CHAPTER 98 Colonic Motor and Sensory Function and Dysfunction
ANATOMY AND BASIC CONTROL MECHANISMS OF THE COLON AND ANORECTUM
STRUCTURE AND ACTIVITY OF COLONIC SMOOTH MUSCLE
ION CHANNELS IN COLONIC SMOOTH MUSCLE
The membrane of colonic smooth muscle cells contains a variety of ion channels, including several types of potassium channels, calcium channels, chloride channels, and nonselective cation channels.2 Although the exact physiologic roles of many of these ion channels are unknown, the high-threshold, voltage-operated calcium channels (l-type calcium channels) do play a crucial role in colonic muscle contractility. These channels open when the membrane potential of smooth muscle cells is depolarized beyond a voltage threshold, and they are responsible for the rapid upstroke of smooth muscle action potentials. The influx of calcium through l-type calcium channels during action potentials is a major trigger for activation of the contractile apparatus. It is not surprising that pharmacologic blockade of l-type calcium channels by dihydropyridine drugs such as nifedipine can reduce the contractility of colonic smooth muscle substantially. Release of calcium from intracellular stores, which is triggered by excitatory neurotransmitters, also may play a role in muscle contraction.
INTERSTITIAL CELLS OF CAJAL
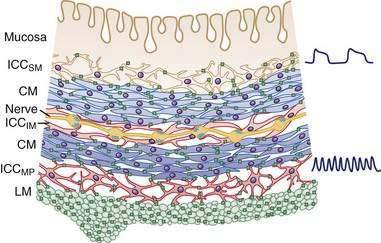
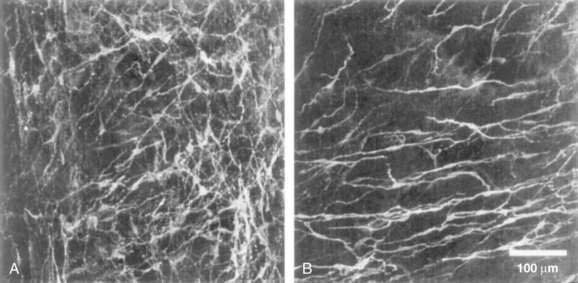
In the human colon, three types of ICC are recognized and are named according to their locations: ICC in the plane of the myenteric plexus (ICCMY), ICC near the submucosal plexus (ICCSM), and intramuscular ICC located between the circular and the longitudinal muscle layers (ICCIM). ICCMY and ICCSM form extensive networks along the colon and are electrically coupled to one another and to the smooth muscle layers by gap junctions (Figs. 98-1 and 98-2). ICCMY probably are the pacemakers for the small, rapid (12-20/min) oscillations in membrane potential (MPOs) of longitudinal and circular smooth muscle layers. ICCSM are the pacemakers for the large-amplitude slow waves (2-4/min) originating in the plane of the submucosal plexus; these slow waves have a powerful influence on the patterning of circular muscle contraction.
The discovery that cellular mechanisms long considered to be the properties of smooth muscle cells actually are mediated by ICC may have important clinical implications. For example, in the distal bowel, reduced numbers of ICC, or a reduction in the total volume of ICC, has been associated with anorectal malformations, colonic manifestations of Chagas’ disease, and possibly some cases of slow-transit constipation.3 Some reports have suggested that the density of ICC may be reduced in aganglionic segments of colon in Hirschsprung’s disease and that this deficit might contribute to diminished propulsive activity; this finding, however, has not been consistent between studies.3
INNERVATION OF THE COLON1
THE ENTERIC NERVOUS SYSTEM
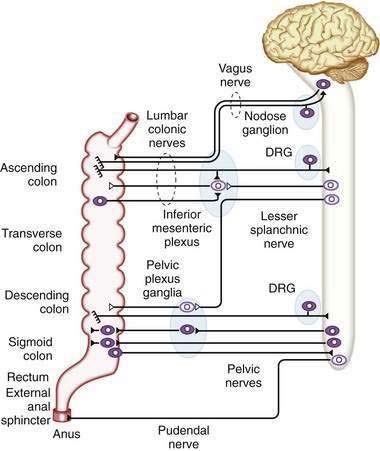
Direct neuronal control of colonic motility is mediated mostly by the enteric nervous system (ENS). Although the ENS is capable of expressing a diverse repertoire of motor patterns, its functions are modulated by sympathetic, parasympathetic, and extrinsic afferent pathways (Fig. 98-3). In terms of numbers of nerve cells, the ENS is by far the largest component of the autonomic nervous system, with considerably more neurons than those of the parasympathetic and sympathetic divisions combined. The nerve cell bodies of the ENS are located in plexuses of myenteric ganglia (Auerbach’s plexus), which lie between the longitudinal and the circular muscle layers of the muscularis externa, or in the submucosal ganglia, which lie between the circular muscle and the mucosa (Fig. 98-4).
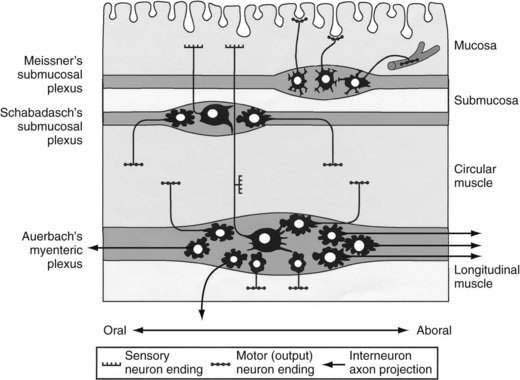
Figure 98-4. Diagram showing the layers and components of the intestinal wall. The lumen is at the top and the longitudinal muscle layer is at the bottom. Auerbach’s myenteric plexus and the submucosal plexuses (Meissner’s and Schabadasch’s plexuses) are shown, along with some of their major classes of enteric neurons. The networks of interstitial cells are shown in Figure 98-1.
Motor Neurons
Enteric motor neurons typically have smaller cell bodies than afferent neurons, with a few short dendrites and a single long axon. Separate populations of motor neurons innervate the circular and longitudinal muscle layers. Excitatory motor neurons synthesize acetylcholine, which they release from their varicose endings in the smooth muscle layers; some also release the tachykinin peptides, substance P and neurokinin A, which excite smooth muscle. Typically, axons of excitatory motor neurons project either directly to the smooth muscle close to their cell bodies or orad for up to 10 mm.4 Once in the smooth muscle layers, the axons turn and run parallel to the smooth muscle fibers for several millimeters; they branch extensively and form many small varicosities, or transmitter release sites, closely associated with intramuscular ICC (ICCIM).
Inhibitory motor neurons typically are slightly larger than excitatory motor neurons, and there are fewer of them. They also have short dendrites and a single axon but, unlike excitatory motor neurons, they project aborally to the smooth muscle layer for distances of 1 to 15 mm in the human colon.4 Once the axon reaches the smooth muscle, it branches extensively to form multiple varicose release sites. Inhibitory motor neurons release a cocktail of transmitters that inhibit smooth muscle cells, including nitric oxide, adenosine triphosphate (ATP), and peptides, such as vasoactive intestinal polypeptide (VIP) and pituitary adenyl cyclase-activating peptide (PACAP). The varicose transmitter release sites of inhibitory motor neurons also are associated with ICCIM, just as are the release sites of excitatory motor neurons. Interstitial cells probably mediate a large component of the electrical effects on smooth muscle of neurotransmitters released by enteric motor neurons. Inhibitory motor neurons usually are tonically active, modulating the ongoing contractile activity of the colonic circular smooth muscle. Inhibitory motor neurons are particularly important in relaxing sphincteric muscles in the ileocecal junction and the internal anal sphincter.
SYMPATHETIC INNERVATION
The major sympathetic innervation of the proximal colon arises from the inferior mesenteric ganglion and projects through the lumbar colonic nerves to the ascending and transverse colon (see Fig. 98-3). A small number of sympathetic neurons in the celiac and superior mesenteric ganglia, in the paravertebral chain ganglia, and in the pelvic plexus ganglia also project to the colon (see Fig. 98-3). These neurons receive a powerful cholinergic drive from preganglionic nerve cell bodies in the intermediolateral column of the spinal cord (segments L2-L5). This is a major pathway by which the central nervous system modifies bowel activity, such as during exercise. Sympathetic efferent neurons also receive input from the enteric viscerofugal neurons and from extrinsic, spinal sensory neurons with cell bodies in the dorsal root ganglia, forming several reflex loops with the distal bowel.
EXTRINSIC AFFERENT PATHWAYS
The entire colon also is innervated by spinal primary afferent neurons with nerve cell bodies in the lumbar dorsal root ganglia. Lumbar spinal afferents project along the lumbar splanchnic nerves, through the prevertebral inferior mesenteric ganglion, and through the lumbar colonic nerves to the colon, where they terminate in sensory endings in the mesentery, serosa, muscular layers, and mucosa throughout the entire colon and rectum. In addition, a population of spinal afferents, with cell bodies in the sacral dorsal root ganglia, projects along the pelvic nerves to the colon and traverses the pelvic plexus en route. Evidence indicates that some of these sacral spinal afferent neurons form a functionally different population from the lumbar spinal afferents, encoding different types of information, particularly from the rectum. Sacral afferents include many mechanoreceptors with a low threshold and wide dynamic range; these mechanoreceptors probably are responsible for graded sensations of rectal filling and for activating defecatory reflexes.5
ANORECTAL ANATOMY AND INNERVATION
As expected, the sources of innervation of the internal and external anal sphincters are different. The internal sphincter directly receives a powerful inhibitory innervation from intrinsic, enteric inhibitory motor neurons and also extrinsic input from lumbar sympathetic and sacral parasympathetic nerves that project via the pelvic plexus ganglia. The external anal sphincter and other pelvic floor muscles are innervated, through the pudendal nerve (S3-S4), by motor neurons with cell bodies in the spinal cord. The rectum and proximal anal canal are richly supplied with sensory receptors that respond to rectal stretch and the composition of the intraluminal contents. These receptors are important for detecting rectal filling, triggering sensations of urgency, facilitating rectal accommodation, and differentiating the composition (stool or gas) of rectal content (see Chapters 96 and 125).
RECOGNIZABLE COLONIC AND ANORECTAL MOTOR PATTERNS AND PUTATIVE FUNCTIONS
NONPROPAGATING MOTOR PATTERNS
Nonpropagating, apparently random activity makes up the majority of the recorded colonic motor activity and is presumed to serve segmenting or mixing functions. The frequency of nonpropagating colonic contractions in vivo is generally 2 to 4 cycles per minute, similar to the frequency of the spontaneous myogenic slow waves generated by ICCSM at the submucosal border of the circular muscle.1 The timing of these nonpropagating contractions is affected relatively little by enteric motor neural activity but is very dependent on the degree of wall distention. This nonpropagating activity also displays a circadian rhythm, being significantly reduced during sleep.
PROPAGATING MOTOR PATTERNS
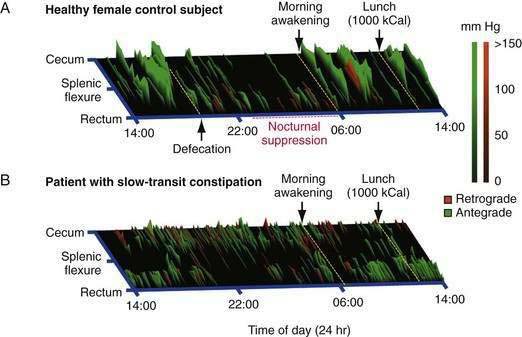
When excitatory motor neurons are active, contractions evoked by MPOs summate, giving rise to powerful lumen-occlusive contractions that can last longer than slow waves and that can propagate substantial distances along the colon. These identifiable colonic motor patterns are commonly referred to as propagating sequences or high-amplitude propagating sequences (also termed propagating contractions or high-amplitude propagating contractions [HAPCs]). Depending upon their direction of propagation, these patterns can be further qualified by the terms antegrade (aboral) or retrograde (orad). In the healthy colon, antegrade propagating sequences are recorded with a three-fold higher frequency than retrograde propagating sequences.6 As with nonpropagating activity, propagating sequences display nocturnal suppression and can be stimulated by a meal.
RECTAL MOTOR COMPLEXES
Periodic contractile activity predominates in the sigmoid colon and rectum. This activity is commonly termed the rectal motor complex (RMC) or periodic rectal motor activity (PRMA). The mean RMC amplitude ranges from 15 to 60 mm Hg with a duration of 3 to 30 minutes.6 In contrast to all other colonic contractile patterns, the circadian trend for RMCs is reversed, i.e., the RMC is more prevalent during sleep, suggesting the relevance of the extrinsic neural control of this pattern. The relationship between the RMC and flow is still incompletely understood. RMCs can be triggered by propagating pressure waves from the proximal colon and by the arrival of stool or gas from the sigmoid colon,7 suggesting the RMC provides a braking mechanism to keep the rectum empty.
REGIONAL VARIATION OF PROPAGATING SEQUENCES
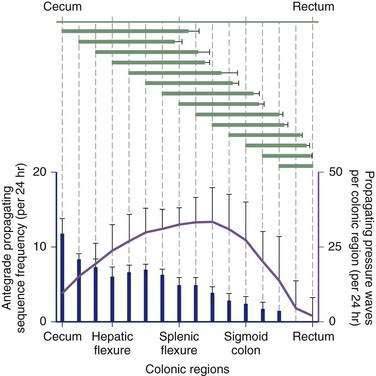
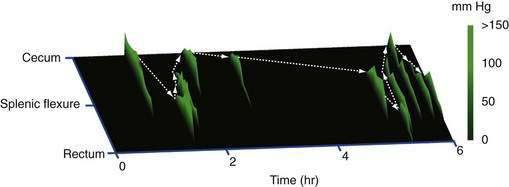
Contractile activity in the human colon demonstrates marked regional variation. For example, propagating pressure waves originate nearly four times as frequently in the proximal colon than in the distal colon (Fig. 98-5). The mean distance covered by antegrade pressure waves arising from the cecum is 50 cm, compared with only 20 cm for sequences originating in the descending colon. Still, pressure waves arising proximally generally do not propagate beyond the mid-colon (see Fig. 98-5). It is now clear that slower propagation rates favor the effective propulsion of contents. The conduction velocity of pressure waves increases as the waves migrate caudally. Indeed, such events often accelerate to the point of synchronicity, which arrests the progress of content moving ahead of the contractile front. In addition, nonpropagating (segmenting) pressure waves make up a higher proportion of activity in the distal colon than in more proximal regions.6 Thus, most motor activity in the distal colon functions to retard forward flow, thereby minimizing challenges to continence.
REGIONAL LINKAGE AMONG PROPAGATING SEQUENCES
In addition to the regional variation in propagating sequence frequency, these sequential motor patterns are linked in an organized spatiotemporal pattern.8 Many of these regionally linked propagating sequences also form series in which three or more consecutive propagating sequences demonstrate a regional shift in the same direction. Each propagating sequence in a linked series originates in either a more proximal or more distal colonic location. Although most single propagating sequences do not span the length the colon, collectively a series of linked propagating sequences can do so (Fig. 98-6), and it is likely that such linkage is important for the transport of content over great lengths of the colon. The mechanisms underlying regional linkage are yet to be determined.
REGULATION OF COLONIC FILLING AND TRANSIT
ROLE OF THE ILEOCECAL JUNCTION
In humans, the ileocecal junction regulates colonic filling and prevents coloileal reflux, thereby preventing contamination of the small bowel with colonic bacteria.9 In the fasting state, cecal filling is slow and erratic, and chyme is retained in the distal ileum for prolonged periods.10 The close physical link between the terminal ileum and the cecum by the ileocecal ligaments behaves functionally as a valve and is responsible in part for continence of the ileocecal junction. A specialized band of muscle forms a low-pressure tonic sphincter11 and prominent 6 cycles-per-minute (cpm) phasic contractions contribute to the regulatory function of the ileocecal junction. Phasic and tonic activity are inhibited concurrently with episodic terminal ileal flow or distention of the ileum, and the tone of the ileocecal junction increases in response to cecal distention.11
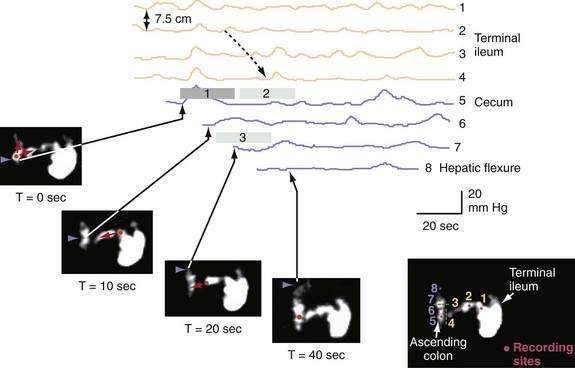
Phase III of the interdigestive motor cycle (IDMC) (or migrating myoelectric-motor complex [MMC]), a motor pattern that occurs every 90 to 120 minutes in the upper intestine during fasting (see Chapter 97), does not contribute to ileocecal transit, because it rarely reaches the terminal ileum in the human. Most ileal chyme, driven by ileal propagating contractions in synchrony with inhibition of phasic contractions of the ileocecal junction, enters the cecum in a pulsatile fashion within 90 minutes of a meal. Prolonged studies, over several hours, correlating ileocecal movement of isotope with intraluminal pressures show that 72% of episodes of ileocecal transport result from monophasic, ileal propagating pressure waves.9 Furthermore, 93% of cecal propagating pressure waves were temporally associated with episodes of cecal filling, a finding that suggests episodic cecal filling is one of the triggers for proximal colonic propagating contractions (Fig. 98-7).9
THE COLON AS A STORAGE ORGAN
The region of preferential storage of colonic content is not entirely settled. In 1902, Cannon proposed on the basis of radiologic observations that the proximal colon is the site of storage and mixing, whereas the distal colon acts as a conduit for expulsion. Subsequent studies, however, found no difference in the dwell time for radiopaque markers in the proximal, middle, and distal colon: roughly 11 hours in each. Composition of the diet influences regional transit and probably accounts for some of the discrepancies among studies. With a liquid diet, the ascending colon empties rapidly, within one to two hours, whereas the transverse colon retains isotope for 20 to 40 hours.12 Solid diet retards transit through the cecum and ascending colon. With a mixed diet, particulate matter and liquids are stored in both the ascending and transverse colon.13
RELATIONSHIPS BETWEEN COLONIC MOTOR PATTERNS AND FLOW
Emptying of the proximal colon occurs more rapidly when wall tone is increased (e.g., by intraluminal fatty acids) than when the tone is low; the volume and consistency of the contents also affect the rate of emptying. Isotonic fluid infused into the proximal colon stimulates proximal colonic emptying, suggesting that distention, per se, can activate propulsive motor patterns. Irritant laxatives (which act by stimulating mucosal receptors) in the proximal colon, however, trigger propagating contractions much more reliably than distention alone.14 Hence, proximal colonic emptying is influenced by a combination of increased wall tone and the initiation of propagating contractions, probably under the influence of both chemical and mechanical factors.
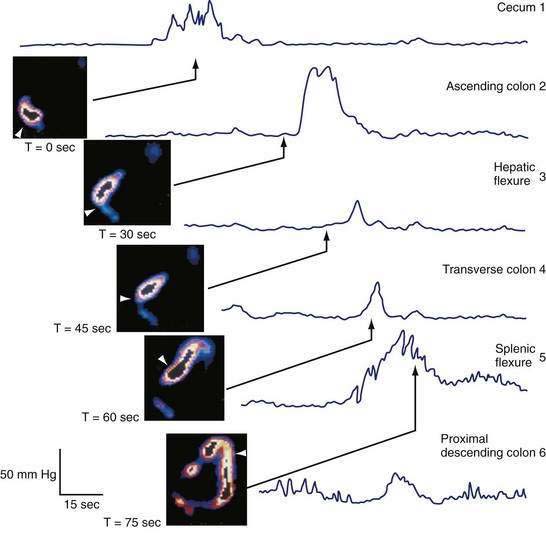
Mass movements, first detected radiologically, are infrequent movements of stool over long distances. More often, movement of colonic content occurs in a stepwise manner over short distances and in both antegrade and retrograde directions.15 Studies combining manometry with radiography in animals and with high frame rate scintigraphy in humans have shown that 93% of all propagating sequences in the proximal colon, regardless of amplitude or polarity, are temporally associated with discrete movements of isotope-labeled colonic contents within the unprepared colon (Fig. 98-8).15 The strength of this pressure-flow relationship is region dependent, being stronger in the transverse colon than in the cecum and ascending colon.
DEFECATION
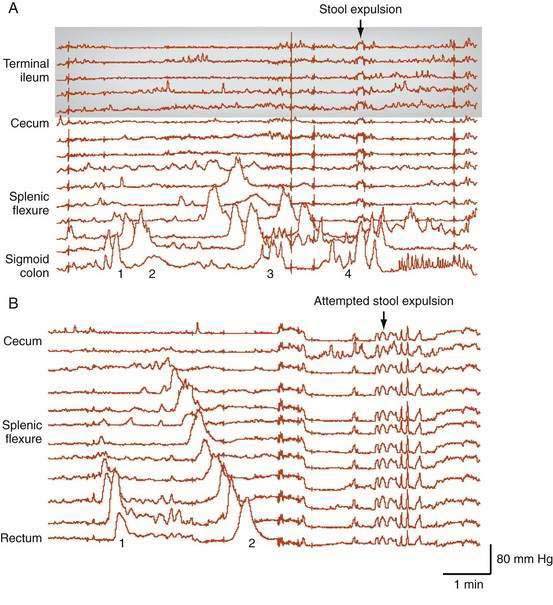
Furthermore, pancolonic manometric studies have demonstrated that the preparatory phase of defecation not only involves the greater part of the colon but also commences up to one hour before stool expulsion.16 In this predefecatory phase, a characteristic progressive increase occurs in the frequency of propagating pressure wave sequences. These sequences start first in the proximal colon, with each successive sequence originating slightly more distal to the preceding one; these priming sequences do not evoke conscious sensation. By contrast, in the 15 minutes leading up to defecation, a dramatic increase occurs in the frequency of these propagating sequences, which leads to a strong defecatory urge. In the last 15 minutes of the predefecatory phase, propagating pressure waves begin to originate in the distal colon; however, in this late phase, each successive propagating sequence originates from a site proximal to the preceding one. Each sequence also tends to run for a slightly longer distance and has a higher amplitude compared with the preceding propagating sequence (Fig. 98-9). These final sequences provide potent forces to fill and distend the rectum, activating specialized low-threshold sacral spinal afferent mechanoreceptors. These mechanoreceptors then give rise to the defecatory urge, prompting the expulsive phase in which the anorectum comes into play.
RECTAL FILLING, CAPACITANCE, AND ACCOMMODATION AND MOTILITY OF THE ANAL SPHINCTERS
A large-volume rectal distention causes an internal sphincter relaxation of longer duration, which is registered consciously and which necessitates extra voluntary contraction of the external anal sphincter to maintain continence while the person decides how best to deal with the intraluminal content (stool or gas). Suppression of the defecation urge at this time, together with receptive accommodation of the rectum (see later), results in temporary storage of stool or gas in the rectum or retrograde transport of the stool or gas back to the sigmoid colon. Although the rectum is usually empty, it has the capacity to temporarily store feces until convenient evacuation can be arranged. More-prolonged rectal storage is made possible by the ability of the rectum to accommodate an increasing volume without a corresponding increase in intrarectal pressure, in a manner similar to gastric fundic relaxation.17 This adaptive increase in rectal compliance, mediated by inhibitory nerves, is important for maintaining continence by permitting prolonged fecal storage without a constant urge to defecate. Such rectal distention also has negative feedback effects on the proximal bowel and inhibits gastric emptying, slows small bowel transit, reduces the frequency of proximal colonic propagating pressure waves, and delays colonic transit.18 Typically, rectal tone is increased following a meal. A pathologic reduction of rectal compliance, such as after pelvic radiotherapy, causes rectal urgency. Conversely, excessive compliance, as in megarectum, attenuates the urge to defecate.
ANORECTAL MOTILITY DURING DEFECATION
If the processes just described give rise to the urge to defecate and the social circumstances are appropriate, the full defecation process is activated. This process involves a combination of pelvic reflexes coordinated in the medulla and pons. Rectal distention by stool stimulates reflex-induced complete relaxation of the internal anal sphincter, and the stool moves into the upper anal canal, heightening the sense of urge. Postural changes and straining facilitate this process in several ways: Sitting or squatting causes descent of the anorectal junction, and straining produces further rectal descent. Both activities serve to increase the anorectal angle, thereby reducing resistance to outflow. At this point, if the person wishes to proceed to expel stool, the external anal sphincter is relaxed voluntarily. At the same time, the puborectalis muscle is relaxed (further increasing the anorectal angle); the levator ani muscles contract; the perineum descends further; and stool is funneled into the anal canal and expelled by increasing strain-induced, intrarectal pressure (Fig. 98-10). Once the expulsion phase has commenced, evacuation of stool can proceed in some cases without further straining, as a consequence of colonic contractions propagating toward the anus (see Fig. 98-9).16 Expulsion of stool is possible in response to strain alone without rectosigmoid contractions, although a contribution from increased rectal wall tone cannot be excluded.
MODULATORS OF COLONIC MOTILITY
PHYSIOLOGIC
Twenty-four hour recordings of myoelectric activity or intraluminal pressure show that colonic phasic and tonic activity predictably are increased one to two hours after a meal (the gastrocolonic response) and are markedly suppressed at night.19 The entire colon responds to the meal, with an increase in colonic wall tone, migratory long spike-bursts, and propagating and segmenting contractile patterns. A minimum caloric load of approximately 300 kcal is required to generate the colonic response to a meal, and a meal of only 200 kcal increases rectal muscle tone.20 The meal response also is highly dependent on the fat content of the caloric load. For example, 600 kcal of fat induces the response, whereas an equicaloric load of protein or carbohydrate does not.
The mechanism of the colonic meal-response remains unclear, although it is known that neither the stomach nor the spinal cord needs to be intact to display the response. Non-nutrient gastric distention, by balloon or water, also can stimulate rectosigmoid motility, yielding a similar response to that following intraduodenal lipid infusion. Both of these responses are markedly attenuated by prior intravenous administration of the 5-hydroxytryptamine-3 (5-HT3) receptor antagonist granisetron, which suggests that 5-HT3 receptors on vagal afferents may be involved in the gastrocolic response.21 Cholecystokinin (CCK), which is released by fats and fatty acids in the duodenum, can replicate the gastrocolic response, but only at doses exceeding those occurring postprandially. The CCK-A antagonist loxiglumide blocks the effects of CCK on the colon but does not abolish the gastrocolic response, thus making CCK an unlikely mediator of the response.
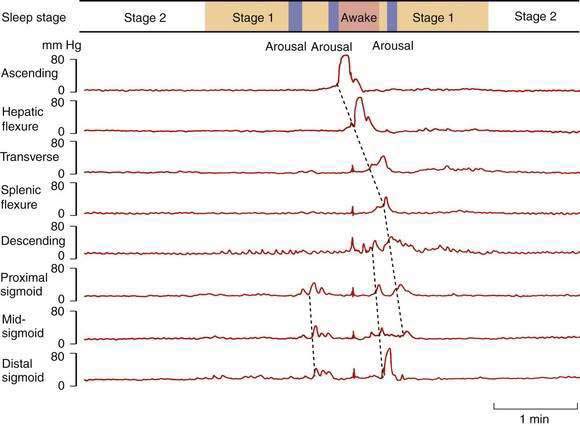
Colonic myoelectric and pressure activities are profoundly suppressed at night.19 During stable sleep, colonic motility virtually ceases (except for the antipropulsive rectal motor complexes, which increase), thereby reducing the challenges to continence at a time when anal sphincter tone and awareness of colorectal sensations are minimal. If the subject shifts to a lighter level of sleep, even without actually awakening, an immediate increase occurs in propagating and nonpropagating pressure waves (Fig. 98-11). Forced awakening at night and spontaneous early-morning awakening both stimulate an immediate increase in colonic propagating pressure waves. This phenomenon clearly is linked with the readily identifiable habit of defecation soon after awakening in the morning and demonstrates the potential for profound modulation of colonic motor activity by the central nervous system.
Stress and emotional factors long have been believed to influence colonic motility, but experimental evidence for this is conflicting, possibly because of a reliance on measurements from the distal colon, which might not be representative. In light of the profound waking-response, it is likely, but unproved, that stress does induce propagating pressure waves. Due to technical difficulties associated with trying to record physical activity and colonic motility simultaneously, data on the colonic response to physical activity are sparse; however, physical exercise, perhaps through increased sympathetic tone, decreases colonic motility.22 The colonic response to stress and exercise highlight the importance of the autonomic nervous system in modulating colonic function. Similarly, autonomic dysfunction, resulting from pelvic surgery, childbirth, or neural degradation, has been implicated in several colonic disorders including slow-transit constipation and irritable bowel syndrome (IBS).23
PHARMACOLOGIC
Colchicine, a natural alkaloid, is well known to cause diarrhea. Colchicine increases the frequency of spontaneous bowel movements and accelerates colonic transit in patients with chronic constipation24; the mode of action is not yet clear but it has been shown to increase prostaglandin synthesis and to promote intestinal secretion, the latter mediated through cyclic AMP. In the rat, small intestinal colchicine stimulates myoelectric activity.
Lubiprostone, a type 2 chloride channel (ClC2) activator, is a member of a new class of compounds known as prostones. Activation of ClC2 increases intestinal chloride secretion resulting in increased intraluminal fluid accumulation, which accelerates intestinal transit, softens stools, and increases spontaneous stool frequency in patients with constipation.25
Serotonin (5-HT) is an important mediator of bowel physiology, and both 5-HT3 and 5-HT4 receptors play a role in colonic peristalsis and transit. For example, the 5-HT3 receptor antagonists granisetron and ondansetron blunt the gastrocolic response and delay colonic transit, respectively.21 Alosetron, another antagonist of the 5-HT3 receptor, exerts a significant constipating affect by slowing colonic transit.26 In contrast, 5-HT4 agonists (e.g., tegaserod, prucalopride, renzapride), act on presynaptic receptors and facilitate release of acetylcholine and CGRP (calcitonin gene-related peptide), thereby inducing colonic propagating contractions and accelerating colonic transit. Although this class of drug shows promise for the treatment of constipation,27 tegaserod, a 5-HT4 agonist, was withdrawn from the market because of concern about associated adverse cardiovascular events (see Chapter 118). Other highly selective 5-HT4 agonists, such as prucalopride, might be attractive options because they do not interact with 5-HT3 or 5-HT1B receptors, and prucalopride does improve stool frequency and symptoms in severe constipation.28 Further trials with these agents are awaited.
Opiates are well known to have an antidiarrheal effect, but their mechanism of action is less clear. In the human colon, morphine increases phasic segmenting activity, reduces colonic tone, and attenuates the bowel’s response to a meal.29 Opiates are known to inhibit presynaptic and postsynaptic enteric neural circuitry. The reduction in neurally dependent propagating contractions and the enhancement of myogenic mixing movements and fluid absorption contribute to the constipating effect of the drug. Specific constipation syndromes, such as opiate-induced constipation or postsurgical ileus, might respond to opiate antagonists such as methylnaltrexone and alvimopan (see Chapter 120).30
Nitric oxide is a potent endogenous inhibitor of colonic propulsive activity and the human colon appears to be under a state of tonic nitrergic inhibition. For example, infusion of the nitric oxide synthase inhibitor, l-NMMA (NG-monomethyl l-arginine), is a potent stimulator of colonic propagating contractions.31 Alternatively, segmental lengthening of the colon induced by the entry of content triggers nitric oxide release from descending pathways, which in turn inhibits colonic propulsive activity.32
NONPHARMACOLOGIC
Probiotics are living organisms that, when ingested in adequate amounts, are claimed to exert a health benefit to the host. Relatively few rigorously designed studies have been conducted with probiotics but some strains have been shown to have a beneficial effect in IBS (see Chapter 118), ulcerative colitis (see Chapter 112), and diarrhea.33 In the colon, probiotics are likely to modulate the inflammatory response through activation of signals with the epithelium and immune system.33 Probiotics may well inflence colonic motility, but this has not been systematically evaluated.
Sacral nerve stimulation modulates the extrinsic nerves innervating the pelvic floor and colon. Electrical stimulation of the S3 sacral root induces a modest increase in external anal sphincter tone and has been used successfully in the management of fecal incontinence. Stimulation of the S3 root also induces propulsive activity throughout the entire colon and has been shown to increase stool frequency in patients with slow-transit constipation.34 Randomized trials of this promising technique for treating slow-transit constipation are in progress; the precise mode of this action remains unknown. The substantial latency between stimulus and pelvic floor or colonic contractile responses is longer than would be expected via a polysynaptic efferent pathway, which suggests possible involvement of extrinsic neural pathways.35 Magnetic stimulation of the sacral nerve S3 also shows promise in modulating colonic and anorectal function.36 Because this approach is less invasive than electrical stimulation of sacral nerves, it may be a reasonable treatment option in children with colonic or anorectal dysfunction.
Acupuncture has been shown to have significant effects upon upper gastrointestinal tract disorders such as nausea and vomiting. Only two studies have evaluated its potential in constipation, one in children and one in adults.37 Acupuncture improved stool frequency in children, but these results weren’t replicated in adults; this warrants further study. Acupuncture is known to activate neural, opioid, humoral, and serotoninergic pathways and potentially has a clinical role in treating disorders such as constipation and IBS.37
Biofeedback has been shown to improve stool frequency and rectal evacuation in patients with pelvic floor dyssynergia, and the technique has been shown to accelerate colonic transit in this subset of patients with constipation (see Chapter 18).38 The mode of action of biofeedback is not fully understood, but evidence suggests that extrinsic autonomic efferent pathways mediate the response.39
DISORDERS OF COLONIC MOTILITY
Disorders attributable to disturbed colonic motor function are discussed elsewhere in this book (Chapter 120). It is useful, however, to consider how disturbances in the mechanisms of colonic motility described in this chapter might relate to symptoms or pathophysiologic phenomena.
CONSTIPATION
In severe slow-transit constipation, prolonged manometric studies have confirmed a reduction in the overall number of high-amplitude propagating pressure waves19; however, the overall number of propagating pressure waves of any magnitude is often normal or increased. Studies examining the spatiotemporal patterning of propagating sequences have revealed colonic regions in which activity is diminished or absent, particularly within the vicinity of the splenic flexure (Fig. 98-12).40 Furthermore, there appears to be a loss of the normal linkage in patients who have sequential progressive systemic sclerosis and constipation.8 The underlying pathogenesis of severe slow-transit constipation is unclear, but changes in enteric excitatory motor innervation of the smooth muscle in patients with severe slow-transit constipation are likely to contribute to this disorder.41
DIARRHEA
Detailed scintigraphic studies in patients with diarrhea have shown the dominant feature to be early and rapid transit through the ascending and transverse colon.42 Normally, propagating sequences are more frequent in these proximal regions than elsewhere. Manometric data from the entire colon in patients with diarrhea would help explain these observations, but are lacking. A relative lack of distal colonic segmenting activity, perhaps in combination with increased proximal colonic propagating pressure waves, might explain this preferential acceleration of proximal colonic transit, but proof of this hypothesis is awaited. Diarrhea is fully discussed in Chapter 15.
IRRITABLE BOWEL SYNDROME
Although colonic transit generally is slower in constipation-predominant IBS and faster in diarrhea-predominant IBS, no colonic motor pattern is specific for IBS.43 Exaggerated responses to stimuli such as meals, CCK, and mechanical stimuli have been reported, but a consistent disturbance has not emerged, probably because of the heterogeneity of the disease and the methodologies used for characterization. In addition, remarkably little study of the proximal colon in IBS has been conducted to date. At present, compelling evidence regarding the pathophysiology of IBS suggests a major contribution by afferent hypersensitivity, in addition to a variable alteration in colonic motor function. IBS is fully discussed in Chapter 118.
COLONIC MOTILITY DISTURBANCES SECONDARY TO NONMOTOR INTESTINAL DISORDERS
Altered motility secondary to underlying inflammation or a hormonal disturbance can contribute to the colonic symptoms of a nonmotor disease. The diarrhea of idiopathic inflammatory bowel disease, for example, results from a combination of enhanced secretion, reduced absorption, and altered colonic motor function. In ulcerative colitis, rectosigmoid-segmenting, nonpropagating pressure waves are diminished, whereas postprandial propagating pressure waves are increased.13 Rectal compliance also is reduced, and together, these effects can exacerbate diarrhea, as suggested by studies demonstrating rapid rectosigmoid transit in ulcerative colitis.13 The motility of the healthy colon also can be perturbed by ileal diseases. For example, exposure of the healthy proximal colon to supranormal concentrations of bile salts, such as from terminal ileal disease or resection, not only stimulates net colonic secretion but also initiates high-amplitude propagating pressure waves, thereby accelerating colonic transit.13
Bampton PA, Dinning PG, Kennedy ML, et al. Spatial and temporal organization of pressure patterns throughout the unprepared colon during spontaneous defecation. Am J Gastroenterol. 2000;95:1027-35. (Ref 16.)
Dickson EJ, Spencer NJ, Hennig GW, et al. An enteric occult reflex underlies accommodation and slow transit in the distal large bowel. Gastroenterology. 2007;132:1912-24. (Ref 32.)
Dinning PG, Szczesniak MM, Cook IJ. Removal of tonic nitrergic inhibition is a potent stimulus for human proximal colonic propagating sequences. Neurogastroenterol Mot. 2006;18:37-44. (Ref 31.)
Dinning PG, Szczesniak MM, Cook IJ. Determinants of postprandial flow across the human ileocecal junction: A combined manometric and scintigraphic study. Neurogastroenterol Mot. 2008;10:1119-26. (Ref 9.)
Di Stefano M, Miceli E, Missanelli A, et al. Meal induced rectosigmoid tone modification: A low caloric meal accurately separates functional and organic gastrointestinal disease patients. Gut. 2006;55:1409-14. (Ref 20.)
Farrugia G. Ionic conductances in gastrointestinal smooth muscles and interstitial cells of Cajal. Ann Rev Physiol. 1999;61:45-84. (Ref 2.)
Hagger R, Kumar D, Benson M, Grunday A. Periodic colonic motor activity identified by 24-h pancolonic ambulatory manometry in humans. Neurogastroenterol Mot. 2002;14:271-8. (Ref 7.)
Hardcastle JD, Mann CV. Physical factors in the stimulation of colonic peristalsis. Gut. 1970;11:41-6. (Ref 14.)
Kamath PS, Phillips SF, O’Connor MK, et al. Colonic capacitance and transit in man: modulation by luminal contents and drugs. Gut. 1990;31:443-9. (Ref 29.)
O’Brien MD, Phillips SF. Colonic motility in health and disease. Gastroenterol Clin North Am. 1996;25:147-62. (Ref 13.)
Porter AJ, Wattchow DA, Brookes SJ, Costa M. The neurochemical coding and projections of circular muscle motor neurons in the human colon. Gastroenterology. 1997;113:1916-23. (Ref 4.)
Rae MG, Fleming N, McGregor DB, et al. Control of motility patterns in the human colonic circular muscle layer by pacemaker activity. J Physiol. 1998;510:309-20. (Ref 1.)
Rao SS, Sadeghi P, Beaty J, Kavlock R. Ambulatory 24-hour colonic manometry in slow-transit constipation. Am J Gastroenterol. 2004;99:2405-16. (Ref 19.)
Sanders KM, Koh SD, Ward SM. Organisation and electrophysiology of intersitial Cajal and smooth muscle cells in the gastrointestinal tract. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. Amsterdam: Elsevier; 2006:533-76. (Ref 3.)
Scott M. Manometric techniques for the evaluation of colonic motor activity: current status. Neurogastroenterol Mot. 2003;15:483-513. (Ref 6.)
1. Rae MG, Fleming N, McGregor DB, et al. Control of motility patterns in the human colonic circular muscle layer by pacemaker activity. J Physiol. 1998;510:309-20.
2. Farrugia G. Ionic conductances in gastrointestinal smooth muscles and interstitial cells of Cajal. Ann Rev Physiol. 1999;61:45-84.
3. Sanders KM, Koh SD, Ward SM. Organisation and electrophysiology of intersitial Cajal and smooth muscle cells in the gastrointestinal tract. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. Amsterdam: Elsevier; 2006:533-76.
4. Porter AJ, Wattchow DA, Brookes SJ, Costa M. The neurochemical coding and projections of circular muscle motor neurons in the human colon. Gastroenterology. 1997;113:1916-23.
5. Lynn PA, Olsson C, Zagorodnyuk V, et al. Rectal intraganglionic laminar endings are transduction sites of extrinsic mechanoreceptors in the guinea pig rectum. Gastroenterology. 2003;125:786-94.
6. Scott M. Manometric techniques for the evaluation of colonic motor activity: current status. Neurogastroenterol Mot. 2003;15:483-513.
7. Hagger R, Kumar D, Benson M, Grunday A. Periodic colonic motor activity identified by 24-h pancolonic ambulatory manometry in humans. Neurogastroenterol Mot. 2002;14:271-8.
8. Dinning PG, Szczesniak MM, Cook IJ. Spatio-temporal analysis reveals aberrant linkage among sequential propagating pressure wave sequences in patients with severe constipation. Neurogastroenterol Mot. 2009. May 14. [Epub ahead of print]
9. Dinning PG, Szczesniak MM, Cook IJ. Determinants of postprandial flow across the human ileocecal junction: a combined manometric and scintigraphic study. Neurogastroenterol Mot. 2008;10:1119-26.
10. Spiller RC, Brown ML, Phillips SF. Emptying of the terminal ileum in intact humans. Influence of meal residue and ileal motility. Gastroenterology. 1987;92:724-9.
11. Dinning PG, Bampton PA, Kennedy ML, et al. Basal pressure patterns and reflexive motor responses in the human ileocolonic junction. Am J Physiol. 1999;276:G331-40.
12. Metcalf AM, Phillips SF, Zinsmeister AR, et al. Simplified assessment of segmental colonic transit. Gastroenterology. 1987;92:40-7.
13. O’Brien MD, Phillips SF. Colonic motility in health and disease. Gastroenterol Clin North Am. 1996;25:147-62.
14. Hardcastle JD, Mann CV. Physical factors in the stimulation of colonic peristalsis. Gut. 1970;11:41-6.
15. Dinning PG, Szczesniak MM, Cook IJ. Proximal colonic propagating pressure waves sequences and their relationship with movements of content in the proximal human colon. Neurogastroenterol Mot. 2008;20(5):512-20.
16. Bampton PA, Dinning PG, Kennedy ML, et al. Spatial and temporal organization of pressure patterns throughout the unprepared colon during spontaneous defecation. Am J Gastroenterol. 2000;95:1027-35.
17. Bell AM, Pemberton JH, Hanson RB, Zinsmeister AR. Variations in muscle tone of the human rectum: recordings with an electromechanical barostat. Am J Physiol. 1991;260:G17-25.
18. Dinning PG, Bampton PA, Kennedy ML, et al. Impaired proximal colonic motor response to rectal mechanical and chemical stimulation in obstructed defecation. Dis Colon Rectum. 2005;48:1777-84.
19. Rao SS, Sadeghi P, Beaty J, Kavlock R. Ambulatory 24-hour colonic manometry in slow-transit constipation. Am J Gastroenterol. 2004;99:2405-16.
20. Di Stefano M, Miceli E, Missanelli A, et al. Meal induced rectosigmoid tone modification: a low caloric meal accurately separates functional and organic gastrointestinal disease patients. Gut. 2006;55:1409-14.
21. Bjornsson ES, Chey WD, Ladabaum U, et al. Differential 5-HT3 mediation of human gastrocolonic response and colonic peristaltic reflex. Am J of Physiol. 1998;275:G498-505.
22. Rao SSC, Beaty J, Chamberlain M, et al. Effects of acute graded exercise on human colonic motility. Am J Physiol. 1999;276:G1221-6.
23. El-Salhy M. Chronic idiopathic slow transit constipation: pathophysiology and management. Colorectal Dis. 2003;5:288-96.
24. Verne GN, Davis RH, Robinson ME, et al. Treatment of chronic constipation with colchicine: randomized, double-blind, placebo-controlled, crossover trial. Am J Gastroenterol. 2003;98:1112-16.
25. Johanson JF, Ueno R. Lubiprostone, a locally acting chloride channel activator, in adult patients with chronic constipation: a double-blind, placebo-controlled, dose-ranging study to evaluate efficacy and safety. Aliment Pharmacol Ther. 2007;25:1351-61.
26. Houghton LA, Foster JM, Whorwell PJ. Alosetron, a 5-HT3 receptor antagonist, delays colonic transit in patients with irritable bowel syndrome and healthy volunteers. Aliment Pharmacol Ther. 2000;14:775-82.
27. Johanson J, Wald A, Tougas G, et al. Effect of tegaserod in chronic constipation: A randomized, double-blind, controlled trial. Clin Gastro Hepatol. 2004;2:796-805.
28. Camilleri M, Kerstens R, Rykx A, Vandeplassche L. A placebo-controlled trial of prucalopride for severe chronic constipation. N Engl J Med. 2008;358:2344-54.
29. Kamath PS, Phillips SF, O’Connor MK, et al. Colonic capacitance and transit in man: modulation by luminal contents and drugs. Gut. 1990;31:443-49.
30. Holzer P. Opioids and opioid receptors in the enteric nervous system: from a problem in opioid analgesia to a possible new prokinetic therapy in humans. Neurosci Let. 2004;361:192-5.
31. Dinning PG, Szczesniak MM, Cook IJ. Removal of tonic nitrergic inhibition is a potent stimulus for human proximal colonic propagating sequences. Neurogastroenterol Mot. 2006;18:37-44.
32. Dickson EJ, Spencer NJ, Hennig GW, et al. An enteric occult reflex underlies accommodation and slow transit in the distal large bowel. Gastroenterology. 2007;132:1912-24.
33. Quigley EM. Probiotics in the management of colonic disorders. Curr Gastroenterol Rep. 2007;9:434-40.
34. Dinning PG, Fuentealba SE, Kennedy ML, et al. Sacral nerve stimulation induces pan-colonic propagating pressure waves and increases defaecation in patients with slow transit constipation. Colorectal Dis. 2007;9:123-32.
35. Kenefick NJ, Emmanuel AV, Nicholls RJ, Kamm MA. Effect of sacral nerve stimulation on autonomic nerve function. Br J Surg. 2003;90:1256-60.
36. Kubota M, Okuyama N, Hirayama Y, et al. Effect of sacral magnetic stimulation on the anorectal manometric activity: a new modality for examining sacro-rectoanal interaction. Pediatr Surg Int. 2007;23:741-5.
37. Ouyang H, Chen JD. Review article: therapeutic roles of acupuncture in functional gastrointestinal disorders. Aliment Pharmacol Ther. 2004;20:831-41.
38. Rao SSC, Seaton K, Miller M, et al. Randomized controlled trial of biofeedback, sham feedback, and standard therapy for dyssynergic defecation. Clin Gastroenterol Hepatol. 2007;5:331-8.
39. Emmanuel AV, Kamm MA. Response to a behavioural treatment, biofeedback, in constipated patients is associated with improved gut transit and autonomic innervation. Gut. 2001;49:214-19.
40. Dinning PG, Zarate N, Hunt L, et al. Twenty-four hour pan-colonic manometry in patients with severe slow transit constipation demonstrates diminished propagating pressure wave activity in the transverse colon. Gastroenterology. 2009;136:A-222.
41. Bassotti G, Villanacci V. Slow transit constipation: a functional disorder becomes an enteric neuropathy. World J Gastroenterol. 2006;12:4609-13.
42. Vassallo MJ, Camilleri M, Phillips SF, et al. Transit through the proximal colon influences stool weight in the irritable bowel syndrome. Gastroenterology. 1992;102:102-8.
43. Clemens CH, Samsom M, Van Berge Henegouwen GP, Smout AJ. Abnormalities of left colonic motility in ambulant nonconstipated patients with irritable bowel syndrome. Dig Dis Sci. 2003;48:74-82.