively in the literature. A significant difference in flexion, abduction, internal rotation, and internal rotation at 90 degrees of flexion angle was detected between the control and study groups, with these angles being significantly reduced for the impingement patients. It could also be shown that the impingement zones automatically computed from the software precisely matched the reported anterosuperior labral and chondral lesion areas observed intraoperatively.
The goal of such preoperative planning systems is to analyze the preoperative impingement situation accurately and plan the location and amount of bony resection, therefore save time in the operating room, by knowing how to address the problem better. However, none of this research discussed has been combined with an effective intraoperative navigation system. The next stage of such programs would be the actual implementation of the planned resections during surgery.
Intraoperative Navigation
One group, based in Boston, published preliminary results using this system for combined impingement types.13 The system was used for acetabular rim and femoral head-neck offset correction. Four patients were reported to have undergone this computer-assisted procedure combined with conventional arthroscopy. The intraoperative registration was successful for all cases and actual tracking of the surgical instruments was performed. However, this system does not allow for intraoperative quantification of bone resection during the procedure, nor can the ROM gain from the current débridement be assessed.
More recently, a similar system was used for a prospective clinical study by a group in Wolhusen, Switzerland.14 The purpose of that study was the evaluation of the impact of a CT-based navigation system from BrainLab on the accuracy of the head-neck offset correction for patients undergoing an arthroscopic procedure for a cam-type impingement lesion. It also sought to evaluate the impact of offset restoration accuracy on early clinical outcomes. The clinical team used preoperative and postoperative MRI scans to evaluate the correction of the alpha angle. A postoperative angle of less than 50 degrees or a correction of more than 20 degrees was considered a successful correction. The intraoperative navigation system also required a preoperative CT scan. The registration was again performed through fluoroscopic image matching. The tracking of surgical instruments by the navigation system was displayed as different three-dimensional views and two-dimensional slices of the patient’s femoral head model. The distance of the instrument tip to the bone surface was computed and displayed, allowing an approximate quantification of the actual bone resection depth. The analysis of the study cases showed no significant statistical difference in the successful correction rate in the navigated or non-navigated groups. Both groups showed 24% insufficient correction of the offset. An average of 25 minutes of extra operating time was observed for the navigated procedures. The current state of the presented CT fluoroscopy–based navigation system does not show any improvement in the correction rate. Moreover, the display of the tracking stage and the presented measures do not help the surgeon assess the amount of bony resection better. Finally, because no preoperative planning can be integrated into the system, there is no predefined target to be reached. The main inconvenience of this adapted system is that it is not fully dedicated to the assistance of FAI arthroscopic procedures. Hence, it cannot provide the specific features required to observe the expected improvements from a navigation system.
Finally, another solution based on computer-assisted encoder linkage navigation concept has been proposed by a team at Carnegie Mellon University (Pittsburgh).15 That system claims to provide assistance first during portal placement to avoid neurovascular structures and then tracking of the surgical instruments. The advantages of using an encoder linkage structure for intraoperative navigation are the elimination of occlusion and distortion problems commonly known with optical and electromagnetic systems. Patients’ preoperative three-dimensional image data have to be obtained from CT or MRI scans or model reconstruction from x-rays. A stiff base pin used to support and attach the tracking linkage is fixed to the patient’s bone and triangulation from intraoperative x-rays is used to register preoperative data to the patient. The linkage arm is mounted to the base pin on one extremity, and the arthroscope or surgical instrument is attached to the other extremity. Two linkage chains are required if tracking of both the arthroscope and surgical tool is to be done. The visualization software displays the relative position of the instrument tip and axis relative to the patient structures. When the arthroscope is navigated, a simulated arthroscopic view is computed that can then be compared with the actual arthroscopic image. A mechanical bench setting has been designed to test the linkage device positioning accuracy. The positioning error has two sources, intrinsic encoder resolution and initialization method of the chain. The overall chain accuracy was show to be within an average of about 5 mm, mainly because of initialization process sensitivity, which needs to be further improved. It was reported that this system provided valuable information while offering a good flexibility required by the narrow space of arthroscopic surgery. All experiences are based only on a phantom hip joint.
Discussion of Current Research Studies
Finally, modeling our clinical problems into a technical system is not trivial, and many issues remain. These dictate extensive work for precise definition of requirements; hopefully we made this clear in the first section of this chapter. Therefore, there is an urgent need to provide a dedicated solution that meets all defined requirements. In that respect, our group is working to develop a new generation of CAS solutions that integrates planning and navigation functions, with the major goals of being user-friendly and not adding operative time. Figures 11-1 and 11-2 illustrate this new concept under development by A2 Surgical (La Tronche, France).
CONCLUSIONS
Complete analysis of the bony anatomy includes circumferential measurements of femoral head-neck offset, acetabular relationships between the anterior and posterior walls, analysis of acetabular coverage in the sagittal and coronal planes, and rotational (version) and angular (varus-valgus) measurements of the femoral neck on the shaft. Each of these parameters will have an effect on the mechanical load across the hip joint, and each must be factored into the surgical solution for the impinging hip. It is naïve to believe that these complexities can be resolved with visual estimation alone. A global and complete approach can be accomplished only with the assistance of computer navigation. Our ability to improve the accuracy and precision of the diagnosis and treatment of FAI will be reflected in continued improvement in patient outcomes and increased longevity of the native hip.
1. Bedi A, Chen N, Robertson W, Kelly BT. The management of labral tears and femoroacetabular impingement of the hip in the young, active patient. Arthroscopy. 2008;24:1135-1145.
2. Heyworth BE, Shindle MK, Voos JE, et al. Radiologic and intraoperative findings in revision hip arthroscopy. Arthroscopy. 2007;23:1295-1302.
3. Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br. 2005;87:1012-1018.
4. Lavallee S, Bainville E, Bricault I. An overview of computer-integrated surgery and therapy (CIST). In: Udupa J, Herman J, editors. 3D Imaging In Medicine. 2nd ed. Boca Raton, Fla: CRC Press; 2000:207-264.
5. Tonetti J, Carrat L, Lavallée S, et al. Percutaneous iliosacral screw placement using image guided techniques. Clin Orthop Rel Res. 1998;354:103-110.
6. Dessenne V, Lavallée S, Orti R, et al. Computer-assisted knee anterior cruciate ligament reconstruction: first clinical tests. J Im Guided Surg. 1995;1:59-64.
7. Stindel E, Briard JL, Merloz P, et al. Bone morphing: 3D morphological data for total knee arthroplasty. Comput Aided Surg. 2002;7:156-168.
8. Lavallée S. Registration for computer integrated surgery: methodology, state of the art. In: Taylor R, Lavallee S, Burdea G, Mosges R, eds. Computer Integrated Surgery. Cambridge, Mass: MIT Press; 1996:77-97.
9. Kilian P, Plaskos C, Parratte S, et al. New visualization tools: computer vision and ultrasound for MIS navigation. Int J Med Robot Comput Assist Surg. 2008;4:23-31.
10. Tannast M, Mistry S, Steppacher SD, et al. Radiographic analysis of femoroacetabular impingement with hip. 2. Norm-reliable and validated. J Orthop Res. 2008;26:1199-1205.
11. Kang MJ, Sadri H, Magnenat-Thalmann N. Computer-assisted pre-operative planning for hip joint-preserving surgery, Paper presented at: 5th Annual Meeting of the International Society for Computer Assisted Orthopaedic Surgery; June 2005, Helsinki, Finland: International Society for Computer Assisted Orthopaedic Surgery; 2005.
12. Tannast M, Kubiak-Langer M, et al. Noninvasive three-dimensional assessment of femoroacetabular impingement. J Orthop Res. 2007;25:122-131.
13. Murphy SB, Ecker TM, Tuma G, Haimerl M. Arthroscopic percutaneous computer assisted FAI relief using a new method of CT-fluoro registration, Paper presented at: 7th Annual Meeting of the International Society for Computer Assisted Orthopaedic Surgery; June 2007, Heidelberg, Germany: International Society for Computer Assisted Orthopaedic Surgery; 2007.
14. Brunner A, Horisberger M, Herzog R. Evaluation of a computer tomography-based navigation system prototype for hip arthroscopy in the treatment of femoroacetabular cam impingement. Arthroscopy. 2009;25:382-391.
15. Monahan E, Shimada K. Computer-aided navigation for arthroscopic hip surgery using encoder linkages for position tracking. Int J Med Robotics Comput Assist Surg. 2006;2:271-278.