Chapter 39 Complications in the Oncologic Patient
Chest
Chemotherapy-Induced Noninfectious Lung Disease
The problem of drugs adversely affecting the lungs remains a major challenge to all primary care physicians. Symptoms are usually nonspecific, with patients presenting with dyspnea, nonproductive cough, and fever, which can begin weeks to years after the medication is first taken. Unfortunately, drug-induced respiratory disease remains a disease of exclusion because the majority of drugs responsible for this effect cannot be diagnosed by any specific test. Diagnosis requires a high index of suspicion because infection, radiation pneumonitis, and recurrence of the underlying disease can manifest clinically and radiographically in a similar manner. There is underreporting of drug toxicity, and thus, the true incidence of drug-induced respiratory disease is unknown, although it is estimated to be less than 10%.1 To complicate matters, only a few patients are treated with single drugs. Thus, how much of an observed reaction is related to one agent or another versus synergy with other drugs, oxygen, or radiation often remains unknown. Prompt diagnosis of drug-induced lung toxicity is important because early drug-induced lung injury will often regress with the cessation of therapy or with initiation of steroid therapy, before the development of pulmonary fibrosis. For an updated list of generic drugs, type of reaction, and radiologic manifestations with references, the reader is referred to the routinely updated website www.pneumotox.com.2 In this reference and in Table 39-1, the reader will find some of the effects of drugs on other chest structures such as those causing pleural effusions, lymphadenopathy, or cardiomyopathy.
Table 39-1 Histologic Patterns Associated with Drug Reactions
| FINDING | DRUG |
|---|---|
| Noncardiogenic pulmonary edema | Carbamazepine, gemcitabine, taxanes, cyclophosphamide, methotrexate, vinblastine |
| Alveolar hemorrhage | Bevacizumab, rituximab |
| Alveolar proteinosis–like reaction | Mitomycin C |
| Diffuse alveolar hemorrhage | Carbamazepine, methotrexate, gemcitabine |
| Diffuse alveolar damage | Gemcitabine, methotrexate, bleomycin, cyclophosphamide, gefitinib, erlotinib |
| Organizing pneumonia | Bleomycin, cyclophosphamide |
| Bronchiolitis obliterans syndrome | Busulfan |
| Usual interstitial pneumonia–like pattern | Bleomycin, gemcitabine, methotrexate, busulfan, cyclophosphamide |
| Diffuse cellular interstitial infiltrates with or without granulomas | Bleomycin, methotrexate |
| Nonspecific interstitial pneumonia | Bleomycin, taxanes, gemcitabine, methotrexate, imatinib |
| Desquamative interstitial pneumonia | Busulfan |
| Acute or chronic eosinophilic pneumonia | Bleomycin, methotrexate |
| Pulmonary veno-occlusive disease | Bleomycin, busulfan |
| Pulmonary nodules | Bleomycin, vinblastine |
| Pneumothorax/pneumomediastinum | Bleomycin |
| Hilar/mediastinal adenopathy | Bleomycin |
| Pleural effusion | Methotrexate, cyclophosphamide, imatinib |
| Cavitations | Bevacizumab |
| Pulmonary thromboembolism | Bevacizumab, thalidomide |
| Cardiomyopathy80 | Doxorubicin, 5-fluorouracil, trastuzumab |
Modified from www.pneumotox.com; and Flieder DB, Travis WD. Pathologic characteristics of drug-induced lung disease. Clin Chest Med. 2004;25:37-45.
The lungs have a limited number of histopathologic responses to injury (see Table 39-1) that mimic many other pulmonary conditions.1 The radiologic appearance of drug toxicity corresponds to the histopathologic finding. Owing to these nonspecific findings, diagnosis rests on correlation with clinical, laboratory, and radiologic information.3 Even bronchoalveolar lavage (BAL) can only suggest an iatrogenic cause by helping to exclude infection and malignancy. Sometimes, cell composition in the lavage and elevation of a specific population (e.g., lymphocytes, neutrophils, or eosinophils) may suggest a more specific diagnosis.
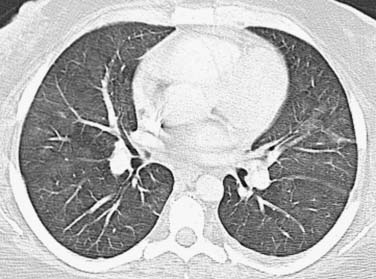
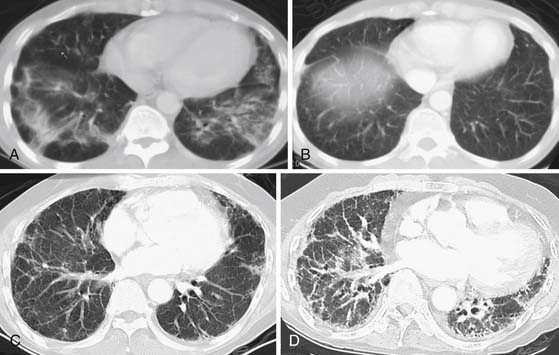
As an aid for interpretation of the chest images, it is helpful to divide drug toxicity into acute and delayed presentation. Acute-onset, chemotherapy-induced lung injury is usually caused by noncardiogenic pulmonary edema/diffuse alveolar damage, hypersensitivity-type reaction, or pulmonary hemorrhage and occurs after the initial dose of chemotherapy. Radiographic findings usually include diffuse or scattered ground-glass opacities (GGOs) or consolidative opacities with or without septal thickening (Figure 39-1).
Delayed-onset, chemotherapy-induced lung injury usually presents more than 2 months after the completion of treatment or during prolonged treatment and is often caused by chronic interstitial pneumonia, which can lead to fibrosis. The more common histologic patterns seen are usual interstitial pneumonia (UIP) or nonspecific interstitial pneumonia (NSIP).3 The pattern most commonly encountered is that of NSIP, which appears as bilateral lower lobe predominant heterogeneous or consolidative opacities on chest radiographs, and on thin-section chest computed tomography (CT), it appears initially as scattered GGOs or consolidative opacities with lower lobe predominance (Figure 39-2). Although intralobular septal thickening and traction bronchiectasis can be seen with NSIP, when present, these findings are much more likely to be from UIP.4,5 Honeycombing is typically seen with UIP. Organizing pneumonia with fibromyxoid connective tissue plugs that fill distal airspaces as well as terminal or respiratory bronchioles is another nonacute form of drug toxicity. When associated with a known causative agent, this finding should not be termed “cryptogenic organizing pneumonia,” but rather identified by its older description: bronchiolitis obliterans organizing pneumonia (BOOP) caused by the name of the drug (e.g., BOOP caused by amiodarone).3–5 Radiographic findings of drug-induced BOOP on chest radiographs are bilateral scattered heterogeneous or consolidative opacities in a peripheral distribution, and on chest CT, there are unilateral or bilateral areas of consolidation, with either peripheral or the more typical peribronchovascular distribution and a lower lobe predominance.6 Rarely, BOOP may have a nodular appearance that can be confused with metastatic disease.7
Key Points Drug toxicity
Chemotherapy-Induced Infectious Lung Disease
Drugs that are used to combat cancer target dividing cells, affect the bone marrow, and cause a decrease in neutrophils resulting in infections. Other drugs often used to treat hematologic malignancies, in conjunction with chemotherapeutic agents, have immunosuppressive properties. Corticosteroids cause a broad suppression of the immune system, whereas the newer targeted monoclonal antibody therapies, such as rituximab, cause specific suppression.8 Diagnosis of pneumonia depends on clinical symptomatology with radiographic findings. The type of pathogen producing the pneumonia, whether bacterial, viral, or fungal, depends mainly on the type of host and combination therapy they received. Given the inability of the severely immunocompromised host (e.g., SCT recipients or prolonged neutropenia in patients with hematologic malignancies) to mount an adequate inflammatory response, the classic radiographic findings of each type of pneumonia may differ from those found in immunocompetent patients, and chest films may even appear normal. CT may disclose more subtle changes, such as minimal GGOs, bronchial thickening, or nodules, and in select cases, may show the typical appearance for a specific group of pathogens.9 Such findings may lead to the correct selection of antibiotic therapy and improved outcomes.10,11
Bacterial Pneumonia
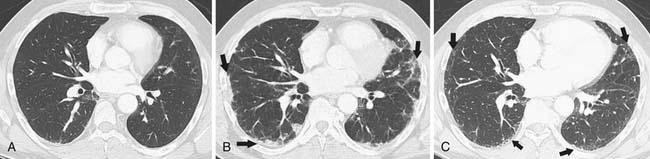
The main source of pathogens are from the patient’s endogenous flora.12 With the introduction of extended-spectrum beta-lactams, there has been a decrease in bloodstream infections due to gram-negative rod bacteremia and an increase in infections due to gram-positive cocci,13 although nosocomial bacterial pneumonias are still predominated by gram-negative rods.14 There is a low predictive yield to determination of the type of bacterial pneumonia from the chest radiographic or even chest CT appearance in the immunocompromised patient population. This is because of coexisting abnormalities such as edema, atelectasis, or aspiration and also because of the diminished immunologic response to infection as the result of therapy.15 In SCT recipients, bacterial pneumonia most commonly manifests as pulmonary nodules (81%), either large or small, in a tree-in-bud distribution, lower lobe predominant, asymmetrically distributed consolidation (69%), and GGOs (35%), usually symmetrically distributed.16 The majority of patients demonstrate a combination of these findings (73%), with another 15% of patients having pulmonary nodules only and 12% consolidation only on CT (Figure 39-3).
Viral Pneumonia
DNA viruses such as herpes simplex, varicella, and cytomegalovirus (CMV) have long been recognized as causing severe respiratory infections in patients with hematologic malignancies.17 These patients are also exposed to community seasonal respiratory viruses such as adenovirus or influenza A, which can be life-threatening in such immunocompromised patients.18 Radiographically, one cannot differentiate one viral infection from another. They tend to be symmetrically distributed in the lung, usually with lower lobe predominance and a combination of GGOs or consolidative airspace disease (Figure 39-4) in addition to small centrilobular nodules and consolidative opacities.18–22 Mortality rate can be high and early diagnosis is essential because early treatment improves survival. Of this group, airspace disease is the most common finding, seen in 90% of patients with CT-documented pneumonia.18,21,22 The early changes of lower lobe–predominant peribronchial thickening and peribronchial GGOs or tree-in-bud opacities are more readily appreciated on a lateral plain film or, with greater sensitivity, by chest CT, although early bacterial pneumonia may have a similar appearance.
Fungal Pneumonia
Opportunistic invasive fungal pneumonias are associated with high morbidity and mortality rates23–25 and are typically found in patients with prolonged, severe immunocompromise, as can be seen with hematologic malignancies and SCT recipients, but is rarely encountered in patients with solid malignancies. Although invasive pulmonary aspergillosis (IPA) and Candida are the most common, other angioinvasive molds, such as Fusarium and Zygomycetes species are increasingly encountered in these severely immunocompromised hosts. Because early institution of high-dose antifungal therapy is associated with improved outcomes,10,26 early recognition of invasive fungal disease is important. However, cultures of respiratory secretions are neither sensitive nor specific, and lavage and invasive procedures often cannot be done in these patients because of coagulation abnormalities and thrombocytopenia.27,28 Thus, diagnosis of invasive pulmonary fungal disease relies heavily on imaging.29 CT is often used in an attempt to identify fungal pneumonia in a timely fashion because some of these typical imaging findings cannot be detected by chest radiographs.
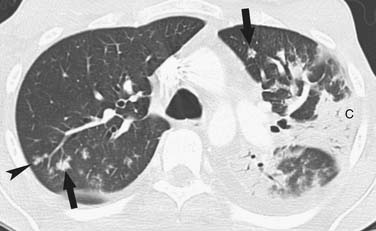
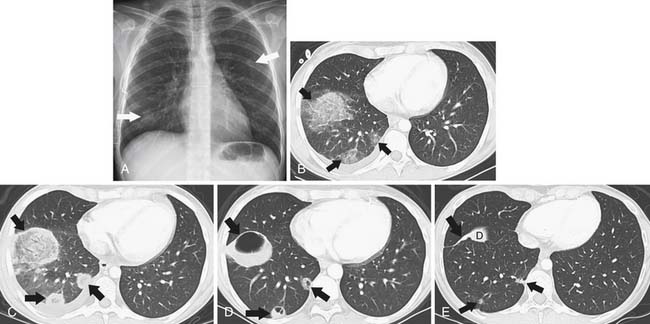
The “halo sign,” a nodule surrounded by GGOs, is seen in 92% of patients with IPA at presentation.10 The presence of large nodules (>1 cm) and visualization of the halo sign are most suggestive of fungal infection.16 There have been attempts to differentiate pulmonary zygomycosis (PZ) from IPA because this distinction has important therapeutic implications: specifically, voriconazole, the preferred drug in the treatment of IPA,30 has no activity in zygomycosis.2 The presence of the “reversed halo sign” (Figure 39-5), a focal round area of ground-glass attenuation surrounded by a ring of consolidation, or the presence of multiple nodules (>10) is more commonly seen with PZ than with other invasive fungi.31,32 Although these CT findings of the reversed halo sign and halo sign are not pathognomonic for fungal infections and can be seen, for example, with cryptogenic organizing pneumonia, infarction, or malignancy, when observed on imaging of a febrile, severely immunocompromised patient, these should be considered invasive fungal pneumonia until proved otherwise.
Pneumocystis pneumonia (PCP), once a major cause of morbidity and mortality in patients treated for hematologic malignancies, has been largely prevented by the use of antimicrobial prophylaxis. Chest radiographs at presentation can be normal and later nonspecific with bilateral, perihilar reticular and poorly defined GGOs, which often progress to alveolar consolidation in 3 to 4 days.33 Thin-section CT usually reveals scattered ground-glass attenuation that can be associated with interlobular septal thickening, findings that can resemble viral pneumonia such as CMV pneumonia. In an attempt to differentiate the CT appearance of CMV from PCP in non–human immunodeficiency virus (HIV) patients, apical distribution and a sharply demarcated mosaic pattern are found more frequently in PCP, whereas small nodules or unsharp demarcation of GGOs and lower lobe predominance are more likely to be seen in CMV pneumonia.34
Tuberculosis
Although patients immunosuppressed from chemotherapy are at risk for tuberculosis (TB), the incidence of tuberculosis in even the most severely immunosuppressed, such as SCT recipients, is proportional to the incidence of TB in the general population. The imaging appearance of TB in this population is identical to that in the general population.35,36
Key Points Chemotherapy-induced pneumonia in the severely immunocompromised host
• Bacterial pneumonia most commonly manifests as a combination of consolidation/GGOs with tree-in-bud lower lobe–predominant nodules.
• Viral pneumonia most commonly manifests as diffuse lower lobe–predominant consolidation/GGOs with or without centrilobular nodules.
• The presence of large nodules (>1 cm) and visualization of the halo sign are most suggestive of fungal infection.
Stem Cell Complications
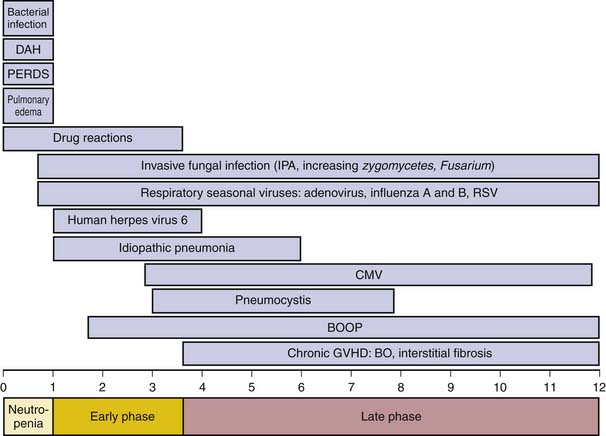
Pulmonary complications develop in 30% to 60% of SCT recipients and are the immediate cause of death in approximately 61% of cases.37–39 The donor source may be the patient (autologous), a sibling or unrelated person (allogeneic), an identical twin (syngeneic), or a genetically unrelated umbilical cord blood sample.40 Although syngeneic grafts have lower treatment-related mortality and do not develop graft-versus-host disease (GVHD), they have a greater risk of malignant recurrence. Thus, the overall mortality is similar for syngeneic and allogeneic SCT. When evaluating the complications associated with SCT, chest imaging findings are generally nonspecific. Often, the patient will have a normal chest radiograph, and thus, the CT findings may prove to be of value. The correct interpretation of the CT scan depends on knowledge of the patient’s immune recovery because specific pulmonary complications tend to occur within well-defined time periods after transplantation.41,42 Despite recovery to normal cell counts within a month, complete immunologic recovery occurs within 1 to 2 years in patients without GVHD. Complications after SCT are categorized into early (≤100 days from transplantation) and late (>100 days from transplantation) complications, according to time of presentation relative to the date of transplantation.
Early Complications
Pre-engraftment Period (Days 1-30)
This period immediately following transplantation with its profound neutropenia is predominated by infectious complications, most commonly bacterial and fungal, with associated imaging findings as described previously. The bacterial pathogens encountered include gram-negative enteric flora, gram-positive cocci, and gram-positive skin commensals along with those related to indwelling catheters. When the period of neutropenia is prolonged, the patient is at high risk for invasive fungal infections, most commonly Candida and Aspergillus.43
Noninfectious complications during this period include pulmonary edema, peri-engraftment respiratory distress syndrome (PERDS), diffuse alveolar hemorrhage (DAH), and drug-induced injury (Figure 39-6). It is difficult to differentiate radiographically between these conditions because the main imaging finding related to these noninfectious complications is that of airspace disease, with bilateral symmetrical GGOs or consolidative opacities, with or without septal thickening.44 Pulmonary edema occurs secondary to the large amounts of intravenous fluids administered with the conditioning regimen prior to SCT. PERDS is seen in approximately 5% of autologous SCT cases and refers to the pulmonary component of the engraftment syndrome.45 Clinically, the syndrome is characterized by rash, fever, and diarrhea, thought to be caused by a complex interaction between the conditioning-related endothelial damage and the cytokine release associated with neutrophil and lymphocyte recovery. Corticosteroid treatment usually leads to rapid recovery.45 DAH is associated with a mortality rate of 40% to 100%, but less than 20% of cases report hemoptysis. Thus, diagnosis of DAH relies on demonstration of more than 20% hemosiderin-laden macrophages in BAL fluid, in the absence of infection.46 Early diagnosis and steroid therapy may improve outcome. Drug-induced injury occurs in 10% of SCT recipients, and its radiographic manifestations are as described previously.
Early Phase (Days 30-100)
Because of the impaired function of the SCT recipient’s marrow cells, infections continue to be a threat during this phase. Bacterial infections are common, particularly in patients with indwelling catheters. Prophylactic antifungal medication has significantly reduced the occurrence of Candida infections, although a wider variety of invasive molds resistant to this prophylaxis is now seen. Nevertheless, IPA continues to be the most common invasive fungus encountered during this period43 (see Figure 39-6). The early phase is the period in which CMV reactivation is common and once led to life-threatening infection. With the availability of effective antiviral medication and initiation of therapy based on CMV viremia before disease manifests, CMV mortality has decreased. Consequently, CMV pneumonia is radiographically less often seen.43 Similarly, PCP, previously encountered during the early phase, is now rarely seen owing to routine antimicrobial prophylaxis.42
Of the noninfectious complications during this period, idiopathic pneumonia syndrome (IPS) is the most common. Hence, IPS is the most common cause of diffuse radiographic abnormalities between 30 to 180 days after SCT, although its incidence has recently decreased to approximately 10%.44,46 CT scan findings include airspace disease with a lower lobe predominance, a pattern seen with noncardiogenic pulmonary edema.44 IPS is a diagnosis of exclusion and is defined by the presence of widespread alveolar injury in the absence of lower respiratory tract infection. The pathogenesis of IPS is attributed to lung tissue injury from cytokine release. Mortality is approximately 70%, and treatment is supportive because IPS does not respond to steroid therapy.44,46
Other complications during this period include a variety of manifestations of early GVHD and are, thus, usually seen in allogeneic SCT recipients. Early GVHD can present in the form of BOOP, characterized by the presence of granulation tissue within the alveolar ducts and alveoli, observed from 1 month to 2 years after transplantation.44,46 Chest radiographs show nonspecific bilateral regions of consolidation, which may be nodular and even migratory. CT scans reveal scattered focal peripheral and/or peribronchovascular consolidation, with a lower lobe predominance.47
Late Complications
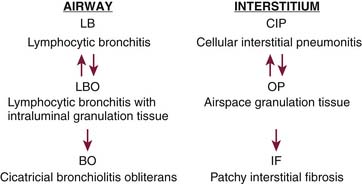
Late complications result from GVHD (see Figure 39-6). Histologically, within the first 6 months, there is infiltration of the perivascular zones and alveolar septa by mononuclear cells and ongoing lymphocyte-mediated injury to the large and small airways (Figure 39-7). The interstitial process will cause exudation of fluid to the alveoli, which may form airspace granulation tissue. If not cleared, it will progress to the irreversible stage of fibrosis. A similar process occurs in the airways. The lymphocytic infiltration causes damage to the bronchus that, if allowed to continue, leads to intraluminal granulation tissue, as seen in BOOP. This process is still reversible but, if not treated, may progress to form concentric or luminal obliterative scars, which will disrupt normal airflow. This effect is known as bronchiolitis obliterans (BO) (Figure 39-8).48 Thus, from about 4 months after SCT, GVHD is to be excluded in a patient with respiratory complaints. Although pulmonary GVHD usually accompanies GVHD in extrathoracic sites, up to 30% of GVHD cases may occur in the lungs alone.48 Early recognition of pulmonary GVHD is important, while the disease is still reversible.
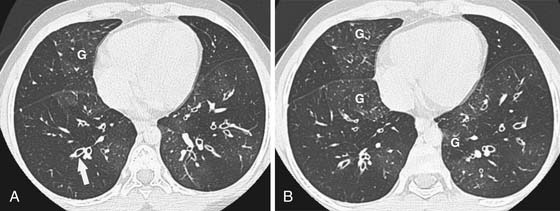
CT plays an important role in late SCT recipients who present with pulmonary complaints. This imaging not only helps to exclude pulmonary infection but may also show findings suggestive of GVHD. Early findings are nonspecific and include scattered GGOs or consolidative opacities, similar to the ones discussed in drug-induced BOOP. Irreversible findings to look for include interstitial thickening/fibrosis or findings seen in BO, namely, bronchiolar thickening, bronchiolectasis, and air trapping (Figure 39-9). Thus, in the search for early pulmonary GVHD, obtaining expiratory CT scans is used to search for air trapping, and prone images are used to differentiate early lower lobe fibrosis from dependent atelectasis.
Pateints with chronic GVHD remain at risk for pulmonary infection because their immune recovery may be prolonged for years.46 Organisms include encapsulated bacteria, as well as invasive fungal molds such as Aspergillus species and Zygomycete, especially in those patients with severe GVHD treated with high-dose corticosteroids and/or other immunosuppressive regimens. CMV reactivation is now more commonly delayed to this late period, but CMV pneumonia is rarely encountered owing to treatment of viremia before the disease manifests clinically.46
Key Points Stem cell transplantation complications
• One to 30 days after SCT, patients are severely neutropenic, and the most common complication is pneumonia: bacterial and fungal.
• Thirty to 100 days after SCT, white blood cell function is impaired; thus, fungal pneumonia is still common but early GVHD in the form of BOOP can be seen.
• Late complications after SCT are due to GVHD, which can manifest as scattered symmetrical consolidation/GGOs, air trapping, interstitial thickening, and fibrosis.
• Because treatment of GVHD is immunosuppressive, patients are at risk for fungal and bacterial pneumonia.
• PCP and CMV pneumonia are less commonly encountered owing to routine prophylactic therapy.
Radiation-Induced Lung Disease
Radiation-induced lung disease (RILD) is common after radiation therapy (RT) but can mimic other inflammatory and infectious pulmonary conditions. To distinguish between these possibilities, one must be familiar with the expected timeline of pulmonary changes after RT and those associated with specific RT plans. When assessing for RILD, one refers to the last day of radiation administration to document changes. Within 4 to 12 weeks, transient radiation pneumonitis is expected histologically. Chest film changes are usually evident 6 to 8 weeks after standard-fractionated radiation doses of above 40 Gy, but similarly fractionated doses below 20 Gy rarely produce pneumonitis.49–51 These changes manifest as heterogeneous opacities within the radiation portal, progressing to consolidation, which is usually most severe 3 to 4 months after RT.52 Because of the greater sensitivity of CT scans, GGOs may be seen within the treatment portal by 3 weeks after RT completion and progress to consolidation. The acute changes of RILD may be homogeneously distributed throughout the radiation portal or be scattered focally within it, sometimes in a nodular fashion, mimicking malignancy.53,54 Rarely, at this stage, RILD can be seen outside of the radiation portal owing to a lymphocyte-mediated hypersensitivity reaction.55,56 Chest radiographs may be normal or show heterogeneous peripheral opacities, but CT shows peripheral or peribronchial multifocal GGOs or consolidative opacities, which may be migratory, that have been biopsy-proven to represent BOOP.56,57
RILD may heal or, more commonly, progress to fibrosis within 6 to 12 months after RT. In most patients, the radiation fibrosis stabilizes 12 months after RT completion, although in some patients, these changes may continue to evolve for up to 2 years before they stabilize.49,58–62 Radiographically, these findings manifest as traction bronchiectasis, retracting consolidative opacities with volume loss. These changes are within the radiation field with a clear boundary between the irradiated and the nonirradiated lung. With conventional two-dimensional RT, radiation is delivered in opposed parallel orientation (e.g., anteroposterior and posteroanterior opposed beams). Because of the limited beam orientations, relatively large volumes of surrounding lung is exposed, and RILD in this setting is identified by pulmonary abnormalities with a sharp border that cross anatomic boundaries, such as the fissure (Figure 39-10). This pattern of lung injury is unusual for other types of pulmonary injury such as that resulting from infection or malignancy.
Since the 1990s, newer RT techniques have been developed with one goal: to ensure the entire target volume is adequately treated or even treated to higher radiation doses, while minimizing the radiation dose to normal structures. These techniques include three-dimensional conformal radiation therapy (3D-CRT), intensity-modulated radiation therapy (IMRT), stereotactic body radiotherapy (SBRT), and heavy particle therapy (e.g., proton therapy).63–66 Regardless of the radiation technique used, the local damage to the lung is histologically and radiographically the same within the radiation field. However, owing to the complex radiation portals produced by these newer techniques, the shape of RILD may be unusual, centered around the tumor, similar to the radiation portal produced by these more targeted radiation techniques, with an end result of either a scarlike or a masslike opacity that must be recognized by the radiologist to avoid misinterpretation of RILD for recurrence of malignancy or infection.67
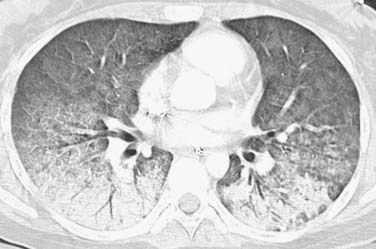
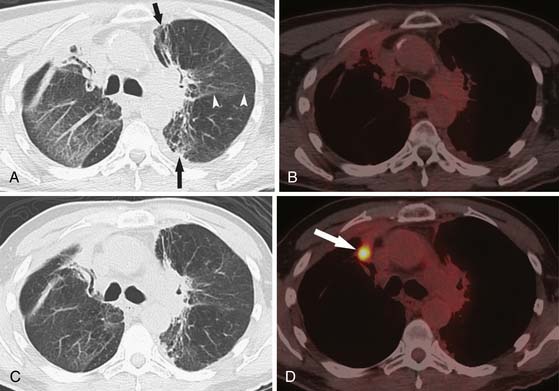
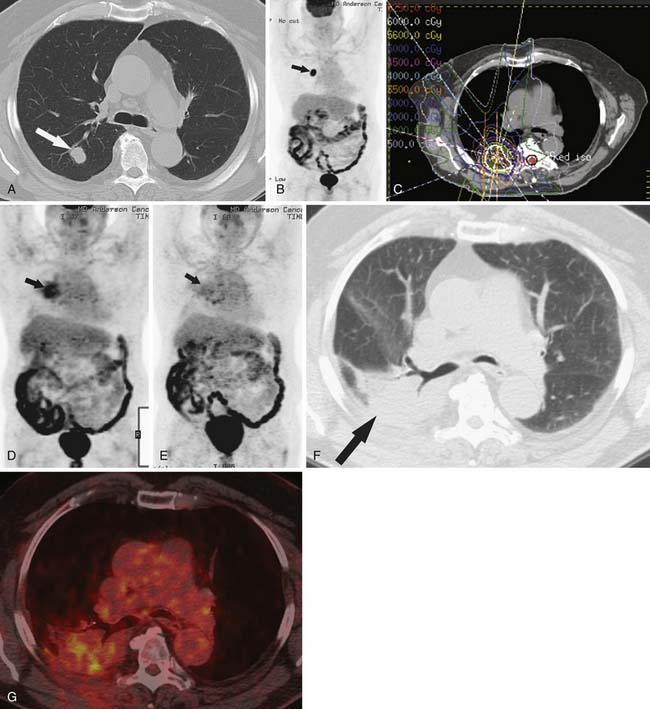
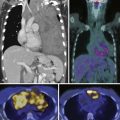
Early identification of recurrence or persistence of malignancy within the radiation field is difficult and requires knowledge of the CT appearance of RILD in conjunction with the date of the last radiation dose. With the newer radiation techniques, it is essential for the radiologist to have the radiation plan if recurrence is to be identified early. PET/CT can also be helpful in this assessment. Although there have been no prospective large studies following patients for RILD with PET/CT scans, smaller studies and our experience show, that, while radiation pneumonitis is evolving, increased fluoro-2-deoxy-D-glucose (FDG) is seen within the radiation field, thereby rendering recurrence difficult to appreciate because hypermetabolically active tumor cannot be easily differentiated from inflammatory changes within the radiation treatment field (Figure 39-11). There is no exact date in which the inflammatory changes become less avid, although 3 to 6 months after radiation, the inflammatory changes typically start to subside and, by 12 months, are not typically present.49 When assessing PET scans for recurrence within the radiation field, one should look not only at the amount of FDG uptake but also its pattern.68 Recurrence is typically focal, whereas the inflammatory RILD changes are mostly diffuse and conform to the radiation portal (see Figure 39-11). Thus, early signs for recurrence include69,70 (1) alteration in stable contours of radiation fibrosis, (2) failure of contracture of an area of radiation pneumonitis 4 months or more after radiation delivery, (3) filling of radiation-induced ectatic bronchi by soft tissue, and (4) focal FDG activity within the radiation field after the more diffuse activity has subsided.
Traditionally, the heart has been viewed as a radiation-resistant organ. However, with improved survival rates of some malignancies, some patients may live to suffer long-term complications including coronary artery disease, valvular disease, conduction abnormalities, pericardial effusion, and cardiomyopathy years after RT.71 Long-term survivors of radiation may also suffer from secondary malignancies within the radiation port including breast cancer, sarcoma, or skin cancers.72
Key Points Radiation-induced lung disease
• RILD can be detected radiographically 6 to 8 weeks after completion of RT above 40 Gy.
• RILD in the form of consolidation within the radiation port is most severe at 3 to 4 months after completion of RT.
• RILD progresses to fibrosis within 6 to 12 months after completion of RT and stabilizes within 2 years.
• RILD from newer radiation may mimick tumor or scar and interpretation is enhanced by viewing the radiation plan.
• On PET, recurrence within the radiation field shows focal FDG activity whereas uptake of RILD is typically more diffuse within the radiation portal.
Treatment-Associated Malignancy
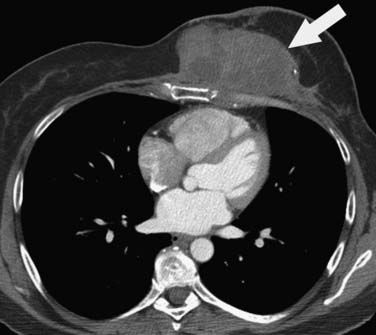
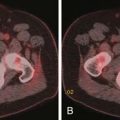
The incidence of a subsequent malignant neoplasm secondary to therapy of a first malignancy is not common, with an approximately 6% absolute risk at 45 years for all diagnoses, which translates into 1.88 absolute excess risk/1000 patient-years follow-up.73,74 The greatest risk is in survivors of Hodgkin’s disease, with a 7.6% cumulative incidence at 20 years and a 5.13 absolute excess risk/1000 patient-years follow-up.74,75 On multivariate analysis, the relative risk of any second malignancy is 2.3, with soft tissue sarcoma having the greatest relative risk (10.3) (Figure 39-12), followed by breast cancer in female survivors (4.9), and then leukemia (4.0).74 Approximately 80% of secondary malignancies are solid tumors, and approximately 50% of secondary solid tumors in female survivors are breast cancers.73 Whereas the risk of secondary leukemia plateaus at 14 years after the primary tumor diagnosis, the risk of solid tumors continues to rise.73,76
There are both patient-related and treatment-related risk factors for development of secondary malignancies. Patient-related factors include age at diagnosis, genetic factors, and possibly gender, although this last factor is disputed in the literature.74,75 The relative risk of second malignancy is inversely related to age at diagnosis, with patients younger than 20 years at first diagnosis having a 10.7 relative risk of developing a second tumor versus a relative risk of 2.4 in patients older than 50 years. The absolute risk of developing any second malignancy (per 10,000 person-years), conversely, is directly related to age at diagnosis, with the younger than 20-year-olds having an absolute risk of 71 versus 211 for the older than 50-year-olds.75 However, both the relative risk and the absolute risk of developing breast cancer as a second malignancy are inversely related to age at diagnosis, with relative risk for survivors of first malignancy diagnosed younger than 15 years being 112 compared with less than 1 if diagnosed older than 40 years, and the absolute numbers being 82 versus 18 for the same patient groups.75
The most common treatment-related risk factors for development of secondary malignancy are RT and chemotherapy. The data for secondary malignancy from radiation have been extrapolated from survivors of radiation injury (e.g., atomic bomb and Chernobyl) and cohort studies. Most radiation-induced second malignancies occur in or near the radiation field, present after a long latency, from a few years to decades, and affect tissues with increased radiation sensitivity (e.g., breast and thyroid).73 The median times to development of leukemia, non-Hodgkin’s lymphoma, and solid tumors are 5.3, 7.1, and 13.8 years, respectively, among patients who developed these second tumors.75 Data for secondary malignancy from chemotherapy have been extrapolated mainly from cohort studies, comparing patient populations in pre- versus postchemotherapy eras. The type of secondary malignancy has also been found to vary depending upon the class of chemotherapeutic agent used, with alkylating agents among the more common offenders. The incidence of chemotherapy-induced secondary malignancy appears to plateau at approximately 5 years after initial diagnosis.73 The effect of combination chemoradiotherapy on the risk of secondary malignancy is unclear because there are reports of both increased risk75,77 and decreased risk78 in the literature.
The recent update of the Childhood Cancer Survivor Study on secondary malignancies in 5-year survivors of childhood cancer79 identified female gender, older age at diagnosis, earlier treatment era, primary diagnosis of Hodgkin’s disease, and treatment with radiation as factors associated with increased risk of subsequent neoplasm. Because of the dramatic improvement of childhood cancer survival, the number of secondary malignancy is anticipated to increase, with substantial increases already being seen, exemplified by the 2.6-fold increase in all subsequent malignant neoplasms.
Key Points Treatment-associated malignancy
• Malignancy develops after a latency of several years to decades.
• More common tumors encountered are thyroid, breast, sarcoma, and leukemia.
• Treatment of first cancer of younger than 20-year-old patients increases the risk for development of treatment-related subsequent cancer.
• Both RT and chemotherapy can cause treatment-associated malignancy.
1. Camus P., Rosenow E.C.3rd. Iatrogenic lung disease. Clin Chest Med. 25, 2004. XIII-XIX
3. Flieder D.B., Travis W.D. Pathologic characteristics of drug-induced lung disease. Clin Chest Med. 2004;25:37-45.
4. Akira M., Inoue Y., Kitaichi M., et al. Usual interstitial pneumonia and nonspecific interstitial pneumonia with and without concurrent emphysema: thin-section CT findings. Radiology. 2009;251:271-279.
5. Silva C.I., Muller N.L., Hansell D.M., et al. Nonspecific interstitial pneumonia and idiopathic pulmonary fibrosis: changes in pattern and distribution of disease over time. Radiology. 2008;247:251-259.
6. Lynch D.A., Travis W.D., Muller N.L., et al. Idiopathic interstitial pneumonias: CT features. Radiology. 2005;236:10-21.
7. Akira M., Yamamoto S., Sakatani M. Bronchiolitis obliterans organizing pneumonia manifesting as multiple large nodules or masses. AJR Am J Roentgenol. 1998;170:291-295.
8. White D.A. Drug-induced pulmonary infection. Clin Chest Med. 2004;25:179-187.
9. Ramila E., Sureda A., Martino R., et al. Bronchoscopy guided by high-resolution computed tomography for the diagnosis of pulmonary infections in patients with hematologic malignancies and normal plain chest X-ray. Haematologica. 2000;85:961-966.
10. Caillot D., Casasnovas O., Bernard A., et al. Improved management of invasive pulmonary aspergillosis in neutropenic patients using early thoracic computed tomographic scan and surgery. J Clin Oncol. 1997;15:139-147.
11. Caillot D., Latrabe V., Thiebaut A., et al. Computer tomography in pulmonary invasive aspergillosis in hematological patients with neutropenia: an useful tool for diagnosis and assessment of outcome in clinical trials. Eur J Radiol. 2010;74:e172-e175.
12. Murono K., Hirano Y., Koyano S., et al. Molecular comparison of bacterial isolates from blood with strains colonizing pharynx and intestine in immunocompromised patients with sepsis. J Med Microbiol. 2003;52:527-530.
13. Rolston K.V.I., Raad I., Whimbey E., Bodey G.P. The changing spectrum of bacterial infections in febrile neutropenic patients. In: Klastersky J.A., editor. Febrile Neutropenia. Berlin: Springer-Verlag; 1997:53-56.
14. Yadegarynia D., Tarrand J., Raad I., Rolston K. Current spectrum of bacterial infections in patients with cancer. Clin Infect Dis. 2003;37:1144-1145.
15. Muller L.M., Franquet T., Lee K.S. Imaging of Pulmonary Infections. Philadelphia: Lippincott Williams & Wilkins, 2007.
16. Escuissato D.L., Gasparetto E.L., Marchiori E., et al. Pulmonary infections after bone marrow transplantation: high-resolution CT findings in 111 patients. AJR Am J Roentgenol. 2005;185:608-615.
17. Ljungman P., Griffiths P., Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002;34:1094-1097.
18. Franquet T., Rodriguez S., Martino R., et al. Thin-section CT findings in hematopoietic stem cell transplantation recipients with respiratory virus pneumonia. AJR Am J Roentgenol. 2006;187:1085-1090.
19. Gasparetto E.L., Ono S.E., Escuissato D., et al. Cytomegalovirus pneumonia after bone marrow transplantation: high resolution CT findings. Br J Radiol. 2004;77:724-727.
20. Kang E.Y., Patz E.F.Jr., Muller N.L. Cytomegalovirus pneumonia in transplant patients: CT findings. J Comput Assist Tomogr. 1996;20:295-299.
21. Franquet T., Lee K.S., Muller N.L. Thin-section CT findings in 32 immunocompromised patients with cytomegalovirus pneumonia who do not have AIDS. AJR Am J Roentgenol. 2003;181:1059-1063.
22. Horger M.S., Pfannenberg C., Einsele H., et al. Cytomegalovirus pneumonia after stem cell transplantation: correlation of CT findings with clinical outcome in 30 patients. AJR Am J Roentgenol. 2006;187:W636-W643.
23. Dasbach E.J., Davies G.M., Teutsch S.M. Burden of aspergillosis-related hospitalizations in the United States. Clin Infect Dis. 2000;31:1524-1528.
24. Lionakis M.S., Kontoyiannis D.P. Fusarium infections in critically ill patients. Semin Respir Crit Care Med. 2004;25:159-169.
25. McAdams H.P., Rosado de Christenson M., Strollo D.C., Patz E.F.Jr. Pulmonary mucormycosis: radiologic findings in 32 cases. AJR Am J Roentgenol. 1997;168:1541-1548.
26. Greene R.E., Schlamm H.T., Oestmann J.W., et al. Imaging findings in acute invasive pulmonary aspergillosis: clinical significance of the halo sign. Clin Infect Dis. 2007;44:373-379.
27. Kontoyiannis D.P., Lionakis M.S., Lewis R.E., et al. Zygomycosis in a tertiary-care cancer center in the era of Aspergillus-active antifungal therapy: a case-control observational study of 27 recent cases. J Infect Dis. 2005;191:1350-1360.
28. Tarrand J.J., Lichterfeld M., Warraich I., et al. Diagnosis of invasive septate mold infections. A correlation of microbiological culture and histologic or cytologic examination. Am J Clin Pathol. 2003;119:854-858.
29. Ascioglu S., Rex J.H., de Pauw B., et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002;34:7-14.
30. Herbrecht R., Denning D.W., Patterson T.F., et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347:408-415.
31. Chamilos G., Marom E.M., Lewis R.E., et al. Predictors of pulmonary zygomycosis versus invasive pulmonary aspergillosis in patients with cancer. Clin Infect Dis. 2005;41:60-66.
32. Wahba H., Truong M.T., Lei X., et al. Reversed halo sign in invasive pulmonary fungal infections. Clin Infect Dis. 2008;46:1733-1737.
33. Rossi S.E., Erasmus J.J., Volpacchio M., et al. “Crazy-paving” pattern at thin-section CT of the lungs: radiologic-pathologic overview. Radiographics. 2003;23:1509-1519.
34. Vogel M.N., Brodoefel H., Hierl T., et al. Differences and similarities of cytomegalovirus and pneumocystis pneumonia in HIV-negative immunocompromised patients—thin-section CT-morphology in the early phase of the disease. Br J Radiol. 2007;80:516-523.
35. Akan H., Arslan O., Akan O.A. Tuberculosis in stem cell transplant patients. J Hosp Infect. 2006;62:421-426.
36. McAdams H.P., Erasmus J., Winter J.A. Radiologic manifestations of pulmonary tuberculosis. Radiol Clin North Am. 1995;33:655-678.
37. Afessa B., Peters S.G. Chronic lung disease after hematopoietic stem cell transplantation. Clin Chest Med. 2005;26:571-586. vi
38. Roychowdhury M., Pambuccian S.E., Aslan D.L., et al. Pulmonary complications after bone marrow transplantation: an autopsy study from a large transplantation center. Arch Pathol Lab Med. 2005;129:366-371.
39. Sharma S., Nadrous H.F., Peters S.G., et al. Pulmonary complications in adult blood and marrow transplant recipients: autopsy findings. Chest. 2005;128:1385-1392.
40. Armitage J.O. Bone marrow transplantation. N Engl J Med. 1994;330:827-838.
41. Wah T.M., Moss H.A., Robertson R.J., Barnard D.L. Pulmonary complications following bone marrow transplantation. Br J Radiol. 2003;76:373-379.
42. Worthy S.A., Flint J.D., Muller N.L. Pulmonary complications after bone marrow transplantation: high-resolution CT and pathologic findings. Radiographics. 1997;17:1359-1371.
43. Hiemenz J.W. Management of infections complicating allogeneic hematopoietic stem cell transplantation. Semin Hematol. 2009;46:289-312.
44. Franquet T., Muller N.L., Lee K.S., et al. High-resolution CT and pathologic findings of noninfectious pulmonary complications after hematopoietic stem cell transplantation. AJR Am J Roentgenol. 2005;184:629-637.
45. Capizzi S.A., Kumar S., Huneke N.E., et al. Peri-engraftment respiratory distress syndrome during autologous hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;27:1299-1303.
46. Afessa B., Peters S.G. Noninfectious pneumonitis after blood and marrow transplant. Curr Opin Oncol. 2008;20:227-233.
47. Yotsumoto S., Okada F., Yotsumoto S., et al. Bronchiolitis obliterans organizing pneumonia after bone marrow transplantation: association with human leukocyte antigens. J Comput Assist Tomogr. 2007;31:132-137.
48. Yousem S.A. The histological spectrum of pulmonary graft-versus-host disease in bone marrow transplant recipients. Hum Pathol. 1995;26:668-675.
49. Choi Y.W., Munden R.F., Erasmus J.J., et al. Effects of radiation therapy on the lung: radiologic appearances and differential diagnosis. Radiographics. 2004;24:985-997. discussion 998
50. Libshitz H.I., Southard M.E. Complications of radiation therapy: the thorax. Semin Roentgenol. 1974;9:41-49.
51. Movsas B., Raffin T.A., Epstein A.H., Link C.J.Jr. Pulmonary radiation injury. Chest. 1997;111:1061-1076.
52. Libshitz H.I. Radiation changes in the lung. Semin Roentgenol. 1993;28:303-320.
53. Pagani J.J., Libshitz H.I. CT manifestations of radiation-induced change in chest tissue. J Comput Assist Tomogr. 1982;6:243-248.
54. Libshitz H.I., Shuman L.S. Radiation-induced pulmonary change: CT findings. J Comput Assist Tomogr. 1984;8:15-19.
55. Roberts C.M., Foulcher E., Zaunders J.J., et al. Radiation pneumonitis: a possible lymphocyte-mediated hypersensitivity reaction. Ann Intern Med. 1993;118:696-700.
56. Arbetter K.R., Prakash U.B., Tazelaar H.D., Douglas W.W. Radiation-induced pneumonitis in the “nonirradiated” lung. Mayo Clin Proc. 1999;74:27-36.
57. Crestani B., Kambouchner M., Soler P., et al. Migratory bronchiolitis obliterans organizing pneumonia after unilateral radiation therapy for breast carcinoma. Eur Respir J. 1995;8:318-321.
58. Chu F.C., Phillips R., Nickson J.J., McPhee J.G. Pneumonitis following radiation therapy of cancer of the breast by tangenital technic. Radiology. 1955;64:642-654.
59. Gross N.J. Pulmonary effects of radiation therapy. Ann Intern Med. 1977;86:81-92.
60. Roswit B., White D.C. Severe radiation injuries of the lung. AJR Am J Roentgenol. 1977;129:127-136.
61. Rubin P., Casarett G.W. Clinical radiation pathology as applied to curative radiotherapy. Cancer. 1968;22:767-778.
62. Smith J.C. Radiation pneumonitis. Am Rev Respir Dis. 1963;87:647-655.
63. Fraass B.A., Kessler M.L., McShan D.L., et al. Optimization and clinical use of multisegment intensity-modulated radiation therapy for high-dose conformal therapy. Semin Radiat Oncol. 1999;9:60-77.
64. Galvin J.M., De Neve W. Intensity modulating and other radiation therapy devices for dose painting. J Clin Oncol. 2007;25:924-930.
65. Timmerman R.D., Forster K.M. Chinsoo Cho L. Extracranial stereotactic radiation delivery. Semin Radiat Oncol. 2005;15:202-207.
66. Welsh J.S. Basics of particle therapy: introduction to hadrons. Am J Clin Oncol. 2008;31:493-495.
67. Koenig T.R., Munden R.F., Erasmus J.J., et al. Radiation injury of the lung after three-dimensional conformal radiation therapy. AJR Am J Roentgenol. 2002;178:1383-1388.
68. Inoue T., Kim E.E., Komaki R., et al. Detecting recurrent or residual lung cancer with FDG-PET. J Nucl Med. 1995;36:788-793.
69. Bourgouin P., Cousineau G., Lemire P., et al. Differentiation of radiation-induced fibrosis from recurrent pulmonary neoplasm by CT. Can Assoc Radiol J. 1987;38:23-26.
70. Libshitz H.I., Sheppard D.G. Filling in of radiation therapy-induced bronchiectatic change: a reliable sign of locally recurrent lung cancer. Radiology. 1999;210:25-27.
71. Stewart J.R., Fajardo L.F., Gillette S.M., Constine L.S. Radiation injury to the heart. Int J Radiat Oncol Biol Phys. 1995;31:1205-1211.
72. Doi K., Mieno M.N., Shimada Y., Yoshinaga S. Risk of second malignant neoplasms among childhood cancer survivors treated with radiotherapy: meta-analysis of nine epidemiological studies. Paediatr Perinat Epidemiol. 2009;23:370-379.
73. Moppett J., Oakhill A., Duncan A.W. Second malignancies in children: the usual suspects? Eur J Radiol. 2001;38:235-248.
74. Neglia J.P., Friedman D.L., Yasui Y., et al. Second malignant neoplasms in five-year survivors of childhood cancer: childhood cancer survivor study. J Natl Cancer Inst. 2001;93:618-629.
75. Ng A.K., Bernardo M.V., Weller E., et al. Second malignancy after Hodgkin disease treated with radiation therapy with or without chemotherapy: long-term risks and risk factors. Blood. 2002;100:1989-1996.
76. Bhatia S., Yasui Y., Robison L.L., et al. High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin’s disease: report from the Late Effects Study Group. J Clin Oncol. 2003;21:4386-4394.
77. Hancock S.L., Tucker M.A., Hoppe R.T. Breast cancer after treatment of Hodgkin’s disease. J Natl Cancer Inst. 1993;85:25-31.
78. Swerdlow A.J., Barber J.A., Hudson G.V., et al. Risk of second malignancy after Hodgkin’s disease in a collaborative British cohort: the relation to age at treatment. J Clin Oncol. 2000;18:498-509.
79. Friedman D.L., Whitton J., Leisenring W., et al. Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2010;102:1083-1095.
80. Broder H., Gottlieb R.A., Lepor N.E. Chemotherapy and cardiotoxicity. Rev Cardiovasc Med. 2008;9:75-83.