66 Community-Acquired Pneumonia
Pneumonia is an infection of the gas-exchanging units of the lung that is most commonly caused by bacteria but occasionally due to viruses, fungi, parasites, and other infectious agents. It is the eighth leading cause of death in the United States and the number one cause of death from infectious diseases.1 When this infection arises in patients who are residing out of the hospital, it is termed community-acquired pneumonia (CAP), although the population included in this definition is expanding. Currently the “community” includes complex patients such as those who have recently been hospitalized, those in nursing homes, and those with chronic diseases who are commonly managed in such facilities as dialysis centers or nursing homes. These patients are now referred to as having “healthcare-associated pneumonia” (HCAP), and it remains controversial whether their treatment should be similar to that for CAP or nosocomial pneumonia.2
 Incidence
Incidence
In 1994, over 5.6 million people were diagnosed with CAP in the United States. The majority, 4.5 million, were treated out of the hospital, and only a minority of hospitalized patients were cared for in the intensive care unit (ICU).1,3 Although the majority of patients with CAP are managed in the outpatient setting, morbidity, mortality, and the major portion of the cost of treatment is focused on hospitalized patients, particularly those admitted to critical care units. In addition, those patients with comorbid illness and those of advanced age make up a large proportion of the hospitalized critically ill population. In particular, the elderly have a higher mortality from CAP than younger patients, generally as a reflection of the fact that they more commonly have comorbid illness.3
Although CAP can vary from being a mild to a severe illness, very few hospitalized patients are severely ill enough to require ICU admission.4–5 Torres, et al. specifically examined all ICU admissions over a 4-year period and found that 10% were related to CAP.4 In that study, CAP patients who required ICU care were admitted directly to the ICU 42% of the time, after admission to another ward 37% of the time, and after transfer from another hospital in 21% of patients.4 In another study of 395 patients admitted to the hospital with CAP, only a total of 64 (approximately 15%) were admitted to the ICU.6 Recently, Woodhead et al. found that CAP accounted for 5.9% of all ICU admissions, but that early admission (within 2 days of hospitalization) was associated with a lower mortality (46.3%) than late admission (>7 days in the hospital, 50.4% mortality).7
Kaplan and colleagues evaluated the cost of care for elderly patients with CAP in the United States.5 Using Medicare data, they evaluated all individuals aged 65 or older admitted to nonfederal hospitals in 1997. A total of 623,718 patients were evaluated, with 86% being aged 70 or older, and the mean age was 77 years. Underlying illness was present in two-thirds, with congestive heart failure, the most common comorbidity, present in 32%. In this population, the use of ICU, mechanical ventilation, or both was common, with 140,226 patients having complex courses of illness. The overall mortality rate was 10.6% but rose higher with advancing age, nursing home residence, and comorbid illness. The mean length of stay was 7.6 days, with a mean cost of $6949, but costs were greater for patients with complex illness and mechanical ventilation and less for those with simple pneumonia. Costs generally paralleled length of stay but were disproportionately high for those needing mechanical ventilation, where the mean length of stay was 15.7 days and the cost $23,961. Interestingly, there was little extra cost for nonsurvivors compared with survivors, except in the group with complex pneumonia as a whole but not in those requiring mechanical ventilation. The findings not only emphasize the high impact of CAP on costs and outcomes in the United States but also demonstrate the disproportionate increase in costs when patients are treated with mechanical ventilation, thereby raising for discussion the ethics and appropriateness of such care in the very elderly. Other studies of CAP have reported that costs are higher for patients with comorbid illness than those without, but in those without comorbid illness, the cost for those who died was less than for those who survived, while the opposite was true when the entire CAP population was considered.8
 Risk Factors for Developing Severe CAP
Risk Factors for Developing Severe CAP
In all studies of CAP, patients who are admitted to the hospital or ICU commonly have a number of coexisting illnesses, suggesting that individuals who are chronically ill have an increased risk of developing severe illness (Box 66-1). In one study, the mean age of all CAP patients was 59 years, coexisting illness was present in 46%, whereas 74% had a history of prior cigarette smoking.6 Patients often have a history of coexisting illness, and the most common chronic illnesses in these patients are respiratory disease, cardiovascular disease, and diabetes mellitus, findings that have been echoed in a number of studies.1,4,9 In studies of severe CAP, serious coexisting illness is present in 46% to 66% of all patients.4,5,9 The most common respiratory illness in CAP patients is chronic obstructive pulmonary disease (COPD), a finding that applies to those with either mild or severe forms of CAP.4 Among those with severe CAP, cigarette smoking and alcohol abuse are also quite common, and cigarette smoking has been identified as a risk factor for bacteremic pneumococcal infection.4,10 Other common illnesses associated with CAP include malignancy, neurologic illness (including seizures), as well as AIDS.1,9,11 One study identified alcohol abuse as a risk factor, along with the failure to receive antibiotic therapy before hospital admission, a finding suggesting that a delay in therapy may convert milder forms of pneumonia into a more severe illness.9,11 In addition, genetic differences in the immune response may predispose certain individuals to more severe forms of infection and adverse outcomes, and may be reflected by a family history of severe pneumonia or adverse outcomes from infection.
 Prognostic Factors
Prognostic Factors
In a meta-analysis of 33,148 patients with CAP, the overall mortality rate (OR) was 13.7%, but those admitted to the ICU had a mortality rate of 36.5%.12 Eleven prognostic factors were significantly associated with mortality:
In other studies, the clinical features that predict a poor outcome (Box 66-2)1 include advanced age (>65 years), preexisting chronic illness of any type, absence of fever on admission, respiratory rate greater than 30 breaths/min, diastolic or systolic hypotension, elevated blood urea nitrogen (>19.6 mg/dL), profound leukopenia or leukocytosis, inadequate antibiotic therapy, need for mechanical ventilation, hypoalbuminemia, and the presence of certain “high-risk” organisms (type III pneumococcus, Staphylococcus aureus, gram-negative bacilli, aspiration organisms, or postobstructive pneumonia). Other studies have found that when CAP patients have a delay in the initiation of appropriate antibiotic therapy, mortality is increased.1,4,11,13
Box 66-2
Risk Factors for a Poor Outcome from Community-Acquired Pneumonia
One study of 3233 patients in Spain found that risk factors for all-cause mortality were a higher severity of illness on admission, need for ICU care, and the presence of multilobar infiltrates. However, late mortality (after at least 3 days) was reduced if blood cultures were negative, antibiotic therapy was consistent with guidelines, and if an etiologic agent was identified.14 Thus severity of illness on admission most affects early mortality, while therapy-related, modifiable risk factors impact late mortality.
One approach to evaluating CAP patients is to use a scoring system to define prognosis and predict the risk of death. The investigators in the Pneumonia Outcomes Research Team (PORT) study have developed a mortality prediction rule that classifies all patients into one of five groups (Pneumonia Severity Index [PSI] classes I to V), each with a different risk for death.15 Patients in classes IV and V have a predicted mortality risk of 8.2% to 9.3% and 27% to 31.1%, respectively, whereas those in classes I and II have a mortality risk of 0.1% to 0.4% and 0.6% to 0.7%, respectively, and those in class III have a risk of death of 0.9% to 2.8%. To use this scoring system, patients have points calculated based on such factors as age, sex, presence of comorbid medical disease, certain physical findings, and certain laboratory data.15
Although the PORT scoring system has been shown to be accurate for predicting mortality and prognosis, it is important to realize that it does not directly measure severity of illness, since many points in the scoring system are for comorbid conditions rather than features of illness. The investigators from the PORT study evaluated the use of ICU by patients with CAP and the ability of the scoring system to predict need for ICU care. From their original database, 170 patients were admitted to the ICU and compared to 1169 who were managed out of the ICU. While the PORT rule was useful for predicting mortality, there was a poor correlation between the need for ICU admission and the risk of death. In fact, 27% of the ICU patients were in PSI risk classes I to III, and this group, although needing intensive care, had a significantly lower mortality than patients in risk classes IV and V.16 In another study, patients in PORT class V were evaluated, and only about 20% needed ICU admission; they had a 37% mortality compared to the 20% mortality of the PSI V patients who did not need the ICU.17 In general, the PSI V patients who needed the ICU tended to get more of their points from acute illness, while those not needing the ICU tended to score points because of chronic disease factors. The findings are quite important for demonstrating that the need for ICU care does not always equate with a high risk of death. In the Infectious Diseases Society (IDSA)/American Thoracic Society (ATS) CAP guidelines, these limitations were discussed, including the fact that age and comorbidity are heavily weighted variables for defining mortality risk, tending to move all older patients into high PORT score classes.1,18 On the other hand, in a young patient without comorbid illness, the pneumonia must be particularly severe to place the patient in a high-mortality risk group, and certain vital sign thresholds must be exceeded to accumulate points toward a poor prognosis. These thresholds are heart rate greater than 125 beats/min, respiratory rate greater than 30 breaths/min, and systolic blood pressure less than 90 mm Hg.
Although prognostic scoring systems can be complex and difficult to apply in clinical practice, the PORT prediction rule has been promoted as a way to avoid overestimating severity of illness, and calculation of the score has been advocated as a way of keeping some patients out of the hospital who have a low risk of death. For the critical care physician, the opposite problem—underestimating severity of illness—is a more serious concern, and the use of the CURB-65 approach, modified from the British Thoracic Society (BTS) rule, is a simple and accurate way to address this issue. CURB-65, an acronym for the clinical features used to assess pneumonia severity and prognosis,18 assigns 1 point, on a 5-point scale, to confusion, blood urea >7 mmol/L (19.6 mg/dL), respiratory rate ≥30 breaths/min, blood pressure <90 mm Hg systolic or ≤60 mm Hg diastolic, and age ≥ 65 years. In one study, when the score was 0 to 1, the mortality rate was 0%, whereas mortality was more than 20% for a score of 3 or higher, and those with a score of 2 had a mortality of 8.3%.
Use of the CURB-65 rules may be a problem in the elderly, reflecting the altered clinical presentations of pneumonia in this population. In one study, a rule similar to CURB-65 had a 66% sensitivity and a 73% specificity for predicting mortality in a population that included 48% of patients who were at least 75 years of age.19,20 Interestingly, although the rule was not optimal in an elderly population and did not work as well as it did in other populations, it had a higher sensitivity for predicting mortality than the Prognostic Scoring Index (PSI) derived from the PORT study.15,20 Some studies have compared the PSI and CURB-65 and found them to be similar for identifying low-risk populations, but the CURB-65 may be more discriminating for identifying poor prognosis in those with severe illness, compared to the PSI.21
Other prognostic scoring systems have been developed to define the presence of severe pneumonia. One called the CUR-XO is based on defining the need for ICU admission by the presence of one of two major criteria: arterial pH < 7.30 or systolic BP < 90 mm Hg.22 In the absence of these criteria, severe CAP can also be identified by the presence of two of six minor criteria including: confusion, BUN > 30 mg/dL, respiratory rate > 30/minute, PaO2/FIO2 ratio < 250, multilobar infiltrates, and age of at least 80. When these criteria were met, the tool was 92% sensitive for identifying those with severe CAP and was more accurate than the PSI or CURB-65 criteria, although not quite as specific as the CURB-65 rule.22 Using this approach, some criteria (acidosis and systolic hypotension) are weighted more heavily than others, which contrasts with the approach of some of the other approaches to define severe CAP.
A different approach than assessing risk for death is to use scoring systems to define the need for ICU interventions such as intensive respiratory and vasopressor support (IRVS). The SMART-COP tool was developed to predict the need for IRVS.23 Using a multi-variate model, there were eight clinical features associated with the need for IRVS: systolic blood pressure <90 mm Hg, multilobar infiltrates, albumin < 3.5 g/dL, respiratory rate elevation (≥25 for those ≤age 50, and ≥30 for those >age 50), tachycardia (>125/min), confusion, low oxygen (<70 mm Hg if ≤age 50 or <60 mm Hg if >age 50), and arterial pH <7.35. The abnormalities in systolic blood pressure, oxygenation, and arterial pH each received 2 points, while the 5 other criteria received 1 point each, and with this system, the need for IRVS was predicted by a SMART-COP score of at least 3 points. Using this cutoff, the sensitivity for need for IRVS was 92.3% and the specificity 62.3%, with a positive and negative predictive value of 22% and 98.6%, respectively. The PSI and CURB-65 did not perform as well overall for predicting the need for IRVS.
 Pathogenesis
Pathogenesis
Pneumonia results when host defenses are overwhelmed by an infectious pathogen. This may occur because the patient has an inadequate immune response, often as the result of underlying comorbid illness (congestive heart failure, diabetes, renal failure, COPD, malnutrition), because of anatomic abnormalities (endobronchial obstruction, bronchiectasis), as a result of acute illness-associated immune dysfunction (as can occur with certain viral infections), or because of therapy-induced dysfunction of the immune system (corticosteroids). Pneumonia can also occur in patients who have an adequate immune system if the host defense system is overwhelmed by a large inoculum of microorganisms (massive aspiration) or if the patient encounters a particularly virulent organism to which he or she has no preexisting immunity or to which the patient has an inability to form an adequate acute immune response.24,25
Recent studies have evaluated the normal lung immune response to infection and have shown that in most patients with unilateral CAP, the inflammatory response is limited to the site of infection, not spilling over to the uninvolved lung or the systemic circulation.26,27 In patients with localized pneumonia, tumor necrosis factor alpha (TNF-α), interleukin (IL)-6, and IL-8 levels were increased in the pneumonic lung and generally not increased in the uninvolved lung or in the serum.26,27 In patients with severe pneumonia, the immune response is characterized by a “spillover” of the immune response into the systemic circulation, reflected by increases in serum levels of TNF-α and IL-6.28 It remains uncertain why localization does not occur in all individuals and why some patients develop diffuse lung injury (e.g., acute respiratory distress syndrome [ARDS]) or systemic sepsis as a consequence of pneumonia. These complications may result from an inability to develop a brisk lung immune response, as a consequence of either specific bacterial virulence factors, inadequate or delayed therapy, or genetic polymorphisms that affect the immune response. In fact, one study suggested that if bacteria persisted in the lung in spite of therapy, then inflammation in the form of IL-1β was persistent and at a high level, presumably being driven by the ongoing presence of the organisms.29 Although there are a large number of genes that have been identified as being able to affect the severity and outcome of CAP by affecting the inflammatory response, the ability to use this information to impact patient management has not emerged.
Pneumonia-associated inflammation may also impact the long-term mortality of CAP. While the in-hospital mortality implications of CAP are well-known, there is also a high incidence of late mortality among hospitalized CAP patients. In one study of elderly patients hospitalized with CAP, the 1-year mortality rate exceeded 40%.30 The explanation for this finding is unclear, but other studies have shown that patients with high levels of systemic inflammation (defined by serum levels of IL-6 and IL-10) on admission and on discharge have an increased mortality at 6 months to 1 year.31 In addition, patients with evidence of cardiac dysfunction complicating CAP, as reflected by high serum levels of B-natriuretic peptide (BNP) are also likely to have increased disease-related mortality.32
 Clinical Features
Clinical Features
Symptoms and Physical Findings
Patients with CAP and an intact immune system have a normal pulmonary response to infection and generally have respiratory symptoms such as cough, sputum production, and dyspnea, along with fever and other complaints. Cough is the most common finding and present in up to 80% of all patients but is less common in the elderly, those with serious comorbidity, or patients coming from nursing homes.33 The elderly generally have fewer respiratory symptoms than younger individuals; as mentioned, the absence of clear-cut respiratory symptoms and an afebrile status have themselves been predictors of an increased risk of death.1,18 Pleuritic chest pain is also common in patients with CAP, and in one study its absence was also identified as a poor prognostic finding.34
In the elderly patient, pneumonia can have a nonrespiratory presentation with symptoms of confusion, falling, failure to thrive, altered functional capacity, or deterioration in a preexisting medical illness such as congestive heart failure.33,35 In one study, delirium or acute confusion were significantly more frequent in the elderly patients with pneumonia than in age-matched controls who did not have pneumonia.35 In that study, there was no association between the type of isolated microorganisms and the clinical presentation of CAP, except for pleuritic chest pain, which was more common in pneumonia caused by bacterial pathogens such as S. pneumoniae. Approximately 16% of elderly patients with pneumonia were considered well nourished, compared with 47% of controls, with kwashiorkor-like malnutrition being the predominant type of nutritional defect and the one associated with delirium on initial presentation. Several other studies have examined the clinical presentation of pneumonia in the elderly and found that a nursing-home elderly population had a substantially higher mortality rate than other individuals with CAP. These findings may be a reflection of the fact that patients residing in a nursing home had a higher frequency of comorbid illness and dementia. Metlay and coworkers studied 1812 patients of all ages and found that with advancing age, patients tended to have a longer duration of symptoms such as cough, sputum production, dyspnea, fatigue, anorexia, myalgia, and abdominal pain.33 In general, overall symptoms were less prominent in patients older than age 65 than in those who were younger.
Another study evaluated 1474 patients with CAP, of whom 305 were older than 80 years of age.36 The population excluded individuals in nursing homes and severe immune suppression (neutropenia, AIDS, and transplant). Clinically, the very elderly had less pleuritic chest pain, headache, and myalgias and were more likely to be afebrile and to have altered mental status on admission. Overall mortality was higher in the older patients (15% versus 6%), as were in-hospital complications and early mortality (within 48 hours). The PSI values, as expected, were higher in the older population, in part because comorbid illness and age itself add to the PSI score; but still, the mortality rate for patients in PSI class V was 24% in the younger population versus 32% in the elderly.
Physical findings of pneumonia include tachypnea, crackles, rhonchi, and signs of consolidation (egophony, bronchial breath sounds, dullness to percussion). Patients should also be evaluated for signs of pleural effusion. In addition, extrapulmonary findings should be sought to rule out metastatic infection (arthritis, endocarditis, meningitis) or to add to the suspicion of an “atypical” pathogen such as M. pneumoniae or C. pneumoniae, which can lead to complications as bullous myringitis, rash, pericarditis, hepatitis, hemolytic anemia, or meningoencephalitis. One of the most important ways to recognize severe CAP early in the course of illness is to carefully count the respiratory rate. In the elderly, an elevation of respiratory rate can be the initial presenting sign of pneumonia, preceding other clinical findings by as much as 1 to 2 days.37 In fact, in one study, tachypnea was the most common finding in elderly patients with pneumonia, being present in over 60% of all patients and occurring more often in the elderly than in younger patients with pneumonia.33
Radiographic Features
For most patients, CAP is defined by a combination of clinical symptoms and the presence of a new radiographic infiltrate, but not all patients with this illness will have this finding when first evaluated. Even when the radiograph is negative, if the patient has appropriate symptoms and focal physical findings, pneumonia may still be present. In one study, 47 patients with clinical signs and symptoms of CAP were evaluated with both chest radiography and high-resolution computed tomography (CT) of the chest.38 Eight patients with a negative chest radiograph were identified by CT to have pneumonia and, in general, more extensive disease was found on CT than on chest radiography.38 The findings of this study confirm the need to repeat the chest film after 24 to 48 hours in certain symptomatic patients with an initially negative chest film. Although some studies have suggested that febrile and dehydrated patients can have a normal chest radiograph when first admitted with pneumonia, the idea of hydrating pneumonia is in the realm of “conventional wisdom” and anecdotal reports.
The presence of alveolar densities (lobar or bronchopneumonic) has been associated with a high likelihood of a bacterial etiology, but it is extremely difficult to distinguish among specific pathogens by using patterns of radiographic abnormalities.39 The chest radiograph may have prognostic value in patients with severe pneumonia, with multilobar infiltrates or rapid progression of infiltrates serving as poor prognostic signs, helping to identify patients who require intensive care.1,4 Chest CT can also have value in the critically ill patient in situations when a noninfectious process is being considered, or when complications such as pneumothorax, empyema, or abscess are suspected. CT can suggest certain alternative noninfectious diagnoses such as granulomatous vasculitis, acute eosinophilic pneumonia, and bronchiolitis obliterans with organizing pneumonia.
Typical Versus Atypical Pneumonia Syndromes
In the past, the clinical and radiographic features of CAP have been organized into patterns of either “typical” or “atypical” pneumonia syndromes, with the idea being that specific patterns could suggest certain etiologic agents. The typical pneumonia syndrome is characterized by sudden onset of high fever, shaking chills, pleuritic chest pain, lobar consolidation, a toxic-appearing patient, and the production of purulent sputum. Although this pattern has been attributed to pneumococcus and other bacterial pathogens, these organisms do not always lead to such classic symptoms, particularly in the elderly. The atypical pneumonia syndrome, which is characterized by a subacute illness, nonproductive cough, headache, diarrhea, or other systemic complaints, is usually due to infection with M. pneumoniae, C. pneumoniae, Legionella species, or viruses. However, patients with impaired immune responses may present in this fashion, even with bacterial pneumonia. Thus, the ability to use the features on clinical presentation to predict the likely etiologic agents is limited and often misleading.1,39–41
In one study examining the microbial etiology and clinical presentation of CAP, clinical features were no more than 42% accurate in differentiating pneumococcus, M. pneumoniae, and other pathogens from one another.40 In another study of 359 patients with CAP, a comparison of patients with S. pneumoniae, H. influenzae, L. pneumophila, and C. pneumoniae revealed no significant differences in their clinical presentations.41 The limitations of clinical features in defining microbial etiology also apply to evaluations of radiographic patterns.39
Using Clinical Features to Define Severe Community-Acquired Pneumonia
Although there is no uniformly accepted definition for severe CAP, this term generally refers to any patient who is admitted to the ICU because of CAP. Most of these patients have “respiratory failure” which is defined by the presence of hypoxemia or hypercarbia, and not all such patients require mechanical ventilation. Some patients with CAP are treated in the ICU because the pneumonia has led to clinical instability of an underlying disease, but the pneumonia itself may not be severe. Bacteremia does not always correlate with more severe illness, and its presence alone is not always a predictor of a poor outcome, with most episodes of bacteremia being due to pneumococcus. However, in the elderly with pneumococcal pneumonia, bacteremia is present in one fourth of patients with CAP and is often associated with azotemia and multilobe involvement.42 When an infection such as pneumonia is complicated by severe sepsis or septic shock (not just bacteremia), outcome is adversely affected, with increases in mortality, length of stay, and costs for survivors.
For nearly 20 years, guidelines have attempted to define when patients should be admitted to the ICU, but the decision is still best made by careful clinical assessment. The 1993 ATS guidelines used the presence of any one of 10 criteria to define the patients who needed ICU admission.1,6 However, subsequent studies showed that 65% of all admitted CAP patients (not needing ICU care) had one of these criteria present, and thus a more specific definition of the need for ICU admission was required.6 Ewig and colleagues evaluated all 10 criteria in a patient cohort and suggested that ICU admission be considered if patients had two of three “minor criteria” present on admission or one of two “major criteria” present on admission or later in the hospital course.6 The minor criteria were systolic blood pressure less than 90 mm Hg, PaO2/FIO2 ratio less than 250, or multilobar infiltrates, while the major criteria were need for mechanical ventilation or septic shock. This definition of need for ICU care had a sensitivity of 78%, a specificity of 94%, a positive predictive value of 75%, and a negative predictive value of 95%. As discussed earlier, another way to identify patients with more severe illness is to apply the BTS rule in its original or modified version. One study found that the use of the revised ATS criteria had a sensitivity of 70.7% and a specificity of 72.4% for predicting need for ICU admission.16 The BTS criteria were much less sensitive with similar specificity, whereas the PORT rule (class IV or V) had similar sensitivity but lower specificity (although this latter rule was very effective at predicting risk of death).
The most recent IDSA/ATS guidelines for CAP suggested that ICU care be considered if the patient had one of two major criteria (need for mechanical ventilation or septic shock with the need for vasopressors), or 3 of 9 minor criteria.1 The minor criteria include: respiratory rate ≥30 breaths/min, PaO2/FIO2 ratio ≤250, multilobar infiltrates, confusion/disorientation, uremia (BUN level >20 mg/dL), leukopenia (WBC count <4000 cells/mm3), thrombocytopenia (platelet count <100,000 cells/mm3), hypothermia (core temperature <36°C), and hypotension requiring aggressive fluid resuscitation. Other factors to consider in the decision making process are hypoglycemia (in a nondiabetic patient), hyponatremia, acute alcohol intoxication, cirrhosis, asplenia, and unexplained metabolic acidosis. The use of these minor criteria to define need for ICU admission requires validation. However, in one study, patients who met only minor criteria for ICU admission did not have an increase in mortality, whereas in another study, presence of four minor criteria was very accurate for defining the need for ICU care.43,44
There is some debate about the benefit of ICU care for patients with CAP, but the benefit seems most certain if patients are admitted early in the course of severe illness, thus emphasizing the need for sensitive criteria to define severe illness. In one recent study, patients with an obvious need for ICU care who were directly admitted to the ICU had a mortality rate of 10.9%, which was significantly lower than the 19.6% mortality rate of those without obvious need for ICU care who had delayed admission.45 The measurement of admission respiratory rate is a simple and reliable assessment, and investigators have observed a linear relationship between admission respiratory rate (once it rose > 30 breaths/min) and mortality.46 If patients are put in the ICU when they meet several “minor” criteria or when they have an elevated respiratory rate, this type of expectant management may have benefits and may keep mortality rates in the 25% to 50% range. This is in marked contrast to the experience in older studies that reported mortality rates above 70% for pneumococcal bacteremia patients admitted to an ICU late in the course of illness, when nearly all admitted patients were mechanically ventilated on arrival to the ICU. In studies of severe CAP with good outcomes, approximately 60% of ICU admitted patients were intubated.4,6
 Etiologic Pathogens
Etiologic Pathogens
Likely Pathogens
Even with extensive diagnostic testing, an etiologic agent is defined in only about half of all patients with CAP, pointing out the limited value of diagnostic testing and the possibility that we do not know all the organisms that can cause CAP.1,41 In the past 4 decades, a variety of new pathogens for this illness have been identified, including L. pneumophila, C. pneumoniae, severe acute respiratory syndrome coronavirus, novel H1N1 influenza, and hantavirus. In addition, antibiotic-resistant variants of common pathogens such as S. pneumoniae have become increasingly common. One of the ways CAP leads to respiratory failure is when it is complicated by ARDS. All of the bacteria and viruses listed here, as well as pneumonia due to aspiration, have been reported to cause ARDS.
The likely pathogens for infection vary depending on patient risk factors for specific microorganisms and the presence of certain comorbid illnesses, but for all patient groups, including those with severe CAP, pneumococcus is the most common pathogen.1 In fact, in one study of lung puncture cultures, this organism was even identified as being common in patients who had no diagnosis established by routine diagnostic testing.47 The incidence of antibiotic-resistant pneumococci has increased in recent years, and up to 40% of these organisms can have reduced sensitivity to penicillin or other antibiotics, although the clinical relevance of in vitro resistance is still uncertain.1,48,49 Identified risk factors for drug-resistant S. pneumoniae (DRSP) include β-lactam therapy in the past 3 months, alcoholism, age older than 65 years, immune suppression, multiple medical comorbidities, and contact with a child in day care.1,50,51 Other common infecting organisms in those with severe CAP include viruses (e.g., influenza, respiratory syncytial virus, and the coronavirus illness of severe acute respiratory syndrome [SARS]), L. pneumophila, M. pneumoniae, M. tuberculosis, and H. influenzae (especially in smokers). In the setting of severe pneumonia, patients can be infected with S. aureus (including methicillin-resistant forms, or MRSA) or enteric gram-negatives and (rarely) anaerobes. In the elderly, including those with aspiration pneumonia, healthcare-associated pneumonia, and in those with underlying cardiopulmonary disease, enteric gram-negative organisms are often seen.
The frequency of gram-negative CAP is difficult to define, but in one study of 559 hospitalized patients with CAP, 60 patients had gram-negative enteric infections, including 39 with P. aeruginosa.1,52 Risk factors for gram-negative organisms were probable aspiration (OR=2.3), previous hospital admission within 30 days of admission (OR=3.5), previous antibiotics within 30 days of admission (OR=1.9), and presence of pulmonary comorbidity (OR=2.8). Risk factors for P. aeruginosa were pulmonary comorbidity (OR=5.8) and previous hospitalization (OR=3.8). Infection with a gram-negative pathogen led to ICU admission and mechanical ventilation more often than infection with other organisms. The mortality rate of CAP due to P. aeruginosa was 28%. In a more recent study from Korea, 10% of 912 CAP patients had gram-negatives, with Klebsiella spp. being twice as common as P. aeruginosa.53 Patients with gram-negatives had a higher mortality than those without, and risk factors for gram-negative infection included septic shock, cardiac disease, smoking, hyponatremia, and dyspnea. Nursing home patients (HCAP) were included in the population of patients studied, again emphasizing the overlap between CAP and HCAP. Another recent study of 3272 episodes of CAP found that 2% were caused by enteric gram-negatives (most commonly P. aeruginosa), and the risk factors for these organisms were COPD, current use of corticosteroids, prior antibiotic therapy, tachypnea ≥ 30/minute, and septic shock on admission.54 Patients with these organisms needed ICU care more often and had a higher mortality and length of stay than those without these pathogens present.
Although aspiration has often been considered a risk factor for anaerobic infection, studies of severe CAP in elderly patients with aspiration risk factors suggested that this population is very likely to have gram-negative infection.55,56 One study evaluated 95 residents of long-term care facilities who had pneumonia requiring ICU admission and risk factors for oropharyngeal aspiration such as swallowing disorders due to neurologic illness, disruption of the gastroesophageal junction, dysphagia, or anatomic abnormalities. Using protected bronchoalveolar lavage (BAL) sampling within 4 hours of admission, a total of 67 pathogens were identified, with enteric gram-negatives in 49%, anaerobes in 16%, and S. aureus in 12%.55 Fifty-five percent of the anaerobes were recovered along with aerobic gram-negative co-infection. The presence of anaerobes did not correlate with oral hygiene but did correlate with functional status, being more common in patients who were totally dependent. Of the seven patients who received inadequate therapy for anaerobes, six recovered, raising a question about whether these organisms really need to be treated. These findings suggest that anaerobes may not really be pathogens but could simply be colonizers in the institutionalized elderly, including those with aspiration risks.55
Primary pulmonary infection with atypical pathogens has been reported for patients with severe CAP for many years. In fact, in one ICU in Spain, atypical pathogens were present in almost 25% of all patients, but the responsible organism varied over time. Legionella was the most common atypical pathogen leading to severe CAP in 14% of patients during one time period, but in the same hospital a decade later, it was seen in only 2%, having been replaced by Mycoplasma and Chlamydophila infection, which were found in 17% of patients compared with only 6% a decade earlier.9,11 Several studies have shown that even if bacterial pathogens lead to CAP, they can be accompanied by atypical pathogens in the form of mixed infection.57,58 Atypical pathogens can include C. pneumoniae, M. pneumoniae, and L. pneumophila, and some recent studies have shown that these infections are common in patients of all ages, not just young and healthy individuals; these organisms have even been reported among the elderly in nursing homes.1,57,59 When mixed infection is present, it may lead to a more complex course and a longer length of stay than if a single pathogen is present, which may explain the increasing number of studies that show a reduction in CAP mortality, including those in the ICU, when initial therapy provides coverage for these organisms, compared with regimens that do not provide coverage.60,61 Interestingly, multiple retrospective studies of pneumococcal bacteremia have shown a reduced mortality when dual therapy (usually involving a macrolide) rather than monotherapy is used, raising the possibility that even these patients have mixed infection with atypical pathogens.62,63 The frequency of atypical pathogens can be as high as 60%, with as many as 40% of all CAP patients having mixed infection.58 These high incidence numbers have been derived with serologic testing, which is of uncertain accuracy.
Atypical organism pneumonia may not be a constant phenomenon, and the frequency of infection may vary over the course of time and with geography. In fact, one study showed that the benefit of providing empirical therapy directed at atypical pathogens was variable, being more important in some calendar years than in others.61 The incidence of Legionella infection among admitted patients has varied from 1% to 15% or more and is also a reflection of geographic and seasonal variability in infection rates, as well as a reflection of the extent of diagnostic testing.
In the past, S. aureus was an uncommon cause of CAP, but it was capable of leading to severe pneumonia. In the past several years, a community-acquired strain of MRSA (CA-MRSA) has emerged as a cause of severe CAP, particularly in patients without a history of previous hospitalization or chronic illness, often as a complication of influenza infection.1,64,65 The organism can lead to a severe bilateral necrotizing pneumonia, often related to toxin production by the organism. This organism is distinct from the nosocomial strain of MRSA and is clonal in origin, usually due to the USA-300 strain.
Risk Factors for Specific Pathogens
Table 66-1 summarizes the common pathogens causing CAP in hospitalized patients, including those admitted to the ICU. The classification is based on the presence of clinical risk factors for specific pathogens, referred to as modifying factors. The modifying factors for DRSP are age older than 65 years, β-lactam therapy within the past 3 months, alcoholism, immune suppressive illness (including therapy with corticosteroids), multiple medical comorbidities, and exposure to a child in day care.1,50 The modifying factors for enteric gram-negatives include residence in a nursing home (now defining the patient as having HCAP), underlying cardiopulmonary disease, multiple medical comorbidities, and recent antibiotic therapy. For the patient with HCAP, resistant gram-negatives and MRSA can occur, particularly if the patient has multiple risk factors in addition to nursing home residence. These risk factors include severe illness, poor functional status, immune suppression, recent antibiotic therapy, and recent hospitalization in the past 3 months.2 In predicting the likely etiologic pathogens for those admitted to the ICU, patients are divided into a population at risk for pseudomonal infection and a population without this organism being likely. The risk factors for P. aeruginosa infection are structural lung disease (bronchiectasis), corticosteroid therapy (>10 mg prednisone/day), broad-spectrum antibiotic therapy for more than 7 days in the past month, and malnutrition.1
TABLE 66-1 Common Pathogens Causing Community-Acquired Pneumonia
| Inpatient with no cardiopulmonary disease or modifying factors | Streptococcus pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae, Chlamydophila pneumoniae, mixed infection (bacteria plus atypical pathogen), viruses (including influenza), Legionella species, and others (M. tuberculosis, endemic fungi, Pneumocystis jirovecii) |
| Inpatient with cardiopulmonary disease and/or modifying factors | All of the above. but drug-resistant S. pneumoniae (DRSP) and enteric gram-negative organisms are more of a concern. |
| Severe community-acquired pneumonia (CAP) with no risks for P. aeruginosa | S. pneumoniae (including DRSP), Legionella species, H. influenzae, enteric gram-negative bacilli, S. aureus (including MRSA), M. pneumoniae, respiratory viruses (including influenza), others (C. pneumoniae, M. tuberculosis, endemic fungi) |
| Severe CAP with risks for P. aeruginosa | All of the pathogens above plus P. aeruginosa |
Table 66-2 shows that certain clinical conditions are associated with specific pathogens, and these associations should be considered in all patients when obtaining a history.1 For example, if the presentation is subacute following contact with birds, rats, or rabbits, the possibility of psittacosis, leptospirosis, tularemia, or plague should be considered. Certain exposures should also raise concern about specific organisms. Thus, Coxiella burnetii (Q fever) is a concern with exposure to parturient cats, cattle, sheep, or goats; Francisella tularensis is a concern with rabbit exposure; hantavirus with exposure to mice droppings; Chlamydophila psittaci with exposure to turkeys or infected birds; and Legionella with exposure to contaminated water sources (saunas). Following influenza, superinfection with pneumococcus, S. aureus (including community-acquired MRSA), and H. influenzae should be considered. With travel to endemic areas in Asia, the onset of respiratory failure after a preceding viral illness should lead to suspicion of SARS or influenza. Endemic fungi (coccidioidomycosis, histoplasmosis, and blastomycosis) occur in well-defined geographic areas and may present acutely as symptoms that overlap with acute bacterial pneumonia.
TABLE 66-2 Clinical Associations with Specific Pathogens
| Condition | Commonly Encountered Pathogens |
|---|---|
| Alcoholism | Streptococcus pneumoniae (including penicillin-resistant), anaerobes, gram-negative bacilli (possibly Klebsiella pneumoniae), tuberculosis |
| Chronic obstructive pulmonary disease/current or former smoker | S. pneumoniae, Haemophilus influenzae, Moraxella catarrhalis |
| Residence in nursing home | S. pneumoniae, gram-negative bacilli, H. influenzae, Staphylococcus aureus, Chlamydia pneumoniae; consider Mycobacterium tuberculosis. Consider anaerobes, but less common. |
| Poor dental hygiene | Anaerobes |
| Bat exposure | Histoplasma capsulatum |
| Bird exposure | Chlamydia psittaci, Cryptococcus neoformans, H. capsulatum |
| Rabbit exposure | Francisella tularensis |
| Travel to southwestern USA | Coccidioidomycosis; hantavirus in selected areas |
| Exposure to farm animals or parturient cats | Coxiella burnetii (Q fever) |
| Postinfluenza pneumonia | S. pneumoniae, S. aureus (including CA-MRSA), H. influenzae |
| Structural disease of lung (e.g., bronchiectasis, cystic fibrosis) | P. aeruginosa, P. cepacia, or S. aureus |
| Sickle cell disease, asplenia | Pneumococcus, H. influenzae |
| Suspected bioterrorism | Anthrax, tularemia, plague |
| Travel to Asia | Severe acute respiratory syndrome (SARS), tuberculosis, melioidosis |
Features of Specific Pathogens
Streptococcus Pneumoniae
The most common pathogen for CAP, S. pneumoniae (synonymous with pneumococcus) is a gram-positive, lancet-shaped diplococcus, of which there are 84 different serotypes, each with a distinct antigenic polysaccharide capsule. Eighty-five percent of all infections are caused by one of 23 serotypes, which are now included in a polysaccharide vaccine. Infection is most common in the winter and early spring, which may relate to the finding that up to 70% of patients have a preceding viral illness. The organism spreads from person to person and commonly colonizes the oropharynx before it leads to pneumonia. Pneumonia develops when colonizing organisms are aspirated into a lung that is unable to contain the aspirated inoculum. The classic radiographic pattern is a lobar consolidation, but bronchopneumonia can also occur, and in some series this is the most common pattern.66 Bacteremia is present in up to 20% of hospitalized patients, and extrapulmonary complications include meningitis, empyema, arthritis, endocarditis, and brain abscess.
Since the mid-1990s, antibiotic resistance among pneumococci has become increasingly common, and penicillin resistance, along with resistance to other common antibiotics (macrolides, trimethoprim/sulfamethoxazole, selected cephalosporins), is present in over 40% of these organisms.1,48–51 Fortunately, most penicillin resistance is of the “intermediate” type (penicillin minimal inhibitory concentration [MIC] of 0.1 to 1.0 mg/L) and not of the high level type (penicillin MIC of 2.0 or more). Although the clinical impact of in vitro resistance is uncertain, one large database has data showing that only organisms with a penicillin MIC of more than 4 mg/L can lead to an increased risk of death.1,48 Recently the definitions of resistance have been changed for non-meningeal infection, with sensitivity being defined by a penicillin MIC ≤2 mg/L, intermediate as a MIC of 4 mg/L, and resistant as a MIC ≥8 mg/L.67 While the clinical impact of resistance on outcomes such as mortality was hard to show using older definitions, with the new definitions of resistance, very few pathogens will be defined as resistant, but those that are may affect outcome.
Although some studies did not show an increased mortality rate in patients infected with resistant strains of pneumococcus after adjusting for disease severity, more recent studies have not been so clear.1,48,68,69 Turrett and colleagues studied a population of 462 patients with pneumococcal bacteremia, of which more than half were HIV positive, and high-level resistance was a predictor of mortality.68 Other investigators did not find an increased risk of death from infection with resistant organisms but did find an enhanced likelihood of suppurative complications (empyema) and a more prolonged hospital length of stay.69 The conflicting data in earlier reports may have been the result of studying relatively few patients. Feikin and colleagues studied the impact of pneumococcal resistance in 5837 patients with bacteremic CAP.48 They found an increased mortality for patients with a penicillin MIC of at least 4 mg/L or greater or with a cefotaxime MIC of 2.0 mg/L or more. However, this increased mortality was only present if patients who died in the first 4 days of therapy were excluded from analysis. One limitation of these data was the failure to account for severity of illness or therapy choices. However, Moroney and associates used both cohort study and matched control methods and found that severity of illness, not resistance or accuracy of therapy, was the most important predictor of mortality.70 Interestingly, in the case-control part of the study, severity of illness was greater in patients without resistant organisms, implying a loss of virulence among organisms that become resistant, a finding echoed in another study that found absence of invasive illness to be a risk factor for pneumococcal resistance.50
The relationship of prior antibiotic use to subsequent pneumococcal resistance has been known, and prior therapy with macrolides, β-lactams, and quinolones has been identified as a predisposing factor for subsequent resistance to the same class of antibiotic.50,71–73 One study related the recent usage of certain specific antibiotic classes to the development of penicillin resistance.73 In this study, 303 patients with pneumococcal bacteremia were evaluated, and 98 had penicillin-nonsusceptible strains. The use of penicillins, sulfonamides, and macrolides within either 1 or 6 months before infection was associated with an increased risk of bacteremia with penicillin-nonsusceptible S. pneumoniae (PNSP). The odds ratio of increased risk was from threefold to sixfold for β-lactams and pneumococci. Interestingly, the risk was no lower for therapy in the past 6 months compared with therapy in the past 1 month. Although quinolones were associated with a slightly increased risk of infection with PNSP, this increase was not statistically significant, but other studies have shown that quinolone therapy can predispose to subsequent pneumococcal resistance to this class of antibiotics.71,72 Prolonged and repeated courses of therapy may be particular risk factors for promoting pneumococcal resistance to β-lactams, sulfonamides, and macrolides.73 In another study of patients with pneumococcal bacteremia, pneumococcal resistance to β-lactams (penicillins and cephalosporins), macrolides, and quinolones was more likely if the patient had received the same agent in the past 3 months.71 Although some studies have shown that discordant therapy of drug-resistant pneumococcus can be a risk factor for mortality, in one study discordant therapy was less likely if patients were treated with ceftriaxone or cefotaxime compared to other therapies.74 Thus in clinical practice, resistance is not likely to affect outcome, since current guidelines for severe CAP recommend the use of these effective agents as empirical therapy. Macrolide-resistant pneumococci have also been described and can be either low- or high-level resistant, depending on whether the mechanism of resistance is efflux or ribosomal alteration, respectively. Although high-level resistance may be clinically relevant, this is generally not an issue in the management of ICU CAP, since all patients who receive macrolide therapy do so in combination with a highly active β-lactam which is effective against pneumococcus even if macrolide resistance is present.
Legionella Pneumophila
This small, weakly staining, gram-negative bacillus was first characterized after an epidemic in 1976 and can occur either sporadically or in epidemic form. Although multiple serogroups of the species L. pneumophila have been described, and these account for 90% of all cases of legionnaires’ disease, serogroup 1 is responsible for the most cases. The other species that commonly causes human illness is L. micdadei. The organism is waterborne and can emanate from air-conditioning equipment, drinking water, lakes and river banks, water faucets, and shower heads.75 Infection is generally caused by inhalation of an infected aerosol generated by a contaminated water source. When a water system becomes infected in an institution, endemic outbreaks may occur. In its sporadic form, Legionella may account for 7% to 15% of all cases of CAP, being a particular concern in patients with severe forms of illness.1,11,75
The classic Legionella syndrome is characterized by high fever, chills, headache, myalgias, and leukocytosis.75 The diagnosis is also suggested by the presence of a pneumonia with preceding diarrhea, along with mental confusion, hyponatremia, relative bradycardia, and liver function abnormalities, but this syndrome is usually not present. Symptoms are rapidly progressive, and the patient may appear to be quite toxic. This classic syndrome is not always present, so this diagnosis should always be considered in patients admitted to the ICU with CAP and in those with rapidly progressive radiographic abnormalities.
To establish this diagnosis serologically, it is necessary to collect both acute and convalescent titers. The urinary antigen test is the single most accurate acute diagnostic test for Legionella but is specific only for serogroup 1 infection. In recent years, most cases have been diagnosed with urinary antigen, and there has been less reliance on serology and culture.76 With this increased reliance on urinary antigen testing, the case fatality rate of legionellosis has fallen, possibly reflecting diagnosis of less severe illness than in the past.76
Staphylococcus Aureus
This organism can lead to severe forms of CAP which can be necrotizing, with a cavitary pneumonia and hematogenous dissemination to multiple sites in the body. The organism can also seed the lung hematogenously from a valvular vegetation in patients with right-sided endocarditis or from septic venous thrombophlebitis (from central venous catheter or jugular vein infection). When a patient develops postinfluenza pneumonia, S. aureus can lead to secondary bacterial infection and, in the United States, community-acquired strains of methicillin-resistant S. aureus (CA-MRSA) have emerged, primarily in skin and soft-tissue infections, but also as a cause of severe CAP. CA-MRSA is a clonal disease, emanating from the USA-300 clone of S. aureus, and is clinically and bacteriologically different from the strains of MRSA that cause nosocomial pneumonia.64 In addition, it can infect previously healthy individuals, and the classic clinical presentation of this pathogen causing CAP is as a complication of a preceding viral or influenza infection. The illness is characterized by a severe bilateral necrotizing pneumonia, which may be related to staphylococcal virulence factors such as the Panton-Valentine leukocidin (PVL). Since the pathogenesis of pneumonia due to this organism may be related to toxin production by the bacteria, therapy may need to involve both an antibacterial agent and an antitoxin-producing agent.65 The frequency of this illness is still relatively low, but it does occur sporadically, with certain geographic areas having a high frequency, especially during influenza season.
Other Organisms, Including Influenza
The incidence of viral pneumonia is difficult to define, but one careful study of over 300 non–immune compromised CAP patients looked for viral pneumonia by paired serologies and found that 18% had viral pneumonia, with about half being pure viral infection and the others being mixed with bacterial pneumonia.77 Influenza (A more than B), parainfluenza, and adenovirus were the most commonly identified viral agents. Influenza should always be considered during epidemic times and can lead to a primary viral pneumonia or to secondary bacterial infection with pneumococcus, S. aureus, or H. influenzae. Viral illnesses that can lead to respiratory failure in addition to influenza include respiratory syncytial virus (which can affect the elderly), varicella (a particular concern in pregnant females with chickenpox), and hantavirus (endemic in the Four Corners area of New Mexico).78
Beginning in April 2009, an outbreak of H1N1 influenza infected approximately 61 million people worldwide, with as many as 13,000 deaths. H1N1 influenza, in contrast to seasonal flu, affected younger people more than the elderly, and high-risk populations included pregnant women and those with obesity. The CDC estimated that 90% of hospitalizations and 87% of deaths occurred in people younger than 65, whereas with seasonal influenza, about 60 percent of flu-related hospitalizations and 90 percent of flu-related deaths occur in people 65 years and older.79 Over 90% of patients with this illness present with cough and fever, but patients may also have chills, muscle aches, and headache. The incubation period is 3 to 7 days, and spread is person to person and via aerosol droplets if the infected person is within 5 to 6 feet. In one series, 12% of all hospitalized patients with H1N1 infection were mechanically ventilated, and 6% of hospitalized patients died.80 When H1N1 infection led to ICU admission, most patients had lung infiltrates which could have been due to viral pneumonia (usually in the first 3-5 days) or secondary bacterial infection (usually after 5-10 days). The frequency of documented bacterial pneumonia complicating this illness varied from less than 5% to more than 25% of patients with radiographic pneumonia. Antiviral therapy with zanamivir and oseltamivir may reduce the severity of illness, particularly if given early. The role of corticosteroids for patients with severe illness is uncertain.81,82
Severe Acute Respiratory Syndrome
In late 2003, a respiratory viral infection caused by a coronavirus emerged in parts of Asia and was termed severe acute respiratory syndrome (SARS). The illness affected people from a variety of endemic areas in Asia, but was seen in North America when an outbreak occurred in Toronto, Canada. Importantly, worldwide as many as 20% of affected patients were healthcare workers, particularly those caring for patients admitted to the ICU. Transmission risk was greatest during emergent intubation and was also possible during noninvasive ventilation, making this latter modality of therapy contraindicated if SARS is suspected.83 Infection control may be quite effective in preventing the spread of SARS to healthcare workers and includes careful handling of respiratory secretions, ventilator circuits, the use of N-95 respirator masks, and careful gowning and gloving.84 Even more elaborate infection control measures, including personal air exchange units, are needed for healthcare workers involved in high-risk procedures such as intubation.
Clinically, SARS patients present after a 2- to 11-day incubation period with fever, rigors, chills, dry cough, dyspnea, malaise, headache, and frequently pneumonia and ARDS. Laboratory data show not only hypoxemia but also elevated liver function tests. In the Toronto experience, about 20% of hospitalized patients were admitted to the ICU, and 15% were mechanically ventilated. Respiratory involvement typically began on day 3 of the hospital stay, but respiratory failure was not until day 8.84 The mortality rate for ICU-admitted SARS patients was over 30%; when patients died, it was generally from multiple-system organ failure and sepsis. There is no specific therapy, but anecdotal reports have suggested a benefit to the use of pulse doses of corticosteroids and ribavirin.
Bioterrorism Considerations
Certain airborne pathogens can cause pneumonia as the result of deliberate dissemination by the aerosol route in the form of a biological weapon, and they present a clinical syndrome of CAP. The pathogens most likely to be used in this fashion and that can lead to severe pulmonary infection are Bacillus anthracis (anthrax), Yersinia pestis (plague), and F. tularensis (tularemia).85–89 The Centers for Disease Control and Prevention (CDC) has classified these agents as category A pathogens because of their high mortality rate and their potential impact on public health.85 Other pneumonic pathogens could also serve as agents of biological warfare but are potentially less serious and are categorized as category B; these include C. bumetii and Brucella species. Certain emerging pathogens are categorized as category C agents and are not widely available as weapons but have the potential for high morbidity and mortality and include hantavirus and multidrug-resistant tuberculosis.84 Some agents of bioterrorism can be spread via the aerosol route but do not generally present as pneumonia; they include smallpox and viral hemorrhagic fevers (Ebola, Marburg).
In the fall of 2001 in the United States, a series of intentional attacks with anthrax led to 11 confirmed cases of inhalational illness.87,88 Anthrax is an aerobic gram-positive, spore-forming bacillus that had rarely led to disease before 2001. Particle size is essential in determining the infectiousness of the spores, and a size of 1 to 5 µm is required for inhalation into the alveolar space, but generally infection requires an inoculum size of 8000 to 40,000 spores. The organisms initially enter alveolar macrophages and are transported to mediastinal lymph nodes, where they can persist and germinate and produce two toxins (lethal toxin and edema toxin). Illness follows rapidly after germination.87,88 Although respiratory symptoms are often present, anthrax is not a typical pneumonic illness but rather a disease characterized by hemorrhagic thoracic lymphadenitis, hemorrhagic mediastinitis, and pleural effusion. Whereas the incubation period of anthrax has varied from 2 to 43 days in prior outbreaks, in the October 2001 series the incubation period was from 4 to 6 days.87 In the U.S. experience, all patients had chills, fever, and sweats and most had nonproductive cough, dyspnea, nausea, vomiting, and chest pain. Chest radiographs were abnormal in all of the first 10 patients, 7 had mediastinal widening, 8 had pleural effusions (generally bloody), and 7 had pulmonary infiltrates.87,88 Blood cultures were positive in all 8 patients in whom they were obtained before therapy, but sputum culture and Gram stain are unlikely to be positive. Five of the 11 patients died.
Therapy for anthrax includes supportive management and antibiotics, with possibly some role for corticosteroids if meningeal involvement or mediastinal edema is present. Recommended therapy is ciprofloxacin (400 mg intravenously (IV) twice daily) or doxycycline (100 mg IV twice daily). Until the patient is clinically stable, one to two additional agents should be added, including clindamycin, vancomycin, imipenem, meropenem, chloramphenicol, penicillin, ampicillin, rifampin, and clarithromycin.87 After an initial response, therapy should be continued with either ciprofloxacin or doxycycline for at least 60 days.87 Postexposure prophylaxis can be done with ciprofloxacin or, alternatively, doxycycline or amoxicillin for a total of 60 days.
 Diagnostic Evaluation
Diagnostic Evaluation
In the patient with severe CAP, diagnostic testing is done to define the presence of pneumonia, the severity of illness and its complications, and the etiologic pathogen. Most studies of severe CAP have not found that establishing an etiologic diagnosis can lead to improved outcome, and mortality is lowest when patients are given empirical therapy that is likely to be effective and leads to a good clinical response within 48 to 72 hours.13 As discussed, the diagnosis of CAP is suggested by the history and physical examination and confirmed by chest radiograph. The history may suggest certain pathogens on the basis of epidemiologic considerations (see Table 66-2), but the clinical features and chest radiograph cannot give an exact etiologic diagnosis. An etiologic diagnosis is best established if blood or pleural fluid cultures identify a pathogen, if bronchoscopic techniques demonstrate an organism in high concentrations, or if serologic testing confirms a fourfold rise in titers to specific pathogens (comparing acute and convalescent samples collected weeks apart).
Although defining a specific etiologic diagnosis of CAP allows for focused antibiotic therapy, most patients do not have a specific pathogen identified. Many who do are diagnosed days or weeks later when the results of cultures or serologic testing become available. In addition, recent studies have emphasized the mortality benefit of prompt administration of effective antibiotic therapy, with a goal of administering IV antibiotics within 4 to 6 hours of admission to the hospital for those with moderate to severe illness.90 Thus therapy should never be delayed for the purpose of diagnostic testing, and the diagnostic workup should be streamlined, with all patients receiving empirical therapy based on algorithms as soon as possible. With such empirical regimens, as many as 90% of admitted patients will have a prompt response to therapy.91
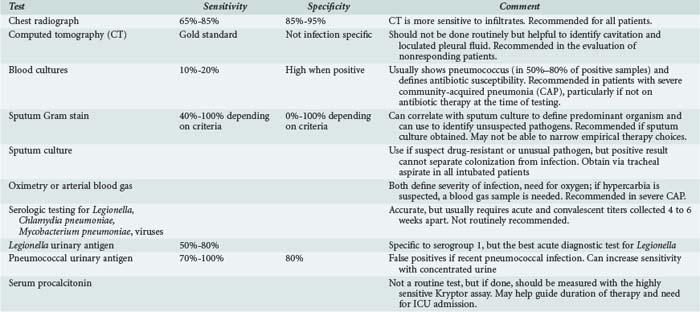
For ICU-admitted patients, after a chest radiograph defines the presence of pneumonia, testing should include an assessment of oxygenation (pulse oximetry or blood gas, the latter if retention of carbon dioxide is suspected), routine admission blood work, and two sets of blood cultures (Table 66-3).1 Although blood cultures are positive in only 10% to 20% of CAP patients, they can be used to define a specific diagnosis and to define the presence of drug-resistant pneumococci.1,48 Blood cultures are not routine for all admitted patients but should be done in those with severe illness, especially if the patient has not received antibiotics prior to admission, since the incidence of a true positive result is high in this population.92 If the patient has a pleural effusion, this should be tapped and the fluid sent for culture and biochemical analysis. Sputum culture can help to identify the presence of a drug-resistant or unusual pathogen and should be obtained from all critically ill patients who are intubated.1 Urinary antigen testing for pneumococcus or Legionella has some potential value for providing a rapid diagnosis. Legionella urinary antigen is specific to serogroup 1 infection and is positive in a little more than half of all infected patients, but it is the test most likely to be positive in the setting of acute illness.93 Pneumococcal urinary antigen has a high sensitivity and specificity for diagnosing pneumococcal pneumonia, especially if concentrated urine is examined; it can be positive even in the presence of antibiotic therapy, but false-positive tests can occur in patients who have had recent pneumococcal infection.94
The role of Gram stain of sputum to guide initial antibiotic therapy is controversial, but this test has its greatest value in guiding the interpretation of sputum culture and can be used to define the predominant organism present in the sample. The role of Gram stain in focusing initial antibiotic therapy is uncertain because the accuracy of the test to predict the culture recovery of an organism such as pneumococcus depends on the criteria used. If the finding of any gram-positive diplococcus is used to define a positive test, the test will be sensitive but not very specific. On the other hand, the finding of a predominance of gram-positive diplococci will be specific but not sensitive for predicting the culture recovery of pneumococcus.1 In one study, the practical limitations of the test were clear: of 116 patients with CAP, only 42 could produce a sputum sample, of which 23 were valid and only 10 samples were diagnostic, with antibiotics directed to the diagnostic result in only 1 patient.95 Even if Gram stain findings were used to focus antibiotic therapy, this would not allow for empirical coverage of atypical pathogens that might be present with pneumococcus as part of a mixed infection. In spite of these limitations, Gram stain can be used to broaden initial empirical therapy by enhancing the suspicion for organisms not covered in routine empirical therapy (such as S. aureus, being suggested by the presence of clusters of gram-positive cocci, especially during a time of epidemic influenza).1
Routine serologic testing is not recommended.1 However, in patients with severe illness, the diagnosis of legionellosis can be made by urinary antigen testing, the test most likely to be positive at the time of admission but specific only for serogroup 1 infection.87 Bronchoscopy is not indicated as a routine diagnostic test and should be restricted to immune-compromised patients and to selected individuals with severe forms of CAP. In the patient admitted to the ICU with CAP, bronchoscopy with quantitative cultures is often done to be sure all efforts are being made to define the etiologic agent, but the benefit of this approach is unclear. As mentioned, several studies13,91 have not shown any improvement in outcome when a specific etiologic diagnosis is made for patients with severe CAP. Rather, outcome is improved if the initial empirical therapy is accurate and the patient has a prompt clinical improvement.13 However, patients who have rapidly progressive lung infection despite therapy may benefit from invasive diagnostic testing, but again a favorable impact of this testing on patient outcome has not been demonstrated. One population that should be considered for invasive testing is the corticosteroid-treated COPD patient who has a slowly responding or nonresponding pneumonia, because these individuals are at risk for infection with Aspergillus, and this organism can be recovered from a bronchoscopic sample. In addition, bronchoscopy may have value for the nonresponding patient or other immune-suppressed individuals; in one study, it provided diagnostically useful information for such patients.96
One study compared the management of CAP with empirical therapy versus a pathogen-directed approach.97 In that study, even with extensive testing, nearly 40% of 262 patients had no etiology established. Although pathogen-directed therapy had no overall impact on mortality or length of stay, it did lead to less adverse events than empirical therapy and also was accompanied by lower mortality for patients admitted to the ICU.97 In patients with severe CAP, diagnostic testing may be valuable for guiding modifications of antibiotic therapy rather than impacting the choice of initial therapy.98 In one study, 214 patients with severe CAP were evaluated, and a microbiologic diagnosis was established in 57.3%. When the yield of specific tests was examined, the investigators found that sputum or tracheal aspirate cultures had the highest yield of any microbiologic investigation, being positive in 44.4% of all patients in which a sample was collected. Blood cultures were positive in 21.1% of the 189 patients sampled, whereas bronchoscopic protected specimen brush was positive in 25% of the 62 patients who were sampled, and bronchoalveolar lavage was positive in 34% of the 41 patients who were sampled. When diagnostic testing identified a cause, antibiotics were changed in 74.3% of patients, compared with 32.7% of patients without an etiologic diagnosis (P < 0.05). In most instances, the change in therapy was a simplification of the initial empirical antibiotic regimen that occurred in 65 patients.98
Although not part of routine management, measurement of serum levels of biomarkers such as C-reactive protein or procalcitonin (PCT) may be valuable in guiding management of antibiotics for CAP. PCT is an acute-phase reactant synthesized in the liver in response to bacterial but not viral infection. Studies in CAP have documented that serial measurement of levels of PCT, using the sensitive Kryptor assay, can guide the duration of antibiotic therapy, allowing cessation of therapy once levels fall and leading to a marked reduction in the duration of therapy, compared to clinical judgment.99,100 In patients with severe CAP, measurement of initial and serial levels can help define those with a poor prognosis, and a low PCT value may distinguish which patients in PSI classes IV and V might be safely managed out of the ICU. In one study, patients with a higher PSI score or with complications or death had significantly higher procalcitonin levels than those with an uncomplicated clinical course.101 In another study, Kruger et al.102 reported that nonsurvivors had significantly higher median PCT levels than survivors (0.88 versus 0.13 ng/mL; P=0.0001). Low PCT accurately predicted patients at very low risk of death, even in patients falling in a high prognostic scoring category by the CURB-65 evaluation. Given its high negative predictive potential (98.9% with PCT level of <0.228 ng/mL), patients with low PCT might be safely treated out of the ICU.102 Huang et al. found that 23.1% (126/546) of high-risk patients defined by PSI had low procalcitonin levels, and this subgroup had very low mortality, similar to low-risk patients.103
 Therapy
Therapy
Initial antibiotic therapy for severe CAP is necessarily empirical, with the goal of targeting the likely etiologic pathogens, based on the considerations in Tables 66-1 and 66-2, which categorize patients on the basis of severity of illness and risk factors for specific pathogens. The likelihood of organisms such as DRSP, enteric gram-negative organisms, and P. aeruginosa is determined by the presence of cardiopulmonary disease or “modifying factors.”1 Although a set of likely pathogens can be predicted for each patient (see Table 66-1), and this information can be used to guide initial empirical therapy, if diagnostic testing shows the presence of a specific pathogen, then therapy can be focused.
In choosing empirical therapy of CAP, certain principles and therapeutic approaches should be followed (Box 66-3). If these principles are followed and patients receive guideline-concordant therapy, outcomes such as duration of mechanical ventilation can be improved.1,104 All individuals should be treated for DRSP and atypical pathogens, but only those with appropriate risk factors (see earlier discussion) should have coverage for P. aeruginosa, and patients with bilateral necrotizing pneumonia after influenza need coverage for CA-MRSA.1 Although macrolide monotherapy (azithromycin) has been documented as effective for some non-ICU admitted patients, all patients admitted to the ICU require combination therapy using a β-lactam with either a macrolide or quinolone, plus the addition of other agents, depending on the clinical setting.1,105 In one study of 529 patients with ICU-admitted CAP, combination therapy with a β-lactam plus either a macrolide or quinolone led to improved survival for the population with shock needing pressors (279 patients), compared to the use of monotherapy.106 This recommendation to avoid monotherapy is based not only on data such as these but also on the fact that the efficacy (especially for meningitis complicating pneumonia), effective dosing and safety of any single agent, including quinolone monotherapy, has not been established for ICU-admitted CAP patients. In one study comparing high-dose levofloxacin to a β-lactam/quinolone combination, the single-agent regimen was overall effective. However, patients in septic shock were excluded, and there was a trend to a worse outcome with monotherapy for individuals receiving mechanical ventilation.105 In another study of severe CAP, use of a β-lactam/macrolide combination had a survival advantage compared to quinolone monotherapy.107 From the available data, it appears that adding either a macrolide or a quinolone leads to similar results, although some data in patients with bacteremic CAP, especially with pneumococcus, suggest that a macrolide may have particular advantages, possibly because of its antiinflammatory effects.62,63 One recent study looking at severe CAP (not all pneumococcal) also confirmed the benefit of adding a macrolide as part of initial empirical therapy, but not a quinolone, for reducing mortality.108 In that study, 165 of the 218 pneumonia patients had sepsis or septic shock, and for these severely ill patients who received a macrolide in a combination regimen, the ICU mortality was 25% compared to a 46% mortality in those getting a quinolone as part of a combination regimen. If Legionella is suspected, the use of a quinolone may be preferable, since these agents have been highly successful in treating pneumonia caused by this organism, possibly more effective than macrolides.109 In addition, the choice between a quinolone and macrolide may best be determined by using a regimen that is different from what the patient has recently received.
Box 66-3
Empirical Therapy Regimens for Severe Community-Acquired Pneumonia
* For patients with normal renal function, the recommended dose of levofloxacin is 750 mg daily. NOTE: Although routine MRSA coverage is NOT recommended for all severe CAP, consider CA-MRSA, especially after influenza and with bilateral necrotizing pneumonia, and if suspected, treat by adding either linezolid or the combination of vancomycin and clindamycin.
For patients with pseudomonal risk factors, therapy can be with a two-drug regimen using an antipseudomonal β-lactam (cefepime, imipenem, meropenem, piperacillin/tazobactam) plus ciprofloxacin (the most active antipseudomonal quinolone) or levofloxacin. Alternatively, a three-drug regimen can be used, combining an antipseudomonal β-lactam plus an aminoglycoside plus either an IV antipneumococcal quinolone (moxifloxacin or levofloxacin) or a macrolide.1 If CA-MRSA is suspected, therapy can be with either vancomycin or linezolid, although other agents might be effective, since this pathogen is not as antibiotic resistant as nosocomial MRSA. However, since CA-MRSA is in part a toxin-mediated illness, the use of an agent that inhibits toxin production along with an antibacterial effect is recommended by some.65 To do this, linezolid can be used alone (since it acts to inhibit protein synthesis), or clindamycin can be added to vancomycin.
Some patients with severe CAP can now be reclassified as having HCAP, because they come to the hospital from a nursing home or have had recent contact with a healthcare environment because of treatment with dialysis or hospitalization in the past 3 months. Some of these patients can be treated the same as other severe CAP patients, but some will need coverage for nosocomial pneumonia pathogens, including multidrug-resistant (MDR) gram-negatives and nosocomial MRSA.2 Those who need coverage for MDR organisms are individuals with severe HCAP who have an additional risk factor (besides just residence in a nursing home), whereas those without such risk factors can receive the severe CAP regimens listed earlier. The risk factors for MDR pathogen infection include poor functional status, immune suppression, recent antibiotic therapy, or recent hospitalization.2 Those at risk for MDR pathogens should receive dual antipseudomonal therapy (β-lactam plus an aminoglycoside) plus MRSA coverage (linezolid or vancomycin).
Although they should not be used as monotherapy for ICU-admitted CAP patients, the antipneumococcal quinolones have assumed great importance because they can cover pneumococcus (including DRSP), nonpseudomonal gram-negative organisms, and atypical pathogens.1 Quinolones penetrate well into respiratory secretions and are highly bioavailable, achieving the same serum levels with oral or IV therapy and thereby allowing rapid switch to oral therapy in responding patients. The available IV agents active against pneumococcus are moxifloxacin and levofloxacin.1 Based on in vitro activity, the recommended doses for moxifloxacin are 400 mg daily and for levofloxacin 750 mg daily, with the need to adjust dosing of levofloxacin (but not moxifloxacin) in patients with renal insufficiency. The higher dose of levofloxacin is recommended because of reports of failures in pneumococcal pneumonia with levofloxacin, which have occurred in patients who were infected with levofloxacin-resistant organisms, particularly after a recent course of quinolone therapy or with the acquisition of resistance during therapy with the 500-mg dose.1,71,72
Timeliness of Initial Therapy of Hospitalized Patients
For inpatients with CAP, the use of timely and accurate therapy is essential to reduce mortality. In patients with severe CAP, improved survival has been shown to occur if initial empirical therapy is accurate and if it leads to a rapid clinical response.13,60,90 In one study, if initial therapy led to a clinical response within 72 hours, mortality of severe CAP was approximately 10%, compared with a mortality rate of 60% in patients who had initially ineffective therapy.13 For CAP in general, early therapy within 4 to 6 hours of arrival to the hospital is associated with reduced mortality compared with therapy given later; but if the patient has pneumonia with sepsis and hypotension, the earlier the therapy is started, the greater the benefit, with mortality rising by nearly 8% for every hour of delay in starting therapy.110
The Need to Treat All Populations for Atypical Pathogen Infection
Although the term atypical does not accurately describe a specific clinical pneumonia syndrome, it can be used to refer to a group of pathogens that includes M. pneumoniae, C. pneumoniae, and Legionella. This group of organisms cannot be reliably eradicated by β-lactam therapy (penicillins and cephalosporins) but must be treated with a macrolide, tetracycline, or a quinolone. In North American CAP guidelines, initial empirical therapy for all patients requires therapy for the possibility of atypical pathogen infection, either as primary infection or as part of a mixed infection, but such therapy is always necessary for ICU patients.1 This recommendation is based on a number of studies, as mentioned earlier, that show a high frequency of these pathogens when using serologic diagnosis, often in the form of mixed infection coexisting with a bacterial pathogen.57,58 In one study of inpatients in the United States, infection with atypical pathogens was more common in older individuals (65-79 years) than in those younger than 35, and other studies have shown these pathogens to be common in patients with severe CAP.11,57
A number of studies of large populations of inpatients, including those with severe CAP, have shown that when therapy includes a macrolide or a quinolone, outcomes including mortality are improved, compared with when a β-lactam is used by itself.60,61,106 Although these findings are not definitive, they do suggest the need for routine therapy of atypical pathogens, a strategy that may even be needed in patients with bacteremic pneumococcal pneumonia. As mentioned, several studies have suggested that when patients with this infection receive a β-lactam alone, the mortality is higher than if they receive a β-lactam combined with a macrolide, and the mortality benefit is particularly high for those in the ICU.62,63
Legionella is a potentially important pathogen in patients with severe CAP, and there are many drugs available with in vitro activity against L. pneumophila, but there are limited prospective comparative data on the role of therapy in the outcome of this infection.75 Retrospective data and clinical experience support the use of erythromycin at a dose of 4 g/day in the hospitalized patient with L. pneumophila. Rifampin should be added in patients with multilobar disease, organ failure, or severe immunosuppression and should be administered for the first 3 to 5 days.1 Other macrolides (clarithromycin and azithromycin) are also effective, and azithromycin is available in an IV form. However, more recent data suggest that quinolones (moxifloxacin, levofloxacin) may be an even more effective alternative, since they have been highly effective in animal models and in treating patients with Legionella, including those in the ICU.109 If the patient has severe CAP following influenza, it may also be necessary to add an antiviral agent such as zanamivir or oseltamivir, which are both effective but not clearly proven to reduce the development of respiratory complications. In patients with documented influenza, including H1N1, early therapy provides the best chance for improved outcome.81,82,111
There is little information on the proper duration of therapy in patients with CAP, especially those with severe illness. Even in the presence of pneumococcal bacteremia, short durations of therapy may be possible, with a rapid switch from IV to oral therapy in responding patients.112 Generally, S. pneumoniae can be treated for 5 to 7 days if the patient is responding rapidly and has received the correct dose of an accurate therapy. The presence of extrapulmonary infection (e.g., meningitis) and identification of certain pathogens (e.g., bacteremic S. aureus and P. aeruginosa) may require longer duration of therapy. Identification of L. pneumophila pneumonia may require at least 14 days of therapy, depending on severity of illness and host defense impairments, but shorter durations with quinolone therapy have been effective. Most therapy in the ICU will be given IV; however, recent studies using a variety of antibiotics have suggested that oral therapy may be instituted after as early as 2 to 3 days of parenteral therapy, assuming the patient’s condition has stabilized and the patient is afebrile.1 The switch to oral therapy, even in severely ill patients, may be facilitated by the use of quinolones that are highly bioavailable and achieve the same serum levels with oral therapy as with IV therapy.
Adjunctive Therapy Measures
In addition to antibiotic therapy, the patient with severe CAP may require chest physiotherapy, especially if either an excessive volume of purulent sputum (>30 mL/day) or severe respiratory muscle weakness resulting in ineffective cough are present.113 Aerosolized humidification has been used to reduce sputum viscosity, thereby enhancing clearance in patients who have generally ineffective cough. However, it is likely that much of the generated water vapor is deposited in the upper airway where it is likely to stimulate cough but unlikely to influence the rheologic properties of sputum. Bronchodilator therapy, which also enhances mucociliary clearance and ciliary beat frequency, is most likely to be of benefit in patients with pneumonia complicating COPD.
Activated protein C (drotrecogin alfa [activated]) infusion has been shown to reduce 28-day mortality in patients with severe sepsis and an APACHE II score of more than 25, but in the original trial, over half of the treated patients had pneumonia as the cause of sepsis, suggesting a role for this therapy in patients with severe CAP.114 However, in a subsequent analysis of these data, although activated protein C had benefit in CAP, this benefit was most evident in patients who received inappropriate empirical antibiotic therapy, questioning the incremental benefit for patients being treated with the correct antibiotics.114
Several studies have looked at the use of adjunctive corticosteroids in patients with severe CAP and have shown possible benefit with no proven harm. However, a recent prospective randomized trial in 213 patients found no difference in clinical cure rates with corticosteroid treatment compared to placebo, although steroid-treated patients had faster defervescence but a higher rate of late failure.115,116 Adjunctive immune therapy with granulocyte colony-stimulating factor (G-CSF) has also been used in severe CAP, with no benefit in mortality or in the course of illness resolution.117 Although the role of corticosteroids as routine therapy of CAP is not established, steroids may be beneficial in patients with sepsis and relative adrenal insufficiency that occurs in a high proportion of patients with severe CAP.118 Salluh et al. have shown that in patients with severe CAP, median cortisol levels were 15.5 µg per dL, and that 65% of patients met the criteria for adrenal insufficiency (cortisol levels less than 20 µg per dL).118 Another setting in which corticosteroids may have benefit is in pneumococcal pneumonia that is complicated by meningitis, where pretreatment with corticosteroids prior to antibiotic therapy may lead to more favorable neurologic outcomes.119
Evaluation of Response to Therapy
The majority of patients will respond rapidly to accurate empirical therapy within 24 to 72 hours. Clinical response is defined as improvement in symptoms of cough, sputum production, and dyspnea, along with ability to take medications by mouth, declining white blood cell count, and an afebrile status on at least two occasions 8 hours apart.1,112 In the critically ill patient, improvement in oxygenation may be one of the earliest signs of response to therapy, although few studies have examined mechanically ventilated patients. When a patient has met criteria for clinical response, it is appropriate to consider a switch to an oral therapy regimen if the patient is otherwise medically and socially stable.1,112 Radiographic improvement lags behind clinical improvement, and in a responding patient, a chest radiograph is not necessary until 2 to 4 weeks after starting therapy. In general, 50% of patients with pneumococcal pneumonia have radiographic clearing at 5 weeks, whereas the majority clear in 2 to 3 months. With bacteremic disease, 50% of patients have a clear chest radiograph at 9 weeks, and most are clear by 18 weeks.120 Radiographic resolution is most influenced by the number of lobes involved and the age of the patient. Radiographic clearance of CAP decreases by 20% per decade after age 20, and patients with multilobar infiltrates take longer to clear than those with unilobar disease.120
If the patient fails to respond to appropriate therapy in the expected time interval, it is necessary to consider infection with a drug-resistant or unusual pathogen (tuberculosis, C. burnetii, Burkholderia pseudomallei, C. psittaci, endemic fungi, or hantavirus); a pneumonic complication (lung abscess, endocarditis, empyema); or a noninfectious process that mimics pneumonia (bronchiolitis obliterans with organizing pneumonia, hypersensitivity pneumonitis, pulmonary vasculitis, bronchoalveolar cell carcinoma, lymphoma, pulmonary embolus).1 The evaluation of the nonresponding patient should be individualized but may include CT of the chest, pulmonary angiography, bronchoscopy, and occasionally open lung biopsy.
 Prevention
Prevention
Prevention of CAP is important for all groups of the population but especially the elderly patient, who is at risk for both a higher frequency of infection and a more severe course of illness. Appropriate patients should be vaccinated with both pneumococcal and influenza vaccines, and cigarette smoking should be stopped in all at-risk patients. Even for the patient who is recovering from CAP, immunization while in the hospital is appropriate to prevent future episodes of infection. Evaluation of all patients for vaccination need and provision of information about smoking cessation are now performance standards used to evaluate the hospital care of CAP patients.1
Pneumococcal Vaccine
Pneumococcal capsular polysaccharide vaccine can prevent pneumonia in otherwise healthy populations, as was initially demonstrated in South African gold miners and American military recruits.1,121 The benefits in individuals of advanced age or with underlying conditions in nonepidemic environments are less clearly defined. The vaccine efficacy has ranged from 65% to 84% in patients with diabetes mellitus, coronary artery disease, congestive heart failure, chronic pulmonary disease, and anatomic asplenia.1,121 In immunocompetent patients over the age of 65, effectiveness has been documented to be 75%. In the immunocompromised patient, effectiveness has not been proven, and this includes patients with sickle cell disease, chronic renal failure, immunoglobulin deficiency, Hodgkin’s disease, lymphoma, leukemia, and multiple myeloma. One retrospective cohort study evaluated 47,365 patients older than 65 years to determine the impact of pneumococcal vaccination on three different clinical events: hospitalization for CA, outpatient therapy for CAP, and documented pneumococcal bacteremia.122 The use of vaccination was associated with a significant reduction in the incidence of pneumococcal bacteremia (OR = 0.56) but no change in the frequency of pneumonia treated in or out of the hospital.
A single revaccination is indicated in a person who is older than age 65 years who initially received the vaccine more than 5 years earlier and was younger than age 65 on first vaccination.1 If the initial vaccination was given at age 65 or older, repeat is not indicated unless the patient has anatomic or functional asplenia or has one of the immune-compromising conditions listed earlier. In these patients, revaccination is indicated, and the second dose is given at least 5 years after the original dose.
The available pneumococcal vaccine is widely underutilized, especially as the 23-valent pneumococcal vaccine contains 23 pneumococcal serotypes that cause 85% of all infections due to pneumococcus. Two protein-conjugated pneumococcal vaccines have been licensed and are more immunogenic than the older vaccine, but they contain only 7 and 13 serotypes, and are approved for use in children but not yet in adults.1 However, the conjugate vaccine has had benefit for adults, even when given to only children, demonstrating a “herd immunity” effect. In one study of the heptavalent conjugated pneumococcal vaccine, benefit in reducing invasive illness occurred not only in the population immunized but in adults, particularly those over age 65, who were not the target of the immunization efforts, possibly by lowering the community incidence and pathogen reservoir and reducing the spread of invasive disease.123 However, more recently, children who have received the 7-valent pneumococcal polysaccharide vaccine have developed infection with strains not included in the vaccine, leading to a higher frequency of severe necrotizing pneumonia, especially with serotype 3 pneumococcus.124
Influenza Vaccination
Influenza epidemics contribute to morbidity and mortality both by causing direct infection and by leading to postinfluenza complications. The influenza vaccine preparations are revised annually to account for changes in the antigenic nature of the virus (antigenic drift) that is present each season. Three strains are represented in each vaccine preparation: an influenza A strain (H3N2); an influenza A strain (H1N1); and one influenza B strain. Vaccination should be given to all patients older than age 65, to those with chronic medical illness (including nursing home residents), and to those who provide health care to patients at risk for complicated influenza.1 It is given yearly, usually between September and mid-November. While the traditional influenza vaccine contains an inactivated virus, there is now an intranasal vaccine containing a live attenuated influenza virus. It is currently approved for individuals aged 5 to 49 years who are not immune suppressed or chronically ill and who do not have asthma.
When the vaccine matches the circulating strain, it can prevent illness in 70% to 90% of healthy persons younger than age 65.1,125 For older persons with chronic illness, the efficacy is less, but the vaccine can still attenuate the influenza infection and lead to fewer lower respiratory tract infections and the associated morbidity and mortality that follow influenza. In many studies, the vaccine has been shown to be cost-effective and able to prevent severe illness and death and reduce the occurrence of secondary pneumonia and hospitalization.125
Key Points
Baddour LM, Yu VL, Klugman KP, et al. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med. 2004;170:440-444.
Charles PG, Wolfe R, Whitby M, Fine MJ, Fuller AJ, Stirling R, et al. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis. 2008;47:375-384.
Christ-Crain M, Stolz D, Bingisser R, et al. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174:84-93.
El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med. 2003;167:1650-1654.
Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589-1596.
Leroy O, Saux P, Bedos JP, Caulin E. Comparison of levofloxacin and cefotaxime combined with ofloxacin for ICU patients with community-acquired pneumonia who do not require vasopressors. Chest. 2005;128:172-183.
Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America; American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44:S27-S72.
Valencia M, Badia JR, Cavalcanti M, Ferrer M, Agusti C, Angrill J, et al. Pneumonia severity index class V patients with community-acquired pneumonia. Characteristics, outcomes, and value of severity. Chest. 2007;132:515-522.
1 Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44:S27-S72.
2 Brito V, Niederman MS. Healthcare-associated pneumonia is a heterogenous disease, and all patients do not need the same broad-spectrum antibiotic therapy as complex nosocomial pneumonia. Curr Opin Infect Dis. 2009;22:316-325.
3 Niederman MS, McCombs JI, Unger AN, et al. The cost of treating community-acquired pneumonia. Clin Ther. 1998;20:820-837.
4 Torres A, Serra-Batlles J, Ferrer A, et al. Severe community-acquired pneumonia: Epidemiology and prognostic factors. Am Rev Respir Dis. 1991;144:312-318.
5 Kaplan V, Angus DC, Griffin MF, et al. Hospitalized community-acquired pneumonia in the elderly: Age- and sex-related patterns of care and outcome in the United States. Am J Respir Crit Care Med. 2002;165:766-772.
6 Ewig S, Ruiz M, Mensa J, et al. Severe community-acquired pneumonia: Assessment of severity criteria. Am J Respir Crit Care Med. 1998;158:1102-1108.
7 Woodhead M, Welch C, Harrison D, et al. Community-acquired pneumonia on the intensive care unit: secondary analysis of 17,869 cases in the ICNARC Case Mix Programme Database. Crit Care. 2006;10(Suppl 2):S1.
8 Colice GL Morley MA, Asche C, Birnbaum HG. Treatment costs of community-acquired pneumonia in an employed population. Chest. 2004;125:2140-2145.
9 Ruiz M, Ewig S, Marcos MA, et al. Etiology of community-acquired pneumonia: Impact of age, comorbidity, and severity. Am J Respir Crit Care Med. 1999;160:397-405.
10 Nuorti JP, Butler JC, Farley MM, et al. The Active Bacterial Core Surveillance Team. Cigarette smoking and invasive pneumococcal disease. N Engl J Med. 2000;342:681-689.
11 Ruiz M, Ewig S, Torres A, et al. Severe community-acquired pneumonia: Risk factors and follow-up epidemiology. Am J Respir Crit Care Med. 1999;160:923-929.
12 Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia: A meta-analysis. JAMA. 1996;275:134-141.
13 Leroy O, Santre C, Beuscart C. A 5-year study of severe community-acquired pneumonia with emphasis on prognosis in patients admitted to an ICU. Intensive Care Med. 1995;21:24-31.
14 Garau J, Baquero F, Perez-Trallero E, et al. Factors impacting on length of stay and mortality of community-acquired pneumonia. Clin Microbiol Infect. 2008;14:322-329.
15 Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243-250.
16 Angus DC, Marrie TJ, Obrosky S, et al. Severe community-acquired pneumonia: Use of intensive care services and evaluation of the American and British Thoracic Society criteria. Am J Respir Crit Care Med. 2002;166:717-723.
17 Valencia M, Badia JR, Cavalcanti M, et al. Pneumonia Severity Index Class V Patients With Community-Acquired Pneumonia: Characteristics, Outcomes, and Value of Severity. Chest. 2007;132:515-522.
18 Niederman MS. Making sense of scoring systems in community acquired pneumonia. Respirology. 2009;14:327-335.
19 Lim WS, Lewis S, Macfarlane JT. Severity prediction rules in community acquired pneumonia: A validation study. Thorax. 2000;55:219-223.
20 Lim WS, Macfarlane JT. Defining prognostic factors in the elderly with community acquired pneumonia: A case controlled study of patients aged > 75 years. Eur Respir J. 2001;17:200-205.
21 Aujesky D, Auble T, Yealy D, et al. Prospective comparison of three validated prediction rules for prognosis in community-acquired pneumonia. Am J Med. 2005;118:384-392.
22 España P, Capelastegui A, Gorordo I, et al. Development and validation of a clinical prediction rule for severe community-acquired pneumonia. Am J Respir Crit Care Med. 2006;174:1249-1256.
23 Charles P, Wolfe R, Whitby M, et al. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis. 2008;47:375-384.
24 Niederman MS, Ahmed QA. Inflammation in severe pneumonia: Act locally, not globally. Crit Care Med. 1999;27:2030-2032.
25 Welsh DA, Mason CM. Host defenses in respiratory infections. Med Clin North Am. 2001;85:1329-1347.
26 Dehoux MS, Boutten A, Ostinelli J, et al. Compartmentalized cytokine production within the human lung in unilateral pneumonia. Am J Respir Crit Care Med. 1994;150:710-716.
27 Boutten A, Dehoux MS, Seta N, et al. Compartmentalized IL-8 and elastase release within the human lung in unilateral pneumonia. Am J Respir Crit Care Med. 1996;153:336-342.
28 Monton C, Torres A, El-Ebiary M, et al. Cytokine expression in severe pneumonia: A bronchoalveolar lavage study. Crit Care Med. 1999;27:1745-1753.
29 Wu CL, Lee YL, Chang KM, et al. Bronchoalveolar interleukin-1B: A marker of bacterial burden in mechanically ventilated patients with community-acquired pneumonia. Crit Care Med. 2003;31:812-817.
30 Kaplan V, Clermont G, Griffin MF, et al. Pneumonia: still the old man’s friend? Arch Intern Med. 2003;163:317-323.
31 Reade MC, Yende S, D’Angelo G, et al. Differences in immune response may explain lower survival among older men with pneumonia. Crit Care Med. 2009;37:1655-1662.
32 Christ-Crain M, Breidthardt T, Stolz D, et al. Use of B-type natriuretic peptide in the risk stratification of community-acquired pneumonia. J Intern Med. 2008;264:166-176.
33 Metlay JP, Schulz R, Li Y-H, et al. Influence of age on symptoms at presentation in patients with community-acquired pneumonia. Arch Intern Med. 1997;157:1453-1459.
34 Fine MJ, Orloff JJ, Arisumi D, et al. Prognosis of patients hospitalized with community-acquired pneumonia. Am J Med. 1990;88:1N-8N.
35 Riquelme R, Torres A, El-Ebiary, et al. Community-acquired pneumonia in the elderly: Clinical and nutritional aspects. Am J Crit Care Med. 1997;156:1908-1914.
36 Fernandez-Sabe N, Carratala J, Roson B, et al. Community-acquired pneumonia in very elderly patients: Causative organisms, clinical characteristics and outcomes. Medicine. 2003;82:159-169.
37 McFadden JR, Price RC, Eastwood HD, Briggs RS. Raised respiratory rate in elderly patients: A valuable physical sign. BMJ. 1982;284:626-627.
38 Syrjala H, Broas M, Suramo I, et al. High resolution computed tomography for the diagnosis of community-acquired pneumonia. Clin Infect Dis. 1998;27:358-363.
39 Macfarlane JT, Miller AC, Smith WHO, et al. Comparative radiographic features of community-acquired legionnaires’ disease, pneumococcal pneumonia, mycoplasma pneumonia, and psittacosis. Thorax. 1984;39:28-33.
40 Farr BM, Kaiser DL, Harrison BW, Connolly CK. Prediction of microbial aetiology at admission to hospital for pneumonia from presenting clinical features. Thorax. 1989;44:1031-1035.
41 Fang GD, Fine M, Orloff J, et al. New and emerging etiologies for community-acquired pneumonia with implication for therapy: A prospective multicenter study of 359 cases. Medicine. 1990;69:307-316.
42 Esposito AL. Community-acquired bacteremic pneumococcal pneumonia: Effect of age on manifestations and outcome. Arch Intern Med. 1984;44:945-948.
43 Liapikou A, Ferrer M, Polverino E, Balasso V, Esperatti M, Piñer R, et al. Severe community-acquired pneumonia: validation of the Infectious Diseases Society of America/American Thoracic Society guidelines to predict an intensive care unit admission. Clin Infect Dis. 2009;48:377-385.
44 Brown SM, Jones BE, Jephson AR, Dean NC. Validation of the Infectious Disease Society of America/American Thoracic Society 2007 guidelines for severe community-acquired pneumonia. Crit Care Med. 2009;37:3010-3016.
45 Renaud B, Santin A, Coma E, et al. Association between timing of intensive care unit admission and outcomes for emergency department patients with community-acquired pneumonia. Crit Care Med. 2009;37:2867-2874.
46 Van Eeden SF, Coetzee AR, Joubert JR. Community-acquired pneumonia—factors influencing intensive care admission. S Afr Med J. 1988;73:77-81.
47 Ruiz-Gonzalez A, Falguera M, Nogues A, Rubio-Caballero M. Is Streptococcus pneumoniae the leading cause of pneumonia of unknown etiology? A microbiologic study of lung aspirates in consecutive patients with community-acquired pneumonia. Am J Med. 1999;106:385-390.
48 Feikin DR, Schuchat A, Kolczak M, et al. Mortality from invasive pneumococcal pneumonia in the era of antibiotic resistance, 1995-1997. Am J Public Health. 2000;90:223-229.
49 Pallares R, Linares J, Vadillo M, et al. Resistance to penicillin and cephalosporin and mortality from severe pneumococcal pneumonia in Barcelona, Spain. N Engl J Med. 1995;333:474-480.
50 Clavo-Sánchez AJ, Girón-González JA, López-Prieto D, et al. Multivariate analysis of risk factors for infection due to penicillin-resistant and multidrug-resistant Streptococcus pneumoniae: A multi-center study. Clin Infect Dis. 1997;24:1052-1059.
51 Ewig S, Ruiz M, Torres A, et al. Pneumonia acquired in the community through drug-resistant Streptococcus pneumoniae. Am J Respir Crit Care Med. 1999;159:1835-1842.
52 Arancibia F, Bauer TT, Ewig S, et al. Community-acquired pneumonia due to gram-negative bacteria and Pseudomonas aeruginosa: Incidence, risk and prognosis. Arch Intern Med. 2002;162:1849-1858.
53 Kang CI, Song JH, Oh WS, et al. Clinical outcomes and risk factors of community-acquired pneumonia caused by gram-negative bacilli: Asian Network for Surveillance of Resistant Pathogens (ANSORP) Study Group. Eur J Clin Microbiol Infect Dis. 2008;27:657-661.
54 Falguera M, Carratalà J, Ruiz-Gonzalez A, et al. Risk factors and outcome of community-acquired pneumonia due to Gram-negative bacilli. Respirology. 2009;14:105-111.
55 El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med. 2003;167:165.
56 El Solh AA, Sikka P, Ramadan F, Davies J. Etiology of severe pneumonia in the very elderly. Am J Respir Crit Care Med. 2001;163:645-651.
57 Marston BJ, Plouffe JF, File TMJr, et al. Incidence of community-acquired pneumonia requiring hospitalization: Results of a population-based active surveillance Study in Ohio. The Community-Based Pneumonia Incidence Study Group. Arch Intern Med. 1997;157:1709-1718.
58 Lieberman D, Schlaeffer F, Boldur I, et al. Multiple pathogens in adult patients admitted with community-acquired pneumonia: A one year prospective study of 346 consecutive patients. Thorax. 1996;51:179-184.
59 Troy CJ, Peeling RW, Ellis AG, et al. Chlamydia pneumoniae as a new source of infectious outbreaks in nursing homes. JAMA. 1997;277:1214-1218.
60 Gleason PP, Meehan TP, Fine JM, et al. Associations between initial antimicrobial therapy and medical outcomes for hospitalized elderly patients with pneumonia. Arch Intern Med. 1999;159:2562-2572.
61 Houck PM, MacLehose RF, Niederman MS, Lowery JK. Empiric antibiotic therapy and mortality among Medicare pneumonia inpatients in 10 Western states: 1993, 1995, 1997. Chest. 2001;119:1420-1426.
62 Weiss K, TIllotson GS. The controversy of combination vs. monotherapy in the treatment of hospitalized community-acquired pneumonia. Chest. 2005;128:940-946.
63 Baddour L, Yu V, Klygkan K, et al. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Resp Crit Care Med. 2004;170:440-444.
64 Deresinski S. Methicillin-resistant Staphylococcus aureus: an evolutionary, epidemiologic, and therapeutic odyssey. Clin Infect Dis. 2005;40:562-573.
65 Micek ST, Dunne M, Kollef MH. Pleuropulmonary complications of Panton-Valentine leukocidin-positive community-acquired methicillin-resistant Staphylococcus aureus: importance of treatment with antimicrobials inhibiting exotoxin production. Chest. 2005;128:2732-2738.
66 Ort S, Ryan JL, Barden G, et al. Pneumococcal pneumonia in hospitalized patients: Clinical and radiological presentations. JAMA. 1983;249:214-218.
67 Clinical and Laboratory Standards Institute. Press release. www.clsi.org, January 2008. On line at
68 Turrett GS, Blum S, Fazal BA, et al. Penicillin resistance and other predictors of mortality in pneumococcal bacteremia in a population with high human immunodeficiency virus seropure valence. Clin Infect Dis. 1999;29:321-327.
69 Metlay JP, Hoffman J, Cetron MS, et al. Impact of penicillin susceptibility on medical outcomes for adult patients with bacteremic pneumococcal pneumonia. Clin Infect Dis. 2000;30:520-528.
70 Moroney JF, Fiore AE, Harrison LH, et al. Clinical outcomes of bacteremic pneumococcal pneumonia in the era of antibiotic resistance. Clin Infect Dis. 2001;33:797-805.
71 Vanderkooi OG, Low DE, Green K, Powis JE, McGeer A, et al. Predicting antimicrobial resistance in invasive pneumococcal infections. Clin Infect Dis. 2005;40:1288-1297.
72 Ho PL, Tse WS, Tsang KW, et al. Risk factors for acquisition of levofloxacin-resistant Streptococcus pneumoniae: A case-control study. Clin Infect Dis. 2001;32:701-707.
73 Ruhe JJ, Hasbu R. Streptococcus pneumoniae bacteremia: Duration of previous antibiotic use and association with penicillin resistance. Clin Infect Dis. 2003;36:1132-1138.
74 Lujan M, Gallego M, Fantanals D, et al. Prospective observational study of bacteremic pneumococcal pneumonia: Effect of discordant therapy on mortality. Crit Care Med. 2004;32:625-631.
75 Stout JE, Yu VL. Legionellosis. N Engl J Med. 1997;337:682-687.
76 Benin AL, Benson RF, Besser RE. Trends in Legionnaires Disease, 1980-1998: Declining mortality and new patterns of diagnosis. Clin Infect Dis. 2002;35:1039-1046.
77 De Roux A, Marcos MA, Garcia E, et al. Viral community-acquired pneumonia in nonimmunocompromised adults. Chest. 2004;125:1343-1351.
78 Duchin JS, Koster FT, Peters CJ, et al. Hantavirus pulmonary syndrome: A clinical description of 17 patients with a newly recognized disease. The Hantavirus Study Group. N Engl J Med. 1994;330:949-955.
79 CDC. http://www.cdc.gov/h1n1flu, 2010. [accessed on May 26th, 2010]
80 Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605-2615.
81 Kumar A, Zarychanski R, Pinto R, et al. Critically ill patients with 2009 influenza A (H1N1) infection in Canada. JAMA. 2009;302:1872-1879.
82 Rello J, Rodriguez A, Ibanez P, et al. Intensive care adult patients with severe respiratory failure caused by influenza A (H1N1) in Spain. Crit Care. 2009;13:R148. (doi:10.1186/cc8044)
83 Lapinsky SE, Hawryluck L. ICU management of severe acute respiratory syndrome. Intensive Care Med. 2003;29:870-875.
84 Fowler RA, Lapinsky SE, Hallett D, et al. Toronto SARS Critical Care Group. Critically ill patients with severe acute respiratory syndrome. JAMA. 2003;290:367-373.
85 Darling RG, Catlett CL, Huebner KD, Jarrett DG. Threats in bioterrorism I: CDC category A agents. Emerg Med Clin North Am. 2002;20:273-309.
86 Moran GJ. Threats in bioterrorism II: CDC category B and C agents. Emerg Med Clin North Am. 2002;20:311-330.
87 Bartlett JG, Inglesby TV, Borio L. Management of anthrax. Clin Infect Dis. 2002;35:851-858.
88 Inglesby TV, O’Toole T, Henderson DA, et al. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA. 2002;287:2236-2252.
89 Dennis DT, Inglesby TV, Henderson DA, et al. Tularemia as a biological weapon: medical and public health management. JAMA. 2001;285:2763-2773.
90 Meehan TP, Fine MJ, Krumholz HM, et al. Quality of care, process, and outcomes in elderly patients with pneumonia. JAMA. 1997;278:2080-2084.
91 Sanyal S, Smith PR, Saha AC, et al. Initial microbiologic studies did not affect outcome in adults hospitalized with community-acquired pneumonia. Am J Respir Crit Care Med. 1999;160:346-348.
92 Metersky ML, Ma A, Bratzler DW, et al. Predicting bacteremia in patients with community-acquired pneumonia. Am J Respir Crit Care Med. 2004;169:342-347.
93 Plouffe JF, File TMJr, Breiman RF, et al. Reevaluation of the definition of legionnaires’ disease: use of the urinary antigen assay. Community Based Pneumonia Incidence Study Group. Clin Infect Dis. 1995;20:1286-1291.
94 Marcos MA, de Anta J, de la Bellacasa JP, et al. Rapid urinary antigen test for diagnosis of pneumococcal community-acquired pneumonia in adults. Eur Respir J. 2003;21:209-214.
95 Ewig S, Schlochtermeier M, Goke N, Niederman MS. Applying sputum as a diagnostic tool in pneumonia: Limited yield, minimal impact on treatment decisions. Chest. 2002;121:1486-1492.
96 Ortqvist A, Kalin M, Lejdeborn L, Lundberg B. Diagnostic fiberoptic bronchoscopy and protected brush culture in patients with community-acquired pneumonia. Chest. 1990;97:576-582.
97 Van der Eeden PDT, van der Eerden MM, Vlaspolder F, de Graaff CS, et al. Comparison between pathogen directed antibiotic treatment and empirical broad spectrum antibiotic treatment in patients with community acquired pneumonia: a prospective randomised study. Thorax. 2005;60:672-678.
98 Rello J, Bodi M, Mariscal D, et al. Microbiological testing and outcome of patients with severe community-acquired pneumonia. Chest. 2003;123:174-180.
99 Niederman MS. Biological markers to determine eligibility in trials for community-acquired pneumonia: a focus on procalcitonin. Clin Infect Dis. 2008;47(Suppl 3):S127-S132.
100 Christ-Crain M, Stolz D, Bingisser R, et al. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174:84-93.
101 Masiá M, Gutiérrez F, Shum C, et al. Usefulness of procalcitonin levels in community-acquired pneumonia according to the patients outcome research team pneumonia severity index. Chest. 2005;128:2223-2229.
102 Krüger S, Ewig S, Marre R, Papassotiriou J, Richter K, von Baum H. Procalcitonin predicts patients at low risk of death from community-acquired pneumonia across all CRB-65 classes. Eur Respir J. 2008;31:349-355.
103 Huang DT, Weissfeld LA, Kellum JA, Yealy DM, Kong L, Martino M, et al. Risk prediction with procalcitonin and clinical rules in community-acquired pneumonia. Ann Emerg Med. 2008;52:48-58.e2.
104 Shorr AF, Bodi M, Rodriguez A, et al. Impact of antibiotic guideline compliance on duration of mechanical ventilation in critically ill patients with community-acquired pneumonia. Chest. 2006;130:93-100.
105 Leroy O, Saux P, Bedos JP, Caulin E. Comparison of levofloxacin and cefotaxime combined with ofloxacin for ICU patients with community-acquired pneumonia who do not require vasopressors. Chest. 2005;128:172-183.
106 Rodriguez A, Mendia A, Sirvent JM, et al. Combination antibiotic therapy improves survival in patients with community-acquired pneumonia and shock. Crit Care Med. 2007;35:1493-1498.
107 Lodise TP, Kwa A, Cosler L, et al. Comparison of beta-lactam and macrolide combination therapy versus fluoroquinolone monotherapy in hospitalized Veterans Affairs patients with community-acquired pneumonia. Antimicrob Agents Chemother. 2007;51:3977-3982.
108 Martin-Loeches I, Lisboa T, Rodriguez A, et al. Combination antibiotic therapy with macrolides improves survival in intubated patients with community-acquired pneumonia. Intensive Care Med. 2010;36:612-620.
109 Yu VL, Greenberg RN, Zadeikis N, et al. Levofloxacin efficacy in the treatment of community-acquired legionellosis. Chest. 2004;125:2135-2139.
110 Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589-1596.
111 Jefferson T, Jones M, Doshi P, et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults: Systematic review and meta-analysis. BMJ. 2009;339:b5106.
112 Ramirez JA, Bordon J. Early switch from intravenous to oral antibiotics in hospitalized patients with bacteremic community-acquired Streptococcus pneumoniae pneumonia. Arch Intern Med. 2001;161:848-850.
113 Graham WG, Bradley DA. Efficacy of chest physiotherapy and intermittent positive-pressure breathing in the resolution of pneumonia. N Engl J Med. 1978;299:624-627.
114 Laterre PF, Garber G, Levy H, et al. Severe community-acquired pneumonia as a cause of severe sepsis: data from the PROWESS study. Crit Care Med. 2005;33:952-961.
115 Salluh JI, Povoa P, Soares M, et al. The role of corticosteroids in severe community acquired pneumonia: a systematic review. Crit Care. 2008:12R76. E pub 2008 Jun11
116 Snijders D, Daniels JM, de Graaff CS, et al. Efficacy of corticosteroids in community-acquired pneumonia: a randomized double-blinded clinical trial. Am J Respir Crit Care Med. 2010;181:975-982.
117 Niederman MS. Granulocyte colony-stimulating factor for severe pneumonia: What do we do when the best laid plans for men (and mice and rats …) fail? Crit Care Med. 2003;31:635-637.
118 Salluh J, Bozza F, Soares M, et al. Adrenal response in severe community-acquired pneumonia: impact on outcomes and disease severity. Chest. 2008;134:947-954.
119 de Gans J, van de Beek D. Dexamethasone in adults with bacterial meningitis. N Engl J Med. 2002;347:1549-1556.
120 Mittl RL, Schwab RJ, Duchin JS, et al. Radiographic resolution of community-acquired pneumonia. Am J Respir Crit Care Med. 1994;149:630-635.
121 Butler JC, Breiman RF, Campbell JF, et al. Pneumococcal polysaccharide vaccine efficacy: An evaluation of current recommendations. JAMA. 1993;270:1826-1831.
122 Jackson LA, Neuzil KM, Yu O, et al. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med. 2003;348:1747-1755.
123 Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737-1746.
124 Bender JM, Ampofo K, Korgenski K, et al. Pneumococcal necrotizing pneumonia in Utah: does serotype matter? Clin Infect Dis. 2008;46:1346-1352.
125 Nichol KL, Margolis KL, Wuorenma J, et al. The efficacy and cost effectiveness of vaccination against influenza among elderly persons living in the community. N Engl J Med. 331, 1994. 778–774