Chapter 10 Common CMR artefacts
Introduction
CMR can provide excellent static and dynamic images but some knowledge of artefacts is important when unusual findings occur or suboptimal images are obtained.
Motion artefact
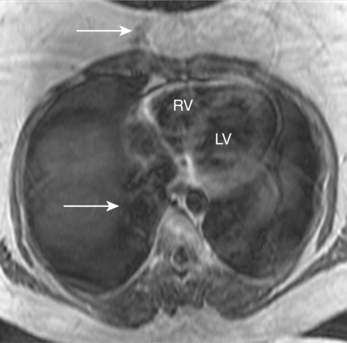
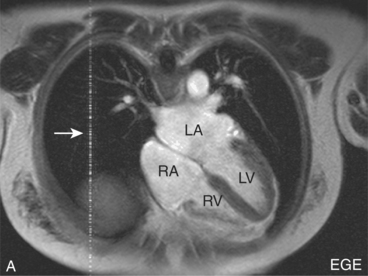
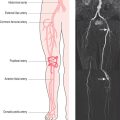
Patient motion artefacts are common, and are also referred to as phase mismapping or ghosting (Figure 10.1). Two important reasons for these artefacts are respiratory and cardiac motion. Other causes are flow and actual patient bodily movement on the table. Image distortion is due to anatomical movement between the application of the phase and frequency encoding gradients, leading to within-view errors, and anatomical motion between each application of the phase encoding gradient, causing view-to-view errors. Motion artefacts always occur along the direction of the phase encoding gradient, the phase encode axis, and appear as blurring across an image. Periodic motion will be located at regular intervals along the phase encode axis with the shape of the ghost reflecting the moving structure. The false images usually have increased signal intensity at the expense of the causal moving structure, from which signal is reduced. There are several ways to reduce motion artefact. General measures include swapping the direction of the phase and frequency encoding gradients so that the ghosting falls outside the area of interest, and vendor-specific gradient moment rephasing methods which can automatically correct altered phases back to their original values. More specific measures directed at respiratory and cardiac motion are discussed below.
Respiratory motion
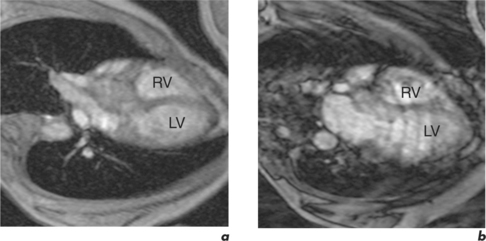
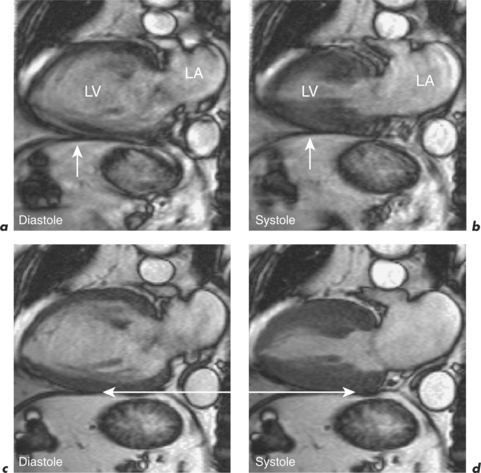
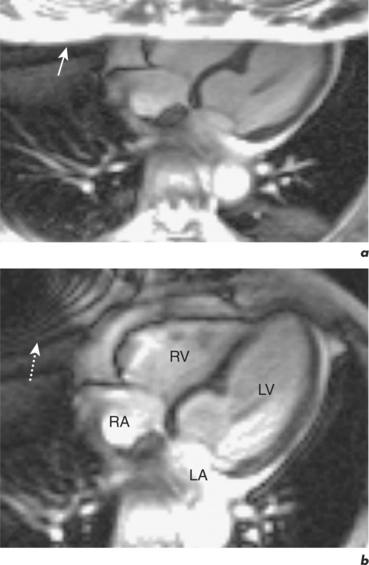
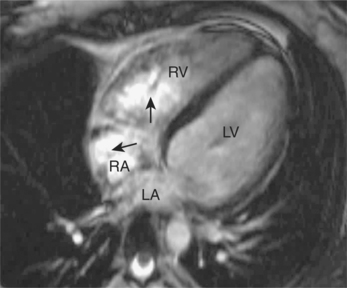
Respiratory motion artefacts are usually eliminated by instructing patients to hold their breath at end expiration throughout the duration of scanner noise (Figure 10.2). If this is unsuccessful, then the operator should firstly reiterate the importance of total suspension of breathing during image acquisition and repeat the scan (Figure 10.3). Following failure of repeated instruction, breath-holding can be tried at maximal inspiration or the scan acquisition time shortened by the addition of parallel acquisition. These methods employ computational techniques and arrays of coils wherein each coil independently and simultaneously images a given volume. Parallel imaging can be used to either reduce the total acquisition time or increase the resolution of a scan. There will be some loss of image quality in return for reduced scan duration. Respiratory navigator techniques can also be tried as in coronary MRA. Additionally, a prepulse RF signal can be directed across the chest wall to reduce or eliminate signal coming from it. Such prepulses are either spatially selective or chemically selective. Chemically selective prepulses tend to remove signal from methylene (CH2) protons in adipose tissue and are therefore means of fat suppression.
Cardiac motion
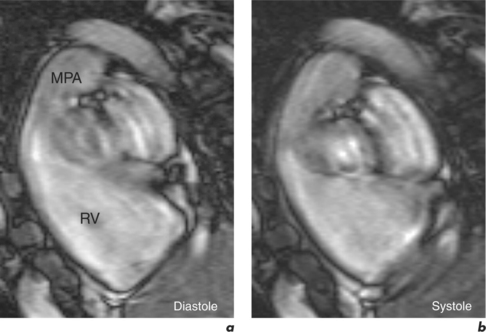
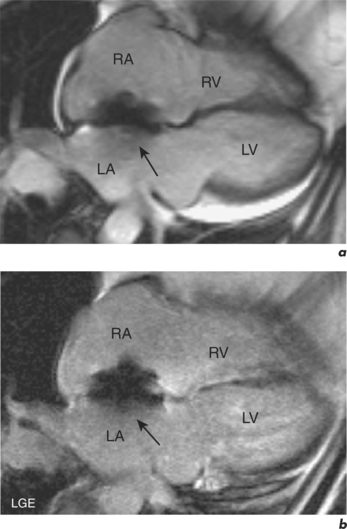
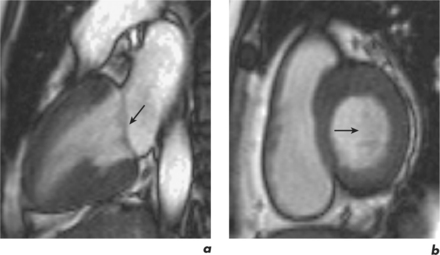
This is generally reduced using ECG-gating, which synchronizes data acquisition with the phases of the cardiac cycle which are identified relative to the R-R interval. R-R signal is detected using externally placed ECG electrodes as part of patient preparation for CMR. Problems from inadequate R-R signal necessitate alternative ECG electrode selection, placement, or further patient skin preparation to increase electrode adhesion, while problems with varying R-R interval are more troublesome. This occurs in arrhythmias such as atrial fibrillation, frequent ventricular ectopics, and ventricular bigeminy (Figure 10.4). Atrial fibrillation requires the use of a variation of the ECG-gating process known as prospective gating. This is as opposed to retrospective gating methods, which acquire data continuously during the cardiac cycle. Retrospective gating is suitable when the R-R interval is regular since the same part of the data is acquired at the same point. When the R-R interval becomes erratic then the shortest interval period is chosen and data are obtained only during that period for each subsequent cycle until the imaging sequence is complete. With frequent ventricular ectopy, a specific arrhythmia rejection program can be instituted to recognize and eliminate the unwanted data. Ventricular bigeminy causes the greatest disruption to image quality and can be counteracted by attempting to exclusively acquire data from the ‘normal’ cardiac cycles, which will prolong scan duration, or pharmacological methods of arrhythmia suppression such as short-acting prior beta-blocker treatment. Parallel acquisition is also useful for reducing cardiac motion artefacts when used to reduce scan duration.
Metallic artefact
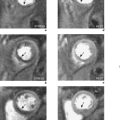
Metallic artefacts on GE sequences appear as varying degrees of signal void and high intensity accompanied by image distortion (Figures 10.5 and 10.6). SE sequences rephase some of the phase incoherence and therefore allow improved imaging (Figure 6.11). Signal void will remain unless the metallic object contains protons, signals from which can be imaged. Whenever possible all metallic items are removed from patients prior to scan initiation.
Wrap-around
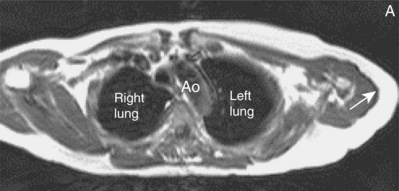
This is a common artefact which occurs when the selected field-of-view of imaging is smaller than the anatomical structure being imaged, leading to details outside the area of interest being mapped onto the final image. With modern scanners wrap-around is usually only problematic in the phase encode axis. The appearance is that a structure from position X is mapped into position Y or one side of the image overlaps the other (Figure 10.7). Reduction of wrap can involve increasing the field-of-view, or reducing signal from structures outside the original field-of-view by placement of spatially selective prepulses. Increasing the field-of-view by alterations to the frequency or phase encode axis can reduce image resolution and increase scan duration respectively. Oversampling of data in the phase encode direction also increases scan time. A certain amount of wrap is acceptable in most clinical imaging as long as there is no ambiguity as to the cause of the artefact, and the area of interest is visualized in full.
Shimming artefact
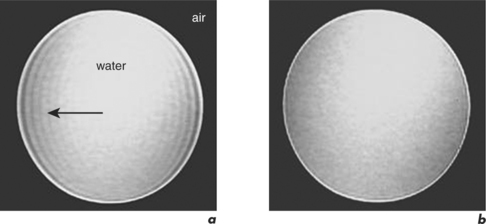
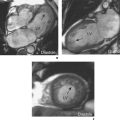
Shimming effects are due to magnetic field inhomogeneities. A shim coil can be placed within the area of inhomogeneity to create evenness, a process referred to as shimming. Individual patient characteristics will result in bright or dark signal inhomogeneities (Figure 10.8). Most shimming is now automated but can still be performed manually if required in a few minutes (Figure 10.9).
Anatomical mimicry
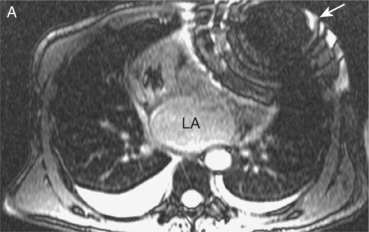
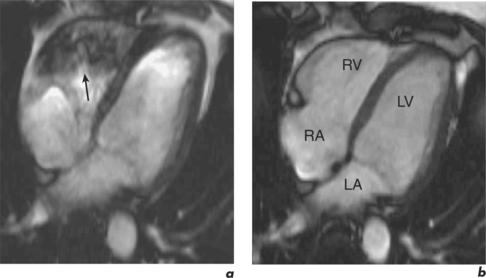
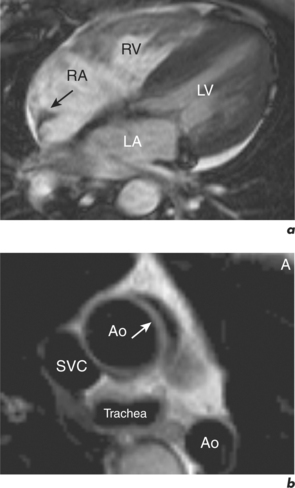
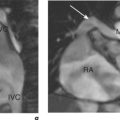
Some routine findings in CMR can appear to represent pathology if not previously encountered, and two important appearances are that of the crista terminalis in the RA and the superior pericardial reflection. The former can be mistaken for a RA mass while the latter can mimic aortic dissection (Figure 10.10).
Chemical shift edge artefacts
Chemical shift misregistration
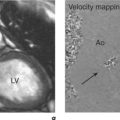
Incorrect mapping of protons with different precessional frequencies leads to signal displacement of fat and water along the frequency encode axis which can appear as high signal bands or a signal void between these materials (Figure 10.11). Resulting artefact is proportional to the strength of the magnetic field, with greater frequency shift and therefore artefact noted at higher field strength. Increasing resolution along the frequency encode axis results in the physical distance corresponding to chemical shift artefact being reduced.
Chemical shift cancellation
When protons within fat and water spin out-of-phase simultaneously, signal intensity is reduced or lost due to signal cancellation. The out-of-phase image produces an asymmetrical edging effect in the phase encode axis and causes a dark ring to form around structures that contain both fat and water. SE sequences use RF rephasing pulses which correct for phase discrepancies and therefore reduce artefact. On GE sequences the echo time parameter can be altered to try to catch the fat and water in-phase, this can lead to a reduction in the number of slices obtained.
External artefact
Devices
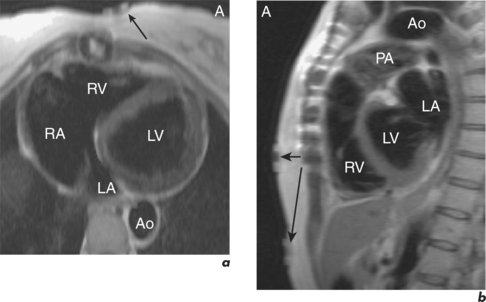
Artefacts from externally placed devices are usually obvious since they are expected following patient positioning in the magnet. A common example is the ECG box required for obtaining ECG-gated images (Figure 10.12).
Partial volume effects
Partial volume effects occur due to the finite limits of image resolution. Image resolution is determined by the size of image voxels. Within a voxel signals cannot be resolved and an average signal intensity is produced. An increase in resolution is required to obtain smaller voxels, at the expense of SNR. Small or thin structures entirely contained within a voxel may disappear following averaging. This can lead to missing stenosis within coronary arteries by coronary MRA, and poor visualization of thin structures such as valves in certain planes (Figure 10.14).
Others
Cross talk
Cross talk occurs as a consequence of interference among adjacent slices. Slice selection profiles are not perfectly rectangular and there is overlap at the edges if they are closely spaced. RF pulses for one slice can then stimulate protons in adjacent slices. Such cross talk often happens in multislice, multiangle acquisitions and gives the appearance of dark bands of signal loss across an image. Insertion of adequate spacing between slices (interslice gap) and obtaining slices in a non-contiguous manner (slice interleaving) both reduce this artefact.
Truncation artefacts
Truncation artefacts are also known as ringing, or Gibbs artefacts. CMR images are normally the result of image approximation by Fourier transformation. Artefacts arise as a fundamental consequence of the Fourier representation of an image when signal intensity is abrupt and not gradual (gets truncated). Truncation artefacts can be in the frequency or phase encode direction. Truncation can give the appearance of multiple, parallel lines adjacent to high contrast interfaces looking like edge ringing or a syrinx-like stripe (Figure 10.15). False widening of the high contrast interface edges is commonly seen and edge enhancement of the interface with adjacent tissue distortion can also occur. Truncation artefacts can be reduced by increasing the spatial resolution or decreasing the interface contrast. The former can be achieved by sampling for a greater time in the phase encode direction and obtaining a greater number of phase encode steps, while an example of the latter is application of fat suppression for truncation artefact adjacent to adipose tissue.