CHAPTER 15 Complications of Supracondylar Fractures of the Elbow
NEUROVASCULAR PROBLEMS ASSOCIATED WITH SUPRACONDYLAR FRACTURES
ANATOMY
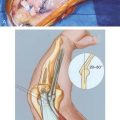
Anterior to the supracondylar area of the distal humerus is the median nerve (Fig. 15-1). In the proximal forearm, the anterior interosseous branch separates to innervate the flexor profundus to the index finger and the flexor pollicis longus and then terminates with the innervation of the pronator quadratus. There is no sensory branch for this nerve. The remainder of the median nerve traverses the forearm and supplies the sensation to the palmar aspect of the thumb, the index finger, the long finger, and the radial aspect of the ring finger.
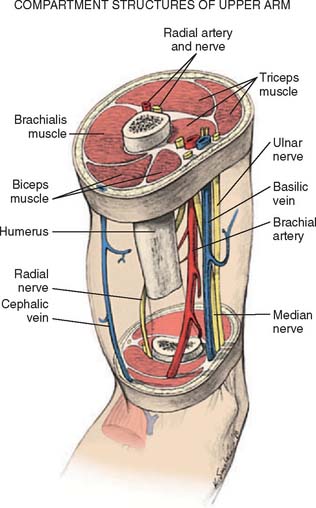
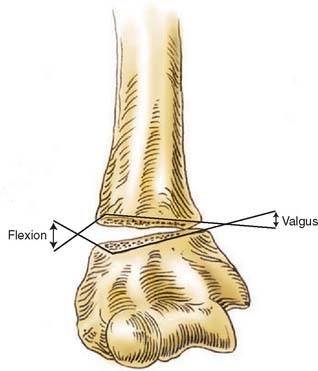
FIGURE 15-1 Major neurovascular structures of the elbow.
(From Mubarak, S. J., and Hargens, A. R.: Compartment Syndromes and Volkmann’s Contracture. Philadelphia, W. B. Saunders, 1981, p. 24.)
The radial nerve lies posterolateral to the usual location of supracondylar fractures and, thus, is less commonly involved (see Fig. 15-1). The ulnar nerve with its posterior location is uncommonly involved with a typical extension-type supracondylar fracture.
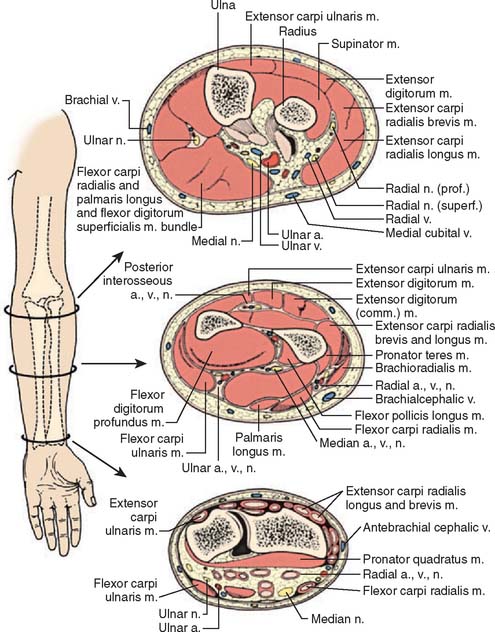
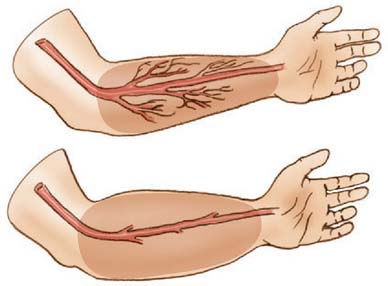
The forearm consists of two basic compartments: volar and dorsal (Fig. 15-2). The volar compartment includes the flexors and pronators of the forearm and wrist, which may be further divided into superficial and deep muscle groups. The superficial muscles include the flexor carpi ulnaris, the palmaris longus, the flexor carpi radialis, and the pronator teres. The deeper group of muscles consists of the flexor digitorum superficialis and profundus, the flexor pollicis longus, and the pronator quadratus.
ETIOLOGY: NERVE INJURY
Most nerve injuries are associated with type III displaced supracondylar fractures. In a recent study by Louahem et al,46 the most commonly injured nerve was the anterior interosseous branch of the median nerve. This is likely due to its anatomic arrangement of the exclusively motor posterior fascicles which are exposed to the zone of injury, and its tight tethering to the proximal forearm musculature. The second-most commonly involved nerve was the ulnar, followed by the radial nerve. Ulnar nerve injury was most commonly associated with posterolateral fracture patterns due to direct contusion and stretching of the nerve from the medially displaced proximal humeral fragment or edema within the cubital tunnel. Radial nerve injury was consistently associated with posteromedial fractures due to contusion and stretching from the laterally displaced proximal humeral fragment.
Ulnar nerve injury also occurred iatrogenically in 5% of patients during medial percutaneous pin placement in a recent large series.69 The causes of iatrogenic ulnar nerve injury include (1) direct penetration of the nerve or its sheath by the medial pin; (2) constriction of the cubital tunnel by the pin while the elbow is in flexion; (3) medial pin injury to an unstable ulnar nerve, which subluxates or dislocates anteriorly when the elbow is in flexion; and (4) nerve contusion and edema.63 In 2001, Skaggs et al reported on 345 extension-type supracondylar humerus fractures in children treated with closed reduction and percutaneous pin fixation. The use of a medial pin was associated with an iatrogenic ulnar nerve injury in 15% of patients in which the pin was placed with the elbow positioned in hyperflexion. Only 4% of patients sustained nerve injury when the medial pin was placed without hyperflexion, and no iatrogenic injuries occurred in patients treated with all lateral entry pin fixation.69 A displaced supracondylar fracture presenting with an absent radial pulse has a 50% to 60% incidence of associated nerve injury at fracture presentation.19
TREATMENT
After reduction of the fracture and stabilization with percutaneous pinning, re-evaluation of the neurovascular examination is mandatory. On rare occasions, the compromised nerve may recover before the patient’s discharge, but in most incidents, the neurapraxia requires observation and will gradually return over the ensuing months. If after 4 to 6 months, no return of function is noted, electromyelographic and nerve conduction studies to evaluate the status of recovery are recommended. Only rarely have cases been reported of permanent nerve deficits requiring later neurolysis, grafting, or tendon transfer. Nearly all nerves will return to normal function within the first 6 months following the injury.19
Advances in surgical techniques with lateral pin entry fixation have demonstrated significant decreases in iatrogenic ulnar nerve injury and satisfactory mechanical stability in Gartland type II, III and IV fractures.43,69,70 Authors recommend two-pin lateral-entry fixation as the primary mode of percutaneous fixation in all unstable supracondylar humerus fractures with the addition of a third lateral-entry pin or medial pin as needed to achieve fracture stability.
ISCHEMIC INJURIES
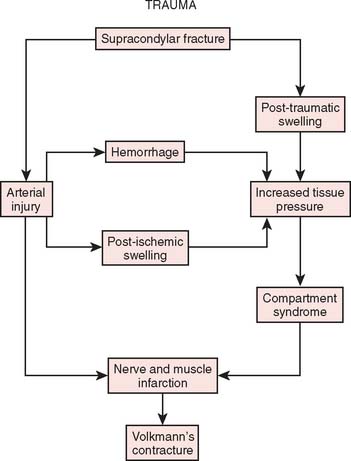
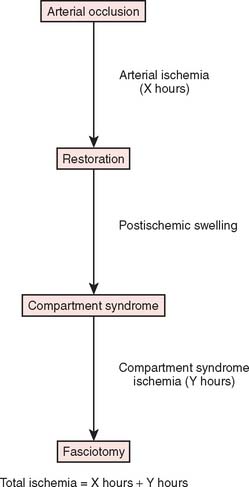
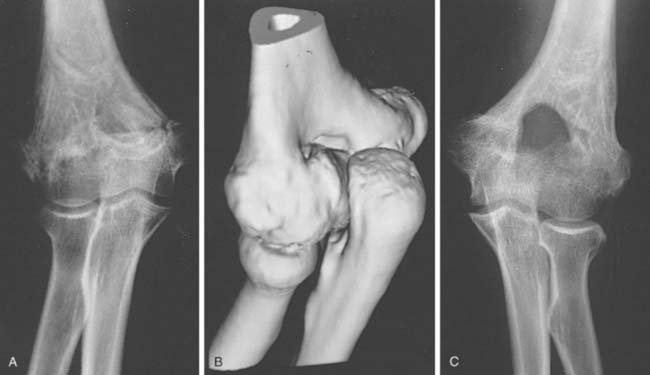
Two basic pathologic processes may result from supracondylar fractures or other injuries to the elbow region that can lead to forearm ischemia: (1) arterial injury and (2) compartment syndrome from hemorrhage or postischemic swelling (Fig. 15-3). An arterial injury may result from laceration, thrombus, embolus, intimal tear, or pseudoaneurysm (Fig. 15-4). Such an injury may cause nerve and muscle ischemia directly or may result in postischemic swelling or hemorrhage, thereby causing a compartment syndrome.
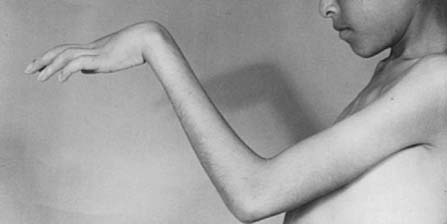
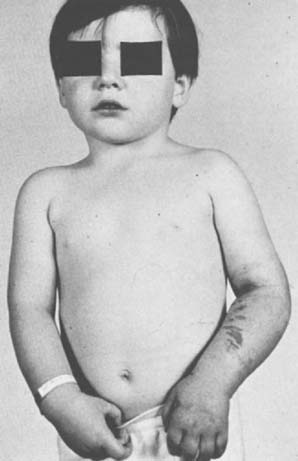
To prevent permanent loss of nerve and muscle function, this condition must be diagnosed promptly and treated correctly. Volkmann’s contracture is the popular term that refers to the end stage of an ischemic injury to the muscles and nerves of the limb (Fig. 15-5). Untreated compartment syndromes and arterial injuries are the primary causes of Volkmann’s contracture. The term Volkmann’s ischemia is nonspecific and should not be used.
ETIOLOGY: ISCHEMIA
In general, the most common traumatic event that produces a compartment syndrome or an arterial injury about the elbow is the supracondylar fracture of the distal humerus (Fig. 15-6). In 1956, Lipscomb noted that supracondylar fractures were the cause of 48% of Volkmann’s contractures in 92 cases from the Mayo Clinic.45 In 1967, Ehrlich and Lipscomb, in a review of 32 more cases of Volkmann’s contracture, reported that 34% were due to supracondylar fractures and 22% were due to forearm fractures.13 In 1979, Mubarak and Carroll, reporting on 58 Volkmann’s contractures in children (Fig. 15-7), found that supracondylar fractures had caused only 16% of these contractures.56 In most recent studies, compartment syndromes are extremely unusual because of the advent of early closed reduction and percutaneous pinning.
An arterial injury can produce nerve and muscle ischemia directly or the additional problem of a compartment syndrome by one of two mechanisms (see Fig. 15-3).57 First, if the major vessel is lacerated, hemorrhage into the compartment may produce the syndrome. Second, a compartment syndrome may result from postischemic swelling if there is inadequate collateral circulation or if the vessel is only partially occluded, for example, from an arterial spasm or an intimal tear. In this situation, the decreased perfusion and ischemia of both capillaries and muscles will cause an increase in the permeability of the capillary walls. The resulting edema will then cause more ischemia, and a vicious circle may ensue. When there is complete arterial occlusion, a compartment syndrome may develop from postischemic swelling or reperfusion injury after the circulation is restored (Fig. 15-8). When complete arterial occlusion is secondary to massive emboli or prolonged use of a tourniquet in which the circulation is not restored, gangrene rather than compartment syndrome will likely result.
CLINICAL DIAGNOSIS
There is an association between supracondylar fractures, an absent radial pulse, and Volkmann’s contracture. When the concepts of compartment syndrome as a cause for Volkmann’s contracture became popular, forearm fasciotomies became the accepted treatment method to prevent this devastating complication. An absent radial pulse, which is most commonly associated with arterial injury, began to merge with the notion of compartment syndrome. This misconception has no doubt caused many physicians to delay treatment for a compartment syndrome while waiting for the radial pulse to disappear. Owing to these misconceptions, the signs and symptoms of arterial injury compared with those of compartment syndrome will be discussed in detail.
Signs of Compartment Syndrome
Except in the presence of major arterial injury or disease, peripheral pulses and capillary filling are routinely intact in compartment syndrome patients. Although intracompartmental pressures may become high enough to cause ischemia of the muscle and nerve by occluding the microcirculation within the compartment, the pressures are rarely high enough to occlude the major arteries (Fig. 15-9). In our experience, the intracompartmental pressures usually do not exceed 80 mm Hg and are more commonly between 40 and 60 mm Hg. It has been suggested that absent pulses may result from vascular spasm secondary to elevated intracompartmental pressures.12 Mubarak and colleagues have demonstrated that pressurization to as high as 80 mm Hg of the entire anterolateral compartment in a number of dogs produced only occasional transient spasm of the midsize vessels on angiography.
DIFFERENTIAL DIAGNOSIS
Many traumatic events that precipitate a compartment syndrome or arterial injury can also produce a painful, swollen extremity. The diagnosis of the underlying problem (e.g., fracture or contusion) is obvious; the diagnosis of a superimposed ischemia is more difficult. Pain out of proportion to that expected for the injury and any sensory deficit must be explained. A compartment syndrome or an arterial injury also must be differentiated from a nerve injury, which is usually a neurapraxia when it is associated with a closed elbow fracture or dislocation. The clinical findings of these three entities overlap, frequently making the diagnosis difficult, if not impossible, by clinical means. All of these problems may be associated with motor or sensory deficits and pain. Careful clinical evaluation is necessary to differentiate these entities (Table 15-1). As noted earlier, an arterial injury usually results in absent pulses, poor skin color, and decreased skin temperature. In contrast, a compartment syndrome routinely presents with intact peripheral circulation unless the underlying etiology is an arterial injury. A diagnosis of nerve injury is usually made by exclusion of the other two entities. Doppler blood flow studies, arteriography, and pressure measurements are frequently required to aid in the differential diagnosis of these three entities, especially if these problems are present in combination.
TABLE 15-1 Typical Clinical Findings of Compartment Syndrome, Arterial Occlusion, and Neurapraxia
| Rights were not granted to include this data in electronic media. Please refer to the printed book. |
From Mubarak, S. J., and Carroll, N. C.: Volkmann’s contracture in children: aetiology and prevention. J. Bone Surg. 61B:290, 1979.
Differentiation of these entities is important because therapy for each is radically different. The neurapraxia accompanying a closed fracture is usually best treated by observation. Arterial injuries warrant immediate operative repair of the vessel, and a compartment syndrome necessitates immediate decompressive fasciotomy.
TREATMENT
Arterial Injury
When an arterial injury associated with a supracondylar fracture is suspected, a Doppler examination should be performed. The velocity Doppler is an integral instrument in assessing the presence of peripheral pulses and is very useful for noninvasive documentation of pulses in the presence of a markedly swollen extremity. A quantitative Doppler technique has been described by Schoenecker and colleagues66 to detect significant asymmetry between the injured and an uninjured extremity in children with type III supracondylar humerus fractures. Arteriography is not recommended in an acute situation.67 Shaw and associates noted the risk of arteriography to be the following: (1) prolongation of ischemic time between fracture and reduction; (2) arterial damage at the catheter insertion site; and (3) allergy to contrast material.67
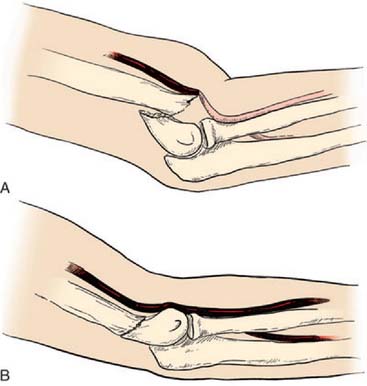
After confirmation of distal forearm ischemia, an attempt to better align the fracture fragments should be made immediately in the emergency room. In extension-type fractures, this is accomplished by extending the elbow, correcting any coronal plane deformity, and reducing the fracture by bringing the proximal fragment posteriorly and the distal fragment anteriorly (Fig. 15-10). Often, this simple maneuver will immediately restore distal circulation.33 If the distal circulation is not restored, a vascular surgeon should be notified, and the patient should be taken immediately to the operating room. All authors agree that the fracture should be reduced and stabilized by percutaneous pinning or, if necessary, open reduction and fixation. If the radial pulse does not return within 30 minutes, and signs of forearm and hand ischemia continue to be evident, then exploration of the brachial artery at the fracture site is recommended. In these circumstances, prophylactic fasciotomy of the forearm should be considered after brachial artery repair if the period of ischemia is more than 4 hours. An algorithm for the treatment of supracondylar humerus fractures associated with forearm and hand ischemia is represented in Figure 15-11.
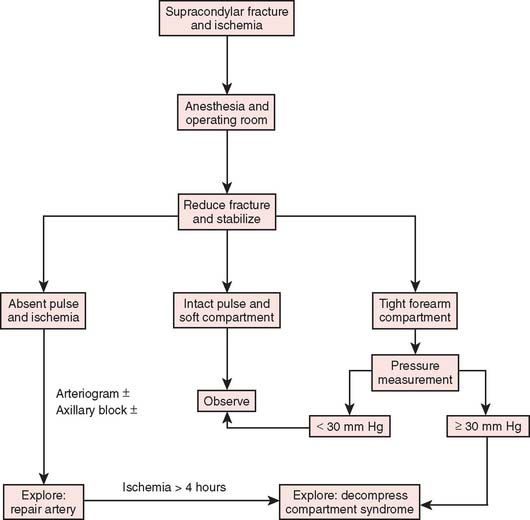
FIGURE 15-11 Scheme for management of supracondylar fractures associated with upper extremity ischemia.
(From Mubarak, S. J., and Hargens, A. R.: Compartment Syndromes and Volkmann’s Contracture. Philadelphia, W. B. Saunders, 1981, p. 144.)
Shaw and colleagues67 explored three cases and documented intimal tears with thrombus obstructing the brachial artery lumen. In two patients, the injured segment was excised and replaced by a saphenous vein graft; and prophylactic fasciotomy was also performed. One patient was noted to have brachial artery entrapment at the fracture site that was appropriately released.
Schoenecker and associates66 recommend brachial artery exploration if Doppler-detectable pulses did not return within 30 minutes after fracture reduction. A vascular surgeon assisted with the exploration. Three of seven patients demonstrated interluminal damage or transsection, requiring saphenous vein graft. Four others demonstrated kinking or entrapment of the artery at the fracture site, with re-establishment of the pulses after mobilization. Garbuz and coworkers19 explored five brachial arteries and found a similar ratio of luminal damage, laceration, and entrapment of the arteries. One patient was treated with ligation, and had long-term claudication symptoms. Eight of 11 patients who initially had an absent radial pulse demonstrated a return of the pulse after the closed reduction. In three children, the radial pulse did not return, but no further treatment was required because the forearm and hand remained pink without any further neurologic deficits. This is known in the orthopedic literature as the “pulseless pink hand.”
The Vancouver study group reviewed the pulseless pink hand in 13 patients following closed reduction and percutaneous pinning of the supracondylar fracture.65 They recommended color flow duplex scanning and MRA as a noninvasive safe technique for evaluating brachial artery patency and collateral circulation around the elbow. The vascular injuries in the 13 patients were studied; four had a thrombus or intimal tear. These patients underwent vein patch graft angioplasty. Urokinase infusion was used intra-arterially in four patients, and open thrombectomy was used in one. At follow-up, MRA studies of these patients showed a high rate of asymptomatic reocclusion and residual stenosis of the brachial artery. Thus, the investigators called into question the need for vascular reconstruction of intimal tears when the patient has a pink, well-perfused hand and MRA or other noninvasive studies that demonstrate adequate collateral circulation. They strongly recommended observation as the mainstay of treatment for these patients.
In a recent survey of pediatric orthopedic surgeons, 60.5% of responding surgeons would monitor a pulseless pink hand for at least 24 hours following reduction and pinning. If the hand remained pulseless after 24 hours, the majority of respondents (61.2%) would continue to observe the extremity and monitor for adequate collateral circulation.49
Compartment Syndrome
Mubarak and colleagues recommend that any intracompartmental pressure greater than 30 to 35 mm Hg be considered for fasciotomy if it is combined with the clinical findings of a compartment syndrome. However, one must remember that any threshold pressure is a relative indication for decompression that should be tempered by the patient’s overall condition, blood pressure, and peripheral perfusion; the trend of the symptoms and signs; the trend of the intracompartmental pressures; and the cooperation and reliability of the patient.57
Bardenheuer2 was the first to report on fasciotomy in the forearm. Eichler and Lipscomb13 described an approach to a patient with a forearm compartment syndrome that included a division of forearm skin, subcutaneous tissue, and fascia. In 1972, Eaton and Green11 described a specific operative technique in which the skin incision began distal to the elbow flexion crease and medial to the bicipital tendon, extending distally in the longitudinal axis of the midforearm to the transverse flexion crease at the wrist. The forearm fascia was incised longitudinally along its full length. The epimysium of all poorly vascularized muscles was sectioned. The fascia was left open, and delayed closure with split-thickness skin grafts and relaxing incisions was performed 48 to 72 hours later.
Neumeyer and Kilgore’s incision began adjacent to the medial epicondyle, extended obliquely across the antecubital fossa over the volar mobile wad, and returned to the midline in the distal forearm.59 It continued in a curvilinear fashion across the carpal canal to the midpalm. This report recommended wide exposure of all three possible areas of involvement: the volar and dorsal compartments of the forearm and the intrinsic compartments of the hand. Closure was accomplished by split-thickness skin grafts after several days.
Whitesides and associates77 described another operative approach in which the incision began above the elbow laterally and was carried transversely across the an-tecubital fossa to the proximal-medial forearm. The incision was continued distally along the ulnar border of the forearm to the wrist, where it curved laterally in the flexor crease of the wrist and extended into the palm in the thenar crease. The fascia was opened from above the elbow to the midpalm. The carpal tunnel and all neurovascular and muscular envelopes were opened fully. They noted that subcutaneous fasciotomy should never be performed in the forearm. The fascia was left open and was closed by split-thickness skin grafts 48 to 72 hours later. Similarly, Matsen and associates used the volar-ulnar approach. They frequently performed carpal tunnel release and epimysiotomy, as recommended by Eaton and Green.11 The advantage of this volar-ulnar approach is that the flexor tendons and median nerve are not left exposed in the distal forearm.
The effectiveness of the volar forearm fasciotomy was evaluated initially in a series of cadaver experiments.21 The incisions used were the volar-ulnar incision described by Whitesides and associates77 and the curvilinear midline volar incision. Both incisions were effective in lowering pressures in the volar forearm, and both also lowered pressure within the mobile wad and dorsal regions in approximately half of the limbs. However, the curvilinear incision allowed easier exposure of the arteries and nerves of the forearm and the mobile wad. The volar forearm pressure generally fell to normal values when the antebrachial fascia had been divided from the lacertus fibrosus to the junction of the middle and distal thirds of the forearm. When the dorsal pressures remained elevated following volar fasciotomy, a dorsal fasciotomy was performed.
A Volkmann contracture is the major complication of a compartment syndrome. Fortunately, the incidence of that complication following supracondylar fractures, as previously noted, has declined significantly in the past 40 years. With proper treatment of the elbow injury and early recognition and treatment of ischemia, Volkmann’s contracture is a rarely seen sequela of a supracondylar fracture. Many large series on the treatment of supracondylar fractures report no cases of Volkmann’s contracture when treated by Dunlap’s traction,10 overhead pin traction,6 or percutaneous pinning.14,43,69,70
Gelberman and colleagues20 reported no cases of Volkmann’s contracture in a study of supracondylar fractures. However, they noted that crush injuries or severe open injuries, when associated with compartment syndromes, resulted in considerable disability with decreased strength and limitation of forearm and hand motion. In these cases, much of the functional loss can be attributed to the crush injury alone; the compartment syndrome is an additional insult.20
A complete discussion of the treatment of an established contracture is covered by Gelberman20,21 and others,57 and is beyond the scope of this discussion.
Authors’ Prefered Fasciotomy Technique
We prefer a single longitudinal curvilinear incision for decompression of the volar forearm (Fig. 15-12). This incision allows an easy approach to the antebrachial fascia and the transverse carpal ligament, as well as to the neurovascular structures of the forearm and the mobile wad. The incision is nearly identical to McConnell’s combined exposure of the median and ulnar neurovascular bundles, as described by Henry.31 A straight longitudinal incision is used for the dorsal compartment of the forearm (see Figs. 15-2 and 15-13). Technique and postoperative care are described in detail elsewhere.20,21,57

FIGURE 15-12 Dorsal and volar forearm incisions.
(From Gelberman, R. H., Garfin, S. R., Hergenroeder, P. T., Mubarak, S. J., and Menon, J.: Compartment syndromes of the forearm: diagnosis and treatment. Clin. Orthop. 161:252, 1981.)
SURGICAL TIMING AND NEUROVASCULAR COMPLICATIONS
A number of recent studies have investigated the association between surgical timing and perioperative complications in the treatment of closed, well-perfused, displaced supracondylar humerus fractures in children.28,51,75 The groups from Cincinnati and Los Angeles were unable to identify any significant differences in the need for open reduction (2% to 13%), or the rates of pin tract infection (1% to 4%), iatrogenic nerve injury (2% to 4%), vascular injury recognized after surgery (2%), and compartment syndrome leading to Volkmann’s ischemic contracture (0%) when comparing patients with closed, well-perfused, displaced supracondylar humerus fractures treated 8 to 12 hours following the initial injury with those treated more than 8 to 12 hours after injury.28,51
More recently, a group from Edinburgh, Scotland, did demonstrate an increased need to perform an open reduction in patients with closed, well-perfused, Gartland type III supracondylar humerus fractures when surgery was delayed more than 8 hours following injury (33%) compared with those treated in less than 8 hours from the time of injury (11.5%), P = 0.05.75
OSSEOUS COMPLICATIONS
Osseous complications of supracondylar humerus fractures include malunion, nonunion of the fracture, avascular necrosis (AVN) of the distal fragment, and myositis ossificans. Techniques for the management of these complications are discussed here. Stiffness after these fractures is discussed at length in Chapter 22.
With current management techniques, good to excellent results should be anticipated in more than 90% of patients with supracondylar fractures.1 The most common significant complication is malunion, which is discussed last and in the greatest detail. Nonunion of the supracondylar fracture is virtually never seen, with only a few scattered anecdotal accounts in the literature. The near absence of nonunion with this fracture may be due to the rich vascular supply in the area, as well as to the fact that the fracture tends to be more metaphyseal in location and extra-articular. The lateral condyle fracture, in which the incidence of nonunion or delayed union is much higher, is an intra-articular fracture, with synovial fluid bathing the surface of the fragments, a phenomenon absent in the supracondylar fracture.
AVASCULAR NECROSIS
Despite the rich vascular supply, AVN has been reported in the distal fragment following a supracondylar humerus fracture.55 AVN of the capitellum as well as the trochlea has been reported, although both are extremely rare. The occurrence of AVN is much more likely to be associated with a condylar fracture, medial or lateral, than with a supracondylar humerus fracture. Condylar fractures require early and accurate diagnosis as well as prompt management to maximize successful osseous healing and minimize the development of future deformity. Even in widely displaced type III supracondylar fractures, Morrissy and Wilkins55 did not find a correlation between severity of the fracture and the extent of avascular changes, which are very rare. Because the capitellar blood supply enters the distal humerus laterally and distally, a fracture that exits the lateral column very distally may lead to avascular changes in this region. Similarly, low fractures exiting medially may be at increased risk for avascularity associated with the trochlea.
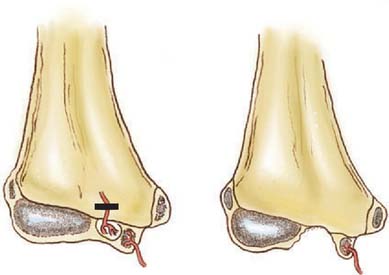
Distal humeral AVN and the fishtail deformity may be associated with decreased range of motion, the development of a cubitus valgus deformity, and, occasionally, a subsequent tardy ulnar nerve palsy. Depending on which of the two vascular supplies to the growth centers is compromised, different deformities may develop. With elimination of the more lateral trochlear blood supply, such as may occur with a supracondylar humerus fracture with T intercondylar extension, a classic fishtail deformity of the distal humerus may occur (Fig. 15-14). The fishtail deformity is more frequently associated with an inadequately treated lateral condyle fracture. The deformity includes the loss of the normal crista dividing the capitellum from the trochlear groove and central involution of the distal articular surface. The central involution allows the proximal ulna to “settle” into the distal humerus. Clinically, the prominence of the olecranon normally seen during maximal flexion of the elbow is absent (Fig. 15-15).
If both vessels feeding the distal humeral fragment have been compromised, complete aplasia of the trochlea may result. This condition will most likely lead to a progressive cubitus varus deformity and potential posterolateral rotatory instability of the ulnohumeral articulation. A tardy ulnar nerve palsy may also develop. Causative factors include instability of the ulnar nerve over a hypoplastic medial condylar region and scarring or tethering as the nerve enters the flexor carpi ulnaris.16 In time, this leads to abnormal traction, stretching, and friction of the ulnar nerve as it moves over the medial epicondyle, producing a gradual progressive ulnar neuropathy.34
Management of the sequelae of distal humeral AVN is rarely satisfying. Surgical intervention for improvement of motion may be indicated in specific cases, if a vigorous physical program is unsuccessful. Cases of tardy ulnar nerve palsy are managed with anterior transposition of the ulnar nerve. Progressive angular deformity of the distal humerus may be attributable to either AVN or physeal injury. Physeal arrest, sporadically reported in conjunction with even mild supracondylar humerus fractures, appears similar to a nonunion but is rare.37,60 If it is detected early and the degree of physeal involvement is limited, physeal bar resection, although technically demanding, may be considered. Although the fishtail deformity itself is not amenable to surgical correction, cubitus varus or valgus, which may occur in conjunction with either physeal arrest or AVN, can be corrected with a supracondylar osteotomy. Because the restoration of growth potential may not be possible in cases of AVN, as in some cases of physeal arrest, recurrent angular deformity may occur, depending on the age of the child, requiring multiple osteotomies over time.
In cases in which the fracture has healed but flexion or extension is believed to be limited structurally by a bony block, excision of the tip of the olecranon with enlargement of the olecranon fossa may occasionally prove beneficial (Fig. 15-16). Osseous impingement is an uncommon cause of motion loss. The technique has been well described for the management of the more common impingement from degenerative changes of the elbow by Morrey.54
MYOSITIS OSSIFICANS
Myositis ossificans is a potential complication of supracondylar humerus fractures, which if extensive, results in poor elbow motion. The phenomenon is not common and may even be self-limiting, with resolution over a 1- to 2-year course (Fig. 15-17). Siris71 found a 2% incidence in a 1939 review of 330 supracondylar humerus fractures, whereas more recent studies have reported an incidence of less than 1% and 0% by most authors. The occurrence and severity would intuitively be expected to be increased in cases treated with open reduction, particularly if the surgery is delayed from the time of the injury. In supracondylar humerus fractures treated with open reduction acutely, only one report identifies myositis as a postoperative complication.22 Even with delayed open management, Lal and Bhan42 found no cases of myositis ossificans in their report of 20 fractures treated with open reduction 11 to 17 days after injury. Surgical excision of the affected tissue should be performed only if the ectopic bone is clearly demonstrated to be causative of motion loss, conservative attempts to regain motion have failed, and adequate time from that of the injury has transpired such that the lesion is quiescent. Bone scan has been recommended in assessing the metabolic activity level of the lesion, but the editor favors the plain radiographic features of clear marginal delineation and trabecularization. Attempts to prevent heterotopic bone formation with low-dose external beam irradiation, and administration of oral nonsteroidal anti-inflammatory agents or oral disphosphonates are not warranted due to the rarity and self-limiting nature of this complication.30
ANGULAR DEFORMITY
Angular deformity of the elbow is the most common significant adverse sequela of a supracondylar humerus fracture. This condition may arise from growth disturbances such as a physeal injury or AVN, as outlined previously, or from the position in which the fracture healed. Malunion may result in either a flexion or extension deformity in the sagittal plane; a varus or valgus deformity in the coronal plane; and rotational deformity in the horizontal plane. Deformity may occur in any single plane or in a combination of planes. Although some remodeling of sagittal plane deformity can be expected, no significant improvement in coronal and horizontal deformity should be anticipated.4,15,40,52
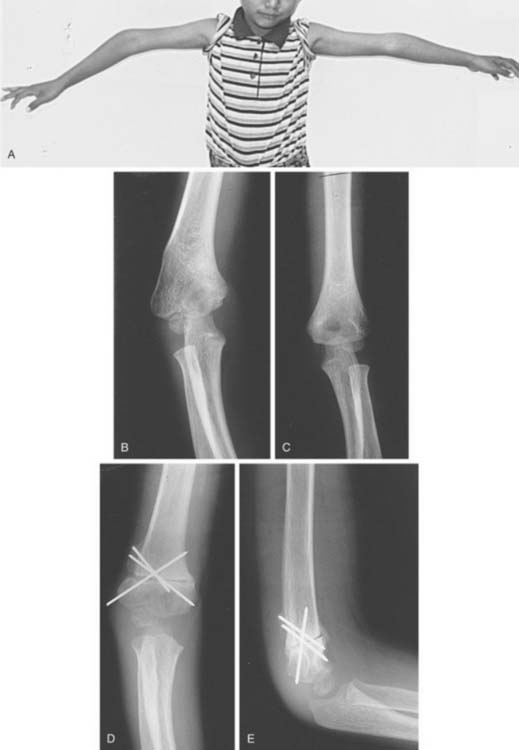
Although cubitus valgus has been reported in association with supracondylar fractures of the humerus,8 cubitus varus is far more commonly reported as a complication of the management of these fractures.
Cubitus varus has been termed a cosmetic sequela by numerous authors. This characterization may limit indications for corrective surgery, particularly in the modern medical environment. Tardy ulnar nerve palsy has been described in association with cubitus varus, although it is more commonly found secondary to cubitus valgus.17 Functional deficits and a potential increased risk of new injury as well as limitations of motion and discomfort have been well described in patients with cubitus varus. A correlation between re-sidual cubitus varus following supracondylar humerus fractures and subsequent lateral condyle fractures has been noted.7 Thus, although the indications for surgical correction of cubitus varus do include poor cosmesis of an unsightly deformity, one must also consider future function of the extremity and the risk for new injuries due to altered biomechanics of the elbow in varus.
INDICATIONS FOR CORRECTIVE SURGERY
Although simple collapse or impaction of the lateral column of the distal humerus will yield a cubitus valgus angular deformity, and the opposite is true for cubitus varus, rotation may contribute to either deformity and should at least be considered in the planning of corrective surgery. Graham24,25 stressed the contribution of rotation to changes in the carrying angle of the arm. Chess and colleagues5 stated that varus malalignment was the most important contributor to postoperative cubitus varus, but that internal rotation combined with varus accentuates the deformity. In apparent contrast, Mahaisavariya and Laupattarakasern48 reviewing anatomic findings at the time of corrective osteotomy concluded that rotation does not contribute to cubitus varus. Mitsunari and coworkers53 found an increased incidence of residual internal rotation deformity in patients with tardy ulnar nerve palsy following a supracondylar humerus fracture. Many techniques have been espoused for the correction of distal humeral malunions. These include closing wedge osteotomies with or without translation of the distal fragment, dome osteotomy, and complex triplane osteotomy, to name a few. The common concept is that each of these techniques addresses the po-tential components of the deformity to varying degrees.
SURGICAL APPROACH
In considering surgical correction of a distal humeral deformity, one of the first steps is deciding on the surgical approach. A medial approach, which allows visualization and protection of the neurovascular bundles, has been advocated by some authors.39 One potential detraction from this approach is that manipulation of the neurovascular structures is necessary to gain access to the distal humerus. Also, particularly for cubitus varus, an osteotomy with lateral closing is difficult from the medial approach.
The posterior approach has been advocated by several authors. This can be accomplished through a triceps-splitting, triceps-tendon transecting,27,61 or triceps-sparing technique.27,61 Avascularity of the distal fragment has been reported to be associated with this tendon transection.27 The posterior approach, particularly the first two techniques, affords excellent visualization of the distal humerus, being used with some frequency in displaced intercondylar distal humeral fractures. A long incision is necessary, as well as significant dissection, which risks postoperative adhesion formation. In one study of supracondylar humeral fractures, stiffness was noted in 21% of those managed with closed techniques but in 50% of those treated with open reduction through a posterior approach.61 In addition, intraoperative assessment of the magnitude of correction of the carrying angle can be difficult in the prone or lateral position.
The lateral approach is the most frequently used and advocated approach to the distal humerus for the correction of cubitus varus.* The dissection is brought to the shaft of the humerus, distal to the area in which the radial nerve exits laterally from around the shaft. A subperiosteal plane is used to expose the humerus, as well as avoid injury to the medial neurovascular structures (Fig. 15-18).
OSTEOTOMY TECHNIQUES
The next step in planning the correction of a distal humeral deformity is the nature of the osteotomy. As already outlined, there are various techniques to consider. The lateral closing wedge osteotomy is the most widely used and recommended technique. With a lateral closing wedge osteotomy, correction of the three aspects of distal humeral deformity following supracondylar fracture malunion are possible: cubitus varus, hyperextension, and rotation (Fig. 15-19). Some authors advocate a simple laterally based closing wedge, leaving the medial cortex intact as a hinge.3,23,39,50,62 Although this approach may have value in certain cases with limited deformity, flexion and extension are not well addressed, nor is rotational deformity. Wong and Balasubramaniam80 argue that correction of the rotational component is unnecessary. Also, as the size of the wedge required to achieve appropriate valgus alignment increases, so does the prominence of the distal fragment laterally.78 Owing to these constraints, a corrective closing wedge osteotomy that is laterally and anteriorly based with medial translation of the distal fragment is preferred to obtain optimal results (Fig. 15-20).* If desired for greater osseous contact, a spike can be left laterally on the distal fragment to interlock with the proximal shaft, similar to a Wiltse distal tibial varus osteotomy.79
Uchida and associates72 described a three-dimensional osteotomy for the correction of cubitus varus. A posterolateral approach is used, and a complex biplane, tridirectional step cut osteotomy is performed, with fixation provided by screws. Although elegant in design and execution, this technically demanding osteotomy differs from a lateral closing wedge flexion osteotomy only in the extent and angle of bone surface contact. If poor postosteotomy healing is a concern, this technique offers extensive surface contact for osseous bridging.
A dome osteotomy has been described that involves a posterior approach, marking the planned dome from a reference Kirschner wire, and uses K-wire fixation.38 This technique allows correction of malrotation and avoids a potentially prominent lateral epicondylar re-gion, but it does not address flexion/extension of the distal fragment. Also, this procedure is more technically demanding than that using the laterally based closing wedge osteotomy. In their report of 11 patients, Kanaujia and associates found that the outcome for all was satisfactory.
FIXATION TECHNIQUES
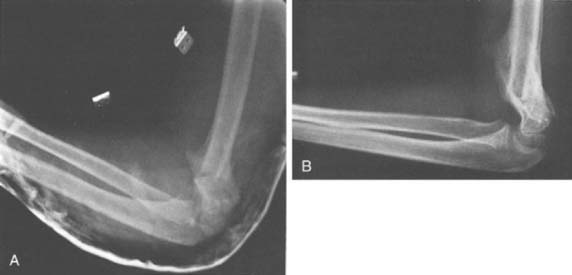
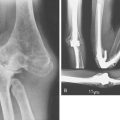
The final consideration in surgical correction of the distal humerus malunion is fixation options for the osteotomy fragments. These include smooth Kirschner wires,62,71 threaded Steinmann pins, screws,72 screws with a wire tension band,3,16,45,50 plates and screws,9,32 and external fixation.42 The smooth Kirschner wire fixation has proven to be less than reliable, with a greater incidence of loss of correction compared with threaded fixation (Fig. 15-21). Adequate fixation and maintenance of alignment is reliably obtained and the buried pins can be retrieved in the future, particularly if the physis was crossed for optimal fixation. In the older, larger child, crossed screws may be used, although great care must be exercised to avoid unplanned translation of the fragments. The technique of screws paralleling the osteotomy, connected with a tension band wire, can be used only if good medial cortical integrity remains following the closing wedge osteotomy. Neither medial translation nor derotation is possible if this fixation is chosen. In the older adolescent, one-third tubular or 3.5-mm pelvic reconstruction plates with screws may be necessary to gain adequate fixation, particularly if early range of motion is planned postoperatively. External fixation is another option, although the size of the distal fragment is such that at most two pins could be used. Also, the inherent difficulties with pin tract care exist, which limits the attractiveness of this option.
1 Alburger P.D., Weidner P.L., Betz R.R. Supracondylar fractures of the humerus in children. J. Pediatr. Orthop. 1992;12(1):16.
2 Bardenheuer L. Die Entstehung und Behandlung der ischamischen Muskelkontractur und Gangran. Dtsch. Z. Chir. 1911;108:44.
3 Bellemore M.C., Barrett I.R., Middleton R.W.D., Scougall J.S., Whiteway D.W. Supracondylar osteotomy of the humerus for correction of cubitus varus. J. Bone Joint Surg. 1984;66B:566.
4 Blount W.P. Fractures in Children. Baltimore: Williams & Wilkins, 1955;26-42.
5 Chess D.G., Leahey J.L., Hyndman J.C. Cubitus varus: significant factors. J. Pediatr. Orthop. 1994;14(2):190.
6 D’Ambrosia R.D. Supracondylar fractures of the humerus: prevention of cubitus varus. J. Bone Joint Surg. 1972;54A:60.
7 Davids J.R., Maguire M.F., Mubarak S.J., Wenger D.R. Lateral condyle fracture of the humerus following posttraumatic cubitus varus. J. Pediatr. Orthop. 1994;14:466.
8 De Boeck H., De Smet P. Valgus deformity following supracondylar elbow fractures in children. Acta Orthop. Belgica. 1997;63:240.
9 Devnani A.S. Lateral closing wedge supracondylar osteotomy of the humerus for post-traumatic cubitus varus in children. Injury. 1997;28:643.
10 Dodge H.S. Displaced supracondylar fractures of the humerus in children: treatment by Dunlop’s traction. J. Bone Joint Surg. 1972;54A:1408.
11 Eaton R.G., Green W.T. Epimysiotomy and fasciotomy in the treatment of Volkmann’s ischemic contracture. Orthop. Clin. North Am. 1972;3:175.
12 Eaton R.G., Green W.T. Volkmann’s ischemia. A volar compartment syndrome of the forearm. Clin. Orthop. 1975;113:58.
13 Eichler G.R., Lipscomb P.R. The changing treatment of Volkmann’s ischemic contractures from 1955 to 1965 at the Mayo Clinic. Clin. Orthop. 1967;50:215.
14 Flynn J.C., Matthews J.G., Benoit R.L. Blind pinning of displaced supracondylar fractures of the humerus in children. J. Bone Joint Surg. 1974;56A:263.
15 France J., Strong M. Deformity and function in the supracondylar fractures of the humerus in children variously treated by closed reduction and splinting, traction, and percutaneous pinning. J. Pediatr. Orthop. 1992;12:494.
16 French P.R. Varus deformity of the elbow following supracondylar fractures of the humerus in children. Lancet. 1959;2:439.
17 Fujioka H., Nakabayashi Y., Hirata S., Go G., Nishi S., Mizuno K. Analysis of tardy ulnar nerve palsy associated with cubitus varus deformity after a supracondylar fracture of the humerus: a report of four cases. J. Orthop. Trauma. 1995;9:435.
18 Gaddy B.C., Manske P.R., Pruitt D.L., Schoenecker P.L., Rouse A.M. Distal humeral osteotomy for correction of posttraumatic cubitus varus. J. Pediatr. Orthop. 1994;14:214.
19 Garbuz D.S., Leitch K., Wright J.G. The treatment of supracondylar fractures in children with an absent radial pulse. J. Pediatr. Orthop. 1996;16:594.
20 Gelberman R.H., Garfin S.R., Hergenroeder P.T., Mubarak S.J., Menon J. Compartment syndromes of the forearm: diagnosis and treatment. Clin. Orthop. 1981;161:252.
21 Gelberman R.H., Zakaib G.S., Mubarak S.J., Hargens A.R., Akeson W.H. Decompression of forearm compartment syndromes. Clin. Orthop. 1978;134:225.
22 Godley D.R., Loeng J.C.Y., Yau A. Open reduction and internal fixation of supracondylar fractures of the humerus in children in Hong Kong: Long term results. Abbot Proc. 1978;9:30.
23 Graham B., Tredwell S.J., Beauchamp R.D., Bell H.M. Supracondylar osteotomy of the humerus for correction of cubitus varus. J. Pediatr. Orthop. 1990;11:228.
24 Graham H.A. Supracondylar fractures of the elbow in children. Part 1. Clin. Orthop. 1967;54:85.
25 Graham H.A. Supracondylar fractures of the elbow in children. Part 2. Clin. Orthop. 1967;54:93.
26 Griffin P.P. Supracondylar fractures of the humerus. Pediatr. Clin. North Am. 1975;2:477.
27 Gruber M.A., Healy W.A. The posterior approach to the elbow revisited. J. Pediatr. Orthop. 1996;16:215.
28 Gupta N., Kay R.M., Leitch K., Femino J.D., Tolo V.T., Skaggs D.L. Effect of surgical delay on perioperative complications and need for open reduction in supracondylar humerus fractures in children. J. Pediatr. Orthop. 2004;24:245-248.
29 Harris I.E. Supracondylar fractures of the humerus in children. Orthopedics. 1992;15:811.
30 Hartigen B.J. Myositis ossificans after a supracondylar fracture of the humerus in a child. Am. J. Orthopedics. 2001;30:152.
31 Henry A.K. Extensile Exposure, 2nd ed. Edinburgh: Churchill Livingstone, 1973.
32 Hernandez M.A., Roach J.W. Corrective osteotomy for cubitus varus deformity. J. Pediatr. Orthop. 1994;14:487.
33 Herring J.A. Tachdijan’s Pediatric Orthopaedics from the Texas Scottish Rite Hospital for Children, 3rd ed., Philadelphia: Elsevier; 2002:2147.
34 Ih J., Oh C.W., Kyung H.S., Park I.H., Kim P.T. Tardy ulnar nerve palsy in cubitus varus deformity associated with ulnar nerve dislocation in adults. J. Shoulder Elbow Surg. 2006;15:474.
35 Ippolito E., Caterini R., Scola E. Supracondylar fracture of the humerus in children. Analysis at maturity of the fifty-three patients treated conservatively. J. Bone Joint Surg. 1986;68A:333.
36 Ippolito E., Moneta M.R., D’Arrigo C. Post-traumatic cubitus varus. J. Bone Joint Surg. 1990;72A:757.
37 Kallio P.E., Foster B.K., Paterson D.C. Difficult supracondylar elbow fractures in children: analysis of percutaneous pinning technique. J. Pediatr. Orthop. 1992;12:11.
38 Kanaujia R.R., Ikuta Y., Muneshige H., Higaki T., Shimogaki K. Dome osteotomy for cubitus varus in children. Acta Orthop. Scand. 1988;59:314.
39 King D., Secor C. Bow elbow (cubitus varus). J. Bone Joint Surg. 1951;33A:572.
40 Kurer M.H.J., Regan M.W. Completely displaced supracondylar fracture of the humerus in children. A review of 1708 comparable cases. Clin. Orthop. 1990;256:205.
41 Labelle H., Bunnell W.P., Duhaime M., Poitras B. Cubitus varus deformity following supracondylar fractures of the humerus in children. J. Pediatr. Orthop. 1982;2:539.
42 Lal G.M., Bhan S. Delayed open reduction for supracondylar fractures of the humerus. Int. Orthop. 1991;15:189.
43 Leitch K.K., Kay R.M., Femino J.D., Tolo V.T., Storer S.K., Skaggs D.L. Treatment of multidirectionally unstable supracondylar humerus fractures in children. A modified Gartland type-IV fracture. J. Bone Joint Surg. 2006;88A:980.
44 Levine M.J., Horn B.D., Pizzutillo P.D. Treatment of posttraumatic cubitus varus in the pediatric population with humeral osteotomy and external fixation. J. Pediatr. Orthop. 1996;16:597.
45 Lipscomb P.R. The etiology and prevention of Volkmann’s ischemic contracture. Surg. Gynecol. Obstet. 1956;103:353.
46 Louahem D.M., Nebunescu A., Canavese F., Dimeglio A. Neurovascular complications and severe displacement in supracondylar humerus fractures in children: defensive or offensive strategy. J. Pediatr. Orthop. B. 2006;15:51.
47 Mahaisavariya B., Laupattarakasem W. Osteotomy for cubitus varus. A simple technique in 10 children. Acta Orthop. Scand. 1996;67:60.
48 Mahaisavariya B., Laupattarakasem W. Supracondylar fracture of the humerus: malrotation versus cubitus varus deformity. Injury. 1993;24:416.
49 Malviya A., Simmons D., Vallamshetia R., Bache C.E. Pink pulseless hand following supra-condylar fractures: an audit of British practice. J. Pediatr. Orthop. B. 2006;15:62.
50 McCoy G.F., Piggot J. Supracondylar osteotomy for cubitus varus. The value of the straight arm position. J. Bone Joint Surg. 1988;70B:283.
51 Mehlman C.T., Strub W.M., Roy D.R., Wall E.J., Crawford A.H. The effect of surgical timing on the perioperative complications of treatment of supracondylar humeral fractures in children. J. Bone Joint Surg. 2001;83A:323.
52 Mehserle W.L., Meehan P.L. Treatment of the displaced supracondylar fracture of the humerus (type III) with closed reduction and percutaneous cross-pin fixation. J. Pediatr. Orthop. 1991;11:705.
53 Mitsunari A., Muneshige H., Ikuta Y., Murakami T. Internal rotation deformity and tardy ulnar nerve palsy after supracondylar humeral fracture. J. Shoulder Elbow Surg. 1995;4:23.
54 Morrey B. Primary degenerative arthritis of the elbow: treatment by ulnohumeral arthroplasty. J. Bone Joint Surg. 1992;74B:409.
55 Morrissy R.T., Wilkins K.E. Deformity following distal humeral fracture in childhood. J. Bone Joint Surg. 1984;66A:557.
56 Mubarak S.J., Carroll N.C. Volkmann’s contracture in children: aetiology and prevention. J. Bone Joint Surg. 1979;61B:285.
57 Mubarak S.J., Hargens A.R. Compartment Syndromes and Volkmann’s Contracture. Philadelphia: W. B. Saunders, 1981.
58 Nassar A. Correction of varus deformity following supracondylar fracture of the humerus. J. Bone Joint Surg. 1974;56B:572.
59 Neumeyer W.L., Kilgore E.S.Jr. Volkmann’s ischemic contracture due to soft tissue injury alone. J. Hand Surg. 1976;1:221.
60 Nwakama A.C., Peterson H.A., Shaughnessy W.J. Fishtail deformity following fracture of the distal humerus in children: Historical review, case presentations, discussion of etiology, and thoughts on treatment. J. Pediatr. Orthop. Part B. 2000;9:309.
61 Omer C., Pestilci F.I., Tuzuner M. Supracondylar fractures of the humerus in children: analysis of the results in 142 patients. J. Orthop. Trauma. 1990;4:265.
62 Oppenheim W.L., Clader T.J., Smith C., Bayer M. Supracondylar humeral osteotomy for traumatic childhood cubitus varus deformity. Clin. Orthop. 1984;188:324.
63 Ozcelik A., Tekcan A., Omerolu H. Correlation between iatrogenic ulnar nerve injury and angular insertion of the medial pin in supracondylar humerus fractures. J. Pediatr. Orthopaedics, Part B. 2006;15(1):58-61.
64 Rang M. Children’s Fractures, 2nd ed. Philadelphia: J. B. Lippincott, 1983.
65 Sabharwal S., Tredwell S.J., Beauchamp R.D., Mackenzie W.G., Jakubec D.M., Cairns R., LeBlanc J.G. Management of pulseless pink hand in pediatric supracondylar fractures of humerus. J. Pediatr. Orthop. 1997;17:303.
66 Schoenecker P.L., Delgado E., Rotman M., Sicard G.A., Capelli A.M. Pulseless arm in association with totally displaced supracondylar fracture. J. Pediatr. Orthop. 1996;10:410.
67 Shaw B.A., Kasser J.R., Emans J.B., Rand F.F. Management of vascular injuries in displaced supracondylar humerus fractures without arteriography. J. Orthop. Trauma. 1991;4:25.
68 Siris J.E. Supracondylar fractures of the humerus analyzed; 330 cases. Surg. Gynecol. Obstet. 1939;68:201.
69 Skaggs D.L., Hale J.M., Bassett J., Kaminsky C., Kay R.M., Tolo V.T. Operative treatment of supracondylar fractures of the humerus in children. The consequences of pin placement. J. Bone Joint Surg. 2001;83A:735.
70 Skaggs D.L., Cluck M.W., Mostofi A., Flynn J.M., Kay R.M. Lateral-entry pin fixation in the management of supracondylar fractures in children. J. Bone Joint Surg. 2004;85A:702.
71 Sweeney J.G. Osteotomy of the humerus for malunion of supracondylar fractures. J. Bone Joint Surg. 1975;57B:117.
72 Uchida Y., Ogata K., Sugioka Y. A new three-dimensional osteotomy for cubitus varus deformity after supracondylar fracture of the humerus in children. J. Pediatr. Orthop. 1991;11:327.
73 Usui M., Ishii S., Miyano S., Narita H., Kura H. Three-dimensional corrective osteotomy for the treat-ment of cubitus varus after supracondylar fracture of the humerus in children. J. Shoulder Elbow Surg. 1995;4:17.
74 Voss F.R., Kasser J.R., Trepman E., Simmons E., Hall J.E. Uniplanar supracondylar humeral osteotomy with preset Kirschner wires for posttraumatic cubitus varus. J. Pediatr. Orthop. 1994;14:471.
75 Walmsley P.J., Kelly M.B., Robb J.E., Annan I.H., Porter D.E. Delay increases the need for open reduction of type III supracondylar fractures of the humerus. J. Bone Joint Surg. 2006;88B:528.
76 Weiland A.J., Meyer S., Tollo V.T., Berg H.L., Mueller J. Surgical treatment of displaced supracondylar fractures of the humerus in children. J. Bone Joint Surg. 1978;60A:657.
77 Whitesides T.E.Jr., Hirada H., Morimoto K. Compartment syndromes and the role of fasciotomy, its parameters and techniques. Instr. Course Lect. 1977;26:179.
78 Wilkins K.E. Residuals of elbow trauma in children. Orthop. Clin. North Am. 1990;21:291.
79 Wiltse L.L. Valgus deformity of the ankle: a sequel to acquired or congenital abnormalities of the fibula. J. Bone Joint Surg. 1972;54A:595.
80 Wong H.K., Balasubramaniam P. Humeral torsional deformity after supracondylar osteotomy for cubitus varus: its influence on the postosteotomy carrying angle. J. Pediatr. Orthop. 1992;12:490.