A. Excessive anxiety and worry (apprehensive expectation), occurring more days than not for at least 6 months, about a number of events or activities (such as work or school performance).
B. The individual finds it difficult to control the worry.
C. The anxiety and worry are associated with three (or more) of the following six symptoms (with at least some symptoms present for more days than not for the past 6 months): (1) restlessness or feeling keyed up or on edge; (2) being easily fatigued; (3) difficulty concentrating or mind going blank; (4) irritability; (5) muscle tension; (6) sleep disturbance (difficulty falling or staying asleep, or restless, unsatisfying sleep).
D. The anxiety, worry, or physical symptoms cause clinically significant distress or impairment in social, occupational, or other important areas of functioning.
E. The disturbance is not attributable to the physiologic effects of a substance (e.g., a drug of abuse, a medication) or another medical condition (e.g., hyperthyroidism).
F. The disturbance is not better explained by another mental disorder (e.g., anxiety or worry about having panic attacks in panic disorder, negative evaluation in social anxiety disorder [social phobia], contamination or other obsessions in obsessive-compulsive disorder, separation from attachment figures in separation anxiety disorder, reminders of traumatic events in posttraumatic stress disorder, gaining weight in anorexia nervosa, physical complaints in somatic symptom disorder, perceived appearance flaws in body dysmorphic disorder, having a serious illness in illness anxiety disorder, or the content of delusional beliefs in schizophrenia or delusional disorder).
Source: Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC, American Psychiatric Association, 2013.
Etiology and Pathophysiology All anxiogenic agents act on the γ-aminobutyric acid (GABA)A receptor/chloride ion channel complex, implicating this neurotransmitter system in the pathogenesis of anxiety and panic attacks. Benzodiazepines are thought to bind two separate GABAA receptor sites: type I, which has a broad neuroanatomic distribution, and type II, which is concentrated in the hippocampus, striatum, and neocortex. The antianxiety effects of the various benzodiazepines are influenced by their relative binding to alpha 2 and 3 subunits of the GABAA receptor, and sedation and memory impairment to the alpha 1 subunit, Serotonin (5-hydroxytryptamine [5-HT]) and 3α-reduced neuroactive steroids (allosteric modulators of GABAA) also appear to have a role in anxiety, and buspirone, a partial 5-HT1A receptor agonist, and certain 5-HT2A and 5-HT2C receptor antagonists (e.g., nefazodone) may have beneficial effects.
PHOBIC DISORDERS
Clinical Manifestations The cardinal feature of phobic disorders is a marked and persistent fear of objects or situations, exposure to which results in an immediate anxiety reaction. The patient avoids the phobic stimulus, and this avoidance usually impairs occupational or social functioning. Panic attacks may be triggered by the phobic stimulus or may occur spontaneously. Unlike patients with other anxiety disorders, individuals with phobias usually experience anxiety only in specific situations. Common phobias include fear of closed spaces (claustrophobia), fear of blood, and fear of flying. Social phobia is distinguished by a specific fear of social or performance situations in which the individual is exposed to unfamiliar individuals or to possible examination and evaluation by others. Examples include having to converse at a party, use public restrooms, and meet strangers. In each case, the affected individual is aware that the experienced fear is excessive and unreasonable given the circumstance. The specific content of a phobia may vary across gender, ethnic, and cultural boundaries.
Phobic disorders are common, affecting ~7–9% of the population. Twice as many females are affected than males. Full criteria for diagnosis are usually satisfied first in early adulthood, but behavioral avoidance of unfamiliar people, situations, or objects dating from early childhood is common.
In one study of female twins, concordance rates for agoraphobia, social phobia, and animal phobia were found to be 23% for monozygotic twins and 15% for dizygotic twins. A twin study of fear conditioning, a model for the acquisition of phobias, demonstrated a heritability of 35–45%. Animal studies of fear conditioning have indicated that processing of the fear stimulus occurs through the lateral nucleus of the amygdala, extending through the central nucleus and projecting to the periaqueductal gray region, lateral hypothalamus, and paraventricular hypothalamus.
STRESS DISORDERS
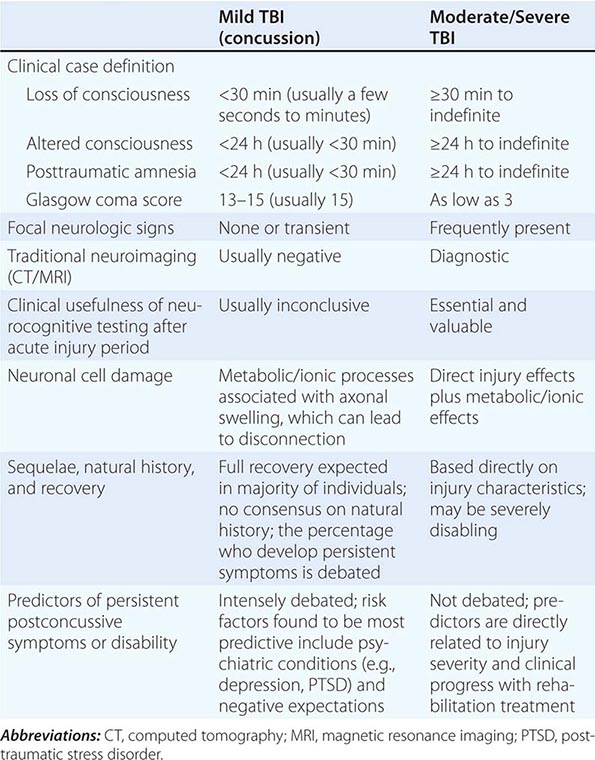
Clinical Manifestations Patients may develop anxiety after exposure to extreme traumatic events such as the threat of personal death or injury or the death of a loved one. The reaction may occur shortly after the trauma (acute stress disorder) or be delayed and subject to recurrence (PTSD) (Table 466-6). In both syndromes, individuals experience associated symptoms of detachment and loss of emotional responsivity. The patient may feel depersonalized and unable to recall specific aspects of the trauma, although typically it is reexperienced through intrusions in thought, dreams, or flashbacks, particularly when cues of the original event are present. Patients often actively avoid stimuli that precipitate recollections of the trauma and demonstrate a resulting increase in vigilance, arousal, and startle response. Patients with stress disorders are at risk for the development of other disorders related to anxiety, mood, and substance abuse (especially alcohol). Between 5 and 10% of Americans will at some time in their life satisfy criteria for PTSD, with women more likely to be affected than men.
|
DIAGNOSTIC CRITERIA FOR POSTTRAUMATIC STRESS DISORDER |
Source: Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC, American Psychiatric Association, 2013.
Risk factors for the development of PTSD include a past psychiatric history and personality characteristics of high neuroticism and extroversion. Twin studies show a substantial genetic influence on all symptoms associated with PTSD, with less evidence for an environmental effect.
Etiology and Pathophysiology It is hypothesized that in PTSD there is excessive release of norepinephrine from the locus coeruleus in response to stress and increased noradrenergic activity at projection sites in the hippocampus and amygdala. These changes theoretically facilitate the encoding of fear-based memories. Greater sympathetic responses to cues associated with the traumatic event occur in PTSD, although pituitary adrenal responses are blunted.
OBSESSIVE-COMPULSIVE DISORDER
Clinical Manifestations Obsessive-compulsive disorder (OCD) is characterized by obsessive thoughts and compulsive behaviors that impair everyday functioning. Fears of contamination and germs are common, as are handwashing, counting behaviors, and having to check and recheck such actions as whether a door is locked. The degree to which the disorder is disruptive for the individual varies, but in all cases, obsessive-compulsive activities take up >1 h per day and are undertaken to relieve the anxiety triggered by the core fear. Patients often conceal their symptoms, usually because they are embarrassed by the content of their thoughts or the nature of their actions. Physicians must ask specific questions regarding recurrent thoughts and behaviors, particularly if physical clues such as chafed and reddened hands or patchy hair loss (from repetitive hair pulling, or trichotillomania) are present. Comorbid conditions are common, the most frequent being depression, other anxiety disorders, eating disorders, and tics. OCD has a lifetime prevalence of 2–3% worldwide. Onset is usually gradual, beginning in early adulthood, but childhood onset is not rare. The disorder usually has a waxing and waning course, but some cases may show a steady deterioration in psychosocial functioning.
Etiology and Pathophysiology A genetic contribution to OCD is suggested by twin studies, but no susceptibility gene for OCD has been identified to date. Family studies show an aggregation of OCD with Tourette’s disorder, and both are more common in males and in first-born children.
The anatomy of obsessive-compulsive behavior is thought to include the orbital frontal cortex, caudate nucleus, and globus pallidus. The caudate nucleus appears to be involved in the acquisition and maintenance of habit and skill learning, and interventions that are successful in reducing obsessive-compulsive behaviors also decrease metabolic activity measured in the caudate.
MOOD DISORDERS
Mood disorders are characterized by a disturbance in the regulation of mood, behavior, and affect. Mood disorders are subdivided into (1) depressive disorders, (2) bipolar disorders, and (3) depression in association with medical illness or alcohol and substance abuse (Chaps. 467 through 471e). Major depressive disorder (MDD) is differentiated from bipolar disorder by the absence of a manic or hypomanic episode. The relationship between pure depressive syndromes and bipolar disorders is not well understood; MDD is more frequent in families of bipolar individuals, but the reverse is not true. In the Global Burden of Disease Study conducted by the World Health Organization, unipolar major depression ranked fourth among all diseases in terms of disability-adjusted life-years and was projected to rank second by the year 2020. In the United States, lost productivity directly related to mood disorders has been estimated at $55.1 billion per year.
DEPRESSION IN ASSOCIATION WITH MEDICAL ILLNESS
Depression occurring in the context of medical illness is difficult to evaluate. Depressive symptomatology may reflect the psychological stress of coping with the disease, may be caused by the disease process itself or by the medications used to treat it, or may simply coexist in time with the medical diagnosis.
Virtually every class of medication includes some agent that can induce depression. Antihypertensive drugs, anticholesterolemic agents, and antiarrhythmic agents are common triggers of depressive symptoms. Iatrogenic depression should also be considered in patients receiving glucocorticoids, antimicrobials, systemic analgesics, antiparkinsonian medications, and anticonvulsants. To decide whether a causal relationship exists between pharmacologic therapy and a patient’s change in mood, it may sometimes be necessary to undertake an empirical trial of an alternative medication.
Between 20 and 30% of cardiac patients manifest a depressive disorder; an even higher percentage experience depressive symptomatology when self-reporting scales are used. Depressive symptoms following unstable angina, myocardial infarction, cardiac bypass surgery, or heart transplant impair rehabilitation and are associated with higher rates of mortality and medical morbidity. Depressed patients often show decreased variability in heart rate (an index of reduced parasympathetic nervous system activity), which may predispose individuals to ventricular arrhythmia and increased morbidity. Depression also appears to increase the risk of developing coronary heart disease, possibly through increased platelet aggregation. TCAs are contraindicated in patients with bundle branch block, and TCA-induced tachycardia is an additional concern in patients with congestive heart failure. SSRIs appear not to induce ECG changes or adverse cardiac events and thus are reasonable first-line drugs for patients at risk for TCA-related complications. SSRIs may interfere with hepatic metabolism of anticoagulants, however, causing increased anticoagulation.
In patients with cancer, the mean prevalence of depression is 25%, but depression occurs in 40–50% of patients with cancers of the pancreas or oropharynx. This association is not due to the effect of cachexia alone, as the higher prevalence of depression in patients with pancreatic cancer persists when compared to those with advanced gastric cancer. Initiation of antidepressant medication in cancer patients has been shown to improve quality of life as well as mood. Psychotherapeutic approaches, particularly group therapy, may have some effect on short-term depression, anxiety, and pain symptoms.
Depression occurs frequently in patients with neurologic disorders, particularly cerebrovascular disorders, Parkinson’s disease, dementia, multiple sclerosis, and traumatic brain injury. One in five patients with left-hemisphere stroke involving the dorsolateral frontal cortex experiences major depression. Late-onset depression in otherwise cognitively normal individuals increases the risk of a subsequent diagnosis of Alzheimer’s disease. All classes of antidepressant agents are effective against these depressions, as are, in some cases, stimulant compounds.
The reported prevalence of depression in patients with diabetes mellitus varies from 8 to 27%, with the severity of the mood state correlating with the level of hyperglycemia and the presence of diabetic complications. Treatment of depression may be complicated by effects of antidepressive agents on glycemic control. MAOIs can induce hypoglycemia and weight gain, whereas TCAs can produce hyperglycemia and carbohydrate craving. SSRIs and SNRIs, like MAOIs, may reduce fasting plasma glucose, but they are easier to use and may also improve dietary and medication compliance.
Hypothyroidism is frequently associated with features of depression, most commonly depressed mood and memory impairment. Hyperthyroid states may also present in a similar fashion, usually in geriatric populations. Improvement in mood usually follows normalization of thyroid function, but adjunctive antidepressant medication is sometimes required. Patients with subclinical hypothyroidism can also experience symptoms of depression and cognitive difficulty that respond to thyroid replacement.
The lifetime prevalence of depression in HIV-positive individuals has been estimated at 22–45%. The relationship between depression and disease progression is multifactorial and likely to involve psychological and social factors, alterations in immune function, and central nervous system (CNS) disease. Chronic hepatitis C infection is also associated with depression, which may worsen with interferon-α treatment.
Some chronic disorders of uncertain etiology, such as chronic fatigue syndrome (Chap. 464e) and fibromyalgia (Chap. 396), are strongly associated with depression and anxiety; patients may benefit from antidepressant treatment or anticonvulsant agents such as pregabalin.
DEPRESSIVE DISORDERS
Clinical Manifestations Major depression is defined as depressed mood on a daily basis for a minimum duration of 2 weeks (Table 466-7). An episode may be characterized by sadness, indifference, apathy, or irritability and is usually associated with changes in sleep patterns, appetite, and weight; motor agitation or retardation; fatigue; impaired concentration and decision making; feelings of shame or guilt; and thoughts of death or dying. Patients with depression have a profound loss of pleasure in all enjoyable activities, exhibit early morning awakening, feel that the dysphoric mood state is qualitatively different from sadness, and often notice a diurnal variation in mood (worse in morning hours). Patients experiencing bereavement or grief may exhibit many of the same signs and symptoms of major depression, although the emphasis is usually on feelings of emptiness and loss, rather than anhedonia and loss of self-esteem, and the duration is usually limited. In certain cases, however, the diagnosis of major depression may be warranted even in the context of a significant loss.
|
CRITERIA FOR A MAJOR DEPRESSIVE EPISODE |
Source: Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC, American Psychiatric Association, 2013.
Approximately 15% of the population experiences a major depressive episode at some point in life, and 6–8% of all outpatients in primary care settings satisfy diagnostic criteria for the disorder. Depression is often undiagnosed, and even more frequently, it is treated inadequately. If a physician suspects the presence of a major depressive episode, the initial task is to determine whether it represents unipolar or bipolar depression or is one of the 10–15% of cases that are secondary to general medical illness or substance abuse. Physicians should also assess the risk of suicide by direct questioning, as patients are often reluctant to verbalize such thoughts without prompting. If specific plans are uncovered or if significant risk factors exist (e.g., a past history of suicide attempts, profound hopelessness, concurrent medical illness, substance abuse, or social isolation), the patient must be referred to a mental health specialist for immediate care. The physician should specifically probe each of these areas in an empathic and hopeful manner, being sensitive to denial and possible minimization of distress. The presence of anxiety, panic, or agitation significantly increases near-term suicidal risk. Approximately 4–5% of all depressed patients will commit suicide; most will have sought help from physicians within 1 month of their deaths.
In some depressed patients, the mood disorder does not appear to be episodic and is not clearly associated with either psychosocial dysfunction or change from the individual’s usual experience in life. Persistent depressive disorder (dysthymic disorder) consists of a pattern of chronic (at least 2 years), ongoing depressive symptoms that are usually less severe and/or less numerous than those found in major depression, but the functional consequences may be equivalent to or even greater; the two conditions are sometimes difficult to separate and can occur together (“double depression”). Many patients who exhibit a profile of pessimism, disinterest, and low self-esteem respond to antidepressant treatment. Persistent and chronic depressive disorders occur in approximately 2% of the general population.
Depression is approximately twice as common in women as in men, and the incidence increases with age in both sexes. Twin studies indicate that the liability to major depression of early onset (before age 25) is largely genetic in origin. Negative life events can precipitate and contribute to depression, but genetic factors influence the sensitivity of individuals to these stressful events. In most cases, both biologic and psychosocial factors are involved in the precipitation and unfolding of depressive episodes. The most potent stressors appear to involve death of a relative, assault, or severe marital or relationship problems.
Unipolar depressive disorders usually begin in early adulthood and recur episodically over the course of a lifetime. The best predictor of future risk is the number of past episodes; 50–60% of patients who have a first episode have at least one or two recurrences. Some patients experience multiple episodes that become more severe and frequent over time. The duration of an untreated episode varies greatly, ranging from a few months to ≥1 year. The pattern of recurrence and clinical progression in a developing episode are also variable. Within an individual, the nature of episodes (e.g., specific presenting symptoms, frequency and duration) may be similar over time. In a minority of patients, a severe depressive episode may progress to a psychotic state; in elderly patients, depressive symptoms may be associated with cognitive deficits mimicking dementia (“pseudodementia”). A seasonal pattern of depression, called seasonal affective disorder, may manifest with onset and remission of episodes at predictable times of the year. This disorder is more common in women, whose symptoms are anergy, fatigue, weight gain, hypersomnia, and episodic carbohydrate craving. The prevalence increases with distance from the equator, and improvement may occur by altering light exposure.
Etiology and Pathophysiology Although evidence for genetic transmission of unipolar depression is not as strong as in bipolar disorder, monozygotic twins have a higher concordance rate (46%) than dizygotic siblings (20%), with little support for any effect of a shared family environment.
Neuroendocrine abnormalities that reflect the neurovegetative signs and symptoms of depression include: (1) increased cortisol and corticotropin-releasing hormone (CRH) secretion, (2) an increase in adrenal size, (3) a decreased inhibitory response of glucocorticoids to dexamethasone, and (4) a blunted response of thyroid-stimulating hormone (TSH) level to infusion of thyroid-releasing hormone (TRH). Antidepressant treatment leads to normalization of these abnormalities. Major depression is also associated with changes in levels of proinflammatory cytokines and neurotrophins.
Diurnal variations in symptom severity and alterations in circadian rhythmicity of a number of neurochemical and neurohumoral factors suggest that biologic differences may be secondary to a primary defect in regulation of biologic rhythms. Patients with major depression show consistent findings of a decrease in rapid eye movement (REM) sleep onset (REM latency), an increase in REM density, and, in some subjects, a decrease in stage IV delta slow-wave sleep.
Although antidepressant drugs inhibit neurotransmitter uptake within hours, their therapeutic effects typically emerge over several weeks, implicating adaptive changes in second messenger systems and transcription factors as possible mechanisms of action.
The pathogenesis of depression is discussed in detail in Chap. 465e.
BIPOLAR DISORDER
Clinical Manifestations Bipolar disorder is characterized by unpredictable swings in mood from mania (or hypomania) to depression. Some patients suffer only from recurrent attacks of mania, which in its pure form is associated with increased psychomotor activity; excessive social extroversion; decreased need for sleep; impulsivity and impairment in judgment; and expansive, grandiose, and sometimes irritable mood (Table 466-8). In severe mania, patients may experience delusions and paranoid thinking indistinguishable from schizophrenia. One-half of patients with bipolar disorder present with a mixture of psychomotor agitation and activation with dysphoria, anxiety, and irritability. It may be difficult to distinguish mixed mania from agitated depression. In some bipolar patients (bipolar II disorder), the full criteria for mania are lacking, and the requisite recurrent depressions are separated by periods of mild activation and increased energy (hypomania). In cyclothymic disorder, there are numerous hypomanic periods, usually of relatively short duration, alternating with clusters of depressive symptoms that fail, either in severity or duration, to meet the criteria of major depression. The mood fluctuations are chronic and should be present for at least 2 years before the diagnosis is made.
|
CRITERIA FOR A MANIC EPISODE |
Source: Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC, American Psychiatric Association, 2013.
Manic episodes typically emerge over a period of days to weeks, but onset within hours is possible, usually in the early morning hours. An untreated episode of either depression or mania can be as short as several weeks or last as long as 8–12 months, and rare patients have an unremitting chronic course. The term rapid cycling is used for patients who have four or more episodes of either depression or mania in a given year. This pattern occurs in 15% of all patients, almost all of whom are women. In some cases, rapid cycling is linked to an underlying thyroid dysfunction, and in others, it is iatrogenically triggered by prolonged antidepressant treatment. Approximately one-half of patients have sustained difficulties in work performance and psychosocial functioning, with depressive phases being more responsible for impairment than mania.
Bipolar disorder is common, affecting ~1.5% of the population in the United States. Onset is typically between 20 and 30 years of age, but many individuals report premorbid symptoms in late childhood or early adolescence. The prevalence is similar for men and women; women are likely to have more depressive and men more manic episodes over a lifetime.
Differential Diagnosis The differential diagnosis of mania includes secondary mania induced by stimulant or sympathomimetic drugs, hyperthyroidism, AIDS, and neurologic disorders such as Huntington’s or Wilson’s disease and cerebrovascular accidents. Comorbidity with alcohol and substance abuse is common, either because of poor judgment and increased impulsivity or because of an attempt to self-treat the underlying mood symptoms and sleep disturbances.
Etiology and Pathophysiology Genetic predisposition to bipolar disorder is evident from family studies; the concordance rate for monozygotic twins approaches 80%. Patients with bipolar disorder also appear to have altered circadian rhythmicity, and lithium may exert its therapeutic benefit through a resynchronization of intrinsic rhythms keyed to the light/dark cycle.
SOMATIC SYMPTOM DISORDER
Many patients presenting in general medical practice, perhaps as many as 5–7%, will experience a somatic symptom(s) as particularly distressing and preoccupying, to the point that it comes to dominate their thoughts, feelings, and beliefs and interferes to a varying degree with everyday functioning. Although the absence of a medical explanation for these complaints was historically emphasized as a diagnostic element, it has been recognized that the patient’s interpretation and elaboration of the experience is the critical defining factor and that patients with well-established medical causation may qualify for the diagnosis. Multiple complaints are typical, but severe single symptoms can occur as well. Comorbidity with depressive and anxiety disorders is common and may affect the severity of the experience and its functional consequences. Personality factors may be a significant risk factor, as may a low level of educational or socioeconomic status or a history of recent stressful life events. Cultural factors are relevant as well and should be incorporated into the evaluation. Individuals who have persistent preoccupations about having or acquiring a serious illness, but who do not have a specific somatic complaint, may qualify for a related diagnosis—illness anxiety disorder. The diagnosis of conversion disorder (functional neurologic symptom disorder) is used to specifically identify those individuals whose somatic complaints involve one or more symptoms of altered voluntary motor or sensory function that cannot be medically explained and that causes significant distress or impairment or requires medical evaluation.
In factitious illnesses, the patient consciously and voluntarily produces physical symptoms of illness. The term Munchausen’s syndrome is reserved for individuals with particularly dramatic, chronic, or severe factitious illness. In true factitious illness, the sick role itself is gratifying. A variety of signs, symptoms, and diseases have been either simulated or caused by factitious behavior, the most common including chronic diarrhea, fever of unknown origin, intestinal bleeding or hematuria, seizures, and hypoglycemia. Factitious disorder is usually not diagnosed until 5–10 years after its onset, and it can produce significant social and medical costs. In malingering, the fabrication derives from a desire for some external reward such as a narcotic medication or disability reimbursement.
FEEDING AND EATING DISORDERS
CLINICAL MANIFESTATIONS
Feeding and eating disorders constitute a group of conditions in which there is a persistent disturbance of eating or associated behaviors that significantly impair an individual’s physical health or psychosocial functioning. In DSM-5 the described categories (with the exception of pica) are defined to be mutually exclusive in a given episode, based on the understanding that although they are phenotypically similar in some ways, they differ in course, prognosis, and effective treatment interventions. Compared with DSM-IV-TR, three disorders (i.e., avoidant/restrictive food intake disorder, rumination disorder, pica) that were previously classified as disorders of infancy or childhood have been grouped together with the disorders of anorexia and bulimia nervosa. Binge-eating disorder is also now included as a formal diagnosis; the intent of each of these modifications is to encourage clinicians to be more specific in their codification of eating and feeding pathology.
PICA
Pica is diagnosed when the individual, over age 2, eats one or more nonnutritive, nonfood substances for a month or more and requires medical attention as a result. There is usually no specific aversion to food in general but a preferential choice to ingest substances such as clay, starch, soap, paper, or ash. The diagnosis requires the exclusion of specific culturally approved practices and has not been commonly found to be caused by a specific nutritional deficiency. Onset is most common in childhood but the disorder can occur in association with other major psychiatric conditions in adults. An association with pregnancy has been observed, but the condition is only diagnosed when medical risks are increased by the behavior.
RUMINATION DISORDER
In this condition, individuals who have no demonstrable associated gastrointestinal or other medical condition repeatedly regurgitate their food after eating and then either rechew or swallow it or spit it out. The behavior typically occurs on a daily basis and must persist for at least 1 month. Weight loss and malnutrition are common sequelae, and individuals may attempt to conceal their behavior, either by covering their mouth or through social avoidance while eating. In infancy, the onset is typically between 3 to 12 months, and the behavior may remit spontaneously, although in some it appears to be recurrent.
AVOIDANT/RESTRICTIVE FOOD INTAKE DISORDER
The cardinal feature of this disorder is avoidance or restriction of food intake, usually stemming from a lack of interest in or distaste of food and associated with weight loss, nutritional deficiency, dependency on nutritional supplementation, or marked impairment in psychosocial functioning, either alone or in combination. Culturally approved practices, such as fasting, or a lack of available food must be excluded as possible causes. The disorder is distinguished from anorexia nervosa by the presence of emotional factors, such as a fear of gaining weight and distortion of body image in the latter condition. Onset is usually in infancy or early childhood, but avoidant behaviors may persist into adulthood. The disorder is equally prevalent in males and females and is frequently comorbid with anxiety and cognitive and attention-deficit disorders and situations of familial stress. Developmental delay and functional deficits may be significant if the disorder is long-standing and unrecognized.
ANOREXIA NERVOSA
Individuals are diagnosed with anorexia nervosa if they restrict their caloric intake to a degree that their body weight deviates significantly from age, gender, health, and developmental norms and if they also exhibit a fear of gaining weight and an associated disturbance in body image. The condition is further characterized by differentiating those who achieve their weight loss predominantly through restricting intake or by excessive exercise (restricting type) from those who engage in recurrent binge eating and/or subsequent purging, self-induced vomiting, and usage of enemas, laxatives, or diuretics (binge-eating/purging type). Such subtyping is more state than trait specific, as individuals may transition from one profile to the other over time. Determination of whether an individual satisfies the primary criterion of significant low weight is complex and must be individualized, using all available historical information and comparison of body habitus to international body mass norms and guidelines.
Individuals with anorexia nervosa frequently lack insight into their condition and are in denial about possible medical consequences; they often are not comforted by their achieved weight loss and persist in their behaviors despite having met previously self-designated weight goals. Recent research has identified alterations in the circuitry of reward sensitivity and executive function in anorexia and implicated disturbances in frontal cortex and anterior insula regulation of interoceptive awareness of satiety and hunger. Neurochemical findings, including the role of ghrelin, remain controversial.
Onset is most common in adolescence, although onset in later life can occur. Many more females than males are affected, with a lifetime prevalence in women of up to 4%. The disorder appears most prevalent in postindustrialized and urbanized countries and is frequently comorbid with preexisting anxiety disorders. The medical consequences of prolonged anorexia nervosa are multisystemic and can be life-threatening in severe presentations. Changes in blood chemistry include leukopenia with lymphocytosis, elevations in blood urea nitrogen, and metabolic alkalosis and hypokalemia when purging is present. History and physical examination may reveal amenorrhea in females, skin abnormalities (petechiae, lanugo hair, dryness), and signs of hypometabolic function, including hypotension, hypothermia, and sinus bradycardia. Endocrine effects include hypogonadism, growth hormone resistance, and hypercortisolemia. Osteoporosis is a longer-term concern.
The course of the disorder is variable, with some individuals recovering after a single episode, while others exhibit recurrent episodes or a chronic course. Untreated anorexia has a mortality of 5.1/1000, the highest among psychiatric conditions. Maudsley family-based therapy has proven to be an effective therapy in younger individuals, with strict behavioral contingencies used when weight loss becomes critical. No pharmacologic intervention has proven to be specifically beneficial, but comorbid depression and anxiety should be treated. Weight gain should be undertaken gradually with a goal of 0.5 to 1 pound per week to prevent refeeding syndrome. Most individuals are able to achieve remission within 5 years of the original diagnosis.
BULIMIA NERVOSA
Bulimia nervosa describes individuals who engage in recurrent and frequent (at least once a week for 3 months) periods of binge eating and who then resort to compensatory behaviors, such as self-induced purging, enemas, use of laxatives, or excessive exercise to avoid weight gain. Binge eating itself is defined as excessive food intake in a prescribed period of time, usually <2 h. As in anorexia nervosa, disturbances in body image occur and promote the behavior, but unlike in anorexia, individuals are of normal weight or even somewhat overweight. Subjects typically describe a loss of control and express shame about their actions, and often relate that their episodes are triggered by feelings of negative self-esteem or social stresses. The lifetime prevalence in women is approximately 2%, with a 10:1 female-to-male ratio. The disorder typically begins in adolescence and may be persistent over a number of years. Transition to anorexia occurs in only 10–15% of cases. Many of the medical risks associated with bulimia nervosa parallel those of anorexia nervosa and are a direct consequence of purging, including fluid and electrolyte disturbances and conduction abnormalities. Physical examination often results in no specific findings, but dental erosion and parotid gland enlargement may be present. Effective treatment approaches include SSRI antidepressants, usually in combination with cognitive-behavioral, emotion regulation, or interpersonal-based psychotherapies.
BINGE-EATING DISORDER
Binge-eating disorder is distinguished from bulimia nervosa by the absence of compensatory behaviors to prevent weight gain after an episode and by a lack of effort to restrict weight gain between episodes. Other features are similar, including distress over the behavior and the experience of loss of control, resulting in eating more rapidly or in greater amounts than intended or eating when not hungry. The 12-month prevalence in females is 1.6%, with a much lower female-to-male ratio than bulimia nervosa. Little is known about the course of the disorder, given its recent categorization, but its prognosis is markedly better than for other eating disorders, both in terms of its natural course and response to treatment. Transition to other eating disorder conditions is thought to be rare.
PERSONALITY DISORDERS
CLINICAL MANIFESTATIONS
Personality disorders are characteristic patterns of thinking, feeling, and interpersonal behavior that are relatively inflexible and cause significant functional impairment or subjective distress for the individual. The observed behaviors are not secondary to another mental disorder, nor are they precipitated by substance abuse or a general medical condition. This distinction is often difficult to make in clinical practice, because personality change may be the first sign of serious neurologic, endocrine, or other medical illness. Patients with frontal lobe tumors, for example, can present with changes in motivation and personality while the results of the neurologic examination remain within normal limits. Individuals with personality disorders are often regarded as “difficult patients” in clinical medical practice because they are seen as excessively demanding and/or unwilling to follow recommended treatment plans. Although DSM-5 portrays personality disorders as qualitatively distinct categories, there is an alternative perspective that personality characteristics vary as a continuum between normal functioning and formal mental disorder.
Personality disorders have been grouped into three overlapping clusters. Cluster A includes paranoid, schizoid, and schizotypal personality disorders. It includes individuals who are odd and eccentric and who maintain an emotional distance from others. Individuals have a restricted emotional range and remain socially isolated. Patients with schizotypal personality disorder frequently have unusual perceptual experiences and express magical beliefs about the external world. The essential feature of paranoid personality disorder is a pervasive mistrust and suspiciousness of others to an extent that is unjustified by available evidence. Cluster B disorders include antisocial, borderline, histrionic, and narcissistic types and describe individuals whose behavior is impulsive, excessively emotional, and erratic. Cluster C incorporates avoidant, dependent, and obsessive-compulsive personality types; enduring traits are anxiety and fear. The boundaries between cluster types are to some extent artificial, and many patients who meet criteria for one personality disorder also meet criteria for aspects of another. The risk of a comorbid major mental disorder is increased in patients who qualify for a diagnosis of personality disorder.
SCHIZOPHRENIA
CLINICAL MANIFESTATIONS
Schizophrenia is a heterogeneous syndrome characterized by perturbations of language, perception, thinking, social activity, affect, and volition. There are no pathognomonic features. The syndrome commonly begins in late adolescence, has an insidious (and less commonly acute) onset, and, often, a poor outcome, progressing from social withdrawal and perceptual distortions to recurrent delusions and hallucinations. Patients may present with positive symptoms (such as conceptual disorganization, delusions, or hallucinations) or negative symptoms (loss of function, anhedonia, decreased emotional expression, impaired concentration, and diminished social engagement) and must have at least two of these for a 1-month period and continuous signs for at least 6 months to meet formal diagnostic criteria. Disorganized thinking or speech and grossly disorganized motor behavior, including catatonia, may also be present. As individuals age, positive psychotic symptoms tend to attenuate, and some measure of social and occupational function may be regained. “Negative” symptoms predominate in one-third of the schizophrenic population and are associated with a poor long-term outcome and a poor response to drug treatment. However, marked variability in the course and individual character of symptoms is typical.
The term schizophreniform disorder describes patients who meet the symptom requirements but not the duration requirements for schizophrenia, and schizoaffective disorder is used for those who manifest symptoms of schizophrenia and independent periods of mood disturbance. The terms “schizotypal” and “schizoid” refer to specific personality disorders and are discussed in that section. The diagnosis of delusional disorder is used for individuals who have delusions of various content for at least 1 month but who otherwise do not meet criteria for schizophrenia. Patients who experience a sudden onset of a brief (<1 month) alteration in thought processing, characterized by delusions, hallucinations, disorganized speech, or gross motor behavior, are most appropriately designated as having a brief psychotic disorder. Catatonia is recognized as a nonspecific syndrome that can occur as a consequence of other severe psychiatric/medical disorders and is diagnosed by the documentation of three or more of a cluster of motor and behavioral symptoms, including stupor, cataplexy, mutism, waxy flexibility, and stereotypy, among others. Prognosis depends not on symptom severity but on the response to antipsychotic medication. A permanent remission without recurrence does occasionally occur. About 10% of schizophrenic patients commit suicide.
Schizophrenia is present in 0.85% of individuals worldwide, with a lifetime prevalence of ~1–1.5%. An estimated 300,000 episodes of acute schizophrenia occur annually in the United States, resulting in direct and indirect costs of $62.7 billion.
DIFFERENTIAL DIAGNOSIS
The diagnosis is principally one of exclusion, requiring the absence of significant associated mood symptoms, any relevant medical condition, and substance abuse. Drug reactions that cause hallucinations, paranoia, confusion, or bizarre behavior may be dose-related or idiosyncratic; parkinsonian medications, clonidine, quinacrine, and procaine derivatives are the most common prescription medications associated with these symptoms. Drug causes should be ruled out in any case of newly emergent psychosis. The general neurologic examination in patients with schizophrenia is usually normal, but motor rigidity, tremor, and dyskinesias are noted in one-quarter of untreated patients.
EPIDEMIOLOGY AND PATHOPHYSIOLOGY
Epidemiologic surveys identify several risk factors for schizophrenia, including genetic susceptibility, early developmental insults, winter birth, and increasing parental age. Genetic factors are involved in at least a subset of individuals who develop schizophrenia. Schizophrenia is observed in ~6.6% of all first-degree relatives of an affected proband. If both parents are affected, the risk for offspring is 40%. The concordance rate for monozygotic twins is 50%, compared to 10% for dizygotic twins. Schizophrenia-prone families are also at risk for other psychiatric disorders, including schizoaffective disorder and schizotypal and schizoid personality disorders, the latter terms designating individuals who show a lifetime pattern of social and interpersonal deficits characterized by an inability to form close interpersonal relationships, eccentric behavior, and mild perceptual distortions.
ASSESSMENT AND EVALUATION OF VIOLENCE
Primary care physicians may encounter situations in which family, domestic, or societal violence is discovered or suspected. Such an awareness can carry legal and moral obligations; many state laws mandate reporting of child, spousal, and elder abuse. Physicians are frequently the first point of contact for both victim and abuser. Approximately 2 million older Americans and 1.5 million U.S. children are thought to experience some form of physical maltreatment each year. Spousal abuse is thought to be even more prevalent. An interview study of 24,000 women in 10 countries found a lifetime prevalence of physical or sexual violence that ranged from 15 to 71%; these individuals are more likely to suffer from depression, anxiety, and substance abuse and to have attempted suicide. In addition, abused individuals frequently express low self-esteem, vague somatic symptomatology, social isolation, and a passive feeling of loss of control. Although it is essential to treat these elements in the victim, the first obligation is to ensure that the perpetrator has taken responsibility for preventing any further violence. Substance abuse and/or dependence and serious mental illness in the abuser may contribute to the risk of harm and require direct intervention. Depending on the situation, law enforcement agencies, community resources such as support groups and shelters, and individual and family counseling can be appropriate components of a treatment plan. A safety plan should be formulated with the victim, in addition to providing information about abuse, its likelihood of recurrence, and its tendency to increase in severity and frequency. Antianxiety and antidepressant medications may sometimes be useful in treating the acute symptoms, but only if independent evidence for an appropriate psychiatric diagnosis exists.
MENTAL HEALTH PROBLEMS IN THE HOMELESS
There is a high prevalence of mental disorders and substance abuse among homeless and impoverished individuals. Depending on the definition used, estimates of the total number of homeless individuals in the United States range from 800,000 to 2 million, one-third of whom qualify as having a serious mental disorder. Poor hygiene and nutrition, substance abuse, psychiatric illness, physical trauma, and exposure to the elements combine to make the provision of medical care challenging. Only a minority of these individuals receives formal mental health care; the main points of contact are outpatient medical clinics and emergency departments. Primary care settings represent a critical site in which housing needs, treatment of substance dependence, and evaluation and treatment of psychiatric illness can most efficiently take place. Successful intervention is dependent on breaking down traditional administrative barriers to health care and recognizing the physical constraints and emotional costs imposed by homelessness. Simplifying health care instructions and follow-up, allowing frequent visits, and dispensing medications in limited amounts that require ongoing contact are possible techniques for establishing a successful therapeutic relationship.
467 |
Alcohol and Alcoholism |
Alcohol (beverage ethanol) distributes throughout the body, affecting almost all systems and altering nearly every neurochemical process in the brain. This drug is likely to exacerbate most medical problems, affect medications metabolized in the liver, and temporarily mimic many medical (e.g., diabetes) and psychiatric (e.g., depression) conditions. The lifetime risk for repetitive alcohol problems is almost 20% for men and 10% for women, regardless of a person’s education or income. Although low doses of alcohol might have healthful benefits, greater than three standard drinks per day enhances the risk for cancer and vascular disease, and alcohol use disorders decrease the life span by about 10 years. Unfortunately, most clinicians have had only limited training regarding alcohol-related disorders. This chapter presents a brief overview of clinically useful information about alcohol use and problems.
PHARMACOLOGY AND NUTRITIONAL IMPACT OF ETHANOL
Ethanol blood levels are expressed as milligrams or grams of ethanol per deciliter (e.g., 100 mg/dL = 0.10 g/dL), with values of ~0.02 g/dL resulting from the ingestion of one typical drink. In round figures, a standard drink is 10–12 g, as seen in 340 mL (12 oz) of beer, 115 mL (4 oz) of nonfortified wine, and 43 mL (1.5 oz) (a shot) of 80-proof beverage (e.g., whisky); 0.5 L (1 pint) of 80-proof beverage contains ~160 g of ethanol (about 16 standard drinks), and 750 mL of wine contains ~60 g of ethanol. These beverages also have additional components (congeners) that affect the drink’s taste and might contribute to adverse effects on the body. Congeners include methanol, butanol, acetaldehyde, histamine, tannins, iron, and lead. Alcohol acutely decreases neuronal activity and has similar behavioral effects and cross-tolerance with other depressants, including benzodiazepines and barbiturates.
Alcohol is absorbed from mucous membranes of the mouth and esophagus (in small amounts), from the stomach and large bowel (in modest amounts), and from the proximal portion of the small intestine (the major site). The rate of absorption is increased by rapid gastric emptying (as seen with carbonation); by the absence of proteins, fats, or carbohydrates (which interfere with absorption); and by dilution to a modest percentage of ethanol (maximum at ~20% by volume).
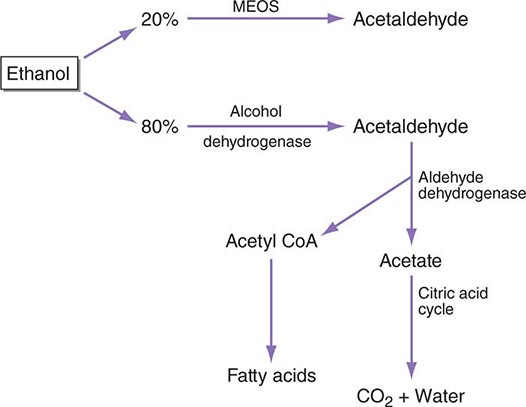
Between 2% (at low blood alcohol concentrations) and 10% (at high blood alcohol concentrations) of ethanol is excreted directly through the lungs, urine, or sweat, but most is metabolized to acetaldehyde, primarily in the liver. The most important pathway occurs in the cell cytosol where alcohol dehydrogenase (ADH) produces acetaldehyde, which is then rapidly destroyed by aldehyde dehydrogenase (ALDH) in the cytosol and mitochondria (Fig. 467-1). A second pathway occurs in the microsomes of the smooth endoplasmic reticulum (the microsomal ethanol-oxidizing system, or MEOS) that is responsible for ≥10% of ethanol oxidation at high blood alcohol concentrations.
FIGURE 467-1 The metabolism of alcohol. CoA, coenzyme A; MEOS, microsomal ethanoloxidizing system.
Although a drink contains ~300 kJ, or 70–100 kcal, these are devoid of minerals, proteins, and vitamins. In addition, alcohol interferes with absorption of vitamins in the small intestine and decreases their storage in the liver with modest effects on folate (folacin or folic acid), pyridoxine (B6), thiamine (B1), nicotinic acid (niacin, B3), and vitamin A.
Heavy drinking in a fasting, healthy individual can produce transient hypoglycemia within 6–36 h, secondary to the acute actions of ethanol on gluconeogenesis. This can result in temporary abnormal glucose tolerance tests (with a resulting erroneous diagnosis of diabetes mellitus) until the alcoholic has abstained for 2–4 weeks. Alcohol ketoacidosis, probably reflecting a decrease in fatty acid oxidation coupled with poor diet or recurrent vomiting, can be misdiagnosed as diabetic ketosis. With the former, patients show an increase in serum ketones along with a mild increase in glucose but a large anion gap, a mild to moderate increase in serum lactate, and a β-hydroxybutyrate/lactate ratio of between 2:1 and 9:1 (with normal being 1:1).
In the brain, alcohol affects almost all neurotransmitter systems, with acute effects that are often the opposite of those seen following desistance after a period of heavy drinking. The most prominent actions relate to boosting γ-aminobutyric acid (GABA) activity, especially at GABAA receptors. Enhancement of this complex chloride channel system contributes to anticonvulsant, sleep-inducing, antianxiety, and muscle relaxation effects of all GABA-boosting drugs. Acutely administered alcohol produces a release of GABA, and continued use increases density of GABAA receptors, whereas alcohol withdrawal states are characterized by decreases in GABA-related activity. Equally important is the ability of acute alcohol to inhibit postsynaptic N-methyl-D-aspartate (NMDA) excitatory glutamate receptors, whereas chronic drinking and desistance are associated with an upregulation of these excitatory receptor subunits. The relationships between greater GABA and diminished NMDA receptor activity during acute intoxication and diminished GABA with enhanced NMDA actions during alcohol withdrawal explain much of intoxication and withdrawal phenomena.
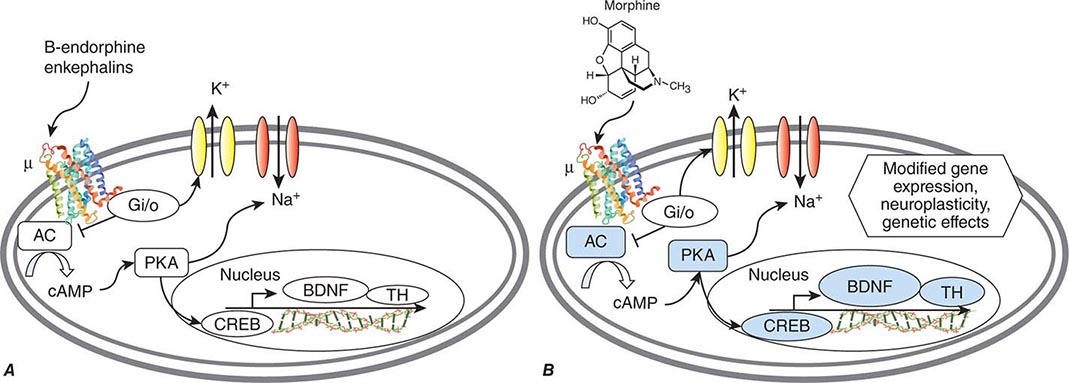
As with all pleasurable activities, alcohol acutely increases dopamine levels in the ventral tegmentum and related brain regions, and this effect plays an important role in continued alcohol use, craving, and relapse. The changes in dopamine pathways are also linked to increases in “stress hormones,” including cortisol and adrenocorticotropic hormone (ACTH) during intoxication and withdrawal. Such alterations are likely to contribute to both feelings of reward during intoxication and depression during falling blood alcohol concentrations. Also closely linked to alterations in dopamine (especially in the nucleus accumbens) are alcohol-induced changes in opioid receptors, with acute alcohol causing release of beta endorphins.
Additional neurochemical changes include increases in synaptic levels of serotonin during acute intoxication and subsequent upregulation of serotonin receptors. Acute increases in nicotinic acetylcholine systems contribute to the impact of alcohol in the ventral tegmental region, which occurs in concert with enhanced dopamine activity. In the same regions, alcohol impacts on cannabinol receptors, with resulting release of dopamine, GABA, and glutamate as well as subsequent effects on brain reward circuits.
BEHAVIORAL EFFECTS, TOLERANCE, AND WITHDRAWAL
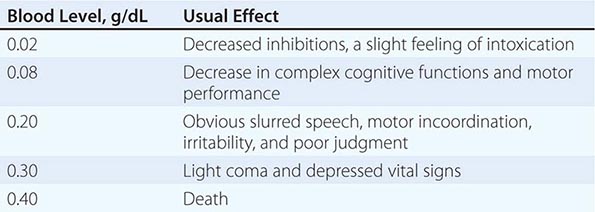
The acute effects of a drug depend on the dose, the rate of increase in plasma, the concomitant presence of other drugs, and past experience with the agent. “Legal intoxication” with alcohol in most states requires a blood alcohol concentration of 0.08 g/dL, but levels of 0.04 are cited in some other countries. However, behavioral, psychomotor, and cognitive changes are seen at 0.02–0.04 g/dL (i.e., after one to two drinks) (Table 467-1). Deep but disturbed sleep can be seen at twice the legal intoxication level, and death can occur with levels between 0.30 and 0.40 g/dL. Beverage alcohol is probably responsible for more overdose deaths than any other drug.
|
EFFECTS OF BLOOD ALCOHOL LEVELS IN THE ABSENCE OF TOLERANCE |
Repeated use of alcohol contributes to acquired tolerance, a phenomenon involving at least three compensatory mechanisms. (1) After 1–2 weeks of daily drinking, metabolic or pharmacokinetic tolerance can be seen, with up to 30% increases in the rate of hepatic ethanol metabolism. This alteration disappears almost as rapidly as it develops. (2) Cellular or pharmacodynamic tolerance develops through neurochemical changes that maintain relatively normal physiologic functioning despite the presence of alcohol. Subsequent decreases in blood levels contribute to symptoms of withdrawal. (3) Individuals learn to adapt their behavior so that they can function better than expected under influence of the drug (learned or behavioral tolerance).
The cellular changes caused by chronic ethanol exposure may not resolve for several weeks or longer following cessation of drinking. Rapid decreases in blood alcohol levels before that time can produce a withdrawal syndrome, which is most intense during the first 5 days, but some symptoms (e.g., disturbed sleep and anxiety) can take up to 4–6 months to resolve.
THE EFFECTS OF ETHANOL ON ORGAN SYSTEMS
Relatively low doses of alcohol (one or two drinks per day) have potential beneficial effects of increasing high-density lipoprotein cholesterol and decreasing aggregation of platelets, with a resulting decrease in risk for occlusive coronary disease and embolic strokes. Red wine has additional potential health-promoting qualities at relatively low doses due to flavinols and related substances. Modest drinking might also decrease the risk for vascular dementia and, possibly, Alzheimer’s disease. However, any potential healthful effects disappear with the regular consumption of three or more drinks per day, and knowledge about the deleterious effects of alcohol can both help the physician to identify patients with an alcohol use disorder and to supply them with information that might help motivate a change in behavior.
NERVOUS SYSTEM
Approximately 35% of drinkers (and a much higher proportion of alcoholics) experience a blackout, an episode of temporary anterograde amnesia, in which the person forgets all or part of what occurred during a drinking evening. Another common problem, one seen after as few as one or two drinks shortly before bedtime, is disturbed sleep. Although alcohol might initially help a person fall asleep, it disrupts sleep throughout the rest of the night. The stages of sleep are altered, and time spent in rapid eye movement (REM) and deep sleep is reduced. Alcohol relaxes muscles in the pharynx, which can cause snoring and exacerbate sleep apnea; symptoms of the latter occur in 75% of alcoholic men older than age 60 years. Patients may also experience prominent and sometimes disturbing dreams later in the night. All of these sleep problems are more pronounced in alcoholics, and their persistence may contribute to relapse.
Another common consequence of alcohol use is impaired judgment and coordination, increasing the risk of injury. In the United States, ~40% of drinkers have at some time driven while intoxicated. Heavy drinking can also be associated with headache, thirst, nausea, vomiting, and fatigue the following day, a hangover syndrome that is responsible for much missed time and temporary cognitive deficits at work and school.
Chronic high doses cause peripheral neuropathy in ~10% of alcoholics: similar to diabetes, patients experience bilateral limb numbness, tingling, and paresthesias, all of which are more pronounced distally. Approximately 1% of alcoholics develop cerebellar degeneration or atrophy, producing a syndrome of progressive unsteady stance and gait often accompanied by mild nystagmus; neuroimaging studies reveal atrophy of the cerebellar vermis. Fortunately, very few alcoholics (perhaps as few as 1 in 500 for the full syndrome) develop Wernicke’s (ophthalmoparesis, ataxia, and encephalopathy) and Korsakoff’s (retrograde and anterograde amnesia) syndromes, although a higher proportion have one or more neuropathologic findings related to these conditions. These result from low levels of thiamine, especially in predisposed individuals with transketolase deficiencies. Alcoholics can manifest cognitive problems and temporary memory impairment lasting for weeks to months after drinking heavily for days or weeks. Brain atrophy, evident as ventricular enlargement and widened cortical sulci on magnetic resonance imaging (MRI) and computed tomography (CT) scans, occurs in ~50% of chronic alcoholics; these changes are usually reversible if abstinence is maintained. There is no single alcoholic dementia syndrome; rather, this label describes patients who have irreversible cognitive changes (possibly from diverse causes) in the context of chronic alcoholism.
Psychiatric Comorbidity As many as two-thirds of individuals with alcohol use disorders meet the criteria for another psychiatric syndrome in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) of the American Psychiatric Association (Chap. 466). Half of these relate to a preexisting antisocial personality manifesting as impulsivity and disinhibition that contribute to both alcohol and drug use disorders. The lifetime risk is 3% in males, and ≥80% of such individuals demonstrate alcohol- and/or drug-related conditions. Another common comorbidity occurs with problems regarding illicit substances. The remainder of alcoholics with psychiatric syndromes have preexisting conditions such as schizophrenia or manic-depressive disease and anxiety syndromes such as panic disorder. The comorbidities of alcoholism with independent psychiatric disorders might represent an overlap in genetic vulnerabilities, impaired judgment in the use of alcohol from the independent psychiatric condition, or an attempt to use alcohol to alleviate symptoms of the disorder or side effects of medications.
Many psychiatric syndromes can be seen temporarily during heavy drinking and subsequent withdrawal. These alcohol-induced conditions include an intense sadness lasting for days to weeks in the midst of heavy drinking seen in 40% of alcoholics, which tends to disappear over several weeks of abstinence (alcohol-induced mood disorder); temporary severe anxiety in 10–30% of alcoholics, often beginning during alcohol withdrawal, which can persist for a month or more after cessation of drinking (alcohol-induced anxiety disorder); and auditory hallucinations and/or paranoid delusions in a person who is alert and oriented, seen in 3–5% of alcoholics (alcohol-induced psychotic disorder).
Treatment of all forms of alcohol-induced psychopathology includes helping patients achieve abstinence and offering supportive care, as well as reassurance and “talk therapy” such as cognitive-behavioral approaches. However, with the exception of short-term antipsychotics or similar drugs for substance-induced psychoses, substance-induced psychiatric conditions only rarely require medications. Recovery is likely within several days to 4 weeks of abstinence. Conversely, because alcohol-induced conditions are temporary and do not indicate a need for long-term pharmacotherapy, a history of alcohol intake is an important part of the workup for any patient with one of these psychiatric symptoms.
THE GASTROINTESTINAL SYSTEM
Esophagus and Stomach Alcohol can cause inflammation of the esophagus and stomach causing epigastric distress and gastrointestinal bleeding, making alcohol one of the most common causes of hemorrhagic gastritis. Violent vomiting can produce severe bleeding through a Mallory-Weiss lesion, a longitudinal tear in the mucosa at the gastroesophageal junction.
Pancreas and Liver The incidence of acute pancreatitis (~25 per 1000 per year) is almost threefold higher in alcoholics than in the general population, accounting for an estimated 10% or more of the total cases. Alcohol impairs gluconeogenesis in the liver, resulting in a fall in the amount of glucose produced from glycogen, increased lactate production, and decreased oxidation of fatty acids. This contributes to an increase in fat accumulation in liver cells. In healthy individuals these changes are reversible, but with repeated exposure to ethanol, especially daily heavy drinking, more severe changes in the liver occur, including alcohol-induced hepatitis, perivenular sclerosis, and cirrhosis, with the latter observed in an estimated 15% of alcoholics (Chap. 363). Perhaps through an enhanced vulnerability to infections, alcoholics have an elevated rate of hepatitis C, and drinking in the context of that disease is associated with more severe liver deterioration.
CANCER
As few as 1.5 drinks per day increases a woman’s risk of breast cancer 1.4-fold. For both genders, four drinks per day increases the risk for oral and esophageal cancers approximately threefold and rectal cancers by a factor of 1.5; seven to eight or more drinks per day produces an approximately fivefold increased risk for many cancers. These consequences may result directly from cancer-promoting effects of alcohol and acetaldehyde or indirectly by interfering with immune homeostasis.
HEMATOPOIETIC SYSTEM
Ethanol causes an increase in red blood cell size (mean corpuscular volume [MCV]), which reflects its effects on stem cells. If heavy drinking is accompanied by folic acid deficiency, there can also be hypersegmented neutrophils, reticulocytopenia, and a hyperplastic bone marrow; if malnutrition is present, sideroblastic changes can be observed. Chronic heavy drinking can decrease production of white blood cells, decrease granulocyte mobility and adherence, and impair delayed-hypersensitivity responses to novel antigens (with a possible false-negative tuberculin skin test). Associated immune deficiencies can contribute to vulnerability toward infections, including hepatitis and HIV, and interfere with their treatment. Finally, many alcoholics have mild thrombocytopenia, which usually resolves within a week of abstinence unless there is hepatic cirrhosis or congestive splenomegaly.
CARDIOVASCULAR SYSTEM
Acutely, ethanol decreases myocardial contractility and causes peripheral vasodilation, with a resulting mild decrease in blood pressure and a compensatory increase in cardiac output. Exercise-induced increases in cardiac oxygen consumption are higher after alcohol intake. These acute effects have little clinical significance for the average healthy drinker but can be problematic when persisting cardiac disease is present.
The consumption of three or more drinks per day results in a dose-dependent increase in blood pressure, which returns to normal within weeks of abstinence. Thus, heavy drinking is an important factor in mild to moderate hypertension. Chronic heavy drinkers also have a sixfold increased risk for coronary artery disease, related, in part, to increased low-density lipoprotein cholesterol, and carry an increased risk for cardiomyopathy through direct effects of alcohol on heart muscle. Symptoms of the latter include unexplained arrhythmias in the presence of left ventricular impairment, heart failure, hypocontractility of heart muscle, and dilation of all four heart chambers with associated mural thrombi and mitral valve regurgitation. Atrial or ventricular arrhythmias, especially paroxysmal tachycardia, can also occur temporarily after heavy drinking in individuals showing no other evidence of heart disease—a syndrome known as the “holiday heart.”
GENITOURINARY SYSTEM CHANGES, SEXUAL FUNCTIONING, AND FETAL DEVELOPMENT
Drinking in adolescence can affect normal sexual development and reproductive onset. At any age, modest ethanol doses (e.g., blood alcohol concentrations of 0.06 g/dL) can increase sexual drive but also decrease erectile capacity in men. Even in the absence of liver impairment, a significant minority of chronic alcoholic men show irreversible testicular atrophy with shrinkage of the seminiferous tubules, decreases in ejaculate volume, and a lower sperm count (Chap. 411).
The repeated ingestion of high doses of ethanol by women can result in amenorrhea, a decrease in ovarian size, absence of corpora lutea with associated infertility, and an increased risk of spontaneous abortion. Heavy drinking during pregnancy results in the rapid placental transfer of both ethanol and acetaldehyde, which may contribute to a range of consequences known as fetal alcohol spectrum disorder (FASD). One severe result is the fetal alcohol syndrome (FAS), seen in ~5% of children born to heavy-drinking mothers, which can include any of the following: facial changes with epicanthal eye folds; poorly formed ear concha; small teeth with faulty enamel; cardiac atrial or ventricular septal defects; an aberrant palmar crease and limitation in joint movement; and microcephaly with mental retardation. Less pervasive FASD conditions include combinations of low birth weight, a lower intelligence quotient (IQ), hyperactive behavior, and some modest cognitive deficits. The amount of ethanol required and the time of vulnerability during pregnancy have not been defined, making it advisable for pregnant women to abstain completely.
OTHER EFFECTS
Between one-half and two-thirds of alcoholics have skeletal muscle weakness caused by acute alcoholic myopathy, a condition that improves but which might not fully remit with abstinence. Effects of repeated heavy drinking on the skeletal system include changes in calcium metabolism, lower bone density, and decreased growth in the epiphyses, leading to an increased risk for fractures and osteonecrosis of the femoral head. Hormonal changes include an increase in cortisol levels, which can remain elevated during heavy drinking; inhibition of vasopressin secretion at rising blood alcohol concentrations and enhanced secretion at falling blood alcohol concentrations (with the final result that most alcoholics are likely to be slightly overhydrated); a modest and reversible decrease in serum thyroxine (T4); and a more marked decrease in serum triiodothyronine (T3). Hormone irregularities should be reevaluated because they may disappear after a month of abstinence.
ALCOHOLISM (ALCOHOL USE DISORDER)
Because many drinkers occasionally imbibe to excess, temporary alcohol-related problems are common in nonalcoholics, especially in the late teens to the late twenties. However, repeated problems in multiple life areas can indicate an alcohol use disorder as defined in DSM-5.
DEFINITIONS AND EPIDEMIOLOGY
An alcohol use disorder is defined as repeated alcohol-related difficulties in at least 2 of 11 life areas that cluster together in the same 12-month period (Table 467-2). Ten of the 11 items were taken directly from the 7 dependence and 4 abuse criteria in DSM-IV, after deleting legal problems and adding craving. Severity of an alcohol use disorder is based on the number of items endorsed: mild is two or three items; moderate is four or five; and severe is six or more of the criterion items. The new diagnostic approach is similar enough to DSM-IV that the following descriptions of associated phenomena are still accurate.
|
DIAGNOSTIC AND STATISTICAL MANUAL OF MENTAL DISORDERS, FIFTH EDITION, CLASSIFICATION OF ALCOHOL USE DISORDER (AUD) |
aMild AUD: 2–3 criteria required; Moderate AUD: 4–5 items endorsed; severe AUD: 6 or more items endorsed.
The lifetime risk for an alcohol use disorder in most Western countries is about 10–15% for men and 5–8% for women. Rates are similar in the United States, Canada, Germany, Australia, and the United Kingdom, tend to be lower in most Mediterranean countries, such as Italy, Greece, and Israel, and may be higher in Ireland, France, and Scandinavia. An even higher lifetime prevalence has been reported for most native cultures, including American Indians, Eskimos, Maori groups, and aboriginal tribes of Australia. These differences reflect both cultural and genetic influences, as described below. In Western countries, the typical alcoholic is more often a blue- or white-collar worker or homemaker. The lifetime risk for alcoholism among physicians is similar to that of the general population.
GENETICS
Approximately 60% of the risk for alcohol use disorders is attributed to genes, as indicated by the fourfold higher risk in children of alcoholics (even if adopted early in life and raised by nonalcoholics) and a higher risk in identical twins compared to fraternal twins of alcoholics. The genetic variations operate primarily through intermediate characteristics that subsequently combine with environmental influences to alter the risk for heavy drinking and alcohol problems. These include genes relating to a high risk for all substance use disorders that operate through impulsivity, schizophrenia, and bipolar disorder. Another characteristic, an intense flushing response when drinking, decreases the risk for only alcohol use disorders through gene variations for several alcohol-metabolizing enzymes, especially aldehyde dehydrogenase (a mutation only seen in Asians), and to a lesser extent, variations in ADH.
An additional genetically influenced characteristic, a low sensitivity to alcohol, affects the risk for heavy drinking and may operate, in part, through variations in genes relating to calcium and potassium channels, GABA, nicotinic, and serotonin systems. A low response per drink is observed early in the drinking career and before alcohol use disorders develop. All follow-up studies have demonstrated that this need for higher doses of alcohol to achieve effects predicts future heavy drinking, alcohol problems, and alcohol use disorders. The impact of a low response to alcohol on adverse drinking outcomes is partially mediated by a range of environmental influences, including the selection of heavier-drinking friends, more positive expectations of the effects of high doses of alcohol, and suboptimal ways of coping with stress.
NATURAL HISTORY
Although the age of the first drink (~15 years) is similar in most alcoholics and nonalcoholics, a slightly earlier onset of regular drinking and drunkenness, especially in the context of conduct problems, is associated with a higher risk for later alcohol use disorders. By the mid-twenties, most nonalcoholic men and women moderate their drinking (perhaps learning from problems), whereas alcoholics are likely to escalate their patterns of drinking despite difficulties. The first major life problem from alcohol often appears in the late teens to early twenties, and a pattern of multiple alcohol difficulties by the midtwenties. Once established, the course of alcoholism is likely to include exacerbations and remissions, with little difficulty in temporarily stopping or controlling alcohol use when problems develop, but without help, desistance usually gives way to escalations in alcohol intake and subsequent problems. Following treatment, between half and two-thirds of alcoholics maintain abstinence for years, and often permanently. Even without formal treatment or self-help groups, there is at least a 20% chance of spontaneous remission with long-term abstinence. However, should the alcoholic continue to drink heavily, the life span is shortened by ~10 years on average, with the leading causes of death being heart disease, cancer, accidents, and suicide.
TREATMENT
The approach to treating alcohol-related conditions is relatively straightforward: (1) recognize that at least 20% of all patients have an alcohol use disorder; (2) learn how to identify and treat acute alcohol-related conditions; (3) know how to help patients begin to address their alcohol problems; and (4) know enough about treating alcoholism to appropriately refer patients for additional help.
IDENTIFICATION OF THE ALCOHOLIC
Even in affluent locales, ~20% of patients have an alcohol use disorder. These men and women can be identified by asking questions about alcohol problems and noting laboratory test results that can reflect regular consumption of six to eight or more drinks per day. The two blood tests with ≥60% sensitivity and specificity for heavy alcohol consumption are γ-glutamyl transferase (GGT) (>35 U) and carbohydrate-deficient transferrin (CDT) (>20 U/L or >2.6%); the combination of the two is likely to be more accurate than either alone. The values for these serologic markers are likely to return toward normal within several weeks of abstinence. Other useful blood tests include high-normal MCVs (≥91 μm3) and serum uric acid (>416 mol/L, or 7 mg/dL).
The diagnosis of an alcohol use disorder ultimately rests on the documentation of a pattern of repeated difficulties associated with alcohol (Table 467-2). Thus, in screening, it is important to probe for marital or job problems, legal difficulties, histories of accidents, medical problems, evidence of tolerance, and so on, and then attempt to tie in use of alcohol or another substance. Some standardized questionnaires can be helpful, including the 10-item Alcohol Use Disorders Identification Test (AUDIT) (Table 467-3), but these are only screening tools, and a face-to-face interview is still required for a meaningful diagnosis.
|
THE ALCOHOL USE DISORDERS IDENTIFICATION TEST (AUDIT)a |

GLOBAL CONSIDERATIONS
![]() As described above, rates of alcohol use disorders differ across sex, age, ethnicity, and country. There are also differences across countries regarding the definition of a standard drink (e.g., 10–12 g of ethanol in the United States and 8 g in the United Kingdom) and the definition of being legally drunk. The preferred alcoholic beverage also varies across groups, even within countries. That said, regardless of sex, ethnicity, or country, the actual drug in the drink is still ethanol, and the risks for problems, course of alcohol use disorders, and approaches to treatment are similar across the world.
As described above, rates of alcohol use disorders differ across sex, age, ethnicity, and country. There are also differences across countries regarding the definition of a standard drink (e.g., 10–12 g of ethanol in the United States and 8 g in the United Kingdom) and the definition of being legally drunk. The preferred alcoholic beverage also varies across groups, even within countries. That said, regardless of sex, ethnicity, or country, the actual drug in the drink is still ethanol, and the risks for problems, course of alcohol use disorders, and approaches to treatment are similar across the world.
468e |
Opioid-Related Disorders |
![]() Opiate analgesics have been abused since at least 300 B.C. Nepenthe (Greek “free from sorrow”) helped the hero of the Odyssey, but widespread opium smoking in China and the Near East has caused harm for centuries. Since the first chemical isolation of opium and codeine 200 years ago, a wide range of synthetic opioids have been developed, and opioid receptors were cloned in the 1990s. Two of the most important adverse effects of all these agents are the development of opioid use disorder and overdose. The 0.1% annual prevalence of heroin dependence in the United States is only about one-third the rate of prescription opiate use and is substantially lower than the 2% rate of morphine users in Southeast and Southwest Asia. Prescription opiates are primarily used for pain management, but due to ease of availability, adolescents procure and use these drugs with dire consequences. In 2011, for example, 11 million individuals in the United States used nonmedically prescribed pain killers that were linked to over 420,000 emergency department visits and nearly 17,000 overdose deaths. Although these rates are low relative to other abused substances, their disease burden is substantial, with high rates of morbidity and mortality; disease transmission; increased health care, crime, and law enforcement costs; and less tangible costs of family distress and lost productivity.
Opiate analgesics have been abused since at least 300 B.C. Nepenthe (Greek “free from sorrow”) helped the hero of the Odyssey, but widespread opium smoking in China and the Near East has caused harm for centuries. Since the first chemical isolation of opium and codeine 200 years ago, a wide range of synthetic opioids have been developed, and opioid receptors were cloned in the 1990s. Two of the most important adverse effects of all these agents are the development of opioid use disorder and overdose. The 0.1% annual prevalence of heroin dependence in the United States is only about one-third the rate of prescription opiate use and is substantially lower than the 2% rate of morphine users in Southeast and Southwest Asia. Prescription opiates are primarily used for pain management, but due to ease of availability, adolescents procure and use these drugs with dire consequences. In 2011, for example, 11 million individuals in the United States used nonmedically prescribed pain killers that were linked to over 420,000 emergency department visits and nearly 17,000 overdose deaths. Although these rates are low relative to other abused substances, their disease burden is substantial, with high rates of morbidity and mortality; disease transmission; increased health care, crime, and law enforcement costs; and less tangible costs of family distress and lost productivity.
The terms “dependence” and “addiction” are no longer used to describe substance use disorders. Opioid-related disorders encompass opioid use disorder, opioid intoxication, and opioid withdrawal. The diagnosis of opioid use disorder as defined in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) requires the repeated use of the opiate while producing problems in two or more areas in a 12-month period. The areas include tolerance, withdrawal, use of greater amounts of opiates than intended, craving, and use despite adverse consequences. This new definition of opiate use disorder, reducing the criteria for diagnosis from three problem areas to two, is not expected to change the rates of these disorders because most individuals using these substances meet more than three criteria.
A striking recent aspect of illicit opiate use has been its marked increase as the gateway to illicit drugs in the United States. Since 2007, prescription opiates have surpassed marijuana as the most common illicit drug that adolescents initially use, although overall rates of opiate dependence are far lower than marijuana. The most commonly used opiates are diverted prescriptions for oxycodone and hydrocodone, followed by heroin and morphine, and—among health professionals—meperidine and fentanyl. Heroin is derived from morphine and acts as a prodrug that more readily penetrates the brain and is converted rapidly to morphine in the body. Two opiate maintenance treatment agents—methadone and buprenorphine—are also misused, but at substantially lower rates, and the partial opiate agonists such as butorphanol, tramadol, and pentazocine are misused even less frequently. Because the chemistry and general pharmacology of these agents are covered in major pharmacology texts, this chapter focuses on the neurobiology and pharmacology relevant to dependence and its treatments. Although the neurobiology of abuse involves all four of the known opiate receptors—mu, kappa, delta, and nociceptin/orphanin—this discussion focuses on the mu receptor, at which most of the clinically used opiates are active.
NEUROBIOLOGY
The neurobiology of opiates and their effects not only include opiate receptors, but also the downstream intracellular messenger systems and ion channels that the receptors regulate. The different functional activities of opiate receptors are summarized in Table 468e-1. Abuse liability of opiates is primarily associated with the mu receptor. All opiate receptors are G protein–linked and coupled to the cyclic adenosine monophosphate (cAMP) second messenger system and to G protein–coupled, inwardly rectifying potassium channels (GIRKs). Opiates activate GIRKs, increasing permeability to potassium ions to cause hyperpolarization, which inhibits the production of action potentials. Thus, opiates inhibit the activity of diverse and widely distributed neuronal types. The major effects of opiates, such as analgesia, sedation, and drug reinforcement are produced through this inhibition of neurons that belong to specific brain pathways.
|
ACTIONS OF OPIOID RECEPTORS |
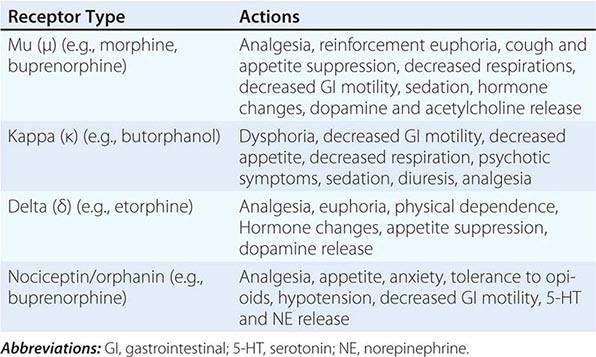
Many opiate actions are related to the specific neuroanatomic locations of mu receptors. Reinforcing and euphoric effects of opiates occur in the mesolimbic dopaminergic pathway from the ventral tegmental area (VTA) to the nucleus accumbens (NAc), where opiates increase synaptic levels of dopamine. This increase is due to inhibition of GABAergic neurons that inhibit both the activity of neurons within the VTA and the NAc. The positive subjective effects of opioid drugs also include mu receptor desensitization and internalization, potentially related to stimulation of beta-arrestin signalizing pathways. However, the “high” only occurs when the rate of change in dopamine is fast. Large, rapidly administered doses of opiates block γ-aminobutyric acid (GABA) inhibition and produce a burst of VTA dopamine neuron activity that is associated with “high” in all abused drugs. Therefore, routes of administration that slowly increase opiate blood and brain levels, such as oral and transdermal routes, are effective for analgesia and sedation but do not produce an opiate “high” that follows smoking and intravenous routes. Other acute effects such as analgesia and respiratory depression involve opiate receptors located in other brain areas such as the locus coeruleus (LC).
Opiate tolerance and withdrawal are chronic effects related to the cAMP-protein kinase A (PKA)-cAMP response-element binding protein (CREB) intracellular cascade (Fig. 468e-1). These effects are also reflective of genetic risk factors for developing opiate use disorder, with estimates of up to 50% of the risk for dependence due to polygenic inheritance. Specific functional polymorphisms in the mu opiate receptor gene appear to be associated with this risk for opiate abuse, including one producing a threefold increase in this receptor’s affinity for opiates and the endogenous ligand beta endorphin. Epigenetic methylation changes also occur on the DNA of the mu receptor gene of opiate addicts, inhibiting gene transcription. This molecular cascade links acute intoxication and sedation to opiate tolerance and withdrawal mediated by the LC. Noradrenergic neurons in the LC mediate activation of the cortical hemispheres. When large opiate doses saturate and activate all of its mu receptors, action potentials cease. When this direct inhibitory effect is sustained over weeks and months of opiate use, a secondary set of adaptive changes occur that lead to tolerance and withdrawal symptoms (Fig. 468e-1). Withdrawal symptoms reflect, in part, overactivity of norepinephrine (NE) neurons in the LC. This molecular model of NE neuronal activation during withdrawal has had important treatment implications, such as the use of the alpha-2 agonist clonidine to treat opioid withdrawal. Other contributors to withdrawal include deficits within the dopamine reward system.
FIGURE 468e-1 Normal mu-receptor activation by endogenous opioids inhibits the cyclic adenosine monophosphate (cAMP)-protein kinase A (PKA)-cAMP response-element binding protein (CREB) cascade in noradrenergic neurons within the locus coeruleus (A) through inhibitory Gi/o protein influence on adenylyl cyclase (AC). Similarly, acute exposure to opiates (e.g., morphine) inhibits this system, whereas chronic exposure to opiates (B) leads to upregulation of the cAMP pathway in an attempt to oppose opiate-induced inhibitory influence. Upregulation of this system is involved in opiate tolerance, and when the opiate is removed, unopposed noradrenergic neurotransmission is involved in opiate withdrawal. Upregulated PKA phosphorylates CREB, initiating the expression of various genes such as tyrosine hydroxylase (TH) and brain-derived neurotrophic factor (BDNF). BDNF is implicated in long-term neuroplastic changes in response to chronic opiates.
PHARMACOLOGY
Tolerance and withdrawal commonly occur with chronic daily use, developing as quickly as 6–8 weeks depending on dose concentration and dosing frequency. Tolerance appears to be primarily a pharmacodynamic rather than pharmacokinetic effect, with relatively limited induction of cytochrome P450 or other liver enzymes. The metabolism of opiates occurs in the liver primarily through the cytochrome P450 systems of 2D6 and 3A4. They then are conjugated to glucuronic acid and excreted in small amounts in feces. The plasma half-lives generally range from 2.5 to 3 h for morphine and more than 22 h for methadone. The shortest half-lives of several minutes are for fentanyl-related opiates and the longest are for buprenorphine and its active metabolites, which can block opiate withdrawal for up to 3 days after a single dose. Tolerance to opioids leads to the need for increasing amounts of drugs to sustain the desired euphoric effects—as well as to avoid the discomfort of withdrawal. This combination has the expected consequence of strongly reinforcing dependence once it has started. Methadone taken chronically at maintenance doses is stored in the liver, which may reduce the occurrence of withdrawal between daily doses. The role of endogenous opioid peptides in tolerance and withdrawal is uncertain.
The clinical features of abuse are tied to route of administration and the rapidity of an opiate bolus in reaching the brain. Intravenous and smoked administration rapidly produces a bolus of high drug concentration in the brain. This bolus produces a “rush,” followed by euphoria, a feeling of tranquility, and sleepiness (“the nod”). Heroin produces effects that last 3–5 h, and several doses a day are required to forestall manifestations of withdrawal in chronic users. Symptoms of opioid withdrawal begin 8–10 h after the last dose; lacrimation, rhinorrhea, yawning, and sweating appear first. Restless sleep followed by weakness, chills, gooseflesh (“cold turkey”), nausea and vomiting, muscle aches, and involuntary movements (“kicking the habit”), hyperpnea, hyperthermia, and hypertension occur in later stages of the withdrawal syndrome. The acute course of withdrawal may last 7–10 days. A secondary phase of protracted abstinence lasts for 26–30 weeks and is characterized by hypotension, bradycardia, hypothermia, mydriasis, and decreased responsiveness of the respiratory center to carbon dioxide.
Besides the brain effects of opioids on sedation and euphoria and the combined brain and peripheral nervous system effects on analgesia, a wide range of other organs can be affected. The cough reflex is inhibited through the brain, leading to the use of some opiates as an antitussive, and nausea and vomiting are due to effects on the medulla. The release of several pituitary hormones is inhibited, including corticotropin-releasing factor (CRF) and luteinizing hormone, which reduces levels of cortisol and sex hormones and can lead to impaired stress responses and reduced libido. An increase in prolactin also contributes to the reduced sex drive in males. Two other hormones affected are thyrotropin, which is reduced, and growth hormone, which is increased. Respiratory depression results from opiate-induced insensitivity of brainstem neurons to increases in carbon dioxide, and in patients with pulmonary disease, this can result in clinically significant complications. In overdoses, aspiration pneumonia is common due to loss of the gag reflex. Opiates reduce gut motility, which is helpful for treating diarrhea, but can lead to nausea, constipation, and anorexia with weight loss. Deaths occurred in early methadone maintenance programs due to severe constipation and toxic megacolon. Opiates such as methadone may prolong QT intervals and lead to sudden death in some patients. Orthostatic hypotension may occur due to histamine release and peripheral blood vessel dilation, which is an opiate effect usefully applied to managing acute myocardial infarction. During opiate maintenance, interactions with other medications are of concern; these include inducers of the cytochrome P450 system (usually CYP3A4) such as rifampin and carbamazepine.
Heroin users in particular tend to use opiates intravenously and are likely to be polydrug users, also using alcohol, sedatives, cannabinoids, and stimulants. None of these other drugs are substitutes for opioids, but they have desired additive effects. Therefore, one needs to be sure that the person undergoing a withdrawal reaction is not also withdrawing from alcohol or sedatives, which might be more dangerous and more difficult to manage.
Intravenous opiate use carries with it the risk of serious complications. The common sharing of hypodermic syringes can lead to infections with hepatitis B and HIV/AIDS, among others. Bacterial infections can lead to septic complications such as meningitis, osteomyelitis, and abscesses in various organs. Off-target effects of opiates synthesized in illicit drug labs can lead to serious toxicity. For example, attempts to illicitly manufacture meperidine in the 1980s resulted in the production of a highly specific neurotoxin, MPTP, which produced parkinsonism in users (Chap. 449).
Lethal overdose is a relatively common complication of opiate use disorder. Rapid recognition and treatment with naloxone, a highly specific reversal agent that is relatively free of complications, is essential. The diagnosis is based on recognition of characteristic signs and symptoms, including shallow and slow respirations, pupillary miosis (mydriasis does not occur until significant brain anoxia supervenes), bradycardia, hypothermia, and stupor or coma. Blood or urine toxicology studies can confirm a suspected diagnosis, but immediate management must be based on clinical criteria. If naloxone is not administered, progression to respiratory and cardiovascular collapse leading to death occurs. At autopsy, cerebral edema and sometimes frothy pulmonary edema are generally found. Opiates generally do not produce seizures except for unusual cases of polydrug use with the opiate meperidine, with high doses of tramadol, or in the newborn.
Opioid Agonist Medications for Maintenance Methadone maintenance substitutes a once-daily oral opioid dose for three- to four-times daily heroin. Methadone saturates the opioid receptors and, by inducing a high level of opiate tolerance, blocks the euphoria from additional opiates. Buprenorphine, a partial opioid agonist, also can be given once daily at sublingual doses of 4–32 mg daily, and in contrast to methadone, it can be given in an office-based primary care setting.
METHADONE MAINTENANCE Methadone’s slow onset of action when taken orally, long elimination half-life (24–36 h), and production of cross-tolerance at doses from 80 to 150 mg are the basis for its efficacy in treatment retention and reductions in IV drug use, criminal activity, and HIV risk behaviors and mortality. Methadone can prolong the QT interval at rates as high as 16% above the rates in non-methadone-maintained, drug-injecting patients, but it has been used safely in the treatment of opioid dependence for 40 years.
BUPRENORPHINE MAINTENANCE While France and Australia have had sublingual buprenorphine maintenance since 1996, it was first approved by the U.S. Food and Drug Administration (FDA) in 2002 as a Schedule III drug for managing opioid use disorder. Unlike the full agonist methadone, buprenorphine is a partial agonist of mu-opioid receptors with a slow onset and long duration of action. Its partial agonism reduces the risk of unintentional overdose but limits its efficacy to patients who need the equivalent of only 60–70 mg of methadone, and many patients in methadone maintenance require higher doses up to 150 mg daily. Buprenorphine is combined with naloxone at a 4:1 ratio in order to reduce its abuse liability. Because of pediatric exposures and diversion of buprenorphine to illicit use, a new formulation, using mucosal films rather than sublingual pills that were crushed and snorted, is now marketed. A subcutaneous buprenorphine implant that lasts up to 6 months has also been tested and is pending FDA approval as a formulation improvement to prevent pediatric exposures and illicit diversion and enhance compliance.
In the United States, the ability of primary care physicians to prescribe buprenorphine for opioid use disorder represents an important opportunity to improve access and quality of treatment as well as reduce social harm. Europe, Asia, and Australia have found reduced opioid-related deaths and drug-injection-related medical morbidity with buprenorphine available in primary care. Retention in office-based buprenorphine treatment has been high as 70% at 6-month follow-ups.
Opioid Antagonist Medications The rationale for using narcotic antagonist therapy is that blocking the action of self-administered opioids should eventually extinguish the habit, but this therapy is poorly accepted by patients. Naltrexone, a long-acting orally active pure opioid antagonist, can be given three times a week at doses of 100–150 mg. Because it is an antagonist, the patient must first be detoxified from opioid dependence before starting naltrexone. It is safe even when taken chronically for years, is associated with few side effects (headache, nausea, abdominal pain), and can be given to patients infected with hepatitis B or C without producing hepatotoxicity. However, most providers refrain from prescribing naltrexone if liver function tests are three times above normal levels. Naltrexone maintenance combined with psychosocial therapy is effective in reducing heroin use, but medication adherence is low. Depot injection formulations lasting up to 4 weeks markedly improve adherence, retention, and drug use. Subcutaneous naltrexone implants in Russia, China, and Australia have doubled treatment retention and reduced relapse to half that of oral naltrexone. In the United States, a depot naltrexone formulation is available for monthly use and maintains blood levels equivalent to 25 mg of daily oral use.
Medication-Free Treatment Most opiate addicts enter medication-free treatments in inpatient, residential, or outpatient settings, but 1- to 5-year outcomes are very poor compared to pharmacotherapy except for residential settings lasting 6 to 18 months. The residential programs require full immersion in a regimented system with progressively increasing levels of independence and responsibility within a controlled community of fellow drug abusers. These medication-free programs, as well as the pharmacotherapy programs, also include counseling and behavioral treatments designed to teach interpersonal and cognitive skills for coping with stress and for avoiding situations leading to easy access to drugs or to craving. Relapse is prevented by having the individual very gradually reintroduced to greater responsibilities and to the working environment outside of the protected therapeutic community.
PREVENTION
Preventing opiate abuse represents a critically important challenge for physicians. Opiate prescriptions are the most common source of drugs accessed by adolescents who begin a pattern of illicit drug use. The major sources of these drugs are family members, not drug dealers or the Internet. Pain management involves providing sufficient opiates to relieve the pain over as short a period of time as the pain warrants (Chap. 18). The patient then needs to dispose of any remaining opiates, not save them in the medicine cabinet, because this behavior leads to diversion by adolescents. Finally, physicians should never prescribe opiates for themselves.
469e |
Cocaine and Other Commonly Abused Drugs |
The abuse of cocaine and other psychostimulants reflects a complex interaction between the pharmacology of the drug, the personality and expectations of the user, and the environmental context in which the drug is used. Polydrug abuse involving the concurrent use of several drugs with different pharmacologic effects is increasingly common. Sometimes one drug is used to enhance the effects of another, as with the combined use of cocaine and nicotine, benzodiazepines and methadone, or cocaine and heroin in methadone-maintained patients. Some forms of polydrug abuse, such as the combined use of IV heroin and cocaine, are especially dangerous and account for many hospital emergency room visits.
![]() Chronic cocaine and psychostimulant abuse may cause a number of adverse health consequences and may exacerbate preexisting disorders such as hypertension and cardiac disease. The combined use of two or more drugs may accentuate medical complications associated with abuse of one drug. Chronic drug abuse is often associated with immune system dysfunction and increased vulnerability to infections, including risk for HIV infection. In addition, concurrent use of cocaine and opiates (the “speedball”) is frequently associated with needle sharing by IV drug users. IV drug abusers continue to be the largest single group of persons with HIV infection in several major metropolitan areas in the United States as well as in many parts of Europe and Asia.
Chronic cocaine and psychostimulant abuse may cause a number of adverse health consequences and may exacerbate preexisting disorders such as hypertension and cardiac disease. The combined use of two or more drugs may accentuate medical complications associated with abuse of one drug. Chronic drug abuse is often associated with immune system dysfunction and increased vulnerability to infections, including risk for HIV infection. In addition, concurrent use of cocaine and opiates (the “speedball”) is frequently associated with needle sharing by IV drug users. IV drug abusers continue to be the largest single group of persons with HIV infection in several major metropolitan areas in the United States as well as in many parts of Europe and Asia.
Stimulants and hallucinogens have been used to induce euphoria and alter consciousness for centuries. Cocaine and marijuana are two of the most commonly abused drugs today. Synthetic variations of marijuana and a variety of hallucinogens have become popular recently, and new drugs are continually being developed. This chapter describes the subjective and adverse medical effects of cocaine, marijuana, and lysergic acid diethylamide (LSD), as well as methamphetamine, 3,4-methylenedioxy-N-methamphetamine (MDMA), synthetic cathinones (bath salts), phencyclidine (PCP), Salvia divinorum, and other drugs of abuse (flunitrazepam, γ-hydroxybutyric acid [GHB], ketamine). Some options for medical management of severe adverse effects are also described.
COCAINE
Cocaine is a stimulant and a local anesthetic with potent vasoconstrictor properties. The leaves of the coca plant (Erythroxylum coca) contain ~0.5–1% cocaine. The drug produces physiologic and behavioral effects after oral, intranasal, IV, or inhalation/smoking routes of administration. The reinforcing effects of cocaine are related to activation of dopaminergic neurons in the mesolimbic system (Chap. 465e). Cocaine increases synaptic concentrations of the monoamine neurotransmitters dopamine, norepinephrine, and serotonin by binding to transporter proteins in presynaptic neurons and blocking reuptake.
PREVALENCE OF COCAINE USE
Cocaine is widely available and is abused in virtually all social and economic strata of society. In 2012, an estimated 1.6 million persons in the United States used cocaine, and 1.1 million abused or were dependent on cocaine. Emergency room admissions involving cocaine totaled 505,224 in 2011. Cocaine abuse is prevalent in the general population and in heroin-dependent persons, including those in methadone maintenance programs. IV cocaine is often used concurrently with IV heroin in a combination called a “speedball.” This combination purportedly attenuates the postcocaine “crash” and substitutes a cocaine “high” for the heroin “high” blocked by methadone.
ACUTE AND CHRONIC INTOXICATION
There has been an increase in both IV administration and inhalation of pyrolyzed cocaine via smoking. Following intranasal administration, changes in mood and sensation are perceived within 3–5 min, and peak effects occur at 10–20 min. These effects rarely last more than 1 h. Inhalation of pyrolyzed materials includes inhaling crack/cocaine or smoking coca paste, a product made by extracting cocaine preparations with flammable solvents, and cocaine free-base smoking. Free-base cocaine, including the free-base prepared with sodium bicarbonate (crack), has become increasingly popular because of its relative high potency and rapid onset of action (8–10 seconds following smoking).
Cocaine produces a brief, dose-related stimulation and euphoria and an increase in cardiac rate and blood pressure. Body temperature usually increases following cocaine administration, and high doses of cocaine may induce lethal pyrexia or hypertension. Because cocaine inhibits reuptake of catecholamines at adrenergic nerve endings, it potentiates sympathetic nervous system activity. Cocaine has a short plasma half-life of approximately 45–60 min. Cocaine is metabolized by plasma esterases, and cocaine metabolites are excreted in urine. The brief duration of the euphorigenic effects of cocaine reported by chronic abusers is probably due to both acute and chronic tolerance. Cocaine may be used as often as two to three times per hour. Alcohol is often used to modulate both the cocaine high and the dysphoria associated with the abrupt disappearance of cocaine’s effects. A metabolite of cocaine, cocaethylene, has been detected in blood and urine of persons who concurrently abuse alcohol and cocaine. Cocaethylene induces changes in cardiovascular function similar to those of cocaine alone, and the pathophysiologic consequences of the concurrent abuse of alcohol plus cocaine may be additive.
Cocaine may cause serious medical consequences by any route of administration. The prevalent assumption that cocaine inhalation or IV administration is relatively safe is contradicted by reports of death from respiratory depression, cardiac arrhythmias, and convulsions associated with cocaine use. In addition to generalized seizures, neurologic complications may include headache, ischemic or hemorrhagic stroke, or subarachnoid hemorrhage. Disorders of cerebral blood flow and perfusion in cocaine-dependent persons have been detected with magnetic resonance spectroscopy (MRS). Inhalation of crack cocaine may lead to severe pulmonary disease due to the direct effects of cocaine and to residual contaminants in the smoked material. Hepatic necrosis may occur following chronic crack/cocaine use. Protracted cocaine abuse may also cause paranoid ideation and visual and auditory hallucinations, a state that resembles alcoholic hallucinosis.
Although men and women who abuse cocaine may report that the drug enhances libidinal drive, chronic cocaine use causes significant loss of libido and adversely affects sexual function. Impotence and gynecomastia have been observed in male cocaine abusers, and these abnormalities often persist for long periods following cessation of drug use. Cocaine abuse may produce major derangements in menstrual cycle function including galactorrhea, amenorrhea, and infertility in women and in a rhesus monkey model of cocaine self-administration. Chronic cocaine abuse may cause persistent hyperprolactinemia as a consequence of disordered dopaminergic inhibition of prolactin secretion by the anterior pituitary. Cocaine abuse by pregnant women, particularly crack smoking, has been associated with both an increased risk of congenital malformations in the fetus and perinatal cardiovascular and cerebrovascular disease in the mother. However, cocaine abuse per se is probably not the sole cause of these perinatal disorders, because maternal cocaine abuse is often associated with poor nutrition and prenatal health care as well as polydrug abuse that may contribute to the risk for perinatal disease.
Psychological dependence on cocaine, indicated by inability to abstain from frequent compulsive use, has been reported. Although the occurrence of withdrawal syndromes involving psychomotor agitation and autonomic hyperactivity remains controversial, severe depression (“crashing”) following cocaine intoxication may accompany drug withdrawal.
MARIJUANA AND CANNABIS COMPOUNDS
Cannabis sativa contains >400 compounds in addition to the psychoactive substance, delta-9-tetrahydrocannabinol (THC). Marijuana cigarettes are prepared from the leaves and flowering tops of the plant, and a typical marijuana cigarette contains 0.5–1 g of plant material. The usual THC concentration varies between 10 and 40 mg, but concentrations <100 mg per cigarette have been detected. Hashish is prepared from concentrated resin of C. sativa and contains a THC concentration of between 8 and 12% by weight. “Hash oil,” a lipid-soluble plant extract, may contain THC between 25 and 60% and may be added to marijuana or hashish to enhance its THC concentration. Smoking is the most common mode of marijuana or hashish use. During pyrolysis, <150 compounds in addition to THC are released in the smoke. Although most of these compounds do not have psychoactive properties, they may have physiologic effects.
THC is quickly absorbed from the lungs into blood and then rapidly sequestered in tissues. THC is metabolized primarily in the liver, where it is converted to 11-hydroxy-THC, a psychoactive compound, and >20 other metabolites. Many THC metabolites are excreted through the feces at a relatively slow rate of clearance compared with most other psychoactive drugs.
Specific cannabinoid receptors (CB1 and CB2) have been identified in the central and peripheral nervous system. High densities of cannabinoid receptors have been found in the cerebral cortex, basal ganglia, and hippocampus. T and B lymphocytes also contain cannabinoid receptors, and these appear to mediate the anti-inflammatory and immunoregulatory properties of cannabinoids. A naturally occurring THC-like ligand has been identified and is widely distributed in the nervous system.
Herbal marijuana alternatives are also available. These are usually a combination of several herbs and synthetic cannabinoids. “Spice” and “K2” are among the best known, but many formulations exist, and marijuana is undetectable by the usual methods. These compounds are marketed on the Internet as containing no illegal ingredients. However a number of synthetic cannabinoids are now classified as Schedule I by the Drug Enforcement Administration due to reports of toxicity.
PREVALENCE OF USE
Marijuana is the most commonly used illegal drug in the United States. In 2012, an estimated 18.9 million people reported using marijuana within the past month. An estimated 7.2% of adolescents age 12 to 17 years reported current use of marijuana. Marijuana is relatively inexpensive and is often considered to be less hazardous than other controlled drugs and substances. Very potent forms of marijuana (sinsemilla) are widely available, and concurrent use of marijuana with other drugs such as cocaine is not uncommon. Due in part to the difficulty of detecting herbal marijuana alternatives, the prevalence of use is unknown.
ACUTE AND CHRONIC INTOXICATION
Acute intoxication from marijuana and cannabis compounds is related to both the dose of THC and the route of administration. THC is absorbed more rapidly from marijuana smoking than from orally ingested cannabis compounds. Acute marijuana intoxication may produce a perception of relaxation and mild euphoria resembling mild to moderate alcohol intoxication. This condition is usually accompanied by some impairment in thinking, concentration, and perceptual and psychomotor function. Higher doses of cannabis may produce more pronounced impairment in concentration and perception, as well as greater sedation. Although the acute effects of marijuana intoxication are relatively benign in normal users, the drug can precipitate severe emotional disorders in individuals who have antecedent psychotic or neurotic problems. Like other psychoactive compounds, both the user’s expectations and the environmental context are important determinants of the type and severity of the effects of marijuana intoxication.
As with abuse of cocaine, opioids, and alcohol, chronic marijuana abusers may lose interest in common socially desirable goals and devote progressively more time to drug acquisition and use. However, THC does not cause a specific and unique “amotivational syndrome.” The range of symptoms sometimes attributed to marijuana use is difficult to distinguish from mild to moderate depression and the maturational dysfunctions often associated with protracted adolescence. Chronic marijuana use has also been reported to increase the risk of psychotic symptoms in individuals with a past history of schizophrenia. Persons who begin marijuana smoking before the age of 17 may have more pronounced cognitive deficits and also may be at higher risk for polydrug and alcohol abuse problems in later life, but the role of marijuana in this sequence is uncertain.
The acute effects of herbal marijuana alternatives are based primarily on case reports and include anxiety, agitation, delusions, paranoia, and psychosis. The extent to which these symptoms reflect drug effects or exacerbation of an underlying psychiatric disorder is often difficult to determine.
PHYSICAL EFFECTS
Conjunctival injection and tachycardia are the most frequent immediate physical concomitants of smoking marijuana. Tolerance for marijuana-induced tachycardia develops rapidly among regular users. However, marijuana smoking may precipitate angina in persons with a history of coronary insufficiency. Exercise-induced angina may increase after marijuana use to a greater extent than after tobacco cigarette smoking. Patients with cardiac disease should be strongly advised not to smoke marijuana or use cannabis compounds.
Significant decrements in pulmonary vital capacity have been found in regular daily marijuana smokers. Because marijuana smoking typically involves deep inhalation and prolonged retention of marijuana smoke, chronic bronchial irritation may develop. Impairment of single-breath carbon monoxide diffusion capacity (DLCO) is greater in persons who smoke both marijuana and tobacco than in tobacco smokers.
Although marijuana has also been associated with a number of other adverse effects, many of these studies await replication and confirmation. A reported correlation between chronic marijuana use and decreased testosterone levels in males has not been confirmed. Decreased sperm count and sperm motility and morphologic abnormalities of spermatozoa following marijuana use have been reported. Prospective studies found a correlation between impaired fetal growth and development and heavy marijuana use during pregnancy. Marijuana has also been implicated in derangements of the immune system; in chromosomal abnormalities; and in inhibition of DNA, RNA, and protein synthesis; however, these findings have not been confirmed or related to any specific physiologic effect in humans. Herbal marijuana alternatives produce many of the effects of marijuana including conjunctival injection and tachycardia.
TOLERANCE AND PHYSICAL DEPENDENCE
Habitual marijuana users may develop tolerance to the psychoactive effects of marijuana, and then smoke more frequently and try to acquire more potent cannabis compounds. Tolerance for the physiologic effects of marijuana develops at different rates; e.g., tolerance develops rapidly for marijuana-induced tachycardia but more slowly for marijuana-induced conjunctival injection. Tolerance for both behavioral and physiologic effects of marijuana decreases rapidly upon cessation of marijuana use.
A distinct withdrawal syndrome has been documented in chronic cannabis users, and the severity of symptoms is related to dosage and duration of use. These symptoms typically reach their peak several days after cessation of chronic use and include irritability, anorexia, and sleep disturbances. Withdrawal signs and symptoms observed in chronic marijuana users are usually relatively mild in comparison to those observed in heavy opioid or alcohol users and rarely require medical or pharmacologic intervention. However, more severe and protracted abstinence syndromes may occur after sustained use of high-potency cannabis compounds. As yet there have been no systematic studies of tolerance and physical dependence to the herbal marijuana alternatives. The large number of synthetic cannabinoids available for combination with about 20 herbs presents a daunting challenge for analysis.
THERAPEUTIC USE OF MARIJUANA
Marijuana, administered as cigarettes or as a synthetic oral cannabinoid (dronabinol), is thought to have a number of clinically useful medicinal properties. These include antiemetic effects in chemotherapy recipients, appetite-promoting effects in AIDS patients, reduction of intraocular pressure in glaucoma, and reduction of spasticity in multiple sclerosis and other neurologic disorders. With the possible exception of AIDS-related cachexia, none of these attributes of marijuana compounds is clearly superior to other readily available therapies.
METHAMPHETAMINE
Methamphetamine is also referred to as “meth,” “speed,” “crank,” “chalk,” “ice,” “glass,” or “crystal.” Methamphetamine is a mixed-action monoamine releaser with activity at dopamine, serotonin, and norepinephrine systems. Methamphetamine was considered second only to cocaine as a drug threat to society by the U.S. Department of Justice in 2009. Hospital admissions for methamphetamine treatment more than doubled between 1998 and 2007, and young adults (age 18–25) have the highest use rates. In 2011, an estimated 439,000 people reported current use of methamphetamine in the United States, and emergency room admissions involving amphetamines/methamphetamine totaled 160,000. Persistent abuse of methamphetamine continues despite drug seizures, closures of clandestine laboratories that produce methamphetamine illegally, and an increase in methamphetamine abuse prevention programs.
Methamphetamine can be used by smoking, snorting, IV injection, or oral administration. Methamphetamine abusers report that drug use induces feelings of euphoria and decreased fatigue. Adverse consequences of methamphetamine use include headache, difficulty concentrating, diminished appetite, abdominal pain, vomiting or diarrhea, disordered sleep, paranoid or aggressive behavior, and psychosis. Chronic methamphetamine abuse can result in severe dental caries, described as blackened, rotting, crumbling teeth. Severe, life-threatening methamphetamine toxicity may include hypertension, cardiac arrhythmia or cardiac failure, subarachnoid hemorrhage, ischemic stroke, intracerebral hemorrhage, convulsions, or coma.
Methamphetamines increase the release of monoamine neurotransmitters (dopamine, norepinephrine, and serotonin) from presynaptic neurons. It is thought that the euphoric and reinforcing effects of this class of drugs are mediated through dopamine and the mesolimbic system, whereas the cardiovascular effects are related to norepinephrine. MRS studies of the brain suggest that chronic abusers have neuronal damage in the frontal areas and basal ganglia.
Treatment of acute methamphetamine overdose is largely symptomatic. Ammonium chloride may be useful to acidify the urine and enhance clearance of the drug. Hypertension may respond to sodium nitroprusside or α-adrenergic antagonists. Sedatives may reduce agitation and other signs of central nervous system hyperactivity. Treatment of chronic methamphetamine dependence may be accomplished in either an inpatient or outpatient setting using strategies similar to those described earlier for cocaine abuse.
MDMA is a derivative of methamphetamine also called Ecstasy or Molly. Reported use of MDMA in the United States has increased from 615,000 persons in 2005, to an estimated 869,000 people in 2012. Emergency ward admissions involving MDMA totaled more than 22,000 in 2011. Ecstasy is usually taken orally but may be injected or inhaled, and its effects last for 3–6 h. MDMA has amphetamine-like effects including vivid visual and auditory hallucinations and other perceptual distortions. Recent studies indicate that MDMA use is associated with cognitive and memory impairment. MDMA can induce hyperthermia and elevated blood pressure, seizures, comma, and death. Withdrawal symptoms after cessation of use may include teeth grinding, anxiety, loss of appetite, insomnia, and fever. The long-term consequences of recreational use of MDMA by young persons are poorly understood.
SYNTHETIC CATHINONES (BATH SALTS)
The rapid emergence of synthetic cathinone abuse during 2010 was accompanied by numerous reports of adverse medical and psychiatric effects, suicides, and deaths. Reports to poison centers and health agencies increased from about 300 in 2010 to over 6000 in 2011. In 2011, the Drug Enforcement Administration classified three commonly abused synthetic cathinones (mephedrone [4-methyl methcathinone], MDPV [3,4-methylenedioxy pyrovalerone], and methylone) as Schedule I compounds with no accepted medical use and a high potential for abuse. However, synthetic cathinones are readily available on the Internet as well as in convenience stores, gas stations, and head shops. These drugs are merchandised under a variety of names such as Vanilla Sky, Purple Wave, Blue Silk, White Lightening, and Snow Leopard. Regulatory constraints are evaded by labeling the products as plant food, insecticides, pond cleaner, and bath salts with the qualifier, “not for human consumption.”
Cathinone is the primary psychoactive ingredient in khat leaves. Chewing the leaves of the khat shrub (Catha edulis) produces mild stimulant and euphoric effects and remains a common practice in east Africa that has persisted for centuries. Cathinone is structurally similar to amphetamine, and mephedrone is structurally similar to methamphetamine. Cathinones, like amphetamines, inhibit dopamine, serotonin, and norepinephrine transporters to varying degrees, and this probably accounts for variations in the behavioral effects observed. The effects of cathinone derivatives are often described as similar to the effects of MDMA or Ecstasy. Synthetic cathinones can be inhaled, snorted, injected, or taken orally. These drugs may be taken repeatedly over several hours in episodes lasting for hours or days. The onset of effects after oral ingestion is relatively rapid for MDPV (15–30 min) and slightly slower for mephedrone and methylone (30–45 min). The duration of action varies from 2 to 5 or 7 h. After mephedrone inhalation, effects occur within minutes and only last for 1 h or less, but mood changes may persist for several days. Evaluation of the neurotoxic effects of prolonged synthetic cathinone abuse is just beginning, and the long-term consequences are unknown.
The reported positive subjective effects of synthetic cathinones include euphoria, improved energy, alertness, sociability, and increased sensitivity to music and other sensory experiences. The reported negative subjective effects include agitation, visual and auditory hallucinations, anxiety and panic attacks, paranoid delusions, disorientation, depression, and suicidal ideation. Observers report irritability, aggression, violent behavior, tremors, and seizures. Medical evidence of adverse effects includes cardiovascular dysfunction and cardiac arrest, hypertension, hyperthermia, nausea and vomiting, and anorexia. There is no specific antagonist for synthetic cathinone intoxication. Patients with severe hyperthermia, seizures, and arrhythmia are medical emergencies and should be treated in a hospital. Sedation with benzodiazepines can be useful for managing agitation, seizures, aggression, and other related symptoms. Antipsychotic medications may be necessary for management of severe and persistent psychiatric symptoms.
LYSERGIC ACID DIETHYLAMIDE (LSD)
Discovery of the psychedelic effects of LSD led to an epidemic of LSD abuse during the 1960s. Imposition of stringent constraints on the manufacture and distribution of LSD (classified as a Schedule I substance by the U.S. Food and Drug Administration [FDA]) and public recognition that psychedelic experiences induced by LSD were a health hazard have resulted in a reduction in LSD abuse. LSD still remains popular among adolescents and young adults, and there are indications that LSD use among young persons has been increasing in some areas in the United States. In 2011, an estimated 358,000 persons used LSD, whereas 200,000 and 271,000 persons reported LSD use in 2003 and 2007, respectively.
LSD is a very potent hallucinogen; oral doses as low as 20 μg may induce profound psychological and physiologic effects. Tachycardia, hypertension, pupillary dilation, tremor, and hyperpyrexia occur within minutes following oral administration of 0.5–2 μg/kg. A variety of bizarre and often conflicting perceptual and mood changes, including visual illusions, synesthesias, and extreme lability of mood, usually occur within 30 min after LSD intake. These effects of LSD may persist for 12–18 h, even though the half-life of the drug is only 3 h.
Emergency ward visits involving LSD totaled nearly 5000 in 2011. The most frequent acute medical emergency associated with LSD use is a panic episode (the “bad trip”), which may persist up to 24 h. Management of this problem is best accomplished by supportive reassurance (“talking down”) and, if necessary, administration of small doses of anxiolytic drugs. Adverse consequences of chronic LSD use include an enhanced risk for schizophreniform psychosis and derangements in memory function, problem solving, and abstract thinking. Treatment of these disorders is best carried out in specialized psychiatric facilities.
Tolerance develops rapidly for LSD-induced changes in psychological function when the drug is used one or more times per day for >4 days. Abrupt abstinence following continued use does not produce withdrawal signs or symptoms. There have been no clinical reports of death caused by the direct effects of LSD.
PHENCYCLIDINE (PCP)
PCP, a cyclohexylamine derivative, is widely used in veterinary medicine to briefly immobilize large animals and is sometimes described as a dissociative anesthetic. PCP binds to ionotropic N-methyl-D-aspartate (NMDA) receptors in the nervous system, blocking ion current through these channels. PCP is easily synthesized and is abused primarily by young people and polydrug users. It is used orally, by smoking, by snorting, or by IV injection. It is also used as an adulterant in THC, LSD, amphetamine, or cocaine. The most common street preparation, angel dust, is a white granular powder that contains 50–100% of the drug. Low doses (5 mg) produce agitation, excitement, impaired motor coordination, dysarthria, and analgesia. Physical signs of intoxication may include horizontal or vertical nystagmus, flushing, diaphoresis, and hyperacusis. Behavioral changes include distortions of body image, disorganization of thinking, and feelings of estrangement. Higher doses of PCP (5–10 mg) may produce profuse salivation, vomiting, myoclonus, fever, stupor, or coma. PCP doses of ≥10 mg cause convulsions, opisthotonus, and decerebrate posturing that may be followed by prolonged coma.
In 2011, more than 75,000 emergency ward admissions involved PCP. The diagnosis of PCP overdose is difficult because the patient’s initial symptoms (anxiety, paranoia, delusions, and hallucinations) may suggest an acute schizophrenic reaction. Confirmation of PCP use is possible by determination of PCP levels in serum or urine. PCP assays are available at most toxicologic centers. PCP remains in urine for 1–5 days following high-dose intake.
PCP overdose requires emergency life-support measures that may involve treatment of coma, convulsions, and respiratory depression in an intensive care unit. There is no specific antidote or antagonist for PCP. PCP excretion from the body can be enhanced by gastric lavage and acidification of urine. Death from PCP overdose may occur as a consequence of some combination of pharyngeal hypersecretion, hyperthermia, respiratory depression, severe hypertension, seizures, hypertensive encephalopathy, and intracerebral hemorrhage.
Acute psychosis associated with PCP use is a psychiatric emergency because patients may be at high risk for suicide or extreme violence toward others. Phenothiazines should not be used for treatment because these drugs potentiate PCP’s anticholinergic effects. Haloperidol (5 mg IM) has been administered on an hourly basis to induce suppression of psychotic behavior. PCP, like LSD and mescaline, produces vasospasm of cerebral arteries at relatively low doses. Chronic PCP use has been shown to induce insomnia, anorexia, severe changes in behavior, and, in some cases, chronic schizophrenia.
SALVIA DIVINORUM
This naturally occurring herb is a recent entry into the spectrum of hallucinogens. Like PCP and ecstasy, this drug can produce profound alterations in mood, hallucinations, and distorted perceptions. This drug is available on the Internet and is known by a variety of names including magic mint, mystic sage, Mariana Pastora, and purple sticky. The drug was first added to the annual National Surveys on Drug Use and Health in 2006, and its use is increasing. Between 2006 and 2011, the number of estimated users in the United States nearly tripled to more than 5000.
The active ingredient is salvinorin A, a selective kappa opioid receptor agonist that has a range of effects including hallucinations, sedation, analgesia, and depression. The hallucinatory symptoms may be associated with intense anxiety and severe agitation that can be managed with benzodiazepines. Importantly, this kappa opioid receptor agonist does not produce respiratory depression, and no significant change in blood pressure or heart rate was reported in a clinical study with healthy subjects.
Salvinorin A extract or crushed leaves of the Salvia divinorum plant can be chewed and absorbed through the buccal membrane or inhaled during smoking. The onset of the acute “high” is within 5–10 min after chewing and 30 s after inhalation. The duration of the effect is relatively brief, usually 15–20 min. However, if the drug is taken with alcohol or other hallucinogens, the duration and intensity of adverse effects may be increased. The effects of the drug are reported to be similar to those of ketamine, LSD, and marijuana.
OTHER DRUGS OF ABUSE
A number of other pharmacologically diverse drugs of abuse are often referred to as “club drugs” because these are frequently used in bars, at concerts, and at rave parties. Commonly abused club drugs include flunitrazepam, GHB, and ketamine and are described below. Methamphetamine, MDMA, and LSD are also considered club drugs and were described earlier in this chapter. Abuse of club drugs at high doses, especially in combination with alcohol, can be lethal and should be treated as a medical emergency. GHB and ketamine can be identified in blood, and flunitrazepam can be identified in urine and hair samples. Flunitrazepam and GHB toxicity can be treated with antagonists at benzodiazepine and γ-aminobutyric acid B (GABAB) receptors, respectively.
Flunitrazepam (Rohypnol) is a benzodiazepine derivative primarily used to treat insomnia, but it has significant abuse potential because of its strong hypnotic, anxiolytic, and amnesia-producing effects. It is a club drug commonly referred to as a “date-rape drug” or “roofies.” The drug enhances GABAA receptor activity, and overdose can be treated with flumazenil, a benzodiazepine receptor antagonist. Flunitrazepam is typically used orally but can be snorted or injected. Concomitant use of alcohol or opioids is common, and this enhances the sedative and hypnotic effects of flunitrazepam and also the risk of motor vehicle accidents. Overdose can produce life-threatening respiratory depression and coma. Abrupt cessation after chronic use may result in a benzodiazepine withdrawal syndrome consisting of anxiety, insomnia, disordered thinking, and seizures.
GHB (Xyrem) is a sedative drug that is approved by the FDA for the treatment of narcolepsy. It is classified as a club drug, is sometimes used in combination with alcohol or other drugs of abuse, and has been implicated in cases of date rape. It is also used by body builders as a growth hormone stimulant. GHB is usually available as a liquid, is taken orally, and has no distinctive color or odor. Its stimulant properties are attributed to agonist activity at the GHB receptor, but it also has sedative effects at high doses that reflect its activity at GABAB receptors. GABAB antagonists can reverse GHB’s sedative effects, and opioid antagonists (naloxone, naltrexone) can attenuate GHB effects on dopamine release. Low doses of GHB may produce euphoria and disinhibition, whereas high doses result in nausea, agitation, convulsions, and sedation that can lead to unconsciousness and death from respiratory depression. In 2011, more than 2400 emergency ward admissions involved GHB.
Ketamine (Ketaset, Ketalar) is a dissociative anesthetic, similar to PCP. In veterinary medicine, it is used for brief immobilization. In clinical medicine, it is used for sedation, analgesia, and to supplement anesthesia. Ketamine increases heart rate and blood pressure, with less respiratory depression than other anesthetics. Ketamine’s popularity as a club drug appears to reflect its ability to induce a dissociative state and feelings of depersonalization, accompanied by intense hallucinations and subsequent amnesia. It can be administered orally, by smoking (usually in combination with tobacco and/or marijuana), or by IV or IM injection. Like PCP, it binds to NMDA receptors and acts as a noncompetitive NMDA antagonist. In 2011, ketamine accounted for 1550 emergency ward admissions. Ketamine has a complex profile of action and appears to be useful as an antidepressant in treatment-resistant patients and as an analgesic in patients with chronic pain. The extent to which chronic recreational use leads to memory impairment remains controversial.
POLYDRUG ABUSE
Although some drug abusers may prefer a particular drug, the concurrent use of multiple drugs is common. Polydrug abuse often involves substances that may have different pharmacologic effects from the preferred drug. For example, concurrent use of such dissimilar compounds as stimulants and opioids or stimulants and alcohol is common. The diversity of reported drug use combinations suggests that achieving a change in subjective state, rather than any particular direction of change (stimulation or sedation), may be the primary reinforcer in polydrug abuse. There is also evidence that intoxication with alcohol, opiates, and cocaine is associated with increased tobacco smoking. Nicotine and cocaine enhance each other’s effects in clinical laboratory studies, and this drug combination maintains significantly higher levels of self-administration than either drug alone in preclinical models of addiction. There are relatively few controlled studies of multiple drug interactions. However, the combined use of cocaine, heroin, and alcohol increases the risk for toxic effects and adverse medical consequences. Similarly, some hallucinogens (MDMA, LSD) and club drugs (GHB, ketamine, flunitrazepam) are used in various combinations with an associated increase in toxic consequences.
One determinant of polydrug use patterns is the relative availability and cost of the drugs. For example, alcohol abuse, with its attendant medical complications, is one of the most serious problems encountered in former heroin addicts participating in methadone maintenance programs. Cocaine abuse often increases during methadone maintenance.
The physician must recognize that perpetuation of polydrug abuse and drug dependence is not necessarily a symptom of an underlying emotional disorder. Neither alleviation of anxiety nor reduction of depression accounts for initiation and perpetuation of polydrug abuse. Severe depression and anxiety are the consequences of polydrug abuse as frequently as they are the antecedents. Interestingly, some adverse consequences of drug use may be reinforcing and contribute to the continuation of polydrug abuse.
Adequate treatment of polydrug abuse, as well as other forms of drug abuse, requires innovative intervention programs. The first step in successful treatment is detoxification, a process that may be difficult when several drugs with different pharmacologic actions (e.g., alcohol, opiates, and cocaine) have been abused. Because patients may not recall or may deny simultaneous multiple drug use, diagnostic evaluation should always include urinalysis for qualitative detection of psychoactive substances and their metabolites. Treatment of polydrug abuse often requires hospitalization or inpatient residential care during detoxification and the initial phase of drug abstinence. When possible, specialized facilities for the care and treatment of drug-dependent persons should be used. Outpatient detoxification of polydrug abuse patients is unlikely to be effective and may increase risk for dangerous medical consequences.
Drug abuse disorders often respond to effective treatment, but periods of relapse may occur unpredictably. The physician should continue to assist patients during episodes of relapse with compassion and understanding. The physician and the patient must recognize that occasional recurrent drug use is not unusual in this complex behavioral disorder.
1Deceased
470 |
Nicotine Addiction |
The use of tobacco leaf to create and satisfy nicotine addiction was introduced to Columbus by Native Americans and spread rapidly to Europe. Use of tobacco as cigarettes, however, only became popular in the twentieth century and so is a modern phenomenon, as is the epidemic of disease caused by this form of tobacco use.
Nicotine is the principal constituent of tobacco responsible for its addictive character, but other smoke constituents and behavioral associations contribute to the strength of the addiction. Addicted smokers regulate their nicotine intake by adjusting the frequency and intensity of their tobacco use both to obtain the desired psychoactive effects and avoid withdrawal.
Unburned cured tobacco used orally contains nicotine, carcinogens, and other toxicants capable of causing gum disease, oral and pancreatic cancers, and an increase in the risk of heart disease. When tobacco is burned, the resultant smoke contains, in addition to nicotine, more than 7000 other compounds that result from volatilization, pyrolysis, and pyrosynthesis of tobacco and various chemical additives used in making different tobacco products. The smoke is composed of a fine aerosol and a vapor phase; aerosolized particles are of a size range that results in deposition in the airways and alveolar surfaces of the lungs. The aggregate of particulate matter, after subtracting nicotine and moisture, is referred to as tar.
The alkaline pH of smoke from blends of tobacco used for pipes and cigars allows sufficient absorption of nicotine across the oral mucosa to satisfy the smoker’s need for this drug. Therefore, smokers of pipes and cigars tend not to inhale the smoke into the lung, confining the toxic and carcinogenic exposure (and the increased rates of disease) largely to the upper airway for most users of these products. The acidic pH of smoke generated by the tobacco used in cigarettes dramatically reduces absorption of nicotine in the mouth, necessitating inhalation of the smoke into the larger surface of the lungs in order to absorb quantities of nicotine sufficient to satisfy the smoker’s addiction. The shift to using tobacco as cigarettes, with resultant increased deposition of smoke in the lung, has created the epidemic of heart disease, lung disease, and lung cancer that dominates the current disease manifestations of tobacco use.
Several genes have been associated with nicotine addiction. Some reduce the clearance of nicotine, and others have been associated with an increased likelihood of becoming dependent on tobacco and other drugs as well as a higher incidence of depression. Rates of smoking cessation have increased, and rates of nicotine addiction have decreased dramatically, since the mid-1950s, suggesting that factors other than genetics are important. It is likely that genetic susceptibility can influence the probability that adolescent experimentation with tobacco will lead to addiction as an adult.
Adult cigarette smoking prevalence has declined to about 19% in the United States, with 20–40% of those smokers not smoking every day. Male smoking prevalence is falling but remains high in most Asian countries, with increasing smoking prevalence among women in those countries. The highest rates of smoking and least cessation are observed in eastern European countries. Of particular concern is the rapidly rising smoking rate observed in the developing world. The World Health Organization Framework Convention on Tobacco Control is encouraging effective tobacco control approaches in these countries with the hope of preventing a future epidemic of tobacco-related illness.
DISEASE MANIFESTATIONS OF CIGARETTE SMOKING
More than 400,000 individuals die prematurely each year in the United States from cigarette use; this represents almost one of every five deaths in the United States. Approximately 40% of cigarette smokers will die prematurely due to cigarette smoking unless they are able to quit.
The major diseases caused by cigarette smoking are listed in Table 470-1. The ratio of smoking-related disease rates in smokers compared to never smokers (relative risk) is greater at younger ages, particularly for coronary artery disease and stroke. At older ages, the background rate of disease in nonsmokers increases, diminishing the fractional contribution of smoking and the relative risk; however, absolute excess rates of disease mortality found in smokers compared to nonsmokers increase with increasing age. The organ damage caused by smoking and the number of smokers who die from smoking are both greater among the elderly, as one would expect from a process of cumulative injury.
|
RELATIVE RISKS FOR CURRENT SMOKERS OF CIGARETTES |
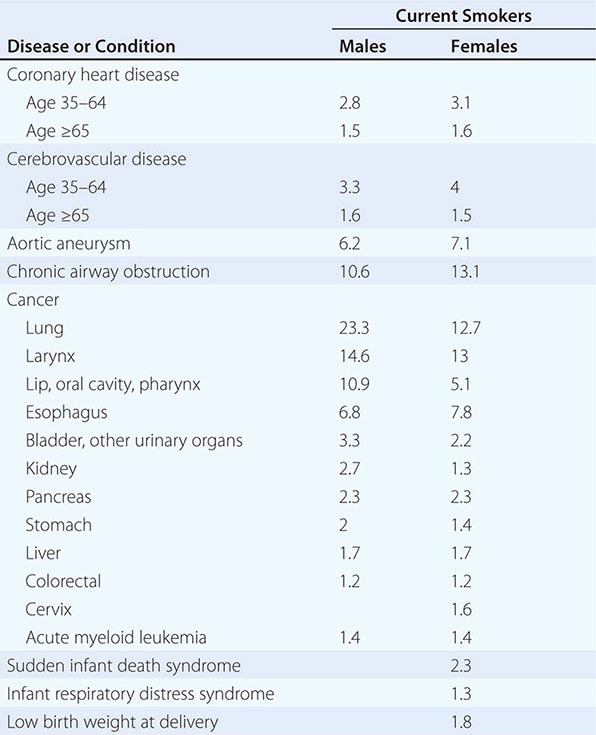
CARDIOVASCULAR DISEASES
Cigarette smokers are more likely than nonsmokers to develop both large-vessel atherosclerosis and small-vessel disease. Approximately 90% of peripheral vascular disease in the nondiabetic population can be attributed to cigarette smoking, as can ~50% of aortic aneurysms. In contrast, 20–30% of coronary artery disease and ~10% of ischemic and hemorrhagic strokes are caused by cigarette smoking. There is a multiplicative interaction between cigarette smoking and other cardiac risk factors such that the increment in risk produced by smoking among individuals with hypertension or elevated serum lipids is substantially greater than the increment in risk produced by smoking for individuals without these risk factors.
In addition to its role in promoting atherosclerosis, cigarette smoking also increases the likelihood of myocardial infarction and sudden cardiac death by promoting platelet aggregation and vascular occlusion. Reversal of these effects on coagulation may explain the rapid benefit of smoking cessation for a new coronary event demonstrable among those who have survived a first myocardial infarction. This effect may also explain the substantially higher rates of graft occlusion among continuing smokers following vascular bypass surgery for cardiac or peripheral vascular disease.
Cessation of cigarette smoking reduces the risk of a second coronary event within 6–12 months; rates of first myocardial infarction and death from coronary heart disease also decline within the first few years following cessation among those with no prior cardiovascular history. After 15 years of abstinence, the risk of a new myocardial infarction or death from coronary heart disease in former smokers is similar to that for those who have never smoked.
CANCER
Tobacco smoking causes cancer of the lung; oral cavity; naso-, oro-, and hypopharynx; nasal cavity and paranasal sinuses; larynx; esophagus; stomach; pancreas; liver (hepatocellular); colon and rectum; kidney (body and pelvis); ureter; urinary bladder; and uterine cervix, and also causes myeloid leukemia. There is evidence suggesting that cigarette smoking may play a role in increasing the risk of breast cancer. There does not appear to be a causal link between cigarette smoking and cancer of the endometrium, and there is a lower risk of uterine cancer among postmenopausal women who smoke. The risks of cancer increase with the increasing number of cigarettes smoked per day and with increasing duration of smoking. Additionally, there are synergistic interactions between cigarette smoking and alcohol use for cancer of the oral cavity and esophagus. Several occupational exposures synergistically increase lung cancer risk among cigarette smokers, most notably occupational asbestos and radon exposure.
Cessation of cigarette smoking reduces the risk of developing cancer relative to continuing smoking, but even 20 years after cessation, there is a modest persistent increased risk of developing lung cancer.
RESPIRATORY DISEASE
Cigarette smoking is responsible for 90% of chronic obstructive pulmonary disease. Within 1–2 years of beginning to smoke regularly, many young smokers will develop inflammatory changes in their small airways, although lung function measures of these changes do not predict development of chronic airflow obstruction. After 20 years of smoking, pathophysiologic changes in the lungs develop and progress proportional to smoking intensity and duration. Chronic mucous hyperplasia of the larger airways results in a chronic productive cough in as many as 80% of smokers >60 years of age. Chronic inflammation and narrowing of the small airways and/or enzymatic digestion of alveolar walls resulting in pulmonary emphysema can result in reduced expiratory airflow sufficient to produce clinical symptoms of respiratory limitation in ~15–25% of smokers.
Changes in the small airways of young smokers will reverse after 1–2 years of cessation. There may also be a small increase in measures of expiratory airflow following cessation among individuals who have developed chronic airflow obstruction, but the major change following cessation is a slowing of the rate of decline in lung function with advancing age rather than a return of lung function toward normal.
PREGNANCY
Cigarette smoking is associated with several maternal complications of pregnancy: premature rupture of membranes, abruptio placentae, and placenta previa; there is also a small increase in the risk of spontaneous abortion among smokers. Infants of smoking mothers are more likely to experience preterm delivery, have a higher perinatal mortality rate, be small for their gestational age, and have higher rates of infant respiratory distress syndrome; they are more likely to die of sudden infant death syndrome and appear to have a developmental lag for at least the first several years of life.
OTHER CONDITIONS
Smoking delays healing of peptic ulcers; increases the risk of developing diabetes, active tuberculosis, rheumatoid arthritis, osteoporosis, senile cataracts, and neovascular and atrophic forms of macular degeneration; and results in premature menopause, wrinkling of the skin, gallstones and cholecystitis in women, and male impotence.
ENVIRONMENTAL TOBACCO SMOKE
Long-term exposure to environmental tobacco smoke increases the risk of lung cancer and coronary artery disease among nonsmokers. It also increases the incidence of respiratory infections, chronic otitis media, and asthma in children and causes exacerbation of asthma in children. Some evidence suggests that environmental tobacco smoke exposure may increase the risk of premenopausal breast cancer. Patients who continue to smoke during treatment for cancer with chemotherapy or radiation have poorer outcomes and reduced survival.
PHARMACOLOGIC INTERACTIONS
Cigarette smoking may interact with a variety of other drugs (Table 470-2). Cigarette smoking induces the cytochrome P450 system, which may alter the metabolic clearance of drugs such as theophylline. This may result in inadequate serum levels in smokers as outpatients when the dosage is established in the hospital under nonsmoking conditions. Correspondingly, serum levels may rise when smokers are hospitalized and not allowed to smoke. Smokers may also have higher first-pass clearance for drugs such as lidocaine, and the stimulant effects of nicotine may reduce the effect of benzodiazepines or beta blockers.
|
INTERACTIONS OF SMOKING AND PRESCRIPTION DRUGS |
OTHER FORMS OF TOBACCO USE
Other major forms of tobacco use are moist snuff deposited between the cheek and gum, chewing tobacco, pipes and cigars, and recently bidi (tobacco wrapped in tendu or temburni leaf; commonly used in India), clove cigarettes, and water pipes. Oral tobacco use leads to gum disease and can result in oral and pancreatic cancer as well as heart disease, with dramatic differences in the risks evident for products used in Africa and Asia as compared to those in the United States and Europe.
All forms of burned tobacco generate toxic and carcinogenic smoke similar to that of cigarette smoke. The differences in disease consequences of use relate to frequency of use and depth of inhalation. The risk of upper airway cancers is similar among cigarette, pipe, and cigar smokers, whereas those who have smoked only pipes and cigars have a much lower risk of lung cancer, heart disease, and chronic obstructive pulmonary disease. However, cigarette smokers who switch to pipes or cigars do tend to inhale the smoke, increasing their risk; and it is likely that comparable inhalation and frequency of exposure to tobacco smoke from any of these forms of tobacco use will lead to comparable disease outcomes.
A resurgence of cigar, bidi, and water pipe use among adolescents of both genders has raised concerns that these older forms of tobacco use are once again causing a public health problem. A variety of devices are currently sold that deliver nicotine by electronically heating materials containing nicotine, the so-called electronic cigarettes. Although these devices are marketed as substitutes for cigarettes and as cessation tools, the composition of the vapor and nicotine delivery varies widely from product to product, raising questions of both safety and efficacy in the absence of regulatory oversight.
LOWER TAR AND NICOTINE CIGARETTES
Filtered cigarettes with lower machine-measured yields of tar and nicotine commonly use ventilation holes in the filters and other engineering designs to artificially lower the machine measurements. Smokers compensate for the lowered nicotine delivery by changing the manner in which they puff on the cigarette or the number of cigarettes smoked per day, and tar and nicotine deliveries are not reduced with use of these products. Cigarette design changes that reduce machine-measured tar and nicotine lead to deeper inhalation of the smoke and an increase in the carcinogenicity of the smoke inhaled by smokers. The presentation of more carcinogenic smoke to the alveolar portions of the lung has resulted in an increase in the risk of lung cancer, and possibly chronic obstructive pulmonary disease, among smokers over the past six decades. This change in cigarette product is also one cause of the dramatic rise in rates of adenocarcinoma of the lung observed over the past half century. There has been no increase in risk of lung cancer or adenocarcinoma of the lung in never smokers over time.
CESSATION
The process of stopping smoking is commonly a cyclical one, with the smoker sometimes making multiple attempts to quit and failing before finally being successful. Approximately 70–80% of smokers would like to quit smoking. More than one-half of current smokers attempted to quit in the last year, but only 6% quit for 6 months, and only 3% remain abstinent for 2 years. Clinician-based smoking interventions should repeatedly encourage smokers to try to quit and to use different forms of cessation assistance with each new cessation attempt rather than focusing exclusively on immediate cessation at the time of the first visit.
Advice from a physician to quit smoking, particularly at the time of an acute illness, is a powerful trigger for cessation attempts, with up to half of patients who are advised to quit making a cessation effort. Other triggers include the cost of cigarettes, media campaigns, and changes in rules to restrict smoking in the workplace.
PHYSICIAN INTERVENTION (TABLE 470-3)
|
CLINICAL PRACTICE GUIDELINES |
aNumerical value following the intervention is the multiple for cessation success compared to no intervention.
All patients should be asked whether they smoke, how much they smoke, how long they have smoked, their past experience with quitting, and whether they are currently interested in quitting. Intensity of smoking and smoking within 30 min of waking are useful measures of the intensity of nicotine addiction. Even those who are not interested in quitting should be encouraged and motivated to quit; provided a clear, strong, and personalized message by the clinician that smoking is an important health concern; and offered assistance if they become interested in quitting in the future. Many of those not currently expressing an interest in quitting may nevertheless make an attempt to quit in the subsequent year. For those interested in quitting, a quit date should be negotiated, usually not the day of the visit but within the next few weeks, and a follow-up contact by office staff around the time of the quit date should be provided. There is a relationship between the amount of assistance a patient is willing to accept and the success of the cessation attempt.
There are a variety of nicotine-replacement products, including over-the-counter nicotine patches, gum, and lozenges, as well as nicotine nasal and oral inhalers available by prescription. These products can be used for up to 3–6 months, and some products are formulated to allow a gradual step-down in dosage with increasing duration of abstinence. Antidepressants such as bupropion (300 mg in divided doses for up to 6 months) have also been shown to be effective, as has varenicline, a partial agonist for the nicotinic acetylcholine receptor (initial dose 0.5 mg daily increasing to 1 mg twice daily at day 8; treatment duration up to 6 months). Severe psychiatric symptoms, including suicidal ideation, have been reported with varenicline, resulting in a U.S. Food and Drug Administration–mandated warning and a recommendation for closer therapeutic supervision, but evidence to establish the frequency of these responses and the specificity of their association with varenicline remains unclear. Some evidence supports the combined use of nicotine-replacement therapy (NRT) and antidepressants as well as the use of gum or lozenges for acute cravings in patients using patches. Pretreatment with antidepressants or varenicline is recommended for 1–2 weeks prior to the quit date, and pretreatment with nicotine-replacement products is also being explored, as is longer duration of nicotine replacement as a maintenance therapy for those who are unsuccessful in quitting with a shorter duration of use. NRT is provided in different dosages, with higher doses being recommended for more intense smokers. Clonidine or nortriptyline may be useful for patients who have failed on first-line pharmacologic treatment or who are unable to use other therapies. Antidepressants are more effective in those with a history of depression symptoms.
Current recommendations are to offer pharmacologic treatment, usually with NRT or varenicline, to all who will accept it and to provide counseling and other support as a part of the cessation attempt. There are some data to suggest that longer term use of NRT may enable cessation in some smokers who are unable to quit with shorter duration use and that some individuals are able to achieve abstinence from tobacco through use of NRT chronically. Cessation advice alone by a physician or his or her staff is likely to increase success compared with no intervention; a more comprehensive approach with advice, pharmacologic assistance, and counseling can increase cessation success nearly threefold.
Incorporation of cessation assistance into a practice requires a change of the care delivery infrastructure. Simple changes include (1) adding questions about smoking and interest in cessation on patient-intake questionnaires, (2) asking patients whether they smoke as part of the initial vital sign measurements made by office staff, (3) listing smoking as a problem in the medical record, and (4) automating follow-up contact with the patient on the quit date. These changes are essential to institutionalizing smoking intervention within the practice setting; without this institutionalization, the best intentions of physicians to intervene with their patients who smoke are often lost in the time crush of a busy practice.
PREVENTION
Approximately 85% of individuals who become cigarette smokers initiate the behavior during adolescence. Factors that promote adolescent initiation are parental or older-sibling cigarette smoking, tobacco advertising and promotional activities, the availability of cigarettes, and the social acceptability of smoking. The need for an enhanced self-image and to imitate adult behavior is greatest for those adolescents who have the least external validation of their self-worth, which may explain in part the enormous differences in adolescent smoking prevalence by socioeconomic and school performance strata.
Prevention of smoking initiation must begin early, preferably in the elementary school years. Physicians who treat adolescents should be sensitive to the prevalence of this problem even in the pre-teen population. Physicians should ask all adolescents whether they have experimented with tobacco or currently use tobacco, reinforce the fact that most adolescents and adults do not smoke, and explain that all forms of tobacco are both addictive and harmful.
471e |
Neuropsychiatric Illnesses in War Veterans |
Neuropsychiatric sequelae are common in combat veterans. Advances in personal protective body armor, armored vehicles, battlefield resuscitation, and the speed of evacuation to tertiary care have considerably improved the survivability of battlefield injuries, resulting in a greater awareness of the “silent wounds” associated with service in a combat zone. Although psychiatric and neurologic problems have been well documented in veterans of prior wars, the conflicts in Iraq and Afghanistan that began after September 11, 2001, were unique in terms of the level of commitment by the U.S. Department of Defense (DoD) and Department of Veterans Affairs (VA), Veterans Health Administration (VHA) to support research as the wars unfolded and to use that knowledge to guide population-level screening, evaluation, and treatment initiatives.
The Iraq and Afghanistan conflicts produced over 2.5 million combat veterans, many of whom have received or will need care in government and civilian medical facilities in the future. Studies clearly showed that service in the Iraq and Afghanistan theaters was associated with significantly elevated rates of mental disorders. Two conditions in particular have been labeled the signature injuries related to these wars: posttraumatic stress disorder (PTSD) and mild traumatic brain injury (mTBI)—also known as concussion. Although particular emphasis will be given in this chapter to PTSD and concussion/mTBI, it is important to understand that the neuropsychiatric sequelae of war are much broader than these two conditions. Wartime service is associated with a number of health concerns that coexist and overlap, and a multidisciplinary patient-centered approach to care is necessary.
EPIDEMIOLOGY OF WAR-RELATED PSYCHOLOGICAL AND NEUROLOGIC CONDITIONS
Service members involved in the Iraq and Afghanistan wars faced multiple deployments to two very different high-intensity combat theaters, and for many veterans, the cumulative strain negatively impacted health, marriages, parenting, educational goals, and civilian occupations. The stresses of service in these conflicts also led to a significant increase in rates of suicide in personnel from the two branches of service involved in the greatest level of ground combat (U.S. Army, Marines).
Service in a war zone can involve extreme physical stress in austere environments, prolonged sleep deprivation, physical injury, exposure to highly life-threatening events, and hazards such as explosive devices, sniper fire, ambushes, indirect fire from rockets and mortars, and chemical pollutants. Certain events, such as loss of a close friend in combat, leave indelible scars. All of these experiences have additive effects on health, likely mediated through physiologic mechanisms involving dysregulation of neuroendocrine and autonomic nervous system (ANS) functions.
Veterans of virtually all wars have reported elevated rates of generalized and multisystem physical, cognitive, and psychological health concerns that often become the focus of treatment months or years after returning home. These multisystem health concerns include sleep disturbance, memory and concentration problems, headaches, musculoskeletal pain, gastrointestinal symptoms (including gastroesophageal reflux), residual effects of wartime injuries, fatigue, anger, hyperarousal symptoms, high blood pressure, rapid heart rate (sometimes associated with panic symptoms), sexual problems, and symptoms associated with PTSD and depression. In order to provide optimal care to veterans with these symptoms, it is important to understand how the symptoms interrelate and to consider the possibility that there may be underlying combat-related physiologic effects.
POSTWAR SYMPTOMS
The overlapping and multisystem health concerns reported by warriors from every generation have been given different labels and have led to debates among medical professionals as to whether these are mediated primarily by physical or psychological causes. For example, World War I produced extensive debate about whether “shell shock,” diagnosed in more than 80,000 British soldiers, was neurologic (“commotional” from the brain being shaken in the skull by concussive blasts) or psychological (“emotional” or “neurasthenia”) in origin. World War II veterans were said to suffer from “battle fatigue,” Korean War veterans developed “combat stress reactions,” and Vietnam veterans developed the “post-Vietnam syndrome.” The role of environmental exposure (e.g., Agent Orange) and psychological causes (e.g., PTSD, depression, substance use disorders) continue to be debated.
Gulf War I (Operation Desert Storm), following the Iraqi invasion of Kuwait in 1990, led to extensive debates as to whether Gulf War syndrome, also known as multisystem illness, was best explained by environmental exposures (e.g., oil fires, depleted uranium, nerve gas, pesticides, multiple vaccinations) or the psychological stress of deployment to a war zone where there was anticipation of high casualty rates from chemical and biologic weapons, repeated stressful alerts, and training exercises involving the use of impermeable full-body protective uniforms (made from rubber, vinyl, charcoal-impregnated polyurethane, and other materials) in desert conditions under extreme temperatures. Although no clinical syndrome was ever definitively confirmed among the nearly 1 million service members who deployed in 1990–1991, studies consistently found that military personnel who served in the Gulf experienced elevations in generalized symptoms across all health domains (e.g., physical, cognitive, neurologic, psychological) compared with service members who deployed elsewhere or did not deploy. In addition, there is good evidence that deployment to the Persian Gulf region during this period was associated with subsequent development of PTSD; other psychiatric disorders including generalized anxiety disorder, depression, and substance use disorders (Chap. 467); functional gastrointestinal symptoms such as irritable bowel syndrome (Chap. 352); and chronic fatigue syndrome (Chap. 464e).
The conflicts in Iraq and Afghanistan led to similar debates as to whether postwar symptoms such as headaches, irritability, sleep disturbance, dizziness, and concentration problems are best attributed to concussion/mTBI or to PTSD. Numerous studies showed that either PTSD or depression explained the majority of the postdeployment “postconcussive” symptoms attributed to concussion/mTBI, a finding not well received by many experts in traumatic brain injury (TBI) but consistent with civilian studies on risk factors for developing persistent symptoms after concussion. As in past wars, the polarized nature of the debate largely focused on only the two conditions of PTSD and concussion/mTBI, which has interfered with full appreciation of how the large spectrum of deployment-related health concerns interrelate and of the clinical implications for designing effective evaluation and treatment strategies.
Veterans understandably may become angry at the suggestion that their postwar health concerns could be “stress-related” or psychological, and thus it is necessary for primary care professionals to be able to discuss the physical toll that war-zone service has on the body, the generalized nature of war-related health concerns, and the likely underlying physiologic neuroendocrine and ANS contributors. Mental health specialists also need to be able to reinforce this message and understand the important role they have in promoting physical health through addressing comorbid health concerns.
PTSD
PTSD (Chap. 466) is the most common mental disorder documented following war-zone service. Studies from the conflicts in Iraq and Afghanistan found PTSD prevalence rates of 2–6% before deployment (comparable to civilian general population samples) and rates of 6–20% after deployment, depending primarily on the level of combat frequency and intensity. Many other veterans experience subclinical PTSD symptoms after war-zone service, sometimes termed posttraumatic stress (PTS) or combat stress. These subclinical symptoms can contribute to distress and affect health, even if overall functioning is not as impaired as in the full disorder.
The definition of PTSD was modified in the fifth edition of the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (2013), although most individuals who had PTSD diagnosed according to the previous criteria also meet the definition under the new criteria. PTSD is defined as persistent (>1 month) symptoms occurring after a traumatic event (involving exposure to actual or threatened death, serious injury, or sexual assault). The symptoms must be associated with significant distress or impairment in social or occupational functioning. Symptoms are grouped into four categories: (1) intrusion/reexperiencing symptoms in which the person has nightmares, flashbacks, or intrusive (often involuntary) memories connected with the traumatic event; (2) avoidance symptoms where the person avoids distressing memories or people, places, situations, or other stimuli that serve as reminders of the traumatic event (for example, a crowded mall that triggers heightened alertness to threat); (3) negative alterations of cognitions or mood (for example, feeling detached or losing interest in things that previously brought enjoyment); and (4) hyperarousal symptoms in which the person is physiologically revved up, hyperalert, startles easily, and experiences sleep disturbance, anger, and/or concentration problems. Although PTSD is a clinical symptom-based case definition, it is best to think of PTSD not as an emotional or psychological/psychiatric condition, but rather as a physiologically-based response to life-threatening trauma that is associated with physical, cognitive, emotional, and psychological symptoms.
PTSD has strong biologic correlates, based in fear-conditioning responses to threat and responses to extreme stress involving neuroendocrine dysregulation and ANS reactivity. Numerous studies have shown that PTSD is highly correlated with generalized physical and cognitive symptoms—including hypertension, chronic pain, and cardiovascular disease—as well as cell-mediated immune dysfunction and shortened life expectancy. PTSD is frequently comorbid with other mental disorders such as major depressive disorder, generalized anxiety, substance use disorders, and risky behaviors (e.g., aggression, accidents); it has been estimated that up to 80% of patients with PTSD exhibit one or more comorbid conditions. Misuse of alcohol or substances is most prevalent, often reflecting self-medication. PTSD is also associated with tolerance and withdrawal symptoms related to prescription pain and sleep medications, as well as nicotine dependence (Chap. 470).
Clinicians should understand how to provide meaningful psychological education in a way that resonates with veterans who may have PTSD symptoms as a result of their military service. There is an important occupational context to consider, which is also applicable to trauma exposures that occur in other first responder professions, such as law enforcement officers and firefighters. Service members and other first responders are trained to respond to traumatic events and effectively learn to override automatic fight-or-flight reflexes in order to carry out their duties. Reactions that are labeled as symptoms of PTSD are based on adaptive survival responses that are beneficial in a combat environment. For example, physiologic hyperarousal, use of anger, and being able to shut down other emotions are very useful skills in combat and can be present even prior to traumatic events during tough realistic training. It is natural for these responses to persist after returning home, and the label of a “disorder” only gets applied when the responses that persist significantly impair functioning.
CONCUSSION/mTBI
TBI (Chap. 457e) gained increased recognition during the conflicts in Iraq and Afghanistan because of the widespread exposure of troops to improvised explosive devices. Many veterans of Iraq and Afghanistan reported experiencing multiple concussions during deployments, and many also reported ignoring concussions and not seeking treatment at the time of injury in order to remain with their unit. However, these legitimate concerns were also counterbalanced and challenged by high prevalence estimates of deployment-related TBI that did not distinguish concussion/mTBI from moderate or severe TBI; data from animal models of blast exposure that did not necessarily extrapolate to human experiences on the battlefield; neuroimaging studies (e.g., diffusion tensor imaging) that attributed putative abnormalities to blast exposure but lacked adequate control comparisons; and fear-provoking speculation that repetitive blast exposure may lead to future dementia, based largely on case series of professional athletes (e.g., boxers, football players) exposed to highly repetitive injuries linked to chronic traumatic encephalopathy (previously termed dementia pugilistica) (Chap. 444e).
TBI includes closed and penetrating head injuries; closed head injuries are categorized as mild (mTBI or concussion), moderate, or severe based on the duration of loss of consciousness, duration of posttraumatic amnesia, and the Glasgow coma score (GCS) (see Table 457e-2). Several studies have estimated that 10–20% of all military personnel deployed to Iraq or Afghanistan sustained one or more concussion/mTBI events during deployment, most commonly from exposure to blasts; however, concussion injuries are also common in nondeployed environments from sports, training (e.g., hand-to-hand combatives), and accidents.
Although there is a neurophysiologic continuum of injury, there are stark clinical and epidemiologic distinctions between concussion/mTBI and moderate or severe TBI (Table 471e-1). Concussion/mTBI is defined as a blow or jolt to the head that results in brief loss of consciousness (LOC) for <30 min (most commonly, only a few seconds to minutes), posttraumatic amnesia (PTA) of <24 h (most commonly <1 h), or transient alteration in consciousness (AOC) without LOC. The majority of concussions in Iraq or Afghanistan involved AOC without LOC or PTA (which soldiers may refer to as getting their “bell rung”). GCSs in concussion/mTBI are usually normal (15 out of 15). Concussion is treated with rest to allow the brain time to heal, and it almost never resulted in air evacuation from Iraq or Afghanistan unless there were other associated injuries.
|
COMPARISON BETWEEN CONCUSSION/MILD TRAUMATIC BRAIN INJURY (TBI) AND MODERATE/SEVERE TBI |
In contrast, moderate, severe, or penetrating TBI, which is estimated to account for <1% of all battlefield head injuries in Iraq and Afghanistan, is characterized by LOC ≥30 min (up to permanent coma), PTA ≥24 h (also may be permanent), and GCSs as low as 3 (the minimum value). These virtually always result in air evacuation from the battlefield and carry a significant risk of severe long-term neurologic impairment and requirement for rehabilitative care.
Symptoms following concussion/mTBI can include headache; fatigue; concentration, memory, or attention problems; sleep disturbance; irritability; balance difficulties; and tinnitus, among other symptoms. Recovery is usually rapid, with symptoms usually resolving in a few hours to days, but in a small percentage of patients, symptoms may persist for a longer period or become chronic (referred to as persistent “postconcussive symptoms” [PCS]).
Establishing a clear causal connection between a deployment concussion injury and persistent PCS months or years after return from deployment has been difficult and often confounded by other postwar conditions that are associated with the same symptoms, including injuries not involving the head, other medical disorders, sleep disorders, PTSD, depression, grief, substance use disorders, chronic pain, and the generalized physiologic effects of wartime service. Contributing to the difficulty in establishing causation is the fact that the concussion/mTBI case definition refers only to the acute injury event and lacks symptoms, time course, or impairment; case definitions for persistent postconcussion syndrome have failed tests of validation. Numerous studies found that PTSD and depression were much stronger predictors of PCS and objective neuropsychological impairment after combat deployment than concussions/mTBIs, and one study even found that bereavement (particularly related to the death of a team member) was as strong a predictor of postdeployment symptoms and poor general health as were symptoms of depression or PTSD. These data do not minimize the importance of concussion/mTBI per se, but highlight the complex interrelationships of war-related health problems and the relatively lower importance of concussion/mTBI in overall postdeployment health than is generally thought.
Studies of veterans who sustained concussions in Iraq or Afghanistan have suggested that blast mechanisms produce similar clinical outcomes as nonblast mechanisms, in contrast to expectations based on some animal models. An explosion can produce serious injury from rapid atmospheric pressure changes (primary blast wave mechanism), as well as from munition fragments/flying debris (secondary blast mechanism) or being thrown into a hard object (tertiary blast mechanism). Secondary and tertiary mechanisms are similar to other mechanical mechanisms of concussions sustained during accidents. It is likely that blast physics explains differences between human clinical studies and experimental animal studies. Because the distribution of munition fragments usually extends well beyond the distribution of the primary blast wave in most explosions, the possibility of a unique head injury solely from the primary blast wave in otherwise uninjured service members appears to be very low.
Multisystem health problems that lack clear case definitions do not lend themselves well to uniform public health strategies such as screening. Nevertheless, mass population screening for concussion/mTBI was mandated for all U.S. service members returning from Iraq or Afghanistan and all veterans presenting for care at VA health care facilities. These screening processes attempt to apply the acute concussion case definition (lacking symptoms, time course, or impairment) months or years after injury, and often involve questions that encourage patients and clinicians to make a direct link between current symptoms and past head injuries that likely have very little to do with the current symptoms. These screening approaches led to sharp criticism that they were encouraging clinicians to misattribute common postwar symptoms to concussion/mTBI. Nevertheless, the screening processes have persisted and are part of an extensive specialty structure of care erected in both the DoD and VA to address health concerns attributed to concussions/mTBIs.
Management of postwar physical and cognitive health concerns is largely symptom focused and ideally carried out within primary care–based structures of care. Studies suggest that optimal strategies for treatment of multisymptom health concerns include regularly scheduled primary care visits with brief physical exam at each visit, protecting patients from unnecessary diagnostic tests and non-evidence-based interventions, judicious use of consultations that protects patients from unnecessary specialty referrals, care/case management, and communication that enhances positive expectations for recovery. Concussion research has shown that negative expectations are one of the most important risk factors for persistent symptoms.
Although many questions remain regarding the long-term health effects of concussions (particularly multiple concussions) sustained during deployment, these are important battlefield injuries that require careful attention. However, they need to be addressed within the context of a much broader approach to other war-related health concerns.
STIGMA AND BARRIERS TO CARE
Stigma and other barriers to care add to the complexity of treating veterans. Despite extensive education efforts among military leaders and service members, perceptions of stigma showed little change over the many years of war; warriors are often concerned that they will be perceived as weak by peers or leaders if they seek care. Studies have shown that less than one-half of service members and veterans with serious mental health problems receive needed care, and upwards of half of those who begin treatment drop out before receiving an adequate number of encounters. Many factors contribute to this, including the pervasive nature of stigma in society in general (particularly among men), the critical importance of group cohesiveness of military teams, the nature of avoidance symptoms in PTSD, perceptions of self-sufficiency (e.g., “I can handle problems on my own”), and sometimes negative perceptions of mental health care and skepticism that mental health professionals will be able to help.
DISCLOSURE
The opinions or assertions contained herein are the private views of the author and are not to be construed as official, or as reflecting the views of the Department of the Army or the Department of Defense.