Chapter 79 Coenzyme Q10
 Introduction
Introduction
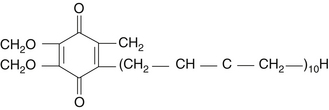
Coenzyme Q10 (CoQ10) is an endogenous proenzyme synthesized naturally in the human body.1 Because of its ubiquitous presence in nature and its quinone structure, CoQ10 is also known as ubiquinone (Figure 79-1). The two major physiologic actions of CoQ10 are (1) as a cofactor in the production of adenosine triphosphate (ATP), and (2) as an antioxidant. Because most cellular functions depend on an adequate supply of ATP, CoQ10 is essential for the health of virtually all human tissues and organs. Cellularly, the highest concentration of CoQ10 is found in the inner mitochondrial membrane, where it facilitates energy production, but CoQ10 is found in the cell membranes of many organelles where it plays a role in membrane stability.2 CoQ10 is the only endogenously synthesized lipid soluble antioxidant.3 In its role in electron transport, the CoQ10 molecule continuously goes through an oxidation-reduction cycle. As it accepts electrons, it becomes reduced to ubiquinol. As it gives up electrons, it becomes oxidized to ubiquinone. In contrast to other antioxidants, this compound inhibits both the initiation and the propagation of lipid and protein oxidation. In its reduced form, ubiquinol, the CoQ10 molecule holds electrons rather loosely, so the CoQ10 molecule will quite easily give up one or both electrons and, thus, act as an antioxidant. It is especially protective against the oxidation of bases of mitochondrial DNA. In addition, ubiquinol is responsible for regenerating vitamin E from the α-tocopheroxyl radical and, thereby interfering with the propagation step of lipid peroxidation.
Biosynthesis of CoQ10 starts from acetyl coenzyme A (CoA) and flows through a multistep process of the mevalonate pathway to produce farnesyl-PP, the direct precursor for not only CoQ10, but also cholesterol, dolichol, and isoprenylated proteins. The long isoprenoid side-chain of CoQ10 is synthesized by trans-prenyltransferase, which condenses farnesyl-PP with several molecules of isopentenyl-PP, all in the trans configuration. The next step involves condensation of this polyisoprenoid side-chain with 4-hydroxybenzoate, catalyzed by polyprenyl-4-hydroxybenzoate transferase. Hydroxybenzoate is synthesized from tyrosine or phenylalanine. In addition to their presence in mitochondria, these initial two reactions also occur in the endoplasmic reticulum and peroxisomes, indicating multiple sites of synthesis in human cells. Nonetheless, numerous conditions are now known to arise in which the body’s synthetic capacity is insufficient to meet CoQ10 requirements. Susceptibility to CoQ10 deficiency appears to be greatest in cells that are the most metabolically active, such as the brain and heart. Tissue deficiencies or subnormal serum levels of CoQ10 have been reported to occur in a wide range of medical conditions and decline with advancing age.3
A need for supplemental CoQ10 could theoretically result from the following:
• Impaired CoQ10 synthesis due to nutritional deficiencies
• A genetic or acquired defect in CoQ10 biosynthesis or utilization
• Increased tissue needs resulting from a particular illness
• The requirement to prevent the side effects of medical intervention
Because oral administration of CoQ10 can increase tissue levels, it is possible to correct CoQ10 deficiency and its associated metabolic consequences by supplementation.4
Coenzyme Q10 Deficiency
Inherited CoQ10 deficiency has been associated with five major clinical phenotypes: (1) encephalomyopathy, (2) severe infantile multisystemic disease, (3) cerebellar ataxia, (4) isolated myopathy, and (5) nephrotic syndrome. In a few patients, pathogenic mutations have been identified in genes involved in the biosynthesis of CoQ10 (primary deficiencies) or in genes not directly related to CoQ10 biosynthesis (secondary deficiencies). Respiratory chain defects, reactive oxygen species production, and apoptosis contribute to the pathogenesis of primary CoQ10 deficiencies.5,6
Acquired CoQ10 deficiency is less well established. There are two major factors that lead to deficiency of CoQ10 in humans: reduced biosynthesis, and increased utilization or need by the body. The typical dietary intake of CoQ10 is 3 to 5 mg,7 so dietary lack is probably not a significant contributor to CoQ10 deficiency. As mentioned previously, endogenous synthesis is a multistep process that can be affected by aging, disease status, and various medications. Some chronic disease conditions (cancer, heart disease, etc.) are not only associated with reduced biosynthesis, but also increased demand for CoQ10. Measurements of plasma CoQ10 levels have been used to detect deficiencies and are by far the most common clinical assessment of CoQ10 status.8 Normal plasma levels are believed to range from 0.45 to 1.5 mcg/mL (or 0.46 to 1.78 µmol/L), with 93% to 100% being the reduced form, ubiquinol.8,9 Some studies used a functional assessment using an assay that measures the citric acid cycle (Krebs cycle) enzyme succinate dehydrogenase-CoQ10 reductase.10 CoQ10 acts as a critical coenzyme to this enzyme, so if the enzyme is fully saturated with CoQ10 in vivo, addition of exogenous CoQ10 does not increase enzyme activity. Additional methods for assessing CoQ10 status are also becoming available.5,11,12
Commercial Forms and Dosage Considerations
A very important consideration in the clinical application of CoQ10 is its pharmacokinetics. CoQ10 as ubiquinone is a crystalline powder that is insoluble in water and has poor absorption characteristics as a result. Ubiquinol has greater solubility and has been promoted as having greater bioavailability that ubiquinone,4,13 but there are limited data currently available and many questions remain to be answered, since ubiquinol is easily oxidized to ubiquinone and absorption studies to date have been suspect. Ubiquinone has an extensive history of use, particularly in oil-based soft-gelatin capsules. Several technologies are now used to enhance the bioavailability of ubiquinone, such as particle size reduction (nanonization) and solubility enhancement via use of emulsifying agents, carriers, and self-emulsifying systems.14 For example, complexing ubiquinone to a soy peptide (BioQ10 SA) has shown exceptional bioavailability because the soy peptide emulsifies the CoQ10 and helps usher it into the bloodstream.15 Given the excellent absorption of this form of ubiquinone, the advantage of ubiquinol over regular ubiquinone appears to have more to do with its improved solubility than because it is in the ubiquinol form.
In light of the pharmacokinetic issues, proper clinical dosing is yet to be determined for CoQ10. Therefore, some have suggested that therapeutic targeting will likely be based upon achieving specific plasma CoQ10 levels (e.g., more than 3.5 mg/mL) or tissue saturation.8,16,17 Prescribing a set dosage, even a milligram per kilogram body weight dosage, at this time is a best guess scenario without confirmation by monitoring CoQ10 blood levels, given the differences noted in absorption rates among the different forms as well as the interindividual variability.
Numerous studies have now been conducted claiming enhanced absorption of one form or another.4 However, there are shortcomings in most of these studies, and some of the studies appear to have been set up to show an advantage for commercial reasons. For example, ubiquinol is being promoted as the best absorbed form of CoQ10, yet the published studies were set up in a curious fashion. In the one study examining ubiquinol absorption, the administration of the ubiquinol was always with a total of CoQ10 capsules that included the emulsification agents diglycerol monooleate, canola oil, soy lecithin, and beeswax, and although the study utilized a placebo, it did not compare ubiquinol with ubiquinone.13 It is possible that ubiquinone might have fared just as well if emulsified as well as the ubiquinol in this study. Absorption studies showed that when CoQ10 was given with food, it was absorbed twice as fast and at least two-fold greater than on an empty stomach.18 It is believed that food-induced secretion of bile acids is responsible for the improved absorption.
What is known based on current absorption studies is that eventually a steady state is produced (usually after 3 to 4 weeks of constant dosing), and the absorption of CoQ10 may be limited in some individuals. Dosages that exceed an individual’s absorptive capacity for CoQ10 may have minimal effect on efficacy and unnecessarily increase the cost of treatment. When single dosages of CoQ10 begin to exceed 300 mg, the percentage of CoQ10 absorbed declines. Plasma CoQ10 levels at a dosage of 900 mg/day (as ubiquinone in a oil suspension in a soft gel capsule) are not significantly greater than a 600 mg/day dosage. Divided dosages (e.g., two or three times a day) result in higher plasma levels compared with single dosages, especially at higher dosage levels.
Despite the challenges, based upon existing data from published studies, an attempt can be made to calculate the approximate plasma levels of CoQ10 for different commercial forms at 100 and 300 mg doses for at least 30 days with several caveats. First, these are estimates only, based upon a typical 75 kg body weight. Keep in mind that absorption of CoQ10 in any form is likely enhanced considerably if taken with a large meal that includes some fat. Lastly, considerable pharmacokinetic studies in humans have indicated significant interindividual variability in CoQ10 absorption, underscoring the need for monitoring CoQ10 plasma levels during clinical studies and perhaps clinical use of CoQ10 as well (Table 79-1).
| DOSE AND FORM | ESTIMATED PLASMA LEVELS IN MICROGRAMs PER MILLILITER |
|---|---|
| 100 mg | |
| Ubiquinone powder in hard gelatin capsule | 1.25 |
| Ubiquinone suspended in oil in soft gelatin capsule with rice bran oil | 1.8 |
| Ubiquinone solubilized in soft gelatin capsule | 2.25 |
| Ubiquinone powder nanonized | 2.25 |
| Ubiquinone emulsified with soy peptide in soft or hard gelatin capsule | 2.50 |
| Ubiquinol in soft gel capsule | 2.50 |
| 300 mg | |
| Ubiquinone powder in hard gelatin capsule | 2.5 |
| Ubiquinone suspended in oil in soft gelatin capsule with rice bran oil | 3.5 |
| Ubiquinone solubilized in soft gelatin capsule | 5.0 |
| Ubiquinone powder nanonized | 5.0 |
| Ubiquinone emulsified with soy peptide in soft or hard gelatin capsule | 7.0 |
| Ubiquinol in soft gel capsule | 7.0 |
The optimal dose of CoQ10 for many clinical indications is not known, and plasma or target tissue levels of CoQ10 are likely the primary determinant of efficacy, rather than the dose.16,19 Current opinion based upon the scientific literature is that an acceptable therapeutic plasma target level of CoQ10 should be at least 2.5 mg/mL,20 but levels higher than 3.5 mcg/mL may be necessary for optimum improvement in neurodegenerative diseases and myocardial function.8,16,19 The usual starting dosage for CoQ10 is generally 100 to 200 mg/day. Where possible, the dosage of CoQ10 should be adjusted according to the response of the patient and, preferably, by monitoring plasma CoQ10 levels for 3 to 4 weeks of constant dosing, when steady-state plasma concentrations occur. Less is known about the ability of CoQ10 and the dose required to reach the central nervous system. Again, divided dosages result in higher plasma levels compared with single dosages, e.g., taking 100 mg twice a day produces higher plasma levels compared with taking 200 mg once a day.21
 Clinical Applications
Clinical Applications
General Antioxidant
Numerous studies have shown CoQ10 can reduce oxidative damage, DNA strand damage, LDL oxidation, and formation of lipid peroxides, thereby supporting its use as a general antioxidant.2,22 In particular, CoQ10 is often used to counteract the reduced synthesis of CoQ10 associated with aging. After the age of 35 to 40 years, humans slowly begin to lose their ability to synthesize CoQ10.23 It has been proposed that the increase in age-associated diseases is due in part to decreased protection afforded by CoQ10 as both an antioxidant and a facilitator of energy production at a cellular level. Therefore, COQ10 is often recommended to counteract this effect.
For example, recent studies identified oxidative stress as a promoting factor for dry mouth (xerostomia) and the development of Sjögren’s syndrome, a condition associated with significant dry mouth. Basically, oxidative damage leads to the inability of salivary cells to produce enough ATP to secrete sufficient amounts of water. CoQ10 exerts antioxidant effects, but its main action in relieving dry mouth may be by increasing energy (ATP) production, allowing the saliva producing cells enough energy to secrete more saliva into the mouth. In one double-blind study, 66 patients, including 31 with dry mouth, were given either ubiquinol or ubiquinone orally at a dosage of 100 mg/day, or a placebo for 1 month.24 Salivary secretion and salivary CoQ10 content were analyzed before and after treatment. Among the dry mouth patients treated with ubiquinone, salivary secretion increased significantly from 0.7 g/2 min before treatment to 1.2 g/2 min after 1 month of treatment. Among the patients treated with ubiquinol, salivary secretion also increased significantly from 0.8 g/2 min before treatment to 1.4 g/2 min after treatment. In normal subjects without dry mouth, salivary secretion increased with ubiquinone (from 4.9 to 5.7 g/2 min) at a statistically significant level, but did not differ significantly after treatment with ubiquinol (from 3.5 to 3.8 g/2 min). Either form of CoQ10 exhibited a marked increase in salivary CoQ10 concentration (ubiquinol more than ubiquionine in dry mouth, ubiquinone more than ubiquinol in normal subjects), suggesting that the observed increase in salivary secretion was attributable to the effect on salivary levels of CoQ10.
Cardiovascular Disease—General Considerations
Enhancing myocardial function is an important, although frequently overlooked, component of the overall prevention and treatment of cardiovascular disease. CoQ10 plays a key role in energy production and is therefore essential for all energy-dependent processes, including heart muscle contraction. CoQ10 deficiency has been documented in patients with various types of cardiovascular disease. Whether a decline in CoQ10 levels is a cause or a consequence of heart disease is unclear. Cardiac cells are highly metabolically active, and thus have higher mitochondrial coenzyme requirements to maintain ATP production. In addition to its role in cellular energetics, exogenously administered CoQ10 acts as an antioxidant to inhibit LDL oxidation,25 decreases proinflammatory cytokines interleukin-6 and tumor necrosis factor-α, and attenuates markers of oxidative and nitrative stress in a dose-dependent manner.2
CoQ10 deficiency is common in patients with various types of cardiovascular disease.2,26 Approximately 75% of patients who had cardiac surgery were shown to be deficient in myocardial CoQ10. Concentrations of CoQ10 declined progressively in both blood and myocardial tissue with increasing severity of heart disease.27 Myocardial deficiencies of CoQ10 were also found in the majority of patients with aortic stenosis or insufficiency, mitral stenosis or insufficiency, diabetic cardiomyopathy, tetralogy of Fallot, atrial septal defects, and ventricular septal defects.28
Cardiomyopathy
A prospective, randomized, double-blind, placebo-controlled trial was conducted in 38 children with idiopathic dilated cardiomyopathy. After 6 months of supplementation, there was a statistically significant (P = 0.011) improvement in diastolic function, leading the authors to conclude that “administration of coenzyme Q10 is useful in ameliorating cardiac failure in patients with idiopathic dilated cardiomyopathy.”29
In one study, 126 patients with dilated cardiomyopathy received 100 mg/day of CoQ10 for up to 66 months. After 6 months of treatment, the mean left ventricular ejection fraction (LVEF) increased from 41% to 59% (P <0.001) and remained stable thereafter with continued treatment. After 2 years, 84% of the patients were still alive, and at 5.5 years, 52% were alive.30 These survival rates were considerably better than the published survival statistics of patients given conventional therapy (i.e., 2-year survival rate of 50% for symptomatic cardiomyopathy, and 1-year survival rate of 50% for decompensated cardiomyopathy).
In another study, 88 patients with cardiomyopathy received 100 mg/day of CoQ10 for 1 to 24 months. Significant improvements in at least two of three cardiac parameters (LVEF, cardiac output, and New York Heart Association [NYHA] class) were seen in 75% to 85% of the patients. Approximately 80% of the patients improved to a lower (i.e., more favorable) NYHA functional class.20
In a double-blind, crossover trial, 19 patients with cardiomyopathy received 100 mg/day of CoQ10 or a placebo, each for 12 weeks. Compared with placebo, CoQ10 treatment significantly increased cardiac stroke volume and LVEF. Eighteen patients reported improvement in activity while taking CoQ10.31
Congestive Heart Failure
The potential of CoQ10 as a treatment for congestive heart failure (CHF) was suggested as early as 1967 by Japanese researchers.32 In 1976, these same investigators administered 30 mg/day of CoQ10 to 17 patients with CHF. All of the patients improved, and nine (53%) became asymptomatic after 4 weeks of treatment.33 Numerous studies since have demonstrated that CoQ10 supplementation resulted in an improvement of stroke volume, LVEF, cardiac output, cardiac index and end-diastolic volume index.2,34,35
In the largest multicenter trial completed to date, 2664 patients in NYHA classes II and III CHF received 50 to 150 mg/day of CoQ10 (78% of patients received 100 mg/day of CoQ10). After 3 months of supplementation, the results showed a low incidence of side effects, and the following proportion of patients had improvements in clinical signs and symptoms36:
The results of these uncontrolled studies were confirmed in several double-blind trials. Some 641 patients with CHF (NYHA classes III or IV) were randomly assigned to receive placebo or CoQ10 (2 mg/kg per day) for 1 year while continuing conventional therapy. The number of patients requiring hospitalization during the study for worsening heart failure was 38% less in the CoQ10 group than in the placebo group (P <0.001), and episodes of pulmonary edema were reduced by about 60% in the CoQ10 group compared with the placebo group (P <0.001).37
These positive results with CoQ10, however, have not been seen in all studies. In one double-blind study, 55 patients with CHF in NYHA classes III and IV, with ejection fractions less than 40%, and peak oxygen consumption less than 50% during standard therapy, were randomly assigned to receive CoQ10 (200 mg) or placebo. Analysis indicated that there were no changes in ejection fraction, peak oxygen consumption, and exercise duration in either group. Possible explanations for failure to achieve a therapeutic benefit in this study (and others) may be the result of CoQ10 not being strong enough to produce significant effects in more severe stages of CHF or the fact that blood levels of CoQ10 did not reach sufficient levels. Although the mean serum concentration of CoQ10 increased from 0.95 to 2.2 mcg/mL, in 19 of 22 patients on CoQ10, blood levels were below the suggested threshold of 2.5 mcg/mL.38
A more recent study administered CoQ10 100 mg three times a day for 4 weeks to 21 patients with CHF in a double-blind, placebo-controlled, crossover design. CoQ10 administration resulted in a threefold increase in plasma CoQ10 levels, improved left ventricular contractility, and subsequently enhanced functional capacity, without any side effects.39
A meta-analysis of randomized controlled trials recently confirmed that CoQ10 supplementation improved LVEF and cardiac output. An ongoing trial, Q-SYMBIO (Symptoms, Biomarker status [BNP], and Long-term Outcome), will address whether CoQ10 supplementation extends survival in patients with CHF.40
An important consideration in patients with CHF in NYHA class IV, is that they often fail to achieve adequate plasma CoQ10 levels (>2.5 mcg/mL) on supplemental ubiquinone at dosages up to 900 mg/day. In one study, seven patients with subtherapeutic plasma CoQ10 levels (mean level, 1.6 mcg/mL, on an average dose of 450 mg of ubiquinone a day [150 to 600 mg/day]) were changed to an average of 580 mg/day of ubiquinol (450 to 900 mg/day) with follow-up plasma CoQ10 levels, clinical status, and ejection fraction measurements by echocardiography.41 Mean plasma CoQ10 levels increased from 1.6 to 6.5 mcg/mL. Mean ejection fraction improved from 22% (10% to 35%) to 39% (10% to 60%) and NYHA class improved from a mean of class IV to a mean of class II (classes I to III). In this study, ubiquinol dramatically improved absorption in patients with severe heart failure, and the improvement in plasma CoQ10 levels was correlated with both clinical improvement and improvement in measurement of left ventricular function.
Protection During Cardiac Surgery
Cardiopulmonary bypass (CPB) is used in coronary artery bypass surgery, cardiac valve repair, and numerous other surgical procedures. CPB, although permitting life-saving surgical procedures, is known to induce oxidative stress. A prospective study of 30 individuals who underwent CPB randomized these patients to oral CoQ10 (150 to 180 mg/day) or placebo for 7 to 10 days preoperatively. The group that received CoQ10 had significantly fewer reperfusion arrythmias, lower requirements for inotropic agents, mediastinal drainage and blood products, and shorter hospital stays compared with the control group.42
Postoperative low cardiac output is a major cause of early death after cardiac surgery. Fifty patients who underwent cardiac surgery for acquired valvular lesions were randomly assigned to receive 30 to 60 mg/day of CoQ10 for 6 days before surgery or to a control group that did not receive CoQ10. Postoperatively, a state of severe low cardiac output developed in 48% of the patients in the control group, compared with only 12% of those in the CoQ10 group. These results suggested that preoperative administration of CoQ10 increased the tolerance of the heart to ischemia during aortic cross-clamping.43
Hypertension
The majority of studies exploring CoQ10 in the treatment of high blood pressure have been uncontrolled, or have used CoQ10 in combination with conventional antihypertensive medical treatments, making these studies difficult to interpret. In one study, 109 patients with essential hypertension received CoQ10 (average dose, 225 mg/day) in addition to their usual antihypertensive regimen. The dosage of CoQ10 was adjusted according to clinical response and blood CoQ10 levels (the aim was to attain blood levels >2 mg/mL). The need for antihypertensive medication declined gradually, and after a mean treatment period of 4.4 months, about half of the patients were able to discontinue between one and three drugs.44 A review of studies on CoQ10 in the treatment of hypertension (12 clinical trials, 362 patients) concluded that in hypertensive patients, CoQ10 has the potential to lower systolic and diastolic blood pressure, without significant side effects. Among all included studies, decreases in systolic blood pressure ranged from 11 to 17 mm Hg and in diastolic blood pressure from 8 to 10 mm Hg.45 In 3 of the 12 studies, CoQ10 was given in addition to existing antihypertensive medication, and in 1 of these, more than 50% of the patients were able to cease taking at least 1 antihypertensive medication during the trial.
The mechanism for the antihypertensive action of CoQ10 is thought to be due to its counteracting vasoconstriction, resulting from impaired ability of the endothelium to induce nitric oxide mediated relaxation of underlying smooth muscle.45
Cancer
Because of its role in enhancing immune function, CoQ10 has been considered as a possible anticancer agent. In one study, 32 women with breast cancer, who were classified as “high risk” because of tumor spread to the axillary lymph nodes, received 90 mg/day of CoQ10, along with vitamins C and E, β-carotene, and essential fatty acids. In six of these women, the tumor became smaller. During the 18-month treatment period, none of the patients died (the expected number of deaths was four), and none showed signs of further distant metastases. Six patients had an apparent partial remission. In addition, patients receiving CoQ10 required fewer pain killers.46 In follow-up, a pilot study using this nutrient cocktail evaluated the survival of 40 patients with end-stage cancer over 9 years. Median survival of individuals receiving the CoQ10-containing nutrient cocktail was 40% longer than median predicted survival using the calculated Kaplan-Meier curve.47
Anthracyclines are a class of drugs used in cancer chemotherapy for a wide range of tumors. The clinical value of these agents is limited by their cardiotoxicity, which is believed to be due to irreversible damage to heart cell mitochondria. These agents cause severe oxidative stress in cardiac inner mitochondrial membranes, resulting in damage to mitochondrial DNA and subsequent myocyte death.48
Research from the 1980s showed that cancer patients receiving doxorubicin (Adriamycin), a commonly used anthracycline, had lower myocardial levels of CoQ10 than did controls, and that the magnitude of CoQ10 depletion was directly related to the severity of cardiac impairment.49 To determine the effect of CoQ10 supplementation on doxorubicin cardiotoxicity, seven patients receiving doxorubicin were also given 100 mg/day of CoQ10, beginning 3 to 5 days before doxorubicin was started. Another seven patients (control group) received doxorubicin without CoQ10. Cardiac function deteriorated significantly in the control group, whereas patients given CoQ10 had little or no cardiotoxicity, although the cumulative dose of doxorubicin in the CoQ10 group was 50% greater than that in the control group.50 These preliminary data, coupled with strong scientific rationale, suggest that CoQ10 supplementation should be considered for the prevention and treatment of anthracycline-induced cardiotoxicity.
Physical Performance
Because CoQ10 is involved in energy production and its concentration in muscle is correlated with performance, it is possible that supplementation may enhance aerobic capacity, muscle performance, and recovery.51–53 In one study, six healthy sedentary men (mean age, 21.5 years) performed a bicycle ergometer test before and after taking CoQ10 (60 mg/day) for 4 to 8 weeks.54 CoQ10 treatment improved certain performance parameters, including work capacity at submaximal heart rate, maximal workload, maximal oxygen consumption, and oxygen transport. These improvements ranged from 3% to 12% and were evident after about 4 weeks of supplementation. In a double-blind study in sedentary men, 100 mg/day of CoQ10 showed performance-enhancing effects during repeated bouts of supramaximal exercises.55
Although these studies suggest that administration of CoQ10 improves physical performance in sedentary individuals, other publications are conflicting. Larger studies are needed to confirm the performance enhancement capacity of CoQ10.56
Diabetes Mellitus
Low tissue levels of CoQ10 have been consistently reported in diabetic patients. There are several mechanisms by which CoQ10 may be of therapeutic value in diabetes.57,58 Improved blood pressure and long-term glycemic control have been reported with CoQ10 supplementation (100 mg twice daily).59 Enhancement of endothelial function may offer some protection against arteriopathies.60 A study of 80 dyslipidemic type 2 diabetics found that CoQ10 (200 mg/day) in combination with fenofibrate (200 mg/day) significantly improved endothelial and nonendothelial forearm vasodilatory function.61 This effect was not found with either therapy alone or in a placebo group. An accumulating body of research demonstrates a link between mitochondrial dysfunction and type 2 diabetes. The mitochondrial enhancement offered by supplemental CoQ10 appears to offer some therapeutic value against a defect in cellular energy production.62,63
Twenty-seven patients with ventricular premature beats (VPBs) and no evidence of organic heart disease received placebo for 3 to 4 weeks, followed by 60 mg/day of CoQ10 for 4 to 5 weeks. The reduction in VPBs was significantly greater after CoQ10 than after placebo. The beneficial effect of CoQ10 was seen primarily in diabetics, in whom the mean reduction in VPB frequency was 85.7%. A significant reduction in VPBs also occurred in 1 (11%) of 9 otherwise healthy patients and in 4 (36%) of 11 patients with hypertension.64
Male Infertility
Sperm dysfunction in infertile men has been associated with lipid peroxidation and decreased antioxidant defenses in spermatozoa.65 A statistically significant correlation between CoQ10 levels and sperm count was found in 32 patients with a history of infertility.
Research suggested that sperm cells with low motility and abnormal morphology have low levels of CoQ10.66 Sperm cells with low CoQ10 concentrations might be less capable of quenching free radicals, and might also be compromised in their ability to produce ATP in these highly metabolically active cells. Some authors suggested using the reduced-to-oxidized CoQ10 ratio as a diagnostic test for asthenozoospermia.67 In a 6-month, uncontrolled open trial, in which infertile men were supplemented orally with CoQ10, sperm cells demonstrated a significant increase in motility, and both seminal plasma and sperm cell quantity of CoQ10 increased.68 This was supported by other studies on both sperm motility and fertilization rates.69,70
Parkinson’s Disease
Excessive free radical production is largely responsible for dopaminergic cell death seen in Parkinson’s disease (PD). Both as a cause and an effect of excessive oxidative stress, there is reduced activity of complex I of the mitochondrial electron transport chain. Given that CoQ10 is essential for the function of complex I and is also a powerful antioxidant, it holds therapeutic potential in the treatment of PD. Reduced levels of CoQ10 were demonstrated in the platelets of individuals with PD, and CoQ10 levels were strongly correlated with activity in complexes I and II/III. Clinically, it was demonstrated that oral CoQ10 increased complex I activity.71
A safety trial of CoQ10 supplementation in PD showed promising results.19 All of the patients had the three primary features of PD—tremor, stiffness, and slowed movements—and were diagnosed with the disease within 5 years of the time they were enrolled. After initial screening and baseline blood tests, the patients were randomly divided into four groups. Three of the groups received CoQ10 at three different doses (300, 600, and 1200 mg/day), whereas a fourth group received a matching placebo for 16 months. The group that received the highest dose of CoQ10 (1200 mg/day) displayed a 44% reduction in PD progression compared to placebo,. The groups that received 300 and 600 mg/day developed slightly less disability than the placebo group, but the effects were less than those in the group that received the highest dosage of CoQ10. Average plasma levels were approximately 1.8, 2.1, and 4.5 mcg/mL, respectively, for the 300, 600, and 1200 mg dosages. These results indicated that the beneficial effects of CoQ10 in PD might require adequate plasma levels. It is important to point out in this study that CoQ10 was administered along with vitamin E at a dosage of 1200 international units (IU) per day. Because high dosages of vitamin E interfere with CoQ10 absorption,7 it might have prevented higher levels of CoQ10 from being achieved. The researchers were aware of this issue, but chose to include the vitamin E, given its apparent protective role against PD at the time (see Chapter 196 on Parkinson’s Disease for more information).
On May 27, 2011, the National Institute of Neurological Diseases and Strokes (NINDS) stopped a Phase III study of CoQ10 for treatment of early stage PD.72 The study enrolled 600 patients with early PD at 67 sites throughout North America. Participants were randomized to receive one of the two dosing levels of active CoQ10 (1200 or 2400 mg/day) or matching placebo. All subjects also received vitamin E at a dosage of 1200 IU/day. Although CoQ10 was shown to be safe, results of an interim analysis showed that it was futile to complete the study because longer patient follow-up was not likely to demonstrate a statistically significant difference between active treatment and placebo. However, at the time of this writing no data from the trial had been published.
Friedreich’s Ataxia
Friedreich’s ataxia (FRDA) is a progressive neurologic disease characterized by loss of myelin in the central nervous system. Evidence indicates that mitochondrial oxidative stress in FRDA may be responsible for the cardiomyocyte hypertrophy associated with this disease.73 In a placebo-controlled trial, idebenone (a synthetic CoQ10 analog) significantly decreased heart hypertrophy (more than 20% decrease) in about 50% of study participants, with no adverse effects.74,75
A second study combined CoQ10 with vitamin E in an attempt to address two of the key features of FRDA—decreased mitochondrial respiratory chain function and increased oxidative stress. This therapy resulted in a rapid and sustained increase in the amount of energy generated by the diseased heart muscle, which returned to near-normal levels. The improvements in skeletal muscle energy generation paralleled those of the heart but were less substantial.76
A 2-year intervention study of CoQ10 with vitamin E in 50 FRDA patients demonstrated that low baseline levels of CoQ10 were the single best predictor of a positive clinical response.77
Muscular Dystrophy
The muscular dystrophies (MDs) are a group of hereditary diseases characterized by the progressive loss of muscle cells. In some types of MD, an impairment of mitochondrial function may contribute to the pathogenesis of the disease. Several studies demonstrated a deficiency of CoQ10 in muscle mitochondria of humans with MD.27 In addition, serum CoQ10 levels were significantly (P <0.05) inversely correlated with the degree of the genetic defect.78 In addition to enhancing mitochondrial function, 90 days of CoQ10 supplementation resulted in markedly reduced inflammatory insult in animal models of MD.79
The first double-blind trial of CoQ10 in the treatment of individuals with MDs and neurogenic atrophies concluded, “Patients suffering from these muscle dystrophies and the like should be treated with vitamin CoQ10 indefinitely.”80 The same group conducted two studies on patients between 7 and 69 years of age who had MDs associated with cardiac diseases, including the Duchenne, Becker, and the limb-girdle dystrophies; myotonic dystrophy; Charcot-Marie-Tooth disease; and the Welander disease. Individuals were treated for 3 months with 100 mg/day CoQ10 or a matching placebo. In both trials, definitely improved physical performance was recorded in the groups receiving CoQ10. Cardiac function was monitored by technicians who were blinded to treatment group. In each case, they correctly identified the treatment group to which the patient had been assigned on the basis of improvement or lack of improvement in cardiac function.
In a smaller double-blind study, 100 mg of CoQ10 was given daily for 3 months to 12 patients with progressive MD. CoQ10 treatment resulted in significant improvements in cardiac output and stroke volume, as well as increased physical well-being in four of eight patients.27 Subjective improvements included increased exercise tolerance, reduced leg pain, better control of leg function, and less fatigue.
Immune Function
A number of studies demonstrated immunomodulatory effects of CoQ10 or its analogs.81–83
In a study of eight chronically ill patients, administration of 60 mg/day of CoQ10 was associated with significant increases in serum levels of immunoglobulin-G after 27 to 98 days of treatment.84 In a cytokine analysis of white blood cells from 19 human volunteers, tumor necrosis factor-α levels were significantly decreased and interleukin-2 levels increased when incubated with CoQ10 in vitro.85
Acquired Immunodeficiency Syndrome
Because oxidative stress is believed to be involved in the pathogenesis of AIDS-related diseases, the antioxidant activity of CoQ10 may be of value for individuals with AIDS.85,86
Early studies suggested blood levels of CoQ10 were significantly lower in patients with AIDS and AIDS-related complex than in healthy controls, and that supplementation with CoQ10 improved ratios of lymphocytes.87 Nucleoside reverse transcriptase inhibitor therapy, a mainstay of conventional human immunodeficiency virus management, has been associated with myopathy due to side effects on myocyte mitochondria. There is one report of CoQ10 alleviating this myopathy, thus permitting continuation of nucleoside reverse transcriptase inhibitor therapy.88
Toxicant Exposure
Many occupational and environmental toxicant exposures affect the mitochondrial respiratory chain.89–93 Damage to the respiratory chain can decrease mitochondrial membrane potential, leading to upregulated apoptosis; CoQ10 demonstrated the ability to reduce this membrane-potential damage.94,95 Although current diagnosis and treatment of environmentally related illnesses remain controversial, cytoprotection with CoQ10 may be an effective therapeutic method to reduce mitochondrial damage due to exposure to toxicants, both acute and chronic, and should be a focus of further research.96
Topical Antiaging Effects
In recent years, makers of several over-the-counter skin creams have begun advertising the addition of CoQ10 as an ingredient capable of curbing the aging process. In a clinical trial, topical CoQ10 was able to protect against ultraviolet A–mediated oxidative stress, suppress the expression of collagenase, and cause a reduction in wrinkle depth.23
Side Effects
CoQ10 appears to be safe and well tolerated in dosages up to 1200 mg/day in adults and up to 10 mg/kg body weight/day in children. Because safety during pregnancy and lactation has not been established, CoQ10 should not be used during these times unless the potential clinical benefit outweighs the risks. CoQ10 is contraindicated in cases of known hypersensitivity. In a series of 5143 patients treated with 30 mg/day of CoQ10, the following incidence of side effects was reported43:
 Drug Interactions
Drug Interactions
Cholesterol-lowering statin drugs such as lovastatin, rosuvastatin, and pravastatin inhibit the enzyme 3-hydroxy-3-methylglutaryl CoA reductase, which is required for biosynthesis of both cholesterol and CoQ10. Thus, administration of these drugs might compromise CoQ10 status by decreasing its synthesis, and statin-associated myopathy has been hypothesized to be related to a depletion of CoQ10. Although some trials demonstrated that statin therapy did reduce serum or muscle levels of CoQ10,97,98 whether or not this CoQ10 depletion caused the myopathy remains controversial.99 Given that statins are typically prescribed to lower cholesterol, with the intention being the prevention and treatment of cardiovascular disease, the concomitant use of Q10 seems well-justified.
The β-blockers propranolol and metoprolol were shown to inhibit CoQ10-dependent enzymes.100 The antihypertensive effect of these drugs might therefore be compromised in the long run by the development of CoQ10 deficiency. In one study, administration of 60 mg/day of CoQ10 reduced the incidence of drug-induced malaise in patients receiving propranolol.101
A number of phenothiazines and tricyclic antidepressants were also shown to inhibit CoQ10-dependent enzymes. It is therefore possible that CoQ10 deficiency might be a contributing factor to the cardiac side effects that are frequently seen with these drugs. In two clinical studies, supplementation with CoQ10 improved electrocardiographic changes in patients on psychotropic drugs.102
Several case reports describing potential interactions between CoQ10 and warfarin have been reported. CoQ10 is structurally related to menaquinone (vitamin K2) and may have procoagulant effects.103 In each of these patients, the international normalized ratio (INR), which had been stable and therapeutic, fell below the therapeutic range within 2 weeks of beginning CoQ10 supplementation (as low as 30 mg/day).104 The INR returned to the therapeutic range after CoQ10 was discontinued. It is recommended that the INR be monitored closely if these agents are to be used concomitantly. However, in a double-blind trial, administration of 100 mg/day of CoQ10 for 4 weeks had no effect on the INR in 21 patients on long-term warfarin therapy.105 Thus, the sporadic case reports of an interaction between CoQ10 and warfarin might have been due to random fluctuations in INR values, rather than to CoQ10.8
1. Bonakdar R.A., Guarneri E. Coenzyme Q10. Am Fam Physician. 2005;72:1065–1070.
2. Kumar A., Kaur H., Devi P., et al. Role of coenzyme Q10 (CoQ10) in cardiac disease, hypertension and Meniere-like syndrome. Pharmacol Ther. 2009;124:259–268.
3. Littarru G.P., Tiano L. Bioenergetic and antioxidant properties of coenzyme Q10: recent developments. Mol Biotechnol. 2007;37:31–37.
4. Bhagavan H.N., Chopra R.K. Plasma coenzyme Q10 response to oral ingestion of coenzyme Q10 formulations. Mitochondrion. 2007;7(Suppl):S78–88.
5. Quinzii C.M., DiMauro S., Hirano M. Human coenzyme Q10 deficiency. Neurochem Res. 2007;32:723–727.
6. Quinzii C.M., Hirano M. Coenzyme Q and mitochondrial disease. Dev Disabil Res Rev. 2010;16:183–188.
7. Pravst I., Zmitek K., Zmitek J. Coenzyme Q10 contents in foods and fortification strategies. Crit Rev Food Sci Nutr. 2010;50:269–280.
8. Steele P.E., Tang P.H., DeGrauw A.J., et al. Clinical laboratory monitoring of coenzyme Q10 use in neurologic and muscular diseases. Am J Clin Pathol. 2004;121(Suppl):S113–120.
9. Miles M.V., Horn P.S., Tang P.H., et al. Age-related changes in plasma coenzyme Q10 concentrations and redox state in apparently healthy children and adults. Clin Chim Acta. 2004;34:139–144.
10. Nakamura R., Littarru G.P., Folkers K., et al. Study of CoQ10-enzymes in gingiva from patients with periodontal disease and evidence for a deficiency of coenzyme Q10. Proc Natl Acad Sci U S A. 1974;71:1456–1460.
11. Morre D.J., Morre D.M. arNOX activity of saliva as a non-invasive measure of coenzyme Q10 response in human trials. Biofactors. 2008;32:231–235.
12. Abdul-Rasheed O.F., Farid Y.Y. Development of a new high performance liquid chromatography method for measurement of coenzyme Q10 in healthy blood plasma. Saudi Med J. 2009;30:1138–1143.
13. Hosoe K., Kitano M., Kishida H., et al. Study on safety and bioavailability of ubiquinol (Kaneka QH) after single and 4-week multiple oral administration to healthy volunteers. Regul Toxicol Pharmacol. 2007;47:19–28.
14. Beg S., Javed S., Kohli K. Bioavailability enhancement of coenzyme Q10: an extensive review of patents. Recent Pat Drug Deliv Formul. 2010;4:245–255.
15. Takeda R., Sawabe A., Nakano R., et al. Effect of various food additives and soy constituents on high CoQ10 absorption. Jpn J Med Pharma Sci. 2011;64:614–620.
16. Sinatra S.T. Coenzyme Q10 and congestive heart failure. Ann Intern Med. 2000;133:745–746.
17. Langsjoen P.H., Langsjoen A.M. Overview of the use of CoQ10 in cardiovascular disease. Biofactors. 1999;9:273–284.
18. Ochiai A., Itagaki S., Kurokawa T., et al. Improvement in intestinal coenzyme Q10 absorption by food intake. Yakugaku Zasshi. 2007;127:1251–1254.
19. Shults C.W., Oakes D., Kieburtz K., et al. Effects of coenzyme Q10 in early Parkinson disease: evidence of slowing of the functional decline. Arch Neurol. 2002;59:1541–1550.
20. Langsjoen P.H., Folkers K., Lyson K., et al. Effective and safe therapy with coenzyme Q10 for cardiomyopathy. Klin Wochenschr. 1988;66:583–590.
21. Singh R.B., Niaz M.A., Kumar A., et al. Effect on absorption and oxidative stress of different oral coenzyme Q10 dosages and intake strategy in healthy men. Biofactors. 2005;25:219–224.
22. Niklowitz P., Sonnenschein A., Janetzky B., et al. Enrichment of coenzyme Q10 in plasma and blood cells: defense against oxidative damage. Int J Biol Sci. 2007;3:257–262.
23. Hojerova J. Coenzyme Q10–its importance, properties and use in nutrition and cosmetics. Ceska Slov Farm. 2000;49:119–123. [Slovak]
24. Ryo K., Ito A., Takatori R., et al. Effects of coenzyme Q10 on salivary secretion. Clin Biochem. 2011 Jun;44(8-9):669–674.
25. Stocker R., Bowry V.W., Frei B. Ubiquinol-10 protects human low density lipoprotein more efficiently against lipid peroxidation than does alpha-tocopherol. Proc Natl Acad Sci U S A. 1991;88:1646–1650.
26. Hanaki Y., Sugiyama S., Ozawa T., et al. Ratio of low-density lipoprotein cholesterol to ubiquinone as a coronary risk factor. N Engl J Med. 1991;325:814–815.
27. Folkers K., Wolaniuk J., Simonsen R., et al. Biochemical rationale and the cardiac response of patients with muscle disease to therapy with coenzyme Q10. Proc Natl Acad Sci U S A. 1985;82:4513–4516.
28. Folkers K., Littarru G.P., Ho L., et al. Evidence for a deficiency of coenzyme Q10 in human heart disease. Int Z Vitaminforsch. 1970;40:380–390.
29. Kocharian A., Shabanian R., Rafiei-Khorgami M., et al. Coenzyme Q10 improves diastolic function in children with idiopathic dilated cardiomyopathy. Cardiol Young. 2009;19:501–506.
30. Langsjoen P.H., Folkers K. Long-term efficacy and safety of coenzyme Q10 therapy for idiopathic dilated cardiomyopathy. Am J Cardiol. 1990;65:521–523.
31. Langsjoen P.H., Vadhanavikit S., Folkers K. Effective treatment with coenzyme Q10 of patients with chronic myocardial disease. Drugs Exp Clin Res. 1985;11:577–579.
32. Yamamura Y.I.T., Yamagami T., Morita Y., et al. Clinical use of coenzyme-Q for treatment of cardiovascular disease. Jpn Circ J. 1967;31:168.
33. Ishiyama T., Morita Y., Toyama S., et al. A clinical study of the effect of coenzyme Q on congestive heart failure. Jpn Heart J. 1976;17:32–42.
34. Soja A.M., Mortensen S.A. Treatment of congestive heart failure with coenzyme Q10 illuminated by meta-analyses of clinical trials. Mol Aspects Med. 1997;18(Suppl):S159–168.
35. Sander S., Coleman C.I., Patel A.A., et al. The impact of coenzyme Q10 on systolic function in patients with chronic heart failure. J Card Fail. 2006;12:464–472.
36. Baggio E., Gandini R., Plancher A.C., et al. Italian multicenter study on the safety and efficacy of coenzyme Q10 as adjunctive therapy in heart failure. CoQ10 Drug Surveillance Investigators. Mol Aspects Med. 1994;15(Suppl):s287–294.
37. Morisco C., Trimarco B., Condorelli M. Effect of coenzyme Q10 therapy in patients with congestive heart failure: a long-term multicenter randomized study. Clin Investig. 1993;71:S134–136.
38. Khatta M., Alexander B.S., Krichten C.M., et al. The effect of coenzyme Q10 in patients with congestive heart failure. Ann Intern Med. 2000;132:636–640.
39. Belardinelli R., Mucaj A., Lacalaprice F., et al. Coenzyme Q10 improves contractility of dysfunctional myocardium in chronic heart failure. Biofactors. 2005;25:137–145.
40. Molyneux S.L., Florkowski C.M., Richards A.M., et al. Coenzyme Q10; an adjunctive therapy for congestive heart failure? N Z Med J. 2009;122:74–79.
41. Langsjoen P.H., Langsjoen A.M. Supplemental ubiquinol in patients with advanced congestive heart failure. Biofactors. 2008;32:119–128.
42. Makhija N., Sendasgupta C., Kiran U., et al. The role of oral coenzyme Q10 in patients undergoing coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2008;22:832–839.
43. Tanaka J., Tominaga R., Yoshitoshi M., et al. Coenzyme Q10: the prophylactic effect on low cardiac output following cardiac valve replacement. Ann Thorac Surg. 1982;33:145–151.
44. Langsjoen P., Willis R., Folkers K. Treatment of essential hypertension with coenzyme Q10. Mol Aspects Med. 1994;15(Suppl):S265–272.
45. Ho M.J., Bellusci A., Wright J.M. Blood pressure lowering efficacy of coenzyme Q10 for primary hypertension. Cochrane Database Syst Rev. 2009. CD007435
46. Lockwood K., Moesgaard S., Folkers K. Partial and complete regression of breast cancer in patients in relation to dosage of coenzyme Q10. Biochem Biophys Res Commun. 1994;199:1504–1508.
47. Hertz N., Lister R.E. Improved survival in patients with end-stage cancer treated with coenzyme Q(10) and other antioxidants: a pilot study. J Int Med Res. 2009;37:1961–1971.
48. Conklin K.A. Coenzyme Q10 for prevention of anthracycline-induced cardiotoxicity. Integr Cancer Ther. 2005;4:110–130.
49. Karlsson J.A.H., Folkers K., Astrom H., et al. Effect of Adriamycin on heart and skeletal muscle coenzyme Q (CoQ10) in man. In: Folkers K., ed. Biomedical and clinical aspects of coenzyme Q. Amsterdam: Elsevier Scientific, 1986.
50. Judy W.V.D.W., Hall J.H., Dugan W., et al. Coenzyme Q10 reduction of Adriamycin cardiotoxicity. In: Folkers K., ed. Biomedical and clinical aspects of coenzyme Q. Amsterdam: Elsevier Scientific, 1984.
51. Mizuno K., Tanaka M., Nozaki S., et al. Antifatigue effects of coenzyme Q10 during physical fatigue. Nutrition. 2008;24:293–299.
52. Jacobson B.H., Smith D.B., Warren A.J., et al. Assessment of the effectiveness of a sublingual, ergogenic spray on muscle strength and power. J Strength Cond Res. 2009;23:2326–2330.
53. Kon M., Kimura F., Akimoto T., et al. Effect of Coenzyme Q10 supplementation on exercise-induced muscular injury of rats. Exerc Immunol Rev. 2007;13:76–88.
54. Vanfraecchhem J. Coenzyme Q10 and physical performance. In: Folkers K., ed. Biomedical and clinical aspects of coenzyme Q10. Amsterdam: Elsevier Scientific; 1981:235–241.
55. Gökbel H., Gül I., Belviranl M., et al. The effects of coenzyme Q10 supplementation on performance during repeated bouts of supramaximal exercise in sedentary men. J Strength Cond Res.. 2010;24:97–102.
56. Malm C., Svensson M., Ekblom B., et al. Effects of ubiquinone-10 supplementation and high intensity training on physical performance in humans. Acta Physiol Scand. 1997;161:379–384.
57. Kishi T., Kishi H., Watanabe T., et al. Bioenergetics in clinical medicine. XI. Studies on coenzyme Q and diabetes mellitus. J Med. 1976;7:307–321.
58. Eriksson J.G., Forsen T.J., Mortensen S.A., et al. The effect of coenzyme Q10 administration on metabolic control in patients with type 2 diabetes mellitus. Biofactors. 1999;9:315–318.
59. Hodgson J.M., Watts G.F., Playford D.A., et al. Coenzyme Q10 improves blood pressure and glycaemic control: a controlled trial in subjects with type 2 diabetes. Eur J Clin Nutr. 2002;56:1137–1142.
60. Watts G.F., Playford D.A., Croft K.D., et al. Coenzyme Q(10) improves endothelial dysfunction of the brachial artery in type II diabetes mellitus. Diabetologia. 2002;45:420–426.
61. Playford D.A., Watts G.F., Croft K.D., et al. Combined effect of coenzyme Q10 and fenofibrate on forearm microcirculatory function in type 2 diabetes. Atherosclerosis. 2003;168:169–179.
62. Lamson D.W., Plaza S.M. Mitochondrial factors in the pathogenesis of diabetes: a hypothesis for treatment. Altern Med Rev. 2002;7:94–111.
63. Silvestre-Aillaud P., BenDahan D., Paquis-Fluckinger V., et al. Could coenzyme Q10 and L-carnitine be a treatment for diabetes secondary to 3243 mutation of mtDNA? Diabetologia. 1995;38:1485–1486.
64. Fujioka T., Sakamoto Y., Mimura G. Clinical study of cardiac arrhythmias using a 24-hour continuous electrocardiographic recorder (5th report)–antiarrhythmic action of coenzyme Q10 in diabetics. Tohoku J Exp Med. 1983;141(Suppl):453–463.
65. Alleva R., Scararmucci A., Mantero F., et al. The protective role of ubiquinol-10 against formation of lipid hydroperoxides in human seminal fluid. Mol Aspects Med. 1997;18(Suppl):S221–228.
66. Mancini A., De Marinis L., Littarru G.P., et al. An update of Coenzyme Q10 implications in male infertility: biochemical and therapeutic aspects. Biofactors. 2005;25:165–174.
67. Balercia G., Arnaldi G., Fazioli F., et al. Coenzyme Q10 levels in idiopathic and varicocele-associated asthenozoospermia. Andrologia. 2002;34:107–111.
68. Balercia G., Mosca F., Mantero F., et al. Coenzyme Q(10) supplementation in infertile men with idiopathic asthenozoospermia: an open, uncontrolled pilot study. Fertil Steril. 2004;81:93–98.
69. Lewin A., Lavon H. The effect of coenzyme Q10 on sperm motility and function. Mol Aspects Med. 1997;18(Suppl):S213–219.
70. Balercia G., Mancini A., Paggi F., et al. Coenzyme Q10 and male infertility. J Endocrinol Invest. 2009;32:626–632.
71. Shults C.W., Haas R.H., Beal M.F. A possible role of coenzyme Q10 in the etiology and treatment of Parkinson’s disease. Biofactors. 1999;9:267–272.
72. NINDS Website. Statement on the Termination of QE3 Study. http://www.ninds.nih.gov/disorders/clinical_trials/CoQ10-Trial-Update.htm. Accessed 06/15/2011
73. Cooper J.M., Schapira A.H. Friedreich’s ataxia: coenzyme Q10 and vitamin Etherapy. Mitochondrion. 2007;7(Suppl):S127–135.
74. Rustin P., Rotig A., Munnich A., et al. Heart hypertrophy and function are improved by idebenone in Friedreich’s ataxia. Free Radic Res. 2002;36:467–469.
75. Hausse A.O., Aggoun Y., Bonnet D., et al. Idebenone and reduced cardiac hypertrophy in Friedreich’s ataxia. Heart. 2002;87:346–349.
76. Cooper J.M., Schapira A.H. Friedreich’s ataxia: disease mechanisms, antioxidant and coenzyme Q10 therapy. Biofactors. 2003;18:163–171.
77. Cooper J.M., Korlipara L.V., Hart P.E., et al. Coenzyme Q10 and vitamin E deficiency in Friedreich’s ataxia: predictor of efficacy of vitamin E and coenzyme Q10 therapy. Eur J Neurol. 2008;15:1371–1379.
78. Siciliano G., Mancuso M., Tedeschi D., et al. Coenzyme Q10, exercise lactate and CTG trinucleotide expansion in myotonic dystrophy. Brain Res Bull. 2001;56:405–410.
79. Potgieter M., Pretorius E., Van der Merwe C.F., et al. Histological assessment of SJL/J mice treated with the antioxidants coenzyme Q10 and resveratrol. Micron. 2011 Apr;42(3):275–282.
80. Folkers K., Simonsen R. Two successful double-blind trials with coenzyme Q10 (vitamin Q10) on muscular dystrophies and neurogenic atrophies. Biochim Biophys Acta. 1995;1271:281–286.
81. Folkers K., Morita M., McRee J., Jr. The activities of coenzyme Q10 and vitamin B6 for immune responses. Biochem Biophys Res Commun. 1993;193:88–92.
82. Bessler H., Bergman M., Blumberger N., et al. Coenzyme Q10 decreases TNF-alpha and IL-2 secretion by human peripheral blood mononuclear cells. J Nutr Sci Vitaminol (Tokyo). 2010;56:77–81.
83. Gazdik F., Pijak M.R., Borova A., et al. Biological properties of coenzyme Q10 and its effects on immunity. Cas Lek Cesk. 2003;142:390–393. [Czech]
84. Folkers K., Shizukuishi S., Takemura K., et al. Increase in levels of IgG in serum of patients treated with coenzyme Q10. Res Commun Chem Pathol Pharmacol. 1982;38:335–338.
85. Sugiyama S., Kitazawa M., Ozawa T., et al. Anti-oxidative effect of coenzyme Q10. Experientia. 1980;36:1002–1003.
86. Folkers K., Langsjoen P., Nara Y., et al. Biochemical deficiencies of coenzyme Q10 in HIV-infection and exploratory treatment. Biochem Biophys Res Commun. 1988;153:888–896.
87. Folkers K., Hanioka T., Xia L.J., et al. Coenzyme Q10 increases T4/T8 ratios of lymphocytes in ordinary subjects and relevance to patients having the AIDS-related complex. Biochem Biophys Res Commun. 1991;176:786–791.
88. Rosenfeldt F.L., Mijch A., McCrystal G., et al. Skeletal myopathy associated with nucleoside reverse transcriptase inhibitor therapy: potential benefit of coenzyme Q10 therapy. Int J STD AIDS. 2005;16:827–829.
89. Greenamyre J.T., Betarbet R., Sherer T.B. The rotenone model of Parkinson’s disease: genes, environment and mitochondria. Parkinsonism Relat Disord. 2003;9(Suppl 2):S59–64.
90. Dorta D.J., Leite S., DeMarco K.C., et al. A proposed sequence of events for cadmium-induced mitochondrial impairment. J Inorg Biochem. 2003;97:251–257.
91. Peraza M.A., Carter D.E., Gandolfi A.J. Toxicity and metabolism of subcytotoxic inorganic arsenic in human renal proximal tubule epithelial cells (HK-2). Cell Biol Toxicol. 2003;19:253–264.
92. Robertson J.D., Orrenius S. Molecular mechanisms of apoptosis induced by cytotoxic chemicals. Crit Rev Toxicol. 2000;30:609–627.
93. Robertson J.D., Orrenius S. Role of mitochondria in toxic cell death. Toxicology. 2002;181-182:491–496.
94. Menke T., Gille G., Reber F., et al. Coenzyme Q10 reduces the toxicity of rotenone in neuronal cultures by preserving the mitochondrial membrane potential. Biofactors. 2003;18:65–72.
95. Kagan T., Davis C., Lin L., et al. Coenzyme Q10 can in some circumstances block apoptosis, and this effect is mediated through mitochondria. Ann N Y Acad Sci. 1999;887:31–47.
96. Merlo Pich M., Castagnoli A., Biondi A., et al. Ubiquinol and a coenzyme Q reducing system protect platelet mitochondrial function of transfusional buffy coats from oxidative stress. Free Radic Res. 2002;36:429–436.
97. Lamperti C., Naini A.B., Lucchini V., et al. Muscle coenzyme Q10 level in statin-related myopathy. Arch Neurol. 2005;62:1709–1712.
98. Paiva H., Thelen K.M., Van Coster R., et al. High-dose statins and skeletal muscle metabolism in humans: a randomized, controlled trial. Clin Pharmacol Ther. 2005;78:60–68.
99. Marcoff L., Thompson P.D. The role of coenzyme Q10 in statin-associated myopathy: a systematic review. J Am Coll Cardiol. 2007;49:2231–2237.
100. Kishi T., Watanabe T., Folkers K. Bioenergetics in clinical medicine XV. Inhibition of coenzyme Q10-enzymes by clinically used adrenergic blockers of beta-receptors. Res Commun Chem Pathol Pharmacol. 1977;17:157–164.
101. Kishi H., Kishi T., Folkers K. Bioenergetics in clinical medicine. III. Inhibition of coenzyme Q10-enzymes by clinically used anti-hypertensive drugs. Res Commun Chem Pathol Pharmacol. 1975;12:533–540.
102. Kishi H. Inhibition of myocardial respiration by psychotherapeutic drugs and prevention by coenzyme Q. In: Folkers K.Y.Y., ed. Biomedical and clinical aspects of coenzyme Q. Amsterdam: Elsevier Scientific; 1984:139–154.
103. Morton R.A. Ubiquinones, plastoquinones and vitamins K. Biol Rev Camb Philos Soc. 1971;46:47–96.
104. Spigset O. Reduced effect of warfarin caused by ubidecarenone. Lancet. 1994;344:1372–1373.
105. Engelsen J., Nielsen J.D., Winther K. Effect of coenzyme Q10 and Ginkgo biloba on warfarin dosage in stable, long-term warfarin treated outpatients. A randomised, double blind, placebo-crossover trial. Thromb Haemost. 2002;87:1075–1076.