CHAPTER 11 Complex special situations
Abdominal hypertension and abdominal compartment syndrome
Pathophysiology
Definitions
IAH is defined by the World Society of Abdominal Compartment Syndrome (WSACS) as a measured IAP of 12 mm Hg or greater, recorded three times using standardized measurement methods 4 to 6 hours apart and/or an abdominal perfusion pressure (APP) of less than 60 mm Hg (mean arterial pressure [MAP] minus intra-abdominal bladder pressure [IABP]), recorded using two standardized measurements 1-6 hours apart). These measurements should be evaluated in the context of clinical symptomatology.
An IAP of greater than 20 mm Hg reflects significant IAH almost universally.
An IAP of 18 mm Hg indicates a high probability of organ compromise.
An IAP of 15 mm Hg reflects moderate probability of organ compromise.
An IAP of 12 mm Hg reflects a lower probability of organ compromise.
Increased IAP may reflect a critical finding in patients with multiorgan dysfunction syndrome (MODS) or multisystem failure, which contributes to global hypoperfusion, aggravating the effects of increased IAP (Table 11-1).
Table 11-1 PRESSURE AND SYMPTOM GRADE FOR INTRA-ABDOMINAL HYPERTENSION
| Graded Measurement | Pressure Measurement and relevance | Physiologic Events and clinical signs |
|---|---|---|
| Pressure Grade I | 12–15 mm Hg Significant in the presence of organ dysfunction |
Cytokine release and capillary leak Third spacing of resuscitative fluid Decreasing venous return and preload Early effects on ICP and CPP Abdominal wall perfusion decreases 42% Marked reduction in intestinal and intra-abdominal organ blood flow leading to regional acidosis and free radical formation. |
| Pressure Grade II | 16–20 mm Hg Significant in most patients |
Markedly decreased venous return, CO and splanchnic perfusion Increased SVR, CVP, PAWP Decreased blood pressure, pulse pressure and particularly systolic blood pressure Decreased TLC, FRC, RV. Increased vent pressures, hypercapnia, hypoxia Reduction to 61% of baseline mucosal blood flow and increasing gut acidosis Oliguria, anuria Increasing ICP and decreasing CPP |
| Pressure Grade III | 21–25 mm Hg Significant in all patients |
Hemodynamic collapse, worsening acidosis, hypoxia, hypercapnia, anuria. Inability to oxygenate, ventilate or resuscitate |
| Pressure Grade IV | >25 mm Hg Significant in all patients |
Hemodynamic collapse, worsening acidosis, hypoxia, hypercapnia, anuria. Inability to oxygenate, ventilate or resuscitate |
If both pressure and clinical symptoms are met for grade III and/or grade IV, patient has abdominal compartment syndrome.
Primary ACS is a condition associated with injury or disease in the abdominopelvic region that frequently requires early surgical or angioradiologic intervention. Any abnormal event that raises abdominal pressure can induce acute IAH, including blunt or penetrating abdominal trauma, abdominal aortic aneurysm (AAA), hemorrhagic pancreatitis, gastrointestinal (GI) obstruction, abdominal surgery resulting in retroperitoneal bleeding or secondary peritonitis, and with tight closure of abdominal incisions. Primary ACS also includes patients with abdominal solid organ injuries who were initially managed medically and then developed ACS. The condition has been relatively well understood by surgeons and their colleagues but is frequently misdiagnosed and/or untreated until surgical intervention is required.
Secondary ACS includes conditions that do not originate from abdominal injury that create IAH, including sepsis or any condition prompting capillary leak (e.g., major burns, and conditions requiring massive fluid resuscitation). A large multicenter study (Malbrain et al. 2005) found the prevalence of IAH was 54% among medical ICU patients and 65% in surgical ICU patients. This was remarkable, as most medical patients are not evaluated for or even considered recipients of IAH and ACS.
Assessment
Vital signs and other values
The following values may be increased:
• Hemodynamic values: Central venous pressure (CVP), pulmonary artery occlusive pressure (PAOP), systemic vascular resistance (SVR), inferior vena cava (IVC) pressure
• Respiratory: Pleural pressure, peak inspiratory pressure
• Screening lab values: PaCO2, serum creatinine, serum blood urea nitrogen (BUN)
Observation
Observe for upward trends in respiratory and heart rates (RR and HR, respectively) and decrease in urine output. Signs and symptoms are nonspecific and subtle and may be attributed to other clinical conditions (Table 11-1). Elevated IAP affects the cardiovascular, pulmonary, renal, and neurologic systems.
Diagnostic tests
Methods of intra-abdominal pressure measurement
1. The nurse will connect a fluid filled pressure system to a transducer, then connect a needle to the distal end of the tubing (farthest from the transducer and after a stopcock).
2. The cable connecting the system to the monitor should allow for visualization of a small pressure (scale either auto or at 30 mm Hg).
3. The connected system will be inserted into the catheter infusion port.
4. After zeroing the system (transducer at the symphysis pubis and the stopcock will be turned off to the patient), the nurse will clamp the catheter drainage system just below the infusion port.
5. Using the stopcock, the system will be turned off to the monitor and 25 mls of sterile fluid (IV fluid is fine) will be injected rapidly into the infusion port on the urinary catheter. The stopcock will be then turned off to the injecting port, leaving a connected pressure system from patient to monitor.
6. Bladder pressure must be read during end expiration and the patient must be as flat as tolerated to facilitate accuracy. There is no dynamic waveform associated with bladder pressure. One should just observe the level of pressure in the first 10–20 seconds after fluid is instilled.
7. A normal value is generally considered between 0 and 5 mm Hg, although levels as high as 15 mm Hg are not unusual in the first 24 hours after abdominal surgery (see Table 11-1). If the pressures are elevated, document and repeat in the next hour using the same techniques. Inform the physician or midlevel practitioner if both measures are elevated.
8. Occlusion is then released and fluid is drained into the urine collection bag. Subtract the amount of fluid from the hourly output.
Collaborative management
Care priorities
1. Prevent abdominal compartment syndrome:
Patients who have a high index of suspicion should have bladder pressure monitoring initiated in order to identify IAH earlier and possibly avoid decompressive laparotomy, which is the only documented evidence-based therapy for ACS (Box 11-1). There are many approaches that may be used to reduce IAH. These strategies are directed at reducing increased abdominal cavity volume or decreasing compliance. Therapies include:
Box 11-1 MANAGEMENT OF ABDOMINAL COMPARTMENT SYNDROME
1. Improvement of abdominal wall compliance
2. Evacuation of intraluminal contents
CT, computerized tomography; US, ultrasound.
Modified from Ivatury et al: In Vincent JL, editor: Yearbook of intensive care and emergency medicine. Berlin, 2008, Springer, p. 554.
Continuous renal replacement therapy (crrt):
Enables minute-to-minute control of intravascular fluid removal and replacement. Fluid management is more exact and CRRT was thought to benefit the patient by removal of cytokines, but more recent evidence indicates that may not be of benefit (see Continuous Renal Replacement Therapies, p. 603).
2. Perform a decompressive laparotomy to relieve acs:
11-1 RESEARCH BRIEF
Tremblay LN, Feliciano DV, Schmidt J, et al: Skin only or silo closure in the critically ill patient with an open abdomen. Am J Surg 182:670, 2001.
CARE PLANS FOR ABDOMINAL COMPARTMENT SYNDROME AND INTRA-ABDOMINAL HYPERTENSION
![]() Fluid Balance; Electrolyte and Acid-Base Balance
Fluid Balance; Electrolyte and Acid-Base Balance
1. Monitor BP at least hourly, or more frequently in the presence of unstable vital signs. Be alert to changes in MAP of more than 10 mm Hg. Even a small but sudden decrease in BP signals the need to consult the physician or midlevel practitioner, especially with the trauma patient in whom the extent of injury is unknown.
2. Once stable, monitor BP at least hourly, or more frequently in the presence of any unstable vital signs. Be alert to changes in MAP of more than 10 mm Hg.
3. If massive fluid resuscitation was necessary for either the trauma patient or a patient with third-spaced fluid, the patient is at higher risk for IAH and should be observed closely for signs of decreased perfusion, respiratory distress, and deterioration in mental status.
4. In the patient with evidence of volume depletion or active blood loss, administer pressurized fluids rapidly through several large-caliber (16-gauge or larger) catheters. Use short, large-bore IV tubing (trauma tubing) to maximize flow rate. Avoid use of stopcocks, because they slow the infusion rate. Fluids should be warmed to prevent hypothermia.
5. Measure central pressures and CO continuously if possible, or at least every 2 hours if blood loss is ongoing. Calculate SVR and PVR if data is available at least every 8 hours—more often in unstable patients. Be alert to low or decreasing CVP and PAWP. Be aware that profound tachycardia (>120 bpm) will decrease the cardiac compliance and therefore normal pressure readings in this instance can be misleading. Also anticipate mild to moderate pulmonary hypertension, especially in patients with concurrent thoracic injury, such as pulmonary contusion, smoke inhalation, or early ARDS. ARDS is a concern in patients who have sustained major abdominal injury, inasmuch as there are many potential sources of infection and sepsis that make the development of ARDS more likely (see Acute Lung Injury and Acute Respiratory Distress Syndrome, p. 365).
6. Measure urinary output at least every 2 hours. Urine output less than 0.5 ml/kg/hr usually reflects inadequate intravascular volume in the patient with abdominal trauma. Decreasing urine output may also signify compression of the renal arteries in ACS.
7. Monitor for physical indicators of arterial hypovolemia, which may include cool extremities, capillary refill greater than 2 seconds, absent or decreased amplitude of distal pulses, elevated serum lactate, and base deficit.
8. Estimate ongoing blood loss. Measure all bloody drainage from tubes or catheters, noting drainage color (e.g., coffee grounds, burgundy, bright red). Note the frequency of dressing changes as a result of saturation with blood to estimate amount of blood loss by way of the wound site.
![]() Electrolyte Management; Fluid Management; Fluid Monitoring; Hypovolemia Management
Electrolyte Management; Fluid Management; Fluid Monitoring; Hypovolemia Management
Ineffective tissue perfusion: gastrointestinal
Tissue Perfusion: Abdominal Organs
Circulatory care: arterial insufficiency
1. Identify patients who are at high risk for IAH.
2. Monitor BP at least hourly, or more frequently in the presence of unstable vital signs.
3. Monitor HR, ECG, and cardiovascular status every 15 minutes until vital signs are stable.
4. Auscultate for bowel sounds hourly during the acute phase of abdominal trauma and every 4 to 8 hours during the recovery phase. Report prolonged or sudden absence of bowel sounds during the postoperative period, because these signs may signal bowel ischemia or mesenteric infarction, which requires immediate surgical intervention.
5. Evaluate patient for peritoneal signs (see Box 3-3, p. 249), which may occur initially as a result of injury or may not develop until days or weeks later, if complications caused by slow bleeding or other mechanisms occur.
6. Ensure adequate intravascular volume.
7. Evaluate laboratory data for evidence of bleeding (e.g., serial Hct) or organ ischemia (e.g., AST, ALT, lactic dehydrogenase [LDH]). Desired values are as follows: Hct greater than 28% to 30%, AST 5 to 40 IU/L, ALT 5 to 35 IU/L, and LDH 90 to 200 U/L.
8. Measure bladder pressure manually: See Diagnostic Tests, Bladder Pressure Measurement, p 864.
9. Prepackaged closed system bladder pressure monitoring: Complete bladder pressure monitoring systems became available in approximately 2004. The system remains completely closed throughout the injection of fluid into the bladder, making it more desirable as part of prevention of catheter-associated urinary tract infections.
10. Assess for changes in level of consciousness, possibly resulting from increased IAP, which may inadvertently affect the draining of the cerebral veins.
Immune Status; Infection Severity
1. Note color, character, and odor of all drainage. Report the presence of foul-smelling or abnormal drainage. See Table 3-2 for a description of the usual character of GI drainage.
2. As prescribed, administer pneumococcal vaccine to patients with total splenectomy to minimize the risk of postsplenectomy sepsis.
3. If evisceration occurs initially or develops later, do not reinsert tissue or organs. Place a saline-soaked gauze over the evisceration, and cover with a sterile towel until the evisceration can be evaluated by the surgeon.
4. For more interventions, see this diagnosis in Abdominal Trauma (p. 245).
Additional nursing diagnoses
Also see Major Trauma, p. 235. For additional information, see nursing diagnoses and interventions in the following sections: Hemodynamic Monitoring (p. 75), Prolonged Immobility (p. 149), Emotional and Spiritual Support of the Patient and Significant Others (p. 200), Peritonitis (p. 805), Enterocutaneous Fistula (p. 778), SIRS, Sepsis and MODS (p. 927), and Acid-Base Imbalances (p. 1).
Drug overdose
Ingestion of unknown substances
Many patients with drug overdose first arrive to be seen with altered mental status and without a useful or reliable history. Identification of the ingested substance is difficult. Lab screening is done for common drugs of abuse, including amphetamines, barbiturates, benzodiazepines, cocaine, opioids, phencyclidine, and cannabinoids. Specific drug levels are available for salicylates, acetaminophen, digoxin, theophylline, iron, and lithium. When one of these drugs is not the offending agent, a number of signs and symptoms should be noted and tests done to determine the list of potential offending agents.
Assessment
• HR and rhythm: Is the patient bradycardic or tachycardic? Is a dysrhythmia present?
• Mental status: Is the patient overall depressed or agitated? Is delirium present?
• Temperature: Is hypothermia or hyperthermia present?
• Seizures: Is the patient having seizures?
• Eyes: Are pupils showing miosis or mydriasis? Is nystagmus present?
• Muscle tone: Is the patient flaccid or rigid? Are dyskinesias present?
• Lungs: If respiratory failure is occurring, is it related to depression, aspiration, edema, hemorrhage, bronchospasm, or cardiac failure?
• Arterial blood gas (ABG): Is the pH acidotic or alkalotic?
• Anion gap: If abnormal, is it increased or decreased?
• Blood glucose: Is the patient hyperglycemic or hypoglycemic?
• Psychosocial: Does the patient have a history of psychiatric disorders, of drug abuse, or of depression? Is the patient on any drugs with narrow therapeutic windows, and is a list of current medications (including over-the-counter [OTC]) available?
| Drug | Diagnostic Lab Tests | Specific Considerations |
|---|---|---|
| Acetaminophen | Serum drug level | Therapeutic level: 10–20mcg/mL Draw level 4 hrs after ingestion. Subsequent levels are drawn according to the Rumack-Mathews nomogram until levels are below the predicted hepatotoxic range. |
| Serum Na+, K+, CO2, BUN, blood glucose, creatinine, liver enzymes, bilirubin; PT, coagulation studies; CBC; protein; amylase; ABGs | ||
| Alcohol Amphetamines Benzodiazepines Phencyclidine |
Blood level; urine drug screen | |
| Amphetamines Cyclic antidepressants |
Serum K+, Na+, CO2, BUN, glucose, creatinine, CBC, liver studies, cardiac enzyme levels with isoenzyme fractionations are monitored. | |
| Barbiturates | Serum drug level | |
| Barbiturates Benzodiazepines Cocaine Hallucinogens Opioids Phencyclidine Salicylates |
Serum K+, Na+, CO2, BUN, glucose, creatinine, CBC, ABGs, liver function studies | |
| Cocaine | Urinalysis provides a quantitative method for identifying the presence of a cocaine metabolite. | Assessing blood levels of cocaine is usually of little diagnostic value. |
| Hallucinogens | Serum plasma drug level. | |
| Opioids | Urine screening | |
| Salicylates | Blood plasma level analyzed for presence of and amount | Repeat every 4 – 6 hours since the patient could have ingested sustained release drug |
| Cyclic Antidepressants | Blood plasma level; urine screen; gastric content analysis |
Treatment options
Extracorporeal removal of toxins
Techniques include all types of dialysis, hemoperfusion, exchange blood transfusion and plasmapheresis. These methods are most often used to remove methanol, ethylene glycol, lithium, theophylline, salicylates, and phenobarbital. May be used in extreme cases when other management strategies are ineffective for drugs which are not highly protein bound, which have a reasonable molecular size, are water soluble (rather than lipid soluble), have a limited volume of distribution, and are not highly charged or ionized. Hemofilters used for continuous renal replacement therapy are often able to accommodate larger sized molecules than conventional hemodialysis therapy.
Commonly abused drugs
Acetaminophen (apap)
Alcohol
Support of cardiovascular and respiratory systems to prevent collapse:
Oxygen supplementation; treatment of ventricular dysrhythmias and bradyarrhythmias according to ACLS guidelines.
Amphetamines
Barbiturates
| Generic Name | Common Brand Name | Half-life (hr) |
|---|---|---|
| Amobarbital | Amytal | 8–42 |
| Secobarbital | Seconal | 19–34 |
| Pentobarbital | Nembutal | 15–48 |
| Phenobarbital | Luminal and others | 24–140 |
| Butabarbital | Butisol | 34–42 |
| Secobarbital/amobarbital | Tuinal | 8–42 |
Note: Withdrawal symptoms can be correlated with the half-life of the drug that was used. Withdrawal from drugs with shorter half-lives produces more intense symptoms that last for shorter periods, whereas withdrawal from drugs with longer half-lives produces less intense symptoms that can be prolonged. Moreover, the severity of the withdrawal is directly related to the drug’s dosage.
Support of cardiovascular and respiratory systems to prevent collapse:
Electrical rhythm is monitored; bradyarrhythmias are treated according to ACLS guidelines. After fluids are replaced, vasopressor therapy (see Appendix 6), including dopamine and norepinephrine bitartrate (Levophed), may be initiated for hypotension. Mechanical ventilation may be required, depending on the degree of hypoxia and CO2 retention.
Sedation for withdrawal symptoms:
Typically the barbiturate that was ingested is tapered gradually to zero.
Benzodiazepines
| Generic Name | Common Brand Name | Half-life (hr) |
|---|---|---|
| Chlordiazepoxide | Librium and others | 7–28 |
| Diazepam | Valium and others | 20–90 |
| Lorazepam | Ativan | 10–20 |
| Oxazepam | Serax | 3–21 |
| Prazepam | Centrax | 24–200* |
| Flurazepam | Dalmane | 24–100* |
| Chlorazepate | Tranxene | 30–100 |
| Temazepam | Restoril | 9.5–12.4 |
| Clonazepam | Klonopin | 18.5–50 |
| Alprazolam | Xanax | 12–15 |
| Halazepam | Paxipam | 14 |
Note: Withdrawal symptoms can be correlated with the half-life of the drug that was used. Withdrawal from drugs with shorter half-lives produces more intense symptoms that last for shorter periods, whereas withdrawal from drugs with longer half-lives produces less intense symptoms that can be prolonged. Moreover, the severity of the withdrawal is directly related to the drug’s dosage.
Cocaine
Cutting agents are substances mixed with pure cocaine to increase bulk. Cutting agents are often unknown but may include procaine, phencyclidine (“angel dust”), amphetamine, quinine, talc, and strychnine. Agents used in the preparation of crack include powdered cocaine, water, baking soda, and lidocaine. Cutting agents can become emboli that shower into cerebral and pulmonary circulation, with subsequent effects.
Treatment of hypotension or hypertension:
Antihypertensives or vasopressors are administered as indicated.
Treatment of volume deficiency:
IV fluid replacement is done, such as with lactated Ringer solution or D5NS.
Cyclic antidepressants
Hallucinogens
Opioids
Phencyclidine
Salicylates
Examples include aspirin, bismuth subsalicylate (Pepto-Bismol), and fendosal.
Specific antidotes for common drug overdoses/toxicities
Table 11-4 gives specific drug treatments for a few of the more common and critical drug overdoses not discussed in the preceding section.
| Target (toxic) Drug or Class | Treatment or Antidote |
|---|---|
| Acetaminophen | N-Acetylcysteine |
| Anticholinergics | Physostigmine |
| Arsenic, lead, mercury, or other heavy metals | Dimercaprol injection |
| Benzodiazepines | Flumezanil |
| Beta-blockers | Glucagon; beta agonists |
| Calcium channel blockers | Calcium IV, glucagon |
| Copper | Trientene |
| Cyanide | Sodium thiosulfate, sodium nitrite, amyl nitrite, hydroxocobalamin |
| Cyclic antidepressants | Sodium bicarbonate |
| Digitalis glycosides | Digoxin immune Fab |
| Heparin | Protamine |
| Insulin | Glucagon, dextrose, octreotide |
| Iron | Deferoxamine |
| Lead | Succimer, edetate calcium disodium (EDTA) |
| Methanol | Fomepizole, ethanol, folinic acid |
| Nitrites | Methylene blue |
| Opiates | Nalmefene, naltrexone, naloxone |
| Organophosphate insecticides | Atropine, pralidoxime |
| Warfarin | Vitamin K (IV or PO) |
CARE PLANS FOR ALL DRUG OVERDOSES
related to presence of tracheobronchial secretions or obstruction; decreased sensorium
Respiratory Status: Airway Patency; Aspiration Prevention; Respiratory Status: Ventilation
1. Assess for respiratory distress hourly and as needed. Note secretions; stridor; gurgling; shallow, irregular, or labored respirations; use of accessory muscles of respiration; restlessness and confusion; and cyanosis (a late sign of respiratory distress).
2. Suction oropharynx, or use suction via endotracheal tube as needed.
3. Administer bronchodilators as appropriate.
4. Monitor ABG values for evidence of hypoxia (PaO2 less than 90 mm Hg) and respiratory acidosis (PaCO2 greater than 45 mm Hg, pH less than 7.35).
5. Monitor respiratory patterns; provide continuous apnea monitoring if available.
6. If patient has been placed on mechanical ventilation, monitor for indicators of airway obstruction (see Mechanical Ventilation, p. 99).
7. Monitor oxygen saturation continuously. Be alert to values less than 95% with response depending on patient’s baseline and clinical presentation.
8. Monitor for nausea and vomiting. Evaluate effects of antiemetics.
related to overdose of cocaine, hallucinogens, phencyclidine, salicylates, or cyclic antidepressants
Optimally, within 24 to 72 hours of intervention, patient becomes normothermic.
1. Monitor for hyperthermia: temperature greater than 38.3°C (greater than 101°F), pallor, absence of perspiration, and torso that is warm to the touch. If means are available, provide continuous monitoring of temperature. Otherwise, measure rectal, core, or tympanic temperature hourly and as needed.
2. Monitor effects of cooling blanket, cooling baths, and ice packs to the axillae and groin.
3. Maintain fluid replacement as prescribed. Monitor hydration status and trend of input and output (I&O).
4. Monitor neurologic status hourly and as needed until stabilized.
5. Monitor vital signs continuously or hourly and as needed until stabilized.
6. Administer and evaluate effects of antipyretic medications.
![]() Fever Treatment; Vital Signs Monitoring; Medication Prescribing
Fever Treatment; Vital Signs Monitoring; Medication Prescribing
related to low intake or losses secondary to vomiting or diaphoresis and shock conditions
1. Monitor hydration status. Note signs of continuing dehydration: poor skin turgor, dry mucous membranes, thirst, weight loss greater than 0.5 kg/day, urine specific gravity greater than 1.020, weak pulse with tachycardia, and postural hypotension.
2. If the patient has a pulmonary artery catheter:
3. Evaluate the effects of fluid therapy.
4. Assess for indicators of electrolyte imbalance, especially the presence of hypokalemia. Be alert to irregular pulse, cardiac dysrhythmias, and serum potassium level less than 3.5 mEq/L.
5. Monitor I&O hourly; assess for output elevated disproportionately to intake, bearing in mind the insensible losses.
6. Monitor laboratory values, including serum electrolyte levels and serum and urine osmolality. Note BUN values elevated disproportionately to the serum creatinine (indicator of dehydration rather than renal disease), high urine specific gravity, low urine sodium, and rising Hct and serum protein concentration. Optimal values are the following: serum osmolality 275 to 300 mOsm/kg, urine osmolality 300 to 1090 mOsm/kg, BUN 10 to 20 mg/dl, serum creatinine 0.7 to 1.5 mg/dl, urine sodium 40 to 180 mEq/24 hr (diet dependent), Hct 37% to 47% (female) or 40% to 54% (male), and serum protein 6 to 8.3 g/dl.
7. Maintain fluid intake as prescribed; administer prescribed electrolyte supplements.
![]() Fluid/Electrolyte Management; Surveillance; Hypovolemia Management
Fluid/Electrolyte Management; Surveillance; Hypovolemia Management
Disturbed sensory/perceptual perception: visual, tactile, auditory, kinesthetic,
related to chemical alterations secondary to ingestion of mind-altering drugs
Cognitive Orientation; Distorted Thought Self-Control
1. Establish and maintain a calm, quiet environment to minimize patient’s sensory overload.
2. Assess patient’s orientation to time, place, and person. Reorient as necessary.
3. Explain procedures before performing them. Include significant others in orientation process.
4. Do not leave patient alone if agitated or confused.
5. Administer antianxiety agents as prescribed.
6. If patient is hallucinating, intervene in the following ways:
![]() Delusion Management; Environmental Management; Fall Prevention; Surveillance: Safety
Delusion Management; Environmental Management; Fall Prevention; Surveillance: Safety
Risk for violence: self-directed and/or other-directed
related to mind-altering drugs or depressed state
Patient, staff, and patient’s significant others are free of injury.
1. If patient’s condition is stable, provide a sitter, or auxiliary staff member, such as an orderly or nursing assistant, to observe patient when awake.
2. Speak with patient in a quiet and calm voice, using short sentences.
3. Establish a therapeutic relationship with patient.
4. Encourage patient to take control over his or her own behavior.
5. Facilitate support by significant others.
7. Administer and evaluate effectiveness of sedation to calm patient.
8. Keep all sharp instruments out of patient’s room. Follow agency protocol accordingly.
9. Develop appropriate behavior expectations and consequences, given the patient’s level of cognitive functioning and capacity for self-control.
![]() Behavior Management: Self-Harm; Substance Use Treatment: Alcohol Withdrawal; Substance Use Treatment: Overdose, Suicide Prevention
Behavior Management: Self-Harm; Substance Use Treatment: Alcohol Withdrawal; Substance Use Treatment: Overdose, Suicide Prevention
Additional nursing diagnoses
See nursing diagnoses and interventions in the following as appropriate: Nutritional Support (p. 117), Mechanical Ventilation (p. 99), Hemodynamic Monitoring (p. 75), Prolonged Immobility (p. 149), Emotional and Spiritual Support of the Patient and Significant Others (p. 200), Acute Lung Injury and Acute Respiratory Distress Syndrome (p. 365), Acute Respiratory Failure (p. 383), Acute Coronary Syndromes (p. 434), Heart Failure (p. 421), Cardiomyopathy (p. 482), Dysrhythmias and Conduction Disturbances (p. 492), Aortic Aneurysm/Dissection (p. 467), Acute Renal Failure (p. 584), Status Epilepticus (p. 668), Hepatic Failure (p. 785), Acute Pancreatitis (p. 762), Fluid and Electrolyte Disturbances, p. 37), and Acid-Base Imbalances (p. 1). In addition, see Traumatic Brain Injury for Impaired Corneal Tissue Integrity (p. 347).
High-risk obstetrics
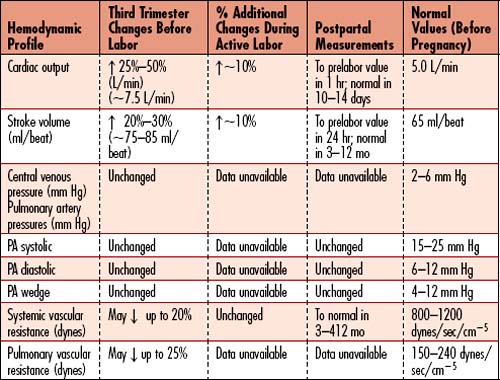
Caring for a pregnant patient presents the unique challenge of caring for the mother and the fetus simultaneously. Although survival of the mother will take precedence over fetal survival in most cases, the optimal outcome is survival of both the mother and fetus. Two basic principles should underlie the care of the pregnant patient. First, maternal anatomic and physiologic changes occur during pregnancy to facilitate adequate blood flow to the fetus and protect the mother after delivery (Box 11-2). The pregnant patient may require hemodynamic monitoring, and the critical care nurse should be familiar with the different hemodynamic changes and pressure values in pregnancy and labor as outlined in Table 11-5. Second, the fetus is totally dependent on the mother for all of his or her oxygenation and growth needs, so any intervention performed on the mother will most likely affect the fetus. Despite the unique challenges presented by the critically ill obstetric patient, transfer of the patient to a critical care unit should not be delayed.
Box 11-2 PHYSIOLOGIC ANATOMICAL CHANGES IN PREGNANCY
Cardiovascular
Heart rate: 10 to 15 bpm increase from prepregnancy rate by 32 weeks
Blood pressure: 10 to 15 mm Hg decrease from prepregnancy value between 14 and 24 weeks returning to prepregnancy value by 37 to 40 weeks’ gestation
Blood volume: 40% to 50% increase by 24 weeks
Hypertensive disorders of pregnancy
Pathophysiology
Hypertensive disease occurs in up to 22% of pregnancies. Preeclampsia, eclampsia, and HELLP (Hemolysis, Elevated Liver enzymes, Low Platelets) are all part of a continuum of hypertensive disorders unique to pregnancy. The etiology of these diseases is probably related to early placental development. Risks to the baby include poor growth and prematurity. Risks to the mother include stroke, pulmonary edema, renal failure, liver rupture, and disseminated intravascular coagulation (DIC). The only definitive cure is delivery of the fetus and placenta. Signs and symptoms usually resolve within 24 to 48 hours after delivery. The overall goal of nursing care of the hypertensive pregnant patient is to avoid complications to the mother and deliver a healthy, mature neonate. (Refer to Box 11-3.)
Box 11-3 CLASSIFICATION OF HYPERTENSIVE STATES OF PREGNANCY
• Gestational: Mild hypertension that develops during pregnancy without additional PIH symptoms
• Preeclampsia: Hypertension that develops during pregnancy after 20 weeks’ gestation (except in molar pregnancies) with proteinuria
• HELLP syndrome: Severe form of preeclampsia with hemolysis, elevated liver enzymes, and low platelets, which may lead to DIC. Rarely patients may be normotensive with HELLP.
• Eclampsia: Preeclampsia complicated by seizures
• Chronic hypertension: Hypertension that is present prior to pregnancy or develops before 20 weeks’ gestation without a molar pregnancy
• Chronic hypertension with PIH: Preeclampsia and/or eclampsia superimposed on chronic hypertension
Preeclampsia
Preeclampsia is defined as hypertension or BP at least 140/90 mm Hg accompanied by proteinuria during the second half of pregnancy. Preeclampsia occurs in 5% to 8% of all pregnancies. Preeclampsia is more common in first pregnancies and in nonwhite women from low socioeconomic backgrounds. Eclampsia is defined as seizures or coma during pregnancy following preeclampsia. Overall, preeclampsia and eclampsia are responsible for 15% of maternal deaths, with most deaths due to complications resulting from eclampsia.
Preeclampsia is more common in African American and Hispanic women and in women experiencing their first pregnancy at ages less than 18 years or older than 34 years. Additional risk factors for preeclampsia are pre-existing hypertension, diabetes, obesity, family history of preeclampsia, multifetal pregnancies, renal disease, connective tissue disease, and anti–phopholipid antibody syndrome.
Severe preeclampsia suggests a worsening degree of vasospasm within the maternal and fetal circulation as evidenced by the presence of headache, blurred vision, scotoma, epigastric pain in the right upper quadrant, altered lab values and indications of fetal status deterioration. Goals of nursing management for severe preeclampsia include controlling maternal BP, avoiding eclampsia and facilitating fetal oxygenation and delivery.
Signs of severe preeclampsia include:
• Hypertension: BP at least 160/110 mm Hg
• Proteinuria: Increased to greater than 5 g in a 24-hour urine specimen
• Interstitial Edema: Third-spaced fluids manifest as edema
• Serum creatinine: Increased to greater than 2 mg/dl
• Platelet count: Rapidly decreasing platelet count
• Oliguria: Decreased to less than 500 ml/24 hr
• Visual or cerebral disturbances: Blurred vision, altered thought processes
• Respiratory distress: Cyanosis or noncardiogenic pulmonary edema
• Intrauterine abnormalities: Fetal growth restriction and/or oligohydramnios (decreased amniotic fluid)
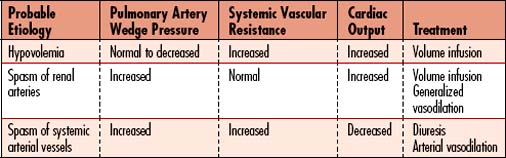
Refer to Table 11-6 for more specific changes in physiology.
Hellp syndrome
Pathophysiology
HELLP syndrome is an acronym for a unique pregnancy condition representing the most severe diagnosis of the preeclampsia continuum. HELLP is an abbreviation for hemolysis, elevated liver enzymes, and low platelets. HELLP syndrome occurs in up to 10% of patients with severe preeclampsia. The etiology of HELLP is uncertain. Worsening of systemic arteriolar vasospasm produces liver damage, subcapsular hematoma, and DIC. DIC in pregnancy may also be caused by placental abruption (abruptio placentae), dead fetus syndrome, septic shock, transfusion reaction, and amniotic fluid embolism. Maternal complications from DIC include ARDS and acute renal failure. The HELLP patient should be closely monitored for right upper quadrant pain or shoulder pain because these complaints may be indicative of liver hematoma or liver rupture. Hemodynamic monitoring may be required to manage patients with HELLP syndrome.
| Test | Findings |
|---|---|
| Complete blood count (CBC) | Hgb and Hct may be elevated (>35 Hct) and steadily rising with severe preeclampsia-eclampsia |
| Peripheral blood smear | Schistocytes or burr cells are present with HELLP syndrome |
| Urinalysis | Positive for protein spillage. |
| 24 Hour urine collection | Mild/moderate: 0.3–5 g protein with normal urine output. Severe preeclampsia-eclampsia: >5 g protein with low urine output. |
| Serum albumin | Decreases as urine protein spillage increases. Severe: <2.5 mg/dl. |
| Liver enzymes | Aspartate transaminase (AST), alanine amino transferase (ALT), and lactate dehydrogenase (LDH) are elevated with severe preeclampsia-eclampsia and HELLP syndrome. Bilirubin may be elevated with HELLP. |
| Renal serum chemistry | Mild-moderate: BUN, creatinine, and uric acid levels may be elevated. Severe preeclampsia-eclampsia: BUN, creatinine, and uric acid will be elevated. |
| Platelet count | Mild-moderate: >100,000/mL. Severe preeclampsia-eclampsia and HELLP syndrome: <100,000/mL |
| Bleeding time | Prolonged when platelet count is <100,000/mm3. |
| Fibrinogen | Decreased (<300 mg/dl). |
| Screening coagulation tests (PT, PTT, thrombin time) | Normal unless the patient develops HELLP syndrome that progresses to DIC, wherein all values are elevated. |
Collaborative care
Preterm complications: timing of delivery
Preeclampsia arising at 34 weeks’ (or more) gestation is generally managed by delivery. Fetal viability, or the gestational age at which the fetus can survive outside the womb, is generally thought to be 24–25 weeks’ gestation. After 34 weeks gestation, the majority of infants will avoid major complications from prematurity. Before 34 weeks, patients with severe preeclampsia-eclampsia require delivery unless the gestational age is less than 26 weeks, wherein attempts to prolong the pregnancy may be initiated. If the mother exhibits signs of HELLP syndrome, such as thrombocytopenia or epigastric or right upper quadrant pain, or has visual disturbances, delivery should be strongly considered regardless of fetal age, since the mother is at risk of life-threatening illness if delivery is delayed. Management of the severely preeclamptic patient will depend on three conditions: maternal status, fetal status, and gestational age. If the pregnancy is at least 34 weeks, delivery is planned as there is little benefit in prolonging gestation. If the pregnancy is 33 to 34 weeks, glucocorticoids are given to the mother to accelerate the development of fetal lung maturity. The glucocorticoids are given intramuscularly in two doses 12 hours apart with maximum effect achieved 24 hours after the second dose. Glucocorticoids are given to facilitate lung maturity. Both the maternal and fetal status must be closely monitored. If either deteriorates, definitive steps must be taken regarding plans for delivery. The medical team carefully and constantly weighs the benefits of delivery for the mother versus the risks of preterm delivery for the fetus. The decision to proceed to delivery in a preterm pregnancy should be made in consultation with the obstetrician, pediatrician, critical care physician, and neonatologist (if available).
• Is continuous electronic fetal monitoring available?
• Are there resources for the administration of and monitoring of medications for labor induction or augmentation, anesthesia, and analgesia?
• Are care providers appropriately trained to provide fetal resuscitation measures?
• Is timely access to operating rooms for emergency cesarean feasible?
• Are care providers able to provide maternal hemodynamic/cardiac/respiratory monitoring and treatment?
• Are there resources available for neonatal support should complications arise?
Preterm labor
1. Routinely screen or observe for the following signs and symptoms of preterm labor:
• Increase or change in vaginal discharge
• Signs and symptoms of urinary tract infection
• Uterine cramping (menstrual-like cramps, intermittent or constant)
• Pelvic pressure or pain (feeling that the baby is “pushing down”)
• Abdominal cramping with or without diarrhea
• If the patient is unconscious, observe for restlessness or nonverbal indications of intermittent pain.
2. If patient has any signs or symptoms of labor, notify the physician or midlevel practitioner and:
• Position mother in the lateral position (turn to the left or right side).
• Encourage bladder emptying every 2 hours.
• Report all new onset of contractions or any change in contraction pattern immediately to the obstetrician or midlevel practitioner, because cervical examination is the most accurate method for diagnosing preterm labor.
3. Monitor uterine contractions (preterm labor) and fetal status:
• Preterm labor contractions may be infrequent, irregular, and painless or frequent, regular, and painful. Preterm labor, left undetected and untreated, can progress into an emergency delivery of a neonate within the critical care unit.
• Tocolytics or medications are used to stop preterm labor, but they must be started early in the labor process to be effective.
4. Provide fetal surveillance:
• On admission: Ultrasound, Doppler flow studies, biophysical profile, and amniotic fluid volume studies are ordered to evaluate fetal well-being. Between 14 to 24 weeks’ gestation, the fetal heart rate (FHR) is generally evaluated with a Doppler or fetoscope. Note the absence of FHR by Doppler does not confirm fetal death.
• Initiating electronic fetal monitoring: Monitoring of the FHR with an electronic fetal monitor typically is not begun until viability has been established. The timing of the initiation of electronic fetal monitoring will vary according to institutional protocol, medical provider, and/or patient situation.
• Fetal nonstress testing: A Non-Stress Test (NST) is a frequently ordered non-invasive test to monitor fetal status. NSTs are performed by the obstetric nurse who is trained in the procedure and interpretation of NST results. This test is used to determine fetal well-being and is reflective of fetal oxygenation and placental function. It is often ordered daily on patients who are stable and not in labor. The NST is an assessment of continuous FHR and uterine activity over a 20- to 30-minute period using an electronic fetal monitor. Test results are interpreted as reactive, nonreactive, equivocal, or unsatisfactory, based on the presence or absence of FHR accelerations or decelerations over a 20-minute period. A reactive NST indicates adequate fetal well-being. A nonreactive NST may indicate a problem with placental functioning or fetal oxygenation and should be followed up with additional testing.
• Biophysical Profile: The Biophysical Profile (BPP) uses a combination of NST and fetal parameters observed via ultrasound to measure fetal well-being. The fetus is scored either 0, 1, or 2 for each of five parameters: fetal breathing movement, gross fetal movement, fetal tone amniotic fluid volume, and NST test results. BPP results of 8–10 indicate a normal fetus (when amniotic fluid volume is adequate). BPP results of 4–5 are considered equivocal and BPP results of < 4 indicate are considered abnormal.
• Amniocentesis: Withdrawal of amniotic fluid under direct ultrasonograpy is called amniocentesis. Examination of amniotic fluid can be used to determine fetal maturity in pregnancies < 34 weeks. An LS (lecithin: sphingomyelin) ratio of > 2:1 represents fetal maturity.
Positioning of the pregnant patient
The supine position should be not be used with pregnant women after 20 weeks’ EGA. At that time, the maternal vena cava is compressed by the growing maternal abdomen when the mother lies in a supine position. This effect is called “supine hypotension” and, if left unchecked, will lead to decreased blood return to the heart, decreased maternal BP, decreased CO, and subsequently to decreased blood flow to the uterus, placenta, and fetus. The appropriate position for the pregnancy patient is lateral positioning, which avoids the risk of supine hypotension and maximizes blood flow to the uterus, placenta, and fetus. Turning some pregnant women on the left side may improve CO more than the right side. Semi-Fowler’s positioning with a hip roll tilting the maternal abdomen off of the vena cava is another acceptable position for the pregnant woman in the latter half of pregnancy.
Blood pressure control and management
• Hydralazine 5 to 10 mg may be given every 10 to 15 minutes. Maximum dosage: 30 mg
• Labetalol 20 to 40 mg may be given every 10 to 15 minutes. Maximum dosage: 220 mg
• Labetalol should be used cautiously in patients with asthma and cardiac failure.
• Nifedipine 10 to 20 mg may be given every 30 minutes. Maximum dosage: 50 mg
Pain management
Neuraxial analgesia techniques (spinal, epidural, or combined spinal-epidural) are commonly used because they are more effective at relieving pain and do not cause respiratory depression in the mother or fetus. Medications used are generally a combination of local anesthetics and opioids, which produce greater pain relief with less motor block than local anesthetics alone. Lumbar epidurals may be given continuously or as a patient-controlled analgesia (PCA). Contraindications to neuraxial analgesia include allergy to local anesthetics, when the last dose of low-molecular-weight heparin has been within 12 hours, coagulation disorders, maternal shock, or infection at the insertion site. Common side effects of neuraxial analgesia include maternal hypotension and itching. Hypotension is generally treated with IV fluid boluses and/or IV Ephedrine. Ephedrine is the preferred vasopressor because it causes peripheral vasoconstriction without affecting the umbilical vessels. Loratadine (Claritin) or Cetirizine (Zyrtec) may be given to alleviate itching.
Prevention of eclampsia/seizure management
![]() Magnesium sulfate is listed as a high-risk medication by The Joint Commission (formerly the Joint Commission on Accreditation of Healthcare Organizations [JCAHO], 2003). Obstetric nurses should be familiar with safety recommendations related to the administration of magnesium sulfate to pregnant or delivered patients.
Magnesium sulfate is listed as a high-risk medication by The Joint Commission (formerly the Joint Commission on Accreditation of Healthcare Organizations [JCAHO], 2003). Obstetric nurses should be familiar with safety recommendations related to the administration of magnesium sulfate to pregnant or delivered patients.
Administration of magnesium sulfate:
• Administer using an infusion pump. Two nurses should verify the infusion pump settings when magnesium is initiated or dosage changed.
• Magnesium solutions should be premixed and labeled in standard solutions.
• An initial loading dose of 4 to 6 g is given over 15 to 30 minutes followed by a maintenance dose of 2 to 3 g/hr.
• Magnesium therapy is continued after delivery for 12 to 24 hours. Side effects of magnesium include flushing, nausea, muscle weakness, headache, and toxicity.
• Magnesium levels greater than 8 mg/dl are associated with signs of toxicity; refer to Table 11-7.
• If toxicity is suspected: Discontinue infusion, administer oxygen, obtain stat magnesium level, and notify physician.
• The antidote for magnesium toxicity is calcium gluconate 10 mg of 10% calcium gluconate solution IVP over 10 minutes and should be readily available.
• Serum magnesium levels are drawn 2 hours following the loading dose, then every 6 hours.
| Serum Magnesium Level | Signs/Symptoms to Watch for |
|---|---|
| 1.7–2.4 mg/dL | Normal |
| 5–8 mg/dL | Therapeutic |
| 8–12 mg/dL | Loss of pateller reflexes |
| 10–12 mg/dL | Somnolence |
| 12–16 mg/dL | Respiratory difficulty and depression |
| 15–17 mg/dL | Muscle paralysis |
| >18 mg/dL | Altered cardiac conduction |
| 30–35 mg/dL | Cardiac arrest |
![]() The patient must be closely monitored during magnesium administration. It is recommended that the obstetric nurse be present at the bedside with the critical care nurse to monitor fetal and uterine response during magnesium therapy. The desired therapeutic level of magnesium is 5 to 8 mg/dl. If magnesium toxicity is suspected, discontinue the magnesium infusion, support oxygenation and notify the physician immediately (Simpson, 2004).
The patient must be closely monitored during magnesium administration. It is recommended that the obstetric nurse be present at the bedside with the critical care nurse to monitor fetal and uterine response during magnesium therapy. The desired therapeutic level of magnesium is 5 to 8 mg/dl. If magnesium toxicity is suspected, discontinue the magnesium infusion, support oxygenation and notify the physician immediately (Simpson, 2004).
Emergency delivery in the critical care unit
CARE PLANS FOR THE HIGH-RISK OBSTETRIC PATIENT IN CRITICAL CARE
Blood Coagulation; Fetal Status: Intrapartum
Electronic fetal monitoring: intrapartum
1. Verify HRs of mother and fetus prior to initiating electronic monitoring.
2. Monitor BP of mother at least every 5 minutes if severe hypertension or seizures have occurred. Monitor FHR for slowing, indicative of fetal distress.
3. Initiate fetal resuscitation measures to treat abnormal fetal heart rhythms, as appropriate. Note response to all supportive interventions.
4. Instruct woman and support person(s) about the need for monitoring and data to be obtained.
5. Keep physician informed of significant changes in FHR, interventions for abnormal patterns, fetal response, labor progress, and maternal response to interventions.
6. Administer anticonvulsive medications as ordered to control seizures.
7. Monitor magnesium levels, and be alert to hypermagnesemia: decreased respiratory rate and depth, loss of deep tendon reflexes.
8. Assist with application of forceps or vacuum extractor, as needed, during delivery.
9. Monitor the patient closely for hemorrhage if HELLP syndrome is present.
10. Note Hgb and Hct levels before and after blood loss, as indicated.
11. Monitor coagulation studies, including prothrombin time (PT), activated partial thromboplastin time (aPTT), fibrinogen, fibrin degradation products (FDPs), fibrin split products (FSPs), and platelet counts as appropriate.
Fetal Status: Antepartum; Blood Coagulation; Fluid/Electrolyte Management
1. Monitor for and document seizures. Protect patient from injury by initiating seizure precautions. When patient is seizing, turn her to the side to promote placental perfusion and prevent aspiration.
2. Assess patient for initial signs of increased ICP, including diminished LOC, headaches, abnormal pupillary responses (i.e., unequal; sluggish/absent response to light), visual disturbances, weakness and paralysis, slow HR, and change in respiratory rate (RR) and pattern. Patient may be experiencing problems related to seizure management, or may experience intracranial bleeding if platelets are extremely low.
3. Monitor trend of Glasgow Coma Scale if patient has difficulty awakening after seizures. If patient exhibits signs of magnesium toxicity, consult physician and consider calcium gluconate administration.
4. Increase frequency of neurologic monitoring as appropriate.
5. Assess the epidural site (as appropriate), if patient received epidural analgesia during labor and delivery. Patients with coagulopathy may develop an epidural hematoma, manifested by sensory or motor deficits, bowel or bladder dysfunction, or back pain. Report problems to the physician immediately. The epidural catheter should remain in place until coagulation studies normalize.
6. Avoid activities that increase ICP.
7. If initial signs of IICP are noted, consult physician immediately. Signs of impending herniation include unconsciousness, failure to respond to deeply painful stimuli, decorticate or decerebrate posturing, Cushing triad (i.e., bradycardia, increased systolic BP, widening pulse pressure), nonreactive/fixed pupils, unequal pupils, or fixed and dilated pupils. See Traumatic Brain Injury, p. 333, for more information about herniation. For additional interventions for IICP, see Box 3-7.
![]() Cerebral Perfusion Promotion; Neurologic Monitoring; Seizure Management
Cerebral Perfusion Promotion; Neurologic Monitoring; Seizure Management
Risk for deficient fluid volume
related to active loss secondary to antepartum, postpartum, intraabdominal, or other bleeding
1. Monitor fluid status, including I&O, signs of hypovolemia, including increases in HR and RR, decreases in BP and urinary output, restlessness, and vital signs.
2. Initiate hemodynamic monitoring if necessary to assess shock state and/or manage hemodynamics.
3. Monitor Hgb and Hct as ordered. Postpartum, the uterus contracts and blood is released into the central circulation as it decreases in size. With a normal 500-ml vaginal delivery blood loss, often the Hgb and Hct increase despite the blood loss.
4. Inspect for bleeding from mucous membranes, bruising after minimal trauma, oozing from puncture sites, and presence of petechiae. Maintain patent IV access.
5. Administer supplemental oxygen as necessary for active bleeding.
6. Perform fundus checks and initiate gentle fundal massage to help the uterus remain firm and promote hemostasis.
7. Initiate bleeding control therapy as ordered. Methylergonovine, ergonovine, and carboprost may be used to manage prolonged bleeding or hemorrhage.
8. Replace lost volume with plasma expanders (e.g., albumin, hetastarch) and/or blood products as indicated. See Table 10-3 for information about blood products.
![]() Fluid Management; Fluid/Electrolyte Management; Surveillance: Late Pregnancy; Bleeding Reduction; Hypovolemia Management
Fluid Management; Fluid/Electrolyte Management; Surveillance: Late Pregnancy; Bleeding Reduction; Hypovolemia Management
Immune Status; Infection Severity
1. Monitor vital signs for evidence of infection (e.g., increases in heart and respiratory rates). Check rectal or core temperature every 4 hours for increases.
2. If temperature elevation is sudden, obtain specimens for blood, sputum, and urine cultures or from other sites as prescribed. Consult physician or midlevel practitioner for positive culture results.
3. Monitor CBC, and consult physician for significant increases in WBCs. Be aware that a normal or mildly elevated leukocyte count may signify infection in patients with liver dysfunction.
4. Evaluate secretions and drainage for evidence of infection (e.g., sputum changes, cloudy urine).
5. Evaluate IV, central line, and other site(s) for evidence of infection (i.e., erythema, warmth, unusual drainage).
6. Provide routine episiotomy care by spraying the area with warm water at least every 4 hours. Sitz baths are usually not possible with critically ill patients. Ice packs and topical anesthetics may be used to enhance comfort.
7. Provide daily breast care by cleansing the breasts with mild soap and water. Breast engorgement should be managed per physician’s orders to help prevent infection and control pain. If left unrelieved, breast engorgement can result in stoppage of lactation. If breast-feeding is planned, breasts can be massaged and milk manually expressed or pumped.
8. Prevent transmission of infectious agents by washing hands well before and after caring for patient and by wearing gloves when contact with blood or other body substances is possible. Dispose of all needles and other sharp instruments in puncture-resistant, rigid containers. Keep containers in each patient room and in other convenient locations. Avoid recapping and manipulating needles before disposal. Teach significant others and visitors proper hand-washing technique. Restrict visitors with evidence of communicable disease.
9. Administer antibiotics as prescribed. Use caution and reduced dosage when administering antibiotics (especially aminoglycosides) to patients with low urinary output or renal insufficiency.
Family Coping; Family Normalization
1. Provide regular updates to family members about the condition of both mother and infant.
2. Invite family members to participate in care of the mother as possible.
3. Promote infant-mother bonding by allowing baby visitations in the ICU as soon as the conditions of mother and infant stabilize. Allow mother to feed baby if possible. Maintain infant safety if mother’s condition is marginally stable.
4. Discuss feelings about labor, delivery, and complications with patient and family members. Relay information about the condition of the infant as often as possible, if mother is unable to visit with the baby.
5. If the infant or mother expires, provide all possible support measures for the patient and significant others, including pastoral care and referrals to community support groups or professional counseling.
![]() Family Involvement Promotion; Family Process Maintenance; Normalization Promotion
Family Involvement Promotion; Family Process Maintenance; Normalization Promotion
Oncologic emergencies
An oncologic emergency is defined as a life-threatening situation occurring as a manifestation of a malignancy or the result of antineoplastic treatment or tumor progression. Such emergencies most commonly arise from the ability of cancer to (1) spread by direct infringement on adjacent structures or metastasize to distant sites leading to thrombosis or hemorrhage, (2) produce abnormal amounts of hormones or cellular products leading to fluid and electrolyte imbalances and organ failure, (3) infiltrate serous membranes with effusion, (4) obstruct vessels, ducts, or hollow viscera, or (5) replace normal organ parenchyma. As more aggressive therapies are used in the treatment of these cancers, side effects are more intense and the use of critical care to manage such side effects will only increase.
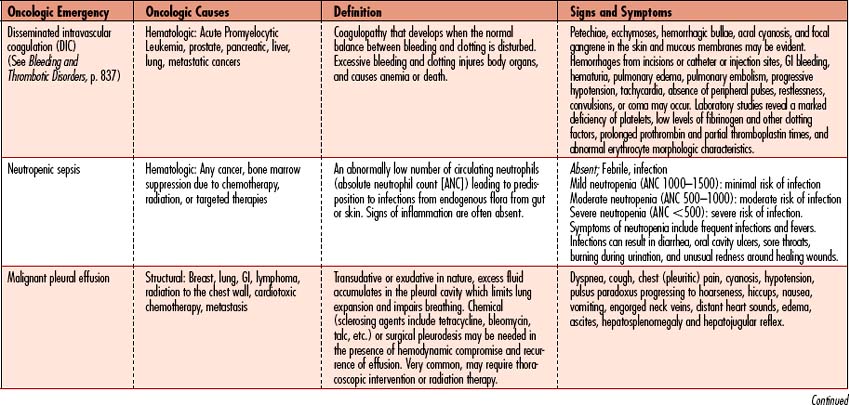
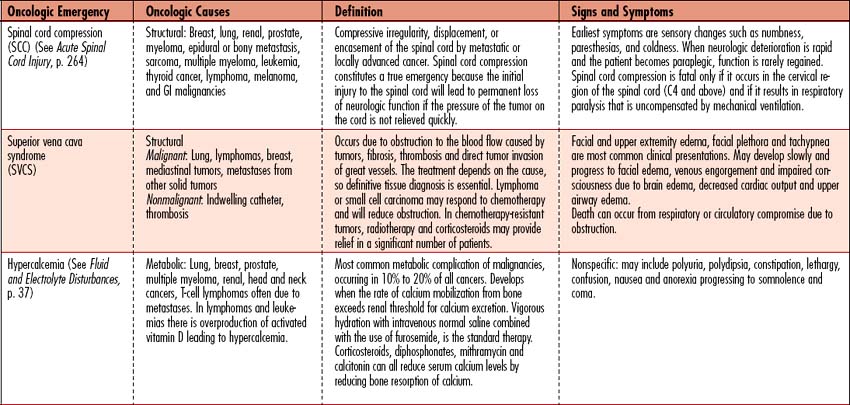
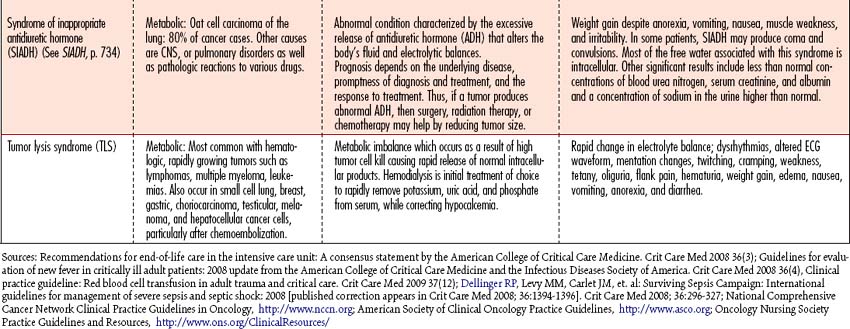
Oncologic emergencies can be classified as hematologic, structural, metabolic, or side effects from treatment such as chemotherapy, radiation therapy, biologic therapy, surgery, or bone marrow transplantation. Neutropenic sepsis, tumor lysis syndrome (TLS), and superior vena cava syndrome (SVCS) will be discussed in this chapter as examples of oncologic emergencies seen in the critical care arena. When recognized early and managed appropriately, these acutely ill individuals have a high likelihood of recovery. An overview of additional oncologic emergencies, associated causes, and signs and symptoms is given in Table 11-8.
neutropenic sepsis
Pathophysiology
• Greater than 1000—Normal protection against infection
• 500 to 1000—Some increased risk of infection
• 200 to 500—Great risk of severe infection
• Lower than 200—Risk of overwhelming infection; requires hospital treatment with antibiotics
Untreated bacteremia in patients with neutropenia is fatal: septic shock is associated with a 50% to 70% mortality rate. Mortality is associated with causative organism, site of infection, and the level and duration of neutropenia. The risk of infection is greater the faster the rate of decline of the neutrophil count and the longer the duration of neutropenia, especially if neutropenia lasts longer than 10 days. Infections in neutropenic patients typically take 2 to 7 days to respond to antimicrobial therapy. Fever (or any inflammatory response related to white cells or cytokines) may not be present in some infected neutropenic patients who are dehydrated or taking steroids or NSAIDs, and the possibility of infection must be considered in any neutropenic patient who is unwell.
Asssessment: neutropenic sepsis
The four stages of sepsis
Observation: rapid progression if untreated
Signs and symptoms of infection may be absent due to decreased WBCs.
• General: fever, hypothermia, chills, pain
• ![]() Neurological: confusion, anxiety, restlessness, decreased level of consciousness, coma
Neurological: confusion, anxiety, restlessness, decreased level of consciousness, coma
• Pulmonary: tachypnea, rales, rhonchi, wheezes, dry cough, cyanosis, hypoxia, ARDS
• Cardiovascular: tachycardia, hypotension, fluctuating pulse pressure, thready pulse
• Digestive: nausea, vomiting, anorexia, decreased GI motility, GI bleed
| Test | Purpose | Abnormal Findings |
| Blood cultures (done baseline and every 24 hours if signs and symptoms of sepsis persist) | Check for bacteria or other microorganisms in a blood sample. | Gram-positive cocci: coagulase-negative staphylococci, Viridans streptococci and Staphylococcus aureus Gram-negative pathogens: Escherichia coli, Klebsiella spp., and Pseudomonas aeruginosa. Fungal: Candida spp., Aspergillus spp. Viral: Herpes simplex; respiratory syncytial virus |
| Cultures (throat, stool, urine, CV catheter, other sites of exudates) prior to antibiotics to determine pathogen | Determines source of infection | As above |
| Chest radiographic examination | Evaluates lung status | Pulmonary infiltrates, pulmonary edema |
| Complete blood count (CBC) | Evaluates the composition and concentration of the cellular components of blood | Increased WBC with infection, decreased WBC with sepsis; chemotherapy, radiation, leukemias |
| Chemistries – electrolytes, liver function tests | Evaluates blood chemistries and liver function | Increased BUN, creatinine reveal dehydration; Increase to decrease in glucose due to shock; increased transaminase, bilirubin, serum lactate in sepsis due to shock |
| Coagulation profile | Examines the factors most often associated with a bleeding problem | Prolonged PT, PTT |
| Pulse oximetry, ABGs | Measures oxygenation, acid-base balance | Respiratory alkalosis followed by metabolic acidosis |
| Electrocardiogram | Records the electrical activity of the heart. | Tachycardia, arrhythmias |
Collaborative management
Because sepsis-related mortality is unacceptably high, the Society of Critical Care Medicine (SCCM) set a quality improvement goal to reduce mortality caused by severe sepsis and septic shock by 25%. Clearer definitions of sepsis, severe sepsis, and septic shock will help in achieving this goal, as will recently updated evidence-based management guidelines for severe sepsis and septic shock. To be effective, these definitions and guidelines need to be applied to the early identification and aggressive treatment of patients who have severe sepsis or septic shock. Early goal-directed therapy to achieve hemodynamic stabilization has been demonstrated to decrease mortality in patients who have septic shock. See SIRS, Sepsis and MODS, p. 927.
Care priorities
Once severe sepsis or septic shock has been identified, the highest management priorities are to establish vascular access and initiate fluid resuscitation to improve tissue perfusion. Failure to manage global hypoxia can lead to MODS. Fluid resuscitation guidelines are defined by the Society of Critical Care Medicine and are a part of early goal-directed therapy (see Sepsis, SIRS, and MODS, p. 927).
2. Restore tissue oxygenation:
Measurements commonly used are SvO2, ScvO2, and lactate levels. Low values indicate several possible causes of poor oxygenation, including inadequate oxygen delivery, high oxygen demand, or inability of tissues to extract oxygen from hemoglobin (see Sepsis, SIRS, and MODS, p. 927).
3. Identify and manage the underlying infection:
CARE PLANS FOR NEUTROPENIA
Ineffective protection: potential for life-threatening infections
related to infection or sepsis-induced myocardial infarction
1. Monitor I&O hourly. Consult physician for a urinary output less than 0.5 ml/kg/hr for 4 consecutive hours. Insert urinary catheter if appropriate.
2. Monitor and document HR, rhythm, pulses, and BP.
3. Evaluate efficacy of volume expansion by closely comparing CVP, PAWP, and CO. Overzealous volume expansion can lead to heart failure and pulmonary edema.
4. Administer diuretics as prescribed in the well-hydrated patient with urine output less than 0.5 ml/kg/hr.
5. Assess patient for volume depletion, including poor skin turgor; dry mucous membranes; hypotension; tachycardia; and decreasing urine output, CVP, and CO.
6. Monitor electrolytes and serum osmolality. A universal increase in electrolytes and osmolality is indicative of dehydration. A universal decrease signals fluid overload.
7. Assess pH (normal range is 7.35 to 7.45) before replacing electrolytes. Acidosis and alkalosis alter electrolyte values. Replace potassium if the pH is outside the normal range.
1. Administer acetaminophen as ordered for fever or mild discomfort.
2. Encourage restful environment.
3. Apply warmth to muscles or joints or cool compress to head as needed.
4. Administer meperidine 12.5 to 25 mg IV or other opiate or benzodiazepine as ordered for chills.
5. Monitor temperature 30 minutes after chill subsides.
6. Bathe patient and change linens after fever subsides.
7. Monitor platelet count as it may decrease with hypermetabolism of hyperthermia.
Additional nursing diagnoses
Refer to nursing diagnoses in Emotional and Spiritual Support of the Patient and Significant Others (p. 200). For patients who manifest activity intolerance, see that nursing diagnosis in Prolonged Immobility (p. 149). For patients with sepsis or septic shock, see SIRS, Sepsis, MODS p. 927.
Superior vena cava syndrome
Pathophysiology
• Failure to establish the correct diagnosis and the underlying etiology
• Failure to initiate immediate treatment
• Failure to recognize a thrombus in the SVC
• Failure to consult a medical oncologist and radiation therapist
• Failure to expeditiously diagnose and appropriately manage heparin-related complications
Assessment
Observation
Other physical findings include laryngeal or glossal edema, mental status changes, and pleural effusion (more commonly on the right side).
| Test | Purpose | Abnormal Findings |
|---|---|---|
| Chest radiograph (CXR) | Evaluates lung status at baseline and every 24 hours if signs and symptoms of sepsis persist | Identify hilar, mediastinal masses, right middle lobe congestion, masses or nodes between clavicle and scapula |
| Spiral chest CT scan with contrast | Further evaluates lung status to help clarify findings from CXR. | Precisely identify location and size of masses around SVC; clarify growth versus compression |
| Magnetic resonance imaging (MRI) of the chest | Provides further data regarding the area of the superior vena cava | Precisely identifies location and size of masses around SVC |
| Doppler ultrasound of the vasculature | Noninvasively assesses the entire vascular tree to identify thrombosis or the effects of thrombolysis | Presence of thrombus or ineffective thrombolysis when therapies are rendered. |
| Contrast and radionuclide venography | Invasively assesses the entire vascular tree to identify thrombolysis; may be used to validate Doppler ultrasound findings | Presence of thrombus or ineffective thrombolysis when therapies are rendered. |
| Complete blood count (CBC): platelet count | Evaluates the composition and concentration of the cellular components of blood, particularly platelets | Declining platelet count may signal the presence of heparin-induced thrombocytopenia (HIT) or “white clot syndrome.” If unmanaged, may lead to extremity gangrene and life-threatening venous thromboembolism. |
Collaborative management
Care priorities
5. Provide additional interventions to assist with maintaining patency of the svc:
Balloon angioplasty is rare but may be considered in patients with SVCS, significant clinical symptoms, and critical SVC obstruction demonstrated by angiography.
CARE PLANS FOR SUPERIOR VENA CAVA SYNDROME
related to reduced venous blood return to the heart
Patient will show evidence of normal tissue perfusion and will not experience upper extremity edema.
Circulatory care: venous insufficiency
1. Monitor I&O hourly. Consult physician for a urinary output less than 0.5 ml/kg/hr for 4 consecutive hours. Insert urinary catheter if appropriate.
2. Monitor and document HR, rhythm, pulses, and BP.
3. Evaluate efficacy of volume expansion by monitoring CVP and CO. Overzealous volume expansion can lead to heart failure and pulmonary edema, with CVP greater than 20% of normal values and CO less than 5 L/min.
4. Administer diuretics as prescribed in the well-hydrated patient with urine output less than 0.5 ml/kg/hr.
5. Assess patient for volume depletion, including poor skin turgor, dry mucous membranes, hypotension, tachycardia, CVP less than 2 mm Hg, and decreasing urine output.
6. Monitor electrolytes and serum osmolality. A universal increase in electrolytes and osmolality is indicative of dehydration. A universal decrease signals fluid overload.
7. Assess skin and mucous membranes for cyanosis and pallor, skin temperature for signs of change in perfusion.
8. Assist in care of patient receiving therapy to treat SVCS.
Impaired gas exchange (or risk for same)
related to decreased blood flow to the lungs
Respiratory Status: Gas Exchange; Vital Signs Monitoring
1. Assess respiratory status every 2 hours, noting rate, rhythm, depth, and regularity of respirations.
2. Monitor for signs of respiratory failure: restlessness, anxiety, and air hunger indicative of hypoxemia; ABG values for increased PaCO2 and decreased pH indicative of hypoventilation; or oxygen saturation less than 90% via pulse oximetry indicative of decreased ventilation. Consult physician if signs of respiratory insufficiency are present.
3. Monitor SvO2: steady increase or decrease from patient’s normal level may indicate deterioration.
4. Assess lungs for bibasilar crackles (rales), indicative of pulmonary edema.
5. Monitor patient’s respiratory secretions. Institute respiratory therapy treatments as needed.
6. Monitor for pulmonary embolus, including sharp, stabbing chest pain, dyspnea, pallor, cyanosis, pupillary dilation, rapid or irregular pulse, profuse diaphoresis, and anxiety. Assess need for supplemental oxygen, and consult physician immediately. Patients with severe pulmonary emboli may require mechanical ventilation. See Pulmonary Embolus, p. 396.
7. Assess patient for changes in sensorium (i.e., confusion, lethargy, somnolence), indicative of inadequate cerebral oxygenation or CO2 retention (respiratory insufficiency).
Tumor lysis syndrome
Pathophysiology
Tumor lysis syndrome (TLS) is most commonly seen in individuals with rapidly growing tumors which are acutely responsive to chemotherapy due to the rapid release of intracellular contents into the bloodstream, leading to life-threatening concentrations of intracellular electrolytes. If the resulting metabolic abnormalities remain uncorrected, patients may develop acute renal failure and sudden death resulting from a lethal dysrhythmia secondary to hyperkalemia.
Assessment
Observation
Signs and symptoms reflect electrolyte imbalance, coupled with those of acute renal failure. The syndrome is characterized by hyperuricemia, hyperkalemia, hyperphosphatemia, hypocalcemia, and oliguric renal failure (see Fluid and Electrolyte Disturbances, p. 37, and Acute Renal Failure, p. 584). The diagnosis of TLS is based on the development of increased levels of serum uric acid, phosphorus, and potassium; decreased levels of serum calcium; and renal dysfunction following chemotherapy
Collaborative care
Care priorities
3. Provide aggressive, immediate treatment of electrolyte imbalances and acute renal failure:
Once tumor lysis is established, treatment is directed at vigorous correction of electrolyte abnormalities, hydration, and hemodialysis (see Fluid and Electrolyte Disturbances, p. 37, and Acute Renal Failure, p. 584).
CARE PLANS FOR TUMOR LYSIS SYNDROME
Electrolyte and Acid-Base Balance
1. Monitor intake and output hourly. Consult physician or midlevel practitioner for a urinary output less than 0.5 ml/kg/hr for 4 consecutive hours. Insert urinary catheter if appropriate.
2. Monitor electrolytes every 6 to 12 hours during high-risk period.
3. Monitor and document HR, rhythm, pulses, and BP.
4. Administer IV fluids before and after cancer treatment.
5. Administer allopurinol as prescribed to manage hyperuricemia.
6. Administer aluminum hydroxide as prescribed to manage hyperphosphatemia.
7. Administer cation-exchange resins as prescribed to manage hyperkalemia.
8. Assist with renal replacement therapies.
9. Administer diuretics as prescribed in the well-hydrated patient with urine output less than 0.5 ml/kg/hr.
10. Monitor electrolytes and serum osmolality. A universal increase in electrolytes and osmolality is indicative of dehydration. A universal decrease signals fluid overload.
11. Assess pH (normal range is 7.35 to 7.45) before replacing electrolytes. Acidosis and alkalosis alter electrolyte values. Replace potassium if the pH is outside the normal range.
12. Teach patients to restrict foods high in potassium and phosphorus and to increase fluid intake.