Chapter 10 Complications of Spinal Injections and Surgery for Disc Herniation
 A detailed history and physical examination of the patient and review of pertinent diagnostic studies and laboratory values in order to identify patient-specific risk factors before intervention is critical.
A detailed history and physical examination of the patient and review of pertinent diagnostic studies and laboratory values in order to identify patient-specific risk factors before intervention is critical. Appreciation of the inherent interindividual variability in the location of critical structures and understanding of the anatomy of the planned procedural approach to the epidural space can help limit complications.
Appreciation of the inherent interindividual variability in the location of critical structures and understanding of the anatomy of the planned procedural approach to the epidural space can help limit complications. Use of fluoroscopic imaging (particularly with continuous fluoroscopy during the instillation of contrast) is essential unless otherwise contraindicated.
Use of fluoroscopic imaging (particularly with continuous fluoroscopy during the instillation of contrast) is essential unless otherwise contraindicated.Introduction
Back pain is among the most common pain-related complaints that lead patients to seek care from physicians. Underlying disc herniation and subsequent radicular symptoms from direct compression of nerve roots or inflammatory mediators related to disc disruption are a frequent cause of the pain leading to these physician visits. The expenditure on care for these patients has been rapidly increasing over the past several years, and one recent study estimates that total health care expenditures for adults with back and neck pain increased by 82% in the United States between 1997 and 2006.1 These costs are attributable to a variety of diagnostic and treatment measures instituted for the alleviation of patient suffering, and a large portion of the costs is clearly related to interventional therapies for back pain such as epidural injections and surgery.
Epidural steroid injections (ESIs) are a commonly instituted therapy for degenerative disc disease with radicular symptoms. The utilization of this type of therapy has also increased substantially in recent years.2 In the same time period, there has been a similarly substantial increase in lumbar surgeries of which lumbar discectomy is the most commonly performed for patients with disc herniation causing lumbar radicular symptoms.3,4 With use of any medical therapy (both pharmacologic and interventional), there is always a degree of risk to the patient, and a comprehensive knowledge of the nature of that risk is paramount to minimizing complications to the greatest extent possible.
Complications of Epidural Steroid Injections
Epidural steroid injections are performed by one of three general techniques: interlaminar, transforaminal, and caudal. The interlaminar and transforaminal approaches are applied in the cervical, thoracic, and lumbar spine for radicular symptoms in the upper and lower extremities, respectively. The caudal approach is, for obvious reasons, only applicable for lumbar radicular symptoms. There is variability in which approach is used to treat cervical or lumbar radicular symptoms between the interlaminar and transforaminal route among interventional pain medicine specialists. The transforaminal approach is advocated to better direct the deposition of corticosteroids at the location of pathology, which is within the neural foramen. However, as discussed later, this anatomic location is also occupied by critical arterial vascular structures that can be inadvertently entered or injured during the injection. The interlaminar approach, on the other hand, is advocated to be safer because of the decreased risk of intraarterial injection and being more in line with the approach used to provide epidural analgesia as an established skill set of the many practitioners who enter pain medicine from anesthesiology backgrounds. Caudal access to the epidural space is among the earliest described methods of providing ESIs and is used by many practitioners as their primary method of administering ESIs for patients with lumbosacral radiculopathy.5 It has an added advantage as well in patients with previous lumbar laminectomy that can make accessing the epidural space from other routes more challenging, particularly from an interlaminar approach.
Broadly speaking, as seen in Table 10-1, the potential complications of ESIs can be differentiated into the following categories: (1) medication reactions, (2) vascular injury or intravascular injection, (3) bleeding, (4) infection, (5) needle trauma (including dural puncture), and (6) miscellaneous.
Table 10-1 Potential Complications of Epidural Steroid Injections
| Category of Complication | Example |
|---|---|
| Medication reactions | Allergic reaction, local anesthetic toxicity, steroid effect |
| Vascular injury or intravascular injection | Artery of Adamkiewicz, vertebral artery |
| Bleeding | Epidural hematoma |
| Infection | Epidural abscess, meningitis |
| Needle trauma | Spinal cord injury, nerve root injury, dural puncture |
| Miscellaneous | Urinary complications, vasovagal reactions |
Medication Reactions
During a typical ESI, three types of medications are administered: a local anesthetic, a corticosteroid preparation, and a contrast agent. Reactions to these medications can be either allergic in nature or related to the known pharmacology of the drugs themselves (Table 10-2).
Table 10-2 Medication Reactions in Epidural Steroid Injections
| Medication | Potential Reaction | Serious Complications |
|---|---|---|
| Local anesthetics | Toxicity | Seizure, cardiac arrest |
| Corticosteroids | Physiologic effects | Hyperglycemia, fluid retention, Cushing syndrome |
| All medications commonly used in epidural steroid injections (local anesthetics, corticosteroids, contrast agents) | Allergic reaction | Anaphylaxis |
With respect to allergic reactions, there is a known incidence of allergy to all of the aforementioned medications used in ESI. In terms of local anesthetics, the most frequently used in the context of ESI are the amide local anesthetics lidocaine and bupivacaine. Allergy to amide local anesthetics is quite rare and usually a contact dermatitis reaction, but it can occasionally be an anaphylactic-type reaction.6 In one such case report of an anaphylactic-type reaction to amide local anesthetics, a patient without a known history of local anesthetic allergy developed an anaphylactic response to lidocaine and bupivacaine administered during a lumbar injection (presumably an ESI, although the report does not mention the exact procedure performed) after two uneventful injections with the same agents previously.6 Corticosteroid preparations have also been reported to cause allergic reactions when administered epidurally, and although symptoms are most frequently seen immediately after administration, they can result in a delayed onset of symptoms up to several hours later.7 Contrast allergy, particularly to iodinated contrast agents, is also reported in the literature, although life-threatening anaphylactic reactions are rare.8 Reported factors that increase the risk of reaction to contrast agents include a history of previous reaction to contrast agent, asthma, atopy, and advanced heart disease.9
Toxicity associated with local anesthetic administration is exceedingly rare with doses commonly administered during ESIs and is more frequently seen with other interventional procedures used in pain medicine (e.g., sympathetic blockade). Inadvertent intravascular injection of local anesthetics into the vertebral artery during a cervical transforaminal ESI, however, has been reported to cause seizures (and even death) at lower doses of local anesthetic than those typically reported to cause seizures when administered intravenously.10 Presumably, the direct access to the cerebral circulation increases the risk of seizure from inadvertent intravascular administration of local anesthetics in the cervical spine.
Vascular Injury and Intravascular Injection
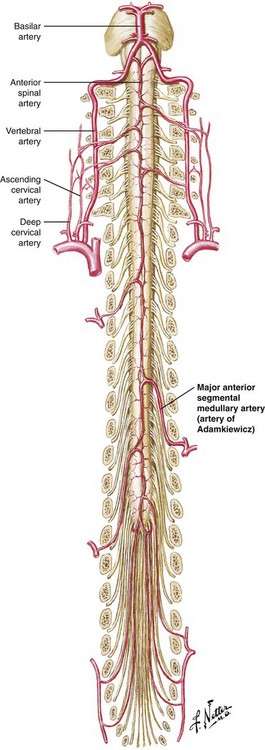
The vascular supply to the spinal cord is commonly understood to consist of paired posterior spinal arteries and a single anterior spinal artery, the latter supplying the anterior two-thirds of the spinal cord. The anterior spinal artery has additional vascular supply from radiculomedullary arteries as it progresses caudally along the spinal cord. In the cervical spine, there is an average of three such radiculomedullary arteries that originate from branches of the vertebral arteries and occasionally have contributions from the ascending and deep cervical arteries as well.11,12 Progressing more caudally, the supply to the anterior spinal artery is more limited, with the primary contribution arising from the artery of Adamkiewicz, a radiculomedullary artery of variable anatomic origin. Most commonly (reportedly 85% of the time), this artery arises between T9 and L2, and 80% of the time, it is located on the left.13 See Fig. 10-1 for a representation of this blood supply.
Because the blood supply to the anterior two-thirds of the spinal cord is dependent primarily on a few radiculomedullary arteries, with the low thoracic cord dependent on a single radiculomedullary artery (the artery of Adamkiewicz), injury to or embolization of any of these arteries can lead to a catastrophic neurologic outcome. Anatomic variation in the location of these arteries compounds the risk and should lead to vigilance in the performance of transforaminal injections. Indeed, there are reports in the literature of paraplegia and anterior spinal artery syndrome resulting from presumed embolism or injury to the artery of Adamkiewicz at almost all lumbar levels with one case as low as S1.11,14
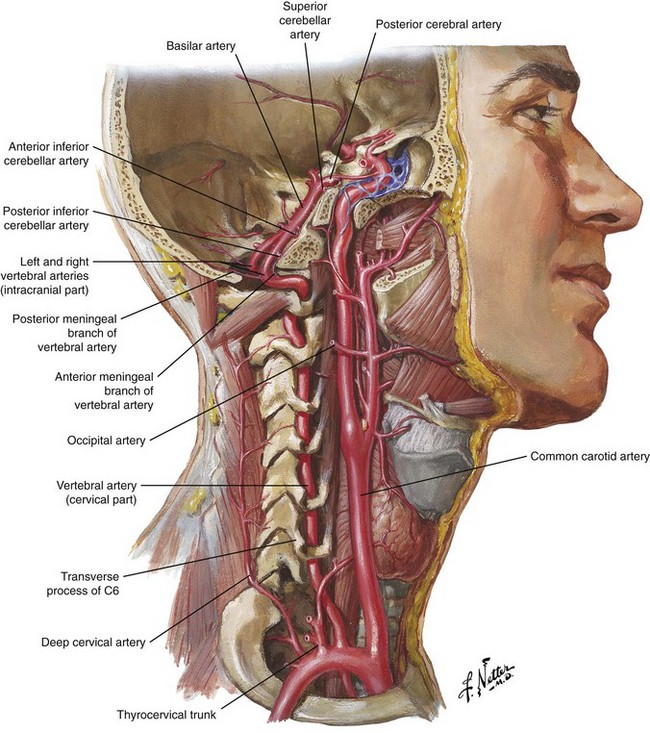
In the cervical spine, as seen in Fig. 10-2, the vertebral artery is found anteriorly within the foramen most frequently at C6 and above.12 Injury to this structure is also associated with catastrophic outcomes, as was reported in one case in which autopsy revealed that a puncture of the vertebral artery during a C7 transforaminal ESI led to a dissection and eventual thrombosis of the artery, causing death of the patient.15 Multiple reports can be found in the literature of brain infarction and spinal cord infarction associated with the transforaminal approach to cervical ESIs with the postulated mechanisms being embolism of particulate steroid particles versus direct injury to the vertebral or radiculomedullary arteries supplying the anterior spinal artery, respectively.10,15–20
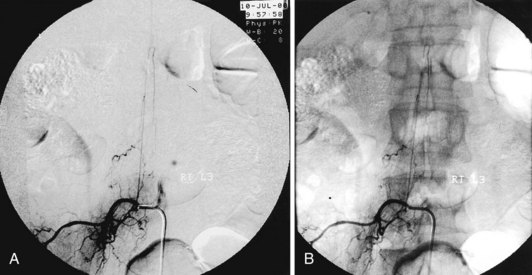
Avoiding these devastating complications is a primary procedural challenge facing interventional pain physicians. With respect to avoiding intravascular injection and subsequent embolic phenomena, it appears that the use of live fluoroscopy (with digital subtraction if available) is an essential step, particularly with the transforaminal approach, even though it may not be 100% effective. Fig. 10-3 illustrates the benefits of digital subtraction angiography in this image that shows the artery of Adamkiewicz in a spinal angiogram at the L3-L4 level. Use of intermittent fluoroscopy (in lieu of live, continuous fluoroscopy) during contrast injection can cause the proceduralist to miss the appearance of vascular uptake.21,22 In addition, it should be noted that a recent study showed an 8.9% incidence of simultaneous epidural and vascular patterns on injection of contrast during lumbar transforaminal injections, a finding that reinforces the critical importance of diligence during contrast injection in transforaminal ESIs, particularly when one considers that a radiculomedullary artery supplying the anterior spinal artery can occur at almost any vertebral level.23
The nature of the agent injected also can be a factor in the outcomes of inadvertent intravascular injection. A study by Tiso et al24 looked at the role of particulate corticosteroids through analysis of particulate size and microscopic characteristics of the particles. In sum, they found that triamcinolone and methylprednisolone had a tendency to form aggregates in excess of 100 µm (which may increase the risk of vascular occlusion after intravascular injection) and recommend that in the case of cervical transforaminal ESIs, that only corticosteroid solutions (dexamethasone and betamethasone sodium phosphate) be used. This sentiment is further supported by review performed by Scanlon et al10 who note that thus far all of the commonly used corticosteroids have been associated with brain or spinal cord infarction except dexamethasone.
Bleeding
Epidural hematoma is a well-known but very rare risk of neuraxial anesthesia and ESI. Estimates of its prevalence vary, but an approximate incidence is fewer than one in 150,000 epidural anesthetics, although there is suggestion that this risk has increased with the advent of newer anticoagulant medications.25 In addition to being associated with neuraxial intervention, epidural hematoma has been reported to occur spontaneously in the general population, often in conjunction with anticoagulation, with one study reporting an incidence of 0.1 per 100,000 people.26,27 Depending on the location, symptoms generally present with an onset of severe back or neck pain and progressive neurologic dysfunction (sensory or motor deficits and occasionally with bowel or bladder dysfunction). A high index of suspicion and rapid diagnosis are the key to better outcomes in the case of epidural hematoma as the definitive treatment of laminectomy and hematoma evacuation has been associated with better recovery of neurologic function when performed promptly within 8 hours of the onset of neurologic symptoms.28,29
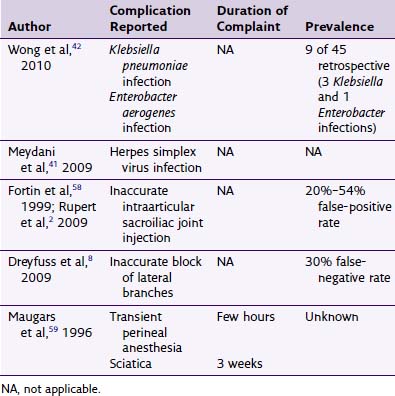
Without question, anticoagulant therapy is the most readily identifiable risk factor for the development of epidural hematoma in conjunction with interventional techniques. With epidural hematoma being such a rare clinical entity, performance of randomized trials to elucidate the best management strategies for anticoagulant use remains a challenging prospect. As such, the large majority of recommendations in this regard are summarized treatment guidelines formulated in the context of the available literature (e.g., case reports), expert opinion, and extrapolation from other clinical knowledge of bleeding risk. The American Society of Regional Anesthesia and Pain Medicine have recently updated one such set of guidelines.25 Although focused primarily on epidural and spinal anesthesia for surgery, they do include case reports (though rare) on epidural hematomas in the context of ESIs. In the absence of more pain medicine–specific guidelines, many practitioners apply these guidelines toward their own practice with regard to ESIs. An adaptation of these recommendations for clinical scenarios commonly encountered in an outpatient pain practice appears in Table 10-3. Specific note is made that this is just an adaptation and that all clinical decision making is best left to the individual practitioner, who would benefit from reading these guidelines directly.
Table 10-3 An Adaptation of the American Society of Regional Anesthesia and Pain Medicine Guidelines on Regional Anesthesia in Patients Receiving Antithrombotic or Thrombolytic Therapy
| Medication | Pre-Block Hold Recommendation | Post-Block Hold Recommendation |
|---|---|---|
| Heparin (subcutaneous)* | 4 hr | 1 hr |
| LMWH: prophylaxis dosing | 10-12 hr | 24 hr |
| LMWH: treatment dosing | 24 hr | 24 hr |
| Coumadin | 4–5 days and normalized INR before proceeding with block | |
| NSAIDs (including aspirin) | No hold recommended | |
| Clopidogrel | 7 days | |
| Ticlopidine | 14 days | |
| Herbal supplements (garlic, ginseng, ginkgo) | No hold recommended |
INR, International Normalized Ratio; LMWH, low-molecular-weight heparin; NSAID, nonsteroidal antiinflammatory drug.
* Recommendations are for standard dosing regimen of 5000 units twice daily. Per the American Society of Regional Anesthesia and Pain Medicine’s guidelines, the safety of 10,000 units daily or more than twice-daily dosing has not been established.
From Horlocker T, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Third Edition). Reg Anesth Pain Med 35(1), 2010.
Note should be made of the fact that the presented guidelines do not address patient-specific factors that may affect their risk of epidural hematoma after ESI. In fact, a case study cited by the authors of the guidelines represents a case of epidural hematoma after an ESI in a patient for whom bridging therapy with enoxaparin was utilized during Coumadin discontinuation for an ESI in which all of the guidelines were followed, and it was hypothesized by the authors that coexistent renal insufficiency was responsible for a prolonged enoxaparin effect.30 Another report documented a similar clinical scenario, with epidural hematoma despite strict adherence to the guidelines in the absence of renal insufficiency.27 Further emphasizing the importance of patient-specific factors is a case report of epidural hematoma resulting in paraplegia in a 67-year-old woman after an ESI with undiagnosed coagulopathy.31 Additionally, it appears that the risk of epidural hematoma is also contributed to by the use of multiple different agents that can all affect the coagulation cascade.
Infection
Although infection is also a possible complication of ESI, serious infection such as epidural abscess is a rare event. There is no commonly agreed upon incidence for this complication in the context of ESI, but some study has been done on the incidence of spontaneous epidural abscess and found it to be 0.88 cases per 100,000 person-years using data from one county’s population in the United States.32 A recent review of the published cases of epidural abscess and meningitis associated with ESI does, however, provide some insight into the characteristics and presentation of these patients. The authors report that the mean age was 54 years and that of the 14 reported cases, nine had a condition that may be expected to decrease immune system function (diabetes mellitus, metastatic cancer, myeloproliferative disorder, oral corticosteroid use). The mean onset of symptoms was 7 days after injection and the most common causative organism was Staphylococcus aureus.33
Their observation of S. aureus as the most common causative organism has been reinforced by several other analyses of epidural abscess data, although gram-negative species (often arising from unintentional bowel penetration) and even fungal species have been reported.20,33–38 The presentation is classically described as involving back pain and fever in the initial stages, with progressive neurologic deficits (motor, bowel and bladder, and sensory) developing later.37 As with epidural hematoma, early diagnosis with advanced imaging (magnetic resonance imaging) and early intervention with antibiotics and surgery are the keys to minimizing morbidity of epidural abscess.
In addition to epidural abscess, other infectious complications of ESI are possible and have been reported. In the event of inadvertent dural puncture during the procedure, there exists the risk of postdural puncture meningitis. An interesting review of this clinical entity was performed by Baer,35 who reported that mouth commensals (e.g., Streptococcus viridans) were the most frequent causative organisms of postdural puncture meningitis and that epidemiologic studies have identified the sources in some cases as being from the mouths and upper airways of medical personnel performing the procedures resulting in dural puncture. Reports also exist of cases of vertebral osteomyelitis (with soft tissue but not epidural abscess) and discitis after an ESI.39,40
Avoidance of infectious complications of ESIs begins with meticulous adherence to aseptic technique, including skin preparation with an appropriate antiseptic, sterile draping of the injection area, and use of sterile gloves and equipment (Table 10-4). Although it is controversial, many argue that wearing a mask while performing procedures that involve entry into the neuraxis is another important step to minimize infectious complications. Careful assessment of the patient for any coexistent infection at the time of the procedure will allow for identification of increased infectious risk and may lead to postponement of the procedure because of the risk of epidural abscess or infection arising from hematogenous spread. Furthermore, a history consistent with potential immunocompromise should reinforce the critical nature of aseptic technique and a risk-to-benefit analysis of the proposed injection and may lead to the decision to provide antibiotic prophylaxis as has been proposed by some interventionalists.
Table 10-4 Avoiding Infectious Complications of Epidural Steroid Injections
Needle Trauma
With any type of interventional procedure, unintended injury to adjacent anatomic structures is possible, and ESIs are no exception. The most common such complication is perhaps inadvertent dural puncture (or “wet tap”), which occurs at a reported frequency of approximately one in 200 epidurals.20 Many of these patients experience postdural puncture headaches (PDPHs) as a result. PDPHs are classically reported as severe dull headaches that are positional (with decreased symptoms in the supine position). Table 10-5 reviews some of the potential treatments for PDPH if it occurs.
Table 10-5 Treatment Options for Postdural Puncture Headache
| Activity Limitation | Medications | Invasive |
|---|---|---|
NSAID, nonsteroidal antiinflammatory drug.
Although most commonly thought of in association with the interlaminar approach to the epidural space, inadvertent dural puncture can also occur with other approaches to the epidural space and may not be associated with cerebrospinal fluid (CSF) flashback on removal of the stylet from the needle, particularly with the transforaminal approach.41 Careful interpretation of contrast pattern will identify the subarachnoid or subdural position of the needle in these circumstances. Failure to recognize the dural puncture and administration of medications intrathecally can be problematic and lead to a potentially high spinal block from local anesthetic and even arachnoiditis (especially if methylprednisolone acetate is administered in this location).9
In the cervical and thoracic spine, direct injury to the spinal cord can occur during an ESI both through interlaminar and transforaminal approaches, and a number of case reports have documented this serious complication.42–45 Too deep a level of procedural sedation has been proposed to be a contributor to this outcome with the hypothesis that the patient would have otherwise reported paresthesia or other symptom upon contact of the needle to the spinal cord. However, in one of the reported cases, the patient was not sedated and did not report any symptoms during intracord injection of steroid and local anesthetic, suggesting that reliance on patient report alone may note be sufficient to guard against this complication.44 Caution must be exercised as well to avoid trauma to the nerve roots themselves because they are clearly not immune to inadvertent injury during an ESI either through the transforaminal or interlaminar approach.46
Minimization of complications resulting from needle trauma is achieved through diligent adherence to proper technique, although even with the utmost of care, these types of events can occur, albeit rarely. One factor that is unquestionably of benefit in avoiding direct injury to the spinal cord and nerve roots is the use of biplanar fluoroscopy and contrast to confirm needle location. Avoiding deep sedation may be of benefit because the patient can report symptoms from pending neurologic injury. Reliance on loss of resistance alone may not be sufficient in the cervical spine; a recent anatomic study of cadavers revealed that there are frequently midline discontinuities in the ligamentum flavum in the cervical and high thoracic spine.47 Obtaining fluoroscopic information of the needle tip position has the potential to prevent advancing the epidural needle too far while attempting to find the loss of resistance in this area.
Miscellaneous
Urinary complications such as urinary retention can occur with local anesthetic administration in the lumbosacral region. In many instances, this is a problem that will resolve along with resolution of the local anesthetic effect. It is reported that urinary complications are more frequently seen in elderly patients (especially men), multiparous women, and patients with a history of inguinal and perineal surgery.20
Vasovagal reactions in the periprocedural period are commonly reported and often resolve without sequelae. One retrospective review found that these reactions were more common in patients undergoing cervical as opposed to lumbar ESI.48 Additionally, common minor post procedural complications include injection site soreness, increased axial neck pain (in the case of cervical ESIs), and facial flushing.49
Complications of Discectomy
Cervical Discectomy
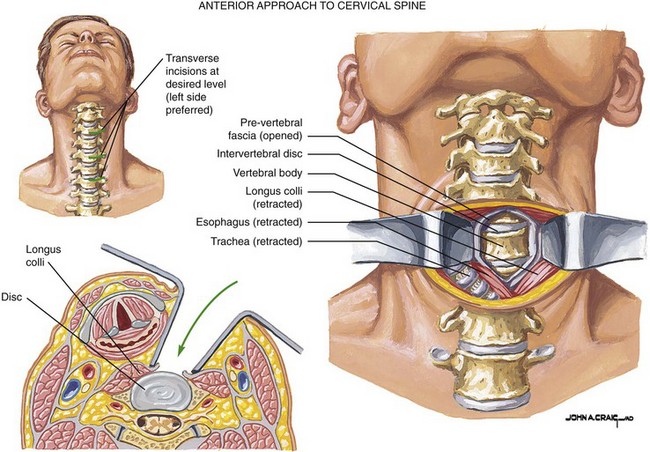
One of the most commonly performed spinal surgical procedures is anterior cervical discectomy and fusion (ACDF), and this represents the most common approach to cervical discectomy (see Fig. 10-4).50 One recent retrospective study analyzed the complication rates of this procedure in 1015 patients undergoing ACDF (note is made that only first-time operations were included in their analysis). Among their findings were that the morbidity rate was 19.3% with the most commonly observed complications being postoperative dysphagia (9.5%), hematoma (5.6%), and recurrent laryngeal nerve palsy (3.1%) (Table 10-6).51 Among the most rare complications observed in their study was also the most catastrophic: esophageal perforation occurred in 0.3%, but one of these cases was fatal. Indeed, injury to adjacent structures is a risk of the ACDF procedure; vascular injury to the carotid artery and jugular vein as well as injury to the thoracic duct have also occurred, although the incidence is rare. These authors in their literature review cited an overall complication rate of ACDF as being between 0.45% and 19.6% based on published case series.51
| More common complications |
From Fountas KN, Zapsalaki EZ, Nikolakakos LG, et al: Anterior cervical discectomy and fusion associated complications. Spine 32(21), 2007.
Lumbar Discectomy
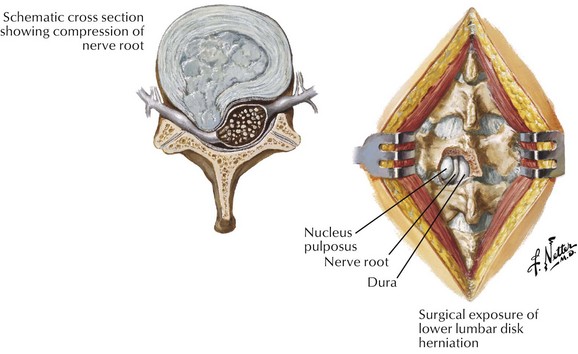
In the lumbar spine, lumbar discectomy is most often performed from a posterior approach (Fig. 10-5). Reports on the overall complication rate vary in the literature with reported values varying from 1.5% to 15.8%.52–56 The most commonly reported complications were dural tears or CSF leak, infection, acute urinary retention, and transient or permanent radiculopathy (Table 10-7). The most catastrophic reported complications are thankfully also among the most rare and involve injury to deep structures in the abdomen, particularly to major vessels and the bowel. With a reported incidence of 0.016% to 0.06%, the majority of intraabdominal injuries are vascular (most often involving the left common iliac artery) and can lead to acute decompensation in the perioperative period (with high mortality rate) or arteriovenous fistula formation.53,55 Interestingly, retroperitoneal hematoma from just such a major vascular injury has even been reported in a patient undergoing the minimally invasive percutaneous endoscopic approach to discectomy, and as such, an awareness of this potential complication is critical regardless of the surgical approach.57
| More common complications |
CSF, cerebrospinal fluid.
Data from Wu X, Zhuang S, Mao Z, Chen H: Microendoscopic discectomy for lumbar disc herniation. Spine 31(23), 2006; Raptis S, Quigley F, Barker S: Vascular complications of elective lower lumbar disc surgery. Aust N Z J Surg 64, 1994; Bell G: Implications of the spine patient outcomes research trial in the clinical management of lumber disk herniation. Cleve Clin J Med 74(8), 2007; Cases-Baldo MJ, Soria-Aledo V, Miguel-Perello JA, et al: Unnoticed small bowel perforation as a complication of lumbar discectomy. Spine J 11(1), 2011; Fallah A, Massicotte EM, Fehlings MG, et al: Admission and acute complication rate for outpatient lumbar microdiscectomy. Can J Neurol Sci 37(1), 2010.
Thoracic Discectomy
Symptomatic thoracic disc herniations are a relatively rare entity, far less common than those seen in the cervical and lumbar levels. This is thought to be related to the lesser mobility of the thoracic spinal segments given their articulation with the rib cage.58 The surgical approaches for these herniations can involve either an anterior approach (via thoracotomy or video-assisted thoracoscopic surgery) or posterior approach (costotransversectomy, minimally invasive discectomy) with the choice often made depending on the location of the herniation (either central or lateral respectively).59 When using the anterior approach, by nature of the surgical technique, the possible complications include those associated with all thoracic surgery (e.g., lung injury, injury to great vessels, chest tube complications). Posterior approaches can mean avoidance of intrathoracic injury but may necessitate large incisions or resection of ribs and can be associated with more manipulation of the spinal cord in order to achieve visualization of the disc material.58
Conclusion
 Obtaining a detailed history and physical examination of the patient and review of pertinent diagnostic studies and laboratory values to identify patient-specific factors that affect the risks of these interventions
Obtaining a detailed history and physical examination of the patient and review of pertinent diagnostic studies and laboratory values to identify patient-specific factors that affect the risks of these interventions A strong foundation in the anatomy of the planned procedural approach to the epidural space with recognition of the inherent interindividual variability in the location of critical structures
A strong foundation in the anatomy of the planned procedural approach to the epidural space with recognition of the inherent interindividual variability in the location of critical structures1 Martin BI, Turner JA, Mirza SK, et al. Trends in health care expenditures, utilization and health status among US Adults with spine problems, 1997-2006. Spine. 2009;34(19):2077-2084.
2 Friedly J, Chan L, Deyo R. Increases in lumbosacral injections in the Medicare population: 1994-2001. Spine. 2007;32(16):1754-1760.
3 Deyo RA, Gray DT, Kreuter W, et al. United States trends in lumbar fusion surgery for degenerative conditions. Spine. 2005;30:1441-1445.
4 Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical vs nonoperative treatment for lumbar disk herniation. JAMA. 2006;296(20):2441-2450.
5 Evans W. Intrasacral epidural injection in the treatment of sciatica. Lancet. 1930;216(5597):1225-1229.
6 Caron AB. Allergy to multiple local anesthetics. Allergy Asthma Proc. 2007;28(5):600-601.
7 Simon DL, Kunz RD, German JD, Zivkovich V. Allergic or pseudoallergic reaction following epidural steroid deposition and skin testing. Reg Anesth. 1989;14(5):253-255.
8 Tramer MR, von Elm E, Loubeyre P, Hauser C. Pharmacologic preventions of serious anaphylactic reactions due to iodinated contrast media: systematic review. BMJ. 2006;333(7570):675-680.
9 Rathmell JP. Atlas of image-guided intervention in regional anesthesia and pain medicine. Philadelphia: Lippincott Williams & Wilkins; 2006. pp 20-21
10 Scanlon GC, Moeller-Bertram T, Romanowsky SM, Wallace MS. Cervical transforaminal epidural injections: more dangerous than we think. Spine. 2007;32(11):1249-1256.
11 Houten JK, Errico TJ. Paraplegia after lumbosacral nerve root block: report of three cases. Spine J. 2002;2(1):70-75.
12 Huntoon MA. Anatomy of the cervical intervertebral foramina: vulnerable arteries and ischemic neurologic injuries after transforaminal epidural injections. Pain. 2005;117:104-111.
13 Lo D, Vallee JN, Spelle L, et al. Unusual origin of the artery of Adamkiewicz from the fourth lumbar artery. Neuroradiology. 2002;44(2):153-157.
14 Lyders EM, Morris PP. A case of spinal cord infarction following lumbar transforaminal epidural steroid injection: MR imaging and angiographic findings. Am J Neuroradiol. 2009;30(9):1691-1693.
15 Rozin L, Rozin R, Koehler SA, et al. Death during transforaminal epidural steroid nerve root block (C7) due to perforation of the left vertebral artery. Am J Forensic Med Pathol. 2003;24(4):351-355.
16 Benny B, Azari P, Briones D. Complications of cervical transforaminal epidural steroid injections. Am J Phys Med Rehabil. 2010;89(7):601-607.
17 Brouwers PJAM, Kottnik EJBL, Simon MAM, Prevo RL. A cervical anterior spinal artery syndrome after diagnostic blockade of the right C6-nerve root. Pain. 2001;91:397-399.
18 Ludwig MA, Burns SP. Spinal cord infarction following cervical transforaminal epidural injection: a case report. Spine. 2005;30(10):E366-E368.
19 Windsor R, Overton A, Sugar R. Cervical transforaminal injection three case reports detailing complications, a review of the literature and a suggested technique. Pain Physician. 2003;6(4):457-465.
20 Windsor RE, Storm S, Sugar R. Prevention and management of complications from common spinal injections. Pain Physician. 2003;6:473-483.
21 Smuck M, Chiodo A, Tong H, et al. Accuracy of intermittent fluoroscopy to detect intravascular injection during transforaminal epidural injections. Arch Phys Med Rehabil. 2006;87:e38.
22 Baker R, Dreyfuss P, Mercer S, Bogduk N. Cervical transforaminal injection of corticosteroids into a radicular artery: a possible mechanism for spinal cord injury. Pain. 2003;103:211-215.
23 Smuck M, Fuller BJ, Yoder B, Huerta J. Incidence of simultaneous epidural and vascular injection during lumbosacral transforaminal epidural injections. Spine J. 2007;7(1):79-82.
24 Tiso RL, Cutler T, Catania JA, Whalen K. Adverse central nervous system sequelae after selective transforaminal block: the role of corticosteroids. Spine J. 2004;4(4):468-474.
25 Horlocker T, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Third Edition). Reg Anesth Pain Med. 2010;35(1):64-101.
26 Baek BS, Hur JW, Kwon KY, Lee HK. Spontaneous spinal epidural hematoma. J Korean Neurosurg Soc. 2008;44:40-42.
27 Xu R, Bydon M, Gokasian ZL, et al. Epidural steroid injection resulting in epidural hematoma in a patient despite strict adherence to anticoagulation guidelines. J Neurosurg Spine. 2009;11(3):358-364.
28 Vandermeulen EP, Van Aken H, Mermylen J. Anticoagulants and spinal-epidural anesthesia. Anesth Analg. 1994;79(6):1165-1177.
29 Stoll A, Sanchez M. Epidural hematoma after epidural block: implications for its use in pain management. Surg Neurol. 2002;57(4):235-240.
30 Ain RJ, Vance MB. Epidural hematoma after epidural steroid injection in a patient withholding enoxaparin per guidelines. Anesthesiology. 2005;102(3):701-703.
31 Yoo HS, Park SW, Han JH, et al. Paraplegia caused by an epidural hematoma in a patient with unrecognized chronic idiopathic thrombocytopenic purpura following an epidural steroid injection. Spine. 2009;34(10):E376-E379.
32 Ptaszynski AE, Hooten WM, Huntoon MA. The incidence of spontaneous epidural abscess in Olmsted County from 1990-2000: a rare cause of spinal pain. Pain Med. 2007;8:338-343.
33 Hooten WM, Kinney MO, Huntoon MA. Epidural abscess and meningitis after epidural corticosteroid injection. Mayo Clin Proc. 2004;79(5):682-686.
34 Knight JW, Cordingley JJ, Palazzo MG. Epidural abscess following epidural steroid and local anaesthetic injection. Anaesthesia. 1997;52(6):576-578.
35 Baer ET. Post-dural puncture bacterial meningitis. Anesthesiology. 2006;105(2):381-393.
36 Saigal G, Donovan Post MJ, Kozic D. Thoracic intradural Aspergillus abscess formation following epidural steroid injection. Am J Neuroradiol. 2004;25(4):642-644.
37 Reihsaus E, Waldbaur H, Seeling W. Spinal epidural abscess: a meta-analysis of 915 patients. Neurosurg Rev. 2000;23(4):175-204.
38 Gaul C, Neundorfer B, Winterholler M. Iatrogenic (para)spinal abscesses and meningitis following injection therapy for low back pain. Pain. 2005;116:407-410.
39 Simopoulos TT, Kraemer JJ, Glazer P, Bajwa ZH. Vertebral osteomyelitis: a potentially catastrophic outcome after lumbar epidural steroid injection. Pain Physician. 2008;11(5):693-697.
40 Hooten WM, Mizerak A, Carns PE, Huntoon MA. Discitis after lumbar epidural corticosteroid injection: a case report and analysis of the case report literature. Pain Med. 2006;7(1):46-51.
41 Goodman BS, Posecion LW, Mallempati S, Bayazitoglu M. Complications and pitfalls of lumbar interlaminar and transforaminal epidural injections. Curr Rev Musculoskelet Med. 2008;1(3-4):212-222.
42 Hodges SD, Castleberg RL, Miller T, et al. Cervical epidural steroid injection with intrinsic spinal cord damage. Spine. 1998;23:2137-2142.
43 Lee JH, Lee JK, Seo BR, et al. Spinal cord injury produced by direct damage during cervical transforaminal epidural injection. Reg Anesth Pain Med. 2008;33(4):377-379.
44 Tripathi M, Nath SS, Gupta RK. Paraplegia after intracord injection during attempted epidural steroid injection in an awake patient. Anesth Analg. 2005;101:1209-1211.
45 Khan S, Pioro EP. Cervical epidural injections complicated by syrinx formation: a case report. Spine. 2010;35(13):E614-E616.
46 Field J, Rathmell JP, Stephenson JH, Katz NP. Neuropathic pain following cervical epidural steroid injection. Anesthesiology. 2000;93:885-888.
47 Lirk P, Kolbitsch C, Putz G, et al. Cervical and high thoracic ligamentum flavum frequently fails to fuse in midline. Anesthesiology. 2003;99(6):1387-1390.
48 Trentman TL, Rosenfeld DM, Seamans DP, et al. Vasovagal reactions and other complications of cervical vs lumbar translaminar epidural steroid injections. Pain Pract. 2009;9(1):59-64.
49 Abbasi A, Malhotra G, Malanga G, et al. Complications of interlaminar cervical epidural steroid injections: a review of the literature. Spine. 2007;32(19):2144-2151.
50 Angevine PD, Arons RR, McCormick PC. National and regional rates and variation of cervical discectomy with and without anterior fusion, 1990-1999. Spine. 2003;28:931-940.
51 Fountas KN, Zapsalaki EZ, Nikolakakos LG, et al. Anterior cervical discectomy and fusion associated complications. Spine. 2007;32(21):2310-2317.
52 Wu X, Zhuang S, Mao Z, Chen H. Microendoscopic discectomy for lumbar disc herniation. Spine. 2006;31(23):2689-2694.
53 Raptis S, Quigley F, Barker S. Vascular complications of elective lower lumbar disc surgery. Aust N Z J Surg. 1994;64:216-219.
54 Bell G. Implications of the spine patient outcomes research trial in the clinical management of lumber disk herniation. Cleve Clin J Med. 2007;74(8):572-576.
55 Cases-Baldo MJ, Soria-Aledo V, Miguel-Perello JA, et al. Unnoticed small bowel perforation as a complication of lumbar discectomy. Spine J. 2011;11(1):e5-e8.
56 Fallah A, Massicotte EM, Fehlings MG, et al. Admission and acute complication rate for outpatient lumbar microdiscectomy. Can J Neurol Sci. 2010;37(1):49-53.
57 Ahn Y, Kim JU, Lee BH, et al. Postoperative retroperitoneal hematoma following transforaminal percutaneous endoscopic lumbar discectomy. J Neurosurg Spine. 2009;10(6):595-602.
58 Sheikh H, Samartzis P, Perez-Cruet MJ. Techniques for the operative management of thoracic disc herniation: minimally invasive thoracic microdiscectomy. Orthop Clin North Am. 2007;38:351-361.
59 Canale ST, Beaty JH. Campbell’s operative orthopedics, ed 11. Philadelphia: Mosby Elsevier; 2008. pp 2195-2199