11 Clinical Trials of Defibrillator Therapy
 Sudden Death: Definition and Implication for Clinical Trials
Sudden Death: Definition and Implication for Clinical Trials
Sudden cardiac death is defined as a death attributable to cardiac cause that occurs soon after the onset of symptoms. A 49% reduction in the incidence of sudden death has been documented over more than 50 years.1 The incidence of out-of-hospital cardiac arrest from ventricular fibrillation (VF) has also been reduced.2 These reductions largely appear to be related to improved management of coronary heart disease risk factors and prevention strategies that include prophylactic insertion of ICD systems.3
Despite these advances, sudden death remains a major public health problem. Worldwide, more than 3 million lives are ended prematurely because of sudden death,4 many in ambulatory populations from sustained ventricular tachycardia (VT) or VF.5,6 Bradyarrhythmic events and electromechanical dissociation may be more common mechanisms in patients with end-stage heart failure.7
A definition of sudden death that includes events that occur within 1 hour of symptom onset has been considered reliable in identifying deaths related to arrhythmias.8 Definitions of sudden death that also incorporate the circumstances surrounding these events may provide additional value in separating arrhythmic from nonarrhythmic causes.9 Despite these refinements, however, misclassification occurs.10 Autopsy data from a large study of patients who survived a myocardial infarction (MI), had residual left ventricular (LV) dysfunction, and later suffered sudden death found that half of deaths categorized as “sudden” were nonarrhythmic. Most of these nonarrhythmic sudden deaths were related to a recurrent MI or cardiac rupture and were more frequent in the initial 6 months after the initial MI.11 Another study found that VT or VF were responsible for most sudden deaths in a group of post-MI patients with moderate LV dysfunction in whom the cardiac rhythm was monitored at the time of these events. Bradyarrhythmias were responsible for a minority of these sudden arrhythmic deaths, and VT or VF was the underlying rhythm for some deaths categorized as nonarrhythmic.6
 Evolution of Therapy: Implication for Clinical Trials
Evolution of Therapy: Implication for Clinical Trials
The first implantation of an automated defibrillator occurred in the 1970s.12 Over the past three decades, improvements have been made in device capability and size. The contemporary tiered-therapy ICD is capable of delivering antitachycardia pacing (ATP), low-energy cardioversion, and high-energy defibrillation therapies. In addition, it provides backup bradycardia pacing. The ICD effectively terminates the vast majority of sustained VT or VF events and most bradyarrhythmias.13 Enhancements that include painless ATP therapies,14,15 algorithms to reduce the likelihood of unnecessary ICD therapies,16 and remote monitoring17,18 have added to the appeal of the ICD. The addition of cardiac resynchronization therapy (CRT) offers the potential to improve LV function, reduce morbidity, and further decrease mortality in select patients.19
Most contemporary ICD systems are placed in a subcutaneous or submuscular position in the chest and use the venous anatomy for lead delivery. The risk of death related to placement of nonthoracotomy, transvenous ICD systems approaches 0%. In contrast, earlier ICD systems that required a thoracotomy or sternotomy for lead placement were associated with a 5% mortality risk in the first 30 postoperative days.20,21 The ease of implantation and relatively low risk of surgical morbidity and mortality with contemporary ICD system placement have altered the direction of studies examining ICD efficacy. Initial trials were limited to patients considered at “very high risk” of cardiac arrest, whereas later trials have included patients at somewhat lower risk.
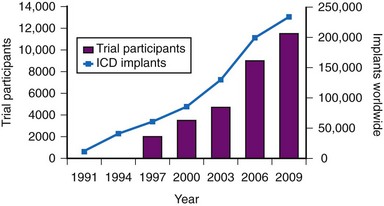
The results of clinical trials over the past two decades have established the ICD as a cornerstone of therapy for patients at risk for sudden death.22,23 The number of ICD implantations worldwide now exceeds 250,000 per year.24 The exponential increase in the number of ICD systems implanted over the past decade mirrors the quantity of patient data obtained from randomized clinical trials4,24 (Fig. 11-1).
 Quality of Life
Quality of Life
In contrast to antiarrhythmic drugs, which are designed to prevent arrhythmias, the ICD treats arrhythmias that have already developed. Because the ICD prolongs life, patients may experience deterioration in underlying health that might not have otherwise occurred. Stress, anxiety, depression, mood disturbance, sexual dysfunction, and a sense of uncertainty or loss of control have been described in ICD recipients.25 The ICD also provides a sense of reassurance to some patients, enhancing their quality of life (QOL). The use of an ICD with CRT may further enhance QOL by reducing heart failure symptoms and preventing hospitalizations.19
Six large trials have published data related to QOL with ICD therapy. Two trials included patients with spontaneous or inducible ventricular arrhythmias and found that ICD therapy and amiodarone were associated with similar alterations in QOL.26,27 Results from the four trials that included patients without a history of these arrhythmias are less consistent. The Coronary Artery Bypass Graft (CABG) Patch trial found reduced QOL with ICD therapy,28 as did the Second Multicenter Automatic Defibrillator Implantation Trial (MADIT-II).29 In contrast, the Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) trial30 and the Sudden Cardiac Death Heart Failure Trial (SCD-HeFT) found no significant difference in QOL over time.31 Details regarding the QOL assessment methods and potential explanations for these differences are provided later in the individual reviews of these trials.
 Limitations of Therapy
Limitations of Therapy
Shocks
Even sporadic ICD shocks are associated with significant, independent reductions in QOL. Compared with patients who did not experience shocks, patients in the Antiarrhythmics versus Implantable Defibrillators (AVID) trial who experienced at least one shock during the initial year of follow-up had reduced QOL, independent of LV ejection fraction (EF), social circumstances, and medication use.26 The reductions in QOL associated with shocks in AVID were similar in magnitude to clinically important adverse effects from amiodarone. In MADIT-II, a higher prevalence of heart failure and the development of shocks in ICD-treated patients were associated with reductions in QOL among ICD recipients.29 ICD shocks were also associated with reduced QOL in the DEFINITE trial.30 In SCD-HeFT, QOL was diminished over the short term following ICD shocks, but subsequently improved.31 This implies a direct association between ICD shocks and reduced QOL.
The development of clusters of life-threatening arrhythmias identifies patients at high risk of death in the near term. Patients in AVID who experienced electrical storm (at least three episodes of VT or VF in a 24-hour period) had a greater than fivefold higher risk of death in the subsequent 3 months.32 The majority of these deaths are related to progressive heart failure, supporting a link between electrical storm and deteriorating LV function. The MADIT-II investigators found that inappropriate ICD shocks were common, representing over 30% of all shocks, and were associated with a twofold higher risk of death. Most of these inappropriate shocks were related to atrial fibrillation or a supraventricular tachycardia.33 In SCD-HeFT, risk of death with appropriate ICD shocks was fivefold higher, and risk of death with inappropriate ICD shocks was twofold higher. Most of the deaths in the patients who received shocks and later died in SCD-HeFT were attributed to progressive heart failure.34
It is not known whether the development of isolated or multiple ICD shocks identifies patients with worsening LV function or whether the ICD therapies themselves contribute to progressive LV dysfunction. Evidence links multiple shocks with myocardial injury and fibrosis.35,36 There is also evidence in animal models that ICD shocks may be proarrhythmic.37 Data from human studies support a proarrhythmic effect of some shocks and suggest that multiple shocks can result in myocardial stunning and electromechanical dissociation.38,39 Also, observational data indicate that ICD shocks, but not ATP therapies, are associated with a higher risk of death.40 However, other studies did not find a difference in outcome if arrhythmias were terminated by ATP or by shocks.32 Nonetheless, strategies to reduce the likelihood of inappropriate and appropriate shocks, and alternative methods to terminate ventricular arrhythmias, are vital avenues of investigation.16
Antiarrhythmic drugs can be used to reduce the likelihood of ICD shocks,41,42 but may negatively affect QOL because of their adverse effects.26 ATP therapies painlessly interrupt reentrant ventricular arrhythmias using brief, rapid bursts of overdrive pacing. ATP has long been used to terminate slower ventricular arrhythmias (rates <188 beats per minute [bpm]) with an efficacy exceeding 90% and a low (<5%) risk of accelerating these slower arrhythmias to a more rapid one that requires a defibrillation shock. ATP has also been shown to be useful for terminating fast VT (188-250 bpm)43 and should be used as the initial treatment for treating these arrhythmias.16 The use of ATP for fast VT has been demonstrated to enhance QOL,43 both in patients with, and in those without, a history of sustained arrhythmias before ICD implantation.44 Extending the time to detect and treat a ventricular arrhythmia, from 4 to 5 seconds to 9 to 12 seconds, also appears to be useful in reducing the number of ICD shocks,45 because even fast VT episodes will spontaneously terminate 30% of the time.43 The combined use of algorithms that avoid shocks from external and intracardiac noise, provide enhanced redetection, and maximize the use of ATP therapies has been estimated to reduce unnecessary ICD shocks by more than 80%.46 It is important to note that the safety and efficacy of delaying the detection of these arrhythmias or the use of multiple algorithms to reduce shocks are not known. Ongoing studies are in progress to address these and other relevant issues.
Reliability
Despite the intuitive appeal of the ICD, the clinician must recognize that it is an imperfect therapeutic device. Present ICD systems have a limited working life and are subject to both complications and unexpected failure over time.47–49 However, the life expectancy of patients receiving ICD systems has improved over time. One study found that 75% of ICD recipients were alive at 5 years and more than 40% were alive at 10 years after ICD placement.48 This is consistent with a 49% survival rate after a median follow-up of over 7 years in MADIT-II ICD recipients.50 A contemporary ICD battery is anticipated to last 4 to 7 years, but clinical experience is that the average in-service time for an ICD is typically 3 to 5 years due to premature battery depletion, device advisories, need for system upgrades, and infections.51 Therefore, recipients are likely to require multiple devices over their lifetime. With recent trials showing benefit in patients with less advanced forms of heart disease, this mismatch will continue to grow. Research related to assessing reliability and longevity is required. Longer-term follow-up of ICD patients in clinical trials is also needed to obtain reliable data on long-term efficacy and cost-effectiveness of ICD therapy.
Cost
Cost-effectiveness is crucial in the setting of limited or restricted health care resources. This is particularly relevant as use of ICD and CRT devices expands to include patients sharing only some similarities with patient populations in whom these devices have been shown to be effective, so-called indication creep or indication extrapolation.52 For example, a large survey of 141 centers in Western Europe found that at least 1 in 5 patients undergoing a CRT procedure had clinical or electrocardiographic characteristics that placed them outside of published guidelines.53 Analysis of data from the National Cardiovascular Data Registry (NCDR) ICD Registry similarly found that over 20% of patients receiving CRT devices did not meet guideline-based indications.54
A major limitation of cost analyses in randomized trials is that these analyses invariably inflate the cost of ICD therapy and devalue the life-years gained. The estimate of life-years gained from randomized trials depends heavily on the duration of follow-up assessing benefit. The cost of an ICD is heavily front-loaded, yet its benefit accrues over time. Thus, short-term data from randomized trials may artificially underestimate the cost-effectiveness of ICD therapy.55 For example, the mortality reduction observed with long-term follow-up in MADIT-II was much larger than that initially observed, despite many patients in the conventional group crossing over to ICD therapy at the conclusion of the trial.50 At a median follow-up of over 7 years, the absolute reduction in mortality with ICD therapy was 13%. This is much larger than the 5.6% reduction in mortality at the trial’s conclusion, where median follow-up was 1.5 years.56
An in-depth analysis of randomized trials of ICD therapy found a modest, incremental cost-effectiveness of ICD therapy that ranged between $25,000 and $50,000 per quality-adjusted life-year.57 Similar estimates have been found in most randomized trials. These data indicate that ICD therapy is cost-effective. However, it is important to recognize that cost-effectiveness likely differs in the real world versus the populations from whom these estimates were derived. For example, only sparse data exist on the cost-effectiveness of ICD therapy in elderlypersons.58 Additional data regarding cost-effectiveness are included in the discussion of individual trials.
Utilization
There are marked differences in the use of ICD therapy in different regions of the world. In the United States, the rate of ICD implantations is approximately 600 implants per 1 million population. In Western Europe and Canada, the rates are approximately one quarter of the U.S. rate.24 The reasons for these differences are complex, but likely relate to differing perceptions of need, variability in guidelines, perceived cost-effectiveness, and concerns over inappropriate ICD therapies and device malfunction.59 This underutilization has also been attributed to a shortage of electrophysiologists, poorly developed local referral strategies and care pathways, and difficult or confusing financial circumstances.24 Solutions to some of these barriers are more obvious than others. For example, an entirely subcutaneous ICD system may address limited implantation resources.60 However, issues then arise related to how these patients and devices will be followed. Studies assessing the long-term effectiveness and safety of subcutaneous ICD systems are in progress. Underuse of ICD therapy is concerning and has significant consequences for patients in whom an ICD is clearly indicated, but not prescribed. Additional efforts to identify and address barriers to patient referral and implantation rates are required.
 Groups at Risk for Sudden Death
Groups at Risk for Sudden Death
Identifying Individuals at Risk
A major barrier to preventing sudden death is the lack of accurate and reliable methods of identifying the majority of individuals at risk and those in whom an ICD would be most beneficial.61 Individuals who can be identified before experiencing sudden death can be categorized as belonging to one of the four groups previously listed. Patients with spontaneous or inducible ventricular arrhythmias are known to be at high risk for cardiac arrest, but they represent only a fraction of those who will experience sudden death62,63 (Fig. 11-2).
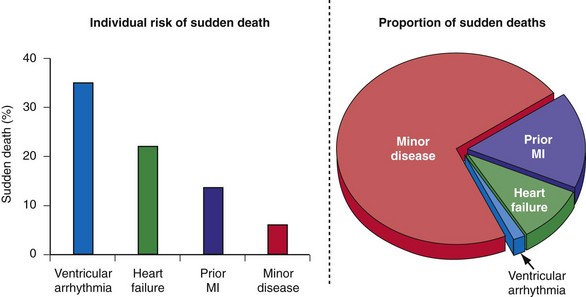
Figure 11-2 Sudden death: individual risk versus population impact.
(Modified from Rea TD, Pearce RM, Raghunathan TE, et al: Incidence of out-of-hospital cardiac arrest. Am J Cardiol 93:1455-1460, 2004.)
Patients with structural heart disease, notably a previous MI or LV dysfunction, represent a significant proportion of those at risk for sudden death and have an intermediate risk of cardiac arrest in the near term. To date, only two approaches have proved useful in guiding prophylactic ICD therapy in this group. LV dysfunction alone (LVEF ≤ 0.35) or in combination with the induction of sustained VT/VF with invasive programmed electrical stimulation.56,64,65 However, invasive programmed electrical stimulation does not appear useful early after MI,66,67 and arrhythmia inducibility is unreliable in predicting long-term outcome.68
Although individuals with LV dysfunction after MI have a heightened risk of sudden death, most will not experience a cardiac arrest in the near term. Further, the majority of sudden deaths occur in post-MI patients with better-preserved LV function69,70 (Fig. 11-3). If a left ventricular ejection fraction cutoff value of 0.35 or less (LVEF ≤ 0.35) is used to identify those at risk, fewer than one third of patients who will suffer sudden death are identified.69–72 In contrast, a cutoff of 0.50 or less (LVEF ≤ 0.50) will identify most patients at risk.69,70 However, an LVEF ≤ 0.50 alone is a nonspecific selection method, and additional risk assessment is required to better identify those at risk for sudden death.
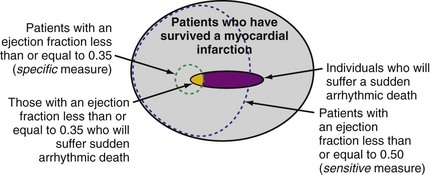
Figure 11-3 Identifying individuals at risk for sudden death: specificity versus sensitivity.
(Modified from La Rovere MT, Bigger JT Jr, Marcus FI, et al. Baroreflex sensitivity and heart rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes after Myocardial Infarction) Investigators. Lancet 351:478-484, 1998; and Exner DV, Kavanagh KM, Slawnych MP, et al: Noninvasive risk assessment early after a myocardial infarction: the REFINE study. J Am Coll Cardiol 50:2275-2284, 2007.)
Individuals with mild or subclinical heart disease account for the majority of sudden deaths,63,73 but the likelihood that an individual in this group will experience a cardiac arrest over a decade is very low (see Fig. 11-2).74 Many of the cardiac arrests in this group appear to be triggered by unstable coronary artery plaques.75 Appropriate attention to therapy with antiplatelet agents, statins, fish oils, and other drugs has the potential to significantly reduce the burden of sudden death.76 The widespread use of automated external defibrillators (AEDs) has been advocated as one method to improve survival from sudden death, but the Home AED Trial (HAT) found that an AED plus cardiopulmonary resuscitation (CPR) offered no advantage over CPR alone in patients at risk for sudden death because of a history of prior MI.77 The efficacy of a wearable AED in patients considered at risk for sudden death, but who are not candidates for an ICD (e.g., LV dysfunction early after MI), is presently under investigation.
Noninvasive methods to assess the location and extent of myocardial scarring,78 cardiac denervation,79 ventricular conduction, cardiac repolarization, and autonomic tone61,80 have been developed to identify patients at risk for sudden death. However, although noninvasive techniques such as T-wave alternans (TWA), heart rate turbulence (HRT), other Holter monitor indices, imaging methods, and genetic assessment may be useful in some patients, these methods have not been useful in identifying groups of patients likely to benefit from an ICD.
The signal-averaged electrocardiogram (signal-averaged ECG) was considered a reliable means to identify patients at risk for sudden death, but failed to predict ICD efficacy in the CABG Patch study.81 Similarly, heart rate variability was regarded as a reliable marker for predicating sudden death after MI, but no survival benefit was identified with ICD therapy in the Defibrillators in Acute Myocardial Infarction Trial (DINAMIT).82 The Microvolt T Wave Alternans Testing for Risk Stratification of Post–Myocardial Infarction Patients (MASTER) study, although not randomized, found that microvolt TWA results were not helpful in identifying post-MI patients with LV dysfunction at risk for arrhythmic death or in predicting ICD effectiveness.83
Relative VS. Absolute Risk Reduction and Number Needed to Treat
When assessing the impact of a therapy, one must consider its relative and absolute benefit. The absolute risk reduction is the difference between control and intervention group rates. For example, ICD therapy reduced mortality by 5.6% (19.8% control vs. 14.2% ICD) after a median follow-up of 1.5 years in MADIT-II. At a median follow-up of more than 7 years, the absolute risk reduction was 13% (62% control vs. 49% ICD).50 The relative risk reduction is the percentage reduction in rates with the intervention. For this same example, the relative risk reduction at 1.5 years in MADIT-II was 31% (5.6% absolute reduction ÷ 19.8% control rate) and at 7 years was 21% (13% absolute reduction ÷ 62% control rate). The number of patients needing to be treated in order to prevent one event is often used to describe the absolute impact of a therapy. The number needed to treat (NNT) is the reciprocal of the absolute risk reduction. In MADIT-II the NNT after 1.5 years was 17.9 (100% ÷ 5.6%) and at 7 years was 7.7 (100% ÷ 13%). This information is important for clinical decision making and health policy.
 Evolution of ICD Therapy
Evolution of ICD Therapy
Initial observational studies and smaller randomized trials suggested that ICD implantation was associated with a greater than 60% relative reduction in mortality compared with conventional strategies.84 Because of limitations in the design of these initial studies, a series of larger, adequately powered, randomized trials were conducted either to confirm or to refute these initial observations. Because of the risk of ICD system placement (via thoracotomy or sternotomy), initial randomized trials focused on patients with the highest individual risk for a fatal cardiac arrest, those with a history of spontaneous ventricular arrhythmias (see Fig. 11-2). The next group of studies evaluated patients with an extremely high risk of a fatal cardiac arrest caused by a history of a prior MI, severe LV dysfunction, and arrhythmias inducible by invasive programmed electrical stimulation. During this time, studies were also designed to minimize the risk of ICD system implantation by limiting therapy to patients who required a sternotomy for another reason (e.g., CABG surgery).81 After the mostly positive results of these early trials, studies in populations with lower but significant risk of sudden death were initiated. These trials have mostly been limited to patients with heart failure and LV dysfunction.
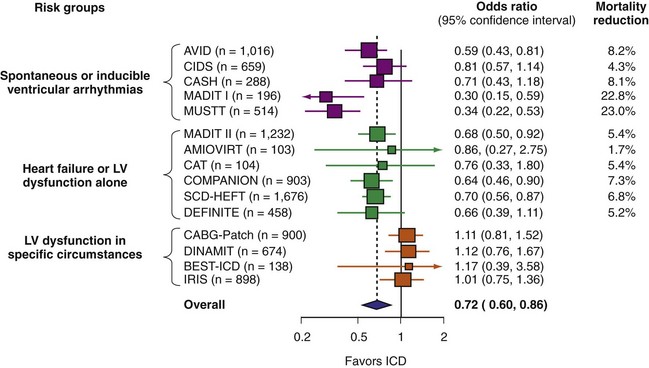
A number of randomized studies have compared ICD therapy with amiodarone or other medical therapy. The relative benefit of ICD was consistent in most of these studies. However, no benefit of ICD therapy was found in four adequately powered trials (Fig. 11-4). These four trials included patients with LV dysfunction in specific circumstances. Three studies enrolled patients very early after MI,82,85,86 and the other included patients undergoing CABG surgery.81 The lack of ICD efficacy in these populations can be explained by (1) post-MI coronary artery revascularization and remodeling dramatically reducing the risk of sudden death, thus reducing ICD efficacy,87 or (2) use of inadequate risk assessment methods to select patients. Given the utility of most of these tests,80 the second explanation appears less likely. The neutral results from these four trials emphasize the need for randomized trials to assess ICD efficacy definitively in new populations and highlight the pitfalls of using a therapy for an indication beyond what has been shown to be efficacious.
 Efficacy: Review of Randomized Trials
Efficacy: Review of Randomized Trials
Patients with Spontaneous or Inducible Ventricular Arrhythmias
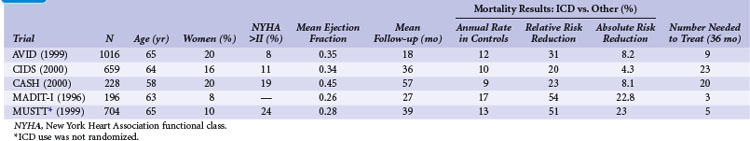
Table 11-1 summarizes the inclusion criteria, primary endpoints, and main results for five studies involving patients with spontaneous or inducible ventricular arrhythmias. Table 11-2 presents details of the study populations, risk of the untreated population (annual control group mortality), and relative and absolute changes in mortality with ICD therapy.
TABLE 11-1 Clinical Trials of ICD Therapy for Spontaneous or Inducible Ventricular Arrhythmias: Inclusion Criteria, Comparison Groups, and Main Results
TABLE 11-2 Clinical Trials of ICD Therapy for Spontaneous or Inducible Ventricular Arrhythmias: Population Details and Mortality Results
Two large randomized trials88,89 and one small trial90 provide convincing evidence that ICD therapy is superior to class III antiarrhythmic drugs in reducing mortality among patients with mostly spontaneous life-threatening arrhythmias. These studies generally included patients who survived a cardiac arrest caused by VT or VF. Another small randomized trial compared ICD therapy with conventional care that generally included amiodarone,64 and a large randomized trial in which ICD therapy was used in a nonrandomized manner91 assessed the efficacy of ICD therapy in patients with LV dysfunction and inducible ventricular arrhythmias. Both trials showed a benefit from ICD therapy similar to that in the three studies of patients who survived a cardiac arrest.
Antiarrhythmics versus Implantable Defibrillators Trial
The Antiarrhythmics versus Implantable Defibrillators (AVID) trial was a large (n = 1016) multicenter trial conducted in the United States and Canada. Patients entered into the trial were resuscitated from VT, had sustained VT with syncope, or had sustained VT with an LVEF of 0.40 or less and symptoms suggesting severe hemodynamic compromise (near-syncope, heart failure, and angina).88 Patients were excluded if the ventricular arrhythmia was attributed to a reversible cause or if the episode of VT was categorized as stable. The LVEF was required to be ≤0.40 in patients who underwent revascularization after the index arrhythmia, regardless of the qualifying arrhythmia. The primary outcome of AVID was all-cause mortality.
The AVID trial began recruitment in mid-1993 and was terminated in 1997, when patients randomly assigned to ICD therapy were found to have a 31% relative reduction in the risk of death compared with patients receiving antiarrhythmic drug therapy, mostly amiodarone (95% confidence interval [CI] = 10% to 52%). This mortality benefit translates into an average life extension of 2.7 months during 18 months of follow-up.92 The NNT over 36 months is approximately 9 (11.3% absolute risk reduction) and the incremental cost of an ICD at 3 years was approximately $67,000 (U.S.) per life-year saved. However, the early termination of the AVID trial has two important implications: (1) premature termination may overestimate the magnitude of benefit with ICD therapy compared with amiodarone,93 and (2) the resultant shorter average duration of follow-up may lead to underestimation of the true cost-effectiveness of ICD therapy.94
Analyses were undertaken to identify groups more likely to benefit from ICD therapy. The 61% of patients with LVEF values less than 0.35 had a large (40%) relative reduction in the risk of death with ICD compared with drug therapy, whereas patients with higher values did not significantly benefit.95 AVID included relatively few patients with advanced heart failure, marked reductions in LVEF, or advanced age and thus had limited power to assess the relative efficacy of ICD therapy versus amiodarone in these and other subgroups.96
As a prespecified secondary endpoint, the AVID investigators also collected QOL data before and at 3, 6, and 12 months after randomization.26 Data were available for 905 of 1016 (89%) patients enrolled. Of these, 800 patients survived 1 year or longer and constituted the QOL study population. Moderate to severe impairment in both physical functioning and mental well-being were evident in both treatment groups at baseline. Patients who received an ICD had better physical functioning over the subsequent year, but no significant changes were noted in mental well-being. No significant difference in QOL over time was observed between the ICD-treated and amiodarone-treated patients. Adverse symptoms were associated with a decrease in QOL in both groups. As previously discussed, there was an independent association between ICD shocks and reduced QOL. The reduction in QOL with multiple ICD shocks was of similar magnitude to the reduction in QOL observed among patients with severe adverse symptoms from amiodarone.
One criticism of AVID was an imbalance in β-blocker use between patients assigned to an ICD (38% at 1 year) versus amiodarone (11% at 1 year). However, β-blocker use did not alter survival among those treated with an ICD or amiodarone. In contrast, patients who were eligible for the AVID trial, but were not randomly assigned to and did not receive amiodarone or an ICD, had a 53% lower risk of death with a β-blocker versus similar patients who did not receive β-blockers.97 These data suggest that β-blockers may reduce sudden death, but this effect is mitigated with the use of an ICD or amiodarone.
Another intriguing result from AVID was that patients with sustained VT or VF caused by a reversible cause had a similar, or perhaps higher, risk of death compared with patients in whom the event was considered a primary episode of VT or VF.98 A separate analysis found that the prognosis of patients with stable VT was similar to those with unstable VT.99 These analyses question the dogma that patients with stable VT and those with a potentially reversible cause of sustained VT or VF have a good prognosis and are not candidates for an ICD.
Canadian Implantable Defibrillator Study
The Canadian Implantable Defibrillator Study (CIDS) was a large (n = 659) multicenter trial conducted in Canada, Australia, and the United States from 1990 to 1997.89 Patients were eligible if they had VF, sustained VT causing syncope, sustained VT and an LVEF ≤ 0.35, or unmonitored syncope with subsequent spontaneous or inducible VT. Patients who had experienced a recent MI (within 72 hours) or had an electrolyte imbalance were excluded. The average follow-up in CIDS (36 months) was twice that of AVID, but the number of deaths was somewhat fewer in CIDS (181) than in AVID (202). The primary outcome in CIDS was all-cause mortality.
This study demonstrated a 20% lower relative (95% CI = 8% increase to 40%) risk of death with an ICD than with amiodarone (P = .14). On the basis of this 4.3% absolute reduction in mortality, the NNT over 36 months was 23. A subsequent single-center analysis of 120 patients found a high rate of cessation of amiodarone because of adverse effects (82% of patients) and a reduction in mortality with an ICD (16 deaths; 27% mortality) versus amiodarone (28 deaths; 47% mortality) over 11 years of follow-up.100
The incremental cost of an ICD was approximately $150,000 (U.S.) per life-year gained.101 Patients in CIDS with low LVEF values benefited from ICD therapy to a greater extent than those with better-preserved LV function. The selective use of an ICD for patients with LVEF values less than 0.35 lowers the incremental cost of an ICD to less than $70,000 per life-year gained.102 Another analysis demonstrated that patients with two or more of three characteristics that included age 70 years or older, LVEF ≤0.35, and New York Heart Association functional class (NYHA Class) greater than II benefited from ICD therapy, whereas patients with one or no characteristics did not.103 However, the CIDS risk score was imperfect when applied to AVID.104
Quality of life was assessed in CIDS using the Rand Corporation’s 38-item Mental Health Inventory and the Nottingham Health Profile.27 Of the initial 400 enrolled patients, only 178 (45%) provided analyzable QOL data at 1 year. Patients randomly assigned to ICD therapy had significant improvements in several QOL measures over 1 year of follow-up. In patients undergoing amiodarone therapy, these QOL measures either did not improve or deteriorated. Patients with at least five ICD shocks during follow-up had reduced QOL. Whether ICD therapy truly provides better QOL than amiodarone therapy is uncertain because of the conflicting results from the AVID trial and the methodologic issues related to the CIDS analysis.105
Cardiac Arrest Study Hamburg
The Cardiac Arrest Study Hamburg (CASH) was a smaller randomized trial (n = 288) that compared the use of an ICD with drug therapy.90 Patients were enrolled from 1987 to 1996. A significant number of patients underwent a thoracotomy or sternotomy for ICD system placement (before July 1991). The study population included patients resuscitated from a cardiac arrest secondary to a documented sustained VT or VF. Patients were excluded if the cardiac arrest occurred within 72 hours of an MI or cardiac surgery or was related to an electrolyte abnormality or proarrhythmic drug. The primary endpoint was all-cause mortality.
Pooled Analysis of the Three Studies
The combined results of the AVID, CIDS, and CASH data demonstrate that mortality is reduced by 27% (95% CI = 13% to 40%; P <.001) with an ICD compared with amiodarone.22 Death attributed to an arrhythmia was reduced by 51% (95% CI = 33% to 64%; P < .001). No significant difference between treatment groups in the risk of death from nonarrhythmic causes was evident. This reduction in mortality corresponded to an average extension in life of 2.1 months. The annual mortality with an ICD was 12.3% per year, versus 8.8% per year with amiodarone. The absolute reduction in mortality (10.5%) over 36 months translates into needing to treat fewer than 10 patients in order to prevent one death.
First Multicenter Automatic Defibrillator Implantation Trial
The First Multicenter Automatic Defibrillator Implantation Trial (MADIT-I) was a small (n = 196) multicenter trial conducted in the United States, Germany, and Italy from December 1990 to March 1996.64 The first one half of the patients received epicardial ICD systems, and the remaining half received nonthoracotomy, transvenous systems. ICD therapy was compared with conventional care, mostly empiric amiodarone therapy, in patients with ischemic LV dysfunction, asymptomatic nonsustained VT (NSVT), and inducible, nonsuppressible, sustained VT/VF with programmed stimulation. Most MADIT-I patients (93%) had nonsuppressible, monomorphic VT.
The MADIT-I demonstrated a large, 54% (95% CI = 18% to 74%) relative reduction in mortality (P = .009). The MADIT-I population has been considered distinct from the patients in the AVID trial, CIDS, and CASH, but the populations are similar. Both CIDS and AVID included patients with VT unrelated to a cardiac arrest. CIDS also included patients with a history of syncope and a low LVEF value plus inducible VT. The average extension of life with an ICD was 10 months. This large reduction in mortality (26.2% absolute reduction over 27 months) represents an NNT of only 3 over 36 months. This translates into a modest incremental cost of $27,000 per life-year gained, making ICD therapy in this population very economically attractive.106
The annual mortality rate in the MADIT-I control group appears to be somewhat higher than that observed in the three trials evaluating ICD therapy in patients with mostly spontaneous sustained ventricular arrhythmias (see Table 11-2). The likely explanation is that all the patients in MADIT-I had LVEF ≤0.35, whereas LVEF was not a primary inclusion criterion in AVID, CIDS, or CASH. If only those patients in AVID, CIDS, and CASH with LVEF ≤0.35 are studied, a similar 16% annual mortality rate is observed in patients receiving amiodarone.22 Moreover, the relative reduction in risk of death with an ICD versus amiodarone among patients with LVEF ≤0.35 in AVID, CIDS, and CASH (44%; 95% CI = 17% to 47%; P <.001) is similar to that found in MADIT-I (54%; 95% CI = 18% to 74% reduction). Thus, patients with spontaneous or inducible sustained ventricular arrhythmias have a similar risk of death and benefit to a similar extent with ICD therapy.
A major limitation of MADIT-I is the lack of information about how many patients with ischemic LV dysfunction and NSVT would have to be screened to identify one patient fulfilling all the inclusion criteria for this trial. This number is estimated to be large because these criteria are present in very few (<2%) patients after MI.107 Also, targeting ICD therapy to only those at highest risk apparently has a marginal effect on reducing the burden of sudden death (see Fig. 11-2). Another concern regarding MADIT-I is the non-protocol-driven use of antiarrhythmic drugs. For example, 23% of patients assigned to conventional therapy were not receiving antiarrhythmic drug therapy at last follow-up, and only 55% of patients were receiving amiodarone at that time. The significance of this issue is questionable, given the results of SCD-HeFT (see later), which found no significant benefit or harm from amiodarone.
Multicenter Unsustained Tachycardia Trial
The randomized Multicenter Unsustained Tachycardia Trial (MUSTT) compared antiarrhythmic therapy with best medical therapy in 704 patients with coronary artery disease, LVEF ≤ 0.40, NSVT, and inducible sustained VT/VF during programmed electrical stimulation.91 It is important to emphasize that although the use or nonuse of ICD therapy in MUSTT was not randomly assigned, the results are often considered when interpreting data from the previously discussed trials.
The prognostic significance of inducible arrhythmias during invasive electrophysiologic testing was also evaluated in MUSTT.108 During 5 years of follow-up, patients with an inducible sustained ventricular arrhythmia had a significantly higher risk of cardiac arrest or death caused by arrhythmia (32%) than patients in whom a sustained arrhythmia was not inducible (24%; P <.001). Overall mortality was also statistically higher in patients in whom a sustained arrhythmia was inducible (48% vs. 44%; P = .005). However, the absolute difference in mortality (4% over 5 years) was small and of questionable clinical significance. Also, LVEF alone was unreliable in predicting long-term outcome.109 Baseline clinical variables (functional class, NSVT, age, QRS duration, inducible VT/VF, and atrial fibrillation) were found to better estimate the risks of arrhythmic death and total mortality than LVEF ≤0.30 versus >0.30.
Patients with Heart Failure or Left Ventricular Dysfunction Alone
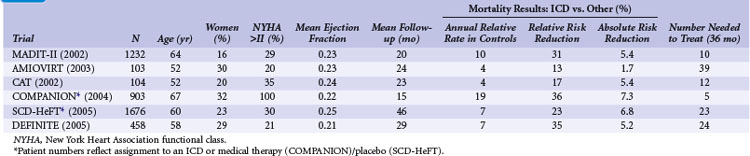
Table 11-3 summarizes the inclusion criteria, primary endpoints, and main results for six studies that involved patients with heart failure or LV dysfunction alone. Table 11-4 presents details of the study populations, the risk of the population untreated (annual control group mortality), and the relative and absolute alterations in mortality with ICD therapy. These trials are often dichotomized on the basis of the underlying etiology of LV dysfunction (ischemic vs. nonischemic). Three trials (CAT, AMIOVIRT, DEFINITE) included only patients with nonischemic LV dysfunction; two (COMPANION, SCD-HeFT) included patients with both ischemic and nonischemic etiologies, and one (MADIT-II) was limited to patients with ischemic LV dysfunction. It is important to recognize that number of patients, duration of follow-up, and resultant statistical power of these trials differ greatly. COMPANION was also different from the other trials in that all patients were required to have a QRS duration of greater than 120 msec and symptoms of advanced heart failure. COMPANION also assessed the combined use of CRT and ICD, whereas the other trials evaluated non-CRT ICD therapy. The two larger and long-term trials that used non-CRT ICD therapy, SCD-HeFT and MADIT-II, provide the most reliable information on the efficacy of ICD therapy in patients with heart failure or LV dysfunction alone.
TABLE 11-3 Clinical Trials of ICD Therapy for Heart Failure or Left Ventricular Dysfunction Alone: Inclusion Criteria, Comparison Groups, and Main Results
TABLE 11-4 Clinical Trials of ICD Therapy for Heart Failure or Left Ventricular Dysfunction Alone: Population Details and Mortality Results
Second Multicenter Automatic Defibrillator Implantation Trial
Although MADIT-I showed a large benefit from ICD therapy, its study population represented a highly select group of patients (see Figs. 11-2 and 11-3). MADIT-II evaluated ICD therapy in a lower-risk group that accounts for a larger proportion of patients who will experience sudden death. MADIT-II enrolled 1232 patients from 71 U.S. centers and five European centers from July 1997 to January 2002.56 ICD therapy was compared with usual care in patients with ischemic LV dysfunction (LVEF ≤0.30). Patients who had experienced a recent MI (within 1 month of evaluation) or revascularization procedure (within 3 months of evaluation) were not eligible. Patients who underwent invasive electrophysiologic testing and had inducible sustained arrhythmias, fulfilling the criteria for MADIT-I, were also ineligible. Angiotensin-converting enzyme (ACE) inhibitors, β-blockers, and lipid-lowering therapies were prescribed to 70%, 70%, and 66% of participants, respectively, on the basis of data from the last follow-up visit. These rates are higher than in the trials evaluating ICD efficacy in patients with spontaneous or inducible ventricular arrhythmias conducted before the wider recognition of the importance of these medications.
The cost-effectiveness of ICD therapy in MADIT-II was assessed using data from 1095 U.S. patients randomized to an ICD or conventional care. Utilization data were converted to costs using national and hospital-specific data. The incremental cost-effectiveness ratio was calculated as the difference in discounted costs divided by the difference in discounted life expectancy within 3.5 years. Secondary analyses included projections of survival (using three alternative assumptions), corresponding cost assumptions, and the resulting cost-effectiveness ratios out to 12 years after randomization. Assumptions included the cost of ICD therapy was $33,000 (device and implant) and a survival rate of ICD recipients ranging from 13% to 26% at 12 years.110 The actual long-term survival of ICD recipients in MADIT-II is in keeping with the more optimistic projection.50 This survival estimate results in an incremental cost-effectiveness ratio in the range of $50,000. This result is similar to that estimated for other trials when results were extrapolated over time.57 These data indicate that ICD therapy is economically beneficial in these patients.
The MADIT-II researchers noted that the effect of ICD therapy was similar in subgroup analyses stratified by age, gender, LVEF, and NYHA class. However, patients with longer QRS durations (>120 msec) had larger absolute and relative risk reductions that trended toward statistical significance. Some considered this finding to indicate that patients with QRS values greater than 120 msec benefit from ICD therapy and that those with shorter QRS values do not, but this remains controversial.111 Delayed ventricular conduction has also been shown to predict a higher risk of death among patients receiving contemporary medical therapy.80 Other noninvasive risk assessment tools, such as T-wave alternans, have been advocated for selecting patients for ICD therapy,112 but their usefulness remains unproved83 (see Ongoing Trials).
The ability of electrophysiologic testing to predict mortality and ICD efficacy was also assessed in MADIT-II.68,113 Electrophysiologic inducibility was performed on 593 patients randomly assigned to ICD therapy. A sustained ventricular arrhythmia was inducible in 36% of patients, who were more likely to demonstrate spontaneous VT in follow-up. In contrast, patients who did not have a sustained inducible ventricular arrhythmia were more likely to experience spontaneous VF. Overall, patients in MADIT-II had a 20% rate of appropriate ICD therapies for VT or VF over 4 years of follow-up. The likelihood of VT or VF was similar for patients with and without inducible arrhythmias at baseline. As with the findings in MUSTT, the MADIT-II data indicate that electrophysiologic testing is suboptimal in identifying patients who will benefit from an ICD.
Analyses from MADIT-II include clinical risk factors associated with benefit from ICD therapy114 and with mortality in ICD recipients115 and utility of noninvasive tests to predict ICD benefit. Although both dynamic alterations in QT duration116 and morphology117 predicted an increased risk of death and arrhythmias in MADIT-II, neither measure was useful in discriminating between patients likely versus unlikely to benefit from an ICD.
A risk score comprised of five clinical factors (NYHA Class >II, age >70, BUN >26 mg/dL, QRS duration >120 msec, atrial fibrillation) was derived after excluding patients with blood urea nitrogen (BUN) values of at least 50 mg/dL and/or serum creatinine values of at least 2.5 mg/dL. Conventional therapy patients with none of these risk factors had an 8% mortality rate, and those with at least one risk factor had a 28% mortality rate. ICD therapy was not associated with a significant alteration in mortality among the 345 patients with no risk factors (hazard ratio 0.96; P = .91), but was associated with a 49% relative reduction in the risk of death in the 786 patients with at least one risk factor (P <.001). This score is interesting in that it is similar to that proposed in CIDS103 and contains some of the same variables that in MUSTT predicted the long-term risks of arrhythmic death and total mortality.109 The utility of the MADIT-II risk score is unclear because it has not been validated, and its components largely overlap with those shown to predict death despite ICD use in MADIT-II participants.115
Another interesting analysis in MADIT-II evaluated the efficacy of ICD therapy based on the time from MI to study enrollment.118 An inclusion criterion for MADIT-II was that patients had their MI at least 1 month before evaluation; however, the average time from MI to enrollment in MADIT-II was much longer (mean, 81 months). Patients were subdivided into quartiles of time from MI to enrollment: less than18 months (300 patients), 18 to 59 months (283), 60 to 119 months (284), and 120 months or longer (292). A survival benefit from ICD therapy was apparent in the three groups with a longer duration between MI and enrollment (relative risk reductions of 38% to 50% with ICD therapy), but no benefit from ICD therapy was evident among patients whose MI events had occurred within 18 months of enrollment (2% reduction). These data, along with those of DINAMIT and IRIS, suggest that ICD therapy may not be effective early after MI. This may relate to the mechanisms of sudden death early after MI,11 a differential risk of sudden versus nonsudden death early after MI, or other factors.119
Amiodarone versus Implantable Cardioverter-Defibrillator Trial
The Amiodarone versus Implantable Cardioverter-Defibrillator Trial (AMIOVIRT) compared ICD therapy versus amiodarone in patients with nonischemic LV dysfunction and asymptomatic nonsustained ventricular tachycardia (NSVT) at 10 U.S. centers from 1996 and 2000.120 Patients were required to have a nonrecent (at least 6 months) diagnosis of LV dysfunction. The primary endpoint was total mortality. A total of 103 patients were enrolled, and average follow-up was 2 years. Of 13 deaths, six were in the ICD group and seven in the amiodarone group (P = .8). AMIOVIRT was terminated early because of futility. The trial was powered to detect a 50% relative (10% absolute) reduction in mortality with an ICD versus amiodarone. The observed mortality rate in amiodarone-treated patients was 12% at 3 years. AMIOVIRT neither supports nor refutes the value of ICD therapy in patients with nonischemic LV dysfunction and NSVT.
Cardiomyopathy Trial
The Cardiomyopathy Trial (CAT), conducted in 15 German centers from 1991 to 1997, compared ICD therapy with standard medical therapy in 104 patients with nonischemic LV dysfunction.121 Unlike the other trials of ICD therapy in patients with heart failure or LV dysfunction alone, patients in CAT were required to have recently diagnosed heart failure (<9 months before enrollment). The primary endpoint of CAT was all-cause mortality. Only 30 deaths were observed over a mean follow-up of 5.5 years. Of these, 13 occurred in the ICD group and 17 in the control group (P = .6). CAT was also terminated because of futility. The observed mortality rate in patients randomly assigned to standard therapy alone at 1 year was 3.7%. This value was much lower than the anticipated mortality rate of 30%. The trial has been interpreted as providing evidence that prophylactic ICD implantation is ineffective in patients with dilated cardiomyopathy and recent onset heart failure. As with AMIOVIRT, however, CAT was grossly underpowered, precluding any firm conclusions to be drawn (see DEFINITE discussion).
Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure
The Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) study evaluated the efficacy of CRT plus ICD (595 patients) versus medical therapy (308), and CRT plus pacemaker (617) versus medical therapy in patients with advanced heart failure (NYHA Class III or IV) and QRS durations over 120 msec.122 The 1520 patients were enrolled at 128 sites in the United States between January 2000 and December 2002. Patients with ischemic (55%) and nonischemic (45%) LV dysfunction were included. Patients with a recent (<2 months before consideration) MI or revascularization were not eligible. The primary endpoint was a composite of death or hospitalization from any cause. Secondary endpoints were mortality and cardiac morbidity. Median follow-up was 12 months in the medical therapy group and 16 months in the pacemaker and ICD groups. ACE inhibitors or angiotensin receptor antagonists (90%), β-blockers (68%), and spironolactone (54%) were prescribed frequently at baseline. Medication use during follow-up was not reported. The remainder of this review focuses on the 903 patients randomly assigned to medical therapy versus CRT plus ICD.
There were 77 deaths (25%) in the medical therapy group and 105 deaths (18%) in the CRT plus ICD group, reflecting a 36% relative risk reduction (95% CI = 14% to 52%; P = .003). The 7% absolute risk reduction indicates that slightly more than 14 patients need to be treated with CRT plus ICD to prevent one death over 12 months. The effects of CRT plus ICD and medical therapy on mortality were compared in a range of patient subgroups. Consistent benefit was evident when data were stratified by age, gender, QRS duration, severity of LV dysfunction, etiology of LV dysfunction, blood pressure, and medication use. The reason for this large reduction in mortality is unclear but may relate to the combined effect of CRT plus an ICD, because large reductions in mortality were observed with CRT plus a pacemaker in COMPANION (4% absolute mortality reduction over 12 months) and the Cardiac Resynchronisation in Heart Failure (CARE-HF) study (10% absolute mortality reduction over 29 months).123
It is important to note that, although most of the baseline characteristics of patients in COMPANION are similar to those of participants in the other trials of ICD in heart failure or LV dysfunction alone (see Table 11-4), the annual control group mortality rate in COMPANION is two to five times greater than that of the other trials. Whether this reflects that all patients in COMPANION had advanced heart failure symptoms, had QRS values above 120 msec, or other factors is not known. The 1-year mortality rate among patients assigned to medical therapy in CARE-HF, involving similar patients, was approximately 13%.123 The brief follow-up in COMPANION or other factors may also explain the high mortality rate in the medical care group.
Sudden Cardiac Death–Heart Failure Trial
The Sudden Cardiac Death–Heart Failure Trial (SCD-HeFT) compared the efficacy of an ICD (829 patients) to placebo (847) and amiodarone versus placebo in patients with heart failure (NYHA II or III).65 The 2521 patients were enrolled at 148 sites in the United States, Canada, and New Zealand from 1997 to 2001. Randomization was stratified according to etiology of LV dysfunction and NYHA functional class. Similar proportions of patients with ischemic (52%) and nonischemic (48%) etiologies of LV dysfunction were enrolled. The majority of patients (70%) had NYHA II functional status limitation at baseline. Patients with a recent (1 month of evaluation) MI or revascularization procedure were not eligible.
Several subanalyses have been performed in SCD-HeFT. One assessed the modes of death and found that ICD therapy reduced cardiac mortality and sudden death overall, but had no effect on heart failure mortality.124 ICD therapy was also not associated with a reduction in mortality in patients with NYHA Class III limitation. Amiodarone had no overall effect on all-cause mortality or its cause-specific components. Amiodarone was associated with an increased risk of noncardiac mortality in NYHA Class III patients. The lack of benefit from ICD therapy and increased risk of noncardiac mortality in Class III patients treated with amiodarone were unexpected. The greater proportion of nonsudden versus sudden deaths in Class III patients, misclassification of deaths due to a lack of ICD data or clinical data, and statistical chance may all explain these findings.
As noted earlier, the impact of ICD shocks on outcome was assessed in SCD-HeFT.34 Of the 829 patients randomized to ICD therapy, 811 had an ICD successfully implanted. More than 99% of patients received a single-chamber ICD system. The ICD systems used were intentionally programmed to intervene only for rapid arrhythmias (cycle lengths ≤320 msec or rates >188 bpm). An episode of tachycardia was defined as lasting at least 18 of 24 bpm. Up to six shocks could be delivered per episode. ATP was not permitted, and the devices had no capability to determine if the episode had spontaneously terminated before delivery of a shock (i.e., no reconfirmation). A group of experts adjudicated all ICD shock episodes. ICD shocks that followed the onset of VT or VF were considered appropriate. Thus, by definition, “appropriate” episodes included longer runs of NSVT that fulfilled the detection criteria, but spontaneously terminated. This is problematic; up to 30% of fast VT episodes will spontaneously terminate.43 All other ICD shocks were considered to be inappropriate.
Quality of life was measured in SCD-HeFT using structured interviews at baseline and at 3, 12, and 30 months.31 The two prespecified primary outcomes were the Duke Activity Status Index (DASI), reflecting cardiac-specific physical functioning, and the Medical Outcomes Study 36-Item Short-Form (SF-36) Mental Health Inventory 5 (MHI-5), reflecting psychological well-being. QOL data collection was 93% to 98% complete. Psychological well-being was significantly improved at 3 months (P = .01) and 12 months (P = .003) but not at 30 months (P = 0.8) with ICD therapy versus medical care. The Minnesota Living with Heart Failure Questionnaire was also used, with scores generally better in the ICD group than the medical care group at each follow-up assessment, but of borderline statistical significance. ICD shocks in the month preceding a scheduled assessment were associated with a decreased QOL in multiple domains. As noted, electrical storm events were not recorded in SCD-HeFT. The use of amiodarone had no significant effects on QOL. An analysis of the impact of significant adverse effects on QOL was not reported. These results provide the largest, most comprehensive assessment of QOL in patients receiving a primary prevention ICD. The results are reassuring in that ICD therapy, including the occurrence of appropriate and inappropriate shocks, is not associated with altered QOL.
The cost-effectiveness of ICD therapy in SCD-HeFT was estimated using hospital billing data and the Medicare Fee Schedule. The “base case cost-effectiveness analysis” used empirical clinical and cost data to estimate the lifetime incremental cost of saving an extra life-year with ICD therapy relative to medical therapy alone. At 5 years, the amiodarone arm had survival equivalent to the placebo arm but higher costs. For ICD relative to medical therapy alone, the base case lifetime cost-effectiveness and cost-utility ratios (discounted at 3%) were $38,389 per life-year saved and $41,530 per quality-adjusted life-year saved, respectively. At 12 years the cost effectiveness ratio was $58,510 per life-year saved. The cost-effectiveness ratio was only $29,872 per life-year saved for NYHA Class II patients, whereas incremental cost but no incremental benefit was observed in Class III patients. These data indicate that ICD therapy is cost-effective. The substantially longer follow-up in SCD-HeFT provides a more reliable and realistic estimate of cost, for reasons previously discussed.125
A substudy in SCD-HeFT included 490 patients at 37 clinical sites and evaluated the utility of microvolt T-wave alternans (TWA).126 Masked readers, using standard criteria, classified the tests as positive (37%), negative (22%), or indeterminate (41%). The composite primary endpoint was the first occurrence of sudden cardiac death, sustained VT or VF, or an appropriate ICD shock. During a median follow-up of 30 months, no significant differences in the composite rate were found in TWA-positive versus TWA-negative patients (hazard ratio 1.2; P = 0.6) or in TWA-nonnegative (positive and indeterminate) versus TWA-negative subjects (hazard ratio 1.3; P = 0.5). Similar results were observed when patients randomized to an ICD and those randomized to amiodarone were separately evaluated. Thus, microvolt TWA assessment was not useful in predicting arrhythmic events death in SCD-HeFT. These results are similar to those in the MASTER study.127 Although unclear, the reasons for this finding support the need for additional studies on noninvasive risk assessment methods. A Holter substudy was also conducted in SCD-HeFT, but those results have not yet been reported.
Another analysis in SCD-HeFT investigated whether defibrillation testing during ICD implantation was associated with outcome.128 This analysis included the 811 patients randomized to ICD therapy and had a device implanted. The testing protocol in SCD-HeFT was designed to limit shock testing. Baseline data were available in 717 patients (88%). All 717 patients were successfully defibrillated with energies of 30 J or less, the maximum output of the ICD systems used. Energies were 20 J or less in 98% of patients. No survival difference between the 547 patients converted with low (≤10 J) versus the 170 patients with higher (>10 J) energy values (P = .4) were observed. The use of routine defibrillation testing in primary prevention patients remains controversial129 and is being addressed in ongoing randomized trials.
Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation
The Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) study compared ICD therapy versus medical care in 488 patients with nonischemic LV dysfunction.130 Patients were enrolled from participating sites in the United States from 1998 to 2003. In addition to an LVEF ≤ 0.35, patients were required to have ambient arrhythmias (frequent premature ventricular beats or NSVT). Unlike CAT, duration of heart failure was neither an inclusion nor an exclusion criterion in DEFINITE.
The primary endpoint was death from any cause. The secondary endpoint was sudden death. Mean follow-up was 29 months. ACE inhibitors (86%), angiotensin receptor blockers (11%), and β-blockers (85%) were prescribed to the majority of patients. As shown in Table 11-4, the mean LVEF in DEFINITE was somewhat lower (0.21) than the average values for patients in CAT (0.24) and SCD-HeFT (0.25). More than half (57%) of the patients in DEFINITE had NYHA Class II symptoms.
Quality of life was assessed using the Medical Outcomes 12-Item Short-Form Survey (SF-12) and the Minnesota Living with Heart Failure Questionnaire in 458 of the 488 (94%) participants.30 QOL was assessed at baseline, 1 month after randomization, and every 3 months thereafter. Overall, there were no significant differences in QOL between patients randomized to an ICD or standard medical therapy. In patients with at least one ICD shock, QOL declined on the emotional scale of the Minnesota Living with Heart Failure Questionnaire (P = .04) and the mental component score of SF-12 (P = .04). As in most other analyses, QOL was similar in patients with and without an ICD, even when shocks are considered.
An interesting analysis of DEFINITE explored the relationship between ICD shocks and otherwise fatal events.131 A masked committee reviewed the shock electrograms and categorized cause of death. There were 15 clinical sudden deaths or cardiac arrests in the conventional group and three in the ICD group. Of the 229 patients randomized to an ICD, 33 patients (14%) received a total of 70 appropriate shocks. The number of events that included syncope plus sudden death or cardiac arrest in the conventional arm and appropriate ICD shocks with syncope in the ICD arm was similar (approximately 7%). Thus, using appropriate ICD shocks as a surrogate for sudden death overestimates the risk twofold. These data highlight the need for caution when “appropriate ICD therapies” are used to estimate survival.
Given the lack of benefit from ICD therapy in CAT, where patients were randomly assigned to therapy within 9 months of the onset of heart failure, a post hoc analysis of data from DEFINITE was undertaken to assess the impact of time from heart failure diagnosis on survival and ICD efficacy.132 Patients were divided into those with a recent (within 3 months) or remote (beyond 9 months) diagnosis of nonischemic cardiomyopathy. Randomization was also stratified by this categorization. Those with recently diagnosed cardiomyopathy had improved survival with an ICD. This benefit was significant at 3 months (P = .05) and of borderline significance at 9 months of follow-up (P = .06). Patients with remotely diagnosed cardiomyopathy did not have a significant survival benefit with an ICD. Thus, patients who have a recent cardiomyopathy diagnosis do not appear to have any less ICD benefit than those with a remote diagnosis. Given the divergent results in DEFINITE and CAT,121 it would reason that recently diagnosed cardiomyopathy should not be used as an exclusion criterion in patients with nonreversible LV dysfunction and LVEF ≤0.35 after an adequate trial of medical therapy.
Pooled Analysis of Trials in Patients with Heart Failure or Left Ventricular Dysfunction Alone
A systematic review found a 26% relative reduction in mortality with ICD therapy when the results of the MADIT-II, AMIOVIRT, CAT, COMPANION, SCD-HeFT, and DEFINITE trials were combined with the MADIT-I results (95% CI = 17% to 33%).133 With the MADIT-I data removed, a smaller but statistically significant relative risk reduction in mortality, 21%, was observed (95% CI = 6% to 34%; P = .009). The absolute mortality reduction in those patients with heart failure or LV dysfunction alone ranged from 5.8% to 7.9%, depending on the trials included. This translates into an NNT of 14 to 18 over 36 months. These absolute mortality reductions with ICD therapy are in addition to the 6.1% absolute risk reduction seen with ACE inhibitors and 4.4% absolute risk reduction seen with β-blockers. Thus, an ICD importantly reduces mortality beyond the benefits of β-blockers, ACE inhibitors, and other medical therapy.
Patients with Left Ventricular Dysfunction in Specific Circumstances
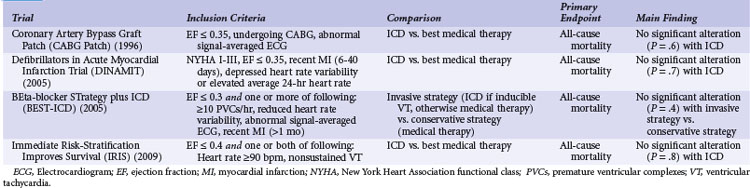
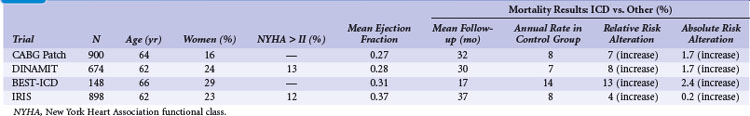
Table 11-5 summarizes the inclusion criteria, primary endpoint, and main result for three studies that included patients with LV dysfunction in specific circumstances. Table 11-6 provides details of the study populations, the risk of the population untreated (annual control group mortality), and the relative and absolute alterations in mortality with ICD therapy. These trials are considered separately because the other studies invariably excluded patients with recent coronary artery bypass surgery or a recent myocardial infarction, the specific populations in which ICD therapy was evaluated in the four studies discussed here.
Coronary Artery Bypass Graft Patch Trial
The CABG Patch trial was conducted at 35 U.S. centers and two German centers between 1990 and 1997. Prophylactic ICD therapy was compared with usual care in 900 patients with LVEF values ≤0.35 and abnormal signal-averaged ECG recordings undergoing CABG surgery.81
No significant reduction in mortality was observed with ICD therapy (relative increased risk 7%; P = .6) over an average follow-up of 32 months. A secondary analysis found that the ICD significantly reduced the risk of sudden death compared with medical therapy. Despite the attempt to identify a group of patients at high risk for sudden death, most of the deaths in CABG Patch (71%) were nonarrhythmic.134 Thus, the lack of benefit from ICD therapy appears to be related to a low risk of sudden death.
The reasons for the divergent results in the CABG Patch trial will likely never be fully understood. Characteristics of the patients in the trial were similar to those of patients with ischemic LV dysfunction and no history of sustained or inducible ventricular arrhythmias who were enrolled in MADIT-II and the other ICD trials, apart from higher mean LVEF values (see Table 11-6). Whether this finding relates to the use of a signal-averaged ECG to select patients, the effect of revascularization, or statistical chance is not known. Early studies suggested that the presence of late potentials on signal-averaged ECG analysis were predictive of death and serious arrhythmias.135 Later data suggested that signal-averaged ECG recordings do not provide useful prognostic information in patients who have undergone revascularization.136 One analysis that evaluated the impact of CABG on outcome in patients with LV dysfunction found that revascularization was associated with a 25% reduction in risk of death and a 46% reduction in risk of sudden death, independent of LVEF and severity of heart failure.87 As baseline LVEF declined, absolute reduction in risk of sudden death with prior CABG rose. When these data were applied to a group of patients with LV dysfunction who had not undergone prior surgery (Coronary Artery Surgery Study Registry), the predicted annual rates of death (8.2%) and sudden death (2.4%) were similar to those observed in CABG Patch (7.9% and 2.3%, respectively).87 An analysis from SCD-HeFT found no relationship between prior CABG and outcome,137 whereas analysis from MADIT-CRT found a significant reduction in the risk of VT/VF or death in patients with (32% risk) versus without (42%) prior coronary revascularization (P = .02).138 Regardless of the reasons why ICD therapy did not alter mortality in CABG Patch, this result highlights the need for restraint in the use of ICD therapy for the prevention of sudden death in patient groups in whom data on ICD efficacy are lacking.
Quality of life was assessed in the CABG Patch trial.28 Six months after surgery, 490 of the 900 enrolled patients completed QOL assessments. Compared with the ICD group, control patients were more likely to think their health status had improved over the preceding year, and controls had both higher emotional role functioning and greater psychological well-being. The ICD and control groups were similar on other QOL measures. These results are at odds with other trials, which found no reduction in QOL with ICD therapy. Whether these differences in QOL with ICD therapy in CABG Patch relate to the population assessed, the use of thoracotomy versus nonthoracotomy ICD systems, rates of incomplete data, or other factors is unknown.
Defibrillators in Acute Myocardial Infarction Trial
The Defibrillators in Acute Myocardial Infarction Trial (DINAMIT) tested the hypothesis that an ICD will reduce mortality in patients with a recent MI who are at high risk of arrhythmic death because of LV dysfunction and impaired autonomic tone, manifesting as low heart rate variability or a high resting heart rate.82 A total of 674 patients were enrolled at 73 sites from 12 countries between 1998 and 2002. Patients were enrolled 6 to 40 days after the index MI. Patients with NYHA Class IV symptoms, or in whom CABG surgery or three-vessel coronary angioplasty had been performed or was planned, were excluded. The primary outcome was death from any cause. Arrhythmic death was a secondary outcome. Mean follow-up was 30 months. Most of the characteristics of patients in DINAMIT (see Table 11-6) were similar to those of patients with ischemic LV dysfunction and no history of sustained or inducible ventricular arrhythmias enrolled in MADIT-II and the other ICD trials involving patients without a history of sustained or inducible ventricular arrhythmias, apart from higher mean LVEF values (see Table 11-6). Mean LVEF values in the CABG Patch trial and DINAMIT were similar.
An important analysis related to the development of arrhythmias requiring appropriate ICD therapies and subsequent mortality in DINAMIT patients has been presented, but not published.139 Over the 2.5 years of follow-up, 18% of patients randomly assigned to receive an ICD in DINAMIT had appropriate ICD therapies. On average, these patients had baseline LVEF values and degrees of autonomic tone impairment similar to patients who did not have appropriate ICD therapies. However, patients who received shocks were more likely to have NSVT. Most patients who received shocks experienced multiple shocks. The mortality rate in those who experienced appropriate ICD therapies (36%) was significantly greater than that in patients who experienced no therapies (15%) or were randomly assigned to not receive an ICD (17%). Three quarters of deaths in patients experiencing appropriate ICD therapies were categorized as nonarrhythmic. As in AVID38 and SCD-HeFT,34 patients who experienced appropriate ICD therapies in DINAMIT were subsequently at high risk of death from progressive heart failure.
The reasons for lack of benefit from ICD therapy in DINAMIT will likely never be fully understood. The inclusion of patients very early after MI may partly explain these results. As discussed, no benefit from ICD therapy was evident among patients within 18 months of an MI in MADIT-II.118 The lack of ICD efficacy may relate to alterations in cardiac structural remodeling,140 electrical remodeling,70 autonomic remodeling,141 or other factors early after MI. Recent insights on the mechanisms of sudden death in early post-MI patients with LV dysfunction are also likely relevant. As noted, almost half of the sudden deaths thought to be arrhythmic were in fact related to recurrent MI events or cardiac rupture.11 The choice of an elevated heart rate or impaired heart rate variability as inclusion criteria may have selected a group of patients at high risk for death but, necessarily, death related to ventricular arrhythmias. Although impaired heart rate variability does identify patients at high risk of presumed arrhythmic death early after MI,69 it is also a potent predictor of death from progressive pump failure in patients with LV dysfunction.142 Moreover, alterations in heart rate variability early versus later after MI provide similar different prognostic information.70 The high risk of nonarrhythmic death after appropriate ICD therapies in DINAMIT also suggests that ventricular arrhythmias in these patients may simply be a marker for progressive heart failure given the population enrolled (severe LV dysfunction). Other studies assessing ICD efficacy early after MI are ongoing (see Ongoing Trials).
BEta-blocker STrategy plus Implantable Cardioverter-Defibrillator Study
The BEta-blocker STrategy plus ICD (BEST-ICD) study evaluated the usefulness of an electrophysiologically guided ICD strategy in patients at high risk of sudden death early (<1 month) after MI.85 Patients were enrolled between 1998 and 2003. The overall study design was complex. Patients were required to have LV dysfunction, to be able to tolerate therapy with a β-blocker, and to have at least one of the following abnormal findings on noninvasive tests: 10 or more premature ventricular complexes per hour (≥10 PVCs/hr), depressed heart rate variability, and an abnormal signal-averaged ECG. Eligible patients who agreed to participate were then randomly assigned (with a ratio of 3 : 2) to usual medical care (conventional therapy) or to an invasive electrophysiologic study (invasive strategy). Patients in the invasive strategy group who had inducible sustained VT/VF received an ICD, whereas those who had no sustained arrhythmia induced were crossed over to receive conventional therapy. On the basis of this algorithm, 59 patients were randomly assigned to conventional therapy and 79 to the invasive strategy. A sustained ventricular arrhythmia was induced in 24 of the 79 patients in the invasive strategy group; these 24 patients received an ICD. The remaining 114 patients received usual medical care. All participants received metoprolol, with an average dose of 68 mg/day at baseline. The majority of patients were prescribed an ACE inhibitor (81% at discharge and 78% at last follow-up) and aspirin (81% at baseline and 82% at last follow-up). The rate of statin use was low (22% at baseline and 25% at last follow-up). A few patients received amiodarone (7% at baseline and 12% at follow-up). Similar to CABG Patch and DINAMIT, patients in the BEST-ICD study had a lesser degree of LV dysfunction than those in MADIT-II and the other trials of patients with heart failure or LV dysfunction alone (see Table 11-6).
A major limitation of the BEST-ICD study is the small number of patients (138), which prevents any conclusion related to the efficacy or lack of efficacy of ICD therapy in this group of patients. Another limitation is that the population was highly selected. More than 15,000 patients were screened. Of these, 8% had an LVEF ≤0.35. The majority (92%) of these patients had at least one abnormal noninvasive test. A large number of patients were excluded for other reasons, including lack of informed consent (40%), intolerance of β-blocker (18%), early revascularization (16%), and NSVT (7%). These data question the external validity of the BEST-ICD results. Further, other data indicate that invasive programmed electrical stimulation does not appear useful early after MI.66,67
Immediate Risk-Stratification Improves Survival Study
The Immediate Risk-Stratification Improves Survival (IRIS) study compared ICD therapy with usual medical care in 898 patients enrolled 5 to 31 days after MI. Participants were considered at risk for sudden death because of LVEF ≤0.40 plus a heart rate of ≥90 bpm on the first available ECG and/or NSVT of at least 150 bpm during Holter monitoring.86 Two thirds of participants (602; 67%) were included because of an elevated ECG heart rate. Remaining patients were enrolled with both elevated heart rate and NSVT (88; 10%) or NSVT alone (208; 23%). Patients were enrolled at 92 European sites between 2002 and 2007. The 898 patients were selected from a group of 62,944 patients with recent MI (screen/enroll ratio 70 : 1). Participants were equally assigned to standard medical care alone (453) or standard care plus an ICD (445). The primary endpoint was all-cause mortality. An independent events committee further categorized deaths as sudden or nonsudden. The trial was extended by the data and safety monitoring board in late 2005 when a lower-than-expected mortality rate was observed.
The results of IRIS mirror those of DINAMIT; inclusion of patients very early after MI may partly explain the lack of benefit.11,70,118,140,141 As noted, post-MI patients with LV dysfunction have a high risk of nonarrhythmic sudden death early after MI,11 and ICD shocks may be particularly deleterious in this patient population. The IRIS results have been criticized for the inclusion of patients with LVEF values outside present guidelines.143 However, IRIS was designed to assess the utility of ICD therapy in patients selected by other criteria. The choice of using an elevated heart rate or NSVT may also explain the clear lack of efficacy in IRIS. Although shown to predict risk, these parameters do not discriminate between sudden death and deaths from progressive heart failure.80 Other studies assessing the efficacy of ICD therapy after MI are ongoing (see Ongoing Trials).
Nonrandomized Studies of Icd Therapy: a Note of Caution
Randomized trials of ICD therapy have not been undertaken in certain situations because of concerns regarding feasibility (e.g., uncommon condition, complex study design, cost) or a lack of equipoise (i.e., unwillingness of physicians to randomize patients or patients to accept no ICD therapy). It is important to note that some of the early landmark trials, including AVID, faced harsh criticism regarding the randomization of patients to no ICD,144 but the investigators persisted,145 altering clinical practice.
In the absence of randomized trial data, nonrandomized studies have been undertaken to inform decision making. These studies have typically used appropriate ICD therapies or a composite of appropriate ICD therapies or death as an outcome.146–150 Findings suggesting that frequent ICD therapies during follow-up justify an ICD in patients with similar characteristics should be interpreted with caution. As discussed, data from DEFINITE131 and other trials151,152 indicate that appropriate ICD therapies are not a reliable surrogate for mortality and overestimate therapeutic benefit. For example, two studies of patients with nonischemic LV dysfunction found high rates (36%-51%) of appropriate ICD therapies over 36 months of follow-up.153,154 However, randomized trials of similar patients found mortality rates of only 12% to 21% over a similar period65,120,130 (see Table 11-3). These data indicate that caution must be used when only nonrandomized data are available to guide clinical decision making.
Patients with Less Common Inherited or Acquired Cardiac Disease
Implantable cardioverter-defibrillator therapy has a clear role in several less common inherited or acquired forms of cardiac disease. These include, but are not limited to, hypertrophic cardiomyopathy, cardiac sarcoidosis, and arrhythmogenic right ventricular cardiomyopathy. These diseases are discussed here with an emphasis on the patient selection for ICD therapy. A more in-depth review of ICD therapy in familial sudden death syndromes can be found in the 2010 Heart Rhythm UK position statement.155
Hypertrophic Cardiomyopathy
Hypertrophic cardiomyopathy (HCM) is usually inherited in an autosomal dominant pattern and is estimated to affect 0.2% of the population. It is characterized by hypertrophy of the interventricular septum, apex, or other LV regions. HCM is the most common cause of sudden death in persons younger than 40, and a cardiac arrest may be the first manifestation of the disease. The arrhythmias that lead to cardiac arrest in HCM are thought to result from cardiac myocyte disarray. The reported annual incidence of sudden death attributed to HCM has declined from 5% to less than 1% per year, likely reflecting enhanced surveillance.155–157
There have been no randomized trials of ICD therapy in HCM, so observational data must be relied on to aid clinical decision making.146,148 Deciding to use an ICD in patients with HCM can be challenging. These patients are often young and active and have paroxysmal atrial fibrillation. Thus, they will require many devices over their lifetime and may be at higher risk for unnecessary shocks.33,51 However, these patients typically have minimal comorbidity and are unlikely to survive a cardiac arrest. Thus, clinicians need to consider risk factors for sudden death in patients with HCM when deciding whether to use an ICD.
Risk factors for HCM fall into three categories: (1) those that mandate an ICD if the patient is otherwise an appropriate candidate, (2) those that favor ICD therapy if the patient is otherwise an appropriate candidate, and (3) less certain risk factors that do not provide clear guidance with respect to ICD insertion. Patients with HCM who have survived a cardiac arrest should receive an ICD,155 as should those with a history of spontaneous sustained VT.155 Less certain risk factors for sudden death in HCM include the presence of atrial fibrillation, LV outflow tract obstruction, myocardial ischemia, presence of a high-risk mutation, and a patient’s desire to pursue competitive physical exercise.155 At present, these less certain risk factors do not aid the decision to insert an ICD in a patient with HCM.
Risk factors that favor insertion of an ICD include a family history of sudden death related to HCM, unexplained syncope, LV wall thickness of 30 mm or greater, a drop in systolic blood pressure with exercise, and NSVT. Some advocate that an ICD is warranted when one of these risk factors is present,148 whereas others believe that at least two of these factors are required.158 The presence of two or more risk factors identifies HCM patients with an annual sudden death risk of about 3%, whereas a single risk factor is associated with an annual risk of about 1%.159 The UK Heart Rhythm position paper states that “patients with HCM and two or more recognized risk factors for sudden death should be considered for ICD implantation. Patients with HCM and a single recognized risk factor for sudden death may, according to individual circumstances, be considered for ICD implantation.” The use of contrast-enhanced cardiac magnetic resonance (CMR) imaging to determine the extent of myocardial scarring and fibrosis has been advocated for sudden death risk assessment in HCM patients160–162 and may be helpful when the decision to use an ICD is unclear.
Cardiac Sarcoidosis
Sarcoidosis is an idiopathic, multisystem disorder characterized by infiltrative, noncaseating granulomas in multiple organ systems. Clinically evident cardiac involvement is observed in about 5% of patients with sarcoidosis and includes tachyarrhythmias, conduction disturbance, heart failure, and sudden death.163 Antiarrhythmic drugs are thought to be of limited value in cardiac sarcoidosis because of poor efficacy, the frequent presence of concomitant conduction disease, and diagnostic issues related to adverse effect surveillance (e.g., amiodarone pulmonary toxicity).164,165 Sudden death in cardiac sarcoidosis is thought to relate to heart block or reentrant ventricular arrhythmias.164
There have been no randomized trials of ICD therapy in cardiac sarcoidosis, and only sparse observational data are available to guide clinical decision making.164,165 Identification of high-risk patients using invasive programmed ventricular stimulation has been advocated, but its sensitivity and accuracy are questionable.166 An invasive study may be useful in patients with cardiac sarcoidosis and unexplained syncope. It is recommended that patients with cardiac sarcoidosis who have survived a cardiac arrest or who have sustained VT should receive an ICD if they are otherwise appropriate candidates.143 It would also seem reasonable to use an ICD for a patient with sarcoidosis and significant LV dysfunction if an otherwise appropriate candidate. Some believe that an ICD is also warranted when chronic pacing is indicated in a patient with cardiac sarcoid.165 It is less certain whether patients with evidence of cardiac sarcoidosis, lesser impairment of LV function, and no indication for pacing warrant an ICD. Contrast-enhanced CMR may be of value in these patients.167 The presence versus absence of significant delayed enhancement, a measure of scarring, was found to have significant prognostic value in a group of 81 patients with biopsy-proven extracardiac sarcoidosis and preserved LV function (median LVEF = 0.56). Significant delayed enhancement on CMR was present in 21 patients (26%). Increased delayed enhancement was associated with a ninefold higher rate of adverse events (death, appropriate ICD shock, or requirement for permanent pacemaker) and an 11.5-fold higher risk of cardiac death. These data indicate that CMR may play a role in selection of patients for prophylactic ICD therapy in the future, but data should be considered preliminary.
Arrhythmogenic Right Ventricular Cardiomyopathy
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is a familial cardiomyopathy characterized by fibrofatty infiltration of the right ventricle and less often the left ventricle. It is typically autosomal dominant with variable penetrance. Disease-causing mutations in genes encoding cell adhesion proteins (e.g., plakoglobin and desmoplakin) indicate a disorder of the cell-to-cell junction. ARVC has a predilection for the thinnest areas, the so-called triangle of dysplasia, which includes the diaphragmatic, infundibular, and apical regions of the right ventricle. These structural changes disrupt normal electrical activity and are thought to form the basis for reentrant VT and VF.155,168
The criteria for diagnosing ARVC were recently revised to improve their clinical utility.168 Briefly, major criteria now include global or regional dysfunction and structural alterations in the right ventricle (regional RV akinesia, dyskinesia, or aneurysm identified using echocardiography, CMR, or RV angiography), abnormal RV endomyocardial biopsy findings (abnormal morphometric analysis with or without fatty infiltration), specific ECG findings (epsilon wave in leads V1 to V3), less specific ECG findings (inverted T waves in leads V1, V2, and V3 in the absence of RBBB), arrhythmias (NSVT or sustained VT of LBB morphology with superior axis), and a family history of confirmed ARVC.
No randomized trials of ICD therapy have been done in ARVC patients, so clinicians must rely on observational data to aid clinical decision making. Observational studies have consistently found a high frequency of appropriate ICD use for life-threatening ventricular arrhythmias and a low rate of arrhythmic death in patients with ARVC treated with ICD therapy.143,147,169 Most experts would agree that patients with ARVC who have survived a cardiac arrest, have poorly tolerated VT, or have syncope, when VT/VF cannot be excluded, should receive an ICD if they are otherwise appropriate candidates.155 ARVC patients presenting with ventricular arrhythmias and severe structural disease should also be considered for an ICD if otherwise appropriate candidates.155 The UK Heart Rhythm position paper further states, “ARVC patients who are asymptomatic with mild disease are at low risk of sudden death, and the risks of ICD therapy may outweigh the benefits in this group.”155 The signal-averaged ECG is helpful in confirming a diagnosis of ARVC, but invasive electrophysiologic assessment and programmed electrical stimulation are of limited value in identifying ARVC patients at risk of sudden death, a result of the low predictive accuracy and unacceptably high false-positive and false-negative results with this assessment method.170
Other Informative Icd Trials
Microvolt T Wave Alternans Testing for Risk Stratification of Post–Myocardial Infarction Patients
Microvolt T Wave Alternans Testing for Risk Stratification of Post–Myocardial Infarction Patients (MASTER) was an observational study that evaluated the association of baseline microvolt TWA results with outcomes.83 A total of 768 patients with ischemic LV dysfunction (LVEF ≤0.35) and no prior sustained ventricular arrhythmia were enrolled. Of these, 392 (51%) received an ICD. The mean follow-up was 27 months. The 514 (67%) patients with nonnegative microvolt TWA results had an 8.6% rate of sudden death, and the remaining 254 patients with negative results had a rate of 5.1%. Patients with nonnegative TWA results had a 19.3% mortality rate, significantly larger than the 11.8% mortality rate in patients with a negative TWA result. A 70% reduction in arrhythmic death was observed with ICD therapy, but the effectiveness of ICD therapy was not consistent across the microvolt TWA subgroups. Data interpretation indicates that microvolt TWA assessment has limited value in identifying patients for ICD therapy and should not be used to decide whether a patient should receive an ICD.
Alternans Before Cardioverter-Defibrillator
The Alternans Before Cardioverter-Defibrillator (ABCD) observational study included 566 patients who underwent microvolt TWA testing and attempted induction of VT/VF with invasive electrophysiologic testing.149 Patients with LVEF < 0.40 values attributable to ischemic heart disease and NSVT were followed for a median of 1.9 years. The primary outcome was ICD shocks, ATP-terminated rhythms, or sudden death. Patients with abnormal microvolt TWA or inducible VT/VF were recommended to receive an ICD; 495 of the 566 patients (87%) received an ICD. A total of 65 events were observed, including 51 ICD shocks, four ATP-terminated rhythms, and 10 sudden deaths. Seven of the 10 sudden deaths occurred despite the patients being treated with an ICD. The event rate in patients with negative TWA and no inducible VT/VF (10.1%) was similar to the event rate in patients with a positive microvolt TWA result but no inducible VT/VF (12%). Patients with inducible VT/VF but a negative microvolt TWA result had a slightly higher event rate (15.5%), as did patients with both a positive microvolt TWA result and an inducible VT/VF (15.7%). Patients with inducible VT/VF and an indeterminate TWA were at highest risk (22.9%). These data suggest that microvolt TWA assessment may provide additive value to the results of invasive electrophysiologic testing.
Inhibition of Unnecessary RV Pacing with AVSH in ICDs
The Inhibition of Unnecessary RV Pacing with AVSH in ICDs (INTRINSIC-RV) study compared dual-chamber rate-responsive (DDDR) with atrioventricular search hysteresis (AVSH) versus backup single-chamber (VVI at 40 bpm) programming. The primary outcome was death or heart failure hospitalization.170a A total of 1530 ICD recipients entered a 1-week period for assessment of percent RV pacing. The 988 patients with less than 20% RV pacing were randomized to DDDR (502) versus VVI (486). LVEF values were not collected. A history of atrial fibrillation or atrial flutter was present in about 12% of participants. Over mean follow-up of 10 months, 32 DDDR (6.4%) and 46 VVI (9.5%) patients died or were hospitalized with heart failure (P = .07) The mean percent RV pacing in the DDDR arm was 10% (median 4%) versus 3% (median 0%) in the VVI arm. Thus, DDDR pacing with AVSH was associated with similar outcomes as VVI backup pacing.
Second Dual Chamber and VVI Implantable Defibrillator Trial
The Second Dual Chamber and VVI Implantable Defibrillator (DAVID-II) trial included 600 patients from 29 sites in North America.171 It assessed whether AAI pacing was a safe alternative to backup VVI ventricular pacing in 600 ICD recipients with LV dysfunction. DAVID-I had found that forced atrial-RV pacing increased the risk of death or heart failure hospitalization in ICD recipients with LV dysfunction.172 Patients receiving an ICD for primary or secondary prevention, with no clinical indication for pacing, were randomly assigned to AAI (70 bpm) versus VVI pacing (at 40 bpm). The mean LVEF of participants was 0.26, a history of atrial fibrillation was present in 14%, and 20% had a PR interval greater than 200 msec. Over a mean follow-up of 2.7 years, the risk of death or heart failure hospitalization was similar in patients assigned to AAI versus VVI pacing (P = .95). No differences in QOL or the rates of atrial fibrillation, syncope, or appropriate or inappropriate shocks were observed. Thus, AAI pacing appears to be a safe alternative when pacing is desired in ICD recipients.
Multicenter Automatic Defibrillator Implantation Trial–Cardiac Resynchronization Therapy
The Multicenter Automatic Defibrillator Implantation Trial–Cardiac Resynchronization Therapy (MADIT-CRT) evaluated the capacity of ICD plus CRT versus ICD therapy alone to reduce the risk of death or heart failure events in 1820 patients with LVEF values ≤0.30, QRS duration ≥130 msec, and NYHA Class I or II limitation.173 Heart failure events were diagnosed by physicians aware of treatment assignment, but adjudicated by a masked committee. During an average follow-up of 2.4 years, the composite outcome occurred in 187 of 1089 patients in the CRT-ICD group (17.2%) and 185 of 731 patients in the ICD group (25.3%), with a resultant hazard ratio of 0.66 (95% CI = 0.52 to 0.84; P = .001). Similar results were observed in patients with ischemic and nonischemic LV dysfunction. CRT was particularly effective in patients with wide QRS durations (≥150 msec). The benefit of CRT was solely attributable to a 41% reduction in the risk of heart failure events. No difference in the risk of death was observed in the two groups (each with 3% annual mortality).
Echocardiographic studies were obtained at baseline and at 1 year in 1372 of the 1820 (75%) patients. The CRT-ICD group had greater improvement in various chamber dimensions versus the ICD group. Most notably, LV end-systolic volume index was reduced by an average of 28.7 versus 9.1 mL/m2, and LVEF increased in absolute terms by 0.11 versus 0.03 in the CRT-D versus ICD groups, respectively. Remodeling at 1 year was predictive of subsequent death or heart failure (P <.001).174 The MADIT-CRT data suggest a benefit from providing CRT earlier in the course of disease. Recent European guidelines now recommend CRT for patients with NYHA Class II limitation175 (see Guidelines for ICD Therapy).
Resynchronization/Defibrillation for Advanced Heart Failure Trial
The Resynchronization/Defibrillation for Advanced Heart Failure Trial (RAFT) compared ICD plus CRT versus an ICD therapy alone in 1798 patients with LVEF values ≤0.30, NYHA Class II or III symptoms, intrinsic QRS duration ≥120 msec, or paced QRS durations ≥200 msec. Patients with ischemic or nonischemic LV dysfunction were eligible. Unlike MADIT-CRT, patients with atrial fibrillation were included if the ventricular rate was controlled.176
The primary objective of RAFT was to determine if the addition of CRT to ICD and optimized medical therapy would reduce total mortality and hospitalizations for heart failure in patients with mild to moderate heart failure symptoms. Patients with NYHA Class II or III limitation were initially eligible, but enrollment was restricted to patients with Class II limitation after completion of CARE-HF.123 The 1798 patients were followed for a mean of 40 months. The primary outcome of death or hospitalization occurred in 297 of 894 patients assigned to ICD-CRT (33.2%) versus 364 of 904 patients in the ICD group (40.3%), which resulted in a 25% relative risk reduction (HR 0.75; 95% CI 0.64 to 0.87; P < 0.001). The 7.1% absolute reduction in the risk of the primary outcome translates into needing to treat 14 patients with CRT-ICD versus an ICD for 40 months to prevent one event. Patients assigned to ICD-CRT also had a 25% lower risk of death from any cause. The 5-year actuarial rate of death in the CRT-ICD group was 28.6% versus 34.6% in the ICD group (HR 0.75; 95% CI 0.62 to 0.91; P = 0.003). Thus, 14 patients would need to be treated for 5 years with ICD-CRT versus an ICD alone to save one life. Further, 32% fewer patients in the ICD-CRT group were hospitalized for heart failure (174 hospitalizations) versus the ICD group (236 hospitalizations; HR 0.68; 95% CI 0.56 to 0.83; P < 0.001). The ICD-CRT patients had more adverse events, largely attributed to left ventricular lead–related complications.
 Guidelines for ICD Therapy
Guidelines for ICD Therapy
The American College of Cardiology (ACC) and the American Heart Association (AHA) have proposed standard definitions for categorizing levels of evidence143 (Table 11-7). The latest published ACC, AHA, and Heart Rhythm Society (HRS) guidelines for the use of ICD therapy are from 2008.143 Table 11-8 summarizes the conditions in which ICD therapy is considered to be useful or may be useful (classes I and II). Table 11-9 summarizes the conditions for which there is evidence or general agreement that ICD therapy is not useful or may be harmful (class III). Some important randomized trials have been published since the 2008 ACC/AHA/HRS guidelines were released, including IRIS and several CRT studies (REVERSE, MADIT-CRT, and RAFT). As noted, recent European Society of Cardiology (ESC) Guidelines now recommend CRT (class I, level A) for patients in sinus rhythm, with LVEF values of 0.35 or greater, NYHA Class II limitation, and QRS duration of 150 msec or longer.175
TABLE 11-7 Standard Definitions: Classification of Recommendations and Level of Evidence
| Classification | Details |
|---|---|
| I | Conditions for which there is evidence and/or general agreement that a given procedure or treatment is beneficial, useful, and effective. |
| II | Conditions for which there is conflicting evidence and/or divergence of opinion about the usefulness/efficacy of a procedure or treatment. |
| IIa | Weight of evidence/opinion is in favor of usefulness/efficacy. |
| IIb | Usefulness/efficacy is less well established by evidence/opinion. |
| III | Conditions for which there is evidence and/or general agreement that a procedure/treatment is not useful/effective and in some cases may be harmful. |
| Level of Evidence | Definition |
| A | Data derived from multiple randomized clinical trials or meta-analyses. |
| B | Data derived from a single randomized trial or nonrandomized studies. |
| C | Only consensus opinion of experts, case studies, or standard of care. |
TABLE 11-8 Indications for ICD Therapy
| Recommendations | Level of Evidence |
|---|---|
| Class I (General Agreement of Benefit with ICD Therapy) | |
| 1. Cardiac arrest due to VF or unstable sustained VT after evaluation in the absence of completely reversible causes. | A |
| 2. Structural heart disease and spontaneous sustained VT, whether hemodynamically stable or unstable. | B |
| 3. Syncope of undetermined origin and clinically relevant, sustained VT or VF induced at electrophysiologic study. | B |
| 4. LVEF ≤0.35 due to prior MI who are at least 40 days post-MI and NYHA II or III. | A |
| 5. LVEF ≤0.35 due to a nonischemic cause and NYHA II or III. | B |
| 6. LVEF ≤0.30 due to prior MI, at least 40 days post-MI and are NYHA I. | A |
| 7. Nonsustained VT due to prior MI, LVEF ≤0.40, and inducible VF or sustained VT at electrophysiologic study. | B |
| Class IIa (Weight of Evidence in Favor of Usefulness of ICD Therapy) | |
| 1. Unexplained syncope, significant LV dysfunction related to a nonischemic cause. | C |
| 2. Sustained VT and normal or near-normal left ventricular systolic function. | C |
| 3. Hypertrophic cardiomyopathy with one or more major risk factors for sudden death (see text). | C |
| 4. Arrhythmogenic right ventricular cardiomyopathy (ARVC) with one or more risk factors for sudden death. | C |
| 5. Long-QT syndrome with syncope and/or VT while receiving β-blockers. | B |
| 6. Nonhospitalized patients awaiting transplantation. | C |
| 7. Brugada syndrome with syncope. | C |
| 8. Brugada syndrome with documented VT that has not resulted in cardiac arrest. | C |
| 9. Catecholaminergic polymorphic VT with syncope and/or documented sustained VT while receiving β-blockers. | C |
| 10. Cardiac sarcoidosis, giant cell myocarditis, or Chagas disease. | C |
| Class IIb (Efficacy of ICD Therapy Is Less Well Established) | |
| 1. LVEF ≤ 0.35 due to nonischemic cause and NYHA I. | C |
| 2. Long-QT syndrome and risk factors for sudden death. | B |
| 3. Advanced structural heart disease and syncope of unknown cause despite invasive and noninvasive investigations. | C |
| 4. Familial cardiomyopathy associated with sudden death. | C |
| 5. Left ventricular noncompaction. | C |
LVEF, Left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association functional class; VF, ventricular fibrillation; VT, ventricular tachycardia.
TABLE 11-9 Circumstances in which ICD Therapy Is Not Indicated
| Recommendations | Level of Evidence |
|---|---|
| Class III (General Agreement that An ICD Is Not Effective and May Be Harmful) | |
| 1. No reasonable expectation of survival for at least 1 year, even when class I or II implantation criteria are present. | C |
| 2. Incessant VT or VF. | |
| 3. Significant psychiatric illnesses that may be aggravated by ICD implantation or that may preclude systematic follow-up. | C |
| 4. Drug-refractory, NYHA IV heart failure and not a candidate for cardiac transplantation or CRT-D. | C |
| 5. Syncope of undetermined without inducible ventricular tachyarrhythmias and without structural heart disease. | C |
| 6. VF or VT is amenable to surgical or catheter ablation (e.g., Wolff-Parkinson-White syndrome, outflow tract VT, idiopathic VT, or fascicular VT without structural heart disease). | C |
| 7. VT or VF caused by a completely reversible disorder in the absence of structural heart disease (e.g., electrolyte imbalance, drugs, trauma). | B |
CRT-D, Cardiac resynchronization therapy–defibrillation; NYHA, New York Heart Association functional class; VF, ventricular fibrillation; VT, ventricular tachycardia.
Whether all patients with the characteristics in Table 11-8 should receive an ICD is controversial.177,178 Some believe that additional risk assessment is required to identify patients who are most likely to benefit from an ICD because of the concerns over device longevity, cost, accessibility, unnecessary shocks, and consequence of shocks. Others believe that ICD therapy saves lives, does not appreciably alter QOL over time (even when ICD shocks are included), and is cost-effective (see previous discussion).
 Ongoing Trials
Ongoing Trials
Table 11-10 provides details on three ongoing trials assessing the efficacy of an ICD or a wearable AED after MI and another evaluating ICD efficacy in nonischemic cardiomyopathy.
 Other Issues
Other Issues
Is There a Need for Additional Risk Assessment?
Controversy surrounds the use of ICD therapy in all patients with an LVEF of 0.35 or less.177,178 Although widespread use of ICD therapy for prevention of arrhythmic death is understandable, this approach still carries the risk of complications related to implantation and subsequent revisions, besides the cost. Although extrapolation of the findings of past studies is reasonable and often necessary, it is prudent to consider a reasonable probability of success, including assessment of sudden death risk versus the risk of competing modes of death. The analyses from CIDS,103 MUSTT,109 and MADIT-II114 indicate that patients with significant comorbidity, particularly chronic renal impairment,179 may benefit from ICD therapy to a lesser extent. Other sources of data, including large registries, may aid in better selection of appropriate candidates for ICD therapy (see Real-World Effectiveness).
In an attempt to include a greater proportion of patients at risk for sudden death (see Fig. 11-2), LV dysfunction has been a major inclusion criterion for most of the completed ICD trials (see Table 11-3). However, many of these patients do not experience life-threatening arrhythmias in the near term. The incidence of appropriate ICD therapies ranges from 28% to 68% over the initial 2 to 5 years after ICD implantation, depending on the population studied and the duration of follow-up.151,154,180 Patients receiving an ICD on the basis of heart failure or LV dysfunction alone have a twofold to threefold lower risk of life-threatening arrhythmias in the initial 2 to 5 years of follow-up than patients with a history of spontaneous sustained VT/VF181–183 or inducible sustained VT/VF.184 Identifying higher-risk groups in those receiving an ICD for heart failure or LV dysfunction alone has been undertaken.103,109,114 Although useful in helping physicians better understand which patients may benefit from an ICD, these clinical scores should be used as “hypothesis-generating” tools, given the lack of prospective validation to date.
Although the relative efficacy of an ICD is similar in most of the ICD trials (see Fig. 11-4), the absolute effect differs substantially. Among sudden death survivors22 and patients with ischemic LV dysfunction and inducible arrhythmias,64,91 the average absolute risk reduction was about 12%. Therefore, the NNT for ICD therapy in this group is approximately 8. In contrast, among patients with only LV dysfunction,23,133 the average absolute benefit was about 6%, translating into an NNT of 16. These data have been used to support the need for methods to better identify patients in whom ICD therapy leads to a greater absolute risk reduction and a lower NNT. The long-term data from MADIT-II identify a large, 13% absolute reduction in mortality with ICD therapy in patients with LV dysfunction.50 This reduction in mortality is similar to that in the randomized trials of ICD therapy in secondary prevention patients, although over shorter follow-up (see Fig. 11-4). The results of RAFT provide further evidence of the cost effectiveness of adding CRT to ICD therapy in patients with prolonged QRS durations and symptomatic heart failure (see Other Informative ICD Trials).
Given the consequence of sudden death, it is imperative that a balance is reached between identifying patients with a significant absolute mortality reduction with ICD therapy (specificity) but not excluding most who will die from sudden death (sensitivity) (see Fig. 11-3). Noninvasive tools have also been advocated to better identify patients likely versus unlikely to benefit from an ICD. As discussed, these methods have not proved useful in guiding ICD therapy to date. Ongoing trials (e.g., DETERMINE, REFINE ICD) are assessing whether these tools can be used to select patients in whom an ICD will reduce mortality. Until these and other trials have been completed, it is unclear what role, if any, these methods have in guiding ICD therapy.
Real-World Effectiveness
The sites participating in the reviewed randomized trials are typically high-volume, academically oriented centers. The success rates and complication rates achieved in these trials may not reflect the results in other centers. Until recently, little was known about the real-world success rates and complications of ICD placement. This issue is particularly relevant when the results of more complex ICD systems that include CRT are applied to less experienced centers, given the steep learning curve associated with implantation of LV leads.185
Large registries have been initiated to assess real-world effectiveness of ICD therapy, most notably the U.S. National Cardiovascular Data Registry (NCDR) Registry. Insights from the NCDR and other registries include improved outcomes when devices are implanted by electrophysiologists versus non-electrophysiologists,186 better outcomes in higher-volume than lower-volume implant centers,187 and significantly impaired outcomes in ICD recipients from comorbidities such as chronic renal disease.188 These data complement the findings from randomized trials. Registry data have also provided insight into implant complications, showing a twofold higher rate of complications with dual-chamber and CRT systems versus single-chamber ICD systems.189 Registry data have also helped in identifying barriers related to device implantation190,191 and the off-label use of devices.53,54 Despite limitations, registry data are providing important insights into real-world application of ICD systems.
1 Fox CS, Evans JC, Larson MG, et al. Temporal trends in coronary heart disease mortality and sudden cardiac death from 1950 to 1999: the Framingham Heart Study. Circulation. 2004;110:522-527.
2 Bunch TJ, White RD, Friedman PA, et al. Trends in treated ventricular fibrillation out-of-hospital cardiac arrest: a 17-year population-based study. Heart Rhythm. 2004;1:255-259.
3 Kuller LH, Traven ND, Rutan GH, et al. Marked decline of coronary heart disease mortality in 35-44-year-old white men in Allegheny County, Pennsylvania. Circulation. 1989;80:261-266.
4 Josephson M, Wellens HJ. Implantable defibrillators and sudden cardiac death. Circulation. 2004;109:2685-2691.
5 Bayes de Luna A, Coumel P, Leclercq JF. Ambulatory sudden cardiac death: mechanisms of production of fatal arrhythmia on the basis of data from 157 cases. Am Heart J. 1989;117:151-159.
6 Gang UJ, Jons C, Jorgensen RM, et al. Heart rhythm at the time of death documented by an implantable loop recorder. Europace. 2010;12:254-260.
7 Luu M, Stevenson WG, Stevenson LW, et al. Diverse mechanisms of unexpected cardiac arrest in advanced heart failure. Circulation. 1989;80:1675-1680.
8 Hinkle LEJr, Thaler HT. Clinical classification of cardiac deaths. Circulation. 1982;65:457-464.
9 Narang R, Cleland JG, Erhardt L, et al. Mode of death in chronic heart failure: a request and proposition for more accurate classification. Eur Heart J. 1996;17:1390-1403.
10 Greenberg H, Case RB, Moss AJ, et al. Analysis of mortality events in the Multicenter Automatic Defibrillator Implantation Trial (MADIT-II). J Am Coll Cardiol. 2004;43:1459-1465.
11 Pouleur AC, Barkoudah E, Uno H, et al. Pathogenesis of sudden unexpected death in a clinical trial of patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. Circulation. 2010;122:597-602.
12 Mirowski M, Mower MM, Langer A, et al. A chronically implanted system for automatic defibrillation in active conscious dogs: experimental model for treatment of sudden death from ventricular fibrillation. Circulation. 1978;58:90-94.
13 Exner DV, Klein GJ, Prystowsky EN. Primary prevention of sudden death with implantable defibrillator therapy in patients with cardiac disease: Can we afford to do it? (Can we afford not to?). Circulation. 2001;104:1564-1570.
14 Wilkoff BL, Ousdigian KT, Sterns LD, et al. A comparison of empiric to physician-tailored programming of implantable cardioverter-defibrillators: results from the prospective randomized multicenter EMPIRIC trial. J Am Coll Cardiol. 2006;48:330-339.
15 Saeed M, Neason CG, Razavi M, et al. Programming antitachycardia pacing for primary prevention in patients with implantable cardioverter defibrillators: results from the PROVE trial. J Cardiovasc Electrophysiol. 2010;21:1349-1354.
16 Schwab JO. Optimizing ICD programming for shock reduction. Fundam Clin Pharmacol. 2010;24:653-659.
17 Steinhaus D, Reynolds DW, Gadler F, et al. Implant experience with an implantable hemodynamic monitor for the management of symptomatic heart failure. Pacing Clin Electrophysiol. 2005;28:747-753.
18 Varma N, Epstein AE, Irimpen A, et al. Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up: the Lumos-T Safely Reduces Routine Office Device Follow-up (TRUST) trial. Circulation. 2010;122:325-332.
19 McAlister FA, Ezekowitz J, Hooton N, et al. Cardiac resynchronization therapy for patients with left ventricular systolic dysfunction: a systematic review. JAMA. 2007;297:2502-2514.
20 Kleman JM, Castle LW, Kidwell GA, et al. Nonthoracotomy-versus thoracotomy-implantable defibrillators: intention-to-treat comparison of clinical outcomes. Circulation. 1994;90:2833-2842.
21 Kim SG, Pathapati R, Fisher JD, et al. Comparison of long-term outcomes of patients treated with nonthoracotomy and thoracotomy implantable defibrillators. Am J Cardiol. 1996;78:1109-1112.
22 Connolly SJ, Hallstrom AP, Cappato R, et al. Meta-analysis of the implantable cardioverter defibrillator secondary prevention trials. AVID, CASH and CIDS studies; Antiarrhythmics vs Implantable Defibrillator study; Cardiac Arrest Study Hamburg; Canadian Implantable Defibrillator Study. Eur Heart J. 2000;21:2071-2078.
23 Ezekowitz JA, Rowe BH, Dryden DM, et al. Systematic review: implantable cardioverter defibrillators for adults with left ventricular systolic dysfunction. Ann Intern Med. 2007;147:251-262.
24 Camm AJ, Nisam S. European utilization of the implantable defibrillator: has 10 years changed the “enigma”? Europace. 2010;12:1063-1069.
25 McCready MJ, Exner DV. Quality of life and psychological impact of implantable cardioverter-defibrillators: focus on randomized controlled trial data. Card Electrophysiol Rev. 2003;7:63-70.
26 Schron EB, Exner DV, Yao Q, et al. Quality of life in the antiarrhythmics versus implantable defibrillators trial: impact of therapy and influence of adverse symptoms and defibrillator shocks. Circulation. 2002;105:589-594.
27 Irvine J, Dorian P, Baker B, et al. Quality of life in the Canadian Implantable Defibrillator Study (CIDS). Am Heart J. 2002;144:282-289.
28 Namerow PB, Firth BR, Heywood GM, et al. Quality-of-life six months after CABG surgery in patients randomized to ICD versus no ICD therapy: findings from the CABG Patch trial. Pacing Clin Electrophysiol. 1999;22:1305-1313.
29 Noyes K, Corona E, Veazie P, et al. Examination of the effect of implantable cardioverter-defibrillators on health-related quality of life: based on results from the Multicenter Automatic Defibrillator Trial-II. Am J Cardiovasc Drugs. 2009;9:393-400.
30 Passman R, Subacius H, Ruo B, et al. Implantable cardioverter defibrillators and quality of life: results from the Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation study. Arch Intern Med. 2007;167:2226-2232.
31 Mark DB, Anstrom KJ, Sun JL, et al. Quality of life with defibrillator therapy or amiodarone in heart failure. N Engl J Med. 2008;359:999-1008.
32 Exner DV, Pinski SL, Wyse DG, et al. Electrical storm presages nonsudden death: the Antiarrhythmics versus Implantable Defibrillators (AVID) trial. Circulation. 2001;103:2066-2071.
33 Daubert JP, Zareba W, Cannom DS, et al. Inappropriate implantable cardioverter-defibrillator shocks in MADIT-II: frequency, mechanisms, predictors, and survival impact. J Am Coll Cardiol. 2008;51:1357-1365.
34 Poole JE, Johnson GW, Hellkamp AS, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359:1009-1017.
35 Epstein AE, Kay GN, Plumb VJ, et al. Gross and microscopic pathological changes associated with nonthoracotomy implantable defibrillator leads. Circulation. 1998;98:1517-1524.
36 Hurst TM, Hinrichs M, Breidenbach C, et al. Detection of myocardial injury during transvenous implantation of automatic cardioverter-defibrillators. J Am Coll Cardiol. 1999;34:402-408.
37 Trayanova N, Eason J. Shock-induced arrhythmogenesis in the myocardium. Chaos. 2002;12:962-972.
38 Pires LA, Lehmann MH, Steinman RT, et al. Sudden death in implantable cardioverter-defibrillator recipients: clinical context, arrhythmic events and device responses. J Am Coll Cardiol. 1999;33:24-32.
39 Mitchell LB, Pineda EA, Titus JL, et al. Sudden death in patients with implantable cardioverter defibrillators: the importance of post-shock electromechanical dissociation. J Am Coll Cardiol. 2002;39:1323-1328.
40 Sweeney MO, Sherfesee L, DeGroot PJ, et al. Differences in effects of electrical therapy type for ventricular arrhythmias on mortality in implantable cardioverter-defibrillator patients. Heart Rhythm. 2010;7:353-360.
41 Pacifico A, Hohnloser SH, Williams JH, et al. Prevention of implantable-defibrillator shocks by treatment with sotalol. d,l-Sotalol Implantable Cardioverter-Defibrillator Study Group. N Engl J Med. 1999;340:1855-1862.
42 Dorian P, Borggrefe M, Al-Khalidi HR, et al. Placebo-controlled, randomized clinical trial of azimilide for prevention of ventricular tachyarrhythmias in patients with an implantable cardioverter defibrillator. Circulation. 2004;110:3646-3654.
43 Wathen MS, DeGroot PJ, Sweeney MO, et al. Prospective randomized multicenter trial of empirical antitachycardia pacing versus shocks for spontaneous rapid ventricular tachycardia in patients with implantable cardioverter-defibrillators: Pacing Fast Ventricular Tachycardia Reduces Shock Therapies (PainFREE Rx II) trial results. Circulation. 2004;110:2591-2596.
44 Sweeney MO, Wathen MS, Volosin K, et al. Appropriate and inappropriate ventricular therapies, quality of life, and mortality among primary and secondary prevention implantable cardioverter defibrillator patients: results from the Pacing Fast VT Reduces Shock Therapies (PainFREE Rx II) trial. Circulation. 2005;111:2898-2905.
45 Wilkoff BL, Williamson BD, Stern RS, et al. Strategic programming of detection and therapy parameters in implantable cardioverter-defibrillators reduces shocks in primary prevention patients: results from the PREPARE (Primary Prevention Parameters Evaluation) study. J Am Coll Cardiol. 2008;52:541-550.
46 Volosin KJ, Exner DV, Wathen M, et al. Combining Shock Reduction Strategies to Enhance ICD Therapy: a role for computer modeling. J Cardiovasc Electrophysiol. 2011;22:280-289.
47 Mehta D, Nayak HM, Singson M, et al. Late complications in patients with pectoral defibrillator implants with transvenous defibrillator lead systems: high incidence of insulation breakdown. Pacing Clin Electrophysiol. 1998;21:1893-1900.
48 Hauser RG. The growing mismatch between patient longevity and the service life of implantable cardioverter-defibrillators. J Am Coll Cardiol. 2005;45:2022-2025.
49 Krahn AD, Champagne J, Healey JS, et al. Outcome of the Fidelis implantable cardioverter-defibrillator lead advisory: a report from the Canadian Heart Rhythm Society Device Advisory Committee. Heart Rhythm. 2008;5:639-642.
50 Goldenberg I, Gillespie J, Moss AJ, et al. Long-term benefit of primary prevention with an implantable cardioverter-defibrillator: an extended 8-year follow-up study of the Multicenter Automatic Defibrillator Implantation Trial II. Circulation. 2010;122:1265-1271.
51 Knops P, Theuns DA, Res JC, Jordaens L. Analysis of implantable defibrillator longevity under clinical circumstances: implications for device selection. Pacing Clin Electrophysiol. 2009;32:1276-1285.
52 Simpson CS, Klein GJ, Hoffmaster B. Expensive medical technologies and “indication extrapolation”: the case of implantable cardioverter-defibrillators. Am Heart J. 2000;140:419-422.
53 Dickstein K, Bogale N, Priori S, et al. The European Cardiac Resynchronization Therapy Survey. Eur Heart J. 2009;30:2450-2460.
54 Fein AS, Wang Y, Curtis JP, et al. Prevalence and predictors of off-label use of cardiac resynchronization therapy in patients enrolled in the National Cardiovascular Data Registry Implantable Cardiac-Defibrillator Registry. J Am Coll Cardiol. 2010;56:766-773.
55 Salukhe TV, Dimopoulos K, Sutton R, et al. Life-years gained from defibrillator implantation: markedly nonlinear increase during 3 years of follow-up and its implications. Circulation. 2004;109:1848-1853.
56 Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877-883.
57 Sanders GD, Hlatky MA, Owens DK. Cost-effectiveness of implantable cardioverter-defibrillators. N Engl J Med. 2005;353:1471-1480.
58 Sanders GD, Kong MH, Al-Khatib SM, Peterson ED. Cost-effectiveness of implantable cardioverter defibrillators in patients ≥65 years of age. Am Heart J. 2010;160:122-131.
59 Myerburg RJ, Reddy V, Castellanos A. Indications for implantable cardioverter-defibrillators based on evidence and judgment. J Am Coll Cardiol. 2009;54:747-763.
60 Bardy GH, Smith WM, Hood MA, et al. An entirely subcutaneous implantable cardioverter-defibrillator. N Engl J Med. 2010;363:36-44.
61 Exner DV. Noninvasive risk stratification after myocardial infarction: rationale, current evidence and the need for definitive trials. Can J Cardiol. 2009;25(Suppl A):21-27.
62 Myerburg RJ. Sudden cardiac death: exploring the limits of our knowledge. J Cardiovasc Electrophysiol. 2001;12:369-381.
63 Rea TD, Pearce RM, Raghunathan TE, et al. Incidence of out-of-hospital cardiac arrest. Am J Cardiol. 2004;93:1455-1460.
64 Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933-1940.
65 Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225-237.
66 Roy D, Marchand E, Theroux P, et al. Programmed ventricular stimulation in survivors of an acute myocardial infarction. Circulation. 1985;72:487-494.
67 Al-Khatib SM, Hafley G, Lee KL, Buxton AE. Relation between time from myocardial infarction to enrolment and patient outcomes in the Multicenter Unsustained Tachycardia Trial. Europace. 2010;12:1112-1118.
68 Daubert JP, Zareba W, Hall WJ, et al. Predictive value of ventricular arrhythmia inducibility for subsequent ventricular tachycardia or ventricular fibrillation in Multicenter Automatic Defibrillator Implantation Trial (MADIT) II patients. J Am Coll Cardiol. 2006;47:98-107.
69 La Rovere MT, Bigger JTJr, Marcus FI, et al. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes after Myocardial Infarction) Investigators. Lancet. 1998;351:478-484.
70 Exner DV, Kavanagh KM, Slawnych MP, et al. Noninvasive risk assessment early after a myocardial infarction the REFINE study. J Am Coll Cardiol. 2007;50:2275-2284.
71 Schmidt G, Malik M, Barthel P, et al. Heart-rate turbulence after ventricular premature beats as a predictor of mortality after acute myocardial infarction. Lancet. 1999;353:1390-1396.
72 Gorgels AP, Gijsbers C, de Vreede–Swagemakers J, et al. Out-of-hospital cardiac arrest: the relevance of heart failure. The Maastricht Circulatory Arrest Registry. Eur Heart J. 2003;24:1204-1209.
73 Traven ND, Kuller LH, Ives DG, et al. Coronary heart disease mortality and sudden death: trends and patterns in 35- to 44-year-old white males, 1970-1990. Am J Epidemiol. 1995;142:45-52.
74 Jouven X, Desnos M, Guerot C, Ducimetiere P. Predicting sudden death in the population: the Paris Prospective Study I. Circulation. 1999;99:1978-1983.
75 Adabag AS, Peterson G, Apple FS, et al. Etiology of sudden death in the community: results of anatomical, metabolic, and genetic evaluation. Am Heart J. 2010;159:33-39.
76 Antman EM, Hand M, Armstrong PW, et al. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2008;51:210-247.
77 Bardy GH, Lee KL, Mark DB, et al. Home use of automated external defibrillators for sudden cardiac arrest. N Engl J Med. 2008;358:1793-1804.
78 Heidary S, Patel H, Chung J, et al. Quantitative tissue characterization of infarct core and border zone in patients with ischemic cardiomyopathy by magnetic resonance is associated with future cardiovascular events. J Am Coll Cardiol. 2010;55:2762-2768.
79 Boogers MJ, Borleffs CJ, Henneman MM, et al. Cardiac sympathetic denervation assessed with 123-iodine metaiodobenzylguanidine imaging predicts ventricular arrhythmias in implantable cardioverter-defibrillator patients. J Am Coll Cardiol. 2010;55:2769-2777.
80 Goldberger JJ, Cain ME, Hohnloser SH, et al. American Heart Association/American College of Cardiology Foundation/Heart Rhythm Society Scientific Statement on Noninvasive Risk Stratification Techniques for Identifying Patients at Risk for Sudden Cardiac Death. A scientific statement from the American Heart Association Council on Clinical Cardiology Committee on Electrocardiography and Arrhythmias and Council on Epidemiology and Prevention. J Am Coll Cardiol. 2008;52:1179-1199.
81 Bigger JTJr. Prophylactic use of implanted cardiac defibrillators in patients at high risk for ventricular arrhythmias after coronary-artery bypass graft surgery. Coronary Artery Bypass Graft (CABG) Patch Trial Investigators. N Engl J Med. 1997;337:1569-1575.
82 Hohnloser SH, Kuck KH, Dorian P, et al. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004;351:2481-2488.
83 Chow T, Kereiakes DJ, Onufer J, et al. Does microvolt T-wave alternans testing predict ventricular tachyarrhythmias in patients with ischemic cardiomyopathy and prophylactic defibrillators? The MASTER (Microvolt T Wave Alternans Testing for Risk Stratification of Post–Myocardial Infarction Patients) trial. J Am Coll Cardiol. 2008;52:1607-1615.
84 Wever EF, Hauer RN, Schrijvers G, et al. Cost-effectiveness of implantable defibrillator as first-choice therapy versus electrophysiologically guided, tiered strategy in postinfarct sudden death survivors: a randomized study [see comments]. Circulation. 1996;93:489-496.
85 Raviele A, Bongiorni MG, Brignole M, et al. Early EPS/ICD strategy in survivors of acute myocardial infarction with severe left ventricular dysfunction on optimal beta-blocker treatment. The BEta-blocker STrategy plus ICD trial. Europace. 2005;7:327-337.
86 Steinbeck G, Andresen D, Seidl K, et al. Defibrillator implantation early after myocardial infarction. N Engl J Med. 2009;361:1427-1436.
87 Veenhuyzen GD, Singh SN, McAreavey D, et al. Prior coronary artery bypass surgery and risk of death among patients with ischemic left ventricular dysfunction. Circulation. 2001;104:1489-1493.
88 AVID Investigators. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. The Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators [see comments]. N Engl J Med. 1997;337:1576-1583.
89 Connolly SJ, Gent M, Roberts RS, et al. Canadian Implantable Defibrillator Study (CIDS): a randomized trial of the implantable cardioverter defibrillator against amiodarone. Circulation. 2000;101:1297-1302.
90 Kuck KH, Cappato R, Siebels J, Ruppel R. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest: the Cardiac Arrest Study Hamburg (CASH). Circulation. 2000;102:748-754.
91 Buxton AE, Lee KL, Fisher JD, et al. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999;341:1882-1890.
92 Larsen G, Hallstrom A, McAnulty J, et al. Cost-effectiveness of the implantable cardioverter-defibrillator versus antiarrhythmic drugs in survivors of serious ventricular tachyarrhythmias: results of the Antiarrhythmics Versus Implantable Defibrillators (AVID) economic analysis substudy. Circulation. 2002;105:2049-2057.
93 DeMets DL, Hardy R, Friedman LM, Lan KK. Statistical aspects of early termination in the beta-blocker heart attack trial. Control Clin Trials. 1984;5:362-372.
94 Stanton MS, Bell GK. Economic outcomes of implantable cardioverter-defibrillators. Circulation. 2000;101:1067-1074.
95 Domanski MJ, Sakseena S, Epstein AE, et al. Relative effectiveness of the implantable cardioverter-defibrillator and antiarrhythmic drugs in patients with varying degrees of left ventricular dysfunction who have survived malignant ventricular arrhythmias. AVID Investigators. Antiarrhythmics versus Implantable Defibrillators. J Am Coll Cardiol. 1999;34:1090-1095.
96 Assmann SF, Pocock SJ, Enos LE, Kasten LE. Subgroup analysis and other (mis)uses of baseline data in clinical trials [see comments]. Lancet. 2000;355:1064-1069.
97 Exner DV, Reiffel JA, Epstein AE, et al. Beta-blocker use and survival in patients with ventricular fibrillation or symptomatic ventricular tachycardia: the Antiarrhythmics versus Implantable Defibrillators (AVID) trial. J Am Coll Cardiol. 1999;34:325-333.
98 Wyse DG, Friedman PL, Brodsky MA, et al. Life-threatening ventricular arrhythmias due to transient or correctable causes: high risk for death in follow-up. J Am Coll Cardiol. 2001;38:1718-1724.
99 Raitt MH, Renfroe EG, Epstein AE, et al. “Stable” ventricular tachycardia is not a benign rhythm: insights from the Antiarrhythmics versus Implantable Defibrillators (AVID) Registry. Circulation. 2001;103:244-252.
100 Bokhari F, Newman D, Greene M, et al. Long-term comparison of the implantable cardioverter defibrillator versus amiodarone: eleven-year follow-up of a subset of patients in the Canadian Implantable Defibrillator Study (CIDS). Circulation. 2004;110:112-116.
101 O’Brien BJ, Connolly SJ, Goeree R, et al. Cost-effectiveness of the implantable cardioverter-defibrillator : results from the Canadian Implantable Defibrillator Study (CIDS). Circulation. 2001;103:1416-1421.
102 Sheldon R, O’Brien BJ, Blackhouse G, et al. Effect of clinical risk stratification on cost-effectiveness of the implantable cardioverter-defibrillator: the Canadian Implantable Defibrillator Study. Circulation. 2001;104:1622-1626.
103 Sheldon R, Connolly S, Krahn A, et al. Identification of patients most likely to benefit from implantable cardioverter-defibrillator therapy: the Canadian Implantable Defibrillator Study. Circulation. 2000;101:1660-1664.
104 Exner DV, Sheldon RS, Pinski SL, et al. Do baseline characteristics accurately discriminate between patients likely versus unlikely to benefit from implantable defibrillator therapy? Evaluation of the Canadian Implantable Defibrillator Study Implantable Cardioverter Defibrillatory Efficacy Score in the Antiarrhythmics versus Implantable Defibrillators trial. Am Heart J. 2001;141:99-104.
105 Exner DV. Quality of life in patients with life-threatening arrhythmias: does choice of therapy make a difference? Am Heart J. 2002;144:208-211.
106 Mushlin AI, Hall WJ, Zwanziger J, et al. The cost-effectiveness of automatic implantable cardiac defibrillators: results from MADIT. Multicenter Automatic Defibrillator Implantation Trial. Circulation. 1998;97:2129-2135.
107 Andresen D, Steinbeck G, Bruggemann T, et al. Can the MADIT results be applied to myocardial infarction patients at hospital discharge? J Am Coll Cardiol. 1998;31:308A.
108 Buxton AE, Lee KL, DiCarlo L, et al. Electrophysiologic testing to identify patients with coronary artery disease who are at risk for sudden death. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 2000;342:1937-1945.
109 Buxton AE, Lee KL, Hafley GE, et al. Limitations of ejection fraction for prediction of sudden death risk in patients with coronary artery disease: lessons from the MUSTT study. J Am Coll Cardiol. 2007;50:1150-1157.
110 Zwanziger J, Hall WJ, Dick AW, et al. The cost effectiveness of implantable cardioverter-defibrillators: results from the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II. J Am Coll Cardiol. 2006;47:2310-2318.
111 Buxton AE, Sweeney MO, Wathen MS, et al. QRS duration does not predict occurrence of ventricular tachyarrhythmias in patients with implanted cardioverter-defibrillators. J Am Coll Cardiol. 2005;46:310-316.
112 Bloomfield DM, Steinman RC, Namerow PB, et al. Microvolt T-wave alternans distinguishes between patients likely and patients not likely to benefit from implanted cardiac defibrillator therapy: a solution to the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II conundrum. Circulation. 2004;110:1885-1889.
113 Moss AJ. Correcting misconceptions. Ann Noninvas Electrocardiol. 2003;8:177-178.
114 Goldenberg I, Vyas AK, Hall WJ, et al. Risk stratification for primary implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol. 2008;51:288-296.
115 Cygankiewicz I, Gillespie J, Zareba W, et al. Predictors of long-term mortality in Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II) patients with implantable cardioverter-defibrillators. Heart Rhythm. 2009;6:468-473.
116 Haigney MC, Zareba W, Gentlesk PJ, et al. QT interval variability and spontaneous ventricular tachycardia or fibrillation in the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II patients. J Am Coll Cardiol. 2004;44:1481-1487.
117 Couderc JP, Zareba W, McNitt S, et al. Repolarization variability in the risk stratification of MADIT-II patients. Europace. 2007.
118 Wilber DJ, Zareba W, Hall WJ, et al. Time dependence of mortality risk and defibrillator benefit after myocardial infarction. Circulation. 2004;109:1082-1084.
119 Goldenberg I, Moss AJ, Hall WJ, et al. Causes and consequences of heart failure after prophylactic implantation of a defibrillator in the Multicenter Automatic Defibrillator Implantation Trial II. Circulation. 2006;113:2810-2817.
120 Strickberger SA, Hummel JD, Bartlett TG, et al. Amiodarone versus Implantable Cardioverter-Defibrillator Trial: randomized trial in patients with nonischemic dilated cardiomyopathy and asymptomatic nonsustained ventricular tachycardia. AMIOVIRT. J Am Coll Cardiol. 2003;41:1707-1712.
121 Bansch D, Antz M, Boczor S, et al. Primary prevention of sudden cardiac death in idiopathic dilated cardiomyopathy: the Cardiomyopathy Trial (CAT). Circulation. 2002;105:1453-1458.
122 Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140-2150.
123 Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539-1549.
124 Packer DL, Prutkin JM, Hellkamp AS, et al. Impact of implantable cardioverter-defibrillator, amiodarone, and placebo on the mode of death in stable patients with heart failure: analysis from the Sudden Cardiac Death in Heart Failure Trial. Circulation. 2009;120:2170-2176.
125 Mark DB, Nelson CL, Anstrom KJ, et al. Cost-effectiveness of defibrillator therapy or amiodarone in chronic stable heart failure: results from the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT). Circulation. 2006;114:135-142.
126 Gold MR, Ip JH, Costantini O, et al. Role of microvolt T-wave alternans in assessment of arrhythmia vulnerability among patients with heart failure and systolic dysfunction: primary results from the T-Wave Alternans Sudden Cardiac Death in Heart Failure trial substudy. Circulation. 2008;118:2022-2028.
127 Chow T, Kereiakes DJ, Bartone C, et al. Prognostic utility of microvolt T-wave alternans in risk stratification of patients with ischemic cardiomyopathy. J Am Coll Cardiol. 2006;47:1820-1827.
128 Blatt JA, Poole JE, Johnson GW, et al. No benefit from defibrillation threshold testing in the SCD-HeFT (Sudden Cardiac Death in Heart Failure Trial). J Am Coll Cardiol. 2008;52:551-556.
129 Healey JS, Birnie DH, Lee DS, et al. Defibrillation Testing at the Time of ICD Insertion: An Analysis from the Ontario ICD Registry. J Cardiovasc Electrophysiol. 2010;21:1344-1348.
130 Kadish A, Dyer A, Daubert JP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151-2158.
131 Ellenbogen KA, Levine JH, Berger RD, et al. Are implantable cardioverter defibrillator shocks a surrogate for sudden cardiac death in patients with nonischemic cardiomyopathy? Circulation. 2006;113:776-782.
132 Kadish A, Schaechter A, Subacius H, et al. Patients with recently diagnosed nonischemic cardiomyopathy benefit from implantable cardioverter-defibrillators. J Am Coll Cardiol. 2006;47:2477-2482.
133 Nanthakumar K, Epstein AE, Kay GN, et al. Prophylactic implantable cardioverter-defibrillator therapy in patients with left ventricular systolic dysfunction: a pooled analysis of 10 primary prevention trials. J Am Coll Cardiol. 2004;44:2166-2172.
134 Bigger JTJr, Whang W, Rottman JN, et al. Mechanisms of death in the CABG Patch trial: a randomized trial of implantable cardiac defibrillator prophylaxis in patients at high risk of death after coronary artery bypass graft surgery. Circulation. 1999;99:1416-1421.
135 Bailey JJ, Berson AS, Handelsman H, Hodges M. Utility of current risk stratification tests for predicting major arrhythmic events after myocardial infarction. J Am Coll Cardiol. 2001;38:1902-1911.
136 Bauer A, Guzik P, Barthel P, et al. Reduced prognostic power of ventricular late potentials in post-infarction patients of the reperfusion era. Eur Heart J. 2005;26:755-761.
137 Al-Khatib SM, Hellkamp AS, Lee KL, et al. Implantable cardioverter defibrillator therapy in patients with prior coronary revascularization in the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT). J Cardiovasc Electrophysiol. 2008;19:1059-1065.
138 Barsheshet A, Goldenberg I, Narins CR, et al. Time dependence of life-threatening ventricular tachyarrhythmias after coronary revascularization in MADIT-CRT. Heart Rhythm. 2010;7:1421-1427.
139 Dorian P, Connolly S, Hohnloser SH. Why don’t ICDs decrease all-cause mortality after MI? Insights from the DINAMIT study. Circulation. 2004;110:502.
140 Savoye C, Equine O, Tricot O, et al. Left ventricular remodeling after anterior wall acute myocardial infarction in modern clinical practice (from the REmodelage VEntriculaire [REVE] study group). Am J Cardiol. 2006;98:1144-1149.
141 Huikuri HV, Exner DV, Kavanagh KM, et al. Attenuated recovery of heart rate turbulence early after myocardial infarction identifies patients at high risk for fatal or near-fatal arrhythmic events. Heart Rhythm. 2010;7:229-235.
142 Nolan J, Batin PD, Andrews R, et al. Prospective study of heart rate variability and mortality in chronic heart failure: results of the United Kingdom Heart Failure Evaluation and Assessment of Risk Trial (UK-Heart). Circulation. 1998;98:1510-1516.
143 Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51:e1-e62.
144 Fogoros RN. An AVID dissent (editorial) [see comments]. Pacing Clin Electrophysiol. 1994;17:1707-1711.
145 Epstein AE. AVID necessity. Pacing Clin Electrophysiol. 1993;16:1773-1775.
146 Maron BJ, Shen WK, Link MS, et al. Efficacy of implantable cardioverter-defibrillators for the prevention of sudden death in patients with hypertrophic cardiomyopathy [see comments]. N Engl J Med. 2000;342:365-373.
147 Piccini JP, Dalal D, Roguin A, et al. Predictors of appropriate implantable defibrillator therapies in patients with arrhythmogenic right ventricular dysplasia. Heart Rhythm. 2005;2:1188-1194.
148 Maron BJ, Spirito P, Shen WK, et al. Implantable cardioverter-defibrillators and prevention of sudden cardiac death in hypertrophic cardiomyopathy. JAMA. 2007;298:405-412.
149 Costantini O, Hohnloser SH, Kirk MM, et al. The ABCD (Alternans Before Cardioverter Defibrillator) Trial: strategies using T-wave alternans to improve efficiency of sudden cardiac death prevention. J Am Coll Cardiol. 2009;53:471-479.
150 Chow T, Kereiakes DJ, Bartone C, et al. Microvolt T-wave alternans identifies patients with ischemic cardiomyopathy who benefit from implantable cardioverter-defibrillator therapy. J Am Coll Cardiol. 2007;49:50-58.
151 Klein RC, Raitt MH, Wilkoff BL, et al. Analysis of implantable cardioverter defibrillator therapy in the Antiarrhythmics versus Implantable Defibrillators (AVID) Trial. J Cardiovasc Electrophysiol. 2003;14:940-948.
152 Wathen M. Implantable cardioverter defibrillator shock reduction using new antitachycardia pacing therapies. Am Heart J. 2007;153:44-52.
153 Grimm W, Flores BT, Marchlinski FE. Shock occurrence and survival in 241 patients with implantable cardioverter-defibrillator therapy. Circulation. 1993;87:1880-1888.
154 Grimm W, Hoffmann JJ, Muller HH, Maisch B. Implantable defibrillator event rates in patients with idiopathic dilated cardiomyopathy, nonsustained ventricular tachycardia on Holter and a left ventricular ejection fraction below 30%. J Am Coll Cardiol. 2002;39:780-787.
155 Garratt CJ, Elliott P, Behr E, et al. Heart Rhythm UK position statement on clinical indications for implantable cardioverter defibrillators in adult patients with familial sudden cardiac death syndromes. Europace. 2010;12:1156-1175.
156 Maron BJ, Olivotto I, Spirito P, et al. Epidemiology of hypertrophic cardiomyopathy-related death: revisited in a large non-referral-based patient population. Circulation. 2000;102:858-864.
157 Maron BJ, Casey SA, Hauser RG, Aeppli DM. Clinical course of hypertrophic cardiomyopathy with survival to advanced age. J Am Coll Cardiol. 2003;42:882-888.
158 Elliott P, Gimeno J, Mahon N, et al. Relation between severity of left-ventricular hypertrophy and prognosis in patients with hypertrophic cardiomyopathy. Lancet. 2001;357:420-424.
159 Maron BJ, Thompson PD, Ackerman MJ, et al. Recommendations and considerations related to preparticipation screening for cardiovascular abnormalities in competitive athletes: 2007 update: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation. 2007;115:1643-1655.
160 O’Hanlon R, Grasso A, Roughton M, et al. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:867-874.
161 Bruder O, Wagner A, Jensen CJ, et al. Myocardial scar visualized by cardiovascular magnetic resonance imaging predicts major adverse events in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:875-887.
162 Gosling OE, Bellenger N, Spurrell P. Risk assessment with cardiac magnetic resonance imaging in hypertrophic cardiomyopathy. Heart. 2009;95:1843.
163 Pierre-Louis B, Prasad A, Frishman WH. Cardiac manifestations of sarcoidosis and therapeutic options. Cardiol Rev. 2009;17:153-158.
164 Syed J, Myers R. Sarcoid heart disease. Can J Cardiol. 2004;20:89-93.
165 Kim JS, Judson MA, Donnino R, et al. Cardiac sarcoidosis. Am Heart J. 2009;157:9-21.
166 Aizer A, Stern EH, Gomes JA, et al. Usefulness of programmed ventricular stimulation in predicting future arrhythmic events in patients with cardiac sarcoidosis. Am J Cardiol. 2005;96:276-282.
167 Patel MR, Cawley PJ, Heitner JF, et al. Detection of myocardial damage in patients with sarcoidosis. Circulation. 2009;120:1969-1977.
168 Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010;121:1533-1541.
169 Dalal D, Nasir K, Bomma C, et al. Arrhythmogenic right ventricular dysplasia: a United States experience. Circulation. 2005;112:3823-3832.
170 Corrado D, Leoni L, Link MS, et al. Implantable cardioverter-defibrillator therapy for prevention of sudden death in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2003;108:3084-3091.
170a Olshansky B, Day JD, Moore S, et al. Is dual-chamber programming inferior to single chamber programming in an implantable cardioverter-defibrillator? Results of the INTRINSIC RV (Inhibition of Unnecessary RV Pacing with AVSH in ICDs) study. Circulation. 2007;115:9-16.
171 Wilkoff BL, Kudenchuk PJ, Buxton AE, et al. The DAVID (Dual Chamber and VVI Implantable Defibrillator) II trial. J Am Coll Cardiol. 2009;53:872-880.
172 Wilkoff BL, Cook JR, Epstein AE, et al. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA. 2002;288:3115-3123.
173 Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart failure events. N Engl J Med. 2009;361:1329-1338.
174 Solomon SD, Foster E, Bourgoun M, et al. Effect of cardiac resynchronization therapy on reverse remodeling and relation to outcome: Multicenter Automatic Defibrillator Implantation Trial: cardiac resynchronization therapy. Circulation. 2010;122:985-992.
175 Dickstein K, Vardas PE, Auricchio A, et al. 2010 focused update of ESC Guidelines on Device Therapy in Heart Failure: an update of the 2008 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure and the 2007 ESC Guidelines for Cardiac Resynchronization Therapy. Developed with the special contribution of the Heart Failure Association and the European Heart Rhythm Association. Eur Heart J. 2010;31:2677-2687.
176 Tang AS, Wells GA, Talajic M, et al. Resynchronization-Defibrillation for Ambulatory Heart Failure Trial Investigators; Cardiac resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. 2010;363:2385-2395.
177 Moss AJ. Should everyone with an ejection fraction less than or equal to 30% receive an implantable cardioverter-defibrillator? Everyone with an ejection fraction ≤30% should receive an implantable cardioverter-defibrillator. Circulation. 2005;111:2537-2549. discussion 2549
178 Buxton AE. Should everyone with an ejection fraction less than or equal to 30% receive an implantable cardioverter-defibrillator? Not everyone with an ejection fraction ≤30% should receive an implantable cardioverter-defibrillator. Circulation. 2005;111:2537-2549.
179 Goldenberg I, Moss AJ. Implantable cardioverter defibrillator efficacy and chronic kidney disease: competing risks of arrhythmic and nonarrhythmic mortality. J Cardiovasc Electrophysiol. 2008;19:1281-1283.
180 Theuns DA, Klootwijk AP, Simoons ML, Jordaens LJ. Clinical variables predicting inappropriate use of implantable cardioverter-defibrillator in patients with coronary heart disease or nonischemic dilated cardiomyopathy. Am J Cardiol. 2005;95:271-274.
181 Capoferri M, Schwick N, Tanner H, et al. Incidence of arrhythmic events in patients with implantable cardioverter-defibrillator for primary and secondary prevention of sudden cardiac death. Swiss Med Wkly. 2004;134:154-158.
182 Wilkoff BL, Hess M, Young J, Abraham WT. Differences in tachyarrhythmia detection and implantable cardioverter defibrillator therapy by primary or secondary prevention indication in cardiac resynchronization therapy patients. J Cardiovasc Electrophysiol. 2004;15:1002-1009.
183 Theuns DA, Thornton AS, Klootwijk AP, et al. Outcome in patients with an ICD incorporating cardiac resynchronisation therapy: differences between primary and secondary prophylaxis. Eur J Heart Fail. 2005;7:1027-1032.
184 Backenkohler U, Erdogan A, Steen-Mueller MK, et al. Long-term incidence of malignant ventricular arrhythmia and shock therapy in patients with primary defibrillator implantation does not differ from event rates in patients treated for survived cardiac arrest. J Cardiovasc Electrophysiol. 2005;16:478-482.
185 Kautzner J, Riedlbauchova L, Cihak R, et al. Technical aspects of implantation of LV lead for cardiac resynchronization therapy in chronic heart failure. Pacing Clin Electrophysiol. 2004;27:783-790.
186 Curtis JP, Luebbert JJ, Wang Y, et al. Association of physician certification and outcomes among patients receiving an implantable cardioverter-defibrillator. JAMA. 2009;301:1661-1670.
187 Freeman JV, Wang Y, Curtis JP, et al. The relation between hospital procedure volume and complications of cardioverter-defibrillator implantation from the Implantable Cardioverter-Defibrillator Registry. J Am Coll Cardiol. 2010;56:1133-1139.
188 Aggarwal A, Wang Y, Rumsfeld JS, et al. Clinical characteristics and in-hospital outcome of patients with end-stage renal disease on dialysis referred for implantable cardioverter-defibrillator implantation. Heart Rhythm. 2009;6:1565-1571.
189 Lee DS, Krahn AD, Healey JS, et al. Evaluation of early complications related to de novo cardioverter-defibrillator implantation: insights from the Ontario ICD database. J Am Coll Cardiol. 2010;55:774-782.
190 Daugherty SL, Peterson PN, Wang Y, et al. Use of implantable cardioverter-defibrillators for primary prevention in the community: do women and men equally meet trial enrollment criteria? Am Heart J. 2009;158:224-229.
191 Curtis AB, Yancy CW, Albert NM, et al. Cardiac resynchronization therapy utilization for heart failure: findings from IMPROVE HF. Am Heart J. 2009;158:956-964.