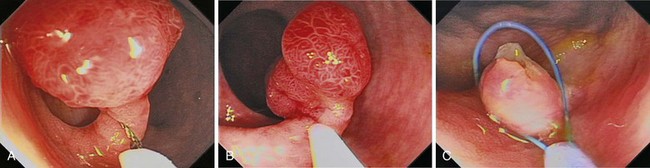
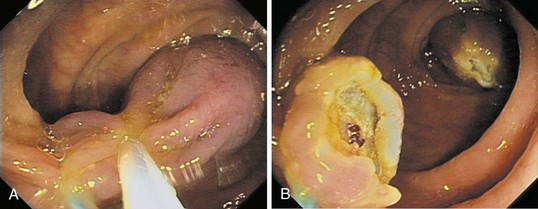
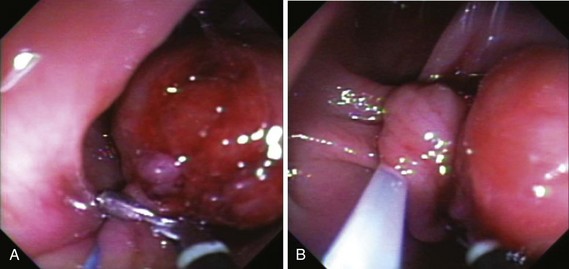
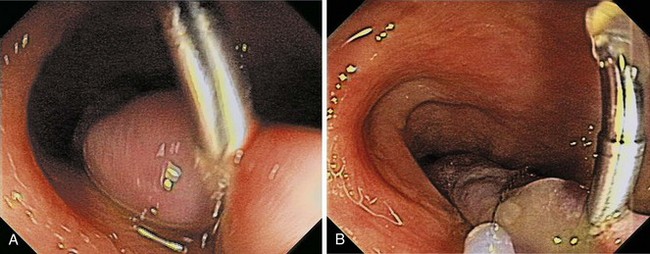
Chapter 36 Colonoscopic Polypectomy, Mucosal Resection, and Submucosal Dissection
Introduction
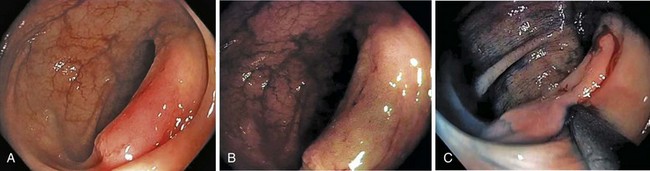
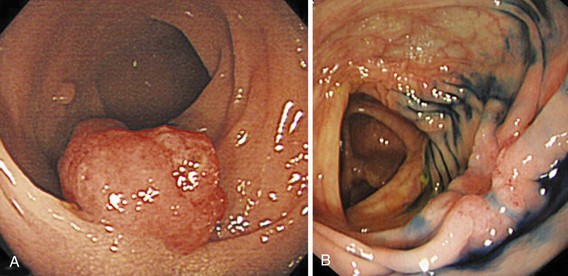
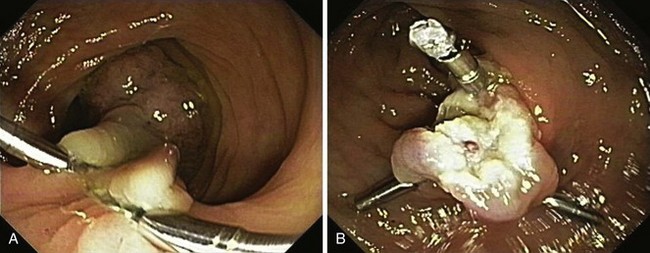
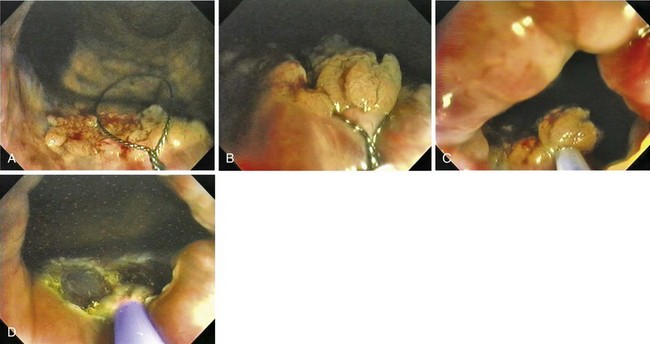
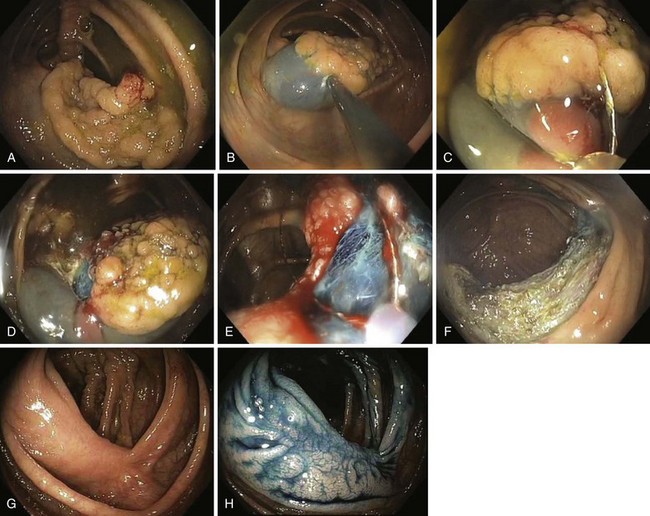
A new wave of change in the field of colorectal neoplasms and colonoscopy is here, leading to the work in colonoscopic resection to begin anew. Nonpolypoid colorectal neoplasms (NP-CRN) are now widely recognized in Western countries (Fig. 36.1).1 This finding requires numerous paradigm shifts in our clinical, educational, and research activities. The detection and diagnosis of NP-CRN necessitates the search for subtle nonprotruding findings during colonoscopy.2 Treatment of NP-CRN requires mucosal resection technique, which is more complex than polypectomy. Because nonpolypoid neoplasms can be found throughout the gastrointestinal (GI) tract, all endoscopists must become familiar with endoscopic mucosal resection (EMR) techniques, and future generations must understand its theoretical basis, obtain the dexterity to perform it, and perfect it. Knowledge about the biology and outcomes of the management of nonpolypoid GI neoplasms is to be acquired. Within the same period of time, new technology—high definition, high magnification, and image-enhanced endoscopy3—that is useful for the detection, diagnosis, and treatment of NP-CRN has become available and is increasingly employed. The technique of submucosal dissection, which has become a standard resection technique for early gastric cancer in Japan, is also being applied for the resection of certain types of colorectal neoplasms to achieve R0 resection—curative en bloc resection with all margins being free of neoplasms (Fig. 36.2)4—without surgery.
Advances in the ability to detect, diagnose, and treat all types of colorectal neoplasms are important. These advances provide hope that the benefit of colonoscopy screening to prevent colorectal cancer, one of the most common causes of cancer death worldwide, can be extended further. In the United States, it was estimated that there would be approximately 146,970 new cases of colorectal cancer in 2009 and 49,920 deaths caused by the disease. The lifetime risk of developing colorectal cancer in the U.S. population is about 6%, with 90% of cases occurring in individuals older than 50 years. The U.S. incidence of colorectal cancer is slightly higher in men than in women, but because women live longer than men, the total number of cases is higher in women. Colorectal cancer incidence and mortality also vary by race and ethnicity, with the highest rate occurring in African Americans; an intermediate rate occurring in whites and Asian/Pacific Islanders; and the lowest rates occurring in American Indians, Alaska Natives, and Hispanics.5 Most colorectal cancer deaths are believed to be preventable with screening colonoscopy and polypectomy.6 The paradigm that a significant majority of colorectal cancers are thought to arise through the adenoma-to-carcinoma sequence7 has been expanded with mismatch repair, serrated, and hybrid pathways.8
Neoplasms can be both polypoid and nonpolypoid, and NP-CRN has a higher risk to contain high-grade dysplasia or submucosal invasion at the time of colonoscopy compared with polypoid neoplasm.1,9,10 It has been estimated that 25% to 40% of adults older than 50 years in the United States have at least one adenoma and that a small fraction of these adenomas progresses to cancer. Because it is impossible to predict which adenoma will become malignant, physicians attempt to remove all adenomas during colonoscopy. The National Polyp Study, which showed that removal of adenomas during screening colonoscopy can decrease the subsequent development of colorectal cancer by 90% compared with historical controls, provided a level of support to this current standard of practice.6 It is our dream to be able to prevent the development of advanced cancer safely and efficaciously in every patient we treat.
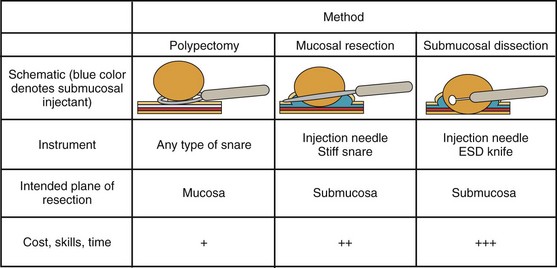
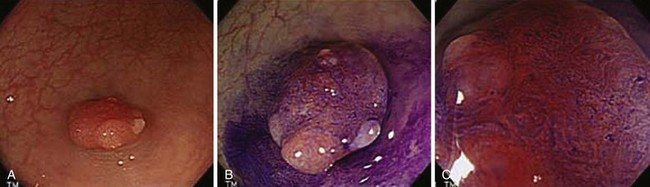
Endoscopically, adenomas and early colorectal cancer can be classified as polypoid (protruded) and nonpolypoid (superficial) types (Fig. 36.3).11 Colonoscopic polypectomy can be used to remove polypoid lesions. Colonoscopic mucosal resection, using the inject and cut technique, is a safe and efficacious technique used to remove nonpolypoid or sessile lesions. Colonoscopic submucosal dissection technique, which is used to remove diseased mucosa by dissecting through the middle to deeper layers of the submucosa, can refine our ability to remove lesions that are difficult, if not impossible, to remove using mucosal resection technique. In this chapter, we provide an in-depth survey of colonoscopic resection of polypoid and nonpolypoid colorectal lesions.
Differential Diagnosis
Careful endoscopic observation of the surface features of the lesion can often allow differentiation of epithelial from nonepithelial origin because nonepithelial lesions are usually covered by normal mucosa. With current videocolonoscopes, and especially with the addition of image enhancement and magnification endoscopy,12 it is increasingly possible to distinguish reliably among hyperplastic polyps, adenomatous polyps, and superficial early adenocarcinomas. Colonoscopic resection may be viewed as a diagnostic procedure. Removal of the entire lesion, when possible, provides the most rigorous evidence that a malignancy was not missed, as it might be because of sampling error with standard biopsies. These procedures provide the definitive treatment when the lesions are removed completely. Subepithelial lesions sometimes can be removed safely when they are located above the muscularis propria, as evidenced by their endoscopic appearance, response to submucosal saline injection, and, if needed, endoscopic ultrasound (EUS). Generally, biopsy should be performed if possible in lesions that are not amendable to endoscopic resection to ascertain their histology, and the lesions should be endoscopically marked for surgical planning with submucosal tattoo and radiopaque clips.
Clinical Features and Pathology
The macroscopic classification of adenomas and early colorectal neoplasms is crucial in the discussion of diagnosis and treatment of early colorectal cancer.11,13 The classification by the Japanese Society for Cancer of the Colon and Rectum can provide a common descriptor of adenomas and early colorectal cancer (mucosal or submucosal cancer, regardless of lymph node status). The Paris classification has been promoted for worldwide use14—although a derivative from the Japanese categorization, its slight alterations in morphologic definitions make it difficult to infer directly the knowledge that has been meticulously collected by our Japanese colleagues. The application of a standard classification of colorectal lesions is the first step in stratifying which lesions are more likely to contain advanced pathology.
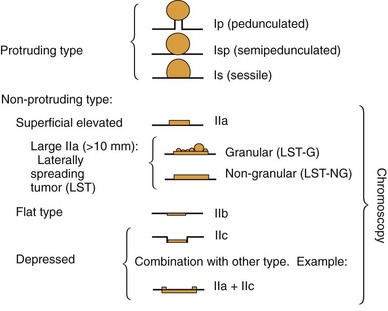
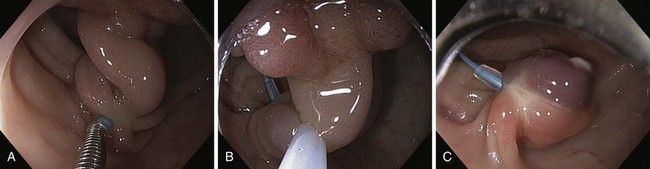
Based on their endoscopic appearance, we classify adenomas and early colorectal cancers as polypoid and nonpolypoid. The polypoid type consists of pedunculated, semipedunculated, and sessile polyps. The nonpolypoid type consists of superficially elevated, flat, and depressed lesions. Excavated superficial colorectal neoplasms are rarely observed. Superficially elevated nonpolypoid lesions are differentiated from sessile polypoid lesions both endoscopically (the height of the lesion is less than half the diameter) and histologically (the thickness of the lesion is less than twice that of the adjacent normal mucosa).15 The term flat is often used to describe superficially elevated lesions. In the colon, however, flat generally connotes that the surface is flat, rather than that the lesion is at the same level as the surrounding mucosa (in the colon and rectum, in contrast to in the esophagus, early neoplastic lesions are rarely at the same level as the surrounding mucosa.)
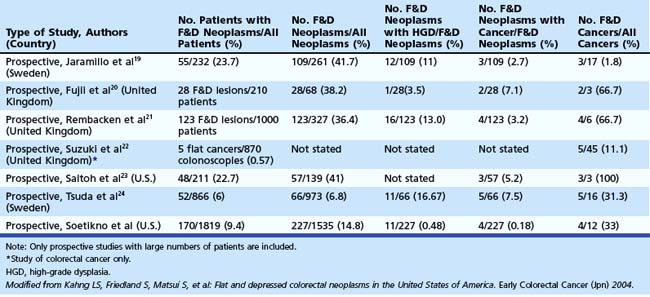
In the Japanese literature and increasingly becoming common worldwide, flat lesions (IIa) larger than 10 mm are also called laterally spreading tumors (LST), although the term refers more to the growth pattern rather than the endoscopic appearance. In the United States, these large flat lesions are often called carpet lesions. LST with nodular or coarsely granular surfaces are called LST-granular. Other LST are called LST-nongranular. This distinction is important because LST-nongranular are more likely to contain invasive cancer, which is more difficult to assess endoscopically. LST-nongranular are also more difficult to resect and may require en bloc resection to ensure cure. In the Paris classification, semipedunculated lesions are lumped into the pedunculated category, and flat lesions are defined as lesions with height less than a closed biopsy forceps. Depressed nonpolypoid lesions, although rare, accounted for almost one-third of the invasive early cancers that were resected endoscopically by Kudo and colleagues,16 who observed more than 14,000 colorectal lesions. Confirmatory reports have been published.12,17 Epidemiology studies of flat and depressed lesions in Western countries have also shown that depressed lesions have a high likelihood of containing invasive cancer (Table 36.1).18 More than 40% of small (6 to 10 mm) depressed lesions contain submucosal invasive cancer; virtually all large (>2 cm) depressed lesions have submucosal invasion (Table 36.2).16 In comparison, submucosal invasive cancer is rare in flat lesions smaller than 10 mm. The risk increases to about 30% in LST larger than 2 cm. Protruding (polypoid) lesions have the lowest rate—slightly greater than 2%—of submucosally invasive cancer.16
Indications and Contraindications
Indications
Endoscopists most commonly resect polypoid lesions using either a snare loop or a biopsy forceps. The malignant potential of an individual polyp is not fully known, and all polyps except diminutive hyperplastic-appearing polyps in the sigmoid and rectum are removed from otherwise healthy patients. However, the risks of polypectomy must also be considered. Treatment decisions must consider whether substantial risks exist and whether the patient’s overall life expectancy is unlikely to be affected by the generally slow progression of colonic adenomas. The application of principles of statistics whereby the risks considered include the confidence interval must be used at the bedside (see later section). The natural history of progression through the adenoma to carcinoma sequence is estimated to be approximately 10 years,26 so patients with advanced comorbid illnesses and limited life expectancy may not benefit from adenoma resection.
Colonoscopic Mucosal Resection and Submucosal Dissection
Colonic mucosal resection is indicated for resection of nonpolypoid and sessile polypoid adenomas in which resection at the submucosal plane is required to obtain accurate pathology and cure. For lesions suspected to contain high-grade dysplasia or superficial submucosal invasive cancer, mucosal resection is indicated if the lesion can be removed en bloc. Otherwise, submucosal dissection or surgery (after confirmatory biopsy) is indicated. The indications of colonoscopic mucosal resection and submucosal dissection are presented in Box 36.1. Nonpolypoid lesions can be difficult to capture with standard snare and polypectomy techniques. It may be impossible to perform en bloc resection of large flat lesions using standard polypectomy techniques. The application of electrocautery may lead to a burn into the muscularis propria. Resection of large sessile lesions carries similar risks. Mucosal resection technique using submucosal injection can ameliorate these technical difficulties and risks. Depressed lesions, including small ones, are most likely to contain submucosally invasive cancer. Complete removal of small depressed lesions is the only way to determine accurately that invasive carcinoma is not present.
Box 36.1 Indications for Colonoscopic Mucosal Resection and Submucosal Dissection
Because Western pathologists rely primarily on evidence of invasion to diagnose invasive carcinoma (as opposed to cellular and glandular morphology, as is common in Japan), mucosal resection technique is more appropriate to obtain a complete sample of small depressed lesions. The superficial submucosa is typically included in these resection specimens, allowing the pathologist to assess for submucosal invasion. Larger true depressed lesions often are invasive carcinoma. After a confirmatory biopsy and tattooing of the site, these lesions may be best managed with surgery. Mucosal resection is also used increasingly to remove submucosal lesions,27 especially small (<1 cm) rectal carcinoids, where the risk of metastasis is low.28,29
Contraindications
Colonoscopy is usually inappropriate in patients who are pregnant30 or have fulminant colitis, suspected intestinal perforation, fresh intestinal anastomosis, or recent myocardial infarction. Polypectomy and mucosal resection generally should not be performed in patients who have uncorrected bleeding disorders. Although polypectomy and mucosal resection were reported in one study to be relatively safe in lesions smaller than 1 cm, the technique used in that study involved submucosal injection before snaring and clipping to approximate the mucosal defect. Good bowel preparation is crucial for detection of subtle lesions and for resection of particularly large or difficult lesions when an elevated risk of perforation exists. Poor bowel preparation is also a contraindication for performance of complex polypectomy or mucosal resection.
Anticoagulation Therapy
Patients need individualized assessment, balancing the risks of interrupting anticoagulation for colonoscopic polypectomy or mucosal resection against the risks of significant bleeding during and after the procedure.31,32 Patients at very high risk for thrombotic events, such as patients with recent coronary stent placements, should simply defer elective endoscopy until the thrombotic risk is lower. The American Society for Gastrointestinal Endoscopy (ASGE) has developed guidelines for management of anticoagulation.33,34 Generally, patients at relatively low risk of thromboembolic complications can discontinue warfarin 5 days before the procedure and resume it shortly after standard polypectomy or 7 to 10 days after complex polypectomy or mucosal resection. The international normalized ratio should be 1.4 or less before polypectomy or mucosal resection of a large lesion. High-risk patients, such as patients with atrial fibrillation and concomitant valvular disease, should receive either standard intravenous heparin until approximately 6 hours before the procedure or low-molecular-weight heparin until approximately 24 hours before the procedure. Warfarin generally can be resumed on the night after the procedure with use of intravenous heparin resumed earlier at 2 to 6 hours after the procedure until the international normalized ratio is therapeutic.
Standard heparin has a short half-life compared with low-molecular-weight heparin, so it permits swift immediate reversal of anticoagulation should patients develop postpolypectomy bleeding. In our published experience of colonoscopic resection of small (<1 cm) colorectal lesions in anticoagulated patients, we withheld warfarin for approximately 36 hours only to avoid supratherapeutic anticoagulation resulting from dietary restriction and bowel purge. In this retrospective series, using various polypectomy techniques, including cold snare, standard snare with cautery, and inject and cut mucosectomy, followed by endoscopic clipping, the risk of major delayed bleeding in the resection of 5.1 ± 2.2 mm lesions was 0.8% (95% confidence interval 0.1% to 4.5%).35
Aspirin, Nonsteroidal Antiinflammatory Drugs, and Antiplatelet Medications
Limited data from the literature suggest that aspirin and other nonsteroidal antiinflammatory drugs (NSAIDs) in standard doses do not increase the risk of significant bleeding after colonoscopic polypectomy. ASGE recommends proceeding with standard polypectomy in patients taking these medications.33,34 We are unaware, however, of any recommendations regarding polypectomy or mucosal resection of large or complex lesions. In our practice, patients with significant coronary disease continue to take aspirin, 81 mg/day, after large polypectomy or mucosal resection. The ASGE guidelines for management of platelet aggregation inhibitors, such as ticlopidine and clopidogrel, recommend discontinuation of these agents for 7 to 10 days. Patients receiving combination therapy (e.g., clopidogrel and aspirin) may be at an additional risk of bleeding. Reinstitution of antiplatelet agents should be individualized. Generally, when we believe that the risk of bleeding after endoscopic removal of a large or complex lesion is significant, we recommend that patients refrain from taking other NSAIDs and platelet inhibitors 7 days before the procedure and for 7 to 14 days after it.
Instruments
Snare Loop
Both the endoscopist and the endoscopy assistant must be familiar with the type of snare used. These individuals must understand and have tactile knowledge of the opening and closing of the snare, the closing pressure required to produce optimal coagulation, and the relationship between the size of the tissue being strangulated and the amount of snare being closed. Various snares, each with a slightly different feature, are used for polypectomy and mucosal resection. The choice is made based on personal preference, the size of the lesion, and the technique being used. The minisnare is often used for small polyps, and larger snares are used for larger polyps. Stiffer snares are used for colonoscopic mucosal resection so that flat or depressed lesions can be captured in the snare.27,37
Electrocautery
High-frequency electrical current is employed to facilitate cutting and to coagulate vessels at the resection margin. Colonoscopic resection is typically performed with a monopolar snare. The metallic conducting snare serves as the active electrode, and the circuit is completed via a conducting grounding pad that is affixed to the patient’s skin. In the case of polypectomy, when the snare grasps the polyp, electrical current is applied as the snare is closed to transect the stalk. Electrical current traveling through tissue heats it. The amount of heat transferred to each point in the tissue (per unit time) is given by the product of the square of the current density and the resistance. The current density is the amount of current passing through a unit area. Although the same total current passes through the stalk of the polyp and the grounding pad, the current density is much higher at the stalk because the cross-sectional area is smaller. As a result, the stalk is cauterized while the bowel wall and the rest of the patient’s body generally are left untouched (Fig. 36.4).38
The use of submucosal injection during mucosal resection promotes a better distribution of current because the current can fan out from the resection site to the wide saline cushion. This fan out reduces thermal damage to the larger submucosal vessel and part of the colon wall immediately beneath the lesion.39
Argon Plasma Coagulation
Argon plasma coagulation (APC) is often used after EMR to cauterize the resection margins.40 APC produces electrically conducting argon plasma by guiding argon gas through a delivery catheter that also contains an electrode for delivery of high-frequency current. APC generally creates uniformly deep zones of desiccation, coagulation, and devitalization that measure less than 3 mm deep in total. Because the argon plasma conducts the current, APC can be applied without tissue contact.41–43 In a randomized, controlled study, prophylactic APC to nonbleeding visible vessels at the en bloc polyp (5 to 20 mm) resection sites did not significantly decrease the rate of delayed postpolypectomy bleeding (4.3% in the control group vs. 2.5% in the APC group). The study excluded resections of pedunculated polyps, polyps smaller than 5 mm or larger than 20 mm, polyps that had immediate bleeding, and polyps with no vessels visible at the postresection site.44
Other Instruments
Other instruments that are often used during polypectomy and mucosal resection include the standard sclerotherapy injection needle, endoclip,44,45 endoloop,46–48 Roth net,49 and Tripod. Detailed examples of use of these instruments, which are important for colonoscopic resection, are described subsequently in the Techniques section.
Techniques
Adequate bowel preparation is important; split dosing of bowel preparation is very useful to clean the bowel adequately for the detection and diagnosis of NP-CRN and resection of all types of appropriate lesions. Techniques to prevent looping of the colonoscope during insertion are essential.50 Familiarity with the patient, staff, equipment, and accessories is required. Various techniques are available to perform sophisticated colonoscopic polypectomy, mucosal resection, and submucosal dissection. These techniques, designed to increase the safety of resection, have allowed resections of lesions that in the past would have been accomplished through surgery. Interpretation of the pathology is vital, and preparation of the pathologic specimen requires the participation of the endoscopists at the completion of the procedure.
Estimation of Malignant Potential
Colonoscopy, in some cases with image enhancement, is generally adequate to assess cancer depth (Fig. 36.5). High magnification colonoscopy can be beneficial but generally is not available in Western countries. Colonoscopy with ultrasound is generally unnecessary because it does not provide a clear advantage in assessing depth of invasion over imaging with image enhancement. The assessment is based on several characteristics.
Appearance
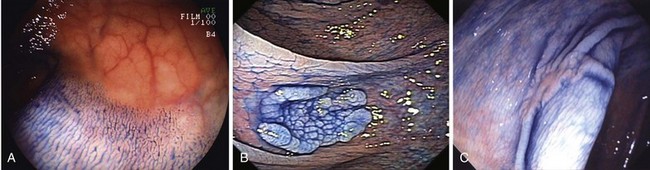
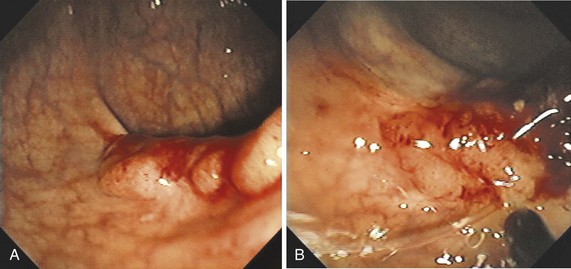
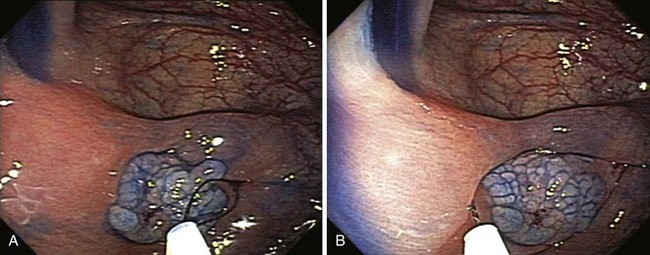
The classification of a lesion helps in providing a general stratification of the risks for the lesion to contain high-grade dysplasia or submucosal invasive carcinoma. NP-CRN has up to 10 times higher risk of containing in situ or submucosal invasive carcinoma than a polypoid lesion of similar size. The larger the size, the more likely is submucosal invasion. The general appearance of the lesion can provide further clues regarding the likelihood for invasive cancer (Figs. 36.6 and 36.7). Firm consistency, adherence, ulceration, and friability are findings suggestive of invasion. In addition, the appearance of expansion of normal tissue immediately surrounding the lesion may indicate the presence of cancer creeping into the surrounding submucosa. Converging folds (two or more) toward the lesion can also predict submucosal invasion. Saitoh and colleagues23 reported the diagnostic operating characteristics of endoscopic findings of depressed colorectal lesions. They observed that patients with one or more findings of expansion appearance, deep depression surface, irregularity of depression surface, or converging folds toward the lesions are more likely to have deep submucosal invasion; the presence of one or more of these findings allowed endoscopists to determine deep submucosal invasion with a 91% accuracy. These investigators used indigo carmine to improve visualization of lesions, as described subsequently.
Image-Enhanced Endoscopy
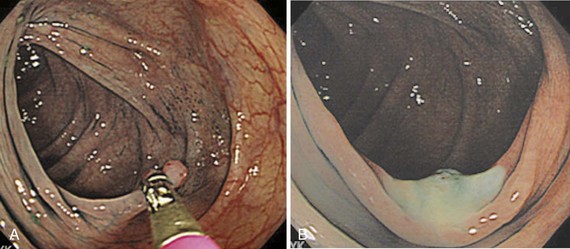
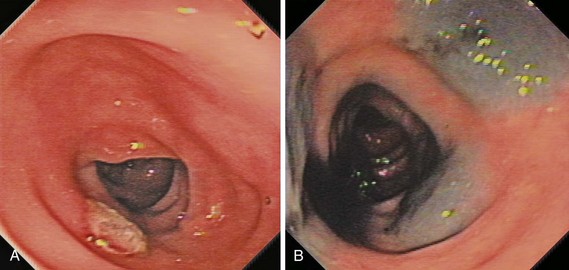
Image-enhanced endoscopy is an integral part of colonoscopic resection of lesions.3 It is part of standard practice in many progressive endoscopy units. Two types of image enhancement are available: equipment-based and dye-based. Equipment-based enhancement includes optical (Narrow Band Imaging; Olympus) or software (Fujinon Intelligent Chromo Endoscopy; Fujinon, and Tone Enhancement; Pentax) methods. Dye-based enhancement includes diluted indigo carmine and crystal violet; dyes are not used in most Western countries (Fig. 36.8). The equipment-based method is part of newer colonoscope systems, and dye-based image enhancement can be readily made available in any unit at low cost. Although equipment-based enhancement is sufficient in most cases, it cannot completely replace dye-based enhancement using diluted indigo carmine. The assessment of depth requires pooling of indigo carmine, and complex surface pattern analysis may require confirmatory dye spray.
Magnification Endoscopy
The ability to select lesions that warrant endoscopic removal is attractive (see Fig. 36.5). Hyperplastic lesions are left alone, adenomas and superficial early carcinomas are resected endoscopically, and invasive cancers are resected surgically. Closer examination of the surface mucosa using magnification endoscopy (100×) allows visualization of the pit pattern, which can provide insights as to the pathology of the lesion. Pit patterns may reflect the tangential structure of the glands of the lesion.51 As the structural organization of the glands becomes disordered or even absent, as it does in invasive carcinoma, the lesion, as seen magnified from its surface, may have a disorderly pattern. Adenomas have sulci patterns. Hyperplastic lesions have a specific, orderly, enlarged round, oval, or stellar pit pattern.52 The original classification of the pit pattern may be too complex to apply in routine clinical practice. A simpler classification that groups the patterns as nonneoplastic, noninvasive, and invasive has been reported but is yet to be used widely.
Nonlifting Sign
Observation of the lesion during and after submucosal saline injection during mucosal resection is a simple but important method to assess the potential for deeply invasive carcinoma (Fig. 36.9).53–55 Lesions may not lift because of desmoplastic reaction, invasion from the lesion itself, or submucosal fibrosis from prior multiple biopsy, cautery, India ink injection, or ulceration. Several studies have reported the diagnostic operating characteristics of the nonlifting sign. The positive predictive value of the nonlifting sign is approximately 80%.56 There is a correlate to the nonlifting sign when submucosal injection is not used; it is typically very difficult to capture in the snare deeply invasive lesions. Difficulties encountered during attempted snare resection should alert the endoscopist to the possibility of deep invasion.
Endoscopic Ultrasound
Generally, endoscopic ultrasound (EUS) is not used in differentiating nonpolypoid mucosal lesions from submucosally invasive ones.57–59 The information needed to decide whether or not to perform mucosal resection can usually be collected by observation during conventional colonoscopy, indigo carmine chromoscopy (with standard magnification), and tests for the nonlifting sign. EUS has been used to locate the blood vessels of large sessile or LST lesions. In this study, the information collected did not change the risks of postpolypectomy bleeding significantly.60
Techniques for Resection
Polypectomy
Diminutive (Baby) Polyps
Diminutive polyps can be removed with various techniques, including single or repeated use of cold biopsy, hot biopsy (Fig. 36.10), cold snare,61 hot snare (Fig. 36.11), or fulguration.62 The optimal technique for complete eradication of all polyp tissue is unknown; the effect of routine removal during colonoscopy of diminutive (≤5 mm) polyps on colon cancer mortality is also unknown. However, documentation of adenomas is important for stratification of need for follow-up colonoscopy, which is based on assessment of risk of developing colorectal cancer.
Pedunculated, Semipedunculated, and Sessile Lesions
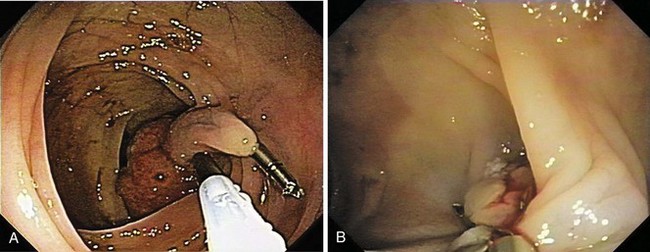
Pedunculated and semipedunculated lesions may be resected by snare-loop polypectomy at the middle or upper stalk. Sessile lesions can be resected by a similar technique at the base. Large polyps (>2 cm) or polyps with a thick stalk carry a higher risk of immediate or delayed bleeding. Prophylactic treatment to strangulate the blood vessels before resection prevents immediate or delayed bleeding (Fig. 36.12).46,47,63–66
One prophylactic method is application of endoloops, which are detachable loops that are applied to the base of the polyp stalk to strangulate the vessels supplying the polyp. Iishi and colleagues63 reported the use of endoloops in 47 patients and compared the rate of bleeding with 42 patients who did not have endoloops placed before polypectomy of pedunculated polyps with heads larger than 1 cm. No immediate or delayed bleeding was observed among patients who had endoloops; five (12%) patients in the control group had bleeding (one immediate, four delayed). Di Giorgio and colleagues67 reported similar results (0% vs. 12% in patients who had and did not have endoloops placed) in a randomized trial with more patients. Another prospective, randomized study of large pedunculated colon polyps showed significantly lower and less severe delayed bleeding in the patients who underwent prophylactic detachable snare placement at the stalk base followed by conventional polypectomy and clip application in the residual stalk compared with the patients who underwent epinephrine injection alone followed by conventional polypectomy.66 However, placement of the endoloop in pedunculated polyps that have short stalks can be difficult (Fig. 36.13). Massive bleeding can occur if the endoloop slips right after snare polypectomy.48,68 In addition, the endoloop is made of nylon, which can be too floppy for ensnaring the polyp.
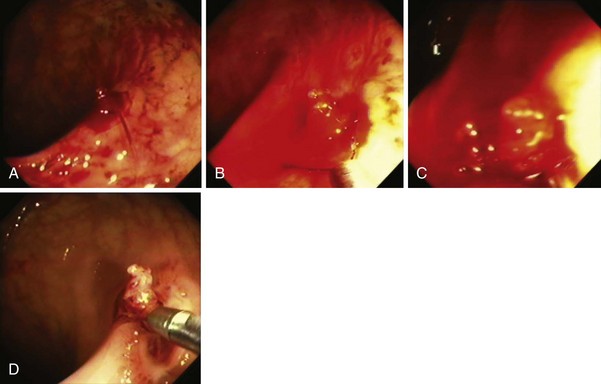
Because massive colonic bleeding can easily cause immediate loss of visualization, other prophylactic techniques may be more appropriate (Figs. 36.14 and 36.15).69 Seitz and colleagues65 used diluted epinephrine to inject to the base of the stalk before polypectomy. Because the effect of epinephrine is transient, the site was then clipped. Other authors have reported safe use of single or multiple deployment of endoclips before polypectomy (Fig. 36.16).63,70 The clips did not conduct current, perhaps because care was taken to avoid contact between the snare and the clips. En bloc resection is a key component to precise pathologic staging in cases of polyps containing invasive cancer. A case series of giant pedunculated polyps described a greater than 80% volume reduction after 4 to 8 mL of 1 : 10,000 epinephrine injections into both the polyp head and the stalk. The dramatic volume reduction decreased the need for piecemeal resection.71 In addition to reducing bleeding, the prophylactic use of the endoloop or endoclip at the base of large pedunculated polyps to facilitate en bloc rather than piecemeal resection has been described. The loop and let go technique may be useful for the treatment of large pedunculated lesions, especially colonic lipomas (Fig. 36.17).72
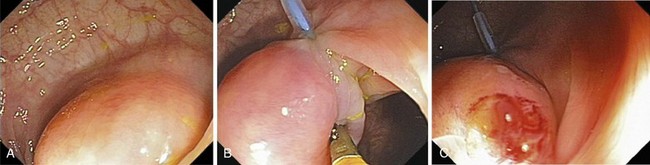
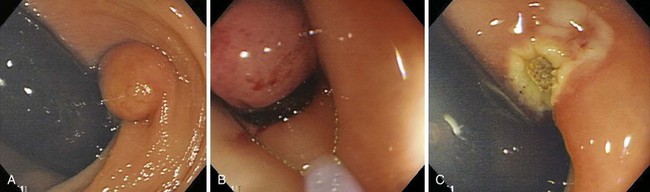
Fig. 36.17 Endoscopic polypectomy of colonic lipoma—well known to be benign—is known to carry a high risk of perforation. The ligate and let go technique is safe and efficacious to treat colonic lipoma.72 A, Typical appearance of a colonic lipoma. Originally, the lesion was sessile (not shown), but by repositioning the patient to make it hanging, the lesion became pedunculated because its weight had pulled it from its point of attachment. B, Ligation of the point of attachment using an endoloop caused the lesion to become ischemic. C, Biopsy on biopsy showed the naked fat, confirming the diagnosis. There was no residual on follow-up colonoscopy a few months later.
Mucosal Resection
Various resection techniques have been described.27,37 The standard inject and cut technique, also known as saline-assisted polypectomy, is most common. The inject, lift, and cut technique is also popular in Japan. The simple suction technique has been used in many patients, some of whom had exceptionally large sessile or flat lesions. The mucosal resection technique using a ligation device is particularly useful for resection of submucosal lesions in the rectum.
Inject and Cut Technique
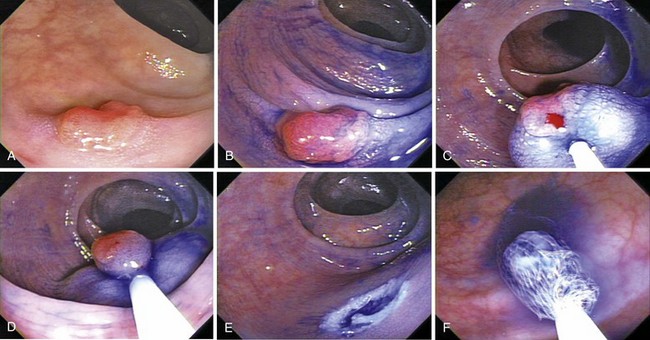
The inject and cut technique requires submucosal injection to lift the diseased mucosa. The key aspects of submucosal injection are to inject a sufficient amount and to recognize the presence of the on-lifting sign. The ideal solution, which would form a substantial bulge and would not dissipate quickly, has not been defined. Physicians in the United States routinely use saline (Figs. 36.18 and 36.21); physicians in Japan use Griseol, a mixture of saline and glycerol with a small amount of indigo carmine. Indigo carmine aids in the assessment of depth during and after resection. The remaining submucosa is blue-green in color, and deeper resections yield visualization of muscularis propria, fat, or other organs. Standard 25-gauge sclerotherapy needles are used. Tumor seeding has been reported in only one patient.73 In our experience, performance of safe and effective mucosal resection demands that all necessary equipment and a trained assistant be present so that resection can be performed immediately after injection. We use a stiff standard snare. Whenever possible, we perform en bloc resections; if we must remove lesions piecemeal, we attempt to do so during a single session. We are able to resect most colorectal lesions using the inject and cut technique.
Simple Suction Technique
The simple suction technique, developed by Soehendra and colleagues,37,74,75 uses a special stiff 0.4-mm monofilament snare. The construction of this snare allows consistent placement of the snare parallel to the bowel wall and, with slight pressure, capture of the diseased mucosa. Piecemeal resections are performed without submucosal injections.
Submucosal Resection with Ligation
Submucosal resection with ligation76 can be particularly useful for resection of submucosal lesions, such as carcinoid tumors in the rectum (Fig. 36.22).28,77 After the endoscope is fitted with the ligation device, the target area is ligated, with or without prior deep submucosal injection. Standard polypectomy is performed below the rubber band. Small submucosal lesions may require prior markings at their periphery, achieved by brief bursts of cautery using the tip of a snare, because such lesions may be difficult to find after the ligation device has been fitted to the endoscope.
Other Techniques
Other techniques that have been described but are not widely used include mucosal resection with cap78–80; inject, lift, and cut technique (double-channel EMR technique); and use of short endoclips to position the snare (Fig. 36.23).81 Although popular and efficacious for resection of superficial early cancer in the esophagus and stomach, EMR with cap or inject, lift, and cut can be risky to use in the colon.78 The thin muscularis propria of the colon can easily be suctioned into the cap, potentially leading to perforation.
Submucosal Dissection
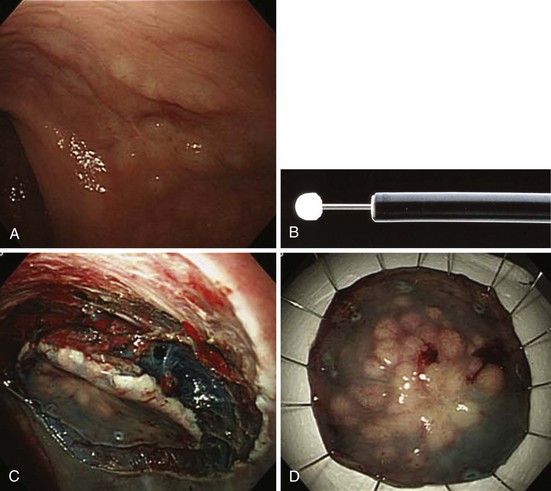
Endoscopic submucosal dissection (ESD) is the latest technique available for the resection of large nonpolypoid GI neoplasms.82 The technique was initially developed for the resection of early gastric cancer that meets specified criteria in which the risks of lymph node metastasis approaches nil. However, in the colon and rectum, most neoplasms are benign and can be resected with minimal risks of recurrence. The indications for colorectal ESD are relatively uncommon (see Box 36.1).4 Colorectal ESD is generally performed using various endoscopic knives, including the flex knife, bipolar knife, hook knife, and flush knife. The submucosal injectants used have also varied: saline with and without diluted epinephrine, glycerol, and hyaluronate. After generous submucosal injection, the procedure is begun with a circumferential cut to isolate the lesion with 3 or 4 mm of surrounding normal mucosa. After further submucosal injection, the submucosa under the lesion is dissected until the submucosa is divided throughout. The lesion is removed en bloc. Numerous techniques have been described. By slow dissection of the submucosa under direct visualization, lesions that otherwise would be unable to be captured by a snare or lesions with submucosa fibrosis can also be removed.
The results of colorectal ESD performed by expert endoscopists are very encouraging. Saito and colleagues83 reported the results of 200 colorectal ESDs measuring 38 mm on average, which took a median time of 90 minutes to resect. These investigators were able to obtain an en bloc resection rate of 84% with curative resection of 83%. They had a 5% perforation rate; except in one case (0.5%), the perforations were able to be treated by endoscopic clipping. Intraprocedure bleeding occurred in most patients and was treated endoscopically. Delayed bleeding occurred within the first 5 days after the procedure. Other authors have reported similar data showing high rates of en bloc resection and perforation, which can typically be treated with clipping, bowel rest, and antibiotics.84
The advantages of colorectal ESD include the ability to obtain a complete and accurate histologic assessment because the tissue is submitted with normal lateral margin and in one piece. In addition, the risk of recurrence in resection of benign lesions with clear margins is nil, and in lesions with minimal submucosal invasive adenocarcinoma, risk of recurrence can be estimated and stratified. Certain cases of well-differentiated adenocarcinoma that are slightly invasive into the submucosa without lymphatic or vascular invasion have minimal risks of invasion. Patients who have smaller risks of metastasis than surgery may be advised to undergo follow-up only. In Japan, ESD for the colon and rectum is indicated as long as the depth of invasion is less than 1000 µm from the muscularis mucosa.85
ESD with snaring, described by Toyonaga and coworkers,86 uses standard snaring technique after circumferential incision and trimming. The same authors have also described the results of EMR with a small incision. In this technique, after submucosal injection, a small mucosal incision was made using the tip of the snare. Snaring was then performed by pushing the snare lightly to the incision. These maneuvers, in effect, allowed the tip of the snare to be fixed and the opened snare to capture the surrounding normal mucosa of the lesion. At the present time, ESD with snaring and EMR with small incision techniques have been reported to lead to shorter procedure times, although data on bleeding and complication rate are still limited.
Techniques for Prevention and Treatment of Residual Lesions
Whenever possible, endoscopic resection should be completed in a single session. Mucosal resection of lesions that are highly suspicious for carcinoma should be resected en bloc. Such en bloc resection, if possible with surrounding normal mucosa, provides the ideal specimen for evaluation of involvement of the lateral and vertical margins. APC has been shown to be effective for treating small amounts of residual lesion.40,87 More than one session may be needed to resect large sessile or flat lesions. Small recurrent lesions are often treated with repeat EMR, application of APC, or surgery.
Techniques for Identifying the Site of a Lesion or Polypectomy
The site of a lesion can be marked with India ink injected into the submucosa or endoscopic placement of a single or multiple radiopaque clips (see Fig. 36.23).88,89 Both techniques are safe and simple to perform, although the endoclip may not be palpable and may not stay in place for a prolonged period.
Techniques for Retrieving the Specimen
The benefits of mucosal resection or polypectomy can be assessed only by a properly prepared pathologic examination. The Roth net is useful in recovering a specimen90 from an en bloc resection of a flat or depressed lesion. Recovering such a specimen through the accessory channel may cause the mucosal resection specimen to be torn into smaller pieces. The Roth net can also aid in efficient recovery of a specimen from piecemeal resection of large sessile or pedunculated lesions. Smaller pieces can be collected through the accessory channel. The net, snare, basket, Tripod, and Pentapod are other accessories that can be useful for removal of large pedunculated polyps.
Management and Surveillance after Polypectomy
Limited data are available to guide recommendations for surveillance after resection of adenomatous polyps.91 The current joint guideline from the American Cancer Society, the U.S. Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology published in 2008 is helpful in directing the interval for patients who have adenomatous lesions.92 Patients who have one or two small (<1 cm) tubular adenomas should have follow-up colonoscopy at 5 to 10 years. Patients who have advanced (villous histology or high-grade dysplasia) or multiple (three or more) adenomas should have follow-up at 3 years. Patients with numerous adenomas, large sessile adenoma, malignant adenoma, or an incomplete colonoscopy should have follow-up examinations at short intervals. Numerous studies have shown that large sessile, flat, and depressed adenomas can be removed endoscopically with relatively good success.16,65,75,93–95 These studies were conducted by expert endoscopists in referral centers, however.
After piecemeal resection, a repeat colonoscopy is typically performed in 3 to 6 months to assess for local recurrence. The postmucosectomy scar site should be examined carefully; image-enhanced endoscopy techniques may be useful to show the presence of the innominate grooves across the scar and normal pit or microvessel patterns. A resected specimen that was noted to contain adenoma at the index examination may contain advanced histology at a follow-up examination.65,96 Khashab and colleagues97 reported a high predictive value for long-term eradication in cases where the postmucosectomy scar site showed both normal macroscopic and microscopic (biopsy) findings. In cases with residual neoplasia, appropriate therapy with biopsy or repeat EMR is prudent, and another surveillance colonoscopy should be performed at 6 months. Subsequent examinations should be performed every 3 to 6 months until long-term eradication is confirmed, and then the patient should resume surveillance at the recommended guideline intervals. The rationale for such an intensive follow-up schedule is the relatively high rate of local recurrence described in the literature, particularly after piecemeal polypectomy.
A series of approximately 300 polyps larger than 3 cm that were resected endoscopically showed a recurrence rate of 17%, with most recurrent lesions successfully treated endoscopically.65 In our efficacy study of standardized inject and cut EMR technique on 125 nonpolypoid lesions (117 flat and 8 depressed), we identified a 10% rate of local recurrence at the prior EMR site at the first surveillance colonoscopy, with ultimate eradication following one or two additional colonoscopies. The recurrence is typically small. No lesion required surgery. Over a 4.5-year follow-up period, no patient developed or died of advanced colorectal cancer or distant metastasis.2 The management of patients who have polypoid lesions containing invasive carcinoma is not straightforward. The risk of metastases of T1 lesions is approximately 10% to 15%.98,99 Because most patients do not develop metastases, the decision of whether to perform surgery is complex. Immediate surgery performed shortly after initial local resection of T1 lesions confers a disease-free 5-year survival rate significantly higher than that of patients who had surgery only after local recurrence or lymph node metastasis from rectal cancer.100
Given the pathology findings, the endoscopist must assess whether the risk for lymph node metastasis is lower than that of partial colectomy. Various stratification methods have been reported; most distinguish patients at high risk and low risk to develop recurrence or metastasis. Published data often are collected from a few patients; even though they indicate that the absolute fraction of patients who developed metastases was low, great care must be exercised because the upper limit of the confidence interval of this fraction is often higher than the risk of surgery. Many studies grouped different types of lesions. The study by Haggitt and colleagues101 that reported the level of invasion as the major prognostic factor is often cited to direct the management of polypoid lesions with submucosal invasion (Fig. 36.24). In this retrospective study, the authors concluded a low risk of metastasis or local recurrence when the level is less than 4. Generalizing their conclusions to endoscopic practice at large should be cautioned, however. The study involved a small number of patients—some had endoscopic treatment and others had surgery. The study lumped all patients who had sessile lesions that contained submucosal invasion into one group (level 4). Finally, Haggitt and colleagues101 did not report the outer limit of the 95% confidence interval of their data (Table 36.3). When analyzed, patients with level 0 to 3 of invasion had up to 2.74% risk of developing metastasis, whereas patients with level 4 had up to 91% risk of favorable outcomes.
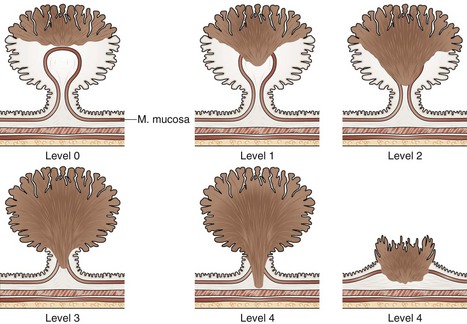
Fig. 36.24 Haggitt and colleagues101 stratified the level of cancer submucosal invasion by the following criteria: level 0, carcinoma in situ (i.e., no extension below the muscularis mucosa); level 1, carcinoma invading through the muscularis mucosa but limited to the head of the polyp (i.e., above the junction between the adenoma and its stalk); level 2, carcinoma invading the level of the neck (i.e., the junction between adenoma and its stalk); level 3, carcinoma invading any part of the stalk; and level 4, carcinoma invading into the submucosa of the bowel wall below the stalk. In malignant sessile lesions, invasive carcinoma is considered as level 4.
| Level of Invasion | Adverse Outcome (95% CI) | Favorable Outcome (95% CI) |
|---|---|---|
| 0–3 | 0.9% (0–2.74) | 99% (97.96–100) |
| 4 | 25% (8.96–41.04) | 75% (58.96–91.04) |
CI, confidence interval.
Because the risk of morbidity from surgery is less than 1% in patients with minimal comorbidity, the data by Haggitt and colleagues101 do not fully support that these patients should be uniformly advised to have watchful waiting. Similarly, a patient who has high risk for surgery with more than 10% risk of mortality may have a higher risk of dying from surgery than from developing metastasis (see Table 36.3). Detailed pathologic studies from Japan have found that the absolute depth of invasion should be considered because there is a gradual increase in the risk of lymph node metastasis with the depth of submucosal invasion.16,102 Kitajima and colleagues85 pooled the data from six institutions in Japan and classified the invasion according to Fig. 36.25. For pedunculated polyp, submucosal invasion was measured from an imaginary line drawn from the level 2 line of Haggitt’s study to the deepest portion of the submucosa invasion. For NP-CRN, the muscularis mucosa was used as baseline, and the depth of invasion was the vertical distance from this line to deepest portion of invasion. When the muscularis mucosa could not be identified, the top of the lesion was used as baseline. For pedunculated lesions, they found that 0 of 53 patients (95% confidence interval 0% to 7%) with invasion limited to the head had lymph node metastasis. For the nonpedunculated lesions, 0 of 123 patients (95% confidence interval 0% to 3%) with depth of invasion less than 1000 µm had lymph node metastasis at the time of surgery. Similar to the data by Haggitt and colleagues,101 this study was limited by the small sample size and length of follow-up.
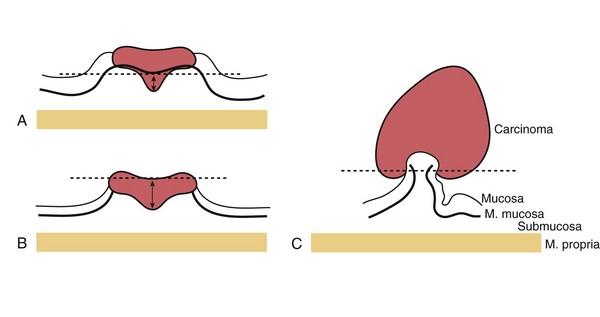
Fig. 36.25 Stratification of submucosal invasion of early colorectal cancer according to the General Rules for Clinical and Pathological Studies on Cancer of the Colon, Rectum and Anus. A, For nonpolypoid and sessile lesions, the vertical depth of the carcinoma is measured from the muscularis mucosa to the deepest point of invasion. B, If the muscularis mucosa has been destroyed by the carcinoma, the measurement is made from the surface of the lesion. Endoscopic therapy without surgery is accepted when the depth of the well-differentiated carcinoma without lymphatic or vascular involvement is less than 1000 µm and the lateral and vertical margins are negative. C, For pedunculated and semipedunculated lesions, the imaginary line is drawn according to level 2 invasion by Haggitt et al101: The lesion is considered to be head invasion only when the carcinoma did not infiltrate below the line. Endoscopic therapy is accepted without surgery in head invasion of well-differentiated carcinoma without lymphatic and vascular involvement.
Kikuchi and colleagues102 stratified the risk of metastasis with the depth of carcinoma invasion from the muscularis mucosa and followed the patients for at least 5 years. Sm1 is slight mucosal invasion from the muscularis mucosa to a depth of 200 to 300 µm, sm2 is intermediate invasion, and sm3 is invasion near the inner surface of the muscularis propria. The investigators found that 64 patients with sm1 invasion (75% of whom had either semipedunculated or sessile lesions) had no evidence of metastasis during follow-up of at least 5 years. Other authors have developed stratification systems that combine depth and histology.98,103 Favorable histology includes well-differentiated and moderately differentiated adenocarcinoma with carcinoma cells at least 2 mm from a clearly visualized margin. Unfavorable histology includes poorly differentiated adenocarcinoma, mucinous adenocarcinoma, and signet-ring cell carcinoma or one with adenocarcinoma cells within 2 mm from a clearly visualized margin. If the margins could not be assessed, the lesion was classified as unfavorable histology. No patients with favorable histology treated with endoscopic polypectomy had an adverse outcome. However, this study also involved only a few patients.
Additional factors have been suggested to be important in the patient risk stratification for local recurrence or metastasis. Tanaka and colleagues104 reported that the absence of lymphatic or vascular involvement is highly predictive of a successful outcome. Masaki and Muto105 showed that unfavorable histology (presence or absence of small nests of cancer cells with poorly differentiated or mucinous histology) at the invasive margin is predictive of adverse outcome. The management of small depressed lesions depends on the histology, lymphovascular invasion, and depth of invasion. Lesions larger than 1 cm often contain invasive cancer; patients who have such lesions are often referred directly to surgery.16
Complications
Postpolypectomy Bleeding
Postpolypectomy bleeding can occur during or after the procedure. The reported incidence varies according to the definition of bleeding, and the size and type of lesions resected. Significant bleeding was observed in 0.4% of hot biopsy specimens of diminutive polyps in one series. Other authors reported no complications in a series of more than 900 hot biopsy specimens of diminutive polyps. The overall risk is approximately 1% to 2% for snare polypectomy. Rosen and coworkers106 reported a 0.4% risk of bleeding requiring hospital admission in a retrospective study involving 4721 patients who had polypectomies. Nivatvongs107 reported 10 episodes of bleeding requiring blood transfusions among 1172 patients. Soehendra and coworkers75 reported a 24% risk of bleeding in a series of 176 large (>3 cm) polypectomies. Most of these hemorrhages occurred during the procedure, and all were successfully treated by endoscopic methods.
A variety of techniques are useful, including application of the endoclip or endoloop, use of APC, injection of diluted epinephrine, cauterization using monopolar or bipolar instruments, and repeat application of the snare or hot forceps biopsy to grasp the remnant stalk of pedunculated polyp. In cases in which endoscopic treatment fails, selective angiogram with the application of absorbable gelatin sponge (Gelfoam) emboli or surgery is employed. Our preferred technique for minor bleeding is APC; for significant bleeding, we use the endoclip or endoloop with or without prior injection of diluted epinephrine (Fig. 36.26). Parra-Blanco and colleagues108 reported a case series showing the efficacy of endoclips to treat postpolypectomy bleeding. In cases with delayed bleeding, we typically would purge the bowel using 4 to 6 L of polyethylene glycol solution within 3 hours, immediately followed by a colonoscopy,109 although Rex and colleagues110 have reported successful colonoscopic treatment of delayed postpolypectomy bleeding without prior bowel purge.
Postpolypectomy Syndrome
Postpolypectomy syndrome, also called transmural burn syndrome, is thought to occur when cautery injury causes full-thickness necrosis of the bowel wall. Patients typically present with fever, localized abdominal tenderness (often with rebound tenderness), and leukocytosis. The onset of symptoms is commonly within a few hours of the polypectomy. The complication occurred in 6 (0.5%) of 1172 patients in one series107 and in 9 (1%) of 777 patients in another.111 Patients who are suspected to have postpolypectomy syndrome must be admitted for close observation by medical and surgical teams, bowel rest, and antibiotics. Most patients recover uneventfully. Abdominal radiographs and computed tomography (CT) scans may show local changes such as air in the bowel wall but not within the peritoneum in the large amounts that would be seen with frank perforation. Localized perforation in the colon that is located in the retroperitoneum also may not be visualized using standard abdominal x-rays or manifested as diffuse free air or peritonitis.
Perforation
Perforation can occur when muscularis propria is included in the tissue grasped by a snare; this accident may happen, for example, when a large sessile polyp that is draped over a fold is grasped in its entirety. Techniques that may decrease the risk of capturing the muscularis propria have been summarized. Endoscopic clipping techniques have been shown to be useful in cases of fresh small perforation or, prophylactically, in cases where the resection appears too deep into the muscularis propria.112 Most patients with colon perforation with diffuse peritonitis were reported to require surgery.113,114 Patients who did not require surgery had smaller perforations owing to polypectomy, mucosal resection, or submucosal dissection rather than the large lacerations that are typically seen with diagnostic colonoscopy perforations. Delayed perforation can also occur as a result of tissue necrosis from cautery and requires surgery.
Future Trends
Advances in the technology and technique of colonoscopy have allowed us to manage increasingly complex colorectal lesions via endoscopy. Learning and using these new technologies and techniques may be demanding, but they enable us to perform endoscopic resections of lesions (Fig. 36.27) that previously would have required major abdominal surgery. The more sophisticated approaches can also improve recognition of the nonpolypoid lesions that were previously underappreciated in the Western countries and can assist in their treatment. Further developments will increase the potential of colonoscopic polypectomy, mucosal resection, and submucosal dissection. Extensive and long-term databases, using a common terminology for endoscopy and pathology,115,116 are needed. Simplifications in the technology and techniques of the resections would allow them to be used more widely and ultimately benefit more patients.
1 Soetikno RM, Kaltenbach T, Rouse RV, et al. Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. JAMA. 2008;299:1027-1035.
2 Kaltenbach T, Friedland S, Maheshwari A, et al. Short- and long-term outcomes of standardized EMR of nonpolypoid (flat and depressed) colorectal lesions > or = 1 cm (with video). Gastrointest Endosc. 2007;65:857-865.
3 Kaltenbach T, Sano Y, Friedland S, et al. American Gastroenterological Association (AGA) Institute technology assessment on image-enhanced endoscopy. Gastroenterology. 134, 2008. 327–140
4 Saito Y, Sakamoto T, Fukunaga S, et al. Endoscopic submucosal dissection (ESD) for colorectal tumors. Dig Endosc. 2009;21:S7-S12.
5 Cancer Facts and Figures 2008. American Cancer Society, Inc, 2009.
6 Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977-1981.
7 Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525-532.
8 Noffsinger AE. Serrated polyps and colorectal cancer: New pathway to malignancy. Annu Rev Pathol. 2009;4:343-364.
9 Kudo S. Early colorectal cancer. Igaku-Shoin; 1996.
10 Shimoda T, Ikegami M, Fujisaki J, et al. Early colorectal carcinoma with special reference to its development de novo. Cancer. 1989;64:1138-1146.
11 Japanese Classification of Colorectal Carcinoma. Kanehara & Co, 1997.
12 Togashi K, Konishi F, Koinuma K, et al. Flat and depressed lesions of the colon and rectum: Pathogenesis and clinical management. Ann Acad Med Singapore. 2003;32:152-158.
13 Schlemper RJ, Hirata I, Dixon MF. The macroscopic classification of early neoplasia of the digestive tract. Endoscopy. 2002;34:163-168.
14 The Paris endoscopic classification of superficial neoplastic lesions: Esophagus, stomach and colon. Gastrointest Endosc. 2003;58:S3-S43.
15 Sawada T, Hojo K, Moriya Y. Colonoscopic management of focal and early colorectal carcinoma. Baillieres Clin Gastroenterol. 1989;3:627-645.
16 Kudo S, Kashida H, Tamura T, et al. Colonoscopic diagnosis and management of nonpolypoid early colorectal cancer. World J Surg. 2000;24:1081-1090.
17 Ajioka Y, Watanabe H, Kazama S, et al. Early colorectal cancer with special reference to the superficial nonpolypoid type from a histopathologic point of view. World J Surg. 2000;24:1075-1080.
18 Soetikno R, Friedland S, Kaltenbach T, et al. Nonpolypoid (flat and depressed) colorectal neoplasms. Gastroenterology. 2006;130:566-576.
19 Jaramillo E, Watanabe M, Slezak P, et al. Flat neoplastic lesions of the colon and rectum detected by high-resolution video endoscopy and chromoscopy. Gastrointest Endosc. 1995;42:114-122.
20 Fujii T, Rembacken BJ, Dixon MF, et al. Flat adenomas in the United Kingdom: Are treatable cancers being missed? Endoscopy. 1998;30:437-443.
21 Rembacken BJ, Fujii T, Cairns A, et al. Flat and depressed colonic neoplasms: A prospective study of 1000 colonoscopies in the UK. Lancet. 2000;355:1211-1214.
22 Suzuki N, Saunders BP, Talbot IC, et al. Small flat colorectal cancer: Experience in 870 consecutive colonoscopies. Gastrointest Endosc. 2000;51:AB149.
23 Saitoh Y, Waxman I, West AB, et al. Prevalence and distinctive biologic features of flat colorectal adenomas in a North American population. Gastroenterology. 2001;120:1657-1665.
24 Tsuda S, Veress B, Toth E, et al. Flat and depressed colorectal tumours in a southern Swedish population: A prospective chromoendoscopic and histopathological study. Gut. 2002;51:550-555.
25 Kahng LS, Friedland S, Matsui S, et al. Flat and depressed colorectal neoplasms in the United States of America. Early Colorectal Cancer (Jpn). 2004.
26 Bond JH. Colon polyps and cancer. Endoscopy. 2003;35:27-35.
27 Soetikno RM, Gotoda T, Nakanishi Y, et al. Endoscopic mucosal resection. Gastrointest Endosc. 2003;57:567-579.
28 Ono A, Fujii T, Saito Y, et al. Endoscopic submucosal resection of rectal carcinoid tumors with a ligation device. Gastrointest Endosc. 2003;57:583-587.
29 Oshitani N, Hamasaki N, Sawa Y, et al. Endoscopic resection of small rectal carcinoid tumours using an aspiration method with a transparent overcap. J Int Med Res. 2000;28:241-246.
30 Qureshi WA, Rajan E, Adler DG, et al. ASGE Guideline: Guidelines for endoscopy in pregnant and lactating women. Gastrointest Endosc. 2005;61:357-362.
31 Hirsh J, Dalen JE, Anderson DR, et al. Oral anticoagulants: Mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 1998;114:445S-469S.
32 Dunn AS, Turpie AG. Perioperative management of patients receiving oral anticoagulants: A systematic review. Arch Intern Med. 2003;163:901-908.
33 Zuckerman MJ, Hirota WK, Adler DG, et al. ASGE guideline: The management of low-molecular-weight heparin and nonaspirin antiplatelet agents for endoscopic procedures. Gastrointest Endosc. 2005;61:189-194.
34 Eisen GM, Baron TH, Dominitz JA, et al. Guideline on the management of anticoagulation and antiplatelet therapy for endoscopic procedures. Gastrointest Endosc. 2002;55:775-779.
35 Friedland S, Sedehi D, Soetikno R. Colonoscopic polypectomy in anticoagulated patients. World J Gastroenterol. 2009;15:1973-1976.
36 Banerjee S, Shen B, Baron TH, et al. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2008;67:791-798.
37 Soehendra N, Binmoeller KF, Seifert H, et al. Therapeutic endoscopy. Thieme; 1998.
38 Farin G, Grund KE. Basic principles of electrosurgery in flexible endoscopy. In: Tytgat GNJ, Mulder CJJ, editors. Procedures in hepatogastroenterology. Kluwer Academic Publishers; 1997:415-436.
39 Norton ID, Wang L, Levine SA, et al. Efficacy of colonic submucosal saline solution injection for the reduction of iatrogenic thermal injury. Gastrointest Endosc. 2002;56:95-99.
40 Regula J, Wronska E, Polkowski M, et al. Argon plasma coagulation after piecemeal polypectomy of sessile colorectal adenomas: Long-term follow-up study. Endoscopy. 2003;35:212-218.
41 Farin G, Grund KE. Technology of argon plasma coagulation with particular regard to endoscopic applications. Endosc Surg Allied Technol. 1994;2:71-77.
42 Grund KE, Storek D, Farin G. Endoscopic argon plasma coagulation (APC) first clinical experiences in flexible endoscopy. Endosc Surg Allied Technol. 1994;2:42-46.
43 Grund KE, Straub T, Farin G. New haemostatic techniques: Argon plasma coagulation. Baillieres Best Pract Res Clin Gastroenterol. 1999;13:67-84.
44 Lee CK, Lee SH, Park JY, et al. Prophylactic argon plasma coagulation ablation does not decrease delayed postpolypectomy bleeding. Gastrointest Endosc. 2009;70:353-361.
45 Soehendra N, Sriram PV, Ponchon T, et al. Hemostatic clip in gastrointestinal bleeding. Endoscopy. 2001;33:172-180.
46 Hachisu T, Ichinose M, Satoh S, et al. A novel detachable snare for hemostasis after polypectomy. Prog Dig Endosc. 1990;36:161-163.
47 Hachisu T. A new detachable snare for hemostasis in the removal of large polyps or other elevated lesions. Surg Endosc. 1991;5:70-74.
48 Soetikno RM, Friedland S, Lewit V, et al. Lift and ligate: A new technique to treat a bleeding polypectomy stump. Gastrointest Endosc. 2000;52:681-683.
49 Faigel DO, Stotland BR, Kochman ML, et al. Device choice and experience level in endoscopic foreign object retrieval: An in vivo study. Gastrointest Endosc. 1997;45:490-492.
50 Miyaoka M, Sudo I. How to manage difficulties with colonoscope insertion. Dig Endosc. 2001;13:111-115.
51 Kudo S, Hirota S, Nakajima T, et al. Colorectal tumours and pit pattern. J Clin Pathol. 1994;47:880-885.
52 Tanaka S, Kaltenbach T, Chayama K, et al. High-magnification colonoscopy (with videos). Gastrointest Endosc. 2006;64:604-613.
53 Uno Y, Munakata A. The non-lifting sign of invasive colon cancer. Gastrointest Endosc. 1994;40:485-489.
54 Kato H, Haga S, Endo S, et al. Lifting of lesions during endoscopic mucosal resection (EMR) of early colorectal cancer: Implications for the assessment of resectability. Endoscopy. 2001;33:568-573.
55 Ishiguro A, Uno Y, Ishiguro Y, et al. Correlation of lifting versus non-lifting and microscopic depth of invasion in early colorectal cancer. Gastrointest Endosc. 1999;50:329-333.
56 Kobayashi N, Saito Y, Sano Y, et al. Determining the treatment strategy for colorectal neoplastic lesions: Endoscopic assessment or the non-lifting sign for diagnosing invasion depth? Endoscopy. 2007;39:701-705.
57 Harada N, Hamada S, Kubo H, et al. Preoperative evaluation of submucosal invasive colorectal cancer using a 15-MHz ultrasound miniprobe. Endoscopy. 2001;33:237-240.
58 Saitoh Y, Obara T, Einami K, et al. Efficacy of high-frequency ultrasound probes for the preoperative staging of invasion depth in flat and depressed colorectal tumors. Gastrointest Endosc. 1996;44:34-39.
59 Friedland S, Soetikno R. Preoperative evaluation of submucosal invasive colorectal cancer using a 15-MHz ultrasound miniprobe. Gastrointest Endosc. 2002;55:959-961.
60 Polkowski M, Regula J, Wronska E, et al. Endoscopic ultrasonography for prediction of postpolypectomy bleeding in patients with large nonpedunculated rectosigmoid adenomas. Endoscopy. 2003;35:343-347.
61 Tappero G, Gaia E, De Giuli P, et al. Cold snare excision of small colorectal polyps. Gastrointest Endosc. 1992;38:310-313.
62 Singh N, Harrison M, Rex DK. A survey of colonoscopic polypectomy practices among clinical gastroenterologists. Gastrointest Endosc. 2004;60:414-418.
63 Iishi H, Tatsuta M, Narahara H, et al. Endoscopic resection of large pedunculated colorectal polyps using a detachable snare. Gastrointest Endosc. 1996;44:594-597.
64 Iida Y, Miura S, Munemoto Y, et al. Endoscopic resection of large colorectal polyps using a clipping method. Dis Colon Rectum. 1994;37:179-180.
65 Seitz U, Bohnacker S, Seewald S, et al. Long-term results of endoscopic removal of large colorectal adenomas. Endoscopy. 2003;35:S41-S44.
66 Kouklakis G, Mpoumponaris A, Gatopoulou A, et al. Endoscopic resection of large pedunculated colonic polyps and risk of postpolypectomy bleeding with adrenaline injection versus endoloop and hemoclip: A prospective, randomized study. Surg Endosc. 2009.
67 Di Giorgio P, De Luca L, Calcagno G, et al. Detachable snare versus epinephrine injection in the prevention of postpolypectomy bleeding: A randomized and controlled study. Endoscopy. 2004;36:860-863.
68 Matsushita M, Hajiro K, Takakuwa H, et al. Ineffective use of a detachable snare for colonoscopic polypectomy of large polyps. Gastrointest Endosc. 1998;47:496-499.
69 Soetikno R, Gotoda T, Barro J, et al. Endoscopic clipping technique. Video Library, American Society of Gastrointestinal Endoscopy; 2003.
70 Hachisu T, Yamada H, Satoh S, et al. Endoscopic clipping with a new rotatable clip-device and a long clip. Dig Endosc. 1996;8:127-133.
71 Hogan RB, Hogan RB3rd. Epinephrine volume reduction of giant colon polyps facilitates endoscopic assessment and removal. Gastrointest Endosc. 2007;66:1018-1022.
72 Kaltenbach T, Milkes D, Friedland S, et al. Safe endoscopic treatment of large colonic lipomas using endoscopic looping technique. Dig Liver Dis. 2008;40:958-961.
73 Zarchy T. Risk of submucosal saline injection for colonic polypectomy. Gastrointest Endosc. 1997;46:89-90.
74 Soehendra N, Binmoeller KF, Bohnacker S, et al. Endoscopic snare mucosectomy in the esophagus without any additional equipment: A simple technique for resection of flat early cancer. Endoscopy. 1997;29:380-383.
75 Binmoeller KF, Bohnacker S, Seifert H, et al. Endoscopic snare excision of “giant” colorectal polyp. Gastrointest Endosc. 1996;43:183-188.
76 Suzuki Y, Hiraishi H, Kanke K, et al. Treatment of gastric tumors by endoscopic mucosal resection with a ligating device. Gastrointest Endosc. 1999;49:192-199.
77 Higaki S, Nishiaki M, Mitani N, et al. Effectiveness of local endoscopic resection of rectal carcinoid tumors. Endoscopy. 1997;29:171-175.
78 Inoue H, Kawano T, Tani M, et al. Endoscopic mucosal resection using a cap: Techniques for use and preventing perforation. Can J Gastroenterol. 1999;13:477-480.
79 Inoue H, Takeshita K, Hori H, et al. Endoscopic mucosal resection with a cap-fitted panendoscope for esophagus, stomach, and colon mucosal lesions. Gastrointest Endosc. 1993;39:58-62.
80 Inoue H, Endo M, Takeshita K, et al. A new simplified technique of endoscopic esophageal mucosal resection using a cap-fitted panendoscope (EMRC) [letter]. Surg Endosc. 1992;6:264-265.
81 Inatsuchi S. Broadening of the indications for endoscopic surgery: Upper gastrointestinal tract. Dig Endosc. 2000;12:S2-S6.
82 Tanaka S, Oka S, Chayama K. Colorectal endoscopic submucosal dissection: Present status and future perspective, including its differentiation from endoscopic mucosal resection. J Gastroenterol. 2008;43:641-651.
83 Saito Y, Uraoka T, Matsuda T, et al. Endoscopic treatment of large superficial colorectal tumors: A case series of 200 endoscopic submucosal dissections (with video). Gastrointest Endosc. 2007;66:966-973.
84 Tamegai Y, Saito Y, Masaki N, et al. Endoscopic submucosal dissection: A safe technique for colorectal tumors. Endoscopy. 2007;39:418-422.
85 Kitajima K, Fujimori T, Fujii S, et al. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: A Japanese collaborative study. J Gastroenterol. 2004;39:534-543.
86 Toyonaga T, Man IM, Morita Y, et al. The new resources of treatment for early stage colorectal tumors: EMR with small incision and simplified endoscopic submucosal dissection. Dig Endosc. 2009;21:S31-S37.
87 Brooker JC, Saunders BP, Shah SG, et al. Treatment with argon plasma coagulation reduces recurrence after piecemeal resection of large sessile colonic polyps: A randomized trial and recommendations. Gastrointest Endosc. 2002;55:371-375.
88 Nizam R, Siddiqi N, Landas SK, et al. Colonic tattooing with India ink: Benefits, risks, and alternatives. Am J Gastroenterol. 1996;91:1804-1808.
89 Kaltenbach T, Friedland S, Barro J, et al. Clipping for upper gastrointestinal bleeding. Am J Gastroenterol. 2006;101:915-918.
90 Miller K, Waye JD. Polyp retrieval after colonoscopic polypectomy: Use of the Roth Retrieval Net. Gastrointest Endosc. 2001;54:505-507.
91 Imperiale TF, Sox HC. Guidelines for surveillance intervals after polypectomy: Coping with the evidence. Ann Intern Med. 2008;148:477-479.
92 Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: A joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130-160.
93 Kanamori T, Itoh M, Yokoyama Y, et al. Injection-incision-assisted snare resection of large sessile colorectal polyp. Gastrointest Endosc. 1996;43:189-195.
94 Tanaka S, Haruma K, Oka S, et al. Clinicopathologic features and endoscopic treatment of superficially spreading colorectal neoplasms larger than 20 mm. Gastrointest Endosc. 2001;54:62-66.
95 Soetikno R, Gotoda T. Con: Colonoscopic resection of large neoplastic lesions is appropriate and safe. Am J Gastroenterol. 2009;104:272-275.
96 Walsh RM, Ackroyd FW, Shellito PC. Endoscopic resection of large sessile colorectal polyps. Gastrointest Endosc. 1992;38:303-309.
97 Khashab M, Eid E, Rusche M, et al. Incidence and predictors of “late” recurrences after endoscopic piecemeal resection of large sessile adenomas. Gastrointest Endosc. 2009;70:344-349.
98 Volk EE, Goldblum JR, Petras RE, et al. Management and outcome of patients with invasive carcinoma arising in colorectal polyps. Gastroenterology. 1995;109:1801-1807.
99 Nivatvongs S. Surgical management of early colorectal cancer. World J Surg. 2000;24:1052-1055.
100 Baron PL, Enker WE, Zakowski MF, et al. Immediate vs. salvage resection after local treatment for early rectal cancer. Dis Colon Rectum. 1995;38:177-181.
101 Haggitt RC, Glotzbach RE, Soffer EE, et al. Prognostic factors in colorectal carcinomas arising in adenomas: Implications for lesions removed by endoscopic polypectomy. Gastroenterology. 1985;89:328-336.
102 Kikuchi R, Takano M, Takagi K, et al. Management of early invasive colorectal cancer: Risk of recurrence and clinical guidelines. Dis Colon Rectum. 1995;38:1286-1295.
103 Nakada I, Tabuchi T, Nakachi T, et al. Histological factors contributing to a high risk of recurrence of submucosal invasive cancer (pT1) of the colon and rectum after endoscopic therapy. Surg Today. 2008;38:675-678.
104 Tanaka S, Haruma K, Teixeira CR, et al. Endoscopic treatment of submucosal invasive colorectal carcinoma with special reference to risk factors for lymph node metastasis. J Gastroenterol. 1995;30:710-717.
105 Masaki T, Muto T. Predictive value of histology at the invasive margin in the prognosis of early invasive colorectal carcinoma. J Gastroenterol. 2000;35:195-200.
106 Rosen L, Bub DS, Reed JF3rd, Nastasee SA. Hemorrhage following colonoscopic polypectomy. Dis Colon Rectum. 1993;36:1126-1131.
107 Nivatvongs S. Complications in colonoscopic polypectomy: An experience with 1,555 polypectomies. Dis Colon Rectum. 1986;29:825-830.
108 Parra-Blanco A, Kaminaga N, Kojima T, et al. Hemoclipping for postpolypectomy and postbiopsy colonic bleeding. Gastrointest Endosc. 2000;51:37-41.
109 Soetikno R, Kaltenbach T, Kelsey P, et al. Endoscopic interpretation and therapy of severe lower gastrointestinal bleeding. American Society of Gastrointestinal Endoscopy Video Learning Library; 2007.
110 Rex DK, Lewis BS, Waye JD. Colonoscopy and endoscopic therapy for delayed post-polypectomy hemorrhage. Gastrointest Endosc. 1992;38:127-129.
111 Waye JD, Lewis BS, Yessayan S. Colonoscopy: A prospective report of complications. J Clin Gastroenterol. 1992;15:347-351.
112 Taku K, Sano Y, Fu KI, et al. Iatrogenic perforation associated with therapeutic colonoscopy: A multicenter study in Japan. J Gastroenterol Hepatol. 2007;22:1409-1414.
113 Orsoni P, Berdah S, Verrier C, et al. Colonic perforation due to colonoscopy: A retrospective study of 48 cases. Endoscopy. 1997;29:160-164.
114 Christie JP, Marrazzo J3rd. “Mini-perforation” of the colon—not all postpolypectomy perforations require laparotomy. Dis Colon Rectum. 1991;34:132-135.
115 Schlemper RJ, Itabashi M, Kato Y, et al. Differences in diagnostic criteria for gastric carcinoma between Japanese and western pathologists [published erratum appears in Lancet 1997 Aug 16;350(9076):524]. Lancet. 1997;349:1725-1729.
116 Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251-255.