Chapter 40 Complications in the Oncologic Patient
Abdomen and Pelvis
Introduction
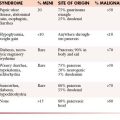
Radiation complications occur in an acute, subacute, or chronic timeframe (Table 40-1).1
Table 40-1 Radiation Effects Can Be Stratified into Specific Time Segments
| EFFECT | TIMEFRAME |
|---|---|
| Acute | <90 days |
| Early delayed | >90 days |
| Late | >90 days |
I Hepatobiliary, Spleen, and Pancreas
Liver
Chemotherapy Change
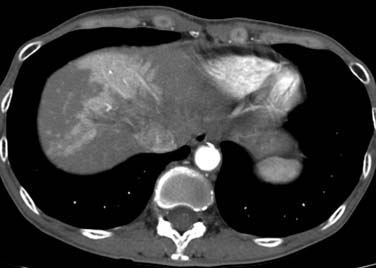
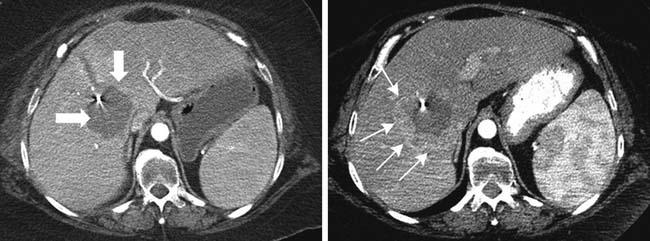
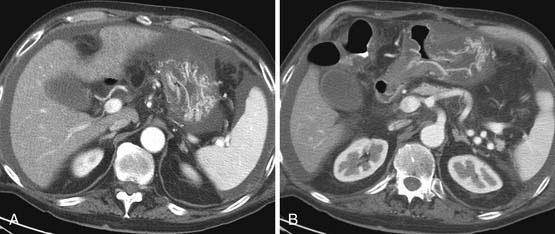
Fatty infiltration of the liver is seen with administration of all chemotherapy agents, especially irinotecan and oxaliplatin.2 It can be focal or diffuse; when diffuse, it is called chemotherapy-associated steatohepatitis. Knowing the different imaging appearances and characteristic sites can aid in differentiating between liver metastases and fatty change. Fatty infiltration can be either focal or diffuse. The focal lesions can sometimes cause difficulty in distinguishing from metastatic disease. Clues that a lesion may represent fatty infiltration are location in the falciform ligament, porta hepatis, and gallbladder fossa and a geometric, well-defined shape. Ultrasound can show a well-defined hypoechoic region. CT and MRI can be used for diagnosis. On CT, there will be a low-attenuation region (Figure 40-1). MRI with in- and out-of-phase imaging is very useful in confirming the diagnosis.2
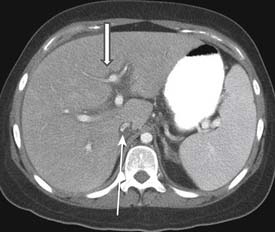
Portal vein thrombosis has also been seen with patients undergoing preoperative chemotherapy.3 Portal vein thrombosis is seen on CT as a filling defect within the portal vein. On ultrasound, there is echogenic thrombus within the portal vein. Branch portal vein thromboses can cause wedge-shaped areas of perfusion change, which need to be distinguished from development of metastatic disease (Figure 40-2). Treatment is conservative and consists of anticoagulation.
Veno-occlusive disease has been described in patients with stem cell transplants and also in patients with colorectal liver metastatic disease taking oxaliplatin.4 Clinically, the patients have hepatic failure and jaundice. CT scan shows a heterogeneous liver with narrowing of the right hepatic vein, periportal edema, and ascites5 (Figure 40-3). Ultrasound examination shows ascites; reversal of hepatic venous flow is a late sign. This can be distinguished from hepatic graft-versus-host disease (GVHD) by its early presentation in the first 20 days after stem cell transplant.6
Changes that have been observed after intra-arterial chemotherapy infusion are chemical hepatitis, gastrointestinal ulceration, sclerosing cholangitis, and biliary cirrhosis.7
Radiation Change
Radiation-induced liver disease (RILD) occurs in approximately 5% to 10% of people who received whole liver irradiation in doses exceeding 30 to 35 Gy.8 Patients usually present 2 to 8 weeks (up to 4 months) after radiation exposure with hepatomegaly, ascites, and elevated liver enzymes.9 Pathologically, there is a picture of veno-occlusive disease with congestion of the central portion of the lobule with sparing of the larger veins. The radiologic picture is that of a clear demarcation—also known as the straight border sign—between the area that has received radiation and that which has not been irradiated.10 This can be differentiated from vascular changes by its nongeographic distribution. In the acute and subacute phases, the area that has been irradiated is seen at CT to be lower in attenuation than the adjacent liver, likely due to edema (Figure 40-4). On MRI, the irradiated area has low T1 signal and high T2 signal due to edema. Chronic changes can occur with fibrosis and volume loss. Usually, patients have resolution of findings in 3 to 5 months, but a small portion progress to liver failure.
Surgical Change
Surgical resection is performed for patients with hepatocellular carcinoma, cholangiocarcinoma, and metastatic disease. Postoperative fluid collections that can be seen at the surgical site are abscess, postoperative seromas, and bile duct leak. At our institution, these are usually drained percutaneously. Over time, these collections and the perfusion change along the hepatic surgical margin will resolve. It is important to distinguish between postsurgical change and recurrence of tumor by reviewing the initial imaging study in the postoperative period. Over time, the remaining liver may regenerate.
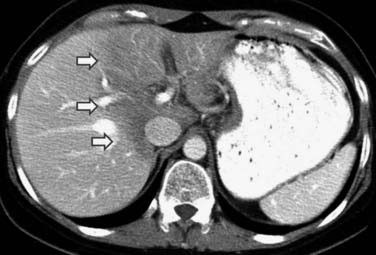
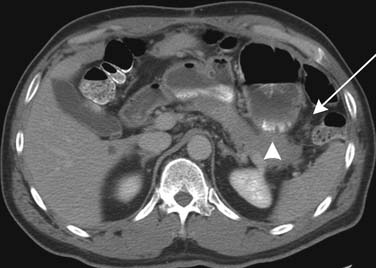
Radiofrequency ablation (RFA) is being increasingly used in the treatment of colorectal metastatic disease to the liver.11 On CT and MRI, the RFA defect site is a low-density area in the place of the treated metastasis. There may be hypervascularity surrounding the surgical site initially, which usually resolves within 3 months. Tumors adjacent to the portal vein pedicle can lead to injury of the bile duct, with stricture and possible sepsis.11 It is important to diagnose recurrence, which is any change in the size of the cavity, mass effect, nodularity, and enhancement (Figure 40-5).
Spleen
Chemotherapy Change
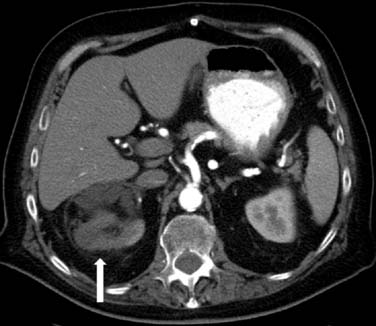
Splenic rupture is a rare complication of stem cell transplant or treatment with granulocyte colony–stimulating factor (G-CSF). G-CSF is given to cancer patients for treatment of therapy-induced neutropenia.12 In splenic rupture, patients usually present with abdominal pain and left shoulder pain. On CT, there is irregularity of the spleen, splenomegaly, and high-density fluid surrounding the spleen and in the abdomen, consistent with hemoperitoneum. Treatment usually consists of intravenous hydration and resection of the spleen.
Surgical Change
Splenectomy is performed for lymphoma, myelodysplastic disease, or other hematologic malignancy to obtain total local control. If the spleen is also adjacent to tumors of the pancreas, retroperitoneum, peritoneum, or kidney, it may be removed as well. Occasionally, in the postsurgical bed, small splenules will develop or remain behind.
Pancreas
Chemotherapy Change
Cytoreductive surgery with intraperitoneal chemotherapy is being used more frequently in order to prolong survival in patients with gastric, ovarian, and pseudomyxoma tumors. Pancreatitis is a complication that occurs approximately 2% to 7% of the time with these procedures.13 Pancreatitis can be focal or diffuse (Figure 40-6).
Surgical Change
CT is frequently used for imaging patients who have undergone pancreaticoduodenectomy. Postoperative changes that are commonly seen are pneumobilia (particularly in the left lobe), normal jejunal loop, and postoperative fluid collections. CT is especially useful for the diagnosis of postoperative abscess, leaks, biloma, biliary obstruction, pancreatitis, and bowel obstruction.14
Key Points Liver, spleen, and pancreas
• Fatty change of liver due to chemotherapy can be diffuse or focal. MRI or ultrasound can be helpful in confirming this diagnosis.
• Radiation change can cause a characteristic appearance in the liver and should not be mistaken for recurrent tumor.
• Enlargement of the spleen can occur with chemotherapeutic agents, and monitoring for portal hypertension should be done.
• Recurrent pancreatic tumor can be distinguished from radiation change with biopsy.
II Digestive System
Chemotherapy Change
Chemotherapeutic agents cause immunosuppression, which can lead to the bowel complications of Clostridium difficile infection, colitis, and typhlitis. C. difficile infection and colitis can present with a spectrum of findings ranging from diarrhea to pseudomembranous colitis to fulminant colitis. Stool culture or endoscopy is used for diagnosis. CT may be helpful, and there is diffuse thickening of the colon on imaging. Treatment is antibiotic therapy with metronidazole or vancomycin.15
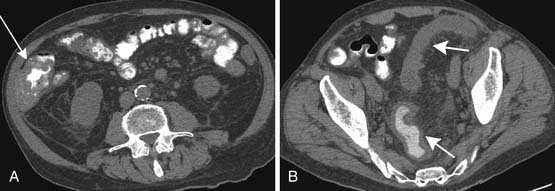
Typhlitis or neutropenic colitis can be seen in children and adults being treated for hematologic malignancies or undergoing immunosuppressive therapies. On CT, typhlitis is seen most commonly as bowel wall thickening greater than 3 mm and inflammation of the terminal ileum and cecum, but it can be seen in the entire bowel. Pneumatosis and fluid/abscess can also be seen. Ultrasound may also be a useful imaging tool. Imaging is also helpful in excluding other etiologies of pain such as appendicitis, other forms of colitis, or obstruction.16 Treatment is supportive and consists of intravenous fluids, antibiotics, and if necessary, G-CSF or surgery for bleeding.
Acute GVHD occurs within the first 100 days after transplant, and chronic GVHD occurs within 100 days after transplant. Acute GVHD clinically presents with cramping and diarrhea, which can be bloody. The diagnosis is usually made by endoscopy, but it can be seen on CT as bowel wall thickening (Figure 40-7). Chronic GVHD affects the small bowel and colon less commonly than acute GVHD. Patients can have malabsorption, sclerosis of the intestine, and fibrosis.15
Targeted therapies are increasingly being used for treatment of many cancers. These drugs target the cell receptors, and examples include vascular endothelial growth factors, epithelial growth factors and platelet-derived growth factors. They affect the blood supply adjacent to the tumor and may have a system-wide thrombogenic effect. Perforation and fistulas are complications of treatment with anti-vascular epithelial growth factor (VEGF) agents17 (Figure 40-8).
Radiation Change
Esophagus
Radiation effects on the esophagus occur at doses greater than 45 Gy and can last weeks to months. The earliest sign is abnormal motility, which can be observed at barium examination. Mucosal edema and fistula may occur within the first month after therapy. Strictures can also be seen as smooth narrowing with margins that are tapered.18 At CT, inflammatory change is seen as enhancement in a central ring. On PET/CT, esophageal radiation change can be FDG-avid and can sometimes be difficult to distinguish from residual tumor and radiation change.19
Stomach and Duodenum
Radiation injury to the stomach and duodenum can occur as a result of therapy directly to these organs or owing to irradiation for tumors of the pancreas. Effects can happen after radiation of 50 Gy, and the fixed position of the duodenum and stomach contributes to the susceptibility to injury.20 Diffuse inflammatory change, ulcers that do not heal, fixed narrowing, and deformity are all seen with radiation injury. CT may show wall thickening and perigastric or periduodenal fluid (Figure 40-9).
Small and Large Bowel
Chronic changes to the small bowel can result in submucosal edema, ulceration, fibrosis, adhesions, and fistula formation. At small bowel studies, there can be diffuse thickening and straightening of the small bowel folds and separation of bowel loops in the acute setting and tethering of bowel loops at the later stages.21 CT is helpful in excluding recurrent disease, identifying fistulas, and evaluating for obstruction. CT findings in radiation injury are similar to those seen at fluoroscopy with bowel wall thickening, fixation, and increased mesenteric density consistent with fibrosis or edema.
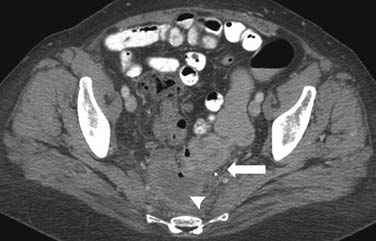
In the treatment of gynecologic and urologic malignancies, the sigmoid and rectum can receive close to the entire dose of radiation and, therefore, rectal injuries are more common than injury to small bowel.22 Patients can present with rectal pain, bleeding, lower abdominal cramping, diarrhea, and obstruction. Fibrosis and ischemia can occur in the late stages. On barium study, strictures, ulcerations, protrusions, and circumferential narrowing can be seen (Figure 40-10). There may be widening of the presacral space.
Surgical Change
CT scan of the abdomen and pelvis is the optimal postoperative tool to evaluate for pneumoperitoneum, obstruction, abscess, or leak. Water-soluble contrast is utilized in cases of possible leak or obstruction. Postsurgical changes can sometimes cause bowel wall thickening and peritoneal stranding, which can confound the picture when searching for recurrent tumor. Alterations in anatomy as a result of surgery may lead to obstruction of bowel loops that are adjacent to recurrent tumor or adjacent to lymph nodes.
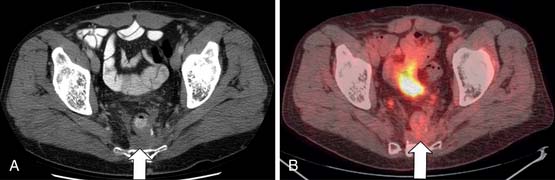
One problematic region is the pelvis in patients after radiation and abdominal pelvic resection. In men, the bladder, prostate, and seminal vesicle can fall into the postsurgical space. In females, the uterus or ovaries can also prolapse into the presacral space. Fibrosis may also develop after surgery. Contrast-enhanced T1- and T2-weighted MRI can be used to aid in diagnosis. In recent times, PET/CT has shown high specificity in evaluating for recurrent tumor (Figure 40-11). However, it should not be performed within 4 months of radiotherapy or surgery.
Key Points Bowel
• Bowel wall perforation and colitis can occur with specific chemotherapeutic agents. Knowledge of therapy can lead to accurate diagnosis and cessation of therapy.
• Radiosensitive bowel areas are most commonly the terminal ileum, cecum, rectum, and sigmoid.
• Radiation can cause increased soft tissue and spiculation, widening the presacral space. This can be distinguished from tumor or abscess by CT or PET/CT.
III Genitourinary System
Chemotherapy Change
Kidney
Renal injury can result from administration of chemotherapy. The most common drug to have this effect is mitomycin C; other drugs that affect the kidneys are methotrexate, bleomycin, cisplatin, 5-fluorouracil (5-FU), and now gemcitabine. The mechanism of injury is intrarenal or systemic microvascular thrombi, resulting in endothelial swelling and microvascular obstruction. Other drugs, such as methotrexate, can cause renal tubular injury that leads to renal failure.23
Stem cell transplant is a confirmed cause of renal injury, occurring most frequently in patients who have received myelosuppressive allogenic transplants. The incidence of acute renal failure requiring dialysis in this population has ranged from 36% to 70%. The major part of the toxicity to the kidneys is due to the myelosuppressive agents. Chronic renal disease as a result of stem cell transplant can occur in 15% to 20% of patients.24
Reproductive System
Chemotherapy may lead to infertility. The testicle is very sensitive to cytotoxic therapies.25 Sterility is a common side effect with cyclophosphamide and mechlorethamine, Oncovin, procarbazine, and prednisole (MOPP) chemotherapy. In women, amenorrhea and early menopause can occur. The ovaries may decrease in size owing to chemotherapy. There is also an increased risk of infections to the genitourinary system as a result of neutropenia and thrombocytopenia.
Radiation Change
Kidney
The kidneys are radiosensitive, and bilateral doses of 28 Gy have been shown to cause complications of renal failure. Acute renal failure was seen at 6 to 12 months, and chronic disease presented at greater than 18 months. On intravenous pyelograms and CT, there are signs of diminished or absent renal function with delayed nephrograms.26 In chronic disease, the kidney will be small and contracted with a smooth border.
Bladder and Ureter
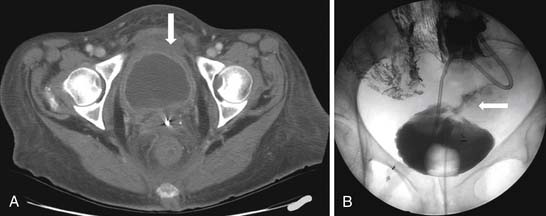
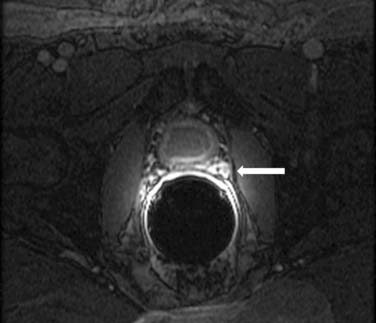
Acute symptoms include urinary frequency, urgency, and pain on urination. The reported incidence varies anywhere from 23% to 80%. Late effects are much more severe and seen in patients receiving radiation therapy for cervix and prostate cancer. Approximately 20% of patients suffer from late effects, with 10% developing the long-term effect of a small and contracted bladder. A small portion of patients require cystectomy. On CT and MRI, a small contracted bladder with wall thickening will be seen (Figure 40-12). Cystogram may show a trabeculated bladder, and perforation may occur owing to friability of the bladder soft tissue. Radiation changes can be distinguished from recurrent tumor by using imaging, of which MRI is superior. There may be high signal intensity in the bladder wall seen on T2-imaging in patients with mild injury. Increased bladder wall thickening with a low-signal inner layer and a higher-signal peripheral layer (a lamellated appearance) may indicate more severe injury. Fistulas and sinus tracts may develop. The bladder wall can enhance with contrast for up to 2 years after irradiation.27
Ureter/Urethra
Strictures and fibrosis of the ureter are uncommon complications of radiation, and the incidence is low in external beam radiation. It does occur in 1% to 3% of patients who receive brachytherapy for gynecologic malignancies. Continued surveillance of renal function is advised because the risk of ureteral narrowing increases from 1% at 5 years to 2.5% at 20 years after therapy. The typical site of strictures is at the ureterovesicular junction, but higher strictures can occur where the ureters cross the iliac vessels. The appearance on imaging is a smooth, long-tapering of the ureter that may exceed 5 cm, but this cannot be distinguished from recurrence on imaging alone. Reflux may also occur as a result of bladder wall fibrosis.
Urethral strictures are also rare and occur in doses greater than 60 to 70 Gy. The risk of radiation injury in patients who have had transurethral resection of the prostate (TURP) is higher, 5% to 15% versus up to 5% in patients who have not had a TURP. Urethral strictures are demonstrated on retrograde urethrograms. Some patients may require surgery for complex or recurrent strictures.28
Prostate and Seminal Vesicles
Posttherapeutic effects that can be seen in the prostate at imaging are a decreased T2 signal intensity, shrinkage of the prostate, and normal zonal anatomy indistinctness. Endorectal coil MRI of the prostate with magnetic resonance spectroscopy is being used for the evaluation of recurrence.29 The seminal vesicles may also decrease in size and also have decreased T2 signal intensity on imaging.
Testes
The testicles are extremely sensitive to radiation and a limited dose should be used whenever possible. Doses as low as 2 to 3 Gy can result in azoospermia. Late recovery can sometimes occur. Sperm banking is encouraged for these patients. On imaging, there may be testicular atrophy.25
Uterus, Cervix, Vagina, and Vulva
The uterus and cervix may receive up to 200 Gy in therapy for gynecologic malignancy. The uterus can decrease in size. Ulceration of the endometrial cavity may result in an indistinct appearance and low T2 signal on MRI. Distention of the endometrial cavity can persist long after radiation has been completed.30 Uterine necrosis is a rare complication.31 Development of a high-grade endometrial cancer or sarcoma may occur many years after therapy.32
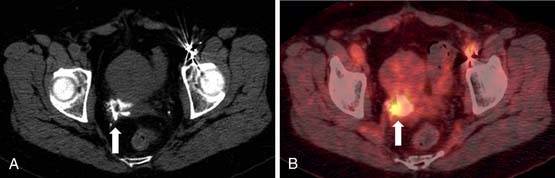
After radiotherapy, the cervix may atrophy and completely disappear with higher doses. Cervical os stenosis is a rare complication and may occur 3 to 6 months after therapy. After 6 months to 2 years, the patients present with cramps and distention due to hematometra and retention of contents. Residual tumor may decrease in size and develop calcification. This needs to be distinguished from recurrence (Figure 40-13).
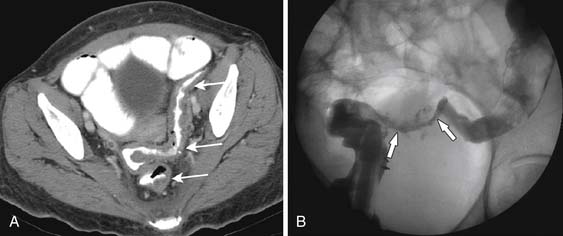
The vagina can tolerate up to 140 Gy to the upper surface and 100 Gy for the surface. Vaginal atrophy, thinning, and fibrosis become more severe with higher doses of radiation therapy. A rectovaginal fistula can develop at doses of 80 Gy, and vesiculovaginal fistulas usually occur at 150 Gy. Fistulas can be evaluated at fluoroscopy with Gastrografin showing the connection between the rectum and the vagina or the colon and the vagina (Figure 40-14). MRI usually shows the fistulous connection best.
Ovaries
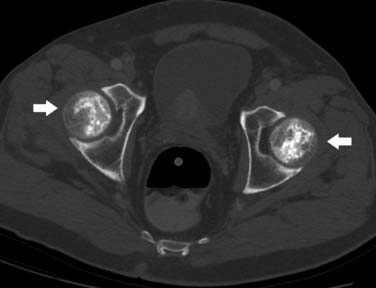
Radiation to the ovaries can result in infertility and endocrine abnormalities. Small doses of 1.8 to 2 Gy per day or a total dose exceeding 24 Gy can lead to permanent ovarian ablation. For this reason, re-implantation of the ovary into the paracolic gutter is performed to reduce the risk of radiation injury.33
Surgical Change
Kidney
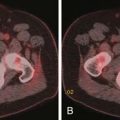
Postoperative complications can arise postnephrectomy, postpartial nephrectomy, RFA, or laparoscopic nephrectomy. The complications consist of ileus, bleeding, urinomas, ureteral injury, or pseudoaneurysm. CT is the best tool for evaluation of these complications and distinguishing postoperative change from recurrence of tumor (Figure 40-15).
Prostate
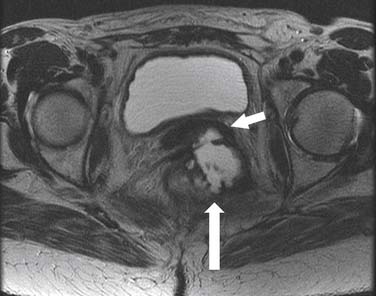
Postsurgical fibrosis caused by radical prostatectomy can sometimes be extensive and a cause of urethral stricture. These changes can be difficult to distinguish from recurrence between the bladder and the membranous urethra. MRI with dynamic enhancement images in the postprostectomy bed may be useful because the recurrent tumor may enhance (Figure 40-16).
Ovaries/Testes
The ovaries may occasionally be transposed into the iliac fossa or higher in the pelvis for patients who require radiation or surgery. This imaging appearance is distinct from recurrent mass. Large postoperative hydroceles can occur after testicular resection and simulate recurrent mass.
Key Points Genitourinary
• Fistulas due to radiation or chemotherapy are better evaluated at MRI.
• Changes of RFA in the kidney can be distinguished from recurrent tumor by use of CT.
• Radiation change can be distinguished from recurrent tumor in the postprostatectomy bed by use of dynamic imaging.
• Ovarian transposition may mimic masses in the upper pelvis and correlation with patient history is essential in avoiding misdiagnosis.
IV Musculoskeletal System
Bone
Chemotherapy Change
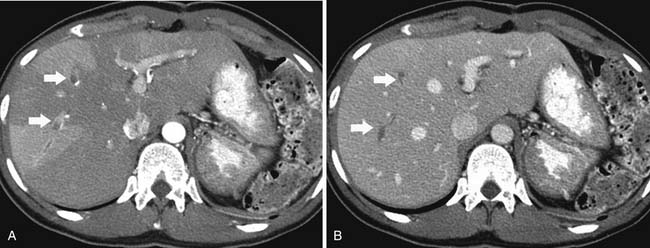
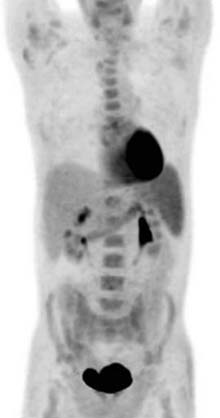
Chemotherapy and bone marrow or stem cell transplantation can cause regenerating hematopoetic marrow. There is reconversion of yellow to red marrow, which contains mature and immature myeloid elements and granulocytes. On MRI, there is decreased T1 signal and increased signal on T2 imaging and short tau inversion recovery (STIR) images. There can also be mild enhancement after contrast administration. This can be difficult to distinguish from metastatic disease on MRI using T1- and T2-weighted imaging. Chemical shift imaging or gradient echo imaging can help differentiate normal marrow from unresponsive disease. On PET/CT, there is diffuse FDG uptake by the marrow, which most likely represents treatment response or marrow-stimulating agents (Figure 40-17).34 If there is persistent concern for recurrence, a bone marrow biopsy can be performed.
Radiation Change
In children, radiation can interrupt the growth plate, interfering with chondrogenesis. This effect is most profound in early childhood and puberty. The changes may not be evident until the child reaches puberty. Radiation to the abdomen for Wilms’ tumors or neuroblastoma can lead to spinal changes, most commonly scoliosis. Initially, the growth arrest lines are apparent and bulbing of the vertebral bodies can appear. In the late stages, there is decreased vertebral body height, anterior beaking, and scalloping of the vertebral bodies. Kyphosis can develop. Children who have long bone irradiation for tumors such as osteosarcoma can have radiation effects at the growth plate with metaphyseal fraying, scoliosis, and epiphyseal widening. Limb length abnormalities may be seen. Orbital irradiation for retinoblastoma can result in abnormal orbital development. Slipped capital femoral epiphyses can occur as a result of radiation therapy. This is seen in patients who receive irradiation to the proximal femurs for abdominal or pelvis malignancy and can present 1 to 8 years posttreatment.35
Osteonecrosis can occur in patients who receive radiation, corticosteroids, chemotherapy, or a combination of therapies. The primary malignancy itself can also be a sole cause. Two types of avascular necrosis (AVN) are seen: an infarct of the trabecular bone in the medullary cavity, which usually has no symptoms, and an infarct of the subchondral cortex, which is very painful. An infarct of the subchondral cortex is commonly seen in the femoral heads and condyles and humeral heads. Unilateral or bilateral AVN has been seen with doses of 30 to 40 Gy 1 to 13 years after radiation therapy. The imaging appearance of osteonecrosis caused by radiation or chemotherapy is similar to that of other etiologies.36 Radiography is nonspecific in the early stages of AVN (Figure 40-18).
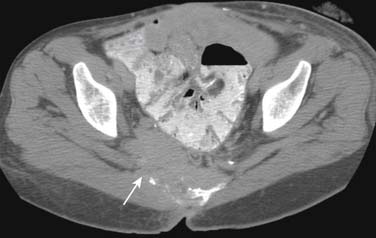
Radiation-induced fractures are caused by normal or physiologic stress on abnormal bone, and most fractures due to radiation are insufficiency fractures. Fractures heal slowly, and they have a high incidence of nonunion. Resorption of fracture fragments may occur. There can be abnormal callous formation. Insufficiency fractures of the sacrum are common in women who have received pelvic irradiation. Bone scan is the most sensitive test, with linear uptake on one or both sides of the sacrum indicating vertical fractures or the characteristic H sign, indicating vertical and horizontal fractures. CT can help distinguish fractures of the sacrum from metastatic disease. MRI may show edema only as decreased T1 signal instead of normal marrow. Insufficiency fractures of the pubis are less common than those of the sacrum. Other fractures that can occur are at the clavicle, rib, humerus, acromion, or any long bone. The imaging findings are as described. Imaging and timeframe may be helpful in excluding radiation-induced sarcomas.37
Muscle
Radiation Change
Radiation-induced changes can be seen as early as 6 weeks at doses of 60 to 65 Gy and usually resolves within 12 to 18 months. In some instances, changes can persist long after therapy has been completed, progressing to atrophy or fibrosis. MRI shows T2 signal increase in the muscles and skin of the radiation field.38 Chronic changes of muscle atrophy such as fatty replacement or asymmetry can be seen on CT.
Vessels
Radiation vasculopathy can develop at doses as low as 20 Gy, and the risk increases with dose and time. This has been seen in patients who undergo pelvic radiation for cervix and uterine cancers. Irradiation leads to an endarteritis and endothelial permeability, which leads to interstitial edema and congestion.30 There can be enhancement of normal tissue due to abnormal vessels for up to 18 months after radiation.
V Secondary Tumors
Radiation-induced osteochondromas (cartilaginous exostosis) are benign tumors that have been reported with an incidence of 6% to 12%. It can occur in children treated with external beam radiation or in adults treated with total body irradiation before transplant. They may be small and can be located within any irradiated bone. They may cause symptoms of pain as they grow and can be resected if symptoms persist or size increases.26
Secondary malignancies can be induced by treatment, especially in children. There is a higher rate of leukemia, lymphoma, myelodysplastic disorders, and solid tumors. Radiation-induced sarcoma is a rare complication that occurs in a very small population of patients who survive 5 years after radiotherapy. It can occur as early as 2 to 3 years after therapy and present as late as 50 years after therapy, with the average latent time of 10 to 15 years. Osteosarcomas are most common after bone irradiation, and malignant fibrous histiocytomas are more common after soft tissue irradiation. Soft tissue secondary tumors are more common. The secondary sarcoma usually occurs within or adjacent to the radiation site. Bony destruction or a soft tissue mass in the radiation field should raise suspicion for secondary malignancy (Figure 40-19). Imaging can be helpful in evaluating for new bone loss, destructive changes, soft tissue masses, or abnormal enhancement within the previously irradiated field. Distinguishing these changes from osteomyelitis may be difficult and require biopsy.
VI Conclusion and Important Points
Key Points Important points
• Splenomegaly and portal hypertension may result from chemotherapy.
• Bowel wall perforation and fistulas occurring during treatment should be conveyed to the clinician as it impacts patient care.
• Radiation to the bowel can result in strictures, bowel wall thickening, dilatation, and fistulas.
• Bladder is the most radiosensitive organ.
• MRI may be helpful in evaluating for radiation injury and fistulas.
• PET/CT can be utilized to distinguish posttreatment effect from recurrent tumor.
• Secondary malignancies that develop after chemotherapy or radiation include hematologic malignancies and solid tumors.
1. Cox J.D., Stetz J., Pajak T.F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341-1346.
2. Kammen B.F., et al. Focal fatty infiltration of the liver: analysis of prevalence and CT findings in children and young adults. AJR Am J Roentgenol. 2001;177:1035-1039.
3. Donadon M., et al. Portal thrombosis and steatosis after preoperative chemotherapy with FOLFIRI-bevacizumab for colorectal liver metastases. World J Gastroenterol. 2006;12:6556-6558.
4. Schouten van der Velden A.P., et al. Hepatic veno-occlusive disease after neoadjuvant treatment of colorectal liver metastases with oxaliplatin: a lesson of the month. Eur J Surg Oncol. 2008;34:353-355.
5. Erturk S.M., et al. CT features of hepatic venoocclusive disease and hepatic graft-versus-host disease in patients after hematopoietic stem cell transplantation. AJR Am J Roentgenol. 2006;186:1497-1501.
6. Lassau N., et al. Hepatic veno-occlusive disease after myeloablative treatment and bone marrow transplantation: value of gray-scale and Doppler US in 100 patients. Radiology. 1997;204:545-552.
7. Ragnhammar P., Hafström L., Nygren P., Glimelius B., SBU-group. Swedish Council of Technology Assessment in Health Care. A systematic overview of chemotherapy effects in colorectal cancer. Acta Oncol. 2001;40(2-3):282-308.
8. Dawson L.A., et al. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys. 2002;53:810-821.
9. Khozouz R.F., Huq S.Z., Perry M.C. Radiation-induced liver disease. J Clin Oncol. 2008;26:4844-4845.
10. Itai Y., Murata S., Kurosaki Y. Straight border sign of the liver: spectrum of CT appearances and causes. Radiographics. 1995;5:1089-1102.
11. Wong S.L., et al. American Society of Clinical Oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. J Clin Oncol. 2010;28:493-508.
12. Masood N., et al. Splenic rupture, secondary to G-CSF use for chemotherapy induced neutropenia: a case report and review of literature. Cases J. 2008;1:418.
13. Sugarbaker P.H. Cytoreductive surgery and perioperative intraperitoneal chemotherapy: a new standard of care for appendiceal mucinous tumors with peritoneal dissemination. Clin Colon Rectal Surg. 2005;18:204-214.
14. Gervais D.A., et al. Complications after pancreatoduodenectomy: imaging and imaging-guided interventional procedures. Radiographics. 2001;21:673-690.
15. Davila M., Bresalier R.S. Gastrointestinal complications of oncologic therapy. Nat Clin Pract Gastroenterol Hepatol. 2008;5:682-696.
16. Khoury N.J., et al. Abdominal complications of chemotherapy in pediatric malignancies: imaging findings. Clin Imaging. 2009;33:253-260.
17. Heinzerling J.H., Huerta S. Bowel perforation from bevacizumab for the treatment of metastatic colon cancer: incidence, etiology, and management. Curr Surg. 2006;63:334-337.
18. Libshitz H.I., Southard M.E. Complications of radiation therapy: the thorax. Semin Roentgenol. 1974;9:41-49.
19. Bruzzi J.F., et al. PET/CT of esophageal cancer: its role in clinical management. Radiographics. 2007;27:1635-1652.
20. Singh A.K., et al. A prospective study of differences in duodenum compared to remaining small bowel motion between radiation treatments: implications for radiation dose escalation in carcinoma of the pancreas. Radiat Oncol. 2006;1:33.
21. Iyer R., Jhingran A. Radiation injury: imaging findings in the chest, abdomen and pelvis after therapeutic radiation. Cancer Imaging. 2006;6:S131-S139.
22. Coia L.R., Myerson R.J., Tepper J.E. Late effects of radiation therapy on the gastrointestinal tract. Int J Radiat Oncol Biol Phys. 1995;31:1213-1236.
23. Humphreys B.D., Soiffer R.J., Magee C.C. Renal failure associated with cancer and its treatment: an update. J Am Soc Nephrol. 2005;16:151-161.
24. Kersting S., et al. Acute renal failure after allogeneic myeloablative stem cell transplantation: retrospective analysis of incidence, risk factors and survival. Bone Marrow Transplant. 2007;39:359-365.
25. Schwartz C.L. Long-term survivors of childhood cancer: the late effects of therapy. Oncologist. 1999;4:45-54.
26. Libshitz H.I., et al. Radiation change in normal organs: an overview of body imaging. Eur Radiol. 1996;6:786-795.
27. Hall T.B., MacVicar A.D. Imaging of bladder cancer. Imaging. 2001;13:1-10.
28. Marks L.B., et al. The response of the urinary bladder, urethra, and ureter to radiation and chemotherapy. Int J Radiat Oncol Biol Phys. 1995;31:1257-1280.
29. Westphalen A.C., et al. Peripheral zone prostate cancer: accuracy of different interpretative approaches with MR and MR spectroscopic imaging. Radiology. 2008;246:177-184.
30. Hricak H., Yu K.K. Radiology in invasive cervical cancer. AJR Am J Roentgenol. 1996;167:1101-1108.
31. Marnitz S., et al. Uterus necrosis after radiochemotherapy in two patients with advanced cervical cancer. Strahlenther Onkol. 2006;182:45-51.
32. Grigsby P.W. Role of PET in gynecologic malignancy. Curr Opin Oncol. 2009;21:420-424.
33. Husseinzadeh N., Van Aken M.L., Aron B. Ovarian transposition in young patients with invasive cervical cancer receiving radiation therapy. Int J Gynecol Cancer. 1994;4:61-65.
34. Strauss L.G. Fluorine-18 deoxyglucose and false-positive results: a major problem in the diagnostics of oncological patients. Eur J Nucl Med. 1996;23:1409-1415.
35. Fletcher B.D. Effects of pediatric cancer therapy on the musculoskeletal system. Pediatr Radiol. 1997;27:623-636.
36. Jones D.N. Multifocal osteonecrosis following chemotherapy and short-term corticosteroid therapy in a patient with small-cell bronchogenic carcinoma. J Nucl Med. 1994;35:1347-1350.
37. Ikushima H., et al. Pelvic bone complications following radiation therapy of gynecologic malignancies: clinical evaluation of radiation-induced pelvic insufficiency fractures. Gynecol Oncol. 2006;103:1100-1104.
38. Shapeero L.G., et al. Post-treatment complications of soft tissue tumours. Eur J Radiol. 2009;69:209-221.