Chapter 37 Common Neck Problems
Introduction and Epidemiology
Successful treatment of painful cervical spinal disorders hinges on the accurate assessment of the underlying tissue injury, which can involve a broad range of biomechanical and biochemical disorders. The clinician needs to conceptualize a process of diagnosis and treatment that incorporates an understanding of the pathophysiology of cervical spine injury and the associated potential symptom manifestations, an awareness of the advantages and disadvantages of the myriad of diagnostic tools, and a knowledge of the potential therapeutic options. The initial step in this process is the history taking. It is important that one distinguish between cervical axial pain (neck pain) and upper limb pain. As eloquently stated by Bogduk,25 the neck is not the upper limb, and the upper limb is not the neck. Pain in the upper limb is not pain in the neck, and vice versa.
Cervical axial pain must not be mistaken for cervical radicular pain. Cervical axial pain is defined as pain occurring in all or part of a corridor extending from the inferior occiput inferiorly to the superior interscapular region, localizing to the midline or just paramidline. The patient perceives it as stemming from the neck. Cervical radicular pain, defined as pain involving the shoulder girdle, distal areas or both, manifests as pain in the upper limb. The etiologies of these two different sets of symptoms vary, as does the diagnosis and management of each. Equating cervical axial and cervical radicular pain can result in misdiagnosis, inappropriate investigation, and institution of suboptimal treatment.25 Such confusion can easily occur because both disorders result from injury to the cervical spine. Each of these injuries or conditions differs in occurrence, mechanism, pathophysiology, treatment, and rehabilitation.
Epidemiologic reports have sometimes clustered neck and limb pain, but neck complaints are ubiquitous. The prevalence of neck pain with or without upper limb pain ranges from 9% to 18% of the general population,130,145,238,248 and one of three individuals can recall at least one incidence of neck pain in their lifetime.130 Cervical pain is more frequently encountered in clinical practice than is low back pain,256 and traumatic neck pain becomes chronic in up to 40% of patients, with 8% to 10% experiencing severe pain.55 The occurrence increases in the workplace, with a prevalence of 35% to 71% among Swedish forest and industrial workers.98,99 The frequency of occupational cervical complaints increases with age. Approximately 25% to 30% of workers younger than 30 years report neck stiffness, and 50% of workers older than 45 years report similar complaints.100,238,248
Cervical radiculopathy occurs less commonly, with an annual incidence of 83.2 per 100,000, and peaks at 50 to 54 years of age.187 Five to ten percent of workers younger than 30 years complain of pain referring into the upper limb, whereas 25% to 40% of those older than 45 years experience pain in the upper limb.100 Overall, 23% of working men have experienced at least one episode of upper limb pain.100 Neck pain, cervical radicular pain, or both are common complaints across different patient profiles.
Pathophysiology and the Significance of Pain Referral Patterns
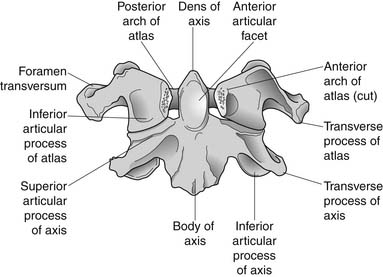
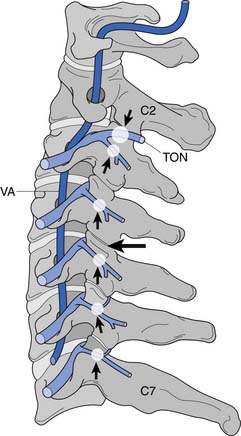
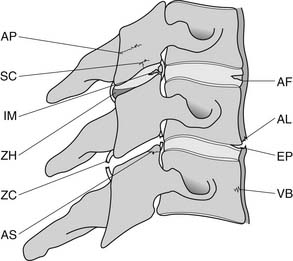
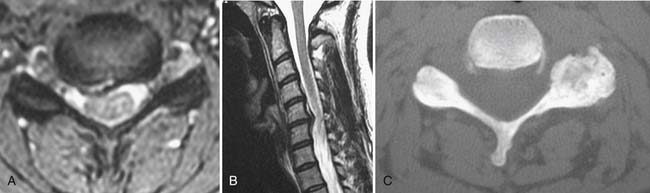
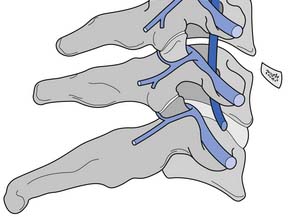
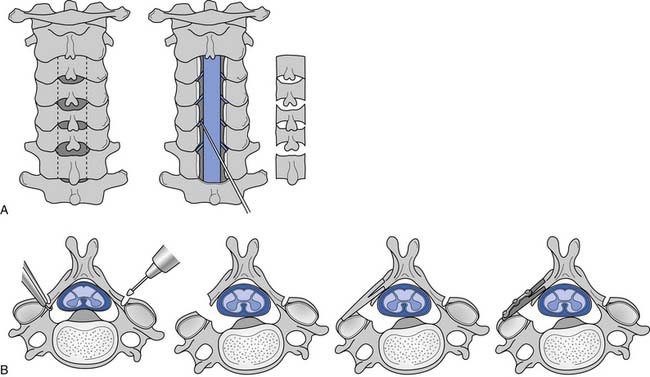
A working knowledge of the anatomic interrelationships within the cervical spine (Figure 37-1) is important, to comprehend the pathomechanisms of cervical spine disorders. The cervical spine is a discrete segment of the axial skeleton (see Figure 37-1), and functions to support and stabilize the head; allow the head to move in all planes of motion; and protect the spinal cord, nerve roots, spinal nerves, and vertebral arteries.163 There are seven cervical vertebrae and eight cervical nerve roots. The atlantooccipital (C0–1) articulation permits 10 degrees of flexion and 25 degrees of extension. The C1–2 level or the atlantoaxial joint (Figure 37-2) forms the upper cervical segment and is responsible for 40% to 50% of all cervical axial rotation, demonstrated clinically by 45 degrees of rotation in either direction.61,62,65,70,249 Below the C2–3 level, lateral flexion of the cervical spine is coupled with rotation in the same direction. This spinal segment marks a transition where permitted motion changes from rotation to flexion, extension, and lateral bending.70,242 This combined motion is facilitated by the 45 degrees sagittal inclination of the cervical zygapophyseal joints (see Figure 37-1).147 The zygapophyseal joints allow motion within the cervical spine, connect each vertebral segment,216 and are innervated by medial branches from the cervical dorsal rami (Figure 37-3).21 In addition, the C0–1 joint is innervated by the C1 ventral ramus,122 and the C1–2 joint by the C2 ventral ramus laterally131 and the sinuvertebral nerves of C1, C2, and C3 medially.120 The greatest amount of flexion occurs at C4–5 and C5–6, while lateral bending occurs primarily at C3–4 and C4–5.70,242 The lower cervical vertebrae (C3–7) have unique synovial joint-like articulations, uncovertebral joints or joints of Luschka, located between the uncinate processes (Figure 37-4).165 These joints commonly develop osteoarthritic changes, which can narrow the diameter of the intervertebral foramina (Figure 37-5).80,97,234 These intervertebral foramina are widest at the C2–3 level and progressively decrease in size to the C6–7 level. The radicular complex of dorsal root ganglia, nerve roots, spinal nerve, and surrounding sheath accounts for 20% to 35% of the cross-sectional area of the intervertebral foramina.80,97,234 The remaining intervertebral foramina volume is filled by loose areolar or adipose tissue, Hoffman’s ligaments, radicular artery, and numerous venous conduits that usually encircle the nerve roots.216 The neuroforamina are bordered anteromedially by the uncovertebral joint, superiorly and inferiorly by successive pedicles, and medially by the edge of the vertebral end plate and intervertebral disk (see Figure 37-5).216
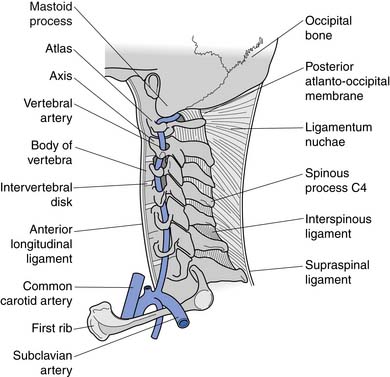
FIGURE 37-1 Anatomic relationship of cervical spine ligaments to other structures in the neck.
(Redrawn from Crafts RC: Textbook of Human Anatomy, ed 2, New York, 1979, John Wiley.)
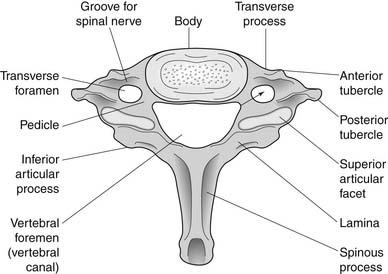
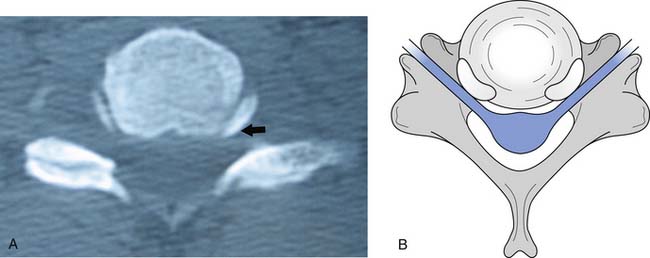
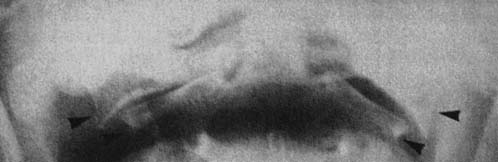
FIGURE 37-5 Axial view of the seventh cervical vertebrae, illustrating the intervertebral foramen (black arrow).
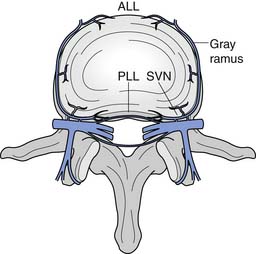
The intervertebral disks are located between the vertebral bodies of C2 through C7. Each is composed of an outer annulus fibrosis innervated posterolaterally by the sinuvertebral nerve, comprising branches from the vertebral nerve and ventral ramus, and innervated anteriorly by the vertebral nerve (Figure 37-6).30 The inner portions of the disks comprise the gelatinous nucleus pulposus, providing transmission of axial loads to dissipate forces throughout various ranges of motion.216 Each intervertebral disk is thicker anteriorly than posteriorly, which contributes to the natural cervical lordotic curvature.216 Normal cervical spine anatomy can undergo degenerative or traumatic changes, leading to various cervical spine disorders.
Three essential requirements are needed for a structure to serve as a source of pain. It must be innervated, capable of producing pain similar to that seen clinically, and susceptible to disease or injury known to be painful.23 Nonneural structures of the neck, such as the intervertebral disk, zygapophyseal joint, posterior longitudinal ligament, and muscles, can serve as a nidus for pain and produce somatic referral of pain into the upper limb.38,69,116–118,128 Classic experiments have demonstrated that stimulation of these posterior midline structures produces local neck pain as well as somatically referred pain into the upper limb.38,69,116–118,128 Kellegren117,118 was first to investigate the pain referral patterns of nonneural spinal structures by stimulating periosteum, fascia, tendon, and muscle with hypertonic saline. He hypothesized that a central nervous system phenomenon mediated the pain referral, because anesthetizing the corresponding peripheral nerve distal to the site of stimulation did not diminish the distally referred pain. These experiments were the first to demonstrate this phenomenon of somatically referred pain, and that cervical spinal disorders could produce pain in the upper limb as well as headache.38,69,116–118,128 These symptoms were produced without irritation of neural tissue. Such pain referral has been termed somatic, previously labeled as sclerotomal, and occurs when a mesodermal structure such as a ligament, joint capsule, intervertebral disk annulus, or periosteum is stimulated, leading to symptoms referred into another mesodermal tissue structure of similar embryonic origin.216 This mechanism of somatically referred symptoms involves convergence.25 Afferents from both the cervical spine and the distal upper limb converge on second-order neurons within the spinal cord, allowing spinal pain to be misperceived as arising from those distal limb sites.25 It is via this mechanism that cervical intervertebral disks and zygapophyseal joints create upper limb symptoms.216
It is thought that biomechanical and/or biochemical insults to nonneural structures can trigger nociceptive nerve fibers, via compression or inflammation, causing pain referral.216 Mechanical stimulation of the cervical zygapophyseal joints or their innervating nerves has been shown to produce head and neck pain with upper limb referral patterns (Figure 37-7).10,59,63,73 Anesthetizing symptomatic joints has revealed similar patterns of symptomatic referral from the cervical joints.28,219
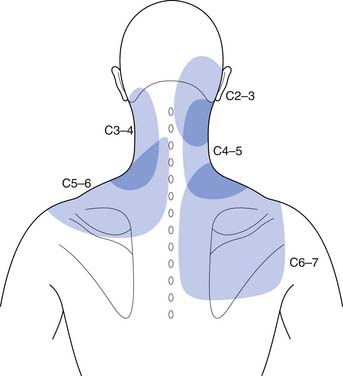
FIGURE 37-7 Pain referral from C2–C3 through C6–C7 facet joints.
(Redrawn from Dwyer A, Aprill C, Bogduk N: Cervical zygapophyseal joint pain patterns. I: A study in normal volunteers, Spine 15:453-457, 1990, with permission.)
Pain emanating from the cervical zygapophyseal joints tends to follow relatively constant and recognizable referral patterns. The C1–2 and C2–3 levels refer rostrally to the occiput. The C3–4 and C4–5 joints produce symptoms over the posterior neck. Pain from the C5–6 joint spreads over the supraspinatus fossa of the scapula, whereas pain from the C6–7 joint spreads further caudally over the scapula.∗ Additionally, C1–2, C2–3, C3–4, and C4–5 zygapophyseal joints can refer pain to the face, and C3–4, C4–5, and C5–6 can refer symptoms to the head.28 Each joint can produce unilateral or bilateral symptomatology. It is not intuitive that a unilateral joint could trigger only contralateral pain, and this manifestation has never been formally investigated.218
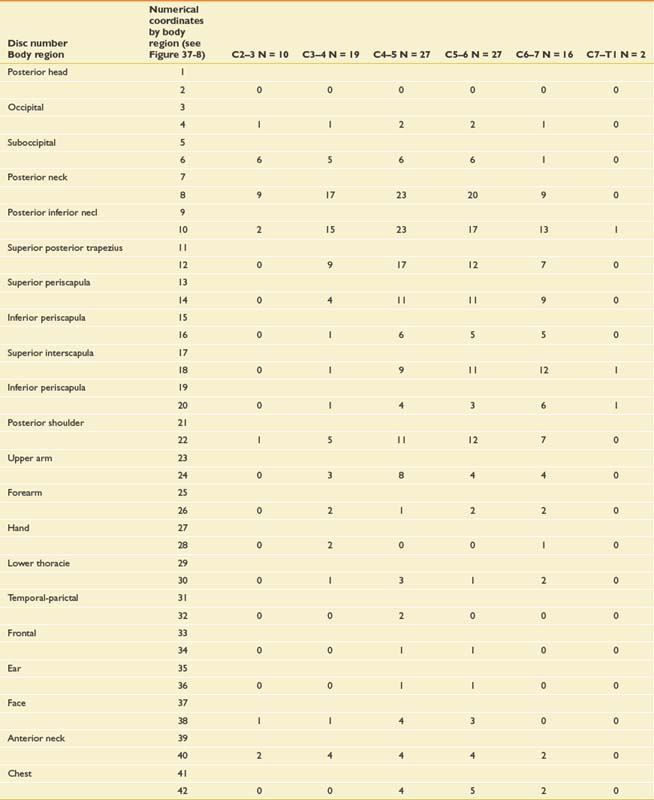
Very similar pain patterns have been produced by mechanical stimulation of the cervical intervertebral disks (Figure 37-8 and Table 37-1).45,78,203,224 In our experience, bilateral paramidline upper neck pain without associated headaches is commonly caused by cervical intervertebral disk disruption (CIDD) rather than zygapophyseal joint–mediated pain. Our observations are supported by Grubb and Kelly’s recent study78 that found that 34% to 50% of cervical disks produced bilateral pain at each cervical disk level. Furthermore, a more detailed study from the Penn Spine Center revealed that 30% to 62% of cervical disks produced bilateral pain during cervical discography.224 When taken together, these findings support the notion that the pattern of pain stemming from a particular structure is a consequence of that structure’s innervation rather than the structure itself.25 In line with this logic is the finding that stimulation of the upper cervical musculature can produce pain in the head.52 Discriminating CIDD-mediated pain from zygapophyseal joint pain or pain emanating from the cervical spine soft tissues requires systematic and meticulous interpretation of history and physical examination findings.
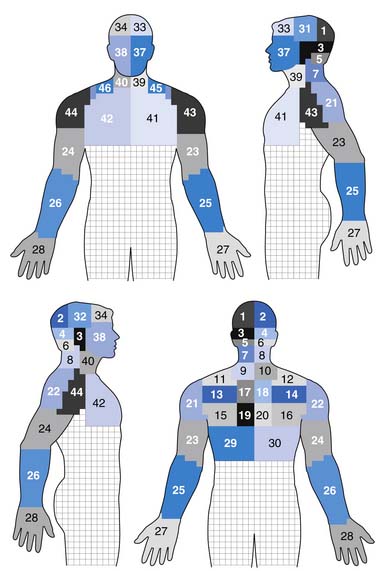
FIGURE 37-8 Pain referral patterns from cervical intervertebral discs stimulated during cervical provocation discography. Pain referral into each area demarked by a number was recorded during disc stimulation (see Table 37-1).
(Redrawn from Slipman CW, Plastaras C, Patel R, et al: Provocative cervical discography symptom mapping, Spine J 5[4]:381-388, 2005, with permission.)
Cervical radicular pain is a fundamentally different clinical picture because the presenting chief complaint is typically upper limb pain more severe than axial pain. The etiology of upper limb symptoms can be confusing when a nonradicular disorder creates symptoms in a radicular distribution, or when a usually radicular disorder causes pain in an uncharacteristic dynatomal pattern (dynatomal pattern—pattern of referred symptoms).225 Regardless, radicular pain is most consistent with upper limb symptoms that are more intense than axial complaints.216 Upper limb pain caused by cervical radiculopathy can refer symptoms into the arm, forearm, and/or hand (Figure 37-9).64 However, periscapular or trapezial pain greater than neck pain can be caused by upper cervical nerve root involvement such as C4 or C5.225 Radicular pain from C5 tends to remain in the arm, but pain from C6, C7, and C8 extends into the forearm and hand. Nevertheless, pain that is primarily in the upper back with or without arm symptoms can emanate from the C4 through C6 roots. When experienced in the middle to lower aspect of the ipsilateral scapula, the C7 or C8 roots could be the culprit.
Nerve vulnerability within the intervertebral foramina arises consequent to changes in one or more of three separate structures: the zygapophyseal joints, the uncovertebral joints, and the intervertebral disk. The most common cause of cervical radiculopathy is a herniated cervical intervertebral disk,102 followed by cervical spondylosis259 with or without cervical myelopathy (Box 37-1). The precise mechanism by which disk herniation or spondylosis causes radicular pain is still somewhat unclear. Direct neurocompression of the nerve root does not necessarily cause pain,96 and pure myotomal weakness can occur.137 Proposed mechanisms for pain in cervical radiculopathy include nerve root inflammation,25 increased discharge of the dorsal root ganglion, mechanosensitivity or chemosensitivity of the nerve root itself, or direct pressure on chronically injured axons or on a normal dorsal root ganglion.96,189 Other potential causes of cervical radiculopathy include tumor,245 trauma,182 sarcoidosis,11 arteritis,200 and athetoid or dystonic cerebral palsy.72
BOX 37-1 Disorders Affecting the Neck
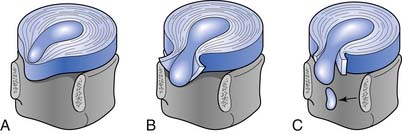
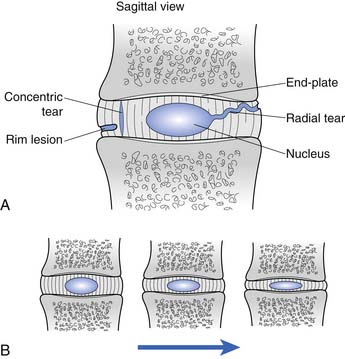
Cervical intervertebral disk injury can be categorized into two broad categories: internal disruption and herniation. Disk herniation is a generalized term, which is further divided into protrusion, extrusion, and sequestration (Figure 37-10). A more thorough discussion of disk herniation follows. Internal disk disruption is a descriptive phrase used to detail derangement of the internal architecture of the nucleus pulposus and/or annular fibers with little or no external deformation.51 The process of disk degeneration occurs over a spectrum of disk abnormalities (Figure 37-11). Initially, circumferential outer annular tears secondary to repetitive microtrauma are associated with interruption in blood and nutritional supply to the disk. These tears eventually coalesce to form radial tears occurring concurrently with a decrement in the water-imbibing ability of the nucleus pulposus. The mechanical integrity of the intervertebral disk suffers as the disk space narrows, more tears develop, and type 2 proteoglycans continue to degrade.121 Biochemical insults have been purported to occur before these biomechanical alterations.198 The end result is a cervical disk that is biomechanically incompetent and prone to biochemical insult.
Cervical zygapophyseal joint injury can occur because of osteoarthritis or trauma resulting from both macrotraumatic and microtraumatic events. Acceleration-deceleration zygapophyseal joint injuries can result in osseous injury to the articular pillars, articular surface, or subchondral bone; intraarticular hemarthrosis; contusion of the intraarticular meniscus; or tears of the zygapophyseal joint capsule (Figure 37-12).∗ We have successfully treated patients with cervical zygapophyseal joint synovitis, who had experienced the onset of symptoms on awakening after sleeping in an awkward position. Painful cervical zygapophyseal joint arthropathy can result as a consequence of cervical intervertebral disk degeneration as well. Biochemical and biomechanical effects can both cause cervical zygapophyseal joint symptom manifestation.
Common Clinical Disorders
Cervical Strain and Sprains
Epidemiology
A cervical strain is a musculotendinous injury produced by an overload injury resulting from excessive forces imposed on the cervical spine.48 In contrast, cervical sprains are overstretching or tearing injuries of spinal ligaments. Muscular strains are seen most frequently because many cervical muscles do not terminate in tendons, but rather attach directly to bone via myofascial tissue that blends seamlessly with periosteum.183 Cervical sprain and strain injuries account for approximately 85% of neck pain resulting from acute, repetitive, or chronic neck injuries.105 These injuries are the most common type of injury to motor vehicle occupants in the United States,185 and are one of the most common causes of pain after noncatastrophic sports injuries.48 Approximately one third of individuals involved in motor vehicle collisions develop neck pain within 24 hours of the injury.207 Automobile-related cervical strain and sprain injuries are more common in Western societies and in metropolitan areas having higher densities of motor vehicles.233 The incidence is higher in women and individuals aged 30 to 50 years.233
Pathophysiology
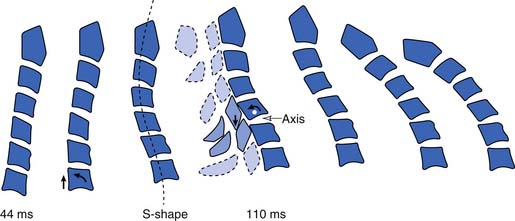
Differing pathomechanisms are causal in cervical strain and sprain injuries, depending on the nature of the abnormal stress applied to the cervical spine. Acceleration-deceleration injuries result in excursions of the cervical spine that result in an S-shaped curvature approximately 100 ms after a rear-end impact.113 By 200 to 250 ms after impact, the head initiates forward flexion of the neck after maximally extending to approximately 45 degrees.154 Posterior neck muscle activation occurs by 90 to 120 ms237 and coincides with the deceleration of the head moving forward.154 As the head continues to move forward, the neck extensors eccentrically contract to decelerate the head, placing them at increased risk for injury (Figure 37-13).168 These experimental findings lend support for a simple muscle or ligamentous strain injury during motor vehicle collisions. Partial and complete muscle tears and hemorrhage have been visualized by ultrasound151 and magnetic resonance imaging (MRI),180 and observed in postmortem examinations.110 Tears of the anterior longitudinal ligament have been reported in surgical explorations34 and identified at postmortem.33 Anatomic studies have demonstrated that the anterior longitudinal ligament merges imperceptibly with the intervertebral disks and can be injured with injury to the cervical disk.190
Physiologic forces acting on a relatively normal cervical spine result in typical soft tissue strain seen in nonathletes. In individuals with thoracic kyphosis and consequential cervical lordosis and extension, strain occurs in the levator scapulae, superior trapezius, sternocleidomastoid, scalene, and suboccipital muscles.48 Traumatic blows often incurred in sporting injuries can result in a more acute cervical strain or sprain.48 Repetitive motions, as occur in recreational activities, can tax shortened and deconditioned cervical rotators, extensors, and lateral flexors that are frequently present in those with cervical spondylosis.48
Diagnosis
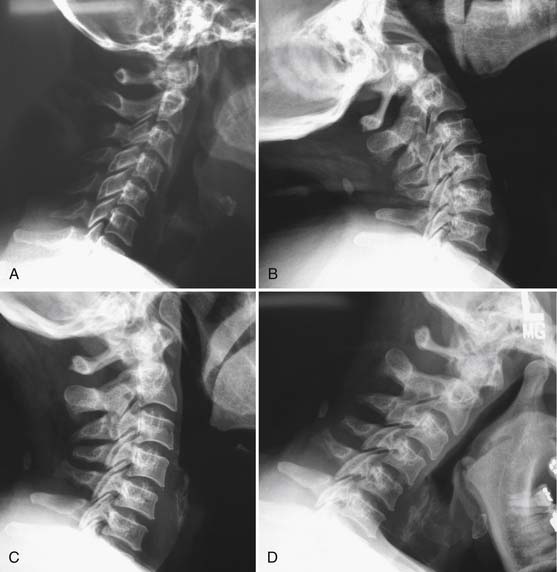
Further diagnostic testing such as imaging or electrodiagnostic evaluations are not indicated unless neurologic or motor abnormalities are detected, or significant pain into the limbs is reported. Plain radiography would be ordered first to evaluate for bony malalignment or fractures. It is reasonable to examine cervical flexion and extension radiographs to evaluate for instability before prescribing functional restoration. In most instances these images are normal or reveal nonspecific loss of cervical lordosis because of muscle splinting (Figure 37-14).
Treatment
Physical modalities such as massage, superficial and deep heat, electrical stimulation, and a soft cervical collar can be used in the treatment program. Light massage causes sedation, reduction of adhesions, muscular relaxation, and vascular changes (see Chapter 20)255 Superficial heat133 and deep heat with ultrasound67 produce analgesia and muscle relaxation, help resolve inflammation, and increase connective tissue elasticity (see Chapter 21).192 Transcutaneous electrical nerve stimulation (TENS) can also be effective in modulating musculoskeletal pain (see Chapter 22).146 A soft cervical collar (Figure 37-15) can be prescribed to ease painful sleep disturbances and reduce further neck strain. The collar can be worn while awake, but should be restricted to the first 72 hours after the injury to minimize interference with healing and prevent development of soft tissue tightening.156 A gradual return to activities should be initiated by 2 to 4 weeks after injury, and should include a functional restoration program to address postural reeducation and functional biomechanical deficits.48
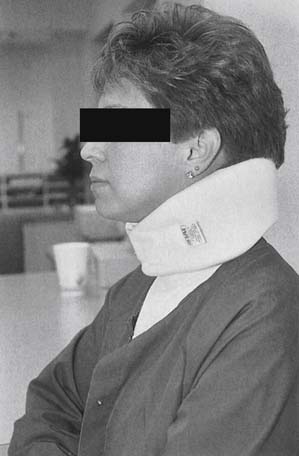
FIGURE 37-15 Cervical orthosis. A cervical soft collar with the widest side posteriorly and the narrowest side anteriorly.
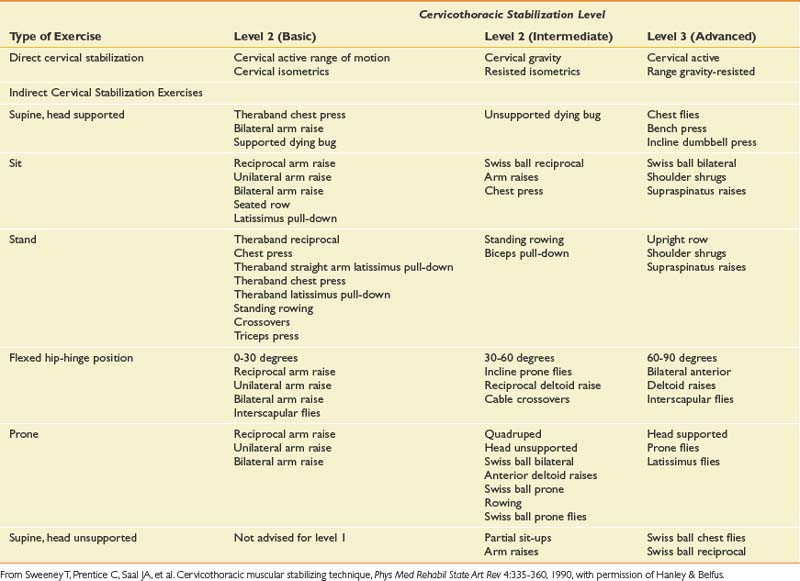
Once the acute pain has improved, proper spinal biomechanics must be restored with the establishment of proper movement patterns. Healthy cervical segmental motion requires efficient stabilization throughout the cervical and thoracic spines. Proprioceptive retraining, balance, and postural conditioning should be incorporated into the exercise regimen. Flexibility and range of motion are improved by mobilization and stretching exercises. Proprioception is improved by using visual feedback during exercises and functional tasks. These should be performed with simultaneous dynamic demands on the patient’s base of support.48,124 Such a program (Table 37-2) enhances the healthy dissipation of forces across the cervical spine with efficient myofascial efforts.
Cervical Radiculopathy and Radicular Pain
Epidemiology
Cervical radiculopathy is a pathologic process involving neurophysiologic dysfunction of the nerve root.64 Signs and symptoms of cervical radiculopathy include myotomal weakness, paresthesias, sensory disturbances, and depressed muscle stretch reflexes.64 Cervical radicular pain represents a hyperexcitable state of the affected nerve root. Cervical radiculopathy, on the other hand, involves reflex and strength deficits marking a hypofunctional nerve root as a result of pathologic changes in nerve root function.25 Separating cervical radicular pain from cervical radiculopathy is important, because treatment strategies will vary depending on the presence or absence of the two conditions.
A large epidemiologic study of 561 patients in Rochester, Minnesota, found an average annual age-adjusted incidence of cervical radiculopathy of 83.2 per 100,000.187 The peak incidence occurred between the ages of 50 and 54 years in the study cohort.187 A history of trauma or physical exertion preceding the onset of symptoms occurred in just under 15% of patients.187 The order of decreasing frequency of involved levels was C7, C6, C8, and C5.187
Pathophysiology
Cervical nerve root injury is most commonly caused by cervical intervertebral disk herniation (CIDH),102 with spondylitic spinal changes the next most common cause (Figure 37-16).167,259 The causal role of neural compression in CIDH-induced radiculopathy was first introduced by Semmes and Murphey208 in 1943. Subsequent radiologic studies have demonstrated the existence of asymptomatic cervical disk abnormalities.20,152,157,240 A growing body of evidence has emerged attesting to the etiologic role of an inflammatory response to a CIDH in some way triggering painful radicular signs and symptoms.74,114,115 Animal studies have shown disrupted nerve root physiology caused by gradient pressure197 and inflammation in the absence of compression.172 Nerve roots are anatomically less resilient than peripheral nerves to both biomechanical and biochemical insults, and respond to each with the same pathologic sequence of events.166
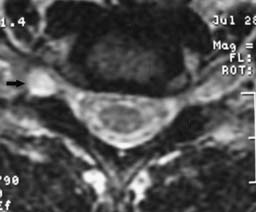
Cervical spondylosis (or degenerative osteoarthritic changes) is manifested by ligamentous hypertrophy, hyperostosis (bony overgrowth), disk degeneration, and zygapophyseal joint arthropathy.259 Hypertrophy of the zygapophyseal joints and uncovertebral joints results in intervertebral foramina stenosis and nerve root impingement.216,259 Vertebral body osteophytes and disk material can form a “hard disk” that can also compress the adjacent nerve root.187,259 Although cervical zygapophyseal joint cysts are rare,142 patients with cervical zygapophyseal joint-induced radiculopathy have been treated at the Penn Spine Center (Figure 37-17). In these cases it is not clear to what extent biochemical versus biomechanical influences affect the neural elements.
Diagnosis
History and Physical Examination
Patients with acute CIDH-related radiculopathy typically report a history of axial cervical pain that is then followed by an explosive onset of upper limb pain. In contrast, spondylitic radicular pain presents more gradually. Cervical radicular pain can masquerade as a deep dull ache or sharp lancinating pain. It can occur in a number of locations, including the medial scapular edge (C5, C6, or C7), superior trapezius (C5 or C6), precordium (C5 or C6), deltoid and lateral arm (C5 or C6), posteromedial arm (C7, C8, or T1), anterolateral forearm (C6 or C7), posterior forearm (C7 or C8), and any of the upper extremity digits (C6, C7, C8, or T1).64,225
Exacerbating factors include activities that raise subarachnoid pressure, such as coughing, sneezing, or Valsalva maneuvers. If a significant component of stenosis is present, cervical extension can amplify the symptoms. Alleviation of the radicular pain by elevating the ipsilateral humerus is known as the shoulder abduction relief sign, which aids in the diagnosis and can be used as a therapeutic manuever.54,68
The physical examination begins with the clinical observation of neck position, as patients characteristically tilt toward the side of the disk herniation. Atrophy can be detected with more severe or longstanding lesions. Muscle wasting in the suprascapular or infrascapular fossae or deltoid suggests C5 or C6 involvement; muscle wasting in the triceps, C7 injury; in the thenar eminence, C8 injury; and in the first dorsal interossei, T1 injury.187 Manual muscle testing has greater specificity than reflex or sensory abnormalities (Table 37-3),257 and might need to be performed repetitively or with the muscle at a mechanical disadvantage to elicit subtle weakness. Severe weakness (<3/5 Medical Research Council [MRC]) is less consistent with a single root lesion and should alert the clinician to the presence of a possible multilevel radiculopathy, α motor neuron disease, plexopathy, or focal peripheral neuropathy. Sensation to light touch, pinprick, and vibration can be altered. The patient should be assessed for the presence of long tract signs such as Hoffman’s sign and Babinski’s response to ensure that there is no spinal cord involvement.
Table 37-3 Nerve Root Levels, Peripheral Nerves, and Muscles of the Upper Limb Commonly Evaluated in the Patient With Neck Pain
| Nerve Root Level | Nerve | Muscle |
|---|---|---|
| C5, C6 | Axillary | Deltoid |
| C5, C6 | Musculocutaneous | Biceps brachii |
| C5, C6 |
Provocative maneuvers such as neuroforaminal closure and root tension signs help localize the lesion to the cervical spine. Spurling’s maneuver,231 cervical extension, lateral flexion, and ipsilateral axial rotation reproducing radicular symptoms are highly specific but not sensitive for cervical radiculopathy.246 Nerve root tension, contralateral cervical axial rotation concurrent with ipsilateral glenohumeral abduction/extension with elbow and wrist extension, can help detect radicular pain. If this examination reveals radicular pain, presumably the imposed neural tension is provocative as a result of nerve root inflammation. Root tension–induced radicular pain might be more sensitive than Spurling’s maneuver but less specific,195 and should be performed bilaterally to ensure absence of contralateral symptoms. Systematic studies comparing the utility of these two examination maneuvers have not been published. L’hermitte’s sign,13526 which is rapid passive cervical flexion while the patient is seated, can produce an electric shock sensation down the spine and occasionally into the limbs in patients with cervical cord involvement as a result of tumor, spondylosis, or multiple sclerosis.176
Imaging Studies
Although plain cervical radiography is not very sensitive in detecting disk pathology, it remains the initial radiographic examination in almost every assessment of musculoskeletal injury.163 Plain films of anteroposterior, lateral, open mouth, and flexion and extension views are indicated to evaluate spinal stability in cases of rheumatoid arthritis or ankylosing spondylitis,64 spondylolisthesis, after fusion, or after traumatic injury.163 Computed tomographic myelography is regarded as the criterion standard against which other imaging modalities ought to be judged in evaluating degenerative cervical spine conditions.196 However, most clinicians reserve unenhanced computed tomography (CT) for the evaluation of osseous details such as foraminal stenosis,163 bone tumors, and fractures.112
MRI is the imaging modality of choice in investigating cervical radiculopathy,112,150 because it details diskal, ligamentous, osseous, and neural tissue very well (see Chapter 7).164 MRI is noninvasive and does not expose the patient to radiation. Although it has become a widely prescribed imaging test, it is expensive, requires patient cooperation to minimize artifact, and is often not tolerated by claustrophobic patients. Patients with embedded metallic objects such as pacemakers or prosthetic heart valves cannot undergo MRI. Contrast-enhanced CT can accurately evaluate disk pathology163 in these cases. Because cervical intervertebral disk abnormalities occur in asymptomatic patients,20,93,152,157,240 the clinical findings have to be correlated with the imaging findings to accurately diagnose the lesion responsible for the patient’s signs and symptoms.
Electrodiagnostic Evaluation
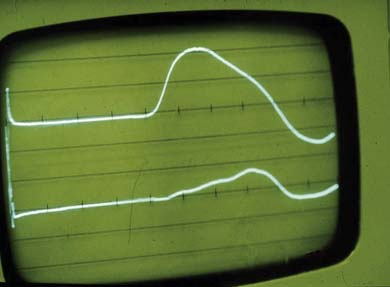
Nerve conduction studies and electromyography can be used to assess the neurophysiologic function of the nerve roots, plexus, and peripheral nerves. Electrodiagnostic examinations, if performed by an appropriately trained physician, can clarify or confirm the suspected diagnosis. Electrodiagnostic examination is also helpful in determining the prognosis of nerve injury. The American Association of Neuromuscular and Electrodiagnostic Medicine guidelines for the electrodiagnostic examination for a radiculopathy include abnormalities in two or more muscles innervated by the same root but different peripheral nerves, provided that normal findings are observed in muscles innervated by adjacent nerve roots.252 At least one corresponding motor and sensory nerve conduction study should be performed in the involved limb to ensure the absence of a concomitant plexus or peripheral process. If abnormalities are found, the correlating contralateral muscle and nerves should be examined to exclude a generalized process such as peripheral neuropathy or motor neuron disease. A screening examination of six upper limb muscles in addition to the cervical paraspinals can identify 94% to 99% of cervical radiculopathies.57 These studies can effectively exclude other diagnoses such as brachial plexus lesions (such as Pancoast tumor or Parsonage-Turner syndrome) and focal peripheral entrapments (such as carpal tunnel syndrome and ulnar entrapment at the elbow or wrist). If the amplitude of the affected muscle’s compound muscle action potential is reduced by less than 50% of that of the contralateral limb (Figure 37-18), functional motor recovery will probably return with conservative care, and studies can be repeated to document neurophysiologic healing (see Chapters 9 through 11).108
Treatment
Physical Medicine and Rehabilitation
The primary objectives of treatment of cervical radiculopathy include the resolution of pain, improvement in myotomal weakness, avoidance of spinal cord complications,64,254 and prevention of recurrence.254 Despite few outcome studies comparing surgical to medical rehabilitation and interventional (conservative) care, accumulated evidence supports the natural resolution of cervical radicular symptoms with conservative care.53,87,199,217 The treatment approach must be molded to the individual patient. A definitive indication for a surgical approach is a progressive neurologic deficit. Otherwise the patient’s necessary level of posttreatment function can help dictate how aggressively to intervene. For example, a relatively sedentary patient might decide to tolerate a low level of discomfort after conservative care. An athlete, on the other hand, might not want to settle for symptoms that are exacerbated by extreme physical activity. The design of the treatment plan has to take into account how the individual functions at home, at work, and in the community.
Modalities
Patient education, activity modification, and relief of pain are the initial treatment steps. The treating physician should explain to the patient the mechanism of how the injury occurred and the most likely treatment outcomes. This explanation should emphasize the importance of proper posture, biomechanics, and the utility of an ergonomic evaluation.254
Thermotherapy is often used to modulate pain and to increase muscle relaxation.134,161 No definitive guideline has been published to date regarding the role of thermal modalities in cervical radiculopathy.191 Cold can be applied for 15 to 30 minutes one to four times a day, and superficial heat can be applied up to 30 minutes two to three times a day. The decision regarding which thermal agent to use is driven by the patient’s perception of which provides the best pain relief.132 Deep heating modalities such as ultrasound should be avoided in the treatment of cervical radiculopathy, because an increased metabolic response and subsequent inflammation can aggravate the nerve root injury.134,191
TENS is helpful in the management of various musculoskeletal and neurogenic disorders (see Chapters 20 through 22). It can be used early in the treatment course of cervical radiculopathy to help modulate pain and enable the patient to engage in other therapeutic modalities. TENS is believed to act via the gate theory. Stimulating large myelinated fibers presumably blocks nociceptive transmission in smaller fibers at the level of the spinothalamic tract neurons.159 Although TENS has been shown to provide some relief of low back pain,229 no studies have been published demonstrating conclusive evidence of its efficacy in cervical radicular pain.
Cervical orthoses function to limit painful range of motion and facilitate patient comfort during the acute injury phase (see Chapter 16).191 Soft cervical collars limit flexion and extension by approximately 26%,109 and are prescribed as kinesthetic reminders of proper cervical positioning.254 The narrower segment should be positioned anteriorly to maintain the neck in the neutral or slightly flexed position (see Figure 37-15).64,199,254 The exceptions to this include patients with a positive L’hermitte’s sign and those with rheumatoid arthritis or atlantoaxial subluxation. The use of a soft collar should be limited to the first week or two of symptoms54,127 to minimize adverse outcomes related to further soft tissue deconditioning.
Cervical traction applies a distractive force across the cervical intervertebral disk space. It is commonly used by patients with cervical radiculopathy, despite a lack of proven efficacy.64,254 It is presumed to work via the decompression of cervical soft tissues and intervertebral disks.47,236 Superficial heat, massage, and/or TENS therapy can be performed before and during traction to relieve pain and to help relax the muscles.46,47 A force of 25 lb is required to distract the midcervical segments when applied for 25 minutes at an angle of pull of 24 degrees.46 Cervical traction can be executed with an intermittent heavyweight or a continuous lightweight regimen in the therapy gym or home setting.47 Traction is contraindicated in patients with myelopathy, positive L’hermitte’s sign, rheumatoid arthritis, or atlantoaxial subluxation (see Chapter 20).47
Medications
A role for antiinflammatory medications is logical in light of the evidence of an inflammatory component to CIDH-related radiculopathy.74,114,115 NSAIDs are the first line of pharmacologic intervention prescribed to treat cervical radiculopathy. At low doses, they provide an analgesic effect, and at high doses an antiinflammatory effect. Side effects associated with the use of NSAIDs relate to their irritation of the gastrointestinal mucosa, platelet inhibition, and renal function. Antiinflammatory agents that target the cyclooxygenase-2 pathway provide similar analgesia and antiinflammatory properties to those of their nonselective counterparts, but with better gastrointestinal tolerability and less antiplatelet effect.85 For these reasons these newer agents may be preferable to traditional NSAIDs for cervical radiculopathy patients, and because diagnostic and therapeutic spinal injections can be performed without interrupting these therapeutic medications. However, recent findings have emerged suggesting that caution should be exercised when prescribing celecoxib at high doses for an extended period, because of an increased cardiovascular risk (National Cancer Institute Adenoma Prevention with Celecoxib trial).
Adjunct medications are often used in conjunction with antiinflammatory medications and include muscle relaxants, tricyclic antidepressants, and antiepileptics. Muscle relaxers are sedating and secondarily relax skeletal muscle. They can be used to aid sleep for 5 to 7 days if sleep is disrupted by painful muscular guarding. Low-dose tricyclic antidepressant medications such as amitriptyline or nortriptyline, prescribed at 10 to 25 mg at bedtime, can be beneficial in decreasing radicular pain and aiding sleep.64 Associated side effects are largely anticholinergic, such as dry mouth, urinary retention, drowsiness, and constipation. Antiepileptic medication such as gabapentin can be effective in modulating neuropathic pain. The therapeutic dose varies from 300 to 900 mg/day up to 3600 mg/day. The most common side effects are lethargy, fatigue, ataxia, and dry mouth. Hence it is recommended to start at a low dose, 300 mg at bedtime, and titrate upward until either symptoms are controlled or side effects curtail the dosage curve. A newer agent, pregabalin (Lyrica), is an option and does not interfere with hepatic metabolism. Other options include tiagabine (Gabitril), zonisamide (Zonegran), and oxcarbazepine (Trileptal). We typically reserve antiepileptic medications for patients suffering with persistent radicular pain postoperatively or who are not appropriate surgical candidates after failure of other therapeutic interventions.
Opiate analgesics can be necessary at times when radicular pain is severe and disrupts sleep. Short-acting opioid-analgesic combination medications can be prescribed to facilitate restorative sleep patterns by controlling the radicular pain (sometimes in conjunction with the use of a soft cervical collar). Opiate medications typically should not be prescribed after the acute phase, and other interventions should be maximized before resorting to long-term use of opioids (see Chapter 42).
Stabilization and Functional Restoration
Cervicothoracic stabilization is the functional restoration of spinal biomechanics, and is used to limit pain, maximize function, and prevent injury progression or recurrence.48,235 Integral parts of this stabilization include restoration of spinal flexibility, postural reeducation, and conditioning. Normal range of motion and good posture are essential to prevent repetitive microtrauma to cervical structures caused by poor movement patterns.36 The patient must be taught how to control activity throughout the kinetic chain. The stabilization program is initiated by establishing the pain-free interval of cervical range of motion, and then progressively adding motion outside this range as the symptoms subside. Any restriction of range of motion in the spine or soft tissues is aggressively treated to achieve full or functional range of motion. Proper cervical biomechanics are restored using passive range of motion, spine and soft tissue mobilization techniques, self-stretching, and correct posture. Improved neuromuscular control is developed first for static positions, and then advanced to dynamic and functional activities.48
Cervical strengthening begins with isometric exercises of the flexor, extensor, rotator, and lateral flexor muscles. The exercises are performed first in the supine position and then progressed to the seated and standing positions.48 The patient is carefully progressed to concentric isotonic exercises, avoiding combined movements unless pain-free. Muscles that are stretched or weakened as a result of poor posture are targeted during this phase.48 One of the main goals of the exercise program is improved muscular balance and flexibility of the cervicothoracic and capital muscle groups.48
Attention is also paid to the shoulder girdles and upper limb conditioning. Mid to upper cervical radiculopathies with myotomal weakness can disrupt scapulothoracic and glenohumeral stabilization. The trapezius, serratus anterior, rhomboid, and rotator cuff muscles must be strengthened.48 Proper scapulothoracic kinetics and glenohumeral coupling allow mechanically efficient spinal posture, as well as efficient dissipation of energy by the upper limbs during functional activities.
Diagnostic Selective Nerve Root Block
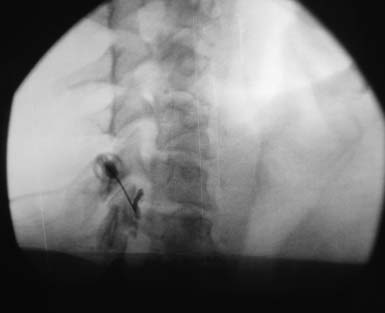
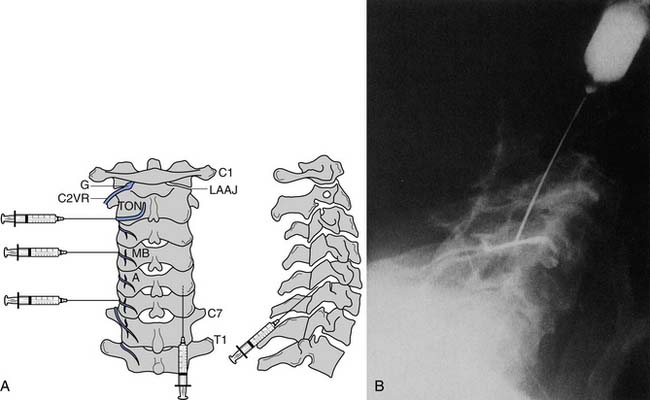
If imaging reveals a structural lesion at the nerve root level that corroborates the physical examination findings, and an electrodiagnostic evaluation confirms the clinical suspicion, therapeutic interventions can be pursued. However, if the physical examination and electrodiagnostic studies are equivocal in the setting of an abnormal MRI, the diagnosis can often be clarified with a fluoroscopically guided diagnostic selective nerve root block (SNRB) at the suspected segmental level (Figure 37-19). If the diagnostic block is negative, the next step in the diagnostic algorithm is investigated.216 The specificity of cervical diagnostic SNRBs has been suggested to range from 87% to 100%,6,119 and the sensitivity has been shown to be 100%.6 The utility of diagnostic lumbar SNRBs has been explored in the literature,∗ and the observed specificity and sensitivity values for lumbar diagnostic SNRBs closely mirror these values for their cervical counterparts. A diagnostic SNRB is a functional diagnostic test, because the patient’s cooperation and understanding is imperative in gaining accurate and valid diagnostic information.202 Because of a relative lack of methodologic investigations configuring the sensitivity and specificity of cervical diagnostic SNRBs, we must extrapolate from the lumbar data (see Chapter 25).
Therapeutic Selective Nerve Root Injection
The natural history of radiculopathy caused by CIDH or spondylosis is a gradual resolution of symptoms with conservative care in 65% to 83% of patients.87,187,199,222 However, two of these studies,199,222 incorporated fluoroscopically guided cervical epidural or selective nerve root injections (SNRIs) of corticosteroids. Heckmann et al.87 retrospectively observed that 65% of 60 patients with cervical radiculopathy (90% caused by CIDH, 10% caused by spondylosis) improved significantly with conservative care that did not involve spinal injections. Several studies have examined the utility of cervical therapeutic SNRIs to treat cervical radiculopathy after failure of more conservative care.† Good to excellent results have ranged from 50% to 83%, with follow-up intervals from 6 to 21 months.‡ More successful results have been obtained in the presence of CIDH35,53,199 than with spondylotic changes.17,53,221,243 Not surprisingly, traumatically induced cervical radiculopathy portends a poorer prognosis.220,222 “Whiplash”-induced cervical radicular pain in the absence of concomitant foraminal stenosis, and traumatic spondylotic cervical radicular pain are successfully treated with conservative care, including up to two to four SNRIs in 14%222 and 20%220 of cases, respectively. In this patient profile, if an initial SNRI achieves no improvement in radicular pain, then further injections might not be warranted, as probably less than 20% of patients will respond to further injections.220,222 More recently, the injection of local anesthetic alone has demonstrated an efficacy equal to that of anesthetic and corticosteroid combined,7 and an equal reduction in avoiding surgery.193
Despite a growing concern regarding the safety of cervical SNRIs, when performed properly,27 they have been demonstrated to be safe104 and offer a valid, minimally invasive treatment intervention to treat painful, nontraumatic cervical radicular symptoms. The goal of SNRI is to modulate the inflammatory response to the CIDH by injecting corticosteroids close to the disk–nerve root interface. This is intended to control pain and to start the process of nerve root healing, while the intervertebral disk herniation naturally resorbs, becomes inert tissue, or both. A proportion of cases will eventually require decompression because of persistent intervertebral disk herniation volume, continuation of the inflammatory response, or both.
Percutaneous Diskectomy and/or Disk Decompression
Percutaneous diskectomy has been investigated as a nonsurgical alternative for treating persistent cervical radiculopathy caused by a corroborative focal herniation.∗ Various technologies have been used to achieve cervical intervertebral disk decompression, including laser,41,83,95,123,211 enzymatic,89 and mechanical41,95 decompression. The follow-up intervals have been relatively short83,89,211 or unspecified.95 The two studies with the longest outcome data demonstrated disparate results41,123 and included a heterogeneous study cohort.123
Nucleoplasty is a technology that uses coblation energy to vaporize nuclear tissue into gaseous elementary molecules. It has recently been developed and applied to the spine.209 In a recent study conducted at the Penn Spine Center,217 nucleoplasty was combined with SNRI to treat both the biomechanical and biochemical causes of CIDH-related cervical radicular pain that had been unresponsive to conservative care. These patients demonstrated a contained CIDH without stenosis and persistent radicular pain, and had been deemed appropriate surgical candidates. At 6 months, 91% to 95% of these 21 patients experienced an average 83% reduction in their pain level, with the greatest rate of reduction occurring within the first 2 weeks.217 At 12 months, 17 of 21 had a good or excellent result. It should be noted that each of the 21 patients in this consecutive cohort had been considered candidates for surgery by a fellowship-trained spine surgeon, yet only two ultimately required surgery. No major complications were encountered, and the procedure was performed with light intravenous sedation on an outpatient basis. These findings have been corroborated by subsequent studies of nucleoplasty performed without an SNRI in which 81% to 85% of patients experienced good to excellent results at 6 to 12 months and significant reduction in radicular pain.31,136 More recently, a randomized, controlled trial of 115 patients demonstrated a significant reduction in pain and disability at 3, 6, and 12 months after nucleoplasty compared with TENS, physical therapy, analgesics, and/or NSAIDs, without complications.39 Percutaneous diskectomy with coblation technology combined with or without SNRI can be safe and effective in relieving cervical radicular pain caused by a corroborative intervertebral disk protrusion. It can be offered as a nonoperative alternative to surgery if more conservative measures fail.
Surgery
Indications for surgical treatment of CIDH or spondylotic-related cervical radiculopathy include intractable pain, severe myotomal deficit (progressive or stable), or progression to myelopathy. Surgical outcome studies have demonstrated good or excellent results in 80% to 96% of patients.4,76,90,122 At 3 months postoperatively, surgical intervention, such as anterior cervical diskectomy and fusion (Figure 37-20) or posterior foraminotomy, has achieved quicker improvement in radicular pain, strength, and sensation than has conservative care.179 These differences between conservative and surgical approaches equalize at 1 year.179 Artificial cervical disk replacement has performed comparably to traditional anterior cervical diskectomy and fusion.202 Our goal is to effectively modulate and enhance the body’s natural response to a CIDH, beginning with conservative antiinflammatory measures, then moving to minimally invasive interventions, and culminating in open surgical decompression if necessary.
Cervical Joint Pain
Epidemiology
The prevalence of cervical facet joint–mediated pain in patients with complaints of neck pain ranges from 36% to 60%.15,141,149,228 The most commonly symptomatic level, determined by controlled diagnostic blocks, is C2-3 (36%), followed by C5–6 (35%) and C6–7 (17%). Joints at C1–2, C3–4, and C4–5 are each symptomatic in less than 5% of cases.49 In patients with cervical facet joint pain, 52% have only one symptomatic joint, and only rarely are two or more consecutive joints symptomatic.49 Similarly, cervical zygapophyseal joints are a common source of chronic posttraumatic neck pain.9 Chronic, traumatic cervical zygapophyseal joint–mediated neck pain has an estimated prevalence of 54% to 64%.15,26,141 Painful cervical zygapophyseal joints most commonly occur in association with a symptomatic intervertebral disk at the same level.26 In patients with chronic zygapophyseal joint pain, 58% to 88% complain of associated headaches.15,140,141 The prevalence of C2–3 zygapophyseal joint pain has been estimated to be 50% to 53% in patients whose chief complaint is posterior headaches after whiplash injury.140,141 Traumatically induced lower cervical pain attributable to a zygapophyseal joint most commonly involves the C5–6 level.15,26,141 More than one structure can be injured in traumatically induced cervical zygapophyseal joint pain.216 Spontaneous (nontraumatic) cervical zygapophyseal joint pain affects usually one joint and can be caused by spondylosis or improper biomechanics.
Diagnosis
History and Physical Examination
A detailed examination should be completed on any patient who sustains a whiplash injury, to understand the mechanism of injury and to exclude spinal cord injury, plexopathy, or traumatic brain injury. Details of the accident, including the neck position at the time of impact, can help predict which structures are the most likely to be injured. However, the clinical history cannot provide pathognomonic findings to distinguish zygapophyseal joint-mediated pain from other sources of axial neck pain.28 In our experience, traumatic upper zygapophyseal joint involvement such as at the C2–3 joint is more likely to cause unilateral occipital headaches rather than neck pain. Unilateral paramidline neck pain, with or without periscapular symptoms, that is more painful than any associated headaches is suggestive of zygapophyseal joint pain rather than disk or root injury.
The physical examination must assess neurologic function and cervical range of motion. The clinician should suspect zygapophyseal joint injury when the patient can pinpoint a localized spot of maximal pain, or define an area of pain typical for the referral distribution of a particular zygapophyseal joint. Focal tenderness to palpation posterolaterally over a joint lends further support for an underlying painful zygapophyseal joint.111 Increased focal suboccipital pain that occurs or is exacerbated with 45 degrees of cervical flexion and sequential axial rotation is suggestive of a painful C1–2 joint.59 Despite these suggestions, to date there have been no well-designed investigations of clinical examination findings that are diagnostic of cervical zygapophyseal joint pain.
Imaging Studies
Cervical zygapophyseal joint subluxation can be detected by plain radiography, and CT can better delineate joint fracture. Soft tissue injury, however, is largely undetected by advanced imaging. This means that imaging has a limited role in determining the pain generator.32,239 Nuclear imaging might demonstrate increased radiotracer uptake if there is an abnormality within the zygapophyseal joint, but it cannot discriminate between symptomatic and asymptomatic abnormalities.
Treatment
Physical Medicine and Rehabilitation
Superficial cryotherapy such as with ice application is preferred to superficial heat, because of its analgesic and antiinflammatory qualities. Although superficial heat has analgesic effects and relaxes muscles, its metabolic effects preclude its use in treating acute cervical zygapophyseal joint injuries. Cryotherapy should initially be applied for 20 minutes three or four times a day to cause vasoconstriction and decrease the release of pain and inflammatory mediators.133 Soft tissue mobilization and massage can help break muscular guarding or splinting, but should not be the mainstay of treatment. Soft cervical collars can be worn for a short period of time, up to 72 hours after the initial injury. These are used for comfort, especially when sleeping. Patient education regarding proper positioning to avoid aggravating factors should occur concurrently with analgesic and antiinflammatory medications.
The restorative phase encompasses stabilization and functional restoration by normalizing the range of motion, soft tissue length, and biomechanical deficits, and strengthening the spinal musculature. Transition to this phase begins after there is a reduction in pain caused by the acute injury. Restoration of cervical spine motion helps achieve a balanced posture that decreases strain of the injured joints and also allows optimal strengthening to occur. Cervicothoracic stabilization addresses flexibility, posture reeducation, and strengthening, all of which reduce pain, improve function, and prevent recurrent injury.235 Proprioceptive skills are implemented during strengthening exercises to achieve these goals.48 The patient is discharged to a home exercise maintenance program to maintain mobility and strength.
Interventional Spine Physiatry
The natural history of whiplash-induced neck pain is of gradual recovery in most patients. It is imperative, however, that an accurate diagnosis is formulated to maximize treatment if a patient’s pain is moderate or severe, or persists beyond this natural historical time frame. Determining which exact joints are the source of symptoms requires the meticulous use of fluoroscopically guided target-specific injections.
Diagnostic Zygapophyseal Joint Blocks
The close anatomic relationship and overlapping referral patterns of spinal structures necessitate the use of fluoroscopically guided diagnostic zygapophyseal joint blocks (Figure 37-21) to confirm a clinically suspected painful joint. Diagnostic blocks offer a definitive means by which to target symptomatic joints. Local anesthetic can be injected into the joint or on the innervating medial branch nerves to the joint to anesthetize a joint. The diagnosis is based on the concept that if the symptomatic structure is blocked, the patient’s pain will be relieved. If the pain is unrelieved, the tested joint is excluded as the source of the symptoms. Approximately one third of responders can represent false-positive results.13 Consequently, comparative blocks139 using anesthetics of varying duration of effect need to be performed before considering more invasive treatment options. Our approach to cervical zygapophyseal joint pain entails instituting therapeutic injections after a positive single intraarticular diagnostic block. If intraarticular injections of corticosteroid are ineffective, comparative blocks incorporating a placebo injection are performed before performing a medial branch neurotomy.
Therapeutic Zygapophyseal Joint Injections
Therapeutic intraarticular cervical zygapophyseal joint injections can be appropriate in individuals who have not improved from pharmacologic and physical modalities. Barnsley et al.14 reported that single intraarticular steroid injections were not effective for the treatment of chronic whiplash-related cervical zygapophyseal joint pain. However, in this study the authors treated patients with only one therapeutic injection, used one outcome measure, and did not restrict physical therapy exercises.14 In our experience, fluoroscopically guided therapeutic intraarticular steroid injections have been efficacious in treating cervical zygapophyseal joint pain. Slipman et al.223 demonstrated good to excellent results in 61% of patients who were having daily whiplash-related occipital headaches originating from the C2–3 joints. Patients who benefited from these injections underwent an average of two therapeutic injections.
Percutaneous Radiofrequency Ablation Medial Branch Neurotomy
If patients do not obtain satisfactory relief from steroid injections, then radiofrequency ablation of the joint’s innervating medial branches can be offered. Lord et al.138 established the efficacy of radiofrequency neurotomy in patients with chronic cervical zygapophyseal joint pain through a randomized, double-blind, placebo-controlled trial. The median time that elapsed before return of pain to preneurotomy level in the treatment group was 9 months, compared with 1 week in the sham control group. A subsequent study by McDonald et al.155 observed that a second neurotomy can provide the same pain relief if the patient’s symptoms returned after an initially successful procedure. However, approximately 50% of patients can require repeat neurotomy procedures.103 Procedures that are repeated achieve similar periods of symptom reduction as the initial treatment procedure.103,155 Radiofrequency neurotomies of the atlantooccipital, atlantoaxial, and C2–3 joints have not been investigated. There is currently no technique for denervating the atlantooccipital and atlantoaxial joints, and denervation of the C2–3 joint has not been studied because of the adverse effect of suboccipital hypoesthesia from neurotomy of the third occipital nerve. We have successfully used radiofrequency neurotomy of the C2–3 zygapophyseal joint, and most patients are not distressed by the suboccipital hypoesthesia.
Cervical Internal Disk Disruption
Epidemiology
Internal disk disruption was first described by Crock51 more than 30 years ago, and indicates that an intervertebral disk has lost its normal internal architecture but maintains a preserved external contour in the absence of nerve root compression. In traumatically induced chronic neck pain, 20% of patients might have CIDD, and another 41% might have CIDD and a concomitant zygapophyseal joint injury.26 Litigation might adversely affect treatment outcomes for CIDD,56 but this potential litigation effect has not been substantiated in other investigations.170,186,201 In nonlitigation cases, nonoperative and operative outcomes are similar to those for CIDD.56
Diagnosis
History and Physical Examination
The symptom complex of CIDD includes posterior neck pain, occipital and suboccipital pain, upper trapezial pain, interscapular and periscapular pain, nonradicular arm pain,224 vertigo, tinnitus, ocular dysfunction, dysphagia, facial pain, and anterior chest wall pain.75,204 Patients often report a history of preceding trauma, such as a motor vehicle collision, with acute onset. In the absence of a precipitating event, CIDD symptoms can start spontaneously and gradually, or explosively. If referral symptoms are present, the patient’s chief complaint is primarily axial pain associated with nondescript upper limb symptoms. Exacerbating factors usually include prolonged sitting, and coughing, sneezing, or lifting. Lying supine with head support typically alleviates the patient’s symptoms.
The physical examination can show only subtle cervical range-of-motion restrictions, unless there has been prior cervical surgery. A thorough neuromusculoskeletal examination should be performed to exclude myelopathy or radiculopathy. If spondylosis is present, cervical extension and lateral bending are more restricted than flexion and axial rotation are. Palpation over the cervical spinous processes of the involved level can elicit pain in that region or a portion of the patient’s axial pain. Separating these patients from those with nonorganic neck pain can be achieved by eliciting nonorganic signs, such as superficial or nonanatomic tenderness, pain with rotation of the head and pelvis together, nonanatomic sensory loss, give-way weakness, and overreaction.227
Imaging Studies
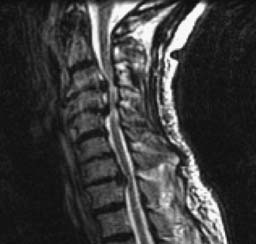
Distinguishing painful from nonpainful cervical disks solely on imaging characteristics can be difficult. Disk abnormalities have been noted in asymptomatic patients,20,152,240 and CIDD by definition displays an age-appropriate appearance on MRI.51 Plain films can reveal hyperostosis and disk space collapse but frequently do not correlate with pain symptoms. Disk desiccation, loss of disk height, annular fissure, osteophytosis, and reactive end-plate changes are markers of disk degeneration (Figure 37-22).163 A decreased intradiskal signal on T2-weighted images correlates well with histologic degeneration of the disk.163 MRI features are not useful, however, in detecting symptomatic cervical disks.203 Consequently, functional diagnostic testing such as provocation diskography is used to diagnose the painful disk level. Our understanding of pain distribution from CIDD or degenerative disks has been expanded by studies of these functional diagnostic tests.45,78,203,224
Treatment
Physical Medicine and Rehabilitation
Physical modalities can be prescribed to modulate pain and transition the patient from passive treatment to active functional restoration. Superficial modalities and TENS therapy can be used for pain. Heat modalities can also be used to increase soft tissue elasticity before and during cervical traction. In uncontrolled studies, electrical stimulation (TENS) has been found to be helpful in cervical pain. Traction might be beneficial by distracting painful intervertebral disks. Traction should be used cautiously, however, because distracting injured annular tissue can provoke painful symptoms. Cervical collars can help maintain comfortable positioning but should not be worn by the patient for more than 72 hours to minimize further deconditioning. These passive modalities should be used early in the treatment process and later, on an as-needed basis, and should not replace activity-based therapies.
As with all other mechanical cervical pain disorders, CIDD requires a thorough evaluation of spinal biomechanics. As the acute pain starts to subside, the patient is enrolled in an active stretching and flexibility program with transition to conditioning and stabilization. The independent effects of exercise and stabilization, specifically in the treatment of cervical disk injury, have not been scientifically validated.230 However, a role for cervical spine stabilization rather than just stretching and strengthening is supported.60,81 The effects can also be inferred from a lumbar stabilization research report that demonstrated a statistically significant reduction in pain and disability in a group of patients with spondylosis and spondylolisthesis.173 The only methodologically correct way to study outcomes in patients with true cervical diskogenic pain would be to enroll and treat a cohort of patients after diskography-proven concordant axial neck pain. No such studies exist.
Interventional Spine Physiatry
Provocation Discography
Provocation discography is a functional diagnostic test in which the accuracy of the investigation relies heavily on patient input. Smith and Nichols226 first described cervical discography in the early 1950s, and its utility has been contested ever since.26 Provocation discography is the only test that can address the symptomatic status of the disk, and is typically used when CIDD is in question. It is also used as a presurgical evaluation. A positive response requires structural evidence of internal derangement that corroborates production of the patient’s usual cervical and referred pain (a concordant response).
Proponents of discography suggest that healthy disks accept a finite volume of contrast and do not produce symptoms with mechanical stimulation.206,251 Discography should be considered valid only when an asymptomatic control disk injection accompanies a concordantly painful disk injection. Although false-positive results have been demonstrated in asymptomatic volunteers,94 these findings can be dismissed because of technical insufficiencies. Cervical provocation discography has not produced false-positive pain responses in asymptomatic volunteers.203 On occasion, a provocative discogram can produce a false-positive response in the presence of a painful cervical zygapophyseal joint.26 Bogduk and Aprill26 warn that deducing that a positive cervical discogram in traumatic chronic cervical pain is conclusive might be misleading, and recommend completing diagnostic zygapophyseal joint blocks at the level of the painful disk before pursuing treatment interventions. Cervical intervertebral disks have been shown to refer pain into the head and face both unilaterally and bilaterally,224 and overlap pain referral patterns produced by painful zygapophyseal joints.63
Transforaminal Epidural Steroid Injections
Instillation of steroids into the anterior epidural space to bathe the posterolateral margins of the annular surface of the intervertebral disks and posterior longitudinal ligament can address biochemically stimulated nociception.216 A C7 transforaminal epidural steroid injection (TFESI) is performed if the symptoms are consistent with diskogenic pain and are primarily located at the base, or refer outward from the base, of the cervical spine. The identical procedure is performed at the C5 or C6 level to treat upper neck pain with or without headaches, and facial or upper limb symptoms.216 If a corticosteroid effect, defined as a 50% reduction in preinjection pain level lasting for 2 days within 7 days after the procedure, is not experienced by the patient, cervical discography or diagnostic zygapophyseal joint blocks are typically pursued.
The efficacy of TFESIs has not been systematically studied. Interlaminar epidural steroid injections have been investigated in a heterogeneous group of patients with axial and radicular pain.42,184,194,210 Most of the patients in these studies had ill-defined symptoms of axial pain, radicular pain, or both. The injections were completed posteriorly via the interlaminar approach without fluoroscopic guidance, using the loss-of-resistance technique. Outcomes were successful in approximately 40% to 84% of treated patients.16,42,184,194,210 Medication injected posteriorly between the laminae might not diffuse anteriorly126 to reach the potential pain generator. Despite the paucity of literature regarding the utility of TFESIs in treating diskogenic cervical pain, we typically perform two injections initially before assessing the clinical response. In our experience, approximately 50% of patients experience significant and lasting relief. The more acute or subacute the symptom duration, the better the results, presumably because of a relatively acute inflammatory disk injury.
Surgery
The only surgical treatment for CIDD or symptomatic cervical degenerative disks is fusion,77,101,149,250 which can be accomplished by anterior cervical diskectomy and fusion or by posterior fusion. The rationale is that by fusing the bony vertebral elements, motion is eliminated, thereby reducing diskogenic pain. The utility of provocation discography to determine the level(s) to fuse is controversial. Some authors have reported “good or excellent” results in 70% to 96% of patients after cervical fusion of levels determined by discography.76,125,206,212,251 Seibenrock and Aebi206 observed a pain reduction of greater than 75% in 96% of 27 patients who underwent cervical fusion of a total of 39 levels. The review was retrospective, and the authors might have included some patients who had primarily radicular complaints. Garvey et al.75 found that 82% of 87 patients reported their self-perceived outcome as good, very good, or excellent at a mean of 4.4 years after fusion. Ninety-three percent of these patients reported a greater than 50% reduction in their pain rating postsurgically. Interestingly, a statistically significant difference was obtained for patients who were treated based on a truly positive diskogram. An extensive literature review concluded that controlled cervical discography improves surgical outcomes.148
Cervical Myelopathy and Myeloradiculopathy
Epidemiology
Cervical spondylitic myelopathy is the most common cervical cord lesion after middle age,253 but not as common as spondylitic cervical radiculopathy.3 The average age at onset is after 50 years, and men predominate.44 Other causes of myelopathy have to be ruled out, including multiple sclerosis, motor neuron disease, vasculitis, neurosyphilis, subacute combined degeneration, syringomyelia, and spinal tumors.259 One of these other conditions can be present in up to 17% of patients having spondylitic myelopathy.37
Diagnosis
History and Physical Examination
Symptom onset is typically insidious, although a minority of patients can experience acute onset with or without a preceding traumatic event. Myelopathic patients often complain of numbness and paresthesias in the distal limbs and extremities, weakness more often in the lower than upper limbs, and intrinsic hand muscle wasting.259 Cervical axial pain can be the primary complaint in up to 70% of patients at one point in the disease course.259 The natural history typically involves an initial deterioration, followed by a static period that can last several years.171 Bladder function disturbances occur in approximately one third of cases and are suggestive of more severe spinal cord injury.259 Patients can concurrently complain of unilateral or bilateral radicular pain caused by nerve root involvement at the stenotic level. The combination of cord and radicular involvement is referred to as cervical spondylitic myeloradiculopathy.
A common examination finding is myelopathic weakness in the lower limbs and, to a lesser extent, in the upper limbs. The upper extremities will demonstrate intrinsic hand muscle weakness and wasting resulting from anterior horn cell damage.259 Pain and temperature disturbances representing injury to the spinothalamic tracts appear as a level of sensory disruption in the thoracic or lumbar region, or “glove and stocking” distribution. Proprioception and vibratory deficits indicating posterior column malfunction are more common in the lower limbs than in the upper.259 Upper motor neuron signs such as Hoffman’s sign, brisk reflexes, and Babinski’s signs are often present. The signs and symptoms might or might not be symmetric, and complete sparing of the upper limbs is rare. The clinical level of spinal involvement might not correlate with the radiologic level of maximum compression, and there can be a difference of one to two segments.259 Asymmetric reflexes in the upper limbs, or myotomal weakness, can indicate concomitant radiculopathy.
Imaging Studies
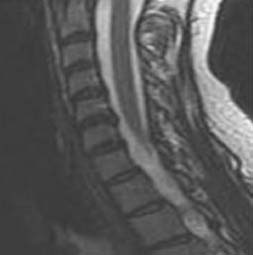
Radiographic evaluations typically demonstrate the cervical cord compression. Most are spondylitic in nature. Other causes include a superimposed CIDH impinging on the thecal sac, or ossification of the posterior longitudinal ligament (the etiology in 27% of middle-aged patients241). Plain radiography provides information regarding the osseous diameter of the central canal and decreased height of the intervertebral disk spaces, and the presence of posterior hyperostosis, foraminal encroachment, and subluxation. A central canal diameter of less than 10 mm in a symptomatic patient supports the existence of myelopathy.259 Asymptomatic central cervical spinal stenosis has been observed in 16% of individuals younger than 64 years.240 A 30% reduction in the cross-sectional area of the cervical spine is the minimum decrement in symptomatic patients.178 To accurately diagnose cervical spondylitic myelopathy, approximately one third of the central canal must be compromised, and objective central canal changes ought to be evident. These include a complete lack of cerebrospinal fluid flow, cord deformation, or intracord signal abnormalities. MRI allows detection of myelomalacia, which reflects progressive cord compression, signal alteration, atrophy,188 and the amount of cerebrospinal fluid volume surrounding the cord (Figure 37-23). The preoperative transverse area of the spinal cord at the site of maximal compression tends to correlate with the eventual clinical outcome, whereas the postoperative dimension of the cord strongly correlates with clinical recovery.166
Electrodiagnostic Evaluation
The utility of somatosensory evoked potentials to evaluate cervical myelopathy has been investigated.40,258 Somatosensory evoked potentials with the median, ulnar, and posterior tibial nerve stimulation are more sensitive in detecting posterior column dysfunction than is clinical testing. Somatosensory evoked potentials can be used to detect subclinical cord involvement, when the chief complaint is cervical axial or radicular pain. Cervical myelopathy can be distinguished from multiple sclerosis by the pattern of abnormalities obtained by somatosensory evoked potentials. Changes in peripheral nerve conduction studies, when combined with central slowing, are indicative of myeloradiculopathy.40,258
Treatment
Nonoperative Care
Conservative care can include physical therapy and cervical orthoses in patients with mild or static symptoms without hard evidence of gait disturbances or pathologic reflexes.66,181 Improvement of sensory and motor deficits occurs in 33% to 50% of patients.37,44,181
Surgery
Surgery is indicated for patients with severe or progressive symptoms, or those for whom conservative measures have been unsuccessful.107 If a CIDH alone is causing cord compression, then anterior cervical diskectomy and fusion at the appropriate level(s) is indicated.91 In the case of impingement from degenerative spondylosis or ossification of the posterior longitudinal ligament, surgical intervention aims to remove either the offending anterior structures (such as osteophytes) or the calcified posterior longitudinal ligament (to decompress posteriorly). Both these interventions allow more space for the cord. Anterior decompression is frequently accomplished by corpectomy, in which a vertebral body is removed in addition to the adjacent intervertebral disks, and the segment is then fused with a fibula autograft or allograft of a bone cage.18 Posterior decompression involves laminectomy with or without fusion,79 or laminoplasty (Figure 37-24).92 Extensive laminectomy without fusion can lead to postoperative deformity and kyphosis.162 This has led to the frequent use of the technique of laminoplasty, in which deformity and kyphosis are much less common, and motion is preserved because of an absence of a fusion.162
The decision to decompress anteriorly or posteriorly is predicated on the number of stenotic levels and the contour of the cervical spine. If three or fewer levels are stenotic, anterior corpectomy and fusion is preferred.91 In cases of three or more stenotic levels with lordosis preserved, laminoplasty is preferred.5,91 If, however, three or more levels are involved, with loss of normal cervical lordosis, laminectomy and posterior fusion is performed to maintain cervical spinal stability.234
Effective treatment is afforded by anterior decompression, with symptomatic improvement in 85% to 99% of patients.143,153 Performing corpectomy at more than two levels can result in a less stable construct, and is generally avoided because of complications arising from dislodgment of the graft91 or nonunion.88 Laminoplasty has been found to be more effective than laminectomy, with fewer complications in treating cervical myelopathy.88
Cervicogenic Headaches
Cervicogenic headaches are a constellation of symptoms that represent the common referral patterns of cervical spinal structures. The term cervicogenic headache was first coined in 1983.215 Its definition has been augmented or adjusted several times since.86,158,160,214 This underscores the myriad of pain generators and manifestations that comprise cervicogenic headaches, and also points out the lack of consensus regarding the definition.
Epidemiology and Pathophysiology
The prevalence of cervicogenic headache has been reported to range from 0.4% to 2.5% in the general population,213 to as high as 36.2% in patients with a complaint of headaches.8,169 Women are more commonly affected (79.1%) than men (20.9%),247 with a mean age of 42.9 years and an average symptom duration of 6.8 years.82
Various spinal structures have been implicated in cervicogenic headache, including nerve roots and spinal nerves, dorsal root ganglia, uncovertebral joints, intervertebral disks, facet joints, ligaments, and muscles.215 Circumstantial evidence supports the convergence theory to explain why cranial symptoms can occur as a result of cervical spine pain generators. Convergence of two separate primary afferents derived from two different body regions on the same second-order intraspinal neurons allows the nociceptive activity of one afferent nerve to be perceived as pain in the distribution of the other afferent nerve.24 The cervicogenic headache can be due to degenerative changes, a direct result of trauma, or occur without any underlying biomechanical insult to the various cervical spinal structures subserved by cervical afferent fibers. The C2–3 zygapophyseal joint140,141 and the C2–3, C3–4, C4–5, and C5–6 intervertebral disks224 have been primarily implicated as sources of cervicogenic headache.
Diagnosis
History and Physical Examination
Support for a structural source within the cervical spine as the etiology of the patient’s headaches is obtained by eliciting any history of prior head or neck trauma such as a whiplash event.214 Whiplash events such as motor vehicle collisions have been associated with injury to the cervical zygapophyseal joints, intervertebral disks, or nerve roots, either in isolation or in combination.15,141,239 Cervicogenic headaches have been conceptualized as being primarily unilateral and stemming from the posterior occipital region. The referral of pain is toward the vertex of the scalp, ipsilateral anterolateral temple, forehead, midface, or ipsilateral shoulder girdle.214,218,219,224 Symptoms can spread to involve the contralateral side,218 but typically the side of the initial referral source remains most intense.214 The character of the pain can vary from a deep ache to sharp and stabbing. The duration of the painful symptoms fluctuates from initial episodic bouts of pain, progressing to more chronic and constant pain. Patients often describe the pain as initiating in the cervical region and traveling to the head and the neck as the pain becomes severe. The cervicogenic headache can then become the primary complaint, overshadowing the original cervical axial pain.
The duration of the symptoms ranges from a few hours to a few weeks, but characteristically lasts longer than that associated with migraine headaches. The pain intensity in cervicogenic headache is less excruciating than for cluster headaches, and is usually nonthrobbing in character.214 Autonomic complaints such as photophobia, phonophobia, and nausea are less apparent than in a migraine attack but can still occur.71,214 Accompanying complaints of dizziness or vertigo sometimes associated with near-syncopal episodes have also been described but are not common.71
Physical examination of the patient with complaints of cervicogenic headache typically reveals a reduced active range of motion because of muscle guarding, arthritic changes, or soft tissue inflexibility. If the cervicogenic headache is being produced by a cervical zygapophyseal joint, the patient can usually pinpoint with one finger or with the palm of the hand a unilateral area of maximal pain. Cervical intervertebral disk–induced cervicogenic headache typically begins as midline pain that spreads across the spine and into the head or face. However, unilateral occipital headache symptoms that are greater than the neck pain after a traumatic event are more suggestive of zygapophyseal joint pain than diskogenic symptoms.15,141 Certain head and neck movements can precipitate painful symptoms, such as axial rotation or cervical extension. We have commonly seen patients report an onset after sleeping in an awkward position. Spurling’s maneuver does not reproduce upper limb radicular symptoms but usually aggravates the axial pain, and patients report reproduction of their paramidline zygapophyseal joint-mediated pain. This pain can also often be reproduced with deep palpation over the involved joint.
Imaging Studies
A history of trauma requires cervical flexion and extension lateral radiographs to detect abnormal segmental motion. It also requires anteroposterior views, including an open mouth view of the odontoid process, to rule out fractures (Figure 37-25).163 Any suspicion for fracture mandates a subsequent cervical CT scan performed with multiplanar reformatted images to better delineate the osseous injury.163 MRI is better than CT at evaluating the intervertebral disks for desiccation, decreased disk height, and frank herniation. However, MRI has a false-positive rate of 51% and a false-negative rate of 27% in detecting painful disks identified by discography.260 Abnormalities seen on imaging studies should be clinically correlated, because such findings can occur in lifelong asymptomatic individuals.20,152,240
Functional Diagnostic Tests and Treatment
Once the etiology of cervicogenic headache has been identified, the offending structure is treated in a similar fashion as that outlined above. Cervicogenic headaches caused by upper cervical zygapophyseal joint pain can be studied with confirmatory diagnostic blockade. The traditional algorithm includes intraarticular diagnostic injections performed sequentially in the C2–3, C3–4, and C1–2 joints.15 After a zygapophyseal joint mapping study, we now also incorporate blockade of C4–5 zygapophyseal joints if the headaches include anterior head or facial symptoms. Once the painful joint is identified, therapeutic injections are performed as outlined in the section on treatment of cervical zygapophyseal joint pain. If patients experience short-term relief from a minimum of two and a maximum of four therapeutic intraarticular injections, a double-block paradigm is exercised to minimize the false-positive rate of single diagnostic injections.13 Radiofrequency ablation of the medial branches to the painful joint can be successful in alleviating cervicogenic headaches caused by cervical zygapophyseal joint injury that does not respond to other measures.138 Radiofrequency ablation of the third occipital nerve to treat C2–3 zygapophyseal joint–mediated headaches has been successfully performed at the Penn Spine Center, but this has not been studied in a large cohort.138
Whiplash Syndrome
Whiplash (hyperflexion-hyperextension) should be conceptualized as having three components. The whiplash event is the biomechanical effect incurred by the occupants of one vehicle when struck by another vehicle. The whiplash injury is the impairment, or injured structure, resulting from the whiplash event. The whiplash syndrome is the set of symptoms arising from the whiplash injury.12 During a whiplash event, the head and neck do not sustain a direct blow, but each undergoes an excursion because of the inertial response of the body to forces imparted on it.12 Rear-end collisions represent the most common pattern of whiplash-related injury, but injury caused by head-on and side collisions can also occur.12
Regardless of the direction of impact, whiplash is defined by the passive movement of the neck. Muscular control to stabilize the cervical spine does not react quickly enough to prevent injurious forces from occurring across the cervical functional spinal units. Both the lack of muscular pillaring and the generation of abnormal forces resulting in movements around abnormal axes of rotation subject the passive restraining structures to abnormal strain.12,113 For example, at 110 ms after rear-end impact, the C5–6 disk space widens anteriorly, causing abutment of the articular processes of the C5–6 zygapophyseal joint113 and posterior shear within the disk.174 The anterior disk, anterior longitudinal ligament, posterior disk or annulus, and cervical zygapophyseal joints are all at risk for injury during a whiplash event.12,113,174 Injury also occurs to the cervical soft tissues, resulting in strain and sprain injuries.168 These injuries typically heal over a relatively short period, as would be expected for soft tissue injury. The most commonly reported symptoms of whiplash injury include neck pain and headaches, followed by shoulder girdle pain, upper limb paresthesias, and weakness. Less common symptoms include dizziness, visual disturbances, and tinnitus.12
Most patients with whiplash syndrome are destined to recover within the first 2 to 3 months after the injury, and after 2 years 82% are symptom free.186 Severely affected patients account for only 6% of all whiplash patients at 3 months, and this percentage decreases minimally to 4% at 2 years.186 Studies have demonstrated that chronicity of whiplash symptoms develops independent of litigation,170 and litigation does not alter the response to treatment.201 The clinician should pursue evaluation and treatment of whiplash-related symptoms, rather than just assume that the pain is a result of mitigating circumstances such as secondary gain considerations.
1. Abel M.S. Occult traumatic lesions of the cervical vertebrae. CRC Crit Rev Clin Radiol Nucl Med. 1975;6:469-553.
2. Abel M.S. The radiology of chronic neck pain: sequelae of occult traumatic lesions. Crit Rev Diagn Imaging. 1982;20:27-78.
3. Adams C. Cervical spondylitic radiculopathy and myelopathy. In: Vinken P.J., Bruyn G.W., editors. Handbook of Clinical Neurology, 26. Amsterdam: North-Holland Publishing; 1977:97-112.
4. Aldrich F. Posterolateral microdiscectomy for cervical monoradiculopathy caused by posterolateral soft cervical disc sequestration. J Neurosurg. 1990;72:370-377.
5. An H.S., Simpson J.M. Surgery of the Cervical Spine. London: Martin-Dunitz; 1994.
6. Anderberg L., Annertz M., Brandt L., et al. Selective diagnostic cervical nerve root block: correlation with clinical symptoms and MRI-pathology. Acta Neurochir. 2004;146(6):559-565.
7. Anderberg L., Annertz M., Persson L., et al. Transforaminal steroids injections for treatment of cervical radiculopathy: a prospective and randomised study. Eur Spine J. 2007;16(3):321-328.
8. Anthony M. Cervicogenic headache: prevalence and response to local steroid therapy. Clin Exp Rheumatol. 2000;18(2 suppl 19):S59-S64.
9. Aprill C., Bogduk N. The prevalence of cervical zygapophyseal joint pain: a first approximation. Spine. 1992;17:744-747.
10. Aprill C., Dwyer A., Bogduk N. Cervical zygapophyseal joint pain patterns. II: A clinical evaluation. Spine. 1990;15:458-461.
11. Atkinson R., Ghelman B., Tsairis P., et al. Sarcoidosis presenting as cervical radiculopathy: a case report and literature review. Spine. 1987;7:412-416.
12. Barnsely L., Lord S., Bogduk N. The pathophysiology of whiplash. Spine State Art Rev. 1998;12(2):209-242.
13. Barnsley L., Lord S.M., Wallis B.J., et al. False-positive rates of cervical zygapophysial joint blocks. Clin J Pain. 1993;9:124-130.
14. Barnsley L., Lord S.M., Wallis B.J., et al. Lack of effect of intraarticular corticosteroids for chronic pain in the cervical zygapophyseal joints. N Engl J Med. 1994;330(15):1047-1050.
15. Barnsley L., Lord S.M., Wallis B.J., et al. The prevalence of chronic cervical zygapophyseal joint pain after whiplash. Spine. 1995;20:20-26.
16. Benyamin R., Singh V., Parr A.T., et al. Systematic review of the effectiveness of cervical epidurals in the management of chronic neck pain. Pain Physician. 2009;12:137-157.
17. Berger O., Dousset V., Delmer O., et al. Evaluation of CT-guided periganglionic foraminal steroid injections for treatment of radicular pain in patients with foraminal stenosis. J Radiol. 1999;80:917-925.
18. Bernard T.N.Jr., Whitecloud T.S.III. Cervical spondylotic myelopathy and myeloradiculopathy: anterior decompression and stabilization with autogenous fibula strut graft. Clin Orthop. 1987;221:149-160.
19. Binet E.F., Moro J.J., Marangola J.P., et al. Cervical spine tomography in trauma. Spine. 1977;2:163-172.
20. Boden S.D., McCowin P.R., Davis D.O., et al. Abnormal magnetic resonance scans of the cervical spine in asymptomatic subjects: a prospective investigation. J Bone Joint Surg. 1990;72(8):1178-1184.
21. Bogduk N. The clinical anatomy of the cervical dorsal rami. Spine. 1982;7:319-330.
22. Bogduk N. Back pain: zygapophyseal joint blocks and epidural steroids. In Cousins M.J., Bridenbaugh P.O., editors: Neural Blockade in Clinical Anesthesia and Pain Management, ed 2, Philadelphia: JB Lippincott, 1988.
23. Bogduk N. Low back pain. In Bogduk N., editor: Clinical anatomy of the lumbar spine and sacrum, ed 2, New York: Churchill Livingstone, 1997.
24. Bogduk N. Cervicogenic headache: anatomic basis and pathophysiologic mechanisms. Curr Pain Headache Rep. 2001;5(4):382-386.
25. Bogduk N. The anatomy and pathophysiology of neck pain. Phys Med Rehabil Clin N Am. 2003;14:455-472.
26. Bogduk N., Aprill C. On the nature of neck pain, discography and cervical zygapophysial joint blocks. Pain. 1993;54:213-217.
27. Bogduk N., Dreyfuss P., Baker R., et al. Complications of spinal diagnostic and treatment procedures. Pain Med. 2008;9(suppl 1):S11-S34.
28. Bogduk N., Marsland A. The cervical zygapophysial joints as a source of neck pain. Spine. 1988;13:610-617.
29. Bogduk N., Twomey L.T. Clinical Anatomy of the Lumbar Spine. New York: Churchill Livingstone; 1991.
30. Bogduk N., Windsor M., Inglis A. The innervation of the cervical intervertebral discs. Spine. 1988;13:2-8.
31. Bonaldi G., Baruzzi F., Facchinetti A., et al. Plasma radio-frequency-based diskectomy for treatment of cervical herniated nucleus pulposus: feasibility, safety, and preliminary clinical results. AJNR Am J Neuroradiol. 2006;27(10):2104-2111.
32. Borchgrevink G.E., Smevik O., Nordby A., et al. MR imaging and radiography of patients with cervical hyperextension-flexion injuries after car accidents. Acta Radiol. 1995;36:425-428.
33. Bucholz R.W., Burkhead W.Z., Graham W., et al. Occult cervical spine injuries in fatal traffic accidents. J Trauma. 1979;119:768-771.
34. Buonocore E., Hartman J.T., Nelson C.L. Cineradiograms of cervical spine in diagnosis of soft tissue injuries. JAMA. 1966;198:143-147.
35. Bush K., Hillier S. Outcome of cervical radiculopathy treated with periradicular/epidural corticosteroid injections: a prospective study with independent clinical review. Eur Spine J. 1996;5:319-325.
36. Cailliet R. Neck and Arm Pain, ed 3. Philadelphia: FA Davis; 1991.
37. Campbell A.M.G., Phillips D.G. Cervical disk lesions with neurologic disorder. Differential diagnosis, treatment and prognosis. Br Med J. 1960;2:481-485.
38. Campbell D.G., Parsons C.M. Referred head pain and its concomitants. J Nerv Ment Dis. 1944;99:544-551.
39. Cesaroni A., Nardi P.V., Casilino P., et al. Plasma disc decompression compared to conservative care for treating symptomatic contained cervical disc protrusion: one year results from a prospective randomized controlled study. Spine J. 2008;8(suppl):78S-79S.
40. Chan K.M., Nasathurai S., Chavin J.M., et al. The usefulness of central motor conduction studies in the localization of cord involvement in cervical spondylitic myelopathy. Muscle Nerve. 1998;21:1220-1223.
41. Chiu J.C., Clifford T.J., Greenspan M., et al. Percutaneous microdecompressive endoscopic cervical discectomy with laser thermodiskoplasty. Mt Sinai J Med. 2000;67(4):278-282.
42. Cicala R.S., Thoni K., Angel J.J. Long term results of cervical epidural steroid injections. Clin J Pain. 1989;5(2):143-145.
43. Clark C.R., Igram C.M., el Khoury G.Y., et al. Radiographic evaluation of cervical spine injuries. Spine. 1988;13:742-747.
44. Clarke E., Robinson P.K. Cervical myelopathy: a complication of cervical spondylosis. Brain. 1956;75:187-225.
45. Cloward R.B. Cervical diskography. A contribution to the aetiology and mechanism of neck, shoulder and arm pain. Ann Surg. 1959;130:1052-1064.
46. Colachis S., Strohm B. A study of tractive forces angle of pull on vertebral interspaces in the cervical spine. Arch Phys Med Rehabil. 1965;46:820.
47. Colachis S.C.Jr., Strohm B.R. Effect of duration of intermittent cervical traction on vertebral separation. Arch Phys Med Rehabil. 1966;47:353-359.
48. Cole A.J., Farrell J.P., Stratton S.A., et al. Functional rehabilitation of cervical spine athletic injuries. In: Kibler W.B., Herring S.A., Press J.M., editors. Functional Rehabilitation of Sports and Musculoskeletal Injuries. New York: Aspen, 1998.
49. Cooper G., Bailey B., Bogduk N. Cervical zygapophysial joint pain maps. Pain Med. 2007;8(4):344-353.
50. Crafts R.C. Textbook of Human Anatomy, ed 2. New York: John Wiley; 1979.
51. Crock H.V. A reappraisal of intervertebral disc lesions. Med J Aust. 1970;1:983-989.
52. Cyriax J. Rheumatic headache. Br Med J. 1938;2:1367-1368.
53. Cyteval C., Thomas E., Decoux E., et al. Cervical radiculopathy: open study on percutaneous periradicular foraminal steroid infiltration performed under CT control in 30 patients. Am J Neuroradiol. 2004;25:441-445.
54. Davidson R.I., Dunn E.J., Metzmaker J.N. Shoulder abduction test in diagnosis of radicular pain in cervical extradural compressive monoradiculopathies. Spine. 1981;6:441-446.
55. Deans G., Magalliard J., Kerr M., et al. Neck pain: a major cause of disability following car accidents. Injury. 1987;18:10-21.
56. DePalma A.F., Rothman R.H., Levitt R.L., et al. The natural history of severe cervical disc degeneration. Acta Orthop Scand. 1972;43:392-396.
57. Dillingham T.R., Lauder T.D., Andary M., et al. Identification of cervical radiculopathies. Optimizing the electromyographic screen. Am J Phys Med Rehabil. 2001;80(2):84-91.
58. Dooley J.F., McBroom R.J., Taguchi T., et al. Nerve root infiltration in the diagnosis of radicular pain. Spine. 1988;13(1):79-83.
59. Dreyfuss P., Michaelsen M., Fletcher D. Atlanto-occipital and lateral atlanto-axial joint pain patterns. Spine. 1994;19:1125-1131.
60. Dusunceli Y., Ozturk C., Atamaz F., et al. Efficacy of neck stabilization exercises for neck pain: a randomized controlled study. J Rehabil Med. 2009;41(8):626-631.
61. Dvorak J., Panjabi M.M., Grob D., et al. Validation of flexion extension radiographs of cervical spine. Spine. 1987;18:120-127.
62. Dvorak J., Panjabi M.M., Novotny J., et al. In vivo flexion/extension of the normal cervical spine. J Orthop Res. 1991;9:828-834.
63. Dwyer A., Aprill C., Bogduk N. Cervical zygapophyseal joint pain patterns. I. A study in normal volunteers. Spine. 1990;15:453-457.
64. Ellenberg M.R., Honet J.C., Treanor W.J. Cervical radiculopathy. Arch Phys Med Rehabil. 1994;75:342-352.
65. Ellis H. Clinical Anatomy: A Revision and Applied Anatomy for Clinical Students, ed 6. London: Blackwell; 1976.
66. Emery S.E. Cervical spondylitic myelopathy: diagnosis and treatment. J Am Acad Orthop Surg. 2001;9(6):376-388.
67. Falconer J., Hayes K.W., Chang R.W. Therapeutic ultrasound in the treatment of musculoskeletal conditions. Arthritis Care Res. 1990;3:85-91.
68. Fast A., Parikh S., Marin E. The shoulder abduction relief sign in cervical radiculopathy. Arch Phys Med Rehabil. 1989;70(5):402-403.
69. Feinstein B., Langton J.B.K., Jameson R.M., et al. Experiments on referred pain from deep somatic tissues. J Bone Joint Surg Am. 1954;36:981-997.
70. Fielding J. Cineroentgenography of the normal cervical spine. J Bone Joint Surg (Am). 1957;39:1280-1284.
71. Fredriksen T., Sjaastad O. Cervicogenic headache (CEH): notes on some burning issues. Funct Neurol. 2000;15(4):199-203.
72. Fuji T., Yonenobu K., Fujiwara K., et al. Cervical radiculopathy or myelopathy secondary to athetoid cerebral palsy. J Bone Joint Surg Am. 1987;69:815-821.
73. Fukui S., Ohseto K., Shiotani M., et al. Referred pain distribution of the cervical zygapophyseal joints and cervical dorsal rami. Pain. 1996;68:79-83.
74. Furusawa N., Baba H., Miyoshi N., et al. Herniation of cervical intervertebral disk: immunohistochemical examination and measurement of nitric oxide production. Spine. 2001;26:1110-1116.
75. Garvey T.A., Transfeldt E., Malcolm J.R., et al. Outcome of anterior cervical discectomy and fusion as perceived by patients treated for dominant axial-mechanical cervical spine pain. Spine. 2002;27(17):1887-1894.
76. Gore D.R., Sepic S.B. Anterior cervical fusion for degenerated or protruded discs: a review of one hundred forty-six patients. Spine. 1984;9:667-671.
77. Gore D.R., Sepic S.B., Gardner G.M., et al. Neck pain: a long term follow up of 205 patients. Spine. 1987;12:1-5.
78. Grubb S.A., Kelly C.K. Cervical discography: clinical implications from 12 years of experience. Spine. 2000;25:1382-1389.
79. Guigui P., Benoist M., Deburge A. Spinal deformity and instability after multilevel cervical laminectomy for spondylotic myelopathy. Spine. 1998;15:440-447.
80. Hadley L.A. Anatomicroradiographic studies of the spine: changes responsible for certain painful back conditions. N Y State J Med. 1939;xxxix:969-974.
81. Hakkinen A., Kautiainen H., Hanonen P., et al. Strength training and stretching versus stretching only in the treatment of patients with chronic neck pain: a randomized one-year follow-up study. Clin Rehabil. 2008;22(7):592-600.
82. Haldeman S., Dagenais D. Cervicogenic headaches: a critical review. Spine J. 2002;2(2):162.
83. Harada J., Dohi M., Fukuda K., et al. CT-guided percutaneous laser disk decompression (PLDD) for cervical disk hernia. Radiat Med. 2001;19(5):263-266.
84. Haueisen D.C., Smith B.S., Myers S.R., et al. The diagnostic accuracy of spinal nerve injections studies: their role in the evaluation of recurrent sciatica. Clin Orthop Relat Res. 1985;198:179-183.
85. Hawkey D.J. Cox-2 inhibitors. Lancet. 1999;353:307-314.
86. Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias, and facial pain. Cephalalgia. 1988;8(suppl 7):1-96.
87. Heckmann J.C., Lang C.J., Zobelein I., et al. Herniated cervical intervertebral discs with radiculopathy: an outcome study of conservatively or surgically treated patients. J Spinal Disord. 1999;12:396-401.
88. Heller J.G., Edwards C.C.II, Murakami H., et al. Laminoplasty versus laminectomy and fusion for multilevel cervical myelopathy: an independent matched cohort analysis. Spine. 2001;26(12):1330-1336.
89. Hellinger J., Linke R., Heller H. A biophysical explanation for Nd:YAG percutaneous laser disc decompression success. J Clin Laser Med Surg. 2001;19(5):235-238.
90. Herkowitz H., Kurz L.T., Overhohlt D.P. Surgical management of cervical soft disc herniation: a comparison between the anterior and posterior approach. Spine. 1990;15:1026-1030.
91. Herkowitz H.N. The surgical management of cervical spondylitic radiculopathy and myelopathy. Clin Orthop. 1989;239:94-108.
92. Hirabayashi K., Watanabe K., Wakano K., et al. Expansive open-door laminoplasty for cervical spine stenotic myelopathy. Spine. 1983;8:693-699.
93. Hitselberger W.E., Witten R.M. Abnormal myelograms in asymptomatic patients. J Neurosurg. 1968;28:204-206.
94. Holt E.P. Fallacy of cervical discography: report of 50 cases in normal subjects. JAMA. 1964;188:799-801.
95. Hoogland T., Scheckenbach C. Low-dose chemonucleolysis combined with percutaneous nucleotomy in herniated cervical disks. J Spinal Disord. 1995;8(3):228-232.
96. Howe J.F., Loeser J.D., Calvin W.H. Mechanosensitivity of dorsal root ganglia and chronically injured axons: a physiological basis for the radicular pain of nerve root compression. Pain. 1977;3:25-41.
97. Hoyland J.A., Freemont A.J., Jayson M.I. Intervertebral foramen venous obstruction: a cause of periradicular fibrosis? Spine. 1989;14(6):558-568.
98. Hult L. Cervical, dorsal, and lumbar spinal syndromes. Acta Orthop Scand Suppl. 1954;17:39-102.
99. Hult L. The Munkfors investigation. Acta Orthop Scand Suppl. 1954;17:1-38.
100. Hult L. Frequency of symptoms for different age groups and professions. In: Hirsch C., Zotterman Y., editors. Cervical Pain. New York: Pergamon Press, 1971.
101. Hunt W.E. Cervical spondylosis: natural history and rare indications for surgical decompression. Clin Neurosurg. 1980;27:466-480.
102. Hunt W.E., Miller C.A. Management of cervical radiculopathy. Clin Neurosurg. 1986;33(29):485-502.
103. Husted D.S., Orton D., Schofferman J., et al. Effectiveness of repeated radiofrequency neurotomy for cervical facet joint pain. J Spinal Disord Tech. 2008;21(6):406-408.
104. Huston C.W., Slipman C.W., Garvin C. Complications and side effects of cervical and lumbosacral nerve root injections. Arch Phys Med Rehabil. 2005;86(2):277-283.
105. Jackson R. Cervical trauma: not just another pain in the neck. Geriatrics. 1982;37:123.
106. Jeffreys E. Disorders of the Cervical Spine, ed 2. Oxford: Butterworth-Heinemann; 1993.
107. Jeffreys R.V. The surgical treatment of cervical myelopathy due to spondylosis and disc degeneration. J Neurol Neurosurg Psychiatry. 1986;49:353-361.
108. Johnson E.W., Melvin J.L. Value of electromyography in lumbar radiculopathy. Arch Phys Med Rehabil. 1971;52:239-243.
109. Johnson R.M., Hart D.L., Simmons E.F., et al. Cervical orthoses: a study comparing their effectiveness in restricting cervical motion in normal subjects. J Bone Joint Surg Am. 1977;59:332.
110. Jonsson H.Jr., Brin G., Rauschning W., et al. Hidden cervical spine injuries in traffic accident victims with skull fractures. J Spinal Disord. 1991;4:251-263.
111. Jull G., Bogduk N.K., Marsland A. The accuracy of manual diagnosis for cervical zygapophysial joint pain syndromes. Med J Aust. 1988;148:233-236.
112. Kaiser J.A., Holland B.A. Imaging of the cervical spine. Spine. 1998;23:2701-2712.
113. Kaneoka K., Ono L., Inami S., et al. Abnormal segmental motion of the cervical spine during whiplash loading. J Jpn Orthop Assoc. 1997;71:S1680.
114. Kang J.D., Georgescu H.I., Larkin L., et al. Herniated cervical intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and PGE2. Spine. 1995;20:2373-2378.
115. Kang J.D., Stefanovic-Racic M., McIntyre L., et al. Toward a biochemical understanding of human intervertebral disc degeneration and herniation: contributions of nitric oxide, interleukins, prostaglandins, and matrix metalloproteinases. Spine. 1997;22:1065-1073.
116. Keegan J.J., Garrett G.D. The segmental distribution of the cutaneous nerves in the limbs of man. Anat Rec. 1948;102:409-437.
117. Kellegren J.H. Observations on referred pain arising from muscle. Clin Sci. 1938;3:175-190.
118. Kellegren J.H. On the distribution of pain arising from deep somatic structures with charts of segmental pain. Clin Sci. 1939;3:35-46.
119. Kikuchi S., Macnab I., Moreau P. Localisation of the level of symptomatic cervical disc degeneration. J Bone Joint Surg Br. 1981;63(2):272-277.
120. Kimmel D.L. Innervation of the spinal dura mater of the posterior cranial fossa. Neurology. 1960;10:800-809.
121. Kirkaldy-Willis W.H. The pathology and pathogenesis of low back pain. In: Kirkaldy-Willis W.H., editor. Managing Low Back Pain. New York: Churchill Livingstone, 1988.
122. Klein B.R., Vaccaro A.R., Albert T.J. Health outcome assessment before and after anterior cervical discectomy and fusion for radiculopathy: a prospective analysis. Spine. 2000;25:801-803.
123. Knight M.T.N., Goswami A., Patko J.T. Cervical percutaneous laser disc decompression: preliminary results of an ongoing prospective outcome study. J Clin Laser Med Surg. 2001;19(1):3-8.
124. Knott M., Voss D. Proprioceptive Neuromuscular Facilitation: Patterns and Techniques. New York: McGraw-Hill; 1956.
125. Kolstad F., Leivseth G., Nygaard O.P. Transforaminal steroid injections in the treatment of cervical radiculopathy: a prospective outcome study. Acta Neurochir (Wien). 2005;147:1065-1070.
126. Kraemer J., Ludwig J., Bickert U., et al. Lumbar epidural perineural injection: a new technique. Eur Spine J. 1997;6:357-361.
127. Krempen J.F., Smith B.S. Nerve root injection: a method for evaluating the etiology of sciatica. J Bone Joint Surg AM. 1974;56:1435-1444.
128. Kuslich S.D., Ahern J.W., Garner M.N.D. An in Vivo, Prospective Analysis of Tissue Sensitivity of Lumbar Spinal Tissues. New York: Presented at the 12th Annual Meeting of the North American Spine Society; 1997.
129. LaRocca H. Acceleration injuries of the neck. Clin Neurosurg. 1978;25:209-217.
130. Lawrence J.S. Disc degeneration. Its frequency and relationship to symptoms. Ann Rheum Dis. 1969;28:121.
131. Lazorthes G., Gaubert J. L’innervation des articulations interapophysaire vertebrales. C R Assoc Anat. 1956;43:488-494.
132. Lehmann J.D., Brunner G.D., Stow R.W. Pain threshold measurements after therapeutic application of ultrasound, microwaves and infrared. Arch Phys Med Rehabil. 1958;39:560-565.
133. Lehman J.F. Therapeutic Heat and Cold, ed 4. Baltimore: Williams & Wilkins; 1990.
134. Lehmann J.F., DeLateur B.J. Therapeutic heat. In Lehmann J.F., editor: Therapeutic Heat and Cold, ed 3, Baltimore: William & Wilkins, 1982.
135. L’Hermitte J. [Etude de la commation de la moele]. Rev Neurol. 1932;1:210-239.
136. Li J., Yan D.L., Zhang Z.H. Percutaneous cervical nucleoplasty in the treatment of cervical disc herniation. Eur Spine J. 2008;17:1664-1669.
137. Liversedge L.A., Hutchinson E.C., Lyons J.B. Cervical spondylosis simulating motor neuron disease. Lancet. 1953;2:652-659.
138. Lord S.M., Barnsley L., Bogduk N. Percutaneous radiofrequency neurotomy in the treatment of cervical zygapophysial joint pain: a caution. Neurosurgery. 1995;36:732-739.
139. Lord S.M., Barnsley L., Bogduk N. The utility of comparative local anaesthetic blocks versus placebo-controlled blocks for the diagnosis of cervical zygapophysial joint pain. Clin J Pain. 1995;11:208-213.
140. Lord S.M., Barnsley L., Wallis B.J., et al. Third occipital nerve headache: a prevalence study. J Neurol Neurosurg Psychiatry. 1994;57:1187-1190.
141. Lord S.M., Barnsley L., Wallis B.J., et al. Chronic cervical zygapophyseal joint pain after whiplash. A placebo-controlled prevalence study. Spine. 1996;21:1737-1745.
142. Lunardi P., Acqui M., Ricci G., et al. Cervical synovial cysts: case report and review of the literature. Eur Spine J. 1999;8:232-237.
143. Macdonald R.L., Fehlings M.G., Tator C.H., et al. Multilevel anterior cervical corpectomy and fibular allograft fusion for cervical myelopathy. J Neurosurg. 1997;26:990-997.
144. Macnab I., McCulloch J. Neck Ache and Shoulder Pain. Baltimore: Williams & Wilkins; 1994.
145. Makela M., Heliovara M., Sievers D., et al. Prevalence, determinants, and consequences of chronic neck pain in Finland. Am J Epidemiol. 1991;134:1356-1367.
146. Malacca R., Jeans M.E., Stratford J.G., et al. Ice massage and transcutaneous electrical stimulation: comparison of treatment for low back pain. Pain. 1980;9:209-217.
147. Malanga G. The diagnosis and treatment of cervical radiculopathy. Med Sci Sports Exerc. 1997;29(suppl):S236-S245.
148. Manchikanti L., Dunbar E.E., Wargo B.W., et al. Systematic review of cervical discography as a diagnostic test for chronic spinal pain. Pain Physician. 2009;12:305-321.
149. Manchikanti L., Singh V., Rivera J., et al. Prevalence of cervical facet joint pain in chronic neck pain. Pain Physician. 2002;5:243-249.
150. Manelfe C. Imaging of the spine and spinal cord. Radiology. 1991;3:5-15.
151. Martino F., Ettore G.C., Cafaro E., et al. [L’ecographia musculo-tendinea nei traumi distorvi acuti del collo]. Radiol Med (Torino). 1992;83:211-215.
152. Matsumoto M., Fujimura Y., Suzuki N., et al. MRI of cervical intervertebral discs in asymptomatic subjects. J Bone Joint Surg Br. 1998;80:19-24.
153. Mayr M.T., Subach B.R., Comey C.H. Cervical spinal stenosis: outcome after anterior corpectomy, allograft reconstruction, and instrumentation. J Neurosurg. 2002;96(1 suppl):10-16.
154. McConnell W.E., Howard R.P., Guzman H.M., et al. Analysis of human test subject kinematic responses to low velocity rear end impacts. Proceedings of the 37th STAPP Car Crash Conference. Warrendale: Society for Automotive Engineers; 1993. paper 930889, 1993
155. McDonald G.J., Lord S.M., Bogduk N. Long-term follow up of patients treated with cervical radiofrequency neurotomy for chronic neck pain. Neurosurgery. 1999;45:61-67.
156. McKinney L.A. Early mobilization of acute sprain of the neck. Br Med J. 1989;299:1006-1008.
157. McRae D.L. Asymptomatic intervertebral disc protrusions. Acta Radiol. 1956;46:9-27.
158. Meloche J., Bergeron Y., Bellavance A., et al. Painful intervertebral dysfunction: Robert Maigne’s original contribution to headache of cervical origin. The Quebec Headache Study Group. Headache. 1993;33(6):328-334.
159. Melzack R., Wall P.D. Pain mechanisms: a new theory. Science. 1965;150:971-979.
160. Merskey H., Bogduk N. Classification of chronic pain: descriptions of chronic pain syndromes and definitions of pain terms. In: Merskey H., Bogduk N., editors. Cervicogenic Headache. Seattle: International Association for the Study of Pain, 1994.
161. Michlovitz S. Cryotherapy: the use of cold as a therapeutic agent. In Michlovitz S., editor: Thermal Agents in Rehabilitation, ed 2, Philadelphia: Davis, 1990.
162. Mikawa Y., Shikata J., Yamamuro T. Spinal deformity and instability after multilevel cervical laminectomy. Spine. 1987;12:6-11.
163. Mink J.H., Gordon R.E., Deutsch A.L. The cervical spine: radiologist’s perspective. Phys Med Rehabil Clin N Am. 2003;14:493-548.
164. Modic M., Masaryk T., Mulopulos G., et al. Cervical radiculopathy: prospective evaluation with surface coil MR imaging, CT with metrizamide, and metrizamide myelography. Radiology. 1986;161:753-759.
165. Moore K.L., Agur A.M. Essential Clinical Anatomy. Baltimore: Williams & Wilkins; 1995.
166. Morio Y., Teshima R., Nagashima H., et al. Correlation between operative outcomes of cervical compression myelopathy and MRI of the spinal cord. Spine. 2001;26:1238-1245.
167. Murphy R.W. Nerve roots and spinal nerves in degenerative disk disease. Clin Orthop Relat Res. 1977;129:46-60.
168. Newham D.J. The consequences of eccentric contractions and their relationship to delayed-onset muscle pain. Eur J Appl Physiol. 1988;57:353-359.
169. Nilsson N. The prevalence of cervicogenic headache in a random population sample of 20-59 year olds. Spine. 1995;20(17):1884-1888.
170. Norris H., Watt I. The prognosis of neck injuries resulting from rear-end vehicle collisions. J Bone Joint Surg Br. 1983;65:608-611.
171. Nurick S. The natural history and the results of surgical treatment of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95:101-108.
172. Olmarker K., Rydevik B., Nordborg C. Autologous nucleus pulposus induces neurophysiologic and histologic changes in porcine cauda equina nerve roots. Spine. 1993;18:1425-1432.
173. O’Sullivan P.B., Phyty G.D., Twomey L.T., et al. Evaluation of specific stabilizing exercise in the treatment of chronic low back pain with radiologic diagnosis of spondylosis or spondylolisthesis. Spine. 1997;22:2959-2967.
174. Panjabi M., Ito S., Pearson A., et al. Injury mechanisms of the cervical intervertebral disc during simulated whiplash. Spine. 2004;29(11):1217-1225.
175. Parke W.W. Applied anatomy of the spine. In Rothman R.H., Simeone F.A., editors: The Spine, ed 3, Philadelphia: Saunders, 1992.
176. Parminder S.P. Management of cervical pain. In: DeLisa J.A., editor. Rehabilitation Medicine: Principles and Practice. Philadelphia: Lippincott, 1988.
177. Pavlov H., Torg J.S. Roentgen examination of cervical spine injuries in the athlete. Clin Sports Med. 1987;6:761.
178. Penning L., Wilmink J.T., Van Woerden H.H., et al. CT myelographic findings in degenerative disorders of the cervical spine: clinical significance. AJR Am J Roentgenol. 1986;7:119-127.
179. Persson L.C., Moritz U., Brandt L. Cervical radiculopathy: pain, muscle weakness and sensory loss in patients with cervical radiculopathy treated with surgery, physiotherapy or cervical collar: a prospective, controlled study. Eur Spine J. 1997;6:256-266.
180. Pettersson K., Hildingsson C., Toolanen G., et al. MRI and neurology in acute whiplash trauma: no correlation in prospective examination of 39 cases. Acta Orthop Scand. 1994;65:525-528.
181. Phillips D.G. Surgical treatment of myelopathy with cervical spondylosis. J Neurol Neurosurg Psychiatry. 1973;36:879-884.
182. Poindexter D.P., Johnson E.W. Football shoulder and neck injury: a study of the ‘stinger’. Arch Phys Med Rehabil. 1984;65:601-602.
183. Press J.M., Herring S.A., Kibler W.B. Rehabilitation of musculoskeletal disorders. In: The Textbook of Military Medicine. Washington, DC: Borden Institute, Office of the Surgeon General; 1996.
184. Purkis I.E. Cervical epidural steroids. Pain Clinic. 1986;1:3-7.
185. Quinlan K.P., Annest J.L., Myers B., et al. Neck strains and sprains among motor vehicle occupants—United States. Accid Anal Prev. 2000;2004(36):21-27.
186. Radanov B.P., Sturzenegger M., Di Stefano G. Long-term outcome after whiplash injury: a 2-year follow-up considering features of injury mechanism and somatic, radiologic, and psychosocial findings. Medicine. 1995;74:281-297.
187. Radharkrishnan K., Litchy W.J., O’Fallon W.M., et al. Epidemiology of cervical radiculopathy: a population-based study from Rochester, Minn, 1976-1990. Brain. 1994;117(pt 2):325-335.
188. Ramanauskas W., Wilner H., Metes J. MR imaging of compressive myelomalacia. J Comput Assist Tomogr. 1989;13:399-404.
189. Rappaport Z.H., Dever M. Experimental pathophysiological correlates of clinical symptomatology in peripheral neuropathic pain syndrome. Stereotact Funct Neurosurg. 1990:54-55, 90-95.
190. Rauschning W., et al. Anatomy of the normal and traumatized spine. In: Sances A., Tomas D.L., Ewing C.L., editors. Mechanisms of Head and Spine Trauma. Deer Park: Aloray, 1986.
191. Redford J.B., Patel A. Orthotic devices in the management of spinal disorders. Spine State Art Rev. 1995;9:673-688.
192. Reitman C., Esses S. Modalities, manual therapy, and education: a review of conservative measures. Spine State Art Rev. 1995;9:661-672.
193. Riew D.K., Kim Y., Gilula L. Can cervical nerve root blocks prevent surgery for cervical radiculopathy? A prospective, randomized, controlled, double-blind study. Spine J. 2006;6(suppl):2S-3S.
194. Rowlingson J.C., Kirschenbaum L.P. Epidural analgesic techniques in the management of cervical pain. Anesth Analg. 1986;65:938-942.
195. Rubinstein S.M., Pool J.J., van Tulder M.W., et al. A systematic review of the diagnostic accuracy of provocative tests of the neck for diagnosing cervical radiculopathy. Eur Spine J. 2007;16(3):307-319.
196. Russell E.J. Computed tomography and myelography in the evaluation of cervical degenerative disease. Neuroimaging Clin N Am. 1995;5(3):329-348.
197. Rydevik B., Brown M., Lundborg G. Pathoanatomy and pathophysiology of nerve root compression. Spine. 1984;9:7-15.
198. Saal J. The role of inflammation in lumbar pain. Spine. 1995;20:1821-1827.
199. Saal J., Saal J., Yurth E. Nonoperative management of herniated cervical intervertebral disc with radiculopathy. Spine. 1996;21(16):1877-1883.
200. Sanchez M.C., Arenillas J.I.C., Gutierrez D.A., et al. Cervical radiculopathy: a rare symptom of giant cell arteritis. Arthritis Rheum. 1983;26:207-209.
201. Sapir D., Gorup J. Radiofrequency medial branch neurotomy in litigant and non litigant patients with cervical whiplash. Spine. 2001;26(12):E268-E273.
202. Sasso R., Heller J., Hacker R., et al. Artificial disc vs. fusion for the treatment of cervical radiculopathy: a prospective randomized study. Spine J. 2005;5:88S.
203. Schellhas K.P., Smith M.D., Gundry C.R., et al. Cervical discogenic pain: prospective correlation of magnetic resonance imaging and discography in asymptomatic subjects and pain sufferers. Spine. 1996;21(3):300-312.
204. Schofferman J., Garges K., Goldthwaite N., et al. Upper cervical anterior diskectomy and fusion improves discogenic cervical headaches. Spine. 2002;27(20):2240-2244.
205. Schutz H., Lougheed W.M., Wortzman G., et al. Intervertebral nerve-root in the investigation of chronic lumbar disc disease. Can J Surg. 1973;16:217-221.
206. Seibenrock K.A., Aebi M. Cervical discography in discogenic pain syndrome and its predictive value for cervical fusion. Arch Orthop Trauma Surg. 1994;113:199-203.
207. Selecki B.R. Whiplash. Aust Fam Phys. 1984;13:243-247.
208. Semmes R.E., Murphey M.D. The syndrome of unilateral rupture of the sixth cervical intervertebral disk with compression of the seventh cervical nerve root: a report of four cases with symptoms simulating coronary disease. JAMA. 1943;121:1209-1214.
209. Sharps L.S., Isaac Z. Percutaneous disc decompression using nucleoplasty. Pain Physician. 2002;5(2):121-126.
210. Shulman M. Treatment of neck pain with cervical epidural injection. Reg Anesth. 1986;11:92-94.
211. Siebert W. Percutaneous laser discectomy of cervical discs: preliminary clinical results. J Clin Laser Med Surg. 1995;13(3):205-207.
212. Simmons E.H., Segil C.M. An evaluation of discography in the localization of symptomatic levels in discogenic disease of the spine. Clin Orthop. 1975;108:57-69.
213. Sjaastad O., Fredriksen T. Cervicogenic headache: criteria, classification and epidemiology. Clin Exp Rheumatol. 2000;18(2 suppl 19):S3-S6.
214. Sjaastad O., Fredriksen T., Pfaffenrath V. Cervicogenic headache: diagnostic criteria. The Cervicogenic Headache International Study Group. Headache. 1998;38(6):442-445.
215. Sjaastad O., Saunte C., Hovdahl H., et al. ‘Cervicogenic’ headache: an hypothesis. Cephalalgia. 1983;3:249-256.
216. Slipman C.W., Chow D.W., Isaac Z., et al. An evidence-based algorithmic approach to cervical spinal disorders. Crit Rev Phys Rehabil Med. 2001;13(4):283-299.
217. Slipman CW, Freg ME, Bhargava A, et al: Outcomes and side effects following percutaneous cervical disk decompression using coblation technology: a pilot study, Spine J 4(5S):71S, 2004.
218. Slipman C.W., Isaac Z., Oleski C., et al. Does the cervical zygapophyseal joint refer symptoms to the neck bilaterally? preliminary data from 100 patients. Arch Phys Med Rehabil. 2002;83(11):1665.
219. Slipman C.W., Isaac Z., Thomas J., et al. Cervical zygapophyseal joint syndrome and referral to the head and face: preliminary data from 100 patients. Arch Phys Med Rehabil. 2002;83(11):1665.
220. Slipman C.W., Lipetz J.S., DePalma M.J., et al. Therapeutic selective nerve root block in the nonsurgical treatment of traumatically induced cervical spondylitic radicular pain. Am J Phys Med Rehabil. 2004;83(6):446-454.
221. Slipman C.W., Lipetz J.S., Jackson H.B., et al. Therapeutic selective nerve root block in the nonsurgical treatment of atraumatic cervical spondylitic radicular pain: a retrospective analysis with independent clinical review. Arch Phys Med Rehabil. 2000;81:741-746.
222. Slipman C.W., Lipetz J.S., Jackson H.B., et al. Outcomes of therapeutic selective nerve root blocks for whiplash induced cervical radicular pain. Pain Physician. 2001;4(2):167-174.
223. Slipman C.W., Lipetz J.S., Plastaras C.T., et al. Therapeutic zygapophyseal joint injections for headaches emanating from the C2–3 joint. Am J Phys Med Rehabil. 2001;80(3):182-188.
224. Slipman C.W., Plastaras C., Patel R., et al. Provocative cervical discography symptom mapping. Spine J. 2005;5(4):381-388.
225. Slipman C.W., Plastaras C.T., Palmitier R.S., et al. Symptom provocation of fluoroscopically guided cervical nerve root stimulation: are dynatomal maps identical to dermatomal maps? Spine. 1998;23:2235-2242.
226. Smith G.W., Nichols P.J. The technique of cervical discography. Radiology. 1957;68:718-720.
227. Sobel J.B., Sollenberger P., Robinson R., et al. Cervical nonorganic signs: a new clinical tool to assess abnormal illness behavior in neck pain patients: a pilot study. Arch Phys Med Rehabil. 2000;81:170-175.
228. Speldewinde G.C., Bashford G.M., Davidson I.R. Diagnostic cervical zygapophysial joint blocks for chronic cervical pain. Med J Aust. 2001;174:174-176.
229. Spitzer W., Leblanc F., Dupuis M., et al. Scientific approach to the assessment and management of activity-related spinal disorder: a monograph for clinicians. Report of the Quebec Task Force on Spinal Disorders. Spine. 1987;12(suppl):S1-S57.
230. Spitzer W.O., Skovron M.L., Salmi L.R., et al. Scientific monograph of the Quebec Task Force on whiplash-associated disorders: redefining ‘whiplash’ and its management. Spine. 1995;20(suppl 8):3S-73S.
231. Spurling R.G., Scoville W.B. Lateral rupture of the cervical intervertebral discs: a common cause of shoulder and arm pain. Surg Gynecol Obstet. 1944;78:350-358.
232. Stanley D., McLaren M.I., Euinton H.A., et al. A prospective study of nerve root infiltration in the diagnosis of sciatica: a comparison with radiculography, computed tomography, and operative findings. Spine. 1990;15(6):540-543.
233. Su H.C., Su R.K. Treatment of whiplash injuries with acupuncture. Clin J Pain. 1988;4:233.
234. Swanberg H. The Intervertebral Foramina in Man. Chicago: Scientific Publishing; 1995.
235. Sweeney T., Prentice C., Saal J.A., et al. Cervicothoracic muscular stabilizing technique. Phys Med Rehabil State Art Rev. 1990;4:335-360.
236. Swezey R.L., Swezey A.M. Efficacy of home cervical traction therapy. Am J Phys Med Rehabil. 1999;78:30-32.
237. Szabo T., Welch J. Human subject kinematic electromyographic activity during low speed rear impacts. In Proc 40th Stapp Car Crash Conp 1996 pp 295–315. Warrendale: Society for Automotive Engineers; 1996.
238. Takala J., Sievers K., Klaukka T. Rheumatic symptoms in the middle-aged population in southwestern Finland. Scand J Rheumatol Suppl. 1982;47:15-29.
239. Taylor J.R., Twomey L.T. Acute injuries to cervical joints: an autopsy study of neck sprain. Spine. 1993;9:1115-1122.
240. Teresi L.M., Lufkin R.B., Reicher M.A., et al. Asymptomatic degenerative disk disease and spondylosis of the cervical spine: MR imaging. Radiology. 1987;164:83-88.
241. Tomita K., Nomura S., Umaeda S., et al. Cervical laminoplasty to enlarge the spinal canal in multilevel ossification of the posterior longitudinal ligament with myelopathy. Arch Orthop Trauma Surg. 1988;107:148-153.
242. Und B., Schlbom H., Nordwall A., et al. Normal range of motion in cervical spine. Arch Phys Med Rehabil. 1989;70:692-695.
243. Vallee J.N., Feydy A., Carlier R.Y., et al. Chronic cervical radiculopathy: lateral approach periradicular corticosteroid injection. Radiology. 2001;218:886-892.
244. VanAkkerveeken P.F. The diagnostic value of nerve root sheath infiltration. Acta Orthop Scand. 1993;251(suppl):61-63.
245. Vargo M.M., Flood K.M. Pancoast tumor presenting as cervical radiculopathy. Arch Phys Med Rehabil. 1990;71:606-609.
246. Viikari-Juntura E., Porras M., Laasonen E.M. Validity of clinical tests in the diagnosis of root compression in cervical disc disease. Spine. 1989;14:253-257.
247. Vincent M. Cervicogenic headache: a comparison with migraine and tension-type headache. Cephalalgia. 1999;1(suppl 25):11-16.
248. Westerling D., Jonsson B.G. Pain from the neck-shoulder region and sick leave. Scand J Soc Med. 1980;8:131-136.
249. White A.A.III, Panjabi M.M. The problem of clinical instability in the human spine: a systematic approach. Clinical Biomechanics of the Spine, ed 2. Philadelphia: Lippincott, 1990.
250. White A.A.III, Southwick W.O., Deponte R.J., et al. Relief of pain by anterior cervical spine fusion for spondylosis: a report of sixty-five patients. J Bone Joint Surg Am. 1973;55:525-534.
251. Whitecloud T.S., Seago R.A. Cervical discogenic syndrome: results of operative intervention in patients with positive discography. Spine. 1987;12:313-316.
252. Wilbourn A.J., Aminoff M.J. AAEM minimonograph 32: the electrodiagnostic examination in patients with radiculopathies. Muscle Nerve. 1998;21:1612-1631.
253. Wilkinson M. The morbid anatomy of cervical spondylosis and myelopathy. Brain. 1960;83:589-616.
254. Wolff M.W., Levine L.A. Cervical radiculopathies: conservative approaches to management. Phys Med Rehabil Clin N Am. 2002;13:589-608.
255. Wood E.C. Beard’s Massage: Principles and Techniques. Philadelphia: Saunders; 1974.
256. Ylinen J., Ruuska J. Clinical use of isometric neck strength measurement in rehabilitation. Arch Phys Med Rehabil. 1994;75:465-469.
257. Yoss R.E., Corbin K.B., McCarthy C.S., et al. Significance of symptoms and signs in localization of involved root in cervical disc protrusion. Neurology. 1957;7:673-683.
258. Yu Y.L., Jones S.J. Somatosensory evoked potentials in cervical spondylosis: correlation of median, ulnar, and posterior tibial nerve responses with clinical and radiological findings. Brain. 1985;108:273-300.
259. Yu Y.L., Woo E., Huang C.Y. Cervical spondylitic myelopathy and radiculopathy. Acta Neurol Scand. 1987;75:367-373.
260. Zheng Y., Liew S., Simmons E. Value of magnetic resonance imaging and discography in determining the level of cervical discectomy and fusion. Spine. 2004;29(19):2140-2145.