Chapter 23 Comparison of Noninvasive Techniques for Myocardial Perfusion Imaging
INTRODUCTION
A variety of noninvasive techniques permit the assessment of regional myocardial perfusion at rest and during exercise or pharmacologic stress. These techniques include radionuclide imaging employing single-photon emission computed tomography (SPECT) or positron emission tomography (PET) methodologies, contrast echocardiography, computed tomography (CT), and cardiac magnetic resonance (CMR) imaging. All have particular strengths and weaknesses. The reader is directed to earlier chapters in this section of the book for detailed descriptions of the use of these perfusion imaging techniques for detecting functionally significant coronary artery stenoses or abnormal coronary flow reserve due to endothelial and microcirculatory abnormalities. In the paragraphs to follow, the major strengths and weaknesses of these diverse approaches to myocardial perfusion imaging (MPI) will be reviewed, with the understanding that advances in technology for all of these imaging methodologies are continuously being reported. Table 23-1 provides a summary of the strengths and limitations of these four diverse imaging technologies used for the assessment of myocardial perfusion under rest and stress conditions.
| Multiple perfusion defects in > 1 coronary supply region (multivessel coronary artery disease scan pattern) |
| An extensive area of stress-induced hypoperfusion, even if confined to the territory of a single coronary artery (e.g., proximal left anterior descending coronary artery scan pattern) |
| A high ischemic burden reflected by multiple reversible defects |
| Transient ischemic left ventricular cavity dilation |
| Multiple abnormal regional wall-motion or thickening abnormalities, even if not associated with perfusion defects |
| A gated SPECT ejection fraction of < 40% |
| Increased diastolic and end-systolic volumes on quantitative SPECT, increased lung-to-heart ratio of thallium uptake when Tl-201 used for exercise imaging |
SPECT, single-photon emission computed tomography.
RADIONUCLIDE MYOCARDIAL PERFUSION IMAGING (See Chapters 14 to 16)
Stress and rest SPECT myocardial perfusion imaging (MPI) is the most commonly performed imaging technique to detect CAD among patients presenting with chest pain or other symptoms thought to be secondary to ischemia. Table 23-1 shows the main strengths and limitations of radionuclide SPECT MPI relative to other noninvasive techniques for assessing myocardial perfusion. The sensitivity, specificity, and normalcy rate for SPECT MPI for detection of coronary artery disease (CAD) are 86%, 74%, and 89%, respectively.1 The prognostic value of SPECT MPI has been proven with multiple studies reported in the literature (see Chapter 16).2 For patients undergoing exercise stress SPECT imaging who have a normal perfusion scan, the annual death or myocardial infarction rate is 0.7% annually, compared to 5.6% for those with an abnormal scan.2 Similarly, for pharmacologic stress SPECT MPI, the annual hard event rate with a normal scan is 1.2% per year versus 8.3% for patients with an abnormal scan.2,3 The higher event rates with pharmacologic stress MPI are attributed to a higher pretest clinical risk in patients deemed unable to exercise. The greater the stress perfusion abnormalities, the higher the event rates.3 The percentage of the left ventricle rendered ischemic with stress has proven clinically useful in separating patients who might benefit from coronary revascularization versus those who have a good outcome beginning medical therapy.4 The percent left ventricular (LV) ischemia can also be used to serially evaluate response to therapy. The COURAGE nuclear substudy showed that patients who did not experience a 5% or more decrease in ischemic defect size after 1 year of therapy had a substantially higher cardiac event rate over 6 to 7 years of follow-up, compared to patients who showed a greater than 5% reduction in ischemic defect size.5 Interestingly, those patients who had no residual ischemia had no future events during follow-up. Patients with diabetes6 and those with chronic kidney disease7 have higher cardiac event rates with ischemic SPECT studies than patients without diabetes or chronic kidney disease. Such patients also have a higher risk of cardiac death or nonfatal myocardial infarction with normal SPECT scans.2
Specificity of SPECT MPI is enhanced when gated images are obtained, since fixed defects due to attenuation artifacts can be distinguished from myocardial scar on stress and rest images.8 Those due to scar (or severe ischemia) will show abnormal wall motion or abnormal wall thickening, whereas those due to attenuation artifacts, as with breast attenuation in women or inferobasilar wall attenuation in men, will show normal regional function. Gated SPECT MPI can provide functional information that contributes to the diagnostic and prognostic value of the test.9 Functional variables include the left ventricular ejection fraction (LVEF), systolic and diastolic volumes at end-systole and end-diastole, and extent of regional wall-thickening or wall-motion abnormalities. Regional wall-motion or thickening abnormalities seen on SPECT images correlate well with measurements made on echocardiography and MRI. Transient ischemic cavity dilation from stress to rest images can also be identified and in most cases represents stress-induced subendocardial ischemia that resolves with rest. It is a scintigraphic marker of high-risk CAD.10
Attenuation correction algorithms have been introduced for clinical SPECT imaging and have enhanced the accuracy of CAD detection, chiefly by reducing false-positive studies that interpret attenuation artifacts as perfusion abnormalities.11 Standardization of attenuation correction methods from one vendor to another has not yet been achieved. Another variable that may confound the interpretation of SPECT tomograms is high visceral activity, which may interfere with the evaluation of the inferior wall. This is more of an issue with vasodilator stress than with exercise stress. Image quality is also diminished with marked obesity, requiring 2-day stress/rest studies to optimize quality by having more activity injected with the rest image than is possible in same-day, low-dose rest and high-dose stress studies. The SPECT artifacts that interfere with accurate interpretation of MPI images are very well summarized in Chapters 6 and 7.
Stress myocardial perfusion imaging, employing the technetium (Tc)-99m-labeled tracers underestimates the extent of significant coronary artery stenoses.12 Some patients with left main and/or three-vessel CAD may have uniform tracer uptake on vasodilator stress perfusion SPECT images if coronary flow reserve is diffusely reduced in the supply regions of all three major coronary vessels. In the study by Lima et al., only a small percentage of patients with angiographic three-vessel disease had perfusion abnormalities in the distribution of all three affected coronary arteries13; on gated SPECT images, 23% had perfusion and/or regional wall-motion abnormalities in the myocardial supply zones of the three stenotic vessels (defined as =50% stenosis). Even using quantitative analysis of relative differences in regional counts on vasodilator stress SPECT, 12% of the patients in this study with three-vessel CAD had no perfusion or function abnormalities on their SPECT studies. Similarly, Berman et al.14 reported that among 101 patients with left main CAD (=50% stenosis) and no clinical evidence of prior infarction, 40% had low-risk SPECT scans with less than 10% LV ischemia, and 15% had no ischemic defects.
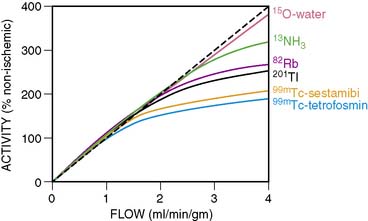
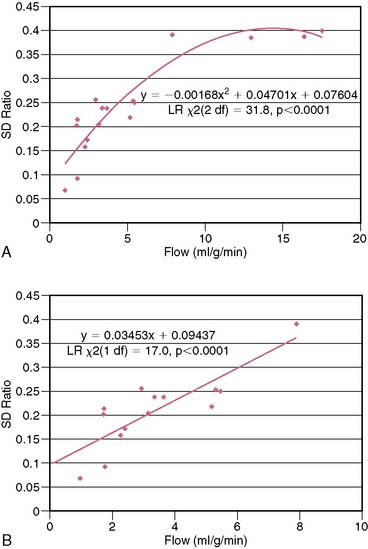
The underestimation of the extent of CAD with SPECT is perhaps more common when 99mTc perfusion agents are used (sestamibi and tetrofosmin) rather than 201Tl.15 The first-pass myocardial extraction fraction is higher with 201Tl (80%) than the other two tracers (55% to 60%), and myocardial uptake is more proportional to flow in the hyperemic flow ranges after vasodilator administration. But in fact all three perfusion agents plateau at high flows (Fig. 23-1). This particularly adversely affects the detection of mild to moderate stenoses.16 Standard SPECT imaging normalizes counts to the region of the myocardium with the highest activity (regional counts). If diffuse multivessel disease is present, that “normal” area may also have subnormal perfusion in response to vasodilator infusion. In this situation, counts in that region may not be higher than in other perfusion zones, since flow reserve is diffusely diminished. When the activity is homogeneous throughout the myocardium in such patients, “balanced ischemia” is often present. If postischemic myocardial stunning or chronic hibernation is present, or if the patient had a prior subendocardial infarction, abnormal regional function can be detected on gated images that would render the interpretation of the stress study as abnormal, despite the absence of focal perfusion abnormalities. Also, transient ischemic dilation of the LV cavity from stress to rest images can at times be observed with normal or minimally abnormal perfusion scans. This finding would hint at the possibility of more extensive underlying CAD.17 A larger LV cavity post stress (compared to rest) is thought to be due to transient stress-induced subendocardial hypoperfusion.
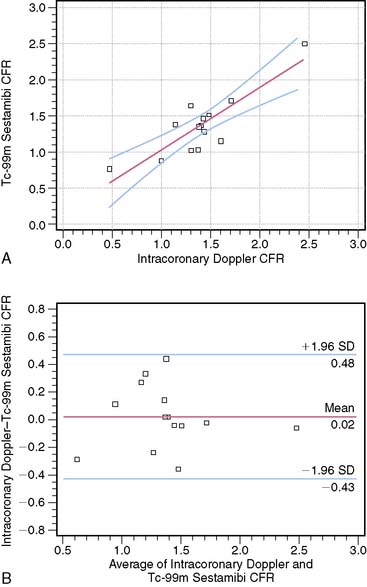
Some attempts have been undertaken to quantitate coronary flow reserve with SPECT perfusion imaging.18 This would address the limitation of standard SPECT in which only relative differences in perfusion from one area of the myocardium to another area are assessed. Estimation of coronary flow reserve (CFR) by SPECT18 involves acquiring anterior planar list-mode images of the heart after intravenous tracer administration. Counts from a right pulmonary artery region of interest are quantified to estimate the arterial input function of the tracer. An estimate of myocardial tissue perfusion is determined by dividing myocardial counts on the SPECT images by the integrated arterial input function. Perfusion values for the stress studies are divided by the corresponding values from the rest studies to obtain myocardial perfusion reserve. Limitations of SPECT that do not allow absolute quantitation of regional myocardial blood flow in mL/min/g are factors such as scatter, attenuation, partial-volume effect, and low resolution. Nevertheless, just being able to evaluate CFR by measuring the ratios of tissue and arterial counts at rest and stress might enhance the detection of multivessel CAD. Figure 23-2 shows the relationship between SPECT-estimated CFR and intracoronary Doppler CFR measurements. Studies continue to be performed to try to achieve absolute quantitation of perfusion with dynamic SPECT imaging.19 Using an experimental canine model, these investigators demonstrated that the kinetic analysis of quantitatively assessed myocardial 201Tl accumulation provided myocardial blood flow measurements that agreed well with flows obtained using radioactive microspheres.
Recent advances have been made in SPECT technology, permitting high-speed imaging using a bank of independently controlled detector columns with large-hole tungsten collimators and multiple cadmium zinc telluride crystal arrays (see Chapter 9).20 Using this high-speed camera, the entire stress/rest 99mTc sestamibi MPI procedure could be completed within 30 minutes. The stress and rest acquisition times were 16 and 12 minutes for conventional SPECT MPI and 4 and 2 minutes for high-speed SPECT. Image quality was high, and myocardial count rates were significantly higher versus conventional SPECT. Diagnostic performance for high-speed SPECT was as good as for conventional SPECT, with summed stress scores and summed reversibility scores correlating extremely well (r = 0.93). Introduction of this new technology will reduce the long procedure time for a full stress/rest study as currently performed with conventional SPECT.
POSITRON EMISSION TOMOGRAPHY (See Chapter 19)
PET MPI is an attractive alternative to SPECT for detecting CAD and determining its extent. PET perfusion tracers used clinically for MPI are rubidium (Rb)-82 and nitrogen (N)-13-ammonia. 82Rb is eluted from a generator and has a short half-life of 78 seconds. It is only used with pharmacologic stress and not exercise, which is a limitation. 13N-ammonia requires a cyclotron for production and has a half-life of 10 minutes. They both show a plateau in myocardial activity with increasing coronary flow, although the flow-uptake relationship is better than that of the 99mTc SPECT perfusion tracers (see Fig. 23-1). In the meta-analysis of clinical studies reported in the literature, the sensitivity and specificity of PET MPI are 89% and 86%, respectively.1 When CT is used for attenuation correction, sensitivity is increased to 93% and 95% for detecting patients with multivessel disease.21 Bateman et al.22 compared SPECT with PET for CAD detection in different groups of patients studied in the same time period. They showed that the sensitivity and specificity were higher for PET (87% versus 82% and 93% versus 73%, respectively), as was the prediction of multivessel disease (71% versus 48%). Some reasons for the improved accuracy of PET over SPECT include higher spatial and contrast resolution and the fact that depth-independent attenuation correction is more easily performed, since it is intrinsic to PET methodology. Since the half-lives of the tracers used for PET MPI are so short, fast sequential evaluations of regional flow can be achieved. This promotes improved throughput of patients in clinical laboratories.
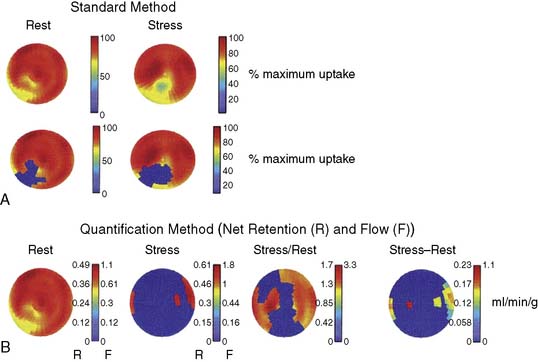
PET has the capability for rapid dynamic imaging of tracer kinetics, permitting absolute quantitation of myocardial blood flow not easily achieved by SPECT technology. This capability is one of the most important advantages of PET over SPECT and other noninvasive technologies for assessing myocardial perfusion. Quantitating regional myocardial blood flow in mL/min/g using tracer kinetic models with rapid dynamic imaging, and obtaining accurate values for CFR rather than just assessing relative differences in perfusion (myocardial counts) from one myocardial region to another, can be achieved. Oxygen-15-labeled water is a positron emitter that is metabolically inert, freely diffusible, and cyclotron-generated. There is virtually complete extraction of this tracer by the myocardium, independent of flow rate and myocardial metabolic state. This makes it the ideal radionuclide for quantitating absolute myocardial blood flow. Quantitation of blood flow with any of the PET tracers includes corrections for the partial volume effect (that causes an underestimation of myocardial tracer concentration), activity spillover from the blood pool to the myocardium, and physical decay. Parkash et al.23 showed that quantitation of 82Rb net retention and estimation of absolute perfusion at rest and with dipyridamole stress defined a greater extent of CAD in patients with three-vessel disease than using the standard approach of assessing relative differences in perfusion (69% of LV sectors versus 44%). The absolute stress-minus-rest perfusion difference in the myocardium is measured in this approach. Figure 23-3 shows an example of the comparison of the standard method for identification of perfusion defects with the quantification method in a patient with stenoses in the left anterior descending (LAD), right, and circumflex coronary arteries. A very good relationship was found between dipyridamole-induced myocardial blood flow in mL/min/g by 82Rb quantitation and stenosis severity in patients with CAD.24 The repeatability of rest and hyperemic myocardial blood flow measurements with 82Rb PET is excellent.25 Myocardial blood flow determinations were highly reproducible in this study of 15 healthy volunteers who underwent two rest and pharmacologic stress studies 60 minutes apart.
PET can be used to assess CFR in the preclinical state in patients with CAD risk factors who may have endothelial and microvascular dysfunction.26–28 Abnormal CFR by PET has been reported in patients with dyslipidemia, diabetic patients, smokers, and patients with hypertension. These risk factors are associated with abnormal vasodilator responses in the coronary circulation. Impaired coronary vasoreactivity may have prognostic implications. PET assessments of CFR with vasodilator stress can be undertaken before and after therapeutic interventions (e.g., statin treatment of hypercholesterolemia) to monitor improvement in myocardial perfusion reserve.26 The normal CFR response to dipyridamole and adenosine is approximately 3.5 to 4.0.29 This hyperemia is attenuated in patients with coronary atherosclerosis in the absence of critical stenoses. Additionally, PET measurement of myocardial blood flow responses to cold pressor testing, causing sympathetic stimulation, identifies a group of patients with an increased cardiac event rate who have normal coronary angiograms.30
Thus, as summarized in Table 23-1, PET has certain advantages over SPECT, particularly when quantitation of perfusion or CFR is accomplished. Disadvantages of PET are limited availability in most institutions for routine clinical imaging, inability to combine PET MPI with exercise stress for 82Rb imaging, and higher cost than SPECT. For tracers other than 82Rb, an on-site cyclotron is needed for tracer production. One exciting advance in PET MPI is the emergence of a new perfusion imaging agent, 18F-BMS-747158-02, a novel pyridaben derivative that binds to the mitochondrial complex, 2(MTC1), of the electron transport chain with very high affinity.31,32 The first-pass extraction fraction of this 18F-labeled PET perfusion tracer is similar to 201Tl, and like sestamibi and tetrofosmin, it does not show redistribution. Extraction of this tracer is high at different flow rates, which makes it suitable for quantitation of absolute blood flow with dynamic PET imaging of tracer kinetics. In a pig model of transient coronary artery occlusion, uptake of 18F-BMS showed a good correlation with regional flow measured by radioactive microspheres (r = 0.88), with flows ranging from 1 mL/min/g to 3 mL/min/g.33 The 109-minute half-life of 18F would permit the use of exercise stress imaging, since adequate time would be available between the time of tracer injection during peak exercise and acquisition of PET MPI images. 18F is also widely available commercially and an in-house cyclotron is not necessary to produce it.
CARDIAC MAGNETIC RESONANCE PERFUSION IMAGING (See Chapter 17)
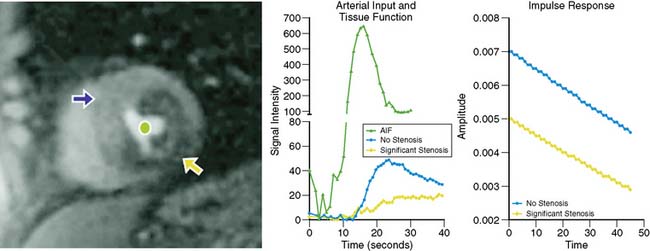
Myocardial perfusion can be assessed at rest and during vasodilator stress, exploiting the first-pass kinetics of T1-weighted CMR imaging of gadolinium (Gd) chelates (e.g., Gd-DTPA) after bolus injection of the contrast agent.34 The basic concept of CMR perfusion imaging by this approach is that signal intensity of the myocardium is proportional to myocardial blood flow as the contrast agent enters the microvasculature and the interstitial space. Normally perfused myocardium shows a fast signal increase per unit of time when contrast is injected during vasodilator stress. Areas perfused by stenotic vessels show a delay in the signal increase. The data acquisition for the first-pass contrast measurement can be obtained in only a few seconds during a single breath hold. The MRI data are collected with ECG gating, which eliminates cardiac motion. Because of the excellent spatial resolution of CMR, the transmurality of perfusion defects can be resolved. Most often, the stress-induced perfusion abnormalities are identified in the subendocardium. Figure 23-4 shows an example of a first-pass CMR perfusion imaging study, with associated time-intensity curves showing hypoperfusion in the stenotic region.34 Late enhancement imaging after the first-pass data are acquired permits the differentiation between myocardial scar, which shows late Gd hyperenhancement, and transient ischemia, where the contrast washes out of the myocardium rather rapidly. It is also possible to measure coronary flow reserve with first-pass CMR by comparing rest and stress CMR data. Table 23-1 lists the strengths and limitations of CMR imaging of myocardial perfusion.
A meta-analysis of CMR perfusion imaging studies demonstrated a sensitivity of 91% and a specificity of 81% for CAD detection.35 The prevalence of CAD in these studies averaged 57%. The MR-IMPACT study compared CMR perfusion imaging with SPECT for detection of CAD.36 They found in a cohort of 241 patients who underwent adenosine stress Gd-DTPA-BMA (Omniscan, GE Healthcare) that perfusion CMR had a similar performance as SPECT by area under the receiver operating characteristic (ROC) curve analysis. The MR-IMPACT study compared the effect of five different contrast doses on image quality and diagnostic ability to detect CAD. The prognostic value of CMR perfusion imaging has not yet been ascertained, although one study37 showed differences in event rates between patients with normal and abnormal scans.
CMR perfusion imaging has some advantages over SPECT and PET MPI:
Improvements in temporal resolution with CMR have permitted acquisition of several tomograms of the heart within one heartbeat. With CMR, myocardial viability, regional function, and global function can be evaluated during the same study in which first-pass perfusion imaging is performed. The ability to quantitate flow in absolute terms is similar to PET MPI. Quantitative perfusion CMR with adenosine, in which the ratio between stress and rest myocardial blood flow determinations by the CMR time intensity curve analysis was derived, correlates well with fractional flow reserve of coronary stenoses determined at cardiac catheterization.38 Thus, hemodynamic significance of coronary stenoses can be assessed by quantitative CMR perfusion imaging. As mentioned, SPECT MPI can underestimate three-vessel CAD because of “balanced ischemia” and diffuse abnormal flow reserve. In such patients, the SPECT perfusion study could show uniform tracer uptake at both rest and stress. With CMR perfusion imaging, diffuse subendocardial ischemia in the setting of three-vessel or left main CAD can be detected both visually and quantitatively because of the excellent spatial resolution of CMR. Absolute perfusion measurements are obtained by deconvolution of the tissue function and the arterial input function, similar to what is accomplished with PET. The microcirculation can also be assessed with CMR perfusion imaging. In patients with no-reflow after reperfusion following an acute myocardial infarction, a characteristic pattern on late Gd enhancement is identified.39,40 Myocardial perfusion reserve abnormalities by CMR were reported in the subendocardium in patients with cardiac syndrome X.41,42
Most CMR studies are performed with a 1.5 tesla (T)–strength magnet. A study by Cheng et al.43 showed that for CMR perfusion imaging, a 3-T field strength was superior to 1.5 T with respect to sensitivity (89% versus 84%), specificity (76% versus 67%), and positive and negative predictive values for CAD detection. In this study, four short-axis images were acquired during every heartbeat using a saturation recovery fast-gradient echo sequence and 0.04 mmol/kg Gd-DTPA bolus injection. The 3-T system provides better signal-to-noise ratio and contrast enhancement, which improves spatial resolution and image quality. The authors of this paper suggested that 3-T may become the preferred CMR field strength for myocardial perfusion assessment in clinical practice.
COMPUTED TOMOGRAPHY PERFUSION IMAGING
Myocardial perfusion can be evaluated by multidetector CT (MDCT) scanning using first-pass, contrast-enhanced methodology similar in principle to first-pass imaging of myocardial perfusion.44 However, for CT perfusion imaging, iodinated contrast agents are injected during vasodilator stress rather than a non–radiation producing agent like Gd-DTPA. With CT perfusion imaging, myocardial signal intensity correlates with myocardial blood flow. The first-pass myocardial extraction fraction of the contrast agent is rather low at 0.33, compared to 0.50 to 0.60 for first-pass CMR and 0.80 for 201Tl. Significant diffusion of contrast moves into the extravascular space. Correction for this diffusion must be undertaken to get accurate measurements of absolute blood flow. In a LAD occlusion model, MDCT first-pass contrast-enhanced perfusion imaging correlated extremely well with microsphere-determined blood flow up to 8.0 mL/g/min (P = 0.001).45 Figure 23-5 shows the data from this animal validation study. A few single-center patient studies of vasodilator stress CT perfusion imaging have been reported, and the findings show promise for using this first-pass perfusion imaging approach for detecting CAD.46,47
The strengths and limitations of CT scanning for myocardial perfusion during stress and rest are listed in Table 23-1. Perfusion imaging with CT may offer some advantages over other techniques so far discussed: high spatial resolution, rapid data acquisition, and the ability to assess coronary anatomy, perfusion, and regional and global ventricular function with one test.48,49 The CT perfusion imaging technique could prove useful in detecting regional myocardial hypoperfusion in patients with acute chest pain presenting to an emergency department. One study of 72 patients who underwent first-pass CT imaging for detection of hypoenhancement found that the technique detected more infarcts than SPECT imaging.50 Like CMR, cardiac CT has the capability to quantitate perfusion and coronary flow reserve. CMR, however, has better temporal resolution than CT. Limitations of cardiac CT are lower-quality images with high heart rates, beam-hardening artifacts resulting in variations of signal intensity in the myocardium, and inability to use high doses of iodinated contrast in patients with renal dysfunction.48 Radiation doses with rest and stress studies using dynamic imaging are quite high with current technology. For CMR perfusion imaging, no radiation exposure occurs. Obviously, CT perfusion imaging is in its infancy, and further studies are warranted to determine its worth over existing technologies that have less radiation exposure and can be undertaken with exercise stress.
PERFUSION IMAGING WITH MYOCARDIAL CONTRAST ECHOCARDIOGRAPHY (See Chapter 18)
Myocardial contrast echocardiography (MCE) images the microcirculation at rest and with vasodilator stress in order to detect functionally significant CAD.51,52,53,54 The gas-filled microbubbles remain in the intravascular space after intravenous administration. Approximately 90% of the microbubbles reside in the capillaries. In the presence of a critical coronary artery stenosis, hyperemia produced by vasodilator stress results in a decrease in capillary volume. This decrease is thought to be secondary to capillary derecruitment in the stenosed bed because of an imbalance between changes in coronary driving pressure and flow during vasodilation.55 The capillaries derecruit to maintain capillary hydrostatic pressure. The resultant decrease in capillary volume in this situation is what produced the perfusion defects on MCE.
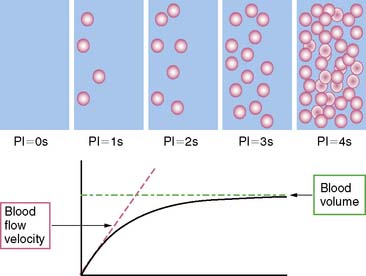
The technique now most often utilized for perfusion imaging with MCE is destroying the microbubbles that are infused to a steady state with high-energy ultrasound and then monitoring the rate of microbubble replenishment within the ultrasound beam (Fig. 23-6).56 When fully replenished, the ultrasound signal represents relative blood volume within the beam. Usually the beam fills within 5 seconds but takes longer when flow is diminished and fills more rapidly with hyperemic flows. The volume of blood in the myocardium, reflected by the microbubbles, is normalized to the signal from the LV cavity. Myocardial blood flow is estimated by the product of the blood volume fraction and the myocardial blood flow velocity. The latter is estimated from rate of bubble reappearance in the myocardium after bubbles were destroyed.56 Only end-systolic images at rest and stress are examined side by side so that the same region within the stress and rest images can be compared at the same pulsing interval. Delayed subendocardial replenishment during hyperemia is reflective of an underlying coronary artery stenosis. Quantitative estimates of regional myocardial blood flow comprise fitting certain parameters to time-intensity curves derived from microbubble replenishment.56
Early studies for CAD detection with MCE showed sensitivities ranging from 75% to 96%, with specificities ranging from 55% to 100%. In most of these studies, SPECT was the gold standard.55 A meta-analysis comparing SPECT to MCE that examined 9 studies comprising 588 patients revealed a concordance of 81% between the two techniques.57 Another meta-analysis of 18 studies in the literature with more than a thousand patients demonstrated an average sensitivity of 82% and specificity of 80% for CAD detection.57 A multicenter study comparing MCE with SPECT found no difference in sensitivity of (84% versus 82%), with similar specificities (56% versus 52%).54 Agreement between MCE and SPECT for presence or absence of CAD was 73%.
There are certain advantages and limitations to the use of stress MCE for detection of CAD (see Table 23-1).48 Advantages of MCE for the noninvasive assessment of myocardial perfusion include: no radiation exposure to the patient; better spatial resolution than SPECT for identifying subendocardial versus transmural hypoperfusion; ability to quantitate absolute flow; wide availability of echocardiography and lower cost than SPECT; CMR or CT for stress and rest perfusion imaging; and more versatile application in the acute setting (e.g., the emergency department). Disadvantages include: difficulty in obtaining high-quality images in some patients; attenuation artifacts, particularly in the lateral-basal myocardial areas of the left ventricle; and getting adequate spatial coverage of all areas of the LV myocardium. Respiratory motion can also cause image-quality problems, as does the presence of chronic pulmonary disease. The technique is very operator dependent, particularly with the need for sustaining a constant image plane during replenishment of the myocardium by the microbubbles.
1. Schuijf J.D., Poldermans D., Shaw L.J., Jukema J.W., Lamb H.J., de Roos A., Wijns W., van der Wall E.E., Bax J.J. Diagnostic and prognostic value of non-invasive imaging in known or suspected coronary artery disease. Eur J Nucl Med Mol Imaging. 2006;33(1):93-104. Review
2. Shaw L.J., Iskandrian A.E. Prognostic value of gated myocardial perfusion SPECT. J Nucl Cardiol. 2004;11(2):171-185. Review. No abstract available
3. Navare S.M., Mather J.F., Shaw L.J., Fowler M.S., Heller G.V. Comparison of risk stratification with pharmacologic and exercise stress myocardial perfusion imaging: a meta-analysis. J Nucl Cardiol. 2004;11(5):551-561.
4. Hachamovitch R., Hayes S.W., Friedman J.D., Cohen I., Berman D.S. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation. 2003;107(23):2900-2907. Epub 2003 May 27
5. Shaw L.J., Berman D.S., Maron D.J., Mancini G.B., Hayes S.W., Hartigan P.M., Weintraub W.S., O’Rourke R.A., Dada M., Spertus J.A., Chaitman B.R., Friedman J., Slomka P., Heller G.V., Germano G., Gosselin G., Berger P., Kostuk W.J., Schwartz R.G., Knudtson M., Veledar E., Bates E.R., McCallister B., Teo K.K., Boden W.E. COURAGE Investigators. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117(10):1283-1291. Epub 2008 Feb 11
6. Anand D.V., Lim E., Hopkins D., Corder R., Shaw L.J., Sharp P., Lipkin D., Lahiri A. Risk stratification in uncomplicated type 2 diabetes: prospective evaluation of the combined use of coronary artery calcium imaging and selective myocardial perfusion scintigraphy. Eur Heart J. 2006;27(6):713-721. Epub 2006 Feb 23
7. Hakeem A., Bhatti S., Dillie K.S., Cook J.R., Samad Z., Roth-Cline M.D., Chang S.M. Predictive value of myocardial perfusion single-photon emission computed tomography and the impact of renal function on cardiac death. Circulation. 2008;118(24):2540-2549. Epub 2008 Dec 1
8. Smanio P.E., Watson D.D., Segalla D.L., Vinson E.L., Smith W.H., Beller G.A. Value of gating of technetium-99m sestamibi single-photon emission computed tomographic imaging. J Am Coll Cardiol. 1997;30(7):1687-1692.
9. Travin M.I., Heller G.V., Johnson L.L., Katten D., Ahlberg A.W., Isasi C.R., Kaplan R.C., Taub C.C., Demus D. The prognostic value of ECG-gated SPECT imaging in patients undergoing stress Tc-99m sestamibi myocardial perfusion imaging. J Nucl Cardiol. 2004;11(3):253-262.
10. Abidov A., Germano G., Berman D.S. Transient ischemic dilation ratio: a universal high-risk diagnostic marker in myocardial perfusion imaging. J Nucl Cardiol. 2007;14(4):497-500. No abstract available
11. Thompson R.C., Heller G.V., Johnson L.L., Case J.A., Cullom S.J., Garcia E.V., Jones P.G., Moutray K.L., Bateman T.M. Value of attenuation correction on ECG-gated SPECT myocardial perfusion imaging related to body mass index. J Nucl Cardiol. 2005;12(2):195-202.
12. Beller G.A. Underestimation of coronary artery disease with SPECT perfusion imaging. J Nucl Cardiol. 2008;15(2):151-153. No abstract available
13. Lima R.S., Watson D.D., Goode A.R., Siadaty M.S., Ragosta M., Beller G.A., Samady H. Incremental value of combined perfusion and function over perfusion alone by gated SPECT myocardial perfusion imaging for detection of severe three-vessel coronary artery disease. J Am Coll Cardiol. 2003;42(1):64-70.
14. Berman D.S., Kang X., Slomka P.J., Gerlach J., de Yang L., Hayes S.W., Friedman J.D., Thomson L.E., Germano G. Underestimation of extent of ischemia by gated SPECT myocardial perfusion imaging in patients with left main coronary artery disease. J Nucl Cardiol. 2007;14(4):521-528.
15. Beller G.A., Bergmann S.R. Myocardial perfusion imaging agents: SPECT and PET. J Nucl Cardiol. 2004;11(1):71-86. Review. No abstract available
16. Shanoudy H., Raggi P., Beller G.A., Soliman A., Ammermann E.G., Kastner R.J., Watson D.D. Comparison of technetium-99m tetrofosmin and thallium-201 single-photon emission computed tomographic imaging for detection of myocardial perfusion defects in patients with coronary artery disease. J Am Coll Cardiol. 1998;31(2):331-337.
17. Abidov A., Bax J.J., Hayes S.W., Hachamovitch R., Cohen I., Gerlach J., Kang X., Friedman J.D., Germano G., Berman D.S. Transient ischemic dilation ratio of the left ventricle is a significant predictor of future cardiac events in patients with otherwise normal myocardial perfusion SPECT. J Am Coll Cardiol. 2003;42(10):1818-1825.
18. Petretta M., Soricelli A., Storto G., Cuocolo A. Assessment of coronary flow reserve using single photon emission computed tomography with technetium 99m-labeled tracers. J Nucl Cardiol. 2008;15(3):456-465. Review
19. Iida H., Eberl S., Kim K.M., Tamura Y., Ono Y., Nakazawa M., Sohlberg A., Zeniya T., Hayashi T., Watabe H. Absolute quantitation of myocardial blood flow with (201)Tl and dynamic SPECT in canine: optimisation and validation of kinetic modelling. Eur J Nucl Med Mol Imaging. 2008;35(5):896-905. Epub 2008 Jan 15
20. Sharir T., Ben-Haim S., Merzon K., Prochorov V., Dickman D., Ben-Haim S., Berman D.S. High-speed myocardial perfusion imaging: Initial clinical comparison with conventional dual detector Anger camera imaging. JACC Cardiovasc Imaging. 2008;1:156-163.
21. Sampson U.K., Dorbala S., Limaye A., Kwong R., Di Carli M.F. Diagnostic accuracy of rubidium-82 myocardial perfusion imaging with hybrid positron emission tomography/computed tomography in the detection of coronary artery disease. J Am Coll Cardiol. 2007;49(10):1052-1058. Epub 2007 Feb 26
22. Bateman T.M., Heller G.V., McGhie A.I., Friedman J.D., Case J.A., Bryngelson J.R., Hertenstein G.K., Moutray K.L., Reid K., Cullom S.J. Diagnostic accuracy of rest/stress ECG-gated Rb-82 myocardial perfusion PET: comparison with ECG-gated Tc-99m sestamibi SPECT. J Nucl Cardiol. 2006;13(1):24-33.
23. Parkash R., deKemp R.A., Ruddy T.D., Kitsikis A., Hart R., Beauchesne L., Williams K., Davies R.A., Labinaz M., Beanlands R.S. Potential utility of rubidium 82 PET quantification in patients with 3-vessel coronary artery disease. J Nucl Cardiol. 2004;11(4):440-449.
24. Anagnostopoulos C., Almonacid A., El Fakhri G., Curillova Z., Sitek A., Roughton M., Dorbala S., Popma J.J., Di Carli M.F. Quantitative relationship between coronary vasodilator reserve assessed by 82Rb PET imaging and coronary artery stenosis severity. Eur J Nucl Med Mol Imaging. 2008;35(9):1593-1601. Epub 2008 Apr 19
25. Manabe O., Yoshinaga K., Katoh C., Naya M., deKemp R.A., Tamaki N. Repeatability of rest and hyperemic myocardial blood flow measurements with 82Rb dynamic PET. J Nucl Med. 2009;50(1):68-71. Epub 2008 Dec 17
26. Campisi R., Di Carli M.F. Assessment of coronary flow reserve and microcirculation: a clinical perspective. J Nucl Cardiol. 2004;11(1):3-11. No abstract available
27. Rimoldi O.E., Camici P.G. Positron emission tomography for quantitation of myocardial perfusion. J Nucl Cardiol. 2004;11(4):482-490. Review. No abstract available
28. Neglia D., L’abbate A. Myocardial perfusion reserve in ischemic heart disease. J Nucl Med. 2009;50(2):175-177. Epub 2009 Jan 21. No abstract available
29. Chareonthaitawee P., Kaufmann P.A., Rimoldi O., Camici P.G. Heterogeneity of resting and hyperemic myocardial blood flow in healthy humans. Cardiovasc Res. 2001;50(1):151-161.
30. Schindler T.H., Nitzsche E.U., Schelbert H.R., Olschewski M., Sayre J., Mix M., Brink I., Zhang X.L., Kreissl M., Magosaki N., Just H., Solzbach U. Positron emission tomography-measured abnormal responses of myocardial blood flow to sympathetic stimulation are associated with the risk of developing cardiovascular events. J Am Coll Cardiol. 2005;45(9):1505-1512.
31. Yalamanchili P., Wexler E., Hayes M., Yu M., Bozek J., Kagan M., Radeke H.S., Azure M., Purohit A., Casebier D.S., Robinson S.P. Mechanism of uptake and retention of F-18 BMS-747158-02 in cardiomyocytes: a novel PET myocardial imaging agent. J Nucl Cardiol. 2007;14(6):782-788. Epub 2007 Oct 22
32. Yu M., Guaraldi M.T., Mistry M., Kagan M., McDonald J.L., Drew K., Radeke H., Azure M., Purohit A., Casebier D.S., Robinson S.P. BMS-747158-02: a novel PET myocardial perfusion imaging agent. J Nucl Cardiol. 2007;14(6):789-798. Epub 2007 Oct 22
33. Nekolla S.G., Reder S., Saraste A., et al. Evaluation of the novel myocardial perfusion PET tracer 18F-BMS-747158-02: comparison to 13N ammonia and validation with microspheres in a pig model. Circulation. 2009. in press
34. Patel A.R., Epstein F.H., Kramer C.M. Evaluation of the microcirculation: advances in cardiac magnetic resonance perfusion imaging. J Nucl Cardiol. 2008;15(5):698-708. No abstract available
35. Nandalur K.R., Dwamena B.A., Choudhri A.F., Nandalur M.R., Carlos R.C. Diagnostic performance of stress cardiac magnetic resonance imaging in the detection of coronary artery disease: a meta-analysis. J Am Coll Cardiol. 2007;50(14):1343-1353. Epub 2007 Sep 17. Review
36. Schwitter J., Wacker C.M., van Rossum A.C., Lombardi M., Al-Saadi N., Ahlstrom H., Dill T., Larsson H.B., Flamm S.D., Marquardt M., Johansson L. MR-IMPACT: comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur Heart J. 2008;29(4):480-489. Epub 2008 Jan 21
37. Bodi V., Sanchis J., Lopez-Lereu M.P., Nunez J., Mainar L., Monmeneu J.V., Husser O., Dominguez E., Chorro F.J., Llacer A. Prognostic value of dipyridamole stress cardiovascular magnetic resonance imaging in patients with known or suspected coronary artery disease. J Am Coll Cardiol. 2007;50(12):1174-1179. Epub 2007 Sep 4
38. Costa M.A., Shoemaker S., Futamatsu H., Klassen C., Angiolillo D.J., Nguyen M., Siuciak A., Gilmore P., Zenni M.M., Guzman L., Bass T.A., Wilke N. Quantitative magnetic resonance perfusion imaging detects anatomic and physiologic coronary artery disease as measured by coronary angiography and fractional flow reserve. J Am Coll Cardiol. 2007;50(6):514-522. Epub 2007 Jul 23
39. Lima J.A., Judd R.M., Bazille A., Schulman S.P., Atalar E., Zerhouni E.A. Regional heterogeneity of human myocardial infarcts demonstrated by contrast-enhanced MRI. Potential mechanisms. Circulation. 1995;92(5):1117-1125.
40. Rochitte C.E., Lima J.A., Bluemke D.A., Reeder S.B., McVeigh E.R., Furuta T., Becker L.C., Melin J.A. Magnitude and time course of microvascular obstruction and tissue injury after acute myocardial infarction. Circulation. 1998;98(10):1006-1014.
41. Petersen S.E., Jerosch-Herold M., Hudsmith L.E., Robson M.D., Francis J.M., Doll H.A., Selvanayagam J.B., Neubauer S., Watkins H. Evidence for microvascular dysfunction in hypertrophic cardiomyopathy: new insights from multiparametric magnetic resonance imaging. Circulation. 2007;115(18):2418-2425. Epub 2007 Apr 23
42. Lanza G.A., Buffon A., Sestito A., Natale L., Sgueglia G.A., Galiuto L., Infusino F., Mariani L., Centola A., Crea F. Relation between stress-induced myocardial perfusion defects on cardiovascular magnetic resonance and coronary microvascular dysfunction in patients with cardiac syndrome X. J Am Coll Cardiol. 2008;51(4):466-472.
43. Cheng A.S., Pegg T.J., Karamitsos T.D., Searle N., Jerosch-Herold M., Choudhury R.P., Banning A.P., Neubauer S., Robson M.D., Selvanayagam J.B. Cardiovascular magnetic resonance perfusion imaging at 3-tesla for the detection of coronary artery disease: a comparison with 1.5-tesla. J Am Coll Cardiol. 2007;49(25):2440-2449. Epub 2007 Jun 11
44. George R.T., Jerosch-Herold M., Silva C., Kitagawa K., Bluemke D.A., Lima J.A., Lardo A.C. Quantification of myocardial perfusion using dynamic 64-detector computed tomography. Invest Radiol. 2007;42(12):815-822.
45. George R.T., Silva C., Cordeiro M.A., DiPaula A., Thompson D.R., McCarthy W.F., Ichihara T., Lima J.A., Lardo A.C. Multidetector computed tomography myocardial perfusion imaging during adenosine stress. J Am Coll Cardiol. 2006;48(1):153-160. Epub 2006 Jun 21
46. Nagao M., Matsuoka H., Kawakami H., Higashino H., Mochizuki T., Murase K., Uemura M. Quantification of myocardial perfusion by contrast-enhanced 64-MDCT: characterization of ischemic myocardium. AJR Am J Roentgenol. 2008;191(1):19-25.
47. Kido T., Kurata A., Higashino H., Inoue Y., Kanza R.E., Okayama H., Higaki J., Murase K., Mochizuki T. Quantification of regional myocardial blood flow using first-pass multidetector-row computed tomography and adenosine triphosphate in coronary artery disease. Circ J. 2008;72(7):1086-1091.
48. Salerno M., Beller G.A. Non-invasive assessment of myocardial perfusion. Circ Cardiovasc Imaging. 2009. in press
49. Cury R.C., Nieman K., Shapiro M.D., Nasir K., Cury R.C., Brady T.J. Comprehensive cardiac CT study: evaluation of coronary arteries, left ventricular function, and myocardial perfusion—is it possible? J Nucl Cardiol. 2007;14(2):229-243. Review
50. Henneman M.M., Schuijf J.D., Dibbets-Schneider P., Stokkel M.P., van der Geest R.J., van der Wall E.E., Bax J.J. Comparison of multislice computed tomography to gated single-photon emission computed tomography for imaging of healed myocardial infarcts. Am J Cardiol. 2008;101(2):144-148.
51. Kaul S. Myocardial contrast echocardiography: a 25-year retrospective. Circulation. 2008;118(3):291-308. No abstract available
52. Lindner J.R., Wei K. Contrast echocardiography. Curr Probl Cardiol. 2002;27(11):454-519. Review. No abstract available
53. Lepper W., Belcik T., Wei K., Lindner J.R., Sklenar J., Kaul S. Myocardial contrast echocardiography. Circulation. 2004;109(25):3132-3135. Review. No abstract available
54. Jeetley P., Hickman M., Kamp O., Lang R.M., Thomas J.D., Vannan M.A., Vanoverschelde J.L., van der Wouw P.A., Senior R. Myocardial contrast echocardiography for the detection of coronary artery stenosis: a prospective multicenter study in comparison with single-photon emission computed tomography. J Am Coll Cardiol. 2006;47(1):141-145. Epub 2005 Dec 15
55. Wei K. Contrast echocardiography: Fulfilling its promise. ACC Curr J Rev. 2005:27-32.
56. Wei K., Jayaweera A.R., Firoozan S., Linka A., Skyba D.M., Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation. 1998;97(5):473-483.
57. Bhatia V.K., Senior R. Contrast echocardiography: evidence for clinical use. J Am Soc Echocardiogr. 2008;21(5):409-416.