Chapter 35 Colorectal Cancer Screening and Surveillance
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer death in North America and Western Europe. Worldwide, there are more than 1 million new cases per year and more than 500,000 deaths. In the United States, 146,970 new cases and nearly 50,000 deaths were estimated to occur in 2009, representing about 14% of cancer deaths.1 In their lifetime, 5% to 6% of adults develop CRC.
Population-based screening can be costly and ineffective. Ideal screening employs relatively simple, inexpensive tests to risk-stratify patients followed by sensitive tests directed at individuals with highest risk. The criteria for population-based screening include factors summarized in Table 35.1. There is compelling evidence that population-based screening can reduce the incidence of and mortality from CRC.2–5 Although there is considerable variation in screening recommendations in high-risk countries, some form of screening is recommended in Europe, Canada, Australia, and the United States.
| Criteria | Colorectal Cancer |
|---|---|
| Disease is common | 5%-6% lifetime risk |
| Early detection can prevent mortality | 5-yr survival |
| Stage I: near 100% | |
| Stage II: 80% | |
| Stage III: 30%-70% | |
| Stage IV: 10% | |
| Screening methods are shown to be effective | See text |
| FOBT RCTs | |
| Sigmoidoscopy and colonoscopy case-control studies | |
| Resources are available to provide screening in U.S. | FOBT: Yes; primary care settingSigmoidoscopy: Yes; primary care or specialty clinicCT colonography: No; limited centers and fully trained radiologistsColonoscopy: Uncertain capacity (see text) |
| Resources are available to provide diagnostic tests for patients with positive screening | Colonoscopy resources are generally available if initial screening test positive |
| Screening is cost-effective | Models show cost-effectiveness |
| Screening methods are accepted by patients and providers | Yes: 50% adherence in U.S. |
CT, computed tomography; FOBT, fecal occult blood test; RCT, randomized controlled trial.
Since 1985, there has been a slow but steady decline in CRC incidence in the United States. During this time period, screening rates have gradually increased to more than 50% of the U.S. population older than 50 years. From 2000–2005, the incidence of CRC declined by 6% in men and 9.4% in women. The annual percent reduction in mortality from 2002–2004 was 4.7%.1
Epidemiology of Colorectal Cancer
Age, Gender, and Race and Ethnicity
CRC represents the third most common form of cancer (excluding skin) among men and women and is the second leading cause of cancer death overall in the United States. There is a progressive increase in risk associated with age (Table 35.2). Age-adjusted incidence and mortality of CRC are higher in men. There is some evidence that natural premenopausal hormones or postmenopausal hormone replacement therapy may exert some protective effect in women.6,7
Table 35.2 Probability of Invasive Colorectal Cancer by Age and Gender
| Male | Female | |
|---|---|---|
| <40 yr | 0.08 (1/1329) | 0.07 (1/1394) |
| 40–59 yr | 0.92 (1/109) | 0.72 (1/138) |
| 60–69 yr | 1.60 (1/63) | 1.12 (1/89) |
| ≥70 yr | 4.78 (1/21) | 4.30 (1/23) |
| Lifetime | 5.65 (1/18) | 5.23 (1/19) |
| Risk of death | 2.45 (1/41) | 2.45 (1/41) |
Data from Jemal A, Siegel R, Ward E, et al: Cancer statistics 2009. CA Cancer J Clin 59:225–249, 2009.
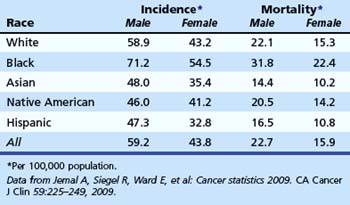
There is strong evidence that African American men and women are more likely to die from CRC than whites (Table 35.3).1 African Americans are more likely to have advanced lesions at the time of diagnosis8 and are more likely to have proximal CRC compared with other races.9,10 The reasons for increased mortality in African Americans are unknown. Delays in diagnosis could be due to socioeconomic status or poor access to health care, although some compelling data suggest that there are biologic differences.11,12 In a study of white and African American patients undergoing screening colonoscopy, African American men and women had higher age-adjusted prevalence of polyps larger than 9 mm.8 These data support the hypothesis of biologic differences.
Lifestyle and Medications
There is strong epidemiologic evidence that lifestyle factors have an impact on CRC risk. Diets that are high in fat and low in fiber and calcium may be associated with increased risk. Obesity, metabolic syndrome, low levels of physical activity, tobacco smoking, and heavy alcohol consumption have been associated with an increased risk of CRC. There is no evidence that modification of these factors reduces CRC risk; however, changes in most of these behaviors are generally good for overall health. There is strong evidence that individuals taking nonsteroidal antiinflammatory drugs and aspirin have a lower risk of developing colon adenomas and CRC.13 However, these drugs have significant adverse effects, and are not recommended for routine CRC prevention.
Polyps and Colorectal Cancer Risk
Most CRC develops from adenoma precursors. Adenoma prevalence increases steadily with age and exceeds 35% in adults older than 75 years. There is a 5% to 6% lifetime risk of CRC, so most patients with adenomas apparently do not develop clinically detectable cancer during their lifetime. Polyp size and histology are directly associated with malignant risk. The risk of high-grade dysplasia in polyps smaller than 5 mm is 1.1%; for polyps 5 to 9 mm, the risk is 4.6%; and in polyps larger than 9 mm, the risk is 20%.14 The risk of invasive cancer is less than 1% in polyps smaller than 1 cm and greater than 10% in larger polyps.15 These data suggest that patients most likely to develop cancer are patients with advanced adenomas. Evidence from the National Polyp Study16 supports the hypothesis that detection and removal of adenomas may prevent cancer incidence. All of these data have important implications for screening. Screening efforts directed at advanced adenomas would target patients with the greatest risk and potentially lead to significant reductions in cancer incidence.
Pathogenesis
Several genetic pathways may result in the development of adenomas and CRC. In 1990, a stepwise model of chromosomal instability was proposed by Fearon and Vogelstein.17 More than 80% of sporadic CRC is associated with mutation of the adenomatous polyposis coli (APC) gene on chromosome 5. The mutation of this tumor suppressor gene most likely promotes the development of adenomas. In familial adenomatous polyposis (FAP), there is a germline mutation of this gene. Normally, this gene acts to phosphorylate beta-catenin, which leads to its destruction. Accumulation of beta-catenin leads to unregulated cell proliferation and suppression of apoptosis.18,19 The progression of adenoma to cancer seems to require additional mutations (K-ras, chromosome 18, TP53). The observation that not all tumors acquire the same mutations strongly suggests that there are several genetic pathways to malignant invasion.
A second genetic pathway (15% to 20%) of sporadic cancer is due to mutation of mismatch repair genes.20 These genes normally repair errors in DNA replication. Germline mutations are found in patients with hereditary nonpolyposis colorectal cancer syndrome (HNPCC). The key feature of this mutation in HNPCC is rapid development of polyps that progress to malignancy. In sporadic cancers, acquired mutation of these genes leads to microsatellite instability and malignant progression. Patients with large serrated adenomas in the proximal colon may have BRAF mutations and hypermethylation, which may result in microsatellite instability and progression to malignancy.21
Another germline mutation in a base-excision repair gene, MYH, has been associated with recessive inheritance of multiple colorectal adenomas. The typical phenotype is a patient with 15 or more adenomas and no germline APC mutation.22
High-Risk Groups
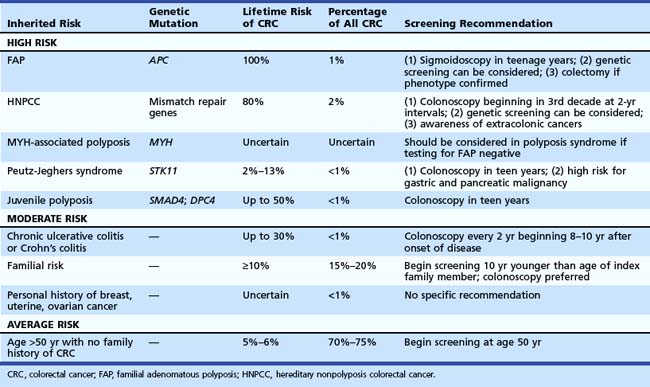
Recognition of inherited syndromes and other high-risk diseases associated with CRC is key to appropriate screening and management (Table 35.4). It is important for primary care providers to recognize these syndromes and refer patients for appropriate screening and surveillance.
Perhaps the most important questions in the medical history are the following: (1) “Do you have a first-degree relative with CRC?” (2) “If yes, did any relative have cancer before age 50 years?” Patients with an index relative younger than 50 years old should be considered at risk for an inherited syndrome and warrant intensive screening at a young age. Recommendations for specific syndromes are summarized in Table 35.4.
Inherited Polyposis Syndromes
Patients with FAP have a germline mutation of APC on chromosome 5, predisposing them to adenoma formation. Most affected patients have more than 100 adenomas. All affected individuals (100%) develop CRC, and when the phenotype is recognized, colectomy should be considered. The average age of adenoma appearance is 16 years, and the age of CRC appearance is 39 years. In a variant of this syndrome, called attenuated FAP, mutations occur at either the 5′ or 3′ end of the gene. Phenotypically, patients have fewer polyps, with delayed onset of adenoma and cancer formation. Familial colon cancer in Ashkenazi Jews may be the result of a specific germline mutation of the APC gene (I1307K). This mutation seems to predispose to sporadic mutations at distant sites resulting in a high malignant risk.19
MYH-associated polyposis leads to development of multiple polyps that may be adenomatous or hyperplastic. Mutations of the MutY human homolog (MYH) gene, which encodes a member of the base excision repair system, occur. This system normally protects cells against the mutagenic effects of aerobic metabolism. Inheritance is autosomal recessive and should be considered in patients with 10 to 100 adenomas or hyperplastic polyps who have negative genetic testing for FAP.22
Hereditary Nonpolyposis Colorectal Cancer Syndrome
HNPCC accounts for about 2% of all colon cancers. Affected individuals inherit one of several mismatch repair gene mutations. There is evidence that regular screening of kindreds can substantially reduce the risk of CRC.23 The clinical definitions have been modified over the years from the original Amsterdam criteria24 to the Bethesda guidelines,25 which provide a much less rigid clinical definition. Clinical suspicion should be high if there is a family member with CRC before age 50, multiple generations with CRC, and relatives with HNPCC-associated cancers (endometrium, small bowel, ureter, or renal pelvis). The findings of the Finnish Cancer Registry26 reinforce the need to be aware of other cancers that may develop in these kindreds, including endometrial (60%); stomach (13%); ovary (12%); bladder, urethra, and ureter (4.0%); brain (3.7%); kidney (3.3%); and biliary tract and gallbladder (2.0%).
Familial Risk
Epidemiologic data27,28 show that individuals with a first-degree relative with CRC have an increased risk of CRC. Twin studies estimated that 35% of CRC arose from inherited factors and 65% arose from environmental factors.29 A meta-analysis considered risk associated with familial risk.30 The relative risk of CRC with an affected first-degree relative was 2.4. With more than one relative, the risk was 4.2. If CRC was diagnosed before age 45 years in the index family member, the relative risk was 3.8; diagnosis at age 45 to 59 years was associated with a relative risk of 2.2. If diagnosis was made after age 59 years, the relative risk was 1.8. The risk associated with second-degree relatives is less certain because this is difficult to study. An analysis from the Utah registry found a risk of 1.5 for patients with second-degree relatives with CRC.
Based on these data, family members should be screened with colonoscopy. If the index family member had cancer after age 60 years, screening should be initiated at age 50 years with colonoscopy and performed every 10 years if colonoscopy is negative. If the family member had cancer before age 60 years, screening should be initiated with colonoscopy at age 40 and then performed every 5 years. There is also evidence that family members of patients discovered to have adenomas before age 60 years may be at increased risk for CRC,31 although this risk may be increased only if the index family member had advanced adenomas.32
Ulcerative Colitis and Crohn’s Colitis
Although patients with inflammatory bowel disease represent less than 1% of all patients who develop CRC, patients with colitis represent a high-risk group. Risk is strongly associated with extent and duration of disease. The risk is very low in the first 8 years of disease. However, in patients with pancolitis, the risk increases by up to 0.5% to 1% per year so that after 35 years of disease, the cumulative risk of CRC may be 35%.33 Patients with severe long-standing disease and primary sclerosing cholangitis may have a higher risk of developing CRC.34
There are no randomized clinical trials to evaluate surveillance. There is some evidence that survival is better in patients enrolled in surveillance.35 Recommendations to perform colonoscopy every 1 to 2 years for life are based on the steadily increasing cumulative risk of CRC with time. Patients with colitis limited to the rectum and left colon have a lower risk. Surveillance beginning at age 15 years is recommended.
Screening Strategies for Average-Risk Individuals
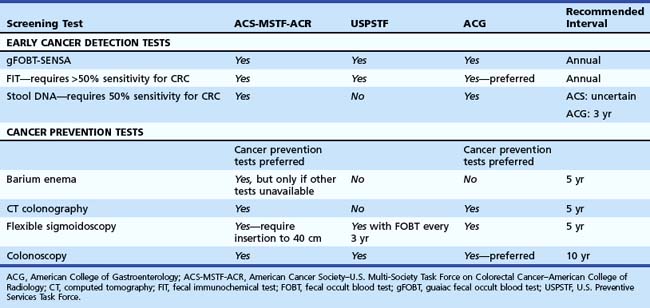
Screening recommendations for average-risk asymptomatic individuals vary around the world. In Asia, there are no specific guidelines. In Europe and Canada, most countries offer fecal occult blood test (FOBT), and some offer sigmoidoscopy (United Kingdom, Italy, Norway) or colonoscopy (Germany, Austria, Poland, Italy). In the United States, three guidelines2–5 were published in 2008 and 2009 (Table 35.5).
Cancer screening is traditionally focused on early cancer detection (i.e., breast, prostate). However, because most CRC develops from adenoma precursors, there is an opportunity to prevent many cancers if precursor lesions are detected and removed.16 More recent guidelines in the United States have preferred the use of tests that are likely to detect polyps and prevent cancer.2,5
Early Cancer Detection Tests
Fecal Occult Blood Test
Three randomized controlled trials showed that among patients who undergo FOBT compared with unscreened controls, cancers are discovered at an earlier stage, and mortality is reduced by 15% to 33%.36–38 Standard guaiac fecal occult blood test (gFOBT) detects peroxidase activity of heme and is not specific for human blood. One-time testing with a standard guaiac test detects only 33% to 50% of patients with cancer (Table 35.6). A more sensitive guaiac test (SENSA) can detect more than 60% of patients with cancer (see Table 35.6).2,4,39 Three separate stool samples per test have superior sensitivity compared with one or two samples. Patients with positive tests have a threefold to fourfold increased risk of cancer and should be referred for colonoscopy.
Table 35.6 Sensitivity (One-Time Test) of Colorectal Cancer Screening Tests
| Cancer | Advanced Adenomas* | |
|---|---|---|
| STOOL-BASED TESTS | ||
| Standard gFOBT39,42,43 | 33%–50% | 11% |
| Sensitive gFOBT2,4,39 | 50%–75% | 20%–25% |
| Fecal immunochemical test3,5 | 60%–85% | 20%–50% |
| Stool DNA—old42 | 51% | 18% |
| Stool DNA—new43,44 | ≥80% | 40% |
| STRUCTURAL EXAMINATIONS OF COLON | ||
| CT colonography48 | Uncertain; likely >90% | 90% if >10 mm |
| Sigmoidoscopy55,57,58 | >95% distal colon | 70%† |
| Colonoscopy45–47 | >95% | 88%–98% |
CT, computed tomography; gFOBT, guaiac fecal occult blood test.
* Advanced adenoma defined as tubular adenoma ≥10 mm; adenoma with villous histology or high-grade dysplasia.
† Assumes that if an adenoma is detected in the distal colon, the patient would have complete colonoscopy, which would result in detection of some proximal advanced adenomas.
From Lieberman D: Screening for colorectal cancer. N Engl J Med 361:1179–1187, 2009.
Fecal immunochemical test uses antibodies specific to human hemoglobin, albumin, or other blood components and is more specific for human blood. There are many commercially available tests, most of which detect more than 50% of cancers with one-time testing. There are several uncertainties, including the number of stool samples, proper preservation of samples while awaiting testing, and the performance characteristics of the test in clinical practice.39,40 It is unknown if fecal immunochemical test is superior to a less costly sensitive gFOBT.
Fecal blood testing has many important limitations. The detection rate for advanced adenomas is only 25% to 30%. One-time testing fails to identify most patients with important cancer precursor lesions. An effective program requires annual repeat testing for negative tests and referral for colonoscopy if tests are positive. Adherence in clinical practice is uncertain.41 Although the initial cost of testing is low, efforts to ensure adherence to repeat testing, referral for colonoscopy, and the management of detected cancers contribute to the programmatic cost. Patients should understand the limitations of the test and the fact that many cancers and high-risk adenomas go undetected.
Stool DNA Test
Gene mutations associated with colonic neoplasia can be detected in stool samples. Proof of principle studies in a large screening cohort showed that more than 50% of cancers could be detected.42 New versions of the test are promising but have not yet been evaluated in screening cohorts.43,44 The stool DNA test fails to identify most patients with advanced neoplasia and should be viewed as an early cancer detection test.
Imaging Studies of the Colon
Barium enema accurately identifies late stage cancer but is a poor test for important cancer precursor lesions and is rarely used for colon cancer screening today. Imaging with computed tomography (CT) colonography has largely replaced barium enema. Helical CT scans render two-dimensional and three-dimensional images of the colon.45–48 CT colonography can identify correctly 90% of patients with polyps 10 mm or larger with a false-positive rate of 14% when performed by properly trained radiologists.48 Quality in other clinical practice settings is unknown.
CT colonography is a new technology with many uncertainties, as follows:
 Who should be referred for colonoscopy? There is consensus that all patients with polyps 6 mm or larger should be offered colonoscopy.2 The management of patients with polyps 1 to 5 mm is controversial. CT colonography cannot accurately identify polyps of this size. Less than 2% of these patients have adenomas with advanced features, and cancer is rare.49,50 No studies have shown the safety of following such patients with repeat CT colonography.
Who should be referred for colonoscopy? There is consensus that all patients with polyps 6 mm or larger should be offered colonoscopy.2 The management of patients with polyps 1 to 5 mm is controversial. CT colonography cannot accurately identify polyps of this size. Less than 2% of these patients have adenomas with advanced features, and cancer is rare.49,50 No studies have shown the safety of following such patients with repeat CT colonography. Appropriate screening intervals after negative examinations or with possible polyps smaller than 6 mm are uncertain.
Appropriate screening intervals after negative examinations or with possible polyps smaller than 6 mm are uncertain. Radiation exposure could increase the risk of developing cancers.52 Although low-dose regimens are used, there is concern about cumulative x-ray exposure, and some countries do not allow imaging for screening purposes.
Radiation exposure could increase the risk of developing cancers.52 Although low-dose regimens are used, there is concern about cumulative x-ray exposure, and some countries do not allow imaging for screening purposes.Flexible Sigmoidoscopy
Case-control studies have found significant reduction in CRC mortality in patients who have undergone sigmoidoscopy—a benefit limited to the portion of the colon examined.53 One small randomized trial showed a reduction in CRC incidence in patients exposed to sigmoidoscopy.54 Screening colonoscopy studies show that sigmoidoscopy would fail to identify 30% or more of individuals with advanced neoplasia. There is evidence that sigmoidoscopy may be less effective with advancing age (>60 years old) because of higher prevalence of proximal neoplasia.55 Large studies of sigmoidoscopy in the United States, United Kingdom, and Italy are not yet completed. Despite evidence of effectiveness, use of flexible sigmoidoscopy in the United States has declined over the past 10 years, coincident with the growth of screening colonoscopy.
Combined Flexible Sigmoidoscopy and Fecal Occult Blood Test
Although several cost-effectiveness analyses endorse the use of combined FOBT (annually) and sigmoidoscopy (every 5 years), there is little clinical experience with combined screening tests. The VA Cooperative Study56 found that the combination of one-time sigmoidoscopy and FOBT would identify 76% of patients with advanced neoplasia, which represented only a small incremental improvement over sigmoidoscopy alone. There are no data regarding adherence to this complex screening program and no evidence that it is being used in clinical practice.
Colonoscopy
Colonoscopy is the most commonly used screening test in the United States. Worldwide experience has shown feasibility in different populations in North America, Asia, and Europe.55,57–60 Among patients age 50 to 75 years being screened for the first time, 5% to 10% have advanced neoplasia, and 0.5% to 1.0% have cancer. Evidence from the National Polyp Study showed that detection and removal of adenomas could result in substantial reduction in incidence of CRC.16 Case-control studies have shown that in patients exposed to colonoscopy, both CRC incidence and mortality are reduced.61–63
 Interval cancers may occur after colonoscopy. In studies of patients with adenomas who had colonoscopy and polypectomy, 0.3% to 0.9% of patients developed cancer within 2 to 5 years of colonoscopy. There are several possible reasons. New fast-growing lesions with microsatellite instability may account for some interval lesions. Incomplete removal of neoplasia at baseline could lead to development of cancer at a prior polypectomy site. However, lesions missed at baseline colonoscopy represent the most common explanation in most interval cancers. Studies using CT colonography to evaluate optical colonoscopy showed that colonoscopy may miss 2% to 12% of polyps 10 mm or larger.45–47 Flat lesions (see Chapter 36) may be difficult to detect at colonoscopy or incompletely visualized without chromoendoscopy.
Interval cancers may occur after colonoscopy. In studies of patients with adenomas who had colonoscopy and polypectomy, 0.3% to 0.9% of patients developed cancer within 2 to 5 years of colonoscopy. There are several possible reasons. New fast-growing lesions with microsatellite instability may account for some interval lesions. Incomplete removal of neoplasia at baseline could lead to development of cancer at a prior polypectomy site. However, lesions missed at baseline colonoscopy represent the most common explanation in most interval cancers. Studies using CT colonography to evaluate optical colonoscopy showed that colonoscopy may miss 2% to 12% of polyps 10 mm or larger.45–47 Flat lesions (see Chapter 36) may be difficult to detect at colonoscopy or incompletely visualized without chromoendoscopy. The 10-year interval after a normal colonoscopy is based on expert opinion derived from indirect data. Although case-control studies have suggested a 10-year benefit from endoscopic examinations of the colon, concerns about interval cancers noted previously have cast some doubt on whether a 10-year interval is appropriate for all patients. Studies of follow-up colonoscopy 5 years after a negative baseline screening examination reveal a low rate of advanced neoplasia.64,65
The 10-year interval after a normal colonoscopy is based on expert opinion derived from indirect data. Although case-control studies have suggested a 10-year benefit from endoscopic examinations of the colon, concerns about interval cancers noted previously have cast some doubt on whether a 10-year interval is appropriate for all patients. Studies of follow-up colonoscopy 5 years after a negative baseline screening examination reveal a low rate of advanced neoplasia.64,65 Quality in clinical practice is uncertain and may contribute to miss rates at colonoscopy. Elements of quality66 are summarized in Box 35.1 and include both reporting indicators (does the element appear in the report, and is it reported in a reproducible manner) and performance indicators (examination completeness, polyp detection and removal, recommendations for follow-up, and adverse events). A study by the Clinical Outcomes Research Initiative67 showed variability in reporting (despite use of a computerized report generator) and key outcomes such as completeness of examinations and polyp detection rates. Studies have shown that spending more time examining the colon during colonoscope withdrawal results in higher rates of polyp detection.68 Further study is needed to validate key quality indicators.
Quality in clinical practice is uncertain and may contribute to miss rates at colonoscopy. Elements of quality66 are summarized in Box 35.1 and include both reporting indicators (does the element appear in the report, and is it reported in a reproducible manner) and performance indicators (examination completeness, polyp detection and removal, recommendations for follow-up, and adverse events). A study by the Clinical Outcomes Research Initiative67 showed variability in reporting (despite use of a computerized report generator) and key outcomes such as completeness of examinations and polyp detection rates. Studies have shown that spending more time examining the colon during colonoscope withdrawal results in higher rates of polyp detection.68 Further study is needed to validate key quality indicators.Box 35.1
Quality Indicators for Colonoscopy
Reporting Indicators (Documentation in the Report)
Procedure indication: Appropriate indication and interval
Precautions requiring interventions (anticoagulation, ventricular defibrillator)
Documentation of bowel preparation quality
Completeness of examination with documentation of landmarks (photographic documentation preferred)
Description of key findings, polypectomy method, and polyp retrieval
Performance Indicators
Unplanned events and interventions
Appropriate follow-up recommendations based on findings and pathology
From Lieberman D, Nadel M, Smith R, et al: Standardized colonoscopy reporting and data system (CO-RADS): Report of the Quality Assurance Task Group of the National Colorectal Cancer Roundtable. Gastrointest Endosc 65:757–766, 2007.
Surveillance after Detection of Neoplasia
Surveillance after detection and removal of colon neoplasia (Table 35.7) should be viewed as part of a screening program. Recommendations for surveillance have an enormous impact on screening program cost and resource use.
| Findings at Baseline Colonoscopy* | Recommended Interval for Colonoscopy |
|---|---|
| No polyps | 10 yr |
| Hyperplastic polyps: rectum-sigmoid | 10 yr |
| 1–2 tubular adenomas <10 mm | 5–10 yr |
| ≥3 tubular adenomas | 3 yr |
| Tubular adenoma ≥10 mm | 3 yr |
| Adenoma with villous histology | 3 yr |
| Adenoma with high-grade dysplasia* | 3 yr |
| Invasive cancer† | 1 yr |
| Incomplete removal of neoplastic lesion | 3 mo |
* Assumes complete examination to cecum, with preparation adequate to detect polyps >5 mm and complete removal of visible polyps.
† Assumes complete examination of the colon before resection of cancer. If complete examination was impossible, colonoscopy should be performed at 3 months to rule out synchronous lesions.
Data from Rex DK, Kahi CJ, Levin B, et al: Guidelines for colonoscopy surveillance after cancer resection: A consensus update by the American Cancer Society and the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 130:1865–1871, 2006; and Winawer SJ, Zauber AG, Fletcher RH, et al: Guidelines for colonoscopy surveillance after polypectomy: A consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology 130:1872–1885, 2006.
Surveillance in Patients with Personal History of Colorectal Cancer
After the diagnosis and treatment of cancer, guidelines recommend surveillance colonoscopy within 1 year of curative resection, and every 3 to 5 years thereafter if the first colonoscopy is negative. This recommendation was based on a literature review of 24 studies, which found that 57 of 137 metachronous CRCs were found within 24 months of resection.69 If a complete examination of the colon was impossible before treatment because of obstruction, a colonoscopy should be completed within 3 to 6 months of surgical treatment to rule out synchronous lesions.
Adenoma Surveillance
The rationale for surveillance after removal of adenomas is based on several lines of evidence, but connecting the dots of evidence is problematic. We know that patients with adenomas have an increased risk of developing CRC. Most CRC has an adenoma precursor lesion. Numerous studies have shown that patients with adenomas who undergo colonoscopy and polypectomy have a high likelihood of having adenomas detected during follow-up examinations.70 Some of these lesions may have been present at the baseline examination but may not have been detected or were incompletely removed, whereas others may represent new lesions. The rationale for adenoma surveillance is based on the premise that adenomas can develop into CRC and that after adenomas are removed at baseline colonoscopy, more adenomas are commonly found during follow-up examinations.
There are several flaws in this reasoning, however. In their lifetime, 30% to 50% of individuals develop adenomas and only 5% to 6% develop cancer. Most patients with adenomas do not develop cancer and would be unlikely to benefit from surveillance. How should patients who are likely to progress to malignancy (and benefit from surveillance) be identified? Risk stratification based on patient characteristics and the index polyp would be ideal. There is some preliminary evidence that patients who have only one or two small tubular adenomas are at low risk for development of new advanced adenomas during 5 to 6 years of follow-up.55 Patients with multiple adenomas, large adenomas (≥10 mm), or adenomas with villous histology have a higher risk of having advanced adenomas during surveillance.55,70,71 These data informed recommendations for adenoma surveillance, which are summarized in Table 35.7.72
Complications of Screening and Surveillance
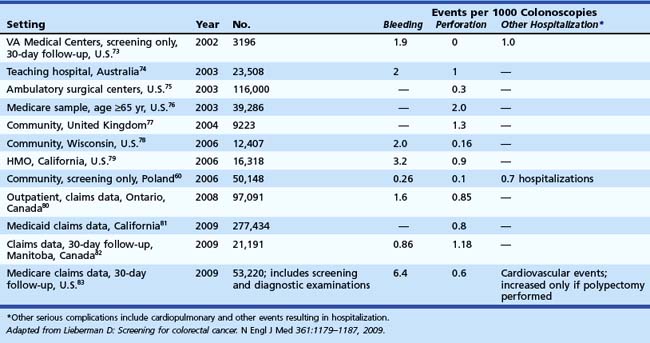
If the initial screening test is positive, all screening programs ultimately lead to colonoscopy. The safety of colonoscopy in diverse practice settings is uncertain. The overall rate of adverse events is 3 to 5 per 1000 colonoscopies.73–83 The clinical studies summarized in Table 35.8 have reported a limited set of adverse events, such as perforation (0.1 to 1 per 1000) and bleeding (0.5 to 6 per 1000). Most of these studies have included patients undergoing screening and diagnostic colonoscopy. Hospitalizations for other episodes such as cardiopulmonary events may occur in 1 in 1000 procedures and have been reported in a few studies. Few studies have fully evaluated events occurring within 30 days of colonoscopy.
Controversies in Screening and Surveillance
Customization of Screening
Women develop CRC and advanced neoplasia at a later age than men. The delay in onset of colon neoplasia may be related to natural hormonal protection before menopause or hormone replacement therapy after menopause. African Americans have a higher CRC incidence and mortality compared with whites1 and have higher age-adjusted cancer precursor lesions compared with whites.84 Current guidelines call for all men and women to be screened beginning at age 50 years, although one expert group advocated initiation of screening at age 45 for African Americans. There is a rational basis for considering customization of screening for women (delay initiation) and for African Americans (early initiation). However, there is also concern that current screening guidelines are complex and that adding another layer of complexity with customization could paradoxically result in lower rates of screening.2 The current guidelines place emphasis on ensuring that all individuals have access to screening.
1 Jemal A, Siegel R, Ward E, et al. Cancer statistics 2009. CA Cancer J Clin. 2009;59:225-249.
2 Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for early detection of colorectal cancer and adenomatous polyps, 2008: A joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570-1595.
3 U.S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2008;149:627-637.
4 Whitlock EP, Lin JS, Liles E, et al. Screening for colorectal cancer: A targeted, updated systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:638-658.
5 Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2008. Am J Gastroenterol. 2009;104:739-750.
6 Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, et al. Women’s Health Initiative Investigators. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med. 2004;350:991-1004.
7 Grodstein F, Martinez E, Platz EA, et al. Postmenopausal hormone use and risk for colorectal cancer. Ann Intern Med. 1998;128:705-712.
8 Lieberman D, Holub J, Moravec M, et al. Prevalence of colon polyps detected by colonoscopy screening in asymptomatic black and white patients. JAMA. 2008;300:1417-1422.
9 Theuer CP, Wagner JL, Taylor TH, et al. Racial and ethnic colorectal cancer patterns affect the cost-effectiveness of colorectal cancer screening in the United States. Gastroenterology. 2001;120:848-856.
10 Nelson RL, Dollear T, Freels S, et al. The relation of age, race and gender to the subsite location of colorectal carcinoma. Cancer. 1997;80:193-197.
11 Chen VW, Fenoglio-Preiser CM, Wu XC, et al. Aggressiveness of colon carcinoma in blacks and whites. Cancer Epidemiol Biomarkers Prev. 1997;6:1087-1093.
12 Weber TK, Chin H-M, Rodriquez-Bigas M, et al. Novel hMLH1 and hMSH2 germline mutations in African Americans with colorectal cancer. JAMA. 1999;281:2316-2320.
13 Hawk ET, Umar A, Viner JL. Colorectal cancer chemoprevention: An overview of the science. Gastroenterology. 2004;126:1423-1447.
14 O’Brien MJ, Winawer SJ, Zauber AG, et al. The National Polyp Study. Patient and polyp characteristics associated with high-grade dysplasia in colorectal adenomas. Gastroenterology. 1990;98:371-379.
15 Muto T, Bussey HJR, Morson BC. The evolution of cancer or the colon and rectum. Cancer. 1975;36:2251-2270.
16 Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med. 1993;329:1977-1981.
17 Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759-767.
18 Calvert PM, Frucht H. The genetics of colorectal cancer. Ann Intern Med. 2002;137:603-612.
19 Chung DC. The genetic basis of colorectal cancer: Insights into critical pathways of tumorigenesis. Gastroenterology. 2001;119:854-865.
20 Chung DC, Rustgi AK. The hereditary nonpolyposis colorectal cancer syndrome: Genetics and clinical implications. Ann Intern Med. 2003;138:560-570.
21 Spring KJ, Zhao ZZ, Karamatic R, et al. High prevalence of sessile serrated adenomas with BRAF mutations: A prospective study of patients undergoing colonoscopy. Gastroenterology. 2006;131:1400-1407.
22 Sieber OM, Lipton L, Crabtree M, et al. Multiple colorectal adenomas, classic adenomatous polyposis and germ-line mutations in MYH. N Engl J Med. 2003;348:791-799.
23 Jarvinen HJ, Aarnio M, Mustonen H, et al. Controlled 15 year trial of screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 2000;118:829-834.
24 Vassen HF, Watson P, Mechlin JP, et al. New clinical criteria for hereditary nonpolyposis colorectal cancer proposed by the International Collaborative Group on HNPCC. Gastroenterology. 1999;116:1453-1456.
25 Rodriquez-Bigas MA, Boland CR, Hamilton SR, et al. A National Cancer Institute Workshop on hereditary nonpolyposis colorectal cancer syndrome: Meeting highlights and Bethesda guidelines. J Natl Cancer Inst. 1997;89:1758-1762.
26 Aarnio M, Sankila R, Pukkala E, et al. Cancer risk in mutation carriers of DNA-mismatch repair genes. Int J Cancer. 1999;81:214-218.
27 Fuchs CS, Giovannucci EL, Colditz GA, et al. A prospective study of family history and risk of colorectal cancer. N Engl J Med. 1994;331:1669-1674.
28 St. John JB, McDermott FT, Hopper JL, et al. Cancer risk in relatives of patients with common colorectal cancer. Ann Intern Med. 1993;118:785-790.
29 Lichtenstein P, Holm NV, Verekasalo PK, et al. Environmental and heritable factors in the causation of cancer—analyses of cohorts of twins from Sweden, Denmark and Finland. N Engl J Med. 2000;343:78-85.
30 Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol. 2001;96:2992-3003.
31 Winawer SJ, Zauber AG, Gerdes H, et al. Risk of colorectal cancer in families of patients with adenomatous polyps. N Engl J Med. 1996;334:82-87.
32 Lynch KL, Ahnen DJ, Byers T, et al. VA Cooperative Study Group # 380. First degree relatives of patients with advanced colorectal adenomas have an increased prevalence of colorectal cancer. Clin Gastroenterol Hepatol. 2003;1:96-102.
33 Ekbom A, Helmick C, Zack M, et al. Ulcerative colitis and colorectal cancer: A population-based study. N Engl J Med. 1990;323:1228-1233.
34 Jess T, Loftus EV, Velayos FS, et al. Risk factors for colorectal neoplasia in inflammatory bowel disease: A nested case-control study from Copenhagen County, Denmark and Olmsted County, Minnesota. Am J Gastroenterol. 2007;102:829-836.
35 Choi PM, Nugent FW, Schoetz DJJ, et al. Colonoscopic surveillance reduces mortality from colorectal cancer in ulcerative colitis. Gastroenterology. 1993;105:418-424.
36 Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. N Engl J Med. 1993;328:1365-1371.
37 Hardcastle JD, Chamberlain J, Robinson MHE, et al. Randomized, controlled trial of fecal occult blood screening for colorectal cancer. Lancet. 1996;148:1472-1477.
38 Kronborg O, Fenger C, Olsen J, et al. Randomized study of screening for colorectal cancer with fecal occult blood test. Lancet. 1996;148:1467-1471.
39 Allison JE, Sakoda LC, Levin TR, et al. Screening for colorectal neoplasms with new fecal occult blood tests: Update on performance characteristics. J Natl Cancer Inst. 2007;99:1-9.
40 Hundt S, Haug U, Brenner H. Comparative evaluation of immunochemical fecal occult blood tests for colorectal adenoma detection. Ann Intern Med. 2009;150:162-169.
41 Nadel MR, Shapiro JA, Klabunde CN, et al. A national survey of primary care physicians’ methods for screening for fecal occult blood. Ann Intern Med. 2005;142:86-94.
42 Imperiale TF, Ransohoff DE, Itzkowitz SH, et al. Fecal DNA vs. fecal occult blood for colorectal cancer screening in an average-risk population. Colorectal Cancer Study Group. N Engl J Med, 351. 2704-2714:2004.
43 Ahlquist DA, Sargent DJ, Loprinzi CL, et al. Stool DNA and occult blood testing for screen detection of colorectal neoplasia. Ann Intern Med. 2008;149:441-450.
44 Itzkowitz SH, Jandorf L, Brand R, et al. Improved fecal DNA test for colorectal cancer screening. Clin Gastroenterol Hepatol. 2007;5:111-117.
45 Pickhardt PJ, Choie R, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003;349:2191-2200.
46 Cotton PB, Durkalski VL, Pineau BC, et al. Computed tomographic colonography (virtual colonoscopy): A multicenter comparison with standard colonoscopy for detection of colorectal neoplasms. JAMA. 2004;291:1713-1719.
47 Rockey DC, Paulson E, Niedzwiecki D, et al. Analysis of air contrast barium enema, computed tomographic colonography and colonoscopy: Prospective comparison. Lancet. 2005;365:305-311.
48 Johnson CD, Chen M-H, Toledano AY, et al. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med. 2008;359:1207-1217.
49 Lieberman DA, Moravec M, Holub J, et al. Polyp size and advanced histology in patients undergoing colonoscopy screening: Implications for CT colonography. Gastroenterology. 2008;135:1100-1105.
50 Butterly LF, Chase MP, Pohl H, et al. Prevalence of clinically important histology in small adenomas. Clin Gastroenterol Hepatol. 2006;4:343-348.
51 Soetikno RM, Kaltenbach T, Rouse RV, et al. Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. JAMA. 2008;299:1027-1035.
52 Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357:2277-2284.
53 Selby JV, Friedman GD, Quesenberry CPJr, et al. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med. 1992;326:653-657.
54 Thiis-Evensen E, Hoff GS, Sauar J, et al. Population-based surveillance by colonoscopy: Effect on the incidence of colorectal cancer. Scand J Gastroenterol. 1999;34:414-420.
55 Lieberman DA, Weiss DG, Bond JH, et al. VACSP Group # 380. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. N Engl J Med. 2000;343:162-168.
56 Lieberman DA, Weiss DG, VACSP #380 Study Group. One-time screening for colorectal cancer with combined fecal occult-blood testing and examination of the distal colon. N Engl J Med. 2001;345:555-560.
57 Imperiale TF, Wagner DR, Lin CY, et al. Risk of advanced proximal neoplasms in asymptomatic adults according to the distal colorectal findings. N Engl J Med. 2000;343:169-174.
58 Schoenfeld P, Cash B, Flood A, et al. Colonoscopic screening of average-risk women for colorectal neoplasia. N Engl J Med. 2005;352:2061-2068.
59 Lieberman DA, Holub J, Eisen G, et al. Prevalence of polyps greater than 9 mm in a consortium of diverse clinical practice settings in the United States. Clin Gastroenterol Hepatol. 2005;3:798-805.
60 Regula J, Rupinski M, Kraszewska E, et al. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med. 2006;355:1863-1872.
61 Muller AD, Sonnenberg A. Prevention of colorectal cancer by flexible endoscopy and polypectomy: A case-control study of 32,702 veterans. Ann Intern Med. 1995;123:904-910.
62 Singh H, Turner D, Xue L, et al. Risk of developing colorectal cancer following a negative colonoscopy examination: Evidence for a 10-year interval between colonoscopies. JAMA. 2006;295:2366-2373.
63 Baxter NN, Goldwasser MA, Paszat LF, et al. Association of colonoscopy and death from colorectal cancer: A population-based, case-control study. Ann Intern Med. 2009;150:1-8.
64 Lieberman DA, Weiss DG, Harford WV, et al. Five year colon surveillance after screening colonoscopy. Gastroenterology. 2007;133:1077-1085.
65 Imperiale TF, Glowinski EA, Lin-Cooper C, et al. Five-year risk of colorectal neoplasia after negative screening colonoscopy. N Engl J Med. 2008;359:1218-1224.
66 Lieberman D, Nadel M, Smith R, et al. Standardized colonoscopy reporting and data system (CO-RADS): Report of the Quality Assurance Task Group of the National Colorectal Cancer Roundtable. Gastrointest Endosc. 2007;65:757-766.
67 Lieberman DA, Faigel DO, Logan J, et al. Assessment of colonoscopy quality: Results from a multi-center consortium. Gastrointest Endosc. 2009;69:645-653.
68 Barclay RL, Vicari JJ, Doughty AS, et al. Adenoma detection rates and colonoscopic withdrawal times during screening colonoscopy. N Engl J Med. 2006;355:2533-2541.
69 Rex DK, Kahi CJ, Levin B, et al. Guidelines for colonoscopy surveillance after cancer resection: A consensus update by the American Cancer Society and the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2006;130:1865-1871.
70 Martinez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses following colonoscopic polypectomy. Gastroenterology. 2009;136:832-841.
71 Winawer SJ, Zauber AG, O’Brien MJ, et al. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. N Engl J Med. 1993;328:901-906.
72 Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: A consensus update by the US Multi-Society Task Force on colorectal cancer and the American Cancer Society. Gastroenterology. 2006;130:1872-1885.
73 Nelson DB, McQuaid KR, Bond JH, et al. Procedural success and complications of large-scale screening colonoscopy. Gastrointest Endosc. 2002;55:307-314.
74 Viiala CH, Zimmerman M, Cullen DJE, et al. Complication rates of colonoscopy in an Australian teaching hospital environment. Intern Med J. 2003;33:355-359.
75 Korman LY, Overholt BF, Box T, et al. Perforation during colonoscopy in endoscope ambulatory surgical centers. Gastrointest Endosc. 2003;58:554-557.
76 Gatto NM, Frucht H, Sundararajan V, et al. Risk of perforation after colonoscopy and sigmoidoscopy: A population-based study. J Natl Cancer Inst. 2003;95:230-236.
77 Bowles CJA, Leicester R, Romaya C, et al. A prospective study of colonoscopy practice in the UK today: Are we adequately prepared for national colorectal cancer screening tomorrow? Gut. 2004;53:277-283.
78 Rathgaber SW, Wick TM. Colonoscopy completion and complication rates in a community gastroenterology practice. Gastrointest Endosc. 2006;64:556-562.
79 Levin TR, Zhao W, Conell C, et al. Complications of colonoscopy in an integrated health care delivery system. Ann Intern Med. 2006;145:880-886.
80 Rabeneck L, Paszat LF, Hilsden RJ, et al. Bleeding and perforation after outpatient colonoscopy and their risk factors in usual clinical practice. Gastroenterology. 2008;135:1899-1906.
81 Arora G, Mannalithara A, Singh G, et al. Risk of perforation from a colonoscopy in adults: A large population-based study. Gastrointest Endosc. 2009;69:654-664.
82 Singh H, Penfold RB, DeCoster C, et al. Colonoscopy and its complications across a Canadian regional health authority. Gastrointest Endosc. 2009;69:665-671.
83 Warren JL, Klabunde CN, Mariotto AB, et al. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med. 2009;150:849-857.
84 Lieberman D, Holub J, Moravec M, et al. Prevalence of colon polyps detected by colonoscopy screening in asymptomatic black and white patients. JAMA. 2008;300:1417-1422.