CHAPTER 127 Complementary and Alternative Medicine
DEFINITION AND EPIDEMIOLOGY
Complementary and alternative medicine (CAM) is defined broadly as medical practices neither taught widely in medical schools nor generally available in U.S. hospitals.1 The prevalence of CAM therapies has increased at an exponential rate both in national and international medical communities. A study by Eisenberg and associates1 demonstrated that among the U.S. population, CAM use increased from 33.8% to 42.1% from 1990 to 1997. Estimated annual expenditures for CAM therapies are in excess of $27 billion, a sum that is equivalent to patients’ out-of-pocket expenditures for all U.S. physician-based services.1
In a study by Ganguli and colleagues, at least 50% of gastroenterology outpatients in a community setting were shown to have implemented CAM therapies to help ameliorate their symptoms.2 Given the widespread use of these modalities and the continuing trend of their increased use, an understanding of CAM therapies, including their potential risks and benefits, is necessary for the practicing gastroenterologist. A thorough knowledge of these practices allows physicians to provide comprehensive medical care and can help further a therapeutic rapport between physicians and their patients.
TYPES OF THERAPIES
There are a wide variety of CAM therapies, and those most commonly employed for gastrointestinal and hepatic disease are defined in Table 127-1. Regardless of the therapy employed, the overall philosophy of CAM takes a uniform holistic approach that all disease results from disturbances at a combination of physical, psychological, social, and spiritual levels. Thus, a CAM modality is used to restore balance and to facilitate the body’s own healing responses, thereby ameliorating troublesome symptoms.3
Table 127-1 Common Complementary and Alternative Therapies for Gastrointestinal and Hepatic Diseases
| Acupuncture |
| Based on the principles of Chinese medicine, qi is energy, which circulates among organs along channels called meridians. Through placement of needles at specifically defined locations (points), the flow of qi is restored to appropriate levels and the health of specific organs is improved. |
| Ayurveda |
| Holistic system of medicine from India that provides diet and lifestyle recommendations to improve overall health. |
| Colonic Irrigation Therapy |
| Cleansing of the colon through various oral and enema preparations to improve “digestive health.” |
| Herbal Medicine |
| Ingestion of various herbal therapies, supplements, or probiotics to improve physiologic function. |
| Homeopathy |
| Based on the principle “like should be cured with like.” Administration of a diluted solution that, when given to a healthy person in an undiluted form, causes symptoms identical to those experienced by the ill person. |
| Hypnosis |
| Induction of a deeply relaxed state during which therapeutic suggestions are made to alter behavior and enhance relief of symptoms. |
| Meditation, Relaxation |
| A process of reflection and contemplation allowing one to focus thoughts to help alleviate symptoms. |
| Reflexology |
| Areas on the feet correspond to organs of the body. Massage and pressure applied to these regions can improve symptoms throughout the body. |
DEMOGRAPHY OF CAM USERS
Certain patients are more likely to use CAM therapies than are others. Women and whites tend to use CAM more often than do men and African Americans, respectively. Patients with higher levels of education, higher annual incomes, and comorbid medical conditions also are more likely to use CAM therapies.1,2 Knowledge of these demographics assists the gastroenterologist in determining which patients are likely to be using these therapies, but it is also important to understand each patient’s rationale and motivation for choosing a particular therapeutic modality.
GASTROINTESTINAL DISORDERS ADDRESSED BY COMPLEMENTARY AND ALTERNATIVE THERAPIES
NAUSEA AND VOMITING
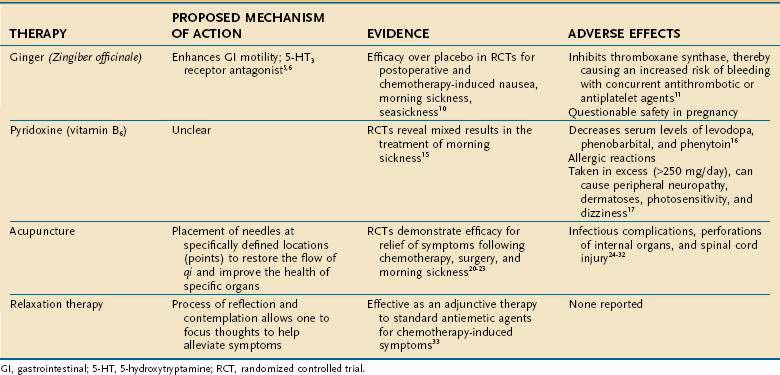
Nausea and vomiting have a wide array of causes ranging from viral gastroenteritis to pregnancy. These symptoms can be quite distressing, and patients often resort to CAM therapies to seek symptomatic improvement. In one study of pregnant women with nausea and vomiting, 61% reported using CAM therapies for relief.4 Several complementary modalities have been used to help ameliorate nausea and vomiting, ranging from herbal medicines to relaxation techniques (Table 127-2).
Ginger
Ginger (Zingiber officinale) is the herbal supplement most commonly employed to relieve nausea and vomiting and derives its name from the Sanskrit word for “horn,” which describes the twisted, gnarled shape of its roots. Several mechanisms have been postulated to explain the antiemetic effect of ginger. Animal studies have demonstrated that one component of the herb, 6-gingerol, improves gastrointestinal motility,5 and another component, galanolactone, is a 5-hydroxytryptamine (HT)3 antagonist,6 similar to ondansetron, an antiemetic agent used to treat chemotherapy-induced nausea and vomiting.
The antiemetic effect of ginger has been studied in various clinical conditions, including morning sickness, seasickness, chemotherapy-induced nausea, and postoperative nausea. Although no more effective than placebo for preventing experimentally induced motion sickness, ginger has been documented to reduce vertigo induced by caloric stimulation of the vestibular apparatus within the inner ear.7–9 In a systematic review of randomized clinical trials evaluating the efficacy of ginger for nausea and vomiting, Ernst and Pittler demonstrated that ginger is superior to placebo and equal in efficacy to metoclopramide for postoperative nausea and emesis. Furthermore, ginger relieved symptoms better than did placebo agents for the treatment of seasickness, morning sickness of pregnancy, and chemotherapy-induced nausea and vomiting.10 The dose of ginger prescribed in most of these studies ranged from 0.5 to 1 g/day.
Although ginger appears to be a natural supplement with therapeutic effect, its potential adverse reactions must be taken into consideration before advocating it for relief of symptoms. First, ginger has been shown to inhibit platelet aggregation by inhibiting thromboxane synthase. Therefore, if patients are taking warfarin, aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs), or clopidogrel concurrently, the risk of bleeding is increased.11 Second, although not proved in animal studies, ginger has been documented to be potentially mutagenic in laboratory assays, thereby raising questions about the safety of the herbal supplement in pregnancy.12,13
Pyridoxine (Vitamin B6)
The water-soluble vitamin pyridoxine is another CAM therapy used to relieve the nausea and vomiting associated with pregnancy,14 and it was one of the most commonly employed CAM agents in a survey of pregnant Canadian women with nausea and vomiting, 29% of whom reported using it.4 Vutyavanich and colleagues reported a significant reduction in nausea, but no statistically significant reduction in vomiting, with pyridoxine, 30 mg daily, in a randomized controlled trial (RCT).15
Although the mechanism of action of pyridoxine is not established, certain drug interactions and adverse effects have been noted. Pyridoxine has been documented to decrease serum levels of levodopa, phenobarbital, and phenytoin when administered with these agents.16 Allergic reactions to pyridoxine also have been documented, and when taken in excess (more than 250 mg/day), pyridoxine has been reported to cause peripheral neuropathy, dermatoses, photosensitivity, and dizziness.17,18
Acupuncture and Acupressure
Acupuncture is another CAM modality commonly used to treat nausea and vomiting. In Chinese subjects, the P6 acupuncture point stimulated for relief of these symptoms is named neiguan, meaning “medial pass.” This acupuncture point is anatomically located three fingerbreadths above the proximal palmar crease on the volar aspect of the wrist in the midline. To date, more than 30 published trials have evaluated the role of stimulating the P6 acupuncture point for relief of nausea and vomiting.19 In a systematic review, acupuncture was demonstrated to be superior to placebo in ameliorating nausea and vomiting; the results were consistent despite numerous investigators, diverse patient populations, and various forms of acupuncture point stimulation.18 Trials have demonstrated that acupuncture effectively relieved nausea and emesis associated with chemotherapy,20 surgery,21,22 and pregnancy.23
Several adverse events have been noted with acupuncture, mainly from infection secondary to improper handling of needles or their reuse without sterilization.24 Such infections have included hepatitis B virus (HBV), hepatitis C virus (HCV), and human immunodeficiency virus (HIV)25–27; bacterial endocarditis secondary to Propionibacterium acnes28; and bacteremia from Staphylococcus aureus and Pseudomonas aeruginosa with a consequent psoas abscess.29 Two fatalities have been documented in which acupuncture was thought to have led to sepsis with staphylococcal organisms.30 Although improperly sterilized needles seem to be the only risk factor for the aforementioned infections, it is difficult to prove such infections were a direct result of acupuncture, because patients might not have divulged other personal potential risk factors such as sexual preference or intravenous drug use.31 Other risks reported to be associated with acupuncture therapy include perforation of an organ during placement of the needles with resultant pneumothorax, hemopericardium with tamponade, and spinal cord injury.24,31,32
Relaxation Therapy
Relaxation therapies have been suggested as a CAM therapy for chemotherapy-induced nausea and vomiting. It has been reported that side effects related to chemotherapy are somewhat conditioned and are developed as a form of associative learning33; the anxiety experienced during chemotherapy sessions can serve as conditioning cues that lead to physiologic reactions. Through progressive muscle relaxation therapy, a patient’s anxiety can be alleviated and physical symptoms averted. Relaxation therapies often are used as an adjunct to standard antiemetic medications.31
FUNCTIONAL DYSPEPSIA
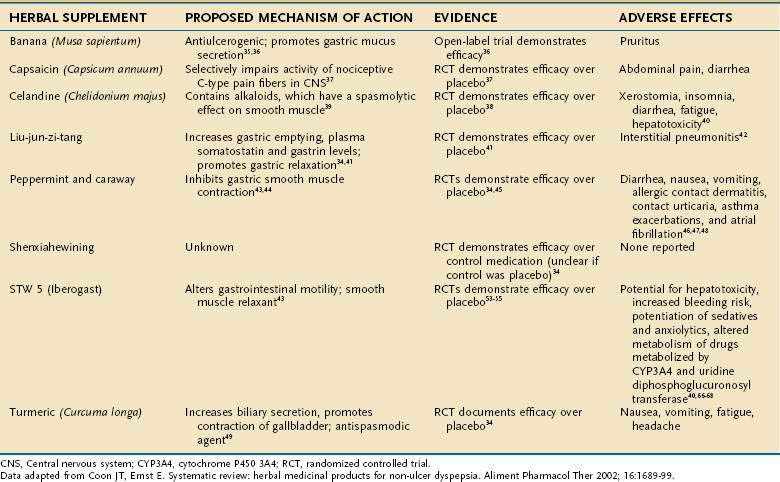
Functional dyspepsia is defined as pain or discomfort in the epigastric area in the absence of demonstrable structural or physiologic abnormalities. Because symptoms tend to be short in duration and relatively mild, dyspepsia often is self-managed34; therefore, CAM therapies clearly are appealing. Herbal therapy has been a mainstay of CAM treatments for functional dyspepsia. The most common supplement therapies for functional dyspepsia, including their active ingredients, proposed mechanisms of action, and adverse effects, are listed in Table 127-3.
Banana (Musa sapientum) has been evaluated for the treatment of functional dyspepsia in prospective open trials. This supplement is thought to promote gastric mucus secretion and has been documented to have antiulcerogenic properties in animals.35 In a study by Arora and Sharma,36 treatment with banana powder resulted in a reduction in symptoms in 75% of patients in the treatment group compared with 25% of those in the placebo group (P < 0.05). Causes of organic dyspepsia were excluded through various endoscopic and laboratory methods before subjects were included in the study. The only adverse effect reported was pruritus in the treatment group.36
Capsaicin, derived from the dried fruit of Capsicum annuum (red pepper), is an herbal supplement. Its mechanism of action is selective impairment of pain (C-type) fibers, which carry pain sensation from the abdominal viscera to the central nervous system.37 In one study, 2.5 mg of red pepper powder given daily improved epigastric pain, nausea, and bloating, whereas placebo did not.37 Although abdominal pain and diarrhea occurred initially in patients treated with capsaicin, these adverse effects were self-limited and of no serious clinical consequence.
Greater celandine (Chelidonium majus) was investigated in functional dyspepsia by Ritter and colleagues in a randomized, double-blind, placebo-controlled trial.38 Celandine accounted for a 34% greater reduction in symptoms compared with placebo (P = 0.003).38 This agent is thought to contain a variety of alkaloids that also have a spasmolytic effect on smooth muscle.39 Despite its apparent efficacy, celandine has many adverse effects, including xerostomia, insomnia, diarrhea, and fatigue. Idiosyncratic hepatotoxicity also has been described with celandine, but it resolved without complication in most cases when the supplement was discontinued.40
Liu-jun-zi-tang, also known as TJ-43, is a Chinese herbal medicine that has been used for relief of functional dyspepsia. The agent is a combination of several extracts including Actractylodis laneae rhizoma, Ginseng radix, Pinelliae tuber, Hoelen, Zizyphi fructus, Aurantii nobilis pericarpium, Glycyrrhizae radix, and Zingiberis rhizoma.34 Multiple mechanisms of action have been proposed, including increased gastric emptying, increased serum levels of gastrin and somatostatin,41 and relaxation of gastric smooth muscle.42 An RCT compared TJ-43, 2.5 g three times per day, with placebo for seven days in patients with functional dyspepsia. The treatment group displayed greater reductions in epigastric fullness, reflux, and nausea compared with the group treated with placebo (P < 0.05).41 The only adverse event noted with TJ-43 was one case of drug-induced interstitial pneumonitis, which resolved after therapy was discontinued.34
Peppermint (Mentha piperita) and caraway (Carum carvi) are the supplements that have been investigated most thoroughly for treating functional dyspepsia. Their proposed mechanism of action is thought to be inhibition of smooth muscle contractions by direct blockade of smooth muscle calcium channels.43,44 Several placebo-controlled trials have compared variable, fixed doses of these agents ranging from 180 to 270 mg for peppermint and 100 to 150 mg daily for caraway. A statistically significant improvement in symptoms such as bloating and epigastric pain was demonstrated when treatment groups were compared with placebo groups in several trials.34,45 Adverse effects seen with these supplements include diarrhea, nausea, vomiting, allergic contact dermatitis, contact urticaria,46 asthma exacerbations, and atrial fibrillation.47,48
Shenxiahewining is a mixture of Chinese herbs, specifically Ginseng radix, Pinelliae tuber, Coptidis rhizoma, Zingiberis rhizoma exsiccatum, and Glycyrrhizae radix, in a 3 : 9 : 3 : 3 : 3 ratio.34 In an RCT performed in China, 92% of patients treated with shenxiahewining reported improvement in symptoms compared with 20% of a control group. No important adverse events were noted.34
Turmeric (Curcuma longa) is an agent that also has been documented to have therapeutic efficacy in alleviating functional dyspepsia. This agent is thought to increase biliary secretion, promote contraction of the gallbladder, and act as an antispasmodic.49 In a placebo-controlled trial performed in Thailand, turmeric (2 g/day) was found to significantly improve dyspeptic symptoms (P = 0.003).34
Another agent that has been studied for the treatment of functional dyspepsia is STW 5, also known as Iberogast. This agent is an herbal preparation composed of bitter candytuft (Iberis umbellata), chamomile (Matricaria chamomilla), peppermint, caraway fruit, licorice root (Glycyrrhiza glabra), lemon balm leaves (Melissa officinalis), celandine (Chelidonium majus), angelica root (Angelica archangelica), and milk thistle (Silybum marianum). In a meta-analysis performed by Melzer and colleagues,50 evaluating three randomized, double-blind, placebo-controlled trials, STW 5 in a dosage of 1 mL three times daily for four weeks was noted to be more effective than placebo with regard to improving the study patients’ most bothersome dyspeptic symptoms. Twenty-six percent of patients in the placebo group compared with 7% in the STW 5 group reported that their symptom remained “severe” or “very severe” after treatment. Specifically, STW 5 appeared more effective in providing symptomatic relief to patients with predominant epigastric pain and gastroesophageal reflux symptoms.50
von Armin and colleagues51 also have demonstrated in the largest randomized, double-blind, placebo-controlled trial evaluating STW 5 that a significantly higher percentage of patients with functional dyspepsia who are prescribed STW 5 are free from their symptoms when compared with a placebo group.51 Pilichiewicz and coworkers showed that STW 5 affects gastric motility in a region-dependent manner, inducing gastric fundic relaxation and antral contraction.52 Although no adverse events have been reported for STW 5, individual components of the preparation are known to have potential toxicities, details of which are addressed in the next section.
The data on these supplement therapies suggest that some of those studied could be useful for patients with functional dyspepsia. Peppermint, caraway, and STW 5 are the most extensively evaluated to date and, given their encouraging safety profiles, warrant further study.34
IRRITABLE BOWEL SYNDROME
IBS is defined as abdominal discomfort and altered bowel function in the absence of structural and biochemical abnormalities (see Chapter 118).53 Symptoms include pain, bloating, cramping, constipation, and diarrhea. Gastroenterologists encounter this disease entity quite commonly. In a systematic review citing only studies that used Rome criteria for diagnosis, IBS prevalence was found to vary between 5% and 10%, with a pooled prevalence of 7%.53 Patients with IBS often are frustrated that laboratory, radiologic, and endoscopic examinations fail to reveal an “organic” source of their discomfort, and they therefore often employ CAM therapies to help ameliorate their symptoms.
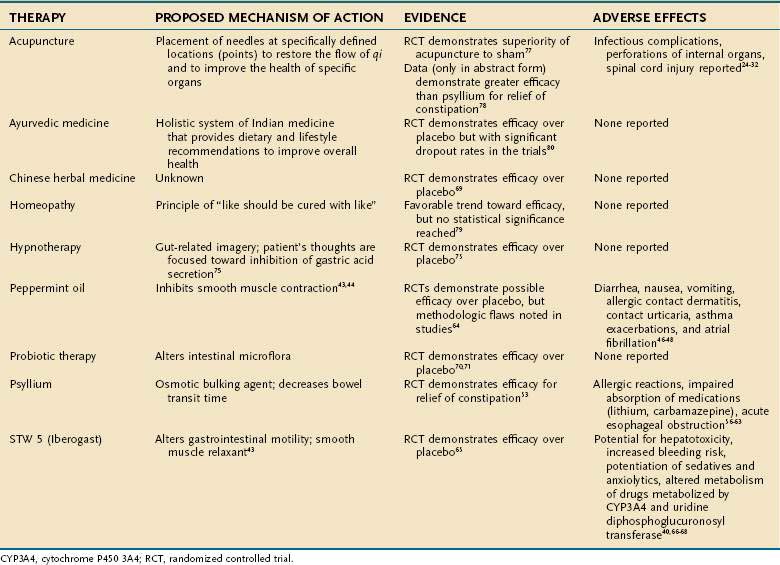
Many CAM therapies have been investigated for the treatment of IBS (Table 127-4). Herbal supplement therapy and the use of probiotics have been evaluated most extensively. Psyllium (Plantago isphagula) is the most commonly prescribed dietary supplement for patients with IBS. This fiber product acts as an osmotic bulking agent and decreases bowel transit time. There have been three placebo-controlled trials of psyllium use in IBS,54–56 but only one fulfilled the five Rome criteria for appropriate study methodology (randomization, concealed allocation, placebo control, double blinding, and appropriate follow-up of study patients). Two additional trials compared psyllium with “active” agents, but neither trial was of high quality.57,58 In general, the evidence that stool frequency, consistency, and ease of passage were better with psyllium than with placebo was modest. There were no statistically significant differences in side effects among psyllium, lactulose, and placebo.
Although psyllium appears to be fairly harmless, allergic hypersensitivity reactions have been documented.59 Impaired absorption of certain medications taken concomitantly, such as lithium and carbamazepine, also has been reported.60,61 Cases of acute esophageal obstruction also have occurred with psyllium-based agents, suggesting that, in certain cases, dysphagia might preclude its use.62
Other supplemental therapies have been used for IBS. Peppermint oil, as previously discussed, is prescribed for its smooth muscle relaxant capabilities. In a meta-analysis by Pittler and Ernst,63 peppermint oil improved symptoms of IBS compared with placebo treatment. Although statistical significance was demonstrated in this study, flaws in the methodology of the trials studied preclude evidence-based acceptance of the efficacy of peppermint oil in the treatment of IBS.
STW 5 also has been used to treat patients with IBS. Placebo-controlled trials have demonstrated that STW 5 improves symptoms of IBS and reduces the severity of abdominal pain.64 Multiple mechanisms of action of STW 5 are postulated; certain components are thought to alter gastrointestinal motility, and others are thought to act as smooth muscle relaxants.60 Although no adverse events have been reported for STW 5, individual components of the preparation are known to have potential toxicities. Specifically, celandine is known to be hepatotoxic at certain doses (greater than 10 mg/day).40 Chamomile is known to contain a coumarin derivative, which increases the risk of bleeding if prescribed concurrently with warfarin, aspirin, or NSAIDs. Chamomile also has been noted to potentiate the central nervous system depressant effects of benzodiazepines and barbiturates,65,66 which often are prescribed to patients with IBS. Milk thistle is known to inhibit cytochrome P-450 3A4 (CYP3A4) and uridine diphosphoglucuronosyl transferase and thus could alter the metabolism of many pharmacologic agents.67
Chinese herbal medicine has been used for IBS symptoms, and RCTs have demonstrated a statistically significant benefit of its use over placebo. Patients treated with Chinese herbal medicine reported improvement in their symptoms and less interference in their daily lives from IBS, with an overall improvement in their quality of life.68
Probiotics are microorganisms that promote health effects through alterations of intestinal microflora.69 Patients with infectious and inflammatory disease states such as pseudomembranous colitis and IBD as well as patients with IBS also have had benefit from these agents. Evidence from RCTs has demonstrated that ingestion of Lactobacillus plantarum resulted in significant reductions in abdominal pain and flatulence in patients with IBS.70,71
A report of fermented milk containing Bifidobacterium animalis demonstrated that this probiotic improves abdominal distention and associated symptoms in patients with constipation-predominant IBS.72 In addition, a systematic review of probiotics demonstrated that Bifidobacterium infantis 35624 (Align) significantly improves abdominal pain, bloating, distention, and the sensation of incomplete evacuation.73
Hypnosis has been documented to have a significant therapeutic effect on symptoms of IBS. Through the use of gut-directed imagery, whereby patients imagine they are inhibiting gastric secretion, an overall symptom improvement rate of 80% has been reported.74 Clinical remission for up to three months has been documented in patients with IBS treated with hypnotherapy. Women and those younger than 50 years seem to respond well to this modality. Hypnotherapy has proved effective in the pediatric IBS population as well, one study having documented that successful treatment of functional abdominal pain or IBS was accomplished in 85% of children treated with hypnotherapy compared with 25% of patients treated with placebo.75
Acupuncture has been shown to be superior to sham therapy and fiber supplementation in patients with IBS.76,77 Homeopathy has demonstrated a trend toward efficacy in IBS.7 Lastly, Ayurvedic medicine (see Table 127-1) also has demonstrated efficacy in relief of symptoms from IBS. The trial of Ayurvedic medicine, however, had a large dropout rate and therefore should be interpreted with caution.78
INFLAMMATORY BOWEL DISEASE
The pathophysiology of Crohn’s disease is not completely understood despite decades of research (see Chapter 111). An overactive intestinal mucosal immune system driven at least in part by a reaction to normal luminal flora is thought to be involved in the pathogenesis,79 facilitated by failure of the mucosal epithelium to serve as an effective barrier to potential dietary and environmental toxins. Given the chronic and persistent nature of Crohn’s disease, many patients turn to CAM therapies when conventional therapies fail.
Probiotics are often employed by certain subgroups of patients with Crohn’s disease, those having had total proctocolectomy and creation of an ileal pouch-anal anastomosis (IPAA) claiming the greatest benefit from these agents. Pouchitis in these patients occurs with a frequency of approximately 50% after 10 years.80 Although the cause of pouchitis remains unknown, alteration in enteric bacterial flora appears to play an important role.80
VSL #3 is a probiotic agent consisting of four strains of Lactobacillus, three strains of Bifidobacterium, and one strain of Streptococcus. VSL #3 is thought to act by increasing tissue levels of interleukin-10 and decreasing levels of proinflammatory cytokines, such as interleukin-1 and tumor necrosis factor (TNF).81 In RCTs, administration of VSL #3 reduced the frequency of pouchitis in IBD patients after IPAA, and it decreased the number of flares of pouchitis in patients known to have chronic pouchitis.77,82 In another RCT, VSL #3 appeared effective compared with placebo for preventing endoscopic recurrence of IBD.83 No adverse events have been noted with administration of VSL #3 in any of the studies evaluated.
Saccharomyces boulardii is a nonpathogenic yeast originally isolated from the litchi fruit, and it is another probiotic that has been shown to decrease the relapse rate of Crohn’s disease.84 S. boulardii exerts its beneficial effect on the intestine by acting as a trophic agent on intestinal mucosa and triggering the release of immunoglobulin (Ig) A.79 In one study, clinical relapses over a six-month period were observed in 37% of patients who received mesalamine alone and in 6% of patients treated with mesalamine plus S. boulardii.79
Another supplemental therapy employed in treating IBD is fish oil. Fish oil contains high amounts of omega-3 fatty acids, which serve as precursors of less proinflammatory cytokines than do other fatty acids commonly found in many foods. In patients with ulcerative colitis (UC) who have frequent disease exacerbations, disease activity scores were improved to a greater extent in patients who received fish oil than in those who consumed other forms of fat85; however, trials have failed to demonstrate that fish oil is effective in maintaining remission of UC.86 Clinical trials evaluating the efficacy of fish oil in the treatment of Crohn’s disease have been disappointing, because no trial has documented fish oil to be effective in maintaining disease remission or lowering an individual patient’s Crohn’s disease activity index (CDAI).85
Turmeric, traditionally used in Indian and Chinese herbal medicine, is a spice that originates from the root Curcuma longa and is a member of the ginger family. This is another complementary agent that has been reported to be effective in the treatment of IBD. A pure curcumin preparation was administered in an open-label study to patients with ulcerative proctitis or Crohn’s disease. All proctitis patients improved, with reductions in concomitant medications in four patients. Four of five patients with Crohn’s disease had lowered CDAI scores and erythrocyte sedimentation rates. This encouraging study warrants further evaluation of this agent with double-blind, placebo-controlled follow-up studies.87 The mechanism of action of turmeric is multifactorial. First, it protects lipids from peroxidation and thereby prevents the formation of free radical species. In addition, it inhibits lipopolysaccharide-induced nitric oxide synthase (iNOS) gene expression, thereby decreasing TNF-α and IL-1β production, as well as inhibiting nuclear factor (NF)-κB activation, cytokines thought to be integral to the pathophysiology of IBD. Lastly, turmeric is thought to inhibit the synthesis of proinflammatory prostaglandins and leukotrienes through inhibiting arachidonic acid uptake by macrophages.88 In safety and toxicity studies of turmeric, curcumin has been found in most studies to be safe even in high doses. In addition, in animal studies, there has been no evidence of mutagenicity or chromosomal damage.89 Turmeric does exhibit an inhibitory effect on platelet aggregation, and therefore, patients who are maintained on agents such as aspirin, clopidogrel, or ticlopidine should be closely monitored.89
DIARRHEA AND CONSTIPATION
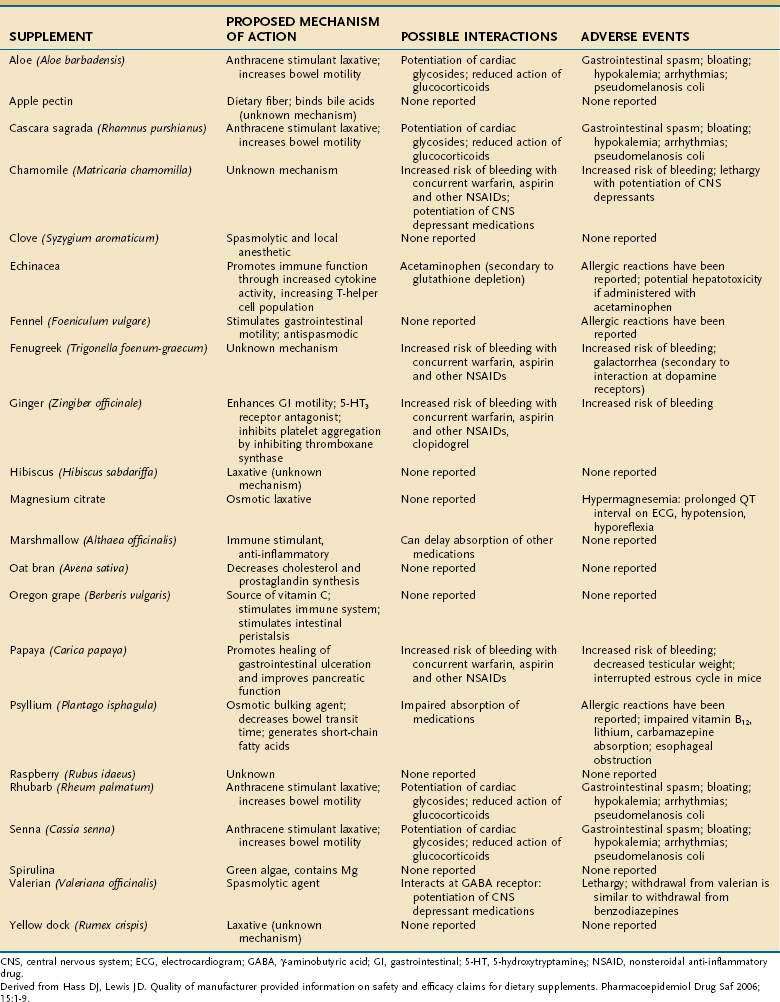
Altered bowel habits often lead to the use of CAM therapies. Within the discipline of CAM, practitioners often group the symptoms of diarrhea and constipation together under the term colonic health. Although several CAM modalities have been reported to promote and improve colonic health, herbal supplements are considered the mainstay of treatment. These supplements range from anthraquinone-based stimulant laxatives, such as aloe (Aloe barbadensis) to osmotic laxatives, such as magnesium citrate, to extracts of papaya (Carica papaya) and raspberry (Rubus udaeus). Those most commonly employed, including their proposed mechanisms of action, possible medication interactions, and reported adverse events, are listed in Table 127-5. As discussed later, because the supplement industry is not regulated, the content and potency of many of these agents are not standardized, which should give practitioners pause before prescribing them.
Probiotics also are employed often to prevent diarrhea. A meta-analysis performed by D’Souza and colleagues described the clinical efficacy of various strains of Lactobacillus (Lactobacillus bulgaricus, Lactobacillus acidophilus, Lactobacillus casei, and Lactobacillus GG) and S. boulardii in the prevention of antibiotic-associated diarrhea.90 Castagliuolo and colleagues described the protective effects of S. boulardii on Clostridium difficile-induced diarrhea in humans; the mechanism of this action is the proteolytic digestion of toxin A and B molecules by a protease secreted by S. boulardii.91 Another study reported that S. boulardii stimulates intestinal IgA in response to infection with C. difficile.92 S. boulardii also has been shown to prevent relapses of pseudomembranous colitis and to maintain intestinal mucosal barrier function against enteropathogenic Escherichia coli.93,94 VSL #3 has been demonstrated in an RCT to be effective in preventing radiation-induced diarrhea.95
Ernst and colleagues have reviewed the various CAM therapies that have been used to treat constipation. Biofeedback, a treatment technique in which people are trained to use signals from their own bodies to help recognize a relaxed state, has demonstrated clear efficacy for the treatment of constipation.96 Pelvic floor dyssynergia, an often-neglected cause of chronic constipation, is a result of the inappropriate contraction or failed relaxation of the puborectalis and external anal sphincter muscles during defecation. Pelvic floor dyssynergia is considered a form of maladaptive learning,97 and biofeedback is thought to help retrain the body to alleviate symptoms.
Sensory training involves simulated defecation through the use of a water-filled balloon that is inserted into the rectum and then slowly withdrawn as the patient is asked to concentrate on relaxing the muscles that are behaving inappropriately.97 Anal manometry and electromyography, which records muscle activity either from intraluminal probes or perianal surface electrodes, are alternative means by which sensory feedback can be provided to the patient with pelvic floor dyssynergia. More than 70% of adult patients with this disorder improve following biofeedback training.97 Further studies are needed, however, to assess the long-term efficacy of biofeedback.
Abdominal massage therapy has shown mixed results in the treatment of constipation. Ernst98 reviewed the data from an RCT for abdominal massage as a treatment for constipation and found that, although some data suggested a significant increase in the number of days with bowel movements, and decrease in the number of episodes of fecal incontinence and number of enemas given, the trials were of poor quality and were methodologically flawed. The trials were not blinded and were subject to observer bias; only one study was randomized. Therefore, further RCTs are needed to determine whether massage is effective in patients with chronic constipation.
Homeopathy has been suggested to have clinical efficacy in the treatment of postoperative ileus. A meta-analysis of studies of patients with ileus after abdominal and gynecologic surgery revealed that homeopathic treatment with agents such as opium poppy (Papaver somniferum L.) and chaparral (Raphanus sativus) significantly reduced the time to normal intestinal peristalsis compared with placebo treatment.99 The underlying principle of homeopathy is “like cures like,” and these supplements, in diluted doses, are thought to ameliorate slowed intestinal transit because they themselves are known to cause constipation. This meta-analysis, however, did not yield definitive conclusions, because several of the trials included were reported in publications that are not peer reviewed, thereby raising suspicion as to the quality of the data.
Lastly, colonic irrigation therapy has gained popularity among patients interested in complementary therapies. “Colonics,” as they are colloquially termed, differ from enemas because they are not self-administered but rather are given by a practitioner who has a certain degree of training, via a device that controls water flow, temperature, and pressure.100 The rationale for this practice comes from the concept of “autointoxication,” a notion popularized by Sir Arbuthnot Lane in the late 1800s and early 1900s that toxins originating in the intestine can enter the circulation and poison the body. There are no large RCTs thoroughly evaluating the efficacy of colonic irrigation therapy. Adverse events of colonic irrigations have been reported, including amebiasis and rectal perforation.101,102
LIVER DISEASE
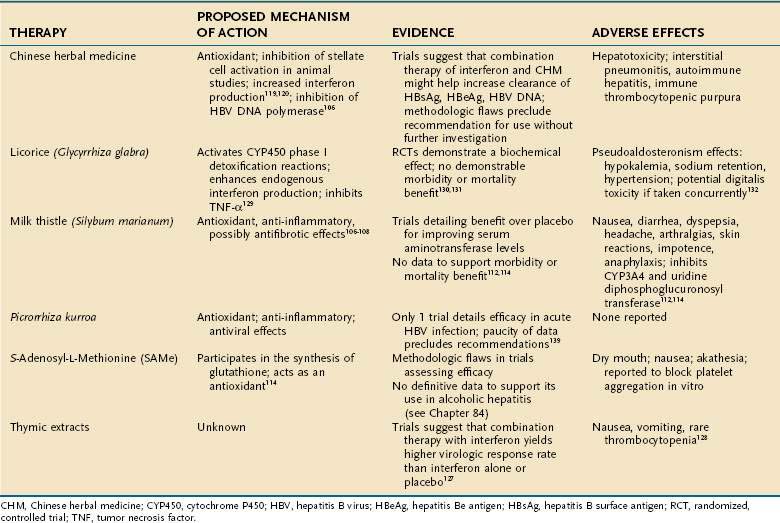
CAM therapies are commonly used to treat conditions such as hepatitis B, hepatitis C, and alcoholic liver disease. One study of U.S. outpatients with chronic liver disease reported that 41% had used some form of CAM therapy in the preceding four weeks.103 As with the other gastrointestinal conditions already discussed, most therapies used for chronic liver disease have been herbal supplements. Table 127-6 details the commonly employed supplements, their mechanisms of action, levels of evidence to support their use, and adverse events.
Silymarin
Milk thistle (Silybum marianum), the CAM compound most commonly used for liver disease, has been employed for many disorders, including alcoholic liver disease, chronic viral hepatitis, and drug-induced hepatitis. Silymarin, the active ingredient, is derived from various parts of the milk thistle plant. Its mechanism of action is not defined fully but appears to be multifaceted. First, it is thought to act as an antioxidant that prevents glutathione depletion.104 Second, it has anti-inflammatory activity and decreases formation of leukotrienes, prostaglandins, and TNF-α.105 Lastly, in several animal studies, silymarin has been shown to block the proliferation of hepatic stellate cells and production of procollagen III, suggesting a role to slow fibrosis in chronic liver disease.106
Silymarin has been evaluated in several trials of alcoholic liver disease. Ferenci and colleagues,107 in an RCT of cirrhotic patients treated with 420 mg of silymarin or placebo, demonstrated an improved four-year survival in the treatment group compared with the placebo-treated group. Patients with alcoholic liver disease and early cirrhosis (Child-Turcotte-Pugh class A) were more likely to benefit than were those with Child-Turcotte-Pugh class B or C. This trial, however, did not confirm a clear benefit of silymarin, because patients were not randomized properly; the placebo group contained patients with more advanced cirrhosis (Child-Turcotte-Pugh class C) than did the treatment group. In addition, the degree of abstinence from alcohol among the study participants was not followed, and the dropout rate was high. A larger, more rigorously defined study by Pares and colleagues108 failed to demonstrate a survival benefit in alcoholic cirrhotic patients treated with 450 mg of daily silymarin compared with a group treated with placebo.
The Hepatitis C Antiviral Long-term Treatment against Cirrhosis (HALT-C) Trial was designed to determine whether maintenance interferon therapy could slow disease progression in patients who had failed to eradicate hepatitis C virus (HCV) during prior interferon treatment (nonresponders). Seeff and colleagues109 examined the use and potential effects of silymarin in the HALT-C patient population. Among all participants, 67% had never used silymarin, 16% used it in the past, and 17% used it at baseline. Silymarin use varied widely with gender and ethnicity; men were more frequent users than women; non-Hispanic whites were more frequent users than African Americans and Hispanics. Silymarin use correlated strongly with higher education. No beneficial effect of silymarin was found on serum alanine aminotransferase or HCV RNA levels. Univariate analysis showed significantly fewer liver-related symptoms and better quality-of-life parameters in users than nonusers.
A systematic review of RCTs of silymarin in various hepatic disease states (hepatitis B, hepatitis C, alcoholic liver disease) also drew no firm conclusions about its therapeutic efficacy. Approximately one half of the trials demonstrated a significant biochemical response to silymarin, specifically a decrease in serum aminotransferase levels; however, this response did not translate into a statistically significant mortality or morbidity benefit. Favorable trends toward a decrease in the frequency of hepatic encephalopathy and gastrointestinal bleeding were suggested by these trials, but a statistically significant difference was not reached between those treated with silymarin and those treated with placebo.110,111
Reddy and colleagues are evaluating the use of silymarin in the SyNCH trial, a randomized, multicenter, double-blind, placebo-controlled trial funded by the National Center for Complementary and Alternative Medicine (NCCAM). This is a phase I/II trial, the objectives of which are to examine the impact of silymarin on noncirrhotic patients who are also nonresponders to traditional antiviral medications for HCV, and to evaluate its impact on patients with nonalcoholic steatohepatitis who have failed to respond to conventional treatment. It is hoped that this study will define the role of silymarin in the treatment of these difficult-to-treat clinical conditions for which conventional medicine has failed to yield therapeutic results.112
The reported adverse effects of silymarin include nausea, diarrhea, dyspepsia, headache, arthralgias, skin reactions, impotence, and anaphylaxis. Most important, milk thistle has been shown to inhibit CYP3A4 and uridine diphosphoglucuronosyl transferase, thereby leading to interactions with traditional prescription medications such as quinine, lidocaine, certain calcium channel–blocking agents, and cyclosporine, all of which are metabolized in part by CYP3A4.110,111
S-Adenosyl-L-Methionine
S-Adenosyl-l-methionine (SAMe) acts as a methyl donor for many biochemical reactions and participates in the synthesis of glutathione, the predominant biochemical antioxidant.113
This compound has been studied best in the treatment of alcoholic liver disease. A systematic review of eight placebo-controlled trials of patients treated for alcoholic liver disease revealed that SAMe had no statistically significant effect on mortality, liver-related mortality, or rate of liver transplantation,111 and the methodologic quality of these trials was poor. Further evaluation of SAMe in more properly designed trials is needed.111
SAMe also has been evaluated in the treatment of cholestasis of pregnancy. In several controlled trials, SAMe reduced pruritus and serum bilirubin levels during pregnancy, thereby suggesting possible efficacy.114 The safety of this agent in pregnancy has been demonstrated in RCTs.115
Chinese Herbal Medicine
Chinese herbal medicine (CHM) is the most common CAM therapy employed for treating HBV, and it is the therapy that has been evaluated most rigorously. HBV is a significant global health problem.116 Given the large number of people affected and high rate of endemic HBV infection in some parts of the world such as Asia, it is not surprising that CAM therapies are often used to treat illness associated with HBV.
Many different herbal combinations have been employed to treat HBV infection. For example, TJ-9, known as xiao-chai-hu-tang in China, is a combination of seven herbs: Bupleurum root, Pinellia tuber, Scutellaria root, jujube fruit, ginger rhizome, ginseng root, and Glycyrrhiza root. This agent is thought to act as an antioxidant as well as an inhibitor of stellate cell fibrosis.117,118 Another example of CHM is Phyllanthus amarus, whose mechanism of action appears to be inhibition of HBV DNA polymerase.119
A systematic review of nine RCTs that evaluated CHM revealed that compared with placebo treatment, the CHM compound fuzheng jiedu tang significantly increased the rate of clearance of hepatitis B surface antigen (HBsAg), hepatitis B e antigen (HBeAg), and HBV DNA. P. amarus and kurorinone were comparable to interferon treatment in clearing these serologic markers.120 The quality of the aforementioned trials was poor, however, and thus no definitive conclusion can be reached at present regarding the efficacy of these agents for chronic HBV infection.
A review of the effects of CHM on asymptomatic HBsAg-positive carriers with normal aminotransferase levels evaluated three RCTs, all of which were of poor methodologic quality. The compound Jianpi Wenshen recipe was found to have beneficial effects on clearance of HBsAg and HBeAg and on seroconversion of HBeAg to antibody to HBeAg.121 Given the flaws in the methodology of the trials evaluated, however, a recommendation for use of this agent cannot be endorsed without further investigation.
A meta-analysis of 27 RCTs compared CHM alone, CHM combined with interferon, and interferon alone for chronic HBV infection.122 The absence of a strict placebo group in these trials is of concern. In China, where most CHM is used for HBV infection, CHM often is used as an adjunct or alternative to interferon therapy. Therefore, these trials were designed to assess the efficacy of CHM in conditions that replicate common clinical practice.118 Patients who received CHM alone were more likely to clear HBsAg than were those treated with interferon alone. CHM was equivalent in efficacy to interferon in achieving clearance of HBeAg and HBV DNA. Patients who received combined therapy were more likely than those treated with interferon alone to achieve seroconversion for HBsAg and HBeAg and to clear HBV DNA.118
Although these trials appear to favor the use of CHM as a potential adjunct therapy to interferon, most of the trials were of poor methodologic quality. In addition, most of the studies had a follow-up of only three months for assessing treatment outcomes. The studies that were reviewed were published in Chinese journals, and many details regarding blinding and randomization of the subjects in the trials were omitted from the publications, raising additional concerns regarding methodologic quality.118
CHM also has been studied for the treatment of HCV infection. A systematic review of 10 randomized trials evaluated the efficacy of CHM in patients with chronic HCV infection. The results of the trials were disappointing in that none of the herbal agents employed was found to increase the rate of HCV RNA clearance. In addition, 9 of the 10 trials showed no improvement in serum aminotransferase levels.123
Adverse effects of CHM include hepatotoxicity; however, given the lack of manufacturing uniformity in content and potency of these agents, definitive causality has not been established.124 Cases of interstitial pneumonitis, autoimmune hepatitis, and acute thrombocytopenic purpura also have been reported.111
Thymic Extract
The efficacy and safety of thymic extract in treating HCV infection has been evaluated in five RCTs. Patients who received thymosin-1, a synthetic polypeptide, in combination with interferon therapy were more likely to have complete virologic response than were those patients treated with interferon alone or with placebo.125 Reported adverse events included nausea and vomiting and one case of thrombocytopenia.126
Licorice (Glycyrrhiza glabra)
Licorice (G. glabra) has been evaluated as a possible CAM therapy for chronic HCV infection. The active component of licorice, glycyrrhizin, is thought to activate cytochrome P-450 phase I detoxification reactions, stimulate endogenous interferon, and inhibit TNF-α.111,127 Several RCTs have evaluated this compound for treating HCV. Suzuki and colleagues128 demonstrated that daily injections of Stronger Neo-Minophagen C, a compound of glycyrrhizin, glycine, and cysteine, decreased serum aminotransferase levels compared with placebo. A morbidity or mortality benefit was not demonstrated. Furthermore, the follow-up period in this trial was only one month, making it extremely difficult to assess any long-term adverse effects of G. glabra. Given that this study was published in 1983, the presence of HCV was not determined in the study population; inclusion criteria merely necessitated histologic evidence of chronic hepatitis. It is not clear, in fact, that the study population had HCV infection.
Another RCT evaluated the effects of G. glabra in patients with chronic HCV infection.129 The efficacy of ursodeoxycholic acid combined with glycyrrhizin was compared with glycyrrhizin alone. There was a statistically significant biochemical improvement in the treatment group, but the trial lacked a placebo arm, and biochemical improvement might not have been the most clinically meaningful parameter to assess.
Adverse events with G. glabra are thought to be secondary to the active metabolite of licorice root, glycyrrhizin, which inhibits 11-β-hydroxysteroid dehydrogenase. This inhibition leads to a pseudoaldosterone effect, resulting in hypokalemia, sodium retention, and hypertension130; hypokalemia can increase the risk of toxicity from some drugs, such as digitalis.
Ayurvedic Medicine
Picrorrhiza kurroa is an Indian herb commonly used in traditional Ayurvedic medicine. It has been used for many gastrointestinal conditions and also is often used for hepatic disease. The active ingredients, picroside and kutkoside,131 are thought to act as antioxidants, anti-inflammatory agents, and inhibitors of proinflammatory cytokines.111 Various studies have described possible cancer chemopreventive and antiviral qualities of these agents.132,133 One trial demonstrated a beneficial biochemical effect of P. kurroa in reducing serum aminotransferase levels in patients with acute HBV infection.134 Clearly, more data are needed before any recommendation can be made regarding this agent.
Liv 52 is an Indian ayurvedic medication that has been marketed specifically for the treatment of liver disease. It is composed of Capparis spinosa (capers), Cichorium intybus (wild chicory), Terminalia arjuna (arjuna), Solanum nigrum (black nightshade), Achillea millefolium (yarrow), and Tamarix gallica (tatarisk).135 This agent has demonstrated efficacy in protecting rats from carbon tetrachloride and alcohol-induced liver injury, as well as improving liver function in patients with acute viral hepatitis. This agent was withdrawn from the U.S. market, however, after an RCT demonstrated decreased survival in patients with alcoholic hepatitis compared with placebo.136
Acupuncture
Acupuncture has been demonstrated in a study by Li and colleagues to have gained popularity in patients with chronic hepatitis. Roughly 9% of patients report implementing this therapy. There are no convincing data detailing the effectiveness of acupuncture in treatment of either chronic hepatitis B or C. Li and colleagues have demonstrated that in patients with pain from hepatocellular carcinoma, acupuncture is useful as an adjunctive therapy for ameliorating postoperative pain.137
GASTROINTESTINAL MALIGNANCIES
Estimates are that up to 64% of adult oncology patients have employed CAM therapies at some point during their treatment.138 The motivation for using these therapies in oncology patients is similar to the rationale cited by other patient populations: CAM therapies are appealing as a result of a failure of conventional medicine to control or cure disease, and they provide patients with a mechanism for feeling in more control of their therapeutic plan. Textbooks have been dedicated to this subject, and the discussion that follows highlights the most commonly employed CAM therapies.
A systematic review of the beneficial effect of green tea consumption in reducing the incidence of gastrointestinal malignancy139 demonstrated that green tea did help prevent colonic adenomatous polyp formation and chronic atrophic gastritis. No definitive supporting evidence, however, was found to conclude that green tea had a similar beneficial effect on the incidence of gastrointestinal malignancy.
Garlic is thought to inhibit the development of gastric cancer through several proposed mechanisms. An antibacterial effect against Helicobacter pylori has been demonstrated and is attributable to the thiosulfinate component of this agent.140 Kaempferol, a flavonol present in high concentration in garlic, also contributes to the detoxification of carcinogenic compounds.141 Published studies suggest that garlic might protect against the development of gastric and colonic carcinomas. Most of the literature, however, consists of observational studies that cannot be used to confirm a therapeutic effect of garlic. Additional therapeutic intervention trials are needed to substantiate the claim that garlic is chemopreventive.142
Vitamins C and E are antioxidants that might reduce the incidence of colorectal cancer. In an epidemiologic study of colorectal cancer patients, long-term use of vitamins C and E did not provide a mortality benefit. In a subgroup analysis, however, use of vitamin C for more than 10 years was associated with a decreased risk of death from colorectal cancer before 65 years of age and a decreased risk of rectal cancer mortality at any age.143
Other dietary factors also could play a role in preventing malignancy. In Mediterranean countries there is a lower incidence of breast, endometrial, colorectal, and prostate cancer compared with Western countries. These cancers have been postulated to have a relationship to diet, in that a low consumption of fruits and vegetables and a high consumption of red meat correlate with cancer incidence. A traditional Mediterranean diet contains low amounts of red meat and high amounts of fruits, vegetables, and olive oil. Some epidemiologists estimate by statistical modeling that up to 25% of colorectal cancer could be prevented in Western countries if diets were changed to reflect Mediterranean practices.144
Several CAM therapies have been implemented to help ameliorate pain in patients with metastatic disease. Acupuncture has shown promise for the treatment of the pain associated with gastric cancer.145 Lycopodium clavatum, a type of fern moss, has been reported to be effective as a homeopathic treatment for rectal cancer pain.146 Meditation and relaxation therapies are practiced commonly by many cancer patients, not only to ameliorate physical pain but also to help cope with the depression that commonly accompanies malignant disease.
SAFETY AND REGULATION OF COMPLEMENTARY AND ALTERNATIVE THERAPIES
With the increasing popularity of CAM therapies, it is important that physicians understand their mechanisms of action as well as the data supporting their efficacy. Although some medical schools and residency programs are increasingly offering education programs in CAM therapies, curriculums should be mandated to include information on these modalities so as to familiarize medical professionals with these practices early in their careers. A study by Mikail and colleagues147 reported that only 16% of medical residents surveyed routinely asked their patients about their use of herbal therapy. It is equally important, however, that health care professionals also understand the regulatory mechanisms, or lack thereof, that are in place for these modalities, so that effective safety measures can be employed to protect the welfare of patients.
Total yearly sales of herbal supplements are approximately $13.9 billion and steadily increasing in the United States. An estimated 15 million adults take prescription medications concurrently with herbal supplements.1 Therefore, the safety of concurrent administration of herbal supplements and traditional allopathic medications is a concern to many physicians. In 1994, the U.S. Congress implemented the Dietary Supplement Health and Education Act (DSHEA). This legislation was developed to prevent the U.S. Food and Drug Administration (FDA) from regulating dietary supplements “excessively” and to ensure that safe and appropriately labeled supplements remain available to those persons who wish to use them.
DSHEA officially defines a “dietary supplement” as
A product (other than tobacco) that is intended to supplement the diet that bears or contains one or more of the following dietary ingredients: a vitamin, a mineral, an herb or other botanical, an amino acid, a dietary substance for use by man to supplement the diet by increasing total daily intake, or a concentrate, metabolite, constituent, extract or combination of these ingredients.148
Additionally, supplement manufacturers are not required to report adverse events that occur with use of their products. It is the responsibility of the FDA to prove that products are unsafe before their use can be restricted.148 The FDA relies on physicians and other health care professionals to report suspected adverse events for an inquiry to be established for a particular agent. Therefore, it is of utmost importance that all health care professionals be aware of their patients’ use of supplements both to provide safe care and to know when to suspect adverse effects or medication interactions. Suspected adverse events or medication interactions can be reported online at http://www.fda.gov/medwatch.
Brandt LJ, Bjorkman D, Fennerty MB, et al. Systematic review on the management of irritable bowel syndrome in North America. Am J Gastroenterol. 2002;97(Suppl 11):S7. (Ref 53.)
Brenner DM, Moeller M, Chey WD. Schoenfeld P. The utility of probiotics in the treatment of irritable bowel syndrome: a systematic review. Am J Gastroenterol. 2007;104:1033-49. (Ref 73.)
Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of turmeric (curcuma longa). J Alt Comp Med. 2003;9:161-8. (Ref 89.)
Coon JT, Ernst E. Systematic review: herbal medicinal products for non-ulcer dyspepsia. Aliment Pharmacol Ther. 2002;16:1689-99. (Ref 34.)
Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990-1997. JAMA. 1998;280:1569-75. (Ref 1.)
Ernst E, Pittler MH. Efficacy of ginger for nausea and vomiting: a systematic review of randomized clinical trials. Br J Anaesth. 2000;84:367-71. (Ref 10.)
Forster HB, Niklas H, Lutz S. Antispasmodic effects of some medicinal plants. Planta Med. 1980;40:309-19. (Ref 44.)
Holt PR, Katz S, Kirshoff R. Curcumin therapy in inflammatory bowel disease: a pilot study. Dig Dis Sci. 2005;50:2191-3. (Ref 87.)
Koretz RL, Rotblatt M. Complementary and alternative medicine in gastroenterology: the good, the bad, and the ugly. Clin Gastroenterol Hepatol. 2004;2:957-67. (Ref 7.)
Levy C, Seeff LD, Lindor KD. Use of herbal supplements for chronic liver disease. Clin Gastroenterol Hepatol. 2004;2:947-56. (Ref 111.)
Madisch A, Holtmann G, Plein K, Hotz J. Treatment of irritable bowel syndrome with herbal preparations: results of a double-blind, randomized, placebo-controlled, multi-center trial. Aliment Pharmacol Ther. 2004;19:271-9. (Ref 64.)
Melzer J, Rosch W, Reichling J, et al. Meta-analysis:phytotherapy of functional dyspepsia with the herbal drug preparation STW 5 (Iberogast). Aliment Pharmacol Ther. 2004;20:1279-87. (Ref 50.)
Seeff LB, Curto TM, Szabo G, et al. Herbal product use by persons enrolled in the Hepatitis C Anti-viral Long term Treatment against Cirrhosis (HALT-C) trial. Hepatol. 2008;47:605-12. (Ref 109.)
Verma S, Thuluvath PJ. Complementary and alternative medicine in hepatology: review of the evidence of efficacy. Clin Gastro Hep. 2007;5:408-16. (Ref 135.)
Whorwell PJ, Prior A, Colgan SM. Hypnotherapy in severe irritable bowel syndrome: further experience. Gut. 1987;28:423-25. (Ref 74.)
1. Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990-1997. JAMA. 1998;280:1569-75.
2. Ganguli SC, Cawdron R, Irvine EJ. Alternative medicine use by Canadian ambulatory gastroenterology patients: secular trend or epidemic? Am J Gastroenterol. 2004;99:319-26.
3. Zollman C, Vickers AJ. What is complementary medicine? In: Zollman C, Vickers AJ, Richardson J, editors. ABC of complementary medicine. New York: BMJ Books; 2000:1-20.
4. Hollyer T, Boon H, Georgousis A, et al. The use of CAM by women suffering from nausea and vomiting during pregnancy. BMC Complement Altern Med. 2002;2:5.
5. Yamahara J, Huang QR, Li Y, et al. Gastrointestinal motility enhancing effect of ginger and its active constituents. Chem Pharm Bull (Tokyo). 1990;38:430-1.
6. Huang Q, Iwamoto M, Aoki S, et al. Anti–5-hydroxytryptamine-3 effect of galanolactone, diterpenoid isolated from ginger. Chem Pharm Bull (Tokyo). 1991;39:397.
7. Koretz RL, Rotblatt M. Complementary and alternative medicine in gastroenterology: the good, the bad, and the ugly. Clin Gastroenterol Hepatol. 2004;2:957-67.
8. Stewart JJ, Wood MJ, Wood CD, Mims ME. Effects of ginger on motion sickness susceptibility and gastric function. Pharmacology. 1991;42:111-20.
9. Grøntved A, Hentzer E. Vertigo-reducing effect of ginger root: a controlled clinical study. Otorhinolaryngol Relat Spec. 1986;48:282-6.
10. Ernst E, Pittler MH. Efficacy of ginger for nausea and vomiting: a systematic review of randomized clinical trials. Br J Anaesth. 2000;84:367-71.
11. Srivastava KC. Aqueous extract of onion, garlic, and ginger inhibit platelet aggregation and alter arachidonic acid metabolism. Biomed Biochim Acta. 1984;43:335-46.
12. Abraham S, Abraham SK, Radhamony G. Mutagenic potential of the condiments, ginger and turmeric. Cytologia. 1976;41:591-5.
13. Soudamini KK, Unnikrishnan MC, Sukumaran K, Kuttan R. Mutagenicity and anti-mutagenicity of selected spices. Indian J Physiol Pharmacol. 1995;39:347-53.
14. Sahakian V, Rouse D, Sipes S, et al. Vitamin B6 is effective therapy for nausea and vomiting of pregnancy. Obstet Gynecol. 1991;78:33.
15. Vutyavanich T, Wongtrangan S, Ruangsri R. Pyridoxine for nausea and vomiting of pregnancy: a randomized double-blind placebo-controlled trial. Am J Obstet Gynecol. 1995;173:881.
16. Vitamin B6: Pyridoxine. In http://ods.od.nih.gov/factsheets/vitaminb6.asp 1978-present
17. Leklem JE. Vitamin B6. In: Machlin LJ, editor. Handbook of vitamins. 2nd ed. New York: Marcel Dekker; 1991:341-378.
18. Schaumburg H, Kaplan J, Windebank A, et al. Sensory neuropathy from pyridoxine abuse: a new megavitamin syndrome. N Engl J Med. 1983;309:445-8.
19. Vickers AJ. Can acupuncture have specific effects on health? A systematic review of acupuncture antiemesis trials. J R Soc Med. 1996;89:303-11.
20. Dundee JW, Ghaly RG, Fitzpatrick KT, et al. Acupuncture to prevent cisplatin-associated vomiting. Lancet. 1987;1:1083.
21. Fry ENS. Acupressure and postoperative vomiting. Anaesthesia. 1986;41:661-2.
22. Ho RT, Jawan B, Fung ST, et al. Electro-acupuncture and post-operative emesis. Anaesthesia. 1990;45:327-9.
23. de Aloysio D, Penacchioni O. Morning sickness control in early pregnancy by Neiguan point acupressure. Obstet Gynecol. 1992;80:852-4.
24. Ernst E, White A. Life-threatening adverse reactions after acupuncture? A systematic review. Pain. 1997;71:123-6.
25. Rampes H, James R. Complications of acupuncture. Acupunct Med. 1995;13:26-33.
26. Kiyosawa K, Tanaka E, Sodeyama T. Transmission of hepatitis C in an isolated area in Japan. Gastroenterology. 1994;106:1596-602.
27. Vittecoq D, Mettetal JF, Rouzioux C, et al. Acute HIV infection after acupuncture treatments. N Engl J Med. 1989;320:250-1.
28. Scheel O, Sundsfjord A, Lunde P, Andersen BM. Endocarditis after acupuncture and injection treatment by a natural healer. JAMA. 1992;267:56.
29. Izatt E, Fairman M. Staphylococcal septicaemia with DIC associated with acupuncture. Postgrad Med J. 1977;53:285-6.
30. Pierik MG. Fatal staphylococcal septicemia following acupuncture: report of two cases. RI Med J. 1982;82:251.
31. Ernst E, Resch KL, White AR. The risks of acupuncture. Int J Risk Saf Med. 1995;6:179.
32. Hasegawa J, Noguchi N, Yamasaki J, et al. Delayed cardiac tamponade and hemothorax induced by an acupuncture needle. Cardiology. 1991;78:58-63.
33. Freeman LW. Mind-body interventions. In: Freeman LW, Lawlis GF, editors. Mosby’s complementary and alternative medicine, a research-based approach. St Louis: Mosby; 2001:146.
34. Coon JT, Ernst E. Systematic review: herbal medicinal products for non-ulcer dyspepsia. Aliment Pharmacol Ther. 2002;16:1689-99.
35. Best R, Lewis DA, Nasser N. The antiulcerogenic activity of unripe plantain banana (Musa species). Br J Pharmacol. 1984;82:107-16.
36. Arora A, Sharma MP. Use of banana in non-ulcer dyspepsia. Lancet. 1990;335:612-13.
37. Bortolotti M, Coccia G, Grossi G, Miglioli M. The treatment of functional dyspepsia with red pepper. Aliment Pharmacol Ther. 2002;16:1075-82.
38. Ritter R, Schatton WFH, Gessner B, et al. Clinical trial on standardized celandine extract in patients with functional epigastric complaints: results of a placebo-controlled double-blind trial. Complement Ther Med. 1993;1:189.
39. Brinker F. Botanical review: Chelidonium majus. J Naturopath Med. 1992;3:93-4.
40. Benninger J, Schneider HT, Schuppan D, et al. Acute hepatitis induced by greater Celandine (Chelidonium majus). Gastroenterology. 1999;117:1234-7.
41. Tatsuta M, Iishi H. Effect of treatment with liu-jun-zi-tang (TJ-43) on gastric emptying and gastrointestinal symptoms in dyspeptic patients. Aliment Pharmacol Ther. 1993;7:459-62.
42. Hayakawa T, Arakawa K, Kase Y, et al. Liu-jun-zi-tang, a kampo medicine, promotes adaptive relaxation in isolated guinea pig stomachs. Drugs Exp Clin Res. 1999;25:211-18.
43. Hillis JM, Aaronson PI. The mechanism of action of peppermint oil on gastrointestinal smooth muscle: An analysis using patch clamp electrophysiology and isolated tissue pharmacology in rabbit and guinea pig. Gastroenterology. 1991;101:55.
44. Forster HB, Niklas H, Lutz S. Antispasmodic effects of some medicinal plants. Planta Med. 1980;40:309-19.
45. May B, Kohler S, Schneider B. Efficacy and tolerability of a fixed combination of peppermint oil and caraway oil in patients suffering from functional dyspepsia. Aliment Pharmacol Ther. 2000;14:1671.
46. Wilkinson SM, Beck MH. Allergic contact dermatitis from menthol in peppermint. Contact Dermatitis. 1994;30:42-3.
47. Spurlock BW, Dailey TM. Shortness of (fresh) breath—toothpaste-induced bronchospasm. N Engl J Med. 1990;323:1845-6.
48. Thomas JG. Peppermint fibrillation. Lancet. 1962;1:222.
49. Chang HM, But PPH. Pharmacology and applications of Chinese Materia Medica. Singapore: World Scientific; 1987.
50. Melzer J, Rosch W, Reichling J, et al. Meta-analysis: phytotherapy of functional dyspepsia with the herbal drug preparation STW 5 (Iberogast). Aliment Pharmacol Ther. 2004;20:1279-87.
51. von Arnim U, Peitz U, Vinson B, et al. STW 5, a phytopharmacon for patients with functional dyspepsia: results of a multicenter, placebo-controlled double-blind study. Am J Gastroenterol. 2007;102(6):1268-75.
52. Pilichiewicz AN, Horowitz M, Russo A, et al. Effects of Iberogast on proximal gastric volume, antropyloroduodenal motility and gastric emptying in healthy men. Am J Gastroenterol. 2007;102:1-8.
53. Brandt LJ, Bjorkman D, Fennerty MB, et al. Systematic review on the management of irritable bowel syndrome in North America. Am J Gastroenterol. 2002;97(Suppl 11):S7-26.
54. Cheskin LJ, Kamal N, Crowell MD, et al. Mechanisms of constipation in older persons and effects of fiber compared with placebo. J Am Geriatr Soc. 1995;43:666.
55. Fenn GC, Wilkinson PD, Lee CE, et al. A general practice study of the efficacy of Reglan in functional constipation. Br J Clin Pract. 1986;40:192.
56. Ashraf W, Park F, Lof J, et al. Effects of psyllium therapy on stool characteristics, colon transit and anorectal function in chronic idiopathic constipation. Aliment Pharmacol Ther. 1995;9:639-47.
57. Dettmar PW, Sykes J. A multi-center, general practice comparison of ispaghula husk with lactulose and other laxatives in the treatment of simple constipation. Curr Med Res Opin. 1998;14:227.
58. Rouse M, Chapman N, Mahapatra M, et al. An open, randomized, parallel group study of lactulose versus ispaghula in the treatment of chronic constipation in adults. Br J Clin Pract. 1991;45:28.
59. Vaswani SK, Hamilton RG, Valentine MD, Adkinson NFJr. Psyllium laxative-induced anaphylaxis. Allergy. 1996;51:266.
60. Perlman BB. Interaction between lithium salts and ispaghula husk. Lancet. 1990;335:416.
61. Skidmore-Rose L. Mosby’s Handbook of Herbs and Natural Supplements. St. Louis: Mosby; 2001.
62. Noble JA, Grannis FW. Acute esophageal obstruction by a psyllium based laxative [Letter]. Chest. 1984;86:800.
63. Pittler MH, Ernst E. Peppermint oil for irritable bowel syndrome: A critical review and meta-analysis. Am J Gastroenterol. 1998;93:1131-5.
64. Madisch A, Holtmann G, Plein K, Hotz J. Treatment of irritable bowel syndrome with herbal preparations: results of a double-blind, randomized, placebo-controlled, multi-center trial. Aliment Pharmacol Ther. 2004;19:271.
65. Gruenwald J, editor. PDR for herbal medicines. Montvale, NJ: Medical Economics, 2000.
66. O’Hara MA, Kiefer D, Farrell K, Kemper K. A review of 12 commonly used medicinal herbs. Arch Fam Med. 1997;7:532.
67. Venkataramanan R, Ramachandran V, Komoroski BJ, et al. Milk thistle, an herbal supplement, decreases the activity of CYP3A4 and uridine diphosphoglucuronosyl transferase in human hepatocyte cultures. Drug Metab Dispos. 2000;28:1270.
68. Bensoussan A, Talley NJ, Hing M, et al. Treatment of irritable bowel syndrome with Chinese herbal medicine: a randomized controlled trial. JAMA. 1998;280:1585-9.
69. Schaafsma G. State of the art concerning probiotic strains in milk products. IDF Nutr Newslett. 1996;5:23.
70. Nobaek S, Johansson M-L, Molin G, et al. Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Am J Gastroenterol. 2000;95:1231.
71. Kim AS, Floch MH. Probiotics in clinical trials from 2004-2007: a review of current literature. (Abstract W1389). Presented at Digestive Disease Week, San Diego, Calif, May 17-22, 2008.
72. Agrawal A, Houghton LA, Morris J, Guyonnet D, et al. Fermented milk containing the probiotic Bifidobacterium animalis, DN-173010 (FM) improves abdominal distention, bloating, and transit in irritable bowel syndrome with constipation (IBS-C) (Abstract T1395). Presented at Digestive Disease Week, San Diego, Calif, May 17-22, 2008.
73. Brenner DM, Moeller M, Chey WD, Schoenfeld P. The utility of probiotics in the treatment of irritable bowel syndrome: a systematic review. Am J Gastroenterol. 2007;104:1033-49.
74. Whorwell PJ, Prior A, Colgan SM. Hypnotherapy in severe irritable bowel syndrome: further experience. Gut. 1987;28:423-5.
75. Vlieger AM, Menko-Frankenhuis C, Wolfkamp SC, et al. Hypnotherapy for children with functional abdominal pain or irritable bowel syndrome: a randomized controlled trial. Gastroenterology. 2007;133:1430-6.
76. Fireman Z, Segal A, Kopelman Y, et al. Acupuncture treatment for irritable bowel syndrome. Digestion. 2001;64:100.
77. Bolin T, Yiu T. Resistant constipation: a role for acupuncture? Aust N Z J Med. 1983;13:102.
78. Yadav SK, Jain AK, Tripathi SN, Gupta JP. Irritable bowel syndrome: Therapeutic evaluation of indigenous drugs. Indian J Med Res. 1989;90:496-503.
79. Podolsky DL. Inflammatory bowel disease. N Engl J Med. 2002;347:417-29.
80. Gionchetti P, Rizzelo F, Venturi A, et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:305.
81. Ulisse S, Gionchetti P, D’Alo D, et al. Expression of cytokines, inducible nitric oxide synthetase and matrix metalloproteinases in pouchitis: Effects of probiotic treatment. Am J Gastroenterol. 2001;96:2691.
82. Gionchetti P, Rizzelo F, Helwig U, et al. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124:1202.
83. Madsen K, Backer JL, Leddin D, et al. A randomized controlled trial of VSL#3 for the prevention of endoscopic recurrence following surgery for Crohn’s disease. Abstract 1207. Presented at Digestive Disease Week, San Diego, Calif, May 17-22, 2008.
84. Guslandi M, Mezzi G, Sorghi M, Testoni PA. Saccharomyces boulardii in maintenance treatment of Crohn’s disease. Dig Dis Sci. 2000;45:1462-4.
85. Koretz RL. Immunonutrition: can you be what you eat? Curr Opin Gastroenterol. 2003;19:134-9.
86. Hawthorne AB, Daneshmend TK, Hawkey CJ, et al. Treatment of ulcerative colitis with fish oil supplementation: a prospective 12-month randomized, controlled trial. Gut. 1992;33:922-8.
87. Holt PR, Katz S, Kirshoff R. Curcumin therapy in inflammatory bowel disease: a pilot study. Dig Dis Sci. 2005;50(11):2191-3.
88. Kaplan M, Mutlu EA, Benson M, et al. Use of herbal preparations in the treatment of oxidant-mediated inflammatory disorders. Comp Ther Med. 2007;15:207-16.
89. Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of turmeric (Curcuma longa). J Alt Comp Med. 2003;9(1):161-8.
90. D’Souza AL, Rajkumar C, Cooke J, Bulpitt CJ. Probiotics in prevention of antibiotic associated diarrhoea: Meta-analysis. BMJ. 2002;324:1361-4.
91. Castagliuolo I, Riegler MF, Valenick L, et al. Saccharomyces boulardii protease inhibits the effects of Clostridium difficile toxins A and B in human colonic mucosa. Infect Immunol. 1999;67:302-7.
92. Qamar A, Aboudola S, Warny M, et al. Saccharomyces boulardii stimulates intestinal immunoglobulin A immune response to Clostridium difficile toxin A in mice. Infect Immunol. 2001;69:2762-5.
93. McFarland LV, Surawicz CM, Greenberg RN, et al. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA. 1994;271:1913-18.
94. Czerucka D, Dahan S, Mograbi B, et al. Saccharomyces boulardii preserves the barrier function and modulates the signal transduction pathway induced in enteropathogenic Escherichia coli–infected T84 cells. Infection Immunol. 2000;68:5998.
95. Delia P, Sansotta G, Donato V, et al. Prevention of radiation-induced diarrhea with the use of VSL #3, a new high-potency probiotic preparation [Letter]. Am J Gastroenterol. 2002;97:2150.
96. Ernst E, Pittler MH, Stevinson C, White AR. The desktop guide to complementary and alternative medicine. Edinburgh: Mosby; 2001.
97. Bassotti G, Chistolini F, Sietchiping-Nzepa F, et al. Biofeedback for pelvic floor dysfunction in constipation. BMJ. 2004;328:393-6.
98. Ernst E. Abdominal massage for chronic constipation: a systematic review of controlled clinical trials. Forsch Komplementärmed. 1999;6:149.
99. Barnes J, Resch KL, Ernst E. Homeopathy for postoperative ileus: a meta-analysis. J Clin Gastroenterol. 1997;25:628-33.
100. Richards DG, McMillin DL, Mein EA, Nelson CD. Colonic irrigations: a review of the historical controversy and the potential for adverse effects. J Alt Comp Med. 2006;12(4):389-93.
101. Istre GR, Kreiss K, Hopkins RS. An outbreak of amebiasis spread by colonic irrigation at a chiropractic clinic. N Engl J Med. 1982;307:339-42.
102. Tan MP, Cheong DM. Life threatening perineal gangrene from rectal perforation following colonic hydrotherapy:a case report. Ann Acad Med Singapore. 1999;28:583-5.
103. Seeff LB, Lindsay KL, Bacon BR, et al. Complementary and alternative medicine in chronic liver disease. Hepatology. 2001;34:596-603.
104. Mira L, Silva M, Manso CF. Scavenging of reactive oxygen species by silibinin dihemisuccinate. Biochem Pharmacol. 1994;48:753-9.
105. Carini R, Comoglio A, Albano E, Poli G. Lipid peroxidation and irreversible damage in the rat hepatocyte model: Protection by the silybin-phospholipid complex IdB 1016. Biochem Pharmacol. 1992;43:2111-15.
106. Boigk G, Stroedter L, Herbst H, et al. Silymarin retards collagen accumulation in early and advanced biliary fibrosis secondary to complete bile duct obliteration in rats. Hepatology. 1997;26:643-9.
107. Ferenci P, Dragosics B, Dittrich H, et al. Randomized controlled trial of silymarin treatment in patients with cirrhosis of the liver. J Hepatol. 1989;9:105.
108. Pares A, Planas R, Torres M, et al. Effects of silymarin in alcoholic patients with cirrhosis of the liver: results of a controlled, double-blind, randomized and multicenter trial. J Hepatol. 1998;28:615.
109. Seeff LB, Curto TM, Szabo G, et al. Herbal product use by persons enrolled in the Hepatitis C Anti-viral Long Term Treatment against Cirrhosis (HALT-C) trial. Hepatol. 2008;47(2):605-12.
110. Mulrow C, Lawrence V, Jacobs B, et al. Milk thistle: effects on liver disease and cirrhosis and clinical adverse effects. Evidence report/technology assessment no. 21. AHRQ publication No. 01-E025. Rockville, Md: Agency for Healthcare Research and Quality; 2000.
111. Levy C, Seeff LD, Lindor KD. Use of herbal supplements for chronic liver disease. Clin Gastroenterol Hepatol. 2004;2:947-56.
112. Reddy KR. Silymarin for the treatment of chronic liver disease. Gastroenterol Hepatol. 2007;3(11):825-6.
113. Rambaldi A, Gluud C. S-adenosyl-l-methionine for alcoholic liver diseasesCochrane Database Syst Rev. 2006; (2):CD002235.
114. Hardy M, Coulter I, Morton SC, et al. S-Adenosyl-l-methionine for treatment of depression, osteoarthritis, and liver disease. Evidence report/technology assessment no. 64, AHRQ Publication No. 02-E034. Rockville, Md: Agency for Healthcare Research and Quality; 2002.
115. Ribalta J. S-adenosyl-l-methionine in the treatment of patients with intrahepatic cholestasis of pregnancy: a randomized, double-blind, placebo-controlled study with negative results. Hepatology. 1991;13:1084-9.
116. Merican I, Guan R, Amarapuka D, et al. Chronic hepatitis B virus infection in Asian countries. J Gastroenterol Hepatol. 2000;15:1356-61.
117. Sakaida I, Matsumura Y, Akiyama S, et al. Herbal medicine sho-saiko-to (TJ-9) prevents liver fibrosis and enzyme-altered lesions in rat liver cirrhosis induced by a choline deficient l-amino acid–defined diet. J Hepatol. 1998;28:298.
118. Kakumu S, Yoshioka K, Wakita T, et al. Effects of TJ-9 sho-saiko-to (kampo medicine) on interferon gamma and antibody production specific for hepatitis B virus antigen in patients with type B chronic hepatitis. Int J Immunopharmacol. 1991;13:141.
119. Blumberg BS, Millman I, Venkateswaran PS, Thyagarajan SP. Hepatitis B virus and primary hepatocellular carcinoma: treatment of HBV carriers with Phyllanthus amarus. Vaccine. 1990;8(Suppl):S86.
120. Liu JP, McIntosh H, Lin H. Chinese medicinal herbs for chronic hepatitis B. Cochrane Database Syst Rev. 2001; (1):CD001940.
121. Liu JP, McIntosh H, Lin H. Chinese medicinal herbs for asymptomatic carriers of hepatitis B virus infection. Cochrane Database Syst Rev 2001; (2):CD002231.
122. McCulloch M, Broffman M, Gao J, Colford JMJr. Chinese herbal medicine and interferon in the treatment of chronic hepatitis B: a meta-analysis of randomized, controlled trials. Am J Public Health. 2002;92:1619-27.
123. Liu JP, Manheimer E, Tsutani K, Gluud C. Medicinal herbs for hepatitis C virus infection. Cochrane Database Syst Rev 2001; (4):CD003183.
124. Stedman C. Herbal hepatotoxicity. Semin Liver Dis. 2002;22:195.
125. Thompson Coon J, Ernst E. Complementary and alternative therapies in the treatment of chronic hepatitis C: a systematic review. J Hepatol. 2004;40:491-500.
126. Raymond RS, Fallon MB, Abrams GA. Oral thymic extract for chronic hepatitis C in patients previously treated with interferon: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998;129:797-800.
127. Abe N, Ebina T, Ishida N. Interferon induction by glycyrrhizin and glycyrrhetinic acid in mice. Microbiol Immunol. 1982;26:535-9.
128. Suzuki H, Ohta Y, Takino T, et al. Effects of glycyrrhizin on biochemical tests in patients with chronic hepatitis. Asian Med J. 1983;26:423-8.
129. Tsubota A, Kumada H, Arase Y, et al. Combined ursodeoxycholic acid and glycyrrhizin therapy for chronic hepatitis C virus infection: a randomized controlled trial in 170 patients. Eur J Gastroenterol Hepatol. 1999;11:1077-83.
130. Conn JW, Rovner DR, Cohen EL. Licorice-induced pseudoaldosteronism. JAMA. 1968;205:492-6.
131. Luper S. A review of plants used in the treatment of liver disease: I. Altern Med Rev. 1998;3:410-21.
132. Rajeshkumar NV, Kuttan R. Protective effect of Picroliv, the active constituent of Picrrrhiza kurroa, against chemical carcinogenesis in mice. Teratog Carcinog Mutagen. 2001;21:303.
133. Mehotra R, Rawat S, Kulshreshtha DK, et al. In vitro studies on the effect of certain natural products against hepatitis B virus. Indian J Med Res. 1990;92:133-8.
134. Vaidya AB, Antarkar DS, Doshi JC, et al. Picrorhiza kurroa (Kutaki) Royle ex Benth as a hepatoprotective agent: experimental and clinical studies. J Postgrad Med. 1996;42:105-8.
135. Verma S, Thuluvath PJ. Complementary and alternative medicine in hepatology: review of the evidence of efficacy. Clin Gastro Hep. 2007;5:408-16.
136. Fleig WW, Morgan MY, Holzer MA, European multicenter study group. The Ayurvedic drug LIV.52 in patients with alcoholic cirrhosis. Results of a prospective, randomized, double-blind, placebo controlled clinical trial [abstract]. and a. J Hepatol, 26(Suppl 1),1997:127
137. Li QS, Cao SH, Xie GM, et al. Combined traditional Chinese medicine and western medicine. Relieving effects of Chinese herbs, ear-acupuncture and epidural morphine on post-operative pain in liver cancer. Chin Med J. 1994;107:289-94.
138. Barraclough J, editor. Integrated cancer care: holistic, complementary, and creative approaches. New York: Oxford University Press, 2001.
139. Borrelli F, Capasso R, Russo A, Ernst E. Systematic review: green tea and gastrointestinal cancer risk. Aliment Pharmacol Ther. 2004;19:467.
140. Jonkers D, van den Broek E, van Dooren I, et al. Antibacterial effect of garlic and omeprazole on Helicobacter pylori. J Antimicrob Chemther. 1999;43:837-9.
141. Hertog MGL, Hollman CH, Katan MB. Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in the Netherlands. J Agric Food Chem. 1992;40:2379.
142. Fleischauer AT, Arab L. Garlic and cancer: a critical review of the epidemiologic literature. J Nutr. 2001;131(3 Suppl):1032S.
143. Jacobs EJ, Connell CJ, Patel AV, et al. Vitamin C and vitamin E supplement use and colorectal cancer mortality in a large American Cancer Society cohort. Cancer Epidemiol Bio-markers Prev. 2001;10:17-23.
144. Trichopoulou A, Lagiou P, Kuper H, Trichopoulos D. Cancer and Mediterranean dietary traditions. Cancer Epidemiol Biomarkers Prev. 2000;9:869.
145. Dang W, Yang J. Clinical study on acupuncture treatment of stomach carcinoma pain. J Trad Chin Med. 1998;18:31-8.
146. Rajendran ES. Homeopathy as a supportive therapy in cancer. Homeopathy. 2004;93:99-102.
147. Mikail CN, Hearney E, Nemesure B. Increasing physician awareness of the common uses and contradictions of herbal medicines: utility of a case-based tutorial for residents. J Alt Comp Med. 2003;9(4):571-6.
148. Lewis JD, Strom BL. Balancing safety of dietary supplements with the free market. Ann Intern Med. 2002;136:616.