Chapter 9 Complications of Nucleus Replacement and Motion-Sparing Technologies
 Fusion has been a long-standing strategy to treat complex degenerative disc disease, and although validated, numerous studies demonstrate increased mechanical stress on adjacent levels and may translate into increased patient morbidity and health care costs.
Fusion has been a long-standing strategy to treat complex degenerative disc disease, and although validated, numerous studies demonstrate increased mechanical stress on adjacent levels and may translate into increased patient morbidity and health care costs. Motion-sparing technologies have been developed to reduce adjacent level degeneration and include artificial discs, nucleus replacement devices, facet replacement devices, and posterior lumbar dynamic stabilization devices.
Motion-sparing technologies have been developed to reduce adjacent level degeneration and include artificial discs, nucleus replacement devices, facet replacement devices, and posterior lumbar dynamic stabilization devices. Lumbar disc arthroplasty has strict patient selection criteria, is poorly validated, and many times these patients respond to nonsurgical management.
Lumbar disc arthroplasty has strict patient selection criteria, is poorly validated, and many times these patients respond to nonsurgical management.Introduction
Surgery for the management of degenerative disorders of the spinal column continues to evolve. Although a majority of these procedures address simple decompression of the neural elements, the use of spinal fusion has been rapidly increasing. Fusion has long been accepted as a means for managing complex spinal deformities and instability. However, the primary reason for its increasing frequency has been its application to the management of degenerative disc disease and the treatment of chronic low back pain. Although this application remains controversial among spinal surgeons, several studies have shown that fusion for degenerative disc disease at one or two levels can improve outcomes compared with the natural history of low back pain.1,2
Although many patients do well after lumbar fusion surgery, some patients can develop further degeneration at spinal levels adjacent to the fusion. Numerous biomechanical studies have demonstrated an increase in mechanical stresses within motion segments adjacent to fused segments.3–5 As adjacent segment degeneration progresses, additional surgery to decompress and stabilize the involved segments may be necessary. This results in increased morbidity and expense of care and can propagate the cycle of additional adjacent segment degeneration and the subsequent need for even more surgery.
Each of these devices allows for some motion at the operated levels, subsequently decreasing the mechanical stresses felt at adjacent levels.6 In theory, this may reduce the incidence of degeneration at adjacent segments caused by fusion, resulting in improved long-term patient outcomes. This technology is attractive to both spinal surgeons as well as informed patients who both recognize the theoretical advantages to a spine fusion alternative. However, these devices are costly, and many lack the long-term follow-up needed to justify the added expense.
Lumbar Arthroplasty
These early failures with artificial disc technology gradually improved aided by advancements in the design and application of artificial hip and knee joints. In the early 1980s Shellnack and Buttner-Janz7 introduced the SB Charité lumbar artificial disc. The device used a sliding, unconstrained polyethylene core placed between two metallic endplates. When inserted into the affected disc space through an anterior surgical approach, it permitted relatively normal motion of the intervertebral segment.
After two early design changes, the SB Charité disc (DePuy Spine, Raynham, MA) eventually underwent a rigorous multicenter, prospective, randomized study with encouraging early and midterm results. It became the first lumbar disc device to receive Food and Drug Administration (FDA) approval. Widespread use of the device began in 2004. The ProDisc device (Synthes Spine, Paoli, PA) also received early FDA approval and is currently in use (Fig. 9-1). Several other lumbar disc replacement devices are currently in varying stages of design, development, clinical analysis, and approval.
Indications and Contraindications
Lumbar disc arthroplasty is approved for use in patients with single-level painful, degenerative disc disease. Bertagnoli8 defined the ideal patient for disc arthroplasty as having a single level of disc disease, greater than 4 mm of retained disc height, no evidence of osteoarthritis of the facet joints, and intact posterior elements. Essentially, this type of patient is young, has an almost normal spine with only single-level disease defined by magnetic resonance imaging or discography, and has no neurologic deficits. This type of patient is relatively rare and is often successfully treated without any type of surgery.
One study evaluated 252 patients who underwent surgery for lumbar spinal disorders. Only 6.3% were deemed potential candidates for disc replacement based on the Charité exclusion criteria. Only one patient was thought by the authors to be an ideal candidate for disc replacement.9
Lumbar disc arthroplasty is contraindicated in patients with painful facet arthropathy, instability (i.e., spondylolisthesis), collapsed disc space, significant neural compression, or neurologic deficits. The clinical trials of the arthroplasty devices also included age older than 60 years as a contraindication to the procedure.10
Clinical Results
Lumbar arthroplasty has been promoted as a replacement for fusion. Preserving intersegmental motion is believed to reduce the potential for the development of adjacent level degenerative changes that can be seen after fusion procedures. Several early studies were retrospective in design and demonstrated superior results for disc replacement.8,11,12 David13 evaluated 106 patients who had received implantation of a Charité disc with a minimum 10-year follow-up. In this study, 87 patients (82.1%) achieved excellent or good clinical outcome. Of the 96 patients working preoperatively, 86 (89.6%) returned to work. A total of 90.6% of the implanted devices were still mobile at long-term follow-up.
The first major randomized, controlled clinical study of lumbar arthroplasty evaluated the use of the Charité device. The control group selected for this study was the use of an anterior lumbar interbody fusion (ALIF) with threaded titanium cages. The selection of this control group has been criticized as an outdated technology not in current use because of relatively high failure rates and better alternatives.14 The use of threaded cages before the study was indicated primarily in patients with a collapsed and painful disc space. However, these patients were excluded from the arthroplasty study. Only patients with preserved disc height were included, a suboptimal criterion for the use of threaded cages.
The study defined success as a 25% improvement in Oswestry Disability Index (ODI), no device failure, no major surgical complication, and no neurologic deterioration.15 With the control group selected for this study, it could be predicted that the patients in this group would not do well. This was the case because only 46.5% of the control group patients were classified as achieving success. This is a poor result compared with other series of ALIF in properly selected patients in which success rates in the 85% to 95% range have been reported.16,17
The arthroplasty group did only slightly better achieving a success rate in only 57.1% of the patients. This modest success rate is further limited by the fact that at 2 years of follow-up, 72.2% of the arthroplasty patients were still on narcotic medication. However, the study did demonstrate safety and effectiveness of the device and ultimately contributed to final FDA approval.15
Complications
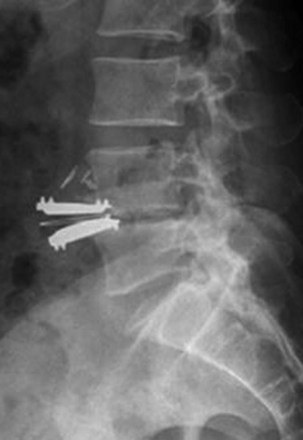
Although relatively rare, migration or extrusion of the implant is the most significant device-related complication that can occur with this procedure (Fig. 9-2). When present, it may result in a spinal deformity or lead to injury of major vascular structures anterior to the lumbar spine. Significant vascular complications can also occur with any anterior revision surgery carried out through a scarred surgical field for the removal of an extruded implant. In a series of 50 patients undergoing lumbar arthroplasty, six patients required anterior revision surgery. One of these patients required three additional vascular procedures because of aortic injury that occurred with the revision surgery.18
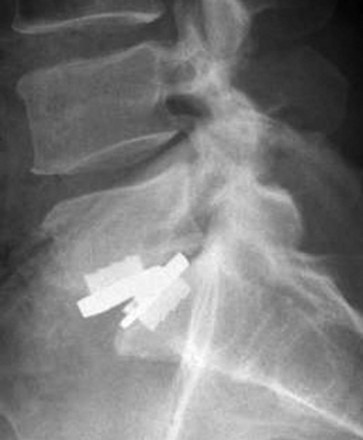
Other device-related complications of lumbar arthroplasty include subsidence of the device through the vertebral endplates, loosening or fracture of the device (Fig. 9-3), and osteolysis of the bone surrounding the device. Malpositioning may result in suboptimal functioning of the device, which can then lead to degenerative changes in the posterior facet joints at the involved or adjacent levels. Suboptimal insertion techniques may also lead to a gradual loss of motion of the interspace or the formation of heterotopic bone bridging the disc space and essentially fusing it.13,19 The impact of metallic and polyethylene wear debris is negligible. In the absence of device extrusion, most device-related complications can be managed by performing a posterior lumbar fusion and fixation procedure, keeping the arthroplasty device in place and avoiding higher risk anterior lumbar revision surgery.
Nucleoplasty
Types of Nucleoplasty Implants
Preformed nucleoplasty implants consist of hydrophilic hydrogels that imbibe water and enlarge within the disc space. Compared with injectable implants, they may provide superior restoration of disc turgor and height. However, because of their physical size and shape, they require a larger annular opening for insertion, increasing the potential for postoperative extrusion. Because they do not completely conform to the adjacent endplates they are in contact with, they may produce higher stress concentrations that result in remodeling of the endplates.20
Indications and Contraindications
Nucleoplasty implants have been used in two different patient populations. The first group consists of patients with degenerated discs and chronic low back pain. These patients have a narrowed disc space without evidence of nerve root compression. They have failed an extensive course of conservative management. Partial disc replacement allows for the removal of the presumed pain generator, the diseased nucleus, and replacement with a device that maintains relatively normal function of the disc space.20,21
A second group of patients presents with radiculopathy, with or without back pain, secondary to a herniated nucleus pulposus. They have failed conservative management and are candidates for a surgical discectomy procedure. Although lumbar discectomy yields consistently good results, it is well documented that a small but significant number of patients who undergo standard discectomy will develop recurrent herniations or progressive degenerative changes with symptomatic low back pain.22–24 The loss of disc space height that frequently occurs after discectomy can promote further degenerative changes in the involved motion segment. Placement of a nucleoplasty device into the disc space may theoretically slow future degenerative changes by maintaining disc space height and normal motion.25,26
Other contraindications for nucleoplasty include associated spinal pathology such as facet arthrosis, central and lateral recess stenosis, instability, deformity, and osteoporosis. Disc space height less than 5 mm has also been shown to be a relative contraindication for nucleoplasty.20 Many of these findings are frequently present in patients with degenerative disc disease, thereby limiting the number of patients who may benefit from nucleoplasty.
Clinical Results
Clinical results of lumbar nucleoplasty remain relatively limited. One study looked at 243 patients who underwent insertion of a preformed hydrogel device for chronic discogenic low back pain. The follow-up period was 2 years, but many patients were lost to follow-up. Only 111 (46%) of the 243 patients provided 6-month follow-up data. Two-year follow-up data were provided by only 23 (9%) patients. The preoperative ODI was 52.7, dropping to 17.2 at 6 months and 9 at 24 months. The Visual Analog Scale was 7.1 preoperatively, 3.0 at 6 months, and 1.8 at 2 years. Sustained increases in disc height were observed at 2 years.20
Complications
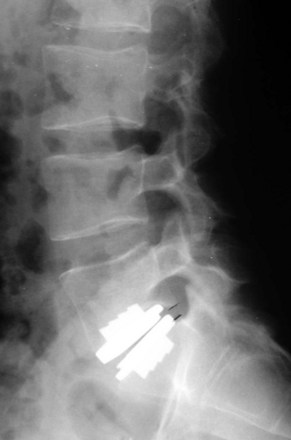
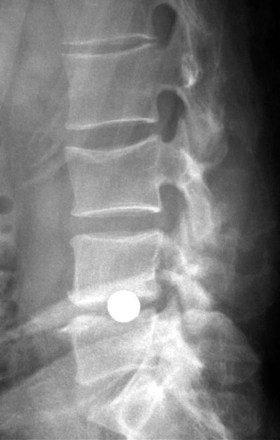
The further investigation and advancement of nucleoplasty technology has been slowed by the persistence of two types of complications seen with this device. The most common complication has been postoperative extrusion of the implanted device (Fig. 9-4). This typically occurs through the annular defect through which the device was inserted. Several design changes have not resulted in any satisfactory reduction in the frequency of this complication.
Bertagnoli and Vazquez27 reported that reoperation for removal of extruded implants was necessary in 12% of their study population. Risk factors for device extrusion were a disc height smaller than 5 mm, insertion of two devices into a disc space with and anteroposterior dimension of larger than 37 mm, a body mass index greater than 30, and implantation through a posterolateral annulotomy.18 Extrusion was not seen when the device was inserted through an anterolateral transpsoas approach.
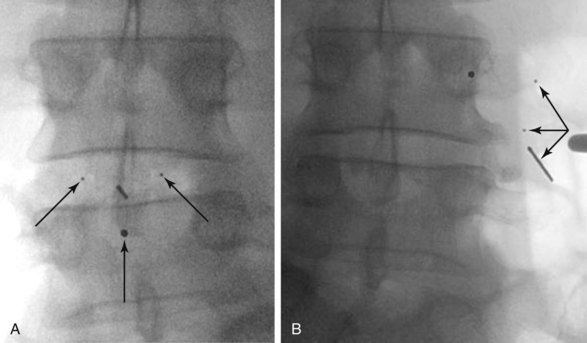
The second complication frequently seen with nucleoplasty devices is their effect on the vertebral endplates. These changes include endplate fracture, subsidence through the endplate (Fig. 9-5), and endplate sclerosis. A study of 46 patients demonstrated implant subsidence into the endplates in nine patients (20%) and endplate sclerosis in 28 patients (61%).20 These changes may limit the theoretical positive effects that nucleoplasty has on the progressive degenerative process by reducing motion or permitting a reduction in disc space height.
1 Fritzell P, Hagg O, Wessberg P, Nordwell A. 2001 Volvo Award Winner in Clinical Studies: lumbar fusion vs nonsurgical treatment for chronic low back pain: a multicenter randomized controlled trial from the Swedish Lumbar Study Group. Spine. 2001;26:2521-2532.
2 Resnick DK, Choudri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine: intractable pain without stenosis or spondylolisthesis. J Neurosurg Spine. 2005;2:670-672.
3 Kim YE, Goel VK, Weinstein JN, Lim TH. Effect of disc degeneration at one level on the adjacent level in axial mode. Spine. 1991;16:331-335.
4 Rao RD, David KS, Wang M. Biomechanical changes at adjacent segments following anterior lumbar interbody fusion using tapered cages. Spine. 2005;30:2772-2776.
5 Weinhoffer SL, Guyer RD, Herbert M, Griffith SL. Intradiscal pressure measurements above an instrumented fusion. A cadaveric study. Spine. 1995;20:526-531.
6 Goel VK, Grauer JN, Patel T, et al. Effects of Charité artificial disc on the implanted and adjacent spinal segment mechanics using a hybrid testing protocol. Spine. 2005;30:2755-2764.
7 Buttner-Janz K, Shellnack K. Principles and initial results with the Charité modular type SB cartilage disk endoprosthesis. Magy Traumatol Orthop Helyreallito. 1988;31:136-140.
8 Bertagnoli R, Kumar S. Indications for full prosthetic disc arthroplasty. A correlation of clinical outcome against a variety of indications. Eur Spine J. 2002;11(suppl 2):131-136.
9 Simmons JW. Inclusion criteria for disc arthroplasty. Philadelphia: North American Spine Society; 2005.
10 Zigler JD, Lorenz MA, Bunch WH. Editorial response to the Charité Lumbar Disc Arthroplasty Study Parts 1 and 2. Spine. 2005;30:E388-E390.
11 Mayer HM, Wiechert K, Korge A. Minimally invasive disc replacement: surgical technique and preliminary surgical results. Eur Spine J. 2002;11(suppl):S124-S130.
12 Lemaire JP, Skalli W, Lavaste F. Intervertebral disc prosthesis. Results and prospects for the year 2000. Clin Orthop. 1997;337:64-76.
13 David T. Long-term results of one-level lumbar arthroplasty. Spine. 2007;32:661-666.
14 Mirza SK. Point of view: commentary on the research reports that led to Food and Drug Administration approval of an artificial disc. Spine. 2005;30:1561-1564.
15 Blumenthal S, McAfee PC, Guyer RD, et al. A prospective, randomized, multicenter Food and Drug Administration investigational device exemptions study of lumbar total disc replacement with the Charité artificial disc versus lumbar fusion: part I: evaluation of clinical outcomes. Spine. 2005;30:1565-1575.
16 Sasso RC, Kitchel SH, Dawson EG. A prospective, randomized controlled clinical trial of anterior lumbar interbody fusion using a titanium cylindrical threaded fusion device. Spine. 2004;29:1113-1122.
17 Burkus JK, Gornet MF, Dickman CA, Zdeblick TA. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech. 2002;15:337-349.
18 Zeegers WS, Bohnen LM, Laaper M. Artificial disc replacement with the modular type SB Charité III: 2-year results in 50 prospectively studied patients. Eur Spine J. 1999;8:210-217.
19 Punt IM, Visser VM, van Rhijn LW, et al. Complications and reoperations of the SB Charité lumbar disc prosthesis: experience in 75 patients. Eur Spine J. 2008;17:36-43.
20 Bertagnoli R, Schonmayr R. Surgical and clinical results with the PDN prosthetic disc-nucleus device. Eur Spine J. 2002;11(suppl 2):S143-S148.
21 Shim CS, Lee SH, Park CW. Partial disc replacement with the PDN prosthetic disc nucleus device: early clinical results. J Spinal Disord Tech. 2003;16:324-330.
22 Lowell TD, Errico TJ, Fehlings MG, et al. Microdiskectomy for lumbar disc herniation: a review of 100 cases. Orthopedics. 1995;18:985-990.
23 Smorgick Y, Floman Y, Millgram MA, et al. Mid to long-term outcome of disc excision in adolescent disc herniations. Spine J. 2006;6:380-384.
24 Yorimitsu E, Chiba K, Toyama Y, Hirabayashi K. Long-term outcomes of standard discectomy for lumbar disc herniations: a follow-up of more than 10 years. Spine. 2001;26:652-657.
25 Jin D, Qu D, Zhao L, et al. Prosthetic disc nucleus (PDN) replacement for lumbar disc herniation: preliminary report with six month follow-up. J Spinal Disord Tech. 2003;16:331-337.
26 Coric D, Mummaneni PV. Nucleus replacement technologies. J Neurosurg Spine. 2008;8:115-120.
27 Bertagnoli R, Vazquez RJ. The anterolateral transpsoatic approach: a new technique for implanting prosthetic disc nucleus devices. J Spinal Disord Tech. 2003;16:398-404.