Chapter 74 Compartment Syndromes and Volkmann Contracture
Anatomy
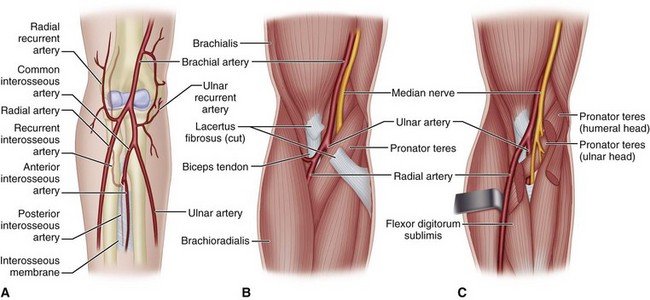
Four interconnected compartments of the forearm are recognized (Fig. 74-1): (1) the superficial volar compartment, (2) the deep volar compartment, (3) the dorsal compartment, (4) and the compartment containing the mobile wad of Henry (brachioradialis and extensor carpi radialis longus and brevis). The volar compartments are most commonly involved, but the dorsal and mobile wad compartments can be involved alone or in addition to the volar compartments. Clinically differentiating isolated or combined involvement of the deep and superficial volar compartments usually is difficult; however, the deep volar compartment (flexor digitorum profundus, flexor pollicis longus, and pronator quadratus) may be solely involved.
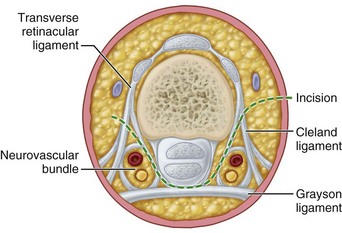
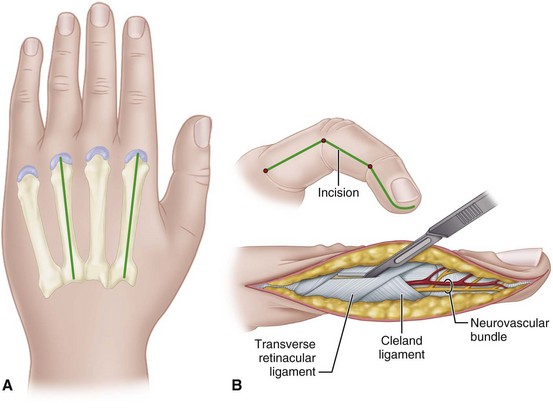
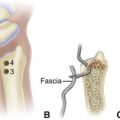
In the hand, each interosseous muscle is surrounded by a tough investing fascial layer, each making an individual compartment as shown by the injection dissections of Halpern and Mochizuki. The adductor pollicis muscle and the thenar and hypothenar muscles form three separate compartments (Fig. 74-2). The neurovascular bundles of each digit also are compartmentalized by fascial layers, making them vulnerable to excessive swelling (Fig. 74-3).
Etiology
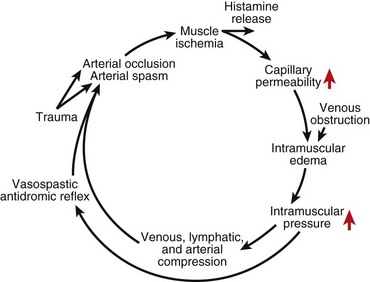
Any situation that causes a decrease in compartment size or increase in compartment pressure can initiate compartment syndrome. As the intracompartmental pressure increases, capillary blood perfusion is reduced to a level that cannot maintain tissue viability. The increase in interstitial pressure overcomes the intravascular pressure of the small vessels and capillaries, causing the walls to collapse and impeding local blood flow. In a canine model, muscle necrosis was shown to occur with a rise in pressure to within 20 mm below diastolic pressure. The local tissue ischemia leads to local edema, which increases intracompartmental pressure. This cycle of increasing muscle ischemia was depicted by Eaton and Green as shown in Figure 74-4.
Diagnosis
Measuring Compartment Pressures in the Forearm and Hand Using a Hand-Held Monitoring Device
Measuring Forearm Compartment Pressure
 Place the compartment to be measured at heart level.
Place the compartment to be measured at heart level.
 Use adequate local analgesia infiltrated into the skin only and avoiding underlying muscle and fascia to control discomfort and pressure spikes.
Use adequate local analgesia infiltrated into the skin only and avoiding underlying muscle and fascia to control discomfort and pressure spikes.
 To measure volar compartment pressure, insert the needle just ulnar to the palmaris longus, to a depth of 1 to 2 cm. Confirm proper needle depth by observing a rise in pressure during external compression of the volar forearm or passive extension of fingers.
To measure volar compartment pressure, insert the needle just ulnar to the palmaris longus, to a depth of 1 to 2 cm. Confirm proper needle depth by observing a rise in pressure during external compression of the volar forearm or passive extension of fingers.
 To measure the dorsal compartment, insert the needle just radial to the border of the ulna to a depth of 1 to 2 cm. Confirm placement by external compression of the dorsal compartment with passive flexion of the wrist.
To measure the dorsal compartment, insert the needle just radial to the border of the ulna to a depth of 1 to 2 cm. Confirm placement by external compression of the dorsal compartment with passive flexion of the wrist.
 To test the mobile wad, identify the radial most portion of the forearm and insert the needle perpendicular to the skin to a depth of 1 to 1.5 cm. A rise in pressure is identified by external pressure or passive flexion of the wrist.
To test the mobile wad, identify the radial most portion of the forearm and insert the needle perpendicular to the skin to a depth of 1 to 1.5 cm. A rise in pressure is identified by external pressure or passive flexion of the wrist.
Measuring hand compartment pressure
 Insert the needle perpendicular to the skin.
Insert the needle perpendicular to the skin.
 Evaluate the compartments individually. Pressure measurements are not obtained from the digits but at the site of maximal swelling of the thenar, hypothenar, and interosseous compartments.
Evaluate the compartments individually. Pressure measurements are not obtained from the digits but at the site of maximal swelling of the thenar, hypothenar, and interosseous compartments.
 If a single compartment pressure is elevated, release all compartments and the carpal tunnel.
If a single compartment pressure is elevated, release all compartments and the carpal tunnel.
 To measure the dorsal interosseous compartment pressure, insert the needle through the dorsal hand 1 cm proximal to the metacarpal head until it rests in the muscle belly. To judge the depth it is helpful to place identifiable marks on the needle at 1.0, 1.5, and 2.0 cm.
To measure the dorsal interosseous compartment pressure, insert the needle through the dorsal hand 1 cm proximal to the metacarpal head until it rests in the muscle belly. To judge the depth it is helpful to place identifiable marks on the needle at 1.0, 1.5, and 2.0 cm.
 To measure the adductor pollicis compartment pressure, insert the needle on the radial side of the second metacarpal in the substance of the thumb-index web space.
To measure the adductor pollicis compartment pressure, insert the needle on the radial side of the second metacarpal in the substance of the thumb-index web space.
 To measure the thenar and hypothenar spaces, insert the needle at the junction of the glabrous and nonglabrous skin over the maximal bulk of the muscle compartment. Advance the needle at least 5 mm below the enveloping fascia for pressure assessment.
To measure the thenar and hypothenar spaces, insert the needle at the junction of the glabrous and nonglabrous skin over the maximal bulk of the muscle compartment. Advance the needle at least 5 mm below the enveloping fascia for pressure assessment.
Management
Acute Compartment Syndrome of the Forearm
Forearm Fasciotomy and Arterial Exploration
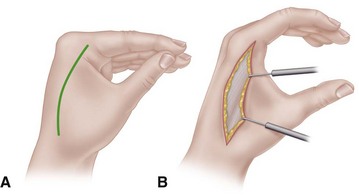
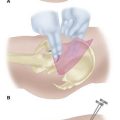
 For the volar fasciotomy, make a curvilinear incision similar to McConnell’s combined exposure of the median and ulnar nerve neurovascular bundles as described by Henry (Fig. 74-5). Make an anterior curvilinear incision medial to the biceps tendon, crossing the elbow flexion crease at an angle. Carry the incision distally into the palm to allow for a carpal tunnel release, but avoid crossing the wrist flexion crease at a right angle.
For the volar fasciotomy, make a curvilinear incision similar to McConnell’s combined exposure of the median and ulnar nerve neurovascular bundles as described by Henry (Fig. 74-5). Make an anterior curvilinear incision medial to the biceps tendon, crossing the elbow flexion crease at an angle. Carry the incision distally into the palm to allow for a carpal tunnel release, but avoid crossing the wrist flexion crease at a right angle.
 Divide the lacertus fibrosus proximally, and evacuate any hematoma.
Divide the lacertus fibrosus proximally, and evacuate any hematoma.
 In patients with suspected brachial artery injury, expose the brachial artery and determine whether there is a free blood flow. If the flow is unsatisfactory, remove the adventitia to expose any underlying clot, spasm, or intimal tear. Resect the adventitia if necessary, and anastomose or graft the artery.
In patients with suspected brachial artery injury, expose the brachial artery and determine whether there is a free blood flow. If the flow is unsatisfactory, remove the adventitia to expose any underlying clot, spasm, or intimal tear. Resect the adventitia if necessary, and anastomose or graft the artery.
 Release the superficial volar compartment throughout its length with open scissors, freeing the fascia over the superficial compartment muscles.
Release the superficial volar compartment throughout its length with open scissors, freeing the fascia over the superficial compartment muscles.
 Identify the flexor carpi ulnaris, and retract it with its underlying ulnar neurovascular bundle medially, and retract the flexor digitorum superficialis and median nerve laterally to expose the flexor digitorum profundus in its deep compartment. Check to see if its overlying fascia or epimysium is tight, and incise it longitudinally.
Identify the flexor carpi ulnaris, and retract it with its underlying ulnar neurovascular bundle medially, and retract the flexor digitorum superficialis and median nerve laterally to expose the flexor digitorum profundus in its deep compartment. Check to see if its overlying fascia or epimysium is tight, and incise it longitudinally.
 If the muscle is gray or dusky, the prognosis for recovery may be poor; however, the muscle may still be viable and should be allowed to perfuse.
If the muscle is gray or dusky, the prognosis for recovery may be poor; however, the muscle may still be viable and should be allowed to perfuse.
 Continue the dissection distally by incising the transverse carpal ligament along the ulnar border of the palmaris longus tendon and median nerve.
Continue the dissection distally by incising the transverse carpal ligament along the ulnar border of the palmaris longus tendon and median nerve.
 In cases of median nerve palsy or paresthesias, observe the median nerve along the entire zone of injury to ensure that it is not severed, contused, or entrapped between the ulnar and humeral head of the pronator teres. If it is, a partial pronator tenotomy is necessary.
In cases of median nerve palsy or paresthesias, observe the median nerve along the entire zone of injury to ensure that it is not severed, contused, or entrapped between the ulnar and humeral head of the pronator teres. If it is, a partial pronator tenotomy is necessary.
 In a patient with a supracondylar fracture, reduce the fracture, pin it with Kirschner wires, and control the bleeding.
In a patient with a supracondylar fracture, reduce the fracture, pin it with Kirschner wires, and control the bleeding.
 Do not close the skin at this time; anticipate secondary closure later.
Do not close the skin at this time; anticipate secondary closure later.
 If the median nerve is exposed within the distal forearm, suture the distal radial-based forearm flap loosely over the nerve.
If the median nerve is exposed within the distal forearm, suture the distal radial-based forearm flap loosely over the nerve.
 Check the dorsal compartments clinically, or repeat the pressure measurements. Usually, the volar fasciotomy decompresses the dorsal musculature sufficiently, but if involvement of the dorsal compartments is still suspected, release them also.
Check the dorsal compartments clinically, or repeat the pressure measurements. Usually, the volar fasciotomy decompresses the dorsal musculature sufficiently, but if involvement of the dorsal compartments is still suspected, release them also.
 Make the incision distal to the lateral epicondyle between the extensor digitorum communis and extensor carpi radialis brevis, extending approximately 10 cm distally. Gently undermine the subcutaneous tissue, and release the fascia overlying the mobile wad of Henry and the extensor retinaculum.
Make the incision distal to the lateral epicondyle between the extensor digitorum communis and extensor carpi radialis brevis, extending approximately 10 cm distally. Gently undermine the subcutaneous tissue, and release the fascia overlying the mobile wad of Henry and the extensor retinaculum.
 Apply a sterile moist dressing and a long-arm splint. The elbow should not be left flexed beyond 90 degrees.
Apply a sterile moist dressing and a long-arm splint. The elbow should not be left flexed beyond 90 degrees.
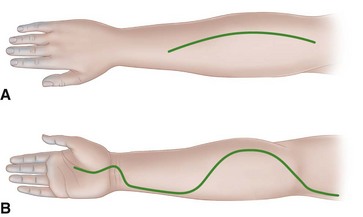
FIGURE 74-5 Incisions used in forearm in severe Volkmann contracture. A, Extensive opening of fascia of dorsum of forearm in dorsal compartment syndromes. B, Incision used for anterior forearm compartment syndromes in which skin and underlying fascia are released completely throughout. SEE TECHNIQUES 74-2, 74-4, AND 74-5.
Postoperative Care
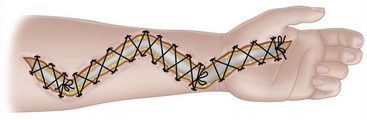
The arm is elevated for 24 to 48 hours after surgery. If closure is not possible within 5 days, a split-thickness skin graft should be applied. Alternatively, closure of fasciotomy wounds can be accomplished gradually with progressive tension using vessel loops. The vessel loops are tightened progressively postoperatively during dressing changes. Wound closure by this method usually can be accomplished in 2 weeks (Fig. 74-6). A vacuum-assisted wound closure system may be used to assist in wound management. The splint is worn until sutures are removed or as determined by fracture care.
Hand Fasciotomies
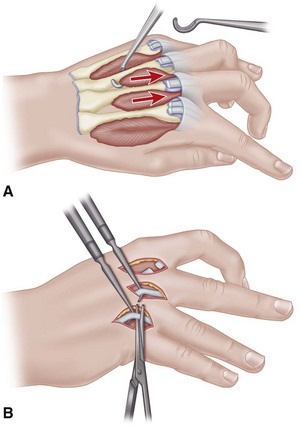
 Make two dorsal parallel incisions through the skin overlying the second and fourth metacarpals, beginning at the level of the metacarpophalangeal joints and extending just distal to the wrist (Fig. 74-7A). Make each incision down to the musculofascial area.
Make two dorsal parallel incisions through the skin overlying the second and fourth metacarpals, beginning at the level of the metacarpophalangeal joints and extending just distal to the wrist (Fig. 74-7A). Make each incision down to the musculofascial area.
 Incise the fascia, and release the compression of the distended muscles by allowing them to extrude into the wound if necessary.
Incise the fascia, and release the compression of the distended muscles by allowing them to extrude into the wound if necessary.
 Identify each muscle individually to ensure that a complete release is done. Passively flex the metacarpophalangeal joints, and extend the proximal interphalangeal joints to stretch the muscles, ensuring that all are adequately released.
Identify each muscle individually to ensure that a complete release is done. Passively flex the metacarpophalangeal joints, and extend the proximal interphalangeal joints to stretch the muscles, ensuring that all are adequately released.
 Release the thenar and hypothenar compartments by making additional palmar radial and palmar ulnar incisions along the glabrous and nonglabrous interval to allow for their separate decompression.
Release the thenar and hypothenar compartments by making additional palmar radial and palmar ulnar incisions along the glabrous and nonglabrous interval to allow for their separate decompression.
 Release the carpal tunnel through a palmar midline incision.
Release the carpal tunnel through a palmar midline incision.
 Do not attempt to débride the interosseous muscles at this point. If the fingers are tensely swollen, and capillary refill is delayed, continue with digital fasciotomies through midlateral incisions along the radial border of the ring and small fingers and the ulnar border of the index and long fingers (Fig. 74-7B).
Do not attempt to débride the interosseous muscles at this point. If the fingers are tensely swollen, and capillary refill is delayed, continue with digital fasciotomies through midlateral incisions along the radial border of the ring and small fingers and the ulnar border of the index and long fingers (Fig. 74-7B).
 In general, it is prudent to release all compartments including the carpal tunnel if any of the hand compartments are involved.
In general, it is prudent to release all compartments including the carpal tunnel if any of the hand compartments are involved.
 Do not attempt to close the wounds at this time. They may be permitted to granulate and heal, or after the swelling has decreased, they can be closed secondarily. A vacuum-assisted wound closure system may be used to assist in wound management.
Do not attempt to close the wounds at this time. They may be permitted to granulate and heal, or after the swelling has decreased, they can be closed secondarily. A vacuum-assisted wound closure system may be used to assist in wound management.
Established Volkmann Contracture of the Forearm
If a compartment syndrome is untreated or inadequately treated, compartment pressures continue to increase until irreversible tissue ischemia occurs. Volkmann ischemic contracture is the result of several different degrees of tissue injury; however, the earliest changes usually involve the flexor digitorum profundus muscles in the middle third of the forearm (Fig. 74-8). The typical clinical picture of established Volkmann contracture includes elbow flexion, forearm pronation, wrist flexion, thumb adduction, metacarpophalangeal joint extension, and finger flexion.
A severe contracture involves the flexors and extensors of the forearm. Fractures of the forearm bones and scars on the skin also may be present. Sensory feedback usually is impaired because the nerves are strangulated by the contracted and scarred muscles surrounding them. The preferred treatment in these instances is early excision of all necrotic muscles, combined with complete median and ulnar neurolysis to restore sensibility and possibly intrinsic function. Although one author recommended this be done no sooner than 3 months but no later than 1 year after the ischemic event, others have recommended surgical intervention within 3 weeks to prevent additional contractures from developing. Tendon transfers to restore function should be performed as a secondary procedure. These may include transfer of the brachioradialis to the flexor pollicis longus and the extensor carpi radialis longus to the flexor digitorum profundus tendons. If motors to restore finger flexion are unavailable, a free innervated muscle transfer using the gracilis muscle may be considered (see Chapter 63). In one long-term study (32 years), substantial improvement was noted with excision of fibrotic muscle, neurolysis, and tendon transfers or free gracilis transfer; however, tendon lengthening alone rarely was satisfactory. Oishi and Ezaki recommended for severe Volkmann ischemic contracture a two-stage procedure with initial muscle débridement and neurolysis followed by a free functioning gracilis transfer after return of sensation and intrinsics to the hand. Satisfactory results also have been reported using a free medial gastrocnemius myocutaneous flap for reconstruction in patients with established Volkmann contracture.
Muscle Sliding Operation of Flexors for Established Volkmann Contracture
The muscle sliding operation was first described by Page in 1923 and was endorsed by Scaglietti in 1957. It has been used for Volkmann and other contractures caused by conditions such as brain damage and burns. In the case of Volkmann contracture, usually the muscle is fibrotic and noncontractile and a muscle sliding operation alone is rarely indicated. For this technique, see Chapter 72.
Excision of Necrotic Muscles Combined with Neurolysis of Median and Ulnar Nerves for Severe Contracture
 Make an extensive volar forearm incision (see Fig. 74-5), and excise all avascular masses of the flexor profundus and sublimis muscles, leaving any muscle that might survive or appears viable.
Make an extensive volar forearm incision (see Fig. 74-5), and excise all avascular masses of the flexor profundus and sublimis muscles, leaving any muscle that might survive or appears viable.
 Perform a neurolysis of the median and ulnar nerves. The median nerve usually is affected and may be found to have an hourglass deformity in the midforearm. Neuroma excision and secondary nerve grafting may be necessary.
Perform a neurolysis of the median and ulnar nerves. The median nerve usually is affected and may be found to have an hourglass deformity in the midforearm. Neuroma excision and secondary nerve grafting may be necessary.
 Correct finger and wrist flexion deformities by dividing the involved flexor tendons at the musculotendinous junctions and excising the fibrotic muscle. At this time, at least the functional position of the hand will have been restored.
Correct finger and wrist flexion deformities by dividing the involved flexor tendons at the musculotendinous junctions and excising the fibrotic muscle. At this time, at least the functional position of the hand will have been restored.
 At a second-stage procedure, any viable extensor muscles can be transferred to the finger flexors. At least one wrist extensor must be retained, however. Otherwise, any wrist flexor or extensor can be transferred to power the profundus and flexor pollicis longus tendons. Most commonly, the brachioradialis is transferred to the flexor pollicis longus and the extensor carpi radialis longus to the flexor digitorum profundus of all four fingers.
At a second-stage procedure, any viable extensor muscles can be transferred to the finger flexors. At least one wrist extensor must be retained, however. Otherwise, any wrist flexor or extensor can be transferred to power the profundus and flexor pollicis longus tendons. Most commonly, the brachioradialis is transferred to the flexor pollicis longus and the extensor carpi radialis longus to the flexor digitorum profundus of all four fingers.
This is a salvage procedure that may result in only modest improvement. If the contracture is diffuse but incomplete throughout all digital and wrist flexors, the muscle sliding technique may be considered (see Chapter 72).
Two-Staged Free Gracilis Transfer
First Stage
 Widely expose the volar forearm compartment from the elbow to the wrist (see Fig. 74-5B) and elevate the skin flaps.
Widely expose the volar forearm compartment from the elbow to the wrist (see Fig. 74-5B) and elevate the skin flaps.
 Identify and mobilize the ulnar nerve at the elbow. After isolating and protecting the median nerve and brachial artery at the antecubital fossa, dissect the median and ulnar nerves and vascular structures all the way from the elbow to the wrist to free adherences to fibrotic necrotic muscle.
Identify and mobilize the ulnar nerve at the elbow. After isolating and protecting the median nerve and brachial artery at the antecubital fossa, dissect the median and ulnar nerves and vascular structures all the way from the elbow to the wrist to free adherences to fibrotic necrotic muscle.
 Débride all the involved muscle, including the deep layers. Sometimes after debridement the only structures remaining are the median and ulnar nerves, the vascular structures, and the tendon ends.
Débride all the involved muscle, including the deep layers. Sometimes after debridement the only structures remaining are the median and ulnar nerves, the vascular structures, and the tendon ends.
 Perform necessary nerve grafting or vascular reconstruction at this point.
Perform necessary nerve grafting or vascular reconstruction at this point.
 Keep the proximal ends of the flexor tendon as long as possible (it is helpful to suture the proximal ends of the flexor digitorum profundus and the flexor pollicis longus together for later identification). In young or small children, suture these ends to an area proximal to the carpal tunnel to prevent retraction into the carpal tunnel.
Keep the proximal ends of the flexor tendon as long as possible (it is helpful to suture the proximal ends of the flexor digitorum profundus and the flexor pollicis longus together for later identification). In young or small children, suture these ends to an area proximal to the carpal tunnel to prevent retraction into the carpal tunnel.
 Close the skin and immobilize the arm in a cast for 3 weeks to allow the wound to heal. After cast removal begin passive range of motion to the fingers and wrist. The patient is observed over the ensuing months (6 months) for muscle and sensory recovery.
Close the skin and immobilize the arm in a cast for 3 weeks to allow the wound to heal. After cast removal begin passive range of motion to the fingers and wrist. The patient is observed over the ensuing months (6 months) for muscle and sensory recovery.
Second Stage
 Identify the brachial artery in the forearm and follow it distally to determine its suitability or that of any branches. Also identify a vein for anastomosis because the vena comitantes or subcutaneous veins may not be suitable.
Identify the brachial artery in the forearm and follow it distally to determine its suitability or that of any branches. Also identify a vein for anastomosis because the vena comitantes or subcutaneous veins may not be suitable.
 Identify the anterior interosseous branch, and in the distal forearm identify and prepare the ends of the flexor digitorum profundus and flexor pollicis longus tendons.
Identify the anterior interosseous branch, and in the distal forearm identify and prepare the ends of the flexor digitorum profundus and flexor pollicis longus tendons.
 In the lower extremity expose the gracilis muscle with or without an accompanying skin paddle. If a skin paddle is necessary, use only the proximal two thirds of overlying skin because the blood supply to the distal third of skin overlying the muscle is unreliable.
In the lower extremity expose the gracilis muscle with or without an accompanying skin paddle. If a skin paddle is necessary, use only the proximal two thirds of overlying skin because the blood supply to the distal third of skin overlying the muscle is unreliable.
 Tag the anterior surface of the gracilis muscle with suture at 2-cm intervals to correctly identify the resting tension of the muscle.
Tag the anterior surface of the gracilis muscle with suture at 2-cm intervals to correctly identify the resting tension of the muscle.
 Identify the neurovascular bundle and dissect it. Careful dissection is mandatory because the anterior branch of the obturator nerve runs superiorly from the muscle.
Identify the neurovascular bundle and dissect it. Careful dissection is mandatory because the anterior branch of the obturator nerve runs superiorly from the muscle.
 When the forearm recipient site has been prepared, release the origin and divide the neurovascular bundle.
When the forearm recipient site has been prepared, release the origin and divide the neurovascular bundle.
 If a vein graft was deemed necessary, the microvascular anastomosis of the vein graft to the gracilis artery can be done on a back table using an operating microscope.
If a vein graft was deemed necessary, the microvascular anastomosis of the vein graft to the gracilis artery can be done on a back table using an operating microscope.
 Suture the proximal gracilis to the medial epicondyle using nonabsorbable suture. Note the location of the ulnar nerve before carrying out this step. Also, take care to position the muscle so as not to cause undue tension on the upcoming microvascular work.
Suture the proximal gracilis to the medial epicondyle using nonabsorbable suture. Note the location of the ulnar nerve before carrying out this step. Also, take care to position the muscle so as not to cause undue tension on the upcoming microvascular work.
 Using an operating microscope, perform anastomoses of the artery, vein(s), and nerve. Examine the anterior interosseous nerve under the operating microscope and cut it back until good fascicles are seen. The closer the nerve coaptation is to the muscle, the shorter the distance necessary for reinnervation.
Using an operating microscope, perform anastomoses of the artery, vein(s), and nerve. Examine the anterior interosseous nerve under the operating microscope and cut it back until good fascicles are seen. The closer the nerve coaptation is to the muscle, the shorter the distance necessary for reinnervation.
 Place an implantable Doppler probe around the artery for postoperative monitoring. After assessment of adequate flow, suture the flexor digitorum profundus ends to each other and to the gracilis muscle at its resting tension (marked earlier).
Place an implantable Doppler probe around the artery for postoperative monitoring. After assessment of adequate flow, suture the flexor digitorum profundus ends to each other and to the gracilis muscle at its resting tension (marked earlier).
 Suture the flexor pollicis longus tendon to a separate portion of the gracilis muscle with the wrist in 10 to 20 degrees of extension and slight overcorrection of the normal finger cascade.
Suture the flexor pollicis longus tendon to a separate portion of the gracilis muscle with the wrist in 10 to 20 degrees of extension and slight overcorrection of the normal finger cascade.
 Flex the wrist to ensure that the tenodesis allows the fingers to extend appropriately.
Flex the wrist to ensure that the tenodesis allows the fingers to extend appropriately.
 Close the skin flaps and immobilize and elevate the upper extremity. Failure to elevate the extremity could jeopardize flap viability.
Close the skin flaps and immobilize and elevate the upper extremity. Failure to elevate the extremity could jeopardize flap viability.
Established Intrinsic Muscle Contractures of the Hand
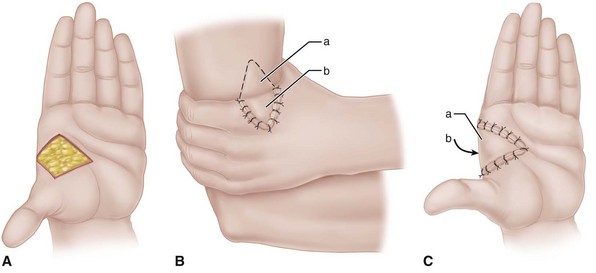
The proper surgical release of established intrinsic muscle contractures depends on the severity of the contractures. When the contractures are mild (Fig. 74-9), the metacarpophalangeal joints can be passively extended completely, but while they are held extended, the proximal interphalangeal joints cannot be flexed (positive intrinsic tightness test); the distal intrinsic release of Littler may be indicated (Fig. 74-10).
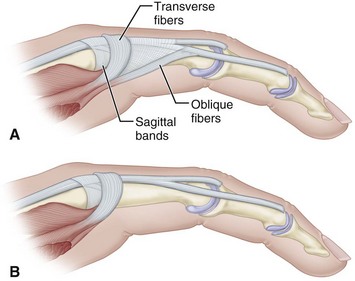
FIGURE 74-10 Littler release of intrinsic contracture. A, Extensor aponeurosis at level of metacarpophalangeal joint consists of long extensor tendon, transverse fibers (which flex metacarpophalangeal joint), and oblique fibers (which extend interphalangeal joint). Crosshatched part is resected from each side of hood. B, Appearance of aponeurosis after release. SEE TECHNIQUE 74-6.
In contractures that are more severe, the interosseous muscles are viable but contracted and the intrinsic tightness test is positive. Active spreading of the fingers may be possible. In these instances, the contracted muscles may be released from the metacarpal shafts by a muscle sliding operation (Fig. 74-11A).
In the most severe contractures, the intrinsic muscles not only may be contracted but also necrotic and fibrosed, so any useful muscle excursion is absent. In these instances, the tendon of each muscle must be divided to release the contractures (Fig. 74-11B). Other procedures, such as capsulotomies and tendon transfers, also may be necessary.
Release of Established Intrinsic Muscle Contractures of the Hand
 The same procedure is done on any finger as needed.
The same procedure is done on any finger as needed.
 Make a single midline incision on the dorsum of the proximal phalanx extending from the metacarpophalangeal joint to the proximal interphalangeal joint to allow good exposure of both sides of the extensor aponeurosis. Incise the insertion of the oblique fibers of the extensor aponeurosis into the extensor tendon; make the incision parallel with the tendon (see Fig. 74-10A).
Make a single midline incision on the dorsum of the proximal phalanx extending from the metacarpophalangeal joint to the proximal interphalangeal joint to allow good exposure of both sides of the extensor aponeurosis. Incise the insertion of the oblique fibers of the extensor aponeurosis into the extensor tendon; make the incision parallel with the tendon (see Fig. 74-10A).
 Preserve the transverse fibers to avoid hyperextension of the metacarpophalangeal joint with its resultant clawhand deformity and limitation of extension of the interphalangeal joints.
Preserve the transverse fibers to avoid hyperextension of the metacarpophalangeal joint with its resultant clawhand deformity and limitation of extension of the interphalangeal joints.
 After adequate excision of the oblique fibers, the proximal interphalangeal joint should have full passive flexion with the metacarpophalangeal joints in neutral (see Fig. 74-10B).
After adequate excision of the oblique fibers, the proximal interphalangeal joint should have full passive flexion with the metacarpophalangeal joints in neutral (see Fig. 74-10B).
 Apply a volar plaster splint from the elbow to the middle of the proximal phalanges, immobilizing the metacarpophalangeal joints in extension and permitting full motion of the interphalangeal joints.
Apply a volar plaster splint from the elbow to the middle of the proximal phalanges, immobilizing the metacarpophalangeal joints in extension and permitting full motion of the interphalangeal joints.
Release of Severe Intrinsic Contractures with Muscle Fibrosis
 Make a dorsal transverse incision just proximal to the metacarpophalangeal joints.
Make a dorsal transverse incision just proximal to the metacarpophalangeal joints.
 Resect the lateral tendons of all the interossei and the abductor digiti quinti at the level of the metacarpophalangeal joints. If these joints remain flexed, retract the sagittal bands distally and divide each accessory collateral ligament at its insertion into the volar plate.
Resect the lateral tendons of all the interossei and the abductor digiti quinti at the level of the metacarpophalangeal joints. If these joints remain flexed, retract the sagittal bands distally and divide each accessory collateral ligament at its insertion into the volar plate.
 Free the volar plate from its attachments to the base of the proximal phalanx, and with a blunt probe, separate any adhesions between the volar plate and the metacarpal head.
Free the volar plate from its attachments to the base of the proximal phalanx, and with a blunt probe, separate any adhesions between the volar plate and the metacarpal head.
 If maintaining extension of the proximal phalanx is difficult after soft tissue release, insert a Kirschner wire obliquely through the metacarpophalangeal joint with the joint in maximal extension. When the phalanx is extended, ensure that its base articulates properly with the metacarpal head before inserting the wire.
If maintaining extension of the proximal phalanx is difficult after soft tissue release, insert a Kirschner wire obliquely through the metacarpophalangeal joint with the joint in maximal extension. When the phalanx is extended, ensure that its base articulates properly with the metacarpal head before inserting the wire.
 If passive flexion of the proximal interphalangeal joints is incomplete with the metacarpophalangeal joints extended, resect the lateral bands at the distal half of the proximal phalanges through separate dorsal incisions.
If passive flexion of the proximal interphalangeal joints is incomplete with the metacarpophalangeal joints extended, resect the lateral bands at the distal half of the proximal phalanges through separate dorsal incisions.
Adducted Thumb
Crushing injuries, infections, or deep burns result in extensive fibrosis within the thumb web that cannot be treated by release of the skin alone; rather, the scarred components of the contracted skin, muscle, fascia, and capsule must be excised with care to avoid damaging the radial artery near the carpometacarpal joint. This excision produces a deep fissure that must be filled with skin and subcutaneous fat to provide an elastic functioning web. Usually this can be accomplished by dorsal rotation or a sliding flap with supplemental skin grafting (Figs. 74-12 and 74-13). If adjacent dorsal skin is unsuitable for transfer, a cross arm flap may be considered. The cross arm flap is fashioned as a double triangle, one on the dorsal surface and one on the volar surface of the web, to eliminate any line of scar paralleling the border of the web. The first and second metacarpals are fixed in the desired position with Kirschner wires. When motion in the carpometacarpal joint can be restored, any necessary tendon transfers for apposition can be done later, but if motion cannot be restored, the carpometacarpal joint must be arthrodesed to maintain the new position of the thumb permanently.
Paralysis of the muscles of apposition can result in secondary contracture of the skin and joint capsule and in contracture of the thumb web, requiring release by a Z-plasty or by a local flap and a skin graft as described by Brand and Milford (see Fig. 74-13). Contracted fascia and bands of muscle must be released, and capsulotomy of the carpometacarpal joint must be done at the same time.
Occasionally, a useless index finger may provide a filleted pedicle with which a satisfactory thumb web can be constructed in one stage. This procedure not only widens the web in that the index metacarpal is excised but also provides skin that can be repositioned over a nearby defect or scar (see discussion of filleted graft in Chapter 65).
Compartment Syndrome and Volkmann Contracture
Boody AR, Wongworawat MD. Accuracy in the measurement of compartment pressures: a comparison of three commonly used devices. J Bone Joint Surg. 2005;87A:2415.
Croutzet P, Chassat R, Masmejean EH. Mini-invasive surgery for chronic exertional compartment syndrome of the forearm: a new technique. Tech Hand Up Extrem Surg. 2009;13:137.
Elliott KG, Johnstone AJ. Diagnosing acute compartment syndrome. J Bone Joint Surg. 2003;85B:625.
Grottkau BE, Epps HR, Di Scala C. Compartment syndrome in children and adolescents. J Pediatr Surg. 2005;40:678.
Gülgönen A. Invited review article. Surgery for Volkmann’s ischaemic contracture. J Hand Surg. 2001;26B:283.
Hovius SER, Ultee J. Volkmann’s ischemic contracture: prevention and treatment. Hand Clin. 2000;16:647.
Hwang RW, de Witte PB, Ring D. Compartment syndrome associated with distal radial fracture and ipsilateral elbow injury. J Bone Joint Surg. 2009;91A:642.
Kalyani BS, Fisher BE, Roberts CS, Giannoudis PV. Compartment syndrome of the forearm: a systematic review. J Hand Surg. 2011;36A:535.
Kumar PR, Jenkins JPR, Hodgson SP. Bilateral chronic exertional compartment syndrome of the dorsal part of the forearm: the role of magnetic resonance imaging in diagnosis: a case report. J Bone Joint Surg. 2003;85A:1557.
Lipschitz AH, Lifchez SD. Measurement of compartment pressures in the hand and forearm. J Hand Surg. 2010;35A:1893.
McQueen MM, Gaston P, Court-Brown CM. Acute compartment syndrome: who is at risk? J Bone Joint Surg. 2000;82B:200.
Oishi SN, Ezaki M. Free gracilis transfer to restore finger flexion in Volkmann ischemic contracture. Tech Hand Upper Extrem Surg. 2010;14:104.
Ronel DN, Mtui E, Nolan WB, 3rd. Forearm compartment syndrome: anatomical analysis of surgical approaches to the deep space. Plast Reconstr Surg. 2004;114:697.
Ultee J, Hovius SER. Functional results after treatment of Volkmann’s ischemic contracture. Clin Orthop Relat Res. 2005;431:42.
Van den Brand JG, Nelson T, Verleisdonk EG, van derWerken C. The diagnostic value of intracompartmental pressure measurement, magnetic resonance imaging, and near-infrared spectroscopy in chronic exertional compartment syndrome: a prospective study in 50 patients. Am J Sports Med. 2005;33:699.
Verleisdonk EJ, van Gils A, van der Werken C. The diagnostic value of MRI scans for the diagnosis of chronic exertional compartment syndrome of the lower leg. Skeletal Radiol. 2001;30:321.
Yang CC, Chang DS, Webb LX. Vacuum-assisted closure for fasciotomy wounds following compartment syndrome of the leg. J Surg Orthop Adv. 2006;15:19.
Yuan PS, Pring ME, Gaynor TP, et al. Compartment syndrome following intramedullary fixation of pediatric forearm fractures. J Pediatr Orthop. 2004;24:370.
Compartment Syndrome and Volkmann Contracture
Allen MJ, Barnes MR. Chronic compartment syndrome of the flexor muscles in the forearm: a case report. J Hand Surg. 1989;14B:47.
Allen MJ, Steingold RF, Kotecha M, et al. The importance of the deep volar compartment in crush injuries of the forearm. Injury. 1985;16:273.
Amendola A, Rorabeck CH, Vallett D, et al. The use of magnetic resonance imaging in exertional compartment syndromes. Am J Sports Med. 1990;18:29.
Asgari MM, Spinelli HM. The vessel loop shoelace technique for closure of fasciotomy wounds. Ann Plast Surg. 1999;43:225.
Broström L-A, Stark A, Svartengren G. Acute compartment syndrome in forearm fractures. Acta Orthop Scand. 1990;61:50.
Brumback RJ. Compartment syndrome complicating avulsion of the origin of the triceps muscle. J Bone Joint Surg. 1987;69A:1445.
Bunnell S. Ischaemic contracture, local, in the hand. J Bone Joint Surg. 1953;35A:88.
Chuang DC, Carver N, Wei FC. A new strategy to prevent the sequelae of severe Volkmann’s ischemia. Plast Reconstr Surg. 1996;98:1023.
Dellaero DT, Levin LS. Compartment syndrome of the hand: etiology, diagnosis, and treatment. Am J Orthop. 1996;25:404.
Eaton RG, Green WT. Epimysiotomy and fasciotomy in the treatment of Volkmann’s ischemic contracture. Orthop Clin North Am. 1972;3:175.
Eichler GR, Lipscomb PR. The changing treatment of Volkmann’s contractures from 1955 to 1965 at the Mayo Clinic. Clin Orthop Relat Res. 1967;50:215.
Fazi B, Raves JJ, Young JC, et al. Fasciotomy of the upper extremity in the patient with trauma. Surg Gynecol Obstet. 1987;165:447.
Gainor BJ. Closed avulsion of the flexor digitorum superficialis origin causing compartment syndrome: a case report. J Bone Joint Surg. 1984;66A:467.
Gelberman RH, Garfin SR, Hergenroeder PT, et al. Compartment syndromes of the forearm: diagnosis and treatment. Clin Orthop Relat Res. 1981;161:252.
Gelberman RH, Zakaib GS, Mubarak SJ, et al. Decompression of forearm compartment syndrome. Clin Orthop Relat Res. 1978;134:225.
Goldie BS, Jones NF, Jupiter JB. Recurrent compartment syndrome and Volkmann contracture associated with chronic osteomyelitis of the ulna. J Bone Joint Surg. 1990;72A:131.
Green TL, Louis DS. Compartment syndrome of the arm: a complication of the pneumatic tourniquet: a case report. J Bone Joint Surg. 1983;65A:270.
Halpern AA, Mochizuki RM. Compartment syndrome of the interosseous muscles of hand: a clinical and anatomic review. Orthop Rev. 1980;9:121.
Harris C, Jr., Riordan DC. Intrinsic contracture in the hand and its surgical treatment. J Bone Joint Surg. 1954;36A:10.
Hastings H, Misamore G. Compartment syndrome resulting from intravenous regional anesthesia. J Hand Surg. 1987;12A:559.
Hazeltine M, Duranceau L, Gariepy G. Presentation of breast carcinoma as Volkmann’s contracture due to skeletal muscle metastases. J Rheumatol. 1990;17:1097.
Henry AK. Exposures of long bones and other surgical methods. Bristol, England: John Wright & Sons; 1927.
Heppenstall RB, Sapega AA, Scott R, et al. The compartment syndrome: an experimental and clinical study of muscular energy metabolism using phosphorus nuclear magnetic resonance spectroscopy. Clin Orthop Relat Res. 1988;226:138.
Hung LK, Kinninmonth WG, Woo ML. Vibrio vulnificus necrotizing fasciitis presenting with compartmental syndrome of the hand. J Hand Surg. 1988;13B:337.
Jones R. Address on Volkmann’s contracture with specific reference to treatment. BMJ. 1928;2:639.
Kline SC, Moore JR. Neonatal compartment syndrome. J Hand Surg. 1992;17A:256.
Kutz JE, Singer R, Lindsay M. Chronic exertional compartment syndrome of the forearm: a case report. J Hand Surg. 1985;10A:302.
Littler JW. The hand and upper extremity. In: Converse, JM, ed. Reconstructive plastic surgery. Philadelphia: WB Saunders, 1977.
Liu XY, Ge BF, Win YM, et al. Free medial gastrocnemius myocutaneous flap transfer with neurovascular anastomosis to treat Volkmann’s contracture of the forearm. Br J Plast Surg. 1992;45:6.
Madigan RR, Hanna WT, Wallace SL. Acute compartment syndrome in hemophilia. J Bone Joint Surg. 1981;63A:1327.
Matava MJ, Whitesides TE, Jr., Seiler JG, 3rd., et al. Determination of the compartment pressure threshold of muscle ischemia in a canine model. J Trauma. 1994;37:50.
Matsen FA, Winquist RA, Krugmire RB. Diagnosis and management of compartmental syndromes. J Bone Joint Surg. 1980;62A:286.
McHale KA, Geissele A, Perlik PD. Compartment syndrome of the biceps brachii compartment following rupture of the long head of the biceps. Orthopedics. 1991;14:787.
Moed BR, Fakhouri AJ. Compartment syndrome after low-velocity gunshot wounds to the forearm. J Orthop Trauma. 1991;5:134.
Mubarak SJ, Carroll NC. Volkmann’s contracture in children: aetiology and prevention. J Bone Joint Surg. 1979;61B:285.
Mubarak SJ, Owen CA, Hargens AR, et al. Acute compartment syndrome: diagnosis and treatment with the aid of the Wick catheter. J Bone Joint Surg. 1978;60A:1091.
Murphy JB. Myositis. JAMA. 1914;63:1240.
Naidu S, Heppenstall RB. Compartment syndrome of the forearm and hand. Hand Clin. 1994;10:13.
O’Neil D, Sheppard JE. Transient compartment syndrome of the forearm resulting from venous congestion from a tourniquet. J Hand Surg. 1989;14A:894.
Page CM. An operation for the relief of flexion-contracture in the forearm. J Bone Joint Surg. 1923;5B:233.
Palumbo RC, Abrams JS. Compartment syndrome of the upper arm. Orthopedics. 1994;17:1144.
Pedowitz RA, Tourounghi FM. Chronic exertional compartment syndrome of the forearm flexor muscles. J Hand Surg. 1988;13A:694.
Ridings P, Gault D. Compartment syndrome of the arm. J Hand Surg. 1994;19B:147.
Rorabeck CH. Tourniquet-induced nerve ischemia: an experimental investigation. J Trauma. 1980;20:280.
Rowland SA, Fasciotomy: the treatment of compartment syndrome. Green, DP, ed. Operative hand surgery, ed 3, New York: Churchill Livingstone, 1993.
Scaglietti O. Sindromi cliniche immediate e tardive da lesioni vascolari nelle fratture degli arti. Riforma Med. 1957;71:749.
Schnall SB, Holtrom PD, Silva E. Compartment syndrome associated with infection of the upper extremity. Clin Orthop Relat Res. 1994;306:128.
Seiler JG, III., Womack S, De L’Aune WR, et al. Intracompartmental pressure measurements in normal forearm. J Orthop Trauma. 1993;7:414.
Sheridan GW, Matsen FA. Fasciotomy in the treatment of acute compartment syndrome. J Bone Joint Surg. 1976;58A:112.
Simpson NS, Jupiter JB. Delayed onset of forearm compartment syndrome: a complication of distal radius fracture in young adults. J Orthop Trauma. 1995;9:411.
Smith RJ, Intrinsic contracture. Operative hand surgery. Green DP, ed. Operative hand surgery, New York, Churchill Livingstone, 1982;vol 1.
Steinberg BD, Gelberman RH. Evaluation of limb compartments with suspected increased interstitial pressure: a noninvasive method for determining quantitative hardness. Clin Orthop Relat Res. 1994;300:248.
Stockley I, Harvey IA, Getty CJM. Acute volar compartment syndrome of the forearm secondary to fractures of the distal radius. Injury. 1988;19:101.
Styf J, Forssblad P, Lundborg G. Chronic compartment syndrome in the first dorsal interosseous muscle. J Hand Surg. 1987;12A:757.
Thomas JJ. Nerve involvement in the ischaemic paralysis and contracture of Volkmann. Ann Surg. 1909;49:330.
Trumble T. Forearm compartment syndrome secondary to leukemic infiltrates. J Hand Surg. 1987;12A:563.
Tsuge K. Treatment of established Volkmann’s contracture. J Bone Joint Surg. 1975;57A:925.
Volkmann R. Die ischaemischen Muskellähmungen und Kontrakturen. Zentralbl Chir. 1881;8:801.
Whitesides TE, Jr., Haney TC, Morimoto K, et al. Tissue pressure measurements as a determinant for the need of fasciotomy. Clin Orthop Relat Res. 1975;113:43.
Whitesides TE, Jr., Harada H, Morimoto K. Compartment syndromes and the role of fasciotomy, its parameters and techniques. Instr Course Lect. 1977;26:179.
Zuker RM, Egerszegi EP, Manktelow RT, et al. Volkmann’s ischemic contracture in children: the results of free vascularized muscle transplantation. Microsurgery. 1991;12:341.
Brand PW, Milford LW, Web deepening with sliding flap for adducted thumb in the hand. Crenshaw, AH, ed. Campbell’s operative orthopaedics, ed 4, St. Louis: CV Mosby, 1963.
Howard LD, Jr. Contracture of the thumb web. J Bone Joint Surg. 1950;32A:267.
Littler JW. The prevention and the correction of adduction contracture of the thumb. Clin Orthop Relat Res. 1959;13:182.