CHAPTER 94 Complications of Spinal Surgery
Pathoanatomy of Neural Injury
The spinal cord lies protected within the spinal canal. The nerve roots exit segmentally through their corresponding neural foramina. Within the spinal canal, the pedicle is a consistent anatomic landmark that can be used to locate the exiting nerve roots and is useful especially in the presence of anomalous anatomy. In the upper cervical spine the hypoglossal nerves (cranial nerve XII) lie 2 to 3 mm lateral to the midpoint of the lateral masses of C1. They are at risk for injury from overpenetration of C1-C2 transarticular screws, which can result in tongue deviation toward the side of injury and dysarthria.1,2 The C2 nerve root exits dorsal to the C1-C2 facet joint and is at risk for injury during placement of C1 lateral mass screws, which can result in pain or paresthesias in the C2 dermatome (i.e., occipital sensation).3 In the subaxial cervical spine the nerve roots exit anterolaterally through the neural foramen and lie within a groove immediately anterior to the superior articular processes, placing them at risk for injury from improperly placed cervical lateral mass and pedicle screws. In anterior approaches to the cervical spine, dysphagia or dysphonia can result from injury to the pharyngeal plexus during approaches to C2-C5, the hypoglossal during approaches above the level of C3, the superior laryngeal nerve during exposure of the C3-4 level, and recurrent laryngeal nerves during approaches to C5-T1.4 The right recurrent laryngeal nerve passes around the subclavian vessels and then ascends in the neck, coursing medially at a variable level (typically C6 to C7) along with the inferior thyroid artery to its destination in the larynx. The left recurrent laryngeal nerve, in contrast, passes around the ligamentum arteriosum at the aortic arch and then predictably ascends within the tracheoesophageal groove, where it remains relatively protected before its destination in the larynx. This distinction is most relevant in surgical approaches to the lower cervical spine, where the variability in the course of the right recurrent laryngeal nerve is greatest, making it at increased risk for injury during dissection. The cervical sympathetic chains lie ventral to the longus colli and course most medially at C5-6, where they are at greatest risk for injury.5 Injury can result in Horner syndrome of ptosis, meiosis, anhidrosis, and enophthalmos. In the thoracic spine the ventral nerve roots continue as the intercostal nerves and lie with the costal groove on the undersurface of the rib. They are at risk for injury during surgical dissection in this area. In the lumbar spine the nerve roots lie along the inferomedial edge of the pedicle and are at risk of injury from medial and inferior pedicle breaches during pedicle screw placement. The lumbar sympathetic chain lies anterior to the psoas muscle and can be injured during retroperitoneal approaches to the lumbar spine manifesting as patient complaints of contralateral foot coolness. The superior hypogastric sympathetic plexus is at risk for injury during approaches to the L5-S1 disc space.1,2 Injury can result in retrograde ejaculation and infertility from lack of proper sphincter control, which misdirects the ejaculate into the bladder instead of through the urethra. This is less of a concern at upper lumbar levels because the fibers of the plexus lie on the anterior aortic wall and only become immediately prevertebral at the level of the L5-S1 disc space. The lateral femoral cutaneous nerve, which provides sensation to the anterolateral thigh, may be injured during harvesting of bone graft.6 Although the nerve typically passes anterior to the anterior superior iliac spine (ASIS), variants may cross posterior to the ASIS, placing the nerve at risk for injury with dissection within 3 cm posterior of the ASIS. Injury to the nerve can result in painful neuromas and meralgia paresthetica. The superior cluneal nerves, cutaneous branches of L1-L3 involved in sensation to the buttock, may be injured during harvesting of bone graft from the posterior iliac crest with dissection more than 6 cm lateral to the posterior superior iliac spine.7
Intraoperative Complications and Their Prevention
Preoperative Patient Evaluation
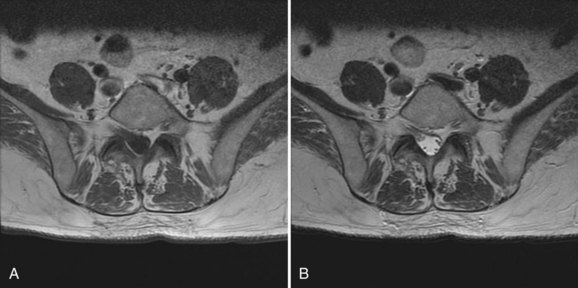
Preoperative evaluation of the patient begins with an accurate diagnosis obtained after a thorough history and physical and review of imaging studies. Preexisting neurologic deficits should be identified, and a baseline neurologic examination obtained and accurately documented. These should be communicated with other health care providers involved in the care of the patient. The preoperative imaging studies should be thoroughly reviewed for anatomic anomalies that may increase the chance for neurologic injury. Dysraphic states can increase the risk of durotomy and complicate exposure. Nerve root anomalies increase the risk of injury if not identified preoperatively (Fig. 94–1). Areas of significant neurologic compression increase the risk of injury to neural structures from surgical decompressive maneuvers because there is less room for error. In these situations, significant stenosis results in loss of the normal subarachnoid space. Normally, the subarachnoid space provides a protective buffer between the dura mater and neural structures. Loss of the normal subarachnoid space increases the chance of contusing neural tissue with surgical maneuvers performed immediately adjacent to the thecal sac (e.g. use of a Kerrison rongeur, sublaminar wiring, epidural bipolar electrocautery during anterior cervical discectomy). Abnormal osseous anatomy (e.g., abnormal pedicle morphology, compressive osteophytes, ossified ligamentum flavum) can complicate decompression, instrumentation, or both and should be identified before surgery because these affect intraoperative tactics. Patients should be instructed to preoperatively discontinue anticoagulant medications to minimize intraoperative and postoperative blood loss and the potential for epidural hematoma formation.
In addition to the specific neurologic risks of a surgical approach, patient factors (e.g., gender, history of prior surgery, preexisting neurologic deficits) and their tolerance for specific injuries may play a role in determining the risk-to-benefit ratio of a given approach; thus it is important to have a preoperative discussion with patients regarding the risks for neurologic injury and determine how willing they are to accept these risks. For example, retrograde ejaculation and male infertility can result from injury to the superior hypogastric plexus during an anterior lumbar approach with transabdominal approaches at especially increased risk in comparison with retroperitoneal approaches.8 Although blunt dissection anterior to the L5 and S1 vertebrae can decrease the chances for injury, it cannot eliminate this risk. Although preoperative cryopreservation (i.e., sperm banking) is an option to retain the possibility of procreation in the event of postoperative infertility, a young male interested in having children may not want to bear this risk. History of prior surgery can also significantly alter the risk-to-benefit ratio of using a given approach because operating through a prior surgical incision (especially during anterior approaches to the spine) considerably increases the risk for complication. This increased risk may be acceptable, however, if the relevant structures have already been compromised. For example, in revising an anterior cervical fusion in a patient with acquired left recurrent laryngeal nerve palsy from a previous left-sided approach, repeat use of a left-sided approach may be preferable because this minimizes the risk of bilateral recurrent laryngeal nerve injury and consequent exacerbation of postoperative dysphagia and/or dysphonia.
Positioning
The surgical and anesthetic teams should work together to ensure proper patient positioning to minimize the chances of neurologic injury. Special attention should be paid to positioning of the neck and limbs with proper padding of superficial nerves. Abnormal cervical postures should be avoided. Patients should be positioned with their heads in a neutral or slightly flexed position without excessive rotation because neurologic injury can occur from cervical extension in myelopathic patients, which narrows the internal diameter of the spinal canal and from excessive neck rotation to one side, which narrows the ipsilateral cervical neuroforamina. Case reports exist of excessive neck rotation resulting in carotid artery occlusion and subsequent stroke.9 In cervical spine surgery, the shoulders are often depressed and taped in position to improve radiographic visualization. If excessive, this can result in a traction injury to the brachial plexus. Placing a patient into the prone position requires a coordinated effort directed by the individual controlling the head. The persons receiving the patient have the important task of controlling the rate of descent of the patient onto the operating room table. In patients with an unstable spine, gentle inline traction can help maintain alignment. The limbs should be positioned to minimize the potential for compression or traction injury to the peripheral nerves and the brachial plexus. Extra care should be taken in patients with stiff shoulders, morbid obesity, and other circumstances that mitigate against correct positioning of the limbs.
Proper positioning also plays an important role in the prevention of ocular complications. Postoperative visual deficits have a reported incidence as high as 0.1% in spinal surgery in the prone position but have also been described in supine patients.10 The most common cause (>80% of cases) is ischemic optic neuropathy associated with surgical blood losses greater than 4 L with a hypotensive event or relative hypotension over an extended period, either from blood loss or deliberate hypotension to minimize blood losses.11 Visual deficits can also result from central retinal artery or vein occlusion from direct pressure to the globe, resulting in increased intraocular pressure and decreased retinal blood flow through the central retinal artery. Transient cortical blindness can result from an isolated stroke that affects the visual cortex typically from an embolic phenomenon or hypoperfusion. In contrast to ischemic optic neuropathy and central retinal vessel occlusion, which are typically irreversible, most cases of cortical blindness resolve. Although the risks of these complications cannot be eliminated, they can be minimized through prevention of direct pressure to the globe (e.g., Mayfield three-point head holder in posterior cervical spine surgeries) and appropriate patient-specific management of hemodynamic parameters with measures aimed at reducing central venous pressure (e.g., reverse Trendelenburg positioning, minimizing abdominal compression).12 These considerations are especially important in high-risk patients (i.e., diabetes, hypertension, glaucoma, large estimated blood losses, systemic hypotension, and long operative times).
Surgical Technique
There is no substitute for good surgical technique and knowledge of surgical anatomy in the prevention of neurologic injury. Proper handling of each surgical instrument is paramount because, if mishandled, any instrument has the potential to cause injury. Instruments should not be passed over an open spinal canal, and all instruments introduced into the wound should be checked to make sure they are not going to come apart and risk blunt or penetrating injury to the thecal sac (e.g., screwdriver shafts should be checked to ensure they are seated within their handles). When possible, instruments should be held with two hands when working around vital structures, with the hand closer to the patient resting against the patient for stability and to prevent “plunging.” In addition, instruments should be directed away from important structures when possible to avoid injury due to inadvertent “past-pointing.” Instruments should be properly sized for the task at hand. Instruments with cutting surfaces should be kept sharp because this allows more control as less force is necessary. A thorough understanding of the local anatomy and familiarity with the instrument allows proper orientation of the instrument in relation to structures at risk. For example, directing the path of a Kerrison rongeur in line with the nerve root can help decrease the chance of inadvertently biting the nerve root during a foraminotomy. When possible, protecting the path of cutting instruments with a cotton pledget or with gentle retraction can be helpful to avoid injury; however, excessive compression or retraction of the neural elements should be avoided. Osteotomes may be useful in cases of severe stenosis because the overlying compressive structures (typically an overgrown facet joint) can be removed without having to introduce an instrument into an already tight spinal canal. Running motorized burrs at high speeds decreases the chances of skipping on sclerotic bone. Drills should ideally be started perpendicular to the bone to prevent skipping; alternatively, a starting indentation may be created with an awl or burr. Past-pointing with the drill should be avoided through the use of tactile and auditory cues to identify when cortical penetration is imminent. It is common to lose focus toward the end of a long spine procedure, especially during the more routine portions of the procedure after the challenging sections have been completed. This is the time when it is most important to remain vigilant because lapses in concentration can risk iatrogenic injury. An inadvertent slip of an instrument into an open spinal canal during final torquing of set screws, for example, is all it takes to ruin an otherwise technically well-executed procedure. Proper placement of retractors is essential to avoid injury. The assistant holding the retractor ideally should have adequate visualization and be free from other duties in order to focus solely on safe retraction. In general, the thecal sac should not be retracted at cord levels. In the lumbar spine at the level of the cauda equina, the thecal sac may be retracted for improved visualization, though excessive retraction risks neurologic injury, especially before decompression in the setting of stenosis. Excessive or poorly controlled intraoperative bleeding risks postoperative epidural hematoma and thecal sac compression. Excessive bleeding should be controlled before closure and drains used when appropriate. Hemostatic agents (e.g., gel foam) and bone wax can create neurologic compression if improperly or inadvertently placed.13
Neuromonitoring
Historically, many intraoperative neurologic complications were not identified until the patient awoke from surgery with a neurologic deficit. Now, with the advent of useful neuromonitoring techniques, perturbations in neural transmission can be identified early, allowing for timely intraoperative correction and mitigation of potential postoperative neurologic deficits. Neuromonitoring is most useful when there is considerable risk of neurologic injury from either patient positioning, planned operative maneuvers, or when the surgical plan calls for significant forces to be applied to the spinal column (e.g., deformity correction).15–17
tcMEPs
TcMEPs allow for a functional evaluation of the motor pathway including the anterior or ventral corticospinal tracts of the spinal cord, plexus, and peripheral nerve. Intermittently applied transcranial electrical stimuli result in evoked potentials that can be recorded either directly from the spinal cord or indirectly from muscle. Direct recording from the spinal cord via electrodes is not commonly used but can be useful for intradural procedures. More commonly, recordings are obtained indirectly from the muscle as compound muscle action potentials (CMAPs) via implanted electrodes. Transcranial MEPs should be distinguished from neurogenic MEPs, an older, less reliable technique that, despite its name, reliably monitors only sensory pathways.18 Transcranial MEPs are a useful complement to SSEPs, allowing for monitoring of both the ventral and dorsal spinal cord tracts. They are sensitive to changes in perfusion, which allows them to be used to titrate the degree of hypotension to spinal cord perfusion. Transcranial MEPs are more sensitive and respond more rapidly than SSEPs to changes in neural transmission, potentially increasing the window of opportunity for intervention in the face of neural injury.17,19
EMG
Electromyography (EMG) is used to evaluate nerve root integrity. There are two types in routine use, mechanically elicited EMG (meEMG) and electrically elicited EMG (eeEMG). MeEMG (also known as spontaneous or free-running) provides continuous monitoring of spinal nerve roots for injury. When a monitored spinal nerve root sustains microtrauma, the subsequent depolarization results in motor unit potentials in the corresponding innervated muscles that can be recorded via surface electrodes. Consequently, meEMG can be useful during placement of implants (e.g., pedicle screws) or when working in close proximity to a nerve root (e.g., posterior cervical foraminotomy), where continuous monitoring for nerve root microtrauma can alert the physician to injury. eeEMG (also known as stimulus-evoked or triggered) is used for intraoperatively evaluating implant placement (typically pedicle screws). eeEMG, in contrast to meEMG, is a static, stimulus-dependent technique and is not able to provide information over the course of an entire surgical procedure. Intermittent stimulation of an implant is performed while the corresponding “at-risk” nerve roots are monitored for signal transmission. Because cortical bone has a high resistance to electrical current flow in contrast to soft tissues, in the absence of a pedicular breach, stimulation of a pedicle screw should not result in signal transmission until the current exceeds an incompletely defined threshold (at our institution we use 10mA).20 When a breach is present, less current (≤5mA) is necessary for signal transmission because the path of least resistance (i.e., the soft tissues accessible via the breach) is taken. Readings between 5 and 10 mA are a gray zone; other factors need to be considered including, but not limited to, the confidence in the accuracy of screw placement, whether the screw placement is harmoniously aligned with the adjacent screws, the importance of the screw to the overall stability of the construct, and the degree of osteopenia. When eeEMG suggests a breach, unless irrefutable evidence is available (e.g., presence of a laminotomy with the ability to palpate and inspect the pedicle within the canal directly for evidence of breach), we would tend to remove the screw, check for a breach, and reposition the screw or opt to leave the screw out. When chronic compression of motor nerve roots exists, these previously mentioned thresholds can be elevated, resulting in false-negative results. In fact, some authors recommend a heightened level of concern when eeEMG testing returns a value less than 60% in comparison with the other screws regardless of absolute value.21 False positives are not infrequent and are an issue because of the need to remove the screw to check for a breach, which can lead to decreased screw purchase on reinsertion.
The amplitude, latency, and waveform morphology criteria for actual or impending neurologic injury with these aforementioned techniques are not clearly defined in the literature. Amplitude change appears to be the most relevant because significant injury is unlikely to occur without it. The most common criterion defines a significant change as greater than or equal to a 50% reduction in amplitude.22 Clinically relevant changes are typically abrupt and associated with a corresponding increase in latency. In contrast, isolated latency changes in the absence of amplitude alterations are often one of a variety of benign evoked potential alterations that can be misinterpreted as actual or impending neurologic injury.
Rationale for the Use of Neuromonitoring Techniques
Indications for monitoring and specific monitoring protocols have not been completely defined and remain controversial, especially in the tort environment of the United States. There are few circumstances wherein spinal cord monitoring is reasonably considered to be part of the so-called standard of care. With this understood, there are circumstances where its use may prove prudent. When there is increased risk of neurologic injury from patient positioning (e.g., unstable spine due to trauma, significant cervical canal stenosis with myelopathy) or from planned surgical maneuvers (e.g., correction of spinal deformity), neuromonitoring may be useful. In general, surgical procedures performed at spinal cord levels may be monitored with some combination of SSEPs, tcMEPs, and EMG.17 Concomitant use of SSEPs and tcMEPS allows monitoring of both the ventral and dorsal spinal tracts and has increased sensitivity and specificity for identifying neural injury. Moreover, changes in one can be compared and correlated with changes in the other, allowing each to effectively serve as a form of internal control. The primary disadvantages of adding tcMEPs include the more frequent need to deal with false-positive events, the need to forgo neuromuscular blockade for at least a portion of the procedure, and the additional expense entailed. In addition, they cannot be used in all patients (e.g., tcMEPS are contraindicated in patients with seizure disorders). Upper limb SSEPs with tcMEPS are typically sufficient to monitor the upper cervical spinal cord. Brainstem AERs can be added for upper cervical procedures to monitor brainstem function because the vertebral arteries are at increased risk for injury. Lower limb SSEPs are added when lower cervical or thoracic spinal cord function is being monitored. In this situation the upper limb SSEPs serve as a useful comparison and afford protection against impending brachial plexus injury. Electromyography is used if root level compromise is a risk. meEMG can be helpful to continuously monitor nerve roots at risk for injury (e.g., posterior cervical foraminotomy). eeEMG is reserved for use evaluating pedicle screw placement. Baseline recordings with tcMEPs or EMG obtained before positioning can allow for identification of positioning-related complications. During instrumentation and corrective maneuvers, frequent signal acquisition of tcMEPs is advised during breaks in surgical activity. If an event does occur, the etiology can then be traced to a set of operative maneuvers.
Coordination Between Surgical and Anesthesia Teams
Coordination of efforts among the surgical, anesthetic, and neuromonitoring teams allows for smooth progress of surgery and minimizes conflict. Important considerations are the choice of anesthetic technique and maintenance of blood pressure. Because inhalational agents tend to produce dose-dependent changes in latency and amplitude of evoked potentials, their use should be avoided or minimized when neuromonitoring is being used.23 Partial substitution with intravenous anesthetic agents can still allow for reliable intraoperative neuromonitoring. Neuromuscular relaxants can compromise techniques that depend on the recording of muscle action potential such as tcMEPs and EMG, so their use should be reserved for the exposure when they are most advantageous and the risk of significant neural injury least. Short-acting neuromuscular blocking agents may also be used at the surgeon’s discretion during the portions of a procedure when sudden and uncontrolled patient movements could result in harm. Mean arterial pressure (MAP) management is critical during spinal procedures as hypertension risks excessive blood loss and hypotension risks ischemic neural injury. This obviously has to be managed appropriately in the context of preexisting cardiovascular disease. Baseline preoperative MAP should be taken into account when deciding on an appropriate MAP to maintain during surgery. Given that the threshold for spinal cord ischemia is approximately 55 mm Hg, maintenance of an MAP of at least 65 mm Hg would be prudent as a rule of thumb in patients without major comorbidities.14,17 The sensitivity of tcMEP to changes in blood pressure can be used to advantage to titrate blood pressure to a level that is adequate for maintenance of adequate spinal cord perfusion.
Surgery
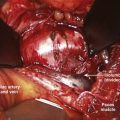
The key to a safe exposure is a thorough understanding of the local anatomy and the relationships of the neurovascular structures within the dissection. Knowing the typical course of neurologic structures within the surgical field and constant vigilance for these structures and the perineural fat that heralds their presence helps avoid injury. During the dissection, certain neural structures may be sacrificed (e.g., lumbar sympathetic chain, C2 nerve root, thoracic nerve roots) while others must be preserved (e.g., cervical sympathetic chain, lumbar nerve roots). Knowledge of the distinction is essential. Anatomic variations may blur this distinction as with a postfixed brachial plexus with contributions from C6-T2, where the T2 nerve root contribution (normally of little relevance) may become functionally important. Neuromonitoring may be of benefit to minimize the chance of certain injuries such as in transpsoas approaches to the lower lumbar intervertebral discs, where injury to the femoral nerve/lumbar plexus, though uncommon, can result in significant functional disability (Fig. 94–2). During posterior spinal exposures, the neural elements are generally not at significant risk until the spinal canal is entered. Care should be taken to respect the interlaminar windows and avoid plunging instruments into the spinal canal during exposure, especially when the spine is flexed (e.g., as on a Wilson frame) and the interlaminar interval widened, in dysraphic states, or in revision surgery with previous laminectomy. Meticulous technique is necessary in these situations with blunt dissection preferred over electrocautery. During exposure it is imperative that the correct level is identified. Transitional spinal segments should be identified preoperatively because these complicate identification of the correct operative level.24 Thoracic procedures, especially, are at increased risk for wrong-level surgery; extra care should be taken when operating on thoracic levels to ensure that the right level is operated on. Once an adequate exposure has been obtained, attention can then be directed toward the definitive surgical procedures.
Decompression
Laminectomies and laminoplasties allow decompression of the neural elements. Cervical laminectomies and laminoplasties are typically performed procedures for spinal cord compression and myelopathy. Part of the surgical technique can include fashioning troughs at the junction of the lamina and lateral mass.25 Appropriate placement of the trough minimizes neurologic risk to the spinal cord and cervical nerve roots. This requires a thorough understanding of the normal anatomy of the lamina-lateral mass junction and the degree to which it can be obscured from degenerative facets or decreased laminar pitch, common anatomic features in patients undergoing these surgeries. Both cervical and thoracic decompressive procedures must take into account the decreased tolerance of the spinal cord for mechanical injury in comparison with the cauda equina of the lumbar spine; thus instruments introduced into the spinal canal should be properly sized, and retraction of the thecal sac should be avoided. In the lumbar spine, it is imperative that the lateral margin of the nerve root (i.e., medial wall of the pedicle) be identified in order to safely proceed with decompression. Nerve roots may be attenuated over a bulging disc and not recognized owing to their peculiar appearance placing them at risk for injury. Nerve root retractors in the lumbar spine should be used cautiously. Excessive retraction of the thecal sac in the lumbar spine, especially before decompression in the setting of spinal stenosis, risks neurologic injury and should be avoided. Forceful retraction of a nerve root lying within a stenotic neural foramen risks nerve root avulsion.
Instrumentation
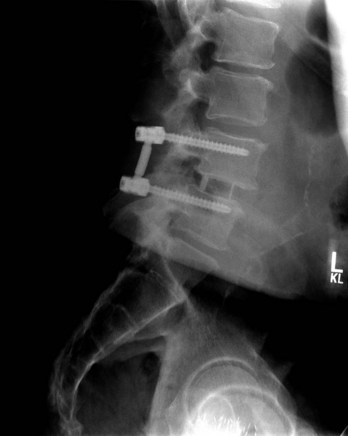
Proper placement of instrumentation minimizes the risk of neurologic injury. Preoperative imaging should be reviewed for anatomic abnormalities that would increase the chances for neurologic injury with hardware placement. Intraoperatively, consideration of anatomic landmarks, use of fluoroscopy, and/or neuromonitoring techniques can be used to confirm appropriate placement. Whatever combination of techniques necessary to ensure safe hardware placement should be used. Screw lengths may be guided by preoperative imaging measurements, intraoperative imaging guidance, and/or intraoperative depth measurements. Screws should be directed such that they remain intraosseous to avoid critical adjacent neurovascular structures (Fig. 94–3). Understanding three-dimensional anatomy is essential. When multiple safe intraosseous screw trajectories may be used, the relative risks and benefits of each technique should be weighed (e.g., cervical pedicle screws versus lateral mass screws with Roy-Camille, Magerl, or An technique). This may necessitate consideration of patient-specific anatomic factors and surgeon training. If necessary, screws may be placed bicortically for improved purchase, but screw lengths should then be checked with intraoperative imaging to avoid neurovascular injury at the point of penetration. Penetration of the anterior cortex of the C1 lateral masses during placement of C1-C2 transarticular screws, for example, can risk injury to the internal carotid artery and hypoglossal nerve, both of which can result in significant neurologic repercussions.2,3 Sublaminar wiring techniques at spinal cord levels can cause cord contusion if the wires are not appropriately passed or if there is inadequate subarachnoid space to accommodate passage of the wires.
Autograft Harvest
Harvesting of anterior or posterior iliac crest or thoracic rib can result in injury to local sensory nerves (i.e., lateral femoral cutaneous, cluneal and intercostal nerves, respectively).6,7 While sensory deficits are typically well tolerated and at least partially compensated by adjacent sensory innervation, painful postoperative neuralgia can result and be a postoperative source of patient discomfort. Avoidance of injury is predicated on a thorough understanding of the local anatomy and proper handling of neural structures.
Durotomy Repair
Incidental durotomies can lead to neurologic complications if not handled properly. Significant neurologic injury can occur from suctioning of exposed rootlets that have escaped the site of a lumbar durotomy. Inadvertent placement of a suture that snares a nerve rootlet during a dural repair can cause new neural deficits or pain. Dural tears at the origin of the root sleeve, especially the axilla, may be a unique challenge. Direct repair of some such tears may create stenosis. Instead a patch graft of locally obtained fat, fascia, or synthetic graft material may be necessary in conjunction with a dural sealant and consideration for placement of a subarachnoid drain. Dural sealants should not be placed into a closed space (e.g., within the neuroforamen) because these can expand and be a source of compression.26,27
Deformity Correction
Surgical management of spinal deformity is associated with an increased risk for neurologic injury.28 Neuromonitoring is especially helpful in these situations in providing a safer surgical circumstance. Anterior exposures may require ligation of multiple adjacent segmental vessels, which carries a theoretical risk of clinically significant spinal cord ischemia. Some authors advocate soft clamping and evaluation of evoked potentials for signal change before ligation.29 Retrospective studies have found that the risk is minimal provided ligations are performed unilaterally and on the convexity.30 In the absence of deformity, animal studies have suggested that ligation of segmentals bilaterally at three levels or less can be safely performed.31 In addition to exposure-related issues, correction of spinal deformity carries significant risks. The considerable amount of force that can be applied with modern instrumentation, if injudiciously applied, can also risk significant neurologic injury. Excessive correction can result in mechanical injury from kinking of the spinal cord or ischemic injury from kinking of the vasculature, resulting in decreased spinal cord perfusion. Traction palsy of the nerve roots after correction is also possible. It is important to understand the goals of correction and balance the degree of correction necessary with the amount of neurologic risk that is acceptable. In addition to the risks associated with sagittal and coronal plane correction, the risks associated with derotational maneuvers should be realized. Direct vertebral derotation techniques through pedicle screws can risk fracture of the pedicle if aggressive forces are applied. Use of this technique on the convexity uses the thicker medial pedicle wall to lever against; however, pedicle failure with medial breach of the pedicle screws in this instance risks injury to the spinal cord. Distributing the derotation forces over several screws with current instrumentation designs may minimize the risk of pedicle failure.
General Response to Real Neurologic Injury or Reported Neuromonitoring Event
When a reproducible neuromonitoring event suggestive of real or impending neurologic injury occurs, benign evoked response alterations should be ruled out first. These can result from technical factors, anesthetic technique changes, and local or systemic changes in temperature as previously described. Reversal of the maneuver that resulted in the event should be considered if possible because neuromonitoring signal changes are often simply a herald for irreversible injury; thus prompt intervention can prevent neurologic sequelae. During this time, adequate oxygenation should be ensured. The fraction of inspired oxygen should be increased to address any ongoing hypoxia and arterial blood gases checked as needed. Judicious use of intravenous fluid replacement, blood transfusion, and vasopressors should follow to allow maintenance of adequate arterial perfusion depending on the hemodynamic status of the patient. The Stagnara wake-up test may be used to evaluate significant unresolved neuromonitoring findings.32 The wake-up test is essentially an intraoperative neurologic examination after reversal of general anesthesia. Because it allows for gross assessment of neural pathway integrity but cannot be expected to allow for a detailed root or peripheral nerve level examination, subtle injury can be missed. The wake-up test is difficult to perform in patients who do not have the capacity to understand the instructions. Complications include self-extubation, loss of safe patient positioning, and postoperative recall of events.
When a root level injury occurs, the patient’s oxygenation and hemodynamic status should be optimized in order to obviate any possible effects from ischemia. Lacerations to a nerve root are not repairable. Judicious intraoperative and perioperative administration of steroids may decrease root inflammation associated with injury or vigorous intraoperative manipulation. Suspected cord level injury can be evaluated with a wake-up test. Alternatively, when there is a high likelihood that a reported event is real (e.g., complete loss of amplitudes and increase in latencies in lower limb SSEPs and MEPs with maintenance of upper limb SSEPs and MEPS immediately after aggressive correction of congenital thoracic kyphosis), reversal of the steps before the event may be done immediately without waiting for a confirmatory wake-up test to minimize the chances of permanent neurologic dysfunction.33 If significant neurologic injury is confirmed before instrumentation and correction, consideration should be given to aborting the remaining portions of the procedure and returning to the operating room after a period of convalescence to avoid further injury. Injuries that occur after instrumentation should have the correction reversed. Removal of hardware at this juncture may assist investigatory postoperative imaging but must be weighed against the problems associated with the increased operative time necessary for their removal and later reimplantation. Ideally, hardware should not be removed if the spine would be left unstable. The use of methylprednisolone dosed according to spinal cord injury steroid protocols, although controversial, is a treatment option for the patient with absence of motor function during the wake-up test after appropriate optimization of oxygenation and hemodynamic parameters and release of correction.34,35 The potential benefits of improved neurologic recovery must be weighed against the significant potential complications that include infection and poor postoperative wound healing. Unfortunately there are few studies in the literature to support an evidence-based approach for intraoperative injuries. Other interventions such as the use of erythropoietin, Rho antagonists, and controlled hypothermia, though promising, remain unproven. Postoperatively, patients should be observed in the intensive care unit (ICU) with close hemodynamic monitoring and frequent neuroexaminations. Adequate oxygen delivery should be ensured. The mean arterial pressures should be maintained above 90 mm Hg again with judicious use of intravenous fluid replacement, blood transfusion, and vasopressors.16 Imaging with computed tomography (CT) or magnetic resonance imaging may be performed to evaluate alignment and hardware placement or for cord signal changes. Return to the operating room may be indicated to alleviate any cord compression identified on imaging.
Postoperative Neurologic Complications
Imaging
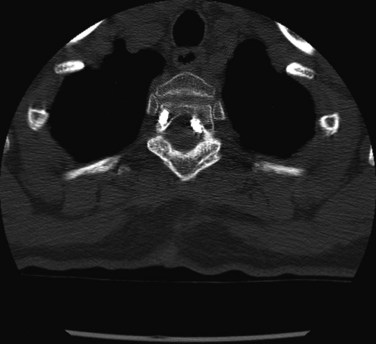
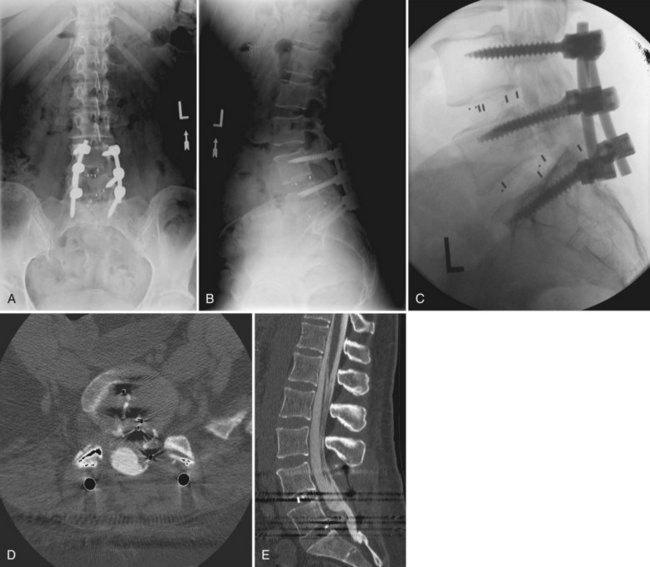
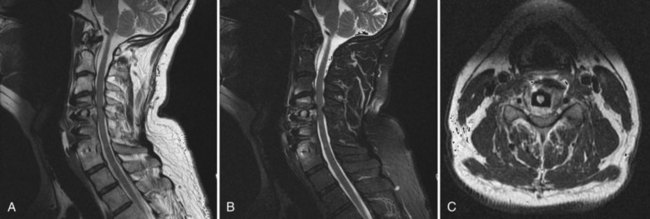
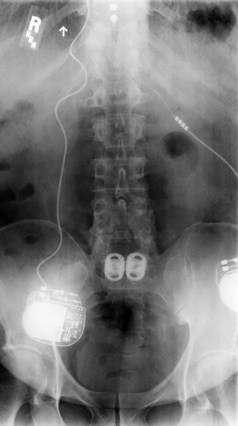
There should be a low threshold for obtaining imaging studies (Fig. 94–4). Plain radiographs are a rapid means to evaluate spinal alignment and gross placement of implanted hardware and to check that surgery was performed at the correct level. CT scans with coronal and sagittal reconstructions are the best study for evaluating implant position and graft placement and looking for residual osseous compression. Magnetic resonance imaging is the most sensitive test for soft tissue concerns and can be useful when looking for cord signal changes, identifying neural compressive lesions (e.g., inadequate decompression), epidural hematoma, and the like (Fig. 94–5). In the immediate postoperative patient, IV gadolinium contrast is unnecessary. With time, contrast becomes more important in order to distinguish vascularized scar tissue from avascular disc material. If metal artifact prevents a useful MRI from being obtained, a CT myelogram should be obtained.
Diagnosis and Treatment
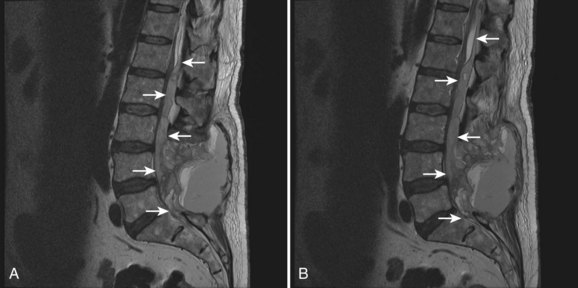
Once non-neurologic etiologies have been ruled out, patients with postoperative acquired neurologic deficits should undergo physical examination with attention paid to determining the neurologic level of the lesion because the distinction among brain, brainstem, spinal cord, nerve root, and plexus and peripheral nerve involvement is critically important when deciding further management. Patients with acute peripheral neuropathies, radiculopathies, or plexopathies typically present with some combination of pain, paresthesias, and motor weakness. It is important to remember that occipital headaches, upper cervical pain, and thoracic pain can be a manifestation of nerve root irritation. Imaging is useful to rule out compressive etiologies such as a foraminal bone fragment causing a radiculopathy or a psoas hematoma causing a lumbar plexopathy (Fig. 94–6). In the absence of correlative imaging findings, the diagnoses of exclusion apply: neural inflammation (e.g., acute radiculitis) or neurologic injury (e.g., traction neuropraxia).36 The postoperative course can help distinguish these because inflammatory etiologies typically resolve with appropriate symptomatic care that can include rest, ice, judicious use of anti-inflammatories, membrane-stabilizing drugs such as gabapentin, and for significant symptoms, use of a steroid taper while persistence of acquired symptoms are associated with neurologic injury (Fig. 94–7). Recovery is, of course, dependent on the etiology of the neurologic lesion.
Special Situations
Dysphagia and Dysphonia
Although any patient undergoing endotracheal intubation (especially those with a difficult airway) may have a sore throat, dysphagia, and/or dysphonia postoperatively to varying degree, these symptoms are most commonly associated with patients undergoing anterior cervical spine surgery, with a substantial percentage (28% to 47%) of patients experiencing some degree of postoperative dysphagia.37–39 At highest risk are older patients, females, patients with longer duration of preoperative symptoms, patients undergoing operations on two or more levels or revision surgery, and use of thicker anterior cervical plates. Swallowing and phonation are complex processes dependent on a variety of neurologic and non-neurologic structures; thus the etiology of postoperative dysfunction is multifactorial. Severe symptoms should be evaluated with lateral plain radiographs or CT scan for bone graft dislodgement and postoperative edema or hematoma. Rapid progression of symptoms can be seen with postoperative epidural hematoma necessitating emergent reexploration. Persistence of symptoms or suspicion of aspiration warrant further evaluation with speech language pathology or otolaryngologic consultation and videofluoroscopic swallow evaluation. Corticosteroids remain of unproven benefit. Fortunately, most patients improve with time.37
Visual Loss
Pearls and Pitfalls
Key Points
1 American Society of Anesthesiologists Task Force on Perioperative Blindness. Practice advisory for perioperative visual loss associated with spine surgery: a report by the American Society of Anesthesiologists Task Force on Perioperative Blindness. Anesthesiology. 2006;104:1319-1328.
2 Bertrand G. The “battered” root problem. Orthop Clin North Am. 1975;6:305-310.
3 Daniels AH, Riew KD, Yoo JU, Ching A, Birchard KR, Kranenburg AJ, Hart RA. Adverse events associated with anterior cervical spine surgery. J Am Acad Orthop Surg. 2008;16:729-738.
4 Lee JY, Hilibrand AS, Lim MR. Characterization of neurophysiologic alerts during anterior cervical spine surgery. Spine. 2006;31:1916-1922.
5 Schwartz DM, Auerbach JD, Dormans JP, et al. Neurophysiological detection of impending spinal cord injury during scoliosis surgery. J Bone Joint Surg Am. 2007;89:2440-2449.
6 Wong DA. Spinal surgery and patient safety: a systems approach. J Am Acad Orthop Surg. 2006;14:226-232.
1 Conroy E, Laing A, Kenneally R, Poynton AR. C1 lateral mass screw-induced occipital neuralgia: a report of two cases. Eur Spine J. 2010;19:474-476.
2 Currier BL, Maus TP, Eck JC, et al. Relationship of the internal carotid artery to the anterior aspect of the C1 vertebra: implications for C1-C2 transarticular and C1 lateral mass fixation. Spine (Phila Pa 1976). 2008;33:635-639.
3 Ebraheim NA, Misson JR, Xu R, Yeasting RA. The optimal transarticular c1-2 screw length and the location of the hypoglossal nerve. Surg Neurol. 2000;53:208-210.
4 Daniels AH, Riew KD, Yoo JU, Ching A, Birchard KR, Kranenburg AJ, Hart RA. Adverse events associated with anterior cervical spine surgery. J Am Acad Orthop Surg. 2008;16:729-738.
5 Ebraheim NA, Lu J, Yang H, et al. Vulnerability of the sympathetic trunk during the anterior approach to the lower cervical spine. Spine (Phila Pa 1976). 2000;25:1603-1606.
6 Mirovsky Y, Neuwirth M. Injuries to the lateral femoral cutaneous nerve during spine surgery. Spine (Phila Pa 1976). 2000;25:1266-1269.
7 Xu R, Ebraheim NA, Yeasting RA, Jackson WT. Anatomic considerations for posterior iliac bone harvesting. Spine (Phila Pa 1976). 1996;21:1017-1020.
8 Sasso RC, Kenneth Burkus J, LeHuec JC. Retrograde ejaculation after anterior lumbar interbody fusion: transperitoneal versus retroperitoneal exposure. Spine (Phila Pa 1976). 2003;28:1023-1026.
9 Wang LC, Liou JT, Liu FC, et al. Fatal ischemia stroke in a patient with an asymptomatic carotid artery occlusion after lumbar spine surgery—a case report. Acta Anaesthesiol Taiwan. 2004;42:179-182.
10 Stevens WR, Glazer PA, Kelley SD, et al. Ophthalmic complications after spinal surgery. Spine (Phila Pa 1976). 1997;22:1319-1324.
11 Dunker S, Hsu HY, Sebag J, Sadun AA. Perioperative risk factors for posterior ischemic optic neuropathy. J Am Coll Surg. 2002;194:705-710.
12 American Society of Anesthesiologists Task Force on Perioperative Blindness. Practice advisory for perioperative visual loss associated with spine surgery: a report by the American Society of Anesthesiologists Task Force on Perioperative Blindness. Anesthesiology. 2006;104:1319-1328.
13 Hoyland JA, Freemont AJ, Denton J, et al. Retained surgical swab debris in post-laminectomy arachnoiditis and peridural fibrosis. J Bone Joint Surg Br. 1988;70:659-662.
14 Lee JY, Hilibrand AS, Lim MR. Characterization of neurophysiologic alerts during anterior cervical spine surgery. Spine. 2006;31:1916-1922.
15 May DM, Jones SJ, Crockard HA. Somatosensory evoked potential monitoring in cervical surgery: Identification of pre- and intraoperative risk factors associated with neurological deterioration. J Neurosurg. 1996;85:566-573.
16 Schwartz DM, Sestokas AK, Turner LA, et al. Neurophysiological identification of iatrogenic neural injury during complex spine surgery. Semin Spine Surg. 1998;10:242-251.
17 Schwartz DM, Auerbach JD, Dormans JP, et al. Neurophysiological detection of impending spinal cord injury during scoliosis surgery. J Bone Joint Surg Am. 2007;89:2440-2449.
18 Toleikis JR, Skelly JP, Carlvin AO, Burkus JK. Spinally elicited peripheral nerve responses are sensory rather than motor. Clin Neurophysiol. 2000;111:736-742.
19 Hilibrand AS, Schwartz DM, Sethuraman V, et al. Comparison of transcranial electric motor and somatosensory evoked potential monitoring during cervical spine surgery. J Bone Joint Surg Am. 2004;86-A:1248-1253.
20 Lenke LG, Padberg AM, Russo MH, et al. Triggered electromyographic threshold for accuracy of pedicle screw placement. An animal model and clinical correlation. Spine (Phila Pa 1976). 1995;20:1585-1591.
21 Raynor BL, Lenke LG, Kim Y, et al. Can triggered electromyograph thresholds predict safe thoracic pedicle screw placement? Spine (Phila Pa 1976). 2002;27:2030-2035.
22 Padberg AM, Wilson-Holden TJ, Lenke LG, Bridwell KH. Somatosensory- and motor-evoked potential monitoring without a wake-up test during idiopathic scoliosis surgery. An accepted standard of care. Spine (Phila Pa 1976). 1998;23:1392-1400.
23 Sloan TB, Heyer EJ. Anesthesia for intraoperative neurophysiologic monitoring of the spinal cord. J Clin Neurophysiol. 2002;19:430-443.
24 Wong DA. Spinal surgery and patient safety: a systems approach. J Am Acad Orthop Surg. 2006 Apr;14(4):226-232.
25 Hirabayashi K, Watanabe K, Wakano K, et al. Expansive open-door laminoplasty for cervical spinal stenotic myelopathy. Spine (Phila Pa 1976). 1983;8:693-699.
26 Mulder M, Crosier J, Dunn R. Cauda equina compression by hydrogel dural sealant after a laminotomy and discectomy: case report. Spine (Phila Pa 1976). 2009;34:E144-E148. 2
27 Thavarajah D, De Lacy P, Hussain R, Redfern RM. Postoperative cervical cord compression induced by hydrogel (DuraSeal): a possible complication. Spine (Phila Pa 1976). 2010;35:E25-E26.
28 Coe JD, Arlet V, Donaldson W, et al. Complications in spinal fusion for adolescent idiopathic scoliosis in the new millennium. A report of the Scoliosis Research Society Morbidity and Mortality Committee. Spine (Phila Pa 1976). 2006;31:345-349.
29 Apel DM, Marrero G, King J, et al. Avoiding paraplegia during anterior spinal surgery. The role of somatosensory evoked potential monitoring with temporary occlusion of segmental spinal arteries. Spine (Phila Pa 1976). 1991;16(8 Suppl):S365-S370.
30 Bridwell KH, Lenke LG, Baldus C, Blanke K. Major intraoperative neurologic deficits in pediatric and adult spinal deformity patients. Incidence and etiology at one institution. Spine (Phila Pa 1976). 1998;23:324-331.
31 Nambu K, Kawahara N, Kobayashi T, et al. Interruption of the bilateral segmental arteries at several levels: influence on vertebral blood flow. Spine (Phila Pa 1976). 2004;29(14):1530-1534.
32 Vauzelle C, Stagnara P, Jouvinroux P. Functional monitoring of spinal cord activity during spinal surgery. Clin Orthop Relat Res. 1973;93:173-178.
33 Cheh G, Lenke LG, Padberg AM, et al. Loss of spinal cord monitoring signals in children during thoracic kyphosis correction with spinal osteotomy: why does it occur and what should you do? Spine (Phila Pa 1976). 2008;33:1093-1099.
34 Bracken MB, Shepard MJ, Holford TR. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury: Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997;277:1597-1604.
35 Sayer FT, Kronvall E, Nilsson OG. Methylprednisolone treatment in acute spinal cord injury: The myth challenged through a structured analysis of published literature. Spine J. 2006;6:335-343.
36 Bertrand G. The “battered” root problem. Orthop Clin North Am. 1975;6:305-310.
37 Lee MJ, Bazaz R, Furey CG, Yoo J. Risk factors for dysphagia after anterior cervical spine surgery: A two-year prospective cohort study. Spine J. 2007;7:141-147.
38 Rihn JA, Kane J, Joshi A, et al: Dysphagia following anterior cervical surgery: A controlled prospective analysis. Paper #3. Presented at the 24th Annual Meeting of the North American Spine Society. San Francisco, Nov. 10-14, 2009.
39 Smith-Hammond CA, New KC, Pietrobon R, et al. Prospective analysis of incidence and risk factors of dysphagia in spine surgery patients: Comparison of anterior cervical, posterior cervical, and lumbar procedures. Spine. 2004;29:1441-1446.