Chapter 8 Complications of Lumbar Spine Fusion Surgery
 Many patients have back and leg pain arising from lumbar spinal stenosis with or without instability.
Many patients have back and leg pain arising from lumbar spinal stenosis with or without instability. Conservative treatment in the form of physical therapy, chiropractic care, medications, and injections should be the first line of care. However, when conservative modalities fail and there is obvious instability or potential for instability, as well as acute neurologic changes, spinal surgery must be considered.
Conservative treatment in the form of physical therapy, chiropractic care, medications, and injections should be the first line of care. However, when conservative modalities fail and there is obvious instability or potential for instability, as well as acute neurologic changes, spinal surgery must be considered. Lumbar surgery often includes decompression of the soft and bony tissues contributing to central and neuroforaminal stenosis that may be compressing the spinal cord or nerve roots. This can reliably relieve pain, numbness, and weakness.
Lumbar surgery often includes decompression of the soft and bony tissues contributing to central and neuroforaminal stenosis that may be compressing the spinal cord or nerve roots. This can reliably relieve pain, numbness, and weakness. It is crucial to understand the specific complications related to lumbar spinal fusion and to recognize and treat them immediately if they occur.
It is crucial to understand the specific complications related to lumbar spinal fusion and to recognize and treat them immediately if they occur. The most commonly discussed complications directly related to lumbar spinal fusion include infection, dural tears, pseudoarthrosis, and adjacent segment degeneration.
The most commonly discussed complications directly related to lumbar spinal fusion include infection, dural tears, pseudoarthrosis, and adjacent segment degeneration. Rarer, more serious complications associated with lumbar spinal fusion include nerve root injury, paralysis, massive blood loss, blindness, heart attack, stroke, and death.
Rarer, more serious complications associated with lumbar spinal fusion include nerve root injury, paralysis, massive blood loss, blindness, heart attack, stroke, and death.Introduction
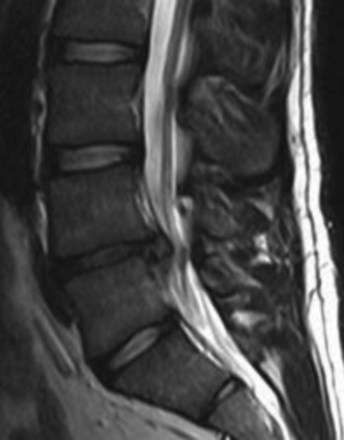
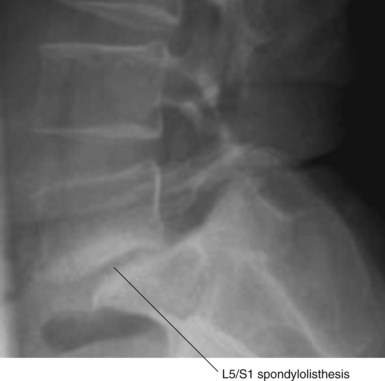
Most lumbar spine surgery involves decompressing the soft tissue, which often includes a herniated disc (Fig. 8-1) or hypertrophied ligamentum flavum. Lumbar spine surgery also involves decompressing the bony elements, which can include hypertrophied facet joints, spinous processes, and lamina that make up the spinal canal. By decompressing both soft and bony tissue, spine surgery can often make patent both central and neuroforaminal narrowing that may be impinging on the spinal cord or nerve roots. Thoroughly decompressing the stenosis and nerve roots can reliably relieve leg pain and its associated symptoms of numbness and weakness. However, there are instances wherein there is obvious instability, spinal spondylolisthesis (Fig. 8-2), or the potential for instability. In this situation, a surgeon must consider both decompressing the stenosis along with stabilizing the affected spinal segments.
Selected Complications
Infection
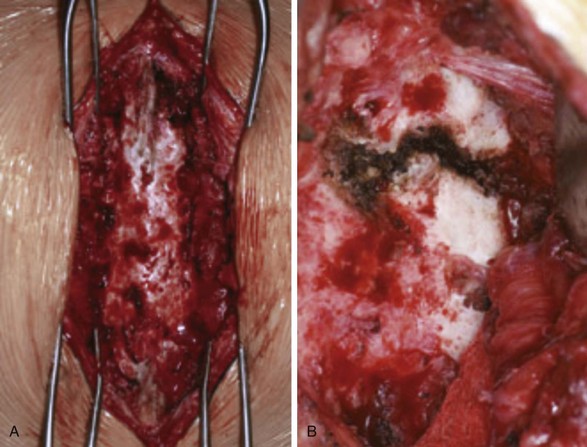
Patients undergoing lumbar spinal fusion typically receive prophylactic intravenous (IV) antibiotics within 1 hour of surgery and postoperatively in several doses. This perioperative regimen, along with proper intraoperative sterility, has decreased infection rates dramatically. However, postoperative spinal wound infection is not an uncommon complication (Fig. 8-3). If not properly recognized and treated, a wound infection can be devastating and affect the ultimate desired surgical outcome.
A review of the literature shows that postoperative wound infections are common and range from 1% to 6% after lumbar spine fusions.1 Staphylococcus aureus was the most common pathogen cultured, but some patients have multiple organisms causing an infection. Multiple studies have assessed risk factors for postoperative wound infections (Table 8-1).
Table 8-1 Patient and Surgical Risk Factors for Postoperative Wound Infections
| Patient Risk Factors for Postoperative Wound Infections | Surgical Risk Factors for Postoperative Wound Infections |
|---|---|
| >60 years of age | Complex spine procedures |
| Smoking | Fusing multiple levels |
| Diabetes | Spinal revisions |
| History of previous surgical infections | Anterior or posterior reconstruction |
| Obesity and increased body mass index | Increased operative times |
| Alcohol abuse |
Age greater than 60 years, smoking, diabetes, previous surgical infection, increased body mass index, and alcohol abuse were statistically significant risk factors. In addition to these factors, more complex spine procedures that involved multiple levels being fused, tumors, surgical revisions, and anterior or posterior reconstructions, also increased the rates of postoperative infections and complications.2
Dural Tear
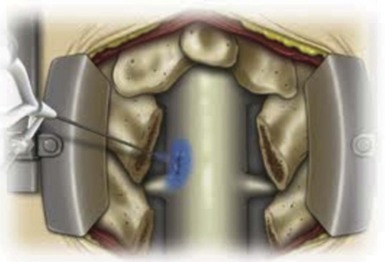
Most lumbar spine fusion cases involve decompression of posterior elements. The soft tissue is often decompressed, which can include a herniated or extruded disc, excessive fat, or hypertrophied ligamentum flavum. Lumbar spine surgery also involves decompressing the bony elements, which often include hypertrophied facet joints, spinous processes, and lamina. The neural elements bathe in CSF and are contained within a thin tissue, the dura mater, before branching out into the neuroforamen to supply motor and sensory function to the body. The dural sac with its neural elements is compressed in central spinal stenosis. There exist central, lateral recess, and foraminal stenoses, and patients may have one or all three of these components comprising their stenosis. To adequately decompress the canal and ligamentum flavum, often the medial aspect of hypertrophied facets joints are excised. In the process of accomplishing this, dural tears may occur secondary to the bone and soft tissue being adherent to the dura from longer standing stenosis or a large herniated disc (Fig. 8-4). While performing a posterior decompression of the lumbar spinal canal, bony spicules may also pierce the dural sac, causing CSF leaks. As a rule, the more severe the spinal stenosis, the higher the likelihood of a dural tear occurring as a result of surgery. In addition, patients who have had previous lumbar spine surgery and have recurrent stenosis also have an increased risk of a dural tear because of formation and adherence of scar tissue.
Dural tears are a common complication of lumbar spine surgery (Fig. 8-5). Khan et al3 reviewed a total of 3183 degenerative lumbar spine cases at one institution, and cases complicated by dural tears were identified. They found that the incidence of dural tears during a primary lumbar surgery was 7.6%. This was significant but appreciably lower than the 15.9% dural tear risk seen with the surgical revision cases. Cammisa et al4 reviewed a total of 2144 patients and found that dural tears occurred in 3.1% of patients during spinal surgery. The incidence of dural tears varied according to the specific procedure performed but was again highest in the group that underwent revision surgery.
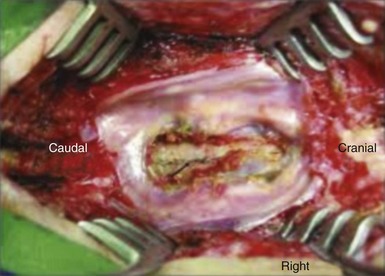
Fig. 8-5 Large dural tear.
(From Pereira Filho Ade A, et al: Symptomatic thoracic spinal cord compression caused by postsurgical pseudomeningocele. Arq Neuropsiquiatr 65[2-A]:279-282, 2007.)
The treatment for dural tears occurring intraoperatively is direct repair of the dura with a thin silk or Prolene suture that nicely approximates the dura tissue edges to each other without impinging the neural elements. Wang et al5 describe primary repair followed by short-term bed rest. This protocol led to no long-term deleterious effects or increased rates of infection, neural damage, or arachnoiditis. In addition, closed suction wound drainage does not aggravate the leak and can safely be used in the presence of a dural repair.
For patients who have continued dural leakage of CSF, other options are available as well. Kitchel et al6 describe the effectiveness and safety of a temporary subarachnoid shunt placed in the upper lumbar spine that is removed after 4 days. These patients must be monitored very carefully. Many of these authors’ patients were treated successfully in this manner with resolution of CSF drainage and symptoms and no permanent sequelae. An undetected postoperative CSF leak or cases with persistent drainage are more commonly treated with reoperation, identification, and repair of the dural leak.
Pseudoarthrosis
Lumbar spine fusion has become a widely used procedure, being performed increasingly over the past 2 decades. Initially, the surgery was only indicated for Potts disease, severe spinal deformity, unstable spine trauma, and tumor excisions leading to instability. The current indications for lumbar spine fusion have broadened and include any spinal instability and painful degenerative disc disease in carefully selected patients. Methods to achieve fusion have evolved and include new instrumentation devices, various biologics, and different approaches. A surgeon can now perform an anterior lumbar interbody fusion or posterior lumbar fusions from midline to true lateral. The lateral approach involves accessing the retroperitoneal space and traversing carefully through the psoas muscle to enter the disc space. Even though technology and biologics to achieve fusion have improved, pseudoarthrosis may still occur. Pseudoarthrosis is defined as an incomplete spinal fusion when a fusion is attempted. A large prospective review of revision surgeries revealed that 23.6% of these revision fusion surgeries were performed for pseudoarthrosis.7 However, this may underestimate the true incidence of pseudoarthrosis because many patients remain asymptomatic despite the lack of a complete fusion.
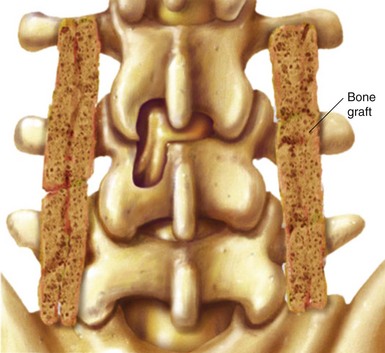
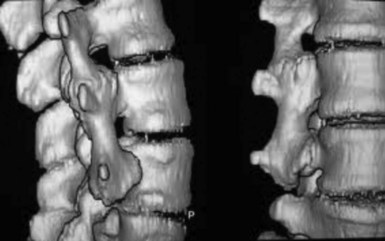
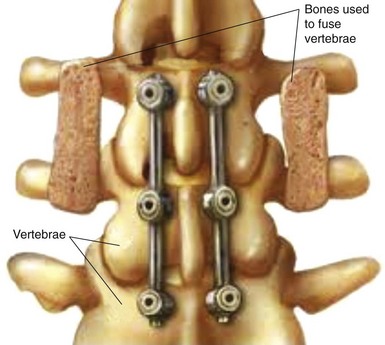
Risk factors for nonunion include metabolic abnormalities, smoking, infection, and excessive motion at the fusion site (Box 8-1). Several studies have shown that not using instrumentation leads to increased pseudoarthrosis as well (Figs. 8-6 and 8-7). Fischgrund et al8 published the results of a prospective, randomized trial comparing decompression and posterolateral fusion with and without instrumentation in patients with degenerative spondylolisthesis and spinal stenosis. Average patient follow up was 2 years with the fusion rate significantly better with instrumentation than without (82% vs. 45%). However, they noted that good to excellent results occurred in both groups whether they fused or not. In 2004 Kornblum et al9 reviewed the noninstrumented fusion patients from the Fischgrund trial with 5 to 14 years of follow-up. They found that there was a significant difference between the long-term results of both groups. The pseudoarthrosis patients ended up having worse clinical outcomes with many of them requiring revision operations. Thus, evidence does support the use of posterior instrumentation (Fig. 8-8) to achieve higher fusion rates and better clinical outcomes.
Similar to the cervical spine, longer segment fusion cases in the lumbar spine have a higher rate of pseudoarthrosis than those with fewer segments fused. Increased rates of nonunion are also seen at the thoracolumbar junction and the lumbosacral junction. Attempts to achieve higher fusion rates at the lumbopelvic junction include the use of interbody fusion at L5–S1 and iliac fixation to protect the S1 promontory screws. As previously mentioned, patients with pseudoarthrosis had significantly worse outcomes than those who had complete fusion. Smoking, the use of allograft, extension of fusion to the sacrum, preexisting hip arthritis, and a thoracoabdominal approach may be associated with increased risk.10
Adjacent Segment Degeneration
After lumbar fusion is attained, an important question arises as to what the future holds at the adjacent levels that have not been operated on. It is unclear if lumbar ASD (Fig. 8-9) occurs as a result of lumbar spinal fusion or is part of a larger preexisting multifactorial disease process. Studies suggest an increase in risk for ASD in patients who underwent spinal fusion compared with patients who received nonfusion or no treatment. Annualized incidence rates for symptomatic ASD requiring reoperation from case series ranged from 0% to 3.9%. Approximately 26% of patients receiving lumbar fusion develop new lumbar ASD within the first 10 years after fusion based on one study.11 The L3–L4 segment appears to be one of the most frequently involved levels, according to multiple studies. Because of this concern for ASD, many technologic advances have been made to decompress and stabilize the lumbar spine without rigid fusion. Some examples include the artificial disc replacement, pedicle-based motion-preserving devices, facet replacements, and interspinous implants. There has been much research done to validate the efficacy of many of these products. However, more time is needed to adequately assess the rates of ASD with these products versus that of lumbar spinal fusion.
1 Weinstein MA, McCabe JP, Cammisa FP. Postoperative spinal wound infection: a review of 2,391 consecutive index procedures. J Spinal Disord. 2000;13(5):422-426.
2 Fang A, Hu SS, Endres N, Bradford DS. Risk factors for infection after spinal surgery. Spine. 2005;30(12):1460-1465.
3 Khan MH, Rihn J, Steele G, et al. Postoperative management protocol for incidental dural tears during degenerative lumbar spine surgery: a review of 3,183 consecutive degenerative lumbar cases. Spine. 2006;31(22):2609-2613.
4 Cammisa FP, Girardi FP, Sangani PK, et al. Incidental durotomy in spine surgery. Spine. 2000;25(20):2663-2667.
5 Wang JC, Bohlman HH, Riew KD. Dural tears secondary to operations on the lumbar spine. Management and results after a two year minimum follow up of eighty-eight patients. J Bone Joint Surg Am. 1998;80(12):1728-1732.
6 Kitchel SH, Eismont FJ, Green BA. Closed subarachnoid drainage for management of cerebrospinal fluid leakage after an operation on the spine. J Bone Joint Surg Am. 1989;71(7):984-987.
7 Martin BI, Mirza SK, Comstock BA, et al. Reoperation rates following lumbar spine surgery and the influence of spinal fusion procedures. Spine. 2007;32:382-387.
8 Fischgrund JS, Mackay M, Herkowitz HN, et al. Volvo Award winner in clinical studies: Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine. 1997;22:2807-2812.
9 Kornblum MB, Fischgrund JS, Herkowitz HN, et al. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective long term study comparing fusion and pseudoarthrosis. Spine. 2004;29:726-733.
10 Kim YJ, Bridwell KH, Lenke LG, et al. Pseudoarthrosis in long adult spinal deformity instrumentation and fusion to the sacrum: prevalence and risk factor analysis of 144 cases. Spine. 2006;31:2329-2336.
11 Park P, Garton HJ, Gala VC, et al. Adjacent segment disease after lumbar or lumbosacral fusion: review of the literature. Spine (Phila PA 1976). 2004;29(17):1938-1944.