Chapter 3 Complications of Cranial Nerve Stimulation
 Appropriate Accreditation Council for Graduate Medical Education mentored subspecialty training in interventional pain management is vital to ensure patient-centered care.
Appropriate Accreditation Council for Graduate Medical Education mentored subspecialty training in interventional pain management is vital to ensure patient-centered care. Accurate and expertly placed needles in appropriately selected patients are essential to ensure accurate diagnosis and successful treatment.
Accurate and expertly placed needles in appropriately selected patients are essential to ensure accurate diagnosis and successful treatment. Stimulation of the trigeminocervical complex is the most described cranial nerve stimulation target to treat pain syndromes.
Stimulation of the trigeminocervical complex is the most described cranial nerve stimulation target to treat pain syndromes. The most common complication for occipital nerve stimulation is lead migration, which may by mitigated by paddle lead choice, retromastoid position lead placement, or implantable pulse generator location in the abdomen or infraclavicular site.
The most common complication for occipital nerve stimulation is lead migration, which may by mitigated by paddle lead choice, retromastoid position lead placement, or implantable pulse generator location in the abdomen or infraclavicular site.Introduction
Cranial nerve stimulation strategies have been used to treat a variety of diverse conditions that sometimes prove to be unresponsive to conservative medical therapies. These range from depression to seizure to congestive heart failure.1–3 More specifically, some other indications include atypical facial pain, terminal branch neuralgias, a variety of headache disorders (i.e., cluster, migraine, trigeminal autonomic cephalalgias, cervicogenic headache, hemicrania continua, trigeminal neuralgia), depression, and postherpetic neuralgia.1,4–24 Stimulation technology has also been used in a case series to treat central hypoventilation syndrome by stimulation of the phrenic nerve.
This chapter focuses on complications reported during peripheral (distal) stimulation of cranial nerves, namely the terminal branches of the trigeminal nerve, the trigeminocervical complex, and the vagus nerve. Although epidural trigeminocervical complex stimulation has been reported to treat trigeminal neuralgia,25 the reader is directed to Chapter 1 for discussion of complications of epidural spinal cord stimulation (SCS).
Although a review of the American Society of Regional Anesthesia’s 2010 guidelines for neuraxial interventions may not directly be applicable to peripheral branch of cranial nerve stimulation interventions, concurrent use of anticoagulants before surgery may increase the bleeding risk. Therefore it is advised that readers familiarize themselves with these guidelines, as well as the guideline statements of perioperative use of anticoagulant therapy.26
Patient selection guides treatment success. Psychometric testing for neuromodulation candidacy deserves special mention. It is well established that concurrent psychiatric illness reduces interventional treatment success rates27 and that approximately 20% to 45% of pain patients have accompanying psychopathology.28 Therefore it is essential that appropriate measures be taken to diagnose, treat, or exclude unsuitable candidates. Instruments described to aid in identifying the presence of clinically significant psychopathology include the Symptom Checklist 90 (SCL-90-R) and the Minnesota Multiphasic Personality Inventory (MMPI-2). Poor treatment outcome was identified in patients with presurgical somatization, depression, anxiety, and poor coping.29
Background
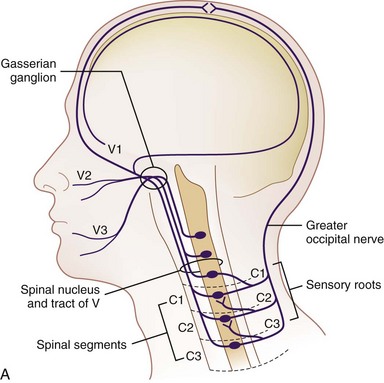
Trigeminal stimulation techniques have been described both centrally and peripherally; however, more reliable stimulation has been achieved in the latter.4 Furthermore, because overall complications of central trigeminal gasserian stimulation have been reported to be near 30% to 40%30–32 and with reduced complications with peripheral branch stimulation, enthusiasm for central stimulation has dwindled. The trigeminal nuclear systems are bilateral structures that span from the midbrain to the medulla. The caudal-most portion, the trigeminal nucleus caudalis, may extend down as far as the second or third cervical level, which has both anatomic and clinical implications. Goadsby33 demonstrated the presence of convergence between the cervical and trigeminal system, forming a trigeminocervical complex. This was characterized by Anthony34 and helped form the basis for greater occipital nerve stimulation for treatment of headache (Fig. 3-1). Traditionally, stimulation was achieved by using either implanted cylindrical or paddle leads. There are some reports of using a small implantable device without the traditional implantable pulse generator (IPG) placement10 along with transcutaneous electrical nerve stimulation unit applications.11
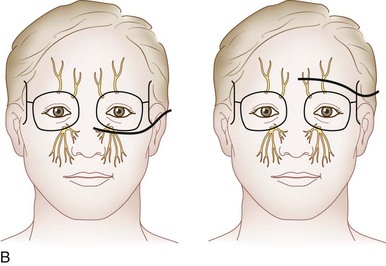
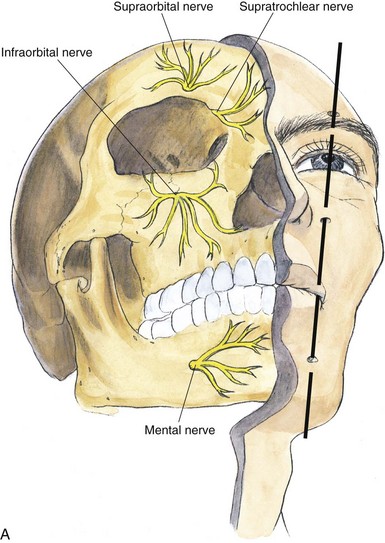
Peripherally, the trigeminal nerve terminates as the supraorbital and supratrochlear nerve from V1, the infraorbital nerve from V2, and the mental nerve from V3. These sites lend themselves to subcutaneous neurostimulatory targets (Fig. 3-2).4,6,12,17,18,24,35,36
Fig. 3-2 A, Terminal branches of the trigeminal nerve.
(From Brown DL: Atlas of regional anesthesia, ed 3, Philadelphia, WB Saunders, 2006.)
Vagal nerve stimulation (VNS) has been approved for drug-refractory epilepsy and is a viable option for those who decline surgery or nonsurgical candidates. Its safety and efficacy have been well established.37 Furthermore, it has been used in the treatment of people with treatment-resistant depression. Commonly, the left vagus nerve is stimulated, not with the commonly used spinal cord stimulator but via a NeuroCybernetic Prosthesis.38 The exact mechanism of action is unknown, although it is postulated to involve vagal sensory afferents. Whereas high-frequency stimulation causes electroencephalographic desynchronization, low-frequency stimulation causes synchronization.39 Typically, the VNS is positioned on the left side because right-sided stimulation causes more cardiac slowing as a consequence of sinoatrial (SA) node stimulation.40 Although this is not commonly used to treat pain, the scope is broadening.35,41,42 Therefore, VNS is discussed here briefly for completeness.
Selected Complications
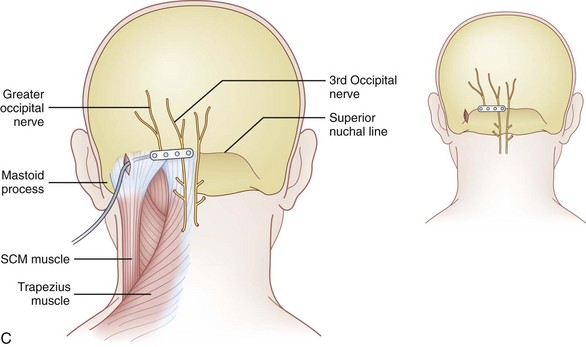
Greater Occipital Nerve Stimulation (Table 3-1)
Lead Tip Erosion
Trentman et al43 in 2008 reported on two cases of lead erosion after greater occipital nerve stimulation occurring months after implantation. One patient was a 27-year-old woman who had intended weight loss after gastric bypass surgery of 52 kilograms; the other case did not have any identifiable risk factors, including smoking, diabetes, or infection. The authors contended that care needs to be emphasized when implanting the paddle or percutaneous electrode, not placing the lead tip too superficial or lateral, lessened by avoiding the lateral decubitus position during implant. Furthermore, extreme weight reduction or patients with very low body mass indexes may be susceptible to eventual lead migration.43
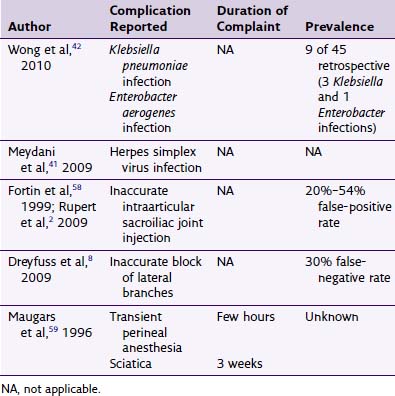
Table 3-1 Reported Greater Occipital Nerve Stimulation Complications
| Complication | Frequency |
|---|---|
| Lead tip erosion | Two reported43 |
| Lead migration | 27.3%,38 33%47* |
| Hardware malfunction | 1.7%,38 1/819 |
| Infection | 5.9%,38 2/7,65 3/166 |
| Lead fracture or disconnect | 2.6%38 |
| Allergy | 0.8%38 |
| Explant | 2.6%38 |
| Contact dermatitis | 7%47 |
| Neck stiffness | 13%47 |
| Pain over the device (IPG or lead) | 1/10,22 3/15,20 7%47 |
| Myofascial pain | 1/11,66 1/15,20 five case reports,44 7%47 |
| Battery depletion | 42% at 3 years47 |
| Stimulation tolerance | Case report53 |
| Overstimulation | 3/610 |
IPG, implantable pulse generator.
* Reported 100% lead migration at 3 years.
Management of lead tip erosion of occipital stimulation leads centers on infection management and revision. Infectious management includes assessment of extent and depth of infection. Generally, two approaches can be used: attempt to salvage the lead and system or explant and reimplant at a future date. Because many patients have greater occipital nerve stimulators secondary to a medically intractable headache, many patients request lead salvage strategies. Trentman et al43 described resection of granulomatous tissue around eroded lead and was sutured to the fascia and closed primarily along with a concurrent postoperative course of appropriately selected bacteria-sensitive antibiotics by culture.
Infection
As one can appreciate, a superficial or deep subcutaneous infection and its management, from a subcutaneously placed lead, are drastically different than an epidural abscess from an epidural lead. That being said, the potential for leads placed near the nuchal ridge can theoretically spread intracranially via the emissary veins.44 As illustrated, device salvage has been described for peripheral nerve (or peripheral branch of cranial nerve) stimulation.
Surgical site wound prevention, however, is largely the same.45 As with any surgical procedure, antimicrobial prophylaxis should occur within 30 minutes of skin incision and cover the most commonly implicated organisms. The decision to provide postoperative prophylactic antibiotics continues to be a controversial topic because many practitioners continue to do so despite only anecdotal evidence. Surgical preparation includes optimizing and mitigating patient comorbidities that increase infectious risk, ensuring proper scrub, and promoting a sterile and accessible operative field.46 Hair removal should be performed immediately before the procedure, and hair should be clipped because shaving increases the chance of infection secondary to microabrasion. Skin preparation is usually accomplished by chlorhexidine gluconate, povidone–iodine, or alcohol-based solutions. Intraoperative strategies to reduce infection include meticulous sterile technique, layered wound closure to avoid dead space, appropriate hemostasis, avoidance of placing sutures overlying the device, and nonpressured irrigation (in clean uncontaminated surgeries).
Lead Migration
Lead migration rates after greater occipital nerve peripheral nerve stimulation have varied in the literature, although it remains the most common complication described. The rate of lead migration seems to be dependent on follow-up length after implant. Schwedt et al47 implanted 15 patients using cylindrical leads placed at C1, with the extension connector placed near the periscapular region and IPG placement in the upper buttocks, abdomen, or infraclavicular region. They reported lead migration of 33% at 6 months to 100% at 3 years follow-up and estimated that “most patients” are expected to have lead migration at 1 year. No comparisons were drawn between the rate and IPG battery site placement.
In an attempt to reduce migration, Trentman et al48 in 2010 investigated the implant pathway associated with the least length change, from occipital lead position to IPG implantation site in the infraclavicular, buttock, or low abdominal region, serial external surface measurements. Flexion and extension pathway changes in the infraclavicular and abdominal sites were associated with less length changes than the periscapular and gluteal sites, respectively. Furthermore, retromastoid lead insertion was hypothesized to reduce lead pathway changes.48
Paddle leads were hypothesized to mitigate migration.49 In a recent review, lead migration was reported to have occurred at approximately a rate of 36% for cylindrical leads, where paddle leads migrated in approximately 5.7%.38 Gofeld50 suggested a distal anchor to reduce percutaneous lead migration. Midline and retromastoid techniques have been described, with a paucity of prospective data regarding migration prevalence. Oh et al22 demonstrated in their review that placing paddle leads significantly reduced migration and loss of therapeutic stimulation. Postulated reasons include anteriorly unidirectional current application and surface area of the lead.
Similarities can be drawn to SCS. Lead migration has been reported to be 11% to 13%.51 Cervical lead migration occurs more often than lumbar or thoracic lead migration, hypothesized to be because of higher mobility in the cervical region. Innately, because the lead placement and anchoring are anatomically in different tissue planes, this is likely as far as the comparison can be made. Regarding SCS, contradictory evidence exists between migration rates of paddle versus percutaneous leads, although the abdominal wall IPG placement fared better than the gluteal region.52
Hardware Malfunction
Hardware malfunctions for ONS have been reported to approximately 2.6%, with lead fracture or disconnects occurring 2.6% of the time,38 which is comparable to spinal cord stimulation (2.9%).52
Battery Depletion
Implantable pulse generator battery depletion is expected when using the device constantly for headache treatment. Schewdt et al47 demonstrated that 42% of implanted nonrechargeable batteries are depleted by 3 years. Trentman et al53 consider battery depletion of less than 1 year a “complication” and suggest a rechargeable IPG to match the potentially required energy requirement.
Muscle Spasm
Muscle spasm associated with occipital nerve stimulation has been reported to occur in as many as 7% of cases.47 Hayek at al33 in 2009 described five cases of unpleasant muscle recruitment causing spasm with reimplantation at the nuchal line as opposed to the C1-C2 level. In all cases, therapeutic stimulation was achieved, and muscle recruitment was stopped. Highlighting the technical considerations of depth of subcutaneous placement, placement that is too superficial may increase propensity for lead erosion and unpleasant burning sensations, and placement that is too deep may cause unpleasant muscle spasms.
Nuchal ridge placement was hypothesized to be superior to the more caudal, traditional C1-C2 placement because less muscle stimulation may be evident when the lead is placed over the aponeurosis of the semispinalis capitis as opposed to the muscle belly.51
Allergy
In Jasper et al’s38 review of occipital nerve stimulation, allergy was reported in 0.8% of those studied (115 patients). However, the patient thought she was allergic to the metal because of development of severe pain at the pulse generator site.22 Commonly, allergy manifests as skin reactions from components of the stimulator device.54,55
Overstimulation
Overstimulation was reported in three of six patients treated for headache disorders and implanted with a bion device, two of which resolved by 4 months of follow-up.10 As stimulation parameters change with distance from the target nerve, recumbence may increase the strength of stimulation. Reprogramming or surgical revision may be required to resolve the stimulation difficulty. The higher the voltage or current programmed, the greater the chance for aberrant structure.
Pain Over the Device
Pain over the device is a relatively common complication, having been reported to be approximately 7%.8,47 Treatment can involve topical or injection therapy, although it typically requires revision. Mitigation of device site pain includes avoidance of placement around friction-generating sites; ensuring adequate distance from osteal structures; and most importantly, a clear consensus with the patient regarding IPG placement preoperatively.
Stimulation Tolerance
Although mentioned in a relatively recent review, peripheral nerve stimulation has not been associated with loss of therapeutic stimulation, or “tolerance” observed in SCS.6
Peripheral Trigeminal Nerve Stimulation (Table 3-2)
Lead Erosion and Infection
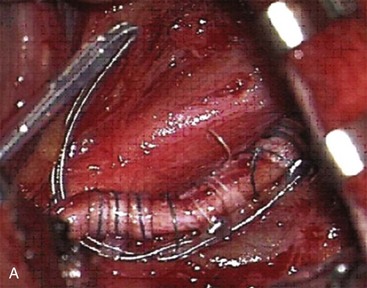
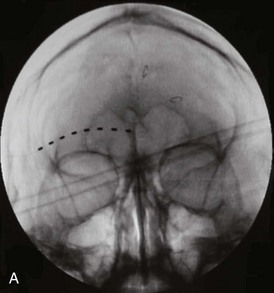
In a case series reported by Amin et al,6 cylindrical lead stimulation placement for supraorbital neuralgia (Fig. 3-3) was complicated by infection in 20% of cases. Interestingly, the infections occurred at the retroauricular connector and extension leads. The authors postulate that the thin dermal and subcutaneous layers results in erosion and skin breakdown.
Table 3-2 Reported Complications of Peripheral Trigeminal Nerve Stimulation
| Complication | Frequency |
|---|---|
| Lead erosion | 1/84 |
| Explant* | 1/84 |
| Infection | 2/106 |
* Loss of therapeutic stimulation after 26 months.
Fig. 3-3 A, Supraorbital cylindrical lead.
(From Amin S, Buvanendran A, Park KS, et al: Peripheral nerve stimulator for the treatment of supraorbital neuralgia: a retrospective case series, Cephalalgia 28:355-359, 2008.)
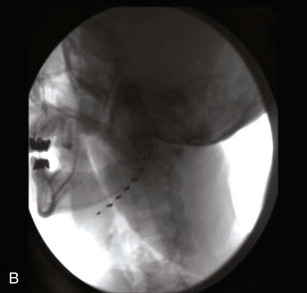
B, V3 cylindrical lead stimulation.
(From Yakovlev AE, Resch BE. Treatment of chronic intractable atypical facial pain using peripheral subcutaneous field stimulation, Neuromodulation 13:137-140, 2010.)
Similarly, Johnson et al56 reported three complications requiring surgical intervention in 10 patients implanted with four contact percutaneous cylindrical leads with an infraclavicular IPG placement site for trigeminal postherpetic and posttraumatic neuropathic pain. All of the complications were located around the retroauricular position of the extension lead and the connector. Two had infection and wound breakdown, and the third patient had pain overlying the site requiring lengthening of the extension.56
Vagal Nerve Stimulation
Overall complication rates of VNS (Table 3-3) have been described to be approximately 13.3%.2 Selected complications are described below.
| Complication | Frequency |
|---|---|
| Voice alteration | 24.1% at 3 mo, 24.8% >12 mo,2 63%,37 66%40 |
| Cough | 10.7% at 3 mo,2 26%37 |
| Respiratory complications | 5/1563 |
| Pain | 20.8% at 3 mo, 10.7% at >12 mo,1 1/902 |
| Dyspnea | 5.1% at 3 mo,1 10%37 |
| Recurrent depression* | 5.6%1 |
| Infection | 7.7%,1 7%6 |
| Interruption of the electrode secondary to trauma | 3/902 |
| Technical malfunction of generator | 1/902 |
| Cardiac arrhythmia | Case report |
| Lead fracture | Case report64 |
| Vocal cord palsy | 1/90,2 case reports57–61 |
| Mania | 1/5937 |
* Indication for implantation.
Hoarseness
Hoarseness associated with VNS typically improves with stimulation titration and is a consequence of activation of the recurrent laryngeal nerve.57 The recurrent laryngeal nerve supplies motor efferents to all intrinsic muscles of the larynx, excluding the cricothyroid muscle. Delayed onset hoarseness and palsy are rare.58–61 Tran et al57 describe a case of traumatic delayed transient vocal cord dysfunction after blunt neck trauma.
Cardiac Arrhythmia
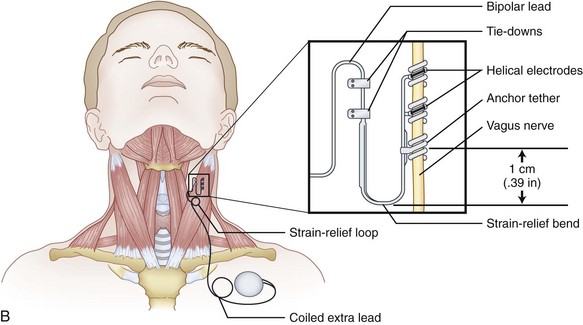
Iriarte et al62 described a report of delayed onset periodic asystole after VNS approximately 9 years later, presenting with dizziness discovered to be stimulation-induced second-degree heart block. This resolved after subsequent explant. Commonly, the parasympathetic, efferent vagal contribution to the atrioventricular node is supplied anatomically by the left vagus nerve (Fig. 3-4), and the right supplies the SA node. Retrograde stimulation is the most plausible explanation.
Dyspnea and Respiratory Complications
Dyspnea from VNS may be related to less mobility of the vocal cords secondary to denervations from chronic nerve stimulation.40 In a study by Marzec et al,63 five of 15 patients developed clinically significant obstructive sleep apnea after device placement. These patients were treated with 9 cm of continuous positive airway pressure.40,63
Hardware Malfunction
Historically, hardware malfunction was largely attributed to lead fracture.57,60 Redesign improved the fracture rate but did not eliminate it. Spitz et al64 reported a discontinuity in the silicone insulation over an electrode of a left vagus nerve stimulator, allowing aberrant leak of current. Ramsay et al61 described a case of an aberrant and intense stimulation-caused permanent vocal cord paralysis.
Spitz et al64 reported a woman that described phonation and breathing difficulty with VNS placement. Surgical exploration revealed silicone insulation disruption, which was adjacent to the phrenic nerve and scalene muscles. The symptoms resolved after lead revision.
Psychiatric Complications
Bajbouj et al1 investigated 2-year follow-up after vagal stimulation for treatment resistant depression, and although 27 patients (of 72) reported 39 adverse events, including worsening depression (33.3%), infection, suicide attempt (5.1%), and overdose (5.1%), 53.1% demonstrated significant improvement in depression via the Hamilton Rating Scale for Depression reduction (>50%) with no escalation in population-specific fatal adverse events.
Bajbouj et al1 described two patients that required explantation in the 2-year follow-up for vagal stimulation for refractory depression. Kuba et al2 investigated 5-year follow-up for patients with VNS for refractory seizure treatment. The study demonstrated a reduction in seizure intensity and frequency, and adverse events occurred in 13.3%, with explant in one patient because of “local complication.”
Vocal Cord Paralysis
Tran et al reported a 19-year-old woman with generalized tonic clonic seizures that was well controlled with VNS who suffered blunt neck trauma. She then had return of previous seizure activity. Interrogation of the device suggested lead fracture; operative findings confirmed the suspicion, with evidence of left vocal cord palsy and compression injury of left vagus nerve. Kalakanis et al58 reported two cases of vagal cord nerve traction injury after manipulation of the IPG in developmentally delayed adults.
Factors to reduce the vocal cord malfunction during VNS therapy include preoperative laryngeal testing before surgery, preservation of the vasa vasorum during dissection of the vagus nerve and placement of the helical electrodes, placement of size appropriate electrode array, and intraoperative stimulation.57
1 Bajbouj M, Merkl A, Schlaepfer TE, et al. Two-year outcome of vagus nerve stimulation in treatment-resistant depression. J Clin Psychopharmacol. 2010;30:273-281.
2 Kuba R, Brazedil M, Kalina M, et al. Vagus nerve stimulation: longitudinal follow-up of patients treated for 5 years. Seizure. 2009;18:269-274.
3 De Ferrari GM, Sanzo A, Schwartz PJ. Chronic vagal stimulation in patients with congestive heart failure. Conf Proc IEEE Eng Med Biol Soc. 2009:2037-2039.
4 Slavin KV, Wess C. Trigeminal branch stimulation for intractable neuropathic pain: technical note. Neuromodulation. 2005;8(1):7-13.
5 Aderjan D, Stankewitz A, May A. Neuronal mechanisms during repetitive trigemino-nociceptive stimulation in migraine patients. Pain. 2010;151(1):97-103.
6 Amin S, Buvanendran A, Park KS, et al. Peripheral nerve stimulator for the treatment of supraorbital neuralgia: a retrospective case series. Cephalalgia. 2008;28:355-359.
7 Asensio-Samper JM, Villanueva VL, Perez AV, et al. Peripheral neurostimulation in supraorbital neuralgia refractory to conventional therapy. Pain Pract. 2008;8(2):120-124.
8 Bartsch T, Paemeleire K, Goadsby PJ. Neurostimulation approaches to primary headache disorders. Curr Opin Neurol. 2009;22:262-268.
9 Broggi G, Messina G, Franzini A. Cluster headache and TACs: rationale for central and peripheral neuromodulation. Neurol Sci. 2009;30(suppl 1):S72-S79.
10 Burns B, Watkins L, Goadsby PJ. Treatment of hemicrania continua by occipital nerve stimulation with a bion device: long-term follow-up of a crossover study. Lancet Neurol. 2008;7:1001-1012.
11 Drummend PD, Treleaven-Hassard S. Electrical stimulation decreases neuralgic pain after trigeminal deafferentation. Cephalagia. 2008;28:782-785.
12 Dunteman E. Peripheral nerve stimulation for unremitting ophthalmic postherpetic neuralgia. Neuromodulation. 2002;5(1):32-37.
13 Finocchi C, Villani V, Casucci G. Therapeutic strategies in migraine patients with mood and anxiety disorders: clinical evidence. Neurol Sci. 2010;31(supp 1):S95-S98.
14 Schoenen J, Allena M, Magis D. Neurostimulation therapy in intractable headaches. Handb Clin Neurol. 2010;97:443-450.
15 Reed KL, Black SB, Banta CJ2nd, Will KR. Combined occipital and supraorbital neurostimulation for the treatment of chronic migraine headaches: initial experience. Cephalalgia. 2010;3:257-259.
16 Dafer RM. Neurostimulation in headache disorders. Neurol Clin. 2010;28(4):835-841.
17 Popeney CA, Alò KM. Peripheral neurostimulation for the treatment of chronic disabling transformed migraine. Headache. 2003;43:369-375.
18 Weiner RL, Reed KL. Peripheral neurostimulation for control of intractable occipital neuralgia. Neuromodulation. 1999;2:217-221.
19 Burns B, Watkins L, Goadsby PJ. Treatment of medically intractable cluster headache by occipital nerve stimulation: long-term follow-up of eight patients. Lancet. 2007;369:1099-1106.
20 Schwedt TJ, Dodick DW, Hentz J, et al. Occipital nerve stimulation for chronic headache—long-term safety and efficacy. Cephalalgia. 2007;27:153-157.
21 Slavin KV, Nersesyan H, Wess C. Peripheral neurostimulation for treatment of intractable occipital neuralgia. Neurosurgery. 2006;58:112-119.
22 Oh MY, Ortega J, Bellotte JB, et al. Peripheral nerve stimulation for the treatment of occipital neuralgia and transformed migraine using a C1-2-3 subcutaneous paddle style electrode: a technical report. Neuromodulation. 2004;7:103-112.
23 Magis D, Allena M, Bolla M, et al. Occipital nerve stimulation for drug-resistant chronic cluster headache: a prospective pilot study. Lancet Neurol. 2007;6:314-321.
24 Holsheimer J. Electrical stimulation of the trigeminal tract in chronic, intractable facial neuralgia. Arch Physiol Biochem. 2001;4:304-308.
25 Tomycz ND, Deibert CP, Moossy JJ. Cervicomedullary junction spinal cord stimulation for head and facial pain. Headache. 2011;51:418-425.
26 Horlocker TT, Rowlingson JC, Enneking FK, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Third Edition). Reg Anesth Pain Med. 2010;35(1):64-101.
27 Fishbain D, Goldberg M, Meagher BR, et al. Male and female chronic pain patients characterized by DSMIII psychiatric diagnostic criteria. Pain. 1986;26:181-197.
28 Gallagher R. Primary care and pain medicine: a community solution to the public health problem of chronic pain. Med Clin North Am. 1999;83:555-583.
29 Celstin J, Edwards RR, Jamison RN. Pretreatment psychosocial variables as predictors of outcomes following lumbar surgery and spinal cord stimulation: a systematic review and literature synthesis. Pain Med. 2009;10(4):639-653.
30 Meglio M. Percutaneously implantable chronic electrode for radiofrequency stimulation of the Gasserian ganglion: a perspective in the management of trigeminal pain. Acta Neurochir (Wien). 1984;33(suppl):521-525.
31 Meyerson BA, Håkansson S. Alleviation of atypical trigeminal pain by stimulation of the Gasserian ganglion via an implanted electrode. Acta Neurochir (Wien). 1980;30(suppl):303-309.
32 Taub E, Munuz M, Tasker RR. Chronic electrical stimulation of the gasserian ganglion for the relief of pain in a series of 34 patients. J Neurosurg. 1997;86:197-202.
33 Goadsby PJ, Hoskin KL. The distribution of trigeminovascular afferents in the nonhuman primate brain Macaca nemestrina: a c-fos immunocytochemical study. J Anat. 1997;190:367-375.
34 Anthony M. Headache and the greater occipital nerve. Clin Neurol Neurosurg. 1992;94(4):297-301.
35 Zitnik RJ. Treatment of chronic inflammatory diseases with implantable medical devices. Ann Rheum Dis. 2011;70(suppl 1):67-70.
36 Levin M. Nerve Bocks in the Treatment of Headache. Neurotherapeutics. 2010;7(2):197-203.
37 Schlaepfer TE, Frick C, Zobel A, et al. Vagus nerve stimulation for depression: efficacy and safety in a European Study. Psychol Med. 2008;38:651-661.
38 Jasper J, Hayek S. Implanted occiptal nerve stimulator. Pain Physician. 2008;11:187-200.
39 Trescher WH, Lesser RP Epilepsies. Bradley (5th ed) Neurology in Clinical Practice, ed 5 Butterworth-Heinemann, Oxford, UK, 2008.
40 Fahy BG. Intraoperative perioperative complications with a vagus nerve stimulation device. Clin Anesthes. 2010;22(3):213-222.
41 Multon S, Schoenen J. Pain control by vagus nerve stimulation: from animal to man … and back. Acta Neurol Belg. 2005;105:62-67.
42 Multon S, Schoenen J. Pain control by vagus stimulation: from animal to man … and back. Acta Neurol Belg. 2005;105:62-67.
43 Trentman TL, Dodick DW, Zimmerman RS, Birch BD. Percutaneous occipital stimulation lead tip erosion: report of 2 cases. Pain Physician. 2008;11:253-256.
44 Hayek SM, Jasper JF, Deer TR, Narouze SN. Occipital neurostimulation-induced muscle spasms: implications for lead placement. Pain Physician. 2009;12:867-876.
45 Mangram AJ, Horan TC, Pearson ML, et al. The Hospital Infection Control Practices Advisory Committee. Guideline for prevention of surgical site infection 1999. Infect Control Hosp Epidemiol. 1999;20:247-280.
46 Lim HB, Hunt K. Anesthetic management for surgical placement of greater occipital nerve stimulators in the treatment of primary headache disorders. J Neurosurg Anesthesiol. 2007;19:120-124.
47 Schwedt TJ, Dodick D, Hentz J, et al. Occipital nerve stimulation for chronic headache—long-term safety and efficacy. Cephalalgia. 2007;27:153-157.
48 Trentman TL, Mueller JT, Shah DM, et al. Occipital nerve stimulator lead pathway length changes with volunteer movement: an in vitro study. Pain Pract. 2010;10(1):42-48.
49 Kapural L, Mekhail N, Hayek SM, et al. Occipital nerve electrical stimulation via the midline approach and subcutaneous surgical leads for treatment of severe occipital neuralgia: a pilot study. Anesth Analg. 2005;10:171-174. table
50 Gofeld M. Anchoring of suboccipital lead: case report and technical note. Pain Pract. 2004;4:307-309.
51 Kumar K, Buchser E, Linderoth B, et al. Avoiding complications from spinal cord stimulation: practical management recommendations an international panel of experts. Neuromodulation. 2007;10:24-33.
52 Cameron T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review. J Neurosurg. 2004;100:254-267.
53 Trentman TL, Zimmerman RS. Occipital nerve stimulation: technical and surgical aspects of implantation. Headache Currents. 2008;48(2):319-327.
54 McKenna KE, McCleane G. Dermatitis induced by a spinal cord stimulator implant. Contact Dermatitis. 1999;41:279.
55 Ochani TD, Almirante J, Siddiqui A, Kaplan R. Allergic reaction to spinal cord stimulator. Clin J Pain. 2000;16:178-180.
56 Johnson MD, Burchiel KJ. Peripheral stimulation for treatment of trigeminal post-herpetic neuralgia and trigeminal posttraumatic neuropathic pain: a pilot study. Neurosurgery. 2004;55:135-142.
57 Tran Y, Shah A, Mittal S. Lead breakage and vocal cord paralysis following blunt neck trauma in a patient with vagal nerve stimulator. J Neurol Sci. 2011;304:132-135.
58 Kalkanis JG, Krishna P, Espinosa JA, Naritoku DK. Self-inflicted vocal cord paralysis in patients with vagus nerve stimulators. Report of two cases. J Neurosurg. 2002;96(5):949-951.
59 Pearl PL, Conry JA, Yaun A, et al. Misidentification of vagus nerve stimulator for intravenous access and other major adverse events. Pediatr Neurol. 2008;38(4):248-251.
60 Rijkers K, Berfelo MW, Cornips EM, Majoie HJ. Hardware failure in vagus nerve stimulation therapy. Acta Neurochir. 2008;150(4):403-405.
61 Ramsay RE, Uthman BM, Augustinsson LE, et al. Vagus nerve stimulation for treatment of partial seizures: 2. Safety, side effects, and tolerability. First International Vagus Nerve Stimulation Study Group. Epilepsia. 1994;35(3):627-636.
62 Iriarte J, Urrestarazu E, Alegre M, et al. Late-onset periodic asystolia during vagus nerve stimulation. Epilepsia. 2009;50:928-932.
63 Marzec M, Edwards J, Sagher O, et al. Effects of vagus nerve stimulation on sleep-related breathing in epilepsy patients. Epilepsia. 2003;44:930-935.
64 Spitz CM, Winston KR, Maa EH, Ojemann SG. Insulation deformity in a vagus nerve stimulator lead: treatable cause of intolerable stimulation-related symptoms. J Neurosurg. 2010;112(4):829-831.
65 Johnstone CSH, Sundaraj R. Occipital nerve stimulation for the treatment of occipital neuralgia—eight case studies. Neuromodulation. 2006;9:41-47.
66 Melvin EAJr, Jordan FR, Weiner RL, Primm D. Using peripheral stimulation to reduce the pain of C2-mediated occipital headaches: a preliminary report. Pain Physician. 2007;10:453-460.