CHAPTER 31 Complications of Shoulder Arthroscopy
The first large-scale report of complication rates was by Small1 in 1986, which included 14,329 shoulder arthroscopic procedures. He noted an overall complication rate of 5.3% with staple capsulorrhaphy but only 0.76% with subacromial surgery. This early study demonstrated the relative safety of shoulder arthroscopy but was limited to relatively simple procedures. Multiple other reviews have been reported since then, but the numbers are difficult to compare for a number of reasons, including differing definitions of what actually constitutes a complication. Berjano and colleagues2 reported on 179 shoulder arthroscopic procedures, noting an overall complication rate of 9.49%. They stated that in procedures that combined arthroscopy with an open procedure, the complication rate was 5.26% versus 10.63% in arthroscopic procedures alone. Interestingly, they did not define shoulder stiffness as a postoperative complication and also noted that few of the complications were serious. Muller and associates3 reported on 846 shoulder arthroscopic procedures, with an overall complication rate of 5.8%. Of the complications in this series, 43% were caused by infections.
What does appear to be consistent across the literature is that the overall complication rate has remained stable, even in the face of the ever-increasing complexity of arthroscopic procedures. Most reviews place this number between 5.8% and 9.5%.1–5 This may be to the result of improvements in arthroscopic instrumentation, techniques, and implants, as well as a better understanding of shoulder pathology and function. These advancements may help offset the potential for increased complication rates associated with more complex procedures. Standardized postoperative rehabilitation programs also have likely decreased some of the complications seen in shoulder arthroscopy.
GENERAL COMPLICATIONS
The incidence of infection after shoulder arthroscopy is low. This may be due to the result of the copious amount of irrigation that is constantly run through the shoulder during the procedure, as well as the minimal exposure of deep tissues to the environment. D’Angelo and Ogilvie-Harris6 reported on 9 cases of septic arthritis after arthroscopy, with an incidence of 0.23%. Based on their studies, they recommended routine antibiotic prophylaxis to prevent these potentially serious complications. Bigliani and coworkers7 reviewed the literature in 1991 and noted infection rates from 0.04% to 3.4%. Other reviews have noted similar infectious rates.2,4 Brislin and colleagues8 published a recent study on 263 patients who had undergone arthroscopic rotator cuff repair; they noted only a single infection in this group, with an incidence of 0.38%. Athwal and associates,9 in 2007, reported on 39 cases of deep infection after rotator cuff repair. They noted that long-term functional limitations in these patients are not uncommon. They emphasized recognizing Propionibacterium acnes as the most likely causative organism and the need for at least 7 days of culture to identify this organism. P. acnes has also been implicated as a potential causative factor for the onset of chondrolysis after shoulder arthroscopy and therefore should be treated aggressively. Regardless of the true incidence, infection after shoulder arthroscopy requires a high index of suspicion with prompt management once deep infection is recognized.
General anesthetic complications germane to any surgical procedure also apply to shoulder arthroscopy. In addition, fatal air embolism10 and pneumothorax,11 possibly related to the use of CO2, pulmonary edema,12 and pneumomediastinum13 have all been reported during shoulder arthroscopic procedures. It is important to educate patients about these risks for them to understand that minimally invasive procedures can still be associated with significant complications.
Of special relevance to shoulder arthroscopy is the increasing use of interscalene anesthesia for intraoperative pain management and postoperative pain control (Fig. 31-1). Although purported to be a safe and effective means for pain control, reports of seizures, cardiovascular collapse, and severe respiratory distress have been noted. Additionally, neurologic complications in the form of transient and even permanent brachioplexopathies are a known risk of regional brachial plexus blockade.14,15 Lenters and coworkers16 have reviewed the experience of a single medical center over a 15-year period and supplemented this data with records from the national American Society of Anesthesiology Closed Claims Database. From the hospital experience, they reported a total of 41 major complications following interscalene block, with 14 of them still present, affecting comfort and function at the most recent follow-up. From the database, they noted 19 permanent complications and 4 deaths attributable to interscalene brachial plexus block. It appears that fewer complications arise as anesthesiologists become more experienced with the techniques and with the use of newer technologies, including ultrasound-guided blocks. However, complications can and do still occur. It is important that the anesthesiologists performing these procedures have significant experience and understand the risks, and that they communicate these to the patients. These procedures must be performed with the patient awake. Ultrasound guidance to direct needle placement is expected to decrease the risk of interscalene blocks.
Deep venous thrombosis (DVT) of the lower extremity after shoulder arthroscopy has rarely been noted in the literature.17,18 Although there have only been a few reported cases of life-threatening pulmonary embolism (PE), the prevalence of these complications are likely much more common than once thought. Standard precautions, such as using mechanical compression devices on the lower extremities during the procedure and early mobilization, may minimize the risk. Currently, there are no guidelines in place suggesting that the use of routine chemoprophylaxis is indicated after shoulder arthroscopy.
COMPLICATIONS SPECIFIC TO SHOULDER ARTHROSCOPY
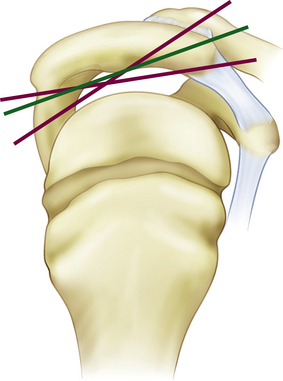
Complications resulting from the two prevalent patient positions used during arthroscopy do occur. In the lateral decubitus position (Fig. 31-2), traction neuropraxia has been reported to occur up to 10% of the time.19 In 1987, Klein and colleagues20 placed strain gauges on the brachial plexus of cadavers and measured the strain on the plexus in the lateral decubitus position with varying amounts of flexion and abduction. They found that increasing flexion and abduction decreased strain on the brachial plexus. Stanish and Peterson21 have reviewed the literature on neurologic injuries after shoulder arthroscopy and suggested that careful patient positioning, minimal traction to distract the joint (no more than 7 kg), limiting the length of the procedure, and accurate portal positioning could minimize neurovascular complications.
The beach chair position (Fig. 31-3) was described in 1988 by Skyhar and associates.22 Its advantages include easier airway access, placing the anatomy in the standard upright position, the ability to move the arm into different positions intraoperatively and decreased risk of traction-related neuropraxia. Several reports of catastrophic complications have been reported recently with the use of the beach chair position. Pohl and Cullen23 reported on four cases of shoulder surgery in the beach chair position that resulted in death in one patient and severe brain damage in three others. Stroke, brain death, loss of vision, and ophthalmoplegia have also been described.24,25 These complications are thought to be attributable to errors in blood pressure reference points leading to decreased cerebral perfusion. In 2008, Papadonikolakis and coworkers26 reviewed the literature and suggested using invasive blood pressure monitoring or placing a blood pressure cuff around the contra lateral brachium (at the level of the heart) to avoid erroneous pressure numbers. Currently, it appears that the choice of position should be left up to the surgeon. Recognition of the potential complications of the preferred position and taking preventative measures can minimize the occurrence of a significant untoward event.
With the increasing complexity of arthroscopic procedures about the shoulder, the need to establish portals that allow for optimal access and precision placement of implants has also increased. Although vascular complications associated with portal placement remain rare,4,27 direct neurologic injury remains a concern. Segmuller and colleagues28 noted direct cutaneous nerve injury in 7% of their 304 patients undergoing shoulder arthroscopy. Most of these injuries involved a cutaneous branch of the axillary nerve at the site of the established lateral portal. This concern over direct nerve injury underscores the importance of accurate portal placement. In 2004, Lo and associates29 studied the neurovascular anatomy in proximity to the most commonly used arthroscopic portals established via an outside-in approach. They noted that with the exception of the cephalic vein, all the neurovascular structures were more than 20 mm away from all the portals evaluated; it was concluded that the standard and accessory arthroscopic portals are safe when established in an outside-in fashion. Other considerations such as careful palpation of bony landmarks, accurate knowledge of shoulder anatomy, accounting for fluid extravasation, and the use of cannulas during portal placement may enhance safety during shoulder arthroscopy.
Iatrogenic tendon injury has been described in the literature. Norwood and Fowler30 described this complication in 1989. The port of Wilmington portal is an accessory portal used primarily for repair of posterior and posterior-superior labral tears. Proper placement of the portal leads to penetration of the muscular portion of the supraspinatus. However, if improperly placed, injury to the tendinous portion of the supraspinatus can occur. It appears that accurate portal placement and a thorough understanding of shoulder anatomy can minimize this complication.
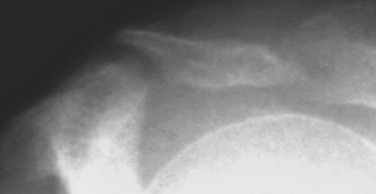
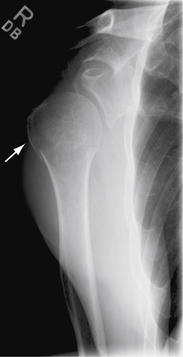
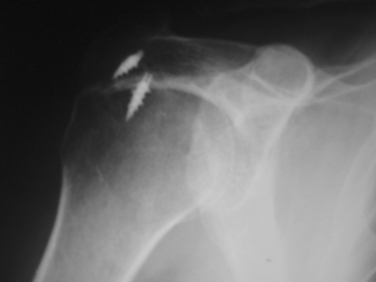
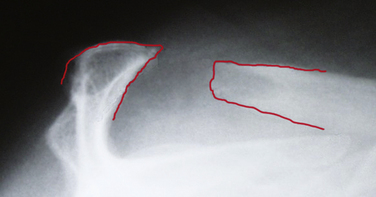
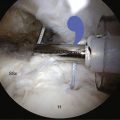
Fractures of the acromion, clavicle, and humerus can occur during shoulder arthroscopy. Fracture of the acromion has been extensively described in the literature.4,5,31 This is usually a result of overzealous and inaccurate resection of the acromial spur, often in the setting of a patient with a thin acromion preoperatively (Fig. 31-4). Simple precautions can be taken to prevent this complication. Measurement of the thickness of the acromion on preoperative radiographs and attention to the depth of resection using the known diameters of the arthroscopic tools as a reference are helpful (Fig. 31-5). Additionally, adequate visualization and exposure of the anterior acromion to prevent beginning the resection too far posteriorly, as well as working from anterior-inferior to superior, will further minimize the chance of excessive resection. Fractures can be treated with screw fixation, tension band fixation, or excision of the remaining bone and repair of the deltoid attachment. Clavicle fractures can occur as a result of the surgeon becoming disoriented during subacromial surgery. At this point, the surgeon may mistakenly resect through the shaft of the clavicle instead of the acromioclavicular (AC) joint because of straying too medially during the procedure. Humeral fractures are also a concern during shoulder arthroscopy. Humeral neck or shaft fractures can occur as a result of excessive manipulation of a stiff shoulder (Fig. 31-6). Greater tuberosity fractures may follow improper instrument penetration (Fig. 31-7) or implant placement. Paying attention to the amount of force imparted during manipulation and careful attention to instrument placement and topography of the tuberosity can minimize the risk of these complications. When such a fracture occurs, it can be treated conservatively or by reduction and internal fixation, depending on the stability of the fracture.
Stiffness is probably the most common complication of any surgery about the shoulder. It can be a significant cause of morbidity, loss of function, and continued disability. Significant restriction of motion has been proposed to occur, ranging from 2.8%5 to 15%3 following shoulder arthroscopy. Stiffness is manifested clinically by decreased active and passive range of motion, often with a very profound loss of external rotation. This is often accompanied by a period of worsening pain after the postsurgical pain has abated. The physical examination demonstrates restricted glenohumeral motion, with a disproportionate amount of motion coming from scapulothoracic rotation. It is important to differentiate postoperative stiffness from restricted motion secondary to complex regional pain syndrome (CRPS). In the latter, physical examination findings of hyperesthesia, profound edema, excessive warmth, and neurologic dysfunction often accompany the lack of motion. If CRPS is suspected, a full workup, usually with the aid of a pain specialist, is required to manage the condition. Postoperative stiffness can be minimized, with early range of motion (ROM) and emphasis on scapular stabilization to encourage smooth glenohumeral motion.
Correctly identifying those patients who are stiff preoperatively is as important as identifying postoperative patients who may develop stiffness. Often, pain restricts a patient’s ROM in the clinical setting, However, if the clinician fails to diagnose early adhesive capsulitis in this setting or if it is, for example, mistakenly attributed to impingement syndrome, the wrong course of treatment may be prescribed. Erroneous diagnosis in this setting may lead to unnecessary surgery and poor postoperative outcomes.
Despite the best efforts on the part of surgeon and patient, a small subset of postoperative shoulders will become stiff and not respond to conservative management. In this group, closed manipulation and/or arthroscopic release is highly effective to restore motion and relieve pain.32,33 Currently, there is no consensus regarding the timing of manipulation or release in this population, with proponents of early (3 month) and late (more than 6 months) very prevalent.
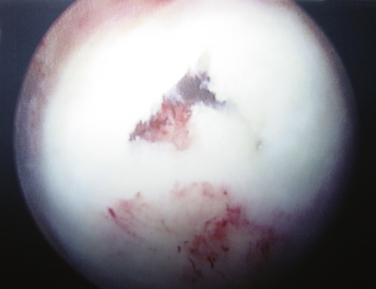
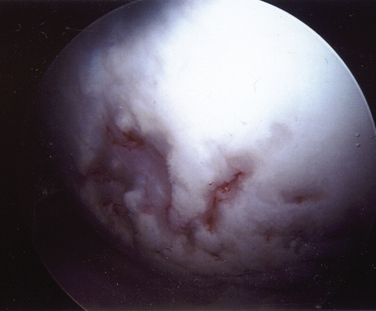
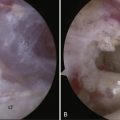
Chondrolysis is a rare but devastating process that has been identified to occur after shoulder arthroscopy (Fig. 31-8). Although no specific cause has been elicited, several potential contributing factors have been explored. Reports of chondrolysis have been made following the use of an intra-articular pain pump.34,35 Uncertainty exists about its contribution to articular cartilage damage. Continuous intra-articular infusion pumps have been studied in the knee and to the best of my knowledge, there have been no reports of chondrolysis. Pain pumps often infuse bupivacaine, a substance that has been implicated as chondrotoxic in various animal and in vitro models.34–36 However, a 2008 study by Dragoo and coworkers37 reported that exposure of cultured chondrocytes to bupivacaine up to a maximum of 48 hours was not significantly chondrotoxic, whereas more prolonged exposure led to increased chondrocyte death. Additionally, bupivacaine injections into the glenohumeral and knee joints are commonly performed in the orthopedically in clinical and operating room settings without significant observed rates of chondrolysis, lending more evidence that significant cartilage damage may not occur after a single exposure to intra-articular bupivacaine.
Several reports have associated chondrolysis with the use of a thermal probe for capsulorrhaphy or capsular release.38–40 In 2009, Good and colleagues41 studied temperatures in the glenohumeral joints of cadavers using a thermal device in different flow states. They noted that the temperature in the joint rose above 45° C in all flow states but that higher flow states led to quicker dispersion of the heat than lower flow states. Irrigant temperatures above 45° C have been shown to result in chondrocyte death. They concluded that the use of a thermal probe might lead to temperatures high enough to cause chondrocyte death and that maintaining adequate fluid pump flow rates may help minimize exposure to high temperatures and protect the articular cartilage.
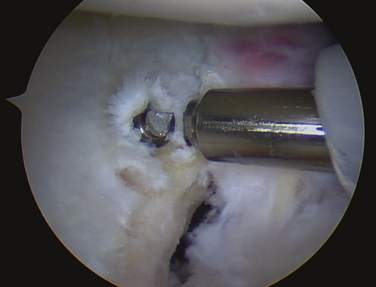
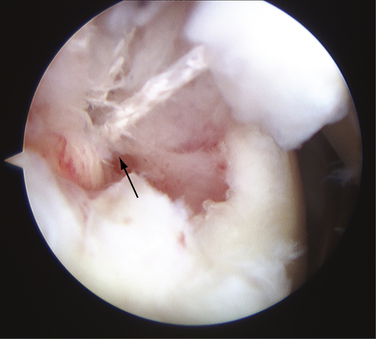
Increasing numbers and types of implants used to stabilize and repair structures about the shoulder are becoming available. These implants have had instrument systems developed to aid their placement arthroscopically. Instruments can break during attempted implantation of an anchor, leading to incarcerated foreign bodies within the glenohumeral joint (Fig. 31-9).
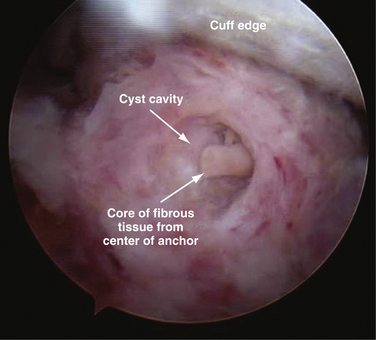
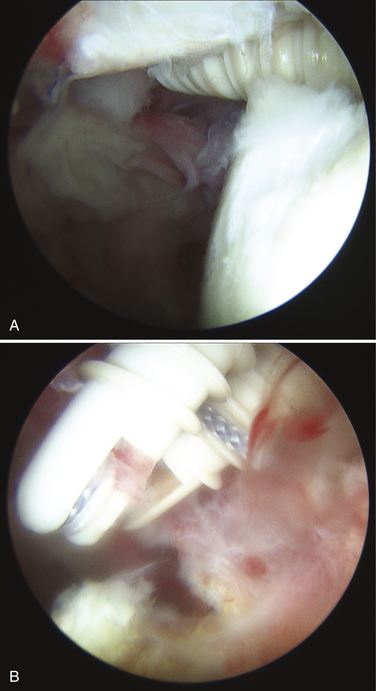
The surgeon has the choice to use metal, bioabsorbable, bioinert, or even recently developed biocompatible implants. Metal implants have the advantage of being able to be followed on routine radiography to confirm placement. Disadvantages include artifact creation on subsequent MRI evaluation, loosening, and migration (Fig. 31-10).42 To address these concerns, bioabsorbable implants have been developed and extensively used in place of metal implants. These implants have been scrutinized for complications, including loose body creation as a result of partial anchor resorption and osteolysis stemming from the biologic response as the anchor resorbs (Fig. 31-11).43,44 Reported complications with the use of bioabsorbable implants are very rare, especially with newer generation implants with more reliable degradation profiles and sturdier suture-eyelet constructs.45 Bioinert and biocompatible implants have also been developed to address concerns about the degradation and osteolysis profile of bioabsorbable implants. When these radiolucent implants are used, if they are inserted in a proud manner or become loose, they may cause significant articular cartilage injury as loose bodies before the cause is recognized (Fig. 31-12). One should harbor a high index of suspicion for this complication in the setting of a patient with mechanical symptoms and pain after implantation of radiolucent anchors.
COMPLICATIONS ASSOCIATED WITH SPECIFIC PROCEDURES
Subacromial Surgery
Overall, subacromial surgery has the lowest rate of complications of all procedures in the shoulder, between 0.76% and 6.5%.1,5 The most common reported cause of failure of arthroscopic acromioplasty is inadequate resection.46 Difficult visualization is a significant factor in most inadequate resections. Visualization can be maximized with extensive bursectomy, meticulous hemostasis, relative hypotensive anesthesia, and adequate hydrostatic pressure. Excessive resection of the acromion should also be avoided (Fig. 31-13). As noted, preoperative planning with careful attention paid to acromial thickness and morphology can minimize its occurrence.
Finally, postoperative AC symptoms can occur when the AC joint is violated during acromioplasty. Whether to leave the AC joint undisturbed, coplane the AC joint, or resect the distal clavicle when violation and inferior osteophytes are encountered remains controversial.46,47 Biomechanical studies have demonstrated increased superior and anterior translation of the clavicle in coplaned specimens48 and AC joint instability symptoms have been reported clinically after coplaning. However, additional studies have noted no increased incidence of AC joint symptoms, compromised clinical results, or additional surgery at an average follow-up of up to 6 years after coplaning the AC joint.49
Rotator Cuff Repair
Arthroscopic rotator cuff repair has gained in popularity recently, with subjective and objective results similar to miniopen rotator cuff repair.50 Failure of the rotator cuff repair remains a concern regardless of the technique employed. In 2007, Cole and associates51 evaluated cuff repair integrity in 49 shoulders at a minimum of 2 years follow-up using MRI and noted that 22% of repairs had a recurrent tear. Brislin and coworkers8 reviewed 263 repairs in 2007 and noted only one reoperation for repair failure. No follow-up imaging was used to identify a retear. Although cuff integrity is not universal following rotator cuff repair in asymptomatic shoulders, it is clearly associated with a better outcome.52 Several recent studies have demonstrated that the tear size at the time of repair and age are critical factors in assessing the risk of failure.51,53 Additionally, other causes for failure can prove to be to the result of concomitant pathology not addressed or identified, including glenohumeral arthritis, impingement, AC joint pain, biceps problems, and even cervical-based pain origins.
During rotator cuff repair, visualization and arm positioning are key to avoid placing anchors too medially into the articular margin (Fig. 31-14). Visualization is improved with careful hemostasis, attention to intraoperative blood pressure, and extensive bursectomy. When placing suture anchors into the medial aspect of the greater tuberosity, the arm should be adducted as much as possible to avoid a shallow approach to the tuberosity and potential penetration of the humeral head. An incision just off the lateral acromion will also improve the trajectory of the anchor and decrease the risk of articular injury.
Instability Repair
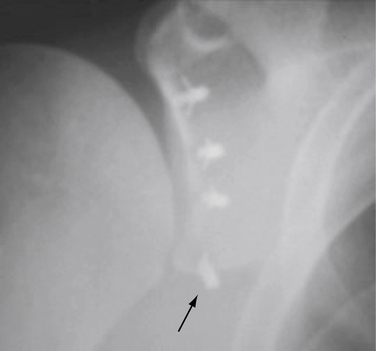
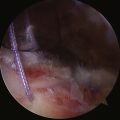
Recurrence after arthroscopic instability surgery remains a relatively common complication. Shaffer and Tibone46 reviewed the literature on rates of recurrence and noted a 16% to 33% rate with staple capsulorrhaphy, 0% to 60% with transglenoid suturing, 0% to 37% with tack stabilization, and 0% to 30% with suture anchor repair. Additionally, each of these techniques has its own unique complications associated with it. Stabilization using a resorbable tack has been reported to demonstrate the rare occurrence of an exuberant synovitis caused by the degradation products of the tack.54,55 Posterior to anterior pin placement with tacks can place the apex of the lung at risk with resultant pneumothorax.56 Transglenoid suture capsulorrhaphy places the suprascapular nerve in danger (Fig. 31-15). The transglenoid method has also been associated with fistula and cyst formation57 and intrathoracic penetration with the guide pin.58
Recently, suture anchor capsulorrhaphy has become the gold standard in arthroscopic stabilization. Several studies have claimed that with newer techniques and improved anchors, outcomes of arthroscopic stabilization are approaching those of open technique, with the potential benefit of less morbidity and improved ROM.59–61 This view is not without some controversy. A meta-analysis performed by Lenters and colleagues62 in 2007 noted that arthroscopic suture anchor techniques are associated with significantly higher risks of recurrent instability and dislocation than open methods. However, the arthroscopic repairs were associated with higher Rowe scores than the open methods, providing evidence that function and motion may be better preserved using arthroscopic approaches.
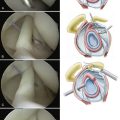
Suture anchor placement during arthroscopic capsulorrhaphy requires a detailed three-dimensional knowledge of glenoid anatomy to prevent anchor malposition (Fig. 31-16). Understanding the slope of the glenoid neck, glenoid version, and angle of approach during anchor placement is critical. Similar to rotator cuff repair, choosing metal or radiolucent implants will determine whether implant position can be followed postoperatively with standard radiography.
SUMMARY
Shoulder arthroscopy appears to be a safe and effective technique based on a review of the literature. Despite the increasing complexity of procedures being done, complication rates have remained steady. However, serious and even life-threatening complications, although rare, can and do occur. Decisions made before (e.g., choice of position, type of anesthesia), during (e.g., choice of implant, use of thermal devices, portal placement) and after surgery (e.g., rehabilitation, use of pain catheters) can affect the complication profile and rate in an individual practice. A thorough understanding of shoulder anatomy, implant design, and patient risk factors can help minimize complications. Finally, when complications do occur, early recognition and management can minimize the morbidity of these procedures.
1. Small NC Complications in arthroscopy: the knee and other joints. Committee on Complications of the Arthroscopy Association of North America. Arthroscopy, 2; 1986:253-258.
2. Berjano P, González BG, Olmedo JF, et al. Complications in arthroscopic shoulder surgery. Arthroscopy. 1998;14:785-788.
3. Muller D, Landsiedl F Arthroscopy of the shoulder joint: a minimal invasive and harmless procedure. Arthroscopy, 16; 2000:425.
4. Curtis AS, Snyder SJ, Delpizzo W, et al. Complications of shoulder arthroscopy. Arthroscopy. 1992;8:395.
5. Rupp S, Seil R, Muller B. Complications after subacromial decompression. Arthroscopy. 1998;14:445.
6. D’Angelo CL, Olgivie-Harris DJ. Septic arthritis following arthroscopy with cost/benefit of antibiotic prophylaxis. Arthroscopy. 1988;4:10-14.
7. Bigliani LU, Flatow EL, Deliz ED. Complications of shoulder arthroscopy. Orthop Rev. 1991;20:743-751.
8. Brislin KJ, Field LD, Savoie FH3rd. Complications after arthroscopic rotator cuff repair. Arthroscopy. 2007;23:124-128.
9. Athwal GS, Sperling JW, Rispoli DM, Cofield RH. Deep infection after rotator cuff repair. J Shoulder Elbow Surg. 2007;16:306-311.
10. Bauereis C, Schifferdecker A, Buttner J, Hempfling H. Fulminant air embolism in arthroscopy of the shoulder using CO2. Anasthesiol Intensivmed Notfallmed Schmerzther. 1996:1654-1657.
11. Burkhart SS, Barnett CR, Snyder SS. Transient postoperative blindness as a possible effect of glycine toxicity. Arthroscopy. 1990;6:112-114.
12. Anderson AF, Alfrey D, Lipscomb AB Acute pulmonary edema, an unusual complication following arthroscopy: a report of three cases. Arthroscopy, 6; 1990:235-237.
13. Lee HC, Dewan N, Crosby L. Subcutaneous emphysema, pneumomediastinum, and potentially life-threatening tension pneumothorax. Pulmonary complications from arthroscopic shoulder decompression. Chest. 1992;101:1265-1268.
14. Weber SC, Jain R Scalene regional anesthesia for shoulder surgery in a community setting: an assessment of risk. J Bone Joint Surg Am, 84; 2002:775-779.
15. Ben-David B, Barak M, Katz Y, Stahl S. A retrospective study of the incidence of neurological injury after axillary brachial plexus block. Pain Pract. 2006;6:119-123.
16. Lenters TR, Davies J, Matsen FA The types and severity of complications associated with interscalene brachial plexus block anesthesia: local and national evidence. J Shoulder Elbow Surg, 16; 2007:379-387.
17. Polzhofer GK, Petersen W, Hassenpflug J. Thromboembolic complication after arthroscopic shoulder surgery. Arthroscopy. 2003;19:129-132.
18. Starch DW, Clevenger CE, Slauterbeck JR. Thrombosis of the brachial vein and pulmonary embolism after subacromial decompression of the shoulder. Orthopaedics. 2001;24:63-65.
19. Pitman MI, Nainzedeh N, Ergas E, Springer S. The use of somatosensory evoked potentials for detection of neuropraxia during shoulder arthroscopy. Arthroscopy. 1988;4:250-255.
20. Klein AH, France JC, Mutschler TA, Fu FH. Measurement of brachial plexus strain in arthroscopy of the shoulder. Arthroscopy. 1987;3:45-52.
21. Stanish WD, Peterson DC Shoulder arthroscopy and nerve injury: pitfalls and prevention. Arthroscopy, 11; 1995:458-466.
22. Skyhar MJ, Altchek DW, Warren RF, et al. Shoulder arthroscopy with the patient in the beach-chair position. Arthroscopy. 1988;4:256-259.
23. Pohl A, Cullen DJ Cerebral ischemia during shoulder surgery in the upright position: a case series. J Clin Anesth, 17; 2005:463-469.
24. Gale T, Leslie K. Anaesthesia for neurosurgery in the sitting position. J Clin Neurosci. 2004;11:693-696.
25. Bhatti MT, Enneking FK. Visual loss and ophthalmoplegia after shoulder surgery. Anesth Analg. 2003;96:899-902.
26. Papadonikolakis A, Wiesler ER, Olympio MA, Poehling GG. Avoiding catastrophic complications of stroke and death related to shoulder surgery in the sitting position. Arthroscopy. 2008;24:481-482.
27. Weber SC, Abrams JS, Nottage WM. Complications associated with arthroscopic shoulder surgery. Arthroscopy. 2002;18(suppl):88-95.
28. Segmuller H, Alfred SP, Zilio G. Cutaneous nerve lesions of the shoulder and arm after arthroscopic surgery. J Shoulder Elbow Surg. 1995;4:254-258.
29. Lo IKY, Lind CCL, Burkhart SS Glenohumeral arthroscopy portals established using an outside-in technique: neurovascular anatomy at risk. Arthroscopy, 20; 2004:596-602.
30. Norwood LA, Fowler HL. Rotator cuff tears. A shoulder arthroscopy complication. Am J Sports Med. 1989;17:837-841.
31. Matthews LS, Blue JM Arthroscopic subacromial decompression: avoidance of complications and enhancement of results. Instr Course Lect, 47; 1998:29-33.
32. Warner JJ, Allen AA, Marks PH, Wong P. Arthroscopic release of postoperative capsular contracture of the shoulder. J Bone Joint Surg Am. 1997;79:1151-1158.
33. Weber SC Arthroscopic management of the stiff shoulder: The first 100 cases. Arthroscopy, 13; 1997:381.
34. Gomoll AH, Kang RW, Williams JM, et al Chondrolysis after continuous intra-articular bupivacaine infusion: an experimental model investigating chondrotoxicity in the rabbit shoulder. Arthroscopy, 22; 2006:813-819.
35. Hansen BP, Beck CL, Beck EP, Townsley RW. Postarthroscopic glenohumeral chondrolysis. Am J Sports Med. 2007;35:1619-1620.
36. Gomoll AH, Yanke AB, Kang RW, et al. Long-term effects of bupivacaine on cartilage in a rabbit shoulder model. Am J Sports Med. 2009;37:72-77.
37. Dragoo JL, Korotkova T, Kanwar R, Wood B. The effect of local anesthetics administered via pain pump on chondrocyte viability. Am J Sports Med. 2008;36:1484-1488.
38. Good CR, Shindle MK, Kelly BT, et al. Glenohumeral chondrolysis after shoulder arthroscopy with thermal capsulorrhaphy. Arthroscopy. 2007;23:797.
39. Ciccone WJ2nd, Weinstein DM, Elias JJ. Glenohumeral chondrolysis following thermal capsulorrhaphy. Orthopedics. 2007;30:158-160.
40. Jerosch J, Aldawoudy AM Chondrolysis of the glenohumeral joint following arthroscopic capsular release for adhesive capsulitis: a case report. Knee Surg Sports Traumatol Arthrosc, 15; 2007:292-294.
41. Good CR, Shindle MK, Griffith MH, et al. Effect of radiofrequency energy on glenohumeral fluid temperature during shoulder arthroscopy. J Bone Joint Surg Am. 2009;91:429-434.
42. Silver MD, Daigneault JP. Symptomatic interarticular migration of glenoid suture anchor. Arthroscopy. 2000;16:102-105.
43. Barber FA. Biodegradable shoulder anchors have unique modes of failure. Arthroscopy. 2007;23:316-320.
44. Spoliti M. Glenoid osteolysis after arthroscopic labrum repair with a bioabsorbable suture anchor. Acta Orthop Belg. 2007;73:107-110.
45. Ozbaydar M, Elhassan B, Warner JJP The use of anchors in shoulder surgery: a shift from metallic to bioabsorbable anchors. Arthroscopy, 23; 2007:1124-1126.
46. Shaffer BS, Tibone JE. Arthroscopic shoulder instability surgery. Complications. Clin Sports Med. 1999;18:737-767.
47. Fischer BW, Gross RM, McCarthy JA, Arroyo JS. Incidence of acromioclavicular joint complications after arthroscopic subacromial decompression. Arthroscopy. 1999;15:241-248.
48. Edwards SG Acromioclavicular stability: a biomechanical comparison of acromioplasty to acromioplasty with coplaning of the distal clavicle. Arthroscopy, 19; 2003:1079-1084.
49. Barber FA. Long-term results of acromioclavicular joint coplaning. Arthroscopy. 2006;22:125-129.
50. Morse K, Davis AD, Afra R, et al Arthroscopic versus mini-open rotator cuff repair: a comprehensive review and meta-analysis. Am J Sports Med, 36; 2008:1824-1828.
51. Cole BJ, McCarty LP3rd, Kang RW, et al Arthroscopic rotator cuff repair: prospective functional outcome and repair integrity at minimum 2-year follow-up. J Shoulder Elbow Surg, 16; 2007:579-585.
52. Harryman DTIII, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FAIII Repairs of he rotator cuff: Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am, 73; 1991:982-989.
53. Nho SJ, Brown BS, Lyman S, et al Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg, 18; 2009:13-20.
54. Edwards DJ, Hoy G, Saies AD, et al. Adverse reaction to and absorbable shoulder fixation device. J Shoulder Elbow Surg. 1994;3:230-233.
55. Burkart A, Imhoff AB, Roscher E. Foreign-body reaction to the bioabsorbable suretac device. Arthroscopy. 2000;16:91-95.
56. Slatery SC, Jobe CM, Watkins B Posterior to anterior transglenoid pins: anatomy at risk. Arthroscopy, 14; 1998:285-288.
57. Moran MC, Warren RF. Development of a synovial cyst after arthroscopy of the shoulder. J Bone Joint Surg Am. 1989;71:127-129.
58. Shea KP, Lovallo LJ Scapulothoracic penetration of a Beath pin: an unusual complication of arthroscopic Bankart suture repair. Arthroscopy, 7; 1991:115-117.
59. Armstrong A, Boyer D, Ditsios K, Yamaguchi K. Arthroscopic versus open treatment of anterior shoulder instability. Instr Course Lect. 2004;53:559-563.
60. Cole BJ, Warner JJ. Arthroscopic versus open bankart repair for traumatic anterior shoulder instability. Clin Sports Med. 2000;19:19-48.
61. Gartsman GM, Roddey TS, Hammerman SM. Arthroscopic treatment of anterior-inferior glenohumeral instability. Two- to five-year follow-up. J Bone Joint Surg Am. 2000;82:991-1003.
62. Lenters TR, Franta AK, Wolf FM, et al. Arthroscopic compared with open repairs for recurrent anterior shoulder instability. J Bone Joint Surg Am. 2007;89:244-254.