Chapter 1 Complications of Spinal Cord Stimulation
 Appropriate Accreditation Council for Graduate Medical Education mentored subspecialty training in interventional pain management is vital to ensure patient-centered care.
Appropriate Accreditation Council for Graduate Medical Education mentored subspecialty training in interventional pain management is vital to ensure patient-centered care. Accurate and expertly placed needles in appropriately selected patients are essential to ensure accurate diagnosis and successful treatment.
Accurate and expertly placed needles in appropriately selected patients are essential to ensure accurate diagnosis and successful treatment. Complications associated with spinal cord stimulation (SCS) rarely impact patient morbidity or mortality.
Complications associated with spinal cord stimulation (SCS) rarely impact patient morbidity or mortality. Approximately 30% to 40% of patients undergoing SCS will have a complication, and nearly 80% of them require a revision.
Approximately 30% to 40% of patients undergoing SCS will have a complication, and nearly 80% of them require a revision. Biologic complications commonly occur within 3 months of implant, although infections can rarely present much later. Technical complications typically occur within 2 years of implant.
Biologic complications commonly occur within 3 months of implant, although infections can rarely present much later. Technical complications typically occur within 2 years of implant.Introduction
Neurosurgical treatments for pain have historically fallen under the headings of anatomic, ablative, or augmentative, and before the gate control theory was proposed by Melzack and Wall,1 little attention was directed toward augmentative therapies. Spinal cord stimulation (SCS) has become one of the most useful interventional therapies to treat neuropathic and ischemic pain since its introduction as an intrathecal device by Shealy et al2 in 1967.
Current estimates suggest that more than 27,000 spinal cord stimulator implants were performed in 2007 alone in the United States,3 and this number will likely increase as SCS is used earlier in treatment algorithms for neuropathic pain,4 supported by cost effectiveness and successful outcomes.5
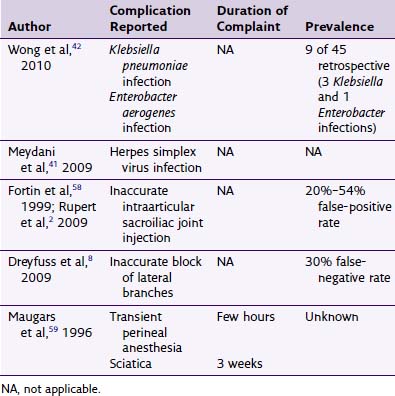
One can appreciate that increased use of the technique also increases the incidence and prevalence of associated complications, which range from the mundane to the devastating. Recent reviews place the overall incidence of complications associated with spinal cord stimulation to be approximately between 14% and 43%.6 Cameron7 performed an extensive review of the literature extending over 3679 patients, concluding an overall mean complication rate with SCS of 36%. Moreover, Kumar et al8 reported that complications requiring revisions were approximately 25% to 33% and approximately 85% of reoperation patients were satisfied with the results.9 Furthermore, Rosenow et al10 retrospectively determined that in 289 patients who underwent spinal cord stimulator implants, 46% required revision, and of those, almost half required more than one.
As with any treatment plan, complication avoidance begins with patient selection. Patients with local infection near the injection site, coagulopathy, allergy to injectate, or comorbidities or conditions that prevent fluoroscopic needle guidance or consent should be avoided. Patient selection regarding psychometric testing deserves special mention. It is well established that concurrent psychiatric illness reduces interventional treatment success rates11 and that approximately 20% to 45% of pain patients have accompanying psychopathology.12 Therefore it is essential that appropriate measures be taken to diagnose, treat, or exclude unsuitable candidates. Instruments described to aid in identifying the presence of clinically significant psychopathology include the Symptom Checklist 90 (SCL-90-R) and the Minnesota Multiphasic Personality Inventory (MMPI-2). Poor treatment outcome was identified in patients with presurgical somatization, depression, anxiety, and poor coping.13
It is also crucial to appropriately use the spinal cord stimulator for the appropriate indications (neuropathic pain states and ischemic pain), as approved by the Food and Drug Administration. Disease states that yield themselves to successful treatment with SCS include postherpetic neuralgia, intercostal neuralgia, postlaminectomy pain, complex regional pain syndrome, phantom limb pain, angina, painful peripheral neuropathy, focal peripheral neuropathies, ischemic limb pain, and radiculitis and radiculopathy.14
A clear understanding of spinal anatomy, utilization of image guidance, and proper surgical technique are vital to ensure both quality treatment and reduced patient morbidity and mortality.15 Furthermore, it should be understood that appropriate training within Accreditation Council for Graduate Medical Education accredited programs and mentorship is pivotal to ensure treatment success and limit iatrogenic morbidity; interventional hobbyists and weekend crusaders only serve to undermine the accessibility of this valuable therapy to patients.
Spinal cord stimulator complications can be broadly divided into those that are technical or those that are biologic or surgical in nature. The consequences of these complications can range from the mundane to the devastating. Most complications of SCS do not impact patient mortality,7 unlike that for intrathecal drug delivery, as recently reported by Coffey et al.16 Although the range of various complications varies from 0% to 81% across studies,17 the frequencies of the complications in the tables in this chapter are presented as the means. Refer to Table 1-1 for reported rates of complications, revisions, and explants associated with SCS.
| Complication | Frequency |
|---|---|
| Overall | 14%–43%6,17 |
| Complication requiring revision | 25%–33%,8 23%17 |
| Complication requiring explant | 11%17 |
Selected Complications
Technical Complications
Innately, the restrictions current technology places on the spinal cord stimulator device plays a role in the associated complications. Advances in system technology have largely reduced many early complications related to device failure, including loss of stimulation. Van Buyten et al18 described such a relationship, commenting in a 5-year prospective study of 84 patients with 85 spinal cord stimulator systems, with overall 54% of patients reporting greater than 50% pain relief at 5 years. Satisfaction and employment of the patient programmer were at least 95% after stimulator therapy began (Table 1-2).
Table 1-2 Technical Complications Reported for Spinal Cord Stimulation
| Complication | Frequency |
|---|---|
| Lead migration (loss of stimulation) requiring revision | 11%,21 13.2%22 |
| Lead integrity violation requiring revision | 6%,21 9%22 |
| Loose connection | 0.4%7 |
| Hardware malfunction, equipment failure | 2.9%,7 6.5%17 |
| Battery failure | 1.6%7 |
| Central nervous system electrical injury from aberrant stimulator activation | Case report25 |
| Misplacement of epidural lead | Case report24 |
Furthermore, stimulation, regardless of the choice of the numerous mechanisms proposed,14 depends on the integrity and characteristics of the “circuit created.” Spinal cord stimulator contact to target structure length varies with patient position, as described by Abejon and Feler,19 and predictively, so too do the circuit created and the resultant therapeutic stimulation. Recumbence reduced impedance and energy requirement (measured in thoracic and cervical implanted leads), and therefore stimulation was perceived to be “stronger,” although the difference was not statistically significant. Clinical implications longitudinally require further study.
Lead Migration
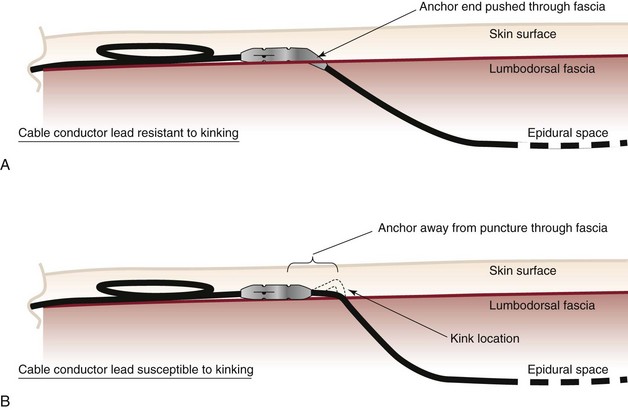
The most common complication of SCS is lead migration7,14,17,20 and loss of therapeutic stimulation. Cameron et al7 reported that 27.2% of spinal cord stimulator cases were technical in nature, and of those, 87% were related to the lead. Estimates place migration incidence to be between 11% and 13%, with a greater propensity for migration in the cervical spine,8 likely related to greater mobility, compared with the lumbar spine.
Understandably, migration occurs when directional forces and tensile load on the electrode exceed the stabilizing forces of the anchor. These forces depend on lead-spanning mobile areas, trajectory into the epidural space, anchor orientation, the type of tissue the anchor is sutured to, suture technique, and the site of battery placement. It has been suggested that percutaneous electrodes are more prone to migration than their laminectomy counterparts and that battery location in the abdomen may be associated with less lead migration than in the gluteal region, however work by Henderson and Kumar suggest otherwise. Those studies have suggested that the closer the implanted pulse generator (IPG) is to the lead insertion site, the lower the risk of lead movement. IPG placement choices include the buttocks, midflank, abdominal wall, or infraclavicular regions.7 Rosenow et al,10 however, in a study of 289 patients, reported that migration was slightly higher in the surgical laminectomy lead.
Mitigating migration involves reducing iatrogenic cofounders. These include the angle of spinal cord lead entry into the epidural space, stress loops, battery site choice, and anchor or suturing technique. Bedder and Bedder15 detail basic surgical technique and nomenclature that will not be further discussed here. Kumar et al8 suggest that proper anchoring and placement of the tip of the anchor into the supraspinal ligament might reduce anchor failure. Strain relief loops of approximately 1 inch in diameter, both near the anchor and at the generator site, may reduce tensile load (Fig. 1-1).
Lead Fracture
Lead fracture occurs approximately 5% to 9%,21–23 with contradictory reports regarding fracture rates with percutaneous versus laminectomy leads. Kumar et al23 described percutaneous lead fracture more often than laminectomy leads; in contradiction, Rosenow et al10 reported more lead fracture with laminectomy. Clearly, lead fracture depends on lead tensile strength, the friction coefficient of the internal wires composing the lead, and a vector of force that challenges the lead integrity. Iatrogenic ways to lessen lead fracture are similar to strategies used to reduce lead migration and include appropriate anchoring, using stress loops, placement of the IPD in the abdomen,8 and avoidance of tunneling around mobile structures (i.e., joints).
Hardware Malfunction and Battery Failure
Exclusive to lead migration of fracture, other hardware complications occur with a frequency of 2.9% and include battery failure (exclusive to predicted nonrechargeable battery depletion) with a frequency 1.6% per Cameron et al.7 Turner et al17 described equipment failure rates of 6.5%.
Misplacement of Epidural Lead
Therapeutic stimulation requires epidural placement of the spinal cord stimulator lead. Intrathecal and subdural placement have been reported.24 Troubleshooting of lead placement centers on fluoroscopic guidance and impedance testing. Anterior epidural lead placement is suggested by muscle contraction of adjacent or distal spinal nerves and painful stimulation within the appropriate expected stimulation parameters. Intrathecal lead placement is suggested by very low-impedance testing, and the hallmark of subdural stimulation is segmental, low-amplitude stimulation that is often uncomfortable to the patient. A case report of intramedullary lead placement is described in the next section.
Aberrant Stimulation
Aberrant activation of stimulation rarely occurs, although an infamous case report is often sited. Eisenberg and Waisbrod25 described a patient who had a cervical spinal cord stimulator that was activated by an antitheft device. Six months after implantation, upon entering a store, the patient felt an electrical shock in the back of his skull and fell unconscious. He awoke in the emergency department with confusion, ataxia, upper extremity intention tremor, weakness, and dysarthric speech. Initial computed tomography (CT) scan and electroencephalogram were normal, although 6 months later, with continued symptoms, follow-up CT demonstrated a left basal ganglia infarct. The authors postulate that the anti-theft device triggered a sudden bolus of current, causing damage that contributed to the injury.
Kumar et al8 reported that whereas device or technical complications typically occur within 2 years of implant, biologic complications of SCS typically occur within 3 months.
Biologic Complications
Biologic complications typically present themselves within 3 months of implant and usually do not cause significant untoward sequelae. Historically, dorsal column stimulation was performed in the subarachnoid space instead of the epidural space, and more devastating complications were reported, including paraplegia and death. As the technology advanced and the procedure evolved, it is well understood that serious biologic consequences are a rarity and usually do not have a significant impact on patient morbidity and mortality.7 However, devastating complications have been reported more recently, warranting attention.8,26–28 The most common biologic complications reported include pain over the device, superficial infection, seroma formation, and dural puncture or leak with frequencies of 5.8%, 5%, 2.5%, and 2%, respectively (Table 1-3).14,17,21,29 Less frequent complications are discussed in turn.
Table 1-3 Reported Biologic Complications of Spinal Cord Stimulation
| Complication | Frequency |
|---|---|
| Infection | 5%,21 3.4%22 |
| Deep infection | 0.1%17 |
| Contact dermatitis | Case report46 |
| Epidural fibrosis | 30 |
| Aseptic meningitis | Case reports7 |
| Allergy | Case report47 |
| Gastrointestinal side effects | Case reports52,53 |
| Renal failure | Case report49 |
| Headache | Case report45 |
| Epidural hematoma | 0.3%,8 case report43 |
| Subdural hematoma | Case report26 |
| Epidural abscess | Case report27 |
| Dural puncture or CSF leak | 2%,14 0.3%8 |
| Seroma | 2.5%29 |
| Pain over device | 0.9%8, 5.8%17 |
| Skin erosion | 0.2%8 |
| Foreign body reaction | Case report48 |
| Micturition inhibition or urologic | Case reports50,51 |
| Quadriparesis after mechanical spinal cord injury | Case report28 |
| Insulin sensitivity | Unpublished Cleveland Clinic Report |
Pain Over the Device
Pain overlying the spinal cord stimulator device components ranges from 0.9% to 5.8%.8,17 Pain has been reported overlying the IPG and the epidural entry site (may be related to anchors between the spinous processes). Treatment can involve topical or injection therapy, although anecdotally, it typically requires revision. Mitigation of device site pain includes avoidance of placement around friction-generating sites; ensuring adequate distance from osteal structures; and most importantly, a clear consensus with the patient regarding IPG placement preoperatively.
Infection
The risk of infection is always a concern when undergoing surgery and even more so when implanting a foreign body. Infections have been reported years after implantation,30 and a high index of clinical suspicion is necessary to make the diagnosis because signs and symptoms are often subtle and vague. Risk factors associated with infections of implanted hardware include immunosuppressive therapy (including steroids), diabetes, rheumatoid arthritis, tobacco, alcohol use, malnutrition, obesity, prolonged hospital stay, multiple surgeries, or perioperative transfusion.15,31 Additional risk factors implicated perioperatively include inappropriate or inadequate timing of preoperative antibiotic therapy, shaving of the operative site (versus clipping), improper surgical site skin preparation, inadequate preoperative hand scrub, and lack of ventilation in the operative suite.15
Superficial and Deep Infections
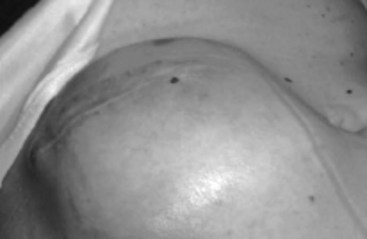
Superficial infections are the most common infectious complication associated with SCS, occurring with a frequency of approximately 5%, with coagulase-negative Staphylococcus spp., Staphylococcus aureus, and Enterococcus spp. being commonly implicated in decreasing order of occurrence.15 Deep infections occur less frequently, and intraspinal infections, including epidural abscesses, are very infrequent and are also caused primarily by S. aureus, although gram-negative, anaerobic, mycobacteria, and fungi have been described.32,33 Torrens et al31 describe a case of methicillin-resistant S. aureus (MRSA) meningitis diagnosed on postoperative day 8 after a superficial infection despite an enteral antibiotic course and removal of the lead (Fig. 1-2).
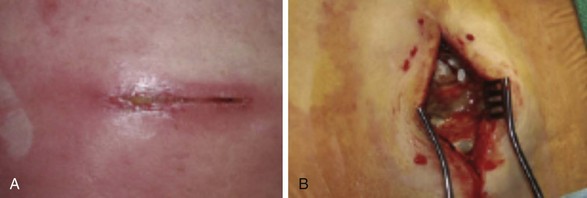
Fig. 1-2 Superficial (A) and deep (B) infection.
(From Deer TR, Stewart CD: Complications of spinal cord stimulation: identification, treatment and prevention, Pain Med 9(suppl):S93-S101, 2008.)
Iatrogenic sources of superficial infections can be reduced by proper patient preparation and operative technique and were reviewed by Bedder and Bedder.15 Antimicrobial prophylaxis should occur within 30 minutes of skin incision and cover the most commonly implicated organisms. First-generation cephalosporins are frequently used (including cefazolin) to cover gram-positive bacteria. Fourth-generation cephalosporins (cefepime) broaden coverage to gram-negative bacteria while maintaining gram-positive coverage, providing treatment for Enterobacter and Pseudomonas spp. Cephalosporins do not cover against enterococci. Clindamycin is an option for patients who require antimicrobial prophylaxis with a severe penicillin allergy. The decision to provide postoperative prophylactic antibiotics continues to be a controversial topic. Many practitioners continue to do so despite only anecdotal evidence.34 Furthermore, unnecessary antibiotic use increases microbial resistance and health care costs.
Surgical preparation includes optimizing and mitigating patient comorbidities that increase infectious risk, ensuring proper scrub, and promoting a sterile and accessible operative field. Hair removal should be performed immediately before the procedure and should including clipping because shaving increases the chance of infection.35 Skin preparation is usually accomplished by chlorhexidine gluconate, povidone–iodine, or alcohol-based solutions. Each deserves special mention. Povidone–iodine preparations need to have sufficient time to be thoroughly dry to maximize their antimicrobial effects. Alcohol is extremely bacteriocidal, but its use may be limited by flammability. Chlorhexidine has a longer duration of action and is more bacteriocidal than iodine preparations.36
Epidural Abscess
Epidural abscess occur spontaneously with a reported incidence of 0.2 to 1.2 per 10,000 hospital admissions.37 Meglio et al27 reported a case of paraplegia after discovery of an epidural and intradural abscess of a patient with a recently explanted spinal cord stimulator. Early diagnosis is paramount to reduce morbidity and mortality.38 Progressive clinical phases have been described for epidural infection leading to epidural abscess, described by Van Zundert.39 Phase I involves backache and local tenderness; phase II is associated with radicular pain, fever, neck stiffness, and rigidity and commonly occurs 2 to 3 days later; phase III presents with sensory, motor, or reflex depression (often 3 to 4 days later); and phase IV is associated with paralysis.39
About 80% to 90% of epidural abscesses are correctly diagnosed using MRI with gadolinium contrast.37 When neurologic deterioration occurs, decompression via laminectomy is warranted and should be used within 36 hours to limit morbidity, although neurologic sequelae can still be significant.40 Intravenous bacteriocidal antibiotics should be started as early as possible and continued for 4 weeks, with enteral or parenteral therapy continued for at least 8 to 12 weeks.41 Therapy should be modified according to speciation and an antibiotic resistance profile, if isolation is achievable.
Seroma
A seroma is a collection of serosanguineous fluid that may occur secondary to friction forces of two tissue planes and excessive surgical trauma (Fig. 1-3).29 Iatrogenic mitigating factors include limiting aggressive blunt dissection, reducing excessive cautery, achieving careful hemostasis, and performing a layered closure to limit dead space. If a seroma forms, treatment includes abdominal binder and drainage, although care must be taken not to introduce infection.
Neuraxial Hematoma
The incidence of epidural hematomas is largely unknown, although it has been estimated to be near one in 150,000 epidurals and one in 222,000 spinal anesthetics.42 Franzini et al43 described a large epidural hematoma after implantation of a laminectomy lead with an immediate postoperative presentation of progressive lower extremity weakness. The authors suggested postoperative vigilance in patients with increased risk. Independent risk factors for epidural hematoma after spinal surgery include male gender (4 : 1) and patients in the fifth or sixth decade of life.
Subdural hematomas have been described as a complication of SCS. Chiravuri et al26 published a case of a subdural hematoma temporally associated with stimulator placement but complicated by a preoperative fall with associated head trauma.
Although a review of the American Society of Regional Anesthesia 2010 guidelines for neuraxial interventions in patients receiving anticoagulation therapy is beyond the scope of this chapter, it is vital that readers use the guidelines.42 Treatment is contingent on hematoma evacuation within 8 hours of neurologic deficit.44
Quadriparesis
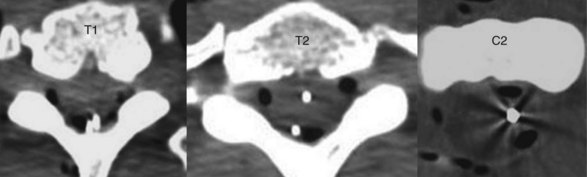
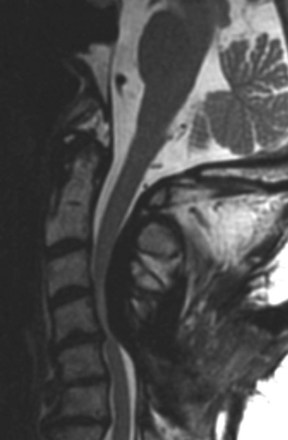
Meyer et al28 report a case describing quadriplegia after inadvertent intramedullary spinal cord stimulator lead placement in a patient under general anesthesia. This unfortunate incident occurred in a 25-year-old woman with chronic regional pain syndrome of the left upper extremity who had an existing stimulator that improved her pain and function but required revision of the IPG for “technical reasons.” The patient was placed under general anesthesia for the procedure. Intraoperatively, it was discovered that her percutaneous leads also needed revision. The existing leads were removed, and new percutaneous leads were introduced via the “standard closed percutaneous technique at T2-3 interspace.” Immediately after surgery, the patient had severe neurologic compromise. Postoperative CT demonstrated a lead within the spinal cord at the tip of C2 (Fig. 1-4).
Dural Puncture
Dural puncture, unfortunately, remains a relatively common complication of SCS30, with estimates centering around 2%. Furthermore, postdural puncture headache (PDPH) after 16- to 18-gauge needle entry is estimated to be as high as 86%.24 Risk factors include previous surgery at needle entry, calcified ligamentum flavum, and an uncooperative patient.30 Very commonly, conservative measures fail to treat the headache, and an epidural blood patch is required, with a success rate of approximately 60% to 70%. Very commonly, when accidental dural puncture occurs, a prophylactic blood patch is placed. The volume of autologous blood depends on the puncture site, but often at least 20 mL or patient discomfort guides the necessary amount. The trial can continue with a more cephalad interspace used for epidural needle placement and entry.30
Headache
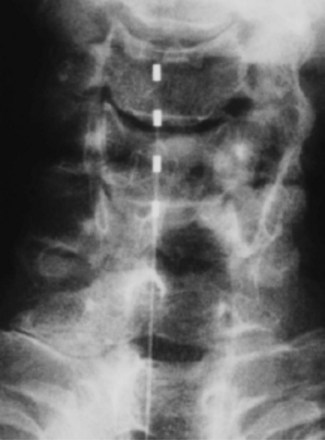
Exclusive to PDPH, SCS has been implicated in headache. Ward and Levin45 described persistent headaches subsequent to placement of a percutaneous cervical spinal cord stimulator at C3 that responded to ergotamine and sumatriptan and resolved with lead repositioning. The authors contend the possibility of migraine (Fig. 1-5).
Immunologic Reactions
Contact dermatitis, allergy, and foreign body reactions have reported as case reports after SCS.46–48 McKenna and McCleane46 reported a patient with a skin reaction overlying the IPG site 1 month after stimulator placement. The authors describe the reaction as an isomorphic response to the expansion of underlying tissues rather than a nickel allergy, however, despite a prior atopy and patch testing suggesting a nickel allergy.
Ochani et al47 described a case of suspected allergic reaction 6 weeks after cervical and lumbar placement for chronic regional pain syndrome after left sympathectomy for atypical face pain after facial trauma. The patient presented 2 weeks after implantation with a burning sensation adjacent to implanted leads and tenderness overlying the generator pocket. Infectious workup was negative. Two weeks later, then patient again presented with generalized hives and edema, which responded to steroid therapy. Patch dermatologic allergy testing revealed sensitization to platinum, silicone, and polyurethane. The stimulator was removed, resolving the complaints.
Lennarson and Guillen48 reported a foreign body reaction to the lead after explant, resulting in symptomatic compression of the spinal canal, necessitating multilevel cervical laminectomy, excision of the mass, and fusion. The diagnosis is clouded, however, because a superficial MRSA infection was discovered at the neck incision, although deep culture results were negative. Histiopathic analysis of the mass excised revealed a giant cell reaction (Fig. 1-6).
Epidural Fibrosis
Epidural fibrosis has also been described as a complication of SCS, although it is inherent to its placement. Scarring begins within 2 weeks of electrode placement and continues throughout the lifetime of the device. The fibrosis may alter the circuit created to produce stimulation, governed by Ohms law, resulting in failed or loss of therapeutic stimulation.30 This phenomenon was described by Kumar et al23 as system tolerance despite continued coverage of the painful area and an intact system.
Urologic Complications
Renal failure, micturition inhibition, and other urologic complications have been reported.49–51 Larkin et al49 reported a case of renal failure temporally associated with placement of a spinal cord stimulator. The patient was anuric and hypotensive, and serum blood urea nitrogen and creatine were 83 mg/dL and 8.1 mg/dL, respectively. The authors suggested the sympathectomy from the SCS might have compromised renal perfusion.
Loubser et al51 described a case report of a patient with partial T12–L1 spinal cord injury and subsequent SCS to treat his neuropathic or central pain. The patient required large amplitudes to produce the desired paresthesias, and at these therapeutic amplitudes, urethral sphincter spasm occurred, preventing self-catheterization. Even after the spinal cord system was explanted, the patient continued to have urinary retention and associated infections attributed to the device. The author suggested routine urologic functional testing to be incorporated during the trial period.
More recently, micturition inhibition complicating SCS was reported by Grua and Michelagnoli.50 The patient had cauda equina syndrome from an angioma at T12. After failing conservative therapy, he underwent percutaneous spinal cord stimulator with cephalad end of the lead left of center at T12. The patient reported complete resolution of his pain but urge incontinence. Only after 30 days from the interrupted trial, the patient had complete return of bladder function. The stimulation was again initiated with immediate reappearance of the bladder dysfunction. The decision was made to explant. The authors postulated the autonomic effects of stimulation may have imbalanced the signals, altering urinary function.
Gastrointestinal Complications
Sporadic case reports of gastrointestinal complications associated with SCS have been described.52,53 Thakkar et al52 described two cases of patients with SCS who experienced symptoms exacerbated by stimulation, namely nausea, diarrhea, worsened gastrointestinal reflux, and flatulence; these were hypothesized to have occurred because of the associated sympathectomy and unopposed parasympathetic gastrointestinal effects.
Kemler et al53 described a case of relapsing colitis caused by SCS. The patient had a spinal cord stimulator system placed with the lead placed at C4 and the IPG placed left lower abdominal quadrant for upper extremity chronic regional pain syndrome. The patient’s ulcerative colitis relapsed and remitted twice with using and discontinuing the device. The authors postulated that there may have been an electromagnetic effect on the colon, aberrancy of the GABAergic system, or an electrical effect on intestinal circulation.
1 Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971-979.
2 Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. Anesth Analg. 1967;46(4):489-491.
3 Prager J Estimates of annual spinal cord stimulator implant rises in the United States. Neuromodulation. 2010;(13):68-69
4 Simpson BA. The role of neurostimulation: the neurosurgical perspective. J Pain Symptom Manage. 2006;4(suppl):S3-S5.
5 North RB, Shipley J, Taylor RS. The cost effectiveness of spinal cord stimulation. In: Neuromodulation. St. Louis: Elsevier; 2009:355-376.
6 Eldrige JS, Weingarten TN, Rho RH. Management of cerebral spinal fluid leak complicating spinal cord stimulator implantation. Pain Practice. 2006;6(4):285-288.
7 Cameron T. Safety and efficacy of SCS for the treatment of chronic pain: a 20-year literature review. J Neurosurg. 2004;100:254-267.
8 Kumar K, Buchser E, Linderoth B, et al. Avoiding complications from spinal cord stimulation: practical management recommendations an international panel of experts. Neuromodulation. 2007;10:24-33.
9 North RB, Kidd DH, Farrokhi F, Piantadosi SA. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery. 2005;56:98-107.
10 Rosenow JM, Stanton-Hicks M, Rezai AR, Henderson JM. Failure modes of spinal cord stimulation hardware. J Neurosurg Spine. 2006;5:183-190.
11 Fishbain D, Goldberg M, Meagher BR, et al. Male and female chronic pain patients characterized by DSMIII psychiatric diagnostic criteria. Pain. 1986;26:181-197.
12 Gallagher R. Primary care and pain medicine: a community solution to the public health problem of chronic pain. Med Clin North Am. 1999;83:555-583.
13 Celstin J, Edwards RR, Jamison RN. Pretreatment psychosocial variables as predictors of outcomes following lumbar surgery and spinal cord stimulation: a systematic review and literature synthesis. Pain Med. 2009;10(4):639-653.
14 Pinzon EG. Spinal cord stimulation. Pract Pain Med. 2005:69-75. May/Jun
15 Bedder MD, Bedder HF. Spinal cord stimulation surgical technique for the nonsurgically trained. Neuromodulation. 2009;12:1-19.
16 Coffey RJ, Woens ML, Broste SK. Medical practice perspective: identification and mitigation of risk factors for mortality associated with intrathecal opioids for non-cancer pain. Pain Med. 2010;11:1001-1009.
17 Turner JA, Loeser JD, Deyo RA, Sanders SB. Spinal cord stimulation for with failed back surgery syndrome or complex regional pain syndrome: a systematic review of effectiveness and complications. Pain. 2004;108:137-147.
18 Van Buyten JP. The performance and safety if an implantable spinal cord stimulation system in patients with chronic pain: a 5 year study. Neuromodulation. 2003;6:79-87.
19 Abejon D, Feler C. Is impedance a parameter to be taken into account in spinal cord stimulation? Pain Physician. 2007;10(4):533-554.
20 Devulder J, Vermeulen H, De Colvenaer L, et al. Spinal cord stimulation in chronic pain: evaluation of results, complications, and technical considerations in sixty-nine patients. Clin J Pain. 1991;7:21-28.
21 Sarubbi F, Vasquez J. Spinal epidural abscess associated with the use of temporary epidural catheters: report of two cases and review. Clin Infect Dis. 1997;25:1155-1158.
22 Kemler MA, De Vet HCW, Barendse GAM, et al. The effect of spinal cord stimulation in patients with chronic reflex sympathetic dystrophy: two years follow-up of the randomized controlled trial. Ann Neurol. 2004;55:13-18.
23 Kumar K, Wilson JR, Taylor RS, Gupta S. Complications of spinal cord stimulation, suggestions to improve outcome, and financial impact. J Neurosurg Spine. 2006;5:191-203.
24 Pope JE, Stanton-Hicks M. Accidental subdural spinal cord stimulator lead placement and stimulation. Neuromodulation. 2010;14(1):30-33.
25 Eisenberg E, Waisbrod H. Spinal cord stimulator activation by an antitheft device. J Neurosurg. 1997;87:961-962.
26 Chiravuri S, Wasserman R, Chawla A, Haider N. Subdural hematoma following spinal cord stimulator implant. Pain Physician. 2008;11:97-101.
27 Meglio M, Cioni B, Rossi GF. Spinal cord stimulation in management of chronic pain. A 9-year experience. J Neurosurg. 1989;70:519-524.
28 Meyer SC, Swartz K, Johnson JO Quadriparesis and spinal cord stimulation: case report. Spine. 2007;(32):E565-E5658
29 Beer GM, Wallner H. Prevention of seroma after abdominoplasty. Aesthet Surg J. 2010;30(3):414-417.
30 Deer TR, Stewart CD. Complications of spinal cord stimulation: identification, treatment and prevention. Pain Med. 2008;9(suppl):S93-S101.
31 Torrens K, Stanely PJ, Ragunathan PL, Bush DJ. Risk of infection with electrical spinal cord stimulation. Lancet. 1997;349:729.
32 Ergan M, Macro M, Benhamou CL, et al. Septic arthritis of lumbar facet joints: a review of six cases. Rev Rhum Engl Ed. 1997;64:386-395.
33 Bruma OJ, Craane H, Kunst MW. Vertebral osteomyelitis and epidural abscess due to mucormycosis, a case report. Clin Neurol Neurosurg. 1979;81(1):39-44.
34 McDonald M, Grabsch E, Marshall C, Forbes A. Single versus multiple dose antimicrobial prophylaxis for major surgery: a systematic review. Aust N Z J Surg. 1999;68:388-396.
35 Alexander JW, Fischer JE, Boyajian M, et al. The influence of hair-removal methods on wound infections. Arch Surg. 1983;118:347-352.
36 Grabsch EA, Mitchell DJ, Hooper J, Turnidge TJ. In-use efficacy of a chlorhexidine in alcohol surgical rub: a comparative study. ANZ J Surg. 2004;74:769-772.
37 Alcock E, Regaard A, Browne J. Facet joint injection: a rare form cause of epidural abscess formation. Pain. 2003;103:209-210.
38 Mackenzie AR, Laing RBS, Kaar GF, Smith FW. Spinal epidural abscess: the importance of early diagnosis and treatment. J Neurol Neurosurg Psychiatry. 1998;65:209-212.
39 Van Zundert A. The epidural abscess: diagnosis and treatment. In: Highlights in regional anaesthesia and pain therapy IX. Rome, Italy: ESRA and Cyprint; 2000:159-162.
40 Danner RL, Hartman BJ. Update of spinal epidural abscess: 35 cases and review of literature. Rev Infect Dis. 1987;9:265-274.
41 Darouiche RO, Hamil RJ, Greenberg, et al. Bacterial spinal epidural abscess: review of 43 cases and literature survey. Medicine. 1992;71:369-385.
42 Horlocker TT, Rowlingson JC, Enneking FK, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Third Edition). Reg Anesth Pain Med. 2010;35(1):64-101.
43 Franzini A, Ferroli P, Marras C, Broggi G. Huge epidural hematoma after surgery for spinal cord stimulation. Acta Neurochir (Wien). 2005;147:565-567.
44 Vandermeulen EP, Van Aken H, Vermylen J. Anticoagulants and spinal-epidural anesthesia. Anesth Analg. 1994;79:1165-1177.
45 Ward TN, Levin M. Case reports: headache caused by spinal cord stimulator in the upper cervical spine. Headache. 2000;40:689-691.
46 McKenna KE, McCleane G. Dermatitis induced by a spinal cord stimulator implant. Contact Dermatitis. 1999;41:279.
47 Ochani TD, Almirante J, Siddiqui A, Kaplan R. Allergic reaction to spinal cord stimulator. Clin J Pain. 2000;16:178-180.
48 Lennarson PJ, Guillen T. Spinal cord compression from a foreign body reaction to spinal cord stimulation: a previously unreported complication. Spine. 2010;35(25):E1516-E1519.
49 Larkin TM, Dragovich A, Cohen SP. Acute renal failure during a trial of spinal cord stimulation: theories as to a possible connection. Pain Physician. 2008;11:681-686.
50 Grua ML, Michelagnoli G. Rare adverse effect of spinal cord stimulation: micturition inhibition. Clin J Pain. 2010;26:433-434.
51 Loubser PG. Adverse effects of epidural spinal cord stimulation on bladder function in a patient with chronic spinal cord injury pain. J Pain Symptom Manage. 1985;13:251-252.
52 Thakkar N, Connelly NR, Vieira P. Gastrointestinal symptoms secondary to implanted spinal cord stimulators. Anesth Analg. 2003;97:547-549.
53 Kemler MA, Barendse GA, van Kleef M. Relapsing ulcerative colitis associated with spinal cord stimulation. Gastroenterology. 1999;117:215-217.