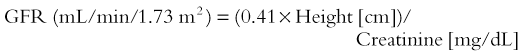Complications of Contrast Media
Allergic-Like Reactions
Incidence
Scant data are available regarding the incidence of contrast reactions in children for at least three reasons: (1) few clinical trials with children as subjects have been performed to obtain federal approval of an agent,1 (2) assessing symptoms, particularly mild ones in very young children, is difficult, and (3) differentiating true contrast reactions from symptoms is difficult because of sedation, synchronous medications, anxiety, and other preexisting diseases.2
In the pediatric population, use of nonionic contrast media is discriminative and exclusive when administered intravenously.3 Administration of nonionic contrast is associated with a much lower incidence of contrast media–related adverse effects.2 Dillman et al4 reported a 0.18% incidence of acute allergic-like reactions to intravenous (IV) administration of nonionic iodinated contrast material in children. This finding is very similar to an incidence of 0.23% in the adult population reported by Cochran et al.5 Most of the contrast reactions are mild in both children4 and adults.2,5–7 Of all the reported allergic reactions in children, 15% (<0.03% overall) were severe in degree.4 Fatal reactions to contrast media have occurred, but they are very rare. A large Japanese study did not blame any fatalities on contrast media in more than 170,000 injections.6 The very low or negligible reported fatalities likely suggest aggressive preventive measures and advancement in management of these reactions.2 Delayed reactions have been described in adults and may occur between 1 hour and 2 days after contrast administration. These reactions are predominantly cutaneous and usually resolve within 7 days.8–10
Risk Factors
1. Known prior reaction to contrast media, which markedly increases the risk of subsequent reactions.6,11–13
2. Known allergies to food products or medications; minor allergies do not pose a significant risk, but a prior anaphylactic reaction to any substance should heighten awareness of the possibility of a similar reaction to contrast media administration.
3. A history of asthma may increase the incidence of contrast reaction.6,13
4. Known renal disease; renal function in such patients can worsen after administration of contrast media.
5. Known heart disease, sickle cell disease, or diabetes mellitus; patients with these diseases may be at increased risk for contrast reactions.
Other disease entities, such as pheochromocytoma, dehydration, heart failure, severe hyperthyroidism, and β-blocker therapy, that are known risk factors in adults have not been studied in the pediatric population (Box 2-1).
Pathogenesis
The exact pathogenesis of untoward events after the administration of contrast media remains obscure and poorly understood. Most of the symptoms resemble an allergic or anaphylactoid reaction to a medication or allergen. However, definitive evidence is lacking that these reactions are truly allergic reactions because antibodies and the typical allergic cascade to these agents have not been identified.13
Classification of Allergic-Like Contrast Reactions
Based on severity, contrast reactions can be classified as mild, moderate, or severe. Flushing and a sensation of warmth are considered physiologic responses (Box 2-2).
Management
In the event of any adverse reaction to contrast media, the IV contrast injection should be discontinued. All reactions and management of the reactions should be documented in the patient care notes, and notation of a contrast allergy should become part of the patient’s permanent medical record. The following protocols closely follow the American College of Radiology (ACR) guidelines for management of acute reactions in children.2 The specific agents used in the management of an adverse reaction are determined by individual institutional pharmacy formulary and policy. Some institutions require the radiology personnel to call for assistance (e.g., a rapid response team) if they administer IV epinephrine for the management of these adverse reactions (given the rare incidence of these events and hence the lack of uniformity in preparedness for these reactions).14,15 To be prepared for such reactions, weight-based dosages of the medications used for management should be posted in clearly visible areas where contrast media are administered to children. Regular review of treatment protocols and practice of contrast reaction scenarios should be performed by radiologists and staff. If at any time a patient does not respond to treatment or the situation seems troublesome, it is appropriate to seek additional medical support immediately. This support may be sought from another radiologist in the department or through activation of an institutional rapid response or code team.
Urticaria
• Red raised wheals that blanch with pressure
• Patchy, symmetric involvement
Mild urticaria is usually self-limiting and does not require treatment.
Hypotension with Bradycardia (Vasovagal Reaction)
Patients may present with pallor, a decreased level of consciousness, diaphoresis, and a decreased heart rate. Management should be initiated with Trendelenburg positioning, securing of the airway, hydration, and administration of atropine if bradycardia persists (Box 2-3).
Delayed Reactions
Delayed reactions appear to occur more frequently than acute/immediate reactions to administration of contrast media. The incidence ranges from 2% to 12%.8,9 No definite data are available on the incidence and symptoms of delayed reactions in children. In adults, rash, itching and other cutaneous manifestations predominate.9 Other symptoms include fevers, chills, nausea, vomiting, headaches, abdominal pain, drowsiness, and dizziness.9 These symptoms can manifest any time from 1 hour to 2 days after administration of the contrast agent and usually resolve spontaneously by 7 days.8–10
Prevention
Ultimately, if the examination is considered absolutely necessary, the patient with known risk factors for adverse reactions should be premedicated with a combination of an antihistamine and corticosteroids. The regimen suggested by the ACR2 is described in Table 2-1.
Table 2-1
Pediatric Premedication Regimen for Prevention of Contrast Reaction
| Medication | Dosage | Timing |
| Prednisone | 0.5-0.7 mg/kg PO (up to 50 mg) | 13, 7, and 1 hour before contrast injection |
| Diphenhydramine | 1.25 mg/kg PO (up to 50 mg) | 1 hour before contrast injection |
From American College of Radiology Committee on Drugs and Contrast Media. ACR manual on contrast media, 7th ed. 2010. Available at http://www.acr.org/~/media/ACR/Documents/PDF/QualitySafety/Resources/Contrast%20Manual/FullManual.pdf. Accessed June 26, 2012.
Extravasation
Extravasation is a well-recognized complication of contrast-enhanced imaging that occurs in approximately 0.7% of all injections.16–19
Risk Factors
Extravasation is more prone to occur in patients who are unable to verbalize their symptoms, such as infants, younger children, and severely ill and unconscious patients.20,21 Increased rates also are noted in patients receiving chemotherapy, likely because of increased friability of the vein wall.21,22,23
Other risk factors include the site of injection, IV access type, and the method of injection.21 Wang et al16 noted that although the antecubital fossa was the single most common extravasation site, most of these events occurred in patients with venous access elsewhere. Increased incidents have been noted with injections at the dorsum of the hand.24 Other risk factors also include venous thrombosis, extremity edema, multiple venous access attempts, and use of a tourniquet.2,20,21 Extravasations are more frequent where preexisting catheters are used as access sites for administration of the contrast media.22 At least two studies did not note any significant difference in the incidence of extravasation with the use of power injectors (Box 2-4).24,25
Presentation
The immediate symptoms of extravasation can be quite variable. Some patients present with a burning sensation, whereas others remain asymptomatic. Close attention should be paid to young children and unconscious patients because they may not be able to express these sensations. On physical examination, the extravasation site may be red, swollen, and tender.2
Most incidents are self-limiting and resolve spontaneously within 1 to 2 days. These incidents usually are restricted to the skin and subcutaneous tissue.2 The injuries can range from transient tightness of the skin to tissue ulceration and necrosis to acute compartment syndromes. Severe reactions have been observed with both small and large volumes of extravasated contrast. However, the majority of severe reactions have been observed with larger volumes (Box 2-5).26
Management
A definite treatment approach has not been accepted because of a lack of consensus on the appropriate management of extravasation.20,27,28 Minor symptoms can be treated with elevation of the affected extremity above the level of the heart to help reduce edema by promoting resorption of the fluid.2,21 Sufficient data are lacking to support either warm or cold compresses over the affected limb.2 Hyaluronidase injected subcutaneously may help speed resorption, but insufficient evidence exists to suggest routine use. Regardless, close monitoring of the patient is warranted. These events should be documented in the patient’s medical records and appropriate directions should be provided to seek medical attention in the event of worsening symptoms.
Severe extravasation injuries require a surgical consultation. Such injuries may present as worsening of pain or swelling, skin blistering, decreased capillary refill, diminished pulses, and a change in sensation of the affected extremity.2,20 Wang et al16 noted in their observations that compartment syndrome can develop even with less than 100 mL of extravasated fluid. Thus it is prudent to conclude that signs and symptoms, rather than the volume extravasated, should be used as a scale for seeking surgical consultation (Box 2-6).
Contrast-Induced Nephropathy
Limited data are available on the nephrotoxic effects of iodinated contrast media in children. The ACR recommends following the principles used for adults in the prevention and management of contrast-induced nephropathy (CIN).2 Risk factors for the development of CIN include preexisting renal insufficiency, diabetes mellitus, and multiple contrast media administrations during the same day.
CIN has been described as either an absolute increase in serum creatinine by at least 0.5 mg/dL within 48 hours of contrast injection29 or an increase of more than 25% within 72 hours of contrast administration.30 However, serum creatinine cannot be reliably used as a measure of renal function in this setting in the pediatric population.2 The estimated glomerular filtration rate (GFR) is used widely for this purpose. The National Kidney Disease Education Program has provided information regarding the measurement of GFR at its website (http://www.nkdep.nih.gov/lab-evaluation/gfr-calculators.shtml) and advocates use of the bedside isotope dilution mass spectrometry–traceable Schwartz GFR calculator for children equation31:
Nephrogenic Systemic Fibrosis
Nephrogenic systemic fibrosis is a disease characterized primarily by skin fibrosis, but it may involve multiple organs. Its association with gadolinium-based contrast agents has been realized and was first documented in 2006.32 Nine pediatric cases have been reported as of 2008, with the patients ranging in age from 8 to 19 years.33 Not enough data exist in the pediatric population to provide guidelines for the prevention of nephrogenic systemic fibrosis in children. The ACR2 recommends following adult guidelines for identifying patients at risk and the use of gadolinium-based contrast media. The suggested risk factors are acute renal failure, decreased renal function with GFR less than 30 mL/min, high doses of administered gadolinium, and postsurgical status.32,34,35 Only a limited number of gadolinium-based contrast agents have been studied and approved by the Food and Drug Administration for use in children younger than 2 years.33 Gadolinium agents should be used with caution in infants and preterm babies because their renal function is immature.33
American College of Radiology Committee on Drugs and Contrast Media. ACR manual on contrast media. 7th ed. http://www.acr.org/~/media/ACR/Documents/PDF/QualitySafety/Resources/Contrast%20Manual/FullManual.pdf, 2010. Available at Accessed June 26, 2012
Brockow, K. Immediate and delayed reactions to radiocontrast media: is there an allergic mechanism? Immunol Allergy Clin North Am. 2009;29(3):453–468.
Mendichovszky, IA, Marks, SD, Simcock, CM, et al. Gadolinium and nephrogenic systemic fibrosis: time to tighten practice. Pediatr Radiol. 2008;38:489–496.
Namasivayam, S, Kalra, MK, Torres, WE, et al. Adverse reactions to intravenous iodinated contrast media: a primer for radiologists. Emerg Radiol. 2006;12:210–215.
Rudnick, MR, Keselheim, A, Goldfarb, S. Contrast-induced nephropathy: how it develops, how to prevent it. Cleve Clin J Med. 2006;73:75–80.
Schaverein, MV, Evison, D, McCulley, SJ. Management of large volume CT contrast medium extravasation injury: technical refinement and literature review. J Plast Reconstr Aesthet Surg. 2008;61(5):562–565.
References
1. Brasch, RC. Contrast media toxicity in children. Pediatr Radiol. 2008;38:281–284.
2. American College of Radiology Committee on Drugs and Contrast Media. ACR manual on contrast media. 7th ed. http://www.acr.org/~/media/ACR/Documents/PDF/QualitySafety/Resources/Contrast%20Manual/FullManual.pdf, 2010. [(website) Accessed June 26, 2012].
3. Cohen, MD, Smith, JA. Intravenous use of ionic and nonionic contrast agents in children. Radiology. 1994;191:793–794.
4. Dillman, JR, Strouse, PJ, Ellis, JH, et al. Incidence and severity of acute allergic-like reactions to i.v. nonionic iodinated contrast material in children. AJR Am J Roentgenol. 2007;118:1643–1647.
5. Cochran, ST, Bomyea, K, Sayre, JW. Trends in adverse events after IV administration of contrast media. AJR Am J Roentgenol. 2001;176:1385–1388.
6. Katayama, H, Yamaguchi, K, Kozuka, T, et al. Adverse reactions to ionic and nonionic contrast media: a report from the Japanese Committee on the Safety of Contrast Media. Radiology. 1990;175:621–628.
7. Mortelé, KJ, Olivia, MR, Ondategui, S, et al. Universal use of nonionic iodinated contrast medium for CT: evaluation of safety in a large urban teaching hospital. AJR Am J Roentgenol. 2005;184:31–34.
8. Yoshikawa, H. Late adverse reactions to nonionic contrast media. Radiology. 1992;183:737–740.
9. Christiansen, C, Pichler, WJ, Skotland, T. Delayed allergy-like reactions to X-ray contrast media: mechanistic considerations. Eur Radiol. 2000;10:1965–1975.
10. Webb, JAW, Stacul, F, Thomsen, HS, et al. Late adverse reactions to intravascular iodinated contrast media. Eur Radiol. 2003;13:181–184.
11. Fischer, H, Doust, V. An evaluation of pretesting in the problem of serious and fatal reactions to excretory urography. Radiology. 1992;103:497–501.
12. Witten, D. Reactions to urographic contrast media. JAMA. 1975;231:974–977.
13. Morcos SK, Thomsen HS. Adverse reactions to iodinated contrast media. Eur Radiol. 2003;13:181-184.
14. Chamberlain, D, Smith, A, Woollard, M, et al. Trials of teaching methods in basic life support: comparison of simulated CPR performance after first training and at 6 months, with a note on the value of re-training. Resuscitation. 2002;53(2):179–187.
15. Wolfram, RW, Warren, CM, Doyle, CR, et al. Retention of pediatric advanced life support (PALS) course concepts. J Emerg Med. 2003;25(4):475–479.
16. Wang, CL, Cohan, RH, Ellis, JH, et al. Frequency, management, and outcome of extravasation of nonionic iodinated contrast medium in 69,657 intravenous injections. Radiology. 2007;243:80–87.
17. Bullard, MA, Cohan, RH, Ellis, JH, et al. Extravasation of intravenous contrast material: incidence, management, outcome. Acad Radiol. 1997;4:711–718.
18. Federle, MP, Chang, PJ, Confer, S, et al. Frequency and effects of extravasation of ionic and nonionic CT contrast media during rapid bolus injection. Radiology. 1998;206:637–640.
19. Jacobs, JE, Birnbaum, BA, Langlotz, CP. Contrast media reactions and extravasation: relationship to intravenous injection rates. Radiology. 1998;209:411–416.
20. Cohan, RH, Ellis, JH, Garner, WL. Extravasation of radiographic contrast material: recognition, prevention and treatment. Radiology. 1996;200:593–604.
21. Bellin, M, Jakobsen, JA, Tomassin, I, et al. Contrast medium extravasation injury: guidelines for prevention and management. Eur Radiol. 2002;12:2807–2812.
22. Sistrom, CL, Gay, SB, Peffley, L. Extravasation of iopamidol and iohexol during contrast-enhanced CT: report of 28 cases. Radiology. 1991;180:707–710.
23. Gault, DT. Extravasation injuries. Br J Plast Surg. 1993;46:91–96.
24. Amaral, JG, Traubici, J, BenDavid, G, et al. Safety of power injector use in children as measured by incidence of extravasation. AJR Am J Roentgenol. 2006;187:580–583.
25. Jacobs, JE, Birnbaum, BA, Langlotz, CP. Contrast media reactions and extravasation: relationship to intravenous injection rates. Radiology. 1998;209:411–416.
26. Upton, J, Mulliken, JB, Murray, JE. Major intravenous extravasation injuries. Am J Surg. 1979;137:497–506.
27. Park, KS, Kim, SH, Park, JH, et al. Methods for mitigating soft-tissue injury after subcutaneous injection of water-soluble contrast media. Invest Radiol. 1993;28:332–334.
28. Yucha, CB, Hastings-Tolsma, TM, Szeverenyi, N. Effects of elevation on intravenous extravasations. J Intraven Nurs. 1994;17:231–234.
29. Solomon, R, Werner, C, Mann, D, et al. Effects of saline, mannitol, and furosemide to prevent acute decreases in renal function induced by radiocontrast agents. N Engl J Med. 1994;331:1416–1420.
30. Porter, GA. Contrast medium-associated nephropathy. Recognition and management. Invest Radiol. 1993;28(suppl 4):S11–S18.
31. Schwartz, GJ, Muñoz, A, Schneider, MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637.
32. Grobner, T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21:1104–1108.
33. Penfield, JG. Nephrogenic systemic fibrosis and the use of gadolinium-based contrast agent. Pediatr Nephrol. 2008;23:2121–2129.
34. Karcaaltincaba, M, Oguz, B, Haliloglu, M. Current status of contrast-induced nephropathy and nephrogenic systemic fibrosis in children. Pediatr Radiol. 2009;39:382–384.
35. Perez-Rodriguez, J, Lai, S, Ehst, BD, et al. Nephrogenic systemic fibrosis: incidence, associations, and effect of risk factor assessment—report of 33 cases. Radiology. 2009;250:371–377.