Chapter 15 Complications of Spinal Cord Stimulation
 Spinal cord stimulation is typically a safe and effective treatment for appropriate patients with chronic intractable pain.
Spinal cord stimulation is typically a safe and effective treatment for appropriate patients with chronic intractable pain. Practitioners must remain vigilant throughout the entire SCS process, including patient selection, surgical preparation, and implantation procedure.
Practitioners must remain vigilant throughout the entire SCS process, including patient selection, surgical preparation, and implantation procedure.Introduction
Spinal cord stimulation (SCS) is a treatment for chronic intractable pain that was introduced by Shealy in 1967.1 With technological advances, SCS has become an increasingly effective treatment for those with many types of chronic pain conditions. In 2007 it is estimated that a total of 27,484 SCS implants occurred over all major payer types in the United States.2 The majority of patients have a positive outcome involving a reduction of pain with no untoward events. Unfortunately complications of SCS occur just as complications occur with all other interventional procedures. The goal of this chapter is provide information regarding the types and prevalence of complications and trends noted. Anecdotal information can also be useful as a way to increase awareness. Increased awareness of potential contributors of untoward events may help to decrease the likelihood of complications.3 When appropriate, recommendations will be proffered.
In an epidemiological report published in 1999, more than 1 million injuries and nearly 100,000 deaths occur annually as a result of errors in medical care.4 According to the systematic review by Turner and associates5 of 22 studies involving patients with diagnoses, including failed back surgery syndrome or complex regional pain syndrome (Table 15-1), complication rates associated with SCS are noted to be 34%.
Cameron6 reported a mean complication rate of 36% in her review of the literature involving 68 studies and 3679 patients. She divided the complications into the categories of technical (27.2%), biological (4%), and others (5%). Different case series report that between one quarter5 and one third7 of patients undergo at least one SCS revision surgery to reverse a complication.8 The majority of complications associated with SCS are treatable conditions that may require simple treatments or minor surgical revisions. Timely recognition and treatment of complications reduce the likelihood that the condition progresses into an untreatable or permanent complication.
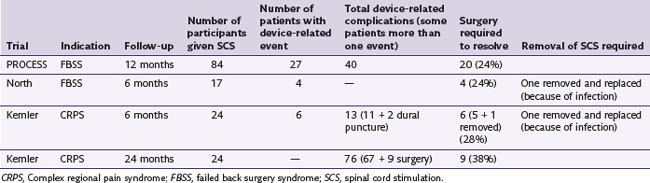
Prospective studies regarding complications of interventional procedures are rare. A summary of device-related complications from three prospective randomized SCS studies is presented in Table 15-2.
Table 15-2 Device-Related Complications from Three Prospective Randomized Spinal Cord Stimulation Studies
Kumar and associates9 published a prospective study regarding SCS in 2007. They compared conventional medical management (CMM) to SCS for patients with failed back surgery syndrome resulting in residual predominant leg pain. One hundred patients were randomized to SCS or CMM, and cross over was permitted. Ultimately 84 patients received an electrode during the 12 months of the study. A total of 27 (32%) patients experienced a total of 40 device-related complications. Surgery was required to resolve the issues of 20 (24%) patients. Hardware-related issues, including lead migration, damage to the leads, and generator migration, accounted for 13 of the events. Six patients had loss of therapeutic effect, loss of paresthesia, or unpleasant paresthesia. Biological complications totaled 16 events and included infection or wound breakdown, pain at the incision site, and fluid collection in the pocket. Iatrogenic issues related to technique numbered five events: dural tear during implant, lead damage during implant, anterior migration of lead during implantation, suboptimal connection of the extension to the generator, and a cap not installed on the generator when one lead was implanted. This prospective study demonstrates the effectiveness of SCS while illustrating the point that complications can be a significant limiting aspect of the modality and must be factored into the decision-making process by both physician and patient when SCS is being considered as a treatment.
Complication rates are often extrapolated from observations in prospective studies, case reports, retrospective reviews, and closed claim studies.10 Avoidance of complications requires awareness and vigilance. Strict adherence to evidence-based surgical techniques is also mandatory to give the patient the best opportunity for a successful outcome.11 Most complications of SCS are not life threatening and can be resolved by explantation of the system.6
Revisions of SCS systems are minimally invasive procedures that are rarely done on an emergent basis, and such procedures are associated with little permanent morbidity.12 However, repeated procedures expose patients to further risk and continuing disability and increase costs to the health care system. In the retrospective analysis by Rosenow and associates,12 the authors reviewed the charts of 289 patients who underwent 577 procedures. Hardware revision was required in 46% of patients, and nearly half (48.9%) of those patients underwent more than one revision.
In the 2-year follow up to the randomized controlled trial of effect of SCS on chronic reflex sympathetic dystrophy, Kemler13 reported that complications occurred in 38% of patients. However, he also states that, because SCS is a lifelong therapy, it is recognized that the incidence of complications is reduced after the first year. He concludes by asserting that SCS is safe and effective if there is careful patient selection and test stimulation.
Furthermore, Kumar14 reported that biological complications are more prevalent within the first 3 months after implantation, whereas hardware-related complications are likely to occur in the first 2 years after implantation. Despite an overall complication rate of 35%, incidence of life-threatening complications has been shown to be very rare in two large retrospective studies.5,6,8
Technical Complications
Technical complications are typically issues related to the hardware itself. The complications may occur because of operator error or negligence, but they also may occur as a result of limitations inherent in the SCS systems that are available at the time of implantation. Technological improvements are capable of reducing the incidence of complications. Van Buyten15 discussed the effect of technological improvements and their effect on the overall complication rate. Devulder and associates16 published a report of 69 patients with implanted SCS systems who were followed for up to 8 years. Of these 69 patients, there were 174 revision surgeries. Battery replacements accounted for 67 of the surgeries. Electrode migration, insufficient stimulation paresthesias in the painful area, technical failure, and electrode breakage made up the remaining 107 surgeries. Most cases of migration of the electrodes occurred in cases in which the electrodes were implanted in the cervical spine. During revision surgeries for lead migration, attempts at anchoring the leads to bony or ligamentous elements helped to ensure that the lead would not migrate again. Epidural fibrosis tended to respond to lead revision as well.
Andersen reviewed the complications associated with 60 patients with intractable angina who had SCS systems implanted.17 The first 22 patients had unipolar electrodes implanted, whereas the remaining 38 had quadripolar systems. The most frequent complication was electrode migration. The unipolar systems migrated in 10 patients; 11 of the quadripolar electrodes migrated. This difference is not statistically significant. All of the unipolar lead migrations required reoperation, but only 4 of the 11 patients with quadripolar electrode migrations underwent reoperation. By increasing the number of contacts, the area that is covered by stimulation increases; thus the placement of the electrode is more forgiving if there is a minor migration. The technological advance of progressing from unipolar to quadripolar electrodes reduces the number of reoperations, which in turn decreases the risk of further surgical complications as a larger area of the spinal cord is covered.
In Cameron’s literature review of 2972 patients treated with SCS,6 the most common complication was electrode migration. Kumar and associates18 noted that electrodes in the cervical spine were twice as likely to migrate as electrodes in the lumbar spine. This occurs because of the increased mobility of the cervical spine compared to the lumbar spine. Directional forces on the lead determine the direction of the displacement, whether laterally or longitudinally. Displacement occurs when the tensile load on the electrode exceeds the capacity of the anchor to stabilize it.18 Tensile load changes with range of motion of the spine, the position of the generator, and the elasticity of the electrode and the surrounding tissues. When the generator was implanted in the gluteal region (3 [21%] of 14), the electrodes were more likely to move than when the generator was implanted in the abdominal wall (15 [10%] of 146). The authors postulate that there is more traction on the electrode with lumbar range of motion if the generator is in the gluteal region.
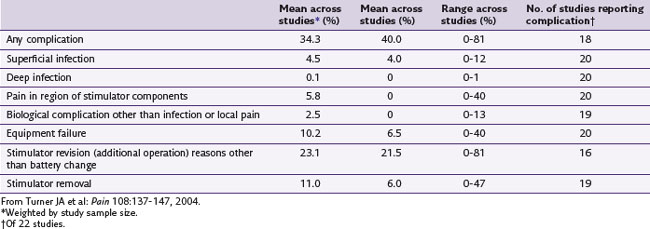
In 2006 Pyles19 presented the notion of a single incision for the implantation of the SCS device in the lumbar spine. Kumar18 suggested that the traditional placement of the generator in the buttock pocket might lead to increased strain and fulcrum effect on the leads and anchors. Having only one incision may decrease the amount of postoperative pain that patients experience11 (Fig. 15-1). There is also reduced operating time that may reduce the likelihood of time-sensitive complications such as infection. A potential complication that is unique to this technique is that revision surgery may be more difficult if the generator is enveloped in the same planes of scar tissue as the electrodes and the anchors.
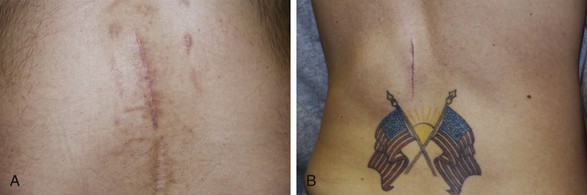
Fig. 15-1 A, One-incision technique above a laminectomy surgical incision. B, One-incision technique.
Another technique to avoid electrode migration was described by Kumar.8 He warns that migration is more likely if the nose of the anchor is not pushed through the deep fascia layer and the anchor is secured to that layer. Adding a strain relief loop may also decrease the likelihood of lead migration by reducing tension on the electrode when the patient is moving.
In his review of 10 years of experience at a single center in Belgium, Van Buyten20 found that most complications were of a technical nature.20 Fracture of the electrode occurred in 5% of the patients; fracture of the extension cable also occurred in 5%. Almost 8% of patients had a fracture in the temporary wires, and just over 8% had a dislocation of the electrode. Eleven percent experienced pain at the site of the electrode-extension connection. One of 24 patients in the Kemler and associates’ study of chronic reflex sympathetic dystrophy had a defective lead implanted that was corrected with a revision surgery.21 In his review of problems encountered with SCS devices published in 1974, Fox21a noted that lead breakage is more likely to occur during revision surgery since the lead is less likely to be identified among scar tissue.
Heidecke22 published a retrospective analysis of a group of 42 failed back surgery syndrome patients using a single percutaneous lead.22 The patients were followed for 6 to 74 months, and 12 of the 42 patients had systems that experienced hardware failures. Eight of the leads broke. The other four hardware issues included two broken extension cables and two cases of receiver insulation failure at the plug connector site. Electrode breakage or a disruption in stimulation should be suspected when a sudden disappearance of stimulation occurs. Six of the cases of electrode breakage were caused by disrupted insulation, and the other two cases of electrode dysfunction were without known cause. One of the eight cases was caused by trauma, whereas the other seven were spontaneous.22
There were two cases of receiver failure because of short circuiting caused by leaks in the insulation at the plug connection.22 Special attention must be paid to all connections. Heidecke22 recommends securing connections with screws while still under pressure. In the cases of the disconnected extension cable at the junction of the lead and cable, the loosening was likely secondary to insufficient tightening of the connector screws that were in use at the time. Another possible problem with that scenario could have been increased traction on the extension cable that could be averted by forming a loop in the course of the electrode. Inadequate connections may be avoided by careful and diligent handling during implantation.
In his retrospective analysis of 160 SCS patients, Kumar and associates18 discovered nine patients whose electrodes were fractured. Of these patients, one had a paddle electrode; the remaining eight patients had percutaneous electrodes. In this series there were a total of 28 paddle electrodes and 132 percutaneous electrodes. The likelihood of an electrode fracture is higher in the percutaneous implants. The fracture developed in the percutaneous electrodes cephalad to where the lead is affixed to the deep fascia at the point at which the electrode enters the spinal canal.
However, in their article reviewing the charts and operative reports of 289 patients who had undergone SCS implantation between 1998 and 2002, Rosenow and associates12 reported that the rate of breakage of electrodes was twofold higher in laminotomy electrodes as compared to the percutaneous variety. They also reported that electrode migration was marginally higher in the laminotomy electrodes.
Eisenberg and Waisbrod23 reported a case in which a patient experienced an electrical injury to the central nervous system as a result of the cervical SCS becoming activated uncontrollably by an antitheft device. Six months after implantation the patient entered a store that was protected by an antitheft device. The patient requested the store representative to deactivate the deterrent system. The patient entered the store at the direction of the representative. The patient recalls a shocklike sensation at the back of his skull before losing consciousness. The patient regained consciousness in the emergency room where he demonstrated moderate confusion, dysarthric speech, gait ataxia, bilateral upper-extremity intention tremor, and weakness of the left upper extremity. Brain computed tomography (CT) and electroencephalography were normal. After 6 months the patient’s condition improved; however, dysarthria, impaired memory, tremor of the right hand, and gait ataxia were still present. CT of the brain revealed an old infarction in the left basal ganglia.
Biological Complications
Infection
Implanting hardware in patients bears a potential risk of infection. Infection in the spinal canal is rare, but superficial infections at the site of the generator or the connector between the generator and the electrode occur more frequently.5 The typical rate of infection is approximately 5%.24 According to Barolat,25 patients may present at any time with an infected device, whether it is within a few days of implantation or a few years. Risk factors for infections of implanted hardware include tobacco and alcohol use and immunosuppressive therapy.26 Co-morbidities such as diabetes mellitus and rheumatoid arthritis may also increase the risk.27
Another risk factor for infection that was illustrated by Barolat28 is multiple surgeries. He reported that 3.4% of patients and 3.7% of implanted electrodes developed infection. In his review of his experience with 509 implanted plate electrodes, 12 patients developed infection involving a total of 19 plate electrodes. In four patients the infection presented after multiple revisions of the electrodes. He adds that two of the four patients had a history of infections in the course of previous surgical interventions.
According to Follett and associates,29 the most commonly reported organism cultured in infected SCS systems is Staphylococcus at 48%, followed by Pseudomonas at 3%. Of the remaining organisms, 24% were unknown or not reported, 18% demonstrated no growth, and 6% of cultures grew multiple organisms. They also found that the location of the infection is most likely to be the generator pocket at 54%, followed by the SCS electrodes at 17% and the lumbar incision site at 8%. Multiple sites were infected in 14%, and 8% were not reported.
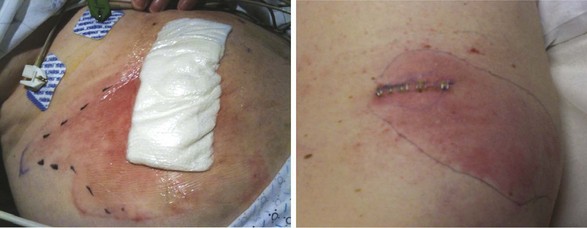
In a study of 84 patients receiving a total of 92 dorsal column stimulators, Pineda30 reported that only one patient experienced an infection. The patient presented  years after implantation with an infection at the site of the generator. S. aureus was isolated from the wound. According to the author, it was likely that the bacteria were sequestered in the hardware. The infection was treated with antibiotics, and the hardware was explanted. Pineda also reported the case of a patient whose electrode extruded through the skin at the site of the generator
years after implantation with an infection at the site of the generator. S. aureus was isolated from the wound. According to the author, it was likely that the bacteria were sequestered in the hardware. The infection was treated with antibiotics, and the hardware was explanted. Pineda also reported the case of a patient whose electrode extruded through the skin at the site of the generator 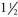 years after the implantation surgery. The extruded electrode ultimately became infected with Staphylococcus, and the system was removed.
years after the implantation surgery. The extruded electrode ultimately became infected with Staphylococcus, and the system was removed.
Kemler and associates21 reported one infected SCS system out of 24 (4%) patients receiving SCS, and the system was explanted. The infection was not confirmed by culture. After the infection had resolved, the patient underwent a successful reimplantation. Devulder and colleagues16 reported that 2 of 69 (3%) patients had infected SCS devices. One of the patients had a successful reimplantation procedure. SCS was abandoned for the other patient since the infection was recurrent.
In Kay and associates’ review of 70 patients who were treated with SCS,31 six patients were diagnosed with an infection. Three of those patients had their SCS system explanted; the others were treated successfully with antibiotics. Prophylactic antibiotics were not administered to these patients, which the authors assert may have contributed to the infection rate of 9%. Three of the infected patients had previously undergone revision surgery. The additional surgical procedures exposed these patients to added risk and increased the likelihood of complications for these patients.
Meglio, Cioni, and Rossi32 reported a 9-year experience in their institution with SCS. Of the 109 patients treated with SCS, seven patients developed an infection related to the implanted hardware. One of the patients became paraplegic within a few days of the explantation of the SCS device. A myelographic block was noted at the level where the electrode had been implanted. A bacterial epidural and intradural abscess was discovered and drained. The recovery of this patient was not complete.
Prophylactic antibiotic administration during implantation procedures is an important step in reducing the likelihood of an infection.11 Optimal prophylaxis takes into consideration the appropriate choice of antibiotic, proper timing of administration, and limiting the duration of antibiosis. In the case of spinal cord stimulator implantation, a single dose of a cephalosporin is generally adequate unless there is history of an allergy to that class of antibiotic agent. To ensure maximal effect, adequate tissue levels must be attained before incision. The usual recommendation is that the drug should be administered between 30 minutes and 2 hours before incision. There is no advantage to prescribing postimplantation antibiotics,33 although this has not been prospectively tested for SCS implantation procedures.
Recommendations to reduce the likelihood of infection also include vigilance when preparing and draping the surgical area and gentle treatment of tissue.34 Electrocautery near the superficial tissues should be limited, and there should be a two- or three-layer closure to carefully approximate the edges of the incision.34 The implanting physician must maintain a low threshold for suspicion with new complaints of increased pain, new neurological deficits, and constitutional signs and symptoms. A significant increase in pain over the baseline level and new neurological deficits in patients with an implanted SCS device are harbingers of infection. Any suspicion of infection should initiate a complete work-up. Useful laboratory studies include a complete blood count with differential, erythrocyte sedimentation rate, C-reactive protein, a gram stain and cultures with sensitivities. A CT scan of the areas in which the hardware is implanted is also indicated. 31
If the infection is superficial, successful treatment is possible with oral or intravenous antibiotics.34 Explantation should not be delayed if there is lack of improvement or if the infection is neuraxial. A consultation from an infectious disease expert is recommended. Typically the device may be reimplanted after a period of 12 weeks. An approach that has been suggested in reducing the risk of infection is to soak the electrode in gentamicin solution before implantation30 (Figs. 15-2 and 15-3).
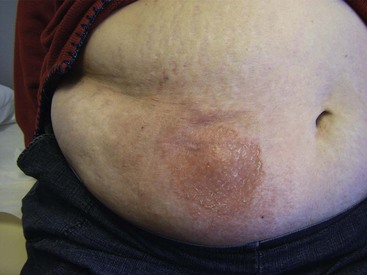
Seroma
Seroma should be part of the differential diagnosis if a patient presents with erythema and edema in the area of the generator incision.34 A seroma is a noninfectious process resulting in a collection of serosanguineous fluid in the pocket of the generator. Patients typically do not present with constitutional signs or symptoms. Culture of a sample does not demonstrate growth. Pineda reports that 10 out of 84 patients who received 92 implants developed a seroma. The seromas were treated successfully with aspiration,30 although this procedure bears the risk of introducing infection into the pocket. Fox21a reported that 5% of patients developed a seroma; these accumulations were treated with one or two aspirations. Open incision and drainage is another treatment option, together with pressure dressings or abdominal binder, depending on the location of the generator (Fig. 15-4).
Postdural Puncture Headache
The most common neurological complication in SCS implantation procedures is inadvertent dural puncture.34 Sundaraj and associates35 report an incidence of 0.46%. In their review of 153 patients in a single center in Belgium, van Buyten20 reported that 9 of 153 (6%) had dural puncture. Kemler and associates21 reported that dural puncture occurred in 2 of the 24 SCS systems implanted for patients with chronic reflex sympathetic dystrophy. One these patients developed a postdural puncture headache, whereas the other did not.
Risk factors for dural puncture are previous surgery at the site of needle placement, obesity, spinal stenosis, calcified ligamentum flavum, and patient movement.34 Risk factors for developing a postdural puncture headache include female gender, young age, pregnancy, and use of a large-bore needle.36
Eldrige and associates36 reported two cases of postdural puncture headache during SCS procedures in which the successful treatment of the headaches was different.36 The first patient was treated successfully with conservative measures, including intravenous caffeine sodium benzoate, placement of abdominal binder, opioids, and hydration. Conservative measures failed to treat the headache of the second patient; so the patient was offered an epidural blood patch. Since the dural puncture occurred during the permanent implantation, particular care had to be taken to avoid interrupting the stimulation. Fluoroscopy was used to guide the needle to the epidural space while the patient was awake, and the SCS remained activated to monitor changes in the stimulation pattern throughout the procedure. The authors were concerned about the mechanical stress that the injected blood may have on the implanted leads and the possibility that the electrodes may be displaced by the volume of the injectate. Other considerations include the risk of infection since blood is a rich bacterial growth medium.34
Kumar and associates18 recommended that an autologous blood patch be administered as soon as a dural puncture is identified. The authors of this analysis also recommend terminating the procedure and rescheduling 3 weeks later to allow the dura enough time to heal from the insult from a large-bore Tuohy needle. This recommendation is made to avoid bathing the electrodes in cerebrospinal fluid (CSF) and increasing the risk of an electrical short circuit.18 If there is no increased risk to the procedure, other authors may continue the procedure at a cephalad level since long-term outcomes have not been affected.34
Wolfensberger and Borruat37 reported a case of sixth nerve palsy after SCS implantation in a 56-year-old male with scleroderma and peripheral limb ischemia. A few days after implantation the patient complained of tinnitus and headache. On postoperative day 5 the patient developed diplopia, especially when looking to the left. Examination revealed a sixth nerve palsy but no other neuroophthalmological abnormalities. The patient was treated conservatively and was free of symptoms at the 3-month follow-up visit.
The authors postulate that the likely etiology of the headache and abducens palsy is secondary to CSF leak at the site of epidural fixation of the electrode. Sixth nerve palsy is a known complication of lumbar puncture.38 A sixth nerve palsy may ensue after a drop in CSF because the sagging of the intracranial contents may induce mechanical pressure on the sixth cranial nerve as it passes over the petrous bone.39
Headache
Ward and Levin40 reported a case in which a patient developed headaches during the use of SCS. The electrodes were placed in the upper cervical spine to treat a bilateral brachial neuritis causing bilateral shoulder pain. The cephalad electrode was placed at the level of C3. Before placement, CT of the brain and magnetic resonance imaging (MRI) of the cervical spine were obtained and read as normal. The patient reported a good response to his shoulder pain with the SCS; however, within 1 week of implantation the patient complained of headaches. He developed a constant mild headache that would occasionally become severe and persist for hours to days before reverting to the baseline headache. The headaches did not respond adequately to acetaminophen. Sumatriptan (Imitrex) gave inadequate relief.
Other Neurological Sequelae
Epidural hematoma is a rare complication in SCS. In a review of 509 consecutive surgical plate electrodes implanted, Barolat28 reported one epidural hematoma. This hematoma was located at the surgical site and resulted in postoperative paraplegia. Pineda30 reported 1 subdural hematoma in 84 patients who received a total of 92 dorsal column stimulator systems.
In 2005 Franzini and colleagues41 published a report of a huge epidural hematoma after implantation of a laminectomy lead at the level of the L1 vertebra. The patient had failed conservative treatment for chronic low back and leg pain and was not a candidate for a surgical intervention. SCS was offered in an attempt to treat the patient’s pain. Immediately after the implantation, the patient had progressive loss of lower-extremity strength that became complete flaccid paraplegia within 2 hours. CT and MRI of the thoracic and lumbar spine demonstrated an epidural hematoma. An 11-level laminectomy successfully decompressed the spinal canal. No coagulopathies were discovered before or after the SCS implantation.
The authors recommend that patients at risk for epidural hematomas should have periodical assessment of motor and sensory function for the first 36 hours after surgery.41 Delays in recovery from anesthesia should prompt a full neurological examination and possibly imaging in the form of a CT or MRI. There is a bimodal age distribution with peaks in childhood and fifth and sixth decades of life. By a ratio of 4 : 1 male gender is more likely to develop an epidural hematoma, as are patients who have had multilevel laminectomies.42
Chiravuri and associates43 described a case of a subdural hematoma following SCS device implantation. The SCS system was implanted to treat chronic intractable angina in a 49-year-old male. An inadvertent dural puncture occurred during placement of the Tuohy needle. During a postprocedure follow-up phone call, the patient complained of a positional headache. The patient was advised to present to emergency room because of a change in the character of his headache. On physical examination there were nonfocal neurological findings and an absence of meningeal signs. Stat CT of the head revealed a subdural hematoma.
The patient underwent an emergency craniotomy. On postoperative day 3 the patient recalled having a fall in which he struck his head but did not lose consciousness in the day preceding the implantation procedure. This patient experienced a blunt head trauma and a dural puncture within 24 hours. The authors propose that the cause of the subdural hematoma may be solely secondary to the drop in intracranial pressure because of the dural puncture or the subdural hematoma may have been subclinical until after the dural puncture. An epidural blood patch is a common treatment for postdural puncture headaches. However, in the setting of a subdural hematoma, an epidural blood patch can cause rebound intracranial hypertension. Increased volume in the epidural space may cause increased intracranial pressure.44
Grillo, Yu, and Patterson45 reported a case of delayed intraspinal hemorrhage 18 months after implantation of a dorsal column stimulator. The patient had a dorsal column stimulator implanted in the subarachnoid space at the C4-C5 level in an attempt to treat her low back and leg pain after the failure of more conservative treatments. The patient used the stimulator for the first 6 months after implantation, but then the pain returned spontaneously. At this point the patient stopped using the stimulator. She woke up with a sudden sharp pain in the right side of her body associated with paralysis of the right upper and lower extremities. She also lost control of her bladder and developed Horner syndrome. Lumbar puncture revealed bloody, xanthochromic cerebral spinal fluid. Myelography demonstrated widening of the spinal cord between C3 and C6 associated with the electrode. The patient underwent surgical exploration, and a 2-mL hematoma beneath the electrode was evacuated. Her neurological status improved with the exception of residual right-hand weakness. The authors suspect that the ultimate cause of the hematoma was a mechanical trauma of the SCS device on a neurovascular structure likely secondary to neck motion that lacerated a pial vessel.45 Electrical damage to the vasculature is unlikely since the device had not been used in approximately 1 year preceding the hemorrhage.
Pineda30 reported that two patients of 84 with 92 systems developed progressive paraparesis with no evidence of arachnoiditis and with no indication of the original pathology that is the source of the pain.30 The patients were advised to stop using their systems. Both continued to use the stimulators because of the relief, despite one relying on a walker for ambulation and the other using a wheelchair. The author stated that it is unclear if the system was responsible for the paraparesis in these patients.
Meyer, Swartz, and Johnson46 reported a case in which the SCS electrode was placed inadvertently in the spinal cord, which resulted in quadriparesis. A 25-year-old female with complex regional pain syndrome in the left upper extremity had improvement in her pain and function after the initial SCS implantation procedure. Six months after the implant she required a revision of the generator for technical reasons. General anesthesia was induced. Intraoperatively it was discovered that the lead connectors also needed to be revised, which required placement of new epidural electrodes. The original electrodes were removed. The new leads were placed through a Tuohy needle by the standard approach. In the postanesthesia care unit the patient exhibited profound weakness in all four extremities. An immediate postoperative CT of the cervical and thoracic spine demonstrated an intramedullary lead with the tip at the level of C2.
A case of severe pain and pseudotabes was reported in the Journal of Neurology, Neurosurgery & Psychiatry in 1987.47 The patient afflicted was a 65-year-old male with failed back surgery syndrome. He experienced severe pain in his back and legs and saddle anesthesia. In 1986 he had an electrode implanted through the T10-T11 interspace and positioned at the level of T7-T8. Radiographic imaging confirmed that the electrode was in the extradural space. Initially extreme pain was felt at the site of the laminectomy, but the patient also developed pain in his legs and the area of his penis and scrotum. The pain was eliminated when the stimulation ceased, and it was reproducible. Despite attempts to adjust the current, the pain persisted, and the system was explanted. The authors described the pain as pseudotabetic because of the location of the pain and the relationship of tabes dorsalis to nerve root or dorsal horn ganglion damage. They suggested that the pain was a result of antidromic conduction of the dorsal tracts of the spinal cord involving neurologically damaged segmental cells that resulted in an altered response.
Devulder and associates16 reported several neurological complications in their experience with 69 SCS patients.16 One patient developed torticollis when the electrode was implanted at the level of T2 until the electrode was revised and placed at T9. The torticollis resolved while the SCS was still effective for the patient’s pain. Two patients experienced cooling in the leg that was confirmed by thermography. The patients perceived the paresthesias in the appropriate areas, but the SCS did not provide relief. In both patients, revising the electrode and implanting the electrode one thoracic segment lower resolved this issue. One patient developed Horner syndrome with the electrode implanted at the paramedian C5 level, which resolved when the electrode was moved to the midline. Five patients had gait problems. Four of these patients had anesthetized legs that resolved immediately with reprogramming the SCS device. One patient had gait difficulties that were improved with reprogramming the SCS device at the expense of relief. The gait problem completely resolved after 3 years without explanation.
In his review of implantation of 509 plate electrodes, Barolat28 reported that four patients complained of radicular-like symptoms following implantation. In three of the patients, the etiology of the pain was spinal stenosis on postoperative CT imaging. The stenosis was in part created by the space occupied by the electrode. The symptoms resolved with limited laminotomies over the site of the electrode. An electrode in the epidural space may result in spinal stenosis even if no stenotic lesion was present before implantation. An MRI of the cervical spine is recommended before implantation of an electrode in the cervical spine.34 Thoracolumbar imaging is not necessary before implantation of electrodes into those areas unless there is suspicion of a potential neurological mass effect secondary to the presence of the electrode.
Epidural fibrosis has been identified as another complication for several reasons. As a space-occupying lesion, the fibrosis may result in stenosis, thereby displacing the neural structures. The fibrosis may also affect the ability of the electrodes to deliver current.34 Surgical revision may be necessary to reinstate stimulation if reprogramming is unsuccessful. Painful stimulation may also occur with fibrosis since the direction of the current may be redirected to nerve roots or other neuraxial structures.34
Turner and colleagues48 published a prospective, population-based controlled cohort study regarding SCS as treatment for failed back surgery syndrome in a workers’ compensation setting.48 Eventually 28 patients underwent a permanent SCS implantation. One patient developed seizures after the SCS implantation. The seizures subsided when the system was turned off and resumed after the stimulator was activated. The SCS system was explanted after 8 months because of the seizure activity and lack of pain relief.
Gastroenterological
Kemler, Barendse, and van Kleef49 reported a case involving the relapse of ulcerative colitis in a patient. The theory that the SCS device was the cause of the relapse of the symptoms was tested twice before attributing SCS to this condition. The symptoms of ulcerative colitis began when the patient was 23 years old. He had two relapses at ages 37 and at 43. At age 46 he developed complex regional pain syndrome in the right upper extremity after a Colles’ fracture. After conservative treatments failed to relieve his pain, the patient underwent a successful SCS trial over 4 days. The SCS system was implanted with a quadripolar lead placed at the level of C4. The pulse generator was implanted in the left anterior abdominal wall.
There were no confounding factors discovered. Possible causes of the relapse of the ulcerative colitis as it related to SCS include the effect that an electromagnetic field may have on the colon. The effect of electricity on colonic circulation was also considered together with effect of SCS on the brain-gut axis through electricity or γ-aminobutyric acid (GABA). It was proposed that the cause might be neurogenic inflammation in a separate article.50
In 2003 Thakkar, Connelly, and Vierira51 published a report of two cases of women developing gastrointestinal symptoms after spinal cord stimulators were implanted. The symptoms ceased after discontinuation of use of the stimulator. The first patient developed nausea and diarrhea 1 month after implantation. The second patient complained of worsened gastroesophageal reflux, flatulence, and diarrhea. The authors propose that a sympathectomy resulting in an unopposed parasympathetic state is the cause of the symptoms that these two patients experienced.
Urologic
In 2008 Larkin, Dragovich, and Cohen published the first case report of acute renal failure occurring during an SCS trial.52 The patient was a 48-year-old male with failed back surgery syndrome resulting in neuropathic leg pain. His past medical history also included hypertension and hepatitis B. He was offered a spinal cord stimulator trial since conservative treatments for his pain had failed. After an uneventful SCS trial procedure, the patient experienced a syncopal episode. He requested that the electrode be removed on the second postoperative day. He did not recall urinating for 2 days. He complained of light-headedness when he was sitting or standing. The electrode was removed, and the patient was referred to the emergency department. He was hypotensive, and his blood urea nitrogen (BUN) and creatinine were 83 mg/dL and 8.1 mg/dL, respectively. The patient was admitted to the medical intensive care unit, where he was monitored and hydrated. His BUN and creatinine normalized within the next few days.
Given the rapid normalization of the laboratory values, the authors surmise that the renal failure was not chronic. They hypothesize that the sympathectomy caused by the SCS may have decreased renal blood flow through peripheral shunting, decreased renal perfusion pressure, and produced an attenuated cardiovascular response.52
Loubser53reported a case in which a patient with chronic pain secondary to spinal cord injury underwent implantation of a SCS system. The patient was a 41-year-old male with an incomplete injury at T12-L1 that resulted in severe cauda equina syndrome. After failing conservative approaches to treat his pain, the patient underwent a successful SCS trial. Within a few months of the implantation of the SCS system, the patient required higher amplitudes to effectively address his pain. At amplitudes such as 6.5 V, the patient was no longer able to self-catheterize because of urethral sphincter spasm. The episodes of urethral sphincter spasm persisted for 3 hours after deactivation of the SCS system. Ultimately the patient’s SCS system was explanted. The urinary retention and recurrent urinary tract infections that ensued were attributed to the SCS system. The author proposed that patients with spinal cord injury should have urodynamic function testing incorporated into the SCS trial.
Allergic Reactions
Allergic reactions to cardiac pacemakers have been documented in the literature.54 A few cases of allergic reactions to components of SCS systems are reported. In his series of 198 patients with SCS systems implanted, Burton55 reported that one patient developed an allergic reaction to the silicone component of the hardware.McKenna and McCleane56 reported a case of a 53-year-old male with intractable angina who was offered an SCS system in an attempt to treat his pain. One month after implantation, the patient developed eczema on the skin of the left lateral chest over the receiver. The receiver circuitry was encased in epoxy resin, with a polyurethane connector block where an insulated stimulating electrode was attached by four titanium screws in silicone rubber grommets. A transmitter was attached to the patient’s belt with a lead that was secured to the patient’s skin by adhesive. The patient had a previous history of rashes from contact with cheap metal. Patch testing revealed a reaction to nickel.
The authors contend that the nickel allergy did not play a role in the dermatitis. They believe that the patient had an isomorphic response to expansion of the underlying tissues by the hard device as has been seen after placement of cardiac pacemakers.57
The Long and Erickson study reviewing the experiences of 69 patients with SCS systems implanted from 1969 to 1973 reported that one patient had the device explanted because of an allergic reaction.58 They did not expound on the nature or circumstances of the allergy except to state that the patient had phantom limb pain that was relieved by the stimulator. Meglio, Cioni, and Rossi32 reported that 2 of 109 patients with SCS devices (1.8%) experienced a rejection phenomenon in which the electrodes were involved. The SCS systems were explanted; no other details were offered.
Ochani and associates59 published a report of an allergic reaction to a spinal cord stimulator in 2000. The patient was a 41-year-old female with a diagnosis of complex regional pain syndrome of the left upper and lower extremities. The patient underwent implantation of electrodes in the cervical and lumbar epidural space. Two weeks after the implantation procedure, she complained of burning and erythema along the tunneled leads that worsened when the stimulator was activated. The SCS device provided relief to the patient and allowed her to improve her function. There were no systemic signs, symptoms, or studies compatible with infection.
The ability to recognize contact sensitivity is important for implanters of SCS devices.59 A level of suspicion must be maintained for contact sensitivity since it may mimic infection. Unnecessary antibiosis and reimplantation would be poor treatment approaches for patients who have contact dermatitis (Fig. 15-5).
Pain at Site of Spinal Cord Stimulation
Alò60 reported outcomes of patients with SCS devices implanted at 24, 30, and 48 months. He found that the most common reason for device explantation was pain at the site of the generator.60 At the 48-month time point, only 30% of patients responded that they would undergo the implantation again; the most common reason for not wanting to repeat the procedure was pain at the site of the generator. On the other hand, Kemler and associates21 reported that 2 of 24 patients had pain at the site of the generator. The generator pockets of these two patients were modified successfully.
Pineda30 encountered patients with pain at the site of the generator or at the site of the lumbar incision. Usually the pain was transitory and treated successfully with lidocaine (Xylocaine) in 12 of 84 patients who had received 92 SCS implants. Pineda reported that three SCS systems were explanted for intractable pain at the site of the generator. One of the patients had intractable pain at the site of the electrode; this system was also explanted. The patients in Pineda’s report underwent dorsal column stimulation that involved an incision into the dura. The dural incision was closed with minimal overlap of edges over the plate electrode to lower the risk of iatrogenic stenosis. The closure itself, with possible predisposing spinal pathology, and not the electrode conceivably could have been the etiology of this patient’s pain.
Pineda30 reported that 1 patient in 84 with 92 implants developed an ulcerative dermatosis that resolved after the patient stopped using the stimulation. A relationship between stimulation and trophic changes of the skin could not be identified.
Other Complications
Long and Erickson58 published a case series involving 69 patients with chronic intractable pain who underwent SCS implantation between December 1969 and January 1973. The authors commented that the major serious complication limiting evaluation of the effectiveness of the device for pain control was failure of stimulation into a painful part. Fox’s 1974 article21a recounting the experience of ten neurosurgeons who had implanted a total of 600 dorsal column stimulators claimed that 20% of patients had stimulation of the incorrect dorsal fibers and 15% had irritating thoracic dermatome stimulation. Most of these patients underwent reoperation. Reoperation exposes patients to increased risk of further complications such as infection or dural puncture. Kemler and associates21 reported that 5 of 24 patients had unsatisfactory positioning of their electrodes. A single operation was successful in four patients; a second revision was required for the other patient. Pineda reported that inappropriate distribution of stimulation occurred in 6 of 84 patients with 92 SCS systems implanted.30 He also reported that eight of these patients had paresthesias in the wrong area. The electrodes were repositioned so these patients had appropriate coverage of their painful areas.
Change of patient position or posture is another factor that may affect stimulation. With the unipolar or bipolar electrodes of dorsal column stimulation, Fox21a wrote that 60% of patients experienced a change in current strength to the dorsal columns with change of position. He adds that most patients adjusted well to the necessity of adjusting the voltage in certain positions. Van Buyten20 reported that 11% of patients had changed perception of paresthesias or changed distribution of paresthesias with changes in position. Kemler13 reported that 19 of 22 patients complained that the paresthesias created by SCS changed with their position, resulting in troublesome amplitude changes.13
Barolat61 reported a case of a patient with positional changes in paresthesias. The patient perceived paresthesias while supine but had reduced paresthesias while standing or sitting. Cameron and Alò62 reported that changes in position may cause spinal cord movement that result in changes of perception threshold of stimulation.62 There are postural effects of SCS for percutaneous electrodes that may or may not exist for plate electrodes that are fixed in place. The mean threshold for paresthesia is lowest in the recumbent position. Another consideration is that the layer of CSF changes in different levels of the spinal cord. Typically the least amount of CSF is present in the thoracic spine. Newer SCS devices that are programmable may allow adjustments in stimulation in response to position changes.
Kay and associates31 reported the experience of 70 patients with SCS systems implanted for a variety of pain states over a 13-year period from 1984 to 1997. There were 72 surgical revisions, including electrode revisions or replacement, generator replacement, cable failure, and explantation. Other than battery replacement because of depletion, the most common reason for a revision surgery was inappropriate area of paresthesias. Ten patients underwent reoperation for revision of the placement of the electrode in an attempt to place the electrode in the correct location. Inadequate paresthesias caused by migration, fibrosis, and unknown causes accounted for a total of 21 patients.
The success of SCS as a treatment depends on multiple factors, but success relies on patients not having an unpleasant response to the paresthesias created by the device. A small percentage of patients find the paresthesias to be unpleasant. The 10 neurosurgeons who responded to Fox’s survey reported that 1% of patients found the paresthesias to be more unpleasant than their pain.21a Pineda30 added that 34% of patients had insufficient relief because no paresthesias were perceived or the paresthesias were unpleasant.Turner and colleagues5 published their systematic review of the effectiveness and complications associated with SCS in 2004. The authors discussed the potential loss of effectiveness over time of SCS to treat the patient’s pain. Alò and associates60 reported the outcomes of patients at 24 months, 30 months, and 48 months. Median pain ratings were higher at 30 and 48 months than at 24 months. The pain ratings at 30 and 48 months were lower than the pre-SCS implantation pain. At 30 months 72% of patients were still using their stimulators, but at 48 months only 37% were still using their device.
Kumar and associates18 describe this phenomenon as system tolerance and define it as progressive loss of pain control despite the presence of a fully functioning stimulating system. There is no specific time at which such tolerance may occur. The etiology may be related to plasticity of pain pathways of the central nervous system.9 Thus far attempts at treatment of system tolerance have failed. Fibrosis at the site of the electrode has also been considered but not proven,18 although Sundaraj and associates35 reported that the electrode of a patient who lost stimulation was surrounded by dense, fibrous tissue. That tissue was presumed to be epidural fibrosis that resulted from a foreign body reaction.
SCS and other implantable devices have eroded through skin. With SCS devices specifically, patients typically have less pain and are able to be more active with activities and exercise. As patients become more active, they may lose weight. The weight loss may change the positioning of the generator relative to body mass, which may result in decreases in subcutaneous tissue between the generator and the surface of the skin. Ohnmeiss, Rashbaum, and Bogdanffy63 reported one patient with pain secondary to diabetic peripheral neuropathy who developed local skin erosion over the SCS device. The SCS system was explanted, and the erosion healed. Another SCS system was eventually reimplanted.
Conclusion
Complications that are unique to SCS include migration, breakage, and malfunction of the implanted hardware and undesirable stimulation and tolerance to the stimulation. Because of the hardware, there is a substantial risk of seroma, infection, and rejection. As the electrodes reside in the epidural space, neurological sequelae have been reported. Two large systematic reviews have demonstrated the overall risk to be 34% and 36%.5,6 The complications of SCS are largely treatable or reversible. If a complication cannot be treated successfully while maintaining the device, the device can be explanted in an attempt to reverse the untoward effect. Direct injuries to neural structures are difficult to treat and often not reversible.
1 Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: a preliminary clinical report. Anesth Analg. 1967;46:489-491.
2 Prager J. Estimates of annual spinal cord stimulator implant rises in the United States. Neuromodulation. 2010;13:68-69.
3 Hayek SM. Complication: a painful entity for patient and physician. Techniques Regional Anesth Pain Manage. 2007;11:121.
4 Kohn LT, Corrigan JM, Donaldson MS. To err is human: building a safer health system. Institute of Medicine: Committee on Quality of Health Care in America. Washington, DC, National Academy Press.; 1999.
5 Turner JA, et al. Spinal cord stimulation for patients with failed back surgery syndrome or complex regional pain syndrome: a systematic review of effectiveness and complications. Pain. 2004;108:137-147.
6 Cameron T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review. J Neurosurg. 2004;100:254-267.
7 Quigley DG, et al. Long-term outcome of spinal cord stimulation and hardware complications. Stereotact Funct Neurosurg. 2003;81:50-56.
8 Kumar K. Avoiding complications from spinal cord stimulation: practical recommendations from an international panel of expert. Neuromodulation. 2007;10:24-33.
9 Kumar K, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007;132:179-188.
10 Fitzgibbon DR, et al. Chronic pain management: American Society of Anesthesiologists Closed Claim Project. Anesthesiology. 2004;100:98-105.
11 Bedder MD, Bedder HF. Spinal cord stimulation surgical technique for the nonsurgically trained. Neuromodulation. 2009;12:1-19.
12 Rosenow JM, et al. Failure modes of spinal cord stimulation hardware. J Neurosurg Spine. 2006;5:183-190.
13 Kemler MA, et al. The effect of spinal cord stimulation in patients with chronic reflex sympathetic dystrophy: two years’ follow-up of the randomized controlled trial. Ann Neurol. 2004;55:13-18.
14 Kemler MA, et al. Avoiding complications from spinal cord stimulation: practical recommendations from an international panel of experts. Neuromodulation. 2007;10:24-33.
15 Van Buyten JP. The performance and safety of an implantable spinal cord stimulation system in patients with chronic pain: a 5-year study. Neuromodulation. 2003;6:79-87.
16 Devulder J, et al. Spinal cord stimulation in chronic pain: evaluation of results, complications, and technical considerations in sixty-nine patients. Clin J Pain. 1991;7:21-28.
17 Andersen C. Complications in spinal cord stimulation for treatment of angina pectoris. Differences in unipolar and multipolar percutaneous inserted electrodes. Acta Cardiol. 1997;52:325-333.
18 Kumar K, et al. Complications of spinal cord stimulation, suggestions to improve outcome, and financial impact. J Neurosurg Spine. 2006;5:191-203.
19 Pyles ST, Khodavirdi A: Placement of a spinal cord stimulation system using a single incision: a novel surgical technique. Poster Presentation at the North American Neuromodulation Society Annual Meeting, 2006. Florida Pain Clinic, Ocala, FL.
20 Van Buyten JP, et al. Efficacy of spinal cord stimulation: 10 years of experience in a pain centre in Belgium. European J Pain. 2001;5:299-307.
21 Kemler MA, et al. Spinal cord stimulation in patients with chronic reflex sympathetic dystrophy. N Engl J Med. 2000;343:618-624.
21a Fox JL. Dorsal column stimulation for relief of intractable pain: problems encountered with neuropacemakers. Surg Neurol. 1974;2:59-64.
22 Heidecke V, et al. Hardware failures in spinal cord stimulation for failed back surgery syndrome. Neuromodulation. 2000;3:27-30.
23 Eisenberg E, Waisbrod H. Spinal cord stimulator activation by an antitheft device: case report. J Neurosurg. 1997;87:961-962.
24 Woods DM, et al. Complications of neurostimulation. Techniques in Regional Anesthesia and Pain Management. 2007;11:178-182.
25 Barolat G. Spinal cord stimulation for chronic pain management. Arch Med Res. 2000;31:258-262.
26 Temel Y, et al. Management of hardware infections following deep brain stimulation. Acta Neurochir (Wien). 2004;146:355-361.
27 Torrens JK, et al. Risk of infection with electrical spinal-cord stimulation. Lancet. 1997;349:729.
28 Barolat G. Experience with 509 plate electrodes implanted epidurally from C1 to L1. Stereotact Funct Neurosurg. 1993;61:60-79.
29 Follett KA, et al. Prevention and management of intrathecal drug delivery and spinal cord stimulation system infections. Anesthesiology. 2004;100:1582-1594.
30 Pineda A. Complications of dorsal column stimulation. J Neurosurg. 1978;48:64-68.
31 Kay AD. Spinal cord stimulation—a long-term evaluation in patients with chronic pain. Br J Neurosurg. 2001;15:335-341.
32 Meglio M, Cioni B, Rossi GF. Spinal cord stimulation in management of chronic pain: a 9-year experience. J Neurosurg. 1989;70:519-524.
33 McDonald M, et al. Single versus multiple dose antimicrobial prophylaxis for major surgery: a systematic review. Aust NZ J Surg. 1999;68:388-396.
34 Deer TR. Complications of spinal cord stimulation: identification, treatment, and prevention. Pain Med. 2008;9:S93-S101.
35 Sundaraj SR, et al. Spinal cord stimulation: a seven-year audit. J Clin Neurosci. 2005;12:264-270.
36 Eldrige JS, Weingarten TN, Rho RH. Management of cerebral spinal fluid leak complicating spinal cord stimulator implantation. Pain Pract. 2006;6:285-288.
37 Wolfensberger TJ, Borruat FX. Sixth nerve palsy following epidural spinal cord stimulation for lower limb ischaemia. Eye (Lond). 2000;14(Pt 5):811-812.
38 Insel TR, et al. Abducens palsy after lumbar puncture. N Engl J Med. 1980;303:703.
39 Fairclough WA. Sixth nerve paralysis after spinal analgesia. Br Med J. 1945;11:801-803.
40 Ward TN, Levin M. Case reports: headache caused by a spinal cord stimulator in the upper cervical spine. Headache. 2000;40:689-691.
41 Franzini A, et al. Huge epidural hematoma after surgery for spinal cord stimulation. Acta Neurochir (Wien). 2005;147:565-567. discussion 567
42 Kou J, et al. Risk factors for spinal epidural hematoma after spinal surgery. Spine (Phila Pa 1976). 2002;27:1670-1673.
43 Chiravuri S, et al. Subdural hematoma following spinal cord stimulator implant. Pain Physician. 2008;11:97-101.
44 Kardash K, Morrow F, Beique F. Seizures after epidural blood patch with undiagnosed subdural hematoma. Reg Anesth Pain Med. 2002;27:433-436.
45 Grillo PJ, Yu HC, Patterson RHJr. Delayed intraspinal hemorrhage after dorsal column stimulation for pain. Arch Neurol. 1974;30:105-106.
46 Meyer SC, Swartz K, Johnson JP. Quadriparesis and spinal cord stimulation: case report. Spine (Phila Pa 1976). 2007;32:E565-568.
47 Cole JD, Illis LS, Sedgwick EM. Pain produced by spinal cord stimulation in a patient with allodynia and pseudo-tabes. J Neurol Neurosurg Psychiatry. 1987;50:1083-1084.
48 Turner JA, et al. Spinal cord stimulation for failed back surgery syndrome: outcomes in a workers’ compensation setting. Pain. 2009;148:14-25.
49 Kemler MA, Barendse GA, Van Kleef M. Relapsing ulcerative colitis associated with spinal cord stimulation. Gastroenterology. 1999;117:215-217.
50 Barbara G, et al. Relapsing ulcerative colitis after spinal cord stimulation: a case of intestinal neurogenic inflammation? Gastroenterology. 1999;117:1256-1257.
51 Thakkar N, Connelly NR, Vieira P. Gastrointestinal symptoms secondary to implanted spinal cord stimulators. Anesth Analg. 2003;97:547-549. table of contents
52 Larkin TM, Dragovich A, Cohen SP. Acute renal failure during a trial of spinal cord stimulation: theories as to a possible connection. Pain Physician. 2008;11:681-686.
53 Loubser PG. Adverse effects of epidural spinal cord stimulation on bladder function in a patient with chronic spinal cord injury pain. J Pain Symptom Manage. 1997;13:251-252.
54 Virbow J. Pacemaker contact sensitivity. Contact Dermatitis. 1985;3:173.
55 Burton CV. Safety and clinical efficacy. Neurosurgery. 1977;1:214-215.
56 McKenna KE, McCleane G. Dermatitis induced by a spinal cord stimulator implant. Contact Dermatitis. 1999;41:229.
57 Wilkerson MG, Jordan WPJr. Pressure dermatitis from an implanted pacemaker. Dermatol Clin. 1990;8:189-192.
58 Long DM, Erickson DE. Stimulation of the posterior columns of the spinal cord for relief of intractable pain. Surg Neurol. 1975;4:134-141.
59 Ochani TD, et al. Allergic reaction to spinal cord stimulator. Clin J Pain. 2000;16:178-180.
60 Alò KM, et al. Four-year follow-up of dual electrode spinal cord stimulation for chronic pain. Neuromodulation. 2002;5:79-88.
61 Barolat G. Epidural spinal cord stimulation in the management of reflex sympathetic dystrophy. Stereotactic Functional Neurosurg. 1989;53:29-39.
62 Cameron T, Alò KM. Effects of posture on stimulation parameters in SCS. Neuromodulation. 1998;1:177-183.
63 Ohnmeiss DD, Rashbaum RF, Bogdanffy GM. Prospective outcome evaluation of spinal cord stimulation in patients with intractable leg pain. Spine (Phila Pa 1976). 1996;21:1344-1350. discussion 1351









 months of continuous stimulation, the ulcerative colitis relapsed. After the patient’s gastroenterologist suggested that there might be an association between the SCS and the ulcerative colitis, the SCS device was turned off. Shortly thereafter the ulcerative colitis went into remission. The SCS was attempted again, but within 2 weeks the ulcerative colitis returned. Placing the battery elsewhere was not attempted.
months of continuous stimulation, the ulcerative colitis relapsed. After the patient’s gastroenterologist suggested that there might be an association between the SCS and the ulcerative colitis, the SCS device was turned off. Shortly thereafter the ulcerative colitis went into remission. The SCS was attempted again, but within 2 weeks the ulcerative colitis returned. Placing the battery elsewhere was not attempted.