Colorectal liver metastases
Introduction
Colorectal cancer is the commonest gastrointestinal malignancy and the second commonest cause of cancer death in Western society. Worldwide there are 1.2 million new cases and 608 000 deaths annually.1 The liver is usually the first site of metastatic disease and may be the only site in 30–40% of patients with advanced disease.2 At the time of initial diagnosis of colorectal cancer, 20–25% of patients will have detectable liver metastases. A further 40–50% will develop liver metastases, usually within the first 3 years of follow-up after successful resection of the primary tumour.3
Without treatment, the median survival for colorectal liver metastases (CRLMs) is just 6–8 months, varying with the extent of disease at presentation. The prognosis is best for those whose metastases are isolated to a single lobe of the liver or are limited in number.3 However, even for the best prognostic groups, very few survive 5 years without treatment. Surgery is the only treatment that offers the prospect of cure for CRLMs. Traditionally, only 10–20% of patients were considered suitable for attempted curative resection; the remaining patients were offered palliative and symptomatic treatment.
Preoperative staging: the key to selection of candidates for curative treatment
On the detection of colorectal liver metastases it is recommended that patients should be fully staged prior to any planned chemotherapy, and the staging and management plan should be coordinated by a specialist multidisciplinary team.4 Individual imaging techniques used in preoperative staging have different strengths and weaknesses, but consensus is now emerging on the optimal choice of technique and the sequence with which it should be employed.5,6 Imaging techniques are often complementary in the management of colorectal liver metastases, and multiple imaging modalities are often employed.
Computed tomography (CT)
CT is considered a standard of care for all patients identified to have hepatic metastasis.4 Intravenous iodinated contrast media should be used routinely. This helps characterise liver lesions based on their enhancement patterns during the various phases of contrast circulation in the liver.7 During the portal venous phase, normal liver parenchyma usually enhances intensely while liver metastases (with their dominant arterial supply) appear as relatively hypodense hypovascular lesions. In small-sized liver metastases, arterial dominant phase imaging may be useful to detect faint peripheral rim enhancement. Delayed images should be obtained 4–5 minutes after contrast injection. This is helpful in differentiating metastases from benign liver lesions, particularly a haemangioma.8 Whilst CT is considered a standard of care, it has limitations, including the need for a high radiation dose and low sensitivity for the detection and characterisation of lesions smaller than 1 cm (Figs 6.1–6.6).
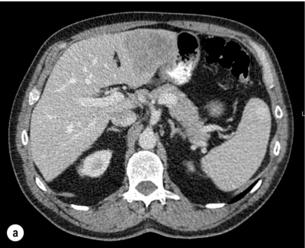
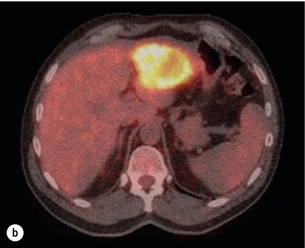
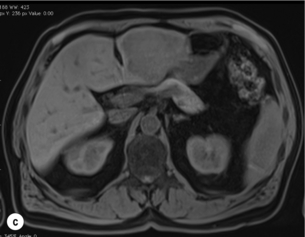
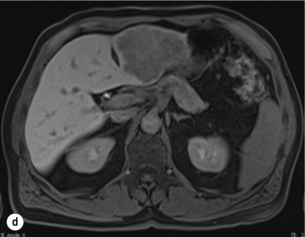
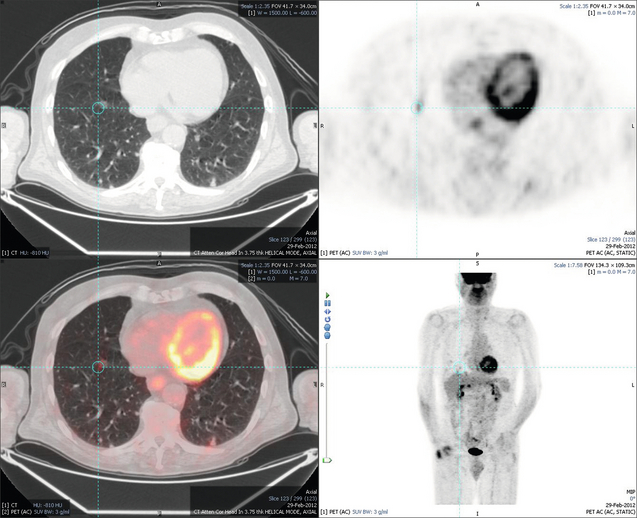
Figure 6.1 (a) CT image in the portal-venous phase demonstrating a hypodense colorectal liver metastasis occupying segments 2 and 3. (b) PET-CT image of the same metastasis demonstrating high uptake of FDG. (c) T1-weighted MRI image of the same metastasis demonstrating a typical hypodense colorectal metastasis. (d) T1-weighted MRI image following primovist contrast administration. Evidence of contrast take-up within the liver and excretion within the common bile duct is observed. No evidence of contrast take-up within the metastasis can be seen.
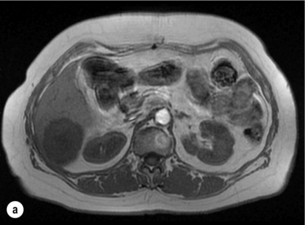
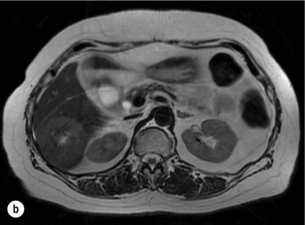
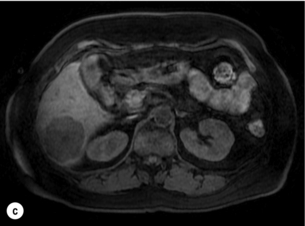
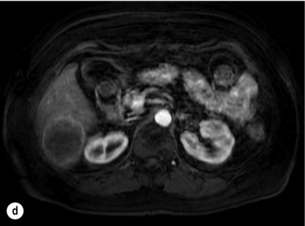
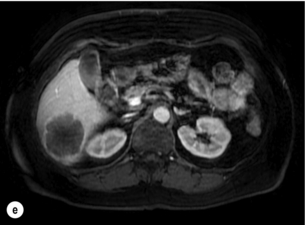
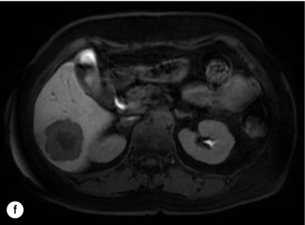
Figure 6.2 (a) T1-weighted MRI image without contrast demonstrating a typical hypodense metastasis in segment 6. (b) T2-weighted MRI image of the same metastasis, where the metastasis is brighter than the surrounding liver. Evidence of central necrosis is seen as a brighter central area of the metastasis. (c) T1-weighted MRI image with fat suppression before contrast administration. (d) T1-weighted MRI image with fat suppression in the arterial phase following primovist contrast administration. The metastasis demonstrates typical rim enhancement. (e) T1-weighted MRI image with fat suppression following primovist contrast administration in the portal venous phase. Good contrast take-up within the liver is observed. (f) T1-weighted MRI image with fat suppression 20 minutes following primovist contrast administration. No evidence of contrast take-up within the metastasis is seen. Evidence of contrast excretion within the gallbladder, common bile duct and kidney can be observed.
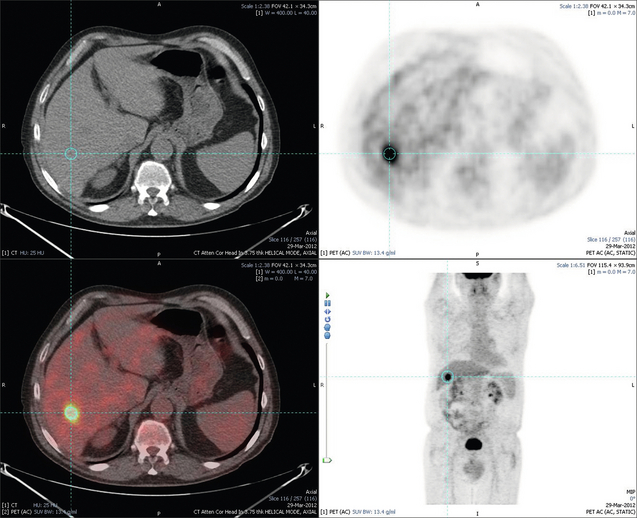
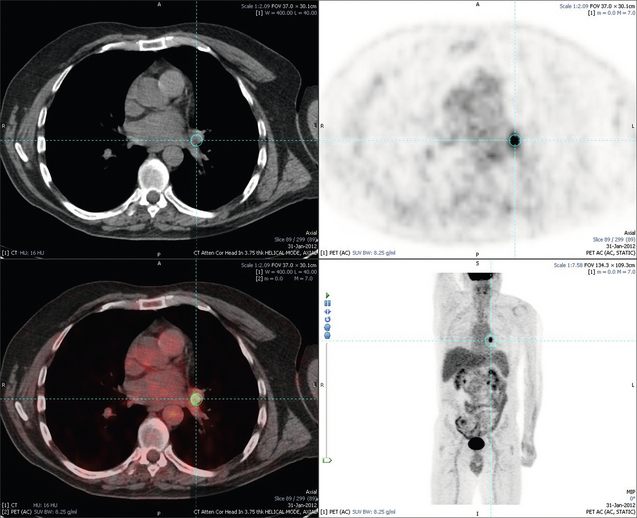
Figure 6.3 Images extracted from a PET-CT scan demonstrating CT, PET and fused PET-CT images of a liver metastasis in the right liver.
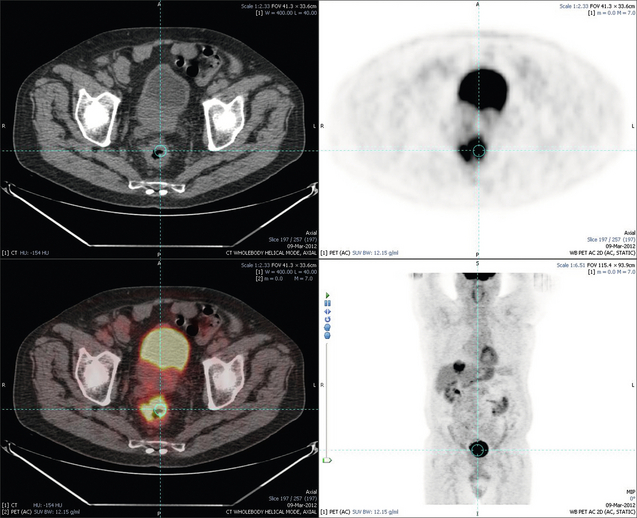
Figure 6.4 Images extracted from a PET-CT scan demonstrating CT, PET and fused PET-CT images of a PET-positive primary rectal cancer. Evidence of a left lobe liver metastasis can be seen in the bottom right image.
Magnetic resonance imaging (MRI)
MRI is a highly effective imaging modality for detecting and characterising liver lesions and provides high lesion-to-liver contrast without using ionising radiation. Typically, CRLMs show low signal intensity on T1-weighted images and moderately high signal intensity on T2-weighted images with fat suppression. Gadolinium, the most commonly used MRI contrast agent, behaves similarly to the iodinated contrast agents used in CT. Liver-specific contrast media such as superparamagnetic iron oxide (SPIO), gadoxetic acid (Primovist®) and Mangafodipir trisodium (Mn DPDP, Teslascan) are not taken up by colorectal hepatic metastases, so may aid in the detection of CRLMs.9,10 These agents are of particular value in the characterisation of liver lesions that are either small or indeterminate on other imaging modalities.8,10
Whilst the benefits of MRI are evident, it does have a number of limitations. MRI has a low sensitivity for detecting extrahepatic disease in the peritoneum and chest, and takes longer to perform than contrast-enhanced CT. There are also a number of contraindications to MRI, including patients with pacemakers, implantable cardiac defibrillators, cochlear implants and metallic orbital foreign bodies.10 However, it can be used safely in patients with allergies to iodinated contrast agents (Figs 6.1 and 6.2).
Positron emission tomography (PET)
PET has emerged as an important diagnostic tool in the evaluation of CRLMs. Colorectal malignancies are often metabolically active and therefore have a greater glucose uptake relative to that of surrounding normal tissues. This can be identified with [18 F]fluoro-2-D-glucose (FDG-PET). This modality is highly sensitive, especially when combined with CT.11 PET-CT is often used in the preoperative assessment of CRLMs, often with the aim of identifying irresectable extrahepatic disease that would make liver resection futile.12 It can sometimes be difficult to differentiate between malignant tissue and other metabolically active tissue, e.g. inflammatory tissue due to infective or postsurgical causes.12 Mucinous colorectal metastases may also prove difficult to detect due to reduced glucose uptake.13 Other disadvantages of PET include high cost and limited sensitivity for lesions smaller than 1 cm (Figs 6.1 and 6.3–6.6).
Staging laparoscopy
The yield of laparoscopy for detecting unresectable disease varies from 6% to 36%.13–19 Staging laparoscopy cannot be performed in 6–16% of patients due to adhesions from previous surgery.13–19 One study suggested that staging laparoscopy had greater value in those patients with a higher clinical risk score (CRS).20 The CRS ranges from 0 to 5 based on the presence of the following characteristics: node-positive primary tumour, prehepatectomy carcinoembryonic antigen (CEA) greater than 200 ng/mL, more than one liver tumour, liver tumour size greater than 5 cm and disease-free interval of less than 1 year. In a Memorial Sloan Kettering Cancer Center study,20 only 4% of patients with CRS of 0–1 were irresectable and none were identified as unresectable at preoperative laparoscopy. At scores of 2–3, 21% of lesions were unresectable and only one-half were found at laparoscopy (yield of 11%). The highest yield was at scores of 4–5, where the yield of laparoscopy was 24%.
Cardiopulmonary exercise testing
Traditionally, selection of patients for resection has been centred on identifying patients with resectable disease. Recently interest has grown in identifying patients who have a higher operative risk, either from reduced fitness or previously unknown cardiorespiratory comorbidities. Cardiopulmonary exercise testing (CPET) has been shown to be useful in quantifying surgical risk in patients undergoing major hepatobiliary surgery.21 Given that patients over the age of 70 are known to have significantly higher operative risk22,23 and that 50% of patients diagnosed with colorectal cancer are over 70, this technique may have a role to play in the appropriate selection and management of patients undergoing liver resection.
Surgery: the old and the new standards for resection
If CRLMs are resectable, patients can look forward to a 5-year survival of 40–50% and a 10-year survival of 24%, with age being no barrier to resection if fit (Fig. 6.7). In the past, liver resection was attempted only in patients who had one to three unilobar metastases, preferably presenting at least 12 months after resection of the primary tumour, whose disease was resectable with at least a 1-cm margin of healthy liver tissue and who had no hilar lymphadenopathy or extrahepatic disease.
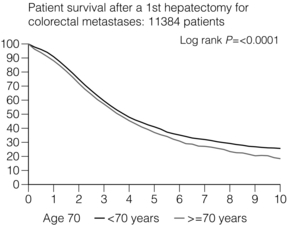
Figure 6.7 LiverMetSurvey. Ten-year survival following hepatectomy for CRLMs comparing patients < 70 years of age with those > 70 years of age. Reproduced with permission.
Recent experience has demonstrated that patients outside these narrow parameters can experience long-term survival following liver resection.24,25 Modern criteria for resection are now based on whether a macroscopically complete resection of the disease can be achieved. Instead of resectability being defined by what is removed, resectability is now being determined by what will remain.
In 2006, consensus statements from the American Hepato-Pancreato-Biliary Association (AHPBA) and a pan-European group changed the criteria for resection.5,26 The American consensus suggested CRLMs should be considered resectable if (i) the disease can be completely resected (regardless of margin), (ii) two adjacent liver segments can be spared with adequate vascular inflow and outflow and biliary drainage, and (iii) the volume of the liver remaining after resection, i.e. the ‘future liver remnant’ (FLR), will be adequate.5 The European group concluded that criteria rendering patients irresectable included invasion of one branch of the liver pedicle and contact with the contralateral branch, contact with the inferior vena cava, invasion of all three hepatic veins, the presence of coeliac lymph nodes and the presence of non-resectable extrahepatic disease.26 These criteria have already been challenged with long-term survival in patients undergoing nodal resection and resection of metastasis involving the inferior vena cava (IVC).27,28 Resection has also been performed for lesions involving all three hepatic veins, though long-term survival data are not available.29
The 2011 UK national guidance recommended that resection should be offered if a patient is fit enough, and complete resection can be achieved whilst leaving an adequate future liver remnant.4 There are no absolute contraindications to resection issued in this guidance, but in normal circumstances they recommend that contraindications to liver resection are:
Surgical strategies to improve resectability
Portal vein embolisation
Portal vein embolisation (PVE) induces atrophy of the liver to be resected and hypertrophy of the liver that will remain (i.e. increases the future liver remnant), with the aim of avoiding post-resection hepatic insufficiency, liver failure and death. A meta-analysis of 1088 patients confirmed that this technique significantly increased the FLR, making more patients suitable for liver resection.30 The overall morbidity rate was 2.2% without mortality. Following PVE, 930 patients (85%) proceeded to laparotomy. Resection was not performed in 158 patients (17%): in 131 because of inadequate hypertrophy of the FLR and in 27 because of disease progression.
Although there is no consensus on what constitutes a safe volume of remnant liver, minimum values of 20–25% for patients with normal livers, 30% following neoadjuvant chemotherapy and 40% in the presence of chronic liver disease have been suggested.5,31,32 PVE also appears to be safe when combined with neoadjuvant chemotherapy.33
Two-stage hepatectomy
Two-stage hepatectomy involves delayed re-hepatectomy after hypertrophy of the residual liver and may be used for large bilateral lesions in which a one-stage resection of all the involved segments would lead to liver failure.34 The first stage involves resection of metastases from the FLR and PVE (or portal vein ligation during surgery), followed by a period of liver regeneration and hypertrophy of the FLR alongside systemic chemotherapy. The second stage is performed 2–3 months later and consists of the major hepatectomy to remove the residual disease. A large series reported 1- and 3-year survival of 70.0% and 54.4%, respectively, in 25 of 33 patients in whom a two-stage hepatectomy could be completed.35 There was no operative mortality; postoperative morbidity was 15.1% and 56.0% after first- and second-stage hepatectomy, respectively.
Repeat hepatectomy
Repeat hepatectomy for patients with colorectal liver metastases is safe and provides survival benefit. A meta-analysis of 21 studies, comprising 3741 patients, showed that there was no difference in perioperative morbidity, mortality or long-term survival between patients undergoing a first or repeat hepatectomy.36 A study looking at 1706 patients undergoing repeat hepatectomy for CRLMs demonstrated similar morbidity and mortality after third and fourth hepatectomies, though 5-year survival decreased from 47.1% for a first resection to 23.8% for a third or fourth resection.37
Extreme liver surgery
Resection of tumours involving the hepatic vascular inflow has been described, including portal vein resection and reconstruction, hepatic artery resection and reconstruction (or arterialisation of the portal vein as an alternative).38 Resections of tumours with involvement of the IVC or the three major hepatic veins have also been performed, using techniques such as total hepatic vascular exclusion, in situ hypothermic perfusion and ex vivo (bench) hepatic resection.39–41 These techniques are at the frontier of what is currently feasible and are associated with significant morbidity and mortality. Nonetheless, this aggressive surgical approach may offer hope for patients with hepatic tumours involving the IVC, who would otherwise have a poor prognosis.
Extrahepatic colorectal disease
Extrahepatic colorectal metastases, such as direct diaphragmatic invasion, adrenal metastases and lung metastases, may be resected with curative intent. Reported long-term survival after pneumonectomy for colorectal metastases mirrors very closely that seen after hepatectomy, with most series quoting a 5-year survival of the order of 40–50%, with similar low operative morbidity and mortality.42–45 More recent series have identified 5-year survival approaching 70%46 and showed that repeat resection of pulmonary metastases is also of benefit, with 5-year survival of 42% following second pneumonectomy.47
Other series looking at liver resection in the presence of extrahepatic disease have demonstrated that there is a role for resection of other limited extraheptic disease, including peritoneal, hepatic pedicle nodal disease, aortocaval nodal disease, ovarian and bone metastases.28,48,49 Five-year survival following limited peritoneal and hepatic pedicle nodal disease is quoted at 27% and 26%, respectively.48 Aortocaval nodal disease is associated with worse long-term survival, with a 5-year survival of just 7%.49
Techniques of surgical resection
Technological innovations in liver surgery have mainly focused on minimising blood loss during transection of the hepatic parenchyma, as blood transfusion is associated with increased postoperative morbidity and mortality, as well as reduced long-term survival.50 Inflow occlusion (Pringle manoeuvre) and low central venous pressure (CVP) anaesthesia minimise blood loss but may cause liver damage by ischaemia and reperfusion injury. Consequently, there has been an interest in devices that facilitate a more bloodless liver transection, obviating the need for inflow occlusion associated with the traditional clamp-crushing technique.
The most popular of these techniques include the ultrasonic aspirating dissector (CUSA) using ultrasonic energy, the Hydrojet using a pressurised jet of water and the dissecting sealer (TissueLink) using radiofrequency energy. These techniques were compared in a randomised controlled trial51 and in a subsequent Cochrane review.52 There was little difference demonstrated between the four techniques, though the clamp-crushing technique was found to be associated with faster tissue transection and lower transfusion requirements. The Cochrane review also found an association with fewer infective complications. Both studies highlighted the significantly reduced cost associated with the clamp-crushing technique, and therefore could not advocate the use of newer techniques in standard practice. A further randomised control trial of radiofrequency-assisted versus clamp-crushing transection in 50 patients showed a higher rate of postoperative complications in the radiofrequency group (20%), compared to none in the clamp-crushing group.53
Fibrin sealants
Fibrin sealants have become popular as a means of improving perioperative haemostasis and reducing biliary leakage after liver surgery. However, a randomised study of 300 patients showed no differences in transfusion requirement, overall drainage, incidence of biliary fistula and postoperative morbidity between those receiving fibrin glue application and controls.54 Similar to the newer transection techniques, there is little evidence to justify the fibrin sealants, especially given the financial pressures on healthcare provision.
Laparoscopic liver surgery: less is more?
Laparoscopic surgery for hepatic neoplasms aims to provide curative resection while minimising complications. There are no randomised controlled trials assessing the use of laparoscopic hepatectomy and the evidence is based on retrospective series. A meta-analysis of series published between 1998 and 200555 included eight non-randomised studies, reporting on 409 resections of hepatic neoplasms, of which 165 (40.3%) were laparoscopic and 244 (59.7%) were open. Operative blood loss and duration of hospital stay were reduced significantly after laparoscopic surgery. These findings remained consistent when considering studies matched for the presence of malignancy and segment resection. There was no difference in postoperative adverse events and extent of oncological clearance. This paper concluded that laparoscopic liver resection has the potential to reduce operative blood loss and allow earlier recovery with oncological clearance comparable with open surgery.
The largest single-centre experience of laparoscopic resection of CRLMs included 83 resections within a series of 133 liver resections.56 Resections comprised 42 wedge excisions, 10 segmentectomies, nine bisegmentectomies, three trisegmentectomies, 30 left lateral segmentectomies, four left hepatectomies, 31 right hepatectomies, three extended right hepatectomies and two caudate lobe resections. The authors reported a median operating time of 210 minutes (30–480 minutes), median blood loss of 300 mL (10–3000 mL) and a median postoperative stay of 4 days (1–15 days). Severe postoperative bleeding occurred in five patients (3.7%), requiring intensive care management or re-operation, and overall serious complications occurred in 16 patients (13%). Microscopically negative margins (R0/R1) were achieved in 96% of patients with CRLMs.
In 2008 a group of 45 experts in hepatobiliary surgery participated in a consensus conference and concluded that the laparoscopic approach to liver resection is a safe and effective technique for appropriately trained surgeons.57
Morbidity, mortality and survival after liver resection for CRLMs
The utility of surgical resection of CRLMs is clearly established. Prospective and retrospective studies consistently show 5-year survival rates following liver resection of 30–50%, depending on selection criteria. A major systematic review of surgical resection for CRLMs was undertaken to assess the published evidence for its efficacy and safety and to identify prognostic factors.58 Thirty independent studies met all the eligibility criteria for the review and data on 30-day mortality and morbidity were included from a further nine studies. The best available evidence came from prospective case series, but only two studies reported outcomes for all patients undergoing surgery. The remainder reported outcomes for selected groups of patients: those undergoing hepatic resection or those undergoing curative resection.
Death within 30 days of hepatic resection was reported by 24 studies and ranged from 0% to 6.6% (median 2.8%). A further nine studies reported perioperative mortality within an undefined time period (1.3–4.6%, median 3.6%) and two studies reported 60-day mortality (3.4–5.5%). Mortality was not reported in four studies. Cause of death was reported in 15 studies for a total of 103 patients. The commonest specified causes of fatal complications were, in descending order of frequency: hepatic failure, postoperative haemorrhage, generalised sepsis, cardiac failure, multiorgan failure, pulmonary embolism, bile leak and anastomotic leak.58
Classification of CRLMs
Staging systems and terminology
The present American Joint Committee on Cancer (AJCC) classifies all colorectal metastasis beyond the local lymphatic basin as stage IV colorectal cancer. This does not allow the distinction between patients who are currently incurable, with a prognosis of less than 6 months, from those who are potentially curable. This has led to the call for a new staging system for colorectal cancer that reflects these differing treatment pathways and prognostic outlook.59 The 2003 French guidelines on the management of CRLMs recommended four categories that could be defined: (1) easily resectable liver metastases, (2) resectable liver metastases involving five to six liver segments and/or contralateral major vascular structures, (3) liver metastases that are initially unresectable but may become resectable after chemotherapy, and (4) definitely unresectable.60 Based on the French classification system, the European Colorectal Metastases Treatment group has proposed a staging system:26
• IVa – easily resectable with curative intent at detection (French classification 1);
• IVb – technically difficult/borderline resectable at detection (French classification 2);
• IVc – potentially resectable after neotherapeutic chemotherapy (French classification 3);
• IVd – little or no hope of being rendered resectable with curative intent after conventional chemotherapy (French classification 4);
Other suggested systems include distinguishing between stage IV-R for patients with resectable disease and stage IV-U for patients with unresectable disease.61 Furthermore, stage IV-R could be further divided into IV-Ra (resectable liver only), IV-Rb (resectable extrahepatic only) and IV-Rc (resectable hepatic and extrahepatic). Stage IV-U could be similarly subdivided, after assessment by an experienced site-specific surgical oncologist.
A number of scoring systems have been developed that take a different approach to the staging of CRLMs and attempt to classify patients based on clinical prognosis. The most popular of these were produced by Fong et al.,20 Nordlinger et al.62 and Rees et al.63 The Fong classification (clinical risk score) was described earlier and is the most widely used owing to its ease of use. Nordlinger’s classification ranges from 0 to 7, with 1 point being awarded for each of the following adverse risk factors:
1. Extension into serosa of primary tumour
2. Lymphatic spread of the primary tumour
3. Delay from primary tumour to resection < 24 months
4. Number of liver metastases in preoperative imaging
5. Largest size of liver metastasis in preoperative imaging ≥ 5.0 cm
6. Preoperatively estimated clearance of normal parenchyma resected with liver metastasis < 1 cm
These two scoring systems have been compared,64,65 with the Fong classification proving to be more appropriate for use in clinical practice and better at differentiating between groups. Both scoring systems exclude patients with extrahepatic disease and fail to take into account many known adverse risk factors, meaning that their clinical utility may be limited.
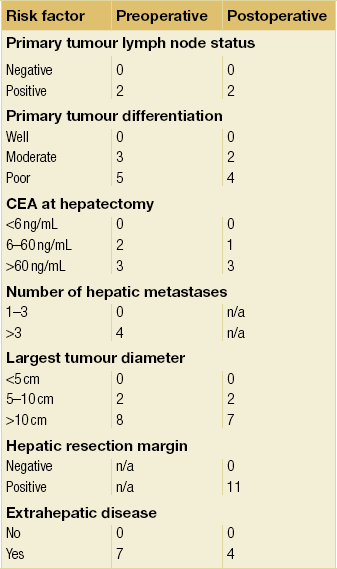
Rees et al. proposed a scoring system that could be used in either the preoperative or postoperative setting.63 In this risk prediction model, points were allocated up to a maximum of 30 (Table 6.1). Patients with a score of 0, 10, 20 and 30 on preoperative scoring had 5-year survival rates of 66%, 35%, 12% and 2%, respectively. This compared very well with the scores determined postoperatively. This scoring system is more complex than previously suggested models, which has limited its uptake as a clinical tool.
Chemotherapy for CRLMs
In the last 10 years, overall survival (OS) in patients with metastatic colorectal cancer has improved substantially,66 reflecting improved chemotherapeutic manipulation of disease. Before 2000, 5-fluorouracil (5-FU) was the only available treatment. With the development of the cytotoxic agents oxaliplatin and irinotecan, doublet regimens are now considered standard therapy. In the last 5 years, major advances in the management of advanced colorectal cancer have been made by harnessing targeted monoclonal antibodies against extracellular receptors.
Extracellular growth factor receptor (EGFR) is a transmembrane glycoprotein that utilises tyrosine kinase activity for signal transduction with downstream signalling intrinsically involved in multiple biological processes essential for tumour survival. Cetuximab is a recombinant human/mouse chimeric antibody that binds specifically to the extracellular domain of human EGFR, inhibiting this pathway. It is now recognised that patients who have a mutation in the downstream KRAS proto-oncogene are resistant to cetuximab therapy, and so KRAS testing is routinely performed prior to commencing treatment.67 Improved understanding of this signalling pathway has identified other common mutations in downstream effectors (including BRAF, NRAS and PIK3CA), which may also confer resistance to anti-EGFR treatments.68
Vascular endothelial growth factor (VEGF) is one of the most important regulators of the dynamic balance between pro- and anti-angiogenic factors that are crucial for tumour growth and metastasis, with signalling leading to angiogenic proliferation and increased microvascular permeability. Bevacizumab is a humanised monoclonal antibody directed against VEGF receptors. Proposed mechanisms of action includes inhibition of vessel development, regression of aberrant tumour vasculature and normalisation of tumour perfusion.69
Clarifying the intent of chemotherapy in CRLMs
Chemotherapeutic manipulation of advanced colorectal cancer has undergone a paradigm shift over the last 15 years. Previously, patients with unresectable disease were treated solely with the aim of prolonging life. However, there is now growing recognition that a subgroup of patients who may not be resectable at presentation become resectable after chemotherapy. This approach is often referred to as ‘induction’ or ‘conversion’ chemotherapy.61
Conversion/induction chemotherapy
Resectability rates after chemotherapy for initially unresectable disease vary widely, with modern regimes achieving conversion rates approaching 60%.70 Attempting to bring unresectable disease to resection is worthwhile, with overall 5-year survival comparable between patients resectable at presentation and those converted to resectability after systemic chemotherapy34 (Fig. 6.8). Response to chemotherapy is known to correlate with resection rate70 and it seems sensible that patients with unresectable liver-only disease should be treated with the most aggressive regimen possible to provide the greatest chance of being bought to potentially curative resection (Fig. 6.9, Table 6.2).
Table 6.2
Response rate and resection rate for key trials of cytotoxic agents with the addition of targeted biological agents in patients with initially irresectable metastatic colorectal cancer
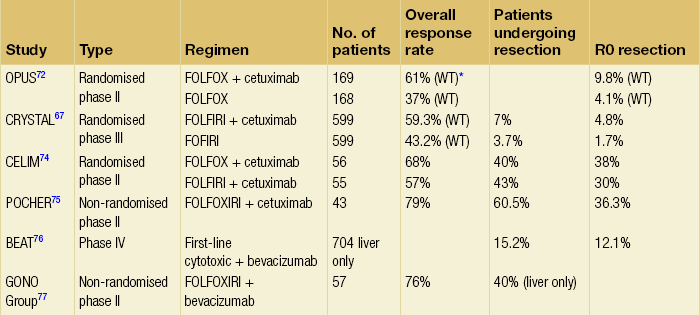
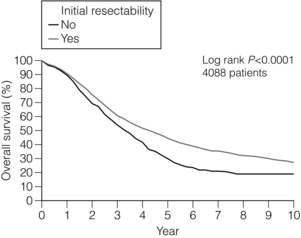
Figure 6.8 LiverMetSurvey. Ten-year survival following hepatectomy for CRLMs comparing those who were initially resectable at presentation with those patients who were considered initially unresectable but were brought to resection using systemic chemotherapy. Reproduced with permission.
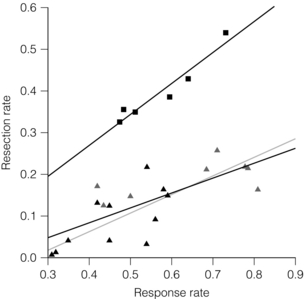
Figure 6.9 Rate of liver resection following systemic chemotherapy for initially unresectable disease. The squares represent patients with non-resectable metastases confined to the liver (‘selected patients’, r = 0.96, P = 0.002). Studies with non-selected patients with colorectal cancer are shown as triangles. Due to the high heterogeneity of these studies, the observed correlation is less strong (r = 0.74, P < 0.001, solid line). A similar correlation was observed when the phase III trials (filled triangles) were separately analysed (r = 0.67, P = 0.024, dashed line). Reproduced from Folprecht G, Grothey A, Alberts S et al. Neoadjuvant treatment of unresectable colorectal liver metastases: correlation between tumour response and resection rates. Ann Oncol 2005; 16(8): 1311–9. With permission from Oxford University Press/European Society for Medical Oncology.
The UK National Institute for Clinical Excellence (NICE) currently recommend the use of 5-FU, leucovorin- and oxaliplatin-based regimens (FOLFOX) as first-line therapy for all patients with non-resectable disease, with irinotecan-based regimens (FOLFIRI) for second-line therapy after failure of first-line treatment. Intensive triplet chemotherapy with FOLFOXIRI has been compared with FOLFIRI alone in a phase III randomised controlled trial. Response rates were higher after FOLFOXIRI, with a secondary resection rate of 36% in patients with liver-limited disease compared to 12% for those treated with standard FOLFIRI (P = 0.017).71 However, toxicity was high and double-agent therapy therefore remains first-line treatment.
There is now growing evidence supporting the addition of targeted biological agents alongside a cytotoxic backbone. The randomised phase II OPUS trial compared FOLFOX with or without cetuximab in 337 patients with metastic colorectal cancer, with response rates of 46% and 36%, respectively (P = 0.064).72 A subgroup analysis of 315 patients assessed KRAS status, demonstrating an overall response rate of 61% in wild-type patients, compared to 37% in KRAS mutants (P = 0.01).
The large CRYSTAL trial randomised 1198 patients to FOLFIRI with or without cetuximab as first-line treatment.67 Retrospective analysis of KRAS status was performed on 1063 patients and found response rates of 59.3% and 43.2% for FOLFIRI and cetuximab compared to FOLFIRI alone in KRAS wild-type patients.73 By contrast, cetuximab offered no survival advantage to the KRAS mutant group. Resectability rates for the entire group (irrespective of KRAS status) were 7% in the FOLFIRI plus cetuximab arm, compared to 3.7% in the FOLFIRI arm, with R0 rates of 4.8% and 1.7% (P = 0.002).
In 2010, the phase II CELIM study assessed FOLFOX/FOLFIRI and cetuximab for a more selected group of patients with unresectable metastatic liver-only disease and found response rates of 68% and 57%, respectively. Forty per cent of the FOLFOX and cetuximab arm underwent resection, compared to 43% of those who received FOLFIRI and cetuximab. In a combined analysis of both arms, 67 patients with KRAS wild-type tumours achieved a response rate of 79%.74
The 2010 phase II POCHER study assessed chronomodulated FOLFOXIRI alongside cetuximab in 43 patients with irresectable liver-only metastases.75 Despite therapy not being allocated on the basis of KRAS status, the authors reported a 60% R0/R1 resection rate after a median of six cycles, with an objective response rate of 79.1%. Two-year survival was 80.6% in resected patients, compared to 47.1% in those who did not undergo resection (P = 0.01).
The largest experience of bevacizumab in unresectable patients remains the BEAT trial.76 This large phase IV trial assessed the addition of bevacizumab to first-line chemotherapy for patients with unresectable metastatic colorectal cancer; 1914 patients were included, with a median progression-free survival (PFS) of 10.8 months (95% confidence interval (CI) 10.4–11.3) and median overall survival (OS) of 22.7 months (95% CI 21.7–23.8). In this unselected group, curative resection was performed in 7.6%. In 704 patients with metastatic disease limited to the liver, resection was achieved in 15.2%. Two-year survival was 89% in those undergoing resection, compared to 54% in those who did not.
The impressive response rates seen following systemic FOLFOXIRI led to the same group performing a single-arm phase II trial of FOLFOXIRI with the addition of bevacizumab.77 Treatment was given as first line to 57 patients, with a 74% PFS at 10 months (95% CI 62–85). Curative resection was performed in 40% of those patients recruited with unresectable liver-only disease, and the same group has now developed a phase III randomised study comparing FOLFOXIRI plus bevacizumb with FOLFIRI plus bevacizumab. The results of this trial (the TRIBE trial) are eagerly awaited.
Perioperative chemotherapy
In 2006, Portier et al. reported the results of the AURC 9002 trial assessing adjuvant 5-FU and leucovorin in patients that underwent liver resection for colorectal metastases, and demonstrated an improved 5-year disease-free survival following chemotherapy (33.5% vs. 26.7%, P = 0.028).78
The EORTC 40983 phase III trial (commonly referred to as EPOC) assessed perioperative chemotherapy by randomising patients to chemotherapy with perioperative FOLFOX and surgery (six cycles before surgery, six cycles after) or surgery alone. Although often criticised, this study clearly demonstrated a significantly improved 3-year progression-free survival in the chemotherapy arm and remains the best available evidence supporting perioperative chemotherapy79 (Fig. 6.10).
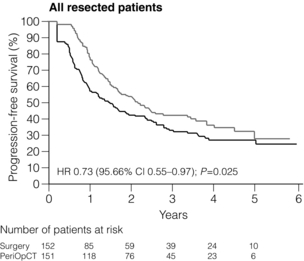
Figure 6.10 The 7.3% improvement in 3-year disease-free survival demonstrated following perioperative chemotherapy (six cycles of FOLFOX before surgery, six cycles of FOLFOX after surgery) compared to surgery alone for CRLM in the EPOC trial (EORTC 40983).79 Reproduced from Nordlinger B, Sorbye H, Glimelius B et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 2008; 371(9617):1007–16. With permission from Elsevier.
Reddy et al. performed a retrospective analysis of 499 patients treated with perihepatectomy 5-FU and leucovorin, and demonstrated a survival advantage to adjuvant but not neoadjuvant therapy.80 The role of neoadjuvant and adjuvant chemotherapy in CRLMs was further clouded by data from the LiverMetSurvey group. Adam et al. assessed 1471 patients with solitary liver metastases, and compared those who underwent resection without chemotherapy and those treated with perioperative chemotherapy followed by resection.22 They found that preoperative chemotherapy had no impact on long-term outcome, but postoperative chemotherapy was associated with better overall and disease-free survival. A meta-analysis of trials assessing postoperative 5-FU based chemotherapy demonstrated a trend towards improved disease-free and overall survival, but did not achieve statistical significance.81
Despite these controversies, expert consensus is that the majority of patients with colorectal liver disease should receive perioperative chemotherapy irrespective of their initial resectability,82 with the rationale that this will result in the destruction of occult disease, allow a test of biology where progression despite chemotherapy signifies poor biology, as well as reduce lesion size, improving resectability.
Pathological response to chemotherapy as a predictor of long-term outcome
It now recognised that patients who exhibit good pathological response to chemotherapy have better overall survival.83 Blazer et al. found complete pathological response to chemotherapy (absence of viable tumour cells on post-resection examination) only occurred in 9% of patients treated with systemic FOLFOX or FOLFIRI, but was associated with a 5-year survival of 75%.84 This finding was supported by Adam et al., who found patients exhibiting complete pathological response had a 5-year survival of 76%, compared to 45% for those without.85 The outstanding survival in this select group of complete pathological responders is similar to stage III and high-risk stage II colorectal disease, i.e. colorectal disease that has not metastasised.86
Complete pathological response is an impressive example of the effectiveness of modern chemotherapeutic regimes. Although oncologically desirable, it creates surgical difficulties. The correlation between complete radiological and pathological response is not clear, with around 80% of lesions showing complete radiological response containing residual disease.87 Trying to locate a lesion that has disappeared is difficult, and results in patients undergoing blind resection on the basis of the last known location of that lesion. The difficulties associated with disappearing lesions highlight the importance of combined surgical and oncological planning to optimise the chemotherapeutic manipulation of disease, as well as the timing of any intervention. Improved preoperative assessment of pathological response by imaging will become increasingly important in deciding which patients can be managed with a ‘watch and wait’ policy.
Chemotherapy-associated hepatotoxicity
Increased use of neoadjuvant treatment has resulted in a rise in chemotherapy-associated hepatotoxicity. Oxaliplatin is associated with sinusoidal obstructive syndrome, characterised by a tender, congested and dilated liver, whilst patients treated with irinotecan develop fatty infiltration and scarring (steatohepatitis)88,89 (Fig. 6.11). Vauthey et al. reported an incidence of 20.2% after a median of 16 weeks of FOLFIRI, compared to 4.4% for chemonaive patients.88 Recognition of the growing number of patients coming to resection with chemotherapy-associated hepatotoxicity has led to growing interest in its impact on surgical outcome.
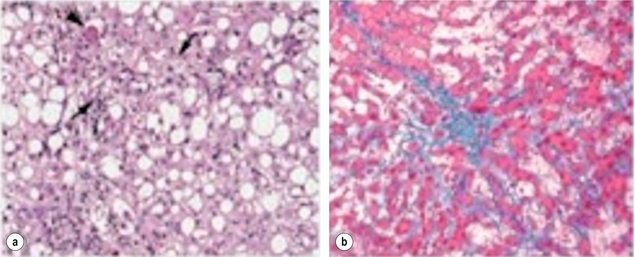
Figure 6.11 Effects of preoperative chemotherapy on the subsequently operated liver. (a) Steatohepatitis seen following excessive pretreatment with irinotecan. The features of ballooned hepatocytes (arrowhead) and Mallory bodies (arrows) are shown. (b) Sinusoidal congestion and thrombosis seen after excessive pretreatment with oxaliplatin.
The EORTC 40983 trial79 comparing upfront surgery and perioperative FOLFOX demonstrated a higher rate of minor complications in the chemotherapy and surgery arm (25% vs. 16%, P = 0.04). The MD Anderson group reported increased mean perioperative blood transfusion requirements in patients with oxaliplatin-induced sinusoidal injury (1.9 vs. 0.5 units, P = 0.03)90 and also demonstrated an increased 90-day mortality in patients who had irinotecan-induced steatohepatitis compared to those who did not (14.7% vs. 1.6%, P = 0.001).88 Other groups have reported similar outcomes91 and marked steatohepatitis is now considered a contraindication to hepatic resection.92
Postoperative morbidity does appear to be related to the duration of neoadjuvant chemotherapy. Karoui et al. demonstrated a higher morbidity in those patients who received more than six cycles (54% vs. 19%, P = 0.047).93 However, this increased risk does appear to be reversible. Welsh et al. showed complication rates of 2.6%, 5.5% and 11% for patients with intervals of 9–12, 5–8 and 4 weeks or less between cessation of chemotherapy and resection (P = 0.009), highlighting the need for close cooperation between medical and surgical oncologists to ensure the optimal timing for any interventions.94
Liver damage is often the result of potentially life-saving chemotherapy and research is therefore needed to elucidate the pathogenesis of this damage, as well as better methods of preoperative prediction that may enable tailoring of chemotherapeutic regimens. Currently, it is prudent to require a larger FLR of at least 30% after resection in patients who have received extensive preoperative systemic chemotherapy to compensate for impaired hepatic function.5 The effects of modern biological therapies on operative morbidity and mortality also require further investigation, although preliminary work does suggest they are safe to use before surgery.95,96
Liver-targeted therapies
The unique blood supply of the liver, with portal flow supplying healthy hepatic parenchyma and arterial flow supplying metastatic disease, has led to the concept of delivering liver-only chemotherapy in an effort to increase metastatic exposure to the agent whilst reducing systemic dose and off-target side-effects. Initial interest focused on hepatic arterial infusion (HAI) as a replacement for systemic chemotherapy. A catheter is inserted at laparotomy into the hepatic artery, through which a portable pump delivers an infusion of chemotherapeutic agent. A meta-analysis by Mocellin et al. found no evidence to support its use instead of systemic chemotherapy in the treatment of irresectable colorectal metastases.97 Interest is now focused on the use of HAI alongside systemic therapies to maximise response in liver dominant disease.
Widescale adoption of HAI has been limited, possibly because of relatively high rates of technical complications. The Memorial Sloan Kettering group reported their experience of 544 consecutive insertions of HAI pump, and found a 16% failure rate within 2 years of insertion.98
Kemeny et al. published data from an early phase I trial of 49 patients treated with systemic oxaliplatin alongside HAI floxuridine in irresectable liver-only disease.99 They reported an 8% complete response rate, an 84% partial response rate and a 47% conversion to resectability, which increased to 57% in chemonaive patients.100,101 The same group recently reported their experience of adjuvant HAI alongside systemic FOLFOX/FOLFIRI in 125 resected patients, and reported improved overall and recurrence-free survival.102 Further randomised studies are needed to accurately define the role for what would appear to be a biologically sensible approach.
Drug-eluting beads for TACE (DEB-TACE)
Drug-eluting beads are compressible microspheres produced from polyvinyl alcohol (PVA) hydrogel loaded with drug (usually irinotecan). DEB-TACE offers a theoretical advantage over HAI of simplified delivery (embolisation and chemotherapy are combined). Common side-effects include post-embolisation syndrome in around 10% of patients, characterised by abdominal pain, pyrexia and a transient rise in liver function tests.103
An international registry reported on 55 patients who had failed first- and second-line systemic therapy for metastatic colorectal cancer and were treated with DEB-TACE.104 Response rates were 66% at 6 months and 75% at 12 months. Median overall survival from time of first treatment was 19 months, with a progression-free survival of 11 months. Six patients (10%) had their disease sufficiently downstaged to allow further treatment, with four undergoing resection and two undergoing radiofrequency ablation. These promising results have led to the development of further studies that aim to better define the precise role of DEBIRI-TACE within the chemotherapeutic armamentarium.
Selective internal radiation treatment (SIRT)
SIRT is the delivery of radiation treatment via intrahepatic arterial administration of yttrium-90 (Y-90) microspheres. Y-90 is a high-energy, beta-particle-emitting isotope bound to resin microspheres that is selectively delivered to a tumour via intra-arterial embolisation. Because of the half-life of Y-90 (2.67 days), 94% of the radiation dose is delivered during the 11 days following treatment.105
In selected patients, radioembolisation can downstage liver metastases so that further treatments, including ablation, are possible.106 Encouraging results have also been reported in heavily pretreated patients with CRLMs.107 Complications of SIRT include transient abdominal pain, fever, lethargy and nausea in up to one-third of patients. Gastroduodenal ulcers have been reported and are avoided by a meticulous administration technique that avoids reflux of Y-90 microspheres into the gastrointestinal vasculature.
Ablative therapies for CRLMs
Ablative therapy takes numerous forms. Cryotherapy, laser hyperthermia and ethanol injection are decreasing in popularity due to high complication rates or lack of efficacy. Radiofrequency ablation (RFA) and microwave ablation (MWA) have significant advantages over older ablative techniques and are increasingly used. However, there remains a lack of clarity surrounding the precise role of ablation compared to surgery. Recent American Society of Clinical Oncology (ASCO) guidelines highlighted the wide variation in overall survival and local recurrence rates after ablation, and suggested that in the absence of adequate data, resection should remain the gold standard treatment for resectable disease.108
Despite these concerns, ablation still has a role as an adjunct to resection. Patients with small-volume resectable metastases who are not sufficiently fit to undergo liver resection should be considered for ablation, as should those with limited liver metastases who have insufficient liver volume to undergo resection.109,110
There is growing interest in the use of ablation alongside systemic chemotherapy for irresectable liver disease. Preliminary results of the EORTC 40004 (CLOCC) trial that compared systemic chemotherapy versus chemotherapy and RFA for unresectable metastatic colorectal liver disease suggested a survival advantage for the combined arm. The final results of this trial are eagerly awaited.111
Radiofrequency ablation
RFA is the most widely used ablative technique and relies on direct current transmission through tissue to generate heat and cause an ablation. Increasing lesion size leads to exponential increases in resistance to current, limiting the size of the effective ablation zone and explaining the increased risk of local recurrence and diminished survival with lesions greater than 3 cm.108
A recent meta-analysis of 95 published series reported a complication rate of 8.9%, with intra-abdominal bleeding, sepsis and biliary tree injury the most common complications.112 Mortality rates range from 0 to 0.5%, with a reported local recurrence rate of 10–31%.113 It is likely this high rate of recurrence is directly related to the type of lesions being treated by RFA. Ablations are often performed on metastases that are adjacent to major vascular structures where blood flow can operate as a heat sink, leading to incomplete ablation and local disease recurrence.
Microwave ablation
MWA has been designed to overcome some of the limitations of RFA. Electromagnetic waves agitate water molecules in tissue without the need for direct current conduction, producing friction and heat causing cell death. MWA offers higher intratumoral temperatures, larger tumour ablation volumes and faster ablation times,114 as well as uniform ablation volumes irrespective of tissue type and moisture content,115 allowing better prediction of ablation volume. Despite this, local recurrence after MWA has been reported between 5% and 13%, with a major complication rate ranging from 3% to 16%.113
Multidisciplinary team approach
The current management of advanced metastatic colorectal cancers is complex and is likely to become increasingly so in the future. Improved chemotherapeutic manipulation of disease and increasing understanding of who and what is technically and oncologically resectable will inevitably lead to more heterogeneous disease management. The rapidly changing and complex management of CRLMs means that these patients must have their treatment managed by highly specialised liver surgeons and oncologists. The UK system of local colorectal multidisciplinary teams (MDTs) organised into cancer networks, with recognised referral pathways to supraregional specialist liver MDTs, is designed to facilitate this process. However, even within this system there remains concern that not all patients with liver-only metastatic disease are being reviewed by appropriate specialists. A 2010 UK population-based study of 114 155 patients who underwent primary colorectal cancer resection between 1998 and 2004 identified 3116 (2.7%) who subsequently underwent resection for CRLMs, with the rate of hepatic resection varying widely between cancer networks (1.1–4.3%) and hospitals (0.7–6.8%).116 The authors suggested that inconsistent use of first-line chemotherapy, or use of different thresholds to determine which patients should be considered for resection, may explain this variability and suggested that direct involvement of appropriate specialists was the only way to address these inequalities.
To help non-experts in decision making, a computer model (OncoSurge) has been created that recommends optimal treatment strategies on a case-specific basis.117 An expert panel rated appropriateness of treatment (chemotherapy, resection or ablation) in 252 cases. A decision model was constructed, consensus measured, and results validated using 48 virtual cases and 34 real cases with known outcomes. Consensus was achieved with overall agreement rates of 93.4–99.1%. This model combines the best available scientific evidence with the collective judgment of worldwide experts to yield a statement regarding the appropriateness of a particular treatment for each patient. The computer program can be accessed at www.evidis.com/oncosurge.
References
1. Ferlay, J., Shin, H.-R., Bray, F., et al, Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. 21351269
2. Weiss, L., Grundmann, E., Torhorst, J., et al, Haematogenous metastatic patterns in colonic carcinoma: an analysis of 1541 necropsies. J Pathol. 1986;150(3):195–203. 3806280
3. Stangl, R., Altendorf-Hofmann, A., Charnley, R.M., et al, Factors influencing the natural history of colorectal liver metastases. Lancet. 1994;343(8910):1405–1410. 7515134
4. Poston, G.J., Tait, D., O’Connell, S., Guideline Development Group, Diagnosis and management of colorectal cancer: summary of NICE guidance. Br Med J 2011; 343:d6751. 22074710
5. Abdalla, E.K., Adam, R., Bilchik, A.J., et al, Improving resectability of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol. 2006;13(10):1271–1280. 16955381
6. McLoughlin, J.M., Jensen, E.H., Malafa, M., Resection of colorectal liver metastases: current perspectives. Cancer Control. 2006;13(1):32–41. 16508624
7. Saini, S., Imaging of the hepatobiliary tract. N Engl J Med. 1997;336(26):1889–1894. 9197218
8. Martínez, L., Puig, I., Valls, C., Colorectal liver metastases: radiological diagnosis and staging. Eur J Surg Oncol. 2007;33(Suppl. 2):S5–16. 18023133
9. Huppertz, A., Balzer, T., Blakeborough, A., et al, Improved detection of focal liver lesions at MR imaging: multicenter comparison of gadoxetic acid-enhanced MR images with intraoperative findings. Radiology. 2004;230(1):266–275. 14695400
10. Xu, L.-H., Cai, S.-J., Cai, G.-X., et al, Imaging diagnosis of colorectal liver metastases. World J Gastroenterol. 2011;17(42):4654–4659. 22180707
11. Israel, O., Mor, M., Gaitini, D., et al, Combined functional and structural evaluation of cancer patients with a hybrid camera-based PET/CT system using 18F-FDG. J Nucl Med. 2002;43(9):1129–1136. 12215549
12. Kochhar, R., Liong, S., Manoharan, P., The role of FDG PET/CT in patients with colorectal cancer metastases. Cancer Biomark. 2010;7(4):235–248. 21576816
13. Berger, K.L., Nicholson, S.A., Dehdashti, F., et al, FDG PET evaluation of mucinous neoplasms: correlation of FDG uptake with histopathologic features. AJR Am J Roentgenol. 2000;174(4):1005–1008. 10749239
14. Mortensen, F.V., Zogovic, S., Nabipour, M., et al, Diagnostic laparoscopy and ultrasonography for colorectal liver metastases. Scand J Surg. 2006;95(3):172–175. 17066612
15. Grobmyer, S.R., Fong, Y., D’Angelica, M., et al, Diagnostic laparoscopy prior to planned hepatic resection for colorectal metastases. Arch Surg. 2004;139(12):1326–1330. 15611458
16. Metcalfe, M.S., Close, J.S., Iswariah, H., et al, The value of laparoscopic staging for patients with colorectal metastases. Arch Surg. 2003;138(7):770–772. 12860759
17. de Castro, S.M.M., Tilleman, EHBM, Busch, O.R.C., et al, Diagnostic laparoscopy for primary and secondary liver malignancies: impact of improved imaging and changed criteria for resection. Ann Surg Oncol. 2004;11(5):522–529. 15123462
18. Koea, J., Rodgers, M., Thompson, P., et al, Laparoscopy in the management of colorectal cancer metastatic to the liver. Aust N Z J Surg. 2004;74(12):1056–1059. 15574147
19. Rahusen, F.D., Cuesta, M.A., Borgstein, P.J., et al, Selection of patients for resection of colorectal metastases to the liver using diagnostic laparoscopy and laparoscopic ultrasonography. Ann Surg. 1999;230(1):31–37. 10400033
20. Fong, Y., Fortner, J., Sun, R.L., et al, Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230(3):309–321. 10493478
21. Snowden, C.P., Prentis, J.M., Anderson, H.L., et al, Submaximal cardiopulmonary exercise testing predicts complications and hospital length of stay in patients undergoing major elective surgery. Ann Surg. 2010;251(3):535–541. 20134313
22. Adam, R., Frilling, A., Elias, D., et al, Liver resection of colorectal metastases in elderly patients. Br J Surg. 2010;97(3):366–376. 20101645
23. de LiguoriCarino, N., van Leeuwen, B.L., Ghaneh, P., et al, Liver resection for colorectal liver metastases in older patients. Crit Rev Oncol Hematol. 2008;67(3):273–278. 18595728
24. Minagawa, M., Makuuchi, M., Torzilli, G., et al, Extension of the frontiers of surgical indications in the treatment of liver metastases from colorectal cancer: long-term results. Ann Surg. 2000;231(4):487–499. 10749608
25. Elias, D., Liberale, G., Vernerey, D., et al, Hepatic and extrahepatic colorectal metastases: when resectable, their localization does not matter, but their total number has a prognostic effect. Ann Surg Oncol. 2005;12(11):900–909. 16184442
26. Van Cutsem, E., Nordlinger, B., Adam, R., et al, Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer. 2006;42(14):2212–2221. 16904315
27. Malde, D.J., Khan, A., Prasad, K.R., et al, Inferior vena cava resection with hepatectomy: challenging but justified. HPB (Oxford). 2011;13(11):802–810. 21999594
28. Carpizo, D.R., Are, C., Jarnagin, W., et al, Liver resection for metastatic colorectal cancer in patients with concurrent extrahepatic disease: results in 127 patients treated at a single center. Ann Surg Oncol. 2009;16(8):2138–2146. 19495884
29. Hemming, A.W., Reed, A.I., Langham, M.R., et al, Hepatic vein reconstruction for resection of hepatic tumors. Ann Surg. 2002;235(6):850–858. 12035042
30. Abulkhir, A., Limongelli, P., Healey, A.J., et al, Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg. 2008;247(1):49–57. 18156923
31. Ribero, D., Abdalla, E.K., Madoff, D.C., et al, Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg. 2007;94(11):1386–1394. 17583900
32. Chun, Y.S., Vauthey, J.N., Extending the frontiers of resectability in advanced colorectal cancer. Eur J Surg Oncol. 2007;33(Suppl. 2):S52–S58. 18006265
33. Covey, A.M., Brown, K.T., Jarnagin, W.R., et al, Combined portal vein embolization and neoadjuvant chemotherapy as a treatment strategy for resectable hepatic colorectal metastases. Ann Surg. 2008;247(3):451–455. 18376189
34. Adam, R., Delvart, V., Pascal, G., et al, Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240(4):644–658. 15383792
35. Jaeck, D., Oussoultzoglou, E., Rosso, E., et al, A two-stage hepatectomy procedure combined with portal vein embolization to achieve curative resection for initially unresectable multiple and bilobar colorectal liver metastases. Ann Surg. 2004;240(6):1037–1051. 15570209
36. Antoniou, A., Lovegrove, R.E., Tilney, H.S., et al, Meta-analysis of clinical outcome after first and second liver resection for colorectal metastases. Surgery. 2007;141(1):9–18. 17188163
37. de Jong, M.C., Mayo, S.C., Pulitano, C., et al, Repeat curative intent liver surgery is safe and effective for recurrent colorectal liver metastasis: results from an international multi-institutional analysis. J Gastrointest Surg. 2009;13(12):2141–2151. 19795176
38. Kondo, S., Hirano, S., Ambo, Y., et al, Arterioportal shunting as an alternative to microvascular reconstruction after hepatic artery resection. Br J Surg. 2004;91(2):248–251. 14760676
39. Azoulay, D., Andreani, P., Maggi, U., et al, Combined liver resection and reconstruction of the supra-renal vena cava: the Paul Brousse experience. Ann Surg. 2006;244(1):80–88. 16794392
40. Hemming, A.W., Reed, A.I., Langham, M.R., et al, Combined resection of the liver and inferior vena cava for hepatic malignancy. Ann Surg. 2004;239(5):712–721. 15082976
41. Lodge, J.P., Ammori, B.J., Prasad, K.R., et al, ex vivo and in situ resection of inferior vena cava with hepatectomy for colorectal metastases. Ann Surg. 2000;231(4):471–479. 10749606
42. Yedibela, S., Klein, P., Feuchter, K., et al, Surgical management of pulmonary metastases from colorectal cancer in 153 patients. Ann Surg Oncol. 2006;13(11):1538–1544. 17009154
43. Shiono, S., Ishii, G., Nagai, K., et al, Predictive factors for local recurrence of resected colorectal lung metastases. Ann Thorac Surg. 2005;80(3):1040–1045. 16122482
44. Pfannschmidt, J., Muley, T., Hoffmann, H., et al, Prognostic factors and survival after complete resection of pulmonary metastases from colorectal carcinoma: experiences in 167 patients. J Thorac Cardiovasc Surg. 2003;126(3):732–739. 14502146
45. Saito, Y., Omiya, H., Kohno, K., et al, Pulmonary metastasectomy for 165 patients with colorectal carcinoma: a prognostic assessment. J Thorac Cardiovasc Surg. 2002;124(5):1007–1013. 12407386
46. Watanabe, K., Nagai, K., Kobayashi, A., et al, Factors influencing survival after complete resection of pulmonary metastases from colorectal cancer. Br J Surg. 2009;96(9):1058–1065. 19672932
47. Kanzaki, R., Higashiyama, M., Oda, K., et al, Outcome of surgical resection for recurrent pulmonary metastasis from colorectal carcinoma. Am J Surg. 2011;202(4):419–426. 21824604
48. Adam, R., de Haas, R.J., Wicherts, D.A., et al, Concomitant extrahepatic disease in patients with colorectal liver metastases: when is there a place for surgery? Ann Surg. 2011;253(2):349–359. 21178761
49. Pulitanò, C., Bodingbauer, M., Aldrighetti, L., et al, Liver resection for colorectal metastases in presence of extrahepatic disease: results from an international multi-institutional analysis. Ann Surg Oncol. 2011;18(5):1380–1388. 21136180
50. Kooby, D.A., Stockman, J., Ben-Porat, L., et al, Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg. 2003;237(6):860–870. 12796583
51. Lesurtel, M., Selzner, M., Petrowsky, H., et al, How should transection of the liver be performed? A prospective randomized study in 100 consecutive patients: comparing four different transection strategies. Ann Surg. 2005;242(6):814–823. 16327491
52. Gurusamy, K.S., Pamecha, V., Sharma, D., et al, Techniques for liver parenchymal transection in liver resection. Cochrane Database Syst Rev. 2009;(1) CD006880. 19160307
53. Lupo, L., Gallerani, A., Panzera, P., et al, Randomized clinical trial of radiofrequency-assisted versus clamp-crushing liver resection. Br J Surg. 2007;94(3):287–291. 17318804
54. Figueras, J., Llado, L., Miro, M., et al, Application of fibrin glue sealant after hepatectomy does not seem justified: results of a randomized study in 300 patients. Ann Surg. 2007;245(4):536–542. 17414601
55. Simillis, C., Constantinides, V.A., Tekkis, P.P., et al, Laparoscopic versus open hepatic resections for benign and malignant neoplasms – a meta-analysis. Surgery. 2007;141(2):203–211. 17263977
56. Abu Hilal, M., Di Fabio, F., Abu Salameh, M., et al, Oncological efficiency analysis of laparoscopic liver resection for primary and metastatic cancer: a single-center UK experience. Arch Surg. 2012;147(1):42–48. 22250111
57. Buell, J.F., Cherqui, D., Geller, D.A., et al, The international position on laparoscopic liver surgery: the Louisville Statement, 2008. Ann Surg. 2009;250(5):825–830. 19916210
58. Simmonds, P.C., Primrose, J.N., Colquitt, J.L., et al, Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer. 2006;94(7):982–999. 16538219
59. Poston, G.J., Figueras, J., Giuliante, F., et al, Urgent need for a new staging system in advanced colorectal cancer. J Clin Oncol. 2008;26(29):4828–4833. 18711170
60. Lazorthes, F., Navarro, F., Ychou, M., ANAES, Therapeutic management of hepatic metastases from colorectal cancers. Gastroenterol Clin Biol 2003; 27 Spec No 2B7. 12637870
61. Poston, G., Adam, R., Vauthey, J.-N., Downstaging or downsizing: time for a new staging system in advanced colorectal cancer? J Clin Oncol. 2006;24(18):2702–2706. 16782909
62. Nordlinger, B., Guiguet, M., Vaillant, J.C., et al, Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer. 1996;77(7):1254–1262. 8608500
63. Rees, M., Tekkis, P.P., Welsh, F.K.S., et al, Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247(1):125–135. 18156932
64. Zakaria, S., Donohue, J.H., Que, F.G., et al, Hepatic resection for colorectal metastases: value for risk scoring systems? Ann Surg. 2007;246(2):183–191. 17667495
65. Merkel, S., Bialecki, D., Meyer, T., et al, Comparison of clinical risk scores predicting prognosis after resection of colorectal liver metastases. J Surg Oncol. 2009;100(5):349–357. 19572329
66. Kopetz, S., Chang, G.J., Overman, M.J., et al, Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27(22):3677–3683. 19470929
67. Van Cutsem, E., Köhne, C.-H., Hitre, E., et al, Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408–1417. 19339720
68. Roock, W.D., Claes, B., Bernasconi, D., et al, Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11(8):753–762. 20619739
69. Ellis, L.M., Mechanisms of action of bevacizumab as a component of therapy for metastatic colorectal cancer. Semin Oncol. 2006;33(5, Suppl. 10):S1–S7. 17145519
70. Folprecht, G., Grothey, A., Alberts, S., et al, Neoadjuvant treatment of unresectable colorectal liver metastases: correlation between tumour response and resection rates. Ann Oncol. 2005;16(8):1311–1319. 15870084
71. Falcone, A., Ricci, S., Brunetti, I., et al, Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the GruppoOncologico Nord Ovest. J Clin Oncol. 2007;25(13):1670–1676. 17470860
72. Bokemeyer, C., Bondarenko, I., Makhson, A., et al, Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27(5):663–671. 19114683
73. Van Cutsem, E., Köhne, C.-H., Láng, I., et al, Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29(15):2011–2019. 21502544
74. Folprecht, G., Gruenberger, T., Bechstein, W.O., et al, Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 2010;11(1):38–47. 19942479
75. Garufi, C., Torsello, A., Tumolo, S., et al, Cetuximab plus chronomodulated irinotecan, 5-fluorouracil, leucovorin and oxaliplatin as neoadjuvant chemotherapy in colorectal liver metastases: POCHER trial. Br J Cancer. 2010;103(10):1542–1547. 20959822
76. Van Cutsem, E., Rivera, F., Berry, S., et al, Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol. 2009;20(11):1842–1847. 19406901
77. Masi, G., Loupakis, F., Salvatore, L., et al, Bevacizumab with FOLFOXIRI (irinotecan, oxaliplatin, fluorouracil, and folinate) as first-line treatment for metastatic colorectal cancer: a phase 2 trial. Lancet Oncol. 2010;11(9):845–852. 20702138
78. Portier, G., Elias, D., Bouche, O., et al, Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol. 2006;24(31):4976–4982. 17075115
79. Nordlinger, B., Sorbye, H., Glimelius, B., et al, Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371(9617):1007–1016. 18358928
80. Reddy, S.K., Zorzi, D., Lum, Y.W., et al, Timing of multimodality therapy for resectable synchronous colorectal liver metastases: a retrospective multi-institutional analysis. Ann Surg Oncol. 2009;16(7):1809–1819. 18979139
81. Mitry, E., Fields, A.L.A., Bleiberg, H., et al, Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. J Clin Oncol. 2008;26(30):4906–4911. 18794541
82. Nordlinger, B., Van Cutsem, E., Gruenberger, T., et al, Combination of surgery and chemotherapy and the role of targeted agents in the treatment of patients with colorectal liver metastases: recommendations from an expert panel. Ann Oncol. 2009;20(6):985–992. 19153115
83. Rubbia-Brandt, L., Giostra, E., Brezault, C., et al, Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol. 2007;18(2):299–304. 17060484
84. Blazer, D.G., Kishi, Y., Maru, D.M., et al, Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008;26(33):5344–5351. 18936472
85. Adam, R., Wicherts, D.A., de Haas, R.J., et al, Complete pathologic response after preoperative chemotherapy for colorectal liver metastases: myth or reality? J Clin Oncol. 2008;26(10):1635–1641. 18375892
86. O’Connell, J.B., Maggard, M.A., Ko, C.Y., Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96(19):1420–1425. 15467030
87. Benoist, S., Brouquet, A., Penna, C., et al, Complete response of colorectal liver metastases after chemotherapy: does it mean cure? J Clin Oncol. 2006;24(24):3939–3945. 16921046
88. Vauthey, J.-N., Pawlik, T.M., Ribero, D., et al, Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24(13):2065–2072. 16648507
89. Rubbia-Brandt, L., Audard, V., Sartoretti, P., et al, Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15(3):460–466. 14998849
90. Aloia, T.A., Vauthey, J.-N., Loyer, E.M., et al, Solitary colorectal liver metastasis: resection determines outcome. Arch Surg. 2006;141(5):460–467. 16702517
91. Fernandez, F.G., Ritter, J., Goodwin, J.W., et al, Effect of steatohepatitis associated with irinotecan or oxaliplatin pretreatment on resectability of hepatic colorectal metastases. J Am Coll Surg. 2005;200(6):845–853. 15922194
92. Chun, Y.S., Vauthey, J.-N., Boonsirikamchai, P., et al, Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA. 2009;302(21):2338–2344. 19952320
93. Karoui, M., Penna, C., Amin-Hashem, M., et al, Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg. 2006;243(1):1–7. 16371728
94. Welsh, F.K.S., Tilney, H.S., Tekkis, P.P., et al, Safe liver resection following chemotherapy for colorectal metastases is a matter of timing. Br J Cancer. 2007;96(7):1037–1042. 17353923
95. Chaudhury, P., Hassanain, M., Bouganim, N., et al, Perioperative chemotherapy with bevacizumab and liver resection for colorectal cancer liver metastasis. HPB (Oxford). 2010;12(1):37–42. 20495643
96. Kesmodel, S.B., Ellis, L.M., Lin, E., et al, Preoperative bevacizumab does not significantly increase postoperative complication rates in patients undergoing hepatic surgery for colorectal cancer liver metastases. J Clin Oncol. 2008;26(32):5254–5260. 18854565
97. Mocellin, S., Pilati, P., Lise, M., et al, Meta-analysis of hepatic arterial infusion for unresectable liver metastases from colorectal cancer: the end of an era? J Clin Oncol. 2007;25(35):5649–5654. 18065736
98. Allen, P.J., Nissan, A., Picon, A.I., et al, Technical complications and durability of hepatic artery infusion pumps for unresectable colorectal liver metastases: an institutional experience of 544 consecutive cases. J Am Coll Surg. 2005;201(1):57–65. 15978444
99. Kemeny, N.E., Melendez, F.D.H., Capanu, M., et al, Conversion to resectability using hepatic artery infusion plus systemic chemotherapy for the treatment of unresectable liver metastases from colorectal carcinoma. J Clin Oncol. 2009;27(21):3465–3471. 19470932
100. Shitara, K., Munakata, M., Kudo, T., et al. Combination chemotherapy with hepatic arterial infusion of 5-fluorouracil (5-FU) and systemic irinotecan (CPT-11) in patients with unresectable liver metastases from colorectal cancer. GanTo Kagaku Ryoho. 2006; 33(13):2033–2037.
101. Gallagher, D.J., Capanu, M., Raggio, G., et al, Hepatic arterial infusion plus systemic irinotecan in patients with unresectable hepatic metastases from colorectal cancer previously treated with systemic oxaliplatin: a retrospective analysis. Ann Oncol. 2007;18(12):1995–1999. 17962209
102. House, M.G., Kemeny, N.E., Gönen, M., et al, Comparison of adjuvant systemic chemotherapy with or without hepatic arterial infusional chemotherapy after hepatic resection for metastatic colorectal cancer. Ann Surg. 2011;254(6):851–856. 21975318
103. Martin, R.C.G., Howard, J., Tomalty, D., et al, Toxicity of irinotecan-eluting beads in the treatment of hepatic malignancies: results of a multi-institutional registry. Cardiovasc Intervent Radiol. 2010;33(5):960–966. 20661569
104. Martin, R.C.G., Joshi, J., Robbins, K., et al, Hepatic intra-arterial injection of drug-eluting bead, irinotecan (DEBIRI) in unresectable colorectal liver metastases refractory to systemic chemotherapy: results of multi-institutional study. Ann Surg Oncol. 2011;18(1):192–198. 20740319
105. Gulec, S.A., Fong, Y., Yttrium 90 microsphere selective internal radiation treatment of hepatic colorectal metastases. Arch Surg. 2007;142(7):675–682. 17638807
106. Hoffmann, R.T., Jakobs, T.F., Kubisch, C.H., et al, Radiofrequency ablation after selective internal radiation therapy with Yttrium90 microspheres in metastatic liver disease – Is it feasible? Eur J Radiol. 2010;74(1):199–205. 19269763
107. Cosimelli, M., Golfieri, R., Cagol, P.P., et al, Multi-centre phase II clinical trial of yttrium-90 resin microspheres alone in unresectable, chemotherapy refractory colorectal liver metastases. Br J Cancer. 2010;103(3):324–331. 20628388
108. Wong, S.L., Mangu, P.B., Choti, M.A., et al, American Society of Clinical Oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. J Clin Oncol. 2010;28(3):493–508. 19841322
109. Oshowo, A., Gillams, A., Harrison, E., et al, Comparison of resection and radiofrequency ablation for treatment of solitary colorectal liver metastases. Br J Surg. 2003;90(10):1240–1243. 14515293
110. Jansen, M.C., van Duijnhoven, F.H., van Hillegersberg, R., et al, Adverse effects of radiofrequency ablation of liver tumours in the Netherlands. Br J Surg. 2005;92(10):1248–1254. 15997440
111. Ruers, T., van Coevorden, F., Pierie, J., et al. Radiofrequency ablation (RFA) combined with chemotherapy for unresectable colorectal liver metastases (CRC LM): interim results of a randomised phase II study of the EORTC-NCRI CCSG-ALM Intergroup 40004 (CLOCC). ASCO Meeting Abstr. 2008; 26(Suppl. 15):4012.
112. Mulier, S., Ni, Y., Jamart, J., et al, Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005;242(2):158–171. 16041205
113. Pathak, S., Jones, R., Tang, J.M., et al, Ablative therapies for colorectal liver metastases (CRLM): a systematic review. Colorectal Dis. 2011;13(9):e252–e265. 21689362
114. Simon, C.J., Dupuy, D.E., Mayo-Smith, W.W., Microwave ablation: principles and applications. Radiographics. 2005;25(Suppl. 1):S69–S83. 16227498
115. Jones, R.P., Kitteringham, N.R., Terlizzo, M., et al, Microwave ablation of ex vivo human liver and colorectal liver metastases with a novel 14.5 GHz generator. Int J Hyperthermia. 2012;28(1):43–54. 22235784
116. Morris, E.J., Forman, D., Thomas, J.D., et al, Surgical management and outcomes of colorectal cancer liver metastases. Br J Surg. 2010;97(7):1110–1118. 20632280
117. Poston, G.J., Adam, R., Alberts, S., et al, OncoSurge; a strategy for improving resectability with curative intent in metastatic colorectal cancer. J Clin Oncol 2005; 23:7125–7134. 16192596