7
Composition and Function of Connective Tissue
1. Outline components of connective tissue.
2. Discuss the sequence of overlapping events of inflammation.
4. Identify the sources of coagulation.
5. Describe and discuss the various cells of inflammation and their function.
6. Discuss the molecular cascade of arachidonic acid metabolic pathways of lipoxygenase and cyclooxygenase.
7. Define cytokines and growth factors, and discuss their various functions.
CONNECTIVE TISSUE PROPERTIES
The functions of various connective tissues are to bind cells together to form and organize tissues, organs, and systems and to provide a mechanical link between musculoskeletal junctions and the articulations of joints. Generally, connective tissues are made up of cells and the extracellular matrix that they produce. Extracellular matrix is defined as the noncellular components of connective tissue.4
Two classic functions of connective tissues are mechanical support for bone and soft tissues and intercellular exchange of oxygen, blood, water, gases, cells, and wastes. Basic mechanical support functions of connective tissues, such as bone, ligament, tendon, muscle, and cartilage,1,3 are to provide stability and shock absorption in joints,2 provide a mechanical link system between bones, and transmit muscle forces.4
Elastin is a noncollagenous glycoprotein in which molecules are arranged randomly as a constituent of extracellular connective tissue matrix. Elastin is found in varying amounts in tissues requiring high levels of physiologic motion (elasticity). Two special amino acids, desmosine and isodesmosine, are found in elastin. They are directly responsible for the cross-linking arrangement of elastin fiber and its unique ability to deform under stress then return to its original orientation and shape. Primarily, elastin fibers contain the amino acids glycine, proline, alanine, and valine. Characteristically, elastin fibers can elongate about 70% without undergoing fiber disruption.4
In contrast to elastin, collagen is the most abundant component of the connective tissue matrix, and 12 to 19 distinct types of collagen exist.4 Types of collagen are classified according to their structure and tissue distribution. Biochemical properties of connective tissues such as ligament, cartilage, tendon, bone, and muscle are dependent on the specific predominant types of collagen found in the extracellular matrix. The characteristic extensive network of cross-links in collagen significantly contributes to the stability and strength of the extracellular matrix. The basic histochemical profile of collagen includes the amino acids glycine, hydroxyproline, proline, and hydroxylysine. Of these amino acids, proline generally is responsible for resisting tensile forces in collagen.4 Fibroblasts stimulate collagen synthesis through assembly of polypeptide chains of proline and lysine, which aggregate into a triple helix monomer.4 Ground substance is an amorphous nonfibrous aqueous–gel component of the connective tissue matrix. Generally this substance is responsible for facilitating intercellular exchange of water, oxygen, cells, and gases, as well as providing mechanical support between various tissues.
Proteoglycans are protein and mucopolysaccharide macromolecules subclassified as glycosaminoglycans. Generally, GAGs are responsible for the compressive strength of the cartilage matrix. Proteoglycans are extremely hydrophilic, so they attract and bind water. The major and distinct types of GAGs found in cartilage are chondroitin sulfate, keratan sulfate, and dermatan sulfate, with chondroitin sulfate representing almost 90% of all GAGs in cartilage. These large proteoglycans, specifically chondroitin and keratan, bind together to form a distinct type of GAG referred to as aggrecan. Various types of connective tissues, such as ligament, cartilage, tendon, and muscle, contain varying amounts of these large proteoglycans that relate directly to the specific biomechanical and biochemical nature of all connective tissues. The networking capacity of proteoglycans and collagen within all forms and types of connective tissue contributes to the classically distinct nature of strength, stiffness, rigidity, and flexibility of connective tissues.4
Lipids represent less than 1% of human articular cartilage matrix. The specific function of lipids and phospholipids is not clearly known. However, the presence of lipids in extracellular connective tissue matrix varies with the onset of osteoarthritis (OA).4
Specific connective tissue organization of muscle fibers is systematically arranged by endomysium connective tissue. Muscle fibers collectively are bound together to form fascicles. These fascicles are supported by perimysium connective tissue. The connective tissue membrane surrounding the entire muscle is called epimysium. Muscle tissue is unique in that it consists of contractile elements that respond to stimuli, as well as passive or elastic elements that resist stretching. Muscle tissue and noncontractile connective tissues such as endomysium, perimysium, and epimysium demonstrate characteristic load deformation viscoelastic properties in response to specific stimuli. Human skeletal muscle exhibits the same viscoelastic properties as other dense connective tissues. In fetal development, these noncontractile connective tissues act as tissue scaffolds to hold, support, and provide continuity of gross form and structure of the muscle’s belly. In addition, loose connective tissue of the perimysium serves as a channel for nutrient arteries and vessels, as well as nerves that supply the muscle fibers.4
REVIEW OF TISSUE HEALING
Inflammatory Response
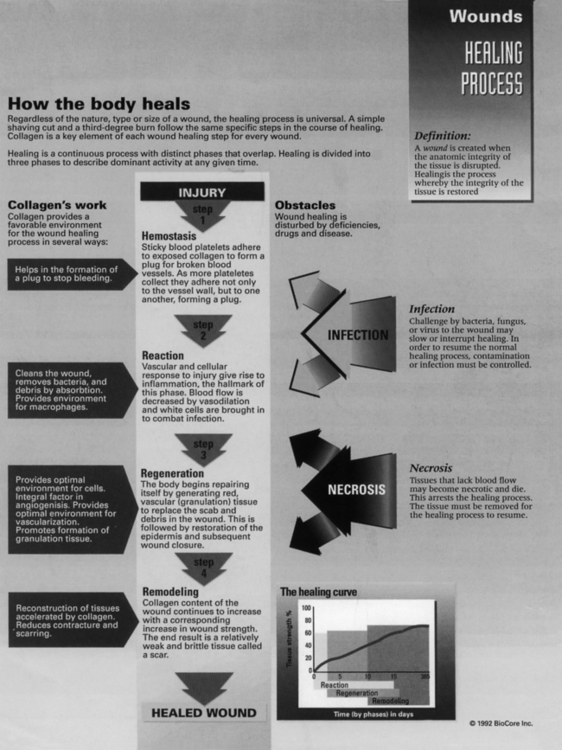
Acute inflammation is a transient initial phase of injury repair that lasts approximately 5 to 7 days. Directly after trauma, platelets migrate to the injury site and release specific growth factors and chemical mediators, which stimulate homeostasis and initiate the repair process. A fibrin scaffold structure is formed within the trauma bed, creating a matrix that allows for platelet aggregation and adherence to the injury site. This process of platelet activation stimulates synthesis of thrombin, fibrin, and the random organization of clot formation. Platelet plug formation is essentially a four-step process: (1) adhesion, (2) aggregation, (3) secretion, and (4) procoagulant activity (Fig. 7-1).
Adhesion of platelets is the deposition of these cells on the subendothelial matrix. Platelets have a surface receptor glycoprotein that binds to a sticky protein substance referred to as von Willebrand factor (vWF) found in the subendothelial matrix. Endothelial cells synthesize vWF, which is released into the circulating plasma, then deposited in the subendothelial matrix in response to exposure from injury.1,3 Aggregation is simply platelet-to-platelet cohesion via the surface fibrinogen receptor complex of the platelets. Secretion is the release of a number of platelet-derived growth factors (PDGFs) by stimulated platelets. The aggregating stimulators of serotonin, thrombospondin, and thromboxane also are secreted. Procoagulant activity refers to the process of thrombin formation and ensures that coagulation occurs at the site of the platelet plug.
Fibroplasia
Several days (5 to 7) after the injury, the relative population of fibroblasts increases, whereas inflammatory cells and proinflammatory factors decrease. At this stage there is a proliferation of reparative cells. Fibroblasts stimulate PDGF and TGF-β among others to synthesize and deposit extracellular matrix constituents of fibronectin, laminin, collagen, and glycosaminoglycans.1,3,4
This phase also includes angiogenesis, the neovascular budding that helps reestablish oxygen-rich and growth factor–rich blood to new, fragile healing tissue. Angiogenic growth factors involved with the stimulation of this neovascularization are fibroblast growth factor (FGF), tumor necrosis factor-β (TNF-β), and wound angiogenesis factor (WAF). Endothelial cells from intact vascular membranes are mobilized to form new tissue from the secretion of specific enzymes and collagens. The end stages of angiogenesis signal vascular capillary and network tube formation, creating new vascular basement membranes that directly communicate with the injury site.1,3,4
Remodeling and Tissue Maturation
The remodeling phase of injury repair is essentially a balance between enzymatic (proteolytic) degradation of excess collagen and the deposition, organization, modification, and maturation of collagen, as well as a systematic regression of inflammatory cells (Fig. 7-2).
Collagenases are enzymes of the metalloproteinase family that act to fragment collagen. The regulation of the rate of collagen synthesis and degradation (turnover) is mediated by specific growth factors such as PDGF, IL, TGF-β, and TNF-β.4
Cummings and Reynolds2 describe remodeling as “the process by which the architecture of connective tissue alters in response to stress.” Collagen fibers align parallel to the direction of applied stress, which increases the strength of the scar tissue union. Remodeling also includes absorption of collagen fibers that lie in opposing directions. If controlled properly, the effect of the remodeling phase is that the fibrous union becomes stronger and more supple with fewer adhesions.
Because of the vascularity, dense cell population of myofibroblasts, and nature of its small, fragile collagen fibers, new scar tissue is able to remodel quickly. Collagen fibers are initially small and disorganized, and they form a spongy randomly oriented tissue. As remodeling continues, larger, more parallel fibers replace the smaller fibers and orient across the wound, which forms a stronger yet supple repair (Fig. 7-3).2
GENERAL CELL TYPES INVOLVED IN INJURY REPAIR
Cellular Structure
Organelles define various component structures within cells. Smooth endoplasmic reticulum as a cell organ is involved with the synthesis of steroid hormones, particularly cholesterol. Rough endoplasmic reticulum organelles are actively involved with protein synthesis and secretion. Within cells the Golgi apparatus functions primarily as a storage and modification organ for various proteins that have been synthesized in the rough endoplasmic reticulum. Lysosomes and peroxisomes are cellular compartment structures that are involved with enzymatic degradation and oxidation of protein and fatty acids. Mitochondria, the powerhouses of the cell, are actively involved with adenosine triphosphate (ATP) production and protein synthesis.1,3
Proteoglycans and Glycoproteins
Proteoglycans are macromolecules consisting of a protein core and bound polysaccharide units referred to as glycosaminoglycans (GAGs). GAGs are major components of ground substance within the extracellular matrix of various connective tissues, such as ligament, capsule, muscle, tendon, cartilage, nerve, vessel, and bone. GAGs are synthesized within the endoplasmic reticulum and Golgi apparatus of the fibroblast cells in connective tissues. Several GAGs are identified in musculoskeletal tissues, which significantly contribute to structure, composition, and function of the extracellular matrix of tissues: (1) chondroitin sulfate, (2) dermatan sulfate, (3) keratan sulfate, (4) hyaluronate, (5) aggregen, (6) decorin, and (7) biglycan.1,3
Generally, GAGs carry a high negative charge that renders GAGs hydrophilic. Within articular cartilage, GAGs are continuously synthesized, assembled, degraded, and secreted by the extracellular matrix to establish a relative homeostatic environment. Enzymatic degradation of GAGs occurs within lysosome organelles of the chondrocytes.1,3,4
CELLS OF INFLAMMATION AND REPAIR
Mononuclear Leukocytes
Mononuclear leukocytes are single-nucleus cells that are derived from pluripotent hematopoietic stem cells, which differentiate into monocytes and lymphocytes. Circulating monocytes are mobilized to damaged tissue, where they are activated and become macrophages. Noted as scavenger cells, macrophages are leukocytes that display three essential functions during the inflammatory process: (1) phagocytosis, (2) antigen presentation, and (3) production of cytokine growth factors.1,3,4
Macrophages synthesize and secrete numerous cytokines, such as TGF-β, PDGF, transforming growth factor-α (TGF-α), and IL-1. The release of these cytokines stimulates proliferation of fibroblasts and collagen deposition and degradation of collagen by secreting enzymes (collagenases) also, which denatures collagen during the inflammatory and remodeling phases.1,3,4
Prostaglandins, Thromboxanes, and Leukotrienes
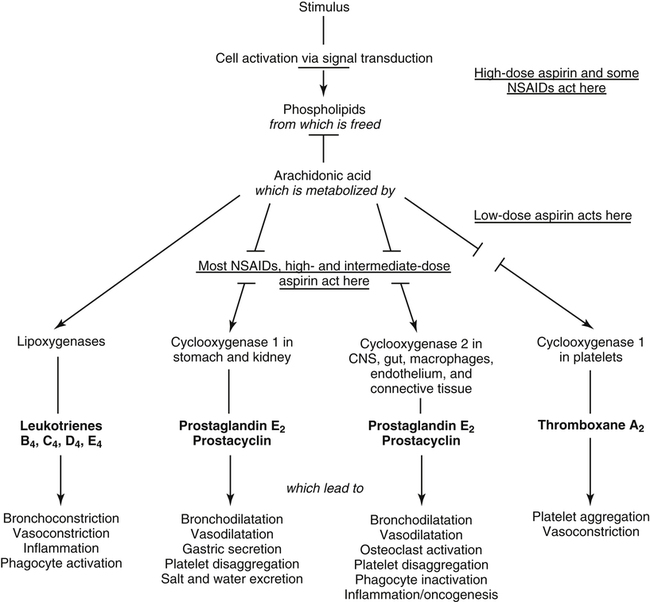
Prostaglandins and thromboxanes are lipid-derived powerful and important mediators of inflammatory reactions (Fig. 7-4), which are metabolized from arachidonic acids within the cells. Arachidonic acid metabolism is initiated by the degradation of cell membrane phospholipids by phospholipase enzymes. This cell membrane degradation releases arachidonic acid–synthesized cyclooxygenase enzymes, such as COX-1 and COX-2, which results in metabolic conversion to prostaglandins and thromboxanes.
Prostaglandins are generally of three forms1,3: (1) prostaglandin E2 (PGE2), which stimulates smooth muscle relaxation and vasodilation; (2) prostaglandin I2 (PGI2), which is synthesized in endothelial cells and incites vascular dilation and inhibition of platelet adhesion; and (3) prostaglandin F2 (PGF2), which is a potent vasoconstrictor and stimulates smooth muscle contractions. These molecules are capable of producing pain and stimulating synthesis of pain-producing chemicals.1,3 Thromboxanes are synthesized by platelets and are products of the COX pathway along with prostaglandins. These cell-signaling molecules are potent vasoconstrictors and smooth muscle contractors. Leukotrienes are products of an alternate arachidonic acid metabolic pathway. In this pathway, lipoxygenase is converted to leukotrienes, which act as smooth muscle contractors; lipoxygenase also stimulates bronchoconstriction and is a strong mediator of other various inflammatory chemicals (chemotactic).1,3
Cytokines
Cytokines, including many growth factors, are a large and complex group (more than 100 identified) of protein-soluble peptide signaling molecules that are synthesized and secreted by all musculoskeletal tissues. They are used for cellular communication and stimulate cell proliferation, differentiation, and regulation of normal growth, homeostasis, injury, disease, and repair (Table 7-1). Generally referred to as mitogenic, cytokines are powerful and important immunologic mediators that coordinate and amplify various repair processes of injured musculoskeletal tissues.1,3
Table 7-1
Types and Functions of Cytokines
| Type | Primary Functions | |
| Interleukins (ILs) | IL-1 | Augments the immune response; inflammatory mediator; promotes maturation and clonal expansion of B cells; enhances activity of NK cells; activates T cells, activates macrophages |
| IL-2 | Induces proliferation and differentiation of T cells; activation of T cells, NK cells, and macrophages; stimulates release of other cytokines (α-IFN, TNF, IL-1, IL-6) | |
| IL-3 (multicolony colony-stimulating factor) | Hematopoietic growth factor for hematopoietic precursor cells | |
| IL-4 | B-cell growth factor; stimulates proliferation and differentiation of B cells; induces differentiation into TH2 cells; stimulates growth of mast cells | |
| IL-5 | B cell growth and differentiation; promotes growth and differentiation of eosinophils | |
| IL-6 | T- and B-cell growth factor; enhances the inflammatory response; promotes differentiation of B cells into plasma cells; stimulates antibody secretion; induces fever; synergistic effects with IL-1 and TNF | |
| IL-7 | Promotes growth of T and B cells | |
| IL-8 | Chemotaxis of neutrophils and T cells; stimulates superoxide and granule release | |
| IL-9 | Enhances T-cell survival; mast cell activation | |
| IL-10 | Inhibits cytokine production by T and NK cells; promotes B-cell proliferation and antibody responses; potent suppressor of macrophage function | |
| IL-11 | Synergistic action with IL-3 and IL-4 in hematopoiesis; is a multifunctional regulator of hematopoiesis and lymphopoiesis; osteoclast formation; elevates platelet count; inhibits proinflammatory cytokine production | |
| IL-12 | Promotes α-IFN production; induction of T helper cells; activates NK cells; stimulates proliferation of activated T and NK cells | |
| IL-13 | B cell growth and differentiation; inhibits proinflammatory cytokine production | |
| IL-14 | Stimulates proliferation of activated B cells | |
| IL-15 | Mimics IL-2 effects; stimulates proliferation of T cells and NK cells | |
| IL-16 | Proinflammatory cytokine; chemoattractant of T cells, eosinophils, and monocytes | |
| IL-17 | Promotes release of IL-6, IL-8, and G-CSF; enhances expression of adhesion molecules | |
| IL-18 | Induces α-IFN, IL-2, and GM-CSF production; important role in development of T helper cells; enhances NK activity; inhibits production of IL-10 | |
| IL-19 | Similar to IL-10 | |
| IL-20 | Similar to IL-10 | |
| IL-21 | Similar to IL-2, IL-4, and IL-5 | |
| IL-22 | Similar to IL-10 | |
| IL-23 | Similar to IL-12; promotes memory T cell proliferation | |
| IL-24 | Similar to IL-10 | |
| IL-25 | Promotes TH2 cytokine production | |
| IL-26 | Similar to IL-10 | |
| IL-27 | Similar to IL-12 | |
| Interferons (IFNs) | α-Interferon (α-IFN) | Inhibit viral replication; activate NK cells and macrophages; antiproliferative effects on tumor cells |
| β-Interferon (β-IFN) | ||
| γ-Interferon (γ-IFN) | Activates macrophages, neutrophils, and NK cells; promotes B cell differentiation; inhibits viral replication | |
| Tumor necrosis factor (TNF) | Activates macrophages and granulocytes; promotes the immune and inflammatory responses; kills tumor cells; is responsible for extensive weight loss associated with chronic inflammation and cancer | |
| Colony-stimulating factors (CSFs) | Granulocyte colony-stimulating factor (G-CSF) | Stimulates proliferation and differentiation of neutrophils; enhances functional activity of mature PMNs |
| Granulocyte-macrophage colonystimulating factor (GM-CSF) | Stimulates proliferation and differentiation of PMNs and monocytes | |
| Macrophage colony-stimulating factor (M-CSF) | Promotes proliferation, differentiation, and activation of monocytes and macrophages | |
| Erythropoietin | Stimulates erythroid progenitor cells in bone marrow to produce red blood cells |
NK, Natural killer; PMN, polymorphonuclear neutrophil.
From Lewis SM. Medical-surgical nursing: assessment and management of clinical problems, ed 7, St Louis, 2008, Mosby.
Transforming growth factor-β is a potent immunosuppressive cytokine with strong anabolic activity in cartilage. TGF-β also reduces enzymatic degradation activity specifically within cartilage. In addition, TGF-β promotes wound healing, bone formation, and neovascular activity.1,3,4
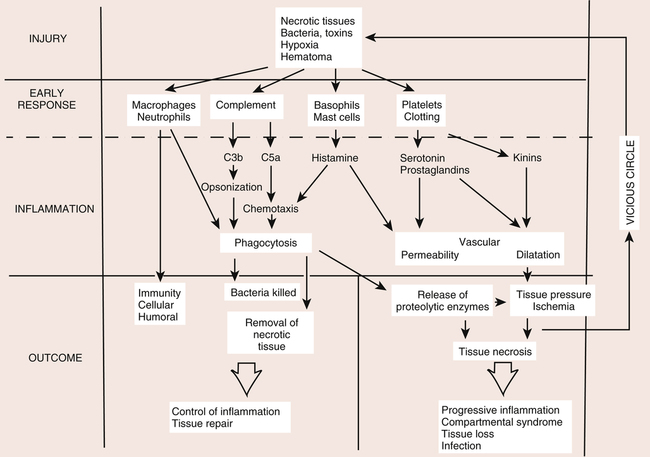
Overall, tissue disruption and subsequent repair processes initiate and propagate complex cellular events mediated by cytokines. Cytokines regulate, stimulate, and express other growth factors to synthesize, proliferate, excrete, and mobilize numerous molecules involved with extracellular matrix deposition, cartilage synthesis, vascular growth, enzymatic degradation, and modulation of inflammatory immune reactions during injury and repair of musculoskeletal and neurovascular repair (Fig. 7-5).1,3
GENERAL CELL INJURY AND REPAIR
Cellular hypertrophy is an adaptive response to a specific applied stimulus when cells and tissues are able to physiologically cope with unusual or stressful demands that do not rupture the cell’s phospholipid membrane or damage the mitochondria. This situation is essentially a reversible cell injury.1,3
However, cells also can become irreversibly damaged. Hypoxia, caused by ischemia, is the most common cause of irreversible cell injury. Tissue or cellular necrosis refers to the aggregate morphologic changes after irreversible cell injury. When cells are damaged, specific organelles (lysosomes) are involved with autolysis, or self-killing, leading to tissue necrosis. Cells of the immune system are involved with the process of heterolysis through phagocytosis and degranulation from active circulating T cells. Apoptosis refers to programmed cellular, organelle, and nuclear disassembly and death. Contrasted with necrosis, in which organelles rupture, cell phospholipid membranes tear from intracellular swelling and inflammatory reactions occur from cell debris. Apoptosis is somewhat more organized and systematic without inducing an inflammatory response. Apoptosis is an efficient, controlled process of orderly cellular, organelle, and nuclear shrinkage and disassembly of intracellular structures.1,3