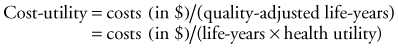CHAPTER 160 Cochlear Implants
Results, Outcomes, Rehabilitation, and Education
Results of Implantation
Factors Related to the Measurement of Auditory Performance
Auditory performance is measured at preimplant and postimplant intervals. Preimplant assessments form the basis of evaluating patient candidacy. Postimplant measures document the progress of the patient, while allowing the clinician to monitor for device-related and environmental factors that can influence long-term outcomes. Measurement variables associated with auditory testing should be standardized as much as possible. Clinicians can choose between closed-set tests (e.g., forced choice of one answer from a list of four) and open-set tests (auditory alone without context) of words or sentences. Closed-set tests are easier for patients than open-set tests, and are susceptible to “ceiling effects,” reflecting high contextual information available when word and sentence material are presented.188 The method of presentation can also affect speech perception scores.1a Live presentation typically produces higher rates of correct responses than taped presentations.
Tests of Implant Performance
The Minimum Speech Test Battery (MSTB) was developed for adult cochlear implant users. The MSTB is a set of high-fidelity disc recordings that provide a standardized set of comprehensive tests of preoperative and postoperative speech recognition.2 To minimize the effects of learning and memorization, MSTB word and sentence tests have different lists for at least six testing trials. The average and range of performance of cochlear implant users are crucial to defining audiologic performance boundaries for implant candidacy.
The major components of the MSTB are the Hearing in Noise Test (HINT) and the Consonant/Nucleus/Consonant Test (CNC). The HINT2 provides a measure of speech recognition ability for sentences in quiet and in noise. The background noise is filtered to match the long-term average spectrum of the sentences. In the MSTB, the HINT sentence lists are presented at 70 +dB in quiet and at 10 +dB signal-to-noise ratio (i.e., noise at 60 +dB). Smaller signal-to-noise ratios (e.g., 5 +dB or 0 +dB) may also be used to avoid ceiling effects. Although normal-hearing listeners can comprehend sentences effectively with signal-to-noise ratios of 3 +dB, implant recipients typically show degraded speech recognition when signal-to-noise ratios are reduced beyond 10 +dB.
The CNC consists of lists of monosyllabic words with equal phonemic distribution, with each list having approximately the same phonemic distribution as the English language.3 Such lists provide material for performance testing that is more likely to represent daily experience with speech stimuli. These tests measure percentage of words correctly recognized. Open-set conditions for monosyllabic word recognition are considered the most difficult challenge to a listener with a cochlear implant, and such tests are useful to compare outcomes in adults.4 Revised CNC lists5 were developed to eliminate uncommon words and proper nouns. Ten lists of 50 words contain monosyllabic words that are employed with a frequency of greater than 4 per 1 million, as calculated in word-frequency tables. As part of the MSTB protocol, during each preoperative and postoperative evaluation, one CNC and one HINT list should be presented in quiet at 70 dB(A). In addition, if the patient performs greater than 30% under quiet conditions, HINT sentences can be presented in noise at 10 +dB signal-to-noise ratio.
Implant Performance in Adults
Improved speech perception is the primary goal of cochlear implantation. Although initial clinical series judged implant efficacy mostly on environmental sound perception and performance on closed-set tests, greater emphasis is now placed on measures of open-set speech comprehension. Speech-perception results from early clinical trials have served to guide the evolution of cochlear implantation. Gantz and colleagues6 provided early comparative data in their assessment of environmental sound and speech perception in a large cohort of nonrandomized single-channel and multichannel implant users. Multichannel implants provided significantly higher levels of performance on all measures. Cohen and associates7 performed the first prospective, randomized trial of cochlear implants through the U.S. Veterans’ Administration hospital system. The trial provided high-quality clinical data and convincing evidence of open-set speech recognition, and established the clear superiority of multichannel over single-channel designs. The study was the first to report a low complication rate and high device reliability in individuals deafened after language acquisition from multiple centers with varying levels of prior programmatic implant experience.
Cohen and Waltzman,8 Skinner and colleagues,9,10 and Wilson and associates11 assessed effects of changing external processing capabilities. They compared the performance of subjects fitted with processors designed to increase the rate of information transfer through higher rates of pulsed stimulation. Each study assessed a different processor, and study design and processing strategy also differed among studies. In each study, the use of a more sophisticated speech processor led to significantly better performance in open-set speech recognition.
Clinical observations in patients with current processors indicate that for patients with implant experience longer than 6 months, the mean score on open-set word testing approximates 25% to 40% (range 0% to 100%).7,12–14 Results achieved with the most recently developed speech processing strategies reveal mean scores greater than 75% on words-in-sentence testing (range 0% to 100%). Although subjects perform substantially poorer on single-word testing, these mean scores continue to improve as speech-processing strategy evolves.10 After implantation, speech recognition by telephone15 and music appreciation are often observed.
Benefits are enhanced through the use of more recently developed processing strategies. Spahr and colleagues16 observed that differences in implant design affect performance, especially in difficult listening situations. Input dynamic range and the method by which compression is implemented seem to be the major factors that account for results in difficult listening situations, with greater performance associated with the expanded dynamic range offered by current processing strategies employed by the Advanced Bionics and MedEl devices.
Predictors of Benefit in Adults
Assessments of speech recognition in adults with cochlear implants offer the opportunity to develop models of benefit prediction. As investigators identify the salient predictive factors, candidacy, device and processing strategy, and the degree of postoperative auditory rehabilitation necessary, they can be better informed. The contributions of modifiers to differences in performance (multivariate analysis) have been addressed in several large studies.6,7,12–14,17–19 The following factors have been evaluated:
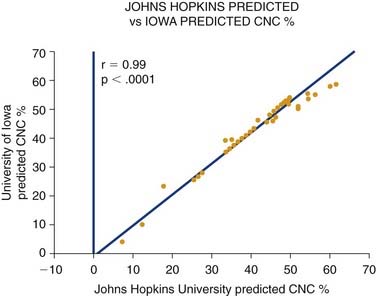
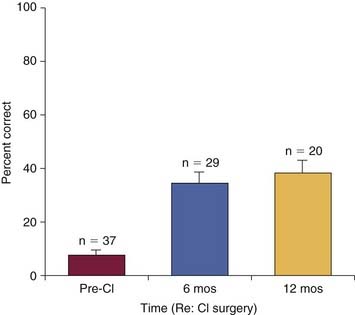
In addition to the factors in the list, the choice of ear to implant is a highly compelling issue. Other studies have emphasized the utility of implanting the better hearing ear.13 At Johns Hopkins Hospital, we have long advocated the implantation of the poorer hearing ear. Although more data are needed, our studies so far reveal no significant difference in implant performance based on whether the better or worse hearing ear is implanted. Figure 160-1 shows a regression plot of the predicted postoperative word scores for each patient as modeled by the Johns Hopkins (implant poorer ear) and Iowa formulas (better ear).17 Virtually identical scores are predicted on the basis of each patient’s duration of deafness and preoperative sentence recognition scores. These data suggest that results obtained through cochlear implantation of the poorer hearing ear are statistically equivalent to results obtained through implantation of the better hearing ear. The similarity of results obtained through both methods suggests that implantation may have a beneficial effect on central auditory pathway development regardless of sidedness.
Another variable that influences speech perception is technologic sophistication of the implanted device. Improvements in speech perception have been associated with generational improvements in signal processing strategies, speech processors, and electrode arrays,20 but may reflect clinical trends and technologic advances.13 Criteria for cochlear implant candidacy are constantly expanding. Research has shown that patients with greater residual hearing show even more promising results. Identification of monosyllabic words for individuals using early generations of the Nucleus (Cochlear Corp., Sydney, Australia) multichannel cochlear implant averaged 16%,6 whereas performance exceeds 50% on similar measures with current technology.10,16 Earlier studies have contrasted performance across different manufacturers of devices. Differences in performance seem more closely related, however, to general demographic differences across patient populations, tests used, and length of experience with the devices than to differences in characteristics of like-generation devices per se.21,22
Cochlear Implantation with Hearing Preservation in Adults
There has been considerable interest in clinical strategies that combine residual acoustic hearing with electric hearing provided by a cochlear implant. Two strategies have been developed, depending on baseline hearing status. A combined mode uses a cochlear implant in one ear and a hearing aid in the other ear.23 A hybrid strategy uses a hearing aid and a cochlear implant in the same ear.24,25
The goal of combining amplification with a cochlear implant in the opposite ear is to preserve perceptual advantages inherent in (predominantly low frequency) acoustic hearing in an attempt to capture at least some binaural cues. In fact, binaural advantages are observed in some patients.23 Tests of speech perception in noise reveal trends toward a binaural advantage for word recognition depending on the direction of origin of noise (with advantages obviated when noise is on the hearing aid side). Localization ability improved with both devices. These data support the use of a hearing aid opposite a cochlear implant for appropriate patients.
Success with hearing preservation26,27 in ears implanted with scala tympani electrodes has encouraged the development of hybrid modes of stimulation—electroacoustic hearing. Various surgical strategies have been adopted in attempts to limit cochlear trauma with scala tympani electrode insertion, enhancing opportunities for preserving residual (typically, <1 kHz) hearing. Hearing preservation procedures are designed to avert basilar membrane rupture and attendant spiral ganglion cell loss. Optimal preservation seems to be achieved with placement of the cochleostomy in a strategic location (away from the basilar membrane) along with cautious insertion of adapted, smaller arrays in a manner that limits impact on the endosteal and basilar membrane surfaces.28
Electroacoustic hearing seems to confer distinct advantages in speech perception and in music listening.25,29 Improvements in speech-in-noise understanding and melody recognition are linked to the ability to distinguish fine pitch differences through preserved, low-frequency acoustic hearing. There may be expanded spectral representation that more faithfully supports higher frequency hearing over time.30 These observations suggest that the preservation of residual low-frequency hearing should be considered in selected patients. Observations of advantages in electroacoustic hearing may expand candidate selection criteria for cochlear implantation.
Although electroacoustic stimulation is a promising treatment for patients with residual low-frequency hearing, a small subset lose residual hearing despite best attempts with preservation. Such patients may obtain expected levels of benefit using the electric mode of the hybrid implant alone. For patients unable to achieve this benefit from an electroacoustic strategy after losing residual hearing, reimplantation with a standard array seems to provide higher levels of speech recognition.31
Implant Performance in Children
Pediatric cochlear implantation began with House-3M, single-channel implants in 1980. Investigational trials with multichannel cochlear implants began with adolescents (10 to 17 years old) in 1985 and with children (2 to 9 years old) in November 1986. Implantation of infants and toddlers younger than 2 years began in 1995.32 Although clinical experience with cochlear implantation is shorter in children than in adults, a large body of clinical reports is now available.33,34 Investigators have evaluated the hearing and speech receptive benefit of cochlear implants in children since 1985.
Auditory Performance Assessments for Children
Methodologic variables must be considered when attempting to rate objectively the effect of cochlear implants on the development of speech perception in deaf children. Difficulties are inherent in objectively rating communication competence in very young children. Older children may exhibit advantages by virtue of greater familiarity with a test’s context, independent of perceptual skills. Objective assessment mandates a structured setting that is not always conducive to eliciting a child’s best cooperative effort. Investigators must also account for discontinuity in the age-appropriate measures necessary for longitudinal assessment.34 Methodologic challenges and developmental considerations inherent in pediatric implant assessment have been examined in detail by Kirk and Diefendorf,35 who categorized variables as relating to the following:
Tests of speech perceptions typically used for childhood assessment have been described in detail,1a,35–37 and usually consist of closed-set tests that assess word identification among a limited set of options with auditory cues only, open-set tests (scored by percentage of individual words correctly repeated), and structured interviews of parents using criteria-based surveys38 to assess response to sound in everyday situations and behaviors related to spoken communication.
Early Studies of Speech Reception in Children with Cochlear Implants
House and colleagues39 found that children with cochlear implants obtain substantial improvement in auditory thresholds, closed-set speech recognition, and limited open-set speech recognition with the House-3M single-channel implant. Miyamoto and coworkers40 provided systematic, well-controlled assessments of childhood cohorts and consistently showed performance advantages of multichannel over single-channel implants. Other early studies by Fryauf-Bertschy,41 Gantz,42 Miyamoto,18 and Waltzman43 and their colleagues observed that implanted children gain substantial speech perceptive capabilities for 5 years after implantation. Because many of the implanted children tracked in these studies were congenitally or prelingually deaf, there are compelling data to indicate that implantation can provide auditory access during critical developmental stages to form the early correlates of spoken language.
The studies we have cited have shown implant benefit in children with total or near-total hearing loss. An important issue for candidacy of children with lesser degrees of hearing impairment is the study of implant benefit relative to controls who have not received implants. Classification of children according to preoperative hearing levels can provide a common ground for comparison. Miyamoto and associates44 described the following candidacy scale for stratifying children according to aided pure-tone detection thresholds: “Gold” class hearing aid users have unaided thresholds of 90 to 100 dB at two of three frequencies (0.5 kHz, 1 kHz, 2 kHz), with a mean of 94 dB. “Silver” class hearing aid users have unaided thresholds of 101 to 110 dB at two of three frequencies, with a mean of 104 dB. “Bronze” class users have unaided thresholds of greater than 110 dB at two of three frequencies, with a mean greater than 110 dB.
Hearing aid users in the “gold” category generally fall into Boothroyd’s45 category of “considerable residual hearing.” In many such cases, hearing aid users that function at this level show substantial capabilities for near-normal trajectories of speech and oral language acquisition. This particular group of profoundly hearing-impaired children sets a comparative standard for speech perception performance. “Silver” category hearing aid users receive few spectral cues and rely heavily on temporal cues for speech perception. “Bronze” category users are considered “totally deaf,” and receive only low-frequency percepts and restricted or no temporal cues.
More Recent Studies of Speech Reception in Children with Cochlear Implants
Between 1994 and 2004, numerous reports documented various levels of gains in speech recognition in young deaf children using multichannel cochlear implants. Miyamoto and coworkers18 noted that in 29 children with 1 to 4 years of experience with a cochlear implant, approximately one half achieved open-set speech recognition. Waltzman and Cohen51 found that 14 children implanted before age 3 years attained high levels of speech perception performance with only 2 years of experience. Fryauf-Bertschy and coworkers52 examined speech perception in cochlear-implanted children over 4 years, and noted significant increases in pattern perception and results of closed-set speech perception tests.
Variability in speech-perception performance across subjects is widely recognized, and is related to several variables, including age of implantation,33,42,52–54 mode of communication,43,55 family support,52 and length of deafness.56,57 Miyamoto and colleagues40 found that duration of deafness, communication mode, age at onset of deafness, and type of processor accounted for approximately 35% of variance in closed-set testing. The length of implant use alone accounted for a larger percentage of variance on all speech perception measures. In a 5-year follow-up study, O’Donoghue and associates53 found that age at implantation was the most significant covariant, and that mode of communication was the most significant between-individuals factor. The latter study showed that young age at implantation and oral communication mode are the most important known determinants of later speech perception in young children after cochlear implantation.
Waltzman and colleagues43 and Brackett and Zara58 reported improved speech reception in children implanted at age 2 to 3 years compared with children implanted at an older age. Multicenter data reported by Osberger and associates59 indicated that implant performance of children implanted before age 2 years is significantly better than implant performance of children implanted between age 2 and 3 years. The authors also identified an important confounding variable, however, that exists in the children who receive a cochlear implant at a younger age: they are more likely to use an oral mode of communication. This, by itself, may be a predictor of higher implant performance—an observation borne out in early studies of a national childhood cohort assembled by Geers and colleagues.55
Osberger and coworkers59 also found that children with more residual hearing relative to earlier cohorts were undergoing implantation. Gantz and colleagues60 compiled data from across centers that indicate children with some degree of preoperative open-set speech recognition obtain substantially higher levels of speech comprehension. Taken together, these studies suggest the strongest potential for benefit exists with implantation at a young age, when intervention is provided early and, in the case of a progressive loss, before auditory input is lost completely.
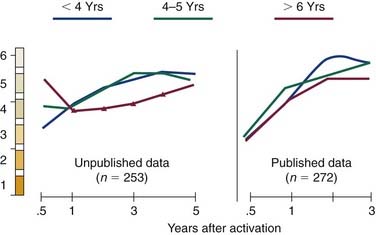
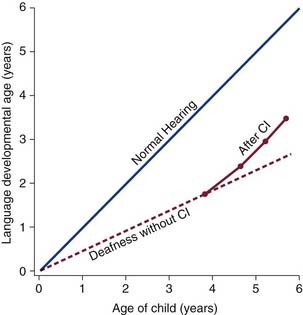
Cheng and associates33 performed a meta-analysis of relevant literature on speech recognition in children with cochlear implants. Of 1916 reports on cochlear implants published since 1966, 44 contained sufficient patient data to compare speech recognition results between published (n = 1904 children) and unpublished (n = 261) trials.33 Meta-analysis was complicated by the diversity of tests required to address the full spectrum of speech reception in children with cochlear implants. An expanded format of the Speech Perception Categories61 was designed to integrate results across studies. The main conclusions of this meta-analysis were that earlier implantation is associated with a greater trajectory of gain in speech recognition (Fig. 160-2), performance comparing deafness of congenital and acquired etiologies levels out over time, and there is an absence of reaching a plateau of speech recognition benefits over time. More than 75% of the children with cochlear implants reported in peer-reviewed publications have achieved substantial open-set speech recognition after 3 years of implant use.
Implantation of Infants
A growing body of evidence supports the early application of cochlear implantation in congenitally deaf children. Meta-analyses of the effects of age at implantation have shown that children undergoing implantation at younger ages achieve greater progress in speech perception skills than children implanted at older ages.33,62,63 Because the development of language follows the same trajectory as that reflecting speech perception, language growth is more favorable in children who receive cochlear implants early in life.64 To the extent that higher language ability results in greater independence in school settings,65 earlier intervention would enhance the cost-effectiveness of the cochlear implant.
Cochlear implantation has been shown to be a safe procedure in children younger than 12 months old.66,67 The motivation to pursue implantation when a child has been deemed an appropriate candidate is supported by compelling data showing the benefits of earlier intervention. Tait and colleagues68 showed that preverbal skills develop much more rapidly in children who receive cochlear implants before 2 years of age compared with children implanted between 2 and 3 years old, or between 3 and 4 years old. Connor and colleagues69 examined latent growth curves and showed the value of very early implantation as it translated into more favorable outcomes. Dettman and colleagues70 in Australia and Lesinski-Schiedat and associates71 in Germany have convincingly shown with longitudinal assessment that the language trajectories of children implanted before age 12 months are greater than those achieved with implantation after 12 months of age.
Nicholas and Geers72 showed that language outcomes for deaf children for whom appropriate intervention occurs within the first months of life are far superior to outcomes associated with later intervention. They noted that the first 3 years in a child’s life are crucial for acquiring information about the world, communicating with family, and developing a cognitive and linguistic foundation from which all further development unfolds.
Bilateral Cochlear Implantation
Normal listeners possess a range of auditory capabilities owing to binaural processing, as follows:
Studies of subjects with unilateral hearing loss illustrate some of the problems that a listener with only one “good” ear may encounter. Background noise often interferes with word recognition in quiet and noisy conditions even when the speech is directed to the good ear. People have reported difficulty hearing and understanding speech even in relatively quiet situations that include low-level noise (e.g., car radios, air conditioners).76 Children with unilateral hearing loss not only have difficulty in noise,77 but also show delays in language development and academic performance relative to their peers with normal hearing.78
In addition to the general advantages of binaural hearing, there may be other compelling reasons for considering bilateral implantation in very young children. Congenitally deaf children depend on their cochlear implants for learning spoken language. Children with normal hearing acquire language through incidental learning—that is, through participation in everyday communication and social experiences, which are often unintended and incidental. Although studies have shown improved language acquisition in children with unilateral implants relative to their preimplant performance with hearing aids,64,79 children still lag behind their peers with normal hearing in mastery of more complex language structures that are requisites for academic success.80 If a young deaf child has two implants, the rate of incidental language learning is likely to be accelerated because they would be better able to understand speech in everyday situations (most of which are noisy), and to localize to the most relevant (speech) events in their environment. The improved benefits with bilateral implants in young deaf children have important implications for language acquisition, academic success, and educational and occupational opportunities.
Providing sound input to both ears in a young deaf child ensures that sound is processed on both sides of the brain. The right and left auditory cortices can develop in a more normal sequence. If a child is implanted on only one side, the parts of the brain that would have been stimulated by the nonimplanted ear do not develop, and eventually plasticity is greatly diminished. Ryugo and colleagues81 reported that electric stimulation of the auditory system largely prevents or reverses the synaptic abnormalities seen in congenitally deaf white cats.
Reports of the bilateral benefits experienced by adult and pediatric patients with two implants continue to increase.82–91 There are few prospective studies of children who receive two implants during early childhood, however.
Although the “era of bilateral implantation” has been introduced, there are few controlled trials to date.92 Preliminary results reveal evidence of an expanded sound field and loudness summation, and some level of sound localization ability to most bilateral recipients. In a few patients, benefits attributable to effective squelch are noted. Such findings show central integration of electric stimulation from the two ears despite an absence of precise tonotopic matching of binaural inputs. Systems that enable integrated speech processing of inputs from the bilateral sound fields have yet to be introduced clinically. It is uncertain whether potential benefits of bilateral implantation could be expanded through such processing.
Language Development in Children
A primary goal of implantation in children is to assist comprehension and expression through the use of spoken language. Language is defined as a vehicle for shaping and relating abstractions for communication.93 The meaning of language-mediated abstraction is independent of the immediate situation. Practical use of language is based on the assignment of a single name to various appearances and situations under varying conditions. Information exchange via spoken language involves a conversion of thought into speech. This conversion relies on mental representations of phonologic (sound) structure and syntactic (phrase) structure.
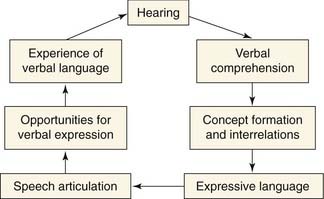
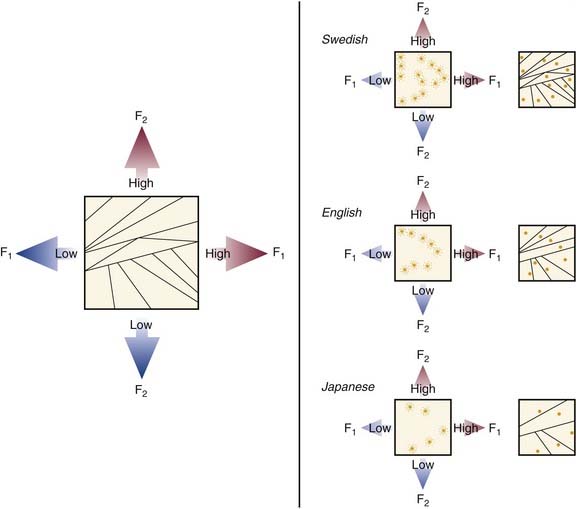
All fundamental models of language development propose that the acquisition of language is the result of progressive organization from an initially undifferentiated state. As incremental differentiation occurs, language skills become more sophisticated and hierarchically integrated. The child first must learn to form internal representations of acoustic speech patterns (Fig. 160-3). These experiences enable a child to identify consistent patterns in phonologic inputs and to develop associations between context and semantic and syntactic patterns. Through increased familiarity, these associations between spoken language and their actual counterparts build a child’s semantic awareness.94 Social cognition and emotional needs are likely to encourage such development, as supported by observations that infants become “tuned” to their language environment in the form of “perceptual maps” (Fig. 160-4),95 and show protolinguistic behaviors with caretakers that are bidirectional.96–98
Early communication between infants and caregivers forms a critical foundation for development. This foundation provides a base on which cognition, affect, and social interaction may evolve.99 If communication is hindered, development fails to proceed normally, with detrimental sequelae in terms of cognitive, behavioral, and social development. Hearing loss and subsequent obstacles to communication threaten optimal development.100–102 Because most children who have sensorineural hearing loss are born to hearing parents, a “mismatch” may exist between child and parent103 that prevents effective communication.104,105 Because sign language skills in hearing parents tend to be absent or basic, such children are at further risk for developmental delay.106,107 Additional evidence confirms that children with sensorineural hearing loss who engage in linguistic interactions with their parents show age-appropriate cognitive, social, and behavioral development.100 More recent theories suggest the processes linking language to cognition, affect, and behavior are dynamic and bidirectional, rather than discrete or independent.108,109
For successful bidirectional communication to occur within the family unit, language must be linked to cognition, affect, and behavior. Visual attention, which may be reliably assessed at 4 months of age, is crucial for guiding development.99,110 Data suggest that visual attention is highly relevant to the development of emotional bonds to caregivers, language learning, and the processing of perceptual information.111–114 In normal development, much of this early visual attention is actually directed by sound. Infants look longer in the direction of sound and longer at visual events whose temporal rhythms match what they hear.115,116 When sound perception is impaired, visual and auditory integration is similarly disrupted, forcing a child with sensorineural hearing loss to rely on vision for communication and cues about when and where to direct attention.117,118 This notion has been confirmed in studies of hearing-impaired children using computerized task performance measures.117,119,120 These impairments have been found to contribute to behavioral problems.120 By comparison, two studies found that children with cochlear implants perform better on this computerized task than children without cochlear implants.117,119
In addition to visual attention, other types of preverbal communication underlie the acquisition of verbal language. Substantial gains in prelinguistic behaviors, including eye contact and turn-taking,121 and in verbal spontaneity122 are observed as early correlates of implant benefit, developing within 6 months of implantation in young children.123 Tait and associates124 found that preverbal measures obtained 12 months after implantation are predictive of late performance on speech perception tasks. They observed a significant association between the preverbal measure of “autonomy” obtained before implantation and later speech perception performance. This latter finding has important theoretic implications for understanding of language development, and suggests that intervention that promotes autonomy in adult-child interaction may lead to improved outcomes. Such intervention can be introduced as soon as deafness is discovered.
Language Acquisition in Children with Cochlear Implants
By improving auditory access, cochlear implants augment sound and phrase structure. Although difficult to characterize, benefits in receptive language skills and language production after implantation are the crucial measure by which effectiveness of implants in young children should be assessed. One approach is to compare language performance on standardized tests. The Reynell Developmental Language Scale evaluates receptive and expressive skills independently.125 These scales have been normalized on the basis of performance levels of hearing children in an age range of 1 to 8 years, and have been used extensively in populations of deaf children. Although deaf children without cochlear implants achieved language competence at half the rate of normal-hearing peers, implanted subjects exhibited language-learning rates that matched, on average, those of their normal-hearing peers.125,126 In a study of 23 children who became deaf before 3 years of age, Svirsky and colleagues126 studied language development after cochlear implantation. The average age of implantation in this cohort was approximately 4 years. These investigators found that speech development progressed at a decreased rate before implantation, but that after implantation, language development in implanted children was parallel to that of normal-hearing children (Fig. 160-5). The implication of this study is that differences in language delay between implanted and normal-hearing children could be explained by the initial period of deafness before implantation, rather than performance or progress after implantation. These findings also emphasize the importance of early implantation. Data are rapidly being gathered to confirm that earlier implantation might limit early language delays to enable more age-appropriate language acquisition.
Robbins and associates125 noted that implantation improved language-learning rates for children in oral communication and total communication settings based on the Reynall Developmental Language Scale. Geers and coworkers,55 also assessing language skills in implanted children enrolled in oral communication and total communication settings, found that the groups did not differ in language level, although the oral group showed significantly better intelligibility in their speech production.
Delays in receptive and expressive language among hearing-impaired children have consistently been documented in the literature, although such delays are not strongly related to degree of hearing loss.127,128 Performance on language measures can be influenced by the child’s mode of communication such that results may not directly reflect the influences of auditory perception or prosthetic intervention. Clinical findings support the hypothesis, however, that some deaf children are able to use the acoustic-phonetic cues provided by the implant in ways that may reduce the language gap between normal-hearing and deaf children.
Central nervous system processing is a primary determinant of the level of verbal language ultimately attained after cochlear implantation.129 Pisoni129 assessed performance in two groups of pediatric cochlear implant users: “Stars” were children whose phonetically balanced kindergarten scores placed them in the top 20%. The second group, children with scores in the bottom 20%, comprised the control group. Speech perception, spoken word recognition, speech intelligibility, and language level were compared between the two groups at preimplantation and at yearly postimplant intervals. Children in the “Stars” group did not score consistently better on all behavioral measures than children in the control group. Differences between the groups were manifest only on specific tests and task demands. Children with superior implant performance were consistently better on measures of speech perception (i.e., vowel and consonant recognition), spoken word recognition, comprehension, language development, and speech intelligibility than were the control children. The two groups did not differ, however, in their vocabulary knowledge, nonverbal intelligence, visuomotor integration, or visual attention. It was concluded that “Star performance” on measures of spoken language processing and speech intelligibility was not attributed to global differences in overall performance, but to differences specifically in the task of processing auditory information provided by the cochlear implant.
The strong relationship between spoken word recognition and speech intelligibility for superior implant performers suggests that (1) a transfer of knowledge exists between speech perception and production, and (2) that speech perception and production share a common system of internal representation. According to Pisoni and Geers,130 the exceptional performance of the “Stars” seems to be a result of their superior abilities to perceive, encode, and retrieve information about spoken words from lexical memory. They described the capacity for processing tasks that require the manipulation and transformation of the phonologic representations of spoken words as “working memory.” Pisoni and Geers130 then investigated the impact of working memory on measures of speech perception, speech intelligibility, language processing, and reading in implanted children with prelingual deafness. The investigators evaluated correlations between children’s ability to recall lists of orally presented digits and their performance on these measures. The authors observed correlations between auditory memory and performance in each outcome area, suggesting that working memory plays an important role in mediating performance across these higher communication tasks.130
An ongoing multicenter study is examining the clinical modifiers of postimplant developmental outcomes, including oral language acquisition, speech recognition skills, selective attention and problem-solving skills, behavioral and social development, parent-child interactions, and quality-of-life measures in children undergoing implantation in six U.S. implant centers.131 It is hoped that a detailed assessment of communicative and psychosocial results will contribute to an understanding of the factors predicting implant-associated language use, communication competence in early childhood, and the perceived value of early cochlear implantation in light of associated costs. It is hoped that conclusions will enable an informed approach to implant candidacy when considering (re)habilitative strategies designed to enhance developmental learning in children with severe to profound sensorineural hearing loss.
Outcomes after Cochlear Implantation
Educational Placement and Support of Implanted Children
A hearing-impaired child is at substantial risk for educational underachievement.132,133 Educational achievement by a hearing-impaired child can be enhanced by verbal communication. Traditional methods of speech instruction are more successful with children who have enough residual hearing to benefit from early devices of hearing rehabilitation.134 Improved speech perception and production provided by cochlear implants offer the possibility of increased access to oral-based education and enhanced educational independence.
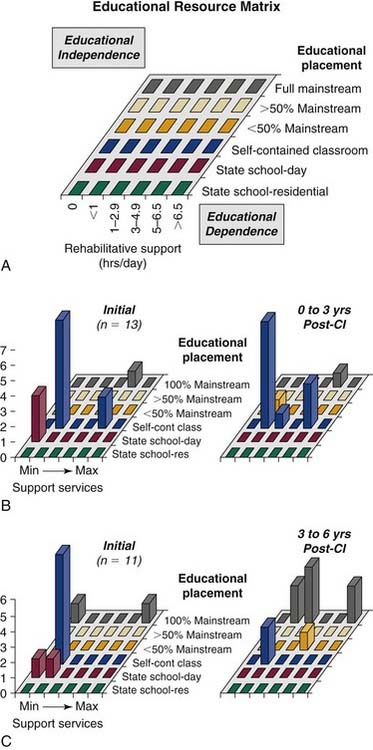
Francis135 and Koch136 and their colleagues tracked the educational progress of implanted children by using an educational resource matrix to map educational and rehabilitative resource use. The matrix was developed on the basis of observations that changes in classroom settings (e.g., into a mainstream classroom) are often compensated by an initial increase in interpreter and speech-language therapy. Follow-up of 35 school-aged children with implants indicated that, relative to age-matched hearing aid users with equivalent baseline hearing, implanted students are mainstreamed at a substantially higher rate, although this effect is not immediate, and requires rehabilitative support to be achieved. Within 5 years after implantation, the rate of full-time assignment to a mainstream classroom increases from 12% to 75% (Fig. 160-6).
Quality of Life and Cost-Effectiveness
Prior studies of the cost-utility of cochlear implants have assessed quality of life and health status to determine the utility gained from cochlear implants.19,137,138 Utility is a concept that reflects the true value of a good or service. Cost-utility methods determine the ratio of monetary expenditure to change in utility as defined by a change in quality of life over a given period. The assessment of cost-utility is based on the following:
Life-years is the mean anticipated number of years of implant experience based on a life-expectancy analysis of the participating cohort. The change in health utility reflects the difference between preimplant and postimplant scores on survey instruments that have been designed and validated to reflect quality of life. In the United States, England, and Canada, health interventions with a cost-utility ratio less than $20,000 (U.S.) are generally considered to represent acceptable value for money expended (i.e., they are “cost-effective”).139–141
Quality-of-Life Studies in Adults and the Elderly
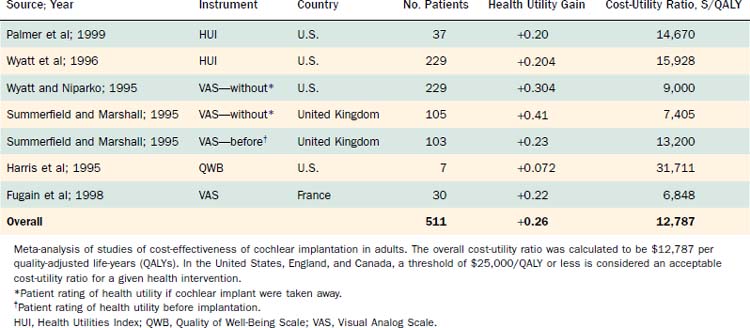
Costs per quality-adjusted life-year (QALY) for cochlear implants in adult users were determined using cost data that account for the preoperative, postoperative, and operative phases of cochlear implantation.19,138 Benefits were determined on the basis of functional status and quality of life. The precise cost-utility results varied among studies, mostly because of methodologic differences in the determination of benefit, level of benefit obtained, and differences in costs associated with the intervention. Nonetheless, these appraisals consistently indicated that the multichannel cochlear implant in adult populations is associated with cost-utility ratios in the range of $14,000 to $18,000/QALY, indicating a highly favorable position in terms of cost-effectiveness (Table 160-1).
Hearing impairment is one of the most common clinical conditions affecting elderly individuals in the United States.142 Hearing loss is so profound in 10% of elderly hearing-impaired individuals that little or no benefit is gained with conventional amplification.143 Assessing the effectiveness of cochlear implants in elderly patients requires consideration of audiologic and psychosocial factors. The social isolation associated with acquired hearing loss in elderly individuals144 is accompanied by a significant decline in quality of life and an increase in emotional handicap.145 The rehabilitation of hearing loss is an important goal in this vulnerable population, providing functional and psychologic contributions to quality of life.
Age-related degeneration of the spiral ganglion146,147 and progressive central auditory dysfunction148,149 raise potential concerns about the efficacy of cochlear prostheses in the elderly. Comparable gains in speech understanding have been reported for elderly and younger groups of implant recipients,150 but the implications of these functional gains on the quality of life of older adults have not been well characterized. The determination of auditory efficacy and quality of life is crucial to any cost-benefit analysis in elderly patients, and may help guide clinical resource allocation, particularly in light of the high costs associated with cochlear implantation as a nonlifesaving intervention.
Reports of quality of life gains in elderly patients with cochlear implants have been favorable,151–153 but are based on questionnaires that are difficult to correlate with function and cost-utility. Francis and colleagues151 evaluated 47 patients with multichannel cochlear implants, 50 to 80 years old, who completed the Ontario Health Utilities Index Mark 3 (HUI3) survey and a quality-of-life survey. This study assessed preimplantation and postimplantation (6 months and 1 year after implantation) responses to questions related to device use and quality of life. A significant mean gain occurred in health utility of 0.24 (SD 0.33) associated with cochlear implantation (P < .0001) (Fig. 160-7). Improvements in hearing and emotional health attributes were primarily responsible for this increase in health-related quality-of-life measure. A significant increase occurred in speech perception scores at 6 months after surgery (P < .0001 for Central Institute for the Deaf sentence and monosyllabic word tests), and there was a strong correlation between the magnitude of health utility gains and postoperative enhancement of speech perception (r = 0.45, P < .05). Speech perception gain was also correlated with improvements in emotional status and the hours of daily implant use. The authors concluded that cochlear implantation has a statistically significant and cost-effective impact on the quality of life of older deaf patients.
Quality-of-Life Studies in Children
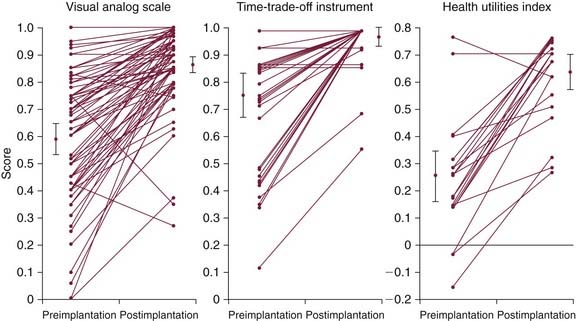
Published cost-utility analyses of the cochlear implant in children have been limited by using either health utilities obtained from adult patients19,139,154 or hypothetically estimated utilities of a deaf child.155–158 These studies yielded cost-utility ratios that spread over a wide range ($3141 to $25,450/QALY). Utility assessments derived from adult patient surveys may not capture the impact of issues unique to childhood deafness.33 To address this issue more rigorously, Cheng and coworkers137 surveyed parents of a cohort of 78 children (average age 7.4 years, with 1.9 years of cochlear implant use) who received multichannel implants at the Johns Hopkins Hospital to determine direct and total cost to society per QALY. Parents of profoundly deaf children (n = 48) awaiting cochlear implantation served as a comparison group to assess the validity of recall. Using the Time-Trade-Off (TTO), Visual Analog Scale (VAS), and HUI3, parents rated their child’s health state “now,” “immediately before,” and “1 year before” the cochlear implant. Mean VAS scores increased 0.27 on a scale of 0 to 1 (from 0.59 to 0.86), TTO scores increased 0.22 (from 0.75 to 0.97), and HUI scores increased 0.39 (from 0.25 to 0.64) (Fig. 160-8). Discounted direct medical costs were $60,228, yielding cost-utility ratios of $9029/QALY using the TTO, $7500/QALY using the VAS, and $5197/QALY using the HUI3. Including indirect costs, such as reduced educational expenses, the cochlear implant yielded a calculated net savings of $53,198 per child. Based on assessments of this cohort from a single center, childhood cochlear implantation produces a positive impact on quality of life at reasonable direct costs and results in societal savings.
The educational resource matrix used by Francis135 and Koch136 and their colleagues offers a basis for assessing overall cost-benefit ratios of the cochlear implant in children. Although educational costs for all implanted students remain static or initially increased, ultimate achievement of educational independence for most implanted children produces net savings that range from $30,000 to $100,000 per child, including the costs associated with initial cochlear implantation and postoperative rehabilitation. Language-related and education-related outcomes in children with cochlear implants have been supplemented with parental perspectives of quality-of-life effects to yield cost-utility ratings.137,154 Despite conservative assumptions, results show that cochlear implantation is, relative to other medical and surgical interventions, highly cost-effective in profoundly hearing-impaired young children.
Auditory Rehabilitation after Cochlear Implantation
Efficacy of Auditory Training in Cochlear Implant Rehabilitation
Auditory training that provides real-world utility to restored audition in implant recipients is necessarily highly individualized. That is, the goals for a patient’s auditory, speech, language, and cognitive development rely on a host of influences unique to the individual. Because of the need for such an individualized approach, studies of treatment efficacy of auditory training with high internal validity are difficult to construct. Carney and Moeller159 noted that earlier efficacy studies target specific treatment areas of perceptual skill development, language development, speech production, academic performance, and social-emotional growth. Treatment goals within various treatment areas often vary, limiting the ability to assess the impact within any single category.
Prior studies have systematically assessed the development of speech perception with training in implant listeners. Watson160 noted that studies of the perception of complex nonspeech sounds have shown that individual parts of spectral-temporal waveforms can become more salient through selective training. One consequence of training is that the same sound can be perceived quite differently, depending on earlier experience as it affects a listener’s expectancy and the psychologic mind-set brought to the task. The time course of learning to identify initially unfamiliar speech sounds by normal-hearing listeners extends through months, possibly even years, before reaching a stable level of performance. If implant users are required to learn to interpret sounds as unfamiliar to them as the nonspeech sounds used in research are to normal-hearing listeners, a similar lengthy experience may be required to reach a plateau of maximum performance. Why this has not been the case in some clinical studies is a puzzle.
Watson160 offered an explanation in that speech perception may depend heavily on abilities other than sensory acuity or tonal resolution. Studies of individual differences in auditory temporal and spectral acuity have not shown strong correlations between individual differences in those abilities and individual differences in speech perception. It is argued that one way to interpret this observation is that differences in the ability to hear speech (as by two hearing-impaired listeners with the same audiogram) may be the result of differences in the way in which central pathways generate the ability to infer an original stimulus from its fragments.
Dawson and Clark161 tested whether the ability to use place-coded vowel formant information would be subject to training effects in a group of congenitally deafened patients with limited speech perception ability. They investigated the relationship between electrode position difference limens and vowel recognition. Young subjects were assessed with synthesized versions of the words “hid,” “head,” “had,” “hud,” “hod,” and “hood.” Performance during a nontraining period was compared with the change in performance after 10 training sessions. Two of four subjects showed significant gains, and improvements were consistent with their electrode discrimination ability. Difference limens ranged from one to three electrodes for these patients, and for two other patients who showed minimal to no improvements. Minimal gains shown by one subject could be partly explained by poorer apical electrode position difference limen. The authors concluded that significant gains in vowel perception occurred after training, and suggested the need for children to continue prolonged aural rehabilitation for a period after implantation. Despite intensive efforts, minimal improvements can sometimes occur, however, and such limited success is not always accounted for by limitations at the level of the auditory periphery.
Svirsky and coworkers162 examined two possible reasons underlying longitudinal improvements in vowel identification by cochlear implant users: improved labeling of vowel sounds and improved electrode discrimination. Improvements in vowel identification were attributed to improved labeling, suggesting cortical learning effects were responsible for the observed changes, as opposed to enhanced electrode discrimination.
Rehabilitation in Adults with Cochlear Implants
After implant activation, adult users must adjust to the novel signals transmitted by the speech processor. Recipients must learn to associate electrically elicited sound patterns with perceptions that were previously meaningful sound sensations. Rehabilitative needs differ in implant recipients depending on their auditory experience before the onset of deafness. For a prelinguistically deafened implant recipient, auditory and speech (articulation) training are crucial in facilitating communication change. For a postlinguistically deafened patient, auditory training often focuses on more complex listening skills—understanding speech in noise, telephone use, and music appreciation.163
Auditory Training in Children with Cochlear Implants
Programs of auditory training in children with implants are often organized with a hierarchic approach by which the child learns to associate meaning with unfamiliar and possibly unnatural sounds.122,164 After showing awareness of sounds (detection), the child is taught to determine whether sounds are the same or different (discrimination), to recognize sounds and to associate meaning with them (identification), and to respond appropriately in verbal discourse (comprehension). Expressively, the child learns to imitate phonemes and syllables and to coarticulate these sounds to produce progressively more difficult words and phrases. Imitation or approximation of speech sounds and words is a strategy used for developing an “auditory feedback loop”—the interrelated function of perception of sound and production of speech. Functional use of spoken language depends on the child’s ability to produce meaningful linguistic utterances spontaneously. This process begins with a single word and progresses through sequential stages to complex phrases and entire stories.165 Boothroyd166 stressed the need for flexibility in guidelines for therapy sessions, noting that “the structure needs to exist in the mind of the teacher, and not be so obvious in the activities done with the child.”
Boothroyd and colleagues167 provided a model of auditory development that highlights the time course of development and identifies component skills that are crucial to effective auditory perception in children. They defined auditory perception as the interpretation of sensory evidence that is derived from sound and is reflective of objects and events that caused the sound. Similar to other perceptual events, auditory perception involves not only sensory evidence, but also contextual evidence, prior knowledge, memory, attention, and processing skills. Auditory speech perception is special because the events to be perceived are those of language. Similarly, the listener’s knowledge base and processing skills must include those related to language in general and to spoken language in particular.
Boothroyd and colleagues167 noted that it is tempting to assume that the sensory evidence available to a developing child is determined only by the functional integrity of the peripheral auditory system, independent of auditory experience. Basic science investigations document the influence of auditory experience on the organization of auditory pathways,168 however, and observe that better organization through experience could increase the sensory evidence made available from patterns of neural excitation produced in the cochlea.
Current cochlear implant technology also provides the potential for deaf children to make use of generalization and incidental learning. Benefits of “hearing” extend well beyond contexts of one-on-one repartees with predictable discourse. Age of onset, degree of hearing loss, and amplification history47 determine the quantity and quality of auditory stimulation received before implantation and readiness to interpret acoustic information. A child experienced in using residual hearing from an early age through amplification is likely to adapt to the sounds provided by the implant more quickly than a child with limited experience in listening.167 The close scrutiny afforded by regular interaction with an auditory training therapist allows for the early detection of either the need for device reprogramming or failure of components of the implant system.
Components of auditory training in children with cochlear implants are designed to foster the development of auditory and speech skills in a manner that simulates a hearing child’s acquisition of spoken language, while addressing remedial needs that arise as a consequence of auditory deprivation during early development.169 Rehabilitative strategies related to auditory, speech, and language skills should seek to achieve overall communicative competence.50 Ideally, rehabilitative strategies do not teach specific, isolated auditory, speech, or language subskills. Because subskills are only a means to an end that is multimodal communicative competence, training in any one individual subskill should not be given too much weight. Programs are most effective when they take an integrated approach to rehabilitation and avoid rote, drill-oriented approaches.
Maintenance of the Device
An important role in developing auditory skills and using a cochlear implant successfully is maintenance of the device itself.155,156 This maintenance includes keeping all external parts in good functioning order, and working with an audiologist who specializes in cochlear implants on a regularly scheduled basis for device programming. In most cochlear implant centers, the audiologist serves as the case manager for the patient during the cochlear implant candidacy process and postimplantation.
If the patient is unable to participate in the judgments needed to create a cochlear implant map, some objective measures may be used. Cochlear implant manufacturers have created software that can measure an electrically evoked compound action potential through the cochlear implant: Neural Response Telemetry (NRT), by the Cochlear Corporation of Englewood, Colorado (manufacturer of the N24 device), and Neural Response Imaging (NRI), by the Advanced Bionics Corporation of Sylmar, California (manufacturer of the Clarion/Hi-Res devices). NRT measures are found at levels between threshold and maximal comfort in an individual’s map. Limitations exist when using these measurements.170,171 Although direct nerve responses seem to have value in some populations, behavioral testing is the tried and true method of programming.
Another objective measurement that has been previously used in device programming is electric acoustic reflex thresholds. Studies have found that the electric acoustic reflex threshold highly correlates with comfort levels on a cochlear implant map.172,173 These measurements are not present in all patients, however, so this option may not always be available. If the reflex is measurable, this can provide valuable information for the audiologist when programming a child. Some children have high tolerance levels for the electric stimulus and do not react negatively if the map is created using inappropriately high levels. If the cochlear implant program is too loud, it can prevent the child from developing auditory skills or responding appropriately.
Educating Children with Cochlear Implants
The options for educational placement today are the following:
These situations are not mutually exclusive, but overlap to a large degree, with wide variations in practice, particularly in degree of support. This overlap makes comparisons about educational independence in varying educational settings complex, and comparing the effectiveness of differing educational settings complex. Francis and colleagues135 produced a useful matrix of educational resource use, illustrating the continuum of support; a child in a mainstream school with full-time support in class may have less educational independence than a child in a class of 10 in a special school.
Advent of Cochlear Implantation
The advent of cochlear implantation was hoped to ameliorate the educational challenges produced by profound deafness.80 In providing useful hearing across the speech frequencies, cochlear implantation facilitates the development of early communication skills and spoken language through interaction with care providers. Early reports of encouraging levels of spoken language perception and production led many authors to predict that mainstream education would be the likelihood for most profoundly deaf children. The success of cochlear implantation has often been measured in terms of access to mainstream education, not least because it has been seen as a means of measuring cost-benefit from cochlear implantation.135,154 Children with implants are attending mainstream schools in greater numbers after implantation,174 and this has been thought to be associated with potential savings in educational costs.154
What are the needs of children with implants, and are they different from the needs of users of traditional hearing aids? Children with implants need the implant system working well and worn consistently, good listening conditions, and good communication opportunities. These needs are not so different from the needs of hearing aid wearers. What cochlear implants have done is enable profoundly deaf children to function as moderately deaf—and many with a unilateral loss, despite the trend to bilateral implantation. Cochlear implants provide useful hearing to enable profoundly deaf children to acquire language through hearing,169 to hear all the grammatical features of speech, and to develop wholly intelligible speech. Cochlear implants require complex technology, which requires a surgical procedure and long-term maintenance. How to ensure that children with implants obtain the maximum benefit from this technology remains a challenge.
It has long been recognized that the long-term management of deaf children with implants is in the hands of educators, and there is a major need for educators responsible for the children on a daily basis to have training and regular updates on this complex technology and the expectations they can have. In a survey of parents, community-based implant professionals, and implant professionals in Europe,175 the most common thread in the responses was the need for long-term support in education for the implant system and for educational services to become more flexible in meeting the needs of this growing group of children. Parents expressed great frustration that their children did not receive the support they needed particularly as they went to secondary or high school, and frustration that there were not greater links between the implant centers and the local educational services. A survey of European teachers of the deaf showed that they were highly interested in receiving information and training about cochlear implants.176
To be successful in mainstream education, it must be ensured that the classroom situation is appropriate, with good acoustics, and that the technology is successfully managed, with high expectations of its use. We know the educational implications of a unilateral or moderate hearing loss in the busy mainstream classroom environment177,178; children would mishear or misunderstand in noise and not follow the fast-moving discourse around the classroom. For some children, implants may work too well: their speech intelligibility may be such that they appear as normally hearing children, and their needs may be overlooked, and for the children themselves it is difficult to articulate their needs. In such situations, misunderstandings would continue, and children would be unlikely to realize their academic potential. For other children, the implant may not have provided the outcomes expected; the range of outcomes from implantation is large. For these children, an auditory processing disorder or a language learning difficulty may be present, which affects the development of spoken language. The educational needs of these children may not be met in mainstream provision, as had been predicted.
Educational Outcomes
With regard to these educational decisions that parents make for their children, there is evidence that cochlear implantation has had an influence. More children with cochlear implants are going to mainstream schools than to schools for the deaf compared with a group of profoundly deaf children with hearing aids of the same age.174 These were children 5 to 7 years old; Thoutenhoofd179 did not find such a trend long-term toward mainstream placement, and there is anecdotal evidence that children with implants are findin g challenges in managing in the secondary or high school environment. Geers and colleagues180 also found a trend toward mainstream education, but again this was in children at the primary stage.
With regard to communication mode and choices of communication after implantation, there is varying evidence of the influence of oral and signing environments. On the whole, the trend is to support the use of oral input,180 but the context in which this is best provided in the long-term is still debated, and the situation is complex. Archbold and colleagues181 showed that the outcomes in terms of speech perception and production 3 years after implantation were not different in children who had begun with some form of signed input and changed to oral, and children who had used oral communication only. Further study showed that children implanted before age 3 change from using signed communication to oral communication,182 and children implanted at a younger age change communication mode faster.68 There is evidence that although young people with implants develop intelligible spoken language, they also value the use of sign language, or signed support.183 If children are to develop spoken language, however, they need to be in an educational environment that values it and promotes its use.
With regard to educational attainments, Stacey and coworkers184 and Thoutenhoofd179 showed that children with cochlear implants had improved educational attainments compared with children with hearing aids. Increasing evidence is showing that children with implants have better reading skills than comparative groups with hearing aids,185 and that children implanted younger are continuing to read at improved rates.186 In providing access to the grammatical features of spoken language through hearing, cochlear implants have enabled profoundly deaf children to have a greater awareness than previously of the phonology of language before coming to the written word. Spencer and Marschark187 stated, “Spoken language development of deaf children may be more possible today than ever before. We are poised on a threshold of what often seem like unlimited possibilities.” This statement reflects the ways in which new hearing technologies, and cochlear implantation in particular, have transformed educational opportunities for deaf children.
Cheng AK, Rubin HR, Powe NR, et al. Cost-utility analysis of the cochlear implant in children. JAMA. 2000;284:850-856.
Francis HW, Buchman CA, Visaya JM, et al. Surgical factors in pediatric cochlear implantation and their early effects on electrode activation and functional outcomes. Otol Neurotol. 2008;29:502-508.
Limb CJ. Cochlear implant-mediated perception of music. Curr Opin Otolaryngol Head Neck Surg. 2006;14:337-340.
Lin FR, Wang NY, Fink NE, et al. Assessing the use of speech and language measures in relation to parental perceptions of development after early cochlear implantation. Otol Neurotol. 2008;29:208-213.
Rubinstein JT. Paediatric cochlear implantation: prosthetic hearing and language development. Lancet. 2002;360:483-485.
Wilson B, Dorman M. Cochlear implants: a remarkable past and a brilliant future. Hearing Res. 2008;242:3-21.
1. Kirk KI. Challenges in the clinical investigation of cochlear implant outcomes. In: Niparko JK, editor. Cochlear Implants: Principles and Practices. Philadelphia: Lippincott Williams & Wilkins; 2000:225-259.
1a. Kirk KI, Pisoni DB, Miyamoto RC. Effects of stimulus variability on speech perception in listeners with hearing impairment. J Speech Lang Hear Res. 1997;40:1395-1405.
2. Nilsson M, Soli SD, Sullivan JA. Development of the Hearing in Noise Test for the measurement of speech reception thresholds in quiet and in noise. J Acoust Soc Am. 1994;95:1085-1099.
3. Lehiste I, Peterson GE. Linguistic considerations in the study of speech intelligibility. J Acoust Soc Am. 1959;31:280-286.
4. Rubinstein JT. Paediatric cochlear implantation: prosthetic hearing and language development. Lancet. 2002;360:483-485.
5. Peterson GE, Lehiste I. Revised CNC lists for auditory tests. J Speech Hear Disord. 1962;27:62-70.
6. Gantz BJ, Tyler RS, Knutson JF, et al. Evaluation of five different cochlear implant designs: audiologic assessment and predictors of performance. Laryngoscope. 1988;98:1100-1106.
7. Cohen NL, Waltzman SB, Fisher SG. A prospective, randomized study of cochlear implants. The Department of Veterans Affairs Cochlear Implant Study Group. N Engl J Med. 1993;328:233-237.
8. Cohen NL, Waltzman SB. Influence of processing strategies on cochlear implant performance. Ann Otol Rhinol Laryngol Suppl. 1995;165:9-14.
9. Skinner MW, Fourakis MS, Holden TA, et al. Identification of speech by cochlear implant recipients with the Multipeak (MPEAK) and Spectral Peak (SPEAK) speech coding strategies, I: vowels. Ear Hear. 1996;17:182-197.
10. Skinner MW, Fourakis MS, Holden TA, et al. Identification of speech by cochlear implant recipients with the multipeak (MPEAK) and spectral peak (SPEAK) speech coding strategies, II: consonants. Ear Hear. 1999;20:443-460.
11. Wilson BS, Finley CC, Lawson DT, et al. Better speech recognition with cochlear implants. Nature. 1991;352:236-238.
12. Gantz BJ, Woodworth GG, Knutson JF, et al. Multivariate predictors of audiological success with multichannel cochlear implants. Ann Otol Rhinol Laryngol. 1993;102:909-916.
13. Rubinstein JT, Parkinson WS, Tyler RS, et al. Residual speech recognition and cochlear implant performance: effects of implantation criteria. Am J Otol. 1999;20:445-452.
14. Waltzman SB, Fisher SG, Niparko JK, et al. Predictors of postoperative performance with cochlear implants. Ann Otol Rhinol Laryngol Suppl. 1995;165:15-18.
15. Cohen NL. Cochlear implant soft surgery: fact or fantasy? Otolaryngol Head Neck Surg. 1997;117:214-216.
16. Spahr AJ, Dorman MF, Loiselle LH. Performance of patients using different cochlear implant systems: effects of input dynamic range. Ear Hear. 2007;28:260-275.
17. Friedland DR, Venick HS, Niparko JK. Choice of ear for cochlear implantation: the effect of history and residual hearing on predicted postoperative performance. Otol Neurotol. 2003;24:582-589.
18. Miyamoto RT, Osberger MJ, Robbins AM, et al. Prelingually deafened children’s performance with the nucleus multichannel cochlear implant. Am J Otol. 1993;14:437-445.
19. Summerfield AQ, Marshall DH. Cochlear Implantation in the UK, 1990-1994. London, England: MRC-INR, Her Majesty’s Stationery Office; 1995.
20. Wilson BS. Niparko JK, editor. Cochlear Implants: Principles and Practices. Philadelphia: Lippincott Williams & Wilkins, 2000.
21. Helms J, Muller J, Schon F, et al. Evaluation of performance with the COMBI40 cochlear implant in adults: a multicentric clinical study. ORL J Otorhinolaryngol Relat Spec. 1997;59:23-35.
22. Kiefer J, Muller J, Pfennigdorff T, et al. Speech understanding in quiet and in noise with the CIS speech coding strategy (MED-EL Combi-40) compared to the multipeak and spectral peak strategies (nucleus). ORL J Otorhinolaryngol Relat Spec. 1996;58:127-135.
23. Tyler RS, Parkinson AJ, Wilson BS, et al. Patients utilizing a hearing aid and a cochlear implant: speech perception and localization. Ear Hear. 2002;23:98-105.
24. Gstoettner W, Kiefer J, Baumgartner WD, et al. Hearing preservation in cochlear implantation for electric acoustic stimulation. Acta Otolaryngol. 2004;124:348-352.
25. Gantz BJ, Turner C, Gfeller KE, et al. Preservation of hearing in cochlear implant surgery: advantages of combined electrical and acoustical speech processing. Laryngoscope. 2005;115:796-802.
26. Luetje CM, Thedinger BS, Buckler LR, et al. Hybrid cochlear implantation: clinical results and critical review in 13 cases. Otol Neurotol. 2007;28:473-478.
27. Briggs RJ, Tykocinski M, Xu J, et al. Comparison of round window and cochleostomy approaches with a prototype hearing preservation electrode. Audiol Neurootol. 2006;11(Suppl 1):42-48.
28. Berrettini S, Forli F, Passetti S. Preservation of residual hearing following cochlear implantation: comparison between three surgical techniques. J Laryngol Otol. 2008;122:246-252.
29. Gantz BJ, Turner C, Gfeller KE. Acoustic plus electric speech processing: preliminary results of a multicenter clinical trial of the Iowa/Nucleus Hybrid implant. Audiol Neurootol. 2006;11(Suppl 1):63-68.
30. Reiss LA, Gantz BJ, Turner CW. Cochlear implant speech processor frequency allocations may influence pitch perception. Otol Neurotol. 2008;29:160-167.
31. Fitzgerald MB, Sagi E, Jackson M, et al. Reimplantation of hybrid cochlear implant users with a full-length electrode after loss of residual hearing. Otol Neurotol. 2008;29:168-173.
32. NIH consensus conference. Cochlear implants in adults and children. JAMA. 1995;274:1955-1961.
33. Cheng AK, Grant GD, Niparko JK. Meta-analysis of pediatric cochlear implant literature. Ann Otol Rhinol Laryngol Suppl. 1999;177:124-128.
34. Wang NY, Eisenberg LS, Johnson KC, et al. Tracking development of speech recognition: longitudinal data from hierarchical assessments in the Childhood Development after Cochlear Implantation Study. Otol Neurotol. 2008;29:240-245.
35. Kirk KI, Diefendorf A, Pisoni DB, Robbins AM. Mendel LL, Danhauer JL, editors. Audiologic Evaluation and Management and Speech Perception Assessment. San Diego: Singular Pub Group, 1997.
36. Osberger MJ, Kalberer A, Zimmerman-Phillips S, et al. Speech perception results in children using the Clarion Multi-Strategy Cochlear Implant. Ann Otol Rhinol Laryngol Suppl. 2000;185:75-77.
37. Tucci DL, Telian SA, Zimmerman-Phillips S, et al. Cochlear implantation in patients with cochlear malformations. Arch Otolaryngol Head Neck Surg. 1995;121:833-838.
38. Osberger MJ, Geier L, Zimmerman-Phillips S, et al. Use of a parent-report scale to assess benefit in children given the Clarion cochlear implant. Am J Otol. 1997;18(6 Suppl):S79-S80.
39. House WF, Berliner KI, Eisenberg LS. Experiences with the cochlear implant in preschool children. Ann Otol Rhinol Laryngol. 1983;92:587-592.
40. Miyamoto RT, Osberger MJ, Todd SL, et al. Variables affecting implant performance in children. Laryngoscope. 1994;104:1120-1124.
41. Fryauf-Bertschy H, Tyler RS, Kelsay DM, et al. Performance over time of congenitally deaf and postlingually deafened children using a multichannel cochlear implant. J Speech Hear Res. 1992;35:913-920.
42. Gantz BJ, Tyler RS, Woodworth GG, et al. Results of multichannel cochlear implants in congenital and acquired prelingual deafness in children: five-year follow-up. Am J Otol. 1994;15(Suppl 2):1-7.
43. Waltzman SB, Cohen NL, Gomolin RH, et al. Long-term results of early cochlear implantation in congenitally and prelingually deafened children. Am J Otol. 1994;15(Suppl 2):9-13.
44. Miyamoto RT, Kirk KI, Todd SL, et al. Speech perception skills of children with multichannel cochlear implants or hearing aids. Ann Otol Rhinol Laryngol Suppl. 1995;166:334-337.
45. Boothroyd A. Hearing aids, cochlear implants, and profoundly deaf children. In: Owens E, Kessler DK, editors. Cochlear Implants in Young Deaf Children. Boston: College-Hill Press; 1989:81-100.
46. Miyamoto RT, Osberger MJ, Robbins AM, et al. Comparison of speech perception abilities in deaf children with hearing aids or cochlear implants. Otolaryngol Head Neck Surg. 1991;104:42-46.
47. Osberger MJ, Miyamoto RT, Zimmerman-Phillips S, et al. Independent evaluation of the speech perception abilities of children with the Nucleus 22-channel cochlear implant system. Ear Hear. 1991;12:66S-80S.
48. Osberger MJ, Maso M, Sam LK. Speech intelligibility of children with cochlear implants, tactile aids, or hearing aids. J Speech Hear Res. 1993;36:186-203.
49. Tobey EA, Hasenstab S. Effects of a Nucleus multichannel cochlear implant upon speech production in children. Ear Hear. 1991;12:48S-54S.
50. Tye-Murray N. Cochlear Implants and Children: A Handbook for Parents, Teachers and Speech and Hearing Professionals. Washington, DC: Alexander Graham Bell Publishing; 1994.
51. Waltzman SB, Cohen NL. Cochlear implantation in children younger than 2 years old. Am J Otol. 1998;19:158-162.
52. Fryauf-Bertschy H, Tyler RS, Kelsay DM, et al. Cochlear implant use by prelingually deafened children: the influences of age at implant and length of device use. J Speech Lang Hear Res. 1997;40:183-199.
53. O’Donoghue GM, Nikolopoulos TP, Archbold SM. Determinants of speech perception in children after cochlear implantation. Lancet. 2000;356:466-468.
54. Shea JJ3rd, Domico EH, Orchik DJ. Speech recognition ability as a function of duration of deafness in multichannel cochlear implant patients. Laryngoscope. 1990;100:223-226.
55. Geers AE, Nicholas J, Tye-Murray N, et al. Effects of communication mode on skills of long-term cochlear implant users. Ann Otol Rhinol Laryngol Suppl. 2000;185:89-92.
56. Meyer TA, Svirsky MA, Kirk KI, et al. Improvements in speech perception by children with profound prelingual hearing loss: effects of device, communication mode, and chronological age. J Speech Lang Hear Res. 1998;41:846-858.
57. Vieu A, Mondain M, Blanchard K, et al. Influence of communication mode on speech intelligibility and syntactic structure of sentences in profoundly hearing impaired French children implanted between 5 and 9 years of age. Int J Pediatr Otorhinolaryngol. 1998;44:15-22.
58. Brackett D, Zara CV. Communication outcomes related to early implantation. Am J Otol. 1998;19:453-460.
59. Osberger MJ, Zimmerman-Phillips S, Koch DB. Cochlear implant candidacy and performance trends in children. Ann Otol Rhinol Laryngol Suppl. 2002;189:62-65.
60. Gantz BJ, Rubinstein JT, Tyler RS, et al. Long-term results of cochlear implants in children with residual hearing. Ann Otol Rhinol Laryngol Suppl. 2000;185:33-36.
61. Geers AE, Moog JS. Predicting spoken language acquisition of profoundly hearing-impaired children. J Speech Hear Disord. 1987;52:84-94.
62. Hammes DM, Novak MA, Rotz LA, et al. Early identification and cochlear implantation: critical factors for spoken language development. Ann Otol Rhinol Laryngol Suppl. 2002;189:74-78.
63. Miyamoto RT, Houston DM, Kirk KI, et al. Language development in deaf infants following cochlear implantation. Acta Otolaryngol. 2003;123:241-244.
64. Svirsky MA, Teoh SW, Neuburger H. Development of language and speech perception in congenitally, profoundly deaf children as a function of age at cochlear implantation. Audiol Neurootol. 2004;9:224-233.
65. Barton GR, Stacey PC, Fortnum HM, et al. Hearing-impaired children in the United Kingdom, II: cochlear implantation and the cost of compulsory education. Ear Hear. 2006;27:187-207.
66. James AL, Papsin BC. Cochlear implant surgery at 12 months of age or younger. Laryngoscope. 2004;114:2191-2195.
67. Waltzman SB, Roland JTJr. Cochlear implantation in children younger than 12 months. Pediatrics. 2005;116:e487-e493.
68. Tait ME, Nikolopoulos TP, Lutman ME. Age at implantation and development of vocal and auditory preverbal skills in implanted deaf children. Int J Pediatr Otorhinolaryngol. 2007;71:603-610.
69. Connor CM, Craig HK, Raudenbush SW, et al. The age at which young deaf children receive cochlear implants and their vocabulary and speech-production growth: is there an added value for early implantation? Ear Hear. 2006;27:628-644.
70. Dettman SJ, Pinder D, Briggs RJ, et al. Communication development in children who receive the cochlear implant younger than 12 months: risks versus benefits. Ear Hear. 2007;28:11S-18S.
71. Lesinski-Schiedat A, Illg A, Heermann R, et al. Paediatric cochlear implantation in the first and in the second year of life: a comparative study. Cochlear Implants Int. 2004;5:146-159.
72. Nicholas JG, Geers AE. Effects of early auditory experience on the spoken language of deaf children at 3 years of age. Ear Hear. 2006;27:286-298.
73. Stevens SS, Newman EB. The localization of actual sources of sound. Am J Psychol. 1936;48:297-306.
74. Middlebrooks JC, Green DM. Sound localization by human listeners. Annu Rev Psychol. 1991;42:135-159.
75. Middlebrooks JC, Makous JC, Green DM. Directional sensitivity of sound-pressure levels in the human ear canal. J Acoust Soc Am. 1989;86:89-108.
76. Giolas TG, Wark DJ. Communication problems associated with unilateral hearing loss. J Speech Hear Disord. 1967;32:336-343.
77. Oyler RF, Oyler A, Matkin ND. Unilateral hearing loss: demographics and educational impact. Lang Speech Hear Serv Schools. 1988;19:201-210.
78. Quigley SP, Thomure RE. Some Effects of Hearing Impairment Upon School Performance. Urbana, IL: Institute for Child Behavior and Development; 1968.
79. Robbins AM, Bollard PM, Green J. Language development in children implanted with the CLARION cochlear implant. Ann Otol Rhinol Laryngol Suppl. 1999;177:113-118.
80. Spencer LJ, Barker BA, Tomblin JB. Exploring the language and literacy outcomes of pediatric cochlear implant users. Ear Hear. 2003;24:236-247.
81. Ryugo DK, Kretzmer EA, Niparko JK. Restoration of auditory nerve synapses in cats by cochlear implants. Science. 2005;310:1490-1492.
82. Kuhn-Inacker H, Shehata-Dieler W, Muller J, et al. Bilateral cochlear implants: a way to optimize auditory perception abilities in deaf children? Int J Pediatr Otorhinolaryngol. 2004;68:1257-1266.
83. Laszig R, Aschendorff A, Stecker M, et al. Benefits of bilateral electrical stimulation with the nucleus cochlear implant in adults: 6-month postoperative results. Otol Neurotol. 2004;25:958-968.
84. Litovsky RY, Parkinson A, Arcaroli J, et al. Bilateral cochlear implants in adults and children. Arch Otolaryngol Head Neck Surg. 2004;130:648-655.
85. Litovsky RY, Johnstone PM, Godar S, et al. Bilateral cochlear implants in children: localization acuity measured with minimum audible angle. Ear Hear. 2006;27:43-59.
86. Litovsky RY, Johnstone PM, Godar SP. Benefits of bilateral cochlear implants and/or hearing aids in children. Int J Audiol. 2006;45:S78-S91.
87. Muller J, Schon F, Helms J. Speech understanding in quiet and noise in bilateral users of the MED-EL COMBI 40/40+ cochlear implant system. Ear Hear. 2002;23:198-206.
88. Peters BR, Litovsky R, Parkinson A, et al. Importance of age and postimplantation experience on speech perception measures in children with sequential bilateral cochlear implants. Otol Neurotol. 2007;28:649-657.
89. Ramsden R, Greenham P, O’Driscoll M, et al. Evaluation of bilaterally implanted adult subjects with the nucleus 24 cochlear implant system. Otol Neurotol. 2005;26:988-998.
90. Schleich P, Nopp P, D’Haese P. Head shadow, squelch, and summation effects in bilateral users of the MED-EL COMBI 40/40+ cochlear implant. Ear Hear. 2004;25:197-204.
91. Tyler RS, Gantz BJ, Rubinstein JT, et al. Three-month results with bilateral cochlear implants. Ear Hear. 2002;23:80S-89S.
92. Murphy J, O’Donoghue G. Bilateral cochlear implantation: an evidence-based medicine evaluation. Laryngoscope. 2007;117:1412-1418.
93. Jackendoff R. Parallel constraint-based generative theories of language. Trends Cogn Sci. 1999;3:393-400.
94. Plaut D, Kello C. The emergence of phonology from the interplay of speech comprehension and production: a distributed connectionist approach. In: MacWhinney B, editor. The Emergence of Language. Carnegie Mellon Symposia on Cognition. Mahwah, NJ: Lawrence Erlbaum Associates; 1999:381-415.
95. Kuhl PK, Williams KA, Lacerda F, et al. Linguistic experience alters phonetic perception in infants by 6 months of age. Science. 1992;255:606-608.
96. Meadow-Orlans K. Effects of mother and infant hearing status on interactions at twelve and eighteen months. J Deaf Stud Deaf Educ. 1997;2:27-36.
97. Pressman L, Pipp-Siegel S, Yoshinaga-Itano C, et al. Maternal sensitivity predicts language gain in preschool children who are deaf and hard of hearing. J Deaf Stud Deaf Educ. 1999;4:294-304.
98. DeCasper AJ, Fifer WP. Of human bonding: newborns prefer their mothers’ voices. Science. 1980;208:1174-1176.
99. Bloom L. Language acquisition in its developmental context. In: Damon W, editor. Handbook of Child Psychology. New York: J. Wiley; 1998:309-370.
100. Marschark M. Origins and interactions in the social, cognitive, and language development of deaf children. In: Marschark M, Clark MD, editors. Psychological Perspectives on Deafness. Hillsdale, NJ: LEA; 1993:7-26.
101. Quittner AL. Coping with a hearing impaired child: a model of adjustment to chronic stress. In: Johnson JH, Johnson SB, editors. Advances in Child Health Psychology. Gainesville: University of Florida Press; 1991:206-223.
102. Stinson MS, Lang HG. Full inclusion: a path for integration or isolation? Am Ann Deaf. 1994;139:156-159.
103. Gregory S, Hindley P. Annotation: communication strategies for deaf children. J Child Psychol Psychiatry. 1996;37:895-905.
104. Meadow-Orlans K. Maternal sensitivity and the visual attentiveness of children who are deaf. Early Dev Parent. 1996;5:213-223.
105. Prendergast SG, McCollum JA. Let’s talk: the effect of maternal hearing status on interactions with toddlers who are deaf. Am Ann Deaf. 1996;141:11-18.
106. Kluwin TN, Stinson MS. Deaf Students in Local Public High Schools: Backgrounds, Experiences, and Outcomes. Springfield, IL: Charles C Thomas; 1993.
107. Vaccari C, Marschark M. Communication between parents and deaf children: implications for social-emotional development. J Child Psychol Psychiatry. 1997;38:793-801.
108. Thelen E, Smith LB. A Dynamic Systems Approach to the Development of Cognition and Action. Cambridge, MA: MIT Press/Bradford Books Series in Cognitive Psychology, MIT Press; 1994.
109. Smith LB, Katz DB. Activity-dependent processes in perceptual and cognitive development. In: Gelman R, Au TK, editors. Handbook of Perception and Cognition. Vol 13, Perceptual and Cognitive Development. San Francisco: Academic Press; 1996:413-445.
110. Bahrick LE, Walker AS, Neisser U. Selective looking by infants. Cognit Psychol. 1981;13:377-390.
111. Butterworth G. The origins of auditory-visual perception and visual proprioception in human infancy. In: Walk RD, Pick HL, editors. Intersensory Perception and Sensory Integrational Development. New York: Plenum Press; 1981:37-70.
112. Morongiello B. Effects of colocation on auditory-visual interactions and cross-modal perception in infants. In: Lewkowicz DJ, Lickliter R, editors. The Development of Intersensory Perception: Comparative Perspectives. Hillsdale, NJ: Lawrence Erlbaum; 1994:235-263.
113. Roberts K, Jacobs M. Linguistic vs. attentional influences on nonlinguistic categorization in 15-month old infants. Cognit Dev. 1991;6:355-375.
114. Roberts K. Categorical responding in 15-month olds: influence of the noun-category bias and the covariation between visual fixation and auditory input. Cognit Dev. 1995;10:21-41.
115. Kuhl PK, Meitzoff A. Speech as an intermodal object of perception. In: Yonas A, editor. Perceptual Development in Infancy. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988:235-266.
116. Walker-Andrews A, Lennon E. Infants’ discrimination of vocal expressions: contributions of auditory and visual information. Infant Behav Dev. 1991;14:131-142.
117. Smith LB, Quittner AL, Osberger MJ, et al. Audition and visual attention: the developmental trajectory in deaf and hearing populations. Dev Psychol. 1998;34:840-850.
118. Swisher M. Learning to converse: how deaf mothers support the development of attention and conversational skills in their young deaf children. In: Meadow-Orlans KP, Spencer PE, Erting C, Marschark M, editors. The Deaf Child in the Family and at School: Essays in Honor of Kathryn P. Meadow-Orlans. Mahwah, NJ: Lawrence Erlbaum Associates; 2000:21-40.
119. Quittner AL, Smith LB, Osberger MJ, Mitchell T, Katz D. The impact of audition on the development of visual attention. Psychol Sci. 1994;5:347-353.
120. Mitchell T, Quittner AL. A multimethod study of attention and behavior problems in hearing-impaired children. J Clin Child Psychol. 1996;25:83-96.
121. Tait M, Lutman ME. Comparison of early communicative behavior in young children with cochlear implants and with hearing aids. Ear Hear. 1994;15:352-361.
122. Schopmeyer B, Mellon N, Dobaj H, et al. Use of Fast ForWord to enhance language development in children with cochlear implants. Ann Otol Rhinol Laryngol Suppl. 2000;185:95-98.
123. Schopmeyer B, Mellon N. Language acquisition. In: Niparko JK, editor. Cochlear Implants: Principles and Practices. Philadelphia: Lippincott Williams & Wilkins; 2000:291-314.
124. Tait M, Lutman ME, Nikolopoulos TP. Communication development in young deaf children: review of the video analysis method. Int J Pediatr Otorhinolaryngol. 2001;61:105-112.
125. Robbins AM, Svirsky M, Kirk KI. Children with implants can speak, but can they communicate? Otolaryngol Head Neck Surg. 1997;117:155-160.
126. Svirsky MA, Robbins AM, Kirk KI, et al. Language development in profoundly deaf children with cochlear implants. Psychol Sci. 2000;11:153-158.
127. Development of language and communication skills in hearing-impaired children. ASHA Monogr. 1987;26:1-158.
128. Osberger MJ. Language and learning skills of hearing-impaired students. ASHA Monogr. 1986;23:3-107.
129. Pisoni DB, Cleary M, Geers A, Tobey E. Individual differences in effectiveness of cochlear implants in children who are prelingually deaf: new process measures of performance. Volta Rev. 2000;101:111-164.
130. Pisoni DB, Geers AE. Working memory in deaf children with cochlear implants: correlations between digit span and measures of spoken language processing. Ann Otol Rhinol Laryngol Suppl. 2000;185:92-93.
131. Fink NE, Wang NY, Visaya J, et al. Childhood Development after Cochlear Implantation (CDaCI) study: design and baseline characteristics. Cochlear Implants Int. 2007;8:92-116.
132. Holt JA. Stanford achievement test, ed. 8. Reading comprehension subgroup results. Am Ann Deaf. 1996;138:172-175.
133. Trybus RJ, Karchmer MA. School achievement scores of hearing impaired children: national data on achievement status and growth patterns. Am Ann Deaf. 1977;122:62-69.
134. Geers AE, Moog JS. Evaluating the benefits of cochlear implants in an education setting. Am J Otol. 1991;12(Suppl):116-125.
135. Francis HW, Koch ME, Wyatt JR, et al. Trends in educational placement and cost-benefit considerations in children with cochlear implants. Arch Otolaryngol Head Neck Surg. 1999;125:499-505.
136. Koch ME, Wyatt JR, Francis HW, et al. A model of educational resource use by children with cochlear implants. Otolaryngol Head Neck Surg. 1997;117:174-179.
137. Cheng AK, Rubin HR, Powe NR, et al. Cost-utility analysis of the cochlear implant in children. JAMA. 2000;284:850-856.
138. Wyatt JR, Niparko JK, Rothman M, et al. Cost utility of the multichannel cochlear implants in 258 profoundly deaf individuals. Laryngoscope. 1996;106:816-821.
139. Summerfield AQ, Marshall DH, Archbold S. Cost-effectiveness considerations in pediatric cochlear implantation. Am J Otol. 1997;18(6 Suppl):S166-S168.
140. Kind P, Gudex C. The role of QALYs in assessing priorities between health-care interventions. In: Drummond MF, Maynard A, Wells NE, editors. Purchasing and Providing Cost-Effective Health Care. Edinburgh: Churchill Livingstone; 1993:94-108.
141. Azimi NA, Welch HG. The effectiveness of cost-effectiveness analysis in containing costs. J Gen Intern Med. 1998;13:664-669.
142. Campbell VA, Crews JE, Moriarty DG, et al. Surveillance for sensory impairment, activity limitation, and health-related quality of life among older adults—United States 1993-1997. MMWR CDC Surveill Summ. 1999;48:131-156.
143. Havlik R. Aging in the eighties: impaired senses for sound and light in persons age 65 years and over. Preliminary data from the supplement on aging to the National Health Interview Survey: United States, January-June 1984. Hyattsville, MD: National Center for Health Statistics, U.S. Department of Health and Human Services; 1986.
144. Weinstein BE, Ventry IM. Hearing impairment and social isolation in the elderly. J Speech Hear Res. 1982;25:593-599.
145. Mulrow CD, Aguilar C, Endicott JE, et al. Association between hearing impairment and the quality of life of elderly individuals. J Am Geriatr Soc. 1990;38:45-50.
146. Ng M, Niparko JK, Nager GT. Inner ear pathology in severe to profound sensorineural hearing loss. In: Niparko JK, editor. Cochlear Implants: Principles and Practices. Philadelphia: Lippincott Williams & Wilkins; 2000:57-87.
147. Otte J, Schunknecht HF, Kerr AG. Ganglion cell populations in normal and pathological human cochleae: implications for cochlear implantation. Laryngoscope. 1978;88:1231-1246.
148. Stach BA, Spretnjak ML, Jerger J. The prevalence of central presbycusis in a clinical population. J Am Acad Audiol. 1990;1:109-115.
149. Welch TM, Church MW, Shucard DW. A method for chronically recording brain-stem and cortical auditory evoked potentials from unanesthetized mice. Electroencephalogr Clin Neurophysiol. 1985;60:78-83.
150. Kelsall DC, Shallop JK, Burnelli T. Cochlear implantation in the elderly. Am J Otol. 1995;16:609-615.
151. Francis HW, Chee N, Yeagle J, et al. Impact of cochlear implants on the functional health status of older adults. Laryngoscope. 2002;112:1482-1488.
152. Horn KL, McMahon NB, McMahon DC, et al. Functional use of the Nucleus 22-channel cochlear implant in the elderly. Laryngoscope. 1991;101:284-288.
153. Thomas JS. Cochlear implantation of the elderly. Ann Otol Rhinol Laryngol Suppl. 1995;166:91-93.
154. O’Neill C, O’Donoghue GM, Archbold SM, et al. A cost-utility analysis of pediatric cochlear implantation. Laryngoscope. 2000;110:156-160.
155. Lea A, Hailey D. The cochlear implant: a technology for the profoundly deaf. Med Prog Technol. 1995;21:47-52.
156. Lea A. Cochlear implants. Health Technology Series, Australian Institute of Health. 6:1991.
157. Hutton J, Politi C, Seeger T. Cost-effectiveness of cochlear implantation of children: a preliminary model for the UK. Adv Otorhinolaryngol. 1995;50:201-206.
158. Carter R, Hailey D. Economic evaluation of the cochlear implant. Int J Technol Assess Health Care. 1999;15:520-530.
159. Carney AE, Moeller MP. Treatment efficacy: hearing loss in children. J Speech Lang Hear Res. 1998;41:S61-S84.
160. Watson CS. Auditory perceptual learning and the cochlear implant. Am J Otol. 1991;12(Suppl):73-79.
161. Dawson PW, Clark GM. Changes in synthetic and natural vowel perception after specific training for congenitally deafened patients using a multichannel cochlear implant. Ear Hear. 1997;18:488-501.
162. Svirsky MA, Silveira A, Suarez H, et al. Auditory learning and adaptation after cochlear implantation: a preliminary study of discrimination and labeling of vowel sounds by cochlear implant users. Acta Otolaryngol. 2001;121:262-265.
163. Edgerton B. Rehabilitation and training of postlingually deaf adult cochlear implant patients. Semin Hear. 1985;6:65-89.
164. Erber NP. Auditory Training. Washington, DC: Alexander Graham Bell Association for the Deaf; 1982.
165. Rice ML, Wexler K, Redmond SM. Grammaticality judgements of an extended optional infinitive grammar: evidence from English-speaking children with specific language impairment. J Speech Lang Hear Res. 1999;42:943-961.
166. Boothroyd A. Auditory development of the hearing child. Scand Audiol Suppl. 1997;46:9-16.
167. Boothroyd A, Geers AE, Moog JS. Practical implications of cochlear implants in children. Ear Hear. 1991;12(4 Suppl):81S-89S.
168. Limb CJ, Ryugo DK. Development of primary axosomatic endings in the anteroventral cochlear nucleus of mice. J Assoc Res Otolaryngol. 2000;1:103-119.
169. Robbins AM. Rehabilitation after cochlear implantation. In: Niparko JK, editor. Cochlear Implants: Principles and Practices. Philadelphia: Lippincott Williams & Wilkins; 2000:323-363.
170. Gordon KA, Ebinger KA, Gilden JE, et al. Neural response telemetry in 12- to 24-month-old children. Ann Otol Rhinol Laryngol Suppl. 2002;189:42-48.
171. Seyle K, Brown CJ. Speech perception using maps based on neural response telemetry measures. Ear Hear. 2002;23:72S-79S.
172. Spivak LG, Chute PM, Popp AL, et al. Programming the cochlear implant based on electrical acoustic reflex thresholds: patient performance. Laryngoscope. 1994;104:1225-1230.
173. Hodges AV, Butts S, Dolan-Ash S, et al. Using electrically evoked auditory reflex thresholds to fit the CLARION cochlear implant. Ann Otol Rhinol Laryngol Suppl. 1999;177:64-68.
174. Archbold SM, Nikolopoulos TP, Lutman ME, et al. The educational settings of profoundly deaf children with cochlear implants compared with age-matched peers with hearing aids: implications for management. Int J Audiol. 2002;41:157-161.
175. Archbold S. Children and adults with cochlear implants: what do they need and what do they get? Presented at BCIG Academic Conference, London, April 2007.
176. Archbold S, O’Donoghue G. Ensuring the long-term use of cochlear implants in children: the importance of engaging local resources and expertise. Ear Hear. 2007;28:3S-6S.
177. Bess FH. The unilaterally hearing-impaired child: a final comment. Ear Hear. 1986;7:52-54.
178. Most T. The effects of degree and type of hearing loss on children’s performance in class. Deaf Educ Int. 2004;6:154-166.
179. Thoutenhoofd E. Cochlear implanted pupils in Scottish schools: 4-year school attainment data (2000-2004). J Deaf Stud Deaf Educ. 2006;11:171-188.
180. Geers AE, Nicholas JG, Sedey AL. Language skills of children with early cochlear implantation. Ear Hear. 2003;24:46S-58S.
181. Archbold S, Nikolopoulos TP, Tait M, et al. Approach to communication, speech perception, and intelligibility after paediatric cochlear implantation. Br J Audiol. 2000;34:257-264.
182. Watson LM, Archbold SM, Nikolopoulos TP. Children’s communication mode five years after cochlear implantation: changes over time according to age at implant. Cochlear Implants Int. 2006;7:77-91.
183. Wheeler A, Archbold S, Gregory S, et al. Cochlear implants: the young people’s perspective. J Deaf Stud Deaf Educ. 2007;12:303-316.
184. Stacey PC, Fortnum HM, Barton GR, et al. Hearing-impaired children in the United Kingdom, I: auditory performance, communication skills, educational achievements, quality of life, and cochlear implantation. Ear Hear. 2006;27:161-186.
185. Vermeulen AM, van Bon W, Schreuder R, et al. Reading comprehension of deaf children with cochlear implants. J Deaf Stud Deaf Educ. 2007;12:283-302.
186. Geers AE. Factors influencing spoken language outcomes in children following early cochlear implantation. Adv Otorhinolaryngol. 2006;64:50-65.
187. Spencer PE, Marschark M. Advances in the Spoken Language Development of Deaf and Hard-of-Hearing Children. Oxford: Perspectives on Deafness, Oxford University Press; 2006.