Complications Associated with Orthognathic Surgery
General Considerations
No matter how accurate the diagnosis and how meticulous the surgeon, complications will occur after orthognathic procedures.* Alternatively, the relative safety of orthognathic procedures has been confirmed by a number of retrospective reviews.13,32,84,93,152,223,245,254,315,353 Panula and colleagues reviewed maxillary and mandibular osteotomy patients at their institution (N = 655) and found only one serious complication (intraoperative bleed). A prospective study of Le Fort I operations by Kramer and colleagues (N = 1000) found a 6.4% incidence of complications that ranged from extensive bleeding (1.1%) to sensory loss in the distribution of the infraorbital nerve.173 A review by Chow and colleagues of a consecutive series of orthognathic patients (N = 1294) documented a relatively high complication rate (9.7%)61; this included mostly manageable “postoperative infections” (7.4%), with no serious or rare events recorded. A large series of orthognathic patients (N = 2049) was reviewed by Van de Perre and colleagues346; those authors identified one patient death and 44 other patients with either primary or secondary complications.
Medical Risk Factors
For middle-aged adults who are considering orthognathic surgery, additional medical risk factors may include cardiovascular disease; obesity or increased body mass index; obstructive sleep apnea; diabetes; pulmonary disease; smoking history; the potential for deep vein thrombosis or pulmonary embolus; and osteoporosis or the use of bisphosphonate treatment (see Chapter 25).30,50,172 Evaluation by the patient’s primary care physician (and, in many cases, by other medical specialists) and appropriate laboratory work (e.g., electrocardiography, chest radiography, chemistries, hematology, thyroid tests, coagulation studies) help to identify areas of concern. The adult patient with an elevated body mass index who is undergoing surgery is at greater than average risk for intraoperative and postoperative complications. The presence of obstructive sleep apnea in the patient with a jaw deformity should be considered and ruled out (see Chapter 26).
Wound-Healing Risk Factors
Specific patient factors that may impair wound healing include diabetes mellitus, smoking, bisphosphonate treatment for osteoporosis, poor nutritional status, and chronic glucocorticoid exposure. Type II diabetes mellitus is frequently a comorbidity of obesity and increased body mass index. Alpha and colleagues documented a 66% incidence of postoperative infections among diabetic patients undergoing maxillary or mandibular osteotomies, despite adequately controlled glucose levels.9
The inhalation of nicotine via cigarettes affects both pulmonary function and wound healing.72,75,95,264,137,149,176 Smoking or nicotine ingestion has been shown to delay the chondrogenic phase of bone (osteotomy) healing and to decrease the circulation of surgically elevated flaps, thereby resulting in impaired healing after wound closure. Cigarette smoke contains nicotine, carbon monoxide, and hydrogen cyanide; each of these chemicals has been shown to impair wound healing by producing relative tissue hypoxia. Nicotine is a vasoconstrictor that diminishes tissue oxygenation, predisposes the individual to microvessel thrombosis (i.e., increased platelet adhesion and direct endothelial cell damage), and diminishes cell proliferation and function. Carbon monoxide reduces oxygen-carrying capacity, and hydrogen cyanide inhibits oxidative metabolism and oxygen transport. These consequences of cigarette smoke tissue ischemia are most evident during healing after surgical procedures, when tissue circulation is at risk for interruption (e.g., after microvascular tissue transfer, during the placement of free [non-pedicled] bone grafts). A number of clinical studies have documented increased morbidity in patients who smoke and later undergo head and neck reconstructive and aesthetic procedures. To minimize postoperative wound difficulties and anesthesia-related pulmonary complications, patients should be counseled to refrain from smoking for at least 6 to 8 weeks preoperatively and for 2 to 4 weeks postoperatively. A serum or urine nicotine concentration can be obtained before surgery to confirm patient compliance.
It is known that bisphosphonate treatment for osteoporosis can affect osteoclast activity and that it may negatively impact successful bone healing. Current guidelines concerning bisphosphonate treatment continue to evolve and should be followed when planning jaw or dentoalveolar procedures.1,85,125,198,209,214,215,220–222,248,283,285,292,294,357,367
Perioperative Airway Risk Factors
Perioperative risk to the airway and its management should be considered during all phases of surgical care, including before nasotracheal intubation, during surgery, at the time of extubation, and soon after extubation (Fig. 16-1).53,61,76,170,175,250 The basic clinical preoperative airway assessment should include 1) measurements of mandibular range of motion (i.e., vertical opening and protrusion); neck mobility 2) Mallampati classification 3) the ratio of the patient’s height to the thyromental distance 4) neck circumference and 5) body mass index. A Mallampati classification of III or IV or a ratio of height to thyromental distance of more than 23.5 is a strong indicator of a difficult airway with conventional direct laryngoscopy. A neck circumference of more than 17 inches in men is a risk factor for obstructive sleep apnea. Individuals who are scheduled to undergo orthognathic surgery may have special medical risk factors that include baseline malformations or syndromes and associated abnormalities that carry the potential for difficult airway control (see Chapter 11). In the high-risk patient, advance discussion with the anesthesia team to arrange for an anesthesiologist who is experienced with difficult away management and who is skilled with awake fiber-optic intubation may be indicated (see Fig. 16-1) (![]() Video 5). After the nasotracheal tube is in place, the surgeon secures it and assists with its management during the operation (see Chapter 15). Complications associated with endotracheal tubes that are placed for orthognathic procedures have included herniation of the airway tube cuff leading to occlusion of the tube lumen; inadvertent surgical sectioning of the endotracheal tube; and the occurrence of contact granuloma of the vocal cords.62,249 Acute intraoperative or postoperative pulmonary edema, postoperative apnea, pneumomediastinum, and pneumothorax have all been reported as complications associated with anesthesia (see Chapter 11).14,33,34,56,91,117,122,153,217,233,245,259,318,321
Video 5). After the nasotracheal tube is in place, the surgeon secures it and assists with its management during the operation (see Chapter 15). Complications associated with endotracheal tubes that are placed for orthognathic procedures have included herniation of the airway tube cuff leading to occlusion of the tube lumen; inadvertent surgical sectioning of the endotracheal tube; and the occurrence of contact granuloma of the vocal cords.62,249 Acute intraoperative or postoperative pulmonary edema, postoperative apnea, pneumomediastinum, and pneumothorax have all been reported as complications associated with anesthesia (see Chapter 11).14,33,34,56,91,117,122,153,217,233,245,259,318,321
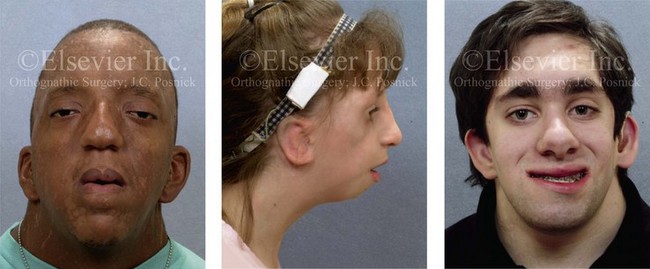
Figure 16-1 Perioperative risk to the airway and its management should be considered during all phases of orthognathic surgery, including before nasotracheal intubation, during surgery, at the time of extubation, and soon after extubation. Individuals who are scheduled for orthognathic surgery and who are recognized to be at high risk for airway compromise should be reviewed in advance with the anesthesia team to arrange for special needs. Three patient examples in which these issues came into play are shown, including an adult with Noonan syndrome, a teenage girl with Treacher Collins syndrome, and a teenager with Klippel–Feil anomaly. Each of these patients also has the potential for cervical spine anomalies with limited neck range of motion. The risk for spinal cord injury should also be evaluated before orthognathic surgery.
Malignant hyperpyrexia involves an abnormality of muscle metabolism of genetic origin that manifests as widespread skeletal muscle contraction, hypercatabolism, and hyperthermia upon exposure to volatile anesthetic agents or succinylcholine.192,223,365 It is a serious condition that requires prompt diagnosis and aggressive management. It may occur during an orthognathic procedure with a typical initial intraoperative presentation of tachycardia and noticeably warm skin.
Decisions about the timing of extubation after surgery should be made by clinicians on the basis of extubation criteria and patient-specific risk factors.159,376 In general, the surgeon is present at the time of extubation in case either emergency tracheostomy or reintubation is required. Our preference is to extubate the patient in the operating room when feasible but only when specific parameters are met. For the patient with an ongoing difficult airway, a more delayed approach to extubation maybe taken. The risk of inadequate ventilation after extubation is highest when pre-extubation parameters are not adequately met and when excess sedation remains (see Chapter 11).
Spontaneous leg compartment syndrome has been reported as a complication after orthognathic surgery.204 The occurrence is thought to be the result of a drug interaction between anesthetic or postoperative medications or the effect of antidepressive drugs that are superimposed on chronic mild exercise-induced compartment syndrome.
Death is recognized as an exceedingly rare complication related to orthognathic procedures. Of the numerous large case series published, only one death has been described; this was reported by Van de Perre and colleagues from a series of 2049 patients.210 It occurred in a 17-year-old male patient who was described as being slightly mentally challenged and who underwent bimaxillary osteotomies. Six hours postoperatively, a cardiac arrest occurred, and attempts to resuscitate the patient failed. The cause was thought to be preexisting cardiomyopathy. A death was also reported by Waack and colleagues as part of a limited series of 63 consecutive Le Fort I osteotomies.216 The death occurred in a healthy 15-year-old female patient on the first postoperative day as a result of unknown causes.
Cervical Spine Risk Factors
As part of the presurgical assessment, the evaluation of the patient’s cervical spine is essential. Congenital malformations, previous trauma, and arthritis are occasional etiologies of cervical spine problems in the orthognathic patient. Patients with specific syndromes (e.g., Klippel–Feil anomaly, hemifacial microsomia, Treacher Collins syndrome, Apert syndrome) are at higher risk for a cervical spine malformation that may be undiagnosed at presentation to the orthognathic surgeon (see Fig. 16-1). Referral for evaluation by an orthopedic spine surgeon, a neurosurgeon and a geneticist as well as appropriate radiographic studies (e.g., magnetic resonance imaging, computed tomography) are carried out when indicated. Controlled positioning with limited flexion, extension, and rotation at the time of intubation, intraoperatively, and during recovery may be required. In some cases, intraoperative neuro-monitoring (e.g., motor and sensory) is useful to confirm minimal spinal compression during surgery.
Psychosocial Risk Factors
To minimize patient and family disappointment after surgery, an informal (or, in some cases, a more rigorous) psychosocial assessment is conducted.24,36,73,81,167,304,317 All parties involved must accept the risks and limitations of the planned surgical procedures. Unfortunately, neither the preoperative psychosocial assessment nor the individual’s biologic wound-healing ability is completely predictable. A small percentage of patients will perceive an unfavorable surgical outcome, even when the clinicians feel that the results are at least satisfactory. Others will not accept a complication, even though the risks were fully explained in advance and all precautions were taken.
Body dysmorphic disorder is a medical condition that can be difficult to recognize before surgery but that will routinely result in postoperative patient dissatisfaction. It is estimated that 7% to 15% of individuals who pursue cosmetic surgery have body dysmorphic disorder (see Chapter 7).
Comprehensive Dental Rehabilitation Needs
Recognizing when a patient who is seeking orthognathic surgery has dental needs that go beyond standard orthodontics is essential to the achievement of an optimal outcome (see Chapter 5). If complex dental restorations and periodontal management are necessary, then planning should coordinate care for these needs at the beginning of treatment (see Chapter 6). This is especially true for the individual with a cleft lip and palate or a craniofacial syndrome (see Chapters 28 through 34). It is also true for the adult who has suffered dental and periodontal sequelae from an uncorrected dentofacial deformity or previous treatment (e.g., orthodontics, restorative care) (see Chapter 25).
Functional Assessment of Head and Neck Structures
The orthognathic surgeon should evaluate the patient’s baseline head and neck functions, including speech, swallowing, mastication, neck and mandibular range of motion, breathing, hearing, vision, cognition, and psychosocial competence. Any of these functions may be negatively affected by the presenting maxillofacial deformity or malformation and influenced (either favorably or unfavorably) by the treatment being contemplated.94,113,139,163,228,232,311,313,320,377 A candid discussion with the patient and the family about the potential impact of the planned procedures on any of these functions should take place before treatment.
Operating Room Team Preparation
Limiting risks and complications requires an informed patient and family, coordinated collaborative comprehensive clinical care, an experienced and focused surgeon, meticulous planning, a dedicated operating room team, effective postoperative hospital care, and appropriate outpatient management. The operating room team includes the surgeon and his or her assistants, the anesthesiologist and his or her assistants, the operating room personnel (e.g., scrub nurse/technician, circulating nurse), and the full complement of sterilized and organized instrumentation (e.g., inventory, sanitation and sterilization personnel). The instrumentation includes power saws and drills, with their associated bits and blades; handheld instruments; and fixation hardware such as plates and screws. Specific medications, sutures, and dressing materials are also required. The confirmation of the prepared operating room team, equipment, instrumentation, and medications should be undertaken before the patient enters the operating room. The anesthesiologist and the surgeon should discuss the patient’s airway and his or her intraoperative and postoperative needs before entering the operating room (see Chapter 11). After the equipment, instrumentation, and medications are set up and checked and the team is briefed, the patient may enter the operating room with the best opportunity for success.
Temporomandibular Disorders
The effects of orthognathic surgery on temporomandibular joint function are often specific to the patient and cannot be fully predicted.11,82,138,139,156,247 Many clinical studies report improvement in temporomandibular joint symptoms in a majority of patients after successful orthodontics and orthognathic surgery, whereas some of the study patients show deterioration. The favorable effects of orthognathic surgery for patients with preexisting temporomandibular joint symptoms may result from the improved occlusion, the corrected facial height and horizontal projection, and the reduced emotional stress (or the placebo effect). Varied degrees of documented radiographic condylar remodeling after orthodontics and orthognathic surgery have been reported in some patients. Interestingly, TMJ radiographic findings do not necessarily correlate with symptomatic temporomandibular disorders (see Chapters 9 and 37).
Management of Third Molars
Clinical studies have now documented no increased risk of unfavorable or so-called “bad splits” when SROs are performed in patients with impacted third molars as compared with those without third molars.90,218,268,276,348 Doucet and colleagues laid this argument to rest with their prospective cohort study that looked at the effects of the presence or absence of third molars during SRO on 1) the frequency of unfavorable fractures 2) the degree of entrapment of the IAN in the proximal segment and 3) the procedural time required to move the teeth.89 The study included patients who were scheduled to undergo SRO to correct a dentofacial deformity. Study patients were divided into two subgroups: Group I was made up of those with an impacted mandibular wisdom tooth at the time of SRO (n = 331; mean age, 19.6 years); Group II included those without a mandibular wisdom tooth at the time of SRO (n = 346; mean age, 30.4 years). The overall incidence of unfavorable fractures was 3.1% (21 out of 677), with frequencies of 2.4% (8 out of 331) in Group I and 3.8% (13 out of 346) in Group II. Interestingly, the rate of IAN entrapment in the proximal segment was significantly lower in Group I (37.2%) than in Group II (46.5%). The removal of third molars at the time of SRO increased procedural time by only 1.7 minutes.
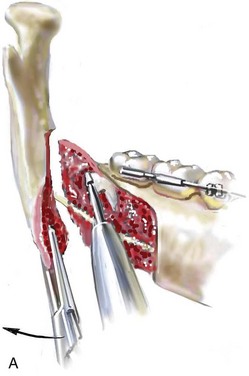
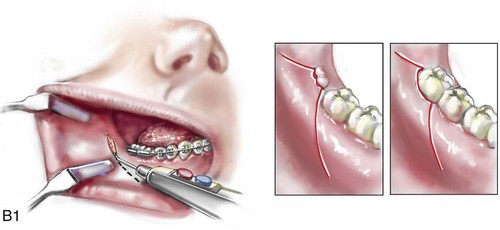
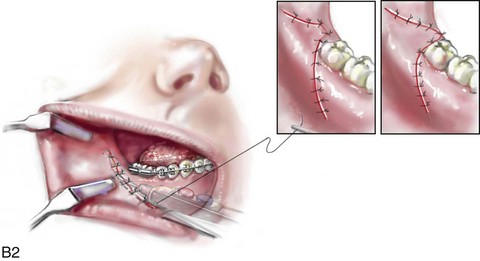
When the removal of erupted or partially erupted mandibular wisdom teeth is carried out at the time of SRO, the standard vestibular incision for SRO is altered to achieve access to the erupted wisdom teeth and then for watertight wound closure (Fig. 16-2, B) (also see Chapter 15). In review, when considering evidence-based medicine, the routine practice of the removal of wisdom teeth before SRO can no longer be justified.
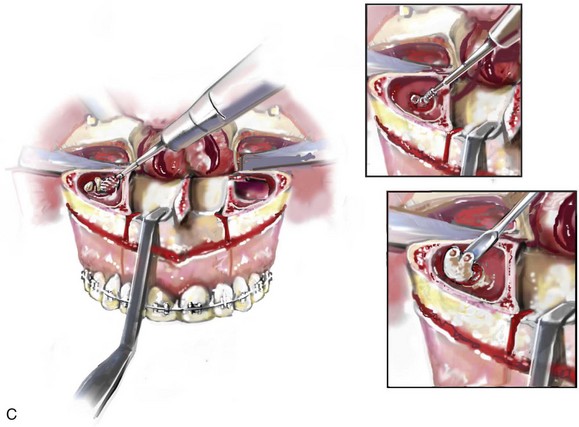
When removing impacted maxillary wisdom teeth, access through the floor of the antrum after down-fracture at the time of Le Fort I osteotomy represents this author’s usual approach (Fig. 16-2, C). The exception is when carrying out segmental osteotomies in a cleft maxilla in conjunction with extensive horizontal advancement of the lesser segment. In these cases, the removal of the wisdom tooth in the lesser segment may significantly diminish bone volume with potential compromise of stabilization and healing.
Fixation Considerations
Need to Remove Plates and Screws
Metal plates and screws have been used to stabilize fractures and osteotomies in the maxillofacial region for more than 40 years.8,9,12,39,46,47,104,131,151,164,225,281,336 The metals that were initially used were stainless steel and vitalium; these were followed by titanium and its alloys.
There is both laboratory and clinical evidence of the tissue compatibility and the high corrosion resistance of titanium and its alloys in the human body. In 1991, the Strasbourg Osteosynthesis Research Group recommended that “the removal of a non-functional plate is desirable provided that the procedure does not cause undue risk to the patient.”32A,355A During earlier years, those who were advocating the routine removal of fixation devices after stable bone union felt that the implant had significant potential to cause problems, so its removal was considered preventative. Today, clinicians agree that the medical risk and financial costs incurred by the routine removal of asymptomatic fixation hardware cannot be justified. It is currently standard practice to remove fixation plates and screws only when clinically indicated.
Becelli and colleagues completed a retrospective analysis of complications of bicortical screws used for sagittal split ramus osteotomy (SSRO) fixation.22 The authors reviewed a consecutive series of patients (N = 241) who were undergoing sagittal ramus osteotomies (N = 482) for skeletal Class III deformities. Complications related to bicortical titanium screws were observed at 3% of the osteotomy sites (Fig. 16-3). The hardware was eventually removed in these patients (15 out of 482), without long-term consequences.
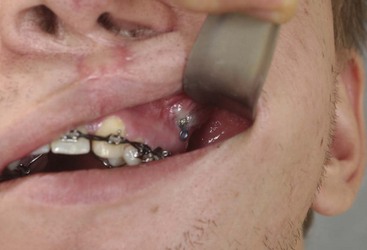
Figure 16-3 An 18-year-old with a bilateral cleft lip and palate jaw deformity underwent cleft–orthognathic surgery including maxillary Le Fort I osteotomy in three segments and interpositional iliac bone grafting. Healing was uncomplicated, with the achievement of the planned improvements. One year after surgery, the patient returned with a complaint of a painless ridge in the left buccal vestibule of the maxilla. Examination confirmed an exposed titanium plate and screw. There were no associated symptoms or signs of infection. During an office procedure, the exposed plate was sectioned with a wire cutter and removed. Healing was uneventful.
Use of Titanium versus Resorbable Fixation
The use of self-reinforced biodegradable bone plates and screws has been advocated for use in orthognathic surgery by a limited number of surgeons throughout the world.92,96,127,130,155,165,178,297,325,344,345 Although this type of fixation is appealing in theory, a greater incidence of postoperative complications has been found with resorbable fixation devices as compared with titanium. Furthermore, no advantages in the achievement of improved skeletal stability have been documented with the use of biodegradable hardware.
Turvey and colleagues reported about 70 patients who were undergoing 194 osteotomies of the maxilla or the mandible and who were stabilized with self-reinforced polylactate bone plates or screws of similar size and configuration to those of titanium systems.344 The placement of the devices was accomplished transorally and transfacially in a way that was consistent with the osteotomy location. Maxillomandibular elastics were used for each patient after surgery to control the position of the jaws. Three out of the 70 patients (4%) experienced immediate postoperative loosening of the resorbable bone screws. Each of the patients required a return to the operating room for more rigid stabilization at the involved osteotomy site. Although all patients eventually achieved acceptable occlusion, a number of fixation sequelae occurred during the healing phase, including prolonged mobility of the maxilla; sterile abscess requiring debridement; swelling with sterile fistula tract formation; and intranasal inflammation requiring fixation removal at the time of secondary septoplasty. Although the authors demonstrated that biodegradable plates and screws could be used for orthognathic surgery, there was no advantage when this was compared with titanium fixation devices, and the complication rate is increased.
In a second study, Turvey and colleagues reported about 69 patients who were undergoing sagittal split ramus osteotomies for mandibular advancement.345 Thirty-four of these patients underwent fixation with self-reinforced biodegradable screws, whereas 35 patients underwent fixation with 2-mm titanium screws. Postoperatively, one of the patients with biodegradable screws required a return to the operating room for improved fixation. Again, no advantage could be demonstrated with the use of biodegradable fixation, whereas a degree of diminished levels of stabilization and increased complications were shown to be present.
Ahn and colleagues completed a study to evaluate the complications of resorbable versus nonresorbable plate and screw fixation in orthognathic surgery.3 Patients were enrolled in the prospective study (N = 272). Titanium plates and screws were used at the osteotomy sites in Group I (N = 152), and resorbable plates and screws were used at the osteotomy sites in Group II (N = 120). In the 152 patients with titanium plates and screws, there was a complication rate of 8.6% (N = 13). Of the 120 patients with resorbable plates and screws, 18.3% (N = 22) developed complications. There was a greater degree of postoperative infections and malocclusions and a trend toward relapse observed in patients with resorbable plate and screw fixation. Three of the patients with resorbable plate and screw fixation required reoperation for residual malocclusion as compared with zero in the titanium group.
Infection Associated with Orthognathic Procedures
Infection after orthognathic surgery is considered by most to be uncommon. However, a review of the literature indicates a wide range in the reported incidence of this complication.* A critical review of these studies provides insight into the true incidence of infection, clinical pitfalls to consider, and methods to use to best limit these sequelae.
All transoral wounds must be considered “clean-contaminated wounds,” with an anticipated theoretical infection rate of as high as 15%.352 It is said that this incidence of infection in a clean-contaminated site can be reduced to as low as 1% with good surgical technique and the appropriate use of prophylactic antibiotics. The question remains of how to best provide this type of antibiotic coverage for the orthognathic surgery patient. According to the infectious disease literature, antibiotic prophylaxis should provide adequate drug levels in the tissue before, during, and for the shortest possible time after surgery to provide a reduced infection rate; be an effective agent against the bacteria that are most likely to cause the infection; and be bactericidal while at the same time being the least toxic agent available.
Spaey and colleagues reported a 4% prevalence rate of infection after orthognathic procedures in their cohort.312 Among the infections that were sustained, 92% occurred at the SSRO site, 1% occurred at the Le Fort I osteotomy site, and only 0.5% involved the chin osteotomy location (Figs. 16-4 through 16-7). In their pilot study group, after completion of an SSRO, the authors placed a drain through the mandibular vestibule mucosa incision; this was removed the following morning. The prophylactic antibiotic regimen that was initially used did not have a drug level in place while the drain remained in or after its removal. Because of the high infection rate observed in the pilot study, the researchers changed the protocol to no longer involve the placement of a drain; to achieve a more meticulous mucosal wound closure; and to extend the time of prophylactic antibiotic use to 5 days after surgery. As a result, the infection rate at the SSRO site was dramatically decreased. The authors concluded that, in addition to an extended use of prophylactic antibiotics, the achievement of a sealed vestibular wound at the SSRO is essential to limit the infection rate.
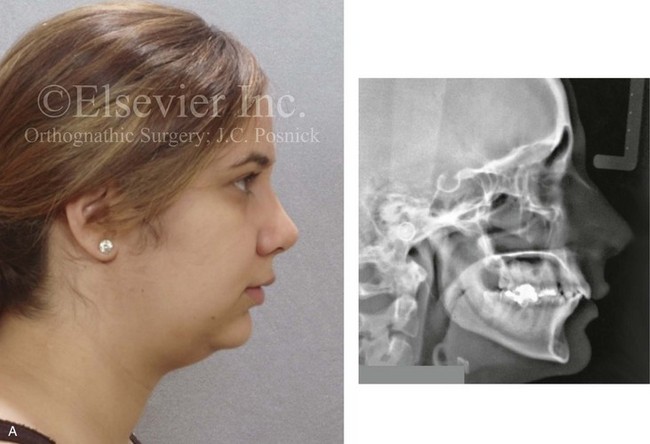

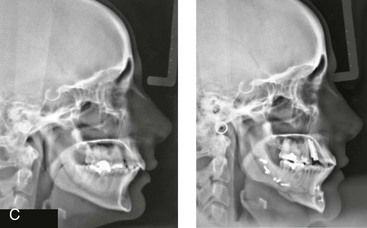
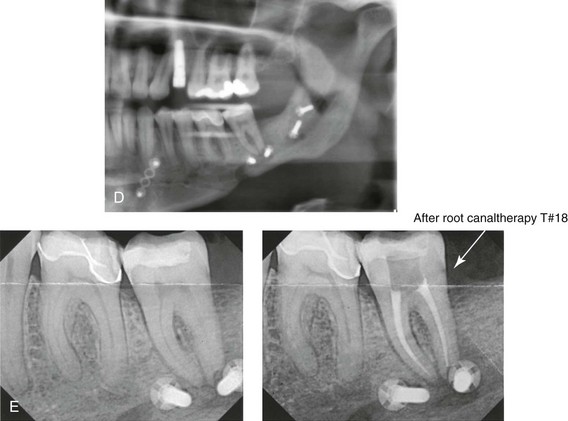
Figure 16-4 A woman in her early 30s with a primary mandibular deficiency growth pattern underwent a comprehensive orthodontic and surgical correction. This required the removal of dental compensation, including opening upper bicuspid extraction spaces for dental implants. Surgery included bilateral sagittal split ramus osteotomies, an osseous genioplasty, and anterior neck soft-tissue procedures. In-hospital and early at-home convalescence proceeded without incident. Five weeks after surgery, the patient returned to a more normal diet, to regular physical activities, and to her orthodontist’s care. Two months after surgery, pain in the left mandibular second molar region with chewing was persistent. The tooth was also tender to percussion. A periapical radiograph revealed the proximity of a bicortical fixation screw to the distal root, with possible apical root resorption. The removal of the involved screw was offered. However, the patient chose root canal therapy followed by the relief of pain and the resolution of percussion tenderness. A, Profile and lateral cephalometric views before treatment. B, Oblique facial views before and after treatment. C, Pretreatment and posttreatment lateral cephalometric radiographs. D, Left side Panorex image 2 months after surgery, with left mandibular second molar pain with chewing. E, A periapical radiograph is shown before and after successful root canal therapy (see Fig. 19-8).
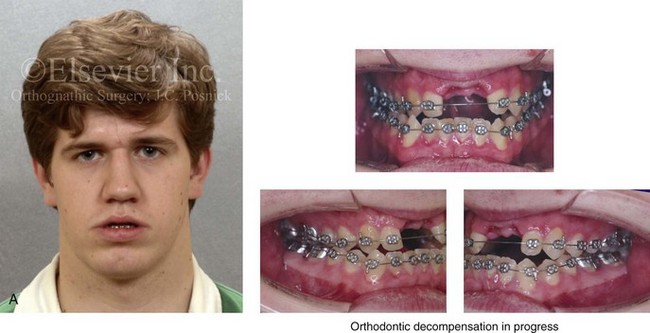
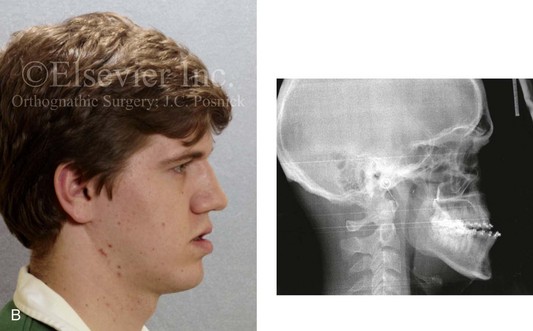
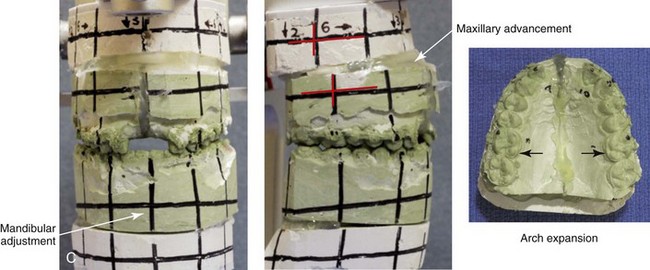
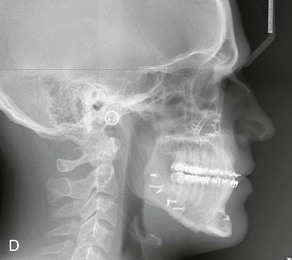
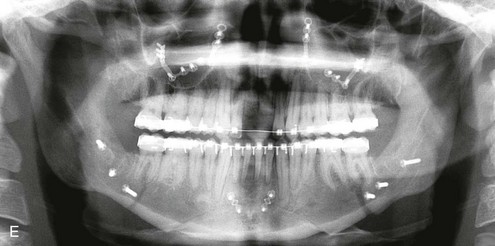
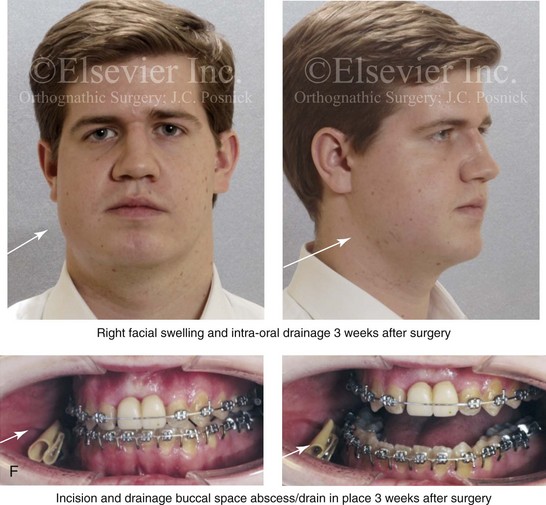
Figure 16-5 A 23-year-old man with a developmental jaw deformity and malocclusion was referred for surgical evaluation. He had also recently sustained trauma to the maxillary anterior region that involved a loss of the central incisors. The jaw deformity was characterized by maxillary deficiency in combination with relative mandibular excess. The patient agreed to a comprehensive surgical and dental rehabilitative approach. He also had lifelong difficulty breathing through his nose. After periodontal and restorative evaluations, the patient underwent orthodontic (dental) decompensation. Surgery included maxillary Le Fort I osteotomy in segments (arch expansion, horizontal advancement, and cant correction); bilateral sagittal split ramus osteotomies (asymmetry correction); osseous genioplasty (horizontal advancement); and septoplasty and inferior turbinate reduction. In-hospital and initial at-home convalescence proceeded without difficulty. Three weeks after surgery, increased swelling and tenderness along the right ramus region were appreciated, and intraoral drainage through the lateral ramus incision was recognized. The patient underwent exploration with Penrose drain placement as an office procedure under local anesthesia, and he was restarted on antibiotics. The drain was removed 1 week later, and the 10-day course of oral antibiotics was completed. Healing at all osteotomy sites remained on track. Five weeks after surgery, the patient returned to a more regular diet and physical activity without delay. The orthodontic appliances were removed 6 months after surgery. A, Presurgical facial and occlusal views with orthodontics in progress. B, Profile and lateral cephalometric views before surgery. C, Articulated dental casts that indicate analytic model planning. D, Three week postoperative lateral cephalometric radiograph. E, Three week postoperative Panorex radiograph. F, Facial and occlusal views 3 weeks after surgery at the time of persistent right facial swelling and intraoral drainage. The patient underwent exploration and Penrose drain placement.
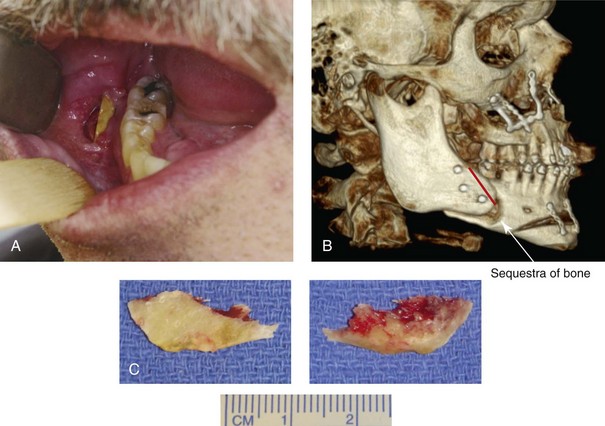
Figure 16-6 A 50-year-old man with a short face growth pattern and obstructive sleep apnea underwent orthognathic surgery, including maxillary Le Fort I osteotomy; bilateral sagittal split osteotomies of the mandible; and an osseous genioplasty. Interpositional allogenic grafting was required in the maxilla and chin regions. At the 2-week postoperative visit, partial dehiscence of the right mandibular vestibular wound was recognized. The distal superior aspect of the proximal segment was visualized through the wound. There was minimal associated facial swelling and no abscess or fluid collection. In the office setting, a rongeur was used to remove the exposed bone, and the patient was restarted on antibiotics. The wound spontaneously closed over the next 10 days. Healing at the osteotomy site remained on track, and there was no long-term sequelae. (Note: If the inferior borders had been more closely aligned before the bicortical screw fixation, then dehiscence with the loss of the bone fragment may not have occurred.) A, Intraoral view of the right mandibular vestibular wound at 10 days after surgery with dehiscence and exposure of the superior aspect of the proximal segment. B, Computed tomography scan demonstrating the portion of the buccal shelf and the proximal segment that was resected with the rongeur. C, Specimen removed with a rongeur.
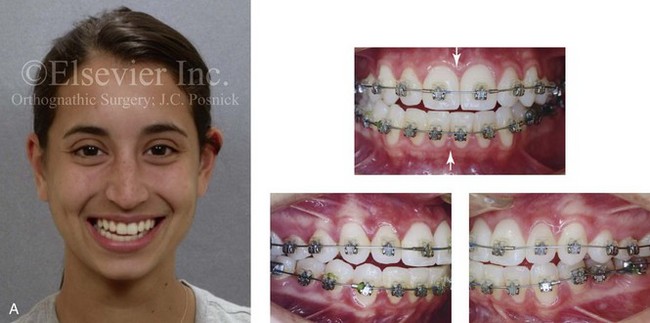


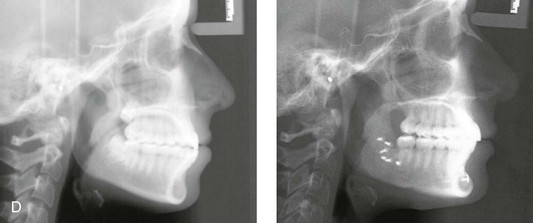
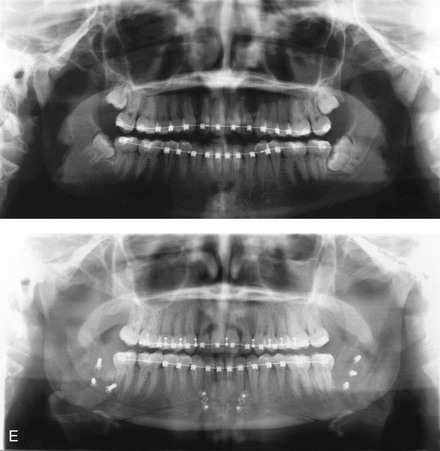
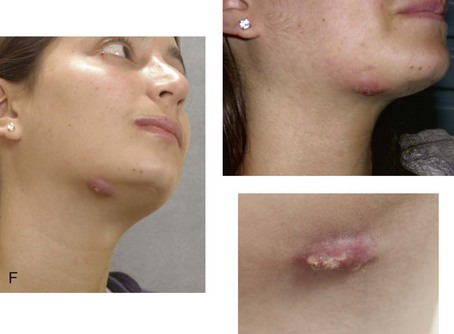
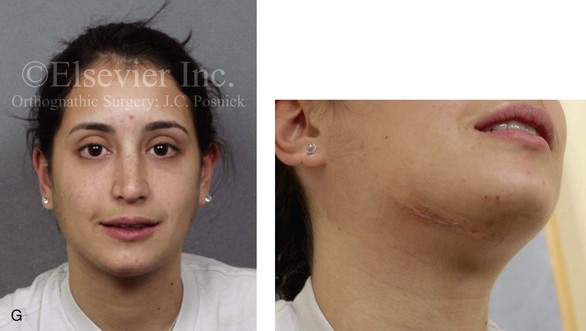
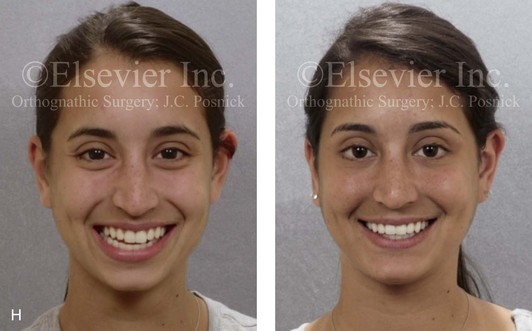
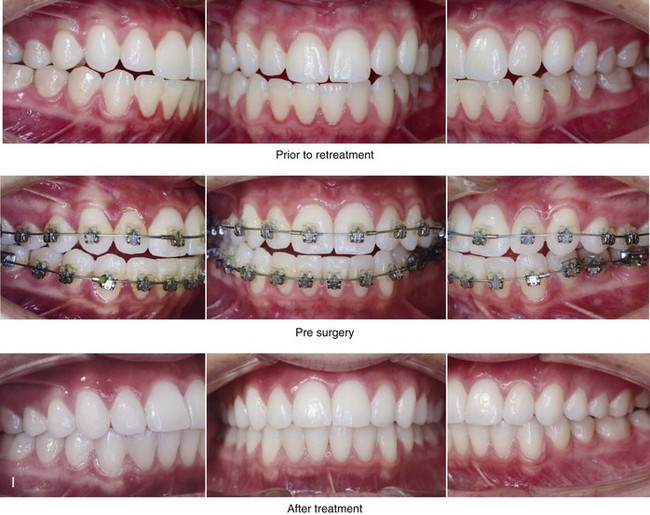
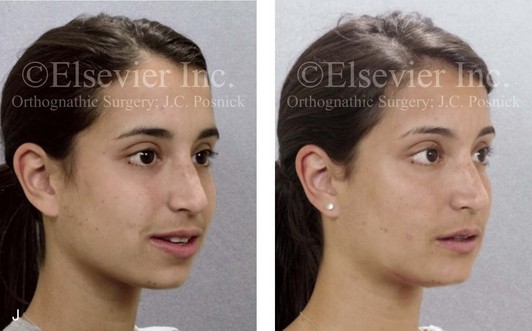
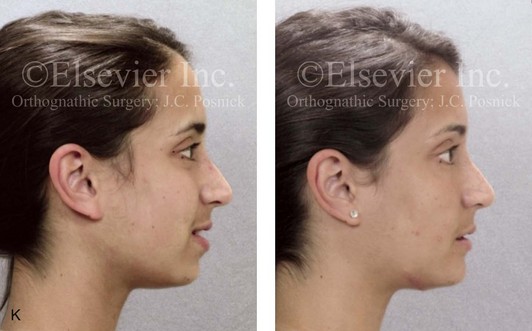
Figure 16-7 A 20-year-old college student with an asymmetric mandibular excess growth pattern (Hemi-mandibular elongation) arrived for surgical evaluation. She had previously undergone orthodontic attempts to neutralize the occlusion without success. She agreed to a combined orthodontic and surgical approach. With the removal of orthodontic (dental) compensation, surgery was carried out. The procedures included bilateral sagittal split ramus osteotomies (asymmetry correction) and osseous genioplasty (horizontal advancement). By 5 weeks after surgery, postoperative swelling had generally subsided. A painless “lymph node” was palpable in the right submandibular region. It was not fixed to the skin or bone. Three months after surgery, what appeared to be a localized lymph node became tender with erythema and then with drainage through the skin. Cultures were carried out and showed no specific growth. An infectious disease consult was obtained, and Gram staining showed gram-positive rods. The patient was placed on amoxicillin and then Augmentin. The localized infection involved intermittent drainage but without a connection down to the bone. Four months after surgery, the patient agreed to undergo excision of the soft-tissue mass with primary closure and the reinstituting of antibiotics. Histopathology was unremarkable, and the cultures remained negative. The wound healed without complications. A, Facial and occlusal views with orthodontics in progress. B, Articulated dental casts that indicate analytic model planning. C, Facial views with smile before and 5 weeks after surgery. D, Lateral cephalometric radiographs before and 5 weeks after surgery. E, Panorex radiographs before and 5 weeks after surgery. F, Right submandibular localized infection demonstrated by 3 months after surgery. G, Four months after surgery, the soft-tissue infection was excised and sent to the pathology and bacteriology departments. H, Frontal views with smile before and after successful treatment. I, Occlusal views before retreatment, just before surgery, and then after successful treatment. J, Right oblique facial views before and after successful treatment. K, Right profile views before and after successful treatment.
In a randomized, double-blind study, Ruggles and colleagues tested two different antibiotic regimens in patients undergoing orthognathic surgery.284 Both groups received preoperative and intraoperative antibiotics, whereas only one group continued 48 hours of postoperative coverage. The group that received no postoperative antibiotics had a 15% infection rate, whereas no infections were found in the group with extended antibiotic coverage.
Danda and colleagues completed a prospective study to evaluate the use of prophylactic antibiotics among patients who were undergoing orthognathic surgery.78 The study patients (N = 150) were divided into two groups. Group I (N = 75) received a single dose of antibiotic prophylaxis. Group II (N = 75) received a full day of antibiotic prophylaxis. In Group I, 9.3% of patients (N = 7) developed infection, whereas only 2.6% (N = 2) of Group II patients did. The results indicate a clinically significant increased rate of infection with single-dose antibiotic prophylaxis as compared with a full day of antibiotic prophylaxis.
Kublefelt and colleagues reported on smoking as a significant risk factor for infection after orthognathic surgery.176A This was a retrospective cohort study of individuals (n = 286) who were undergoing one-jaw orthognathic surgery (i.e., bilateral SSRO [BSSRO] or Le Fort I osteotomy) during a 7-year period. All patients received a 1-week course of antibiotics. Patients ranged in age from 17 to 56.5 years (mean, 34.8 years). The overall infection rate was 9.1%. The only statistically significant identified risk factor for infection in these study groups was smoking history. The incidence of infection was 14.4% among smokers and 7.0% among non-smokers. The site of infection was primarily the ramus region followed by the Le Fort I osteotomy region.
Interestingly, six cases of actinomycosis after orthognathic procedures have been reported.216,293 Ozaki and colleagues reported a case of actinomycosis that occurred in the left submandibular area after SSRO.249 The infection resolved after 2 months of antibiotic therapy.
Instrument Breakage: Foreign-Body Reaction
Manikandban and colleagues reviewed the incidence and consequence of bur breakage within the surgical wound during orthognathic surgery.210 The authors found that surgical bur breakage was not uncommon (5 out of 76 patients; 6.6%). They concluded that bur retrieval was not always advisable, because collateral damage to the hard and soft tissues may occur in the process. They recommended radiographic confirmation immediately after surgery and then clinical follow up for at least 1 year to confirm that no foreign body reaction or infection has occurred (Fig. 16-8).
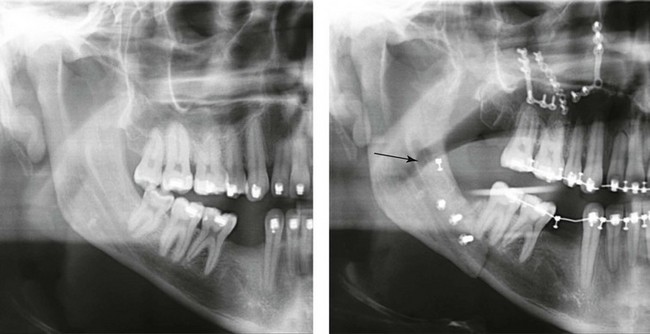
Figure 16-8 Individuals who are undergoing orthognathic surgery may experience intraoperative bur breakage. It is also not uncommon for orthodontic brackets, hooks, or ligatures to loosen with the potential for loss in the surgical field. Because of the small size of the lost objects within deep wounds, retrieval is not always advisable or possible. These foreign bodies can be visualized radiographically. Clinical follow up for at least 1 year is recommended to confirm that no foreign body reaction or infection has occurred. A Panorex radiograph is shown before and after orthognathic surgery. An orthodontic hook was lost in the right mandibular vestibular wound and can be seen on the Panorex radiograph. No negative sequelae resulted.
Risks and Complications Specific to Sagittal Split Ramus Osteotomies of the Mandible
Unfavorable Sagittal Split Ramus Osteotomy
When completing an SRO, an unfavorable fracture (i.e., a “bad split”) can occur.23,42,139,162,206,243,334 The definition of a bad split varies from clinician to clinician. This author reserves the term bad split only for those SROs in which the condyle either remains with the distal segment, thus requiring a separate osteotomy (i.e., third piece), or shatters into a separate component (i.e., third piece) on its own (Fig. 16-9). Other less than ideal splits may include the following: 1) a separate fracture or piece of the buccal plate 2) a separate fracture or piece of the lingual plate posterior to the second molar; or 3) a separate fracture or piece of the coronoid process. All three of these splits are generally manageable without alteration of the expected postoperative recovery. When the buccal plate separates from the proximal segment, the fragment is either removed or additional plate and screw fixation is used to secure it. When the lingual plate separates from the distal segment, no additional fixation is generally required. The lingual bone fragment is either removed or left in place. When the coronoid process separates from the proximal segment, it is usually removed to limit the chance of ankylosis during the healing process.
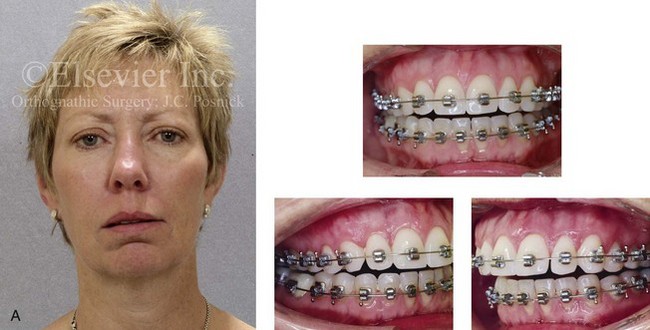
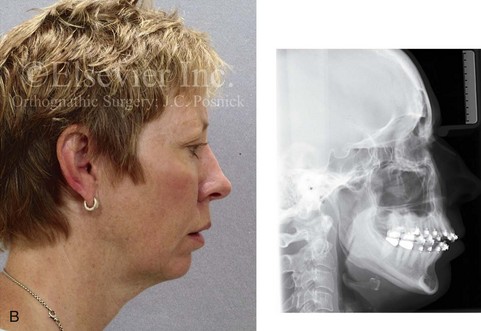
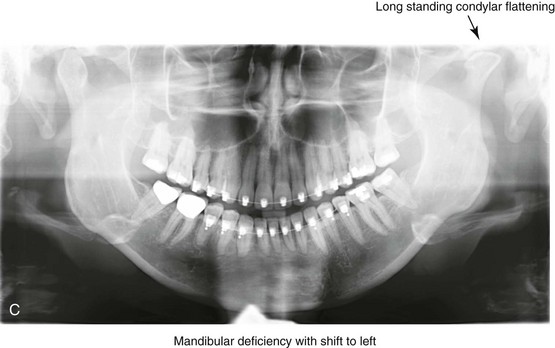
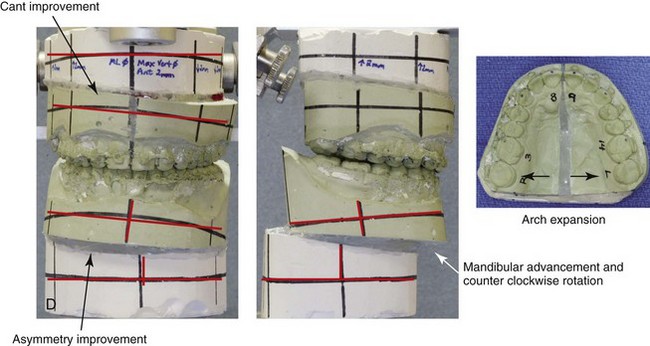
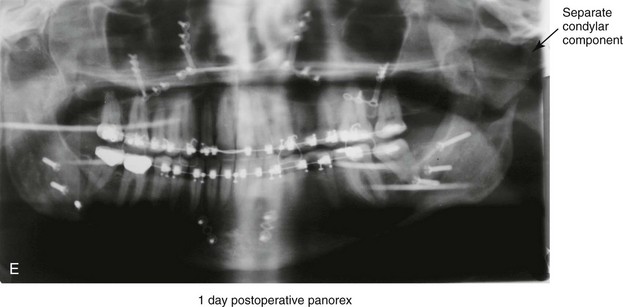
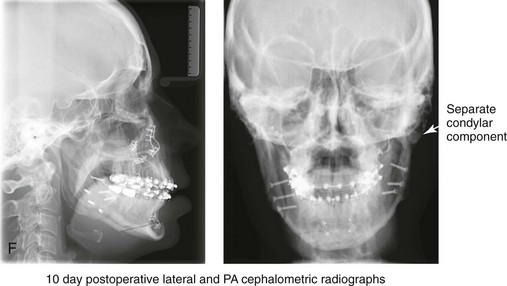
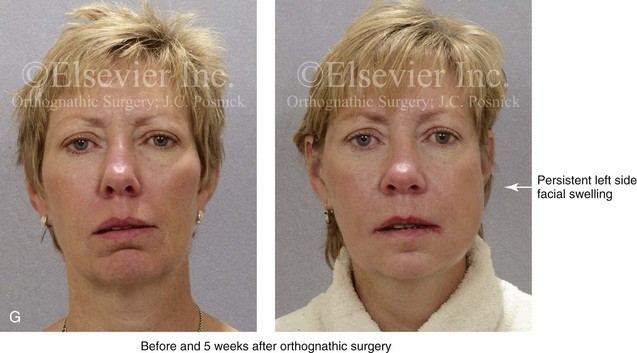
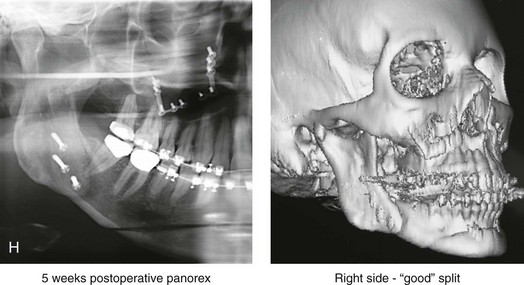
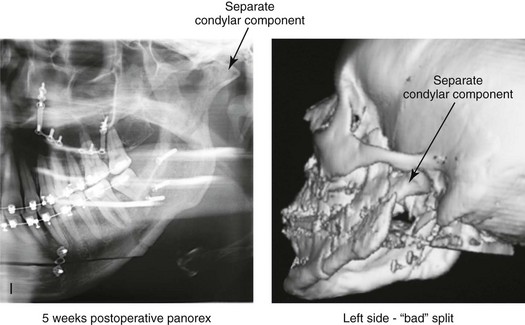
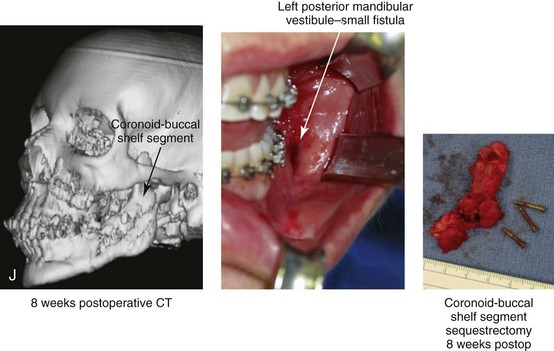
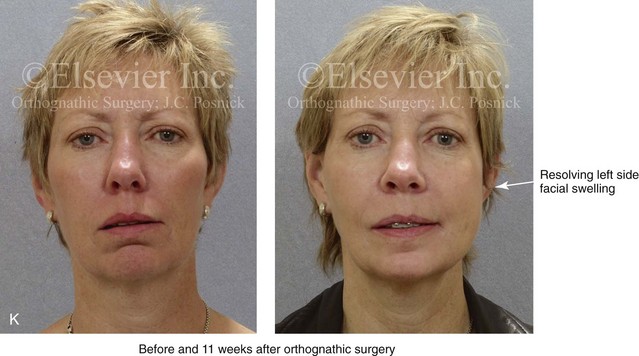
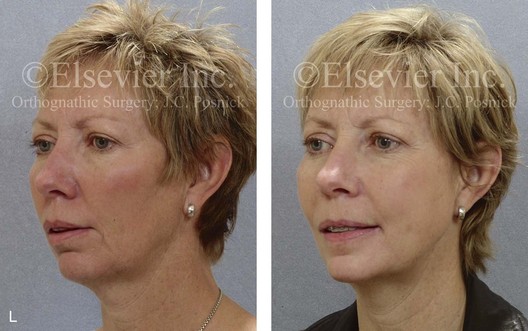
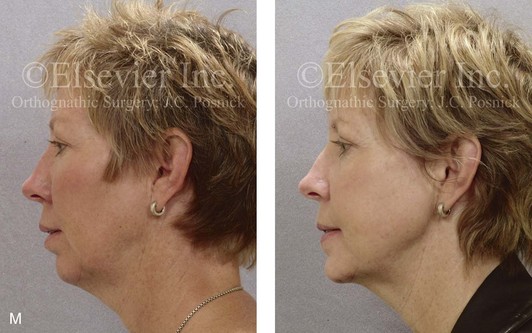
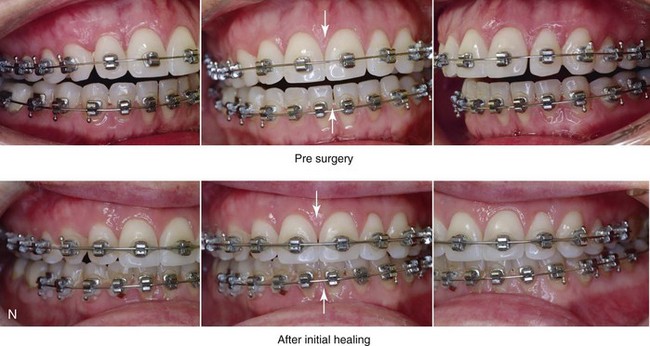
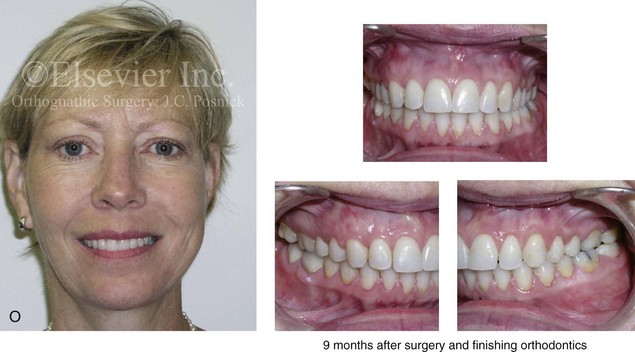
Figure 16-9 A woman in her mid 40s was referred for the surgical evaluation of asymmetric mandibular deficiency with secondary deformities of the maxilla. A longstanding flattening of the left condylar head with a loss of posterior facial height was felt to be responsible. There were no current symptoms of temporomandibular disorders, and the condyle was felt to be stable. The patient had an asymmetric Class II excess overjet anterior open-bite malocclusion. An orthodontic and surgical approach was selected. With orthodontic (dental) decompensation complete, surgery included maxillary Le Fort I osteotomy in segments (arch expansion and cant improvement); bilateral sagittal split ramus osteotomies (minimal horizontal advancement and asymmetry improvement); osseous genioplasty (horizontal advancement); redo septoplasty; and neck liposuction. During surgery, a “bad split” was appreciated on the left side. Nevertheless, the ramus osteotomy was secured with three bicortical screws. Radiographs the day after surgery confirmed a separate condylar component in the left ramus region. Posteroanterior and lateral cephalometric and Panorex radiographs were again taken 10 days after surgery, and these were suggestive of adequate segment approximation. At 5 weeks after surgery, left lateral mandibular facial swelling with mild tenderness persisted. At 8 weeks after surgery, a left posterior mandibular vestibular fistula was appreciated. In the operating room, the wound was opened to remove a buccal shelf and coronoid fragment as well as the fixation screws. Cultures confirmed Escherichia coli, Enterobacter, alpha-hemolytic Streptococcus, and Candida. Oral antibiotics were initiated, including clindamycin, ciprofloxacin, and Diflucan. At 11 weeks after surgery, there was a functional occlusion with good vertical mouth opening and a mild shift to the left. At 9 months after surgery, facial morphology and occlusion remained acceptable, and the orthodontic appliances were removed. A, Facial and occlusal views with orthodontics in progress. B, Profile and lateral cephalometric views before surgery. C, Panorex radiograph before surgery that indicates longstanding left condylar head flattening with an intact cortical rim. There is mandibular deficiency and a shift to the left. D, Articulated dental casts that indicate analytic model planning. E, Panorex radiograph 1 day after surgery suggests a separate condylar component from the left ramus. F, Lateral and posteroanterior cephalometric radiographs taken 10 days after surgery indicate the same. G, Facial views before and 5 weeks after surgery. Persistent left side facial swelling is appreciated. H, Panorex and computed tomography scan views of the right ramus 5 weeks after surgery. I, Panorex and computed tomography scan views of the left ramus region 5 weeks after surgery confirm a “bad split” that demonstrates the expected anatomy. J, Left ramus computed tomography scan, left intraoral vestibular view, and sequestrectomy specimen with removed fixation screws. K, Frontal views in repose before and 11 weeks after surgery. L, Left facial oblique views before and then 11 weeks after surgery. M, Left profile views before and 11 weeks after surgery. N, Occlusal views before surgery and then 11 weeks postoperatively. O, Nine months after the completion of treatment, frontal facial and occlusal views demonstrate the results.
When the condyle separates as a third piece, intraoperative decisions will affect the postoperative recovery and occlusion (see Fig. 16-9). From the perspective of the occlusion, if the condyle or the medial pole remains with the distal segment, it will be as if no osteotomy on the ipsilateral side of the mandible was carried out. If no further action is taken, an intraoperative malocclusion should be recognized. To fully complete the ramus osteotomy, the surgeon must then separate (i.e., osteotomize) the condyle from the distal segment (i.e., create a third piece). The osteotomy should be carried out, with as much ramus left on the condylar segment (i.e., the third piece) as possible. Even when this type of bad split occurs, unless significant mandibular advancement is required for the correction of the jaw deformity, the successful healing of the segments will generally occur (see Figs. 16-9 and 16-10).
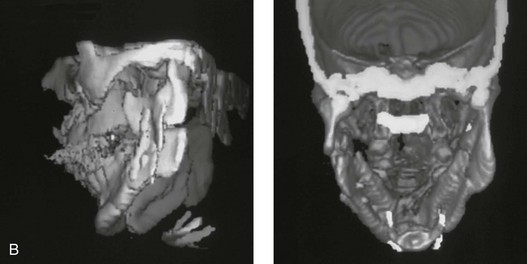
Figure 16-10 A teenager with a cleft jaw deformity underwent orthognathic surgery that included a maxillary Le Fort I osteotomy in segments; bilateral sagittal split osteotomies of the mandible; and an osseous genioplasty. The sagittal split on the left side separated unfavorably. The proximal segment includes a condyle coronoid upper ramus component; an angle ramus component; and a buccal shelf component. The three components of the proximal segment were secured together and fixed to the distal segment with a combination of titanium plates and screws. Fortunately, the reduction was adequate, so healing occurred with favorable facial aesthetics, occlusion, and temporomandibular joint function. A and B, Three-dimensional computed tomography scan views of a “bad split” fixed into acceptable alignment during orthognathic surgery.
If the condyle separates (i.e., if a third piece is created) “high up,” the result is similar to that of a high condylar neck fracture. There will not be a stable posterior stop unless the surgeon is able to carry out plate and screw fixation of the condylar component (i.e., the third piece) to the rest of the proximal segment. This may not be practical, and it will depend on the morphology of the condylar piece. The incidence of postoperative malocclusion will also be dependent on the directional change planned for the distal mandible. If the mandible is to be advanced a significant amount, there is a greater probability that the minimally stabilized condylar segment (i.e., the third piece) will not heal in an ideal location relative to the residual proximal and distal segments. Alternatively, if the mandible is to remain relatively neutral or is set back, there is a higher probability that the condylar segment will heal to the residual proximal and distal segments in a favorable way (see Fig. 16-9). As long as healing is associated with adequate mobility (i.e., mouth opening), then any residual malocclusion can typically be managed 6 to 12 months later with redo SROs, if desired (see Chapter 35).
The occurrence of a bad split is greatly reduced by keeping the medial osteotomy “short (not to far back) and low” (close to the occlusal surface of the mandibular molars) (Fig. 16-11). Using this technique a bad split as described should be a rare event (see Chapter 15).
Figure 16-11 Illustration of this author’s preferred “short and low” medial osteotomy of the sagittal split to minimize an incidence of a “bad split.”
Veras and colleagues evaluated risk factors and the functional and radiographic long-term results of a bad split.350 They also completed detailed temporomandibular joint examinations on patients with bad splits. Interestingly, they did not find the simultaneous extraction of a mandibular third molar in conjunction with an SSRO to be a risk factor for the development of a bad split. This is consistent with the research reported by Kriwalsky and colleagues,174 Precious and colleagues,268 and others (see discussion earlier in this chapter).
Inferior Alveolar Nerve and Mental Nerve Injury
Facial sensibility of the area affected by either an SSRO or a chin osteotomy occurs through the mandibular nerve, which enters the mandibular foramen at the medial surface of the ramus.* This area is adjacent to and in front of the inferior alveolar artery and vein and just inferior to the occlusal plane of the mandibular molar teeth. The mandibular nerve becomes the IAN, and it runs with its vessels within the canal and supplies sensation to the three molars and the two premolar teeth. It then divides into two terminal branches. The incisive branch supplies sensation to the canine and incisor teeth, whereas the mental nerve exits from the foramen to supply sensibility to the chin, the lower lip mucosa and skin, and the adjacent gingiva. Damage to the IAN during either the ramus osteotomies or when completing an oblique osteotomy of the chin may occur. During SSRO of the mandible, the IAN can be damaged by a bur on a rotary drill, a saw blade on a reciprocating saw, an osteotome, or during the placement of plate and screw fixation (Fig. 16-12).
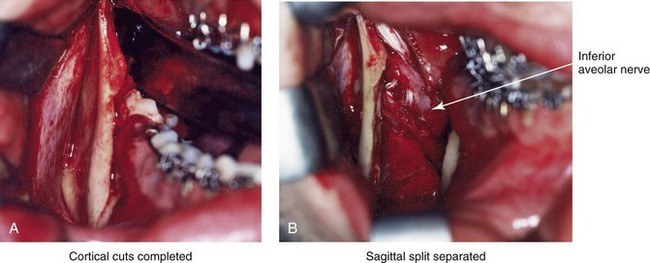
Figure 16-12 Intraoperative views of a right sagittal split ramus osteotomy. The demonstration of cortical cuts completed A, before and B, after the splitting (separation) and mobilization of the segments. The inferior alveolar nerve is visualized in the surgical field.
Posnick and colleagues completed a study to clarify normal chin, lower lip, and gingival sensibility in adolescents and then compared the normal values with sensibility measurements in three distinct groups of adolescents 1 year after these individuals had undergone mandibular osteotomy.261,263 One hundred fifteen subjects (230 mental nerves) were included in the study and divided into four groups. The first group included 67 individuals (Group I: n = 134 nerves; mean age, 18 years) who were undergoing orthodontic treatment; none had undergone orthognathic surgery. This group served as controls to determine the normal sensibility values of the chin, the lower lip, and the gingiva in adolescents. The remaining three groups were made up of adolescents who had previously undergone BSSRO of the mandible (Group II: n = 14 nerves; mean age, 19 years), osteoplastic genioplasty (Group III: n = 40 nerves; mean age, 19 years), or a combination of BSSRO and osteoplastic genioplasty (Group IV: n = 42 nerves; mean age, 19 years). Testing of Groups II, III, and IV was performed 1 year after the patients had undergone the indicated mandibular procedures. At the time of the 1-year postoperative examination, each patient was also asked to detail subjective impressions of current altered sensibility in the mandibular vestibular mucosa, the gingiva, the lower lip, and the chin region. The subjective questioning and objective testing were carried out by an occupational therapist who was trained in facial sensibility testing and familiar with the surgical procedures carried out. Fixed and reproducible coordinates in the area corresponding to the mental nerve were used for objective testing (Fig. 16-13). Three sensory modalities were tested at each coordinate for each involved nerve. Static two-point discrimination was measured with a MacKinnon–Dellon Disk-Criminator (Richardson Productions, Inc, Frankfort, Ill); vibratory thresholds were determined with a biothesiometer; and cutaneous pressure thresholds were measured with a set of Semmes–Weinstein monofilaments. Sensibility values at 1 year after operation for Groups II, III, and IV were compared with the mean normal control values established for Group I. A subjective awareness of alterations in the sensibility of the chin, lip, or gingiva region 1 year after BSSRO was reported in 2 patients in Group II (29%), in 2 patients in Group III (10%), and in 14 patients in Group IV (67%). Interestingly, only 2 of these 18 patients considered the residual sensory loss problematic. The mean objective sensibility values of the three sensory modalities tested were highest among patients from Group IV. Significant differences were found only between the mean two-point discrimination of Group IV patients as compared with the control group in the chin skin area. Thus, 1 year after BSSRO, only 29% of patients had residual subjective awareness of sensory alteration along the distribution of the mental nerve. After an osseous genioplasty, there was only a 10% incidence of long-term subjective diminished sensibility. One year after a combination of BSSRO and osteoplastic genioplasty, 67% of patients experienced varying degrees of residual subjective loss of sensation in the chin and lip region.
Figure 16-13 Illustration of sites for sensibility testing in the inferior alveolar and mental nerve distribution. In a study carried out by Posnick and colleagues, coordinates 6, 8, and 10 located within each of the circled areas were tested bilaterally.
Espelan and colleagues completed a 3-year postoperative survey of patients who had undergone SSRO (N = 583 subjects) and found that that 36.8% of the study patients reported impaired sensation over the long term.96A Westermark and colleagues completed a retrospective study of patients who had undergone SSRO and concluded that nearly 100% of the patients experienced impaired sensation and sensory function.358 This is also consistent with the findings of Essick and colleagues97 and Teerijoki-Oksa and colleagues.332,333 The exact reasons why, over time, most patients experience only a degree of simple loss of sensation—whereas some continue to experience active sensations that are not normally present and an even smaller percentage of those with active sensations subjectively describe them as uncomfortable or painful (dysesthesia)—are not known. Zuniga and colleagues reported a 5% incidence of dysesthesia of the IAN after SSRO.380,381
Turvey reported a 5.5% incidence of IAN lacerations at the time of SSRO.343 Van Merkesteyn and colleagues documented a visible injury in the IAN at the time of SSRO in 7 out of 124 patients (6%).347 The term traumatic neuroma is used to describe the formation of a bulbous mass that develops at the end of a proximal nerve stump after partial or complete nerve transection. The lesion represents an exaggerated relative hyperplasia (i.e., healing) response to a nerve injury. Neuroma symptoms range from paresthesia to severe paroxysmal or persistent pain. Despite the incidence of partial or complete transection of the IAN at the time of SSRO, few cases of traumatic neuroma have been documented. This is likely because the healing transected nerve remains deep in the medullary cavity of the mandible. It is known that a healing nerve stump within bone is rarely painful. The few reported cases of neuroma after SSRO have confirmed the location to be within the soft tissues along the inferior border of the mandible.120,121
Essick and colleagues completed a clinical trial that determined that the magnitude and duration of the patient-reported burden of altered sensation after SSRO was lessened when facial sensory retraining exercises were performed in conjunction with standard mouth-opening exercises as compared with the group of patients who performed only mouth-opening exercises.97 The authors defined the term burden as the person’s reporting of subjective impressions of numbness or unusual sensations (i.e., dysesthesia) in the face, the perioral region, and or oral region. One interpretation of the study findings is that patients who perform facial sensory retraining become more introspective with regard to their altered sensation, which leads to a greater acceptance of their sensory loss by 6 months after surgery. Consistent with this interpretation was the finding that sensory retrained patients initially viewed numbness and decreased sensitivity as more problematic early during recovery but as less problematic later during recovery than did the group of patients who did not undergo retraining.258 The retraining sessions were held at 1 week, 1 month, and 3 months after surgery. The levels of instruction for the sensory retraining were designed to increasingly challenge patients’ sensory discrimination abilities in a manner similar to that of the early and late phases of sensory retraining commonly used after injuries to the sensory nerves of the hand.120,121 Through sensory retraining, individuals learn to discriminate moving touch from non-moving touch; the orientation of moving touch; and the direction of moving touch (see Chapter 11). Poort and colleagues completed a literature review of methods that are used to test for sensory loss and recovery after IAN injury and recommended that clinicians and researchers use the following two measurement tools: 1) a light touch test with Semmes–Wienstein monofilaments for grading sensation; and 2) a visual analog scale–based questionnaire to evaluate the individual’s subjective sensibility.261A If clinicians were to adopt consistent measuring techniques, improved communication with regard to sensory loss and level of recovery after interventions would likely follow.
A dilemma that surgeons continue to face is what to do in the operating room when the laceration of the IAN is witnessed. The question raised asks if there is any significant long-term advantage for the patient to undergo either primary or delayed micro repair of the lacerated IAN. Although the concept of the immediate repair of a witnessed IAN transection is appealing, the practicality of this maneuver and the patient’s long-term improved results (i.e., greater sensory return and decreased dysesthesia) have not been statistically confirmed. The increased operative time and the potential risks of losing focus on the primary orthognathic objectives must also be considered. Tay and colleagues reported four cases of immediate micro-neural repair of an IAN that was transected during SSRO.334 This represented 4 out of 260 (2%) SSROs completed at their center. One of the four patients who sustained IAN laceration was apparently lost to follow up. Interestingly, the location of IAN transection in the remaining three study patients was at the anterior and inferior aspect of the osteotomy along the buccal shelf between the first and second molars; this is a recognized “danger zone” for IAN transection during SSRO. When there is a moderate to severe degree of mandibular hypoplasia with the need for buccal shelf extension of the proximal segment to achieve successful approximation of the bone after advancement, this risk may increase. In the study by Tay and colleagues, the three study patients underwent immediate repair of the IAN (i.e., neurorrhaphy) by a trained microsurgeon and then received follow for 1 year.334 The microsurgeon’s technique included the complete surgical release of the nerve from the mandibular foramen to the mental foramen followed by approximation and repair with the use of 6-0 microfilament suture under microscope magnification. The increased time of the surgery was approximately 3 to 4 hours. Although the authors felt favorable regarding the outcomes of their patients, the level of sensory return that was documented was not proven to be significantly better than expected after the simple approximation of the nerve endings within the ramus of the mandible.
Lingual Nerve Injury
The incidence of injury to the lingual nerve during SSRO is not precisely known, but it is assumed to be rare.35,132,145,147,279,289–291,327 Becelli and colleagues completed a retrospective analysis of complications after SSRO and found a 0.6% rate of lingual nerve dysfunction among 482 osteotomy patients during the first postoperative week. The authors documented complete resolution of this injury after 12 months of follow up in the study group. Interestingly, lingual nerve injury at the time of the removal of mandibular wisdom teeth as an isolated procedure is also estimated to be in the same range (i.e., <1%).260 When a lingual nerve injury does occur with SSRO, it likely occurs during subperiosteal dissection along the medial ramus; when using a rotary drill (e.g., with a Lindeman bur); or when using the reciprocating saw (e.g., during a medial ramus cortical cut). It may also occur with use of the electrocautery to control bleeding, during screw fixation, or when closing the wound if the lingual nerve is caught within the suture tie (Fig. 16-14).
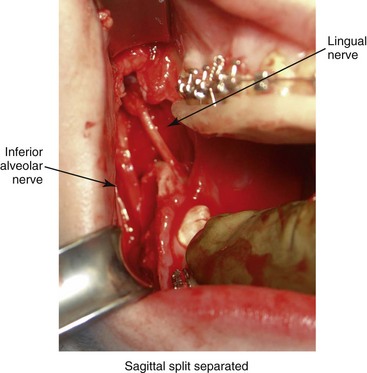
Figure 16-14 A completed right sagittal split ramus osteotomy. The separation of the segments demonstrates both the inferior alveolar and lingual nerves. The lingual nerve is only occasionally visualized in the surgical field.
Pogrel and Hung Le completed a cadaver study that demonstrated that different modalities (e.g., sharp scalpel laceration, fissure bur injury, retraction stretch injury) produced different types of nerve injury, both clinically and histologically.261 The clinical recovery of the injured nerve is believed to be primarily related to the type of injury sustained. For this reason, the treatment recommended should also vary in accordance with the type of injury sustained. Data reveal that 57% of lingual nerve injuries were unknown to the surgeon at the time; therefore, the type of injury could not be accurately ascertained.86
Unfortunately, many of the published lingual nerve injury studies are retrospective case series or questionnaire surveys.260 The studies also frequently combine both lingual and IAN injuries. The literature recommends that observed injuries and those involving a foreign object causing compression or laceration (e.g., endodontic filling material, bone fragment, broken instrument) should be treated immediately. Caution is warranted when interpreting two-dimensional radiographic views when making decisions about impingement on the nerve by a foreign object (e.g., a fixation screw). Three-dimensional computed tomography scans may be useful to help make the determination. Patients with severe hypoesthesia who are demonstrating no sensory improvement at approximately 3 months after treatment may be surgical candidates. Those with at least some documented continuous sensory improvement are best managed conservatively.
Susarla and colleagues used a retrospective cohort study design to address whether the early repair of a lingual nerve injury improves functional sensory recovery.324 The study sample was composed of 64 subjects with lingual nerve injuries who underwent repair with a mean time between injury and repair of 153 days (31 to 1606 days). Twenty-two percent of the subjects underwent early repair (i.e., <90 days after injury). The nerves were repaired either through direct suture (77%) or surgical exploration with decompressive neurolysis (23%). Ninety-three percent of the study subjects in the early repair group achieved functional sensory recovery within 1 year as compared with only 63% in the late repair group. Although early repair shows statistical benefits for the achievement of functional sensory recovery, it must also be assumed that a percentage of those individuals who submitted to early repair would have gone on to achieve adequate recovery through the natural course of events. A randomized clinical trial would be required to clarify any clear advantage of early repair.
A study by Bagheri and colleagues suggests that, for some individuals, secondary repair of a lingual nerve or IAN injury will result in the regaining of acceptable sensory function as classified by the Medical Research Council Scale.17 However, at least some of those who are undergoing secondary repair will show little improvement, and the prospect of deterioration is not insignificant. The evaluation and management of both lingual and IAN dysfunction after an SSRO remains a challenging clinical dilemma. The specific etiology and incidence of persistent sensory deficit and neuropathic pain after SSRO remains poorly understood. Nevertheless, prudent care suggests that, when a lingual nerve injury occurs during a ramus osteotomy, the surgeon is obliged to offer evaluation by a knowledgeable peripheral nerve surgeon within 3 to 6 months. This will ensure that the injured individual receives the information needed to make a personal decision about how to proceed.
Facial Nerve Injury
Reports of injury to other than sensory nerves are primarily restricted to the facial nerve, and these mainly occur during mandibular procedures.60,68,77,79,226,272,287 A retrospective study conducted by de Vries and colleagues after SSRO (N = 1747) found an incidence of seventh nerve palsy of 0.26% (9 patients).83 Choi and colleagues found an incidence of facial nerve palsy of 0.1% among 3105 patients who underwent sagittal split ramus osteotomies.60
Significant Bleeding
In current practice, significant bleeding is rarely encountered at the time of SSRO.16,64,177,184,185,191,207,253,288,303,306,326,372 In 1972, a 38% incidence of major intraoperative hemorrhage was reported; in 2005, an incidence of only 1% was reported.184,257 The vessels to consider include the maxillary artery and its branches; the retromandibular vein; and the facial artery and vein. Behrman reported about two patients with excessive bleeding in which he carried out ligation of the external carotid artery, although doing so was relatively ineffective for controlling the hemorrhage.23 In 1985, Turvey reported the incidence of troublesome hemorrhage to be 1.2%; the inferior alveolar and facial arteries were the sources.322 Acebal-Bianco and colleagues reported about the laceration of the facial artery during SSRO in two patients with successful management.2 Van Merkesteyn and colleagues discussed difficult-to-control bleeding from the inferior alveolar and facial arteries in two patients.257 Teltzrow and colleagues reported bleeding from the retromandibular vein to be the most common source of severe hemorrhage after SSRO.335
The occurrence of a false aneurysm (i.e., pseudoaneurysm or traumatic aneurysm) after an elective mandibular ramus osteotomy is uncommon. Precious and colleagues reported three cases of false aneurysm after SSRO and reviewed the pertinent literature.269 A false aneurysm will present as a delayed phenomenon, usually 1 to 16 weeks after injury. These conditions are thought to result from a partial transaction of an artery (i.e., a facial artery) that leads to the extravasation of blood under pressure into the surrounding soft tissues. The resultant hematoma organizes, and this is followed by fibrous pseudocapsule and then endothelialization. A pulsatile and expanding mass characterizes a false aneurysm. The difficulty of this situation is related to the making of an accurate and timely diagnosis. The treatment approaches include primary surgical resection, arterial embolization, or arterialization followed by surgical resection. Superselective arterial embolization alone is the current preferred approach, whenever feasible.
Risks and Complications Specific to Le Fort I Osteotomy
Infraorbital Nerve Injury
Sensory innervation of the area affected by the Le Fort I osteotomy is mainly through the maxillary division of the trigeminal nerve. During the Le Fort I osteotomy, the three superior alveolar nerves on each side are transected as part of the osteotomy, and the terminal labial branches of the infraorbital nerve are transected as part of the mucosal incision. After the infraorbital nerve emerges from the infraorbital foramen, it is also subject to trauma. A degree of injury to the infraorbital nerve at the time of Le Fort I osteotomy that results in temporary paresthesia is expected, whereas permanent dysesthesia of the cheek skin, the upper lip, the palate mucosa, and the gingiva is thought to be less common.* The nerve injury may result from compression or stretching, and direct injury may result from saw blades, burs, or surgical instruments (Fig. 16-15). The patient’s age and gender will also be factor when considering nerve recovery and the occurrence of dysesthesia.
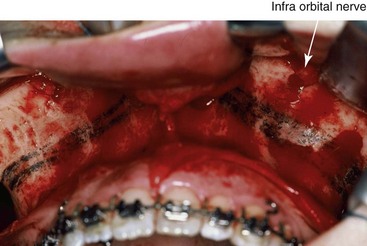
Figure 16-15 Intraoperative view after a circumvestibular incision for exposure to complete a Le Fort I osteotomy. The infraorbital nerve is visualized.
Posnick and colleagues objectively measured facial sensibility in adolescents (with and without cleft lip and palate) 1 year after Le Fort I osteotomy.263 Fifty-nine individuals (118 infraorbital nerves) were included in the study and divided into three groups. The first group (N = 30) included subjects with unilateral cleft lip and palate (mean age, 18 years; with jaw deformity). The second group (N = 12) included subjects with bilateral cleft lip and palate (mean age, 19 years; with jaw deformity). All of these patients had undergone cleft lip and palate repair earlier during their lives; none had an associated craniofacial syndrome, developmental delay, or systemic neurologic impairment. The third group (N = 17) consisted of subjects without clefts or syndromes (mean age, 19 years) but with a developmental jaw deformity. Facial sensibility testing was conducted in the three groups 1 year after the Le Fort I osteotomy. Each patient was also asked about his or her subjective impressions of altered sensibility in the maxillary vestibular mucosa, the gingiva, the upper lip, or the cheek region at the 1-year examination. Previously defined fixed and reproducible coordinates were used for objective testing in the area that corresponds with the infraorbital nerve (Fig. 16-16).267 Three sensory modalities were tested bilaterally at each coordinate. Static two-point discrimination was measured with a MacKinnon–Dellon Disk-Criminator. Vibratory thresholds were determined with the biothesiometer, and cutaneous pressure thresholds were measured with a set of Semmes–Weinstein monofilaments. The data was compared with the mean normal values that had been previously established for adolescents with and without cleft lip and palate before any orthognathic surgery.
Figure 16-16 Illustration of the infraorbital nerve sensibility distribution. Coordinates 3, 4, 5, 7, and 9 are fixed sites that were used by Posnick and colleagues for sensibility testing.
Thygesen and colleagues recently looked at risk factors for paresthesia and dysesthesia of the infraorbital nerve after Le Fort I ostoeomy.337 Their results are somewhat different from those of Posnick and colleagues. The authors found objective changes in somatosensory function 12 months after Le Fort I osteotomy in 7% to 60% of patients, depending on the site at which the sensory measurement was carried out (i.e., skin, gingiva, or palatal mucosa). The authors did not report instances of significant dysesthesia associated with the sensory loss in any patient. Despite a degree of subjective sensory loss at 12 months after surgery, all of the study patients were satisfied with the results of their operation, with 100% saying that they would do it again.
Visual Disturbances
Blindness
Decreased visual acuity (blindness) is a rare complication of Le Fort I osteotomy.* Although the exact mechanism of injury to the optic nerve has not been clarified, atypical fracture patterns that extend upward to the skull base from the pterygomaxillary (PM) separation at the time of down-fracture have been implicated.180,309,340,366 Girotto and colleagues reported on three cases of visual loss after elective standard Le Fort I osteotomy.115 All three cases were presumed to be straightforward and were performed by experienced surgeons. The authors hypothesized that the unpredictable nature of the PM dysjunction may result in the extension of fracture to the skull base or generate deforming forces to the optic canal, with compression and pressure placed on the optic nerve. Lanigan reviewed the literature up through 1993 and reported two cases of blindness after Le Fort I osteotomy.188 Bendor-Samuel and colleagues reported one additional case of blindness.28 Lo and colleagues reported two cases of monocular blindness in 94 consecutive cleft palate patients who underwent mixed dentition maxillary (Le Fort I) osteotomy with osteodistraction with the use of the RED II Distraction System (KLS Martin, Mühlheim, Germany) during a 4-year period.154 Although the exact etiology could not be clarified by Lo and colleagues, they stated that the PM disjunctions were performed at a higher level to avoid tooth bud injury in their patients with cleft palates during the mixed dentition. Therefore, the osteotomies were closer to the skull base and the orbit. The authors also speculated that the hard palate in the tuberosity region and the PM junction may have been more sclerotic as a result of the repaired cleft palate, thereby making precise PM disjunction less predictable.
Of the nine reported cases of blindness after Le Fort I osteotomy, five were of unknown cause, one was due to arterial aneurysm, two were attributed to propagation of the PM disjunction fracture through the skull base, and one was the result of the hypoperfusion of the optic nerve. There was no recovery in three cases, light perception returned in three, hand movements were visible in one, fingers could not be counted in one, and visual acuity showed much improvement in one. Interestingly, a majority of the reported cases of monocular blindness were in individuals born with cleft lip and palate. These patients had all undergone primary lip and palate repair, and several had previously undergone unsuccessful Le Fort I osteotomies. This likely represents a higher than average risk group for the rare complication of monocular blindness.194
Extraocular Cranial Nerve Dysfunction
Newlands and colleagues identified five cases of abducens palsy, three cases of oculomotor palsy, and one case in which both palsies were present together.129,135,199,237,307,356 Most reported cases of extraocular nerve dysfunction after Le Fort I osteotomy occur immediately after surgery, although it has been reported up to 5 days postoperatively.
Nasolacrimal Apparatus Dysfunction
Tearing dysfunction has been reported after Le Fort I osteotomy by a number of authors; this involves either a lack of or excessive tearing.202,210,211,300,338 A lack of tearing is thought to have occurred as a result of damage to the greater petrosal or Vidian nerve, which could interrupt parasympathetic supply to the lacrimal gland. Excessive tearing is thought to be related to nasolacrimal duct damage.
Significant Bleeding
Vascular complications (i.e., excessive bleeding)—either immediate or delayed—after Le Fort I osteotomy are rare.* The reported cause of the delayed bleeding is generally a partial laceration of a vessel with an aneurysm or an arteriovenous fistula formation (i.e., false aneurysm). The confirmation of a traumatic vascular abnormality is best made by angiography. This condition most frequently occurs in the internal maxillary artery or in the internal carotid artery region. The presentation of bleeding from the nose and occasionally from the oral cavity early after surgery is the typical clinical event. When massive epistaxis occurs soon after Le Fort I osteotomy, the differential diagnosis may include coagulopathy, bleeding from a partially resected inferior turbinate, or bleeding from a large artery (i.e., the internal maxillary artery). Angiography with the possible need for embolization should be considered. A frequent palliative (but temporary) treatment approach is the packing of the nose. This is likely to immediately control bleeding; however, over time, a false aneurysm may form, and rebleeding (i.e., recurrent epistaxis) is typical.
Procopio and colleagues described one patient (a 37-year-old woman who had undergone Le Fort I down-fracture) whose epistaxis occurred 2 hours after surgery and who was treated with anterior nasal packing.270 Seven days later, she was taken back to surgery to reposition her maxilla for the treatment of malocclusion. No source of excessive bleeding was found. Further epistaxis occurred on days 21, 53, 63, 85, and 115. These episodes were treated with nasal packing, which resulted in resolution for short periods of time followed by rebleeding. On day 115, angiography and a computed tomography scan were completed. A false aneurysm of the sphenopalatine artery was identified and successfully embolized.
Aseptic Necrosis of Maxilla
A rare but serious complication of Le Fort I osteotomy is ischemic necrosis.25,27,38,44,86,87,124,128,179,182,235,251,271,298,373 The severity of this postoperative complication is related to the degree of vascular compromise of the down-fractured maxillary segments. Reported sequelae of vascular compromise include infection, mucosa sloughing, periodontal defects, pulpal changes, malunion or nonunion, and the partial or complete loss of the maxilla and dentition (Figs. 16-17, 16-18, and 16-19).
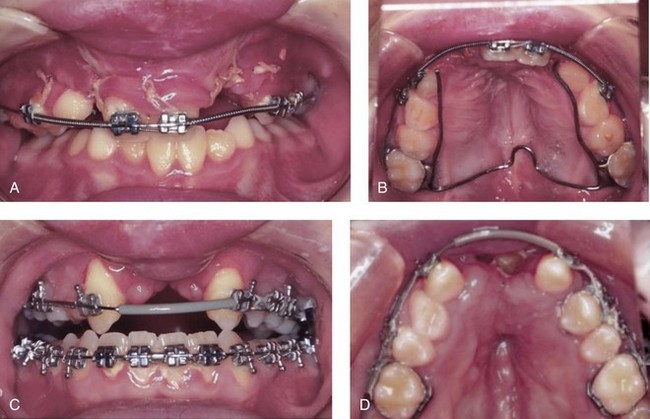
Figure 16-17 An individual who was born with a bilateral cleft lip and palate was treated at another institution. During the mixed dentition, he underwent unsuccessful iliac (hip) bone grafting and fistula closure. The premaxilla remained mobile, with residual alveolar clefts. When he was 17 years old, he underwent maxillary Le Fort I osteotomy, also at another institution. Unfortunately, given the residual alveolar clefts, the circumvestibular incision that was used resulted in circulation injury to the premaxilla (i.e., aseptic necrosis). Unless the bilateral cleft is successfully grafted through at least one of the alveolar sites, a circumvestibular incision that cuts through the labial vestibule of the premaxilla will predictably result in aseptic necrosis. A and B, Occlusal and palatal views are shown during the mixed dentition after unsuccessful grafting. C and D, The same views are shown during the teenage years after unsuccessful Le Fort I osteotomy with aseptic necrosis of the premaxilla and the resulting oronasal fistula.
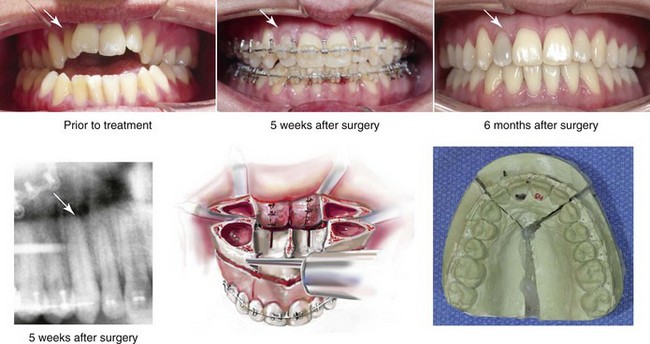
Figure 16-18 A man in his 20s presented with a maxillary deficiency and a relative mandibular excess growth pattern. He underwent orthodontics and reconstruction, including a Le Fort I osteotomy in three segments (i.e., interdental cuts between the lateral incisor and the canine on each side). Approximately 3 months after surgery, the right lateral incisor crown was noted to be dark in color. No other symptoms occurred. A postoperative radiograph shows the interdental osteotomy site. There was no direct injury to the dental root on either side of the osteotomy. The pulp of the tooth was treated with root canal therapy. Bleaching is planned to whiten the crown. Occlusal views are shown before orthodontics, at 5 weeks after surgery, and after successful reconstruction. An illustration and a maxillary model show the location of segmental osteotomies.
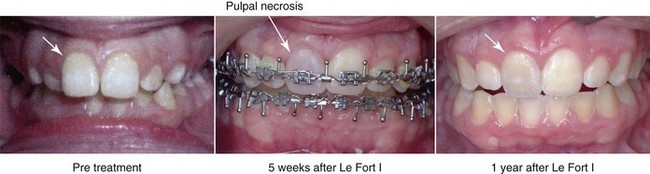
Figure 16-19 Occlusal views are shown of a teenage girl with a long face growth pattern. She underwent an orthodontic and surgical approach. The surgery included maxillary Le Fort I osteotomy without segmentation. By 5 weeks after surgery, the right maxillary central incisor was noted to be dark in color. No other symptoms occurred. A postoperative radiograph showed no signs of root injury or periapical pathology. The pulp of the tooth was treated with root canal therapy followed by bleaching.
The biologic basis for maxillary (i.e., isolated segmental, down-fracture Le Fort I, and down-fracture Le Fort I with segmentation) osteotomies used in orthognathic surgery was extensively investigated in experimental animals by Bell and colleagues with the use of microangiographic and histologic techniques to study revascularization and wound healing (see Chapter 2).25 Meyer and colleagues also completed animal studies involving radioactive microsphere methods to quantify preoperative and postoperative blood flow after classic osteotomies.219 The results of Bell’s microangiographic and histologic studies showed minimal transient vascular ischemia, minimal osteonecrosis, and early osseous union, with the maxilla pedicled essentially to the palatal mucosa. The preservation of the integrity of the descending palatine arteries was not found to be essential to maintain circulation to the down-fractured maxilla. The periosteal vascular bed provides adequate reserve blood supply after down-fracture, even with ligation of the named vessels. Alteration of the hemodynamics within the intramedullary cavity after total maxillary down-fracture was felt to produce only transient and clinically insignificant intraosseous ischemia (see Chapter 2).
In clinical practice, controversy continues to exist regarding the management of the descending palatine artery (DPA) during Le Fort I osteotomy. Some surgeons advocate the preservation of the DPA, whereas others ligate the vessel routinely. Dodson and colleagues completed a prospective randomized clinical study of patients (N = 34) who underwent Le Fort I osteotomy.86,87 The individuals were randomly assigned to either Study Group 1 (N = 26), in which the DPA was ligated, or Study Group 2 (N = 18), in which the DPA was preserved. The researchers measured maxillary gingival blood flow during the operation with the use of a laser Doppler flow meter, and they found “no statistically significant differences in mean maxillary gingival blood flow between patients having the DPA ligated and those having the DPA preserved.”87 The maintenance of adequate flap circulation through the palatal mucosa attached to the hard palate is the key to the avoidance of this problem.
Documented cases of aseptic necrosis after maxillary osteotomies primarily come from the questionnaire survey conducted by Lanigan and colleagues.182 The authors sent out 5000 questionnaires to oral and maxillofacial Surgeons, and 800 replies were received. Fifty-one cases of aseptic necrosis after maxillary surgery were reported from 44 surgeons who had encountered this specific complication. The number of cases reported was assumed to be less than the magnitude of the clinical problem in practice. The results of the questionnaire suggested that, at the time of the survey (i.e., in 1986), a Le Fort I osteotomy performed in multiple segments in conjunction with superior repositioning and transverse expansion (especially when significant palatal perforations occurred) was more likely to result in a tenuous blood supply to the anterior maxilla, with resulting aseptic necrosis. More recently, Singh and colleagues described a single patient from the United Kingdom who underwent maxillary Le Fort I osteotomy with down-fracture (without segmentation) and who sustained aseptic necrosis of approximately two thirds of the total maxilla.305 The patient’s risk factors included nicotine abuse, the use of deliberate hypotensive anesthesia in the presence of baseline hypertension, and uncontrolled intraoperative bleeding. In 2010, Pereira and colleagues reported a case of maxillary aseptic necrosis after Le Fort I osteotomy.255 The patient was 52 years old, with baseline hypertension, an ongoing smoking history of two packs per day, and chronic generalized periodontal bone loss and gingival recession. He underwent Le Fort I down-fracture osteotomy with horizontal advancement. Uncommon bleeding was noticed in the posterior region intraoperatively, and this was treated with gauze compression. On the seventh postoperative day, the researchers reported that the “[m]ucosa overlying the maxilla was ischemic and covered with a pseudomembrane.”255 The patient was treated with 20 sessions of hyperbaric oxygenation. The maxilla and the associated dentition were preserved, but further alveolar bone loss occurred.
Palatal Fistula
The tearing of the palatal mucosa can occur at the time of either surgically assisted rapid palatal expansion (SARPE) or standard parasagittal segmental osteotomies carried out through the Le Fort I down-fracture. A tear is more likely to become clinically apparent when significant transverse expansion is undertaken. Intraoperative palatal mucosa tears at the time of segmental osteotomy will occur and are best managed by meticulous nasal mucosa closure and by interposing a middle layer to limit epithelialization with fistula tract formation during the healing of the palatal mucosa. This can be accomplished with a biocompatible material (e.g., Surgicel, fibrin glue, acellular collagen matrix) that is placed through the down-fractured maxilla directly onto the bony palate (i.e., the floor of nose side) after stabilization of the segments into the prefabricated splint and before the placement of maxillary plate and screw fixation. By instituting precautions to limit pressure gradients across the opening and by avoiding hard food boluses on the roof of the mouth during initial healing, spontaneous closure is typically seen. Under these circumstances, long-term persistent fistulization of a palatal mucosal tear after segmental osteotomies is uncommon. When a palatal (i.e., oronasal) fistula remains and is of clinical concern as a result of a loss of fluid and air, it can be closed with the use of local palatal flaps (Fig. 16-20). Surgical closure should be postponed until 6 to 12 months after Le Fort I osteotomy to ensure adequate maxillary revascularization. During the interim, a custom acrylic palatal plate can be used to both obturate the palatal–nasal opening and to assist with the maintenance of the new arch form.
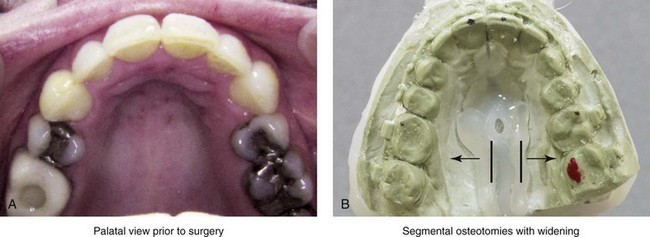
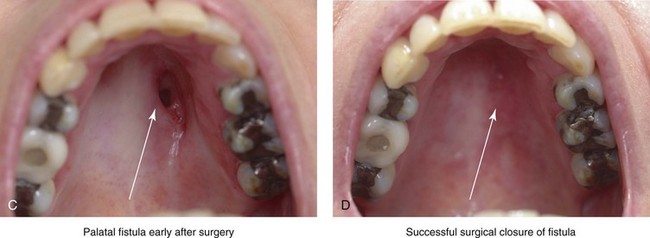
Figure 16-20 A 48-year-old woman with a long face growth pattern and Angle Class II anterior open-bite malocclusion is shown. The patient underwent four bicuspid extractions and orthodontic treatment during childhood as a compensatory approach. She was referred by a restorative dentist to an orthodontist and then to this surgeon for evaluation. She was found to have obstructive sleep apnea, chronic obstructive nasal breathing, and a long face growth pattern with a Class II tendency. She underwent a comprehensive surgical, orthodontic, and dental rehabilitative approach. The procedures included maxillary Le Fort I osteotomy in two segments (interdental osteotomy between the central incisors and then parasagitally down the hard palate). The maxillary segmentation was to widen the posterior arch by several millimeters. When placing the maxillary segments into the intermediate splints, a tear in the palatal mucosa at the parasagittal osteotomy location was appreciated. This was managed with Gelfoam and tissue glue through the down-fracture along the floor of the nose and with meticulous nasal mucosa suturing. A fistula persisted at 1 year after surgery, with air loss affecting her speech (i.e., hypernasal speech) and fluid loss occurring into the nose when she was drinking liquids. She underwent outpatient surgery to elevate the palatal flaps, to separate the nasal and palatal mucosa, to suture closed the nasal side, and then to suture closed the palatal side. She was placed on a mechanical soft diet and sinus precautions for 4 weeks. The fistula was successfully closed. A, Palatal view before surgery. B, Model planning indicating two-segment maxillary osteotomy with widening at the molar region. C, Palatal view 1 year after surgery indicating a persistent oronasal fistula. D, Palatal view 3 months after the successful surgical closure of the fistula.
Fibrous Union (Painless Mobile Maxilla)
The fibrous union of the maxilla after Le Fort I osteotomy is an infrequent complication.78,162,200,342,350 Risk factors should be recognized, and surgical techniques and postoperative management may then be adjusted to limit this complication. The combination of extensive horizontal advancement and vertical lengthening results in large osteotomy site defects with limited bone contact. Failure to form adequate bony bridges at the healing sites is likely to result in delayed healing, fibrous union, or late relapse. Influences on bone healing at the Le Fort I osteotomy site may include the following: 1) injury to flap circulation 2) thin osteoporotic bone at the buttress and the pyriform rims with inadequate stabilization (fixation) 3) the decision being made to not place interpositional grafts when the bone gaps are of critical size (as described previously) 4) infection or wound dehiscence after surgery 5) the presence of a heavy occlusion (e.g., grinding, clenching) 6) too rapid of a return to normal chewing forces 7) excessive mouth opening against intermaxillary elastics and 8) stress fracture of the fixation plates. Systemic risk factors for poor bone healing include 1) diabetes; 2) collagen disease; 3) vascular disease; 4) osteoporosis and the use of bisphosphonates; 5) nicotine use; 6) poor nutritional status; and 7) prior radiation.
This author has experience with four of his own patients who underwent Le Fort I osteotomy and then developed fibrous union of the maxilla (Fig. 16-21). This represents a small percentage (i.e., <0.5%) of the total group of Le Fort I osteotomies performed. In each case, initial healing seemed unremarkable, with no infection or wound dehiscence. There were no signs of vascular compromise at the time of osteotomy or after surgery. None of the patients were felt to have more than average residual mobility at the 5-week postoperative interval upon return to their orthodontist’s care. They were then encouraged to gradually return to a regular diet and the usual physical activities as well as to work toward normal mouth opening. The four affected individuals were as follows:
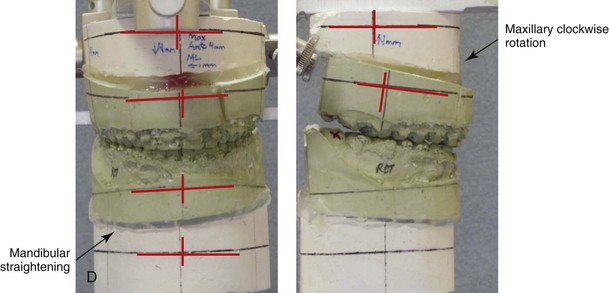
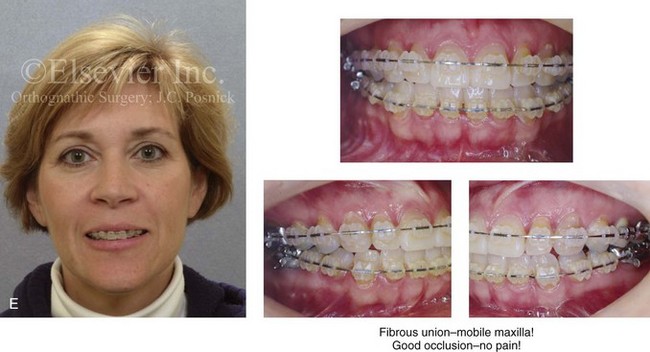
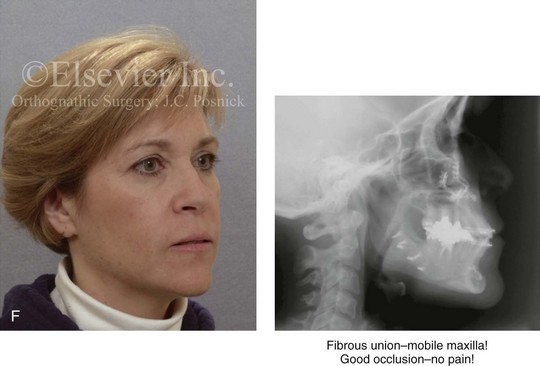
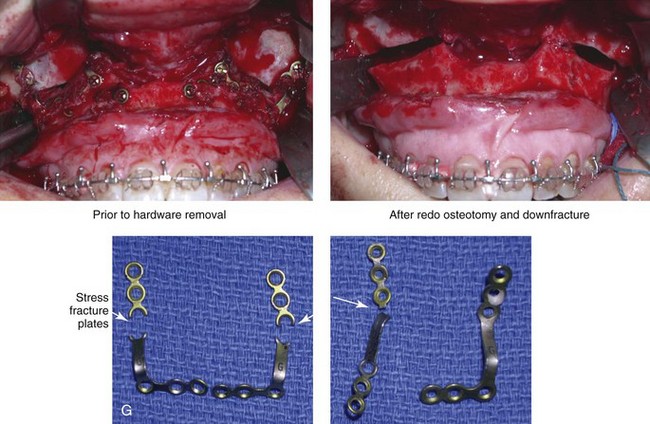
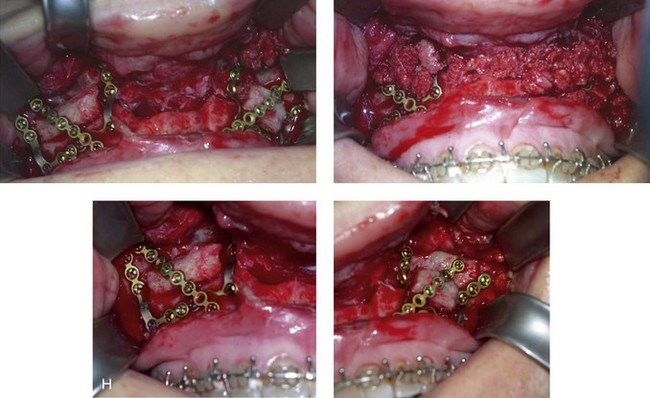

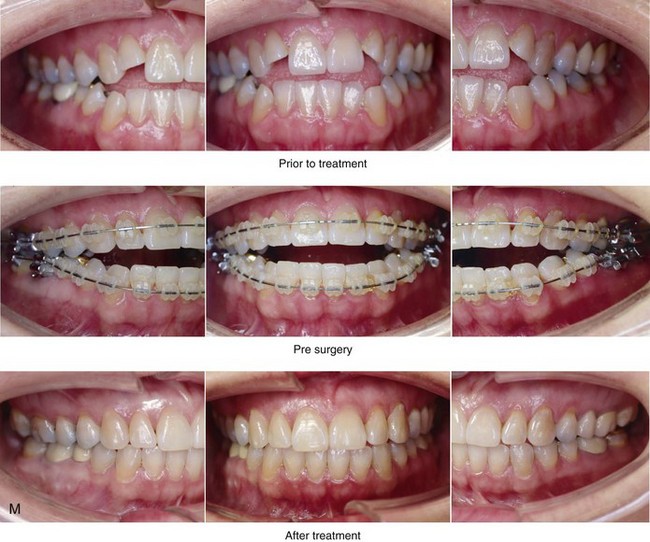
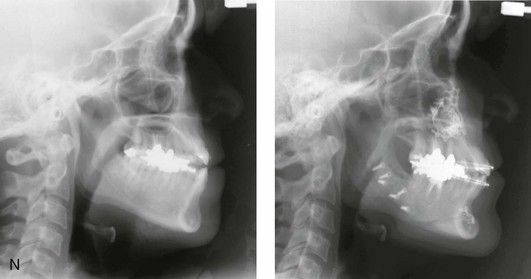
Figure 16-21 A woman in her mid 40s with an asymmetric mandibular excess growth pattern arrived for surgical evaluation. The longstanding asymmetric Angle Class III negative overjet anterior open-bite malocclusion had been detrimental to her dentition. She required crown restorations and had a need for root canal therapy in several of the posterior teeth. She desired the correction of the malocclusion (i.e., improved chewing and speech articulation) and improved lip closure (i.e., relief of mentalis strain). She had a lifelong history of obstructed nasal breathing and was found to have septal deviation and inferior turbinate enlargement. She agreed to an orthodontic and surgical approach. Orthodontic (dental) decompensation was followed by surgery that included maxillary Le Fort I osteotomy (horizontal advancement, clockwise rotation, and vertical lengthening); bilateral sagittal split ramus osteotomies (asymmetry correction); osseous genioplasty (horizontal advancement); and septoplasty and inferior turbinate reduction. The quality of the maxillary buttress bone was noted to be thin, and it did not hold the fixation plates and screws firmly in all locations as compared with bone of average thickness; however, it was felt to be adequate. No interpositional graft was used, and initial healing was on track. Five weeks after surgery, the patient progressed to a normal diet and her regular physical activities. She also returned to the care of her orthodontist. Postoperative radiographs (lateral cephalometric and Panorex) did not look out of the ordinary. Three months after surgery, during a routine orthodontic evaluation, she was found to have mobility of the maxilla but without pain and with a good occlusion. The diagnosis of fibrous union at the Le Fort I osteotomy site was made. The patient was placed on a liquid diet, told to avoid clenching and heavy biting forces, and asked to stop all use of interarch elastics. Despite a waiting period of an additional 2 months, a painless mobile maxilla (i.e., fibrous union) remained. The patient was returned to the operating room, where she underwent the following:
• Standard circumvestibular incision and subperiosteal anterior maxillary exposure
• Removal of all maxillary fixation plates and screws
• Down-fracture and complete disimpaction
• Removal and cleaning out of all fibrous tissue along the osteotomy sites
• Placement of the maxilla into the preferred occlusion through a prefabricated splint
• Adjustment of the maxilla to the preferred anterior vertical height
• Placement of new titanium bone plates and screws at each zygomatic buttress and pyriform aperture
• Harvesting and crafting of the iliac (hip) corticocancellous graft
• Placement of a bloc graft in each bony gap between the zygomatic buttress and the pyriform plates
• Securing of each graft with an additional titanium microplate and screws
• Packing of additional cancellous bone along the osteotomy sites
1. A 15-year-old Caucasian individual with Down syndrome who underwent Le Fort I osteotomy, SSRO of the mandible, and oblique osteotomy of the chin. The maxilla underwent horizontal advancement and vertical lengthening without interpositional grafting. Maxillary stabilization was performed with a titanium plate placed at each pyriform aperture, and the zygomatic buttress region was secured with screws. The quality of the bone was thin and did not hold the fixation screws as firmly in all locations as is typical. Initial healing was felt to be normal. The patient returned to the orthodontist’s care at 5 weeks without special concerns.
2. A 25-year-old Asian graduate student who presented with congenital facial palsy that resulted in maxillofacial skeletal asymmetry. This patient underwent Le Fort I osteotomy, SSRO of the mandible, and oblique osteotomy of the chin. The maxilla underwent cant correction and intrusion with minimal horizontal advancement. Stabilization was obtained with a titanium plate placed at each zygomatic buttress, and the pyriform aperture was secured with screws. The quality of the bone was thin and did not hold the fixation screws as firmly at all locations as is typical. Initial healing was felt to be normal. The patient returned to her orthodontist’s care at 5 weeks without special concern.
3. A 45-year-old woman with a history of osteoporosis that had previously been treated with oral bisphosphonates. This patient’s jaw deformity was characterized by maxillary deficiency with relative mandibular excess. She underwent Le Fort I osteotomy, SSRO, and oblique osteotomy of the chin. The maxilla was horizontally advanced and vertically lengthened. Stabilization was performed with a titanium bone plate placed at each zygomatic buttress, and the pyriform aperture was secured with titanium screws. The quality of the bone was thin and did not hold the fixation screws as firmly as is usual at all locations, but it was felt to be adequate. No interpositional graft was placed, and initial healing was felt to be normal. The patient returned to her orthodontist’s care at 5 weeks without special concerns.
4. A 45-year-old woman with an asymmetric mandibular excess growth pattern (see Fig. 18-20). This patient underwent Le Fort I osteotomy, SSRO, and oblique osteotomy of the chin. The maxilla was horizontally advanced and vertically lengthened. Stabilization was with a titanium plate placed at each pyriform aperture, and the zygomatic buttress was secured with screws. The quality of the bone was thin; it did not hold the fixation screws firmly at all locations as is average, but it was felt to be adequate. No interpositional graft was placed, and initial healing was felt to be normal. The patient returned to her orthodontist’s care at 5 weeks without special concerns.
1. Return to the operating room with general anesthesia through standard nasotracheal intubation
2. Maxillary circumvestibular incision and standard subperiosteal dissection for Le Fort I exposure
3. Removal of all maxillary fixation plates and screws
4. Down-fracture and complete disimpaction
5. Removal of all fibrous tissue at the osteotomy sites
6. Placement of the maxilla into the preferred occlusion with the mandibular dentition using a prefabricated splint; adjustment of the maxilla to the preferred vertical height
7. Placement of new titanium plates and screws at each zygomatic buttress and pyriform aperture
8. Harvesting of iliac (hip) corticocancellous graft.
9. Crafting of a corticocancellous (iliac) bloc graft and placement in the bony gaps between the zygomatic buttress and the pyriform plates on each side
10. Securing of each corticocancellous graft with an additional titanium plate and screws
11. Packing of additional cancellous bone along the osteotomy sites
Interestingly, in the three patients who received additional treatment, the fracture of most of the titanium plates was recognized at the time of the redo Le Fort I osteotomy (see Fig. 16-21D). One explanation is that the titanium plate failure occurred soon after surgery. If so, then the fractured plates would be responsible for inadequate immobilization at the osteotomy site, thereby resulting in fibrous union. Another explanation is that bony union did not occur at the usual time (i.e., approximately 4 to 5 weeks after surgery). The initially successful plate and screw fixation masked the fibrous union for several months; this was followed by the eventual stress fracture of the titanium plates in response to the continuous masticatory compressive occlusal forces. Painless mobility of the maxilla eventually became noticeable to the patient (i.e., 4 to 6 months after surgery), and this was then brought to the surgeon’s attention.
Acute and Chronic Nasal Obstruction
Buckling and deviation of the nasal septum can occur during the superior repositioning of the maxilla after Le Fort I osteotomy (see Fig. 16-22). When vertically intruding the maxilla, adequate resection of the inferior aspect of the cartilaginous and bony septum is required to avoid this complication.208 If the septum is properly managed at operation, the occurrence of a “crooked nose” after the removal of the nasotracheal tube should be uncommon. Visual inspection of the nose is necessary after maxillary repositioning just before and again after extubation to confirm that the septum remains straight. If septal displacement is observed after extubation and is simply due to nasotracheal tube pressure, then limited manual manipulation in the recovery room will suffice. If the surgeon did not adequately resect (i.e., manage) the inferior aspect of the septum when completing a Le Fort I intrusion, then a return to the operating room to do so will be required.
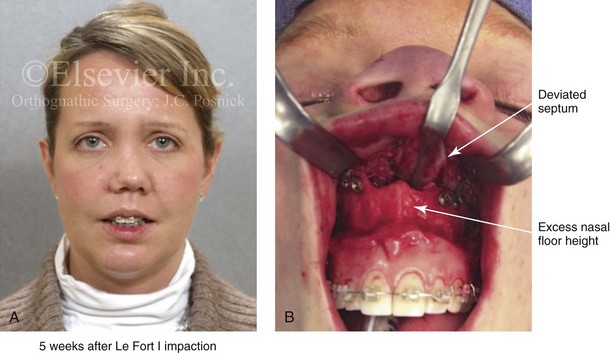
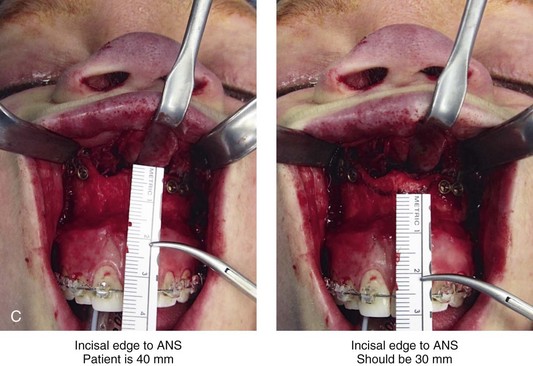
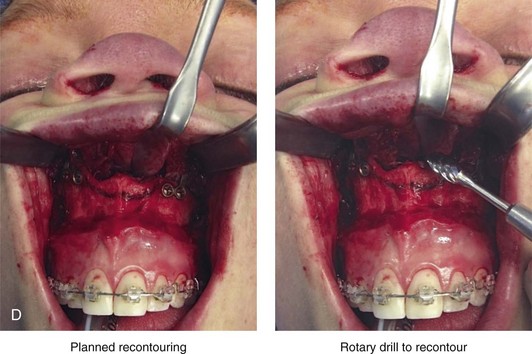
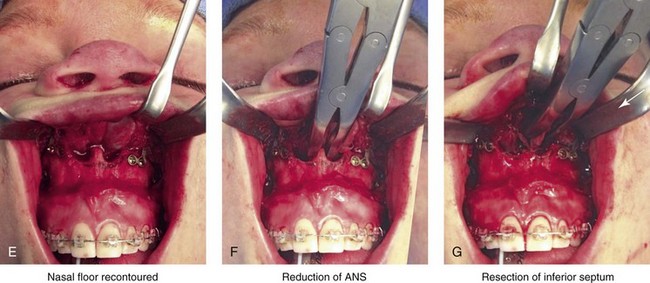
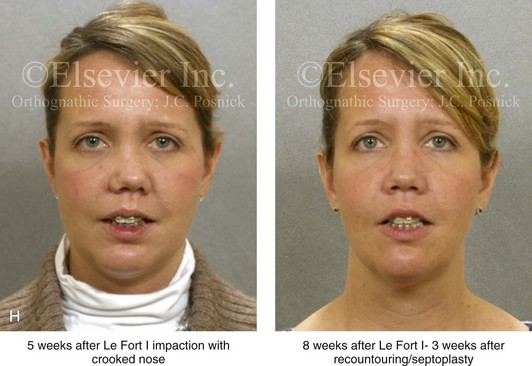
Figure 16-22 A woman in her mid 30s was diagnosed with a long face growth pattern and mild obstructive sleep apnea. She underwent perioperative orthodontics and orthognathic procedures, including maxillary Le Fort I osteotomy (horizontal advancement and vertical intrusion); bilateral sagittal split osteotomies of the mandible (advancement); and osseous genioplasty (horizontal advancement). The surgery was carried out by another surgeon. Immediately after surgery, in the recovery room, significant asymmetry and distortion of the base of the nose and nasal tip were recognized. Although the patient generally convalesced well, there was persistent obstructed nasal breathing and noticeable nasal base and tip asymmetry and distortion. At 5 weeks after surgery, consultation with this surgeon occurred. Clinical and radiographic examination confirmed uncorrected excess anterior alveolar maxillary height as well as a buckled and deviated quadrangular cartilage. Visually, there was marked distortion of the base of the nose and nasal tip as well as blocked nasal airflow. The patient was taken to the operating room and underwent general anesthesia through orotracheal intubation. A limited intraoral circumvestibular incision was made with the dissection of the anterior maxilla, the floor of the nose, and the anterior aspect of the septum. The maxillary incisal edge to the anterior nasal spine measured 40 mm (elongated by three standard deviations) and confirmed vertical excess. The quadrangular cartilage was buckled and deviated, which further contributed to an obstructed left nasal passage. Using a rotary drill with a watermelon bur and a rongeur, the nasal rims, the floor of the nose, and the anterior nasal spine were recontoured and lowered toward normal morphology. The incisal edge to the anterior nasal spine now measured 30 mm. The inferior aspect of the quadrangular cartilage was resected and repositioned to the midline. A, Frontal facial view at 5 weeks after initial surgery indicating distortion and asymmetry of the nasal base and tip. B, Intraoperative view of anterior vertically maxillary excess and buckled quadrangular cartilage at redo surgery. C, Measurement of the incisal edge to the anterior nasal spine (40 mm) before recontouring confirms the baseline elevation of the nasal floor. D, Pencil marks indicate the planned lowering of the floor of the nose. E, The floor of the nose is recontoured (lowered) with a rotary drill. F, This is followed by rongeur resection and refinement of the nasal spine. G, A rongeur was then used to resect the inferior aspect of the quadrangular cartilage. H, Facial views of the patient 5 weeks after the initial orthognathic procedure and then 3 weeks after secondary revision (i.e., recontouring of the floor of the nose and septoplasty). The patient’s nose is no longer crooked, and the nasal airway is now patent.
The most common reason for persistent postoperative nasal obstruction is preoperative chronic nasal obstruction that was not recognized and addressed (see Chapter 10). The second likely cause of persistent nasal obstruction is inadequate recontouring of the nasal floor, the pyriform rims, and the anterior nasal spine in conjunction with maxillary intrusion.
Acute and Chronic Maxillary Sinusitis
The occurrence of persistent maxillary sinusitis that requires treatment after Le Fort I osteotomy is not thought to be common, but the true incidence has not been fully studied.256,374 When maxillary sinusitis after Le Fort I osteotomy does occur, it may be due to the following: 1) changes in the clearance mechanism of sinus mucous; 2) the retention of a blood clot in the sinus cavity; 3) dental infection; 4) the presence of foreign bodies (e.g., grafts, loose fixation devices) in the sinus cavity; or 5) the anatomic blockage of the osteomeatal opening (Fig. 16-23). A blocked ostium can generally be assessed with fiber-optic nasoendoscopy in the office setting. The occurrence of a loose displaced graft that cannot be expressed through the ostium and that then becomes chronically contaminated with bacteria should also be ruled out. A sinus computed tomography scan is helpful to clarify these issues. Standard titanium fixation screws are expected to perforate through the anterior wall into the sinus. They are often presumed to be the source of sinusitis; however, this author’s experience, that is rarely the case. Interestingly, the most common reason for postoperative chronic sinusitis is preoperative chronic sinusitis that was not recognized and addressed.
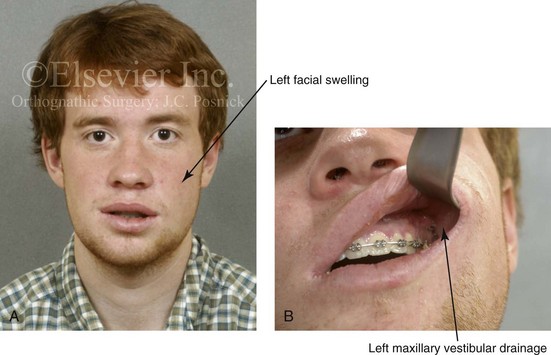
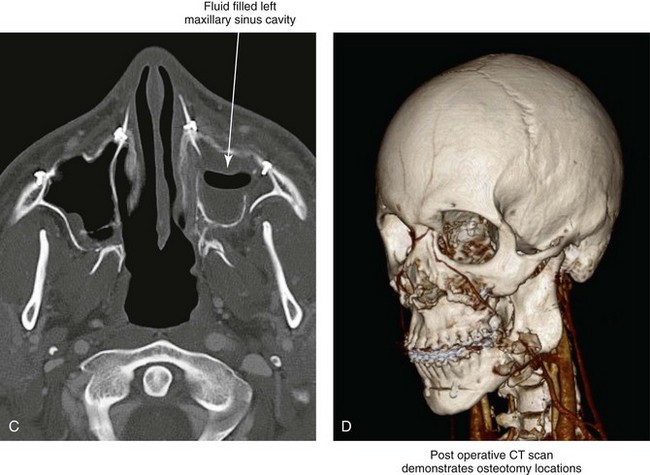
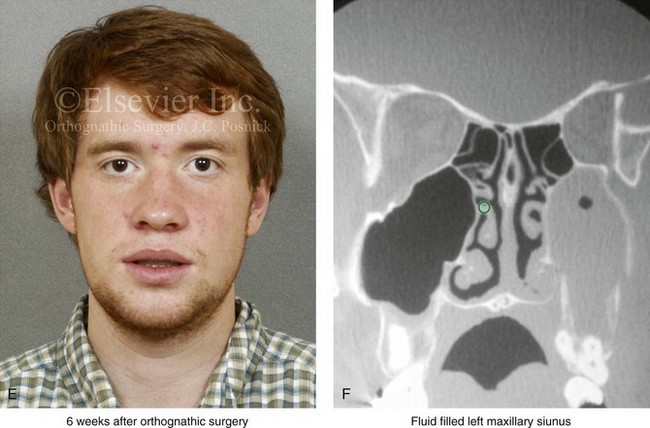

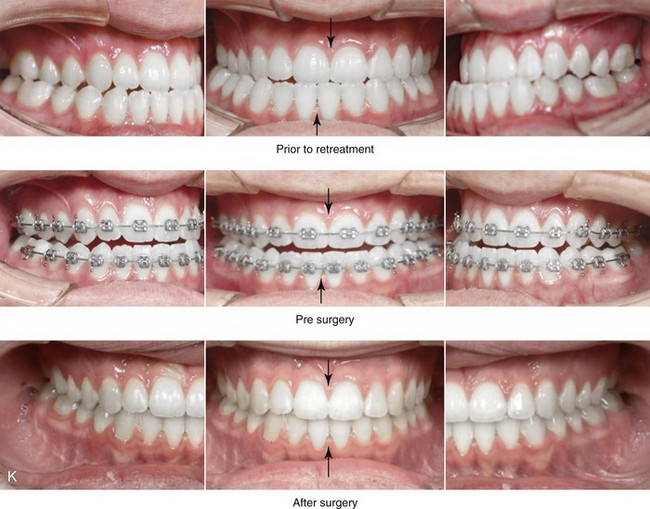
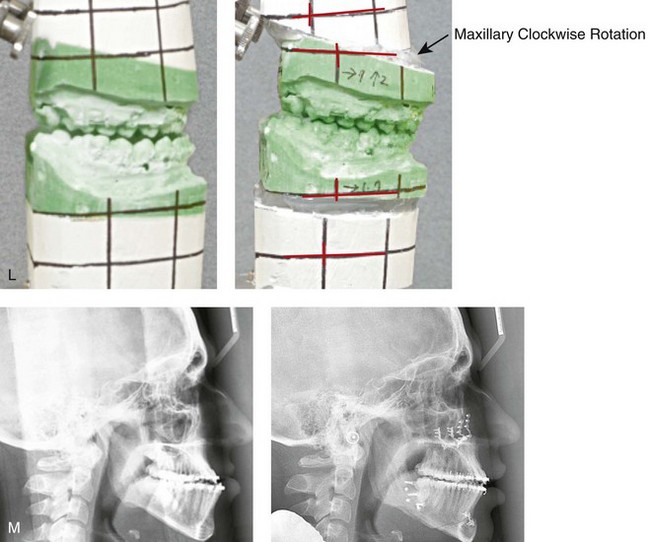
Figure 16-23 A 19-year-old man with a maxillary deficiency in combination with asymmetric mandibular excess presented for treatment. He also had chronic obstructed nasal breathing. He was referred for orthodontic and surgical correction. His surgery included maxillary Le Fort I osteotomy (horizontal advancement and clockwise rotation); bilateral sagittal split osteotomies of the mandible (correction of asymmetry); osteotomy of the chin (horizontal advancement); septoplasty; and inferior turbinate reduction. In the presence of a penicillin allergy, prophylactic clindamycin antibiotic was initiated just before surgery and continued for 5 days. Approximately 2 weeks after surgery, left cheek region swelling and intraoral purulent drainage were noted. A computed tomography scan confirmed a fluid-filled left maxillary sinus. The patient was restarted on clindamycin (600 mg by mouth four times daily), and a Penrose drain was inserted into the left maxillary vestibular wound. The cultures confirmed beta-hemolytic streptococci (Group C). Sensitivities indicated that ciprofloxacin was the antibiotic of choice, so the patient was started on a 10-day course. He was also placed on nasal decongestants. Within days, the drainage subsided, and the swelling diminished. The Penrose drain was removed 1 week after insertion. At 5 weeks after surgery, the healing of the osteotomies remained on track, and the patient resumed a regular diet. At 6 weeks, left cheek swelling recurred. A computed tomography scan confirmed a fluid-filled maxillary sinus without adequate drainage into the nose. Nasoendoscopy confirmed a blocked ostium. The endoscope was used to create an ostium to drain the sinus. No further sequelae occurred. A, Frontal facial view 2 weeks after surgery showing left cheek swelling. B, Left maxillary vestibular wound indicating the site of drainage. C, A computed tomography scan taken 2 weeks postoperatively confirmed a fluid-filled left maxillary sinus. D, A computed tomography scan taken 2 weeks after surgery indicated the osteotomy locations and the repositioning of the jaws. E and F, Frontal facial and sinus computed tomography scan views at 6 weeks indicating recurrent left maxillary sinus blockage and mucocele. G, Frontal views in repose before and 6 months after treatment. H, Frontal views with smile before and 6 months after reconstruction. I, Oblique facial views before and 6 months after treatment. J, Profile views before and 6 months after treatment. K, Occlusal views before redo orthodontics, with orthodontics in progress, and after treatment. L, Articulated dental casts indicate analytic model planning. M, Lateral cephalometric radiographs before and after reconstruction.
Risks and Complications Specific to the Oblique Osteotomy of the Chin (see Chapter 38)
Mental Nerve Injury
The mental nerve is at risk for injury in association with an osseous genioplasty.114,238,349 When the soft-tissue dissection and retraction carried out for the exposure of the chin are carefully completed, the mental nerves will be stretched but rarely lacerated or avulsed. If this is the case, the observed immediate loss of sensibility in the lip and chin region should be temporary. The mental nerve can be cut during the soft-tissue incision. Care is taken with the lateral aspect of the incision to avoid this problem. The nerve can be stretched excessively or avulsed from the mental foramen during retraction at the time of osteotomy (see Chapter 15). It is important to remember that the nerve dips below the mental foramen posteriorly. The nerve also extends anteriorly (i.e., the incisal nerve) and may be injured in this location. After genioplasty, approximately 10% of patients will experience a degree of permanent loss of sensibility in the distribution of the mental nerve on one or both sides.
Wound Complications
The osseous genioplasty is completed through an intraoral vestibular incision.63 To limit wound-healing difficulties, the incision should be made in the depth of the vestibule. The maintenance of a thick cuff of mucosa and muscle tissue adjacent to the cervical margins of the teeth for a relaxed wound closure in two layers (i.e., muscle and mucosa) limits wound dehiscence, muscle dysfunction, chin ptosis, and long-term mucogingival problems. Placing the soft-tissue incision too high in the vestibule can negatively affect wound closure, thereby resulting in dehiscence or scar bands with periodontal sequelae (i.e., mucogingival pull) (![]() Videos 11 and 12).
Videos 11 and 12).
Injury to the Teeth
The location of the chin osteotomy should be based on the patient’s chin dysmorphology and a review of the radiographs of the anterior teeth. Injury to the teeth at the time of osteotomy should be an uncommon occurrence. Dental injury may also occur from the fixation screws. If a dental injury is sustained, evaluation by the patient’s dentist, referral to an endodontist, and a discussion between the surgeon and the patient are essential, because root canal therapy and other forms of treatment may be indicated (Fig. 16-24).
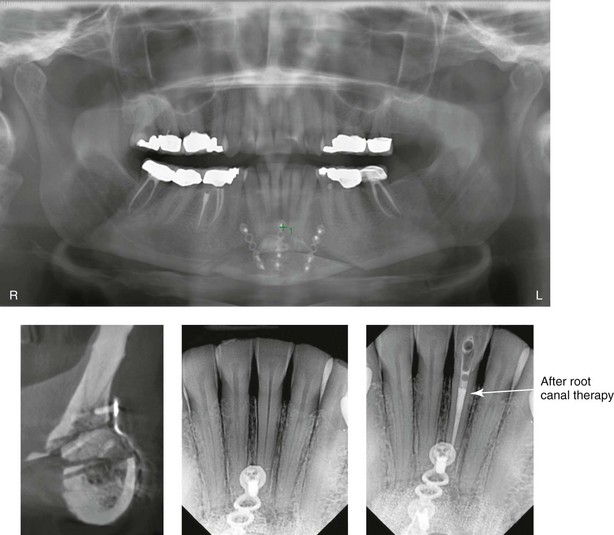
Figure 16-24 An osseous genioplasty was carried out for the vertical lengthening (interpositional graft) and horizontal advancement of the chin. Postoperative radiographs indicate the closeness of screw fixation (1.7 mm wide and 4 mm in length) to the lateral incisor but without root resorption. The darkening of the crown indicated pulpal injury. Root canal therapy and internal bleaching were carried out.
Suboptimal Facial Aesthetics
1. Having a detailed discussion with the patient concerning his or her objectives and reviewing the patient’s overall pretreatment facial and chin morphology and aesthetics (i.e., the recognition of any associated dentofacial deformity) as well as any presenting head and neck dysfunction (e.g., lip incompetence, mentalis strain, obstructive sleep apnea)
2. Making sure that there is a clear understanding between the surgeon and the patient with regard to the operative objectives and any compromises in treatment
3. Having the surgeon meticulously execute the procedures with the patient under controlled anesthesia and with competent assistance and proper instrumentation
4. Having the surgeon perform an objective assessment and then discussing with the patient the results achieved and any shortcomings perceived
References
1. Abu-Id, MH, Warnke, PH, Gottschalk, J, et al. “Bis-phossy jaws” high and low risk factors for bisphosphonate-induced osteonecrosis of the jaw. J Craniomaxillofac Surg. 2008; 36:95–103.
2. Acebal-Bianco, F, Vuylsteke, PLPJ, Mommaerts, MY, De Clerq, CAS. Perioperative complications in corrective facial orthognathic surgery: A 5-year retrospective study. J Oral Maxillofac Surg. 2000; 58:754–760.
3. Ahn, YS, Kim, SG, Baik, SM, et al. Comparative study between resorbable and nonresorbable plates in orthognathic surgery. J Oral Maxillofac Surg. 2010; 68:287–292.
4. Albernaz, VS, Tomsick, TA. Embolisation of arteriovenous fistulae of the maxillary artery after Le Fort I osteotomy: Report of two cases. J Oral Maxillofac Surg. 1995; 53:208.
5. Al-Bishri, A, Barghash, Z, Rosenquist, J, et al. Neurosensory disturbance after sagittal split and intraoral vertical ramus osteotomy: As reported in questionnaires and patients’ records. Int J Oral Maxillofac Surg. 2005; 34:247.
6. Al-Bishri, A, Rosenquist, J, Sunzel, B. On neurosensory disturbance after sagittal split osteotomy. J Oral Maxillofac Surg. 2004; 62:1472–1476.
7. Al-Din, OF, Coghlan, KM, Magennis, P. Sensory nerve disturbance following Le Fort I osteotomy. Int J Oral Maxillofac Surg. 1996; 25:13.
8. Alpert, B, Seligson, D. Removal of asymptomatic bone plate used for orthognathic surgery and facial fractures. J Oral Maxillofac Surg. 1996; 54:618.
9. Alpha, C, O’Ryan, F, Silva, AC, et al. The incidence of postoperative wound healing problems following sagittal ramus osteotomies stabilized with miniplates and monocortical screws. J Oral Maxillofac Surg. 2006; 64:659.
10. Appiah-Anane, S. Amputation neuroma: A late complication following sagittal split osteotomy of the mandible. J Oral Maxillofac Surg. 1991; 49:1218.
11. Aragon, SB, Van Sickels, JE, Dolwick, MF, et al. The effect of orthognathic surgery on mandibular range of motion. J Oral Maxillofac Surg. 1985; 43:938.
12. August, M, Marchona, J, Donady, J, Kaban, L. Neurosensory deficit and functional impairment after sagittal ramus osteotomy: A long-term follow-up study. J Oral Maxillofac Surg. 1998; 56:1231–1235.
13. Ayoub, AF, Lalani, Z, Moos, KF, et al. Complications following orthognathic surgery that required early surgical intervention: Fifteen years’ experience. Int J Adult Orthodon Orthognath Surg. 2001; 16:138.
14. Aziz, SR, Agnihotri, N, Ziccardi, VB. Lobar collapse immediately after orthognathic surgery. J Oral Maxillofac Surg. 2010; 68:2335.
15. Baddour, HM, Watson, J, Erwin, BJ. Life-threatening hemorrhage from a Le Fort I osteotomy. J Oral Maxillofac Surg. 1982; 40:117.
16. Baddour, HM, Watson, J, Erwin, BJ. Injuries to the internal carotid artery following orthognathic surgery. J Oral Maxillofac Surg. 1988; 3:215.
17. Bagheri, S, Meyer, RA, Khan, HA, et al. Microsurgical repair of the peripheral trigeminal nerve after mandibular sagittal split ramus osteotomy. J Oral Maxillofac Surg. 2010; 68:2770–2782.
18. Bailey, PH, Bays, RA. Evaluation of long-term sensory changes following mandibular augmentation procedures. J Oral Maxillofac Surg. 1984; 42:722.
19. Baker, SB, Weinzweig, J, Bartlett, SP, et al. Brain abscess as a complication of orthognathic surgery: Diagnosis, management and pathophysiology. Plast Reconstr Surg. 1999; 104:480.
20. Baqain, ZH, Hyde, N, Patrikidou, A, et al. Antibiotic prophylaxis for orthognathic surgery: A prospective, randomized clinical trial. Br J Oral Maxillofac Surg. 2004; 42:506.
21. Becelli, R, Renzi, G, Carbomni, A, et al. Inferior alveolar nerve impairment after mandibular sagittal split osteotomy: An analysis of spontaneous recovery patterns observed in 60 patients. J Craniofac Surg. 2002; 13:315–320.
22. Becelli, RB, Fini, G, Renzi, G, et al. Complications of bicortical screw fixation observed in 482 mandibular sagittal osteotomies. J Craniofac Surg. 2004; 15:64–68.
23. Behrman, SJ. Complications of sagittal osteotomy of the mandibular ramus. J Oral Surg. 1972; 30:554.
24. Bell, RB, Phillips, C, Manente, SJ. Patients’ expectations following video imaging prior to orthognathic surgery. J Oral Maxillofac Surg. 1997; 55(supplement 3):88–89.
25. Bell, WH. Biologic basis for maxillary osteotomies. Am J Phys Anthropol. 1973; 38:279.
26. Bell, WH, Schendel, SA. Biologic basis for modification of the sagittal ramus split operation. J Oral Surg. 1977; 35:362–369.
27. Bell, WH, You, ZH, Finn, RA, et al. Wound healing after multisegmental Le Fort I osteotomy and transection of the descending palatine vessels. J Oral Maxillofac Surg. 1995; 53:1425.
28. Bendor-Samuel, R, Chen, YR, Chen, PK. Unusual complications of the Le Fort I osteotomy. Plast Reconstr Surg. 1995; 96:1289.
29. Bentley, KC, Head, TW, Aiello, GA. Antibiotic prophylaxis in orthognathic surgery: A 1 day versus 5-day regimen. J Oral Maxillofac Surg. 1999; 57:226.
30. Bercault, N, Boulain, T, Kuteifan, K, et al. Obesity-related excess mortality rate in an adult intensive care unit: A risk-adjusted matched cohort study. Crit Care Med. 2004; 32:998–1003.
31. Berlin, R. Injuries to the optic nerve and ophthalmic artery from fracture of the optic canal. London: Klockman; 1881.
32. Bhaskaran, AA, Courtney, DJ, Anand, P, et al. A complication of Le Fort I osteotomy. Int J Oral Maxillofac Surg. 2010; 39:292.
Bhatt, V, Langford, RJ. Removal of miniplates in maxillofacial surgery: University Hospital Birmingham experience. J Oral Maxillofac Surg. 2003; 61:553–556.
33. Bigoli, SJ, Dumont, L, Mattys, M, et al. A serious anesthetic complication of a Le Fort I osteotomy. Eur J Anaesthesiol. 1999; 16:201.
34. Blackburn, TK, Pritchard, K, Richardson, D. Symptomatic venous thromboembolism after orthognathic operations: An audit. Br J Oral Maxillofac Surg. 2006; 44:389.
35. Blakey, GH, 3rd., Zuniga, JR. Lingual nerve injury associated with superior border wire fixation. Int J Adult Orthodon Orthognath Surg. 1992; 7:115.
36. Blinder, D, Rotenberg, L, Taicher, S. Conversion disorder after maxillofacial trauma and surgery. Int J Oral Maxillofac Surg. 1996; 25:116.
37. Blomqvist, JE, Alberius, P, Isaksson, S. Sensibility following sagittal split osteotomy in the mandible: A prospective clinical study. Plast Reconstr Surg. 1998; 102:325–333.
38. Bloomquist, DS. Intraoperative assessment of maxillary perfusion during Le Fort I osteotomy (discussion). J Oral Maxillofac Surg. 1994; 52:831.
39. Bouwman, JPB, Husak, A, Putman, GD, et al. Screw fixation following bilateral sagittal ramus osteotomy for mandibular advancement—complications in 700 consecutive cases. Br J Oral Maxillofac Surg. 1995; 33:231–234.
40. Brady, S, Courtemanche, A, Steinbok, P. Carotid artery thrombosis after elective mandibular and maxillary osteotomies. Ann Plast Surg. 1981; 6:121.
41. Braun, TW, Sotereanos, GC. Vascular changes in the pterygopalatine fossa after craniofacial dysjunction surgery. J Oral Surg. 1979; 37:88.
42. Broadbend, TR, Woolf, RM. Our experience with sagittal split osteotomy for retrognathia. Plast Reconstr Surg. 1977; 60:860.
43. Brown, RH, Schauble, JF, Miller, NR. Anemia and hypotension as contributors to perioperative loss of vision. Anesthesiology. 1994; 80:222.
44. Browne, RM, Brady, CL, Frame, JW. Tooth pulp changes following Le Fort I maxillary osteotomy in a primate model. Br J Oral Maxillofac Surg. 1990; 28:1.
45. Brusati, R, Fiamminghi, L, Sesenna, E, et al. Functional disturbances of the inferior alveolar nerve in sagittal osteotomy of mandibular ramus: Operating technique for prevention. J Maxillofac Surg. 1981; 9:123.
46. Buijs, GJ, Van Der Houwen, EB, Stegenga, B, et al. Mechanical strength and stiffness of biodegradable and titanium osteofixation systems. J Oral Maxillofac Surg. 2007; 65:2148.
47. Buijs, GJ, van der Houwen, EB, Stegenga, B, et al. Torsion strength of biodegradable and titanium screws: A comparison. J Oral Maxillofac Surg. 2007; 65:2142.
48. Burden, DW. Principles of antimicrobial prophylaxis. World J Surg. 1981; 6:262.
49. Burns, SJ, Dippe, SE. Postoperative wound infections detected during hospital stay and after discharge in a community hospital. Am J Infect Control. 1982; l:60.
50. Calle, EE, Thun, MJ, Petrelli, JM, et al. Body-mass index and mortality in a prospective cohort of US adults. N Eng J Med. 1999; 341:1097–1105.
51. Campbell, RL, Shamaskin, RG, Harkins, SW. Assessment of recovery from injury to inferior alveolar and mental nerves. Oral Surg Oral Med Oral Pathol. 1987; 64:519.
52. Canavan, D, Graff-Radford, S, Gratt, BM. Traumatic dysesthesia of the trigeminal nerve. J Orofac Pain. 1994; 8:391.
53. Caplan, RA, Posner, KL, Ward, RJ, et al. Adverse respiratory events in anesthesia: A closed analysis. Anesthesiology. 1990; 72:828.
54. Carr, RJ, Gilbert, P. Isolated partial third nerve palsy following Le Fort I maxillary osteotomy in a patient with cleft lip and palate. Br J Oral Maxillofac Surg. 1986; 24:206.
55. Chau, MNY, Jönsen, E, Lee, KM. Traumatic neuroma following sagittal mandibular osteotomy. Int J Oral Maxillofac Surg. 1989; 18:95.
56. Chebel, NA, Ziade, D, Achkouty, R. Bilateral pneumothorax and pneumomediastinum after treatment with continuous positive airway pressure after orthognathic surgery. Br J Oral Maxillofac Surg. 2010; 48:e14.
57. Chen, N, Neal, CE, Lingenbrink, P, et al. Neurosensory changes following orthognathic surgery. Int J Adult Orthodon Orthognath Surg. 1999; 14:259.
58. Chen, YR, Fisher, DM. Blindness as a complication of Le Fort I osteotomies: Role of atypical fracture patterns and distortion of the optic canal (discussion). Plast Reconstr Surg. 1998; 102:1422.
59. Cheng, HC, Chi, LH, Wu, JY, et al. Blindness and basal ganglia hypoxia as a complication of Le Fort I osteotomy attributable to hypoplasia of the internal carotid artery: A case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007; 104:e27.
60. Choi, BK, Goh, RCW, Chen, PKT, et al. Facial nerve palsy after sagittal split ramus osteotomy of the mandible: Mechanism and outcomes. J Oral Maxillofac Surg. 2010; 68:1615–1621.
61. Chow, LK, Singh, B, Chiu, WK, et al. Prevalence of postoperative complications after orthognathic surgery: A 15 year review. J Oral Maxillofac Surg. 2007; 65:984.
62. Chua, W, Chidambaram, A, Doyle, PT, et al. Voicing concern: An unusual sequelae of orthognathic surgery. Prog Orthod. 2006; 7:220.
63. Clark, CL, Baur, DA. Management of mentalis muscle dysfunction after advancement genioplasty: a case report. J Oral Maxillofac Surg. 2004; 62:611.
64. Clark, R, Lew, D, Giyanani, VL, et al. False aneurysm complicating orthognathic surgery. J Oral Maxillofac Surg. 1987; 45:57.
65. Coghlan, KM, Irvine, GH. Neurological damage after sagittal split osteotomy. Int J Oral Maxillofac Surg. 1986; 15:369–371.
66. Colella, G, Cannavale, R, Vicidomini, A, et al. Neurosensory disturbance of the inferior alveolar nerve after bilateral sagittal split osteotomy: A systematic review. J Oral Maxillofac Surg. 2007; 65:1707.
67. Connolly, SE, Gordon, KB, Horton, JC. Salvage of vision after hypotension-induced ischemic optic neuropathy. Am J Ophthalmol. 1994; 117:235.
68. Consolo, U, Salgarelli, A. Transient facial nerve palsy following orthognathic surgery: A case report. J Oral Maxillofac Surg. 1992; 50:77.
69. Costas, PD, Heatley, G, Seckel, BR. Normal sensation of the human face and neck. Plast Reconstr Surg. 1994; 93:1141.
70. Cruz, AA, dos Santos, AC. Blindness after Le Fort I osteotomy: A possible complication associated with pterygomaxillary separation. J Craniomaxillofac Surg. 2006; 34:210.
71. Cunningham, LL, Jr., Tiner, BD, Clark, GM, et al. A comparison of questionnaire versus monofilament assessment of neurosensory deficit. J Oral Maxillofac Surg. 1996; 54:454–459.
72. Cunningham, SJ, Crean, SJ, Hunt, NP, Harris, M. Preparation, perception and problem: A long term follow up of orthognathic surgery. Int J Adult Orthodon Orthognath Surg. 1996; 11:41.
73. Cunningham, SJ, Feinmann, C, Horrocks, EN. Psychological problems following orthognathic surgery. J Clin Orthod. 1995; 29:755.
74. D’Orta, JA, Shatney, CH. Post-traumatic pseudoaneurysm of the internal maxillary artery. J Trauma. 1982; 22:161.
75. Daftari, TK, Whitesides, TE, Heller, JG, et al. Nicotine on the revascularization of bone graft: An experimental study in rabbits. Spine. 1994; 19:904.
76. Dan, AE, Thygesen, TH, Pinholt, EM. Corticosteroid administration in oral and orthognathic surgery: A systematic review of the literature and meta-analysis. J Oral Maxillofac Surg. 2010; 68:2207–2220.
77. Danda, AK, Ravi, P. Effectiveness of postoperative antibiotics in orthognathic surgery: A meta-analysis. J Oral Maxillofac Surg. 2011; 69:2650–2656.
78. Danda, AK, Wahab, A, Narayanan, V, Siddareddi, A. Single-dose versus single-day antibiotic prophylaxis for orthognathic surgery: A prospective, randomized, double-blind clinical study. J Oral Maxillofac Surg. 2010; 68:344–346.
79. Dendy, RA. Facial nerve paralysis following sagittal split mandibular osteotomy: A case report. Br J Oral Surg. 1973; 11:101.
80. De Mop van Otterloo, JJ, Tuinzing, DB, Greebe, RB, et al. Intra and early postoperative complications of the Le Fort I osteotomy: A retrospective study on 410 cases. J Craniomaxillofac Surg. 1991; 19:217.
81. De Sousa, A. Psychological issues in oral and maxillofacial reconstructive surgery. J Oral Maxillofac Surg. 1998; 46:661.
82. Dervis, E, Tuncer, E. Long-term evaluations of temporomandibular disorders in patients undergoing orthognathic surgery compared with a control group. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002; 94:554.
83. De Vries, K, Devriese, PP, Hovinga, J, et al. Facial palsy after sagittal split osteotomies: A survey of 1747 sagittal split osteotomies. J Craniomaxillofac Surg. 1993; 21:50.
84. Dimitroulis, G. Complications of orthognathic surgery. Aust Orthod J. 1996; 14:158.
85. Dimopoulos, MA, Kastritis, E, Anagnostopoulos, A, et al. Osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates: Evidence of increased risk after treatment with zoledronic acid. Haematologica. 2006; 91:968–971.
86. Dodson, TB, Kaban, LB. Recommendations for management of trigeminal nerve defects based on a critical appraisal of the literature. J Oral Maxillofac Surg. 1997; 55:1380.
87. Dodson, TB, Neuenschwander, MC. Maxillary perfusion during Le Fort I osteotomy after ligation of the descending palatine artery. J Oral Maxillofac Surg. 1997; 55:51.
88. Donoff, RB. Surgical management of inferior alveolar nerve injuries (Part I): The case for early repair. J Oral Maxillofac Surg. 1995; 53:1327.
89. Doucet, JC, Morrison, AD, Davis, BR, et al. The presence of mandibular third molars during sagittal split osteotomies does not increase the risk of complications. J Oral Maxillofac Surg. 2012; 70:1935–1943.
90. Doucet, JC, Morrison, AD, Davis, BR, et al. Concomitant removal of mandibular third molars during sagittal split osteotomy minimizes neurosensory dysfunction. J Oral Maxillofac Surg. 2012; 70:2153–2163.
91. Edwards, DB, Scheffler, RB, Jackler, I. Postoperative pneumomediastinum and pneumothorax following orthognathic surgery. J Oral Maxillofac Surg. 1986; 44:137.
92. Edwards, RC, Kiely, KD, Eppley, BL. The fate of resorbable poly-L-lactic/polyglycolic acid (LactoSorb) bone fixation devices in orthognathic surgery. J Oral Maxillofac Surg. 2001; 59:19.
93. El Deeb, M, Wolford, L, Bevis, R. Complications of orthognathic surgery. Clin Plast Surg. 1989; 16:825.
94. Ellis, E, III., Dechow, PC, Carlson, DS. A comparison of stimulated bite force after mandibular advancement using rigid and non rigid fixation. J Oral Maxillofac Surg. 1988; 46:26.
95. El-Zawawy, HB, Gill, CS, Wright, RW, et al. Smoking delays chondrogenesis in a mouse model of closed tibial fracture healing. J Orthop Res. 2006; 24:2150.
96. Eppley, BL. Bioabsorbable plate and screw fixation in orthognathic surgery. J Craniofac Surg. 2007; 18:818.
Espeland, L, Hogevold, HA, Stenvik, A. A 3-year patient-centered follow-up of 516 consecutively treated orthognathic surgery patients. Eur J Orthod. 2008; 30:24–30.
97. Essick, GK. Comprehensive clinical evaluation of perioral sensory function. Oral Maxillofac Surg Clin North Am. 1992; 4:503.
98. Essick, GK, Austin, S, Phillips, C, et al. Short-term sensory impairment after orthognathic surgery. Oral Maxillofac Surg Clin North Am. 2001; 13:295.
99. Essick, GK, Phillips, C, Turvey, TA, et al. Facial altered sensation and sensory impairment after orthognathic surgery. Int J Oral Maxillofac Surg. 2007; 36:577.
100. Evans, GRD, Crawley, W, Dellon, AL. Inferior alveolar nerve grafting: An approach without intermaxillary fixation. Ann Plast Surg. 1994; 33:221.
101. Ferdousi, AM, MacGregor, AJ. The response of the peripheral branches of the trigeminal nerve to trauma. Int J Oral Surg. 1985; 14:41.
102. Fiamminghi, L, Aversa, C. Lesions of the inferior alveolar nerve in sagittal osteotomy of the ramus: Experimental study. J Maxillofac Surg. 1979; 7:125.
103. Flynn, NM, Lawrence, RM. Antimicrobial prophylaxis. Med Clin North Am. 1979; 63:1225.
104. Francel, TJ, Birely, BC, Ringelman, PR, Manson, PN. The fate of plates and screws after facial fracture reconstruction. Plast Reconstr Surg. 1992; 90:568.
105. Fridrich, KL, et al. Preoperative antibiotic prophylaxis: A randomised, double-blind and placebo-controlled clinical study. J Oral Maxillofac Surg. 1999; 57:1406–1407.
106. Fridrich, KL, Holton, TJ, Pansegrau, KJ, Berckley, MJ. Neurosensory recovery following the mandibular bilateral sagittal split osteotomy. J Oral Maxillofac Surg. 1995; 53:1300–1306.
107. Fridrich, KL, Partnoy, BE, Williamson, LW. Closed intraoral wound suction: A prospective analysis of 65 cases and a review of the literature. Int J Adult Orthodon Orthognath Surg. 1990; 5:183–187.
108. Fridrich, KL, Partnoy, BE, Zeitler, D. Prospective analysis of antibiotic prophylaxis for orthognathic surgery. Int J Adult Orthodon Orthognath Surg. 1994; 9:129–131.
109. Fujioka, M, Hirano, A, Fujii, T. Comparative study of inferior alveolar disturbance restoration after sagittal split osteotomy by means of bicortical versus monocortical osteosynthesis. Plast Reconstr Surg. 1998; 102:37–41.
110. Gallagher, DM, Epker, BN. Infection following intraoral surgical correction of dentofacial deformities. J Oral Surg. 1980; 38:117–120.
111. Garner, JS, Jarvis, WR, Emori, TG, et al. CDC definitions for nosocomial infections 1988. Am J Infect Control. 1988; 16:128.
112. Geha, HJ, Gleizal, AM, Nimeskern, NJ, et al. Sensitivity of the inferior lip and chin following mandibular bilateral sagittal split osteotomy using piezosurgery. Plast Reconstr Surg. 2006; 118:1598.
113. Gent, JF, Shafer, DM, Frank, ME. The effect of orthognathic surgery on taste function on the palate and tongue. J Oral Maxillofac Surg. 2003; 61:766.
114. Gianni, AB, D’Orto, O, Biglioli, F, et al. Neurosensory alterations of the inferior alveolar and mental nerve after genioplasty alone or associated with sagittal osteotomy of the mandibular ramus. J Craniomaxillofac Surg. 2002; 30:295.
115. Girotto, JA, Davidson, J, Wheatly, M, et al. Blindness as a complication of Le Fort osteotomies: Role of atypical fracture patterns and distortion of the optic canal. Plast Reconstr Surg. 1998; 102:1409.
116. Goffinet, L, Laure, B, Tayeb, T, et al. An arteriovenous fistula of the maxillary artery as a complication of Le Fort I osteotomy. J Craniomaxillofac Surg. 2010; 38:251.
117. Goodson, ML, Manemi, R, Paterson, AW. Pneumothorax after orthognathic surgery. Br J Oral Maxillofac Surg. 2010; 48:180.
118. Grammer, FC, Meyer, MW, Ricater, KC. A radioisotope study of the vascular response to sagittal split osteotomy of the mandibular ramus. J Oral Surg. 1974; 32:578.
119. Greenberg, RN, James, RN, Marier, RL, et al. Microbiologic and antibiotic aspects of infections in the oral and maxillofacial region. J Oral Surg. 1979; 37:873.
120. Gregg, JM. Studies of traumatic neuralgias in the maxillofacial region: Surgical pathology and neural mechanisms. J Oral Maxillofac Surg. 1990; 48:228.
121. Gregg, JM. Surgical management of inferior alveolar nerve injuries (Part II): The case for delayed management. J Oral Maxillofac Surg. 1995; 53:1330.
122. Grime, PD, Tyler, C. An obstructed airway: Cuff herniation during nasotracheal anesthesia for a bimaxillary osteotomy. Br J Oral Maxillofac Surg. 1991; 29:14.
123. Gruber, EA, Bhaskaran, A, Anand, P, et al. A complication of Le Fort I osteotomy—a case report and review of the literature. Br J Oral Maxillofac Surg. 2008; 46:e25.
124. Gunaseelan, R, Anantanarayanan, P, Veerabahu, M, et al. Intraoperative and perioperative complications in anterior maxillary osteotomy: A retrospective evaluation of 103 patients. J Oral Maxillofac Surg. 2009; 67:1269.
125. Gutta, R, Louis, PJ, Birmingham, AL. Bisphosphonates and osteonecrosis of the jaws: Science and rationale. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007; 104:186–189.
126. Habal, M. A carotid cavernous sinus fistula after maxillary osteotomy. Plast Reconstr Surg. 1986; 77:981.
127. Haers, PE, Sailer, HF. Biodegradable self-reinforced poly-L/DL-lactide plate and screws in bimaxillary orthognathic surgery: short term skeletal stability and material related failures. J Craniomaxillofac Surg. 1998; 26:363.
128. Hall, HD, Apparent ischemic necrosis after maxillary osteotomy: Report of three cases. Case reports and outlines of selected scientific session. 1978.
129. Hanu-Cernat, LM, Hall, T. Late onset of abducens palsy after Le Fort I maxillary osteotomy. Br J Oral Maxillofac Surg. 2009; 47:416.
130. Harada, K, Enomoto, S. Stability after surgical correction of mandibular prognathism using the sagittal split ramus osteotomy and fixation with poly-L-lactic acid screws. J Oral Maxillofac Surg. 1997; 55:464.
131. Haug, RH. Clinical controversies in oral and maxillofacial surgery: I. Retention of asymptomatic bone plates used for orthognathic surgery and facial fractures. J Oral Maxillofac Surg. 1996; 54:611.
132. Hegtvedt, AK, Zuniga, JR. Lingual nerve injury as a complication of rigid fixation of the sagittal ramus osteotomy: Report of a case. J Oral Maxillofac Surg. 1990; 48:647.
133. Heit, JM, Farhood, VW, Edwards, RC. Survey of antibiotic prophylaxis for intraoral orthognathic surgery. J Oral Maxillofac Surg. 1991; 49:340–342.
134. Hemmig, S, Johnson, RS, Ferraro, N. Management of a ruptured pseudoaneurysm of the sphenopalatine artery following a Le Fort I osteotomy. J Oral Maxillofac Surg. 1987; 45:533.
135. Herold, J, Falworth, M. Sub-total unilateral oculomotor nerve palsy in Le Fort I osteotomy. Br J Oral Maxillofac Surg. 1996; 34:104.
136. Hes, J, de Man, K. Carotid-cavernous sinus fistula following maxillofacial trauma and orthognathic surgery. Int J Oral Maxillofac Surg. 1988; 17:295.
137. Hollinger, JO, Schmitt, JM, Hwang, K, et al. Impact of nicotine on bone healing. J Biomed Mater Res. 1999; 45:294.
138. Hori, M, Okaue, M, Hasegawa, M, et al. Worsening of pre-existing TMJ dysfunction following sagittal split osteotomy: A study of three cases. J Oral Sci. 1999; 41:133.
139. Hovinga, J, Kraal, ER, Roorda, LAM. A follow-up of osteotomies for dysgnathia. J Maxillofac Surg. 1979; 7:271.
140. Humber, CC, Lanigan, DT, Hohn, FI. Retrograde hemorrhage (hemolacria) from the lacrimal puncta after a Le Fort I osteotomy: A report of 2 cases and a review of the literature. J Oral Maxillofac Surg. 2011; 69:520.
141. Jaaskelainen, SK, Peltola, JK, Forssell, K, et al. Evaluating function of the inferior alveolar nerve with repeated nerve conduction tests during mandibular sagittal split osteotomy. J Oral Maxillofac Surg. 1995; 53:269.
142. Jaaskelainen, SK, Peltola, JK, Lehtinen, R. The mental nerve blink reflex in the diagnosis of lesions of the inferior alveolar nerve following orthognathic surgery of the mandible. Br J Oral Maxillofac Surg. 1996; 34:87.
143. Jaaskelainen, SK, Teerijoki-Oksa, T, Forssell, K, et al. Intraoperative monitoring of the inferior alveolar nerve during mandibular sagittal-split osteotomy. Muscle Nerve. 2000; 23:368.
144. Jaaskelainen, SK, Teerijoki-Oksa, T, Virtanen, A, et al. Sensory regeneration following intraoperatively verified trigeminal nerve injury. Neurology. 2004; 62:1951.
145. Jacks, SC, Zuniga, JR, Turvey, TA, et al. A retrospective analysis of lingual nerve sensory changes after mandibular bilateral sagittal split osteotomy. J Oral Maxillofac Surg. 1998; 56:700.
146. Jackson, L. Outpatient orthognathic surgery: Review of 205 cases (discussion). J Oral Maxillofac Surg. 1997; 55:563.
147. Jerjes, W, Swinson, B, Moles, DR, et al. Permanent sensory nerve impairment following third molar surgery: A prospective study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006; 102:e1.
148. Johnson, MW, Kincaid, MC, Trobe, JD. Bilateral retrobulbar optic nerve infarctions after blood loss and hypotension: A clinicopathologic case study. Ophthalmology. 1987; 94:1577.
149. Jones, JK, Triplett, RG. The relationship of cigarette smoking to impaired intraoral wound healing: A review of evidence and implications for patient care. J Oral Maxillofac Surg. 1992; 50:237.
150. Jones, JK, Van Sickels, JE. Facial nerve injuries associated with orthognathic surgery: A review of incidence and management. J Oral Maxillofac Surg. 1991; 49:740.
151. Jorgenson, DS, Mayer, MH, Ellenbogen, RG, et al. Detection of titanium in human tissues after craniofacial surgery. Plast Reconstr Surg. 1997; 99:976.
152. Junior, SML, Granato, R, Marin, C, et al. Analysis of 40 cases of intraoral verticosagittal ramus osteotomies to treat dentofacial deformities. J Oral Maxillofac Surg. 2009; 67:1840.
153. Kademani, D, Voiner, JL, Quinn, PD. Acute hypertensive crisis resulting in pulmonary edema and myocardial ischemia during orthognathic surgery. J Oral Maxillofac Surg. 2004; 62:240.
154. Kahnberg, KE, Engstrom, H. Recovery of maxillary sinus and tooth sensibility after Le Fort I osteotomy. Br J Oral Maxillofac Surg. 1987; 25:68.
155. Kallela, I, Laine, P, Suuronen, R, et al. Assessment of material- and technique-related complications following sagittal split osteotomies stabilized by biodegradable polylactide screws. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005; 99:4.
156. Karabouta, J, Martis, C. The TMJ dysfunction syndrome before and after sagittal split osteotomy of the rami. J Maxillofac Surg. 1985; 13:185.
157. Karas, ND, Boyd, SB, Sinn, DP. Recovery of neurosensory function following orthognathic surgery. J Oral Maxillofac Surg. 1990; 48:124.
158. Keighley, MRB. A rational approach to antimicrobial prophylaxis in surgery. World J Surg. 1982; 6:327.
159. Keiser, GJ, Bozentka, NE, Gold, BD. Laryngeal granuloma: A complication of prolonged endotracheal intubation. Anesth Prog. 1991; 38:232.
160. Kersarwani, A, Antonyshyn, O, Mackinnon, SE, et al. Facial sensibility testing in the normal and post traumatic population. Ann Plast Surg. 1989; 22:416.
161. Kim, H-S, Kho, H-K, Kim, S-W, et al. Reliability and characteristics of current perception thresholds in the territory of the infraorbital and inferior alveolar nerves. J Orofac Pain. 2000; 14:286.
162. Kim, SG, Park, SS. Incidence of complications and problems related to orthognathic surgery. J Oral Maxillofac Surg. 2007; 65:2438.
163. Kim, YG, Oh, SH. Effect of mandibular setback surgery on occlusal force. J Oral Maxillofac Surg. 1997; 55:121.
164. Kim, YK, Yeo, HH, Lim, SC. Tissue response to titanium plate: A transmitted electron microscopic study. J Oral Maxillofac Surg. 1997; 55:322.
165. Kin, BC, Padwa, BL, Park, HS, Jung, YS. Stability of maxillary position after Le Fort I osteotomy using self-reinforced biodegradable poly-70L/30DL-lactide miniplates and screws. J Oral Maxillofac Surg. 2011; 69:1442–1446.
166. Kipp, DP, Goldstein, BH, Weiss, WW. Dysesthesia after mandibular third molar surgery in retrospective study and analysis of 1,377 surgical procedures. J Am Dent Assoc. 1980; 100:185.
167. Kiyak, HA, McNeill, RW, West, RA. The emotional impact of orthognathic surgery and conventional orthodontics. Am J Orthod. 1985; 88:224.
168. Klemm, E, Stösslein, F, Mürbe, B. Arteriovenous fistula of the maxillary artery, eustachian tube dysfunction and tinnitus after Le Fort I osteotomy [article in German]. HNO. 2001; 49:216.
169. Kobayashi, A, Yoshimasu, H, Kobayashi, J, et al. Neurosensory alteration in the lower lip and chin area after orthognathic surgery: Bilateral sagittal split osteotomy versus inverted L ramus osteotomy. J Oral Maxillofac Surg. 2006; 64:778.
170. Kohno, M, Nakajima, T, Someya, G. Effects of maxillomandibular fixation on respiration. J Oral Maxillofac Surg. 1993; 51:992.
171. Kooke, R, Egyedi, P. Postoperative contamination of mandibular osteotomy sites with saliva. Int J Oral Maxillofac Surg. 1987; 16:554.
172. Kopelman, PG. Obesity as a medical problem. Nature. 2000; 404:635–643.
173. Kramer, FJ, Baethge, C, Swennen, G, et al. Intra-and perioperative complications of the Le Fort I osteotomy: A prospective evaluation of 1000 patients. J Craniofac Surg. 2004; 15:971.
174. Kriwalsky, MS, Maurer, P, Veras, RB, et al. Risk factors for a bad split during sagittal split osteotomy. Br J Oral Maxillofac Surg. 2008; 46:177–179.
175. Krobbuaban, B, Diregpoke, S, Kumkeaw, S, et al. The predictive value of the height ratio and thyromental distance: Four predictive tests for difficulty laryngoscopy. Anesth Analg. 2005; 101:1542.
176. Krueger, JK, Rohrich, RJ. Clearing the smoke: The scientific rationale for tobacco abstention with plastic surgery. Plast Reconstr Surg. 2001; 108:1063.
Kuhlefelt, M, Laine, P, Suominen, AL, et al. Smoking as a significant risk factor for infections after orthognathic surgery. J Oral Maxillofac Surg. 2012; 70:1643–1647.
177. Lai, JP, Hseih, CH, Chen, YR, et al. Unusual late vascular complications of sagittal split osteotomy of the mandibular ramus. J Craniofac Surg. 2005; 16:664.
178. Landes, CA, Ballon, A. Skeletal stability in bimaxillary orthognathic surgery: P(L/DL)LA-resorbable versus titanium osteofixation. Plast Reconstr Surg. 2006; 118:703.
179. Lanigan, DT. Wound healing after multisegmental Le Fort I osteotomy and transection of the descending palatine vessels (discussion). J Oral Maxillofac Surg. 1995; 53:1433.
180. Lanigan, DT. The inherent risks of the pterygomandibular dysjunction and maxillary downfracture. Jpn J Jaw Deform. 2000; 10:158.
181. Lanigan, DT, Guest, P. Alternative approaches to pterygomaxillary separation. Int J Oral Maxillofac Surg. 1993; 22:131.
182. Lanigan, DT, Hey, JH, West, RA. Aseptic necrosis following maxillary osteotomies: Report of 36 cases. J Oral Maxillofac Surg. 1990; 48:142.
183. Lanigan, DT, Hey, JH, West, RA. Major vascular complications of orthognathic surgery: Hemorrhage associated with Le Fort I osteotomies. J Oral Maxillofac Surg. 1990; 48:561.
184. Lanigan, DT, Hey, JH, West, RA. Hemorrhage following mandibular osteotomies: A report of 21 cases. J Oral Maxillofac Surg. 1991; 49:713.
185. Lanigan, DT, Hey, JH, West, RA. Major vascular complications of orthognathic surgery: False aneurysms and arteriovenous fistulas following orthognathic surgery. J Oral Maxillofac Surg. 1991; 49:571.
186. Lanigan, DT, Hohn, FI. Facial nerve injuries after sagittal split mandibular ramus osteotomies for advancement: A report of 2 cases and review of the literature. J Oral Maxillofac Surg. 2004; 62:503.
187. Lanigan, DT, Loewy, J. Postoperative computed tomography scan study of the pterygomaxillary separation during the Le Fort I osteotomy using a micro-oscillating saw. J Oral Maxillofac Surg. 1995; 53:1161.
188. Lanigan, DT, Romanchuk, K, Olson, CK. Ophthalmic complications associated with orthognathic surgery. J Oral Maxillofac Surg. 1993; 51:480.
189. Lanigan, DT, Tubman, DE. Carotid-cavernous sinus fistula following Le Fort I osteotomy. J Oral Maxillofac Surg. 1987; 45:969.
190. Lanigan, DT, West, RA. Management of postoperative hemorrhage following the Le Fort I maxillary osteotomy. J Oral Maxillofac Surg. 1984; 42:367.
191. Lanigan, DT, West, RA. Aseptic necrosis of the mandible: Report of two cases. J Oral Maxillofac Surg. 1990; 48:296.
192. Laureano-Filho, JR, de Oliveiro Neto, PJ, Duarte, N, et al. Successful management of malignant hyperthermia during orthognathic surgery: A case report. J Oral Maxillofac Surg. 2008; 66:1485.
193. Lee, JG, Kim, SG, Lim, KJ, et al. Thermographic assessment of inferior alveolar nerve injury in patients with dentofacial deformity. J Oral Maxillofac Surg. 2007; 65:74.
194. Lee, SH, Lee, SH, Mori, Y, et al. Evaluation of pterygomaxillary anatomy using computed tomography: Are there any structural variations in cleft patients? J Oral Maxillofac Surg. 2011; 69:2644–2649.
195. Leira, JI, Gillhuus-Moe, OT. Sensory impairment following sagittal split osteotomy for correction of mandibular retrognathism. Int J Adult Orthodon Orthognath Surg. 1991; 6:161–167.
196. Lemke, RR, Clark, GM, Bays, RA, et al. Effects of hypesthesia on oral behaviors of the orthognathic surgery patient. J Oral Maxillofac Surg. 1998; 56:153.
197. Lemke, RR, Rugh, JD, Van Sickels, J, et al. Neurosensory differences after wire and rigid fixation in patients with mandibular advancement. J Oral Maxillofac Surg. 2000; 59:1354–1359.
198. Lenz, JH, Steiner-Krammer, B, Schmidt, W, et al. Does avascular necrosis of the jaws in cancer patients only occur following treatment with bisphosphonates? J Craniomaxillofac Surg. 2005; 33:395–403.
199. Li, KK, Meara, JG, Rubin, PAD. Orbital compartment syndrome following orthognathic surgery. J Oral Maxillofac Surg. 1995; 53:964.
200. Li, KK, Stephens, W. Fractures of the atrophic, edentulous maxilla during Le Fort I osteotomy. Int J Oral Maxillofac Surg. 1996; 25:430.
201. Lindeboom, JA, Baas, EM, Kroon, FH. Prophylactic single-dose administration of 600 mg clindamycin versus 4-time administration of 600 mg clindamycin in orthognathic surgery: A prospective randomised study in bilateral mandibular sagittal ramus osteotomies. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003; 95:145–149.
202. Little, C, Mintz, S, Ettinger, AC. The distal lacrimal ductal system and traumatic epiphora. Int J Oral Maxillofac Surg. 1991; 20:31.
203. Lo, LJ, Hung, KF, Chen, YR. Blindness as a complication of Le Fort I osteotomy for maxillary distraction. Plast Reconstr Surg. 2002; 109:688.
204. Lowry, JC. Thromboembolic disease and thromboprophylaxis in oral and maxillofacial surgery: Experience and practice. Br J Oral Maxillofac Surg. 1995; 33:101.
205. Lustbader, DP, Schwartz, MH, Zito, J, et al. The use of percutaneous transcatheter embolisation to control postoperative bleeding following Le Fort I osteotomy: Report of three cases. J Oral Maxillofac Surg. 1991; 49:426.
206. Macintosh, RB. Experience with the sagittal osteotomy of the mandibular ramus: A 13 year review. J Oral Maxillofac Surg. 1981; 9:151.
207. Madani, M, Veznedaroglu, E, Pazoki, A, et al. Pseudoaneurysm of the facial artery as a late complication of bilateral sagittal split osteotomy and facial trauma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010; 110:579.
208. Mainous, EG, Crowell, NT. Nasal septum perforation following total maxillary osteotomy: Report of a case. J Oral Surg. 1973; 31:869.
209. Malmgren, B, Aström, E, Soderhall, S. No osteonecrosis in jaws of young patients with osteogenesis imperfecta treated with bisphosphonates. J Oral Pathol Med. 2008; 37:196–200.
210. Manikandhan, R, Anantanarayanan, P, Mathew, PC, et al. Incidence and consequences of bur breakage in orthognathic surgery: A retrospective study with discussion of 2 interesting clinical situations. J Oral Maxillofac Surg. 2011; 69:2442–2447.
211. Marais, J, Brookes, GB. Secretomotor rhinopathy after Le Fort I maxillary osteotomy. Case report. Int J Oral Maxillofac Surg. 1993; 22:17.
212. Martis, CS. Complications after mandibular sagittal split osteotomy. J Oral Maxillofac Surg. 1984; 42:101–107.
213. Martis, CS, Karabouta, I. Infection after orthognathic surgery with and without preventive antibiotics. Int J Oral Surg. 1984; 13:490.
214. Marx, RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J Oral Maxillofac Surg. 2003; 61:1115–1117.
215. Marx, RE, Cillo, JE, Jr., Ulloa, JJ. Oral bisphosphonate-induced osteonecrosis: Risk factors, prediction of risk using serum CTX testing, prevention, and treatment. J Oral Maxillofac Surg. 2007; 65:2397–2410.
216. Maurer, P, Otto, C, Eckert, AW, et al. Actinomycosis as a rare complication of orthognathic surgery. Int J Adult Orthodon Orthognath Surg. 2002; 17:230.
217. McKenzie, WS, Rosenberg, M. Iatrogenic subcutaneous emphysema of dental and surgical origin: A literature review. J Oral Maxillofac Surg. 2009; 67:1265.
218. Mehra, P, Castro, V, Freitas, RZ, Wolford, LM. Complications of the mandibular sagittal split ramus osteotomy associated with the presence or absence of third molars. J Oral Maxillofac Surg. 2001; 59:854–858.
219. Meyer, MW, Cavanaugh, GD. Blood flow changes after orthognathic surgery: Maxillary and mandibular subapical osteotomy. J Oral Surg. 1976; 34:495.
220. Migliorati, CA, Schubert, MM, Peterson, DE, Seneda, LM. Bisphosphonate-associated osteonecrosis of mandibular and maxillary bone: An emerging oral complication of supportive cancer therapy. Cancer. 2005; 104:83–93.
221. Milles, M, Desjardins, J. Reduction of postoperative facial swelling by low-dose methylprednisolone: An experimental study. J Oral Maxillofac Surg. 1993; 51:987.
222. Mirzai, R, Chang, C, Greenspan, A, Gershwin, ME. The pathogenesis of osteonecrosis and the relationships to corticosteroids. J Asthma. 1999; 36:77–95.
223. Monaghan, A, Hindle, I. Malignant hyperpyrexia in oral surgery: Case report and literature review. Br J Oral Maxillofac Surg. 1994; 32:190.
224. Morris, DE, Lo, LJ, Margulis, A. Pitfalls in orthognathic surgery: Avoidance and management of complications. Clin Plast Surg. 2007; 34:17.
225. Mosbah, MR, Oloyede, D, Koppel, DA, et al. Miniplate removal in trauma and orthognathic surgery—a retrospective study. Int J Oral Maxillofac Surg. 2003; 32:148–151.
226. Motamedi, MH. Transient temporal nerve paresis after intraoral subcondylar ramus osteotomy. J Oral Maxillofac Surg. 1997; 55:527.
227. Mucci, SJ, Dellon, AL. Restoration of lower-lip sensation: Neurotization of the mental nerve with the supraclavicular nerve. J Reconstr Microsurg. 1997; 13:151.
228. Nagler, RM, Peled, M, Laufer, D. Prolonged dysphagia after orthognathic surgery: Report of a case and review of the literature. J Oral Maxillofac Surg. 1996; 54:523.
229. Nakagawa, K, Ueki, K, Matsumoto, N, et al. The assessment of trigeminal sensory nerve paraesthesia after bilateral sagittal split osteotomy: Modified somatosensory evoked potentials recording method. J Oral Maxillofac Surg. 1997; 25:97.
230. Nakagawa, K, Ueki, K, Takatsuka, S, et al. Somatosensory-evoked potential to evaluate the trigeminal nerve after sagittal split osteotomy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001; 91:146.
231. Nakagawa, K, Ueki, K, Takatsuka, S, et al. Trigeminal nerve hypesthesia after sagittal split osteotomy in setback cases: Correlation of postoperative computed tomography and long-term trigeminal somatosensory evoked potentials. J Oral Maxillofac Surg. 2003; 61:898.
232. Nanda, R, Sugawara, J. Mandibular adaptations following total maxillary osteotomy in adolescent monkeys. Am J Orthod. 1983; 83:485.
233. Nanni, V, Sachs, S. Mediastinal emphysema following Le Fort I osteotomy: Report of a case. Oral Surg Oral Med Oral Pathol. 1986; 62:508.
234. Naples, RJ, Van Sickels, JE, Jones, DL. Long-term neurosensory deficits associated with bilateral sagittal split osteotomy versus inverted “L” osteotomy. Oral Surg Oral Med Oral Pathol. 1994; 77:318.
235. Nelson, RL, Path, MG, Ogle, RG, et al. Quantitation of blood flow after Le Fort I osteotomy. J Oral Surg. 1977; 35:10.
236. Newhouse, RF, Schow, SR, Kraut, RA, et al. Life-threatening hemorrhage from a Le Fort I osteotomy. J Oral Maxillofac Surg. 1982; 40:117.
237. Newlands, C, Dixon, A, Altman, K. Ocular palsy following Le Fort I osteotomy: A case report. Int J Oral Maxillofac Surg. 2004; 33:101.
238. Nishioka, GJ, Mason, M, Van Sickels, JE. Neurosensory disturbance associated with the anterior mandibular horizontal osteotomy. J Oral Maxillofac Surg. 1988; 46:107.
239. Nishioka, GJ, Zysset, MK, Van Sickels, JE. Neurosensory disturbance with rigid fixation of the bilateral sagittal split osteotomy. J Oral Maxillofac Surg. 1987; 45:20.
240. Nout, E, Mathijssen, IM, van der Meulen, JJ, et al. Internal carotid dissection after Le Fort III distraction in Apert syndrome: A case report. J Craniomaxillofac Surg. 2010; 38:529–533.
241. O’Regan, B, Bharadwaj, G. Prospective study of the incidence of serious posterior maxillary hemorrhage during a tuberosity osteotomy in low level Le Fort I operations. Br J Oral Maxillofac Surg. 2007; 45:538.
242. O’Ryan, F. Complications of orthognathic surgery. Part II. Maxillary and two-jaw surgery. Sel Readings Oral Maxillofac Surg. 1989; 1:21–30.
243. O’Ryan, F. Complications of orthognathic surgery. Oral Maxillofac Surg Clin North Am. 1990; 2:602.
244. O’Ryan, F, Complications with orthognathic surgery. Oral and maxillofacial surgery. Ch 30. Fonseca, R, Turvey, T, eds. Oral and maxillofacial surgery. Ch 30; Vol III. Missouri: Saunders Elsevier Co, St Louis, 2009.
245. O’Ryan, F, Ebker, BN. Prolonged apnea after orthognathic surgery due to atypical cholinesterase. Int J Oral Surg. 1981; 10:338.
246. O’Ryan, F, Poor, DB, Hattori, M. Intraoperative angioedema induced by angiotensin-converting enzyme inhibitors: Overview and case report. J Oral Maxillofac Surg. 2005; 63:551.
247. Olson, RE, Laskin, DM. Expectations of patients from orthognathic surgery. J Oral Surg. 1980; 38:283.
248. Ortega, C, Montemurro, F, Faggiuolo, R, et al. Osteonecrosis of the jaw in prostate cancer patients with bone metastases treated with zoledronate: A retrospective analysis. Acta Oncol. 2007; 46:664–668.
249. Ozaki, W, Abubaker, AO, Sotereanos, GC, Patterson, GT. Cervicofacial actinomycosis following sagittal split ramus osteotomy: A case report. J Oral Maxillofac Surg. 1992; 50:649.
250. Pagar, DM, Kupperman, AW, Stern, M. Cutting of nasoendotracheal tube: An unusual complication of maxillary osteotomies. J Oral Surg. 1978; 36:314.
251. Pames, El, Becker, ML. Necrosis of the anterior maxilla following osteotomy: Report of a case. Oral Surg. 1972; 33:326.
252. Panula, K, Finne, K, Oikarinen, K. Incidence of complications and problems related to orthognathic surgery: A review of 655 patients. J Oral Maxillofac Surg. 2001; 59:1128–1136.
253. Pappa, H, Richardson, D, Niven, S. False aneurysm of the facial artery as a complication of sagittal split osteotomy. J Craniomaxillofac Surg. 2008; 36:180.
254. Patel, PK, Morris, DE, Gassmann, A. Complications of orthognathic surgery. J Craniofac Surg. 2007; 18:975.
255. Pereira, FL, Yaedu, RY, Sant’Ana, AP, Sant’Ana, E. Maxillary aseptic necrosis after Le Fort I osteotomy: A case report and literature review. J Oral Maxillofac Surg. 2010; 68:1402–1407.
256. Pereira-Filho, VA, Gabrielli, MF, Gabrielli, MA, et al. Incidence of maxillary sinusitis following Le Fort I osteotomy: Clinical, radiographic, and endoscopic study. J Oral Maxillofac Surg. 2011; 69:346–351.
257. Peterson, LJ, Booth, DF. Efficacy of antibiotics prophylaxis in intraoral orthognathic surgery. J Oral Surg. 1976; 34:1088.
258. Phillips, C, Essick, G, Blakey, G, III., et al. Relationship between patients’ perceptions of postsurgical sequelae and altered sensations after bilateral sagittal split osteotomy. J Oral Maxillofac Surg. 2007; 65:597.
259. Piecuch, JF, West, RA. Spontaneous pneumomediastinum associated with orthognathic surgery: A case report. Oral Surg Oral Med Oral Pathol. 1979; 48:506.
260. Pogrel, MA, Jergensen, R, et al. Long-term outcome of trigeminal nerve injuries related to dental treatment. J Oral Maxillofac Surg. 2011; 69:2284–2288.
261. Pogrel, MA, Le, H. Etiology of lingual nerve injuries in the third molar region: A cadaver and histologic study. J Oral Maxillofac Surg. 2006; 64:1790–1794.
Poort, LJ, van Neck, JW, van der Wal, KG. Sensory testing of inferior alveolar nerve injuries: A review of methods used in prospective studies. J Oral Maxillofac Surg. 2009; 67:292–300.
262. Posnick, JC, Orthognathic Surgery: General considerations; complications; and patient educational material. Craniofacial and maxillofacial surgery in children and young adults. Posnick, JC, eds. Craniofacial and maxillofacial surgery in children and young adults; 42. WB Saunders Co, Philadelphia, 2000:1103–1112.
263. Posnick, JC, Al-Qattan, MM, Pron, G. Facial sensibility in adolescents with and without clefts 1 year after undergoing Le Fort I osteotomy. Plast Reconstr Surg. 1994; 94:431.
264. Posnick, JC, Al-Qattan, MM, Pron, GE, Grossman, JA. Facial sensibility in adolescents born with cleft lip after undergoing repair in infancy. Plast Reconstr Surg. 1994; 93:682.
265. Posnick, JC, Al-Qattan, MM, Stepner, NM. Alteration in facial sensibility in adolescents following sagittal split and chin osteotomies of the mandible. Plast Reconstr Surg. 1996; 97(5):920–927.
266. Posnick, JC, Grossman, JAI. Facial sensibility testing: A clinical update. Plast Reconstr Surg. 2000; 106(4):892–894.
267. Posnick, JC, Zimbler, AG, Grossman, JA. I. Normal cutaneous sensibility of the face. Plast Reconstr Surg. 1990; 86:429.
268. Precious, DS, Lung, KE, Pynn, BR, Goodday, RH. Presence of impacted teeth as a determining factor of unfavourable splits in 1256 sagittal-split osteotomies. Oral Surg. 1998; 85:362–365.
269. Precious, DS, Powell, JE, Tuzuner, A, et al. False aneurysms after sagittal split ramus osteotomies. J Oral Maxillofac Surg. 2012; 70:e58–e65.
270. Procopio, O, Fusetti, S, Liessi, G, Ferronato, G. False aneurysm of the sphenopalatine artery after a Le Fort I osteotomy: Report of 2 cases. J Oral Maxillofac Surg. 2003; 61:520–524.
271. Quejada, JG, Kawamura, H, Finn, RA, et al. Wound healing associated with segmental total maxillary osteotomy. J Oral Maxillofac Surg. 1986; 44:366.
272. Rai, KK, Shivakumar, HR, Sonar, MD. Transient facial nerve palsy following bilateral sagittal split ramus osteotomy for setback of the mandible: A review of incidence and management. J Oral Maxillofac Surg. 2008; 66:373.
273. Rasmussen, OC. Painful traumatic neuromas in the oral cavity. Oral Surg Oral Med Oral Pathol. 1980; 49:191.
274. Reiner, S, Willoughby, JH. Transient abducens nerve palsy following a Le Fort I maxillary osteotomy: Report of a case. J Oral Maxillofac Surg. 1988; 46:699.
275. Renton, T, Hankins, M, Sproate, C, et al. A randomised controlled clinical trial to compare the incidence of injury to the inferior alveolar nerve as a result of coronectomy and removal of mandibular third molars. Br J Oral Maxillofac Surg. 2005; 43:7.
276. Reyneke, JP, Tsakiris, P, Becker, P. Age as a factor in the complication rate after removal of unerupted/impacted third molars at the time of mandibular sagittal split osteotomy. J Oral Maxillofac Surg. 2002; 60:654–659.
277. Robinson, PP. Observations on the recovery of sensation following inferior alveolar nerve injuries. Br J Oral Maxillofac Surg. 1988; 26:177.
278. Robinson, PP, Hendy, CW. Pterygoid plate fractures caused by the Le Fort I osteotomy. Br J Oral Maxillofac Surg. 1986; 24:198.
279. Robinson, RC, Williams, CW. Documentation method for inferior alveolar and lingual nerve paresthesias. Oral Surg Oral Med Oral Pathol. 1986; 62:128.
280. Rogers, SN, Patel, M, Beirne, JC, Nixon, TE. Traumatic aneurysm of the maxillary artery: The role of interventional radiology. A report of two cases. Int J Oral Maxillofac Surg. 1995; 24:336.
281. Rosenberg, A, Gratz, KW, Sailer, HF. Should titanium miniplates be removed after bone healing is complete? Int J Oral Maxillofac Surg. 1993; 22:185.
282. Rosendorf, LL, Octavia, J, Estes, JP. Effect of methods of postdischarge wound infection surveillance on reported infection rates. Am J Infect Control. 1983; 11:226.
283. Ruggiero, SL, Mehrotra, B, Rosenberg, TJ, Engroff, SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: A review of 63 cases. J Oral Maxillofac Surg. 2004; 62:527–534.
284. Ruggles, JE, Hann, JR. Antibiotic prophylaxis in intraoral orthognathic surgery. J Oral Maxillofac Surg. 1984; 42:797–801.
285. Russell, RG, Rogers, MJ. Bisphosphonates: From the laboratory to the clinic and back again. Bone. 1999; 25:97–106.
286. Sakai, Y, Kobayashi, S, Sekiguchi, J, Ohmori, K. New method of endoscopic pterygomaxillary disjunction for a Le Fort type I osteotomy. J Craniofac Surg. 1996; 7:111.
287. Sammartino, G, Califano, L, Grassi, R, et al. Transient facial nerve paralysis after mandibular sagittal osteotomy. J Craniofac Surg. 2005; 16:1110.
288. Sanni, K, Campbell, R, Rosner, M, Goyne, WB. Internal carotid arterial occlusion following mandibular osteotomy. J Oral Maxillofac Surg. 1984; 42:394.
289. Schow, SR, Triplett, RG, Solomon, JM. Lingual nerve injury associated with overpenetration of bicortical screws used for fixation of a bilateral mandibular sagittal split ramus osteotomy. J Oral Maxillofac Surg. 1996; 54:1451.
290. Schultze-Mosgau, S, Krems, H, Ott, R, Neukam, FW. A prospective electromyographic and computer-aided thermal sensitivity assessment of nerve lesions after sagittal split osteotomy and le Fort I osteotomy. J Oral Maxillofac Surg. 2001; 59:128–139.
291. Schultze-Mosgau, S, Reich, RH. Assessment of inferior alveolar and lingual nerve disturbances after dentoalveolar surgery, and of recovery of sensitivity. Int J Oral Maxillofac Surg. 1993; 22:214.
292. Schwartz, HC. Osteonecrosis and bisphosphonates: Correlation versus causation. J Oral Maxillofac Surg. 2004; 62:763–764.
293. Schwartz, HC, Wilson, MC. Cervicofacial actinomycosis following orthognathic surgery: Report of 2 cases. J Oral Maxillofac Surg. 2001; 59:447.
294. Schwartz, S, Joseph, C, Iera, D, Vu, DD. Bisphosphonates, osteonecrosis, osteogenesis imperfecta and dental extractions: A case series. J Can Dent Assoc. 2008; 74:537–542.
295. Seo, K, Tanaka, Y, Terumitsu, M, et al. Efficacy of steroid treatment for sensory impairment after orthognathic surgery. J Oral Maxillofac Surg. 2004; 62:1193.
296. Seo, K, Tanaka, Y, Terumitsu, M, et al. Characterization of different paresthesias following orthognathic surgery of the mandible. J Oral Maxillofac Surg. 2005; 63:298.
297. Shand, JM, Heggie, AA. Use of a resorbable fixation system in orthognathic surgery. Br J Oral Maxillofac Surg. 2000; 38:335.
298. Sher, MR. A survey of complications in segmental orthognathic surgical procedures. Oral Surg Oral Med Oral Pathol. 1984; 58:537.
299. Sheridan, SM. Traumatic neuroma following sagittal split mandibular osteotomy. Br J Oral Surg. 1983; 21:198.
300. Shoshani, Y, Samet, N, Ardekian, L, et al. Nasolacrimal duct injury after Le Fort I osteotomy. J Oral Maxillofac Surg. 1994; 52:406.
301. Sickels, JE. Neurosensory recovery following the mandibular bilateral sagittal split osteotomy (discussion). J Oral Maxillofac Surg. 1995; 53:1306.
302. Siebert, JW, Angrigiani, C, McCarthy, JG, et al. Blood supply of the Le Fort I maxillary segment: An anatomic study. Plast Reconstr Surg. 1997; 100:843.
303. Silva, AC, O’Ryan, F, Beckley, ML, et al. Pseudoaneurysm of a branch of the maxillary artery following mandibular sagittal split ramus osteotomy: Case report and review of the literature. J Oral Maxillofac Surg. 2007; 65:1807.
304. Sinclair, P, Kilpelanien, P, Phillips, C, et al. The accuracy of video imaging in orthognathic surgery. Am J Dentofac Orthop Orthod. 1995; 107:177–185.
305. Singh, J, Doddrige, M, Broughton, A, Goss, A. Reconstruction of post-orthognathic aseptic necrosis of the maxilla. Br J Oral Maxillofac Surg. 2008; 46:408–410.
306. Singhal, A, Golomb, M, Mochida, G, et al. Another case of internal carotid artery dissection after mandibular osteotomy. J Oral Maxillofac Surg. 1998; 56:115.
307. Sirikumara, M, Sugar, AW. Adie’s pupil following Le Fort I maxillary osteotomy. A complication of coincidence? Br J Oral Maxillofac Surg. 1990; 28:306.
308. Smith, IM, Anderson, PJ, Wilks, MJ, et al. Traumatic arteriovenous malformation following maxillary Le Fort I osteotomy. Cleft Palate Craniofac J. 2008; 45:329.
309. Smith, KS, Heggie, AAC. Vomero-sphenoidal disarticulation during the Le Fort I maxillary osteotomy; report of a case. J Oral Maxillofac Surg. 1995; 53:465.
310. Solomons, NB, Blumgart, R. Severe late-onset epistaxis following Le Fort I osteotomy: Angiographic localization and embolization. J Laryngol Otol. 1988; 102:260.
311. Song, HC, Throckmorton, GS, Ellis, E, III., et al. Functional and morphologic alterations after anterior or inferior repositioning of the maxilla. J Oral Maxillofac Surg. 1997; 55:41.
312. Spaey, YJ, Bettens, RM, Mommaerts, MY, et al. A prospective study on infectious complications in orthognathic surgery. J Craniomaxillofac Surg. 2005; 33:24.
313. Stacy, GC. Recovery of oral opening following sagittal ramus ostectomy for mandibular prognathism. J Oral Maxillofac Surg. 1987; 45:487.
314. Stajcic, Z, Roncevic, R. Facial nerve palsy following combined maxillary and mandibular osteotomy. J Craniomaxillofac Surg. 1990; 18:192.
315. Steel, BJ, Cope, MR. Unusual and rare complications of orthognathic surgery: A literature review. J Oral Maxillofac Surg. 2012; 70:1678–1691.
316. Stern, NS, Shensa, DR, Trop, RC. Cavernous sinus thrombosis: A complication of maxillary surgery. J Oral Surg. 1981; 39:436.
317. Stewart, TD, Sexton, J. Depression: A possible complication of orthognathic surgery. J Oral Maxillofac Surg. 1987; 45:847.
318. St-Hilaire, H, Montazem, AH, Diamond, J. Pneumomediastinum after orthognathic surgery. J Oral Maxillofac Surg. 2004; 62:892.
319. Stone, HH, Haney, BB, Kolb, LD, et al. Prophylactic and preventive antibiotic therapy: Timing, duration and economics. Ann Surg. 1979; 189:691–699.
320. Storum, K, Bell, WH. Hypomobility after maxillary and mandibular osteotomies. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1984; 57:7.
321. Stringer, DE, Dolwick, MF, Steed, DL. Subcutaneous emphysema after Le Fort I osteotomy: Report of two cases. J Oral Surg. 1979; 37:115.
322. Sun, JR, Kim, SG. Facial nerve injuries following bilateral sagittal osteotomies. Hosp Dent (Tokyo). 2007; 19:13.
323. Sunderland, S. A classification of peripheral nerve injuries producing loss of function. Brain. 1951; 74:491.
324. Susarla, SM, Kaban, LB, Donoff, RB, Dodson, TB. Does early repair of lingual nerve injuries improve functional sensory recovery? J Oral Maxillofac Surg. 2007; 65:1070–1076.
325. Suuronen, R, Ashammakhi, N, Peltoniemi, H, et al. Developments in craniomaxillofacial surgery: Use of self-reinforced bioabsorbable osteofixation devices. Plast Reconstr Surg. 2001; 108:167.
326. Suzuki, H, Saito, E, Hashimoto, K. Dissecting aneurysm of the internal carotid artery after a mandibular osteotomy. J Oral Maxillofac Surg. 1997; 55:747.
327. Svartz, K, Ahlborg, G, Finne, K, Nethander, G. Nerve disturbances after sagittal split osteotomy. Int J Oral Surg. 1983; 12:279.
328. Taher, AAY. Facial palsy: A complication of sagittal ramus osteotomy (Obwegeser-Dal Pont technique): Report of a case. Quintessence Int. 1988; 19:229.
329. Takasaki, Y, Noma, H, Masaki, H, et al. A clinical analysis of the recovery from sensory disturbance after sagittal splitting ramus osteotomy using a Semmes-Weinstein pressure aesthesiometer. Bull Tokyo Dent Coll. 1998; 39:189.
330. Takeuchi, T, Furusawa, K, Hirose, I. Mechanism of transient mental nerve paraesthesia in sagittal split mandibular ramus osteotomy. Br J Oral Maxillofac Surg. 1994; 32:105–108.
331. Tay, AB, Go, WS. Effect of exposed inferior alveolar neurovascular bundle during surgical removal of impacted lower third molars. J Oral Maxillofac Surg. 2004; 62:592.
332. Tay, AB, Poon, CY, Teh, LY. Immediate repair of transected inferior alveolar nerves in sagittal split osteotomies. J Oral Maxillofac Surg. 2008; 66:2476–2481.
333. Teerijoki-Oksa, T, Jääskeläinen, S, Forssell, H, et al. Risk factors of nerve injury during mandibular sagittal split osteotomy. Int J Oral Maxillofac Surg. 2002; 31:33–39.
334. Teerijoki-Oksa, T, Jääskeläinen, S, Forssell, K, et al. An evaluation of clinical and electrophysiological tests in nerve injury diagnosis after mandibular sagittal split osteotomy. Int J Oral Maxillofac Surg. 2003; 32:15–23.
335. Teltzrow, T, Kramer, FJ, Schulze, A, et al. Perioperative complications following sagittal split osteotomy of the mandible. J Craniomaxillofac Surg. 2005; 33:307.
336. Theodossy, T, Jackson, O, Petrie, A, et al. Risk factors contributing to symptomatic plate removal following sagittal split osteotomy. Int J Oral Maxillofac Surg. 2006; 35:598.
337. Thygesen, TH, Bardow, A, Norholt, SE, et al. Surgical risk factors and maxillary nerve function after Le Fort I osteotomy. J Oral Maxillofac Surg. 2009; 67:528.
338. Tomasetti, BJ, Broutas, M, Gormley, M, et al. Lack of tearing after Le Fort I osteotomy. J Oral Surg. 1976; 34:1095.
339. Tomazzoli-Gerosa, L, Marchini, G, Monaco, A. Amaurosis and atrophy of the optic nerve: An unusual complication of mandibular-nerve anesthesia. Ann Ophthalmol. 1988; 20:170.
340. Trimble, LD, Tideman, H, Stoelinga, PJ. A modification of the pterygoid separation in low-level maxillary osteotomies. J Oral Maxillofac Surg. 1983; 41:544.
341. Tuinzing, DB, Greebe, RB. Complications related to the intraoral vertical ramus osteotomy. Int J Oral Surg. 1985; 14:319.
342. Tung, TC, Chen, YR, Bendor-Samuel, R. Surgical complications of the Le Fort I osteotomy: A retrospective review of 146 cases. Changgeng Yi Xue Za Zhi. 1995; 18:102.
343. Turvey, TA. Intraoperative complications of sagittal osteotomy of the mandibular ramus: Incidence and management. J Oral Maxillofac Surg. 1985; 43:594.
344. Turvey, TA, Bell, BB, Tejera, TJ, Proffit, WR. The use of self-reinforced biodegradable bone plates and screws in orthognathic surgery. J Oral Maxillofac Surg. 2002; 60:59–65.
345. Turvey, TA, Bell, RB, Phillips, C, Proffit, WR. Self-reinforced biodegradable screw fixation compared with titanium screw fixation in mandibular advancement. J Oral Maxillofac Surg. 2006; 64(1):40–46.
346. Van de Perre, JP, Stoelinga, PJ, Blijdorp, PA, et al. Perioperative morbidity in maxillofacial orthopedic surgery: A retrospective study. J Craniomaxillofac Surg. 1996; 24:263.
347. Van Merkesteyn, JPR, Groot, RH, van Leeuwaarden, R, et al. Intra-operative complications in sagittal and vertical ramus osteotomies. Int J Oral Maxillofac Surg. 1987; 16:665.
348. Van Sickels, JE. Concomitant removal of mandibular third molars during sagittal split osteotomies minimizes neurosensory dysfunction: Comment. J Oral Maxillofac Surg. 2012; 70:2164.
349. Van Sickels, JE, Hatch, JP, Dolce, C, et al. Effect of age, amount of advancement and genioplasty on neurosensory disturbance after a bilateral sagittal split osteotomy. J Oral Maxillofac Surg. 2002; 60:1012.
350. Van Sickels, JE, Tucker, MR. Management of delayed union and nonunion of maxillary osteotomies. J Oral Maxillofac Surg. 1990; 48:1039.
351. Vaughan, TC, Cronin, A. Neurosurgery required following a sagittal split osteotomy. Br J Oral Maxillofac. 2009; 47:e23.
352. Veterans Administration Ad Hoc Interdisciplinary Advisory Committee on Antimicrobial Drug Usage. Prophylaxis in surgery. J Am Med Assoc. 1977; 237:1003–1008.
353. Waack, D. Perioperative complications associated with Le Fort I osteotomies. J Oral Maxillofac Surg. 1994; 52(suppl 2):92.
354. Walter, JM, Jr., Gregg, JM. Analysis of postsurgical neurologic alteration in the trigeminal nerve. J Oral Surg. 1979; 37:410.
355. Wang, JH, Waite, DE. Evaluation of the surgical procedure of sagittal split osteotomy of the mandibular ramus. Oral Surg Oral Med Oral Pathol. 1974; 38:167.
Ward Booth, P. Discussion: Risk factors contributing to symptomatic plate removal in orthognathic surgery patients. J Oral Maxillofac Surg. 1999; 57:682.
356. Watts, PG. Unilateral abducent nerve palsy: A rare complication following a Le Fort I maxillary osteotomy. Br J Oral Maxillofac Surg. 1984; 22:212.
357. Weber, CR, Griffin, JM. Evaluation of dexamethasone for reducing postoperative edema and inflammatory response after orthognathic surgery. J Oral Maxillofac Surg. 1994; 52:35.
358. Westermark, A, Bystedt, H, von Konow, L. Inferior alveolar nerve function after mandibular osteotomies. Br J Oral Maxillofac Surg. 1998; 36:425–428.
359. Westermark, A, Bystedt, H, von Konow, L. Inferior alveolar nerve function after sagittal split osteotomy of the mandible: Correlation with degree of intraoperative nerve encounter and other variables in 496 operations. Br J Oral Maxillofac Surg. 1998; 36:429–433.
360. Westermark, A, Bystedt, H, von Konow, L. Patient’s evaluation of the final result of sagittal split osteotomy: Is it influenced by impaired sensitivity of the lower lip and chin? Int J Adult Orthodon Orthognath Surg. 1999; 14:135.
361. Westermark, A, Englesson, L, Bongenhielm, U. Neurosensory function after sagittal split osteotomy of the mandible: A comparison between subjective evaluation and objective assessment. Int J Adult Orthodon Orthognath Surg. 1999; 14:268–275.
362. Westwood, RM, Tilson, HB. Complications associated with maxillary osteotomies. J Oral Surg. 1975; 33:104.
363. Willmar, K. On Le Fort I osteotomy. Scand J Plast Reconstr Surg Suppl. 12, 1974.
364. Willmar, K, Hogeman, KE, Thiseus, S. Sagittal split osteotomy in our experience: A follow-up study of 100 operated patients. Scand J Plast Reconstr Surg. 1979; 13:445.
365. Wilson, ME, Ellis, FR. Predicting malignant hyperpyrexia. Br J Anaesth. 1979; 51:66.
366. Wilson, MW, Wheatley, MJ, Shults, WT. Secondary fractures of Le Fort I osteotomy. Ophthal Plast Reconstr Surg. 2000; 16:258.
367. Woo, SB, Hellstein, JW, Kalmar, JR. Systematic review: Bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006; 144:753–761.
368. Ylikontiola, L, Kinnunen, J, Laukkanen, P, et al. Prediction of recovery from neurosensory deficit after bilateral sagittal split osteotomy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000; 90:275.
369. Ylikontiola, L, Kinnunen, J, Oikarinen, K. Comparison of different tests assessing neurosensory disturbances after bilateral sagittal split osteotomy. Int J Oral Maxillofac Surg. 1998; 27:417.
370. Ylikonitiola, L, Kinnunen, J, Oikarinen, K. Factors affecting neurosensory disturbance after mandibular bilateral sagittal split osteotomy. J Oral Maxillofac Surg. 2000; 58:1234.
371. Yoshida, T, Nagamine, T, Kobayashi, T, et al. Impairment of the inferior alveolar nerve after sagittal split osteotomy. J Craniomaxillofac Surg. 1989; 17:271.
372. You, ZH. A study of maxillary and mandibular vasculature in relation to orthognathic surgery [article in Chinese]. Zhonghua Kou Qiang Yi Xue Za Zhi. 1991; 26:263.
373. You, ZH, Zhane, ZK, Zhane, XE. Le Fort I osteotomy with descending palatal artery intact and ligated: A study of blood flow and quantitative histology. Contemp Stomatol. 1991; 5:71.
374. Young, RA, Epker, BN. The anterior maxillary osteotomy: A retrospective evaluation of sinus health, patient acceptance, and relapse. J Oral Surg. 1972; 30:69.
375. Yrastorza, J. Indications for antibiotics in orthognathic surgery. J Oral Surg. 1976; 34:514.
376. Yun, KI, Lee, JA, Park, JU. Intubation granuloma: Report of a case. J Oral Maxillofac Surg. 2008; 66:1263.
377. Zarrinkelk, HM, Throckmorton, GS, Ellis, E, III., et al. A longitudinal study of changes in masticatory performance of patients undergoing orthognathic surgery. J Oral Maxillofac Surg. 1995; 53:777.
378. Zaytoun, HS, Jr., Phillips, C, Terry, BC. Long-term neurosensory deficits following transoral vertical ramus and sagittal split osteotomies for mandibular prognathism. J Oral Maxillofac Surg. 1986; 44:193.
379. Zijderveld, SA, Smeele, LE, Kostense, PJ, Tuinzing, DB. Preoperative antibiotic prophylaxis: A randomized, double-blind and placebo-controlled clinical study. J Oral Maxillofac Surg. 1999; 57:1403–1406.
380. Zuniga, JR, Essick, GK. A contemporary approach to the clinical evaluation of trigeminal nerve injuries. Oral Maxillofac Surg Clin North Am. 1992; 4:353.
381. Zuniga, JR, Meyer, RA, Gregg, JM, et al. The accuracy of clinical neurosensory testing for nerve injury diagnosis. J Oral Maxillofac Surg. 1998; 56:2.
*References 2, 23, 25–27, 42, 53, 78, 80, 83, 118, 146, 150, 157, 162, 186, 203, 206, 212, 224, 242–244, 246, 252, 262, 266, 314, 322, 328, 334, 335, 341–343, 345, 347, 355, 362–364.
*References 19, 20, 29, 48, 49, 77, 103, 105, 107, 108, 110, 111, 119, 133, 158, 171, 201, 213, 257, 279, 282, 284, 312, 319, 350, 375, 379.
*References 3, 5, 6, 10, 12, 17, 18, 21, 37, 45, 51, 52, 55, 57, 65, 66, 69, 71, 88, 97–102, 106, 109, 112, 114, 121, 141–144, 160, 161, 166, 169, 193, 195, 196, 197, 227, 229–231, 234, 239, 258, 263, 265, 275, 277, 295, 296, 299, 301, 305, 327, 329–334, 344, 351, 354, 358–361, 369, 370, 368, 374, 376, 378, 380.
*References 7, 52, 57, 71, 97–99, 120, 154, 160, 161, 261, 264, 265, 267, 273, 295, 323, 380, 381.
*References 28, 31, 43, 54, 58, 59, 67, 70, 115, 148, 188, 198, 199, 274, 278, 286, 339.
*References 4, 15, 40, 41, 64, 74, 116, 123, 126, 134, 136, 140, 168, 181, 183, 185, 187, 189, 190, 204, 205, 219, 236, 240, 241, 280, 302, 306, 308, 310, 316.