CHAPTER 8 Complications and Adverse Sequelae of Sclerotherapy
Adverse Sequelae
Postsclerotherapy hyperpigmentation
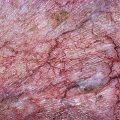
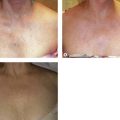
Pigmentation is usually temporary. Physicians report a 1% to 2% incidence of pigmentation persisting after 1 year.1,2 Pigmentation is usually linear along the course of the treated blood vessel. We use the term ghost of the blood vessel to explain to patients that it represents a resolving and not functioning vessel. However, in addition to linear lines of pigmentation, osmotic sclerosing solutions may produce punctate pigmentation at points of injection, which may be related to their mechanism of action through an osmotic gradient that produces maximal osmolality and resultant endothelial destruction at the injection site. In contrast, detergent-type sclerosing solutions destroy the treated vessel for a few centimeters along its length, producing a more linear golden brown color (Figs 8.1 and 8.2). Cutaneous pigmentation is to some degree a relatively common occurrence after sclerotherapy with any sclerosing solution.3 It has been reported in 11% to 80%4–6 of patients treated with sodium tetradecyl sulfate (STS). One study found that a 0.1% concentration of STS resulted in pigmentation in 11% of patients. The incidence of pigmentation with hypertonic saline (HS) has been reported to range from 10% to 30%.6–10 Patients treated with polidocanol (POL) have a reported incidence of pigmentation from 6.7%11,12 to 31%.8,13,14 A 35% incidence has been reported in 7200 patients treated with POL, ethanolamine oleate, or iodine-iodide solution.15 Post-sclerotherapy hyperpigmentation has a reported incidence of 15.7% with Sclerodex (dextrose with sodium chloride),12 and 32% with Sclerodine (iodine and sodium iodide).12 A 2% incidence of hyperpigmentation was reported from one series of patients treated with POL, chromated glycerin (CG), and sodium salicylate.1 A 2%–4% incidence was reported from another series of 102 patients treated with either STS, POL, or CG.16
Between 2003 and 2008, 1187 of our patients underwent sclerotherapy treatment. Of this group, 351 had been treated with foam or liquid STS and were available for follow-up. Thirty-five percent of these patients experienced hyperpigmentation following sclerotherapy. However, hyperpigmentation was graded as minimal to mild. Furthermore, no hyperpigmentation was evident in any patient 1 year after treatment. Of note, the ‘hyperpigmentation’ reported by many patients was actually a post-treatment coagulum.17
Etiologic factors
The cause of this pigmentation most likely results from a combination of both postinflammatory hyperpigmentation (incontinence of melanin pigment) and hemosiderin deposition.18–20 However, histologic examination has demonstrated that this pigmentation is caused only by hemosiderin staining of the dermis, irrespective of the type of sclerosing solution used, pigmentation of the patient, or length of time after injection (Fig. 8.3, Table 8.1).21–24 Defects in iron storage and/or transport mechanisms have also been found in a significant number of patients who have developed pigmentation after sclerotherapy.25
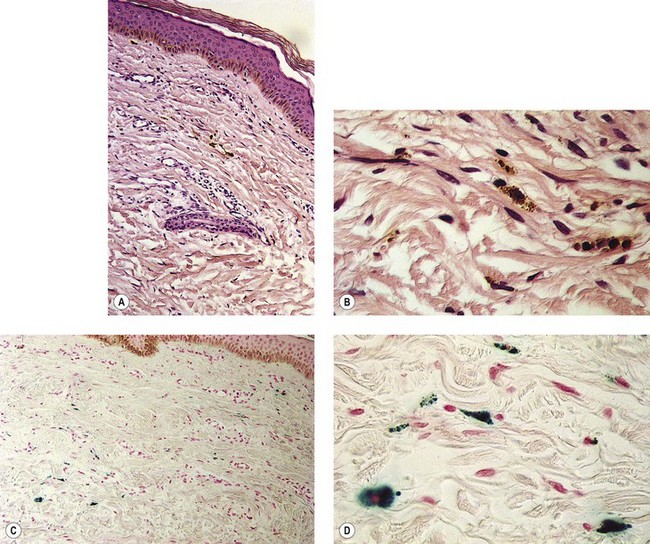
Figure 8.3 Section stained with hematoxylin–eosin taken 6 months after injection with polidocanol 0.75%. Note scattered foci of golden brown pigment. A, Original magnification ×50. B, Perls-stained section from the same patient as in Figure 8.1. Note scattered foci of green-blue granules within siderophages. Original magnification ×200. C, Original magnification ×350. D, Original magnification ×3200.
(From Goldman MP, Kaplan RP, Duffy DM: J Dermatol Surg Oncol 13:547, 1987.)
Hemosiderin deposition occurs predominantly in the superficial dermis, although it may be present in periadnexal and mid-dermal locations, particularly near the ankle. This phenomenon probably occurs when RBCs extravasate into the dermis after the rupture of treated vessels.26 Erythrocyte diapedesis also may occur after inflammation of the vessel and is commonly seen after thrombophlebitis. Perivascular inflammation is presumed to promote degranulation of perivascular mast cells. Released histamine leads to endothelial cell contraction, which results in widening of endothelial gaps through which extravasation of RBCs can occur.27–31 Thus, injecting a sclerosing solution dilates the vessel both directly through pressure generated by the syringe and indirectly through histamine-induced endothelial cell contraction.
Perivascular phagocytosis of RBCs occurs either by intact cells or piecemeal after fragmentation by macrophages.32,33 The intracellular fragments in the macrophage cytoplasm are further compartmentalized into hemoglobin-containing globules. They are referred to as secondary lysosomes. Since hemosiderin is an indigestible residue of hemoglobin degradation, it may appear as aggregates up to 100 µm in diameter.34 Hemosiderin has a variable concentration of these aggregates. Iron concentrations vary from 24% to 36%.35 Iron hydroxide contained in hemosiderin occurs in different forms, with differing amounts of ferritin.36 On unstained tissue it appears golden and is 30% iron by weight. Its elimination from the area through phagocytosis may take years, if it ever occurs.
In addition to being insoluble, hemosiderin may directly affect cellular function. Histologic examination with X-ray fluorescence analysis of patients with varicose ulceration disclosed an elevation of mean iron levels in periulcerated skin.37 The authors speculate that free radical formation resulting from local iron accumulation may cause melanocytic stimulation, thereby augmenting brown pigmentation. Indeed, multiple authors have demonstrated melanin incontinence in the presence of venous stasis, complicated by extravascular RBCs.38–40 Whether melanocytic stimulation plays a role in the early appearance of postsclerotherapy pigmentation is unlikely, but it may contribute to the persistence of pigmentation in certain patients, especially in Fitzpatrick skin types V and VI.
Solution Type and Concentration
The type and concentration of the sclerosing solution affect the degree of endothelial destruction. The extent of endothelial destruction with resulting inflammation and extravasation of RBCs is thought to influence the development of postsclerotherapy hyperpigmentation. The increased incidence of pigmentation with certain concentrations of STS and HS, which produce a greater reaction than POL, confirms this hypothesis.4,41–43 In fact, when excessive concentrations of POL are used to treat telangiectasias (1%), the pigmentation rate is even higher than with 20% HS.44 It is therefore not surprising that sclerosing solutions reported to have the lowest incidence of postsclerotherapy pigmentation – CG,1,41,45–49 glycerin alone,50 and sodium salicylate1,20 – also produce minimal inflammation.
A higher concentration of the same sclerosing solution produces increased inflammation.51 Thus, the inflammatory response after treatment should be kept to a minimum, and sclerosing solutions and concentrations should be altered for each treatment session so that the minimal effective sclerosant concentration is used.
Foam sclerosants are stronger than liquids for an identical concentration. Therefore, when foam is used, special attention should be directed towards reducing the strength or concentration of the agent. This is especially true for treatment of reticular and spider veins.52 Recently published analyses of large numbers of patients treated with foam sclerotherapy estimate the incidence of post-inflammatory hyperpigmentation to be between 10% and 30%.53–55 Furthermore, Alos et al noted that – although the overall incidence of pain with sclerotherapy using 0.5% POL is rare – foam is more often associated with pain than is liquid.56
Technique
Optimal technique consists of limiting pressure into damaged (sclerosed) veins to prevent extravasation of RBCs. To limit the degree of intravascular pressure, larger feeding varices, incompetent varices, and points of high pressure reflux should be treated first. A greater incidence of pigmentation occurs if vessels distal to the saphenofemoral junction (SFJ) are treated before successful closure of the junction, with a decreased incidence of pigmentation when treatment is from proximal to distal.57
The average piston radius is 8 mm for a 2-mL syringe and 5 mm for a 1-mL syringe. The calculated pressure with an implied force of 250 g is 180 mmHg for a 2-mL syringe and more than 300 mmHg for a 1-mL syringe.58 This is one reason we recommend using a 3-mL syringe for sclerotherapy (Fig. 8.4).
Gravitational and Other Intravascular Pressures
Postsclerotherapy pigmentation appears most commonly in vessels treated below the knee20 but can occur anywhere on the leg, probably as a result of a combination of increased capillary fragility and increased intravascular pressure by gravitational effects in this location. Pigmentation has been observed once in our practice after sclerotherapy treatment of hand veins (Fig. 8.5). Duffy et al59 note that pigmentation did not develop after treating 100 patients with dilated hand veins with either 0.5% STS, 1.5% POL, or 3% POL.
Vessel Diameter
It is commonly observed that telangiectasias that have the maximal incidence of pigmentation are between 0.6 and 1.2 mm in diameter. This could be related to an increased incidence of microthrombi in these vessels. Chatard20 also has observed an increased incidence of pigmentation in the treatment of blue venulectases as opposed to the treatment of red telangiectasias. The reason behind this latter observation is unknown but may be related to vessel diameter, since blue telangiectasias are usually of larger diameter than red telangiectasias. An evaluation of 113 patients treated with sclerotherapy demonstrated pigmentation only rarely in vessels less than 1 mm in diameter.60
Predisposition to Pigmentation
Certain individuals appear to be predisposed to the development of pigmentation through a variety of genetic mechanisms. Pigmentation has been reported as more common and pronounced in patients with dark hair and ‘dark-toned’ skin.18 This may be caused by an increased incidence of postinflammatory hyperpigmentation in patients with these colorings However, Chatard20 reported that pigmentation is unrelated to skin or hair color. We are not aware of the number of Type V and Type VI patients Chartard has treated, but in our experience (RAW, MPG) it is clear that patients with darker skin coloring and Asians do have an increased incidence of post-sclerotherapy pigmentation.
Pigmentation resolves from a gradual resorption of ferritin particles from macrophage digestion. It is hypothesized that the patient’s iron storage and transport mechanisms may influence the rate of clearance of dermal hemosiderin.55 A preliminary study of 16 patients with age-matched controls disclosed that pigmentation developed in patients who had higher serum ferritin levels. Serum ferritin levels correlate with total body iron stores.61 To clarify the relationship between serum ferritin and postsclerotherapy pigmentation, a prospective study of 233 consecutive patients was conducted.62 A linear relationship between the occurrence of pigmentation and pretreatment serum ferritin levels was found at each post-treatment assessment date. This supports the hypothesis that high total body iron stores increase the susceptibility toward hyperpigmentation. However, serum ferritin levels are not an absolute predictor for the development of hyperpigmentation. In a patient with hemochromatosis having a serum ferritin level of 1200 ng/mL, pigmentation did not develop after sclerotherapy of telangiectasia 0.6 mm in diameter with 0.2% STS.63 The explanation may be that this patient’s physician probably used outstanding technique to avoid extravasation of RBCs.
If histamine-induced endothelial contraction promotes extravasation of RBCs or hemosiderin, or both, histamine antagonists should prevent or limit its occurrence. The catecholamines norepinephrine (noradrenaline) and isoproterenol antagonize histamine-induced edema in canine brachial artery preparations.64 Similarly, corticosteroids decrease the size of histamine-induced endothelial gap junctions.65 Terbutaline also inhibits macromolecular leakage from postcapillary venules in hamster femoral veins.66 Cimetidine blocks histamine-induced widening of endothelial gaps in rat femoral veins.67 Therefore, patients who have developed postsclerotherapy pigmentation in past treatments may be pretreated with one or a combination of these medications to block or limit histamine effects.
Vessel fragility may also result in an innate predisposition toward pigmentation. Capillary strength is related to both menstrual cycles and circulating estrogen.68 Decreased capillary strength occurs 3 to 5 days before and 2 days after menses and during ovulation. Fragility has been found to improve with intravenous (IV) and oral administration of conjugated equine estrogen (Premarin) in postmenopausal women.
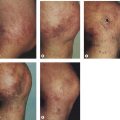
Patients taking minocycline may have an increased risk for postsclerotherapy pigmentation.69,70 This propensity may be related to the inflammatory effects of sclerotherapy. Unlike the golden to deep brown color characteristic of typical sclerotherapy-induced pigmentation, pigmentation associated with minocycline use is most commonly blue–gray (Fig. 8.6). Minocycline, known to have increased tissue distribution due to its lipophilicity, produces pigmentation in a variety of organs and structures.71,72 The most common form of minocycline-related pigmentation appears as bluish-black or bluish-gray macules within acne scars or at other sites of inflammation.10,73–77 Other forms of minocycline-related pigmentation have been described on the skin of the lower legs, including that associated with sites of UV-exposure,78 as well as with sites of pre-existing capillaritis.79 The pigment involved in minocycline hyperpigmentation is most likely a drug metabolite–protein complex chelated with calcium, or an insoluble minocycline–melanin complex.75,78–83 It is hypothesized that minocycline or a metabolite interferes with degradation of hemosiderin through lysosomal disruption, leading to macrophage death and deposition of pigment.77 Recently, four cases of minocycline-related hyperpigmentation involving the subcutaneous fat were reported.84 Histopathologically, tissue exhibited the previously well-described deposition of brown/black, Fontana-Masson and Perls’ positive granules along elastic fibers in the papillary dermis as well as within dermal macrophages located near vessels and eccrine glands. In addition to these dermal findings, tissue from the four patients also showed green–gray, nonrefractile, flocculent globules within macrophages in the subcutaneous tissue. Also, two of the four patients demonstrated the distinctive finding of pigment-related lipomembranous changes. Although further studies are needed to establish a direct relationship, it may be prudent to withhold minocycline therapy in sclerotherapy patients. Of note, successful lightening of minocycline-induced, post-traumatic pigmentary deposition has been described with successive Q-switched Nd:YAG laser treatments.85 Often discontinuation of minocycline will result in spontaneous clearing of minocycline pigmentation within 6 to 12 months.
Postsclerotherapy Coagula
Removal of postsclerotherapy coagula may decrease the incidence of pigmentation. Thrombi to some degree are thought to occur after sclerotherapy of all veins, regardless of size, because of the inability to occlude the vascular lumen completely with external pressure. The persistence of a small vascular lumen, even with maximal external pressure, has been predicted with experimental models of vein wall.86 This has also been directly observed with fiberoptic varicography (Muntlak H, personal communication, 1989).
Persistent thrombi are thought to produce a subacute ‘perivenulitis’ that can persist for months.87–89 The perivenulitis favors extravasation of RBCs through a damaged endothelium or by an increase of the permeability of treated endothelium. In addition, intratissue fixation of hemosiderin may occur.20 This provides a rationale for drainage of all foci of trapped blood 2 to 4 weeks after sclerotherapy. Sometimes blood can be released even 2 months after sclerotherapy.
Thrombi are best removed by gentle expression of the liquefied clot through a small incision made with a 21-gauge needle (Figs 8.7 and 8.8). A no. 11 blade or lancet or 18 gauge no core needle have also been recommended by some, but, since this results in a larger incision and often requires pretreatment with a local anesthetic, we favor the more conservative 21-gauge needle approach.
In a multicentered, randomized, controlled study, 101 patients with varicose veins were treated at 1–3 weeks with microthrombectomy in one half of the treated veins.90 Photographs of the sclerotherapy-treated areas were evaluated at 16 weeks. Veins ≤1 mm in diameter had less pigmentation when drained, but veins ≤3 mm did not show any benefit from microthrombectomy.
Perchuk91 raised the possibility of infection occurring from stab incisions. This danger was presumed to be caused by the presence of bacteria in varicose veins – a belief commonly held by physicians 40 to 50 years ago.92,93 However, there have been no reports in the modern medical literature of infections occurring in patients treated with stab incisions into postsclerotherapy clots. This problem has not occurred in our practice, in which this procedure is used routinely.
Duration
Despite therapeutic attempts, pigmentation often lasts from 6 to 12 months.23 Rarely, pigmentation may last more than 1 year. Georgiev2 estimates that 1% of his patients, and Duffy6 that up to 10% of his patients, have pigmentation lasting more than 1 year. Izzo1 reports a 2% incidence after 1 year. Our experience parallels that of Georgiev.
Persistent telangiectasia may be caused by factors other than sclerotherapy itself. In certain patients, pigmentation may be present over superficial varicosities and telangiectasias before sclerotherapy is performed.2,20 Hyperpigmentation as a result of ‘physiologic’ diapedesis of RBCs through fragile vessels is common in patients with venous stasis, or over varicose veins. Therefore, preoperative documentation, including photographs, may be beneficial during follow-up patient visits.
Prevention and minimization
Thibault and Wlodarczyk62 recommend that patients avoid taking all iron supplements during the course of treatment and for 1 month after treatment. This presumably decreases serum ferritin levels. Alternatively, patients’ serum ferritin levels may be assessed before sclerotherapy to determine if iron chelation therapy is warranted. Obviously, this latter recommendation awaits further study. We think it is prudent to ask our patients who have developed pigmentation if they are taking iron supplementation, and, if so, discontinue it before future treatments.
Preventing formation of postsclerotherapy-related ecchymoses would theoretically prevent postinflammatory hyperpigmentation through avoidance of dermal hemosiderin deposition. Although Arnica montana is routinely used by many surgeons to prevent perioperative bruising, the efficacy of this homeopathic product has not been scientifically proven. A recent randomized, prospective, double-blind study evaluated the perioperative use of A. montana (SinEcch) in 29 patients undergoing face-lifts.94 Interestingly, the amount, duration, and degree of bruising did not differ between those treated with 10 days of A. montana and those in the placebo group. Although further procedure-specific studies would be necessary, it is likely that this lack of efficacy would be similar if A. montana was used in conjunction with sclerotherapy.
While laser treatment of telangiectatic leg veins is said not to be associated with pigmentation, we have documented numerous patients treated with a variety of lasers who developed prolonged pigmentation (Fig. 8.9).
Treatment
Treatment of pigmentation, once it occurs, is often unsuccessful unless one has access to a Q-switched laser. Because this pigmentation is caused primarily by hemosiderin deposition and not melanin incontinence, bleaching agents that affect melanocytic function are usually ineffective. Exfoliants (trichloroacetic acid) may hasten the apparent resolution of this pigmentation by decreasing the overlying cutaneous pigmentation or promoting the exfoliation of hemosiderin, but they carry a risk of scarring, permanent hypopigmentation, and postinflammatory hyperpigmentation. However, some physicians have reported apparent success with this therapeutic method.1,95 The combination of 20% trichloroacetic acid with retinoic acid and hydroxyquinoline has also been reported to totally fade pigmentation in 76% of patients whose pigmentation persisted from 6 months to 5 years.1 Pigmentation decreased in the remaining patients. Treatments were given every 7 to 10 days from 4 to 12 weeks.
Chatard20 has found that using light cryotherapy to exfoliate the epidermis and ‘evict the pigment’ is helpful. We have not found cryotherapy useful in our practice.
A seemingly logical form of treatment would be chelation of the subcutaneous iron deposition. Myers96 reported the use of a 150 mg/ mL ointment of disodium ethylenediamine tetraacetic acid (EDTA) in the treatment of 10 patients with pigmentation after sclerotherapy or vein stripping or with pigmentation in chronic postphlebitic legs. He reported a consistent reduction in the shade of the pigmentation in every patient treated. Unfortunately, this was an uncontrolled study, and there have been no further reports of this form of treatment since its presentation in 1965. In our experience, intradermal injections of deferoxamine in an attempt to cause chelation of the hemosiderin appear to be somewhat effective but are painful and expensive. The timing of injections and the concentration and quantity of deferoxamine injected have not been systematically studied.
The topical iron chelator 2-furildioxime (FDO) has been found to provide a level of photoprotection and theoretically may also be useful to treat cutaneous hemosiderin pigmentation.97 However, at the time of that research proposal, Proctor and Gamble had no interest in providing this agent for clinical testing of postsclerotherapy pigmentation (personal correspondence, Oct 1994).
Graduated elastic compression with coadministration of the anabolic steroid stanozolol decreases pigmentation in patients with lipodermatosclerosis who also have varicose veins.98 Skin pigmentation did not change when patients used graduated compression stockings alone. Stanozolol may exert its effect through reduction in perivascular fibrin from fibrinolytic enhancement. In addition, compression alone improves lipodermatosclerotic skin changes, including hyperpigmentation (Partsch H, personal communication, 1992). Although it seems reasonable to promote the wearing of graduated support stockings after treatment, further studies are needed before recommending systemic stanozolol therapy.
A study on the use of 20- to 30-mmHg compression stockings after sclerotherapy treatment of telangiectasia and reticular veins 0.4 to 3 mm in diameter found a decreased incidence of pigmentation when compression was used. Compression for 3 days resulted in a 20% decrease in pigmentation; compression for 1 week produced a 60% decrease in pigmentation compared with no compression; and compression for 3 weeks resulted in limited pigmentation in only 2 of 10 patients.99 This follows the logic of compression reducing vessel lumen size, resulting coagula, and hydrostatic pressure. However, Guex found an even lower incidence of residual pigmentation in a prospective study on reticular and spider veins without compression.100
Finally, laser treatment has been efficacious in 45%24 to 69%101 of patients with pigmentation of 12 or 6 months’ duration, respectively. Hemosiderin has an absorption spectrum that peaks at 410 to 415 nm, followed by a gradually sloping curve throughout the visible spectrum (Fig. 8.10)102,103 The copper vapor laser (CVL) at 511 nm in a continuous airbrush technique and the flashlamp-excited pulsed dye laser (PDL) at 510 nm should interact relatively specifically with the hemosiderin absorption spectrum. Competition from oxygenated hemoglobin (peak absorption at 577 nm) should be low, but interaction with epidermal melanin, which has a higher absorption rate at these wavelengths, may be significant. These lasers are thought to result in physical fragmentation of pigment granules, which are later removed by phagocytosis. However, penetration of laser energy at 510 and 511 nm is limited to 1 mm below the granular layer. Since hemosiderin may occur up to 2.8 mm below the granular layer, nonthermal effects may result in clinical resolution. An inflammatory reaction from thermal or photoacoustic effects may stimulate hemosiderin absorption. Although CVL-treated pigmentation responded better than that treated with PDL therapy, thermal relaxation times used by the latter laser system should be more selective.
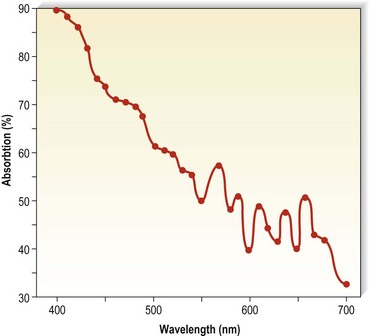
Figure 8.10 Absorption spectra for hemosiderin (freshly frozen, average of two determinations).
(From Wells CI, Wolken JJ: Nature 193:977, 1962.)
The Q-switched ruby laser (694 nm) is also effective in removing recalcitrant pigmentation. Hemosiderin has a peak at 694 nm, and the Q-switching impulse at 20 to 30 nanoseconds is effective in removing tattoo granules. In addition, 694 nm is not absorbed to a significant extent by epidermal melanin or hemoglobin and thus has a relative specificity for dermal hemosiderin. An older ruby laser (Laseaway, England) was been found to effectively remove or minimize pigmentation in a number of patients (Fig. 8.11)104 In a study of eight patients with pigmentation still present 1 to 2 years after sclerotherapy, 92% of the lesions lightened with treatment; 58% of lesions demonstrated significant (75–100%) resolution after one to three (average 1.7) treatments. The Laseaway ruby laser was used with a 4-mm beam size and a fluence range of 5.6 to 10.5 J/cm2. Weiss and Weiss105 could achieve 90% resolution of pigmentation that had been present for an average duration of 18 months with 3.2 treatments using a Q-switched ruby laser as well (Ruby Star, Aesclepion-Meditec AG, Jena, Germany) at 5 to 10 J/cm2 with a 2-nsecond pulse and a 4- to 5-mm diameter spot. We now use a Q-switched ruby laser (Sinon) from WaveLight Laser Technologies (Erlangen, Germany) at 4 to 7 J/cm2 with a 20-nsecond pulse and a 4- to 5-mm diameter spot size. Treatments are performed every 4 weeks until resolution. Care is taken to use the minimal fluence required to produce a whitening of the skin without causing bleeding. Our patients require one to two treatments for complete resolution.
Weiss106 has reported successful clearing with the use of intense pulsed light (IPL; Photoderm PL, Lumenis, Santa Clara, Calif.) in 10 patients with pigmentation persisting after 1 to 2 months. He used the IPL at 30 to 40 J/cm2 given in a single 4-msec pulse with a 590-nm cut-off filter. Significant lightening occurred in 6 of the 10 patients.
The most recent Q-switched laser used to treat postsclerotherapy pigmentation was a novel 532/1064-nm Nd:YAG laser (Palomar Med Tech, Burlington, Mass.). Ten patients who had had persistent pigmentation for an average of 18 months were treated at 2-nsecond pulse with 75% 1064 nm and 25% 532 nm simultaneously with a 6-mm diameter spot at 2 J/cm2. There was a 75% resolution in an average of 2.8 treatments.107
Temporary swelling
Etiologic factors
Multiple factors are responsible for swelling of a treated area. These factors include changes in the pressure differential between the intravascular and perivascular space and changes in endothelial permeability. Edema is most common after treatment of varicose veins or telangiectasias below the ankle, because of the increase in gravitational intravascular pressure in this area and the relative scarcity of perivascular fascia at the ankle. Riddock108 speculates that edema is caused by an unduly prolonged reflex spasm spreading to some of the subfascial (deep) veins.
The extent of edema appears to be related to the strength of the sclerosing solution. This result apparently correlates with the degree of perivascular inflammation produced by the sclerosing solution. The byproducts of inflammation, including release of histamine and various mediators, increase endothelial permeability. In addition to the degree of inflammation induced by sclerotherapy itself, the innate sensitivity of a patient’s perivascular mast cells (possibly related to their atopic or asthma history), concomitant medications that may promote or inhibit mast cell degranulation (e.g. corticosteroids, antihistamines, nonsteroidal anti-inflammatory agents), and previous exposure sensitivity to the sclerosing agent may all contribute to edema. Duffy8 and Goldman13 estimate that the occurrence of pedal edema ranges from 2% to 5%.
Prevention and treatment
Two techniques may decrease temporary swelling. First, perivascular inflammation must be limited, as previously described (see the sections ‘Recommended sclerosing solution amounts and concentrations’ and ‘Postsclerotherapy compression’ in Chapter 9). Ankle edema occurs much less frequently if the quantity of sclerosing solution is limited to 1 mL per ankle.
One method that we have found helpful is topical application of a strong-potency corticosteroid cream, lotion, or gel. Methylprednisolone acts both to stabilize mast cell membranes, preventing histamine release, and to exert part of its anti-inflammatory action directly on the endothelial cell, rendering it less responsive to various mediators.109 Ruscus extract inhibits macromolecular permeability, increasing the effect of histamine in the hamster cheek pouch model.110 This effect is due to stabilization of endothelial pore size. Beta-receptor agonists such as terbutaline and theophylline counteract histamine-induced venular permeability. This effect also occurs with verapamil and glucocorticoids.111
A second method for limiting the degree of pedal or ankle edema is application of a graduated pressure stocking routinely after injections in this area.112 One study compared the use of postsclerotherapy graduated compression for 3 days to 3 weeks and reported that none of 10 patients complained of edema when stockings were worn for 3 weeks; 40% of patients who did not wear post-treatment compression stockings had edema, with 30% having edema if stockings were worn for only 3 days and 20% complaining of ankle/pedal edema if stockings were worn for 1 week.99
Telangiectatic matting
The new appearance of previously unnoticed, fine red telangiectasias occurs in a number of patients after either sclerotherapy or surgical ligation of varicose veins and leg telangiectasias (Figs 8.12 and 8.13). This occurrence has been termed flares by Arenander and Lindhagen,113 distal angioplasia by Terezakis,114 blushing by many, and telangiectatic matting (TM) by Duffy.6 The reported incidence varies from 5%13 to 75%.14,43,113 Two retrospective analyses of 2120 and 7200 patients with leg telangiectasia each reported a 16% incidence.15,115 This incidence has been confirmed in a random sample of 113 female sclerotherapy patients.42
Although the average severity was considered minimal, telangiectatic matting was noted in approximately 70% of our patients treated with foam versus 84% of those treated with liquid STS sclerotherapy.17 Of note, however, all evidence of new vessel formation had resolved completely within several months (on average within 3–6 months). Reasons for the development of TM are multiple. Recovery from an ischemic injury such as closing blood vessels with sclerotherapy may produce a hypoxia-induced neovascularization. In addition, injury to endothelial cells may stimulate the release of a variety of growth factors.
These responses are probably a fundamental feedback response, acting to satisfy tissue needs for oxygenation. For example, this response is commonly seen in myocardial collateralization. Circulating endothelial progenitor cells, elevated with estrogen therapy, may also lead to neoangiogenesis.116 Given these protective factors, it is curious that the incidence of TM after sclerotherapy is not higher; therefore, other innate factors must predispose to the development of TM.
The incidence of TM increased with increasing patient age in one report,113 but this correlation was not observed in another report.115 Although most authors do not comment on a gender predisposition,8 we have seen the development of TM in only one male patient with leg telangiectasia. Because fewer men than women seek treatment for leg telangiectasia, an accurate appraisal of the gender incidence of TM cannot be stated.
TM may appear anywhere on the leg but we have never seen it occur on the face, hand, or chest after sclerotherapy treatment. One detailed study found that most TM occurred on the medial ankle and the medial and lateral calves.113 However, TM also has been reported to occur more frequently on the thighs.117 Duffy has reported that in 80% of his patients TM developed within 10 inches (25 cm) above the knees (personal communication, Oct 1994). Our experience is similar to Duffy’s. Duffy postulates that relative ischemia occurs in this area from tissue hypoxia that results from the thighs and knees pressing on each other during sleep when persons lie on their side. Hypoxia has been found both in the retina and around compressive tumors to promote vascular endothelial growth.118–120 Although a 14.5% incidence of TM has been reported when treating hand veins,59 we have not seen this in hundreds of hand veins treated by sclerotherapy.
Etiologic factors
Postsclerosis TM was first described in the 1960s by Ouvry and Davy.121 They observed that the incidence of matting was proportional to the degree of inflammation and thrombus formation. The etiology of TM is probably related either to angiogenesis8,122 and/or to a dilation of existing subclinical blood vessels by the promotion of collateral flow through arteriovenous anastomoses.122,123
Probable risk factors for the development of TM in patients with leg telangiectasia include obesity, use of estrogen-containing hormones, pregnancy, and a family history of telangiectatic veins. Excessive standing does not appear to influence the incidence of TM.115 Excessive postsclerotherapy inflammation also may predispose toward development of TM.
After sclerotherapy, the development of TM occurs rapidly; often patients report the development over a few days at 3 to 6 weeks after treatment. Normally, the more than one trillion endothelial cells that line blood vessels have a turnover time of more than 1000 days.124 However, under appropriate conditions, new vessels can develop in 2 to 3 days. Observations of mammalian systems have demonstrated the development of a vein from a capillary, an artery from a vein, a vein from an artery, or from either back to a capillary.125,126 In coronary vessels, the number of arterioles and capillaries increases within 1 week after injury.127
Angiogenesis
Angiogenesis is a complex process in which capillary blood vessels grow in an ordered sequence of events. Angiogenic factors act either directly on the endothelium to stimulate locomotion and mitosis or indirectly by mobilization of host helper cells (mast cells and macrophages) with release of endothelial growth factors (see below). When a new capillary sprout grows from the side of a venule, endothelial cells degrade basement membrane, migrate toward an angiogenic source, proliferate, form a lumen, join the tips of two sprouts to generate a capillary loop, and manufacture new basement membrane.128 Obstruction of outflow from a vessel (which is the end result of successful sclerotherapy) is one of the most important factors contributing to angiogenesis.129 Initiation of angiogenesis also follows disruption of endothelial continuity or intercellular contact. This contact results in endothelial cell sprouting and migration.130
Hypoxia with a decrease in the partial pressure of cellular oxygen also is a potent stimulator of neovascularization. With ischemia, one of the first genes upregulated is the gene encoding hypoxia-inducible factor-1 (HIF-1).131 This factor activates genes involved in angiogenesis, glycolysis, modulation of vascular tone, and erythropoiesis.131–133
In addition, endothelial damage leads to the release of heparin and other mast cell factors that both promote the dilation of existing blood vessels and stimulate angiogenesis.134–137 Finally, neovascularization may be promoted by numerous other angiogenic factors, including, but not limited to, heparin-binding fibroblast growth factor (FGF),2,138,139 tumor necrosis factor (TNF),135,140,141 platelet-derived endothelial mitogen,142 endothelial cell growth factor (ECGF),143 and other macrophage-derived growth factors.144,145 These factors and many others are released from perivascular mast cells.146 FGF is released at cell death and is essential for the stimulation of angiogenesis and wound repair.147,148 Thus, sclerotherapy, through endothelial damage, promotes the release of both endothelial angiogenic factors and perivascular mast cell angiogenic factors that provide multiple mechanisms for the formation of new blood vessels. Indeed, it is remarkable that postsclerosis TM does not occur more frequently.
Sclerotherapy-induced perivascular inflammation also may promote TM.26 Inflammation may be considered a hypermetabolic state, with new vessel growth occurring as a result of increased metabolic demand.149 In addition, mast cells are found in increased numbers in inflammatory states, such as allergic contact dermatitis or delayed hypersensitivity reactions.150 Since mast cell heparin is one factor responsible for capillary endothelial cell migration,124 the degree of inflammation should be limited as much as possible to decrease angiogenic stimuli. This is achieved by choosing an appropriate solution concentration for each type of vessel treated, limiting the quantity of solution to the amount that will not produce excessive endothelial damage, and limiting the size of postsclerotherapy thrombosis. This was confirmed by Weiss and Weiss,42 who found that in a random sample of 113 sclerotherapy patients, TM developed in 10 with injection of POL 1% into vessels less than 1 mm in diameter. When POL 0.5% was used for subsequent treatments in these patients, further areas of TM did not develop. The use of low concentrations of POL was found in a multicenter report of 16,804 legs to have an incidence of TM of 0.04%.151 However, it is unclear how closely patients in this large prospective clinical trial were followed. A comparison of POL 1% with HS 20% in treating leg telangiectasia showed that the incidence of TM was higher with the more caustic POL (36%) than with HS (31%) (Fig. 8.14).44
Another group of investigators reported TM in 12% of patients treated with Sclerodine 0.25%, in 17% treated with Sclerodex, and in 15% treated with 0.25% POL.12 These agents should all be comparatively similar in their sclerosing power despite their different mechanism of action on endothelial cells. Interestingly, although their effectiveness in eliminating telangiectasia varied from 73% for POL to 44% for Sclerodine and Sclerodex, the incidence of TM was relatively similar.
A study comparing different times of postsclerotherapy compression in treating leg telangiectasia also demonstrated a decrease in TM when compression was maintained for 1 to 3 weeks (5%) versus 3 days (30%) or no compression (40%).152 This is most likely a reflection of a decrease in intravascular thrombosis with prolonged graduated compression, which results in a decreased phlebitic effect with decreased inflammation.
As noted previously, assuming a role for the perivascular mast cell in the etiology of TM is intriguing. With aging, cutaneous mast cells decrease by 50%, associated with a 35% decrease in subepidermal venules.60 Thus, if TM develops predominantly from mast cell factors, its incidence should be decreased in the elderly; however, this has not been observed in two studies.113,115 Cutaneous mast cells usually occur perivascularly, with a distribution ranging from 7000/mm to 20,000/mm.7,8,153–156 This represents 0.2% to 0.7% of normal skin. In telangiectatic macules associated with mastocytosis, mast cells account for 3.5% (±1.8 SEM) of cells, whereas telangiectasia not associated with mastocytosis has a mast cell volume of 0.4% (±0.1 SEM).157 An analysis of mast cell content in TM lesions is needed.
Estrogen may play a role in the development of TM. It appears that the incidence of persistent TM may be increased in patients taking systemic estrogen preparations.8,42,115 Weiss and Weiss42 found a relative risk of 3.17 (P < 0.003) for development of TM while patients were receiving exogenous estrogen. Sadick also found an increased incidence of TM (10% vs 4%) in patients on oral contraceptive agents or hormone replacement therapy.158 The mechanism for promotion of TM by estrogen is speculative but may be the result of its effect on modulating mast cell responses.159
Estrogen receptors have been found in a number of tumors, including angioma of the nose, soft tissue sarcoma, breast carcinoma, endometrial carcinoma, and unilateral nevoid telangiectasia syndrome. Estrogens also play a role in the development of vascular tissues. In vitro, estrogen and estradiol have promoted endothelial cell migration and proliferation.160,161 Spider angiomas develop during pregnancy and resolve after delivery.162,163 Spider nevi also occur in patients with hepatic cirrhosis associated with elevated serum estradiol levels.164 In addition, Davis and Duffy105 have reported on the virtual disappearance of leg telangiectasia and TM in a 51-year-old woman with estrogen-receptor-positive breast carcinoma after initiation of antiestrogen therapy with tamoxifen citrate (nolvadex). This may be due to the inhibition of angiogenesis by tamoxifen.165,166 However, although it seems logical that estrogen plays a role in the development of TM, estrogen receptors could not be demonstrated in biopsy specimens from leg telangiectasia.167 An evaluation of estrogen receptors in 10 patients with TM lesions did not demonstrate estrogen/progesterone receptors as assayed by ERICA/PRICA technique.168 The limiting factor of this study as stated by the authors was the small size of the study group, as well as the possibility that the immunocytochemical and radioligand assays may not have been sensitive enough to document a small number of estrogen or progesterone receptors. In addition, a selective estrogen-receptor modulator that is specific for blood vessels has yet to be identified.169 Further, circulating endothelial progenitor cells are also elevated with estrogen therapy and may lead to neoangiogenesis.116 Since estrogen receptors have been implicated in the promotion of angiogenesis,170 it may be prudent, albeit premature, to withhold estrogen therapy during sclerotherapy treatment until double-blind controlled studies have been performed.
Prevention and treatment
Regardless of the cause of TM, since patients seek treatment to eliminate leg telangiectasia, it is disconcerting for the sclerotherapist to produce new areas of telangiectasia. Unfortunately, even in the most expert hands, TM occurs in a significant percentage of patients. Fortunately, TM usually resolves spontaneously over 3 to 12 months.8,42 It has been estimated that 10% to 20% of patients will have persistent TM.115,171 Our experience is that less than 1% of patients will have TM persisting for 1 year.
Various vascular-specific lasers and IPL sources may be useful in treating these vessels.172–176 In our practice, at least 75% of patients with persistent TM partially or completely improve after laser or IPL treatment. Interestingly, individual TM lesions may respond better to one laser or IPL than another. Reasons for the variable response are speculative. The 532-nm long-pulse Nd:YAG laser set at the highest fluence and pulse durations available has been found to be most effective on the most recalcitrant lesions. However, persistent and, rarely, permanent hypopigmentation may occur. The use of the PDL may also be effective but result in long-term hyperpigmentation. Glaich et al recently reported successful treatment of postsclerotherapy matted telangiectasias using fractional photothermolysis.177 Specifically, marked improvement was noted in these telangiectasias, which had been present for longer than a year, after five successive treatments with a 1550 nm fractional photothermolysis laser at 4 week intervals. The reason for this reported success is questioned as in this study resolution required 6 months, which is the time course of spontaneous resolution of TM. In addition, this was a single case report. We therefore can not recommend fractional photothermolysis as effective at this time. (A complete discussion of laser treatment is found in Chapter 13.) Unfortunately, even with the aformentioned therapeutic approaches, rare TM may remain resistant to treatment, possibly because in certain cases TM may have a feeding arteriolar network that prevents persistent vessel elimination.
In patients who demonstrate a propensity for the development of TM, additives to the sclerosing solutions or topical agents may minimize this complication. Protamine blocks the ability of mast cells and heparin to stimulate migration of capillary endothelial cells.178 Protamine also prevents the neovascularization induced by an inflammatory agent when it is applied locally or given systemically.179 It has no effect on established capillaries that are not proliferating.180 In addition, beta-cyclodextrin tetradecasulfate administered with cortexolone is a potent inhibitor of angiogenesis.181
Systemic treatment before or during sclerotherapy also may be helpful in limiting TM. Through suppression of TNF synthesis, pentoxifylline (Trental) may minimize angiogenesis.182,183 Inhibition of mast cell mediators with the cell wall-stabilizing medication ketotifen also may help prevent TM, edema, and localized urticaria. Ketotifen, a benzocycloheptathiophene derivative, has H1 antihistaminic properties in addition to decreasing mast cell mediator release.184,185 Ketotifen may also exert its effect by depleting mediators in cutaneous mast cells and so requires multiple doses over a few days for maximal effect.186 Its clinical beneficial effect in patients with chronic idiopathic urticaria, cutaneous mastocytosis, and urticaria pigmentosa has been established.186–190
Pain
Prevention
Since most patients who seek treatment of leg telangiectasia require multiple treatment sessions, each consisting of numerous injections, an attempt should be made to minimize the unpleasantness of the procedure. Certain areas are slightly more painful, especially the ankles, feet, upper medial thighs, and medial knees. The authors have not found it necessary to utilize topical anesthetic creams and indeed a commonly used anesthetic cream, EMLA (eutectic mixture of lidocaine and prilocaine) has been found to produce vessel contraction, making it more difficult to perform treatment (Fig. 8.15). Two variables that can be adjusted to minimize pain are the type and size of the needle and the type of sclerosing solution.
Technique
With sclerosing solutions that are inherently painful to inject (e.g. hypertonic solutions), slow infusion can minimize pain.6,8 Slow injection produces a slower distention of tissue and may minimize endothelial cell separation, which may decrease perivascular nerve stimulation.
Type of Sclerosing Solution
Hypertonic solutions are notorious for causing pain on injection. The cramping pain that may develop after correct IV injection usually occurs a few minutes after injection. Weiss and Weiss6 reported that 72% of their patients injected with HS 23.4% felt pain that lasted less than 5 minutes; 4.5% of patients had pain that lasted more than 5 minutes. This pain probably occurs at the time the hypertonic solution reaches the nerve fibers of the adventitia, either through the wall of the vein or through the capillaries. Subsequently, because of stimulation of sympathetic perivenous nerve fibers, an active contraction of the muscle occurs that may also produce a cramping pain.191 In addition, vascular spasm caused by direct effects of the hypertonic solution itself may occur.
Hypertonic solutions also produce muscle cramping after injection. With the injection of 5 to 10 mL of heparsal (HS 20% plus heparin, 10 U/mL) per injection site into varicose veins, Chou et al192 noted that 16% of 310 patients could not tolerate the pain associated with the procedure. Duffy8 estimated that 82% of his patients treated with HS reported moderate cramping or aching. This can be limited somewhat by keeping the volumes injected to 0.1 mL or less per injection site and by massaging the area immediately after injection.
Adding lidocaine to the sclerosing solution may lessen muscle cramping and allow placement of additional injections into the same area with less pain.8,9 A comparison of HS 23.4% with HS 19% diluted from HS 23.4% with a 2% lidocaine solution demonstrated a significant decrease in pain with the lidocaine solution.14 In the HS 23.4% group, 61.9% of patients rated their pain as none to mild, whereas in the group treated with the HS 19%/lidocaine solution, 90.5% of patients reported none to mild pain. There was no difference in efficacy between the two solutions. However, McCoy et al44 found that even with the addition of 2% lidocaine to HS, patients reported significantly more pain with injection as compared to POL 1%.
Chromated, or plain, glycerin solutions are also painful during injection and may produce mild muscle cramping if more than 1 mL of solution is injected into a single vein.47 We have found that the addition of lidocaine 1% to the glycerin solution minimizes pain on injection similar to its effect with HS. Two relatively painless solutions are POL and STS. The advantage of STS is that it is painful only when it is injected into perivascular tissues, thereby providing a noticeable check on inadvertent perivascular injection. Polidocanol is painless with both intradermal and IV injection. Therefore, one does not have the additional sign of pain to ensure accurate placement of the sclerosing solution. In a double-blind comparison of STS, HS, and POL, patients preferred injection with POL.193 Although relatively painless to inject, STS sometimes produce a dull ache a few minutes after injection. This ache resolves in a few minutes and is most likely related to damaged endothelial cells, which release a variety of factors promoting perivascular edema and inflammation; there is no post-treatment aching with POL.
Despite optimal technique and mild sclerosing agents, post-treatment soreness for 1 or 2 weeks after injection occurs in 20% of patients.8 With the use of nonosmotic sclerosing solutions and the use of graduated compression stockings after treatment, we have not seen soreness in most patients after treatment. If patients do complain of soreness, the cause usually is secondary to thrombosed or inflamed vessels or even a poorly fitted graduated compression stocking.
Localized urticaria
Localized urticaria occurs after injection of any sclerosing solution (Fig. 8.16). It is usually transient (lasting approximately 30 minutes) and probably is the result of endothelial irritation with release of perivascular mast cell histamine. Localized urticaria is not an allergic response, since it occurs even after injection of unadulterated HS 23.4%. It is an example of physical urticaria and is frequent in patients demonstrating dermographism.
Urticaria may occur as the earliest manifestation of perivascular inflammation, through release of endothelial- or platelet-derived factors that lead to perivascular mast cell degranulation194 (see previous discussion in sections on pigmentation and edema).
Approximately 40% of patients studied by Norris et al43 described temporary itching after injections with POL, regardless of drug dosage. Duffy8 reported an almost 100% occurrence of urticaria with injection of either POL or HS–heparin–lidocaine solutions. The urticaria is usually more intense when more concentrated solutions are used.8,13 Urticaria also may be more intense with repeat injection sessions, especially when POL or STS is used.
Treatment
In our experience, localized urticaria and itching can be diminished by applying topical steroids immediately after injection and by limiting the injection quantity per injection site. This is particularly helpful in patients who will wear a graduated support stocking after treatment. High-potency topical corticosteroids, such as clobetasol propionate, have been shown to rapidly decrease histamine-induced pruritus.195 Therefore we recommend application of a non-greasy, fast-absorbing, high-potency corticosteroid to all treated areas after sclerotherapy. A secondary effect of the topical corticosteroid is vasoconstriction, which also may help with vessel resolution, reduction of TM and minimize post-treatment thrombosis with its sequelae.
Tape compression blister
Tape compression blisters (Fig. 8.17) occur when a tape dressing is applied to an area of tissue movement or to thin, elderly skin. The blister usually appears as a flaccid fluid-filled sac overlying normal-appearing skin. It usually is not associated with induration or erythema of the adjacent skin. Common sites of occurrence are the posterior calf, medial thigh, and popliteal fossa.
Tape compression folliculitis
Occlusion of any hairy area can promote the development of folliculitis (Fig. 8.18). If patients do not have secondary alopecia associated with chronic venous insufficiency, men seeking treatment for varicose veins usually have hairy legs. If a tape dressing is placed over foam or cotton ball pads under a graduated compression stocking, a follicular inflammation or infection may occur. Folliculitis is more likely to occur in the summer months or when patients are active and perspire under the dressing.
Other skin disorders
More benign than blisters is skin suntan fading (Fig. 8.19), which is due to the removal of more superficial skin layers of tanned skin. Although completely harmless, these little inconveniences can be misinterpreted by certain patients; fortunately, their duration is limited to a couple of weeks. In some cases, the tape can cause a dehydration of superficial skin layers and be responsible for an allergic-like reaction (Fig. 8.20).
Morpheas
Presenting like morphea observed in scleroderma, this can appear after injection of subcutaneous varicose veins. The etiology is unknown and the incidence is rare. Some patients suffering from scleroderma have been treated by sclerotherapy without presenting morphea; conversely, patients without scleroderma have developed morphea (Fig. 8.21).
Recurrence
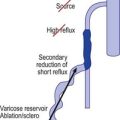
Although we believe that recurrence is really the formation of new vessels in the same region that was previously treated, recurrence of sclerotherapy-treated vessels has been estimated to occur in 3% to nearly 100% of leg telangiectasias at 5-year follow-up (Figs 8.22–8.24).196,197 Recanalization of initially thrombosed leg veins is procedure dependent. The larger the extent of intravascular thrombosis, the greater the likelihood of recanalization of the thrombosis during organization.198,199 The recanalization of injected varices without subsequent compression or with inadequate compression is caused by the following:
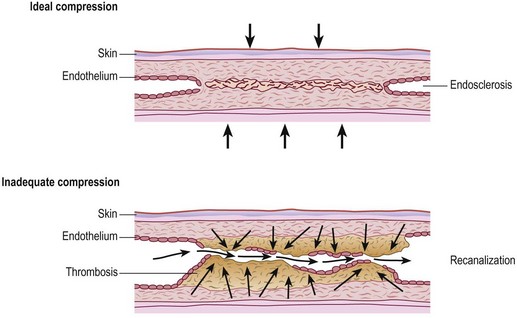
Figure 8.25 Diagrammatic representation of recanalization of a varicose vein through a sclerotherapy-induced thrombosis.
(Redrawn from Wenner L: Vasa 15:180, 1986.)
Treatment
Tournay201 was the first physician to stress the importance of postinjection removal of blood clots, in 1938. The importance of draining these postsclerotherapy thrombi has since been emphasized by Sigg,202 Pratt,203 Hobbs,204 and Orbach.198
With proper technique, varicose veins only rarely recur. A biopsy study of six patients with ‘recurrence’ of previously treated veins demonstrated that the veins thought to have recurred were in reality new varicose veins.205 Raymond-Martimbeau and Dupuis197 have reported a 3.6% recurrence rate with 2-year follow-up of 884 sites of telangiectasia in 525 patients. When high recurrence rates are reported, the patients have usually been treated with minimal compression.198 For example, in one recent study of 310 patients, 83% required reinjection of a treated varicosity. These patients received only 48 hours of compression with elastic bandages.192
Unlike recanalization through a varicose vein cord, recanalization is not common through a sclerosed telangiectasia. Post-treatment histologic studies have demonstrated only fibrosis in an area treated with sclerotherapy.7 One study of telangiectasia found a ‘recurrence rate’ of 56% when patients were evaluated 5 years after sclerotherapy. In 48% of patients affected by a recurrence, the additional telangiectasias were of minimal extent, requiring little if any treatment.206 Examination of telangiectasia present 1 year after treatment most likely indicated either untreated telangiectasia or new telangiectasia, and not recurrent veins. Our experience in observing before and after images on thousands of patients with multiple treatments over two decades confirms that telangiectasias are typically not recurrent but new.
Stress-related symptoms
Vasovagal reflex
The vasovagal reflex (neurocardiogenic syncope) is a common adverse sequela of any surgical or invasive procedure. A survey conducted in an ambulatory care center revealed an incidence of 10.6% during vein cannulation in 1500 patients.207 It has been estimated to occur in 1% of patients during sclerotherapy208 and is more frequent when using the technique of Fegan or Sigg, which requires patients to stand on insertion of needles.209 Duffy,8 who performs sclerotherapy with 30-gauge needles in reclining patients, estimates the incidence of vasovagal reactions as 0.001%. Interestingly, the percentage of men who have this response far exceeds the percentage of women. We (MPG, RAW) have seen a patient with a vasovagal reaction only twice in over 20 years of performing sclerotherapy in reclining patients, and, interestingly, have seen vasovagal reactions many times in male patients just being examined with duplex ultrasound or hearing the Doppler flow sound.
Vasovagal reactions have typical clinical findings. The usual symptoms include light-headedness, nausea, and sweating. The patient also may have shortness of breath and palpitations. Syncope may occur and usually provokes the most concern in the physician and staff. With progression of the reaction, a seizure may occur, as well as cardiac arrhythmia with a rapid decrease in cardiac output and even cardiac arrest.210 Vasovagal reactions most often are preceded by painful injection but may even occur from the patient seeing the needle or smelling the topical isopropyl alcohol or sclerosing solution.
Treatment
The patient should be placed in the Trendelenburg position and observed. If the reaction persists or intensifies, consider a subcutaneous injection of 1 mL atropine 0.4 mg/mL.211 This safe and effective treatment rapidly reverses the vasovagal reaction and prevents its progression. Although a medical workup is rarely necessary, the reader is referred to an excellent review article on this subject.212
Urticaria
Rarely, an urticarial reaction has been noted with use of graduated compression stockings. In one patient, a diffuse urticarial eruption occurred under the compression stocking only on the leg treated with sclerotherapy that was compressed with the stocking (Fig. 8.28). This ruled out a systemic reaction from the sclerosing solution, making the most probable cause the compression stocking itself. In another patient, a dermatopathic urticarial reaction was observed in the skin under contact with the silicone band of the compression stocking (Fig. 8.29). Both of these patients did well when a different brand of compression stocking was used. Detailed communication with the stocking company whose product caused the reaction failed to disclose a definite etiologic factor.
Localized hypertrichosis
Localized hypertrichosis developing after sclerotherapy with the use of multiple sclerosing agents has been described. The cause may be multifactorial. The most logical explanation of increased hair growth appears to be improved cutaneous oxygen content. Other factors may also stimulate increased hair growth. A longstanding low-grade inflammatory reaction may increase vascularity, as well as release various cytokines and growth factors. Vascular endothelial growth factor serves as a growth factor for hair follicle dermal papilla cells.213 Mast cell histamine release is associated with the release of various neuropeptides that may also have a direct effect on the isthmus and bulge region of the hair follicle.214 Clinically, patients with chronic venous insufficiency have been reported to develop localized hair growth after surgical treatment.215
Localized hair growth has been reported from a variety of sclerosing solutions. Hair growth at the site of injection has been described in three patients treated with STS.216 All patients were given injections of 1 to 6 mL of STS over 5 to 10 sessions. Localized hair growth developed 4 to 7 months after the last injection. The site of hair growth was related to the area of skin most damaged by venous incompetence. Another report of localized hypertrichosis occurring 9 months following a patient’s (second) STS sclerotherapy session was notable in that it is the first published case to our knowledge of this phenomenon occurring after use of the foam technique.217 Weissberg218 also has reported the development of localized hypertrichosis in 1 of 62 patients treated with STS. The hair growth occurred at the site of injection 1 month after treatment. It lasted for 4 months and then subsided. A 44-year-old Korean woman also developed localized hypertrichosis on the shin 1 month after sclerotherapy with 3% STS for recurrent varicose veins.219 Sclerotherapy with polyiodinated iodine has also been associated with hypertrichosis at the injection site in three cases.205 Two cases of hypertrichosis have been noted after sclerotherapy with POL.220 Duplex-guided sclerotherapy of the SFJ, as well as sclerotherapy of the posterior arch vein, has also produced temporary hypertrichosis in two patients.221 Therefore, the sclerosing solution itself is most likely not the cause of localized hypertrichosis; its stimulation of the surrounding microcirculation and/or induction of inflammation produces this effect. Although there are multiple case reports of hypertrichosis, this effect must be very rare, since we have seen it in only two patients despite having performed thousands of sclerotherapy treatments over the past 25 years.
Complications
Complications have been observed and described since the very beginning of the technique but their precise incidence remained unclear until recently.222–224, The actual number of complications in a collective of sessions including all types of sclerotherapy (liquid, foam, with ultrasound guidance or not; varicose, reticular and spider veins) is presented in Table 8.2. Interestingly, certain complications previously presented as frequent, such as allergy or skin necrosis, have not been observed in this study. This might demonstrate the progress of the technique: improvement in quality of sclerosing agents, improvement in phlebologists’ training and subsequent skill, impact of ultrasound guidance and foam sclerosants, better knowledge of indications, etc. Regardless, analysis and knowledge of complications is an important part of sclerotherapy.
Table 8.2 Complications observed in a prospective French registry of 12,173 sclerotherapy sessions*
| IMMEDIATE COMPLICATIONS | ||
| Liquid (T = 12) | Foam (T = 28) | |
| Anaphylactic shock | – | – |
| Intra-arterial injections | – | – |
| Vasovagal fainting (VVF) alone | 4 | 6 |
| Headaches alone | – | – |
| Paresthesias alone | 2 | 1 |
| Nausea, vomiting alone | 1 | 0 |
| Visual disturbances alone | 4 | 8 |
| Visual disturbances associated with one or more of: headache nausea, VVF | – | 8 |
| Others | 1 | 5 |
| DELAYED COMPLICATIONS | ||
| Liquid (T = 0) | Foam (T = 9) | |
| Deep vein thrombosis | – | 1 |
| Muscular vein thrombosis | – | 1 |
| Muscular vein extension | – | 1 |
| Perforating vein thrombosis | – | 3 |
| Intense superficial thrombophlebitis | – | 3 |
| Skin necrosis | – | – |
T, total number of complications per type of agent form (liq or foam)
* 5434 (44.6%) with liquid, 6395 (52.5%) with foam, and 344 (2.8%) using both.
Adapted from Guex J-J, Allaert F-A, Gillet J-L, Chleir F: Dermatol Surg 31:123, 2005.
Cutaneous necrosis
Etiology
Cutaneous necrosis may occur with the injection of any sclerosing agent, even under ideal circumstances, and does not necessarily represent physician error (Fig. 8.30) Fortunately, its occurrence is both rare and usually of limited sequelae. Its cause may be the result of:
Extravasation
Clinically, bright erythema is present in the skin overlying the extravasated solution (Fig. 8.31). With certain extravasation injuries, the formation of epidermal blistering may occur but does not predict a partial-thickness injury, although it may precede eventual full-thickness necrosis.225
During injection of an abnormal vein or telangiectasia, even the most adept physician may inadvertently inject a small quantity of sclerosing solution into the perivascular tissue (Fig. 8.32). A tiny amount of sclerosing solution may be left in the tissue when the needle is withdrawn, and sclerosing solution may leak out of the injected vessel, which has been traumatized by multiple or through-and-through needle punctures. Rarely, the injection of a strong sclerosing solution into a fragile vessel may lead to endothelial necrosis and rupture producing a ‘blow-out’ of the vessel and perivascular extravasation of sclerosing solution (Fig. 8.33). Therefore, injection technique is an important but not foolproof factor in avoiding this complication, even under optimal circumstances.
With an osmolality greater than that of serum (281–289 mOsm/L), hyperosmotic agents can cause tissue damage as a result of the osmotic gradient. Epidermal necrosis has even occurred from extravasation of solutions containing 10% dextrose.226 Hypertonic saline 23.4% is a caustic sclerosing agent, as demonstrated in intradermal injection experiments. Clinically, small punctate spots of superficial epidermal damage occur at points of injection, especially when a small bleb of the solution escapes from the vein. However, subcutaneous injection of up to 1 mL of HS 23.4% (by mistake) in lieu of lidocaine into the neck or cheek has been reported to result in no adverse sequelae.227 In this situation, cutaneous necrosis was most likely avoided by rapid physiologic dilution of the HS. Alternatively, dermal tissue may be more resistant to the caustic effects of hypertonic solutions. However, the increasing frequency of cutaneous necrosis occurring after extravasation of inadvertent subcutaneous injection of HS has moved the US Department of Health and Human Services and the product manufacturer (American Regent Laboratories, Inc.) to recommend that HS be stored only in pharmacies where all dilutions would be performed before dispensing. This would eliminate the possibility of an iatrogenic medication error outside the pharmacy (Mary Helenek, American Regent Laboratories, Inc., written communication, May 1990). It is recommended that HS be stored in a location separate from other injectable solutions to prevent this potential complication.
Experimentally, POL apparently is minimally toxic to subcutaneous tissue. Duffy228,229 has reported injecting 0.5 mL of a 3% solution of POL directly into his own forearm skin without the development of an ulceration. Although some physicians advocate the use of intradermal POL 0.5% to treat tiny telangiectatic leg veins,230,231 POL in sufficient concentration causes cutaneous necrosis. Solutions of POL greater than 1.0% may produce superficial necrosis with intradermal injection.230 This unfortunately occurred with the mistaken injection of 0.1 mL POL 5% solution into a leg telangiectasia 0.2 mm in diameter in our practice. This injection resulted in extensive overlying cutaneous necrosis that took 8 weeks to heal. Therefore, POL is not without the risk of cutaneous necrosis if a strong enough concentration is injected.
Of note, several cases of Nicolau’s livedoid dermatitis (NLD) following sclerotherapy have been reported.232–235 Although most commonly seen after intramuscular injections, NLD manifests as pain at the injection site followed by the development of a livedoid plaque, often progressing to cutaneous necrosis. One case of delayed NLD following ultrasound-guided sclerotherapy with POL 2% foam was described recently.235 This particular case was unique in that, not only did it follow an intravenous injection but it also did not manifest until 4 days after the procedure, when the patient first experienced acute pain followed by subsequent cutaneous ischemia. Interestingly, in each reported case of NLD following sclerotherapy, duplex scans failed to show evidence of thromboses in major arteries or deep veins. The author hypothesized that NLD most likely occurred via perivascular spreading of the sclerosant, after leakage from a site of intravenous injection. Subsequently, POL probably acted as a local irritant, inducing arterial or arteriolar vasospasm. Concentrations of POL associated with sclerotherapy-induced NLD ranged from 0.5% to 3%, disfavoring a concentration-dependent phenomenon. One unifying theme among these isolated cases of NLD is that each occurred after injection into veins known to be in close proximity to rich arterial or arteriolar networks. Examples of these at-risk sites include the inguinal fold, medial knee, and medial ankle. Although the limited number of cases of NLD following sclerotherapy reflects its rarity, the clinician is encouraged to remain aware of this potential complication.
Even when sclerotherapy is performed with expert technique, using the safest sclerosing solutions and concentrations, cutaneous ulceration may occur (Figs 8.30, 8.34, and 8.35). Therefore, it appears that extravasation of caustic sclerosing solutions alone is not totally responsible for this complication.
Arteriolar Injection
De Faria and Moraes236 have observed that 1 in 26 leg telangiectasias is associated with a dermal arteriole. Bihari and Magyar237 have found pulsatile flow in 68.9% of patients in 16 of 18 biopsies (2.5 × 1.5 cm) taken from the pulse-positive telangiectasia in patients demonstrating arteriovenous (AV) microshunts. This gives a 61% incidence of AV microshunts in patients with leg telangiectasia. An expanded study of 155 patients with leg telangiectasia by the same group demonstrated a 72.2% incidence of pulsatile flow.238 We believe that the incidence seen in our patients is considerably lower and may represent only 10% or less and might be attributed to a different age or subset of patients, or perhaps to the more physiologic conditions seen with Duplex ultrasound rather than biopsy. The higher incidence found in the later two studies is probably caused by the larger biopsy specimens taken. Of the 22 Doppler-positive telangiectasias, 19 demonstrated AV microshunts on biopsy. Thus, it is likely that rapid injection or large-volume injection into leg telangiectasias that are associated with microshunts will force the sclerosing solution into the arterial circulation. It is our opinion that inadvertent injection into or near this communication is the most common cause of cutaneous ulcerations.
It has been shown by Duffy and by our experience that when POL is injected intradermally to effect sclerosis of TM, cutaneous ulceration does not occur, even with the injection of 0.5 mL of a 0.75% solution. However, we have noted the development of 3- to 6-mm diameter ulcerations in approximately 0.0001% of injections with POL 0.5%. Five consecutive ulcerations that appeared over the course of 12 months were excised. In these patients, each cutaneous ulceration developed as the result of the occlusion of the feeding dermal arteriole. This produced a classic wedge-shaped arterial ulceration (Fig. 8.36). The Australian Polidocanol Open Clinical Trial at 2 years reported 43 ulcers on 32 legs after sclerotherapy treatment of varicose and telangiectatic leg veins on 12,544 legs, for an incidence of 0.23%.239 Therefore, it appears that rare cases of small ulcerations may be unavoidable to some extent, especially in the pretibial and malleolar areas.
Vasospasm
Rarely, after injection of the sclerosing solution, an immediate porcelain-white appearance is noted at the site of injection (Fig. 8.37). A hemorrhagic bulla usually forms over this area within 2 to 48 hours (Fig. 8.38) and progresses to an ulcer.87 This cutaneous reaction might represent an arterial spasm. Duffy229 reported this effect when injecting facial telangiectasia.
Vasospastic reactions of arteries occur in predisposed individuals for unknown reasons.240–242 This may occur even with puncture of the artery without injection of sclerosing solution.242 Thus, small vessels, when irritated in susceptible patients, may spasm.
The major systemic action of nitrates is a direct reduction in venous smooth muscle tone.243 Nitrates also relieve spasm of angiographically normal and diseased arteries.244 Topical nitroglycerin ointment has been reported as beneficial in treating both dopamine extravasation and vasoconstriction necrosis.245,246 Although more experience from other investigators needs to be reported, it seems prudent to use this technique.
Another technique that may help in reversing vasospasm is the topical application of nitric-oxide-generating gel. This gel has been found to increase baseline blood flow in the fingers of patients with Raynaud’s syndrome.247 Since patients with Raynaud’s syndrome have abnormal digital vasoconstriction, the improvement found in application of this gel may crossover to potential improvement in sclerotherapy-induced vasospasm. The gel is prepared by mixing a solution of KY jelly and sodium nitrate (5% weight per volume (w/v)) with a solution of KY jelly and ascorbic acid (55% w/v).
Arterial spasm also may explain the development of cutaneous ulceration upstream from the injection site (see Fig. 8.34). In this latter case, 2 mL of POL 0.25% was injected into a feeding reticular vein (arrow Fig. 8.34). That was the only injection given to the patient in that sclerotherapy session. This has also been reported by Rabe and termed embolia cutis medicamentosa.248
Lymphatic Injection
Injection into a lymphatic vessel also may lead to cutaneous necrosis. Histologic studies have disclosed evidence of lymphovenous anastomoses in humans.249 It is possible that injection into such an anastomosis could result in necrosis of the associated lymphatic vessel and infiltration of the sclerosing solution extravascularly. If the sclerosing solution is caustic to extravascular tissues, tissue necrosis may result.
Subcutaneous Injection
Parmentier observed a case of necrotizing panniculitis after an accidental, large volume, subcutaneous injection of 0.5% POL.235 We have seen a similar patient develop a small necrotizing panniculitis after injection with HS (Fig. 8.39).
Excessive Localized Compression
Excessive compression of the skin overlying the treated vein may produce tissue anoxia with the development of localized cutaneous ulceration (Fig. 8.40). Subcutaneous tissue flow in the leg is decreased when cutaneous pressure exceeds 20 mmHg.250 In addition, external pressure greater than 30 mmHg reduces muscle blood flow in some patients.251 Therefore, excessive compression may produce tissue ischemia. However, both these studies used indirect measurements of subcutaneous tissue flow and calf muscle blood flow and thus must be viewed with caution. A more physiologic method for measuring the effect of compression on blood flow was recently performed through determination of femoral blood flow.252 These authors demonstrated that in the recumbent patient, static, graduated external compression of approximately 20 mmHg at the ankle, reduced to approximately 10 mmHg in the upper thigh, produces an increase in femoral flow of up to 75%. However, if calf pressures exceed 30 mmHg when the patient is recumbent, a progressive fall in subcutaneous tissue flow and deep venous velocity occurs. Therefore it is recommended that patients not wear a graduated compression stocking of greater than 30 to 40 mmHg when lying down for prolonged periods.
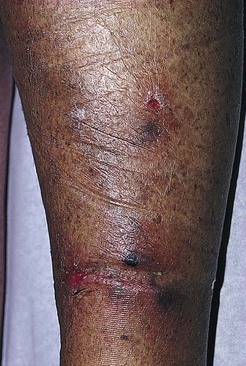
Figure 8.40 Cutaneous ulceration developed 2 days after application of a compression bandage in a nongraduated manner.
One method for applying compression to treated veins that could be varied with patient position consists of a double layer of graduated compression stockings. This would ensure that maximal pressure over the vein is maintained while the patient is ambulatory. When the patient is recumbent, the outer stocking is removed, thereby decreasing the cutaneous pressure to 20 to 30 mmHg at the ankle, which should prevent a reduction in cutaneous and subcutaneous blood flow. Another method described in Chapter 6 is to use foam or rolled cotton wool directly over the treated varicose vein, which increases the pressure applied by the foam by over 50%.
Hypercoagulable State
Two separate case reports of ulceration following sclerotherapy with appropriate concentrations and amounts of STS and POL into leg telangiectasia have been reported, with both patients having an underlying hypercoagulable state.253,254 In the first patient, a mutation in factor V Leiden was uncovered, and in the second, a primary antiphospholipid syndrome. Interestingly, these patients, 45 and 49 years old, had no previous history of ulcerations until the sclerotherapy treatments. The hypercoagulable states in these women promoted excessive thrombosis in perivenous arterioles, causing cutaneous ulcerations.
Pyoderma Gangrenosum
Pyoderma gangrenosum (PG) is an inflammatory skin condition that results in the development of an ulcer at sites of minor trauma and in surgical wounds. It is usually associated with autoimmune conditions, such as inflammatory bowel disease, and hematologic malignancies, but may also be idiopathic. There is one report of the development of a 1.5 cm painful ulceration 4 weeks after ultrasound guided sclerotherapy to the great saphenous vein (GSV) and anterior thigh circumflex vein in a patient with a past history of PG.255 This resolved after treatment with systemic prednisone as well as a topical steroid. Therefore, sclerotherapy should be undertaken carefully in patients with a history of PG, and nonhealing ulcerations after sclerotherapy should be evaluated by a dermatologist to rule out other etiologies.
Prevention
Detergent sclerosing solutions of adequate strength also may be toxic to tissues. Dilution is again of paramount importance. Dilution with hyaluronidase in normal saline solution limits the extent and prevents development of cutaneous necrosis from STS 3%.256 Hyaluronidase (Wydase, lyophilized, 150 USP U/mL) enzymatically breaks down hyaluronic acid in connective tissue. This is hypothesized to disrupt the normal interstitial fluid barrier to allow rapid diffusion of solution through tissues, thereby increasing the effective absorption.257,258 This beneficial effect has been demonstrated in limited IV extravasation injuries from 10% dextrose, calcium and potassium salts, contrast media, sodium bicarbonate, aminophylline, hyperalimentation solution, and doxorubicin.257,259–262 In addition to its enhanced dilutional ability, hyaluronidase may have an independent cellular preservation function.
Hyaluronidase injection improves skin flap survival263 and reduces myocardial infarction.264 This has been postulated to occur through enhanced nutritive flow. Enhanced healing with resolution of painful induration was observed when 250 U of hyaluronidase was injected in an area where neoarsphenamine and oxophenarsine (mapharsen) were extravasated subcutaneously.265 Finally, hyaluronidase promotes wound repair in fetal skin, contributing to scarless repair of wounds by as of yet unclear mechanisms.266 In summary, accelerated dilution, cellular stabilization, and wound repair properties of hyaluronidase appear useful in preventing cutaneous necrosis from inadvertent sclerosing solution extravasation.
Side effects from hyaluronidase use are rare and generally of the urticarial type.267,268 Because of its limited stability, it should be reconstituted with 0.9% sodium chloride solution immediately before use. The ideal concentration and quantity to inject after extravasation has been reported to be 75 U in a volume of 3 mL. Higher doses did not appear to improve clinical outcome after intradermal infiltration of 0.25 mL of HS 23.4%.269 For maximum effectiveness, we recommend injecting the diluted solution into multiple sites around the extravasated area. Studies have demonstrated that hyaluronidase solution must be injected within 60 minutes of extravasation to be effective.270
Treatment
Whatever the cause of the ulceration, it must be dealt with when it occurs. Fortunately, ulcerations, when they do occur, are usually fairly small, averaging 4 mm in diameter in our practice. At this size, primary healing usually leaves an acceptable scar (Fig. 8.41). In addition to various topical therapies directly applied to the ulcer, elevation of the affected extremity and systemic pentoxifylline may be helpful in minimizing the ulcer size. In a series of 26 extravasation sloughs, less tissue damage occurred when limbs were elevated.271
Pentoxifylline may decrease tissue injury of ischemia–reperfusion by inhibiting the production of platelet-activating factor during reperfusion.272 Pentoxifylline should improve microcirculatory dysfunction observed during reperfusion of ischemic tissues. Pentoxifylline causes increased deformability of RBCs and lowers blood viscosity.273–275 From experimental studies in the canine gracilis muscle model, the optimal dose for protective effects appears to be 25 mg/kg. However, the dose that produces maximal protective effects in humans is unknown.
Bodian,117 who uses HS 23.4%, notes that ulceration usually takes  months to heal, even when judicious wound care is provided. He advocates treatment with a daily application of 20% benzoyl peroxide powder (Vanoxide Acne Lotion) under moist dressings cut to fit snugly over the ulcer. However, benzoyl peroxide is cytotoxic for newly growing epidermis and therefore cannot be recommended by us. We have found that the use of occlusive or hydrocolloid dressings results in an apparent decrease in wound healing time. Occlusive dressings do not speed healing of full-thickness ulcers until granulation tissue has formed. Hydrocolloid gel dressings enhance debridement of wounds, possibly through their pectin–gelatin base. Non-gelatin, non-pectin hydrocolloid dressings only act to stimulate fibrin lysis. Thus, its enhanced efficacy may be related to wound debridement, which should always be used either medically or surgically to promote granulation tissue formation. More important, the use of occlusive dressings decreases the pain associated with an open ulcer. Dressings must be changed every 3 to 4 days, and necrotic tissue should be sharply debrided every week or two, as needed, to promote granulation tissue.
months to heal, even when judicious wound care is provided. He advocates treatment with a daily application of 20% benzoyl peroxide powder (Vanoxide Acne Lotion) under moist dressings cut to fit snugly over the ulcer. However, benzoyl peroxide is cytotoxic for newly growing epidermis and therefore cannot be recommended by us. We have found that the use of occlusive or hydrocolloid dressings results in an apparent decrease in wound healing time. Occlusive dressings do not speed healing of full-thickness ulcers until granulation tissue has formed. Hydrocolloid gel dressings enhance debridement of wounds, possibly through their pectin–gelatin base. Non-gelatin, non-pectin hydrocolloid dressings only act to stimulate fibrin lysis. Thus, its enhanced efficacy may be related to wound debridement, which should always be used either medically or surgically to promote granulation tissue formation. More important, the use of occlusive dressings decreases the pain associated with an open ulcer. Dressings must be changed every 3 to 4 days, and necrotic tissue should be sharply debrided every week or two, as needed, to promote granulation tissue.
Systemic allergic reaction or toxicity
Systemic reactions caused by sclerotherapy treatment occur very rarely.
Minor reactions
Bronchospasm has been estimated to occur after sclerotherapy in 0.001% of patients.8 It usually responds to the addition of an inhaled bronchodilator or IV aminophylline, 6 mg/kg over 20 minutes, or to the antihistamine–corticosteroid regimen already noted.
Major reactions
Anaphylaxis is a systemic hypersensitivity response caused by exposure or, more commonly, re-exposure to a sensitizing substance. Anaphylaxis is usually an IgE-mediated, mast cell-activated reaction that occurs most often within minutes of antigen exposure. Other classes of immunoglobulin such as IgG may also produce anaphylaxis.276 Since the risk of anaphylaxis increases with repeated exposures to the antigen, one should always be prepared for this reaction in every patient.277
Allergic reactions to sclerosing agents
Sodium morrhuate
Although promoted by the manufacturer as ‘the natural sclerosing agent’, sodium morrhuate causes a variety of allergic reactions, ranging from mild erythema with pruritus278–280 to generalized urticaria280–282 to GI disturbances with abdominal pain and diarrhea278,279 to anaphylaxis. It has been estimated that ‘unfavorable reactions’ from the treatment of varicose leg veins occur in 3% of patients.283 The incidence of allergic reactions in the treatment of esophageal varices ranges from 11% to 48%.284 The reason for the high number of allergic reactions with this product may be related to the inability to remove all the fish proteins present in sodium morrhuate. In fact, 20.8% of the fatty acid composition of the solution is unknown.285
Many cases of anaphylaxis have occurred within a few minutes after injection or, more commonly, when therapy is reinstituted after a few weeks.280,286–288 Most of these cases occurred before 1950. Rarely, anaphylaxis has resulted in fatalities,282,283,289 many of which have not been reported in the medical literature.278
Bronchospasm developed in one patient while being treated with the twelfth injection under anesthesia. This responded readily to antihistamine and epinephrine. The patient was subsequently treated with STS without an adverse reaction.290
Pleural effusions with pulmonary edema and acute respiratory failure appearing as adult respiratory distress syndrome (ARDS) are common with esophageal injection.285 It has been estimated that pleural effusions occur in 46% of patients with an esophageal injection.291 With injection into esophageal varices, the sclerosing solution rapidly enters the pulmonary circulation, causing increased permeability of the pulmonary microvasculature.285 There have been no reports of pleural effusions with injection into varicose veins of the legs.
Prolonged dysrhythmia requiring placement of a permanent pacemaker has been reported in two cases.292 This complication has been attributed to a direct cardiotoxic effect of sodium morrhuate.
Ethanolamine oleate
Ethanolamine oleate (Ethamolin; EO) is a synthetic mixture of ethanolamine and oleic acid with an empirical formula of C20H41NO3. It is a salt of an unsaturated fatty acid that acts as a sclerosant when administered intravenously.293 The minimal lethal IV dose in rabbits is 130 mg/kg.281 The oleic acid component is responsible for the inflammatory action. Oleic acid may also activate coagulation in vitro by release of tissue factor and Hageman factor. Biegeleisen294 observed no toxic effects in 500 injections, and EO is thought to have a lesser risk of causing allergic reactions compared with sodium morrhuate or STS.295 However, pulmonary toxicity and allergic reactions have been associated with this sclerosing agent.
Pleural effusion, edema, and infiltration and pneumonitis have been demonstrated in human trials with the injection of esophageal varices. Pleural effusion or infiltration has been estimated by the product manufacturer to occur in 2.1% of patients and pneumonia in 1.2% of patients. One study of 75 patients treated for esophageal varices disclosed abnormal chest X-ray films showing infiltration or effusion in 45 patients, for an incidence of 60%.296 These conditions usually resolve spontaneously within 48 hours.296
The product manufacturer has reported anaphylactic shock after injection in three cases (product information (1989) from Glaxo Pharmaceuticals, Research Triangle Park, N.C.). Another case of a nearly fatal anaphylactic reaction during the fourth treatment of varicose leg veins with 1 mL of solution has also been reported.297 In one additional case, a fatal reaction occurred in a man with a known allergic disposition (product information (1989), Glaxo Pharmaceuticals). Another episode of a fatal anaphylactic reaction occurred in a woman having her third series of injections.278 This represented one reaction in 200 patients from that author’s practice. Generalized urticaria occurred in approximately 1 in 400 patients; this symptom responded rapidly to an antihistamine.298
Acute renal failure with spontaneous recovery occurred after injection of 15 to 20 mL of EO in two women.299 A hemolytic reaction occurred in five patients in a series of over 900 patients, with injection of over 12 mL of 0.5% EO per patient per treatment session.298 The patients were described as ‘having pain in the loins and passing red–brown urine. All rapidly recovered with bed rest and were perfectly normal the next day.’ Injections of less than 12 mL per treatment session have not caused this reaction.
Transient chest pain also has been reported in 13 of 23 patients treated for esophageal varices.300 However, pyrexia and substernal chest pain are considered common sequelae of esophageal varices injection with any sclerosing agent.295
The EO mixture has also been tested for carcinogenic activity in the albino rat by intradermal injection without induction of tumors.301 Recently, EO was found to be a successful sclerosing agent in 21 patients treated for reactive vascular lesions.302 Interestingly, the authors of this study found that pain developed when the sclerosant permeated the normal dermis before leaking out through the epidermis and thus stopped the injections at the first sign of pain development.
Sodium tetradecyl sulfate
A synthetic detergent developed in the 1940s, STS has been used throughout the world as a sclerosing solution. A comprehensive review of the medical literature (in multiple specialties and languages) until 1987 disclosed a total of 47 cases of nonfatal allergic reactions in a review of 14,404 treated patients; this included six case reports.303 A separate review of treatment in 187 patients with 2249 injections disclosed no evidence of allergic or systemic reactions.304 An additional report of 5341 injections given to an unknown number of patients found ‘no unfavorable reaction’.305 Fegan306 has reviewed his experience with STS in 16,000 patients. He reported 15 cases of ‘serum sickness, with hot, stinging pain in the skin, and an erythematous rash developing 30 to 90 minutes after injection’. These patients subsequently underwent additional uneventful treatment with STS after premedication with antihistamines. In 10 additional patients, ‘mild anaphylaxis’ developed that required treatment with an injection of epinephrine. If one were to combine only those reviews of over 1000 patients, the incidence of nonfatal allergic reactions would be approximately 0.3%.9,298,307–309
Bioniche Pharma Group, the manufacturer of Sotradecol, notes at least six fatalities associated with the use of Sotradecol, two from the sclerotherapy procedure itself and not specifically related to STS. One fatality occurred in a patient who was receiving an antiovulatory agent. Another death (fatal pulmonary embolism) was reported in a 36-year-old woman who was not taking oral contraceptives. Bioniche Pharma Group was also required through a warning letter by the FDA to disseminate information to providers regarding four of the six fatalities related to Sotradecol administration, which resulted from anaphylactic shock after sclerotherapy treatment with STS.310 One of the four patients had an underlying history of asthma, which is a contraindication to the administration of Sotradecol. Similarly, four deaths attributed to anaphylactoid reactions were reported to the Committee on Safety of Medicines for the United Kingdom between 1963 and 1988, with 22 nonfatal allergic reactions such as urticaria noted over the same period.311
A fatality has been reported after a test dose of 0.5 mL of STS 0.5% was given to a 64-year-old woman.312 An autopsy performed by the Hennipin County, Minnesota, Coroner’s Office revealed no obvious cause of death. Subsequently, mast cell tryptase studies were performed on blood collected approximately 1 hour after the reaction while the patient was receiving life support. A normal tryptase level is less than 5 ng/mL; in experimental anaphylactic reactions induced in the laboratory, levels up to 80 ng/mL have been observed. In this patient, the levels were extremely high at 6000 ng/mL, suggesting that an anaphylactoid reaction had caused her death. Since all reported cases of allergic reactions are of the IgE-mediated immediate hypersensitivity type, it is recommended that patients remain in or near the office for 30 minutes after sclerotherapy when STS is used. However, allergic reactions also may develop hours or days after the procedure.309,313 For example, urticaria occurred 8 hours after treatment in one patient313 and 2 weeks after treatment in two other patients.309 Therefore, patients should be warned about the possibility of allergic reactions and how to obtain care should a reaction occur. In a review of 2300 patients treated over 16 years, four cases of allergic reactions were reported (0.17% incidence).314 Reactions in this study were described as periorbital swelling in one patient and urticaria in three. All reactions were easily treated with oral antihistamines. It is of interest that French phlebologists have advocated a 3-days-before and 3-days-after treatment course with an antihistamine. P. Flurie noted no episodes of allergic reactions in 500 patients treated in this manner.315
In a 2-year prospective study of 2665 patients treated with STS by Paul Thibault,316 there were four cases of anaphylactoid reactions (0.15%). These occurred 10 to 30 minutes after injection of 3% solution, with patients having facial flushing, urticaria, dizziness, tachycardia, shortness of breath, and, finally, GI symptoms of nausea, vomiting, and abdominal pain. All four patients responded well to a subcutaneous injection of 0.5 mL of 1 : 1000 epinephrine followed by promethazine HCl 25 to 50 mg IM. Urticaria occurred in an additional two patients (0.07%).
The reports of the Clinical Drug Safety Surveillance Group of Wyeth-Ayerst Laboratories, the prior manufacturer of Sotradecol are compiled from voluntary reporting to the manufacturer or the FDA, or both. The following are summaries of those reports. January to July 1991 disclosed one episode of erythema multiforme, one episode of ARDS, one episode of fever, lymphadenopathy, and rash, and three episodes of abdominal pain, nausea, vomiting, and diarrhea. The case report of erythema multiforme was reported in a woman after her thirteenth sclerotherapy treatment.317 Pruritus developed the morning after the last injection, with a generalized eruption beginning on the legs 4 days later. This was followed by fever the following day. A rapid tapering course of oral prednisone was given, with complete resolution of the rash in 2 weeks. From September 1991 to November 1992 there were five reports of urticaria and one episode of ARDS. From December 1992 to September 1993 there was only one case of a maculopapular rash. From September 1993 through October 1994 there was one case of angioedema, and generalized weakness was reported in one patient after receiving 10 mL of 3% STS. From November 1994 through January 1996 there was one case of anaphylaxis. From January 1996 through December 1996, there was one case of allergic vasculitis. From November 1997 through October 1999 there were three cases of urticaria and four cases of nonspecific hypersensitivity reactions. These reactions voluntarily reported to Wyeth-Ayerst occurred with approximately 500,000 2-mL ampules of 1% and 3% being sold yearly within the United States. Thus, the incidence of adverse reactions is rare. (Most information regarding adverse reactions from Sotradecol was provided by Paul Minicozzi, Ph.D., Wyeth-Ayerst Laboratories, through yearly correspondence.)
In the French registry of 12,173 sessions of sclerotherapy, no allergic reaction has been reported.224 In France, sclerosing agents are STS, POL, and CG.
The most common systemic reaction consists of transient low-grade fever and chills lasting up to 24 hours after treatment.318 This has also been seen in one of our patients. Of note is that three patients with allergic systemic reactions to monoethanolamine oleate had no evidence of allergy to STS.318
Reactions can occur with any sclerosing solution; they are not allergic in nature but represent the effect of the sclerosing solution on the vascular system. One such reaction is hemolysis that occurs through lysis of RBCs that are present in the treated vein. A hemolytic reaction occurred in five patients in a series of more than 900 patients with injection of more than 8 mL of STS 3%.298 Like a similar reaction that occurred with EO, patients were described as ‘…feeling generally unwell and shivery, with aching in the loins and passage of red-brown urine. All rapidly recovered with bed rest and were perfectly normal the next day.’ Injections of less than 8 mL per treatment session did not result in this reaction. Intravascular hemolysis was also reported to the FDA after injection of STS into a hepatic artery feeding a hepatic tumor.
Although the lethal dose in humans has never been reported, the IV median lethal dose (LD50) in mice is 90 mg/kg.319 The lethal volume after IV injection in the rat is approximately four to six times as high for STS as for POL in equivalent concentrations.320 In our practice, it is not uncommon for patients to be treated with up to 30 mL of 0.5% STS. We have not observed an adverse reaction from this dose of STS.
The experience of two of the authors (MPG, RAW) over 20 years in an estimated 40,000 patients is that no patient has developed a serious allergic reaction from the use of STS. Since STS from various sources may have a variable purity (see Chapter 7) it appears possible that allergic reactions may occur from the impurities, such as carbitol, and not STS itself.321 This may explain the decreased reported incidence of allergic reactions with the use of Fibro-Vein (STD Pharmaceuticals) as compared with Sotradecol (Bioniche Pharma Group) and/or Trombovar (Laboratoires Innothéra, Arcueil, France).
Polidocanol
Kreussler Pharma has sold over 250,000,000 mL of polidocanol (Aethoxysklerol) for sclerotherapy from 1987 to 2010 and estimates that this translates to between 40 and 45,000,000 treatments (personal communication Christian Freyberg, International Sales and Marketing Director, January 2010). Allergic reactions to POL are quite rare and had been reported in only four patients in a review of the world’s literature up to 1987, with an estimated incidence of 0.01%.303 Amblard322 reported no allergic reactions in over 250 patients, including no allergic reactions in two patients who were intolerant to STS. Hoffer231 reported no allergic reactions in over 19,000 cases. In addition, patients who are allergic to STS or iodine have no allergic manifestations to injections of POL.322–324 However, rare allergic reactions have been reported, including a case of nonfatal anaphylactic shock to 1 mL of POL 2% injected into a varicose vein during the fourth treatment session.314,325–327 Also, Ouvry et al328 reported generalized urticaria with cough and dyspnea in a patient after receiving 2 mL of POL 2%; the condition resolved in 30 minutes with IV corticosteroid therapy.
Since the previous review, additional cases of allergic reaction have been reported. Guex58 reported seven cases of minor general urticaria in nearly 11,000 patients treated over 12 years. These patients cleared completely in 1 to 2 days with antihistamine and topical corticosteroid therapy, with one patient requiring systemic corticosteroids. He has more recently reported only one possible case of benign allergy and no cases of anaphylaxis in 3357 patients treated.329
A detailed account of three serious cases of anaphylaxis was reported from the Netherlands.330 These patients were anaphylactic within 15 minutes after injection of POL. Two of them had received the drug for the first time. One patient, a 70-year-old woman with a complicated medical history of two heart operations, two cerebrovascular accidents, and hyperthyroidism, was successfully resuscitated after cardiac arrest. She was receiving multiple medications, including digoxin, carbimazole, captopril, furosemide, mebeverine, and acenocoumarol. She had been treated with POL without complications on four previous occasions. The second patient showed signs of ARDS after being treated with epinephrine and systemic methylprednisolone for ‘shock’. The third patient developed urticaria, dyspnea, paresthesia, headache, and chest pain with electrocardiographic (ECG) findings of cardiac ischemia. No further studies were performed on these patients.
The Australian Polidocanol Open Clinical Trial at 2 years, with over 8000 treated patients, reported nine local urticarial reactions and three generalized reactions, with two patients developing a rash, for an incidence of approximately 0.2%. There were no cases of anaphylaxis.239 After a further 8804 patients were evaluated, an additional three patients developed urticaria, again without any additional significant adverse sequelae.151 A 5-year experience in 500 patients treated with POL 3% reported five cases of allergic reaction (1% incidence); one patient had nonfatal anaphylactic shock, with the other patients experiencing urticaria.331
Two of 689 sequential patients were reported who developed an immediate-type hypersensitivity reaction with systemic pruritus and urticaria.332 This represented an incidence of 0.3% in their patient population and 0.91% for the ‘true’ population. These two reactions occurred without prior exposure to POL as a sclerosing agent. Since POL is used as an emulsifying agent in preprocessed foods, patients may have been exposed previously through ingestion. Both patients responded easily to either a single dose of oral diphenhydramine, 50 mg, or 0.3 mL of subcutaneous epinephrine plus 50 mg of diphenhydramine IM.
Jaquier and Loretan230 believe that the decrease in antigenicity is the result of the absence of a benzene nucleus and a paramine group and the presence of a lone free alcohol group. Dexo SA Pharmaceuticals, the product manufacturer in France, recommends that this substance not be used in patients with an allergic diathesis (e.g. asthma) (product description of hydroxypolyethoxydodecane (May 1985) received with correspondence from Dexo SA Pharmaceuticals, France). Thus, allergic reactions to POL are similar to those reported with STS.
Other unusual reactions reported to Kreussler (correspondence, January 1994) include rare episodes of short convulsive coughing, acute pressure sensation in the chest, acute difficulty with breathing, and one case of stabbing chest pain without demonstrable ECG changes or myoglobin band creatine phosphokinase fluctuations. One specific case report describes a 30-year-old woman who underwent four separate sclerotherapy sessions with POL. On the fourth session, 3 mL of POL 1.5% and 12 mL of POL 0.5% were administered. The patient complained of chest heaviness and constriction, which had also appeared after two of her other sessions but had not been brought to the attention of the medical staff. During the fourth episode, she lost consciousness and was found without a pulse or blood pressure, with dilated pupils. Spontaneous respiration occurred after 2 to 3 minutes; she began to vomit and complained of headache and earache. She recovered and was discharged after 10 hours well, but returned the next day with dysosmia which lasted 6 weeks. Although a brain CT scan was normal, the presumed cause was cerebral.333
Like EO and sodium morrhuate, POL has demonstrated a dose-dependent cardiac toxicity when injected into esophageal varices. POL has a negative inotropic, chronotropic, and dromotropic effect, reducing atrioventricular and intraventricular conduction, as well as lowering blood pressure. Animal studies have demonstrated a reversible, dose-dependent decrease in myocardial contractility, blood pressure and pulse rate, and a prolongation of the PQ interval.334 This effect may explain the cause of heart failure in three elderly patients with severe liver failure who were given massive quantities of POL during esophageal sclerotherapy (760 mg in a 74-year-old woman, 600 mg in a 70-year-old woman, and 750 mg in a 76-year-old woman).335,336 In addition, a case of sinus bradycardia with eventual asystolic cardiac arrest occurred after administration of 4 mL of 1% POL into a 5-year-old girl under general anesthesia with sevoflurane 2% with Klippel-Trénauny syndrome. After appropriate emergency care, cardiac function was restored without adverse sequelae.337
Polidocanol, being a local anesthetic, demonstrates a systemic toxicity level similar to that of lidocaine and procaine; when combined with other anesthetics, an additive risk to the cardiovascular system occurs.338,339 Specifically, when administered concomitantly with other local anesthetics, additional proarrhythmic effects were observed. The LD50 in rabbits at 2 hours is 0.2g/kg, which is three to six times greater than the LD50 for procaine hydrochloride.340 The LD50 in mice is 110 mg/kg.341 Finally, the teratogenicity was evaluated by Fournier in rabbits and rats.342 The pregnant animals received 2.7 to 4.5 mg/kg per day and did not demonstrate a significant increase in the number of abnormal fetuses.343 One severe malformation was seen in a rat pup out of 530 normal pups (0.08%) whose mother received 4.5 mg/kg per day. This is far in excess of the recommended doses of POL in humans. Kreussler has reported that POL does cross the placental barrier in rats, but that perinatal and postnatal development and behavior were not impaired in rats whose mothers received intravenous POL every other day during late gestation as well as in the lactation period.339 However, no formal studies in pregnant women have been reported. Sigg344 has reported treating 3600 pregnant women with 34,000 injections without fetal injury or abortion.
Chromated glycerin/glycerin
Chromated glycerin 72% (Sclérémo) is a sclerosing solution with a very low incidence of side effects (Sclérémo product information (1987)).21,86,345 Hypersensitivity is a very rare complication.346,347 Contact sensitivity to chromium occurs in approximately 5% of the population.348 Intravenous potassium dichromate leads to complete desensitization in chromium-sensitized guinea pigs. This effect occurs because chromium needs to bind to skin proteins to become an effective antigen.349 This may be related to the necessity for epidermal Langerhans’ cells to produce an allergic response, whereas T lymphocyte accessory cooperation is not optimal with IV injection and its resulting endothelial necrosis. Thus it is more common for a sclerotherapist to develop an allergic contact dermatitis to CG than it is for a patient to have an allergic reaction to IV use of CG. Indeed, Ouvry (personal communication, 1995) has developed an allergic contact dermatitis from CG injected without the use of protective gloves.
Ramelet et al350 have reported seven patients who developed an allergic reaction to CG. One patient had a vasculitis, and six patients had an eczematous reaction. All allergic patients demonstrated a sensitivity to topically applied chromium.
One case of fatal allergic reaction has been described recently.351 The patient, a 51-year-old female, developed a laryngeal edema immediately after the end of the first sclerotherapy session with undiluted Sclérémo, and died after 2 hours of appropriate resuscitation. The patient had declared a past history of asthma, but no cutaneous allergy. One patient, treated with 72% glycerin with lidocaine and epinephrine for spider veins, developed leg swelling with 3–4+ pitting edema several days following sclerotherapy (personal email communication with Joseph Ang, October 2007). In this case, 18 mL of non-foam sclerosant was used in the treatment of one leg, the patient wore compression stockings following treatment, and there was no evidence of an underlying DVT. The etiology of this lower extremity edema is unclear. Regardless, due to the potential for hemolysis, we recommend limiting the total volume of glycerin per treatment session to 10 mL.
Hematuria accompanied by ureteral colic has been reported to occur transiently after injection of large doses of CG. Ocular manifestations, including blurred vision and a partial visual field loss, have been reported by a single author, with resolution in less than 2 hours.352 Glycerin (or any sclerotherapy) induced hemolysis may not be a benign event. Hemoglobin can exert direct cytotoxic, inflammatory and pro-oxidant effects that adversely affect endothelial function.353 Hemoglobin from destroyed RBCs dimerizes and is rapidly bound by the serum protein haptoglobin. The haptoglobin–hemoglobin complex causes endocytosis and degradation, which can lead to a variety of adverse effects.354
Although transient hemoglobinuria is common in athletes and is without known long-term adverse effects, hemoglobulinemia can cause renal failure.355 More commonly, hemoglobulinemia can cause a dose-related gastrointestinal dystonia and pain including esophageal spasm and dysphagia.354 The reader is referred to an excellent recent review which details more clinical manifestations of hemoglobinemia.356
An additional case was reported of transient hypertension and visual disturbance after the injection of 12 mL of 50% CG into spider and ‘feeder’ leg veins in a fourth treatment session.357 These symptoms occurred  hours after treatment and lasted more than 3 hours without treatment. This may have represented a retinal spasm or an ophthalmic migraine. Since we have been using glycerin alone without chromium but mixed 2 : 1 with lidocaine 1% with or without 1 : 100,000 epinephrine, we have yet to see an allergic reaction. We have also yet to see hemoglobinuria or adverse effects with the use of up to 12 mL of this glycerin mixture, except for a minute or two of epinephrine-induced ‘rush’ which can occur in rare patients who have a sensitivity to epinephrine.
hours after treatment and lasted more than 3 hours without treatment. This may have represented a retinal spasm or an ophthalmic migraine. Since we have been using glycerin alone without chromium but mixed 2 : 1 with lidocaine 1% with or without 1 : 100,000 epinephrine, we have yet to see an allergic reaction. We have also yet to see hemoglobinuria or adverse effects with the use of up to 12 mL of this glycerin mixture, except for a minute or two of epinephrine-induced ‘rush’ which can occur in rare patients who have a sensitivity to epinephrine.
Polyiodinated iodine
Polyiodinated iodine (Varigloban, Variglobin, Sclerodine 6) is a stabilized water solution of iodide ions, sodium iodine, and benzyl alcohol. Sigg et al358 reported on their experience with over 400,000 injections with Variglobin. Paravenous injections readily produced tissue necrosis. In 1975, Sigg and Zelikovski359 reported an incidence of 0.13 allergic cutaneous reactions per 1000. No systemic allergic reactions were observed. Obvious contraindications to the use of polyiodinated iodine are hyperthyroidism and allergies to iodine and benzyl alcohol.
High concentrations of iodine solutions may also produce bronchiomucosal lesions. Therefore, Wenner360 recommends that a maximum of 5 mL of 12% solution be used in a single sclerosing session.
Hypertonic saline
As discussed previously, hematuria can occur with any sclerosing agent. Dodd361 has reported painless hematuria in five patients injected with HS. Sometimes blood appears in the urine after one or two acts of micturition and occasionally at other times throughout the day. Usually there are no other ill effects, and the hematuria resolves spontaneously. Hematuria probably occurs because of hemolysis of RBCs during sclerotherapy.
Heparin in hypertonic saline
Whereas unadulterated HS solutions in concentrations from 15% to 30% are used for the treatment of varicose and telangiectatic leg veins, adding heparin to the solution to prevent the theoretic risk of embolization associated with sclerotherapy has been advocated.22 Although the necessity for heparin has been discounted in a well-controlled 800-patient randomized study,24 Foley’s solution, Heparsal, consisting of HS 20% with heparin, 100 U/mL, and procaine 1%, is commonly used in the treatment of telangiectatic leg veins. Therefore, the risk of adverse reactions to heparin should be mentioned.
Commercial preparations of heparin consist of straight-chain anionic polysaccharides of variable molecular weight (usually 7000 to 40,000). Heparin prepared from different tissues also appears to vary: more protamine is required to neutralize a unit of beef lung heparin than porcine mucosal heparin. Plasma lipolytic activity, antifactor Xa activity, and activated partial thromboplastin time ratios are significantly different.362
Fever, urticaria, and anaphylaxis occur occasionally after administration of heparin.362–366 Necrosis has been reported in patients receiving subcutaneous heparin,358,367–370 and has usually occurred 3 to 10 days after multiple subcutaneous injections. Pruritus, local tenderness, and burning sensations associated with large, indurated, erythematous plaques have also been reported to occur in six patients 10 to 20 days after beginning prophylactic doses of heparin.371 Thus, there is a measurable risk of toxicity to heparin.
Although heparin is not a totally benign substance, those who use Heparsal have not reported any adverse reactions that could be attributed to the heparin component.8,22,24,192 The absence of side effects has been reported in one series of 310 patients when volumes of 10 to 20 mL have been injected into varicose veins.192 In the doses used, heparin in Heparsal may be without significant side effects, but one must be aware of the potential dangers associated with its use.
Lidocaine in hypertonic saline or glycerin
Alderman9 and Duffy8 advocate the addition of lidocaine (to achieve a 0.4% final concentration) to minimize patient discomfort during injection of HS. This addition introduces a potentially allergenic sclerosing mixture. Although the most common cause of patient-reported ‘allergic reactions’ to lidocaine is psychogenic or vasovagal phenomena,372 allergic reactions may occur. Vasovagal reactions are due to emotional or physical stimuli and are not directly due to the local anesthetic. Besides vasovagal reactions, the majority of side effects to lidocaine are localized and include bruising and edema at the injection site.373 A report on 28 patients who had a variety of adverse reactions to lidocaine, including skin reactions, loss of consciousness, and vasovagal symptoms, were evaluated with skin testing and found not to be allergic to lidocaine. Nineteen of these patients were subsequently treated with lidocaine without an adverse reaction.374 In a more recent study, 183 patients with a reported allergy to lidocaine were patch tested and those patients who tested positive received a 0.1 mL intradermal challenge with lidocaine 1%. Of the 183 patients tested, four had positive reactions to lidocaine, two of whom had sensitivity to local injections of lidocaine manifested by dermatitis.375 Impending anaphylaxis must be distinguished from vasovagal reactions, which occur more commonly. One important distinction is that in vasovagal reactions the patient demonstrates bradycardia, whereas a patient with anaphylaxis exhibits tachycardia.376 As described previously in this chapter, vasovagal reactions also commonly include lightheadedness, hypotension, and diaphoresis.
Unlike the ester class of anesthetics, which are much more commonly shown to cause allergic reactions, members of the amide class of anesthetics, to which lidocaine belongs, have a very low risk of allergic reaction.377–382 There are two types of allergic reaction to local anesthetics: a type I immediate hypersensitivity or anaphylactic reaction and a type IV delayed hypersensitivity reaction. The former is mediated by antibodies and the latter by cell-mediated immunity. Allergy is most likely caused by the methylparabens or sodium metabisulfite that is used as a preservative in anesthetic solutions.377,378,383–385 To keep the allergic risk as low as possible, single-dose vials of lidocaine without preservatives should be used.
Despite the low risk of allergenicity to lidocaine, a few true allergic reactions, including anaphylaxis, have been reported.386–393 The prevalence of anaphylactic reactions to anesthetics is approximately 1 : 3500 to 1 : 20,000, with a mortality of between 3% and 6%.394 One patient demonstrated a type I hypersensitivity reaction to lidocaine after undergoing intralesional injections to his keloids with a total of 0.5 mL of Kenalog diluted to 10 mg/mL with lidocaine 1% and epinephrine 1 : 100,000.373 He was fine when leaving the office, but approximately 15 minutes later began to experience wheezing, lightheadedness, and periorbital edema. Symptoms resolved several hours after treatment in the emergency room with an albuterol nebulizer and oral famotidine. Interestingly, this patient subsequently had a positive wheal and flare response to intradermal lidocaine 1% at a dilution of 1 : 10,000 during allergy testing. Thus, HS or glycerin, when adulterated with lidocaine, may place the patient at risk for allergic reactions.
Superficial thrombophlebitis
Before the advent of modern-day sclerotherapy, which uses graduated compression to limit thrombosis, thrombophlebitis, both superficial and deep, occurred in a significant number of sclerotherapy patients.395 In fact, because phlebitis was such a common sequela of sclerotherapy, some doubted early on whether sclerotherapy was a legitimate treatment for varicose veins.396 With the use of compression and the realization that many adverse effects result from thrombus formation in treated veins, the incidence of thrombophlebitis has greatly decreased.
Numerous underlying conditions may predispose a patient to develop thrombophlebitis through induction of a state of hypercoagulability (see ‘Pulmonary embolism and deep venous thrombosis’ section). The most common of these risk factors appears to be elevated factor VII.397 In addition, the persistence of significant reflux into a vein that has been treated can also lead to phlebitis. Fegan398 states that this complication most commonly occurs when perforator veins in the region of treatment are not diagnosed and treated. When eosinophilia accompanies superficial venous thrombophlebitis, the practitioner should consider an underlying malignant blood disorder, neoplasia, vasculitis, or hypereosinophilic syndrome (HES).399 We have seen two patients who developed superficial thrombophlebitis as the presenting sign of ovarian cancer (RAW) and recurrent breast cancer (MPG). This has been reported by others as well.400
Etiology
Superficial thrombophlebitis appears 1 to 3 weeks after injection as a tender erythematous induration over the injected vein (Fig. 8.42). Duffy8 estimates that it occurs in 0.5% of his patients who have primarily small-vessel varicose veins and telangiectasia. Mantse309 reported an incidence of 1% in the treatment of varicose (nontelangiectatic) veins despite the use of a tensor bandage for 6 weeks. Mantse notes that the patients who developed superficial thrombophlebitis found the bandage was too tight and reapplied it too loosely at home. In our recent 5-year retrospective, this complication occurred to some degree in approximately 1.7% of patients (6 of 351). Most patients have minimal phlebitis and require either no treatment or simple drainage of an associated coagulum with a 22-gauge needle.17 Rarely, in symptomatic patients, treatment with graduated compression and anti-inflammatory agents is given (0.001% of patients totaling more than 10,000 separate injections).
In certain cases it presents as an extensive pigmented cord along the superficial venous pathway (Fig. 8.43). The content of the venous lumen can vary according to the etiology, emphasizing the need to distinguish between ‘phlebitis’ and ‘thrombosis’. The presented case represents obviously a pure ‘phlebitis’ (Fig. 8.44).
Sometimes confused with superficial thrombophlebitis, patients may, although rarely, develop a chemical lymphangitis. It typically appears within 24 hours after treatment but resolves spontaneously within 72 hours. It is not associated with pain or tenderness. Shown in Figure 8.45, this occurred after treatment with 1% STS liquid.
Prevention
The cause of thrombophlebitis is related in part to treatment technique as well as to lack of adequate post-treatment compression. In our experience, a decreased incidence of superficial thrombophlebitis may result from a greater degree and length of compression used after sclerotherapy is performed. An inadequate degree or length of compression results in excessive intravascular thrombosis. Sigg401 notes that perivenous inflammation is observed only at those parts of the limb not covered by a compression dressing. Thus, to avoid this complication, one should prevent or minimize the development of postsclerosis thrombosis by using compression pads and hosiery over the entire leg and not just over the treated veins.
However, even when appropriate compression is used, thrombosis and perivascular inflammation may still occur (Fig. 8.46). Ascending phlebitis in the small saphenous vein (SSV) or its long tributaries, starting at the upper edge of the compression stocking, is relatively common. Here, the sclerosing action continues up the abnormal vessel (even beyond what apparently is the extent of the abnormality). It is thought that the sclerosing solution destroys damaged endothelium to a greater extent than normal endothelium (product description on polidocanol received with correspondence, May 1985). Therefore, the placement of a foam pad extending above the compression stocking or bandage to create a gradual transition of pressure from compressed to noncompressed vein may provide a safety margin, as well as prevent damage to the vein by the otherwise abrupt cut-off of the pressure stocking.
Thrombophlebitis is a complication that should not be taken lightly. If untreated, the inflammation and clot may spread through perforating veins to the deep venous system. This extension may lead to valvular damage and possible pulmonary embolic events.402–407 A study of a group of 145 patients with superficial thrombophlebitis found that 23% of affected limbs had proximal extent into the SFJ.408 Pulmonary embolism (PE) was found in 7 of 21 patients (33.3%) with thrombophlebitis of the GSV above the knee;409 17 of the 21 patients had varicose veins. Interestingly, in this study, clinical symptoms suggestive of PE were present in only one of the seven patients. The occurrence of DVT in patients with below-knee superficial vein thrombosis (SVT) was 32% in one study of 78 patients.410
Treatment
In addition to adequate compression, drainage of thrombi after liquefaction (approximately 2 weeks after sclerotherapy) hastens resolution of the otherwise slow, painful resorption process.401 Adequate compression and frequent ambulation should be maintained until the pain and inflammation resolve. Aspirin or other nonsteroidal anti-inflammatory agents may be helpful in limiting both the inflammation and pain.
Patients with extensive involvement of leg varices should receive anticoagulation, particularly if the thrombosis extends into the proximal part of the SFJ. In addition to propagation of the thrombus through the SFJ, 11% to 40% of patients with SVT at the SFJ have evidence of concurrent DVT.409–412 In these patients, anticoagulation for 6 months achieved resolution of the DVT/SVT while preventing PE. This success occurred despite duplex evidence of progression of SVT to DVT in 2 of 20 patients.411 Of interest is the fact that the incidence of coincidental DVT with SVT was greater in patients without varicose veins (60% versus 20%), thus indicating that other innate factors place patients with SVT at additional risk for DVT.
In addition, the use of low-dose low-molecular weight heparin (LMWH) in patients with SVT may decrease perivascular inflammation. LMWH has been demonstrated to limit neutrophil extravasation.413 Thus, LMWH has anti-inflammatory properties in addition to anticoagulant properties.
Other methods for enhancing thrombolysis may be considered for SVT as well as for DVT. Ultrasound-enhanced systemic thrombolysis has recently been found to augment arterial recanalization.414 This procedure utilizes a 2-MHz Doppler ultrasound in conjunction with systemic tissue-type plasminogen activator (t-PA). Ultrasound increases enzymatic fibrinolysis through mechanisms that include improving drug transport, altering the fibrin structure, and increasing the binding of t-PA to fibrin.415
Recently, a liposomal heparin spray (Lipohep Forte Spraygel) applied after the onset of SVT has been found to improve symptoms as well as hasten resolution of the SVT.416 This spray was as effective as LMWH injections. Finally, distinguishing between hypereosinophilic syndrome-related and idiopathic superficial venous thrombophlebitis is important, since anticoagulant therapy is probably indicated in the former, which carries an increased thrombotic risk.399
Arterial injection
The most feared complication in sclerotherapy is inadvertent injection into an artery, but fortunately this complication is very rare.417–419 Five examples of this complication had been reported to the Medical Defence Union in Great Britain in 1985.420 Cockett421 reported 18 cases, including those reported to the two medical protection societies in the United Kingdom, over a 10-year period. However, Cockett believes even this number is too low because many cases never reach the courts or the medical literature. Biegeleisen et al422 reported seven cases in their practice history of more than 10 years. Forty cases over 17 years have been reported from France.423 Bergan et al424 more recently reported on a series of cases from the United States.
Arterial injection of a sclerosing solution causes the development of an embolus. This has been experimentally confirmed in the canine femoral artery.425 These experiments demonstrate little effect on the artery itself with injection – spasm does not occur. The sclerosing solution acts to denature blood and endothelial cells in smaller arteries, producing a sludge embolus that obstructs the microcirculation (Fig. 8.47).426 Stagnant blood flow, secondary thrombosis, and necrosis soon follow.
Occlusion of small arteries may lead to the development of a compartmental syndrome. Intracompartmental pressures of 30 mmHg or more in humans usually produce neural deficits characterized by pain during stretching of the muscles involved, paresthesia, and then paresis and anesthesia in the sensory distribution of the nerve that courses through the affected compartment. The end result is paralysis.313 Muscle necrosis then leads to leg atrophy. Sensory deficit in the first web space (deep peroneal nerve) implicates anterior compartment syndrome. Sensory deficit on the dorsum of the foot (superficial peroneal nerve) implicates the lateral compartment. The superficial posterior compartment is indicated by sensory deficit of the sural nerve distribution (lateral foot). Deep compartment syndrome is likely if the sole of the foot is affected (posterior tibial nerve).
Unless large arteries are blocked, peripheral pulses will be intact, and capillary filling is easily identified because the compartmental pressure must be greater than 80 mmHg to occlude large artery flow.427 This may lull the physician into a false sense of security, and the underlying compartmental syndrome may not be recognized. Intracompartmental pressures between 30 and 40 mmHg for 6 to 12 hours can cause irreversible damage to the nervous tissue because of impairment of microcirculation.313 Magnetic resonance imaging (MRI) examination of the affected limb has been able to demonstrate muscle destruction (Fig. 8.48).
Etiology
Arterial injection occurs most commonly in the posterior or medial malleolar region, particularly when an effort is made to inject the internal ankle perforator vein, specifically in the posterior tibial artery.398,419,421,428 Other areas predisposed to inadvertent arterial injection include the groin and the back of the knee, but any injected area can be prone to arterial injection.429 The patient will usually, but not always, note immediate pain (it can be supposed that since it has a local anesthetic effect, intra-arterial injection of POL could be painless during the first days – see Fig. 8.49). Evidence is still lacking and the decrease in incidence of these complications with the use of ultrasound guidance and foam will probably (and fortunately) not allow one to draw more precise conclusions. Pain then typically slowly propagates down into the foot and outer toes over the following 2 to 6 hours post-injection. During this time, arterial pedal pulses are palpable. At 10 to 12 hours later, the four outer toes are white, with the sole of the foot becoming painful. Cutaneous blanching of the injected area usually occurs in an arterial pattern associated with a loss of pulse and progressive cyanosis of the injected area.
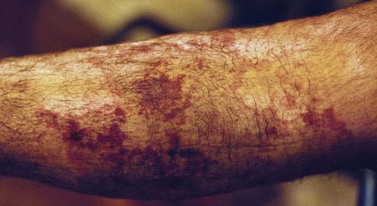
Figure 8.49 Postsclerotherapy necrosis at an early stage: 8 days after injection of 3 × 0.5 cm3 of polidocanol 0.5% in medial leg postsurgical residual varicose veins (38-year-old male). Extreme pain appeared only 3–4 days after the injections. Limited surgical excising was carried out after 30 days, leaving small scars and no other sequelae. This complication is rare in this area. Despite the pejorative initial aspect of the leg and the extreme pain, necrosis was limited to three zones smaller than 1 cm2 each. The injection of an aberrant superficial skin artery was suspected; the alternative explanation being embolia cutis medicamentosa.248
Natali and Farman429 reported 40 cases of arterial injection in their 20-year experience and review of the French medicolegal system. They found that injection of the SSV was the most common preceding event. Seven patients required amputations (two above the knee and five below the knee), six required peripheral amputations of one or more toes, and 27 had abnormalities of the triceps and/or sural muscles. Muscle abnormalities consisted of infarction with subsequent fibrosis and retraction of the affected area, with or without limb atrophy. In one case, POL 0.5% was injected into the SSV in the popliteal fossa. An arteriogram showed thrombosis of the peroneal artery with clinical symptoms of a pale, numb foot with muscular necrosis.430
Another area in which the artery and veins are in close proximity is the junction of the femoral vein and GSV. A review of one sclerotherapy practice spanning more than 20 years disclosed two cases of accidental arterial injection in this area, requiring thigh amputations.431 This also has been the experience of Biegeleisen et al,422 who reported seven cases of arterial injection during attempts to inject the GSV or SSV at the groin or popliteal areas, even under color-flow ultrasound control. In this location, the external pudendal artery bifurcates and may surround the GSV shortly after the location of its connection with the femoral vein. In addition, the junction of the GSV with the popliteal vein has been demonstrated to have a tortuous and variably located satellite arteriole.432 Because these collateral arteries vary anatomically in these locations, duplex scanning may be useful before sclerosing these vessels but does not guarantee absolute safety, as detailed in a series of case reports.424
With the onset of duplex-assisted sclerotherapy, small arteries in the superficial and deep aspects of the thigh and calf have been inadvertently injected (Fig. 8.50).424 As described previously, the usual sequelae are both loss of tissue (cutaneous and subcutaneous) and nerve damage, which may result in muscle atrophy or necrosis, or both. Color-flow duplex scanning and the use of open needles or a long catheter when sclerosing these very tricky areas are recommended but still do not guarantee a completely risk-free procedure. While the development of solid-state high-resolution duplex has allowed for more precise anatomic visualization, Duplex-guided injection should be performed only by experts with experience of thousands of sclerotherapy procedures. A physician should be present at all times to give immediate treatment if needed.
Reports cited in the previous discussion assume direct arterial injection as the cause of extensive tissue necrosis. However, another explanation may be valid. De Takats433 described and illustrated preferential arterial and venous connections in the precapillary circulation by stating: ‘These shunts permit more direct transmission of pressure from the arterial to the venous system. They offer less resistance to flow than the capillaries and help maintain venous pressure and flow.’ In patients with prolonged chronic venous hypertension who develop dilated and elongated varicose and subcutaneous veins, free flow of blood from the venous to the arterial system may occur in this manner. Thus, injection into a varicose vein near these shunts may allow the sclerosing solution direct access to the arterial circulation (Fig. 8.51).
Prevention and treatment
Arterial injection is a true sclerotherapy emergency. The extent of cutaneous necrosis is usually related to the amount of solution injected. Therapeutic efforts to treat this complication are usually unsatisfactory but should be attempted.419 Because its occurrence is extremely rare, we recommend that an emergency flow sheet be readily accessible (Box 8.1). Hobbs (personal communication, 1989) recommends that, on realization of arterial injection, blood and sclerosing solution should be aspirated back into the syringe to empty the needle of solution. In addition, aspiration of the injected artery as rapidly and completely as possible, if performed immediately, may help remove the injected sclerosing solution. The needle should not be withdrawn, but the syringe should be replaced with one containing 10,000 U of heparin, which should be injected slowly into the artery. Unfortunately, this maneuver is difficult to accomplish because the patient usually is in considerable pain and may find it difficult to hold still (Hobbs, personal communication, 1991) and it also assumes that arterial injection has typical manifestations which make it recognizable while the vein is still cannulated.
Rarely, patients have no complaints of pain and demonstrate only a mild, sharply demarcated erythema that becomes dusky and cyanotic after a few hours.422 More practical treatments include periarterial infiltration with procaine 3%, 1 mL, which will form a complex with STS and render it inactive.398,428 The foot should be cooled with ice packs to minimize tissue anoxia. Immediate heparinization (continued for 6 days) and administration of IV dextran 10%, 500 mL per dose, for 3 days is recommended. Use of IV streptokinase or another thrombolytic agent may also be considered if there are no contraindications for its use. Finally, use of oral prazosin, hydralazine, or nifedipine for 30 days should be considered. Cockett421 has found that treatment that is delayed for more than an hour has no effect in limiting damage to the foot.
Use of IV heparin followed by subcutaneous heparin has been found to avert skin necrosis.422 It was observed that warfarin (Coumadin) did not appear as effective as subcutaneous heparin in these patients. The protective effect of heparin may be unrelated to its anticoagulant activity.434 Postischemic endothelial cell dysfunction was prevented with heparin perfusion in the rat hind limb model.435 Heparin infusion at 0.5 U/mL resulted in endothelial-dependent vasodilation. Serum levels less than 0.5 U/mL (the average serum heparin level in a patient undergoing therapeutic anticoagulation) were less effective. It is postulated that direct interactions of heparin with the endothelium, inducing maintenance of a strong luminal charge, may produce beneficial membrane-stabilizing effects. Heparin may also modulate TNF activity, contributing to this effect.436 In addition, a favorable resolution of arterial injection occurred in one patient with injection of t-PA.437 In this patient, promazine was injected into an artery. Use of heparin, axillary plexus blockade, and IV sodium nitroprusside was not successful. Brachial artery injection of t-PA (Actilyse, 50 mg over 8 hours) resulted in therapeutic efficacy. Thus, local fibrinolytic therapy should be considered if conservative treatments prove ineffective.
Given the known relationship of injection site to the development of this problem, one must consider the necessity for injecting vessels in the vicinity of the medial malleolus. The British Medical Journal published an interesting report of a legal case brought against a physician for performing such an injection, which resulted in a transmetatarsal amputation.417 The case was found in favor of the physician after several renowned sclerotherapists stated that the injection should be attempted if the patient would benefit, since the risks are infrequent and the benefits significant.
Prevention of this dreaded complication is best accomplished by visualization of the blood emanating from the needle. If it is pulsatile and continues to flow after the leg is horizontal, injection should not be attempted at this site. Mantse309 advises that when the medial malleolar area is treated, placement of the sclerosing needle should be performed while the patient is standing, so that the varix is bulged and the distance between the artery and vein is increased.
Emboli without pulmonary involvement
Reversible ischemic neurologic defects have been reported following sclerotherapy.438 In one case, 2 mL of POL 3% and 1 mL of POL 1% were injected into the varicose leg veins of a 41-year-old woman. One hour later, paresthesia developed in the right half of her body, with loss of visual field in the right upper quadrant. The neurologic signs and symptoms resolved within 2 days. There was no evidence of DVT, and all coagulation factors were normal except for a transient elevation of antithrombin III immediately after sclerotherapy, and this resolved within 5 days. Unfortunately, the patient was not evaluated for a patent foramen ovale (PFO), which was the probable causative event for this temporary cerebral vascular accident.
Another patient sustained an immediate hemiparesis that lasted for 24 hours after injection of a left leg varix with a hypertonic solution.439 An open atrial septal defect was demonstrated on ultrasound; however, thrombosis was not detected in either the varicose or deep veins of the leg or in the cerebral circulation.
A third patient was recently reported to have developed right hemiparesis soon after undergoing sclerotherapy of the right GSV with 0.5% POL foam.440,441 In this 61-year-old patient, an immediate carotid duplex scan as well as an MRI of the brain was performed, both of which yielded normal results. A subsequent echocardiogram revealed a PFO as well as an associated atrial septal aneurysm and a right-to-left shunt. The authors assumed that the stroke, which resulted in residual impairment in the patient’s fine motor skills, was the result of a paradoxical embolism of the foam through the PFO. The ischemia could have resulted from either an air embolism or an arterial spasm induced by the POL sclerosant.
Since 20% to 30% of unselected patients can be found to have a PFO with current ultrasound techniques,442 this complication may occur more often than reported. Several recently published studies have shown that, when evaluating a patient for a PFO, use of a contrast transcranial Doppler (cTCD) yields highly sensitive and specific results.443,444 When compared to the transesophageal echocardiogram (TEE), which is currently the gold-standard in PFO detection, cTCD is cheaper as well as easier to administer. The only disadvantage to the cTCD is that it can – in rare cases – occasionally fail to detect very small-diameter PFOs. However, current consensus does not advocate routine pretreatment PFO screening in sclerotherapy patients since some reported serious adverse events occurring around the time of sclerotherapy are probably coincidental.54 Specifically, adult patients choosing to undergo sclerotherapy usually fall within the same age range as do those at highest risk for first manifesting pathologic cardiovascular and neurologic events. However, any patient who reports or manifests neurologic symptoms should be evaluated carefully for leg and cerebral thrombosis, as well as a PFO. Consideration should be given in these patients for anticoagulation therapy.
Pulmonary embolism and deep venous thrombosis
Pulmonary embolism and DVT occur very rarely after sclerotherapy. The French registry reported one case of DVT after 12,173 sessions, and no PE; an incidence lower than 1 per 10,000 sessions can be estimated.224 It is hard to tell how many DVTs were to be expected in such a population during the time of the registry; thus it is still difficult to ascertain whether sclerotherapy has some responsibility. However, in the same registry, several cases of thrombotic complications have been reported that do not require the same management, but which must be identified and followed carefully (especially gastrocnemius vein thrombosis). An excellent review by Feied445 summarizes the major reports and risk factors for DVT.
The diagnosis of DVT is often clinically difficult, with up to 50% of cases going unnoticed. The most common clinical finding is mild enlargement of the calf or thigh, leg pain, pitting edema, warmth, dilated superficial veins, and erythema. Unfortunately, these findings are not sensitive or specific for DVT and may be caused by other disease processes.446–449 Plasma D-dimers are sensitive markers for thrombosis but lack specificity. They therefore cannot be used to make a positive diagnosis of DVT but have a high negative predictive value and are useful as an exclusionary test.450 The reader is referred to a review of laboratory markers which may be useful in the diagnosis of venous thromboembolism.451 An excellent decision-tree diagnostic approach evaluating a systematic review of the patient’s risk factors, symptoms, and physical signs, in addition to noninvasive testing, has been demonstrated to help guide further diagnostic testing and treatment strategies.452
Embolization of a thrombus occurs in more than 50% of patients453 and is not diagnosed in up to 70% of cases,454 with mortality approaching 35% without treatment.455 Clinical suspicion and the use of precautionary measures are important in preventing this complication. Establishing the diagnosis of DVT on clinical grounds is accurate only 50% of the time as compared to the use of fibrinogen scanning.456 Venous Doppler examination has a 30% to 96% accuracy, depending on the experience of the investigator.457–459 Likewise, impedance phlebography has a 40% accuracy.459 Thus, the reported incidence of DVT after sclerotherapy treatment may be greatly underestimated.
In the 1930s and 1940s, PE after sclerotherapy of varicose veins occurred in, at most, 0.14% of patients.460,461 In a series of 45,000 injections given to 7500 patients, only one episode of PE was reported.462 Sicard, in 1928, reported 325,000 injections without a pulmonary infarction.463 Linser and Vohwinkel464 reported four cases of PE after 75,000 injections, only one of which was fatal. With the introduction of compression techniques in combination with sclerotherapy, this complication has become even less common. Sigg465 has reported PE occurring only once with 42,000 injections. A French vascular surgeon who treats approximately 75 sclerotherapy patients a week, amounting to 25,000 yearly injections, reported only one case of PE in 20 years.318 Fegan307 reported having never seen conclusive evidence of DVT after injection treatment of 16,000 patients in his clinic. The 2-year Australian Polidocanol Trial reported one case of major DVT and two cases of minor DVT that occurred after injection of 4 mL of POL 0.5%, 4 mL of POL 0.5%, and 0.5 mL of POL 1%, respectively, in 12,544 injected legs, for an incidence of approximately 0.02% (Fig. 8.52).239 In a review of 28 cases of PE after sclerotherapy, most cases occurred in patients at bed rest and occurred 1 to 21 days after the treatment session. The incidence of PE in this series was estimated as 1 per 10,000 patients.466
To evaluate the true incidence more accurately, Stevenson et al467 used continuous-screening ascending venography to study 13 patients undergoing compression sclerotherapy with Fegan’s technique. Not more than 1 mL of STS 3% was injected, with the total amount not exceeding 5 mL. Patients were seen in follow-up 1 week later for comparison venography. Radiographic studies of the patients showed no evidence of DVT.
An additional study using impedance plethysmography and Doppler ultrasonic examination was performed before and after sclerotherapy treatment by the classic Fegan technique in 67 legs.468 This study confirmed no alterations in deep venous blood flow at 1 and 2 weeks after injection treatment. This confirmed the clinical experience that sclerotherapy using the Fegan technique is highly unlikely to be complicated by the development of DVT. Venographic evaluation of the injection of 0.5 to 1.0 mL of sclerosing solution in 15 patients with incompetent perforator veins failed to demonstrate extension of radiocontrast media into the deep venous system.467
Another reason why sclerotherapy treatment does not usually result in DVT may be related to platelet inhibition by sclerosing solutions.469 It has been shown that platelet aggregation, the first step in thrombogenesis, is inhibited by the concentrations of sclerosing solution that usually occur in the deep venous system after sclerotherapy of superficial varicosities.470,471 However, an additional study with serial measurement of thrombin–antithrombin III complexes (TAT), D-dimer, fibrinogen, and C-reactive protein (CRP) before and on the 7th and 28th days after sclerotherapy treatment in 23 patients contradicts the latter three studies.472 Here, a higher incidence of superficial thrombosis was observed, and the TAT concentration at day 7 and day 28 was higher than the preoperative level. The sclerosing agent used was HS 14.6%, with a maximum amount of 20 mL injected followed by an unspecified type of compression. Thus, latent activation of the coagulation system does occur with sclerotherapy, possibly as a result of damage of both superficial and deep venous endothelial cells. Sclerotherapy may be a risk factor for venous thromboembolism, especially in patients with an underlying hypercoagulability.
Cause
The cause of DVT, with or without PE, after sclerotherapy is unclear. The three major factors responsible for DVT were first elucidated more than 100 years ago by Virchow:473
Sclerotherapy treatment always produces the first two causes in the triad, with coagulability changes related to endothelial damage, as well as to the coagulability properties of sclerosing solutions and predisposing hypercoagulability factors in the patient. Chemical endophlebitis produced by sclerotherapy should anchor the thrombus to the site of injection. Histologic examination of treated varicose veins has demonstrated that firm thrombosis occurs only on the damaged endothelium. Nonadherent thrombosis occurs on normal endothelium.474 Therefore, the most logical explanation for the development of emboli is damage to the deep venous system by migration of sclerosing solution or a partly attached thrombosis into deep veins from treated superficial veins. This may occur as a result of either injection of excessive volumes of sclerosing solution or physical inactivity after injection.475
An additional possibility concerns injection of tributary perforator veins, which may directly communicate with the deep venous system. In this situation, inadequate compression or injection of even 0.5 mL of sclerosing solution may force nonadherent thrombi into the deep circulation with muscle contraction. Ascending venography and digital subtraction phlebography in women with leg telangiectasia found that 0.7 mL of contrast medium injected into telangiectatic branches spread into the saphenous system in 8 of 15 patients.476 Two of the eight patients had telangiectasia as the only clinically perceptible abnormality. Therefore it is possible that up to 13% of patients with telangiectatic veins are at risk for the spread of sclerosing solution directly into the deep venous system. This further emphasizes the importance of limiting injection volumes.
Amount of Injection per Site
The circulation and direction of blood flow in varicose veins have been determined radiographically as stagnant or reversed (away from the heart), so the chemically induced thrombus is forced distally toward the smaller branching veins.477 However, cinematographic studies documented that a small amount of sclerosing solution entered the deep circulation after injection of 0.5 to 1.0 mL of solution into a superficial varicosity during 7 of 15 injections in nine subjects (Figs 8.53 and 8.54).478 No adverse effects were noted in these patients treated with POL 2%, probably because of rapid compression and ambulation of treated individuals and the resulting rapid dilution of the sclerosing agent within the deep venous system.

Figure 8.53 Injected contrast media, 1.5 mL, shown 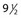 seconds after injection into a varicose vein with the patient supine.
seconds after injection into a varicose vein with the patient supine.
(From Goldman MP, Weiss RA, Bergan JJ, editors: Varicose veins and telangiectasias: diagnosis and treatment, St Louis, 1999, Quality Medical Publishing.)
Although studies demonstrating the rarity of DVT after sclerotherapy are reassuring, DVT and embolic episodes usually occur 4 to 28 days after the sclerotherapy treatment session,460 and therefore longer follow-up is necessary. In addition, nearly 40% of patients with DVT have symptomatic PE.479
Both DVT and PE have been reported to occur with injection of large quantities of sclerosing solution (12 mL) in a single site.480 Two separate case reports of this complication, occurring with injection of less than 0.5 mL of POL 1% in two patients in each report, have been published.239,481 In both reports, the injected veins were leg telangiectasia. In addition, the injection of 0.5 to 1 mL of sclerosing solution above the midthigh resulted in a presumed thrombus at the SFJ with resulting PE.298 This prompted Reid and Rothnie298 to recommend that injections not be given above the midthigh.
Finally, sclerotherapy of pedal veins may be hazardous, since the lack of valves or reversal of valves allows inward flow from superficial to deep veins,482 which may produce DVT. Therefore, when treatment of pedal veins is necessary, phlebectomy may be preferable to sclerotherapy.
Combining Sclerotherapy with Surgical Procedures
As stated previously, endothelial damage, vascular stasis, and changes in coagulability are the major predisposing factors for DVT. Since sclerotherapy produces endothelial damage and the sclerosing agent changes coagulability, the addition of surgical immobilization with relative vascular stasis places the patient at risk for DVT and PE. A case report that illustrates this problem was recently published.483 Here, the patient had a high ligation of the GSV at the SFJ under local anesthesia, followed by sclerotherapy of distal varices with POL 3%. A total of 3 mL was injected into six sites. Compression was applied, and the patient was ambulatory but remained in the hospital after treatment. On the first postoperative day, the patient collapsed after starting to walk, and massive PE was diagnosed and appropriately treated. Obviously, the sclerosing solution traveled into the deep venous system through perforating veins not ligated during the surgical procedure. We have evaluated similar cases that occurred in the United States under similar circumstances. The common denominator in all these cases is the combination of sclerotherapy with surgery. Therefore it appears prudent to separate these two procedures by an appropriate time interval to allow the patient’s coagulation system to normalize, as well as to allow the patient sufficient time to resume normal ambulation.
Inappropriate Compression
The inappropriate tourniquet effect of an excessively tight wrap on the thigh is a rare cause for the development of DVT.484 Occlusion of deep venous flow results in popliteal vein thrombosis, which can be resolved with conservative treatment. The adverse effects of nongraduated compression emphasize the importance of using properly fitted graduated support stockings in sclerotherapy treatment.
Hypercoagulable States
A number of primary and secondary hypercoagulable states exist that can be ruled out through the use of an appropriate patient history and a review of systems in the presclerotherapy treatment evaluation (Box 8.2). The prevalence of inherited thrombotic syndromes in the general population is 1 in 2500–5000, but increases to 4% in patients with a past history of thrombosis.485 In addition, a past history of DVT raises the likelihood of new postoperative venous thrombosis from 26% to 68%, whereas a past history of both DVT and PE gives a near 100% incidence of thrombosis.486 Thus, sclerotherapy should be undertaken cautiously in patients with previous DVT. Further detail on all hypercoagulable states is beyond the scope of this chapter. The most common states are discussed below. The reader is referred to multiple review articles on this important subject.445,485,487,488
Box 8.2
Hypercoagulable states
Secondary hypercoagulable states
Modified from Thomas JH: Am J Surg 160:547, 1990; and from Samlaska CP, James WD: J Am Acad Dermatol 23:1, 1990.
Oral Contraceptive Agents and Estrogen Replacement Therapy
The mechanism for thromboembolic disease in women who use oral contraceptives is multifactorial. Both estrogens and progestogens have been implicated in promoting thrombosis despite low-dose therapy.489–491 All studies have indicated that the increased risk occurs only while the preparations are actually in use and perhaps for 1 week or so after discontinuation.492,493 Total correction of the potentially hemostatic changes that occur during oral contraceptive therapy requires 4 weeks of abstinence.494
The highest rate of thromboembolism occurs with the use of higher levels of estrogen;489–492495 some studies show an 11-fold increase.493,496 Nevertheless, the risk of postoperative PE still appears increased in women who use oral contraceptive agents with minimal amounts of estrogen.497 Interestingly, a detailed review of the statistical methodologies of these studies did not prove a definite risk.498 The following is a brief review on this topic.
The incidence of DVT after oral contraceptive use varies depending on the type and concentration of estrogen. In addition, there is at least a 200-fold difference in potency between the native estrogens, estrone, estradiol, and ethinyl estradiol and the estrogens found in oral contraceptive agents.499 Hormone replacement therapy with 0.625 mg of conjugated equine estrogens and 2.5 mg of medroxyprogesterone has an elevated risk of DVT of 2.0 to 3.6 times higher than among nonusers.500
It has been estimated that oral contraceptives are responsible for one case of SVT or DVT per 500 women users per year.501 This estimate of hypercoagulability may be low, since an examination of plasma fibrinogen chromatography demonstrated a 27% incidence of silent thrombotic lesions in 154 new contraceptive users of either mestranol, 100 mg, or ethinyl estradiol, 50 mg.502 Oral contraceptive users as a group have numerous alterations in their coagulation system that promote a hypercoagulable state. They include hyperaggregable platelets, decreased endothelial fibrinolysis,503 decreased negative surface charge on vessel walls and blood cells,504 elevated levels of procoagulants, reduced RBC filterability,505 increased blood viscosity secondary to elevated RBC volume,506 and decreased levels of antithrombin III.507–509 Any of these factors, alone or in combination, may predominate in women taking oral contraceptives. The extent of this derangement in the hemostatic system determines whether thrombosis will occur. When endothelial damage with sclerotherapy is initiated in this population, an increased incidence of thrombosis may result.
The most important factors preventing clot propagation are antithrombin III and vascular stores of t-PA.507,510–512 Antithrombin III levels have been demonstrated as 20% lower in some women taking oral contraceptive agents510 and estrogen replacement therapy.513 Of women using oral contraceptive agents who have thromboembolic events, 90% have a 25-fold decrease in releasable t-PA;507,510,511 51.6% have an abnormally low plasminogen activator content in the vein walls.512 Therefore, a certain subgroup of women taking birth control pills is at particular risk for thromboembolic disease.
In addition, increased distensibility of peripheral veins may occur with use of systemic estrogens and progestins.514 This may promote valvular dysfunction and a relative stasis in blood flow to add to the hypercoagulable state. Since it is practically impossible and also impractical at this time to determine which women are at risk, it would appear prudent to recommend that patients consider discontinuing this medication before sclerotherapy treatment.
Alternatively, since oral contraceptive agents and estrogens have peak levels 3–5 hours after administration, alternative routes of administration, such as transdermal and vaginal, should be considered. For women who cannot discontinue oral estrogen replacement therapy, the use of transdermally administered 17β-estradiol may be an option.515 By delivering estrogen directly into the peripheral circulation, the ‘first-pass effect’ of liver metabolism is eliminated. This decreases hepatic estrogen levels with subsequent minimization of estrogen-induced alteration of coagulation proteins. Thus it is recommended that transdermal estrogen be used in patients at risk for thromboembolism, since alterations in blood clotting factors have not been demonstrated during such treatment.516 In addition, the use of esterified estrogens as opposed to conjugated estrogens has been shown not to increase the incidence of venous thrombosis in perimenopausal and postmenopausal women.517

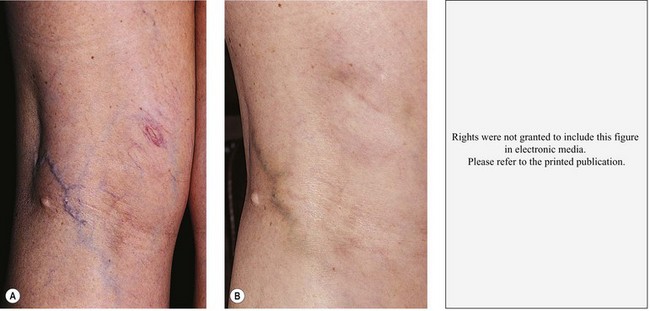
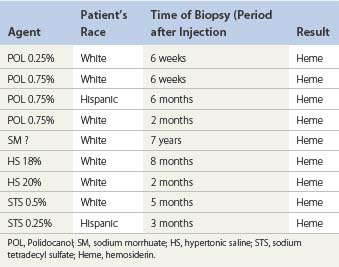
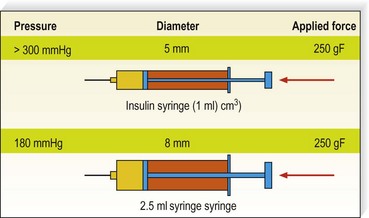
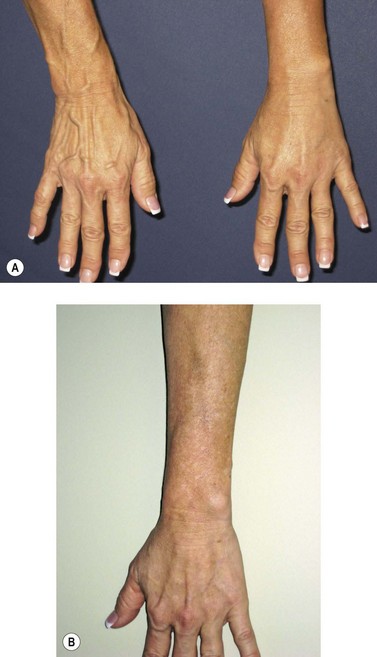
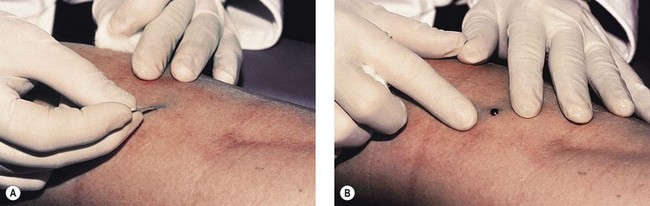
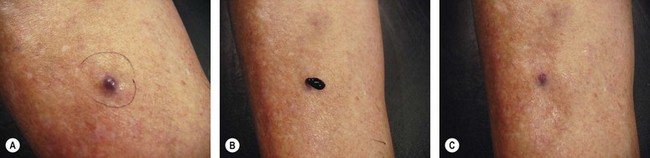
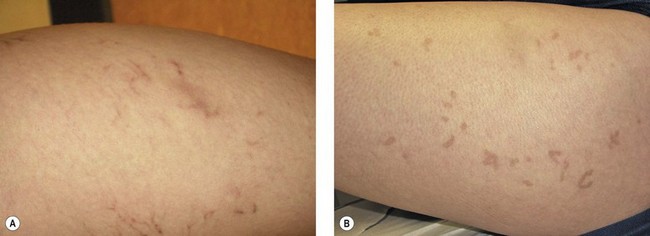
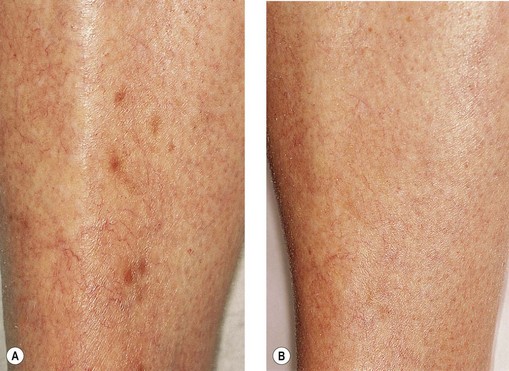
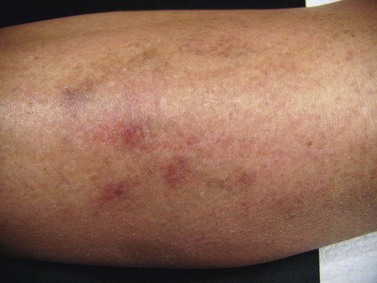
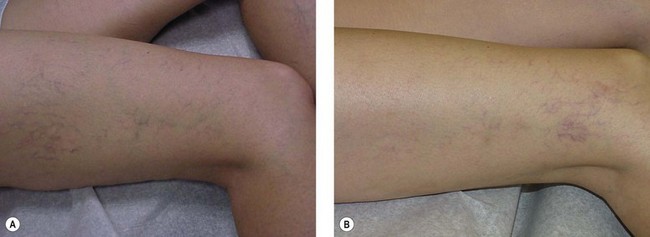
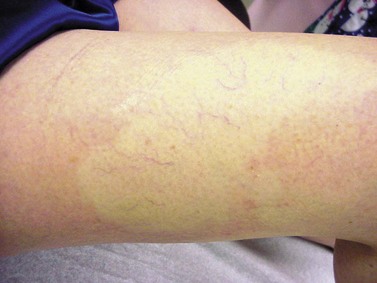
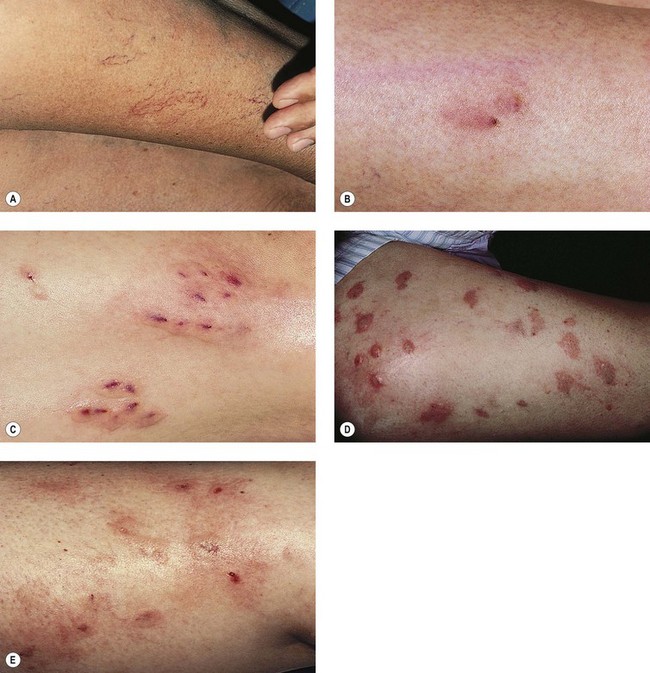
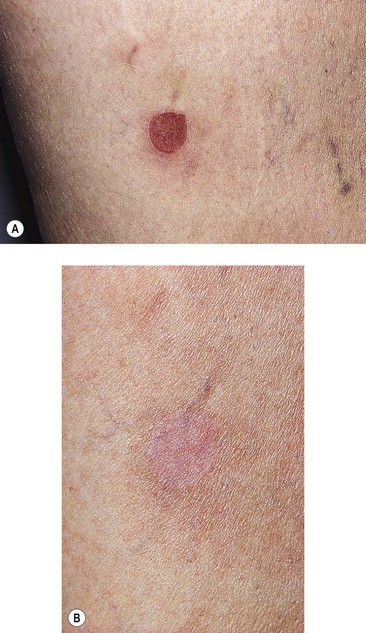
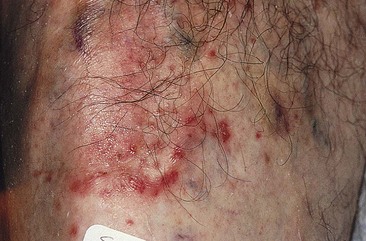
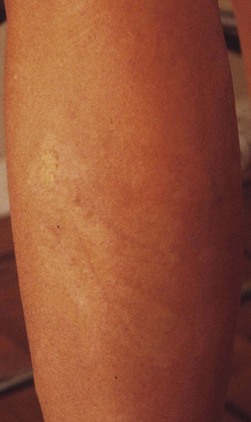
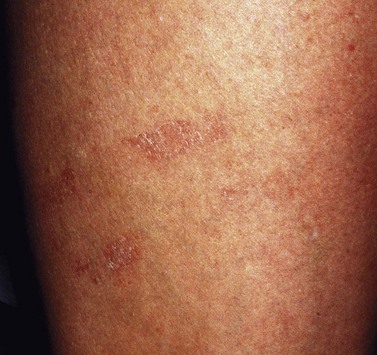
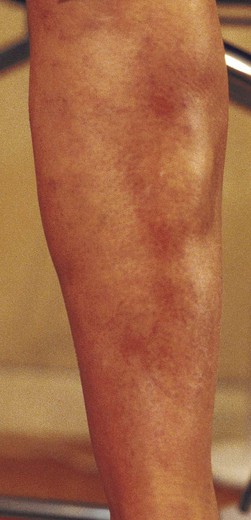
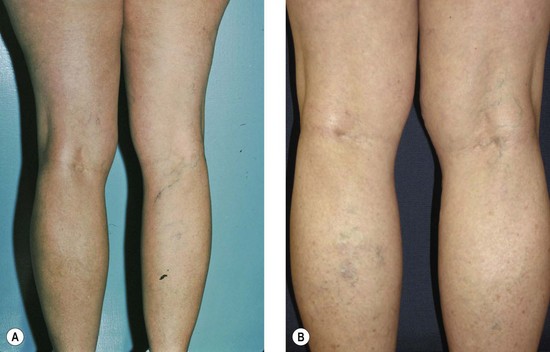
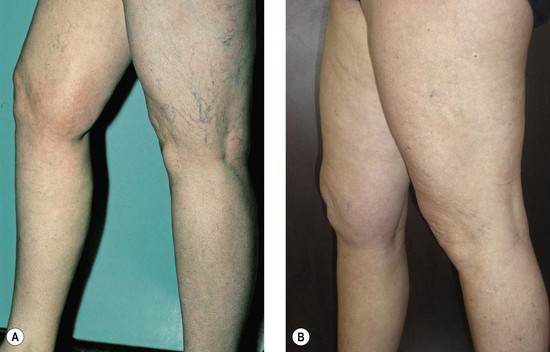
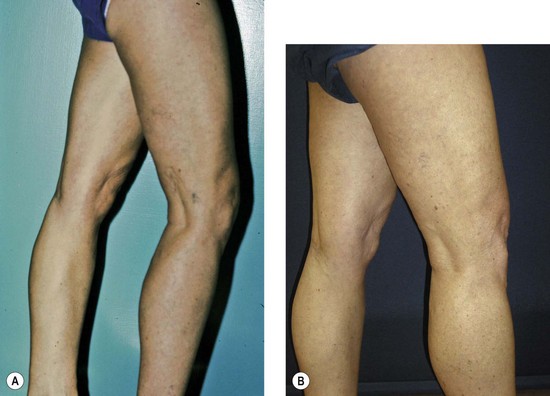
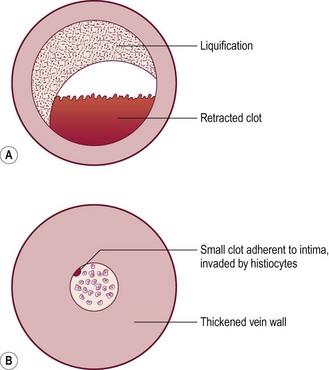
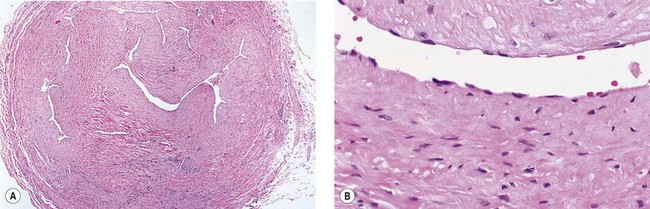
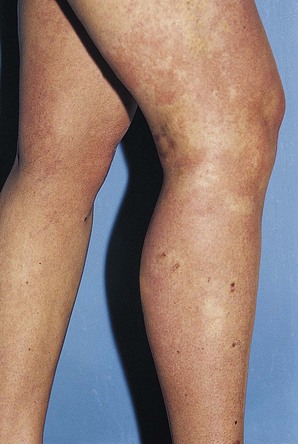
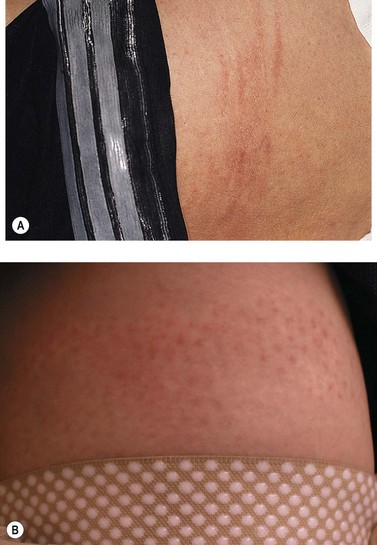
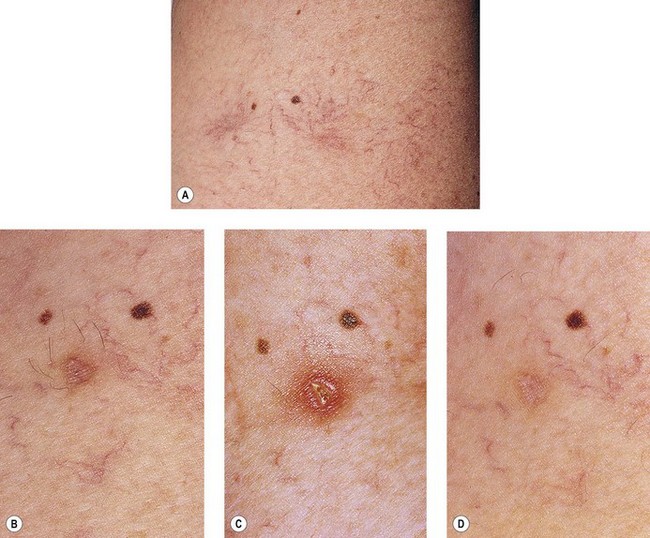
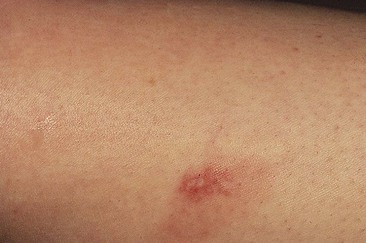
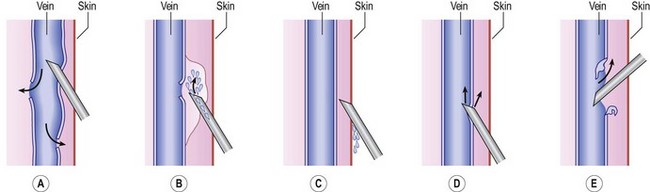
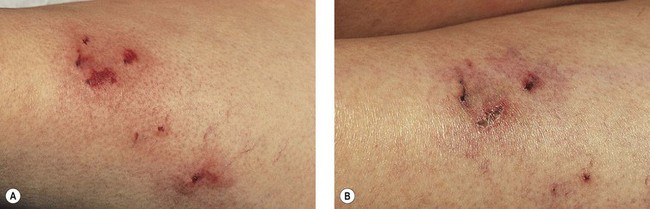
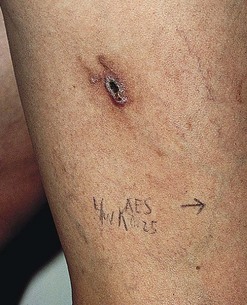
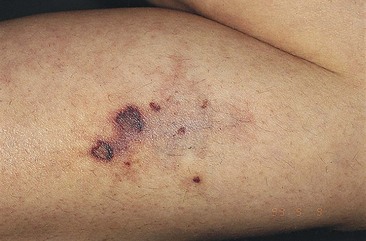
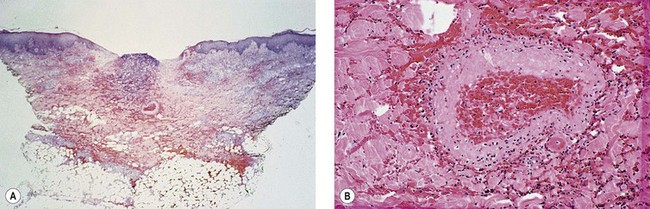
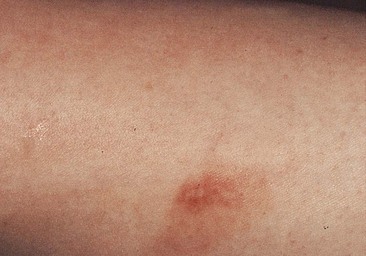
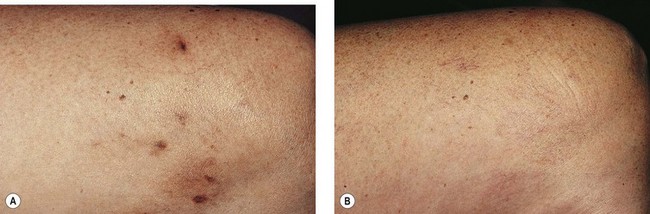
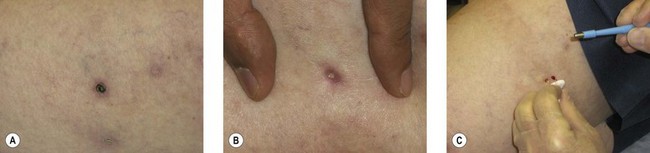
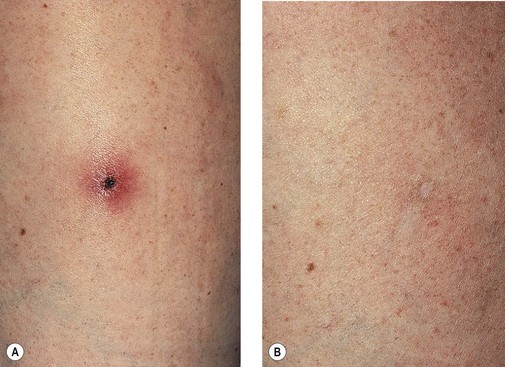
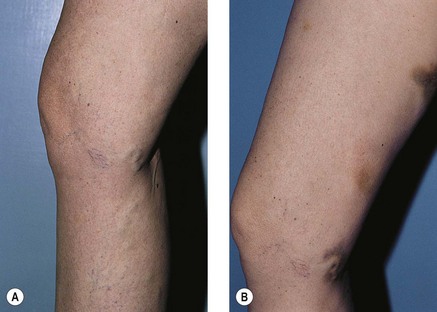
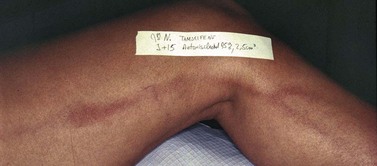
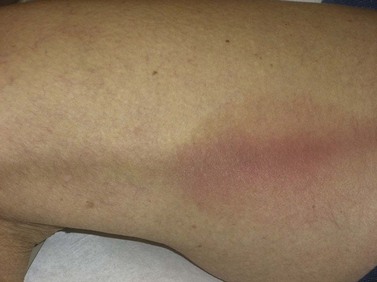
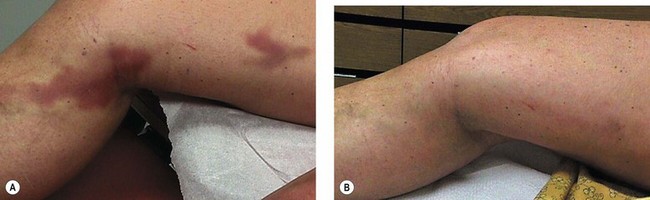
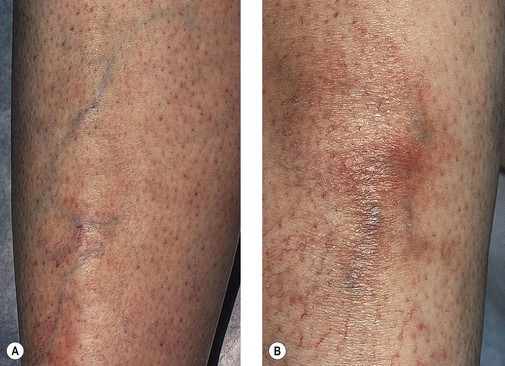
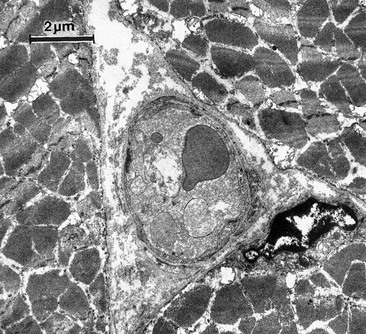
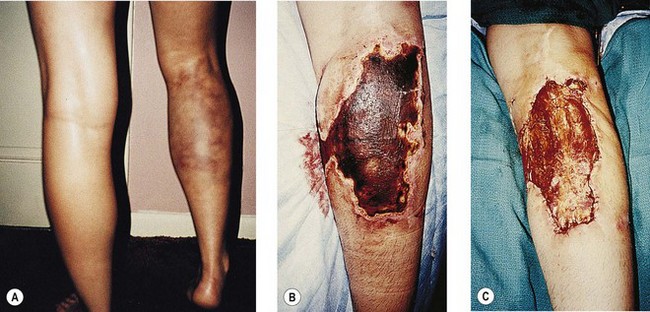


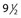 seconds after injection into a varicose vein with the patient standing.
seconds after injection into a varicose vein with the patient standing.