9 Comparison of botulinum toxins
Summary and Key Features
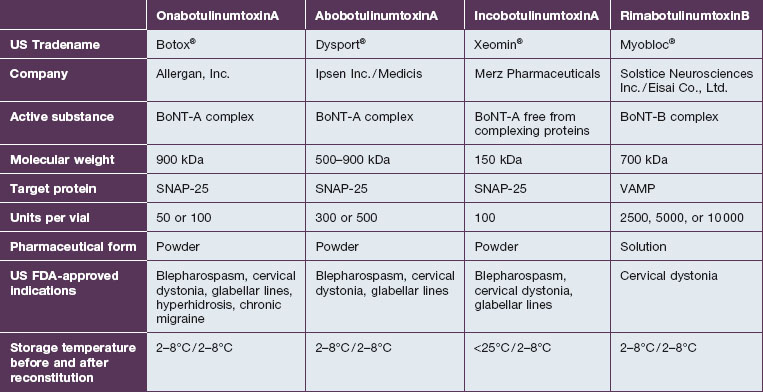
• Botox® (onabotulinumtoxinA), Dysport® (abobotulinumtoxinA) and Xeomin® (incobotulinumtoxinA) are the three commercially available formulations of BoNT-A existing in the US today
• All three formulations are FDA approved for the treatment of glabellar lines; in addition, all are highly effective in treatment of dynamic rhytides of the face and neck
• The botulinum neurotoxin molecule is a single-chain polypeptide found in its native state surrounded by varying amounts of complexing proteins; Botox® contains a larger number of complexing proteins than Dysport®; Xeomin® does not contain complexing proteins
• Although the exact dosing ratio is unclear and may vary depending on application, the ratio between Botox® : Dysport® : Xeomin® is likely to be around 1 : 2.5 : 1
• Clinically the duration of action appears to be similar for all currently available BoNT-A preparations; however, further investigation is needed
• Some evidence suggests that the degree of diffusion may vary between formulations when used to treat hyperhidrosis; however, these differences, if any, are subtle
• Therapeutic failure of BoNT-A in cosmetic use is very rare and the role of blocking antibodies remains unclear
• The safety of Botox® and Dysport® has been well established and Xeomin®, while a newer product, appears to have a similar safety profile
• Vials of Botox® and Dysport® must be kept refrigerated prior to reconstitution; however, Xeomin® may be stored at room temperature prior to use
Types of botulinum toxin A
There are multiple formulations of BoNT-A available in the US and internationally; however, the practitioner must be aware that these products are not interchangeable and important differences exist. As such, in 2009 the US FDA established unique drug names to ‘help ensure patient safety and reduce confusion’ (Table 9.1). What follows is a review of available products and their known and theoretical differences.
There are currently three formulations of BoNT-A available in the US today for cosmetic applications: onabotulinumtoxinA or Botox® (Allergan, Inc, Irvine, California, USA), abobotulinumtoxinA or Dysport® (Ipsen Biofarm Ltd, Berkshire, UK) and incobotulinumtoxinA or Xeomin®® (Merz Pharmaceuticals, Frankfurt am Main, Germany). For ease of discussion, the products will be referenced by their trade names moving forward. Botox® (Fig. 9.1) was the first to become available and continues to dominate the worldwide cosmetic market. In the USA it is approved for the treatment of glabellar lines and hyperhidrosis. It has also been shown to be highly effective for such ‘off-label’ uses as the treatment of dynamic rhytides of the forehead, periorbital area, midface, perioral area, and neck.
Dysport® (Fig. 9.2), another preparation of BoNT-A, is approved for the treatment of glabellar lines. It has become increasingly popular worldwide since its introduction and has been shown to have similar cosmetic applications to Botox®.
Compositional differences
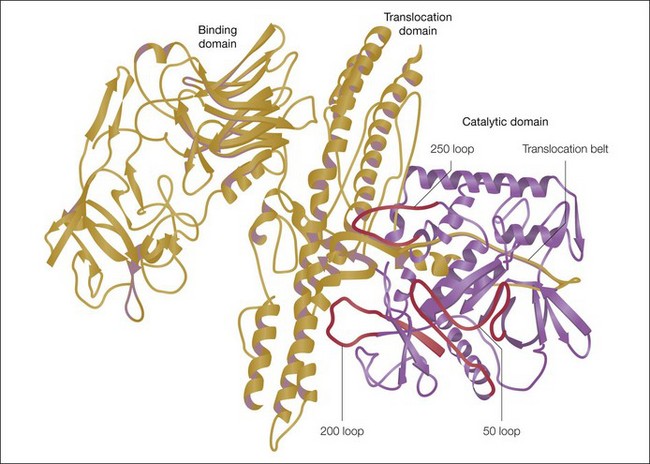
The botulinum neurotoxin molecule is a 150 kDa single-chain polypeptide consisting of a 100 kDa heavy chain and a 50 kDa light chain. These chains are held together by a disulfide bond and are associated with a zinc atom (Fig. 9.3). In its native state the 150 kDa toxin is found complexed with large protective proteins. These complexing proteins, which are primarily hemagluttinins, act to protect ingested toxin from degradation within the gastrointestinal tract. In neutral to basic pH environments the BoNT-A complex dissociates into the free 150 kDa neurotoxin and high-molecular-weight hemagglutinin components.
The three available formulations of BoNT-A vary in the presence, absence, and amount of complexing proteins. Botox® is the largest of the available preparations at 900 kDA (Fig. 9.4) and is associated with the most complexing proteins. Due to the method employed in its production, Dysport® may be associated with a variable number of proteins with a molecular weight ranging from 500 to 900 kDa. Uniquely in the group, Xeomin® is composed only of the 150 kDa neurotoxin, free of any complexing proteins. Purtox® will also be a non-complexed formulation of botulinum toxin A.
Benecke R, Jost WH, Kanovsky P, et al. A new botulinum toxin type A free of complexing proteins for treatment of cervical dystonia. Neurology. 2005;64(11):1949–1951.
Binder WJ. Long-term effects of botulinum toxin type A (Botox) on facial lines: a comparison in identical twins. Archives of Facial Plastic Surgery. 2006;8(6):426–431.
Brin MF. Basic and clinical aspects of BOTOX. Toxicon. 2009;54(5):676–682.
Carli L, Montecucco C, Rossetto O. Assay of diffusion of different botulinum neurotoxin type a formulations injected in the mouse leg. Muscle Nerve. 2009;40:374–380.
Carruthers A, Carruthers J. History of cosmetic use of botulinum A exotoxin. Dermatologic Surgery. 1998;24:1168–1170.
Carruthers A, Carruthers J. Botulinum toxin type A. Journal of the American Academy of Dermatology. 2005;53(2):284–290.
Carruthers JA, Lowe NJ, Menter MA, et al. A multicentre, double-blind, randomized, placebo-controlled study of efficacy and safety of botulinum toxin type A in the treatment of glabellar lines. Journal of the American Academy of Dermatology. 2002;46:840–849.
Carruthers JD, Carruthers JA. Treatment of glabellar frown lines with C. botulinum-A exotoxin. Journal of Dermatologic Surgery and Oncology. 1992;18:17–21.
De Boulle K, Fagien S, Sommer B, et al. Treating glabellar lines with botulinum toxin type A-hemagglutinin complex: a review of the science, the clinical data, and patient satisfaction. Clinical Interventions in Aging. 2010;5:101–118.
Dressler D. Clinical presentation and management of antibody induced failure of botulinum toxin therapy. Movement Disorders. 2004;19(8 suppl):S92–S100.
Dressler D, Eleopra R. Clinical use of non-A botulinum toxins: botulinum toxin type B. Neurotoxicity Research. 2006;9(2-3):121–125.
Dressler D, Wohlfahrt K, Meyer-Rogge E, et al. Antibody induced failure of botulinum toxin a therapy in cosmetic indications. Dermatologic Surgery. 2010;36(suppl 4):2182–2187.
Eisele KH, Fink K, Vey M, et al. Studies on the dissociation of botulinum neurotoxin type A complexes. Toxicon. 2011;57(4):555–565.
Flynn TC. Botulinum toxin: examining duration of effect in facial aesthetic applications. American Journal of Clinical Dermatology. 2010;11(3):183–199.
Hevia O. Retrospective review of 500 patients treated with abobotulinumtoxin A. Journal of Drugs in Dermatology. 2010;9:1081–1084.
Hsu TS, Dover JS, Arndt KA. Effect of volume and concentration on the diffusion of botulinum exotoxin A. Archives of Dermatology. 2004;140(11):1351–1354.
Inoue K, Fujinaga Y, Watanabe, et al. Molecular composition of Clostridium botulinum type A progenitor toxins. Infection and Immunity. 1996;64:1589–1594.
Jost WH, Kohl A, Brinkmann S, et al. Efficacy and tolerability of a botulinum toxin type A free of complexing proteins (NT 201) compared with commercially available botulinum toxin type A (BOTOX) in healthy volunteers. Journal of Neural Transmission. 2005;112(7):905–913.
Karsai S, Raulin C. Current evidence on the unit equivalence of different botulinum neurotoxin A formulations and recommendations for clinical practice in dermatology. Dermatologic Surgery. 2009;35(1):1–8.
Karsai S, Raulin C. Botox and Dysport: is there a dose conversion ratio in dermatology and aesthetic medicine? Journal of the American Academy of Dermatology. 2010;62(2):346–347.
Lee SK. Antibody-induced failure of botulinum toxin type A therapy in a patient with masseteric hypertrophy. Dermatologic Surgery. 2007;33(1 spec. no.):S105–S110.
Meunier FA, Schiavo G, Molgo J. Botulinum neurotoxins: from paralysis to recovery of functional neuromuscular transmission. Journal of Physiology (Paris). 2002;96:105–113.
Middlebrook JL. Cell surface receptors for protein toxins. In: Simpson LL, ed. Botulinum neurotoxin and tetanus toxin. San Diego: Academic Press; 1989:95–119.
Naumann M, Carruthers A, Carruthers J, et al. Meta-analysis of neutralizing antibody conversion with onabotulinumtoxinA (BOTOX(r)) across multiple indications. Movement Disorders. 2010;25(13):2211–2218.
Roggenkämper P, Jost WH, Bihari K, et al. Efficacy and safety of a new Botulinum Toxin Type A free of complexing proteins in the treatment of blepharospasm. Journal of Neural Transmission. 2006;113(3):303–312.
Sattler G. Current and future botulinum neurotoxin type A preparations in aesthetics: a literature review. Journal of Drugs in Dermatology. 2010;9(9):1065–1071.
Schiavo G, Matteoli M, Montecucco C. Neurotoxins affecting neuroexocytosis. Physiological Reviews. 2000;80:717–766.
Sharma SK, Singh BR. Enhancement of the endopeptidase activity of purified botulinum neurotoxins A and E by an isolated component of the native neurotoxin associated proteins. Biochemistry. 2004;43:4791–4798.
Simpson LL. Molecular pharmacology of botulinum toxin and tetanus toxin. Annual Review of Pharmacology and Toxicology. 1986;26:427–453.
Trindade de Almeida AR, Marques E, et al. Pilot study comparing the diffusion of two formulations of botulinum toxin type A in patients with forehead hyperhidrosis. Dermatologic Surgery. 2007;33(1 spec. no.):S37–S43.
Trindade DE Almeida AR, Secco LC, et al. Handling botulinum toxins: an updated literature review. Dermatologic Surgery. 2011;37(11):1553–1565.
Tsai YC, Maditz R, Kuo CL, et al. Targeting botulinum neurotoxin persistence by the ubiquitin–proteasome system. Proceedings of the National Academy of Sciences USA. 2010;107:16554–16559.
Wabbels B, Reichel G, Fulford-Smith A, et al. Double-blind, randomised, parallel group pilot study comparing two botulinum toxin type A products for the treatment of blepharospasm. Journal of Neural Transmission. 2011;118(2):233–239.

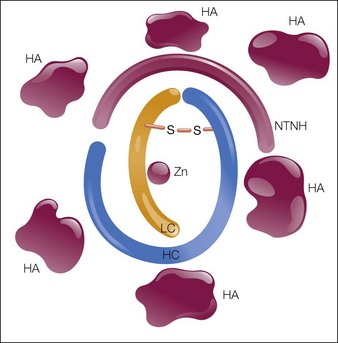
 S = disulfide bond; Zn = zinc.)
S = disulfide bond; Zn = zinc.)