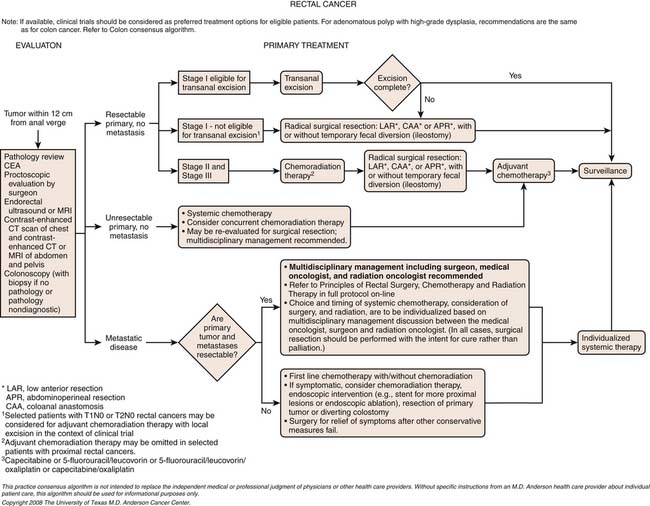
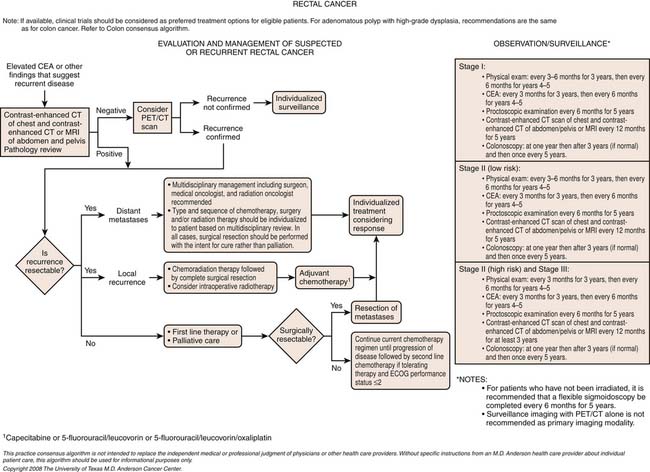
Chapter 17 Colorectal Cancer
Introduction*
Colorectal cancer (CRC) is one of the most common adult cancers.1 Imaging plays an important role in the management of CRC, including screening, staging, and surveillance. Understanding the anatomy of the colon and rectum is important when interpreting radiologic examinations pertaining to CRC. This especially applies when evaluating for the pattern and extent of disease spread.
Double-contrast barium enema (DCBE) and computed tomographic colonography (CTC) are recommended imaging screening tests for CRC.2 Staging involves accurate depiction of disease based on the tumor, node, and metastasis (TNM) classification system. Endoscopic ultrasound (EUS) plays an important role in the staging of rectal cancer. Advancements in miniprobe examination may increase its role for the more proximal cancers.3 Computed tomography (CT) remains the mainstay for CRC imaging owing to its widespread availability. Its value lies more in the assessment of nodal and distant disease rather than of the primary tumor itself. Magnetic resonance imaging (MRI), with its superior soft tissue resolution capability, has a role for both primary evaluation of rectal cancers and assessment of distant metastatic disease in the liver.4 Positron-emission tomography (PET)/CT provides both functional and anatomic information. It allows for whole body assessment in a single examination, making it valuable for detection of unsuspected sites of disease.
Surveillance by imaging is usually carried out in tandem with serum carcinoembryonic antigen (CEA) assays. In the appropriate setting, surgical resection of locally recurrent disease in the pelvis as well as metastatic disease in the liver and lungs has been shown to improve survival.5,6 Therefore, when CEA levels show a rise during postoperative surveillance, it is important to identify the presence and location of recurrent and distant disease to allow for prompt treatment. PET/CT has emerged as an indispensable modality in this regard, and its use has increased tremendously since its introduction in 2001.
Epidemiology and Risk Factors
Approximately 1 in 19 men and women will be diagnosed with cancer of the colon and rectum during their lifetime.1 In the United States, CRC is the second leading cause of death from cancer, with an estimated 50,000 cases in 2009.7 The majority of CRC patients are older than age 50, with relatively equal gender incidence.4 Recent declines in CRC incidence and mortality are attributable to reduced risk factor exposure, early detection and prevention through polypectomy, and improved treatment.8
Most CRCs develop from adenomatous polyps through a process known as the adenoma-carcinoma sequence. Malignant potential of a polyp increases with size. Polyps smaller than 0.5 cm have virtually no risk, whereas those greater than 2 cm have a 40% risk for invasive carcinoma.9 Although previously thought to be benign, the serrated form of hyperplastic colonic polyps have recently been shown to be associated with malignant transformation.10
The most common form of hereditary CRC is hereditary nonpolyposis CRC (Lynch’s syndrome). Others include familial adenomatous polyposis (FAP), juvenile polyposis, and Peutz-Jeghers.11 Specific genetic mutations linked to CRC include p53, APC, and K-ras mutations.12 With inflammatory bowel disease (ulcerative colitis or Crohn’s disease), the risk of CRC increases with duration and anatomic extent of colitis, degree of inflammation, and concomitant family history of CRC.13 Dietary and lifestyle factors that have been implicated as risk factors include obesity, hyperglycemia, consumption of red and processed meat and animal fat, and high alcohol intake.14,15
Anatomy and Pathology
The large intestine is about 1.5 m long, being one fifth of the whole extent of the intestinal canal. The large intestine is divided into the cecum, colon, rectum, and anal canal. It is generally accepted that tumors with epicenters located more than 2 cm proximal to the dentate line are rectal, whereas those that are distal to this are considered anal.16 This distinction is important for staging. Based on the American Joint Committee on Cancer (AJCC) staging system, the T stage of CRC is determined by degree of involvement of the bowel wall whereas that of the anus is determined by the size of the primary tumor. Also, the N stage of CRC is defined by the number of involved nodes, as opposed to the location of nodes in anal cancer.
Tumor size has not been shown to carry prognostic significance in CRC.17,18 Other than the prognostic indicators defined by the AJCC staging system (which are discussed in the “Staging” section, later), histologic subtype, tumor grade, lymphovascular involvement, tumor border invasion, and host lymphoid response to tumor also affect prognosis.
The World Health Organization (WHO) classifies the CRCs into the following: adenocarcinoma, medullary, mucinous (colloid) adenocarcinoma (>50% mucinous), signet-ring cell carcinoma (>50% signet-ring cells), squamous cell (epidermoid) carcinoma, adenosquamous carcinoma, small cell (oat cell) carcinoma, undifferentiated carcinoma, and others (e.g., papillary carcinoma). Adenocarcinomas make up more than 95% of colorectal primaries. Of the remaining primaries, neuroendocrine (1.8%), lymphoma (0.6%), squamous cell carcinoma (0.3%), and sarcoma (<0.1%) are considered rare. Among the histologic subtypes of CRC, carcinoid and neuroendocrine carcinoma show the best and the worst relative 5-year survival rates, respectively. Also, both malignant lymphoma and squamous cell carcinoma patients have significantly worse overall 5-year and early-stage (localized and regional) survival rates than adenocarcinoma.19 The signet-ring cell type of adenocarcinoma and small cell (oat cell) carcinoma are the only histologic types of colonic carcinoma that consistently have been found to have a stage-independent adverse effect on prognosis. The prognostic significance of mucinous carcinoma remains controversial. Conversely, medullary carcinoma is prognostically favorable.20
In terms of tumor grade, a two-tier system of low- (well- and moderately differentiated) and high- (poorly and undifferentiated) grade tumors has been proposed to reduce interobserver variability.17 Multivariate analysis has also shown the presence of lymphovascular invasion to be an adverse prognostic indicator.17,18,20 The configuration of the tumor at the advancing edge (irregular and infiltrating pattern) has been shown to be an independent adverse prognostic factor as well. In contrast, the presence of lymphocytic infiltration of tumor or peritumoral tissue at pathology implies a host immunologic response to the tumor and, therefore, is a favorable prognostic factor. Use of molecular markers as prognostic indicators is currently still the subject of research and no known marker has been validated for routine patient care.16
Clinical Presentation
Symptoms commonly associated with CRC include abdominal pain, change in bowel habits, hematochezia or anemia, weakness or malaise, and weight loss.21,22 Symptoms depend on tumor size and location. The left colonic lumen has a smaller caliber than the right and contains better-formed stool owing to water absorption by the proximal colon. This explains why left-sided colonic cancers have a higher chance of causing obstructive symptoms and change in bowel habits. This may also be seen in bulky exophytic tumors.23 Distal tumors can cause rectal bleeding, whereas proximal tumors are more likely to result in occult bleeding and symptoms of anemia.
Clinical signs do not manifest until late in disease.24 The primary mass rarely presents as an abdominal mass.25 Digital rectal examination may reveal the presence of a low rectal tumor. Systemic effects of malignancy include muscular wasting and hypoalbuminemia (manifests as anasarca in severe cases). Hepatomegaly and enlarged Virchow’s (supraclavicular) lymph node may be encountered in bulky metastatic disease. Iron deficiency anemia results from gastrointestinal bleeding, with stigmata such as koilonychia, cheilitis, and pallor.
Although elevated serum CEA levels are often present in CRC they are of little use in detecting early CRC and, therefore, are not a recommended tool for screening asymptomatic patients.26 The accuracies of fecal occult blood tests (FOBTs) and fecal immunochemical tests (FITs) depend on the frequency of testing, specimen collection, and analysis technique.27 For the detection of occult gastrointestinal bleeding, brush-sampling FIT has been shown to be more sensitive than guaiac-based FOBT.28 Newer methods that detect DNA mutations in the stool (sDNA) may be more specific but require validation.29 The current American Cancer Society guidelines for CRC screening are presented in Table 17-1.
Table 17-1 Testing Options for the Early Detection of Colorectal Cancer and Adenomatous Polyps for Asymptomatic Adults Aged 50 Years and Older
| TESTS THAT DETECT ADENOMATOUS POLYPS AND CANCER |
|---|
| Flexible sigmoidoscopy every 5 yr, or Colonoscopy every 10 yr, or Double-contrast barium enema every 5 yr, or Computed tomographic colonography every 5 yr |
| Tests that Primarily Detect Cancer |
| Annual guaiac-based fecal occult blood test with high test sensitivity for cancer, or Annual fecal immunochemical test with high test sensitivity for cancer, or Stool DNA test with high sensitivity for cancer, interval uncertain |
Adapted from Levin B, Lieberman DA, McFarland B, et al; American Cancer Society Colorectal Cancer Advisory Group; US Multi-Society Task Force; American College of Radiology Colon Cancer Committee. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570-1595.
Patterns of Tumor Spread
Local Spread
Locally, tumor can spread either intramurally or transmurally. Intramural spread of tumor can occur longitudinally along the bowel wall or superficially toward the serosa. Tumor preferentially grows circumferentially, resulting in luminal narrowing. Longitudinal spread is not common and is generally approximately 2 cm from the primary site of disease. This supports the practice of a 5-cm surgical margin.30 Transmural spread is present in more advanced tumors. The depth of invasion within the bowel wall determines prognosis, as detailed in standard staging classification systems (see section on “Staging,” later). The ascending colon, descending colon, and rectum, which are primarily retroperitoneal in location, can directly invade the adjacent retroperitoneal organs such as the kidneys and pancreas via this mode of spread. Locally advanced rectal cancer often involves the pelvic viscera including the bladder base, prostate gland, and vagina.31 These may present with rectovaginal or rectovesical fistulas. Invasion of adjacent organs depends upon the site of primary tumor and mandates en bloc resection to achieve curative surgery.32
Disease can also spread along the neurovascular structures. Extramural venous invasion is associated with increased metastastic burden and poorer overall prognosis. This has been shown in multiple studies (see section on “Imaging,” later). Perineural spread in rectal cancer can result in tumor deposits along the perineural spaces more than 10 cm from the primary tumor. This has been reported in as many as 35% of cases.33
Nodal
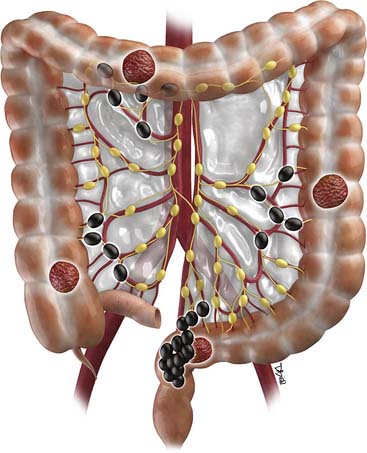
Distant spread of disease most commonly occurs in the liver, followed by lymph nodes.34 The nodal pathways of spread for CRC are illustrated in Figure 17-1.35 Nodal spread from carcinomas of the right colon follow along the marginal vessels of the cecum and ascending colon and then along the ileocolic vessels to the root of the superior mesenteric artery. Tumors of the proximal transverse colon tend to spread along the marginal vessels on the mesocolic side of the colon. These marginal vessels in turn drain to the right or middle colic vessels and to the root of the mesocolon, anterior to the head of the pancreas. Lymphatics from the distal transverse colon and splenic flexure follow the left middle colic vessels to the inferior mesenteric vein just caudal to the body and tail of the pancreas. Cancers of the descending colon and sigmoid colon will spread to nodes along the left ascending colic and sigmoidal vessels that can then be followed to the origin of the inferior mesenteric artery.36 Proximal rectal tumors spread cranially via the superior hemorrhoidal nodes antegradely to the inferior mesenteric lymph nodes. The more distal rectal tumors spread laterally along the internal iliac lymph nodes antegradely to the common iliac and retroperitoneal chains. Those located at the rectosigmoid junction tend to spread to the perirectal lymph nodes rather than along the sigmoid mesenteric chain.37
Peritoneal
Peritoneal spread of disease is seen in up to 43% of patients.31 Tumor cells can spread throughout the peritoneal cavity and implant on the omentum and peritoneal surfaces. This pattern of spread is seen more commonly in the intraperitoneal portions of the colon, including the cecum and transverse and sigmoid colon.31 The ovaries are a common site of involvement by peritoneal dissemination.30 In animal models, injection of neoplastic cells into the mesenteric border tend to give rise to nodal metastases whereas injection into the antimesenteric border gives rise to peritoneal metastases, alluding to the role of tumor location to the method of dissemination.38 The presence of peritoneal involvement predicts for higher local recurrence and is strongly associated with a mucinous tumor phenotype (signet-ring feature).39
Hematogenous
Ten percent to 25% of patients harbor detectable metastases at the time of initial diagnosis.40 Hematogeneous spread of colonic and upper rectal tumors initially occur via the portal circulation. The liver is often the first site of metastatic disease and may be the only site of spread in as many as 30% to 40% of patients with advanced disease.41 Twenty percent to 25% of patients will have clinically detectable liver metastases at the time of the initial diagnosis and a further 40% to 50% of patients will eventually develop liver metastases after resection of the primary. Approximately 20% to 30% of patients with metastatic CRC have disease that is confined to the liver and is potentially resectable.42 In low rectal tumors, venous drainage occurs through the systemic circulation via the iliac vessels. This may explain the higher propensity of pulmonary metastases in low rectal cancers compared with tumors from the more proximal parts of the colon and rectum.31 In the appropriate clinical setting, aggressive surgical resection of distant metastases (including the liver, lung, adrenal gland, and spleen) has been shown to confer survival benefit.43 Therefore, early detection of metastastic disease by imaging is important.
Key Points Tumor Spread
• CRCs can spread locally or through lymphatic, peritoneal, and hematogenous routes.
• Nodal disease has predictable spread.
• Peritoneal disease occurs in up to 43% in patients, is a predictor of local recurrence and mucinous histology. There is 10% to 25% hematogenous spread at diagnosis
• CRCs can spread locally or through lymphatic, peritoneal, and hematogenous routes.
Staging
The TNM staging system of the AJCC and the International Union Against Cancer (UICC) is currently the standard for CRC staging recommended by the National Cancer Institute and tumor registries worldwide.44,45
Clinical classification (cTNM) is based on clinical, radiologic, and surgical information. Pathologic classification (pTNM) is based on a previously untreated primary tumor. cTNM, once assigned, is not changed on the basis of subsequent information, even when pTNM becomes available and may be considered more accurate.44 The posttreatment status of the tumor (yTNM) carries prognostic importance as well. For multiple synchronous tumors, the lesion with the highest T category determines the stage.
Because the TNM staging is applicable only to primary carcinomas of the colon and rectum, other tumors such as colorectal lymphomas, carcinoid tumors, and metastases are excluded.46 The staging classification is summarized in Figure 17-2.
T Stage
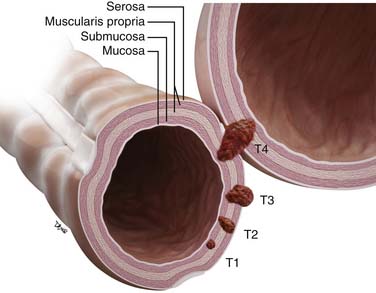
Tis (carcinoma in situ) refers to disease that is superficial to the muscularis mucosa. Penetration of the muscularis mucosa and invasion of the submucosa is classified as T1. CRC that has penetrated into, but not completely through, the muscularis propria is classified as T2.
T3 disease includes all transmurally invasive tumors confined to the perimuscular soft tissue (i.e., that have neither violated the serosal surface nor infiltrated an adjacent structure).47 Extramural tumor nodules discontinuous from the primary tumor mass that are irregular in shape are also included.47 Nodules with smooth contours are regarded as lymph nodes.
Direct invasion of adjacent organs or structures or other segments of the colorectum by way of the serosa or mesocolon (e.g., invasion of the sigmoid colon by carcinoma of the cecum) is classified as T4 disease. Intramural (longitudinal) extension of tumor from one subsite (segment) of the large intestine into an adjacent subsite (either the terminal ileum or the anal canal) does not affect the T classification.47 Serosal penetration (T4b disease) is associated with significantly shorter median survival time than T4 tumors without serosal involvement (T4a disease).47 The prognostic significance of this may supersede even that of regional lymph node metastasis (N category).48
N Stage
The definition of regional nodes is covered in Table 17-2. Because it has been shown that many nodal metastases in CRC are found in small lymph nodes (<5 mm in diameter), a minimum of 12 lymph nodes is considered adequate for curative surgical resection.49–51 Regional nodal involvement is classified as N disease (see Table 17-2), whereas all other nodal metastases are classified as M1. As mentioned, extramural tumor nodules of smooth contours are regarded as replaced regional lymph nodes.44,45,47 The number of pericolonic tumor deposits has been shown to correlate inversely with disease-free survival.52
Table 17-2 Definitions of Regional Lymph Node Groups in Anatomic Subsites of the Colon and Rectum
| COLON AND RECTUM SUBSITE | DEFINITION |
|---|---|
| Cecum | Anterior cecal, posterior cecal, ileocolic, right colic |
| Ascending colon | Ileocolic, right colic, middle colic |
| Hepatic flexure | Middle colic, right colic |
| Transverse colon | Middle colic |
| Splenic flexure | Middle colic, left colic, inferior mesenteric |
| Descending colon | Left colic, inferior mesenteric, sigmoid |
| Sigmoid colon | Inferior mesenteric, superior rectal sigmoidal, sigmoid mesenteric* |
| Rectosigmoid colon | Perirectal,† left colic, sigmoid mesenteric, sigmoidal, inferior mesenteric, superior rectal, middle rectal |
| Rectum | Perirectal,† sigmoid mesenteric, inferior mesenteric, lateral sacral, presacral, internal iliac, sacral promontory, superior rectal, middle rectal, inferior rectal |
* Lymph nodes along the sigmoid arteries are considered pericolic nodes, and their involvement is classified as pN1 or pN2 according to the number involved.
† Perirectal lymph nodes include the mesorectal (paraproctal), lateral sacral, presacral, sacral promontory (Gerota), middle rectal (hemorrhoidal), and inferior rectal (hemorrhoidal) nodes. Metastasis in the external iliac or common iliac nodes is classified as pM1.
Adapted from Compton CC, Greene FL. The staging of colorectal cancer: 2004 and beyond. CA Cancer J Clin. 2004;54:295-308.
In rare cases, the regional nodes of the primary tumor site are free of malignancy but the nodes in the drainage area of an organ directly invaded by the primary tumor contain metastases. When this occurs, the lymph nodes of the invaded site are considered as those of the primary site and are also classified under the N category.47
Isolated tumor cells (ITCs) are defined as tumor cells measuring 0.2 mm or less. According to current recommendations, ITCs are classified as N0 or M0, as appropriate.44,47,53 The biologic significance of ITCs is unknown.54 In contrast, metastatic tumors that measure greater than 0.2 mm but less than 2.0 mm are defined as micrometastases and classified as N1 or M1.
Surgical Resection Margin
The pertinent margins of a CRC resection specimen include the proximal and distal transverse margins, the mesenteric margin, and in rectal tumors, the circumferential margin. When the distance between the tumor and the nearest transverse margin is 5 cm or more, anastomotic recurrences are very rare.55 For rectal tumors in which sphincter preservation is an important consideration, a margin of 2 cm is accepted as adequate, especially for T1 and T2 tumors.
The circumferential resection margin (CRM) is defined by the distance between the retroperitoneal or the peritoneal adventitial soft tissue margin closest to the furthest advancing edge of the tumor. This applies to all segments of the large intestine that are either incompletely encased (ascending colon, descending colon, upper rectum) or not encased (lower rectum) by peritoneum. Multivariate analyses have suggested that tumor involvement of the CRM is the most critical factor in predicting local recurrence in rectal cancer.56,57 Emerging data on CRM involvement in the colon suggest likewise.56
Based on published data from clinical trials, the risk of local recurrence is strongly increased if the CRM is less than 1 mm.57 Thus, the current recommendation is for a clearance of 2 mm or less to be considered a positive CRM.58 Currently, if a tumor is present on a peritonealized surface, it is categorized as T4 but may not require adjuvant radiation if the resection margins (including the CRM) are free of tumor (R0). However, if a tumor is present on a nonperitonealized surface (i.e., the CRM), adjuvant radiation may be appropriate irrespective of the T category of the tumor.46
Key Points Staging
• The CRM is defined by the distance between the retroperitoneal or the peritoneal adventitial soft tissue margin closest to the furthest advancing edge of the tumor.
• Serosal penetration (T4b) associated with shot survival times metastases is not uncommon in even small nodes; therefore, minimum resection for cure should include 12 nodes.
Lymphovascular Invasion
Venous invasion by tumor has been demonstrated repeatedly to be a stage-independent adverse prognostic factor by either multivariate or univariate analyses.59–62 Mixed results have also been reported for lymphatic invasion, although the general consensus is that lymphovascular invasion is a negative prognostic factor.63,64 Interestingly, the presence of tumor cells within the lymphatics or veins does not affect the T stage. Intravascular spread via lymphatic or venous vessels is classified as L1 and V1, respectively. Tumor within the lymphatics of a distant organ is classified as M1 disease.
Imaging
Screening
Double-Contrast Barium Examination
Five-yearly DCBE is acceptable for screening, even though use of DCBE is on the decline. Most studies evaluating DCBE are limited in that they are retrospective reviews of known CRC patients for prior DCBE. DCBE has sensitivity of between 85% and 97% for cancer.2 Sensitivity decreases with lesion size. In symptomatic patients, for lesions more than 1 cm in size, the reported sensitivities are 75% to 90%, whereas those smaller than 1 cm range from 50% to 80%.65,66 For asymptomatic patients, lower sensitivity rates of 48% and 32%, respectively, are reported.67 There have been no randomized, controlled trials evaluating the efficacy of DCBE as a primary screening modality to reduce incidence or mortality from CRC.2
The advantages of DCBE is that it provides screening for the whole colon and is relatively safe, with a perforation rate of 1 in 25,000 and an associated mortality rate of 1 in 250,000,68 compared with 1 in 1000 patients undergoing colonoscopy.69 The disadvantages of DCBE include need for rigorous bowel preparation, discomfort during the procedure, and operator and reader dependency on interpretation of results.
Computed Tomography Colonography
The ACRIN (American College of Radiology Imaging Network) National CT Colonography Trial, which studied 2500 patients across 15 institutions in the United States, has shown comparable accuracy between CTC and standard colonoscopy.70 Using fecal tagging and primary 3D polyp detection in asymptomatic adults, Pickhardt and coworkers71 reported a sensitivity of 89% for adenomas 6 mm or larger. For invasive CRC, the pooled CTC sensitivity was higher at 96%. As with other screening techniques, CTC accuracy improves with lesion size. Other than detection, consistent polyp size measurement is important because it will influence referrals for polypectomy. Use of 3D reconstructed images or 2D assessment in the lung window is recommended for accurate polyp size measurement.72 All patients with one or more polyps 10 mm or larger or three or more polyps 6 mm or larger should be referred for colonoscopy.73 The management of patients with fewer polyps (<3) in which the largest is 6 to 9 mm or smaller remains controversial.74,75
The main drawback of CTC is radiation exposure. One CTC study in a 50 year old results in an estimated organ dose to the colon of 7 to 13 mSv, an additional 0.044% to the lifetime risk of colon cancer.76 More efficient low-dose protocols have demonstrated decreased estimated organ dose ranges of 5 to 8 mSv.77 The risk for colonic perforation during screening is extremely low, 0.005% for asymptomatic patients and higher for symptomatic patients (0.03-0.06%), and may be safer with carbon dioxide delivery rather than with room air.78,79
Cancer Evaluation
Plain Radiography
Plain films have little role in the diagnosis of CRC, except to demonstrate acute complications of obstruction or perforation. In bowel obstruction, isolated dilatation of the colon is unusual. More commonly, both the small and the large bowels are dilated. If the ileocecal valve is patent, small bowel dilatation only may manifest, due to decompression of air retrogradely from the proximal colon. Rarely, a soft tissue mass may be visualized, although additional imaging is often required to confirm the diagnosis. In the mucus-secreting (colloid) variant, calcified liver or nodal metastases may be evident. These are usually associated with a poor prognosis.80
Endoscopic Ultrasound
The role of EUS is largely confined to local staging for rectal cancer because more proximal tumors are difficult to assess with the rigid probe, although this may change with the use of the miniprobe ultrasound examination.3
EUS provides accurate depiction of the rectal wall layers. Most practices use a 7.5- or 10-MHz rigid transducer with a saline-filled balloon tip, allowing a 360-degree field of view. Tumor most commonly appears as a hypoechoeic mass disrupting the rectal wall layers.3 Early rectal cancer is demonstrated by tumor ingrowth into the superficial layers of the rectal wall, with staging accuracy ranging from 69% to 97%.81,82 A meta-analysis by Bipat and colleagues,83 comparing CT, EUS, and MRI, showed that endorectal ultrasound may be the imaging modality of choice for rectal cancer.
Owing to the similar appearances between peritumoral inflammatory changes and extramural extension, overstaging T2 tumors as T3 tumors is common.84 Perirectal nodal involvement is also limited. Bipat and colleagues83 showed overall sensitivity of 67% and specificity of 78% for nodal involvement. This is related to its limited acoustic window, depth of penetration, and small field of view, which also limits evaluation of the distance between the tumor and the mesorectal fascia. Operator dependence, need for bowel preparation, and intraprocedural pain due to stenotic lesions are other limitations.
Computed Tomography
Clinical use of CT for rectal cancer staging is limited, owing to the lack of contrast resolution to discriminate between tumor and normal visceral soft tissue, with accuracy of approximately 70%.85 In the study by O’Neil and associates,86 CT consistently overestimated tumor volume and underestimated distance from the anal verge compared with MRI. It is also poor for assessment of levator ani invasion in low rectal lesions.87 For the more proximal colon, however, CT may be the modality of choice, given its high temporal resolution.
In general, CT is more useful for nodal and metastases staging. However, the optimal strategy for the elective distant staging of CRC is controversial. For instance, in patients with CRC, CT chest often detects indeterminate lung lesions, of which only a small proportion develops into definite metastases.88 Similarly, in rectal cancer, if pelvic MRI has already been performed, CT including the pelvis does not provide additional value.89 Therefore, further studies are required to define optimal preoperative imaging.
Novel techniques such as perfusion CT and combined perfusion CT-PET/CT show promise but require further validation.90–92 Preoperative PET/CT colonography may yield valuable information on the presence of synchronous tumors and for surgical planning.93
Magnetic Resonance Imaging
The advent of high-resolution phased array coils in combination with improvements in MRI sequences has led to significant improvements in rectal cancer staging. The Magnetic Resonance Imaging and Rectal Cancer European Equivalence (MERCURY) study, which recruited 679 patients, showed that MRI is accurate in preoperative evaluation of rectal cancer.4 Currently, MRI allows for accurate detection of extramural extension, venous invasion, nodal involvement, and at times, peritoneal dissemination. High-resolution T2-weighted fast spin echo (T2W-FSE) imaging using a phased array coil is the sequence of choice owing to superior discrimination between hyperintense mesorectal fat, hypointense rectal wall, and mesorectal fascia and intermediate signal of tumor.94 Use of endorectal coil MRI has not been shown to be significantly better than EUS.3 MRI accurately predicts T stage and CRM status. It predicts histopathologic involvement of the CRM to within 0.5 mm.4 Using 1 mm as a cutoff to determine clear margins from the mesorectal fascia, the authors showed that MRI has a specificity of 92%.95 Furthermore, MRI has been shown to be superior to CT in the assessment of pelvic floor muscle as well as sacral bone invasion.96 Diffusion-weighted imaging (DWI) may improve liver metastasis detection with greater sensitivity compared with CT.97
MRI is limited in nodal evaluation, for which it is no better than CT.98 Using a short-axis-diameter cutoff of 5 mm, the nodal status accuracy rate of MRI lies between 59% and 85%.99,100 Spiculation, indistinct margins, lobulation, round configuration, and mixed signal intensity on T2-weighted imaging are other suspicious features. Ultrasmall superparamagnetic iron oxide (USPIO) improves negative predictive value, with benign nodes showing central or uniform low signal in 96% of patients. Tumor infiltrated nodes fail to take up iron particles and, therefore, appear hyperintense on T2*-weighted imaging.101 Whole body MRI is a feasible and promising method for examining colon cancer patients for recurrence and metastatic disease but cannot displace the present role of PET/CT.102
Key Points Endoscopic Ultrasound, Computed Tomography, and Magnetic Resonance Imaging
• EUS provides accurate depiction of the rectal wall layers. Tumor most commonly appears as a hypoechoeic mass disrupting the rectal wall layers.
• CT is most useful for nodal and metastases staging.
• Preoperative PET/CTC may yield information in synchronous tumor.
• Radiology report should include location and size of tumor, presence and distribution of nodes, presence or absence of metastases.
Positron-Emission Tomography
18F-fluoro-2-deoxy-D-glucose (FDG) is the most widely used substrate for PET imaging. Fusion PET/CT combines the functional evaluation by PET with the anatomic detail provided by CT. Contrast-enhanced PET/CT and PET/CTC show promise for improving accuracy.103,104
For local disease, PET/CT can improve preoperative target volume delineation by CT for conformal radiation therapy in rectal cancer.105 However, by far the greatest value of PET in CRC lies in whole body lesion detection. It can reveal unsuspected disease and modify the scope of surgery in approximately 10% of patients.106 In one study, FDG PET/CT altered treatment plans in 38% of patients largely through the detection of unsuspected lymphadenopathy.107 PET/CT also shows high accuracy in detection of liver metastases, with reported accuracy up to 99%, sensitivity up to 100%, and specificity up to 98%. It resulted in a change of management in approximately 30% of cases.108,109
Physiologic uptake in the gastrointestinal tract (due to lymphoid, glandular, and muscular tissues), infection, and inflammation can result in false positives. Benign colonic adenomas may also not be distinguished from FDG-avid carcinomas.110 PET/CT is relatively insensitive to mucinous colorectal tumors owing to paucicellularity.111 Urinary excretion of tracer can potentially be confused with tumor spread or recurrence in the retroperitoneum or pelvis. Most current PET scanners have a spatial resolution of 5 mm, which is below that of other imaging modalities. Even with fusion PET/CT, the various layers of the colonic wall are not distinguishable, limiting ability for T staging. Furthermore, small nodal metastases in the vicinity of the primary lesion may be missed.112 Despite these limitations, PET/CT has become an indispensible imaging tool in CRC management.
Therapies
Surgery
Surgical technique influences local recurrence and survival in the treatment of CRC; hence, a standardized operative technique is important. Strict adherence to the principles of en bloc resection and complete surgical removal of tumor along with the regional lymphatics is the best prevention strategy against local recurrence and affords the best prognosis.113 Proximal and distal margins of 5 cm are the standard of care for colonic tumors.114 For rectal tumors, a distal margin of 2 cm is adequate.115
The extent of colonic resection and location of the anastomosis is based on the tumor location and corresponding colonic blood supply. Ligation of the vascular pedicles at their origin is important. Cecal and ascending colonic tumors require right hemicolectomy with ligation of the ileocolic and right colic vessels. Hepatic flexure tumors require an extended right hemicolectomy with additional ligation of the middle colic artery. Transverse colonic tumors will require either subtotal colectomy or extended right hemicolectomy with preservation of the left colic vessels.114,116 Splenic flexure tumors require either subtotal colectomy or extended left hemicolectomy with ligation of the inferior mesenteric vessels. Descending or sigmoid colonic tumors require extended left hemicolectomy with ligation of the inferior mesenteric vessels.
Rectal tumors require either anterior or abdominoperineal resection, depending on the proximity to the sphincter.117 Sharp dissection along the mesorectal fascia with en bloc resection of the tumor and its draining lymph nodes (total mesorectal excision [TME]) is currently the gold standard technique in rectal surgery.117 An adequate CRM of more than 2 mm is important.118 Preservation of the anal sphincter, bladder, and sexual function is important but must not compromise adequate resection margins, unless performed for palliation. For those with impaired preoperative anorectal function, radical resection and permanent colostomy may be preferred. Less invasive procedures such as transanal excision for T1 tumors and laparoscopic-assisted TME surgery are feasible in select patients.119–121 For T3 disease, a negative CRM after TME is associated with a lower risk of local recurrence.122 The more conservative approaches of transanal excision or observation for patients with a complete biochemical response to neoadjuvant therapy is not favored owing to the difficulty in precisely determining tumor response to chemoradiation and assessing residual mesorectal lymph node involvement. Currently, there are no data to support the routine use of transanal excision in these patients, and therefore, TME remains to be the definitive treatment.
Lymph node status is one of the most important prognostic factors in CRC. Inconsistencies in number of nodes harvested can affect staging accuracy. Accurate nodal staging allows upstaging of patients and consideration for adjuvant treatment. Therefore, a minimum of 12 lymph nodes is advocated.123 Interestingly, long-term survival increases along with the number of harvested nodes, independent of the proportion of involved nodes.124
Hepatic and pulmonary metastectomy in selected patients improves survival.125 The presence of extrahepatic disease such as the spleen and adrenal glands is no longer considered a contraindication to hepatic metastastectomy.43 For liver metastases, patients with unilobar disease, fewer than four lesions and requiring limited resection, fare better.126 Portal vein embolization and two-stage resections may be considered in select cases to optimize outcome. Conversely, increased size and number of lesions, margins, extrahepatic disease, poorly differentiated tumor, and high CEA levels negatively affect prognosis.127 Underlying disease such as decompensated liver cirrhosis would, however, preclude liver resection. Criteria for resection of pulmonary metastases from CRC include fewer than three lesions in either lung, absence of extrapulmonary disease (other than resectable hepatic metastases), and adequate cardiorespiratory reserve. Size of metastases has been shown to correlate inversely with mean survival time.128
Radiofrequency ablation (RFA) has been reserved for cases in which complete resection is not possible, either alone or in combination with surgery. However, it does not provide survival comparable with that of resection and provides survival slightly superior to that of nonsurgical treatment.129
Radiotherapy
Neoadjuvant chemotherapy and radiation therapy (CRT) are increasingly administered preoperatively to downstage rectal tumors that are classified as T3 or greater; this approach allows more sphincter-preserving low anterior resections with a decreasing incidence of recurrence. Preoperative radiation, combined with chemotherapy, for rectal cancer is administered at doses on the order of 45 to 54 Gy. Most neoadjuvant chemotherapy protocols use 5-fluorouracil or capecitabine.130
Chemotherapy
Node-negative patients are usually not treated with adjuvant chemotherapy because of the lack of definitive evidence of survival benefit. Patients with nodal disease, conversely, should be treated with adjuvant chemotherapy because of the potential reduction of mortality up to 33%.131 Less toxicity and better local control may be achieved when chemoradiation is given preoperatively instead of postoperatively.132 The benefits of neoadjuvant chemoradiation therapy include tumor downstaging, increased tumor resectability, and a higher rate of sphincter preservation. There is also significant reduction in both local recurrence rate and treatment toxicity. Patients who demonstrate complete response to neoadjuvant chemoradiation show improved survival compared with those who do not.
Bevacizumab is an anti-VEGF (vascular endothelial growth factor) antibody that is increasingly used in CRC treatment. Recent trials suggest that it may be used to downstage irresectable liver metastases and, therefore, increase the number of candidates suitable for liver resection. When combined with conventional chemotherapy, it may prolong survival in patients with advanced CRC treated in a palliative setting.133
Key Points Therapies
• Neoadjuvant CRT is increasingly administered preoperatively to downstage rectal tumors.
• Use a standardized operative technique; en bloc resection is critical.
• Optional minimal surgery is the best prevention against local recurrence.
• Adequate sampling of nodes is critical for prognostic assessment.
• Hepatic and pulmonary “metastectomy” in selected patients affects survival.
Clinical Perspectives
Surgeon
EUS is the most accurate way to determine depth of penetration (T stage), particularly for lower-stage mobile tumors; however, it is unreliable for evaluating the degree of residual disease after neoadjuvant treatment.134 It also has limited accuracy in bulky T4 tumors. CT scan can provide useful information about the local extent of disease but cannot distinguish between tumor and inflammation/fibrosis.135 CT is excellent in the evaluation of distant disease. MRI has high accuracy in the assessment of the fascia propria and positive CRM as well as detecting tumor invasion into the genitourinary tract. Coronal, axial, and particularly sagittal views make it the modality of choice for the local staging of advanced rectal tumors.136 PET/CT has a growing role in the evaluation of local recurrence and distant disease but has a limited role in the preoperative staging of colorectal malignancies as compared with EUS, CT, or MRI.106
Radiation Oncologist
Patients with stage II and III (T3-4 and/or N1-2) rectal cancer are now commonly treated with preoperative chemoradiation. Imaging studies are critical in identifying the patients who are appropriate for preoperative chemoradiation. EUS can help determine T and N group, CT can evaluate for nodal involvement and distant metastases, and MRI can determine T and N group and also evaluate extramural depth of invasion and predict CRM status.4,85,137 Imaging studies are also invaluable for radiation therapy planning. In particular, information on the location and extent of the primary rectal cancer, involvement of adjacent structures, and location of involved lymph nodes can guide the design of radiation therapy fields. For patients with colon and rectal cancer who are treated postoperatively with radiation therapy, preoperative imaging studies can play an important role in radiation field design.
Role of Radiation Therapy in the Treatment of Colorectal Cancer
Multiple randomized trials have established the role of postoperative radiation therapy for stage II and III rectal cancer.138–140 More recently, a randomized trial from Germany showed that preoperative chemoradiation leads to higher rates of locoregional control and sphincter preservation and lower rates of acute and long-term toxicity compared with postoperative chemoradiation.133 Moreover, a randomized trial from the United States showed that preoperative chemoradiation improves disease-free survival compared with postoperative chemoradiation.141 Based on these studies, preoperative chemoradiation has been widely accepted as a standard of care for stage II and III rectal cancer. Preoperative chemoradiation is typically delivered with a dose of 45 to 54 Gy, delivered in 1.8- to 2-Gy fractions, over 5 to 6 weeks. An alternative to preoperative chemoradiation is preoperative short-course radiotherapy alone, with a dose of 25 Gy, delivered in 5-Gy fractions, in a single week. A randomized Swedish trial showed that preoperative short-course radiotherapy improves survival compared with surgery alone,142 and a randomized Dutch trial showed that this regimen of preoperative radiotherapy improves local control even in patients undergoing TME.143 Hence, preoperative short-course radiotherapy is considered a standard of care for rectal cancer in many countries.
Controversies exist about the role of radiation therapy for colon cancer. Retrospective studies have shown that postoperative radiation therapy improves local control in patients with T4 tumors, tumors associated with abscess or fistula, and tumors with residual disease after surgery.144 A randomized trial did not show any differences in overall survival or disease-free survival in T3-4 colon cancer patients treated with CRT compared with those treated with radiotherapy alone; however, this trial had many limitations.145 Selected T4 colon cancer patients could potentially benefit from radiation therapy. Furthermore, radiation therapy could play a role in selected colon and rectal cancer patients with isolated recurrences or metastases.
Surveillance
Monitoring Tumor Response
Owing to its ability to depict the rectal wall, EUS has high accuracy (93%) in detecting residual early-stage (T1 and T2) disease, although high-positive rates occur owing to overlap with scar and edema. Therefore, the decision to limit surgical intervention after neoadjuvant therapy cannot be based upon EUS findings.146,147 As with pretreatment assessment, EUS is limited by its small field of view for distant disease. For both local and nodal assessment of rectal cancer after neoadjuvant CRT, CT is not able to reliably predict pathologic response, with a tendency to overstage disease.148
MRI in rectal cancer (performed ≤ 12 wk after CRT) correlates well with histopathology, with accuracies of 82% for local tumor staging and 88% for nodal staging.149 Advanced techniques such as USPIO and dynamic contrast-enhanced MRI have been reported to improve detection of residual disease after treatment.150,151 Mesorectal fascia invasion post CRT is determined by the presence of diffuse iso- or hyperintense tissue in the treated region. Conversely, the development of a fat pad larger than 2 mm is associated with treated disease.152 For CRM involvement, post-CRT restaging MRI had an accuracy of 81% and a high negative predictive value of 91%.153 Nevertheless, MRI still cannot reliably discriminate residual tumor from posttreatment fibrosis because they both appear as hypointense soft tissue thickening on T2-weighted images.154 Therefore, if R0 resection is the goal, pretreatment MRI is preferred to determine the extent of surgical resection.155
PET/CT provides both functional and anatomic information. In the study by Capirci and coworkers,156 taking a decrease in SUV of more than 65% to define metabolic response, the accuracy of PET for detection of residual rectal cancer after neoadjuvant CRT was 81%. Furthermore, FDG-PET can aid prognostication.157 Guillem and colleagues158 showed that rectal cancer patients who remained disease free at a median follow-up of 42 months showed a greater reduction in maximum standardized uptake value (SUVmax) after neoadjuvant treatment than patients who eventually developed recurrence. Quantitative assessment based on tumor volume and SUV (PET metabolic volume) are significantly correlated with CEA levels after surgery.159 It may also enable titration of treatment based on individual response.160
In one study, PET/CT correctly assessed response of liver metastases to bevacizumab-based therapy in 70% of cases compared with 35% by CT.161 For evaluating liver metastases after RFA, MRI and PET/CT are comparable. In the study by Keuhl and associates,162 the accuracy and sensitivity for detection of liver metastases were 91% and 83% for PET/CT and 92% and 75% for MRI, respectively. After treatment of liver metastases with yttrium-90 microspheres, metabolic response on PET/CT correlates better with CEA levels than anatomic response with both CT and MRI.163 Nevertheless, it must be noted that complete metabolic response on FDG-PET after neoadjuvant chemotherapy may not necessarily imply complete pathologic response. Therefore, currently, curative resection of liver metastases should not be deferred solely on the basis of FDG-PET findings.164,165
Detection of Recurrence
Nearly 85% of recurrences occur within the first 3 years after surgery and none after 5 years. Therefore, most surveillance strategies focus resources on the first 3 years. The meta-analysis by Bruinvels and coworkers166 involving 3923 patients showed that, in patients with intensive follow-up that included CEA assays, there were more asymptomatic recurrences that were amenable to surgery, leading to 9% better 5-year survival rates than those with minimal or no follow-up. For routine surveillance, the American Society of Clinical Oncology (ASCO) currently recommends CEA assays every 3 months for the first 3 years; CT scan of the chest, abdomen, and pelvis annually for the first 3 years; and colonoscopy at 3 years in patients with stage 2 and stage 3 CRC.167
As a result of its superior soft tissue resolution, MRI is better than CT at detecting local disease recurrence in rectal cancer, especially in differentiating normal pelvic soft tissue structures from that of recurrent tumor.168,169 For the more proximal colon, CT is probably better than MRI because of its higher temporal resolution. On CT, recurrence is demonstrated by serial progression of a mass, nodular configuration, and invasion of adjacent structures.170 MRI also relies on anatomic evaluation, although tumor tissue can be differentiated from fibrous tissue when it shows hyperintense signal on T2-weighted images.
PET/CT is increasingly shown to be superior to the other imaging modalities in demonstrating recurrent disease activity and has become an integral part of the surveillance strategy. It has the potential to replace CT as the first-line diagnostic tool for restaging patients for recurrent CRC.171 PET/CT can distinguish between tumor recurrence and postsurgical scar, as well as pinpoint the site of recurrence in cases of unexplained rise in serum CEA.172 It is recommended for evaluation of equivocal findings on serial CT and MRI.173 For recurrent nodal disease, PET/CT is superior to MRI, with a sensitivity of 93%.174 PET/CT is superior to contrast-enhanced CT in detecting local recurrences at the colorectal anastomosis, intrahepatic recurrences, and extrahepatic disease, with sensitivity rates of close to or exceeding 90%.175 Quantitative measurements of SUV and tumor volume may be used as a marker of tumor burden in cases of tumor recurrence.176 Note that PET/CT should be performed more than 6 weeks after surgery because inflammatory changes can result in false positives.
Key Points Surveillance
• Routine surveillance: The ASCO currently recommends CEA assays every 3 months for the first 3 years; CT scan of the chest, abdomen, and pelvis annually for the first 3 years; and colonoscopy at 3 years in patients with stage 2 and stage 3 CRC.167
• PET/CT is increasingly shown to be superior to the other imaging modalities in demonstrating recurrent disease activity and has become an integral part of the surveillance strategy.
Complications of Therapy
Chemotherapy
Chemotherapy-associated steatohepatitis may not be distinguishable from other forms of steatohepatitis on imaging.177 With the platinum-based chemotherapy agents such as oxaliplatin, hepatotoxicity in the form of sinusoidal obstruction can present with heterogeneous appearance of the liver, portal vein dilatation, and cirrhosis in late stage.178 This may be associated with nodular regenerative hyperplasia.179 Severe enteropathy may also occur.180
With bevacizumab, an antiangiogenic agent, there is reported increased incidence of gastrointestinal perforation (0.9%) among patients receiving bevacizumab, with a mortality rate of approximately 20%.181 Other complications include venous thromboembolism (6-12%) and a dose-dependent increase in risk of proteinuria and hypertension.182,183
Radiation Therapy
The overall incidence of chronic radiation injury to the bowel after radiotherapy to the pelvis is about 1% to 5%.184 This can manifest acutely with edema and dilatation, followed by stricture formation. Complex fistulas may also develop. Atrophy of the gynecologic organs may result. Only a minority of urologic complications such as radiation cystitis can be ascribed to the effects of radiation alone because chemotherapy is often given together and can have additive effects. Demineralization, spontaneous fractures, aseptic necrosis, and rarely, radiation-induced sarcomas may develop within the pelvic bones.185 Prior knowledge of the boundaries of the radiation port and the time interval between the initiation and the cessation of therapy can be extremely helpful for making the diagnosis of radiation-induced changes. This is especially in the context of intensity-modulated radiation therapy (IMRT), in which complex and irregular clinical target volumes are irradiated.
New Therapies
For rectal cancer, TME is now a standardized procedure with reproducible and improved cancer-specific outcomes.186 Less invasive methods such as transanal excision may be performed to reduce morbidity associated with radical surgery, but this can be applied only to selected T1 tumors without high-risk features.187 It may also be an option for palliation in patients with locally advanced disease unsuitable for radical resection. Transanal endoscopic microsurgery (TEM) is an option for lesions too proximal to be resected by standard transanal excision.188
Laparoscopic surgery is currently considered an acceptable alternative to open resection, with measurable short-term benefits such as decreased postoperative use of narcotic analgesics, earlier return of bowel function, shorter length of stay, and better cosmesis.189 Sentinel lymph node mapping (ultrastaging) may improve lymph node yield from surgery, but its effects on disease prognostication require validation.190,191 Aggressive surgical resection of liver and lung metastases improves survival. For the majority of patients with liver metastases who have unresectable disease, RFA shows slight improvement in outcome compared with other nonsurgical therapies.129 The survival benefits of selective internal radiation therapy (SIRT) with yttrium-90 microspheres remains controversial.192
Preoperative radiotherapy in combination with 5-fluorouracil is now standard treatment for locally advanced rectum carcinoma.193 Capecitabine is an oral chemotherapeutic that is converted to 5-fluorouracil in vivo, with decreased toxicity. Among the antiangiogenic agents, bevacuzimab (Avastin) may help to downstage irresectable liver metastases. On the horizon are cyclooxygenase-2 (COX-2) inhibitors, possessing both antiangiogenic and apoptotic effects on human colon cancer cells.194 They may have the potential to reduce recurrence but require validation for clinical use.
1. Horner M.J., Ries L.A.G., Krapcho M., et al. SEER Cancer Statistics Review. Bethesda, MD: National Cancer Institute; 1975-2006. http://seer.cancer.gov/csr/1975_2006.. Available at Based on November 2008 SEER data submission, posted to the SEER website, 2009
2. Levin B., Lieberman D.A., McFarland B., et al. American Cancer Society Colorectal Cancer Advisory Group; US Multi-Society Task Force; American College of Radiology Colon Cancer Committee. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570-1595.
3. Wald C., Scheirey C.D., Tran T.M., Erbay N. An update on imaging of colorectal cancer. Surg Clin North Am.. 2006;86:819-847.
4. MERCURY Study Group. Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer: results of the MERCURY study. Radiology. 2007;243:132-139.
5. Adam R. The importance of visceral metastasectomy in colorectal cancer. Ann Oncol. 2000;11(Suppl 3):29-36.
6. Goldberg R.M., Fleming T.R., Tangen C.M., et al. Surgery for recurrent colon cancer: strategies for identifying resectable recurrence and success rates after resection. Eastern Cooperative Oncology Group, the North Central Cancer Treatment Group, and the Southwest Oncology Group. Ann Intern Med. 1998;129:27-35.
7. Jemal A., Siegel R., Ward E., et al. Cancer statistics. CA Cancer J Clin. 2008;58:71-96.
8. Espey D.K., Wu X.C., Swan J., et al. Annual report to the nation on the status of cancer, 1975-2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007;110:2119-2152.
9. Nusko G., Mansmann U., Partzsch U., et al. Invasive carcinoma in colorectal adenomas: multivariate analysis of patient and adenoma characteristics. Endoscopy. 1997;29:626-631.
10. Higuchi T., Jass J.R. My approach to serrated polyps of the colorectum. J Clin Pathol. 2004;57:682-686.
11. Jeter J.M., Kohlmann W., Gruber S.B. Genetics of colorectal cancer. Oncology (Huntingt). 2006;20:269-276. discussion 285-286, 288-289
12. Conlin A., Smith G., Carey F.A., et al. The prognostic significance of K-ras, p53, and APC mutations in colorectal carcinoma. Gut. 2005;54:1283-1286.
13. Triantafillidis J.K., Nasioulas G., Kosmidis P.A. Colorectal cancer and inflammatory bowel disease: epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer Res.. 2009;29:2727-2737.
14. Chung Y.W., Han D.S., Park Y.K., et al. Association of obesity, serum glucose and lipids with the risk of advanced colorectal adenoma and cancer: a case-control study in Korea. Dig Liver Dis. 2006;38:668-672.
15. Gonzalez C.A. Nutrition and cancer: the current epidemiological evidence. Br J Nutr. 2006;96(Suppl 1):S42-S45.
16. Compton C.C. Colorectal carcinoma: diagnostic, prognostic, and molecular features. Mod Pathol. 2003;16:376-388.
17. Compton C.C., Fielding L.P., Burgart L.J., et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:979-994.
18. Compton C., Fenoglio-Preiser C.M., Pettigrew N., Fielding L.P. American Joint Committee on Cancer Prognostic Factors Consensus Conference: Colorectal Working Group. Cancer. 2000;88:1739-1757.
19. Kang H., O’Connell J.B., Leonardi M.J., et al. Rare tumors of the colon and rectum: a national review. Int J Colorectal Dis. 2007;22:183-189.
20. Compton C.C. Pathology report in colon cancer: what is prognostically important? Dig Dis. 1999;17:67-79.
21. Cappell M.S. The pathophysiology, clinical presentation, and diagnosis of colon cancer and adenomatous polyps. Med Clin North Am.. 2005;89:1-42.
22. Speights V.O., Johnson M.W., Stoltenberg P.H., et al. Colorectal cancer: current trends in initial clinical manifestations. South Med J. 1991;84:575-578.
23. Cappell M.S. From colonic polyps to colon cancer: pathophysiology, clinical presentation, and diagnosis. Clin Lab Med. 2005;25:135-177.
24. Cappell M.S., Goldberg E.S. The relationship between the clinical presentation and spread of colon cancer in 315 consecutive patients. A significant trend of earlier cancer detection from 1982 through 1988 at a university hospital. J Clin Gastroenterol. 1992;14:227-235.
25. Cappell M.S. Pathophysiology, clinical presentation, and management of colon cancer. Gastroenterol Clin North Am.. 2008;37:1-24.
26. Duffy M.J. Carcinoembryonic antigen as a marker for colorectal cancer: is it clinically useful? Clin Chem.. 2001;47:624-630.
27. Allison J.E., Tekawa I.S., Ransom L.J., Adrain A.L. A comparison of fecal occult-blood tests for colorectal-cancer screening. N Engl J Med. 1996;334:155-159.
28. Smith A., Young G.P., Cole S.R., Bampton P. Comparison of a brush-sampling fecal immunochemical test for hemoglobin with a sensitive guaiac-based fecal occult blood test in detection of colorectal neoplasia. Cancer. 2006;107:2152-2159.
29. Levin B., Brooks D., Smith R.A., Stone A. Emerging technologies in screening for colorectal cancer: CT colonography, immunochemical fecal occult blood tests, and stool screening using molecular markers. CA Cancer J Clin. 2003;53:44-55.
30. Sarela A., O’Riordain D.S. Rectal adenocarcinoma with liver metastases: management of the primary tumour. Br J Surg. 2001;88:163-164.
31. Niederhuber J.E. Colon and rectum cancer. Patterns of spread and implications for workup. Cancer. 1993;71(Suppl 12):4187-4192.
32. Weiss E.G., Laverly I., et al. Colon cancer evaluation and staging. In: Wolff B.G., Fleshman J.W., Beck D.E. The ASCRS Textbook of Colon and Rectal Surgery. New York: Springer Science + Business Media; 2007:388-389.
33. Knudsen J.B., Nilsson T., Sprechler M., et al. Venous and nerve invasion as prognostic factors in postoperative survival of patients with resectable cancer of the rectum. Dis Colon Rectum. 1983;26:613-617.
34. Giess C.S., Schwartz L.H., Bach A.M., et al. Patterns of neoplastic spread in colorectal cancer: implications for surveillance CT studies. AJR Am J Roentgenol. 1998;170:987-991.
35. Iyer R.B., Silverman P.M., DuBrow R.A., Charnsangavej C. Imaging in the diagnosis, staging and follow-up of colorectal cancer. AJR Am J Roentgenol. 2002;179:3-13.
36. Charnsangavej C. Pathways of lymph node metastases in cancer of the gastrointestinal and hepatobiliary tracts. In: Myers M.A., editor. Dynamic Radiology of the Abdomen. 5th ed. New York: Springer-Verlag; 2000:287-308.
37. Park I.J., Choi G.S., Lim K.H., et al. Different patterns of lymphatic spread of sigmoid, rectosigmoid, and rectal cancers. Ann Surg Oncol. 2008;15:3478-3483.
38. Boni L., Benevento A., Dionigi G., et al. Injection of colorectal cancer cells in mesenteric and antimesenteric sides of the colon results in different patterns of metastatic diffusion: an experimental study in rats. World J Surg Oncol. 2005;3:69.
39. Shepherd N.A., Baxter K.J., Love S.B. The prognostic importance of peritoneal involvement in colonic cancer: a prospective evaluation. Gastroenterology. 1997;112:1096-1102.
40. Kemeny N. Systemic and regional chemotherapy in advanced colorectal carcinoma. In: MacDonald J.S., editor. Gastrointestinal Oncology: Basic and Clinical Aspects. Boston: Martin Nijhoff, 1987. :235-251
41. Weiss L., Grundmann E., Torhorst J., et al. Haematogenous metastatic patterns in colonic carcinoma: an analysis of 1541 necropsies. J Pathol. 1986;150:195-203.
42. Stangl R., Altendorf-Hofmann A., Charnley R.M., et al. Factors influencing the natural history of colorectal liver metastases. Lancet. 1994;343:1405-1410.
43. Garden O.J., Rees M., Poston G.J., et al. Guidelines for resection of colorectal cancer liver metastases. Gut. 55(Suppl 3), 2006. iii1-iii8
44. Evans D.B., Catalano P., Charnsangavej C.. Digestive system. Greene F.L., Page D.L., Fleming I.D., et al. AJCC Cancer Staging Manual, 6th ed., New York: Springer, 2002. :89-164
45. Colon and rectum.. Sobin L.H., Wittekind C. TNM: Classification of Malignant Tumors, 6th ed., New York: Wiley-Liss, 2002. :72-76
46. Compton C.C., Greene F.L. The staging of colorectal cancer: 2004 and beyond. CA Cancer J Clin. 2004;54:295-308.
47. Colon and rectum.. Wittekind C., Greene F.L., Henson D.E. TNM Supplement: A Commentary on Uniform Use, 3rd ed., New York: Wiley-Liss, 2003. :41-44
48. Shepherd N., Baxter K., Love S. The prognostic importance of peritoneal involvement in colonic cancer: a prospective evaluation. Gastroenterology. 1997;112:1096-1102.
49. Herrera-Ornelas L., Justiniano J., Castillo N., et al. Metastases in small lymph nodes from colon cancer. Arch Surg. 1987;122:1253-1256.
50. Brown H.G., Luckasevic T.M., Medich D.S., et al. Efficacy of manual dissection of lymph nodes in colon cancer resections. Mod Pathol. 2004;17:402-406.
51. Compton C.C., Fielding L.P., Burgart L.J., et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:979-994.
52. Goldstein N.S., Turner J.R. Pericolonic tumor deposits in patients with T3N+M0 colon adenocarcinomas. Markers of reduced disease free survival and intra-abdominal metastases and their implications for TNM classification. Cancer. 2000;88:2228-2238.
53. Singletary S.E., Greene F.L., Sobin L.H. Classification of isolated tumor cells: clarification of the 6th edition of the American Joint Committee on Cancer Staging Manual. Cancer. 2003;98:2740-2741.
54. Compton C.C., Fenoglio-Preiser C.M., Pettigrew N., Fielding L.P. American Joint Committee on Cancer Prognostic Factors consensus conference: Colorectal Working Group. Cancer. 2000;88:1739-1757.
55. Sloane J.P. Minimum dataset for colorectal cancer histopathology reports. In Standards and Minimum Datasets for Reporting Common Cancers. Minimum Datasets for Reporting Common Cancers. London: The Royal College of Pathologists; 1998. Royal College of Pathologists Working Group on Cancer Services:1-10
56. Petersen V.C., Baxter K.J., Love S.B., Shepherd N.A. Identification of objective pathologic prognostic determinants and models of prognosis in Dukes’ B colon cancer. Gut. 2002;51:65-69.
57. Stocchi L., Nelson H., Sargent D.J., et al. Impact of surgical and pathological variables in rectal cancer: a United States community and cooperative group report. J Clin Oncol. 2001;19:3895-3902.
58. Mulcahy H.E., Skelly M.M., Husain A., O’Donoghue D.P. Long-term outcome following curative surgery for malignant large bowel obstruction. Br J Surg. 1996;83:46-50.
59. Chapuis P.H., Dent O.F., Fisher R., et al. A multivariate analysis of clinical and pathological variables in prognosis after resection of large bowel cancer. Br J Surg. 1985;72:698-702.
60. Newland R., Dent O., Lyttle M., et al. Pathologic determinants of survival associated with CRC with lymph node metastases. A multivariate analysis of 579 patients. Cancer. 1994;73:2076-2082.
61. Talbot I., Ritchie S., Leighton M.H., et al. The clinical significance of invasion of veins by rectal cancer. Br J Surg. 1980;67:439-442.
62. Horn A., Dahl O., Morild I. Venous and neural invasion as predictors of recurrence in rectal adenocarcinoma. Dis Colon Rectum. 1991;34:798-804.
63. Takahashi Y., Tucker S., Kitadai Y., et al. Vessel counts and expression of vascular endothelial growth factor as prognostic factors in node-negative colon cancer. Arch Surg. 1997;132:541-546.
64. Minsky B., Mies C., Recht A., et al. Resectable adenocarcinoma of the rectosigmoid and rectum. II. The influence of blood vessel invasion. Cancer. 1988;61:1417-1424.
65. Fork F.T. Double contrast enema and colonoscopy in polyp detection. Gut. 1981;22:971.
66. Steine S., Stordahl A., Lunde O.C., et al. Double-contrast barium enema versus colonoscopy in the diagnosis of neoplastic disorders: aspects of decision-making in general practice. Fam Pract. 1993;10:288.
67. Winawer S.J., Stewart E.T., Zauber A.G., et al. A comparison of colonoscopy and double-contrast barium enema for surveillance after polypectomy. National Polyp Study Work Group. N Engl J Med. 2000;342:1766-1772.
68. Blakeborough A., Sheridan M.B., Chapman A.H. Complications of barium enema examinations: a survey of UK consultant radiologists 1992 to 1994. Clin Radiol. 1997;52:142-148.
69. Levin T.R., Zhao W., Conell C., et al. Complications of colonoscopy in an integrated health care delivery system. Ann Intern Med. 2006;145:880-886.
70. Johnson C.D., Chen M.H., Toledano A.Y., et al. Accuracy of CT colonography in the detection of large adenomas and cancers. N Engl J Med. 2008;359:1207-1217. Erratum in N Engl J Med. 2008;359:2853
71. Pickhardt P.J., Choi J.R., Hwang I., et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003;349:2191-2200.
72. Young B.M., Fletcher J.G., Paulsen S.R., et al. Polyp measurement with CT colonography: multiple-reader, multiple-workstation comparison. AJR Am J Roentgenol. 2007;188:122-129.
73. Kim D.H., Pickhardt P.J., Hoff G., Kay C.L. Computed tomographic colonography for colorectal screening. Endoscopy. 2007;39:545-549.
74. Kim D.H., Pickhardt P.J., Taylor A.J. Characteristics of advanced adenomas detected at CT colonographic screening: implications for appropriate polyp size thresholds for polypectomy versus surveillance. AJR Am J Roentgenol. 2007;188:940-944.
75. Moravec M., Lieberman D., Holub J., et al. Rate of advanced pathologic features in 6-9 mm polyps in patients referred for colonoscopy screening. Gastrointest Endosc. 2007;65:822.
76. Brenner D.J., Georgsson M.A. Mass screening with CT colonography: should the radiation exposure be of concern? Gastroenterology. 2005;129:328-337.
77. Macari M., Bini E.J., Xue X., et al. Colorectal neoplasms: prospective comparison of thin-section low-dose multi-detector row CT colonography and conventional colonoscopy for detection. Radiology. 2002;224:383-392.
78. Pickhardt P.J. Incidence of colonic perforation at CT colonography: review of existing data and implications for screening of asymptomatic adults. Radiology. 2006;239:313-316.
79. Burling D., Halligan S., Slater A., et al. Potentially serious adverse events at CT colonography in symptomatic patients: national survey of the United Kingdom. Radiology. 2006;239:464-471.
80. Kelvin F.M., et al. Diagnosis of colorectal cancer by conventional radiology. In: Meyers M.A., editor. Neoplasms of the Digestive Tract. 1st ed. Philadelphia:: Lippincott-Raven; 1998:221-223.
81. Akasu T., Kondo H., Moriya Y., et al. Endorectal ultrasonography and treatment of early stage rectal cancer. World J Surg. 2000;24:1061.
82. Beets-Tan R.G., Beets G.L. Rectal cancer: review with emphasis on MR imaging. Radiology. 2004;232:335.
83. Bipat S., Glas A.S., Slors F.J., et al. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging meta-analysis. Radiology. 2004;232:773.
84. Heriot A.G., Grundy A., Kumar D. Preoperative staging of rectal carcinoma. Br J Surg. 1999;86:17.
85. Kwok H., Bissett I.P., Hill G.L. Preoperative staging of rectal cancer. Int J Colorectal Dis. 2000;15:9.
86. O’Neill B.D., Salerno G., Thomas K., et al. MR vs CT imaging: low rectal cancer tumour delineation for three-dimensional conformal radiotherapy. Br J Radiol. 2009;82:509-513.
87. Wolberink S.V., Beets-Tan R.G., de Haas-Kock D.F., et al. Multislice CT as a primary screening tool for the prediction of an involved mesorectal fascia and distant metastases in primary rectal cancer: a multicenter study. Dis Colon Rectum. 2009;52:928-934.
88. Brent A., Talbot R., Coyne J., Nash G. Should indeterminate lung lesions reported on staging CT scans influence the management of patients with colorectal cancer? Colorectal Dis. 2007;9:816-818.
89. Adeyemo D., Hutchinson R. Preoperative staging of rectal cancer: pelvic MRI plus abdomen and pelvic CT. Does extrahepatic abdomen imaging matter? A case for routine thoracic CT. Colorectal Dis. 2009;11:259-263.
90. Wu G.Y., Ghimire P. Perfusion computed tomography in colorectal cancer: protocols, clinical applications and emerging trends. World J Gastroenterol. 2009;15:3228-3231.
91. Goh V., Halligan S., Wellsted D.M., Bartram C.I. Can perfusion CT assessment of primary colorectal adenocarcinoma blood flow at staging predict for subsequent metastatic disease? A pilot study. Eur Radiol. 2009;19:79-89.
92. Veit-Haibach P., Treyer V., Strobel K., et al. Feasibility of integrated CT-liver perfusion in routine FDG-PET-CT. Abdom Imaging. 2010;35:528-536.
93. Nagata K., Ota Y., Okawa T., et al. PET-CT colonography for the preoperative evaluation of the colon proximal to the obstructive colorectal cancer. Dis Colon Rectum. 2008;51:882-890.
94. Brown G., Daniels I.R., Richardson C., et al. Techniques and trouble-shooting in high spatial resolution thin slice MRI for rectal cancer. Br J Radiol. 2005;78:24.
95. MERCURY Study Group. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ. 2006;333:779.
96. Beets-Tan R.G., Beets G.L., Borstlap A.C., et al. Preoperative assessment of local tumor extent in advanced rectal cancer: CT or high-resolution MRI? Abdom Imaging. 2000;25:533-541.
97. Shinya S., Sasaki T., Nakagawa Y., et al. The efficacy of diffusion-weighted imaging for the detection of colorectal cancer. Hepatogastroenterology. 2009;56:128-132.
98. Lahaye M.J., Engelen S.M., Nelemans P.J., et al. Imaging for predicting the risk factors—the circumferential resection margin and nodal disease—of local recurrence in rectal cancer: a meta-analysis. Semin Ultrasound CT MR. 2005;26:259-268.
99. Ferri M., Laghi A., Mingazzini P., et al. Pre-operative assessment of extramural invasion and sphincteral involvement in rectal cancer by magnetic resonance imaging with phased-array coil. Colorectal Dis. 2005;7:387.
100. Brown G., Radcliffe A.G., Newcombe R.G., et al. Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging. Br J Surg. 2003;90:355.
101. Koh D.M., Brown G., Temple L., et al. Rectal cancer: mesorectal lymph nodes at MR imaging with USPIO versus histopathologic findings initial observations. Radiology. 2004;231:91.
102. Squillaci E., Manenti G., Mancino S., et al. Staging of colon cancer: whole-body MRI vs. whole-body PET-CT—initial clinical experience. Abdom Imaging. 2008;33:676-688.
103. Badiee S., Franc B.L., Webb E.M., et al. Role of IV iodinated contrast material in 18F-FDG PET-CT of liver metastases. AJR Am J Roentgenol. 2008;191:1436-1439.
104. Veit-Haibach P., Kuehle C.A., Beyer T., et al. Diagnostic accuracy of colorectal cancer staging with whole-body PET-CT colonography. JAMA. 2006;296:2590-2600.
105. Bassi M.C., Turri L., Sacchetti G., et al. FDG-PET-CT imaging for staging and target volume delineation in preoperative conformal radiotherapy of rectal cancer. Int J Radiat Oncol Biol Phys. 2008;70:1423-1426.
106. Llamas-Elvira J.M., Rodríguez-Fernández A., Gutiérrez-Sáinz J., et al. Fluorine-18 fluorodeoxyglucose PET in the preoperative staging of colorectal cancer. Eur J Nucl Med Mol Imaging. 2007;34:859-867.
107. Gearhart S.L., Frassica D., Rosen R., et al. Improved staging with pretreatment positron emission tomography/computed tomography in low rectal cancer. Ann Surg Oncol. 2006;13:397-404.
108. Nahas C.S.R., Akhurst T., Yeung H., et al. Positron emission tomography detection of distant metastatic or synchronous disease in patients with locally advanced rectal cancer receiving preoperative chemoradiation. Ann Surg Oncol. 2007;15:704-711.
109. Wiering B., Krabbe P.F., Jager G.J., et al. The impact of fluoro-18-deoxyglucose-positron emission tomography in the management of colorectal liver metastases. Cancer. 2005;104:2658-2670.
110. Yasuda S., Fujii H., Nakahara T., et al. 18F-FDG-PET detection of colonic adenomas. J Nucl Med. 2001;42:989-992.
111. Berger K.L., Nicholson S.A., Dehadashti F., et al. FDG-PET evaluation of mucinous neoplasms: correlation of FDG uptake with histopathologic features. AJR Am J Roentgenol. 2000;174:1005-1008.
112. Kosugi C., Saito N., Murakami K., et al. Positron emission tomography for preoperative staging in patients with locally advanced or metastatic colorectal adenocarcinoma in lymph node metastasis. Hepatogastroenterology. 2008;55:398-402.
113. Bokey E.L., Chapuis P.H., Dent O.F., et al. Surgical technique and survival in patients having a curative resection for colon cancer. Dis Colon Rectum. 2003;46:860-866.
114. Fengler S.A., Pearl R.K. Technical considerations in the surgical treatment of colon and rectal cancer. Semin Surg Oncol. 1994;10:200-207.
115. Pollett W.G., Nicholls R.J. The relationship between the extent of distal clearance and survival and local recurrence rates after curative anterior resection for carcinoma of the rectum. Ann Surg. 1983;198:159-163.
116. Ruo L., Guillem J.G. Surgical management of primary CRC. Surg Oncol. 1998;7:153-163.
117. Lange M.M., Rutten H.J., van de Velde C.J. One hundred years of curative surgery for rectal cancer: 1908-2008. Eur J Surg Oncol. 2009;35:456-463.
118. Bernstein T.E., Endreseth B.H., Romundstad P., Wibe A. Norwegian CRC Group. Circumferential resection margin as a prognostic factor in rectal cancer. Br J Surg. 2009;96:1348-1357.
119. Doornebosch P.G., Tollenaar R.A., De Graaf E.J. Is the increasing role of transanal endoscopic microsurgery in curation for T1 rectal cancer justified? A systematic review. Acta Oncol. 2009;48:343-353.
120. Leroy J., Jamali F., Forbes L., et al. Laparoscopic total mesorectal excision (TME) for rectal cancer surgery: long-term outcomes. Surg Endosc. 2004;18:281-289.
121. Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050-2059.
122. Nagtegaal I.D., Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol. 2008;26:303-312.
123. Compton C.C. Updated protocol for the examination of specimens from patients with carcinomas of the colon and rectum, excluding carcinoid tumors, lymphomas, sarcomas, and tumors of the vermiform appendix: a basis for checklists. Cancer Committee. Arch Pathol Lab Med. 2000;124:1016-1025.
124. Chang G.J., Rodriguez-Bigas M.A., Skibber J.M., Moyer V.A. Lymph node evaluation and survival after curative resection of colon cancer: systematic review. J Natl Cancer Inst. 2007;99:433-441.
125. Neeff H., Hörth W., Makowiec F., et al. Outcome after resection of hepatic and pulmonary metastases of CRC. J Gastrointest Surg. 2009;13:1813-1820.
126. Wanebo H.J., Chu Q.D., Vezeridis M.P., Soderberg C. Patient selection for hepatic resection of colorectal metastases. Arch Surg. 1996;131:322-329.
127. Rees M., Tekkis P.P., Welsh F.K., et al. Evaluation of long-term survival after hepatic resection for metastatic CRC: a multifactorial model of 929 patients. Ann Surg. 2008;247:125-135.
128. Vogelsang H., Haas S., Hierholzer C., et al. Factors influencing survival after resection of pulmonary metastases from CRC. Br J Surg. 2004;91:1066-1071.
129. Abdalla E.K., Vauthey J.N., Ellis L.M., et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818-825.
130. Meade P.G., Blatchfor G.J., Thorson A.G., et al. Preoperative chemo-radiation downstages locally advanced ultrasound-staged rectal cancer. Am J Surg. 1995;170:609-613.
131. Wolmark N., Rockette H., Fisher B., et al. The benefit of leucovorin-modulated fluorouracil as postoperative adjuvant therapy for primary colon cancer: results from National Surgical Adjuvant Breast and Bowel Project protocol C-03. J Clin Oncol. 11, 1993. 1879-1887
132. Sauer R., Becker H., Hohenberger W., et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 351, 2004. 1731-1740
133. Giantonio B.J., Catalano P.J., Meropol N.J., et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539-1544.
134. Vanagunus A., Lin E., Stryker S. Accuracy of endoscopic ultrasound for restaging rectal cancer following neoadjuvant chemoradation therapy. Am J Gastroenterol. 2004;99:109-112.
135. Farouk R., Nelson H., Radice E., et al. Accuracy of computed tomography in determining resectability for locally advanced primary or recurrent colorectal cancers. Am J Surg. 1998;175:283-287.
136. Brown G., Davies S., Williams G., et al. Effectiveness of preoperative staging in rectal cancer: digital rectal examination, endoluminal ultrasound or magnetic resonance imaging? Br J Cancer. 2004;91:23-29.
137. Smith N., Brown G. Preoperative staging of rectal cancer. Acta Oncol. 2008;47:20-31.
138. Prolongation of the disease-free interval in surgically treated rectal carcinoma. Gastrointestinal Tumor Study Group. N Engl J Med. 1985;312:1465-1472.
139. Douglass H.O.Jr., Moertel C.G., Mayer R.J., et al. Survival after postoperative combination treatment of rectal cancer [letter]. N Engl J Med. 1986;315:1294-1295.
140. Wolmark N., Wieand H.S., Hyams D.M., et al. Randomized trial of postoperative adjuvant chemotherapy with or without radiotherapy for carcinoma of the rectum: National Surgical Adjuvant Breast and Bowel Project Protocol R-02. J Natl Cancer Inst. 2000;92:388-396.
141. Roh M.S., Colangelo L.H., O’Connell M.J., et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol. 2009;27:5124-5130.
142. Improved survival with preoperative radiotherapy in resectable rectal cancer. Swedish Rectal Cancer Trial. N Engl J Med. 1997;336:980-987.
143. Kapiteijn E., Marijnen C.A., Nagtegaal I.D., et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638-646.
144. Willett C.G., Fung C.Y., Kaufman D.S., et al. Postoperative radiation therapy for high-risk colon carcinoma. J Clin Oncol. 1993;11:1112-1117.
145. Martenson J.A.Jr., Willett C.G., Sargent D.J., et al. Phase III study of adjuvant chemotherapy and radiation therapy compared with chemotherapy alone in the surgical adjuvant treatment of colon cancer: results of intergroup protocol 0130. J Clin Oncol. 2004;12:12.
146. Meyenberger C., Huch Böni R.A., Bertschinger P., et al. Endoscopic ultrasound and endorectal magnetic resonance imaging: a prospective, comparative study for preoperative staging and follow-up of rectal cancer. Endoscopy. 1995;27:469-479.
147. Maor Y., Nadler M., Barshack I., et al. Endoscopic ultrasound staging of rectal cancer: diagnostic value before and following chemoradiation. J Gastroenterol Hepatol. 2006;21:454-458.
148. Huh J.W., Park Y.A., Jung E.J., et al. Accuracy of endorectal ultrasonography and computed tomography for restaging rectal cancer after preoperative chemoradiation. J Am Coll Surg. 2008;207:7-12.
149. Johnston D.F., Lawrence K.M., Sizer B.F., et al. Locally advanced rectal cancer: histopathological correlation and predictive accuracy of serial MRI after neoadjuvant chemotherapy. Br J Radiol. 2009;82:332-336.
150. Lahaye M.J., Beets G.L., Engelen S.M., et al. Locally advanced rectal cancer: MR imaging for restaging after neoadjuvant radiation therapy with concomitant chemotherapy. Part II. What are the criteria to predict involved lymph nodes? Radiology. 2009;252:81-91.
151. de Lussanet Q.G., Backes W.H., Griffioen A.W., et al. Dynamic contrast-enhanced magnetic resonance imaging of radiation therapy-induced microcirculation changes in rectal cancer. Int J Radiat Oncol Biol Phys.. 2005;63:1309-1315.
152. Vliegen R.F., Beets G.L., Lammering G., et al. Mesorectal fascia invasion after neoadjuvant chemotherapy and radiation therapy for locally advanced rectal cancer: accuracy of MR imaging for prediction. Radiology. 2008;246:454-462.
153. Kulkarni T., Gollins S., Maw A., et al. Magnetic resonance imaging in rectal cancer downstaged using neoadjuvant chemoradiation: accuracy of prediction of tumour stage and circumferential resection margin status. Colorectal Dis. 2008;10:479-489.
154. Suppiah A., Hunter I.A., Cowley J., et al. Magnetic resonance imaging accuracy in assessing tumour down-staging following chemoradiation in rectal cancer. Colorectal Dis. 2009;11:249-253.
155. Larsen S.G., Wiig J.N., Emblemsvaag H.L., et al. Extended total mesorectal excision in locally advanced rectal cancer (T4a) and the clinical role of MRI-evaluated neo-adjuvant downstaging. Colorectal Dis. 2009;11:759-767.
156. Capirci C., Rubello D., Pasini F., et al. The role of dual-time combined 18-fluorodeoxyglucose positron emission tomography and computed tomography in the staging and restaging workup of locally advanced rectal cancer, treated with preoperative chemoradiation therapy and radical surgery. Int J Radiat Oncol Biol Phys. 2009;74:1461-1469.
157. Dirisamer A., Halpern B.S., Flöry D., et al. Performance of integrated FDG-PET/contrast-enhanced CT in the staging and restaging of colorectal cancer: comparison with PET and enhanced CT. Eur J Radiol. 2010;73:324-328.
158. Guillem J.G., Moore H.G., Akhuurst T., et al. Sequential preoperative fluorodeoxyglucose-positron emission tomography assessment of response to preoperative chemoradiation: a means for determining long term outcomes of rectal cancer. J Am Coll Surg. 2004;199:1-7.
159. Choi M.Y., Lee K.M., Chung J.K., et al. Correlation between serum CEA level and metabolic volume as determined by FDG PET in postoperative patients with recurrent colorectal cancer. Ann Nucl Med. 2005;19:123-129.
160. Vriens D., de Geus-Oei L.F., van der Graaf W.T., Oyen W.J. Tailoring therapy in colorectal cancer by PET-CT. Q J Nucl Med Mol Imaging. 2009;53:224-244.
161. Goshen E., Davidson T., Zwas S.T., Aderka D. PET/CT in the evaluation of response to treatment of liver metastases from colorectal cancer with bevacizumab and irinotecan. Technol Cancer Res Treat. 2006;5:37-43.
162. Kuehl H., Antoch G., Stergar H., et al. Comparison of FDG-PET, PET/CT and MRI for follow-up of colorectal liver metastases treated with radiofrequency ablation: initial results. Eur J Radiol. 2008;67:362-371.
163. Wong C.Y., Salem R., Raman S., et al. Evaluating 90Y-glass microsphere treatment response of unresectable colorectal liver metastases by [18F]FDG PET: a comparison with CT or MRI. Eur J Nucl Med Mol Imaging. 2002;29:815-820.
164. Tan M.C., Linehan D.C., Hawkins W.G., et al. Chemotherapy-induced normalization of FDG uptake by colorectal liver metastases does not usually indicate complete pathologic response. J Gastrointest Surg. 2007;11:1112-1119.
165. Lubezky N., Metser U., Geva R., et al. The role and limitations of 18-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) scan and computerized tomography (CT) in restaging patients with hepatic colorectal metastases following neoadjuvant chemotherapy: comparison with operative and pathological findings. J Gastrointest Surg. 2007;11:472-478.
166. Bruinvels D.J., Stiggelbout A.M., Kievit J., et al. Follow-up of patients with colorectal cancer. A meta-analysis. Ann Surg. 1994;219:174-182.
167. Desch C.E., Benson A.B.3rd, Somerfield M.R., et al. Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2005;23:8512-8519.
168. Pema P.J., Bennett W.F., Bova J.G., Warman P. CT vs MRI in diagnosis of recurrent rectosigmoid carcinoma. J Comput Assist Tomogr. 1994;18:256-261.
169. Beets-Tan R.G., Beets G.L., Borstlap A.C., et al. Preoperative assessment of local tumor extent in advanced rectal cancer: CT or high-resolution MRI? Abdom Imaging. 2000;25:533-541.
170. McCarthy S.M., Barnes D., Deveney K., et al. Detection of recurrent rectosigmoid carcinoma: prospective evaluation of CT and clinical factors. AJR Am J Roentgenol. 1985;144:577-579.
171. Soyka J.D., Veit-Haibach P., Strobel K., et al. Staging pathways in recurrent colorectal carcinoma: is contrast-enhanced 18F-FDG PET-CT the diagnostic tool of choice? J Nucl Med. 2008;49:354-361.
172. Vriens D., de Geus-Oei L.F., van der Graaf W.T., Oyen W.J. Tailoring therapy in colorectal cancer by PET-CT. Q J Nucl Med Mol Imaging. 2009;53:224-244.
173. Potter K.C., Husband J.E., Houghton S.L., et al. Diagnostic accuracy of serial CT/magnetic resonance imaging review vs. positron emission tomography/CT in colorectal cancer patients with suspected and known recurrence. Dis Colon Rectum. 2009;52:253-259.
174. Schmidt G.P., Baur-Melnyk A., Haug A., et al. Whole-body MRI at 1.5 T and 3 T compared with FDG-PET-CT for the detection of tumour recurrence in patients with colorectal cancer. Eur Radiol. 2009;19:1366-1378.
175. Selzner M., Hany T.F., Wildbrett P., et al. Does the novel PET/CT imaging modality impact on the treatment of patients with metastatic colorectal cancer of the liver? Ann Surg. 2004;240:1027-1034.
176. Choi M.Y., Lee K.M., Chung J.K., et al. Correlation between serum CEA level and metabolic volume as determined by FDG PET in postoperative patients with recurrent colorectal cancer. Ann Nucl Med. 2005;19:123-129.
177. Morris-Stiff G., Tan Y.M., Vauthey J.N. Hepatic complications following preoperative chemotherapy with oxaliplatin or irinotecan for hepatic colorectal metastases. Eur J Surg Oncol. 2008;34:609-614.
178. Kandutsch S., Klinger M., Hacker S., et al. Patterns of hepatotoxicity after chemotherapy for colorectal cancer liver metastases. Eur J Surg Oncol. 2008;34:1231-1236.
179. Canon J.L., Gigot J.F. Nodular regenerative hyperplasia: a deleterious consequence of chemotherapy for colorectal liver metastases? Liver Int. 2007;27:938-943.
180. Kuebler J.P., Colangelo L., O’Connell M.J., et al. Severe enteropathy among patients with stage II/III colon cancer treated on a randomized trial of bolus 5-fluorouracil/leucovorin plus or minus oxaliplatin: a prospective analysis. Cancer. 2007;110:1945-1950.
181. Hapani S., Chu D., Wu S. Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: a meta-analysis. Lancet Oncol. 2009;10:559-568.
182. Nalluri S.R., Chu D., Keresztes R., et al. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA. 2008;300:2277-2285.
183. Zhu X., Wu S., Dahut W.L., Parikh C.R. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis. 2007;49:186-193.
184. DuBrow R.A. Radiation changes in the hollow viscera. Semin Roentgenol. 1994;29:38-52.
185. Iyer R., Jhingran A. Radiation injury: imaging findings in the chest, abdomen and pelvis after therapeutic radiation. Cancer Imaging. 2006;6:S131-S139.
186. Kapiteijn E., Putter H., van de Velde C.J. Impact of the introduction and training of total mesorectal excision on recurrence and survival in rectal cancer in The Netherlands. Br J Surg. 2002;89:1142-1149.
187. Paty P.B., Nash G.M., Baron P., et al. Long-term results of local excision for rectal cancer. Ann Surg. 2002;236:522-529.
188. Neary P., Makin G.B., White T.J., et al. Transanal endoscopic microsurgery: a viable operative alternative in selected patients with rectal lesions. Ann Surg Oncol. 2003;10:1106-1111.
189. van der Voort van Zijp J., Hoekstra H.J., Basson M.D. Evolving management of colorectal cancer. World J Gastroenterol. 2008;14:3956-3967.
190. Saha S., Seghal R., Patel M., et al. A multicenter trial of sentinel lymph node mapping in colorectal cancer: prognostic implications for nodal staging and recurrence. Am J Surg. 2006;191:305-310.
191. Des Guetz G., Uzzan B., Nicolas P., et al. Is sentinel lymph node mapping in colorectal cancer a future prognostic factor? A meta-analysis. World J Surg. 2007:311304-311312.
192. Townsend A., Price T., Karapetis C. Selective internal radiation therapy for liver metastases from colorectal cancer. Cochrane Database Syst Rev.. 4, 2009. CD007045
193. Bosset J.F., Collette L., Calais G., et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114-1123.
194. C. Waskewich, R.D. Blumenthal, H. Li. et al. Celecoxib exhibits the greatest potency amongst cyclooxygenase (COX) inhibitors for growth inhibition of COX-2-negative hematopoietic and epithelial cell lines. Cancer Res... 2002;62:2029-2033.