CHAPTER 122 Colonic Polyps and Polyposis Syndromes
COLONIC POLYPS
Colonic polyps may be divided into two major groups: neoplastic (the adenomas and carcinomas) and non-neoplastic (Table 122-1). The adenomas and carcinomas share a characteristic—cellular dysplasia—but they may be subdivided according to the relative contribution of certain microscopic features. The non-neoplastic polyps may be grouped into several distinct categories: hyperplastic polyps (including serrated polyps), “mucosal polyps,” juvenile polyps, Peutz-Jeghers polyps, inflammatory polyps, and others. Submucosal lesions also can impart a polypoid appearance to the overlying mucosa and therefore are briefly mentioned even though they are not true polyps.
| Neoplastic Mucosal Polyps |
| Benign (Adenoma) |
NEOPLASTIC POLYPS
Pathology
Histologic Features
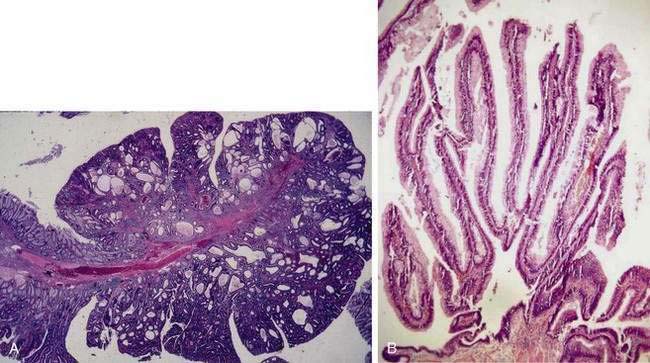
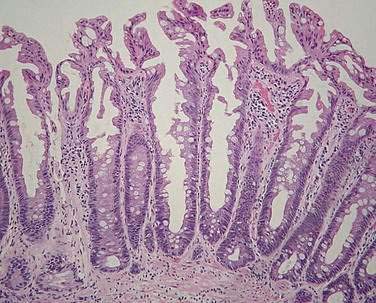
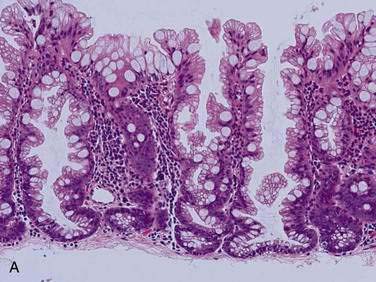
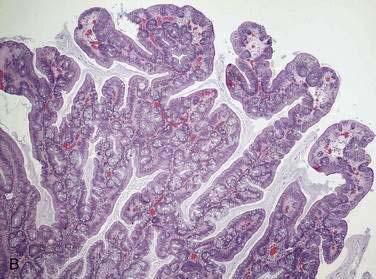
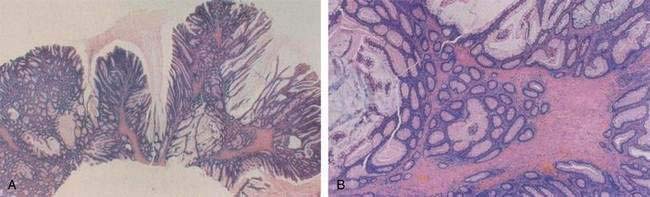
Adenomatous polyps are tumors of benign neoplastic epithelium that can either be pedunculated (i.e., attached by a stalk) or sessile (i.e., attached by a broad base with little or no stalk). The neoplastic nature of adenomas is apparent by histologic examination of their glandular architecture. Tubular adenomas are the most common subgroup and are characterized by a complex network of branching adenomatous glands (Fig. 122-1A). In villous adenomas, the adenomatous glands extend straight down from the surface to the center of the polyp, thereby creating long, finger-like projections (see Fig. 122-1B). Tubulovillous (villoglandular) adenomas manifest a combination of these two histologic types.
A polyp is assigned a histologic type on the basis of its predominant glandular pattern, and in practice, pure villous adenomas are quite rare. According to the World Health Organization, adenomas are classified as tubular if at least 80% of the glands are of the branching, tubule type and as villous if at least 80% of the glands are villiform.1 Of all adenomatous polyps, tubular adenomas account for 80% to 86%, tubulovillous for 8% to 16%, and villous adenomas for 3% to 16%.2,3 Tubular adenomas usually are small and exhibit mild dysplasia, whereas villous architecture is more often encountered in large adenomas and tends to be associated with more severe degrees of dysplasia (Table 122-2).
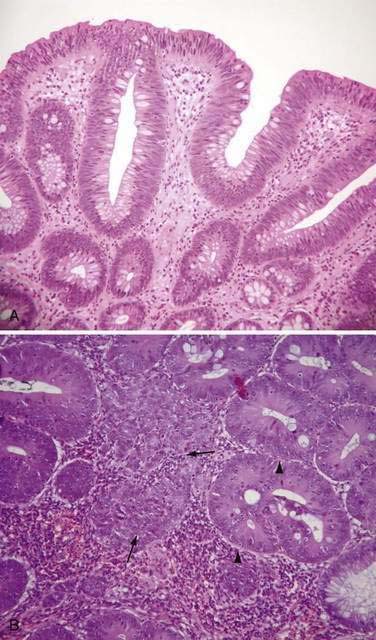
The dysplasia exhibited by all adenomas can be graded subjectively on the basis of certain cytologic and architectural features into three categories: mild, moderate, and severe. Some polyps contain the entire spectrum from mild to severe dysplasia, but in all cases, the adenoma is classified according to its most dysplastic focus. In cells that exhibit mild dysplasia, the nuclei in the cell maintain their basal polarity but are hyperchromatic, slightly enlarged, and elongated, yet uniform in size, without prominent nucleoli (Fig. 122-2A). There often is loss of goblet cell mucin. Architecturally, the glands manifest branching and budding and become more crowded. With moderate dysplasia, nuclei become stratified and pleomorphic, with prominent nucleoli, along with further loss of goblet cell mucin and increased glandular crowding. Severe dysplasia (see Fig. 122-2B) is characterized by further stratification and pleomorphism of nuclei, more numerous and prominent nucleoli, increased nucleus-to-cytoplasm ratios, and extreme glandular crowding.
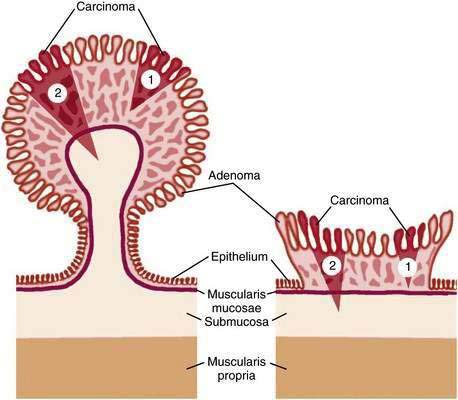
With further cell proliferation within the crypt, cells pile up, lose polarity, and create glands within glands, giving a disorderly cribriform appearance termed carcinoma in situ (see Fig. 122-2B). Most pathologists group severe dysplasia and carcinoma in situ, considering them both high-grade dysplasia2; one reason for this grouping is to avoid using the term carcinoma for these lesions because they often can be managed endoscopically rather than surgically (see later). Indeed, it is now common practice to categorize dysplasia in colorectal adenomas into only two grades: low-grade dysplasia, which includes mild and moderate dysplasia, and high-grade dysplasia, which comprises severe dysplasia and carcinoma in situ.
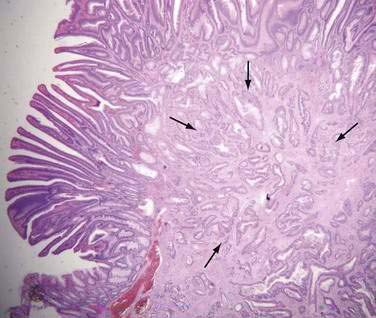
Carcinoma in situ is characterized by intracryptal cell proliferation that leaves intact the basement membrane surrounding the gland. If a focus of neoplastic cells grows beyond the basement membrane and into the lamina propria of the mucosa, the lesion is termed intramucosal carcinoma (see Fig. 122-2B). Both carcinoma in situ and intramucosal carcinoma are noninvasive lesions without metastatic potential, because lymphatics are not present in the colonic mucosa above the level of the muscularis mucosae.4 Because clinical confusion often arises on encountering these two entities, it has been suggested that both carcinoma in situ and intramucosal carcinoma be reported as “noninvasive carcinoma” to avoid unnecessarily aggressive management. Only when a focus of neoplastic cells has spread through the muscularis mucosae is the lesion considered invasive carcinoma (Fig. 122-3). An adenoma that contains a focus of invasive carcinoma commonly is referred to as a malignant polyp (see later).
Of all adenomatous polyps, mild dysplasia is found in 70% to 86% moderate dysplasia in 18% to 20%, severe dysplasia (carcinoma in situ) in 5% to 10%, and invasive carcinoma in 5% to 7%.3,5,6 Higher grades of dysplasia are more common in adenomas of larger size and greater villous content,2 and adenomas with severe dysplasia are more likely to contain foci of invasive cancer.
Adenoma Size
Adenomas are categorized into three size groups: less than 1 cm, 1 to 2 cm, and greater than 2 cm.5 Overall, most adenomas are smaller than 1 cm, but the size distribution of adenomas can vary greatly among studies, depending on study design, age of the study population, and location of the adenomas within the colon. Thus, in autopsy series, which describe a presumably asymptomatic population dying of other causes, only 13% to 16% of adenomas are larger than 1 cm,7–9 whereas surgical and colonoscopic series that include symptomatic or higher-risk patients report a higher prevalence (26% to 40%) of adenomas larger than 1 cm.2,3,5 In countries where the prevalence of colon cancer is high, adenomas tend to be larger than in low-prevalence countries.10,11 Adenoma size increases as a function of age,8,12,13 even in low-prevalence countries,10 and larger adenomas are more common in distal colonic segments.2,5,8
Diminutive Polyps
Diminutive polyps measure 5 mm or less in diameter and are commonly encountered during endoscopy. An earlier concept that these lesions were almost always non-neoplastic has been revised based on several flexible sigmoidoscopic and colonoscopic studies in which 30% to 50% of diminutive polyps were found to be adenomatous14–18; despite the frequency of adenomatous change, however, they represent little if any threat of cancer. Earlier studies found that less than 1% of diminutive polyps were villous or contained a focus of severe dysplasia and that they almost never harbored invasive carcinoma.14–16,18 In an analysis of 4381 diminutive polyps, 4.4% contained severe dysplasia or villous components, although still only 0.1% had invasive carcinoma.19 Moreover, in a retrospective study of predominantly asymptomatic people with diminutive adenomas found on flexible sigmoidoscopy, full colonoscopy identified a synchronous proximal adenoma in only 33% of subjects, and most of the proximal lesions were smaller than 5 mm.20 Likewise, prospective colonoscopic studies confirm only a 24% to 34% prevalence of proximal adenomas in asymptomatic patients with distal diminutive polyps (of all histologic types)21,22; the likelihood of finding proximal adenomas is greater when the distal polyp is larger than 5 mm.21 Diminutive adenomas manifest little, if any, appreciable growth over time.23,24 A population-based study that involved fulgurating small polyps (even those up to 1 cm) without obtaining initial histologic identification reported that the subsequent risk for colorectal cancer and overall survival was no worse than in the general population.25
Malignant Potential of Adenomatous Polyps
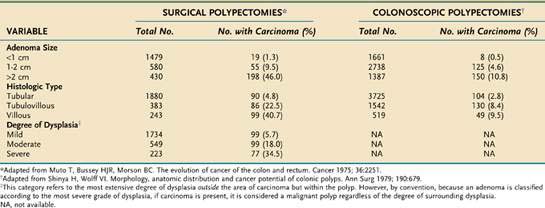
The three principal features that correlate with malignant potential for an adenomatous polyp are size, histologic type, and degree of dysplasia (Table 122-3). Although higher rates of malignant transformation are found when the source of the pathologic material is mainly from surgical polypectomies5 rather than colonoscopic polypectomies,6 the malignant potential is correlated directly with larger adenoma size, more villous histology, and higher degrees of dysplasia. These three histopathologic criteria usually are interdependent, so it is difficult to assign a primary premalignant role to any one of them. For example, although only 1.3% of all adenomas smaller than 1 cm harbor a cancer (see Table 122-3), if these small lesions have a predominant villous component or contain a focus of severe dysplasia, the cancer rate rises to 10% or 27%, respectively (Table 122-4). A small (<1 cm), tubular, mildly dysplastic adenoma is highly unlikely to harbor a focus of invasive cancer. Nonetheless, although this type of lesion is innocuous in itself, once removed, it often is considered a marker of a person who is at (low) risk for developing a recurrent adenoma (discussed later). Because adenomas that are larger than 1 cm, have villous architecture, or manifest high-grade dysplasia or carcinoma represent a more biologically hazardous group, the term adenoma with advanced pathology (AAP) often is applied to adenomas that display any of these features.
Other Adenoma Variants
Flat Adenomas
A subset of adenomas, termed flat adenomas by Muto and coworkers,26 is receiving increasing attention as a potentially important lesion. Macroscopically, a flat adenoma is either completely flat or slightly raised and can contain a central depression. By definition of the Japanese Society for Cancer of the Colon and Rectum, the diameter of this polyp is more than twice its thickness. Typically smaller than 1 cm in diameter, these lesions can be easily missed at endoscopy. This potential risk has prompted investigators, particularly in Japan, to adapt better methods of detection that involve dye-spraying (chromoendoscopy) to generate a contrast relief-map image of the mucosa, or magnification colonoscopy, for enhanced visualization.27 In studies without such specialized endoscopic techniques, flat adenomas accounted for 8.5% to 12% of all adenomas and could be multiple.28
Prospective studies of Western populations aided by the use of chromoendoscopy found that 6.8% to 36% of all detected adenomas were flat. Compared with lesions that were polypoid, these flat polyps tended to be smaller and to have increased rates of high-grade dysplasia and early cancer.29–31 The largest study to date looked at over 1800 veterans undergoing colonoscopy. The prevalence of flat or nonpolypoid neoplasms was 9.4%. These lesions were 10 times more likely to harbor a carcinoma, although the rate of carcinoma was quite low.32 Indeed, it has been suggested that flat adenomas can have distinct biologic and chromosomal profiles.30,33 In contrast, re-evaluation of adenomas removed during the National Polyp Study found no increased risk of high-grade dysplasia in polyps classified as flat, based on histologic features.34 Future studies might help define whether broader acceptance of advanced endoscopic techniques such as chromoendoscopy or narrow-band imaging by endoscopists in Western countries will result in higher detection rates of flat adenomas, lower colorectal cancer incidence, or both following colonoscopy.35 A hereditary flat adenoma syndrome in four families described by Lynch and colleagues36 subsequently was confirmed to be a variant of familial adenomatous polyposis (FAP) (see later).
The natural history of flat adenomas is not known. It is possible that they give rise to typical polypoid adenomas. Alternatively, the facts that residual flat adenoma tissue can be found adjacent to flat carcinomas, that some studies have observed a substantial incidence of high-grade dysplasia in these small lesions, and that flat adenomas have a lower incidence of K-ras mutations compared with polypoid adenomas, suggest that malignant progression from flat adenomas might not necessarily involve a polypoid phase.37 It is possible that flat adenomas are the precursors of the long-recognized, but uncommon, small de novo colon carcinomas.38
Aberrant Crypts
Investigations of human and carcinogen-treated rat colonic mucosa have disclosed a putative preneoplastic lesion called the aberrant crypt.39 Found within macroscopically normal mucosa, aberrant crypts can occur individually or as small, slightly raised foci. They can be identified in methylene blue-stained whole mounts of colonic mucosa using a low-power lens or with a magnifying endoscope.40 When viewed from above, the lumina of aberrant crypts are elliptical and irregular rather than circular. Aberrant crypt foci have become useful biomarkers in animal studies of colon carcinogenesis and chemoprevention. Human aberrant crypts often are hyperplastic; however, when they are dysplastic, they can represent the earliest detectable preneoplastic lesions. This notion is supported by molecular studies indicating that dysplastic, but not hyperplastic, aberrant crypts manifest mutations in the adenomatous polyposis coli (APC) gene (see later).41
In one study, patients who had aberrant crypt foci in the rectum underwent repeat colonoscopy in one year42; at which time 60% of patients had aberrant crypt foci, but less than half had the same foci re-identified and 50% had new foci; this suggests that the progression of aberrant crypt foci is a dynamic process. In a large study using high-magnification chromoendoscopy to identify aberrant crypt foci in the rectum, the number of aberrant crypt foci increased in a stepwise fashion in normal patients, patients with a flat adenoma, and patients with a flat carcinoma.43 Despite these findings, it remains to be fully elucidated if aberrant crypt foci are useful biomarkers for adenomas.
Pathogenesis
Histogenesis
Adenomatous polyps are thought to arise from a failure in a step, or steps, of the normal process of cell proliferation and cell death (apoptosis). The initial aberration appears to arise in a single colonic crypt in which the proliferative compartment, instead of being confined to the crypt base, is expanded throughout the entire crypt. This disturbance results in a unicryptal adenoma. The DNA-synthesizing cells at the surface are not sloughed into the lumen, as normally occurs, and they accumulate in a downward infolding manner, interposing themselves between normal preexisting crypts. New adenomatous glands then are created either by further infolding or by branching. Thus, the unicryptal adenoma is believed to arise from a monoclonal expansion of an abnormal cell, and as the adenoma enlarges, the adenomatous cell population becomes polyclonal. Evidence for this concept comes from studying intestinal tissues from an extremely rare patient with FAP who was an XO/XY mosaic.44 Analysis of Y chromosome expression in the intestinal mucosa of this patient revealed that normal crypts of the small and large intestine and even unicryptal adenomas were monoclonal (either XO or XY), whereas at least 76% of very small microadenomas were polyclonal. Whether the same situation also applies to sporadic adenoma development is not clear at present.
Adenoma-Carcinoma Hypothesis
Epidemiologic Evidence
The prevalence of adenomas within a population, and the prevalence of people with multiple adenomas, geographically parallel the prevalence of colon cancer.10 Indeed, adenoma prevalence increases in migrants from low-risk to high-risk colon cancer regions (see Chapter 123). The prevalence rates for both adenomatous polyps and cancer increase with age, and age distribution curves indicate that the development of adenomas precedes that of carcinomas by 5 to 10 years.5,45
Clinicopathologic Evidence
The most compelling evidence for the adenoma-carcinoma sequence is the fact that in patients with FAP who have hundreds to thousands of adenomas, the development of colorectal cancer is inevitable. For persons in the general population without an inherited predisposition to colon cancer, perhaps the best evidence that adenomas give rise to carcinomas comes from endoscopic intervention studies. The National Polyp Study (see later) demonstrated that colonoscopic removal of adenomas results in a much lower than expected incidence of subsequent colorectal cancer.46 In addition, screening proctosigmoidoscopy can lower the expected incidence of47 and mortality from48 rectal cancer. Pathology-based studies often describe the presence of remnant adenoma tissue within colon cancers. Conversely, small foci of cancer are extremely rare in normal mucosa but commonly are found in adenomas, particularly in those that are larger, more dysplastic, and more highly composed of villous elements (see Tables 122-3 and 122-4). Furthermore, the site distribution within the colon is similar for large adenomas and colon cancers. In addition, adenomatous polyps are found in one third of surgical specimens that contain a single colon cancer and in more than two thirds of specimens that contain more than one synchronous cancer.
Molecular Genetic Evidence
Molecular genetic studies provide some of the strongest experimental support for the adenoma-carcinoma hypothesis. The progression from adenoma to carcinoma results from an accumulation of molecular genetic alterations involving, among other changes, activation of oncogenes, inactivation of tumor suppressor genes, and participation of stability genes (see Chapter 123).49 The K-ras oncogene commonly undergoes point mutations at particular sites within the gene, thereby endowing it with the ability to transform cells. Only 9% of small adenomas exhibit K-ras gene mutations, compared with 58% of adenomas larger than 1 cm and 47% of colon cancers50; therefore, K-ras activation can act at an intermediate stage in tumorigenesis, perhaps contributing to a polypoid growth pattern. The fact that a large number of adenomas and cancers do not have K-ras gene mutations indicates that other genetic events also must play a role.
Tumor suppressor genes that normally function to suppress tumor development commonly are inactivated in colorectal neoplasms by mutation or allelic deletion, thereby promoting tumorigenesis. The loss of function of tumor suppressor genes on chromosomes 5q, 18q, and 17p is critical for colorectal tumorigenesis. The APC (adenomatous polyposis coli) gene, which resides on the long arm of chromosome 5, is considered the gatekeeper for the process of colon carcinogenesis.51 Mutation or loss of this gene is believed to be the crucial first step that confers susceptibility to colonic adenomas in patients with FAP as well as in people with sporadic adenomas. The APC protein plays an important role in colonic epithelial cell homeostasis (see later). Other tumor suppressor genes are located on chromosome 18q, in a region where the DCC (deleted in colon cancer) gene resides. Loss of function of DCC, or other nearby tumor suppressor genes, seems to contribute to later stages of adenoma progression, because allelic deletion at this locus occurs in only 11% to 13% of small tubular or tubulovillous adenomas, but increases to 47% of adenomas with foci of cancer and 73% of frank colon cancers.52 Allelic deletion of chromosome 17p, at the locus that contains the TP53 gene, is the most common region of allelic loss in colorectal cancers. Because adenomas seldom manifest 17p deletion,52 this alteration probably occurs as a late step in the adenoma-carcinoma progression. Perhaps the most compelling molecular evidence that colon carcinomas arise from previous adenomas is that when cancer cells arise in a malignant adenoma, their pattern of molecular alterations is identical to that of the neighboring adenoma cells, but in addition, they have acquired further mutations that are presumably critical for malignant behavior.52
Oncogenes and tumor suppressor genes enhance the adenoma-carcinoma process by directly stimulating cell proliferation and inhibiting cell death; however, stability genes, or caretakers, normally keep genetic alterations to a minimum, and thus, when they are inactivated by mutation or loss, they permit mutations in other target genes to occur at a higher rate.49 Examples of stability genes include the DNA mismatch repair (MMR) and base-excision repair (BER) genes responsible for repairing subtle mistakes that are made during DNA replication. Germline mutations of DNA MMR genes (such as hMLH1, hMSH2, hMSH6) occur in persons with HNPCC, whereas inheritance of a mutated BER gene (e.g., MUTYH, also known as MYH) is responsible for a type of attenuated adenomatous polyposis (see later).
Pathways of Colon Carcinogenesis
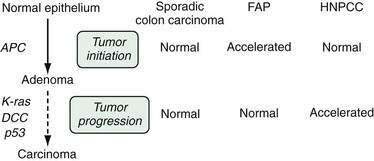
It is useful to consider the process of colon carcinogenesis in two general stages: the formation of the adenoma, termed tumor initiation, and the progression of the adenoma to carcinoma, termed tumor progression (Fig. 122-4). It is believed that most, if not all, adenomas arise from an initial loss of APC gene function, and for that to happen, epithelial cells must lose the function of both APC alleles (two hits). In patients with FAP, one APC allele is inherited in a mutated form (germline mutation) from the affected parent. Adenomas arise when the second, normal copy of the APC gene from the unaffected parent either is lost or mutated (somatic mutation). Because persons with FAP are born with the first hit, they develop polyps at a much younger age and in much greater number than does the general population; thus, FAP can be considered a condition of accelerated tumor initiation. Despite this abnormal initiation rate, once adenomas form in patients with FAP, it is believed that each adenoma tends to display a normal progression to carcinoma. Thus, the inevitable progression to cancer in FAP is more a consequence of the numerous polyps than of any increased premalignant potential of the individual adenoma. In the general population, sporadic adenomas arise as a consequence of two acquired somatic mutations of the APC gene. Because two acquired hits are statistically less likely than one acquired hit, sporadic adenomas tend to occur later in life and to be fewer than the polyps in patients with FAP.
Another major molecular pathway for colon carcinogenesis involves mutations in DNA MMR genes (see Chapter 123); this is the predominant pathway in patients with HNPCC. Mutations in these enzymes result in a characteristic molecular phenotype termed microsatellite instability (MSI), a phenomenon that is observable in colon cancer cells from approximately 85% of HNPCC colon cancers but in only 15% of sporadic colon cancers. Although its name implies a lack of polyps, HNPCC colon cancers do arise from preexisting adenomas. It is believed that the numbers of adenomas that occur in patients with HNPCC are similar to those in the general population but that HNPCC is marked by an accelerated tumor progression stage, so the few adenomas that do arise often manifest advanced pathology (villous features, high-grade dysplasia) even at small sizes.53 Indeed, adenomas in patients with HNPCC often manifest MSI54 even in their earliest stages of formation. Because these adenomas tend to progress more rapidly to carcinoma,55,56 surveillance intervals for colonoscopy following removal of adenomas in HNPCC patients should be shortened (see later; see also Chapter 123).
Epidemiology and Etiology
Prevalence
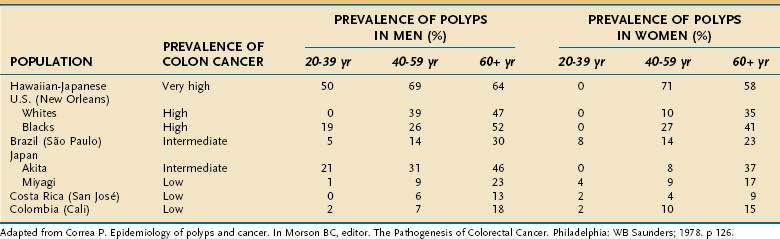
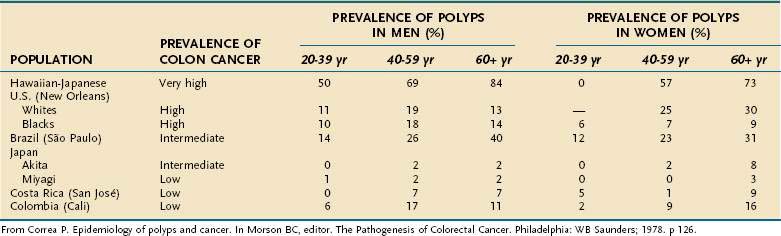
The prevalence of adenomatous polyps is affected by four major factors: the inherent risk for colon cancer in the population, age, sex, and family history of colorectal cancer. The frequency of colon adenomas varies widely among populations, but it tends to be higher in populations at greater risk for colon cancer (Table 122-5).10 One illustrative example is to compare the very high adenoma prevalence in Japanese living in Hawaii (a very high risk area for colon cancer) with the much lower adenoma prevalence in Japanese who still reside in Japan, an area of much lower risk. Even within Japan itself, adenoma prevalence correlates quite well with colon cancer prevalence in different prefectures of the country. Data from autopsy series provide an approximation of adenoma prevalence. In populations at low risk for colon cancer, adenoma prevalence rates are lower than 12% (see Table 122-5), whereas in most intermediate- and high-risk populations, adenomas are found in 30% to 40% of the population, and rates as high as 50% to 60% have been observed.7,57 One half to two thirds of people older than 65 years in high-risk areas can harbor colonic adenomas.7,57
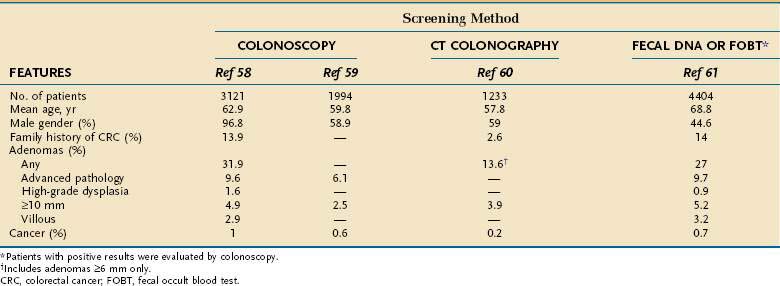
The true prevalence rate of adenomatous polyps within an asymptomatic population is only now being elucidated because, until recently, colonoscopy was not performed on healthy persons in the absence of gastrointestinal symptoms. Approximately 27% to 32% of asymptomatic average-risk persons older than 50 years undergoing screening colonoscopy will have an adenoma, and 6% to 10% will have an AAP (Table 122-6).58–61 By comparison, colonoscopic screening of asymptomatic persons between 40 and 49 years of age revealed prevalence rates of only 8.7% for tubular adenomas and 3.5% for AAP or cancer.62 Colonoscopic series indicate that men have a 1.5 relative risk of adenomas compared with age-matched women,62,63 thus confirming earlier observations in autopsy series.8,11 Men also have slightly higher rates of advanced adenomas than do women, with a relative risk of approximately 1.5.64 A large database study found a higher rate of polyps larger than 9 mm in African Americans undergoing screening colonoscopy compared with age-matched whites.65
The prevalence of adenomas is higher in older people, particularly those older than 60 years. In fact, age is the single most important independent determinant of adenoma prevalence7,11,12,57,62,63,66,67 in high-risk and low-risk regions of the world (see Table 122-5). Not only is advancing age associated with a higher prevalence rate of adenomas, but it also correlates with a greater likelihood for multiple polyps, adenomas with more severe degrees of dysplasia, and, in some studies, larger adenomas.
Adenoma prevalence also is higher in persons with a family history of colorectal cancer and adenomas,67,68 particularly if more than one relative is affected with colorectal neoplasia and if the affected relative is young.
Incidence
Estimating the incidence of new adenomas requires examining the colon at more than one point in time. Two types of endoscopic studies lend themselves to this analysis: surveillance studies following polypectomy (or following cancer resection) and interval examinations in persons who initially had a negative examination. Of course, for both types of studies, the small but measurable miss rate of adenomas can influence the rate of apparent incident adenomas (see “Detection,” later). For the purposes of this discussion, adenomas found in persons after polypectomy are considered recurrences (see “Postpolypectomy Management”), whereas those found in persons after an initial negative colonoscopy are considered incident adenomas.
The incidence of new adenomas varies from 24% to 41%.63 In one study, patients underwent colonoscopy twice on the same day to clear the colon of all potentially missed adenomas, and 38% were found to have new adenomas at colonoscopy two years later.69 Three-year follow-up sigmoidoscopy after an initial negative examination in the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial found a 3.1% incidence of all adenomas and 0.8% incidence of advanced adenomas or cancer.70 A large surveillance study looked at findings on follow-up colonoscopy performed within 5.5 years of the index colonoscopy. Patients who had one or two tubular adenomas less than 10 mm were no more likely to have advanced neoplasia than patients with negative baseline colonoscopies. In average-risk, asymptomatic persons with no adenomas at baseline colonoscopy, repeat colonoscopy within five years detects an adenoma in approximately 16% to 27%71,72 and an AAP in approximately 1% to 2.4%.71–73
Anatomic Distribution
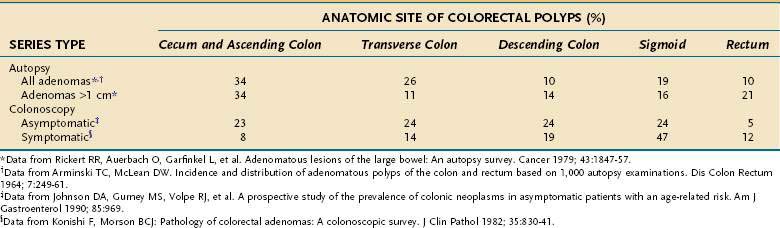
The distribution of adenomatous polyps within the colon differs, depending on the method of investigation (Table 122-7). In autopsy series in which the normal distribution is approximated in presumably asymptomatic subjects, adenomas are distributed uniformly throughout the colorectum; this even distribution has been confirmed in colonoscopic investigations of asymptomatic subjects.23,57 Large adenomas in autopsy series have a distal predominance, in the region where most colon cancers arise, thereby supporting the adenoma-carcinoma hypothesis. Likewise, adenomas detected in surgical and colonoscopic studies of symptomatic people also display a left-sided predominance, indicating that distal adenomas are more likely to come to clinical attention. In older persons, particularly those older than 60 years, distribution of adenomas demonstrates a shift toward more proximal colonic locations. This phenomenon, which is based on autopsy11,12 and on colonoscopic studies of symptomatic74 and asymptomatic22,58,59,66,75 subjects, has importance for choosing appropriate colon cancer screening approaches (see Chapter 123). Some data suggest that African Americans have a greater proportion of proximal adenomas compared with whites, especially in persons older than 60 years.65
Risk Factors for Susceptibility to Adenomas
Evidence is mounting to suggest that both heredity and environment contribute to susceptibility to colonic adenomas. Indeed, the interplay between genetic predisposition and environmental factors supports a hypothesis proposed by Hill many years ago concerning adenoma causation, which was based mainly on epidemiologic and histopathologic observations.76 He postulated that genetic susceptibility to colonic adenomas is extremely common throughout the world. For adenomas to form and then progress to cancer, several environmental factors, presumably dietary, must act in concert. One factor would be responsible for the initial development of adenomas, another would enhance the growth of adenomas, and one or more carcinogens or tumor promoters would finally give rise to cancer.
Inherited Susceptibility to Adenomatous Polyps
There is a strong genetic component to the well-defined hereditary polyposis (FAP) and nonpolyposis (HNPCC) colon cancer syndromes that exhibit a Mendelian pattern of inheritance (see later); however, 95% of common (sporadic) adenomas and carcinomas arise in people who do not have these syndromes. In the past, this observation was interpreted to mean that genetic predisposition played only a minor role in most colonic neoplasms. Epidemiologic studies, however, have revealed a two- to three-fold increased risk for colon cancer in probands who have a first-degree relative affected by colonic cancer or adenoma67; a similarly increased risk also has been found for adenomas in first-degree relatives of people with adenomas.67 Moreover, data from the National Polyp Study indicate that siblings and parents of patients with adenomatous polyps are at an increased risk for colon cancer, particularly when the adenoma proband was younger than 60 years at diagnosis.77 There is even a suggestion that adenomas in patients with a family history of colorectal cancer have faster growth rates.78 Of patients with HNPCC, 11% were found to have at least one adenoma on first colonoscopy; 5% had at least one hyperplastic polyp; and the frequency of adenomas, but not hyperplastic polyps, increased with older age.79
It is now estimated that as much as 10% to 30% of colon cancers are familial, implying the possibility of susceptibility genes that give rise to common colon cancers.72 Despite epidemiologic data suggesting an increased risk in patients with family members who have colon cancer or adenomas, however, it has been difficult to fully elucidate the causative genetic basis. Several genes have been identified that can contribute to common familial risk. These include a germline mutation in the APC gene at codon 1307 (I1307K) that appears to predispose Ashkenazi Jewish populations to colon cancer; mutations of the DNA MMR gene hMSH6, a type I transforming growth factor (TGF)-β receptor allele TbR-I(6A); and polymorphisms of certain genes involved in the metabolism of nutrients and environmental agents, such as methylenetetrahydrofolate reductase and N-acetyltransferase-1 and -2.67,80 A specific mutation in methylenetetrahydrofolate reductase has been found to be protective against colon cancer risk.81 Germline mutations of APC and other genes involved in the APC pathway, such as β-catenin and AXIN1, as well as the DNA mismatch repair genes hMLH1 and hMSH2, have been implicated in the predisposition to multiple adenomas.82 The identification of genes responsible for common susceptibility to colonic adenoma and carcinoma, particularly using polymorphisms of candidate genes, is an area of considerable research.83
Dietary and Lifestyle Risk Factors
Although genetic predisposition clearly plays a role in colorectal carcinogenesis, diet and life-style factors also contribute. It is estimated that as much as a third to a half of colon cancer risk and a fourth to a third of distal colon adenoma risk might be avoidable by modification of dietary and life-style habits.84 For the most part, dietary factors that correlate with a predisposition to colon cancer (see Chapter 123) also are associated with a risk for colonic adenomas.84,85 Factors that have each been correlated with an increased adenoma risk include excess dietary fat, excess alcohol intake, obesity, and cigarette smoking.86 Curiously, low calcium intake, despite being associated with increased risk for colon cancer, does not appear to confer risk for adenoma (although as discussed later, calcium supplementation does seem to lower adenoma recurrences).
Factors that have shown the most consistent protective effect against adenomas in epidemiologic studies include dietary fiber, plant foods, and carbohydrate. Indeed, analysis of dietary questionnaires from patients in the prospective PLCO Trial found that those with the highest fiber intake had a 27% lower risk of adenomas compared with those who had the lowest fiber intake.87 Other protective measures include increased physical activity, increased intake of calcium, and high intake of folate. An analysis of an asymptomatic, predominantly male veteran population undergoing screening colonoscopy found that smoking and moderate-to-heavy alcohol use increased risk, whereas cereal fiber intake, vitamin D intake, and use of nonsteroidal anti-inflammatory drugs (NSAIDs) decreased risk for advanced colonic neoplasia (AAPs and colon cancer).88
Unfortunately, the rather attractive hypothesis that “proper” diets would reduce colon cancer risk has not been substantiated when tested in prospective, interventional studies.89 Dietary changes maintained over two to four years have failed to significantly reduce recurrent or incident adenomas in several studies that tested reductions in fat with increases in fiber, fruits, and vegetables; combinations of low-fat with wheat bran or beta-carotene supplements, or both; supplements of wheat bran fiber with vitamins C and E; and a complex supplement of calcium, vitamin C, vitamin E, and selenium.
Unlike these null studies, four classes of chemopreventive compounds have shown protective effects against colon adenomas or cancers: NSAIDs, calcium, hormone replacement therapy (HRT), and selenium. Of these, NSAIDs including aspirin are the most well-established agents. Greater than 90% of the more than 110 studies of various animal models and more than 35 epidemiologic studies confirm a significant reduction in colorectal adenomas, cancers, and cancer-associated mortality among users of aspirin or NSAIDs.89 For example, the Nurses’ Health Study, a prospective cohort study of 27,077 nurses, found that regular use of aspirin was associated with a lower risk of developing colorectal adenomas.90
Three prospective randomized trials investigating the use of aspirin to prevent colorectal adenomas have shown decreased rates of adenoma recurrence in the study groups compared with placebo. In one trial, which was terminated early because of significant results, 635 patients with a history of curative resection of colorectal cancer and randomized to 325 mg/day of aspirin (or placebo) were found to have a significant reduction in incident adenomas after a mean of 12.8 months.91 Another trial in patients with prior colorectal adenomas compared 81 mg/day or 325 mg/day of aspirin with placebo and found that the 81-mg dose reduced the relative risk of developing adenomas and advanced adenomas by 19% and 41%, respectively, with a similar, but not significant, trend found with the higher dose.92 The one-year results of a trial comparing two doses of lysine acetylsalicylate with placebo found a significant reduction in recurrence of adenomas larger than 5 mm in both treatment groups but a greater effect with the higher dose.93 In contrast, in the Physicians’ Health Study, administration of aspirin 325 mg every other day over five years showed no reduction compared with placebo in advanced adenomas or colon cancers.94 A meta-analysis pooled data from four randomized trials of aspirin at doses from 81 mg to 325 mg daily compared with placebo. More than 2900 patients with a history of an adenoma were included. At a median follow-up of 33 months, the rate of recurrent adenoma was 37% in the placebo group and 33% in the aspirin group. The absolute risk reduction with aspirin use was 6.7%.95
NSAIDs act by inhibiting cyclooxygenase (COX)-1 and COX-2 enzymes, which thereby reduces cellular proliferation, enhances apoptosis, and reduces angiogenesis; other COX-independent effects also are operative. Based on these observations as well as studies showing that selective COX-2 inhibition reduces the number of adenomas in patients with FAP (see later), three large-scale prospective studies of COX-2 inhibitors (celecoxib and rofecoxib) have been undertaken.96 The APPROVe, APC, and PreSap trials randomized more than 5000 patients with a history of adenomas to treatment with celecoxib or rofecoxib or placebo. In all three studies, celecoxib or rofecoxib was superior to placebo in preventing recurrent adenomas and, specifically, advanced adenomas; enthusiasm for these agents was tempered by the finding of increased cardiovascular adverse events in the COX-2 arms. The APPROVe study was terminated early and rofecoxib was withdrawn from the market, but the studies illustrated proof of principle that COX-2 inhibition leads to decreased polyp burden.97
Calcium supplements have been shown in two randomized, placebo-controlled phase III studies to reduce adenoma recurrence by approximately 19% to 34%, with effects noticed even after one year of supplementation.98,99 It has even been suggested that calcium supplements might have a more pronounced effect on advanced adenomas.100 The mechanism for this protective effect likely is multifactorial, because calcium has been shown to decrease proliferation of colonic epithelial cells and to inhibit mucosal injury induced by bile acids and carcinogens in the fecal stream. Calcium can act by neutralizing the mutagenic effects of bile acids on the colonic mucosa or by directly inhibiting epithelial cell proliferation. Data suggest that the benefit of calcium in reducing the adenoma burden is maintained for at least five years after stopping calcium therapy.101
In a study investigating chemoprevention of skin cancer, selenium supplements were associated with a 58% reduction in colon cancer incidence as a secondary end-point.89 This result has prompted additional trials to evaluate the effects of selenium on recurrent formation of adenoma. HRT has been associated in many studies with an approximately 20% reduction in colon cancer risk and a protective effect against colonic adenomas.89 As with NSAIDs, the adverse side effects of HRT often outweigh the beneficial chemopreventive effects of these agents. A randomized, placebo-controlled trial in patients with a history of adenomas found that use of ursodeoxycholic acid at a dose of 8 to 10 mg/kg over three years did not lead to a statistically significant decrease in recurrent adenomas; however, there was a statistically significant 39% decrease in adenomas with high-grade dysplasia.102 Initial evidence from multiple cohort studies, including the Nurses’ Health Study and Canadian National Breast Cancer Screening study, suggested a 40% lower risk of colorectal adenomas in women with the highest levels of folate intake. A randomized, controlled trial of folate alone or in combination with aspirin, however, did not show a decreased risk of polyps or advanced neoplasia in the folate arms.97 Currently, it is unclear if folate has a true benefit in reducing polyp burden.
Difluoromethylornithine (DFMO) suppresses polyamine levels in rectal mucosa likely by modulating ornithine decarboxylase, which can be abnormally expressed in the presence of an APC mutation. DFMO in combination with sulindac has been compared with placebo in patients with a history of an adenoma. At the three-year colonoscopic follow-up, the rate of adenomas was 41% in the placebo group and 12% in the treatment group. The rate of advanced adenoma was 8% in the placebo group and 0.7% in the treatment group.103 DFMO is relatively well tolerated, although ototoxicity is a concern at higher doses. Further work is needed to confirm the efficacy of this agent.
Conditions Associated with Adenomatous Polyps
Ureterosigmoidostomy Sites
Patients who have undergone a urinary diversion procedure with implantation of the ureters into the sigmoid colon are at particularly high risk for developing neoplastic lesions at the ureterosigmoidostomy sites.104 At least 29% of such patients develop colonic neoplasms, usually close to the stoma, after this procedure. Adenomatous polyps and carcinomas have been found after mean latent periods of 20 and 26 years, respectively. Lesions that resemble juvenile polyps and inflammatory polyps also have been reported at ureterosigmoidostomy sites. It has been suggested that these lesions are produced by the generation of N-nitrosamines from urinary amines in the presence of fecal flora. In view of the extremely high frequency of neoplasia in this setting, these patients should undergo lifelong colonoscopic surveillance, recognizing the long latent period between the implantation of the ureters and the subsequent development of colonic neoplasia.
Acromegaly
Patients with acromegaly have an increased tendency to develop colon cancers and adenomas.105–107 Although these studies involved few subjects, consistently high prevalence rates of 5% to 25% for colon cancer and 14% to 35% for adenomatous polyps have been observed in patients with acromegaly. A meta-analysis found the pooled odds ratio for adenomas was 2.4 and for colorectal cancers was 4.3 in patients with acromegaly compared with controls.108 The risk for colonic neoplasia may be higher in younger acromegalics105 and in those with a family history of colon cancer,106 multiple skin tags (acrochordons),107 or previous colorectal adenomas.109 The mechanism for enhanced colonic neoplasia in this disease is not clear but probably relates to increased growth hormone and/or insulin-like growth factor (IGF)-1 levels. In patents without acromegaly, high serum levels of IGF-1 have been correlated with an increased risk of colorectal cancer.110 In patients with acromegaly, high serum IGF-1 levels have been correlated with increased epithelial cell proliferation111 and increased recurrence rates of colorectal adenomas.109 Other studies in acromegalics, however, have not found that blood levels of growth hormone or IGF-1 correlated with the presence of neoplasms107 and that the risk of neoplasia actually was greater in cured acromegalics than in those with active disease.106
Streptococcus bovis Bacteremia and JC Virus
Bacteremia and endocarditis caused by S. bovis have been associated with colorectal carcinoma, adenomatous polyps, and even FAP.112,113 The fecal carriage rate of this organism is higher in people with adenomas or carcinomas than in those with benign colon diseases or in normal controls.114 It has been suggested that patients with S. bovis bacteremia undergo thorough colonic examination to exclude a neoplasm. Endocarditis caused by Streptococcus agalactiae (an organism that seldom is pathogenic in adults) has been reported in two patients, each of whom had a rectal villous adenoma with foci of carcinoma.115 JC virus, an oncogenic polyomavirus that blocks tumor suppressor genes, also has been associated with colonic adenomas and carcinomas.114
Cholecystectomy
In some studies, cholecystectomy has been associated with an increased risk for colon cancer, although this increase is only modest and applies mainly to women and to lesions in the proximal colon.116 It is postulated that in the absence of the gallbladder, there is enhanced delivery of bile acids to the colon and possibly a shift from primary to secondary bile acids that enhances the proliferative activity of the colonic mucosa. In general, however, case-control studies have not found an increased risk for adenomatous polyps among patients who have had cholecystectomy,117 nor was this a risk factor for advanced adenomas or colon cancer among asymptomatic male veterans.88
Clinical Features
Most patients with colonic polyps either have no symptoms referable to the gastrointestinal tract or have nonspecific intestinal symptoms. In persons with symptoms that can be attributed to colonic polyps, the most common presenting symptom is occult or overt rectal bleeding. Histopathologic observations suggest that in contrast to colonic carcinomas, which exhibit considerable surface erosion, the generally less rigid adenomas maintain the integrity of their surface epithelium but can bleed into the polyp stroma.118 These findings help explain the clinical impression that bleeding from polyps is intermittent and usually does not cause fecal occult blood loss or anemia.
A syndrome of secretory diarrhea with considerable and sometimes life-threatening water and electrolyte depletion occasionally has been observed in patients with villous adenomas.119 Tumors that produce this syndrome are typically larger than 3 to 4 cm in diameter and are almost always located in the rectum or rectosigmoid, with little surface area distal to the tumor for reabsorption of fluid and electrolytes. In contrast to the absorption of water and sodium and secretion of potassium exhibited by normal colonic mucosa, secretory villous adenomas have a net secretion of water and sodium and an exaggerated secretion of potassium.120
Detection
Colorectal polyps usually are clinically silent. They typically are detected either in asymptomatic people being screened for colorectal neoplasia or incidentally during investigation for symptoms ostensibly referable to the colon or evaluation of unexplained iron deficiency anemia. A more complete discussion of colorectal cancer screening can be found in Chapter 123. This section addresses the issue of adenoma detection using the available screening modalities.
Fecal Occult Blood Testing
The actual frequency of bleeding from adenomas is difficult to determine. A significant adenoma (i.e., >1 cm or carcinoma in situ) is the cause in less than 10% of people who report frank rectal bleeding.121 In general, polyps smaller than 1 cm do not bleed. This dictum is supported by quantitative measurements of fecal blood loss in people with known adenomas; these measurements indicate that only patients with adenomas larger than 1.5 to 2.0 cm lose more than the usual amounts of blood, regardless of the location of the polyp within the colon.122 Thus, less than 40% of patients with known adenomas show positive fecal occult blood test (FOBT) results, and the higher rates occur primarily in patients with larger and distal polyps.122
When asymptomatic persons older than 40 years of age undergo colon cancer screening with guaiac-based FOBTs, about 1% to 3% will have a positive result.123 Upon colonoscopic evaluation, less than half of these people will have a colorectal neoplasm, and among the lesions found, adenomas outnumber carcinomas by 3 : 1. Thus the proportions of all positive guaiac tests attributable to colonic neoplasms (i.e., positive predictive values) are 30% to 35% for adenomas and 8% to 12% for cancer.124 Despite the predominance of adenomas among lesions detected, 75% of adenomas still may be missed by guaiac testing (i.e., false-negative values) unless they are large or located in the distal portion of the colon. Positive test results for occult blood one to two years after a negative search will detect some of these missed polyps.125 Because small polyps seldom bleed and their rate of detection by occult blood testing is low, sigmoidoscopy or colonoscopy has been recommended to complement FOBTs.
Fecal Immunochemical Testing
Two types of FIT studies have been performed: comparison studies to FOBT, in which all patients received a colonoscopy, and in vitro studies to determine at what level FIT can detect blood in various samples. Like the guaiac-based FOBT, FIT detects more cancers than adenomas. Most studies report sensitivities of 30% to 60% for detecting either cancer or advanced neoplasia.126,127 Few studies have looked at the sensitivity for only adenomas. One large study analyzed seven different FIT tests in a screening population of more than 1300 patients. FITs were better than guaiac-based FOBTs.128 The two best-performing assays had sensitivities of 25% to 27% for advanced adenomas. Specificity was high (97% for all adenomas and 93% for advanced adenomas). When quantitative FIT tests were set at detecting 50 ng Hb/mL in stool, the sensitivity for advanced adenomas was 55%, and higher rates of detection correlated with larger polyps and multiple adenomas.129 At present, although FIT is an improvement over guaiac-based FOBT, it remains inadequate for adenoma screening and is best used as a screening test primarily for colorectal cancer.
Sigmoidoscopy
For several decades, sigmoidoscopy was the mainstay of endoscopic colon cancer screening. Rigid sigmoidoscopy would detect polyps (of all histologic types) in about 7% of asymptomatic persons older than 40 years,130 whereas the flexible sigmoidoscope would find polyps in 10% to 15%, principally because a greater length of bowel could be examined.131 Screening sigmoidoscopy reduced mortality from distal rectosigmoid cancers by as much as 60% to 75%, in several retrospective case-control studies.68 Conclusions regarding mortality reduction from three large-scale prospective studies of screening sigmoidoscopy—the PLCO Study in the United States,70 the United Kingdom Flexible Sigmoidoscopy Screening Trial,132 and a “once-only” sigmoidoscopy trial in Italy133—are awaited. Baseline findings of the U.K. study were that 12% and 0.3% of subjects between ages 55 and 64 years were found to have distal adenomas and distal cancers, respectively.132 The Italian study found an 11% prevalence of distal adenomas in the same age group. In the meantime, the increasing use of colonoscopy in the United States has resulted in a marked reduction in screening sigmoidoscopy examinations.
Barium Enema
Detecting adenomas by barium enema depends on their size. In the National Polyp Study, the detection rates of adenomas smaller than 6 mm, 6 to 10 mm, and larger than 10 mm were 32%, 53%, and 48%, respectively.134 Common sources of error include inadequate cleansing of the colon, which contributes to the 5% to 10% false-positive rate, and diagnostic difficulty caused by the presence of diverticulosis, redundant bowel, or poor mucosal coating, which results in a 10% false-negative rate. Because of these issues, as well as the fact that barium enema never has been formally tested as a colon cancer screening tool, the use of barium enema for colon cancer screening has all but been abandoned in favor of colonoscopy or CT colonography (see later).
Colonoscopy
Colonoscopy is preferred to double-contrast barium enema examination for detecting adenomas because it has enhanced diagnostic accuracy as well as therapeutic capability. This diagnostic superiority has been demonstrated in studies of patients with known polyps134,135 as well as in symptomatic patients who have negative findings on proctosigmoidoscopic and barium enema examinations.136 Colonoscopy has become the preferred colon cancer screening test in many settings.137 Despite its reputation as the gold standard for detecting adenomas, colonoscopy does have some limitations.138 Colonoscopy fails to reach the cecum in up to 10% of cases, it usually requires sedating the patient, and it carries a higher cost than FOBT, FIT, or sigmoidoscopy. Colonoscopy also can miss neoplasms, especially those located at flexures or behind folds. In general, adenomas that are missed tend to be small. Studies using a tandem colonoscopy design demonstrate adenoma miss rates of 0% to 6%, 12% to 13%, and 15% to 27% for adenomas larger than 1 cm, between 6 and 9 mm, and less than 6 mm, respectively.138 CT colonography reveals that colonoscopy can miss 12% to 17% of adenomas larger than 1 cm.138
Given the concern about polyp miss rates, there has been increasing attention to quality measures for colonoscopy. Key measures of high-quality colonoscopy include adequacy of preparation, cecal intubation rate, withdrawal time, and adenoma detection rate. Inadequate preparation contributes to prolonged procedure times, decreased detection of lesions, and the need for repeat colonoscopy before recommended surveillance intervals.139 Colonoscopy is not considered complete unless cecal intubation is accomplished. The majority of screening colonoscopy studies report a cecal intubation rate greater than 95%. Current guidelines suggest that cecal intubation rates should be greater than 90% for all colonoscopies and greater than 95% in screening colonoscopies.139 Most screening colonoscopy studies report adenoma detection rates of 25% to 40%. Men have been consistently found to have a higher burden of adenomas than women. Current guidelines suggest that adenoma detection rates should be at least 15% in women and 25% in men.139
A key factor in adenoma detection rate is colonoscopic withdrawal time. A large study examined the effect of withdrawal time in more than 7800 colonoscopies performed by 12 endoscopists. The adenoma detection rate was 28.3% among endoscopists with a withdrawal time of six minutes or more compared with 11.8% when the withdrawal time was shorter than six minutes. The respective detection rates for advanced adenomas were 6.4% and 2.6%140; slower withdrawal time has been validated by the same investigators in a follow-up study of over 2300 colonoscopies.141 Current recommendations suggest that a withdrawal time of at least six minutes is necessary to maximize detection of adenomas. Continued emphasis on adhering to quality measures and detailed elucidation of the reasons lesions are missed can serve to improve colonoscopy further.
Computed Tomography Colonography
In this rapidly emerging field, a number of studies have compared the performance characteristics of CT colonography with standard optical colonoscopy for detecting polyps.142 Factors affecting detection rates include the polyp prevalence rate in the population being studied, the experience of the radiologist, and technical aspects including bowel preparation techniques, software, and the use of single-row or multi-row scanners. In high-prevalence populations that included symptomatic patients, the sensitivity for detecting polyps ranged from 29% to 59% for small polyps, 47% to 82% for medium polyps, and 63% to 92% for large polyps (Fig. 122-5). Studies of cohorts with low polyp prevalences fared less well, with sensitivities ranging from 32% to 58% and specificities of 90% for polyps larger than 6 mm in diameter.
In the first large study to involve a pure asymptomatic screening population, CT colonography had a sensitivity of 86% for polyps 5 to 9 mm and 92% for polyps larger than 10 mm in the hands of highly skilled radiologists and with patients ingesting oral contrast prior to the study (fecal tagging) to facilitate distinguishing stool from mucosal abnormalities60; a subsequent meta-analysis found very similar results.143 A large multicenter study described a sensitivity of 90% for polyps larger than 10 mm and 78% for polyps between 6 mm and 9 mm.144 In all studies, the detection of polyps smaller than 5 mm by CT colonography has been consistently low.
Concerns have been raised about radiation exposure in healthy screening populations. It has been estimated that the lifetime risk of cancer in any organ associated with a screening CT at age 50 years is 0.14%.137 Given that CT colonography images the entire abdomen, incidental extracolonic findings are often encountered; the rate is as high as 70%, and up to 11% of these incidental findings are clinically significant.137 These incidental findings can prompt additional testing, cost, and anxiety. Lastly, questions remain about the frequency of CT colonography surveillance intervals.
Newer Methods for Detecting Adenomas
Based on our knowledge of molecular genetic alterations in colon carcinogenesis, a noninvasive method for detecting altered human DNA in stool has been developed.145 In initial studies of patients with colon cancer, the sensitivity of the test was approximately 65%. In a large multicenter study of average-risk, asymptomatic persons older than 50 years, the sensitivity for colon cancer was 52% and specificity was 95%.61 This study demonstrated that stool DNA analysis was three to four times more sensitive than guaiac-based FOBT for detecting invasive cancers and adenomas with high-grade dysplasia. A second-generation stool DNA assay was found to have a sensitivity of 83% and a specificity of 82% for cancer.146 A similar stool DNA test detected 46% of adenomas larger than 1 cm compared to a detection rate of 10% to 17% with guaiac-based FOBT.147 Currently, stool DNA testing, like all noninvasive stool-based screening tests, has a low rate of adenoma detection. New technology, however, is already detecting 59% of advanced adenomas148 and is expected to improve in the years ahead.
Treatment
Natural History
The Untreated Adenoma
Longitudinal studies of people with untreated adenomas afford the most direct picture of the natural history of adenomas. In general, however, studies of this kind have been retrospective and either involved few patients or suffered from a lack of histologic confirmation of the index polyp. Despite these drawbacks, it appears that the adenoma-to-carcinoma progression is rather slow, requiring several years to unfold. Muto and associates reported that in 14 persons with nonresected polyps, it took at least five years and often more than 10 years for histologically proven adenomas to progress to cancer.5 The size of the index polyp affects the interval to carcinoma, because larger adenomas are more likely than smaller ones to develop or already contain a focus of cancer. But even starting with a 1-cm polypoid tumor of unknown histology, serial barium enema measurements have suggested that it can take two to five years for cancer to develop149 and that the cumulative risk of cancer at the polyp site is 2.5% at five years, 8% at 10 years, and 24% at 20 years after diagnosis.150 Other radiologic studies indicate that in adenomas with growth rates that are as rapid as those of cancer, doubling times are still longer than four to six months.151
Smaller polyps are likely to require even more time to progress to cancer, and even after several years, many adenomas do not enlarge. For example, in a study involving 213 asymptomatic people with rectal polyps of unknown histology ranging in size from 0.2 to 1.5 cm, serial rigid sigmoidoscopies over three to five years detected only two cancers, and only 4% of the polyps grew larger; the other 96% of polyps remained unchanged, got smaller, or disappeared.152 Also, over a three-year period, adenomas smaller than 1 cm did not significantly change size, and those that were 5 to 9 mm actually showed slight regression.153 In another endoscopic study, histologically proven diminutive adenomas were left in place for two years, after which time only half of them enlarged, but none grew to more than 0.5 cm or developed severe dysplasia or cancer.23 By contrast, other investigators reported that of 30 rectosigmoid polyps 3 to 9 mm that were left in place but measured every six months for two years, none regressed in size, although only three showed a fast growth rate (2 to 4 mm per year).154
A mathematical model, using assumptions based on doubling-time calculations from serial barium enemas, predicts that a diminutive polyp (<0.5 cm) requires two to three years to reach 1-cm size.155 A computational analysis of data from the National Polyp Study, where initial adenoma rates were high but incidence rates were low, also supports the notion that adenomas can regress.156 Despite evidence of polyp regression or slow growth rates, from a clinical management standpoint, any polyp that is detected should be removed.
Age Distribution Studies
Additional, albeit indirect, support of the rather slow growth of adenomas comes from studies that have compared the mean age of people with adenomas with that of people with carcinomas. For instance, studies from St. Mark’s Hospital in London and from the National Polyp Study in the United States indicate that the mean age of people with a single adenoma is about four to five years younger than those with a colon cancer.5,157 A similar analysis in FAP patients has shown that patients with adenomas are about 12 years younger than those with colon cancer.5 The mean age of male veterans with AAP or colon cancer found by screening colonoscopy was 65.1 years, compared with 62.7 years for those without any polyps.88 Kozuka and colleagues estimated that the transition period for adenomas with mild dysplasia to cancer is eight years, whereas the time for adenomas with severe dysplasia to become malignant is 3.6 years.45 Finally, Eide calculated that, over a 10-year period, there is only a 2.5% risk that an adenoma-bearing person will develop colon cancer, but this risk would be greater if the adenoma were large or villous.158
Multiple Adenomas and Carcinomas
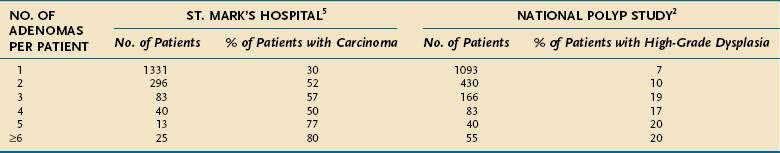
The adenomatous polyp itself commonly is regarded as a marker of a neoplasm-prone colon. Indeed, 30% to 50% of colons with one adenoma contain at least one other synchronous adenoma, especially in the older age groups.5,12,16 The risks of colon cancer and of high-grade dysplasia both rise with the number of adenomas present (Table 122-8) and approach 100% in people with FAP.
Table 122-8 Correlation between the Number of Adenomas per Patient and Associated Carcinomas or High-Grade Dysplasia
A synchronous adenoma can be found in 30% of colons that harbor a carcinoma5,159–161 and in 50% to 85% of those that harbor two or more synchronous cancers.5,159,162 If the synchronous adenoma is diagnosed preoperatively and is distant from the carcinoma, the surgical approach might have to be adapted to the particular circumstances.159 For this reason, before surgical resection of any colorectal carcinoma, a thorough examination of the colon by preoperative colonoscopy or CT colonography is strongly recommended. Moreover, the presence of a synchronous adenoma in a patient with colon cancer increases the risk for developing subsequent colon cancer.5,160 Likewise, a synchronous adenoma in a patient with a colonic polyp places that person at greater risk for developing metachronous polyps163 and cancer.163,164
Initial Treatment
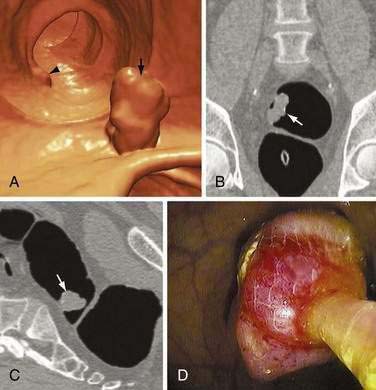
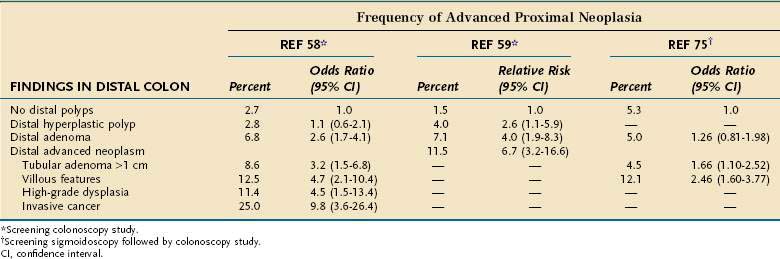
If a polyp is detected by barium enema or CT colonography, a colonoscopy is recommended to afford the simultaneous opportunities to remove the polyp and search for synchronous neoplasms. There is some debate as to whether rectosigmoid adenomas found at sigmoidoscopy are markers for proximal colonic neoplasia and thereby prompt the need for a full colonoscopy. This controversy applies particularly to patients who have only a single small tubular adenoma with low-grade dysplasia. Some studies indicate that in this subset of patients, colonoscopy does not discover a substantial number of proximal AAPs or cancers,165–167 nor is there a subsequent risk of developing proximal cancer among those who do not undergo colonoscopy.168,169 In contrast, two screening colonoscopy studies suggest that the odds of finding advanced proximal neoplasia, even with a single small tubular adenoma, are 2.6-fold to 4-fold that of finding no distal pathology (Table 122-9).58,75
Some authors have estimated that by not doing colonoscopy for a distal nonadvanced adenoma, 36% of advanced proximal neoplasms would be missed.170 Furthermore, 52% of patients with advanced proximal neoplasia have no distal adenoma,58 and 70% of proximal colon cancers lack a distal marker lesion.171 These observations support the use of colonoscopy as a primary screening modality. Because risk factors for finding advanced proximal neoplasia are increasing age,58,59,75 a positive family history of colorectal cancer,58,75 and male gender,59 performing colonoscopy in these persons on the basis of finding a distal small tubular adenoma would seem prudent. A risk index that stratified patients into low, intermediate, or high risk based on the type and size of distal colonic polyps as well as patient age and male sex was able to predict the risk of proximal advanced colonic neoplasia.172
There is little debate that if sigmoidoscopy reveals more than one adenoma or a distal AAP, a full colonoscopy is warranted because of the higher likelihood of finding synchronous proximal advanced neoplasms (see Table 122-9). Because negative biopsy results from a fractional sample of a polyp cannot possibly rule out cancer, total excision of a polyp is the only method of providing a thorough and accurate histologic diagnosis. For larger polyps, this can require piecemeal excision; for sessile growths, injection of saline into the polyp base can assist with complete endoscopic resection. After apparent complete removal of a large adenoma, it is advisable to repeat the colonoscopy in three to six months to document the completeness of the excision. If a polyp cannot be completely excised after two or three endoscopic sessions, surgical therapy is recommended.
Based on reasons listed in the previous discussion, screening colonoscopy is gaining acceptance in the United States as a primary screening tool, although many other countries with more limited resources do not yet subscribe to this philosophy. Two large-scale European trials are under way to evaluate the use of selective colonoscopy in patients based on the findings of a single sigmoidoscopy performed at about age 60 years.132,133 After screening sigmoidoscopy, 5% to 8% of patients were referred for colonoscopy based on the finding of high-risk lesions or multiple polyps. Of these patients, 15% to 18% had proximal adenomas, 5% had advanced proximal adenomas, and fewer than 1% had cancer. Follow-up data regarding the incidence and mortality of colorectal cancer in these patients, as well as the results of the PLCO Cancer Screening Trial,70 will provide additional data from prospective trials on the utility of screening sigmoidoscopy.
Management of the Malignant Polyp
Complete endoscopic removal of an adenoma with noninvasive carcinoma is curative. The decision regarding therapy becomes much more difficult when polyps contain invasive carcinoma. Although most of these lesions are treated adequately by endoscopic polypectomy, approximately 10% of patients experience an adverse outcome,173 defined as residual cancer in the bowel wall or in lymph nodes either at the time of polypectomy or on follow-up examination. This rate of failure is comparable to that of the precolonoscopy era when adenomas were larger and patients underwent more complete surgical resections.173 Because malignant polyps account for only 5% of all adenomas, and because not all patients who have had colonoscopic removal of malignant polyps have had surgical resection or are available for follow-up, conclusions often are based on small numbers.
Notwithstanding these limitations, combined experience has identified certain favorable and unfavorable histopathologic features of a colonoscopically resected malignant polyp that can be used to classify a patient as being at low or high risk for an adverse outcome (see later) (Table 122-10). If none of these unfavorable risk factors is found, the patient is considered to have been cured by the endoscopic polypectomy. This principle applies even to endoscopically removed polypoid carcinomas, which have been associated with a surprisingly good outcome when not complicated by any unfavorable histopathologic features. If one or more unfavorable features is found in a malignant polyp, the chance of an adverse outcome rises to about 10% to 25%.174 In such cases, surgical resection usually should be performed, taking into account the risk of operative mortality in elderly patients with comorbid illnesses.
Table 122-10 Favorable and Unfavorable Features for Adverse Outcomes in Patients with Malignant Polyps
| Feature | FAVORABLE | UNFAVORABLE |
|---|---|---|
| Degree of differentiation | Well or moderate | Poor |
| Invasion of veins or lymphatics | Absent | Present |
| Polypectomy margin | Clear or >2-mm margin | Involved |
| Invasion of submucosa of bowel wall | Absent | Present |
Several aspects of defining these risk factors demand close collaboration between endoscopists and pathologists. First, some malignant polyps contain only a small focus of poorly differentiated carcinoma, and therefore meticulous pathologic analysis is essential. Second, identification of vessel invasion by cancer cells in malignant polyps may be difficult, in which case special stains of vascular endothelium can be used for clarification. Third, although the polypectomy margin may be found on microscopy to contain cancer cells, some studies suggest that if the endoscopist believes that a complete excision was achieved, surgical resection might not be necessary because the electrocautery might have effectively destroyed residual tumor in the bowel wall.175 Finally, when judging the adequacy of endoscopic polypectomy, the issue of submucosal invasion is important.
A pedunculated polyp differs anatomically from a sessile polyp in that the submucosa of the former projects up into the stalk, whereas the submucosa of the latter is in direct continuity with the bowel wall proper (Fig. 122-6). If cancer in a pedunculated polyp is confined to the submucosa of the stalk and all other histologic features are favorable, surgery is not indicated because the chance of an adverse outcome from endoscopic polypectomy is less than the operative mortality. Once the submucosa of the bowel wall is involved with cancer (a situation that occurs more readily in sessile polyps), the chance of an adverse outcome often outweighs the operative mortality, thereby justifying surgical resection. Furthermore, because endoscopic technique purposely avoids cutting deep into the bowel wall submucosa, there are few published examples of sessile lesions that have been completely excised endoscopically with clear margins and no other unfavorable features.
Deciding on the optimal plan of management after polypectomy involves weighing the risks of morbidity and mortality from potential residual or recurrent cancer against the risks of morbidity and mortality from a surgical attempt to cure any such residual disease or lymph node metastasis. A few general recommendations, however, can be offered. If excision of an adenoma is complete, endoscopic polypectomy alone is adequate therapy for adenomas that contain carcinoma in situ, pedunculated adenomas that harbor well-differentiated or moderately differentiated invasive carcinoma, and polypoid carcinomas. Resectional surgery is indicated for malignant polyps in which the invasive carcinoma is poorly differentiated, involves endothelium-lined channels (lymphatics, blood vessels), extends to or within 2 mm of the polypectomy margin, or involves the submucosa of the colonic wall (including all sessile adenomas). Studies have examined the depth of submucosal invasion as a predictor of lymph node metastasis. Two studies found the rate of lymph node metastasis was zero when depth of submucosal invasion was less than 1 mm in one study and 2 mm in the other.176
Postpolypectomy Management
Polyp Recurrence Rates
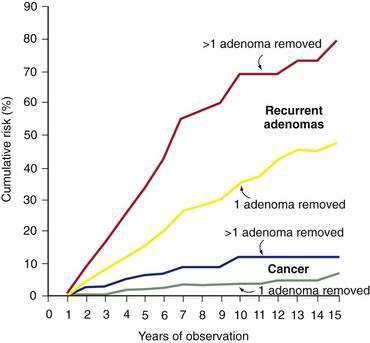
Although patients in whom a colorectal adenoma has been excised completely are likely to develop subsequent (metachronous) neoplasms, the frequency and time course of these occurrences are not well understood. In long-term retrospective studies, the cumulative risk of adenoma recurrence is nearly linear, being 20% at five years after polypectomy and rising to 50% after 15 years (Fig. 122-7).177 Information on this subject is inexact because earlier studies were primarily retrospective, involved short follow-up periods, and differed in endoscopic indications. Moreover, recurrence rates tend to be somewhat inflated because lesions missed during the index colonoscopy might be considered recurrences. With these caveats in mind, it is estimated that one third of people who have undergone polypectomy develop recurrent adenomas.178–180 The recurrence rate at one year is as low as 5% to 15%177,180 but, more realistically, it ranges from 30% to 45% based on prospective colonoscopy studies.178,181–184 In the National Polyp Study, the overall adenoma recurrence rate was 42% for patients who underwent surveillance colonoscopy at one and three years after index polypectomy compared with 32% in the group that was examined only at three years.46 There is general consensus that recurrent adenomas typically are smaller and less likely to harbor advanced pathology than are index adenomas.
Can histopathologic features of adenomas at the time of the index polypectomy be used to help predict recurrence of adenomas? Virtually all studies agree that the presence of multiple index adenomas is an important predictor of subsequent recurrence of adenoma (and carcinoma) (see Fig. 122-7).177,178,180,185,186 This dictum applies despite negative findings on colonoscopy one year after polypectomy.181 Some studies suggest that polyp size greater than 1 cm,179,180,186 severe dysplasia,187 villous histology,180,185 and older age178,180 also are risk factors for adenoma recurrence, but the independent relative importance of each of these factors is uncertain.
It can be argued that the most clinically important recurrence is an AAP. Some studies have reported a 6.3% to 7.0% recurrence rate for AAP over a four-year follow-up period.183 In the National Polyp Study, the recurrence rate of advanced adenomas was 3.3%, regardless of whether patients underwent colonoscopy at one and three years, or only three years, after polypectomy. The cumulative incidence of AAP was 4% at three years and 8% at six years of follow-up. Independent predictors for AAP at follow-up included more than three adenomas at the index colonoscopy, and age older than 60 years at the time of adenoma diagnosis, with a parent who had colorectal cancer.188 In the presence of these two risk factors, cumulative AAP recurrence rates rose to 10% at three years and 20% at six years of follow-up. The persons at lowest risk for developing AAP were those with a single adenoma diagnosed when they were younger than 60 years. Another colonoscopic follow-up study reported that multiple adenomas at the index colonoscopy increased the risk of AAP, but it also found that adenoma size greater than 1 cm and proximal location were additional risk factors.186 A pooled analysis of eight prospective trials with more than 9000 patients found that more than five adenomas, size greater than 20 mm, proximal location, and the presence of villous features were the strongest predictors of recurrent advanced adenomas.189 Interestingly, the presence of high-grade dysplasia was not associated with recurrent adenomas.
Effect of Polypectomy on Colorectal Cancer Incidence
If adenomas are the precursor to colon cancer, then removing them should decrease the subsequent incidence of colon cancer. Indeed, both uncontrolled and case-control series strongly suggest that distal colorectal cancers can be prevented and mortality reduced by screening proctosigmoidoscopy.47,48 Other studies found that removing adenomas by polypectomy was associated with an increased incidence of colon cancer.190–192 Because these retrospective studies did not establish a polyp-free colon (or always consider adenoma size and histology), however, the higher colon cancer rate might have reflected malignant progression of other adenomas that had not been removed; two studies from the Mayo Clinic also did not establish a polyp-free colon at the time of polypectomy.25,164 Despite these observations, if the removed polyps were small (<1 cm), there was no greater risk for subsequently developing colon cancer; in contrast, removal of large adenomas was associated with a greater risk of subsequent colorectal cancer, again supporting the concept that advanced neoplasms at index polypectomy are predictors of subsequent neoplasia. Important observations also come from the St. Marks Hospital study in which rectal adenomas were removed without any subsequent examinations.168 If the index polyp was a small, tubular adenoma, there was no greater risk of subsequent cancer anywhere in the colon, whereas the risk was significantly increased if the index adenoma was large or contained villous elements. Other studies have shown a marked reduction in colon cancer incidence related to polypectomy.193
The most valid study design to address this issue is a prospective colonoscopic study in which an adenoma-free colon is established and patients are followed for subsequent development of colonic neoplasms. The National Polyp Study is a landmark study in this regard. This was a prospective, multicenter, randomized trial in which a cohort of 1418 patients underwent a clearing colonoscopy to remove one or more adenomas; patients were then followed at specific intervals for a mean of 5.9 years. During the follow-up period, five early asymptomatic cancers were detected, representing only 10% to 24% of the expected incidence compared with three historical reference groups.46 Two other studies (from Norway and Italy) confirm that colonoscopic polypectomy was associated with a 75% to 80% reduction in subsequent colorectal cancer incidence.194,195 In a smaller study, the Funen Adenoma Follow-up Study, it was found that although the incidence of subsequent colon cancers was not reduced by polypectomy, the mortality was reduced.196 In summary, these studies indicate that the adenoma is a marker of a neoplasm-prone colon and that colonoscopic clearing of all adenomas is of considerable benefit; however, the data are heterogeneous in describing both the level and duration of protection following a clearing colonoscopy. Furthermore, the number, size, and location of removed polyps plays in a role in determining the level of protection afforded by a clearing colonoscopy. The uneven benefit in preventing subsequent colorectal cancers afforded by colonoscopy is a major impetus in the drive to enhance the quality of colonoscopic examinations.
Frequency of Surveillance Colonoscopy
The National Polyp Study has taught us that performing follow-up colonoscopy at one year is a low-yield proposition, as others have observed using sigmoidoscopy.193 It also is worth realizing that an endoscopic examination of the colorectum can afford protection against colon cancer for 6 to 10 years.47,48 Moreover, it has been proposed that a single lifetime sigmoidoscopy at about age 55 to 60 years could be standard policy for colon cancer screening, with referral of patients for colonoscopy and subsequent surveillance only if the sigmoidoscopy discloses a distal adenoma with advanced features.131
A consensus statement for postpolypectomy surveillance guidelines has been generated by the U.S. Multi-Society Task Force on Colorectal Cancer and the American Cancer Society.197 (Table 122-11). A complete colonoscopy should be performed at the time of polypectomy, clearing the colon of all existing adenomas; this can take more than one session for large or multiple polyps. The interval before the next surveillance colonoscopy is based on the patient’s category of recurrent adenoma risk and features of the adenomatous polyps.
Table 122-11 Surveillance Recommendations after Colonoscopic Polypectomy
| FINDINGS ON COLONOSCOPY | NEXT COLONOSCOPY | COMMENT |
|---|---|---|
| Small rectal hyperplastic polyps | 10 years | Considered equivalent to normal colonoscopy |
| One or two small (<1 cm) tubular adenomas with only LGD (low-risk adenoma) | 5-10 years | Specific follow-up interval is determined by prior colonoscopic findings, family history, patient preference, and clinical judgment |
| 3-10 adenomas, any adenoma ≥1 cm, or any adenoma with villous features or HGD | 3 years | If the 3-year examination is normal, or shows only a low-risk adenoma, repeat again in 5 years |
| >10 adenomas at one examination | <3 years | Use clinical judgment; consider familial syndrome |
| Suggestive of HNPCC* | 2-3 years | Consider more frequent intervals |
| Piecemeal removal of a sessile adenoma | 2-6 months | Follow-up interval based on clinical judgment |
HGD, high-grade dysplasia; HNPCC, hereditary nonpolyposis colorectal cancer; LGD, low-grade dysplasia.
* Features suggestive of HNPCC include right-sided cancers that are poorly differentiated and mucus-producing tumors with intense lymphocytic infiltrates; tumors demonstrate microsatellite instability.
Modified from Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: A consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. CA Cancer J Clin 2006; 56:143-59.
NON-NEOPLASTIC POLYPS
Pathologically, whereas neoplastic polyps are part of an identifiable spectrum, non-neoplastic polyps fall into several distinct and unrelated groups, including hyperplastic polyps, mucosal polyps, juvenile polyps, Peutz-Jeghers polyps, inflammatory polyps, and many other submucosal lesions (see Table 122-1).
Hyperplastic Polyps and Serrated Polyps
Histologic Features
Hyperplastic polyps typically are small sessile lesions that are grossly indistinguishable from small adenomatous polyps. Microscopically, the colonic crypts are elongated and the epithelial cells assume a characteristic papillary configuration (Fig. 122-8). The epithelium is made up of well-differentiated goblet and absorptive cells. The cytologic atypia that is characteristic of adenomatous polyps is not seen. Mitoses and DNA synthesis are limited to the base of the crypts, and orderly cell maturation is preserved. The epithelial cell and attendant pericryptal sheath fibroblast make up an epithelial-mesenchymal unit that migrates up the colonic crypt. In contrast to adenomatous polyps, in which the epithelium and fibroblast appear to be immature, this tissue is more differentiated, and abundant collagen is synthesized in the basement membrane.198 It is thought that the migration of epithelial cells up the colonic crypt is slow and that hyperplastic polyps develop from the failure of mature cells to detach normally.
For the most part, true sporadic hyperplastic polyps are considered to have little if any intrinsic malignant potential; however, it is important to consider that hyperplastic polyps and neoplastic lesions can appear in the same colon, suggesting that the two may be pathogenetically related. Indeed, a germline mutation of the APC gene has been associated with an unusually large number of colorectal hyperplastic polyps in association with adenomas.199 Coexisting hyperplastic and adenomatous polyps also are common in the setting of a strong family history of colorectal cancer, including HNPCC.200 It appears, however, that in patients with sporadic adenomas, the coexistence of hyperplastic polyps does not confer an increased risk of recurrent adenomas during a three-year follow-up period.201
Prevalence
The prevalence of hyperplastic polyps is not known with precision, but these growths are common. In colonoscopic examinations of asymptomatic patients older than 50 years, hyperplastic polyps were found in 9% to 10%,66 although this frequency was higher (30% to 31%) among male veterans.202 Sigmoidoscopic screening of asymptomatic relatives of adenoma-prone kindreds revealed 26% with hyperplastic polyps, a prevalence that was essentially identical (28%) to that of asymptomatic spouse controls.203 Autopsy data report a prevalence rate of 20% to 35%.8,12
The incidence of hyperplastic polyps depends largely on the site of the colon being examined and on the age of the patient. Autopsy studies repeatedly observe a distal predominance of hyperplastic polyps.8,11,12 Of course, sigmoidoscopic studies, which focus on the distal colon and rectum, find many hyperplastic polyps, but even screening colonoscopy studies detect more hyperplastic polyps in the distal than in the proximal colon.62 Among all diminutive polyps (<5 mm) removed during colonoscopy, hyperplastic polyps outnumber adenomatous polyps in the rectum and sigmoid, and adenomas predominate in the remainder of the colon.15,204 The prevalence of hyperplastic polyps increases with age.10,53 There also is an association between prevalences of hyperplastic polyps and colon cancer (Table 122-12), although this correlation is not as firm as the association between adenomas and colon cancers, nor does it necessarily imply any premalignant potential of the hyperplastic polyp itself. Data suggest that current smoking is a risk factor for hyperplastic polyps.88
Treatment
Hyperplastic polyps remain small, usually are sessile, and seldom, if ever, cause symptoms. Inasmuch as they are not likely to give rise to cancer, little is gained by removing them, but because they cannot be distinguished from neoplastic or serrated polyps simply by gross examination, they usually are removed. Given their usual predominance in the distal colorectum, finding hyperplastic polyps in this location is not an alarming finding, particularly in the elderly. Because sigmoidoscopic findings sometimes are used to decide the need for a full colonoscopy, the importance of the distal hyperplastic polyp as a marker of proximal neoplasia has been questioned. A systematic review of the subject found that in asymptomatic persons, a distal hyperplastic polyp is associated with a 21% to 25% risk for any proximal neoplasia and a 4% to 5% risk of advanced proximal neoplasia, thus justifying colonoscopy.205 Thus, although most patients with a distal hyperplastic polyp likely will undergo colonoscopy, current guidelines suggest that if no adenomas are found, such patients can subsequently be followed at intervals similar to those without any polyps.197
Serrated Polyps and the Serrated Neoplasia Pathway
Evidence is mounting to support the concept of a distinct serrated neoplasia pathway.206,207 Polyps that display features of both hyperplastic and adenomatous transformation have been described; these mixed hyperplastic-adenomatous serrated polyps account for about 13% of hyperplastic polyps.208 It has become clear that polyps with a serrated phenotype, instead of representing simple hyperplastic polyps, might represent more clinically significant lesions. Serrated polyps are now classified into hyperplastic polyps (with two subtypes: goblet cell and microvesicular), and two types of serrated adenomas: traditional serrated adenoma (TSA) and sessile serrated adenoma (SSA, sometimes referred to as sessile serrated polyp) (Fig. 122-9).209
SSAs are considered the precursor lesions for the large hyperplastic polyps found in the proximal colon of patients with hyperplastic polyposis.209 When sporadic, SSAs are found more commonly in the proximal colon. By contrast, TSAs look and behave like conventional adenomas: They are often pedunculated, have unequivocal adenomatous dysplasia (albeit with crypt serration), and are found more often in the distal colon. At the molecular level, SSAs often have extensive BRAF mutations and extensive DNA methylation, whereas TSAs usually do not have BRAF mutations and have only infrequent mutations of APC, TP53, and MSI, but they can have K-ras mutations.209
Serrated adenomas are much less common than hyperplastic polyps, accounting for less than 1% of all polyps and between 1% and 11% of adenomas.207 The prevalence of high-grade dysplasia and cancer in these lesions, however, may be as high as 5% to 16%.207 The risk of colorectal cancer may be as high as 1 in 17 in a patient with a proximal sessile serrated adenoma. There are no guidelines for the management of SSAs. It is generally recommended that surveillance intervals for serrated adenomas, although not yet defined, should follow that of other adenomas, bearing in mind their possibly more rapid progression.209
Juvenile Polyps
Juvenile polyps (Fig. 122-10) are mucosal tumors that consist primarily of an excess of lamina propria and dilated cystic glands, rather than an overabundance of epithelial cells as seen in adenomatous and hyperplastic polyps; therefore they are classified as hamartomas. The appearance of distended, mucus-filled glands, inflammatory cells, and edematous lamina propria has prompted some observers to call these lesions retention polyps.
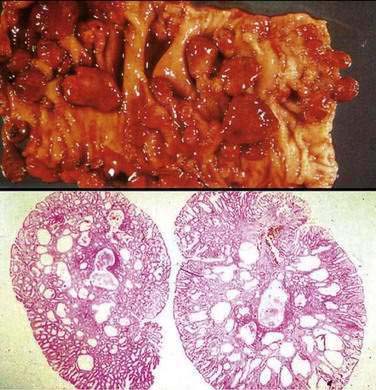
(From Demetris AJ, Finkelstein SD, Nalensnik MA, et al. Slide carousel of GI pathology course for medical students. Available at: http://www.pathology.pitt.edu/lectures/gi/. Copyright © Department of Pathology, University of Pittsburgh School of Medicine.)
Juvenile polyps have essentially no malignant potential when single,210 and they tend not to recur. Although approximately 20% of individual juvenile polyps in the rectum may be associated with proximal polyps, proximal adenomas are rare, and the subsequent rate of dying of or developing colorectal cancer is no greater than these rates for the general population, even without specific surveillance.210 When juvenile polyps are multiple (see “Juvenile Polyposis Syndrome,” later), however, the risk of developing cancer is present, either because adenomatous epithelium may be present in some juvenile polyps or because of a coexistent adenoma.
Peutz-Jeghers Polyps
The Peutz-Jeghers polyp is a unique hamartomatous lesion characterized by glandular epithelium supported by an arborizing framework of well-developed smooth muscle that is contiguous with the muscularis mucosae (Fig. 122-11). The smooth muscle bands fan out into the head of the polyp and become progressively thinner as they project toward its surface. A Peutz-Jeghers polyp differs from a juvenile polyp in that the lamina propria is normal, and the characteristic architecture of the lesion derives chiefly from its abnormal smooth muscle tissue. These polyps almost always are multiple, and their distinctive appearance, in association with the extraintestinal manifestations, makes Peutz-Jeghers syndrome easily identifiable. This type of polyp seldom is found in the colon in the absence of generalized polyposis (see later).
Inflammatory Polyps (Pseudopolyps)
Any form of severe colitis, including chronic inflammatory bowel disease (ulcerative colitis, Crohn’s disease),211 amebic colitis,212 ischemic colitis, or bacterial dysentery, can give rise to inflammatory polyps. In chronic schistosomiasis, multiple inflammatory polyps that contain granulation tissue, eggs, or adult worms commonly are seen.213 The significance of these polyps, which have no intrinsic neoplastic potential, is that they often appear in diseased colons that are at high risk for developing colon cancer (ulcerative colitis, schistosomiasis); therefore, they must be distinguished from neoplastic lesions that do carry premalignant potential. Giant or grouped pseudopolyps can cause colonic obstruction.
Rare cases of multiple and recurrent inflammatory gastrointestinal polyps that produce pain and obstruction have been reported on a sporadic and even a familial basis.214 These lesions are found primarily in the ileum, may be very large, and can even cause intussusception. Cap polyposis is another rare condition, characterized by inflammatory polyps with elongated crypts, a mixed inflammatory infiltrate in the lamina propria, and a surface cap of fibrinopurulent exudate.215 Cap polyposis may be confused endoscopically with pseudopolyps in inflammatory bowel disease. Mucosal prolapse has been suggested as a possible underlying etiology.
SUBMUCOSAL LESIONS
Colitis Cystica Coli
Colitis cystica profunda (see Chapter 124) is a rare lesion consisting of dilated, mucus-filled glands in the submucosa that can form solitary or multiple polyps. The typical lesion is a solitary polyp smaller than 3 cm that most commonly is found in the rectum in the setting of chronic inflammation. Prior surgical procedures and ulcerative proctitis have been linked to the pathogenesis of this abnormality. The involved epithelium shows no evidence of dysplasia. The primary significance of this lesion is that it must be recognized and distinguished from colloid carcinoma, which can look similar histologically, because a mistaken diagnosis of colloid carcinoma could lead to inappropriate radical surgery.216 The lesion is presumably caused by displacement of normal colonic glands to beneath the epithelium during the healing of a surgical wound or inflammation. Rarely, the polyps become large or recur and can even obstruct the colon. It has been suggested that the pathologic picture and clinical presentation of colitis cystica profunda are similar to those of the solitary rectal ulcer and that both may be produced by rectal prolapse.216
Pneumatosis Cystoides Coli
Multiple gas-filled cysts are occasionally encountered within the submucosa of the colon (and small intestine) and can produce a polypoid appearance. The diagnosis of pneumatosis cystoides intestinalis (see Chapter 124) may be made on full-thickness pathologic sections or by the characteristic radiologic or endoscopic appearance of the intramural gas-filled cysts. The diagnosis is substantiated at endoscopy if the cysts collapse after aspiration with a sclerotherapy needle or by unroofing them with a biopsy forceps. This condition can produce a variety of symptoms, some of which suggest colitis, but it also may be associated with vague symptoms or can remain asymptomatic.217
Two forms of pneumatosis intestinalis have been recognized. One type (pneumatosis linearis) may be associated with a fulminant mucosal process, such as inflammatory or ischemic bowel disease in adults or necrotizing enterocolitis in children. In these fulminant settings, in which the condition often is fatal, it is thought that the cysts result from invasion of the submucosa by gas-forming bacteria. The more common type, pneumatosis cystoides intestinalis, is seen in adults and is more typically a chronic or incidental finding; it even may be associated with an asymptomatic pneumoperitoneum. Pneumatosis cystoides intestinalis is associated with chronic obstructive pulmonary disease and may be seen in patients with scleroderma. The genesis of the gas-filled cysts in these benign settings is incompletely understood, but it has been demonstrated that gas within the bowel lumen diffuses into the cysts, which can contribute to their maintenance. Oxygen therapy results in the resolution of these cysts,218 but the pathophysiologic basis of this response is by no means clear. The natural history can be deduced from only a small number of cases, but the disease can persist for months.217–219 A single course of oxygen therapy (often as little as 5 to 6 L/min of oxygen) can result in resolution of symptoms for a long time. Antibiotic treatment is of no benefit.
Other Submucosal Lesions
Any lesion beneath the colon mucosa can elevate the overlying epithelium to produce a polypoid appearance. Lymphoid tissue is present throughout the colon, and hypertrophied follicles may be mistaken for a pathologic mucosal process. Benign lymphoid polyps can grow large enough to produce symptoms (pain, bleeding) or can become pedunculated. Multiple benign lymphoid polyps may be found as normal variants, particularly in children. The principal importance of benign lymphoid polyps is in their distinction from malignant lymphoid lesions. Malignant lymphoma220 and chronic lymphocytic leukemia221 can manifest as multiple colonic polyposis.
The colon is the most common gastrointestinal site for lipomas, which tend to be solitary but may be multiple submucosal lesions. Lipomas usually are asymptomatic and detected incidentally. The low density of fat can give the lesions a characteristic radiologic appearance, and their soft, deformable nature is helpful to the colonoscopist in making the diagnosis grossly. Colonic lipomas are most common in the right colon and tend to occur on or near the ileocecal valve.222 Removal of these lesions usually is not necessary.