Chapter 392 Community-Acquired Pneumonia
Epidemiology
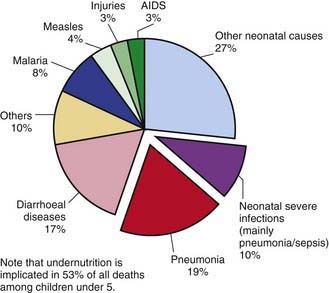
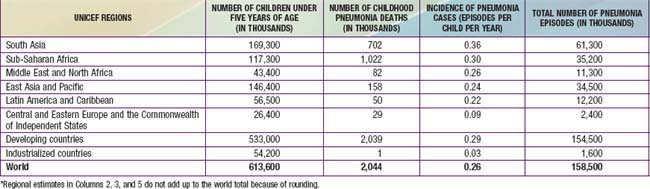
Pneumonia—inflammation of the parenchyma of the lungs—is a substantial cause of morbidity and mortality in childhood throughout the world, rivaling diarrhea as a cause of death in developing countries (Fig. 392-1). With ≈158 million episodes of pneumonia per year, of which ≈154 million are occurring in developing countries, pneumonia is estimated to cause ≈3 million deaths, or an estimated 29% of all deaths, among children younger than 5 yr worldwide. The incidence of pneumonia is more than 10-fold higher (0.29 episodes versus 0.03 episodes), and the number of childhood-related deaths due to pneumonia ≈2000-fold higher, in developing than in developed countries (Table 392-1).
Table 392-1 INCIDENCE OF PNEUMONIA CASES AND PNEUMONIA DEATHS AMONG CHILDREN UNDER FIVE, BY UNICEF REGION*
In the USA from 1939 to 1996, pneumonia mortality in children declined by 97%. It is hypothesized that this decline is attributable to the introduction of antibiotics, vaccines, and the expansion of medical insurance coverage for children. Haemophilus influenzae type b (Hib) (Chapter 186) was an important cause of bacterial pneumonia in young children but has become uncommon with the routine use of effective vaccines. The introduction of heptavalent pneumococcal conjugate vaccine and its impact on pneumococcal disease (Chapter 175) has reduced the overall incidence of pneumonia in infants and children in the USA by ≈30% in the 1st yr of life, ≈20% in the 2nd yr of life, and ≈10% in children >2 yr of age. In developing countries, the introduction of measles vaccine has greatly reduced the incidence of measles-related pneumonia deaths.
Etiology
Although most cases of pneumonia are caused by microorganisms, noninfectious causes include aspiration of food or gastric acid, foreign bodies, hydrocarbons, and lipoid substances, hypersensitivity reactions, and drug- or radiation-induced pneumonitis. The cause of pneumonia in an individual patient is often difficult to determine because direct culture of lung tissue is invasive and rarely performed. Cultures performed on specimens obtained from the upper respiratory tract or “sputum” often do not accurately reflect the cause of lower respiratory tract infection. With the use of state-of-the-art diagnostic testing, a bacterial or viral cause of pneumonia can be identified in 40-80% of children with community-acquired pneumonia. Streptococcus pneumoniae (pneumococcus) is the most common bacterial pathogen in children 3 wk to 4 yr of age, whereas Mycoplasma pneumoniae and Chlamydophila pneumoniae are the most frequent pathogens in children 5 yr and older. In addition to pneumococcus, other bacterial causes of pneumonia in previously healthy children in the USA include group A streptococcus (Streptococcus pyogenes) and Staphylococcus aureus (Chapter 174.1) (Table 392-2).
| BACTERIAL | |
| Common | |
| Streptococcus pneumoniae | Consolidation, empyema |
| Group B streptococci | Neonates |
| Group A streptococci | Empyema |
| Mycoplasma pneumoniae* | Adolescents; summer-fall epidemics |
| Chlamydophila pneumoniae* | Adolescents |
| Chlamydia trachomatis | Infants |
| Mixed anaerobes | Aspiration pneumonia |
| Gram-negative enterics | Nosocomial pneumonia |
| Uncommon | |
| Haemophilus influenzae type b | Unimmunized |
| Staphylococcus aureus | Pneumatoceles, empyema; infants |
| Moraxella catarrhalis | |
| Neisseria meningitidis | |
| Francisella tularensis | Animal, tick, fly contact; bioterrorism |
| Nocardia species | Immunosuppressed persons |
| Chlamydophila psittaci* | Bird contact (especially parakeets) |
| Yersinia pestis | Plague; rat contact; bioterrorism |
| Legionella species* | Exposure to contaminated water; nosocomial |
| Coxiella burnetii* | Q fever; animal (goat, sheep, cattle) exposure |
| VIRAL | |
| Common | |
| Respiratory synctial virus | Bronchiolitis |
| Parainfluenza types 1-3 | Croup |
| Influenza A, B | High fever; winter months |
| Adenovirus | Can be severe; often occurs between January and April |
| Human metapneumovirus | Similar to respiratory syncytial virus |
| Uncommon | |
| Rhinovirus | Rhinorrhea |
| Enterovirus | Neonates |
| Herpes simplex | Neonates |
| Cytomegalovirus | Infants, immunosuppressed persons |
| Measles | Rash, coryza, conjunctivitis |
| Varicella | Adolescents or unimmunized |
| Hantavirus | Southwestern USA, rodents |
| Coronavirus (severe acute respiratory syndrome) | Asia |
| FUNGAL | |
| Histoplasma capsulatum | Ohio/Mississippi River valley; bird, bat contact |
| Blastomyces dermatitidis | Ohio/Mississippi River valley |
| Coccidioides immitis | Southwest USA |
| Cryptococcus neoformans | Bird contact |
| Aspergillus species | Immunosuppressed persons; nodular lung infection |
| Mucormycosis | Immunosuppressed persons |
| Pneumocystis jiroveci | Immunosuppressed, steroids |
| RICKETTSIAL | |
| Rickettsia rickettsiae | Tick bite |
| MYCOBACTERIAL | |
| Mycobacterium tuberculosis | Travel to endemic region; exposure to high-risk persons |
| Mycobacterium avium complex | Immunosuppressed persons |
| PARASITIC | |
| Various parasites (e.g., Ascaris, Strongyloides species) | Eosinophilic pneumonia |
* Atypical pneumonia syndrome; may have extrapulmonary manifestations, low-grade fever, patchy diffuse infiltrates, poor response to beta-lactam antibiotics, and negative sputum Gram stain.
From Kliegman RM, Greenbaum LA, Lye PS: Practical strategies in pediatric diagnosis & therapy, ed 2, 2004, Philadelphia, Elsevier, p 29.
S. pneumoniae, H. influenzae, and S. aureus are the major causes of hospitalization and death from bacterial pneumonia among children in developing countries, although in children with HIV infection, Mycobacterium tuberculosis (Chapter 207), atypical mycobacteria, Salmonella (Chapter 190), Escherichia coli (Chapter 192), and Pneumocystis jiroveci (Chapter 236) must be considered. The incidence of H. influenzae has been significantly reduced in areas where routine Hib immunization has been implemented.
Viral pathogens are a prominent cause of lower respiratory tract infections in infants and children <5 yr of age. Viruses are responsible for 45% of the episodes of pneumonia identified in hospitalized children in Dallas. Unlike bronchiolitis, for which the peak incidence is in the 1st yr of life, the highest frequency of viral pneumonia occurs between the ages of 2 and 3 yr, decreasing slowly thereafter. Of the respiratory viruses, influenza virus (Chapter 250), and respiratory syncytial virus (RSV) (Chapter 252) are the major pathogens, especially in children <3 yr of age. Other common viruses causing pneumonia include parainfluenza viruses, adenoviruses, rhinoviruses, and human metapneumovirus. The age of the patient may help identify possible pathogens (Table 392-3).
Table 392-3 ETIOLOGIC AGENTS GROUPED BY AGE OF THE PATIENT
| AGE GROUP | FREQUENT PATHOGENS (IN ORDER OF FREQUENCY) |
|---|---|
| Neonates (<3 wk) | Group B streptococcus, Escherichia coli, other gram-negative bacilli, Streptococcus pneumoniae, Haemophilus influenzae (type b,* nontypable) |
| 3 wk-3 mo | Respiratory syncytial virus, other respiratory viruses (parainfluenza viruses, influenza viruses, adenovirus), S. pneumoniae, H. influenzae (type b,* nontypable); if patient is afebrile, consider Chlamydia trachomatis |
| 4 mo-4 yr | Respiratory syncytial virus, other respiratory viruses (parainfluenza viruses, influenza viruses, adenovirus), S. pneumoniae, H. influenzae (type b,* nontypable), Mycoplasma pneumoniae, group A streptococcus |
| ≥5 yr | M. pneumoniae, S. pneumoniae, Chlamydophila pneumoniae, H. influenzae (type b,* nontypable), influenza viruses, adenovirus, other respiratory viruses, Legionella pneumophila |
From Kliegman RM, Marcdante KJ, Jenson HJ, et al: Nelson essentials of pediatrics, ed 5, Philadelphia, 2006, Elsevier, p 504.
* H. influenzae type b is uncommon with routine H. influenzae type b immunization.
Pathogenesis
Recurrent pneumonia is defined as 2 or more episodes in a single year or 3 or more episodes ever, with radiographic clearing between occurrences. An underlying disorder should be considered if a child experiences recurrent pneumonia (Table 392-4).
Table 392-4 DIFFERENTIAL DIAGNOSIS OF RECURRENT PNEUMONIA
HEREDITARY DISORDERS
DISORDERS OF IMMUNITY
DISORDERS OF CILIA
ANATOMIC DISORDERS
From Kliegman RM, Marcdante KJ, Jenson HJ, et al: Nelson essentials of pediatrics, ed 5, Philadelphia, 2006, Elsevier, p 507.
Diagnosis
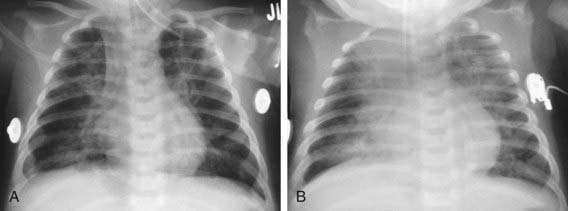
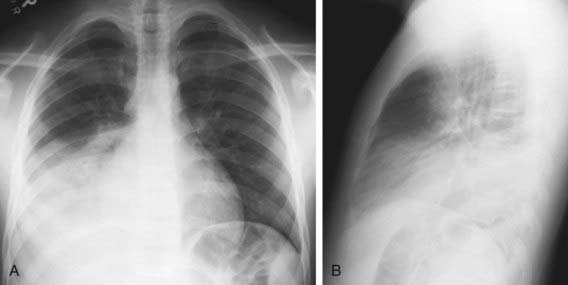
An infiltrate on chest radiograph supports the diagnosis of pneumonia; the film may also indicate a complication such as a pleural effusion or empyema. Viral pneumonia is usually characterized by hyperinflation with bilateral interstitial infiltrates and peribronchial cuffing (Fig. 392-2). Confluent lobar consolidation is typically seen with pneumococcal pneumonia (Fig. 392-3). The radiographic appearance alone is not diagnostic, and other clinical features must be considered. Repeat chest radiographs are not required for proof of cure for patients with uncomplicated pneumonia.
Treatment
Indications for admission to a hospital are noted in Table 392-5. In developing countries, oral zinc (20 mg/day) helps accelerate recovery from severe pneumonia. The optimal duration of antibiotic treatment for pneumonia has not been well-established in controlled studies. For pneumococcal pneumonia, antibiotics should probably be continued until the patient has been afebrile for 72 hours, and the total duration should not be less than 10 to 14 days (or 5 days if azithromycin is used). Available data do not support prolonged courses of treatment for uncomplicated pneumonia.
Table 392-5 FACTORS SUGGESTING NEED FOR HOSPITALIZATION OF CHILDREN WITH PNEUMONIA
Adapted from Baltimore RS: Pneumonia. In Jenson HB, Baltimore RS, editors: Pediatric infectious diseases: principles and practice, Philadelphia, 2002, WB Saunders, p 801.
Complications
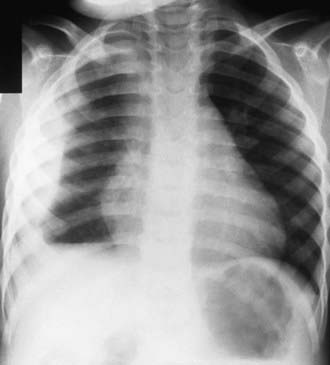
Complications of pneumonia are usually the result of direct spread of bacterial infection within the thoracic cavity (pleural effusion, empyema, pericarditis) or bacteremia and hematologic spread (Fig. 392-4). Meningitis, suppurative arthritis, and osteomyelitis are rare complications of hematologic spread of pneumococcal or H. influenzae type b infection.
S. aureus, S. pneumoniae, and S. pyogenes are the most common causes of parapneumonic effusions and of empyema (Table 392-6). The treatment of empyema is based on the stage (exudative, fibrinopurulent, organizing). Imaging studies including ultrasonography and CT are helpful in determining the stage of empyema. The mainstays of therapy include antibiotic therapy and drainage with tube thoracostomy. Additional approaches include the use of intrapleural fibrinolytic therapy (urokinase, streptokinase, tissue plasminogen activator) and selected video-assisted thoracoscopy (VATS) to debride or lyse adhesions, and drain loculated areas of pus. Early diagnosis and intervention, particularly with fibrinolysis or VATS, may obviate the need for thoracotomy and open debridement. Fibrinolysis may be more cost effective than VATS.
| TRANSUDATE | EMPYEMA | |
|---|---|---|
| Appearance | Clear | Cloudy or purulent |
| Cell count (per mm3) | <1000 | Often >50,000 (cell count has limited predictive value) |
| Cell type | Lymphocytes, monocytes | Polymorphonuclear leukocytes (neutrophils) |
| Lactate dehydrogenase | <200 U/L | >1000 U/L |
| Pleural fluid/serum LDH ratio | <0.6 | >0.6 |
| Protein >3g | Unusual | Common |
| Pleural fluid/serum protein ratio | <0.5 | >0.5 |
| Glucose* | Normal | Low (<40 mg/dL) |
| pH* | Normal (7.40-7.60) | <7.10 |
| Gram stain | Negative | Occasionally positive (less than one-third of cases) |
* Low glucose or pH may be seen in malignant effusion, tuberculosis, esophageal rupture, pancreatitis (positive pleural amylase), and rheumatologic diseases (e.g., systemic lupus erythematosus).
From Kliegman RM, Greenbaum LA, Lye PS: Practical strategies in pediatric diagnosis & therapy, ed 2, Philadelphia, 2004, Elsevier, p 30.
Addo-Yoba E, Chisaka N, Hassan M, et al. Oral amoxicillin versus injectable penicillin for severe pneumonia in children aged 3 to 59 month: a randomized multicentre equivalency study. Lancet. 2004;364:1141-1148.
Asghar R, Banajeh S, Egas J, et al. Chloramphenicol versus ampicillin plus gentamicin for community acquired very severe pneumonia among children aged 2–59 months in low resource settings: multicentre randomized controlled trial (SPEAR study). BMJ. 2008;336:80-84.
Avansino JR, Goldman B, Sawin RS, et al. Primary operative versus nonoperative therapy for pediatric empyema: a meta-analysis. Pediatrics. 2005;115:1652-1659.
Baumer JH. Parapneumonic effusion and empyema. Arch Dis Child. 2005;90:ep21-ep24.
Berjohn CM, Fishman NO, Joffe MM, et al. Treatment and outcomes for patients with bacteremic pneumococcal pneumonia. Medicine. 2008;87:160-166.
Bhutta ZA. Childhood pneumonia in developing countries. BMJ. 2006;333:612-613.
Bhutta ZA. Managing severe pneumonia in children in developing countries. BMJ. 2008;336:57-58.
Bradley JS, Arguedas A, Blumer JL, et al. Comparative study of levofloxacin in the treatment of children with community-acquired pneumonia. Pediatr Infect Dis J. 2007;26:868-878.
Bradley JS, McCracken GH. Unique considerations in the evaluation of antibacterials in clinical trials for pediatric community-acquired pneumonia. Clin Infect Dis. 2008;47:S241-S248.
Brooks WA, Yunus M, Santosham M, et al. Zinc for severe pneumonia in very young children: double-blind placebo-controlled trial. Lancet. 2004;363:1683-1688.
Cantin AM. Childhood pneumonia and oxygen treatment. Lancet. 2008;372:1278-1280.
Caracciolo S, Minini C, Colombrita D, et al. Human metapneumovirus infection in young children hospitalized with acute respiratory tract disease. Pediatr Infect Dis J. 2008;27:406-412.
Cardoso MRA, Nascimento-Carvalho CM, Ferrero F, et al. Adding fever to WHO criteria for diagnosing pneumonia enhances the ability to identify pneumonia cases among wheezing children. Arch Dis Child. 2011;96:58-61.
Centers for Disease Control and Prevention. Acute respiratory disease associated with adenovirus serotype 14—four states, 2006–2007. MMWR Morb Mortal Wkly Rep. 2007;56:1181-1184.
Centers for Disease Control and Prevention. Hantavirus pulmonary syndrome in five pediatric patients, four states, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1409-1412.
Centers for Disease Control and Prevention. Pneumonia hospitalizations among young children before and after introduction of pneumococcal conjugate vaccine—United States, 1997–2006. MMWR Morb Mortal Wkly Rep. 2009;58:1-4.
Centers for Disease Control and Prevention. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep. 2008;57(RR07):1-60.
Cohen E, Weinstein M, Fisman DN. Cost-effectiveness of competing strategies for the treatment of pediatric empyema. Pediatrics. 2008;121:e1250-e1257.
Dagan R, Bhutta ZA, de Quadros CA, et al. The remaining challenge of pneumonia. Pediatr Infect Dis J. 2011;30(1):1-2.
Duke T, Wandi F, Jonathan M, et al. Improved oxygen systems for childhood pneumonia: a multihospital effectiveness study in Papua New Guinea. Lancet. 2008;372:1328-1333.
El Arifeen S, Baqui AH. Treating severe pneumonia in children: we can do better. Lancet. 2008;371:7-8.
Flood RG, Badik J, Aronoff SC. The utility of serum C-reactive protein in differentiating bacterial from nonbacterial pneumonia in children. Pediatr Infect Dis J. 2008;27:95-99.
Frist B, Sezibera R. Time for renewed global action against childhood pneumonia. Lancet. 2009;374:1485-1486.
Garau J, Calbo E. Community-acquired pneumonia. Lancet. 2008;371:455-458.
Gupta A, Khaw FM, Stokle EL, et al. Outbreak of Streptococcus pneumoniae serotype 1 pneumonia in a United Kingdom school. BMJ. 2009;338:950-952.
Haider BA, Saeed MA, Bhutta ZA: Short-course versus long-course antibiotic therapy for non-severe community-acquired pneumonia in children aged 2 months to 59 months, Cochrane Database Syst Rev (16):CD005976, 2008.
Hazir T, Fox LM, Nisar YB, et al. Ambulatory short-course high-dose oral amoxicillin for treatment of severe pneumonia in children: a randomized equivalency trial. Lancet. 2008;371:49-56.
Kusel MMH, de Klerk NH, Holt PG, et al. Role of respiratory viruses in acute upper and lower respiratory tract illness in the first year of life: a birth cohort study. Pediatr Infect Dis J. 2006;25:680-686.
Le Monnier A, Carbonnelle E, Zahar JR, et al. Microbiological diagnosis of empyema in children: comparative evaluations by culture, polymerase chain reaction, and pneumococcal antigen detection in pleural fluids. Clin Infect Dis. 2006;42:1135-1140.
Li STT, Gates RL. Primary operative management for pediatric empyema. Arch Pediatr Adolesc Med. 2008;162:44-48.
Li STT, Tancredi DJ. Empyema hospitalizations increased in US children despite pneumococcal conjugate vaccine. Pediatrics. 2010;125:26-33.
Mandell L. Community acquired pneumonia: new guidelines on management in primary care. BMJ. 2010;341:59-60.
Michelow IC, Olsen K, Lozana J, et al. Epidemiology and clinical characteristics of community-acquired pneumonia in hospitalized children. Pediatrics. 2004;113:701-707.
Panitch HB. Evaluation of recurrent pneumonia. Pediatr Infect Dis J. 2005;24:265-266.
Paludo C, Zhang L, Lincho CS, et al. Chest physical therapy for children hospitalized with acute pneumonia: a randomized controlled trial. Thorax. 2008;63:791-794.
Philipps B. Towards evidence based medicine for paediatricians. Are oral antibiotics as efficacious as intravenous antibiotics for the treatment of community acquired pneumonia? Arch Dis Child. 2011;96(1):103-104.
Picard E, Joseph L, Goldberg S, et al. Predictive factors of morbidity in childhood parapneumonic effusion-associated pneumonia. Pediatr Infect Dis J. 2010;29(9):840-843.
Ponce CA, Gallo M, Bustamante R, et al. Pneumocystis colonization is highly prevalent in the autopsied lungs of the general population. Clin Infect Dis. 2010;50:347-356.
Ranganathan SC, Sonnappa S. Pneumonia and other respiratory infections. Pediatr Clin North Am. 2009;56:135-156.
Regamey N, Kaiser L, Roiha HL, et al. Viral etiology of acute respiratory infections with cough in infancy. Pediatr Infect Dis J. 2008;27:100-104.
Rours GIJG, Hammerschlag MR, Van Doorman GJJ, et al. Chlamydia trachomatis respiratory infection in Dutch infants. Arch Dis Child. 2009;94:705-707.
Schuetz P, Christ-Crain M, Thomann R, et al. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections. JAMA. 2009;302:1059-1066.
Shah SS, DiCristina CM, Bell LM, et al. Primary early thoracoscopy and reduction in length of hospital stay and additional procedures among children with complicated pneumonia. Arch Pediatr Adolesc Med. 2008;162:675-681.
Sorde R, Falco V, Lowak M, et al. Current and potential usefulness of pneumococcal urinary antigen detection in hospitalized patients with community-acquired pneumonia to guide antimicrobial therapy. Arch Intern Med. 2011;171(2):166-172.
Srinivasan R, Asselin J, Gildengorin G, et al. A prospective study of ventilator-associated pneumonia in children. Pediatrics. 2009;123:1108-1115.
Steinhoff M, Black R. Childhood pneumonia: we must move forward. Lancet. 2007;369:1409-1410.
St. Peter SD, Tsao K, Harrison C, et al. Thoracoscopic decortication vs tube thoracostomy with fibrinolysis for empyema in children: a prospective, randomized trial. J Pediatr Surg. 2009;44:106-111.
Tsolia MN, Psarras S, Bossios A, et al. Etiology of community-acquired pneumonia in hospitalized school-age children: evidence for high prevalence of viral infections. Clin Infect Dis. 2004;39:681-686.
van der Poll T, Opal SM. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet. 2009;374:1543-1554.
van Gils EJM, Veenhoven RH, Hak E, et al. Effect of reduced-dose schedules with 7-valent pneumococcal conjugate vaccine on nasopharyngeal pneumococcal carriage in children. JAMA. 2009;302:159-166.
Wacogne I, Negrine RJS. Are follow up chest x ray examinations helpful in the management of children recovering from pneumonia? Arch Dis Child. 2003;88:457-458.
Wardlaw T, Salama P, White Johansson E. Pneumonia: the leading killer of children. Lancet. 2006;368:1048-1050.
Weltz T. Inhaled corticosteroids in COPD and the risk of pneumonia. Lancet. 2009;374:668-670.
Wong CL, Holroyd-Ledue J, Straus SE. Does this patient have a pleural effusion? JAMA. 2009;301:309-316.
Zhang Q, Guo Z, MacDonald NE. Vaccine preventable community-acquired pneumonia in hospitalized children in Northwest China. Pediatr Infect Dis J. 2011;30(1):7-10.