Complications of Local Flaps
Introduction
Complications associated with surgery are perhaps nowhere more apparent and devastating than when they occur on the face. Conley spoke of surgical complications by saying, “These untoward events, unpredictable and unwanted, lurk in all surgical arenas, in the biological process and in the patient. They give credence to our frailty, from which there is no escape.”1 It is essential that all facial surgeons become familiar with the potential complications associated with facial reconstructive surgery. Furthermore, it is of utmost importance that surgeons anticipate these undesirable events and take proper measures to help prevent their occurrence.
Establishing a consistent perioperative routine is the first step in preventing complications. Appropriate preoperative counseling of the patient will reduce the likelihood of complications. Informed consent should include an open discussion of potential problems that may occur. Hand scrubbing, sterile surgical attire, and surgical site preparation are important in reducing the likelihood of infection.2–6 Immediate and regular postoperative evaluations of the patient will facilitate early recognition and treatment of complications. Some complications are reversible, and expeditious treatment may prevent a reversible complication from becoming an irreversible one. This chapter discusses complications inherent to surgery, those related to the medical conditions of patients, and complications associated with surgical technique and local flap design.
Complications Inherent to Surgery
Adherence to well-established surgical principles is essential for minimizing the incidence of complications in performing facial reconstructive surgery. Conley writes that “hemostasis should be absolute … all flaps should be viable and tissue should not be closed with undue wound closure tension. The type and size of the suture material should be appropriate for the job it has to do. Compliance with these fundamental principles establishes the groundwork for healing … the incidence of complications will develop in direct proportion to the violation of these principles.”1
Bleeding
The most common causes of bleeding from facial reconstruction with local flaps are drug-associated coagulopathy and inadequate hemostasis.7 Patients are questioned preoperatively concerning use of all prescription and over-the-counter medications that may contribute to perioperative bleeding. All aspirin-containing products, nonsteroidal anti-inflammatory medicines, vitamin E, and most herbal remedies are discontinued 1 week or more before surgery. The exception to this policy is patients with cardiac stents. These patients continue to take aspirin if it is prescribed by their cardiologist. Cold and sinus medications are avoided because many contain substances that may affect clotting or cause adverse elevation in blood pressure. Discontinuation of any medications prescribed by a physician to reduce blood clotting is coordinated with the patient’s primary physician or cardiovascular specialist. Bleeding may also be associated with hypertensive events, hepatic or renal failure, vomiting, straining, or alterations in the hematopoietic system.
Hematomas may cause compromise of local flap vascularity by inducing vasospasm, stretching the subdermal plexus, or separating the flap from the surface of the recipient site.8–10 Furthermore, iron compounds in a hematoma may promote free radical production, leading to flap necrosis.11,12 Hematoma formation also predisposes to infection, which may compromise flap vascularity secondary to inflammatory edema.13,14
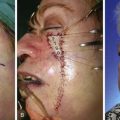
If a hematoma occurs, patients usually complain of significant pain. The wound may appear mottled, pale, or bluish, and palpation of the skin in the area of the hematoma usually reveals a tight, tense flap with oozing of blood from the suture lines. Small (5-10 mL) hematomas may sometimes be aspirated through the suture line by use of a 25-mL syringe attached to an 18-gauge needle. A compression dressing is then applied and the patient is re-examined in 24 to 48 hours. If the hematoma recurs, it may be necessary to return the patient to the operating room to properly drain it. There, the wound is opened at a dependent portion, the hematoma is evacuated, and bleeding vessels are controlled. A compression dressing is reapplied. The patient is examined within 24 hours. Expediency in treatment of large hematomas is essential to avoid compromise of flap vascularity and skin slough. Typically, within the first 48 hours after surgery, hematomas consist of a fresh clot of gel or liquid consistency. As the clot matures during the next several days, it becomes firmer and adherent to the underlying wound bed and cannot be easily aspirated. After approximately 2 weeks, fibrinolysis begins, and the hematoma liquefies (Fig. 26-1). At this point, repeated aspiration or drainage may be necessary to facilitate adherence of the flap to the wound bed (Fig. 26-2).
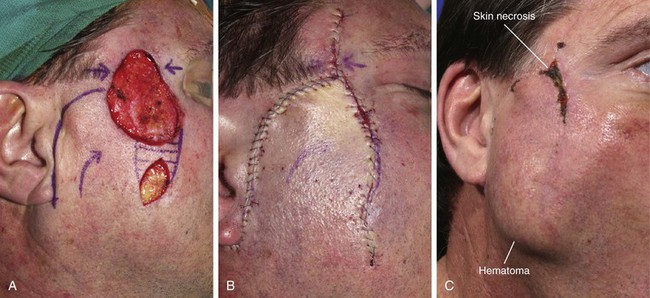
FIGURE 26-1 A, Rotation flap designed to repair 5 × 4-cm cutaneous defect of the temple. Partial primary wound closure was also planned at superior aspect of defect. Repair of smaller skin defect occurring in area of anticipated standing cutaneous deformity of rotation flap is noted. B, Wound repaired. C, Postoperative result at 2 weeks. Flap necrosis at peripheral border may have been related to development of hematoma at the base of flap. (Courtesy of Shan R. Baker, MD.)
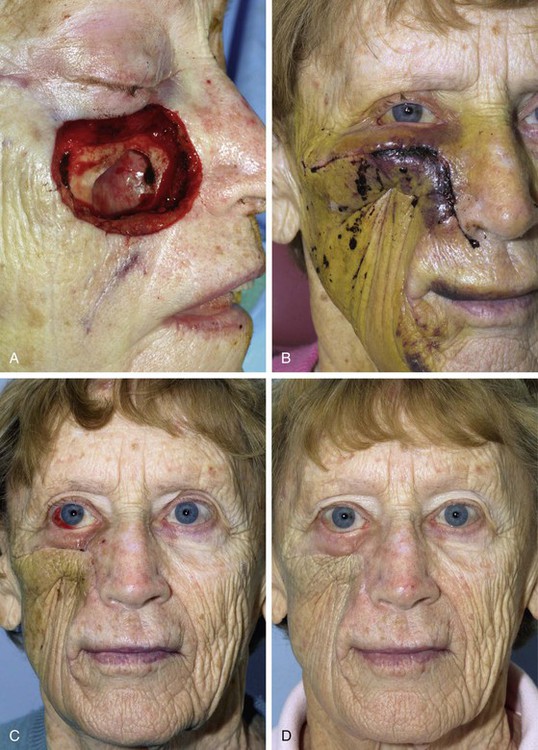
FIGURE 26-2 A, Midface soft tissue and bone defect after resection of skin malignant neoplasm. B, Hematoma developed 1 week postoperatively, after restarting of anticoagulant medication (warfarin). C, One month after evacuation of hematoma. Lower eyelid retraction noted. Massage of lower eyelid initiated. D, Postoperative result at 6 months.
Injury of Adjacent Anatomic Structures
Sensory nerves are also susceptible to injury during facial reconstructive procedures. Extensive undermining along the superior bony orbital rim may injure the supraorbital and supratrochlear nerves, leading to dysesthesias in their sensory distributions.15 Similarly, the deep branch of the supraorbital nerve runs within the galea aponeurotica and may be injured when the frontalis muscle is undermined in the subgaleal plane while defects of the lateral forehead skin are being closed. The infraorbital and mental nerves are less susceptible to injury but may be compromised during dissection in their respective areas. The great auricular nerve may be injured during subcutaneous dissection in the periauricular area. This nerve is identified and preserved when skin is dissected away from its attachment to the superior aspect of the sternocleidomastoid muscle.
Injury to eyelid structures may lead to devastating consequences, including lagophthalmos, lacrimal duct obstruction, visual field obstruction, and vision loss. Facial surgeons should be familiar with the cross-sectional anatomy of the upper and lower eyelids, especially the connective tissue support apparatus and the position and location of the levator muscle. Injury to these structures is best avoided because treatment is extremely difficult if scarring has occurred.
Infection
Fortunately, infection during facial surgery is reported to be as low at 2.8%, perhaps secondary to the rich vascularity of facial tissues.16–20 However, the infection rate associated with facial flaps is increased in certain circumstances. Higher infection rates are associated with wounds that are repaired in a delayed fashion, as is often the case with facial flaps used to repair wounds created by micrographic surgery.21 Ischemia of flaps is likely to increase the risk for infection because tissue oxygenation is extremely important in prevention of wound bacterial colonization.16,22,23
Wound infection is associated with poor outcomes. Infection of a cutaneous flap is usually associated with distortion from inflammatory edema. Release of toxic substances and free radicals from inflammatory mediators leads to decreased collagen production and early degradation of suture materials with potential wound dehiscence. Necrosis of all or part of the flap may develop, with the final scar being widened or thickened. Systemic dissemination of bacteria may occur if wound infections are not treated promptly.24
Although perioperative antibiotic prophylaxis is controversial in clean wounds, it has been shown to be effective in decreasing wound infection rates when it is used for clean-contaminated wounds.16,21,22 Antiseptic solutions for skin preparation, sterile technique, proper scrubbing, and surgical attire are key elements in helping prevent infection. It is also important to avoid crushed, charred, or excessively thinned tissue. Staphylococcus aureus is the most common single pathogen causing wound infections, but streptococci, gram-negative bacteria, and oral anaerobes may also be isolated from infected wounds.21 The author uses parenteral antibiotics when mucosal surfaces are incised, when cartilage grafting is performed, and during ear reconstruction to help prevent perichondritis. They are also employed in patients with underlying medical conditions that may predispose to infection, such as diabetes and immunosuppression. In these cases, a preoperative dose of intravenous cephalosporin is administered, followed by 5 days of an oral cephalosporin. In penicillin-allergic patients, clindamycin is used. In patients with open wounds older than 3 days, a 5-day course of oral cephalosporin is recommended before the surgical repair. This will decrease the bacterial colonization of the granulation tissue that has developed in the depths of the wound.
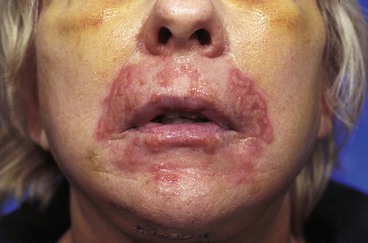
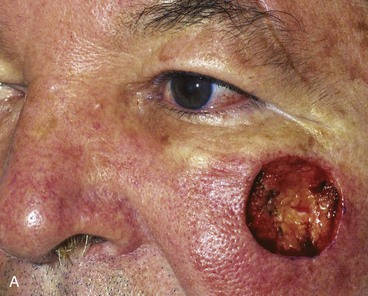
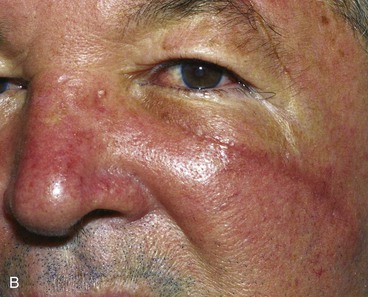
Excessive pain or erythema at the wound site may herald an infection (Fig. 26-3). These clinical signs usually appear between the fourth and eighth days after wound closure. Topical allergies to antibiotic ointments and creams applied to the wound may be confused with wound infection. Topical allergic reactions are manifested as erythema, often with vesicle formation and exudates forming on all areas treated with the topical medication (Fig. 26-4). Treatment consists of discontinuation of the offending agent and cleaning of the wound with soap and water to remove all residual topical medication.
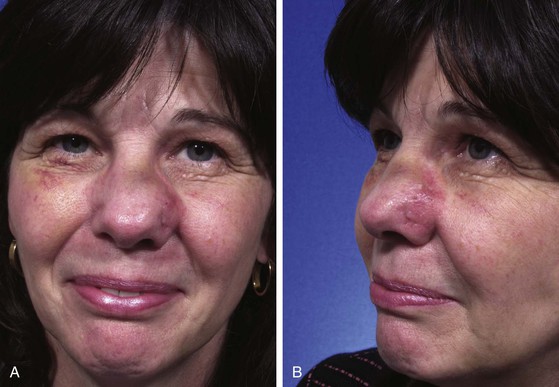
FIGURE 26-3 A, B, Infected nasal wound after contouring procedure to correct trap-door deformity of bilobe nasal flap. (Courtesy of Shan R. Baker, MD.)
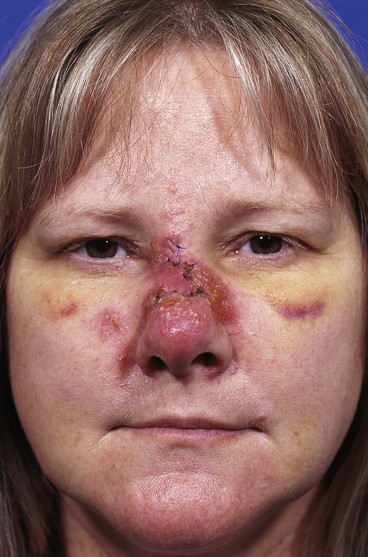
FIGURE 26-4 Topical allergic reaction to antibiotic ointment causing erythema and vesicles of skin. (Courtesy of Shan R. Baker, MD.)
If a wound infection does develop, Gram stain and culture of any drainage from the wound are performed. The patient is treated with an appropriate broad-spectrum antibiotic until culture results and sensitivities are available. Any wound fluctuance is drained and the wound irrigated. Significant fluid collections beneath a cutaneous flap usually require the placement of a drain or wick to facilitate further drainage and adherence of the flap to the underlying wound bed. The presence of excessive granulation tissue may require débridement to reduce the bacterial load. Topical antibiotic preparations may also be employed, and superficial sutures are removed to eliminate foreign bodies in the wound.25 Open wounds are allowed to heal by secondary intention. The resulting scar is addressed at a later time once the infection has been adequately treated and scarring has matured.
Viral and fungal infections of facial wounds are rare and almost always are associated with skin resurfacing procedures, such as dermabrasion and laser peels. Although these infections may occur anywhere on the face where resurfacing has been performed, they occur predominantly in the area of the lips when this facial region is treated (Fig. 26-5). Patients undergoing dermabrasion or laser treatment to scars of the lip should receive antiviral therapy before treatment and postoperatively until the treated area has completely re-epithelialized.
Abnormal Scarring
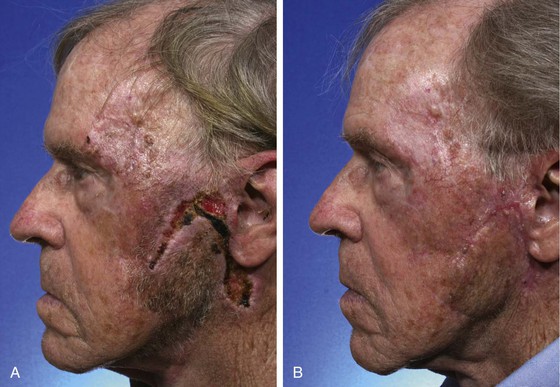
Patients with darker skin and those with a family history of excessive scarring are prone to the development of hypertrophic scars and keloids after surgery. Although numerous treatment modalities have been proposed, there is no definitive method of managing or preventing these problems.26 Intralesional triamcinolone acetonide (10 mg/mL) is often helpful in reducing the volume of scar tissue within a hypertrophic or keloid scar. If the scar is responsive to the injections, surgical excision may be performed after a preoperative injection 1 week before surgery. Several injections are usually required postoperatively every 2 months after excision of the scar for an indeterminate time interval (Fig. 26-6). Rarely, irradiation is considered for extremely problematic keloids.
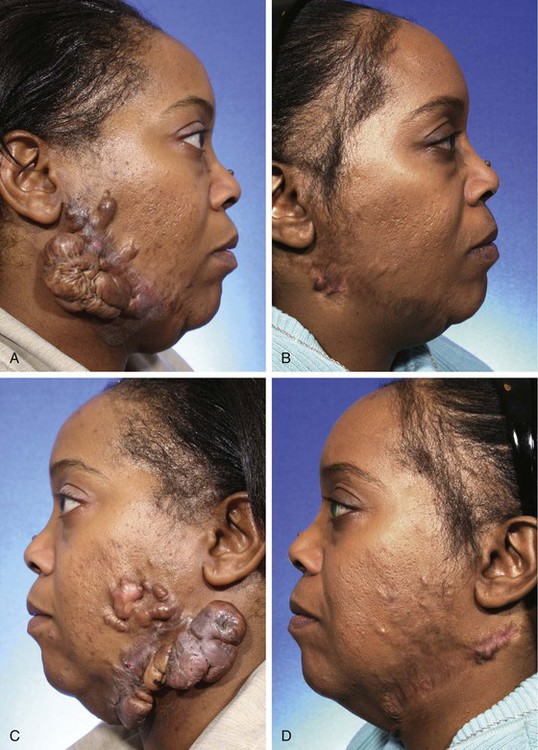
FIGURE 26-6 A-D, Preoperative views and postoperative result 1 year after keloid excisions. Postoperative injection of triamcinolone acetonide (10 mg/mL) into the surgical site was performed every 2 months. (Courtesy of Shan R. Baker, MD.)
Glucocorticoids have a profound anti-inflammatory effect. The most common form used is triamcinolone acetonide suspension, which is injected into the depths of the scar. Injections are used to soften firm scars, to lower raised keloids, and to prevent their recurrence. Steroids decrease fibroblast proliferation, reduce blood vessel formation, and interfere with fibrosis by inhibiting extracellular matrix protein gene expression. When steroid injection is used at the time of keloid excision, it is injected at the wound margins immediately after excision. Repeated injection is performed at 3- to 6-week intervals. Multiple injections are usually necessary. Excessive steroid injection of scars may lead to skin atrophy, hypopigmentation, and telangiectasia (Fig. 26-7).
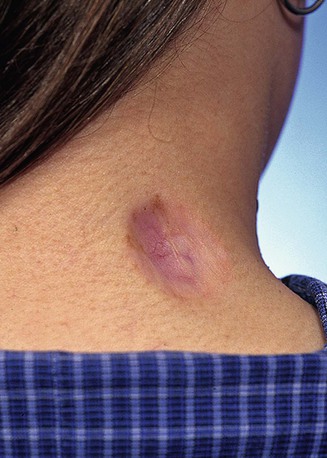
FIGURE 26-7 Skin atrophy with telangiectasia after steroid injection of scar. (Courtesy of Shan R. Baker, MD.)
Scar hyperpigmentation may be seen in patients who tan easily, particularly if the patient has excessive sun exposure during the immediate postoperative period. Hyperpigmentation improves over time and with sun avoidance. Use of topical hydroquinone gel with or without the administration of an anti-inflammatory drug may be helpful in preventing permanent discoloration of the scar (Fig. 26-8).
Wound Dehiscence
Wound dehiscence may develop secondary to infection, hematoma, or skin necrosis (Fig. 26-9). In addition, dynamic motion or trauma to wounds may also lead to wound separation. Typically, wounds have 3% to 5% of normal skin tensile strength at 2 weeks after surgery, emphasizing the need for subcutaneous sutures. At 1 month, only 35% of the maximum wound tensile strength has been gained. However, at this point, the wound is usually able to withstand the forces of normal physical activity. During the succeeding months, wound tensile strength increases to 80% of the strength of normal tissue. Wound separations in uncomplicated, clean wounds may be repaired with sutures if this is performed within 24 hours of wound dehiscence. Wound separations that have been present for more than 24 hours are best left to heal by secondary intention, with scar revision reserved for the future if necessary.24,27
Medical Conditions
Patients are questioned preoperatively concerning their use of tobacco and alcohol. Heavy alcohol consumption will dilate blood vessels, predisposing to hematoma formation. Avoidance of alcohol during the perioperative period is recommended. Ideally, tobacco and nicotine products should be avoided, at least 4 to 8 weeks before and after surgery. Even smoking cessation for 2 days before surgery and 7 days after surgery has been shown to have beneficial effects on flap survival.28 Smokers develop skin necrosis three times more frequently than nonsmokers do, and the extent and depth of skin slough are more severe.28 Nicotine causes systemic vasoconstriction through activation of the adrenergic nervous system, which may lower tissue oxygenation pressure by more than 50%. This occurs within 10 minutes of smoking a cigarette and lasts approximately 50 minutes. Smoking also produces carbon monoxide, which has a higher affinity for hemoglobin than oxygen, thereby producing high levels of carboxyhemoglobin.29–33 This means that less oxygen can be delivered to the tissues by the vascular system. When possible, consideration is given to delaying surgical procedures until smoking cessation can be ensured.
Surgical Technique and Flap Design
Ischemia
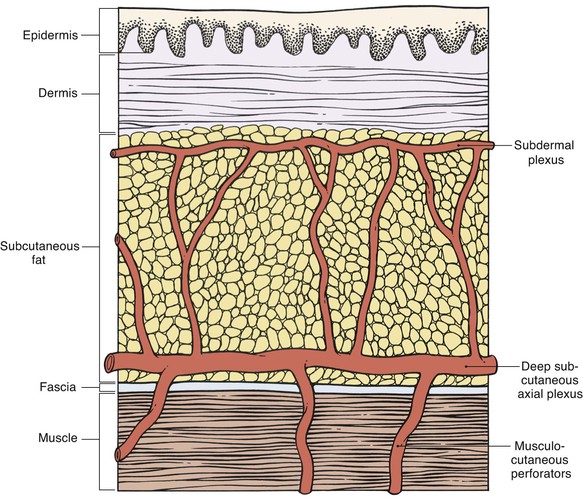
Appropriate flap design is essential to ensure sufficient perfusion pressure of the entire flap.34 According to their vascularity, local and regional flaps may be classified as random pattern or axial pattern flaps. Flap dissection should proceed at the appropriate tissue depth to preserve the blood supply to the skin (Fig. 26-10). Random pattern flaps require an intact subdermal plexus, and dissection is performed in the subcutaneous tissue plane. Too superficial dissection or improper flap design may lead to compromise of the flap’s vascularity. Random patterned flaps have a critical length beyond which the perfusion of the distal flap does not occur. Although increasing the width of a flap’s pedicle potentially increases the number of vessels contained in the subdermal plexus of the flap, it does not increase the perfusion pressure to the distal portion of the flap. Therefore, within certain limits, widening the pedicle does not increase the survival of the distal flap. Axial pattern flaps are nourished by a named artery that is located within the tissues of the flap and is parallel to the linear axis of the flap.
Design of axial pattern flaps requires a thorough knowledge of the location and orientation of the arterial vessels supplying the skin of the donor area to properly align the flap with the axis of the artery and to avoid inadvertent injury to the flap’s blood supply. Careful dissection is performed around the flap’s pedicle. Increasing the width of the pedicle of axial flaps does not necessarily improve perfusion.35,36 In fact, it is more prudent to raise a thicker flap that contains deeper subcutaneous vessels than to design the flap with a wider base (Fig. 26-11). An excessively wide pedicle may also complicate the use of the donor site for repair of future defects (Fig. 26-12).
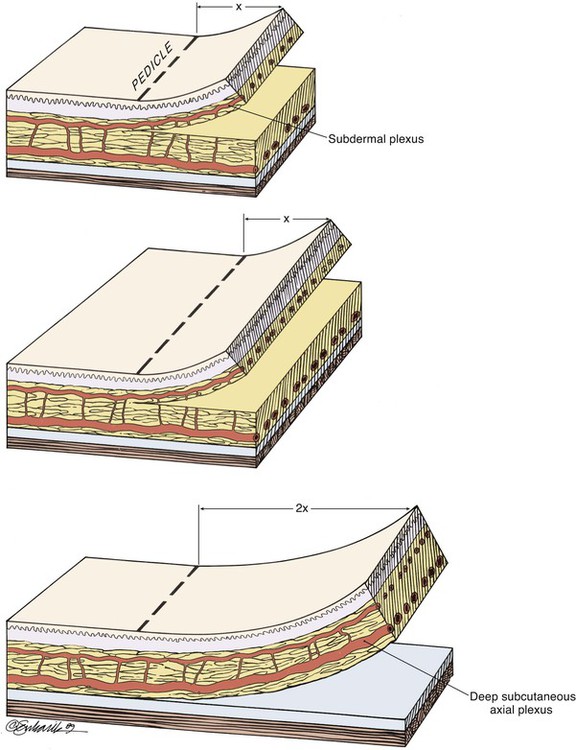
FIGURE 26-11 Widening pedicle of flap does not increase distal flap perfusion, but increasing thickness of flap to include deeper, larger vessels does.
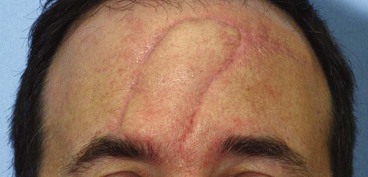
FIGURE 26-12 Patient with history of prior nasal reconstruction by inappropriately designed paramedian forehead flap. Majority of flap returned to donor forehead site. Patient referred with recurrence of malignant disease requiring resection and reconstruction with second forehead flap. Design of second paramedian forehead flap complicated by first flap, which had excessively wide pedicle and was oriented obliquely toward contralateral paramedian forehead region.
Ischemia is defined as vascular perfusion insufficient to provide the required oxygenation of tissue.25 Cutaneous flaps are more vulnerable to tissue ischemia than are wounds closed primarily, given that the vascularity is isolated to the pedicle of the flap used for repair of the wound.37 This is especially true in the distal portion of the flap.38,39 Dissection of flaps causes release of catecholamines from severed sympathetic nerves, thromboxane A from platelet microthrombi, and oxygen free radicals. All of these substances cause vasoconstriction, which can enhance tissue ischemia.40,41 The type of flap also is a factor influencing the degree of tissue ischemia. Transposition flaps generate less distal wound tension than rotation or advancement flaps do. This is because the greatest wound closure tension is at the donor site when transposition flaps are used, whereas wound tension is greatest at the distal borders of advancement and rotation flaps42 (Fig. 26-13). Because rotation and advancement flaps have innate wound closure tension at the distal closure site, they likely benefit from multiple, precisely placed subcutaneous “tacking” sutures to evenly distribute wound tension along the distal suture line (see Chapter 6).
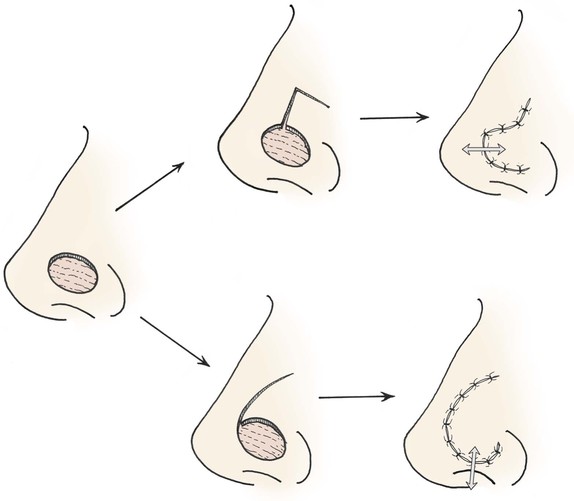
FIGURE 26-13 Transposition flap is pushed into recipient site (arrow) by closing donor defect. Greatest wound closure tension is at donor site. Rotation and advancement flaps tend to pull back toward donor site (arrow), and greatest wound closure tension is at recipient site.
With proper technique, cutaneous flap necrosis is uncommon because most tissue can survive on a fraction of its average blood flow,37 and eventual neovascularization reduces flap dependency on the pedicle’s blood supply.10,36,42,43 However, reversible ischemia may become irreversible if improper surgical technique or postoperative wound care causes additional vascular compromise. Excessive flap thinning, aggressive electrocautery, crush injury with surgical instruments, and excessive wound closure tension may worsen flap ischemia44,45 (Fig. 26-14). Injection of local anesthetic containing vasoconstrictor agents is used judiciously and avoided in wounds in which vascular perfusion is a concern.46,47 Infection is treated expeditiously because edema from inflammation may decrease tissue perfusion. The effects of excessive wound closure tension, tight compression dressings, bacterial contamination, and blood beneath the flap may all cause irreversible ischemia of the flap.24 When flap ischemia is evident, local wound factors and factors related to the patient are assessed and medical conditions are optimized as discussed previously. In such situations, use of nicotine by the patient must be avoided.
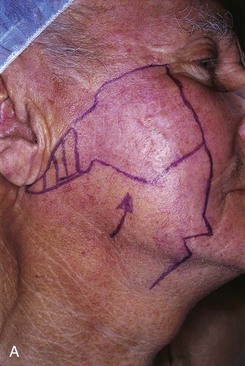
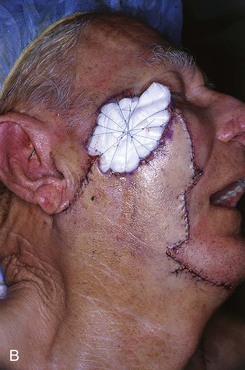
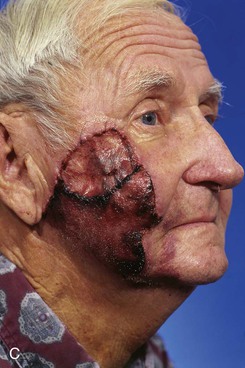
FIGURE 26-14 A, Melanoma in situ of cheek marked for excision. Advancement flap designed for reconstruction of face after resection of melanoma. B, Defect repaired with advancement flap combined with full-thickness skin graft (beneath bolster dressing). C, Five days after surgery. Excessive wound closure tension has caused limited necrosis of borders of flap. (Courtesy of Shan R. Baker, MD.)
Ischemia of a skin flap is signaled by color changes of the flap. Pale flaps and slow capillary refill when the skin of the flap is compressed are signs of impending ischemia. A reduced flap temperature below ambience and the absence of bleeding after pinprick of the flap suggest arterial insufficiency. Flaps with edema, purple-blue color, and brisk bleeding of dark blood after pinprick suggest venous congestion. Flaps may survive arterial insufficiency for up to 13 hours, but venous congestion can cause necrosis in as quickly as 3 hours.25 No widely accepted methods of monitoring flap vascularity have been established, and most surgeons rely on careful and frequent examinations of the flap when ischemia is a concern. Repeated pinpricks of the flap or a trial of medicinal leeches may be helpful in treating flaps with venous insufficiency.48,49 Aeromonas infection secondary to treatment with leeches may occur and should be treated promptly with appropriate antibiotic coverage.50,51
Arterial insufficiency of a flap may be caused by kinking of the flap’s pedicle, an excessively tight compression dressing, or excessive wound closure tension. If these causative factors are corrected early in the postoperative period, the ischemia is usually reversible. Correction of these conditions should improve the vascularity of the flap. In the case of excessive wound closure tension, the flap may be returned to its donor site and allowed to remain in situ for a few days. This has the effect of delaying the flap, which will cause an enhanced blood supply to the flap. The flap is then usually transferred to the recipient site without ischemia.24 There are numerous reported experimental treatments for flap ischemia with pharmacologic agents, such as vasodilators, sympatholytics, and free radical scavengers; however, there is no widely accepted agent for treatment of arterial insufficiency.52–55 Skin blanching may become progressively prolonged with digital compression until capillary refill cannot be elicited, signaling flap failure.34,55 When ischemia causes epidermolysis or skin necrosis, the tissue is allowed to demarcate because the more proximal portion of the flap is usually viable (Fig. 26-15). Devitalized tissue that is easily removed from the wound bed is débrided. Tissue adherence signifies some underlying viable tissue and is left as a biologic wound dressing. Patients are instructed to keep the wound moist with petroleum or water-based antibiotic ointment, and the wound is allowed to heal by secondary intention. Frequent follow-up examination is essential for maintaining appropriate wound care and providing reassurance to the patient.
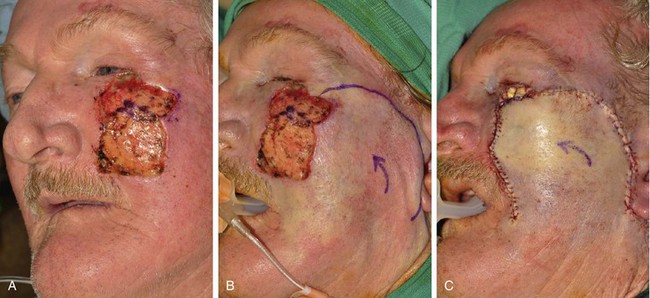
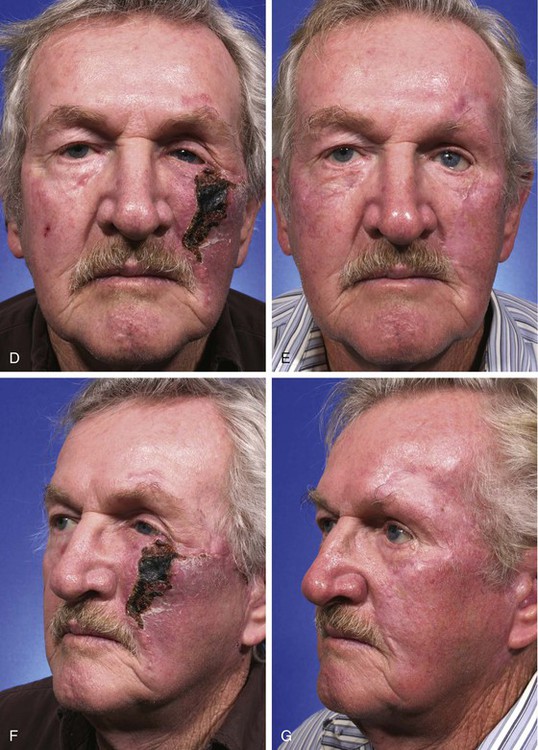
FIGURE 26-15 A, A 3 × 2.5-cm cutaneous defect of cheek and a 2 × 1-cm defect of lower eyelid. B, C, Defects repaired with rotation cheek flap and full-thickness skin graft (lower eyelid). D-G, Eschar resulting from flap necrosis left in situ. Wound healed beneath eschar by secondary intention. Patient developed ectropion of lower eyelid that did not require treatment because left eye was artificial. (Courtesy of Shan R. Baker, MD.)
Trap-Door Deformity
Trap-door deformity may result from persistent edema and poor lymphatic drainage of flaps with incisions on three borders (Fig. 26-16). Flaps with curvilinear borders are particularly prone to development of trap-door deformity. The scar around the border of the flap contracts in a concentric fashion. This concentric contraction combines with contraction of the scar sheet beneath the flap to push the skin upward in a mushroom effect.56–58 Bilobe flaps of the nasal skin are particularly susceptible to development of trap-door deformity because of the two circular lobes used for construction of the flap. The deformity is even more likely to occur when a bilobe flap is used to repair a nasal cutaneous defect in which the nasal skin is thick and has sebaceous gland hypertrophy (Fig. 26-17). Proper flap design and adequate tissue undermining reduce the incidence of trap-door deformity. Wide skin undermining around the border of the recipient site of the flap is thought to limit trap-door deformity. Wide undermining presumably reduces scar contractile vectors toward the center of the flap by enlarging the area of the underlying sheet of scar.56,57,59,60 Flaps that are superiorly based are more prone to development of trap-door deformity than inferiorly based flaps because lymphatic channels of the face drain in an inferior direction.
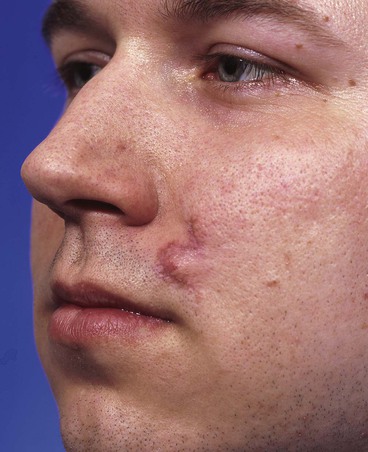
FIGURE 26-16 Small transposition flap manifesting trap-door deformity. (Courtesy of Shan R. Baker, MD.)
Unsightly Scars
Scars continue to change and to improve during the remodeling phase of wound healing. This includes collagen remodeling and collagen fiber reorientation for up to 18 months. This process may be longer in children. Young patients may have an exaggerated healing reaction with increased scar erythema and hypertrophy, so scar revisions in children should be delayed as long as feasible. Immature scars tend to be erythematous, which will usually fade over time. The scar’s appearance will also improve as it matures. There are a number of factors during wound healing that influence the ultimate appearance of a scar. Wounds subjected to excessive wound closure tension typically result in scars that are hypopigmented and often hypertrophied. Incisions made in thick skin, especially if there is sebaceous gland hypertrophy, often heal with depressed or widened scars (Fig. 26-18). Patients who can bend their thumb back to the volar surface of their forearm or touch their tongues to their noses (Gorlin’s sign) are more flexible than the average person. These individuals possess more elastin within the dermis and are more likely to spread their scars (Fig. 26-19).
Distortion of Facial Features
Scar contracture may lead to poor aesthetic and functional outcomes, especially in regions of the face with mobile facial structures, such as the eyelids, lips, and nasal alae. This is especially a problem in the periocular and oral area, where inadequate lip or eyelid closure leads to problems with eating, phonation, excessive tearing, and dry eye symptoms. Excessive wound closure tension may also lead to flap compromise and distortion of adjacent facial structures. Cervical facial flaps used to repair cutaneous defects near the lower eyelids should be secured to the periosteum of the maxilla or nasal bones with a tacking suture to reduce wound closure tension on the lower eyelid. This in turn reduces the risk for development of lower eyelid retraction. A temporary Frost suture may also be used to limit the downward pull of the flap on the eyelid skin. In spite of these precautionary measures, reconstruction of large cutaneous defects adjacent to the lower eyelid is often associated with lower eyelid retraction or ectropion. This is especially true in elderly patients because they have less muscle tone of the orbicularis oculi and less overall connective tissue to support the eyelid (Fig. 26-20). In the case of nasal reconstruction, flaps used for repair of the nose are designed sufficiently large to avoid alar retraction. For defects adjacent to the alar rim, cartilage grafting is routinely employed to provide a strut against the contractive forces of wound healing.
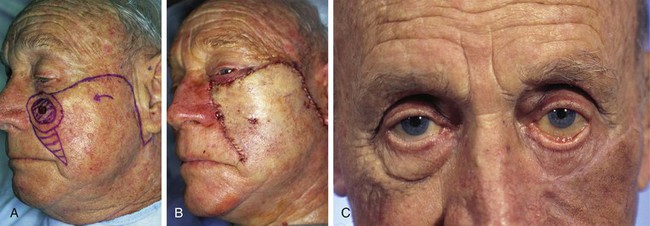
FIGURE 26-20 A, Squamous cell carcinoma of skin marked for excision. Rotation flap designed for reconstruction after resection. Horizontal lines mark anticipated standing cutaneous deformity. B, Flap in place after resection of skin cancer. C, Postoperative result at 2 months. Wound repair has caused ectropion of lower eyelid. (Courtesy of Shan R. Baker, MD.)
Distorted anatomic structures may respond to massage but may also require surgical revision, sometimes with cartilaginous grafts for support (Fig. 26-21). Like the nose, the lip is vulnerable to distortion by scars because of the mobile nature of the lips and the discrete borderline between the skin and vermilion. During healing, vertically oriented scars of the upper lip require only minimal contraction to cause notching or distortion of the vermilion-cutaneous border. Such distortion is quite noticeable to the observer, particularly when the vermilion is well-developed (Fig. 26-22). As with all lip wound closures, tension should be minimized. Precise alignment of the opposing edges of the lip wound in the vicinity of the vermilion-cutaneous borders is extremely important in repair of lacerations or cutaneous defects of the lip.
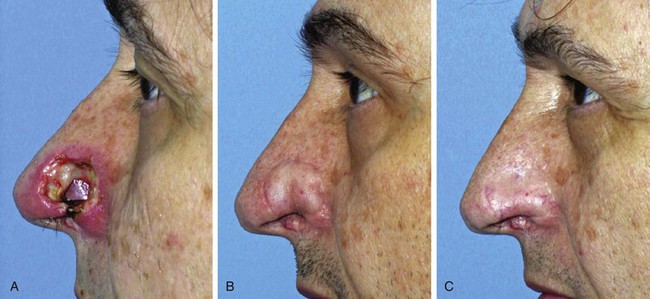
FIGURE 26-21 A, Patient with full-thickness alar defect after excision of skin malignant neoplasm. Defect repaired with ipsilateral septal mucoperichondrial hinge flap, auricular cartilage graft, and paramedian forehead flap. B, Two months after inset of forehead flap. Alar retraction observed despite cartilage graft used for framework. C, Six months after contouring procedure, release of scar contracture, and insertion of cartilage graft at alar rim.
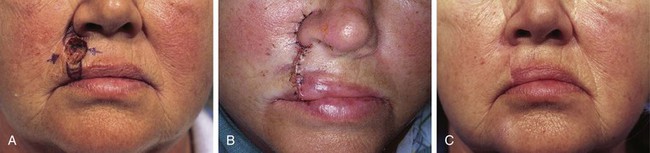
FIGURE 26-22 A, A 1 × 1.5-cm skin defect of upper lip. Primary wound closure planned. Anticipated standing cutaneous deformities marked with horizontal lines. B, Wound repaired. Deformities excised. C, Postoperative result at 4 months. Notching of vermilion-cutaneous border noted. (Courtesy of Shan R. Baker, MD.)
Facial Aesthetic Regions and Units
It is important to respect the borders of facial aesthetic regions and units when designing cutaneous flaps to avoid unsightly scars. This is particularly true of the alar-facial sulcus, which is an important boundary between the aesthetic regions of the nose, cheek, and upper lip. Larger defects of the ala are best reconstructed with interpolated cheek flaps that cross over the alar-facial sulcus rather than with transposition or advancement cheek flaps because these flaps obliterate this aesthetic boundary line (Fig. 26-23).
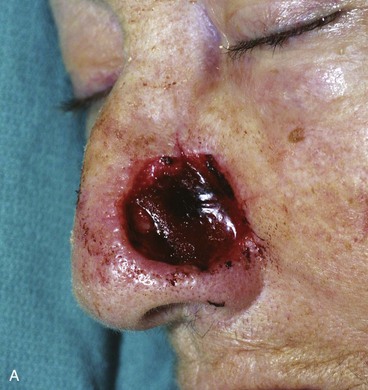
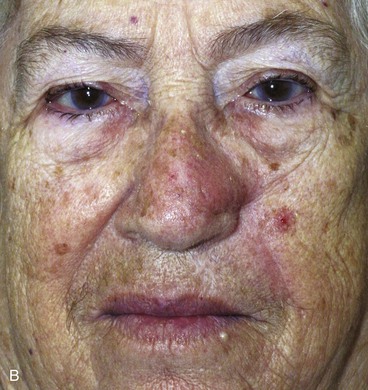
FIGURE 26-23 A, Elderly, frail patient with cutaneous defect of ala and caudal nasal sidewall after excision of skin malignant neoplasm. Patient desired simple, one-stage reconstruction. Cheek transposition flap performed. B, Postoperative result at 3 months. Flap has caused obliteration of superior aspect of alar-facial sulcus.
Cutaneous defects that extend from the nose to the medial cheek or to the upper lip are best repaired with separate flaps for each aesthetic region. Individual flaps are designed to repair the nasal component of the defect and the cheek or lip component of the defect. This approach positions some of the borders of the flaps in aesthetic boundary lines that help camouflage scars. More important, the concave topography of the sulcus separating the nose from the cheek and the nose from the lip is not obliterated by advancing or transposing skin from the cheek to the nose (Fig. 26-24).
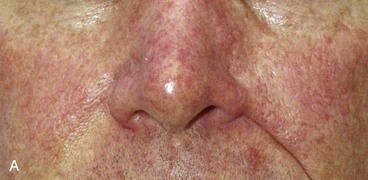
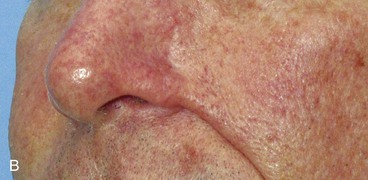
FIGURE 26-24 A, B, Patient with obliteration of alar-facial sulcus secondary to cheek advancement flap employed to repair alar defect.
The principle of employing separate flaps is equally important in reconstructing skin defects that cross over the melolabial crease from the lip to the cheek. When a cheek advancement flap is used to repair both the lip and cheek component of the defect, the flap inevitably obscures the melolabial crease, which is the aesthetic boundary line between the lip and cheek aesthetic regions (Fig. 26-25).
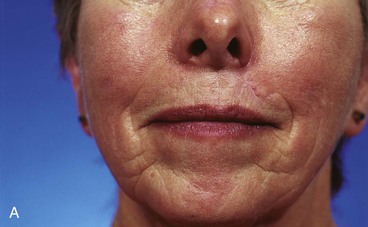
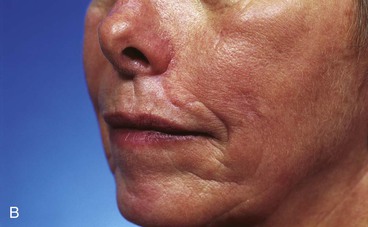
FIGURE 26-25 A, B, Obliteration of superior aspect of melolabial crease has occurred from use of cheek advancement flap to repair upper lip skin defect. (Courtesy of Shan R. Baker, MD.)
Standing cutaneous deformities result from transfer of local flaps or from wound closures that result in unequal wound borders. In the majority of cases, these deformities may be safely excised at the time of wound repair. It is a common error to underestimate the size of standing cutaneous deformities and to excise too little skin. This is particularly true for wounds repaired on the cheek and lips (Fig. 26-26). This may in part be related to the swelling of the soft tissues in the vicinity of the flap donor site during dissection and transfer of the flap. This swelling may prevent an accurate estimate of the size of the deformity and thus leads to an error in the amount of skin excised (Fig. 26-27).
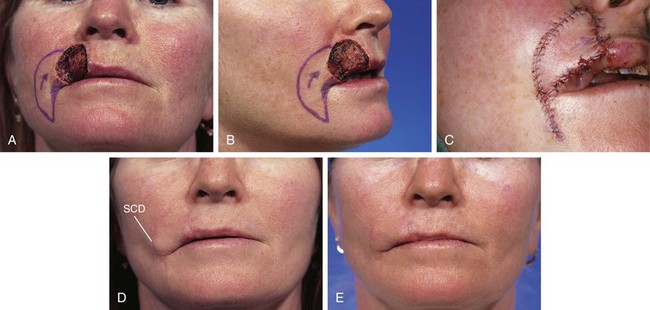
FIGURE 26-26 A, B, A 4 × 4-cm skin defect of lip. Subcutaneous tissue pedicled island advancement flap designed for repair. Shaded area at commissure represents anticipated standing cutaneous deformity forming from pivotal movement of flap. C, Deformity excised and flap in place. D, Postoperative result at 4 months. Fullness at oral commissure represents persistent standing cutaneous deformity (SCD). E, Four months after excision of redundant skin at commissure. (Courtesy of Shan R. Baker, MD.)
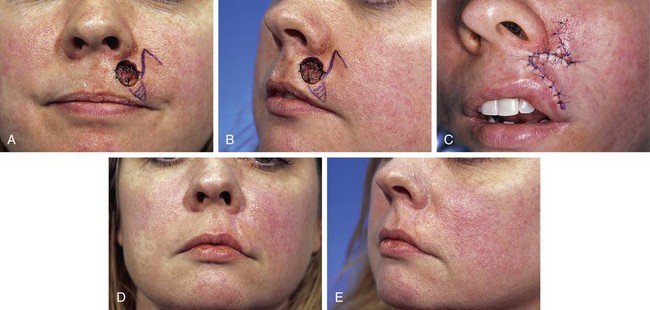
FIGURE 26-27 A, B, A 1 × 1-cm skin defect of lip. Transposition note flap (see Chapters 6 and 8) designed for repair. Anticipated standing cutaneous deformity marked with horizontal lines. C, Flap in place. Deformity excised. D, E, Postoperative result at 10 months. Insufficient excision of standing cutaneous deformity has pushed lateral vermilion downward, causing loss of visible vermilion border near commissure. (Courtesy of Shan R. Baker, MD.)
Case Reports
Excessive Wound Closure Tension
Case 1
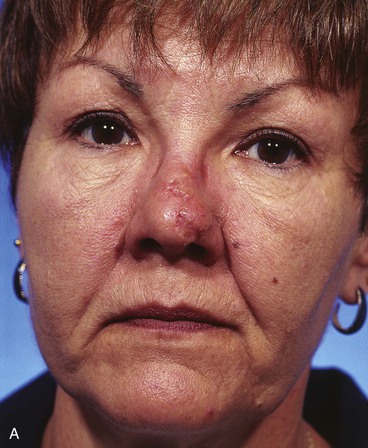
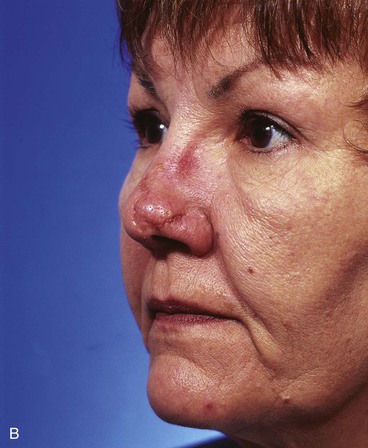
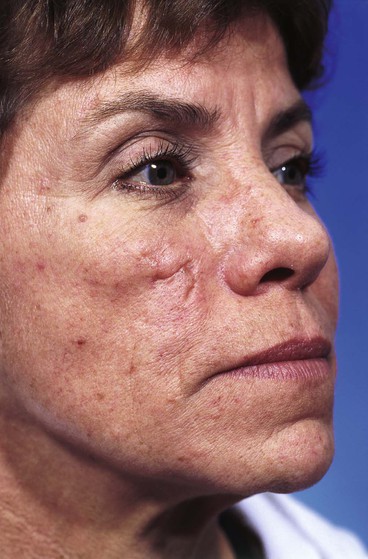
A 45-year-old patient developed a melanoma in situ of the left superior cheek. Margins around the lesion were ensured by the square technique as discussed in Chapter 8, Case 3. The area requiring excision measured 4 × 2-cm. A laterally based transposition cheek flap was designed for reconstruction after excision of the melanoma (Fig. 26-28). Figure 26-28B shows the flap sewn into the recipient site. There is marked pallor indicating ischemia of the distal portion of the flap due to excessive wound closure tension. The majority of this apparent ischemia proved to be reversible and was in part related to the vasoconstrictive effects of the anesthetic injected into the area in anticipation of reconstructing the cheek. However, a small area of the distal flap suffered irreversible ischemia, and this resulted in necrosis of the skin, causing the eschar shown in Figure 26-28C. The closure of the flap donor site was also under sufficient wound closure tension to cause a small area of skin necrosis.
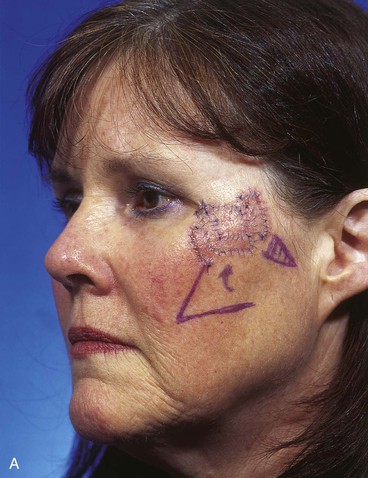
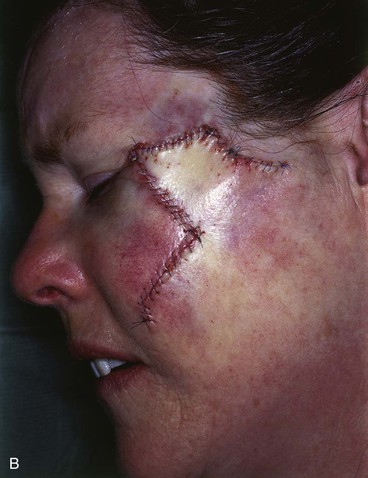
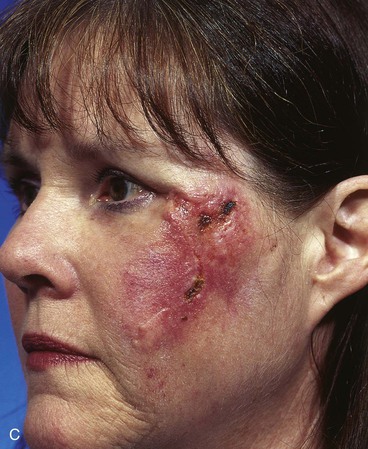
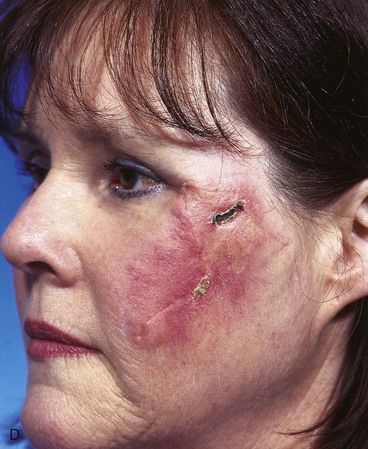
FIGURE 26-28 A, Melanoma in situ, 4 × 2-cm area marked for excision. Transposition flap marked for repair after resection. B, Melanoma excised. Flap in place. Distal flap has pallor, suggesting ischemia. C, Postoperative result at 2 weeks. Small area of necrosis of distal flap and at donor site wound has occurred. D, Postoperative result at 1 month. (Courtesy of Shan R. Baker, MD.)
Case 2
A 75-year-old patient underwent micrographic surgical resection of a large recurrent basal cell carcinoma that demonstrated aggressive growth patterns. The tumor extended to the periosteum of the zygomatic arch and required excision of a large portion of the posterior and superior cheek skin during a few days of surgical excision (Fig. 26-29). Because the wound had been open for several days during multiple staged surgical excisions of the tumor, it was elected to allow the wound to granulate for 2 weeks before reconstruction. A large inferiorly based pivotal advancement flap was designed to recruit skin for construction of the flap from the postauricular and superior regions of the neck. A small portion of the donor site wound posterior to the ear was closed with a full-thickness skin graft. The flap was transposed into the recipient site and advanced considerably for the flap to reach the most superior border of the defect. The extreme advancement necessary for wound repair resulted in excessive wound closure tension along the distal suture line. This in turn resulted in a limited necrosis of the distal border of the flap. The area of necrosis healed by secondary intention, and revision surgery was not necessary. The complication of flap necrosis could likely have been avoided by combining the flap with a full-thickness skin graft to assist with the wound repair. The graft could have been used to cover the superior portion of the defect, thereby reducing the degree of flap advancement required in this case. The technique of and indications for combining local flaps with skin grafts are discussed in detail in Chapter 16.
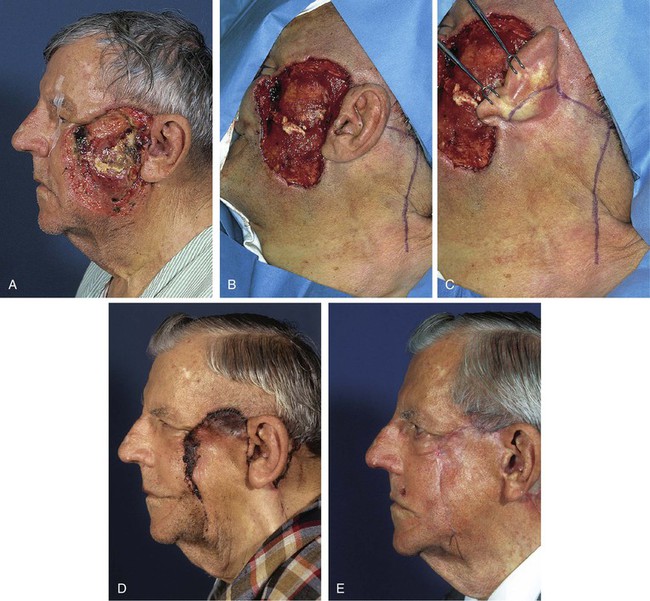
FIGURE 26-29 A, A 75-year-old man after micrographic excision of extensive basal cell carcinoma. Wound allowed to granulate for 2 weeks. B, C, Pivotal advancement flap designed for repair of defect. D, Postoperative result at 1 month. Necrosis of distal borders of flap has occurred. E, Necrotic wound allowed to heal by secondary intention. No revision surgery performed. (Courtesy of Shan R. Baker, MD.)
Case 3
A 35-year-old patient presented with a 1 × 1-cm defect of the skin and vermilion of the upper lip after micrographic excision of a basal cell carcinoma. An A-T repair of the defect was planned with bilateral unipedicled advancement flaps (Fig. 26-30). However, at the time of surgery, it was elected to use a V-Y island advancement flap based on an orbicularis oris muscle pedicle. This was an error in judgment because the orbicularis muscle did not provide sufficient mobility to the island flap. This in turn resulted in excessive wound tension on the vermilion. As the wound healed, scar contracture and the excessive wound closure tension led to notching and superior migration of the vermilion-cutaneous border. Subsequent surgery was necessary to rectify the situation. Two Z-plasties were performed on either side of the inferior border of the flap to lengthen the scars and to release the superior pull on the vermilion by the flap (Fig. 26-31). It was also necessary to resect a small ellipse of mucosa along the wet line of the lip in the area of the reconstruction. This allowed better symmetry of vermilion vertical height in the area of reconstruction. Fortunately, the revision surgery provided the patient with an acceptable appearance of the lip.
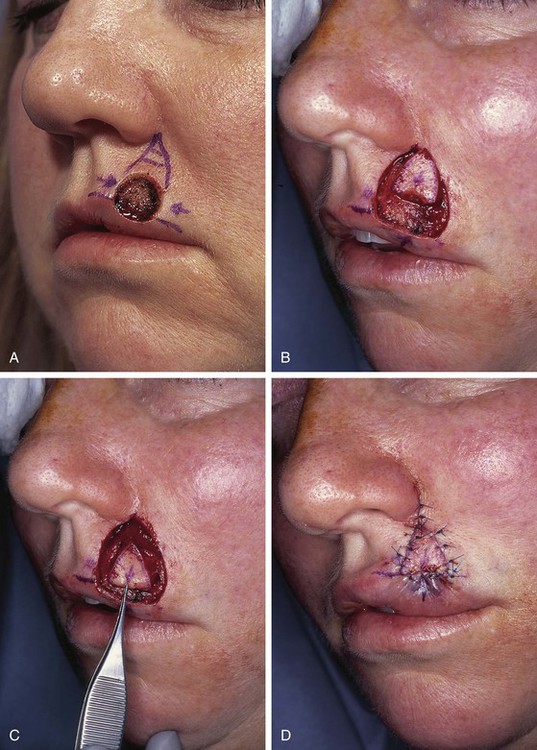
FIGURE 26-30 A, A 1 × 1-cm skin defect of lip and vermilion. A-T repair planned. B-D, Subcutaneous tissue pedicled island advancement flap used for reconstruction instead of A-T repair.
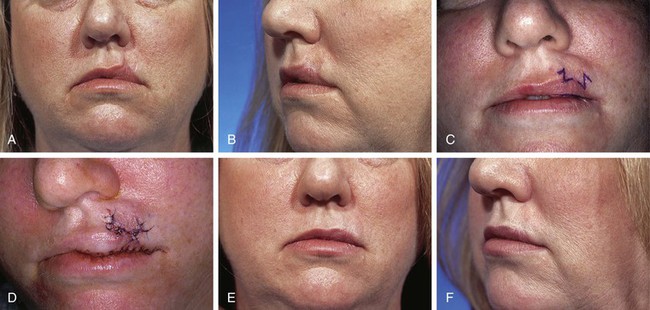
FIGURE 26-31 A, B, Same patient as shown in Figure 26-30, postoperative view at 5 months. Distortion of vermilion-cutaneous border resulting from excessive wound closure tension. C, Z-plasties at base of flap and elliptical excision of mucosa along wet line of lip planned to correct lip deformity. D, Revision surgery completed. E, F, One year after revision surgery. (Courtesy of Shan R. Baker, MD.)
V-Y island advancement flaps are best used in areas of the face with ample subcutaneous fat that can provide a pedicle of sufficient mobility to enable the flap to be advanced into a recipient site without excessive wound closure tension. For this reason, island advancement flaps work best for repair of defects of the medial cheek, which has the greatest amount of subcutaneous fat of the face. The skin of the lips is tightly attached to the underlying muscle, and there is no significant subcutaneous fat on which to create a pedicle for an island flap. For this reason, use of V-Y island advancement flaps of the lip should be confined to the repair of very small defects in the midline of the lip between the philtral ridges. Island advancement flaps are discussed in detail in Chapters 6 and 9.
Case 4
A 32-year-old patient had micrographic surgery to remove two basal cell carcinomas involving the lower lip and chin. The defect of the lip measured 2 × 2-cm and involved skin and vermilion of the lip. The skin defect of the chin measured 1 × 1-cm (Fig. 26-32). It was elected to repair both defects simultaneously with bilateral unipedicled advancement flaps. The vermilion component of the lip defect was reconstructed with a separate mucosal advancement flap. The mucosal flap was constructed from mucosa on the inner aspect of the lower lip and was advanced outward to cover the orbicularis muscle exposed by the vermilion defect. Although the surgical plan was sound, the skin of the lip is inelastic and without redundancy. For this reason, cutaneous advancement flaps of the lips are typically subjected to greater wound closure tension than cutaneous flaps used to repair similar-sized defects elsewhere on the face. In this case, the wound tension from closure of the lip defect resulted in a small area of skin necrosis. The involved area was allowed to heal by secondary intention. On healing, some deformation of the vermilion occurred, but the severity of the distortion did not require revision surgery.
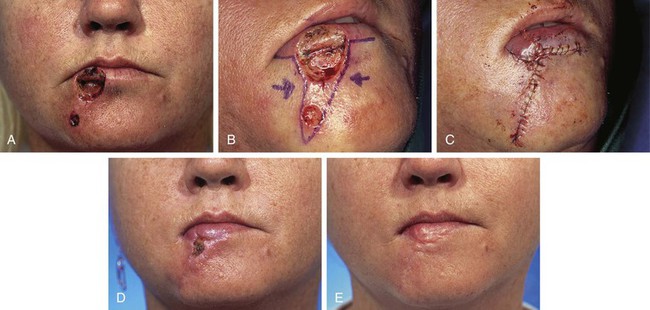
FIGURE 26-32 A, A 2 × 2-cm skin and vermilion defect of lip and a 1 × 1-cm skin defect of chin. B, C, Defects repaired with bilateral unipedicled advancement flaps. Mucosal advancement flap harvested from inner aspect of lip used to repair vermilion component of lip defect. D, Excessive wound closure tension caused necrosis of small area of opposing borders of advancement flaps. Area healed by secondary intention. E, Postoperative result at 5 months. Hypertrophic scar noted in area of necrosis. (Courtesy of Shan R. Baker, MD.)
Improper Flap Design
Case 5
A 47-year-old man developed a melanoma in situ of the superior medial cheek immediately inferior to the eyelid. Tumor-free margins around the lesion were ensured by the square technique. The area requiring resection measured 2 × 1.75-cm and was rhombus shaped. A superiorly based Dufourmentel flap was designed for repair of the wound resulting from resection of the melanoma (Fig. 26-33). The flap healed without causing distortion of the lower eyelid. However, the flap was harvested in the hair-bearing region of the cheek and transferred to an area that was not hair bearing. In fair-skinned men, this does not cause a very noticeable deformity. The patient in this case was dark complected and had dense, dark facial hair. This quality caused a noticeable discrepancy in appearance between the skin of the flap and the adjacent skin of the superior cheek. For this complication to be avoided, it may have been wiser to use a rotation flap to reconstruct the cheek. The rotation flap could have been designed to recruit skin for construction of the flap from the non–hair-bearing skin immediately lateral to the tumor resection.
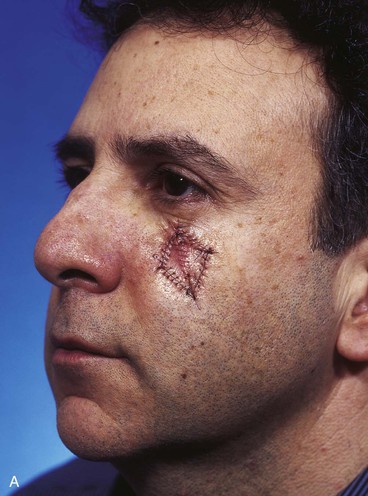
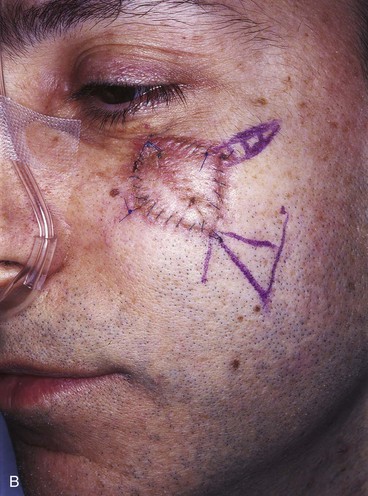
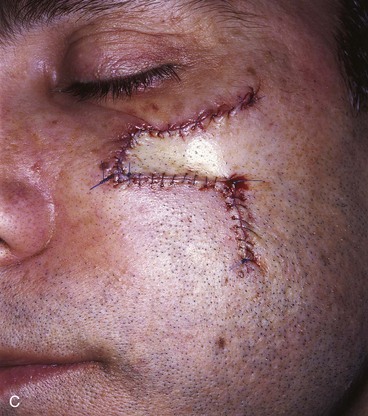
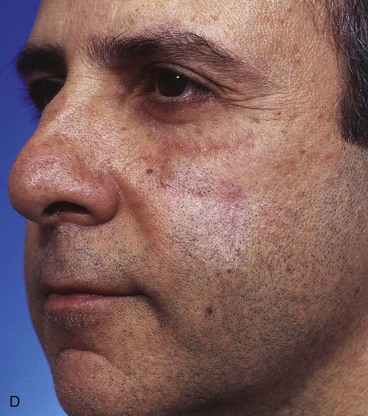
FIGURE 26-33 A, Melanoma in situ marked for excision by square technique. B, Dufourmentel flap designed for wound repair after excision of melanoma. Anticipated standing cutaneous deformity marked with vertical lines. C, Melanoma excised. Flap in place. D, Postoperative result at 6 months. Hair-bearing flap has caused noticeable discrepancy in appearance between skin of flap and adjacent non–hair-bearing skin of cheek. (Courtesy of Shan R. Baker, MD.)
Case 6
A 67-year-old patient developed a melanoma in situ of the cheek requiring the square technique to achieve tumor-free margins around the lesion. The planned resection measured 3.5 × 2.5-cm and involved excision of the skin over the malar eminence (Fig. 26-34). A transposition flap was designed for repair of the defect resulting from resection of the melanoma. Because the flap was too small to cover the entire defect, a full-thickness skin graft harvested from the standing cutaneous deformity formed by pivoting the flap was used to cover the superior portion of the defect. In spite of the use of a skin graft to assist with wound closure, a portion of the distal flap suffered necrosis, although the skin graft survived completely. The area of necrosis healed by secondary intention, and the result after wound healing was acceptable but less than ideal.
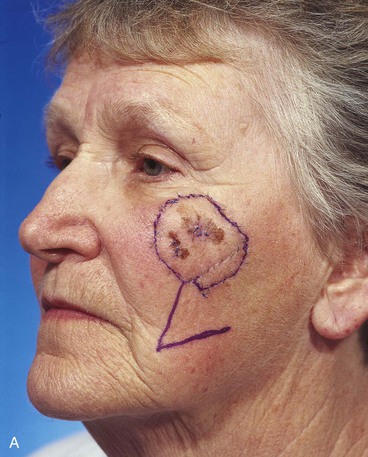
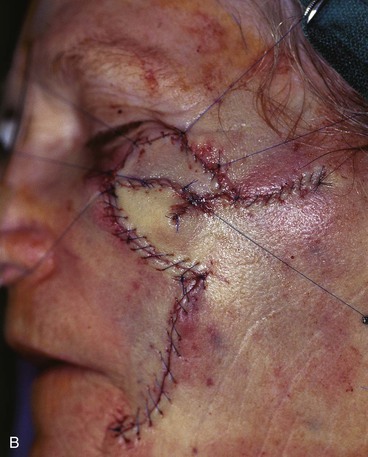
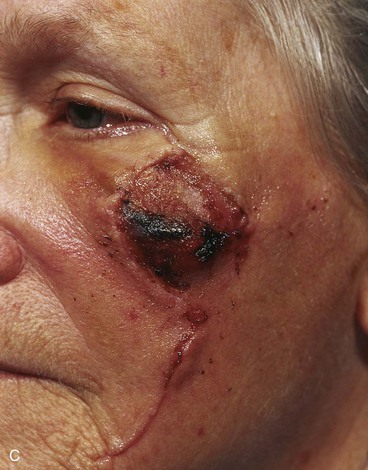
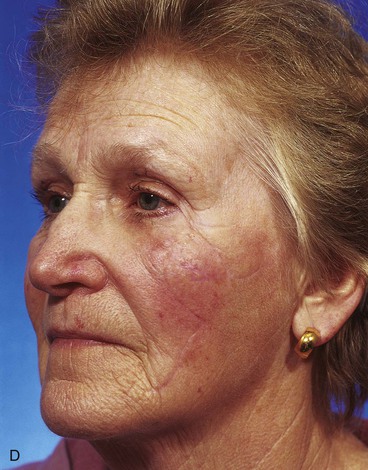
FIGURE 26-34 A, Melanoma in situ, 3.5 × 2.5-cm area marked for excision. Transposition flap designed for repair. B, Melanoma excised. Flap transferred. Full-thickness skin graft combined with flap for wound repair. C, Postoperative result at 2 weeks. Skin graft survived, but portion of flap suffered necrosis. Wound healed by secondary intention. D, Postoperative result at 1 year. No revision surgery performed. (Courtesy of Shan R. Baker, MD.)
Case 7
A 64-year-old patient was treated for melanoma in situ of the cheek by the square technique to ensure tumor-free margins around the lesion. The necessary area of resection measured 3 × 2.5-cm and required excision of the skin covering the malar eminence. Similar to Case 6, a transposition flap was selected for repair of the cheek after tumor excision (Fig. 26-35). The flap was inferiorly based and was harvested from the melolabial fold. As with the previous case, the flap proved to be too small to cover the entire defect resulting from excision of the melanoma. Therefore, a full-thickness skin graft was used to repair a portion of the defect adjacent to the lower eyelid. The distal portion of the flap suffered necrosis, and unlike in the previous case, the skin graft did not survive. The complete loss of the skin graft and the necrosis of the distal flap resulted in a poor outcome. The patient developed severe lower eyelid ectropion and hypertrophic scarring of the cheek.
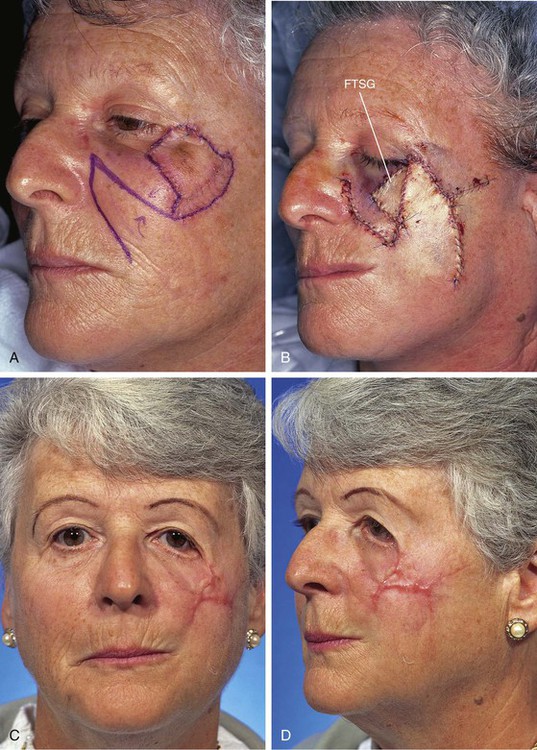
FIGURE 26-35 A, Melanoma in situ, 3 × 2.5-cm area marked for excision. Transposition flap designed for repair of wound after resection of melanoma. B, Melanoma excised. Flap in place. Full-thickness skin graft (FTSG) combined with flap for wound repair. C, D, Skin graft did not survive and distal portion of flap underwent necrosis. This resulted in ectropion of lower eyelid and hypertrophic scarring. (Courtesy of Shan R. Baker, MD.)
Even when a local flap is designed sufficiently large to cover a defect over the malar eminence, flap necrosis may occur. This is more likely in smokers and when defects are large, requiring movement of flap skin for considerable distance. An example of this is shown in Figure 26-36. An area of melanoma in situ measuring 6 × 5-cm over the malar eminence is marked with sutures. After excision of the melanoma, a large pivotal advancement flap was used to reconstruct the defect. The flap recruited redundant skin from the superior cervical area and a Z-plasty was designed at the base of the flap. On transfer of the flap, it was not necessary to use the entire length of the planned incision line. Therefore, the Z-plasty was positioned more superiorly over the body of the mandible.
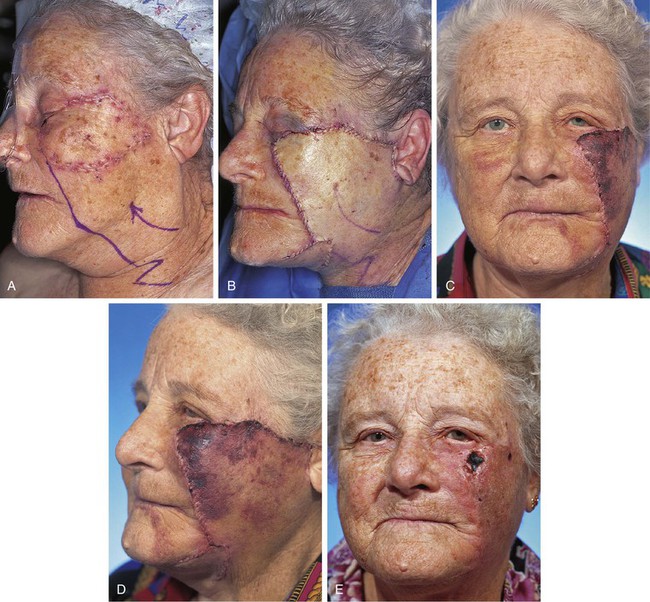
FIGURE 26-36 A, Melanoma in situ, 6 × 5-cm area marked by sutures for excision. Pivotal advancement flap designed for repair. B, Melanoma excised and flap transferred to recipient area. Z-plasty positioned over body of mandible because entire length of planned flap incision was not required. C, D, Postoperative result at 1 week. Flap demonstrates signs of ischemia. E, Postoperative result at 3 weeks. Full-thickness necrosis of most distal portion of flap observed. (Courtesy of Shan R. Baker, MD.)
Postoperatively, ischemia of the distal flap and subsequent full-thickness necrosis of the most distal corner of the flap developed. The necrosis resulted in scarring, which in turn led to the development of lower eyelid ectropion (Fig. 26-37). Because the area of necrosis occupied the lateral aspect of the anterior lamellae of the lower eyelid, the ectropion was severe and required surgical treatment. Correction of the ectropion was accomplished by excision of the scar and reconstruction of the area with a full-thickness skin graft. A lateral canthoplasty was also performed (Fig. 26-37).
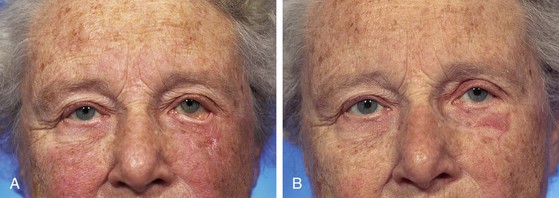
FIGURE 26-37 A, Same patient shown in Figure 26-36. Flap necrosis caused severe ectropion of eyelid. B, Ectropion corrected with full-thickness skin graft and lateral canthoplasty. (Courtesy of Shan R. Baker, MD.)
References
1. Conley JJ, ed. Complications of head and neck surgery. Philadelphia: WB Saunders, 1979.
2. Leshin, B, Whitaker, DC, Swanson, NA. An approach to patient assessment and preparation in cutaneous oncology. J Am Acad Dermatol. 1981; 19:1081.
3. Nichols, RL. Techniques known to prevent postoperative wound infection. Infect Control. 1982; 3:34.
4. Sebben, JE. Avoiding infection in office surgery. J Dermatol Surg Oncol. 1982; 8:455.
5. Sebben, JE. Sterile technique and the prevention of wound infection in office surgery: part I. J Dermatol Surg Oncol. 1988; 14:1364.
6. Sebben, JE. Sterile technique and the prevention of wound infection in office surgery: part II. J Dermatol Surg Oncol. 1989; 15:38.
7. Salasche, SJ, Grabski, WJ. Complication of flaps. J Dermatol Surg Oncol. 1991; 17:132.
8. Diaz, DD, Freeman, SB, Wilson, JF, et al. Hematoma-induced flap necrosis and free radical scavengers. Arch Otolaryngol Head Neck Surg. 1992; 118:516.
9. Hillelson, RL, Glowacki, J, Healey, NA, et al. A microangiographic study of hematoma-associated flap necrosis and salvage with isoxsuprine. Plast Reconstr Surg. 1980; 66:528.
10. Kaufman, T, Angel, MF, Eichenlaub, EH, et al. The salutary effects of the bed on the survival of the experimental flaps. Ann Plast Surg. 1985; 14:64.
11. Angel, MF, Narayanan, K, Swartz, WM, et al. The etiologic role of free radicals in the hematoma-induced flap necrosis. Plast Reconstr Surg. 1986; 77:795.
12. Mulliken, JB, Healey, NA. Pathogenesis of skin flap necrosis from an underlying hematoma. Plast Reconstr Surg. 1979; 63:540.
13. Krizek, TJ, Davis, JH. The role of the red cell in subcutaneous infection. J Trauma. 1965; 5:85.
14. Polk, HC, Miles, AA. Enhancement of bacterial infection by ferric iron. Kinetics, mechanisms and surgical significance. Surgery. 1971; 70:71.
15. Becker, FF. Facial reconstruction with local and regional flaps. New York: Thieme-Stratton; 1985.
16. Sylaidis, P, Wood, S, Murray, DS. Postoperative infection following clean facial surgery. Ann Plast Surg. 1997; 39:342.
17. Pearl, RM, Johnson, D. The vascular supply to the skin: an anatomical and physiological reappraisal: part I. Ann Plast Surg. 1983; 11:99.
18. Pearl, RM, Johnson, D. The vascular supply to the skin: an anatomical and physiological reappraisal: part II. Ann Plast Surg. 1983; 11:196.
19. Pearl, RM. A vascular approach to the prevention of infection. Ann Plast Surg. 1985; 14:443.
20. Tran, DT, Miller, SH, Bucks, D, et al. Potentiation of infection by epinephrine. Plast Reconstr Surg. 1985; 76:993.
21. Sebben, JE. Prophylactic antibiotics in cutaneous surgery. J Dermatol Surg Oncol. 1985; 11:901.
22. Bumpous, JM, Johnson, JT. The infected wound and its management. Otolaryngol Clin North Am. 1995; 28:987.
23. Pearl, RM, Arnstein, D. A vascular approach to the prevention of infection. Ann Plast Surg. 1985; 14:443.
24. Salische, SJ. Complications of local flaps. In: Baker SR, Swanson NA, eds. Local flaps in facial reconstruction. New York: Mosby; 1995:545–585.
25. Vural, E, Key, JM. Complications, salvage, and enhancement of local flaps in facial reconstruction. Otolaryngol Clin North Am. 2001; 4:739.
26. Niessen, FB, Spauwen, PHM, Schalkwijk, J, et al. On the nature of hypertrophic scars and keloids: a review. Plast Reconstr Surg. 1999; 104:1435.
27. Edlich, RF, Friedman, HI, Haines, PC, et al. Biology of wound repair: its influence on surgical decision. Facial Plast Surg. 1984; 1:169.
28. Golminz, D, Bennett, RG. Cigarette smoking and flap and full thickness graft failure. Arch Dermatol. 1991; 127:1012.
29. Craig, S, Rees, TD. The effect of smoking on experimental flaps in hamsters. Plast Reconstr Surg. 1985; 75:842.
30. Forrest, C, Pang, C, Lindsay, W. Dose and time effects of nicotine treatment on the capillary blood flow and viability of random pattern flaps in the rat. Br J Plast Surg. 1987; 40:295.
31. Jensen, JA, Coodson, WH, Hopf, HW, et al. Cigarette smoking decreases tissue oxygen. Arch Surg. 1991; 126:1131.
32. Nolan, J, Jenkins, RA, Kurihara, K, et al. The acute effects of cigarette exposure on experimental skin flaps. Plast Reconstr Surg. 1985; 75:544.
33. Kinsella, JB, Rassekh, CH, Wassmuth, ZD, et al. Smoking increases skin flap complications. Ann Otol Rhinol Laryngol. 1999; 108:139.
34. Kerrigan, CL, Daniel, FI. Monitoring acute skin flap failure. Plast Reconstr Surg. 1983; 71:519.
35. Milton, SH. Pedicled skin flaps: the fallacy of the length:width ratio. Br J Surg. 1970; 57:502.
36. Daniel, RK, Kerrigan, CL. Skin flaps: an anatomical and hemodynamic approach. Clin Plast Surg. 1979; 6:181.
37. Myers, B. Understanding flap necrosis. Plast Reconstr Surg. 1986; 78:813.
38. Cutting, CB. Critical closing and perfusion pressures in flap survival. Ann Plast Surg. 1982; 9:534.
39. Suzuki, S, Isshiki, N, Ogawa, Y, et al. The minimal requirement of circulation for survival of undelayed flaps in rats. Plast Reconstr Surg. 1986; 78:221.
40. Angel, MR, Narayanan, K, Swartz, WM, et al. Deferoxamine increases skin flap survival: additional evidence of free radical involvement in ischemic flap surgery. Br J Plast Surg. 1986; 39:469.
41. Angel, MF, Ramasastry, SS, Swartz, WM, et al. The critical relationship between free radicals and degree of ischemia: evidence for tissue intolerance of marginal perfusion. Plast Reconstr Surg. 1988; 81:233.
42. Angel, MF, Kaufman, T, Swartz, WM, et al. Studies of the nature of flap/bed interactions in rodents: part I. Ann Plast Surg. 1986; 17:317.
43. Angel, MF, Ramasastry, SS, Narayanan, K, et al. Studies on the nature of flap/bed interactions in rodents: part II. Ann Plast Surg. 1986; 17:434.
44. Larrabee, WF, Jr., Holloway, GA, Jr., Trachy, RE, et al. Skin flap tension and wound slough: correlation with laser Doppler velocimetry. Otolaryngol Head Neck Surg. 1982; 90:185.
45. Larrabee, WF, Jr., Holloway, GA, Jr., Sutton, D. Wound tension and blood flow in skin flap. Ann Otol Rhinol Laryngol. 1984; 93:112.
46. Pang, CY, Neligan, PC, Forrest, CR, et al. Hemodynamics and vascular sensitivity to circulating norepinephrine in normal skin and delayed and acute random skin flaps in the pig. Plast Reconstr Surg. 1986; 78:75.
47. Wu, G, Calamel, PM, Shedd, DP. The hazards of injecting local anesthetic solutions with epinephrine into flaps. Plast Reconstr Surg. 1978; 62:396.
48. Dabb, RW, Malone, JM, Leverett, LC. The use of medicinal leeches in the salvage of flaps with venous congestion. Ann Plast Surg. 1992; 29:250.
49. Utley, DS, Koch, RJ, Goode, RL. The failing flap in facial plastic and reconstructive surgery: role of the medicinal leech. Laryngoscope. 1998; 108:1129.
50. Lineaweaver, WC, Hill, MK, Buncke, GM, et al. Aeromonas hydrophila infections following use of medicinal leeches in replantation and flap surgery. Ann Plast Surg. 1992; 29:238.
51. Mackay, DR, Manders, EK, Saggers, GC, et al. Aeromonas species isolated from medicinal leeches. Ann Plast Surg. 1999; 42:275.
52. Cherry, GW. Pharmacologic treatment of the failing skin flap [discussion]. Plast Reconstr Surg. 1982; 70:549.
53. Kerrigan, CL, Daniel, RK. Pharmacologic treatment of the failing skin. Plast Reconstr Surg. 1982; 70:541.
54. Pratt, MF. Evaluation of random skin flap survival in a porcine model. Laryngoscope. 1996; 106:700.
55. Salasche, SJ. Acute surgical complications: cause, prevention, and treatment. J Am Acad Dermatol. 1986; 15:1163.
56. Hosokawa, K, Susuki, T, Kikui, T, et al. Sheet of scar causes trap-door deformity: a hypothesis. Ann Plast Surg. 1990; 25:134.
57. Kaufman, AJ, Kiene, KL, Moy, RL. Role of tissue undermining in the trap-door effect of transposition flaps. J Dermatol Surg Oncol. 1985; 19:128.
58. Koopman, DF. Cutaneous wound healing: an overview. Otolaryngol Clin North Am. 1995; 28:835.
59. Koranda, FC, Webster, RC. Trap-door effect in nasolabial flaps. Arch Otolaryngol. 1985; 111:421.
60. Webster, RC, Benjamin, BJ, Smith, RC. Treatment of trap-door deformity. Laryngoscope. 1978; 88:707.